Abstract
Background
Preoperative lymphocyte count (pre-LC) predicts the relapse and survival in patients with esophageal squamous cell carcinoma (ESCC), the clinical application of postoperative lymphocyte count (post-LC) change (LCc) remains unclear.
Methods
A retrospective analysis of patients with newly diagnosed ESCC who received curative resection from 2008 to 2015 was conducted. Complete blood counts from preoperative within seven days to postoperative within seven days were analyzed. LCc was defined as LCc increased (post-LC higher than pre-LC) and LCc decreased (post-LC lower than pre-LC). LCc was evaluated for an association with disease-free survival (DFS).
Results
A total of 677 patients who reach the standard were enrolled into the study. There were 579 (85.5%) male and 98 (14.5%) female patients with ESCC. The median age was 61 years (range, 39–84 years). In univariate analysis, LCc significantly correlated to DFS (P=0.006, HR =0.593, 95% CI: 0.409–0.862). In multivariate analysis (COX model), LCc was an independent prognostic factor for DFS (P=0.011, HR =0.617, 95% CI: 0.424–0.897).
Conclusions
The dynamic change of LC after surgery may serve as a simple and new prognostic factor in patients with newly diagnosed ESCC.
Keywords: Postoperative lymphocyte count change, esophageal squamous cell carcinoma (ESCC), prognosis, disease-free survival (DFS)
Introduction
Esophageal cancer (EC) is the seventh most common cancer and the sixth cause of cancer-related death worldwide. EC is the fifth most common cancer and the fourth most frequent cause of cancer-related death in China (1). Esophageal squamous cell carcinoma (ESCC) is the main pathological type with 90% of all EC cases in China (2,3). Curative resection is one of the most effective therapies for ESCC. However, the clinical outcome in patients with ESCC has remained far from satisfactory (4). Therefore, it is critical to define novel and reliable prognostic factors that may help to classify patients who at high risk of recurrence. Moreover, this group of patients in ESCC might benefit from postoperative adjuvant therapies including chemotherapy and radiotherapy against recurrence.
Plentiful studies have reported that cancer was infiltrated by white blood cells, especially lymphocytes. These findings have suggested that lymphocytes identify and destroy malignant cells (5,6). Retrospective studies have demonstrated that patients with tumor tissue infiltrated by inflammatory cells, particularly lymphocytes, have better clinical outcome than patients with immune cells infiltration (7-10). Lower pre-treatment lymphocyte count predicts worse survival in various cancers including breast cancer, colorectal cancer, and lung cancer (11-14). Lymphopenia prior to initiating treatment has been associated with poor prognosis in patients with ESCC (15). These studies focused on primarily on preoperative lymphocyte count (pre-LC), while the dynamic change of lymphocyte on survival after surgery has not been addressed. This research was undertaken to evaluate the relationship between dynamic change of lymphocyte count and survival in patients with ESCC treated with surgery.
Methods
Study population
We enrolled 677 patients with newly diagnosed ESCC treated with surgery at Zhejiang Cancer Hospital, Hangzhou, China between 2008 and 2015. There were 23 cases with sweet, 476 cases with Ivor-lewis, 108 cases with McKeown, and 70 cases with hybrid esophagectomies. All tumor specimens were histologically proven to be ESCC after curative resection. The blood routine was checked within 7 days before operation, which included lymphocyte count. The postoperative lymphocyte count (post-LC) was obtained within 7 days after surgery. All patients had complete laboratory and clinical data. Data on lymphocyte counts were checked in clinical laboratory. And per L was used as the unit of measurement. The latest lymphocyte counts were selected before surgery. To reduce the influence of postoperative stress response, we chose the lymphocyte counts farthest during 7 days after surgery. To ensure that the lymphocyte count represent the normal baseline value, we excluded patients who had any coexistent hematological illness or active infection before the surgery. In addition, patients with neoadjuvant chemotherapy were excluded, because chemotherapy may influence the lymphocyte count. Approval for the present study was obtained from the Ethics Committee of Zhejiang Cancer Hospital (Hangzhou, China), and informed consent was obtained.
