Abstract
Lung cancer is the leading cause of cancer-related death worldwide and lobectomy remains the standard of care for patients with early-stage non-small cell lung cancer (NSCLC). The combination of an aging population and the implementation of low-dose CT for lung cancer screening is leading to an increase in diagnosis of early stage NSCLC in medically “inoperable” patients. The recommended treatment for this latter group of patients is stereotactic body radiation therapy (SBRT). However, many patients cannot undergo SBRT because they have received prior radiation or because the tumor is located next to vital structures. Percutaneous ablative therapies have become an alternative to SBRT but, unfortunately, they all violate the pleura and are associated with high rate of pneumothorax. With a more favorable safety profile and the ability to provide also diagnosis and nodal staging, bronchoscopic ablation is hence emerging as a potential future therapeutic alternative for these patients. Herein we review the current state of the art including animal and human data that exists thus far. We also discuss technical and research challenges as well as future directions that this exciting new technology may take.
Keywords: Bronchoscopy, ablation, lung cancer
Introduction
The last several years have seen a substantial rise in the detection of pulmonary nodules. This is likely due to a combination of factors such as the recommendation for lung cancer screening by multiple medical societies and the wide-spread use of chest CT scans (1-7). As our population ages, we are bound to diagnose more and more often early stage lung cancer in medically inoperable patients. The current standard-of-care for treatment of early stage lung cancer in such patients is radiation therapy, specifically stereotactic body radiation therapy (SBRT) (8). However, there is controversy as to whether survival and recurrence rates after SBRT are equivalent to those after surgical resection (9-12). SBRT is also costly, carries its own risks of toxicity, requires multiple sessions, and some patients are not candidates for this modality due to prior radiation treatment or tumor location (13,14). Percutaneous thermal ablation has been described as an alternative to SBRT. Radiofrequency ablation (RFA), microwave ablation (MWA), and cryotherapy can be delivered percutaneously to treat both lung cancer and lung metastases (15). Unfortunately, the percutaneous approach inevitably violates the visceral pleura and pneumothorax has been described in as many as 50% of cases (15). Since the majority of medically inoperable patients have a poor lung reserve, pneumothorax can have severe consequences and it is of great concern. Other documented complications with the percutaneous approach include bronchopleural fistula, pleural effusion, bleeding, thermal injury to nearby structures, and needle tract seeding (16-22). Safer therapeutic modalities for the treatment of early stage unresectable lung cancer are hence highly desirable, and bronchoscopic ablation is emerging as a plausible candidate. The bronchoscopic approach may prove to have several potential advantages compared to other strategies. Since the pleural space is not violated, pneumothorax and bronchopleural fistulas could be largely minimized. Respiratory motion should not be a problem as it is with a percutaneous approach since lesions are reached via the airways which move in concert with the parenchyma during the respiratory cycle. Most promising of all, bronchoscopy could become a “one-stop shop” where patients with peripheral lung cancers are diagnosed, staged (nodal staging with endobronchial ultrasound), and treated all within a single procedure.
Bronchoscopic ablation is currently at a very early investigational stage. A brief summary of published animal and human studies and on-going registered trials (ClinicalTrials.gov) is depicted in Tables 1,2. The purpose of this review is to provide a broad description of the various bronchoscopic ablation techniques currently under investigation. Results from the available literature will be examined in detail followed by a discussion of technical and research challenges as well as future directions. It is the hope of the authors that our work will spur further interest in this exciting new frontier in the treatment of lung cancer.
Table 1. Available animal and human data on bronchoscopic ablation of peripheral tumors.
| Subject type | Ablative technique | Number of ablations/subjects | Authors |
|---|---|---|---|
| Animal (sheep) | RFA | 12/6 | Tsushima et al. (23) |
| Animal (swine) | MWA | 12/3 | Ferguson et al. (24) |
| Animal (swine) | TVA | 84/7 | Henne et al. (25) |
| Animal (canine) | PDT | 3/3 | Musani et al. (26) |
| Animal (swine) | BLITT | 10/5 | Casal et al. (27) |
| Human | RFA | 10/10 | Tanabe et al. (28) |
| Human | RFA | 28/20 | Koizumi et al. (29) |
RFA, radiofrequency ablation; MWA, microwave ablation; TVA, thermal vapor ablation; PDT, photodynamic therapy; BLITT, bronchoscopic thermal interstitial therapy.