Statistical analysis
Statistical analysis was examined using SPSS, version 19.0 (SPSS, Chicago, IL, USA). The Pre-LC and Post-LC were analyzed as continuous variables that do not meet a normal distribution. The continuous data were presented as median and interquartile range. All clinical features were counted as categorical variables, which were expressed as numbers and percentage. Categorical variables were compared using the chi-square test. Disease-free survival (DFS) was calculated between the date of surgery and the date of recurrence. Disease free survival curve was plotted using the Kaplan-Meier method and compared by the log-rank test. The survival curve was analyzed using GraphPad Prism 7 software. Univariate and multivariate COX regression analyses were utilized to investigate independent prognostic factors. P<0.05 was regarded as statistical significance.
Results
Patient characteristics
There were 579 (85.5%) male and 98 (14.5%) female patients with ESCC. There were 366 (54.1%) old patients (>60 years) and 311 (45.9%) young patients (≤60 years). 295 (43.6%) patients without lymph node metastasis, 213 (31.5%) patients with one lymph node metastasis, 117 (17.3%) patients with two lymph node metastasis, and 52 (7.7%) patients with three lymph node metastasis. There were 458 (67.7%) patients who only received surgery, 155 (22.9%) patients with postoperative chemotherapy, and 64 (9.5%) patients with chemotherapy and radiotherapy after surgery. The median value of pre-LC was 1.7 (range from 1.3 to 2.0). Seven days after surgery, the median value of post-LC was 1.1 (range from 0.9 to 1.5). LCc was decreased in 582 (86.0%) patients and increased in 95 (14.0%) patients. Details of features are listed in Table 1.
Table 1. Demographic and clinical data of 677 ESCC patients according to LC change.
| Characteristics | Total (N=677), % | LC change | P | |
|---|---|---|---|---|
| Increased (N=95), % | Decreased (N=582), % | |||
| Sex | 0.238 | |||
| Male | 579 (85.5) | 85 (89.5) | 494 (84.9) | |
| Female | 98 (14.5) | 10 (10.5) | 88 (15.1) | |
| Age (years) | 61 [56–67] | 62 [57–69] | 61 [55–66] | 0.029 |
| Pathology grade | 0.338 | |||
| Well differentiated | 49 (7.4) | 4 (4.3) | 45 (7.9) | |
| Middle differentiated | 447 (67.3) | 61 (64.9) | 386 (67.7) | |
| Poorly differentiated | 166 (25.0) | 29 (30.9) | 137 (24.0) | |
| Undifferentiated | 2 (0.3) | 0 (0) | 2 (0.4) | |
| Depth of tumor | 0.926 | |||
| T1a–1b | 64 (9.5) | 9 (9.5) | 55 (9.5) | |
| T2 | 131 (19.4) | 17 (17.9) | 114 (19.6) | |
| T3 | 482 (71.2) | 69 (72.6) | 413 (71.0) | |
| Lymph node metastasis | 0.907 | |||
| N0 | 295 (43.6) | 44 (46.3) | 251 (43.1) | |
| N1 | 213 (31.5) | 27 (28.4) | 186 (32.0) | |
| N2 | 117 (17.3) | 17 (17.9) | 100 (17.2) | |
| N3 (≥3) | 52 (7.7) | 7 (7.4) | 45 (7.7) | |
| Pathological stage | 0.928 | |||
| 1a–1b | 116 (17.1) | 17 (17.9) | 99 (17.0) | |
| 2a–2b | 228 (33.7) | 33 (34.7) | 195 (33.5) | |
| 3a–3c | 333 (49.2) | 45 (47.4) | 288 (49.5) | |
| Vessel invasive | 0.664 | |||
| Yes | 208 (30.7) | 31 (32.6) | 177 (30.4) | |
| No | 469 (69.3) | 64 (67.4) | 405 (69.6) | |
| Nerve infiltration | 0.708 | |||
| Yes | 252 (37.2) | 37 (38.9) | 215 (36.9) | |
| No | 425 (62.8) | 58 (61.1) | 367 (63.1) | |
| Treatment regimen | 0.998 | |||
| S | 458 (67.7) | 64 (67.4) | 394 (67.7) | |
| S plus postoperative C | 155 (22.9) | 22 (23.2) | 133 (22.9) | |
| S plus postoperative CRT | 64 (9.5) | 9 (9.5) | 55 (9.5) | |
| Hospital time (days) | 11 [10–14] | 12 [10–18] | 11 (10–13.3) | 0.020 |
| Pre-LC (109/L), median | 1.7 (1.3–2.0) | 1.3 (1.0–1.6) | 1.7 (1.4–2.1) | <0.001 |
| Post-LC (109/L), median | 1.1 (0.9–1.5) | 1.6 (1.3–2.0) | 1.1 (0.9–1.4) | <0.001 |
The data were shown as n (%) or median (interquartile range). P<0.05 marked in italic. ESCC, esophageal squamous cell carcinoma.
Relationship between clinical features and dynamic change of lymphocyte count
The clinical features of patients with two LCc groups are summarized in Table 1. None of the popularly used clinical features (gender, pathology grade, depth of tumor, lymph node metastasis, pathological stage, vessel invasive, nerve infiltration, and treatment regimen) were significantly disparate between LCc increased group and LCc decreased group. There were significant difference in age and hospital times between LCc increased group and LCc decreased group. The median pre-LC was higher (P<0.001) and the median Post-LC was lower (P<0.001) in LCc decreased group than in LCc increased group. Patients with LCc decreased tend to have shorter hospital time, but do not reach statistically different (P=0.055).
Risk factors for prognosis of ESCC undergoing curative resection
Univariate analysis revealed that LCc (decreased vs. increased) (P=0.006), lymph node metastasis (P<0.05), pathological stage (P<0.05), nerve infiltration (P=0.001), and treatment regimen (P<0.05) predict worse DFS. On multivariate analysis, five parameters including LCc (decreased vs. increased) (P=0.011), lymph node metastasis (P<0.05), nerve infiltration (P=0.012), and surgery (P=0.007) could be independent prognostic factors of worse DFS (Table 2). DFS of patients with LCc increased group were significantly worse than patients with LCc decreased group (P=0.009) (Figure 1). To correct for the occurrence of complications including infections, leaks, 578 cases without complications were analyzed. DFS of patients were significantly worse in LCc increased group compared with LCc decreased group (P=0.002) (Figure 2).
Table 2. Disease-free survival analyses according to LC change in 677 patients with ESCC.
| Variables | Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | ||
| LCc (decreased vs. increased) | 0.593 | 0.409–0.862 | 0.006 | 0.617 | 0.424–0.897 | 0.011 | |
| Sex (male vs. female) | 1.201 | 0.782–1.844 | 0.404 | ||||
| Age (>60 vs.≤60) | 0.943 | 0.707–1.258 | 0.690 | ||||
| Pathology grade | |||||||
| Well differentiated | 0.147 | 0.018–1.202 | 0.074 | ||||
| Middle differentiated | 0.274 | 0.038–1.968 | 0.198 | ||||
| Poorly differentiated | 0.427 | 0.059–3.090 | 0.399 | ||||
| Undifferentiated | 1.000 | ||||||
| Depth of tumor | |||||||
| T1a–1b | 0.899 | 0.535–1.509 | 0.686 | ||||
| T2 | 0.726 | 0.492–1.071 | 0.107 | ||||
| T3 | 1.000 | ||||||
| Lymph node metastasis | |||||||
| N0 | 0.222 | 0.135–0.364 | <0.001 | 0.316 | 0.135–0.739 | 0.008 | |
| N1 | 0.348 | 0.213–0.568 | <0.001 | 0.387 | 0.234–0.641 | <0.001 | |
| N2 | 0.519 | 0.310–0.870 | 0.013 | 0.526 | 0.313–0.882 | 0.015 | |
| N3 | 1.000 | 1.000 | |||||
| Pathological stage | |||||||
| 1a–1b | 0.507 | 0.327–0.787 | 0.002 | 1.155 | 0.493–2.705 | 0.740 | |
| 2a–2b | 0.483 | 0.343–0.680 | <0.001 | 0.873 | 0.460–1.657 | 0.677 | |
| 3a–3c | 1.000 | 1.000 | |||||
| Vessel invasive (absence vs. presence) | 1.280 | 0.939–1.743 | 0.118 | ||||
| Nerve infiltration (absence vs. presence) | 1.624 | 1.214–2.171 | 0.001 | 1.483 | 1.092–2.014 | 0.012 | |
| Treatment regimen | |||||||
| S | 0.472 | 0.308–0.725 | 0.001 | 0.550 | 0.355–0.852 | 0.007 | |
| S plus postoperative C | 1.123 | 0.718–1.758 | 0.611 | 1.063 | 0.677–1.670 | 0.791 | |
| S plus postoperative CRT | 1.000 | 1.000 | |||||
| Hospital time (days) (>14 vs.≤14) | 1.195 | 0.842–1.696 | 0.319 | – | – | – | |
P<0.05 marked in italic. ESCC, esophageal squamous cell carcinoma.