Table 2. Available human trials of bronchoscopic ablation of peripheral tumors registered in ClinicalTRials.gov.
| Ablative technique | Ablation followed by surgical resection | Region | ClinicalTrials.gov identifier |
|---|---|---|---|
| PDT | No | United States, Canada | NCT02916745 |
| PDT | Yes | United States | NCT03344861 |
| PDT | No | Taiwan | NCT03211078 |
| TVA | Yes | Australia | NCT03198468 |
| TVA | No | Austria, Italy | NCT03514329 |
| BLITT | Yes | United States | NCT03707925 |
| RFA | No | China | NCT03009630 |
| RFA | Yes | China | NCT03272971 |
| RFA/MWA | No | China | NCT02972177 |
| MWA | No | United States | NCT03713099 |
| MWA | No | United Kingdom | NCT03569111 |
RFA, radiofrequency ablation; MWA, microwave ablation; TVA, thermal vapor ablation; PDT, photodynamic therapy; BLITT, bronchoscopic thermal interstitial therapy.
Techniques
RFA
In RFA, a radiofrequency generator produces an alternating high frequency electrical current of nearly 500 kHz that is passed through an electrode into tissue (30,31). This current causes ions in the target tissue to oscillate, generating frictional heat (32). The goal temperature is between 65 and 105 °C. Within this range, protein denaturation occurs leading to coagulation necrosis; above this range, there is carbonization of tissue. The latter event increases tissue impedance reduces current flow, and thus becomes counterproductive (33).
Tsushima et al. published the only animal study to date of bronchoscopic RFA (23). This was a safety and feasibility study comparing two different RFA catheters for ablation of normal sheep lung. A non-cooled RFA catheter resulted in significant bronchial bleeding as well as an increase in impedance due to build-up of coagulated necrotic tissue around the overly heated catheter tip. The researchers then tested a catheter that was internally cooled with water and studied the effects of different combinations of power output and water flow rate. Based on their findings, a power output of 30 watts and flow rate of 30–40 mL/min achieved the greatest amount of coagulation necrosis of lung tissue while causing no complications.
These same authors then translated these findings into a human “ablate and resect” study (28). Ten patients with T1N0M0 non-small cell lung cancer (NSCLC) received bronchoscopic RFA of their respective peripheral tumors with one of three different internally-cooled catheters. There were no complications after the bronchoscopic intervention. They subsequently underwent previously planned surgical resection and the ablated areas were examined histologically. The catheter with the largest active tip (10 mm) and the longest ablation time (50 seconds) produced the greatest ablation area with destruction of the alveolar space and good coagulation necrosis. However, some viable tumor cells remained in the periphery of the ablation zone.
In the only other human study of bronchoscopic RFA, Koizumi et al. applied these prior findings to patients with T1–2N0M0 NSCLC who were either medically inoperable or refused surgery (29). Twenty patients underwent 28 RFA procedures for 23 lesions. No serious adverse events were noted. Two patients developed chest pain and three developed fever, but all resolved with conservative management. Median follow-up was 46 months. Overall, 19/23 (82.6%) lesions either decreased in size or remained stable at initial follow-up. During further follow-up, local progression was noted in 12 lesions. Five of these cases underwent repeat RFA; three underwent SBRT and one had chemotherapy. The median progression-free interval was 35 months (95% CI: 22–45 months) with a 5-year survival rate of 61.5% (95% CI: 36–87 months). Of the 6 patients that died during the study, 3 died due to progression of the original lung cancer. Of note, at enrollment, patients were determined to have N0 disease based on imaging only (CT and/or positron emission tomography); no invasive staging was done. Thus, it is possible that some patients had been under-staged.