Figure 1.
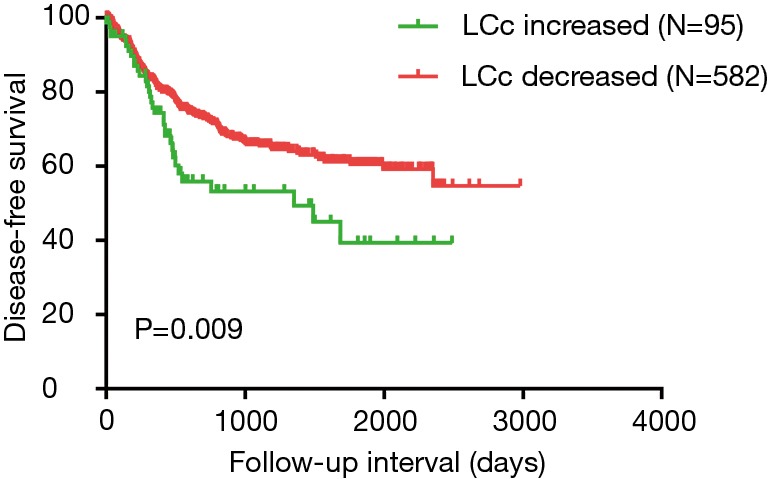
Disease-free survival analysis in all 677 patients with ESCC according the postoperative absolute lymphocyte count change. ESCC, esophageal squamous cell carcinoma.
Figure 2.
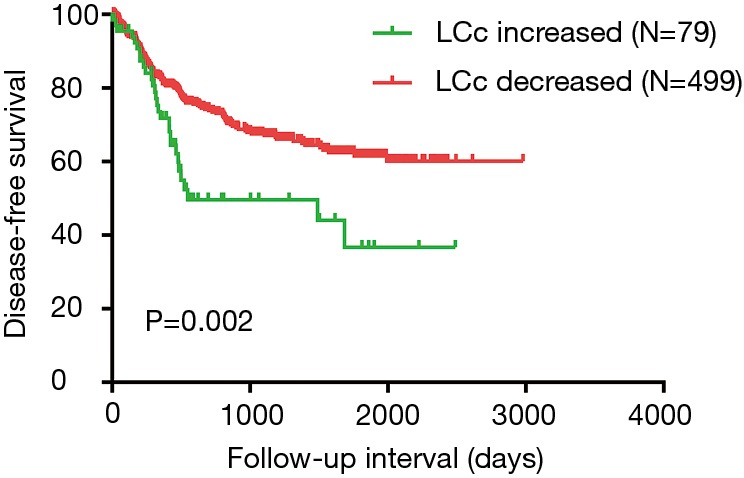
Disease-free survival analysis in 578 cases without complications according the postoperative absolute lymphocyte count change.
Subgroup analysis according to lymph node metastasis
To clarify the subgroups of ESCC affected by dynamic change of lymphocyte count, we classified patients according to pathological stage (1a–1b, n=116; 2a–2b, n=228; 3a–3c, n=333) and lymph node metastasis (lymph node negative, n=295; 1 lymph node, n=213; 2 lymph node, n=117; 3 lymph node, n=52). DFS of lymph node negative patients and 1 lymph node were significantly worse for those with LCc increased (P=0.035 and P=0.004), but DFS did not differ neither 2 lymph node subgroup nor 3 lymph node subgroup (data not shown) (Figure 3). There was no difference within pathological stage subgroup (data not shown).
Figure 3.
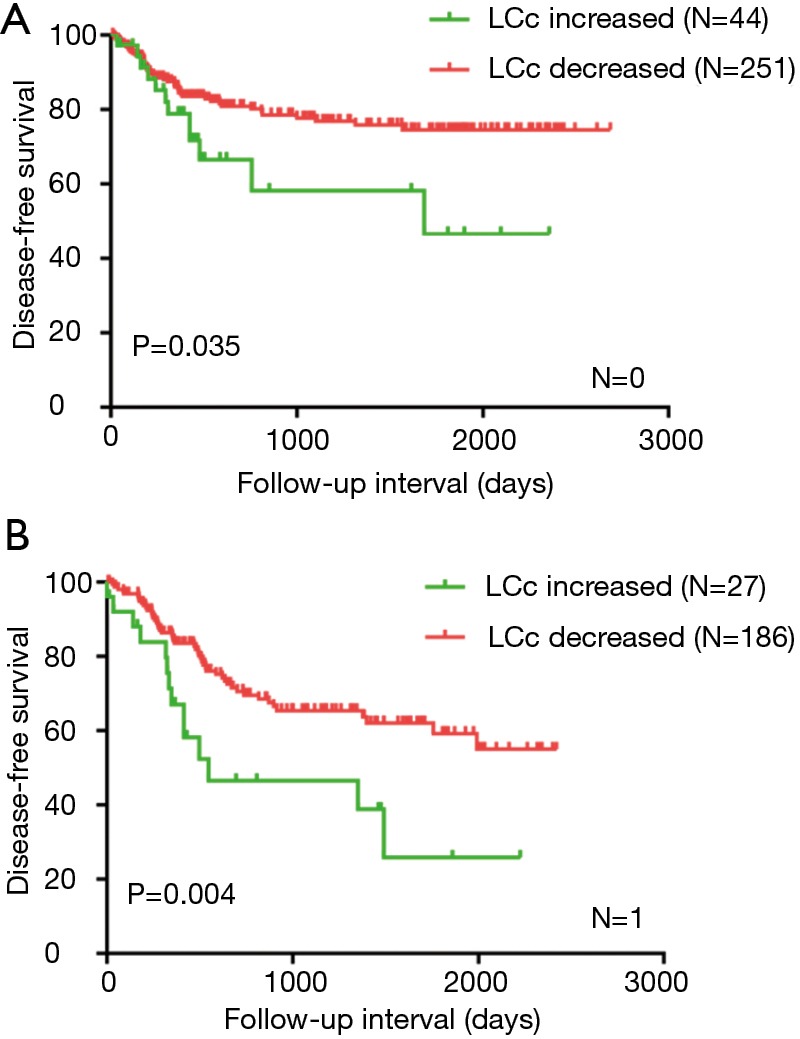
Disease-free survival analysis in ESCC with no lymph node metastasis and 1lymph node metastasis according the postoperative absolute lymphocyte count change (A,B). ESCC, esophageal squamous cell carcinoma.
Discussion
While multiple researches have reported that pre-LC could predict survival in various cancers including ESCC (11-15), the dynamic change of lymphocyte count after surgery has not been studied. Besides, we think there was no common cutoff value exists that was typically obtained by the receiver operating characteristics curve (ROC), and cutoff Finder (16-19). Therefore, the optimal cutoff value of Pre-LC differed in different studies (20-22). It is difficult to evaluate which way is the best to obtain cutoff value. In the present study, we divided the patients into two groups by the dynamic change of lymphocyte count from preoperative to postoperative.
Our study examined the dynamic change of lymphocyte count and the impact of LCc on survival in patients with ESCC undergoing curative resection. To the best our knowledge, we firstly demonstrated that dynamic change of lymphocyte count was associated with survival in patients with ESCC undergoing surgery. However, Zhao reported that patients following palliative care with increased LC had significantly better survival compared to patients with decreased LC (23). This inconsistent result was due to the different stage of patients that focused on the advanced cancer patients, and the various type of cancer that included all kinds of cancers. From subgroup analysis we found that LCc increased predicted worse survival in lymph node negative and 1 lymph node subgroup, but not in other subgroup. This finding suggested that LCc could be prognostic factor particularly in patients with less lymph node metastasis. There was no association between clinical features and LCc in patients with ESCC undergoing curative resection. Because of the enrolled patients were mainly in early and middle stage but not in advanced stage.
Although the mechanisms of the relationship between LCc increased and worse survival remains unclear, the possibilities are as follows: (I) the low pre-LC may be related to a preexisting weak immunity condition that suggested the host with an inadequate immune reaction; (II) the increased post-LC may be influenced by bleeding, and sepsis. Therefore the LCc increased, which means low preoperative lymphocyte and high post-LC, predicted shorter survival time. There were some biological and molecular studies showed that lymphocyte act as anti-tumor by inhibiting tumor cell proliferation and migration (24). Moreover, cytotoxic lymphocytes, particularly cytotoxic T cells, play a crucial part in clearing residual tumor cell and being used in immunotherapy (25,26). The lymphocyte count is commonly examined as a component of a complete blood cell count with differential. It is easy, reliable and inexpensive to check the lymphocyte count. The clinical application of this index would assist the doctors to classify the patients who are possible to recur after surgery and make follow up frequently.