RFA is the most studied of the bronchoscopic ablative techniques for peripheral tumors. Thus far, it seems to have a good safety profile, and to have outcomes—albeit based on a single study—that are similar to other currently used treatments for inoperable patients with early stage lung cancer.
Photodynamic therapy (PDT)
PDT involves the use of a photosensitizing agent followed by activation of the drug with light of a certain wavelength (34). The most commonly used agent is dihematoporphyrin ether, better known as porfimer sodium (Photofrin; Pinnacle Biologics, Bannockburn, IL, USA) which is administered intravenously and allowed to distribute throughout the body for approximately 48 hours. The drug concentrates in tumor tissue but also is taken up by normal tissues. During the interval period, levels of the drug that accumulated in normal cells fall but remain elevated in tumor due to selective retention (34). Flexible bronchoscopy is then performed with light activation through a diffuser laser fiber. This generates a photochemical (rather than a thermal) effect. Porfimer sodium within exposed cells absorbs the light, generating superoxide and hydroxyl radicals which leads to apoptosis (Photofrin package insert, Pinnacle Biologics, Bannockburn, IL, USA). Cell death is accentuated via ischemic necrosis from vascular occlusion triggered by release of thromboxane A2. It is also thought that this process triggers a prolonged immune response leading to suppression of remaining tumor (34).
Initial applications of PDT were in breast, bladder, and skin cancers in the 1960s–1970s, but with further development of endoscopes in the 1980s, PDT has since been used for endobronchial and esophageal malignancies. However, until recently, PDT in the lungs has been limited to central lesions that can be directly visualized with the bronchoscope. With new advances in bronchoscopy that have increased our reach of peripheral tumors, PDT of these more distant lesions is being explored.
To that end, Musani et al. conducted an experiment of PDT in dogs (26). Unlike all other animal studies described in this manuscript in which normal lung parenchyma was ablated, this study involved ablation of naturally occurring lung cancer in dogs. Three dogs with solitary peripheral lung adenocarcinomas were administered porfimer sodium followed by navigational bronchoscopy to localize their lesions and perform photoillumination 48 hours later. Tumor size ranged from 2.8 to 5.1 cm in longest dimension. Each tumor received photoillumination at two or three locations adjacent to or within the tumor. One-week post-photoillumination they underwent lobectomy and the affected tissues were examined. Tissue specimens showed sharply demarcated areas of coagulative necrosis known as “kill zones” which are indicative of complete cell death. Kill zones in all 3 dogs extended to a diameter of approximately 1.5 cm but viable tumor cells were noted to still be present along the periphery of this area. Damage to normal lung parenchyma around the tumor was felt to be mild and limited. With regards to complications of PDT, two dogs developed cough after photoillumination with imaging suggesting mild pneumonitis. Two also developed signs of photosensitivity, one in an eye and the other on the skin. Though safe and feasible, a small kill zone was achieved, and the relatively large tumors included in the study were incompletely ablated.
One of the primary disadvantages of PDT is the photosensitivity of the patient following administration of the photosensitizing chemical. According to the manufacturer of Photofrin, patients are to avoid exposure of skin and eyes to direct sunlight or bright indoor light for at least 30 days following injection and for as much as 90 days or more if they have liver impairment (Photofrin package insert, Pinnacle Biologics, Bannockburn, IL, USA). There is a second-generation agent that has been used with which patients need only avoid light exposure for one week (Radachlorin; Rada-Pharma, Russia); however, there is limited experience with this drug thus far (35). When PDT is utilized in the central airways there is resultant sloughing of necrotic tissue which typically requires repeat bronchoscopy for debridement (34). It is unlikely that this will be a significant issue with peripheral tumors given the distal location but that remains to be proven.
Photodynamic therapy has been used effectively for localized, central lung cancers for several years. It remains to be seen if this success can be translated to the periphery and several studies are currently underway (Table 2).