There were several limitations in the present study: first, a main drawback is that a single center and retrospective design study. More prospective and multicenter studies are needed to prove the universal association between LCc and clinical outcome; Second, the patients did not divided into the training set and the validation set, which could strengthen the suggestion of LCc increased to be a prognostic factor; Third, the adjuvant therapies including chemotherapy and radiotherapy, which may potentially influence the lymphocyte count, were not taken into the subgroup because the low patients; Third, we could not have access to the lymphocyte counts on specific days before and after surgery. The window of post-LC is very wide, as the postoperative stress response occurs during the first days, and complications, such as anastomotic leaks often during days 5–7. In our future study, we hope to check the lymphocyte counts in the routine follow-up on specific days such as during 14 days. Fourth, the information about preoperative weight-loss and malnourishment, which are known to affect patient’s immunity and peripheral blood cell counts, were not available. Despite these shortcomings, this is the first study to evaluate the association between dynamic change of lymphocyte count and prognosis in patients who received curative resection.
Conclusions
In conclusion, the present study is the first to demonstrate that the dynamic change of lymphocyte count after surgery could be the disease free survival predictor in patients with ESCC. LCc could assistant the doctors to predict the easy relapse by providing crucial information. Future studies are warranted to confirm our findings.
Acknowledgments
Funding: This study was funded by National Natural Science Foundation of China (contract/grant number: 81502603), General research program of Health Department of Zhejiang Province (contract/grant number: 2016KYB048) and Zhejiang Provincial Medical Youth Talent (2019RC026).
Ethical Statement: All procedures in the present study were performed in accordance with the ethical standards of the World Medical Association Declaration of Helsinki. The study approval was obtained from ethics committee at Zhejiang Cancer Hospital and informed consents were informed from all participants.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 2.Arnold M, Laversanne M, Brown LM, et al. Predicting the Future Burden of Esophageal Cancer by Histological Subtype: International Trends in Incidence up to 2030. Am J Gastroenterol 2017;112:1247-55. 10.1038/ajg.2017.155 [DOI] [PubMed] [Google Scholar]
- 3.Tran GD, Sun XD, Abnet CC, et al. Prospective study of risk factors for esophageal and gastric cancers in the Linxian general population trial cohort in China. Int J Cancer 2005;113:456-63. 10.1002/ijc.20616 [DOI] [PubMed] [Google Scholar]
- 4.Gertler R, Stein HJ, Langer R, et al. Long-term outcome of 2920 patients with cancers of the esophagus and esophagogastric junction: evaluation of the New Union Internationale Contre le Cancer/American Joint Cancer Committee staging system. Ann Surg 2011;253:689-98. 10.1097/SLA.0b013e31821111b5 [DOI] [PubMed] [Google Scholar]
- 5.Smyth MJ, Dunn GP, Schreiber RD. Cancer immunosurveillance and immunoediting: the roles of immunity in suppressing tumor development and shaping tumor immunogenicity. Adv Immunol 2006;90:1-50. 10.1016/S0065-2776(06)90001-7 [DOI] [PubMed] [Google Scholar]
- 6.Shankaran V, Ikeda H, Bruce AT, et al. IFNgamma and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature 2001;410:1107-11. 10.1038/35074122 [DOI] [PubMed] [Google Scholar]
- 7.Mahmoud SM, Paish EC, Powe DG, et al. Tumor-infiltrating CD8+ lymphocytes predict clinical outcome in breast cancer. J Clin Oncol 2011;29:1949-55. 10.1200/JCO.2010.30.5037 [DOI] [PubMed] [Google Scholar]
- 8.Dahlin AM, Henriksson ML, Van Guelpen B, et al. Colorectal cancer prognosis depends on T-cell infiltration and molecular characteristics of the tumor. Mod Pathol 2011;24:671-82. 10.1038/modpathol.2010.234 [DOI] [PubMed] [Google Scholar]
- 9.Hiraoka K, Miyamoto M, Cho Y, et al. Concurrent infiltration by CD8+ T cells and CD4+ T cells is a favourable prognostic factor in non-small-cell lung carcinoma. Br J Cancer 2006;94:275-80. 10.1038/sj.bjc.6602934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lohr J, Ratliff T, Huppertz A, et al. Effector T-cell infiltration positively impacts survival of glioblastoma patients and is impaired by tumor-derived TGF-β. Clin Cancer Res 2011;17:4296-308. 10.1158/1078-0432.CCR-10-2557 [DOI] [PubMed] [Google Scholar]
- 11.Ownby HE, Roi LD, Isenberg RR, et al. Peripheral lymphocyte and eosinophil counts as indicators of prognosis in primary breast cancer. Cancer 1983;52:126-30. [DOI] [PubMed] [Google Scholar]
- 12.Ray-Coquard I, Cropet C, Van Glabbeke M, et al. Lymphopenia as a prognostic factor for overall survival in advanced carcinomas, sarcomas, and lymphomas. Cancer Res 2009;69:5383-91. 10.1158/0008-5472.CAN-08-3845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xie QK, He WZ, Hu WM, et al. Tumor-infiltrating lymphocyte as a prognostic biomarker in stage IV colorectal cancer should take into account the metastatic status and operation modality. Cancer Manag Res 2018;10:1365-75. 10.2147/CMAR.S162147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang J, Huang SH, Li H, et al. Preoperative lymphocyte count is a favorable prognostic factor of disease-free survival in non-small-cell lung cancer. Med Oncol 2013;30:352. 10.1007/s12032-012-0352-3 [DOI] [PubMed] [Google Scholar]
- 15.Feng JF, Liu JS, Huang Y. Lymphopenia predicts poor prognosis in patients with esophageal squamous cell carcinoma. Medicine (Baltimore) 2014;93:e257. 10.1097/MD.0000000000000257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang SH, Waldron JN, Milosevic M, et al. Prognostic value of pretreatment circulating neutrophils, monocytes, and lymphocytes in oropharyngeal cancer stratified by human papillomavirus status. Cancer 2015;121:545-55. 10.1002/cncr.29100 [DOI] [PubMed] [Google Scholar]
- 17.Wang L, Wang C, Wang J, et al. A novel systemic immune-inflammation index predicts survival and quality of life of patients after curative resection for esophageal squamous cell carcinoma. J Cancer Res Clin Oncol 2017;143:2077-86. 10.1007/s00432-017-2451-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang H, Shang X, Ren P, et al. The predictive value of a preoperative systemic immune-inflammation index and prognostic nutritional index in patients with esophageal squamous cell carcinoma. J Cell Physiol 2019;234:1794-802. 10.1002/jcp.27052 [DOI] [PubMed] [Google Scholar]
- 19.Feng JF, Chen S, Yang X. Systemic immune-inflammation index (SII) is a useful prognostic indicator for patients with squamous cell carcinoma of the esophagus. Medicine (Baltimore) 2017;96:e5886. 10.1097/MD.0000000000005886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mehrazin R, Uzzo RG, Kutikov A, et al. Lymphopenia is an independent predictor of inferior outcome in papillary renal cell carcinoma. Urol Oncol 2015;33:388.e19-25. 10.1016/j.urolonc.2014.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu ES, Oduyebo T, Cobb LP, et al. Lymphopenia and its association with survival in patients with locally advanced cervical cancer. Gynecol Oncol 2016;140:76-82. 10.1016/j.ygyno.2015.11.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Feng F, Zheng G, Wang Q, et al. Low lymphocyte count and high monocyte count predicts poor prognosis of gastric cancer. BMC Gastroenterol 2018;18:148. 10.1186/s12876-018-0877-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhao W, Wang P, Jia H, et al. Lymphocyte count or percentage: which can better predict the prognosis of advanced cancer patients following palliative care? BMC Cancer 2017;17:514. 10.1186/s12885-017-3498-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bastid J, Bonnefoy N, Eliaou JF, et al. Lymphocyte-derived interleukin-17A adds another brick in the wall of inflammation-induced breast carcinogenesis. Oncoimmunology 2014;3:e28273. 10.4161/onci.28273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nazir T, Islam A, Omer MO, et al. Lymphocytopenia; induced by vinorelbine, doxorubicin and cisplatin in human cancer patients. Breast Dis 2015;35:1-4. 10.3233/BD-140386 [DOI] [PubMed] [Google Scholar]
- 26.Martínez-Lostao L, Anel A, Pardo J. How Do Cytotoxic Lymphocytes Kill Cancer Cells? Clin Cancer Res 2015;21:5047-56. 10.1158/1078-0432.CCR-15-0685 [DOI] [PubMed] [Google Scholar]