MWA
MWA uses electromagnetic waves to generate heat. These microwaves (usually 900–2,500 MHz) oscillate, causing water molecules—which are polar—to have to realign constantly in a rapid flip motion (30,36). This increase in kinetic energy produces heat that reaches cytotoxic levels. Compared to RFA, which only allows heating of tissues adjacent to the electrode, heat from MWA radiates into deeper tissues. Moreover, because MWA does not rely on an electric current as in RFA, it is not affected by conductivity of tissues such as the air surrounding lung lesions or the increase in impedance from carbonization and desiccation of tissue (30,36).
MWA has been used percutaneously to treat neoplasms in various organs, including the lung, but there is no human data as of yet for bronchoscopic delivery of this technique (36-38). The only study of bronchoscopic MWA has been reported in abstract form describing the development of microwave antennas that were directed bronchoscopically into the lung parenchyma of pigs (24). Four MWAs using different power and time settings were performed in each of three pigs with the ablation zones resected and examined thereafter. The ablation zone was proportionate to the power used—as large as 3.7 cm in diameter with a relatively oblong shape. Pneumothorax occurred in 3 of the 12 ablations.
MWA is in its early stages relative to RFA. Given some theoretical advantages, further studies are needed and are currently underway, including a prospective comparison of RFA versus MWA for peripheral lung cancer (Table 2).
Thermal vapor ablation (TVA)
TVA was initially described for bronchoscopic lung volume reduction (BLVR) (39,40). In BLVR, a catheter is introduced bronchoscopically into the target airway segment which is then isolated by an occlusion balloon (40). A precise amount of heated water vapor is delivered, leading to a localized inflammatory response that ultimately causes fibrosis and atelectasis. As a result, hyperinflation of the treated segment(s) is reduced.
Recently, Henne et al. adapted this technology with the goal of potentially treating peripheral lung tumors (25). In a safety and feasibility study in normal healthy pigs, rather than targeting segmental airways as in BLVR, the investigators delivered vapor at subsegmental levels in an attempt to completely perfuse the distal lung parenchyma with a higher density of thermal energy. The rationale was that this would yield a uniform field of necrosis along the natural anatomic boundaries of that subsegment. This ablated area would not only encompass a given lesion but also the surrounding parenchyma, reducing concerns about leaving a positive margin of residual tumor. Moreover, the authors hypothesized that since the supporting vasculature in this territory was also to be ablated, any portions of the lesion that were not directly ablated would eventually die due to ischemia (25). Eleven pigs underwent TVA with different levels of energy (125–390 cal) in different non-adjacent segments. Five pigs underwent necropsy two hours post-treatment (the ‘acute’ group); six were kept alive for 10, 21, or 32 days before being euthanized and examined (the ‘chronic’ group). Pigs in the acute group had approximately five times as many subsegments ablated as pigs in the chronic group. A total of 84 ablations were performed. Pneumothorax occurred in 3 out of 5 pigs in the acute group but treatments were generally well tolerated otherwise. Treatment energy levels of 330 cal and above were required to achieve uniform necrosis in 75–88% of the ablations. Unfortunately, these energy levels were associated with approximately a 70% rate of development of pneumatoceles. Histopathology primarily revealed necrosis in the ablation zones; in the animals sacrificed at 32 days, most necrosis had been replaced by fibrotic tissue. Necrosis was most often found at the higher energy levels. It was hypothesized by the authors that the high rate of pneumatoceles was related to the lack of collateral ventilation between different segments found in pigs. The same authors then tested bronchoscopic TVA in explanted human lungs to determine if anatomically confined and uniform ablations could be obtained with levels of energy comparable with their swine and human emphysema studies (41). Non-transplantable human lungs excised en bloc were obtained from a tissue procurement agency (MedCure, Portland, OR). Ten human lungs received a total of 107 ablation treatments. At 330cal and above, ≥67% of the ablations achieved a sharp boundary, and these boundaries were demarcated by subsegmental anatomy. In sharp contrast to their swine study, only 4 pneumatoceles were observed in 107 ablations.
Taken together, the authors responsible for these preclinical data on bronchoscopic TVA conclude that if a nodule were to be present in the ablated areas, it likely would have been destroyed, and most likely with a low rate of complications. A potential advantage that we see of this technique over others is the that tumors do not necessarily need to be directly accessed. Reaching a subsegmental airway proximal to the tumor may be enough, and, hence, more patients can potentially be treated bronchoscopically. Two human studies are currently underway (Table 2).
Bronchoscopic laser interstitial thermal therapy
Laser therapy has been a mainstay in the treatment of centrally located endobronchial pathology for many years (42). With more recent technological advancements allowing the ability to reach distal lesions, this therapy is now being attempted in the periphery. However, some adaptations need to be made. One is regarding the aperture of the laser delivery fiber (LDF). With conventional use of laser in the central airways, a very narrow beam of thermal energy is emitted. The flexible bronchoscope is then manipulated to direct the beam in various directions to various targets; this movement is possible because of the relatively large central airways. In the periphery where a bronchoscope or LDF will be in a much smaller/tighter airway, there is little room for changing the direction of laser emission. Thus, in order to ablate a tumor that is larger than a given peripheral airway, the laser must be emitted through a wider aperture that will cause the beam to fan out and produce a wider ablation zone. To that end, Casal et al. recently published the first investigation of a technique they named Bronchoscopic Laser Interstitial Thermal Therapy (BLITT) (27). This feasibility and safety study had two components: ex vivo and in vivo portions. The ex vivo study was undertaken to first identify an LDF that produced the largest and most spherical ablation zone in explanted normal pig lung parenchyma. The five different LDF that were tested were: frosted-tip, ball-tip, round-tip, capped round-tip, and cone-tip. The LDF was passed bronchoscopically, advanced through the bronchial wall and into the lung parenchyma where laser ablations were carried out for 30, 60, 90, and 120 seconds at 25 W in different parts of the lung. The round tip produced the most spherical ablation zone, and this was chosen for the in vivo study. In the in vivo study, 5 pigs underwent two ablations each. A chest CT was performed within 30 min of ablation and then the animals were monitored for 3 days post-ablation before undergoing repeat chest CT and necropsy. One pig sustained a pneumothorax that was briefly aspirated with a pleural catheter (not left in place); otherwise, there were no complications. Chest CTs, both at day 1 and day 3, identified each targeted location as an area of cavitation surrounded by a combination of ground-glass attenuation and consolidation. On gross pathology, the ablated areas consisted of cavitation and charred tissue that varied in size from 1 to 3.5 cm in greatest diameter. On histologic examination, variable degrees of necrosis were noted around these charred areas. Thus, the wide aperture allowed for relatively large ablation zones that may be able to encompass small lung tumors. Previous percutaneous laser approaches with standard LDF have either yielded much smaller ablation zones or required multiple simultaneous needles to achieve similar results (43). A human study of ablation followed by resection for early NSCLC and metastases is currently underway (Table 2).
Technical challenges
As with any developing technology, bronchoscopic ablation of peripheral nodules still has several technical issues that need to be addressed. One of the primary concerns is our ability to navigate to the targets. Bronchoscopic techniques for such purpose include navigational (electromagnetic and non-electromagnetic) bronchoscopy, radial-probe endobronchial ultrasound (RP-EBUS), and—most recently—robotic bronchoscopy. However, despite all these advances, our ability to reach such lesions remains suboptimal with ample room for improvement (44,45). As important as navigating to our target lesions is the ability to confirm that we have certainly reached them. None of the techniques described above for navigation can truly do so. One particular modality that can both aid in navigating to a nodule and also confirm that we have reached the target is cone beam CT (CBCT) (46) (Figure 1). Mounted on a C-arm, CBCT scanner can provide projection radiography, fluoroscopy, and volumetric CT data in a single rotation around our patient (47). With CBCT the bronchoscope and bronchoscopic catheters/probes can be left in place while the scanner rotates around the immobile patient. In addition to accurately confirming in real-time the position of the ablating tools with respect to the target, it also provides a view of nearby anatomic structures; this could be quite useful in preventing complications, particularly when using thermal energy (Figure 2). Another potential advantage of CBCT is that it could be used to assess ablation effects and intraoperative complications. Of course, these are theoretical advantages; data on the use of CBCT in bronchoscopic ablation trials is needed and it is underway.
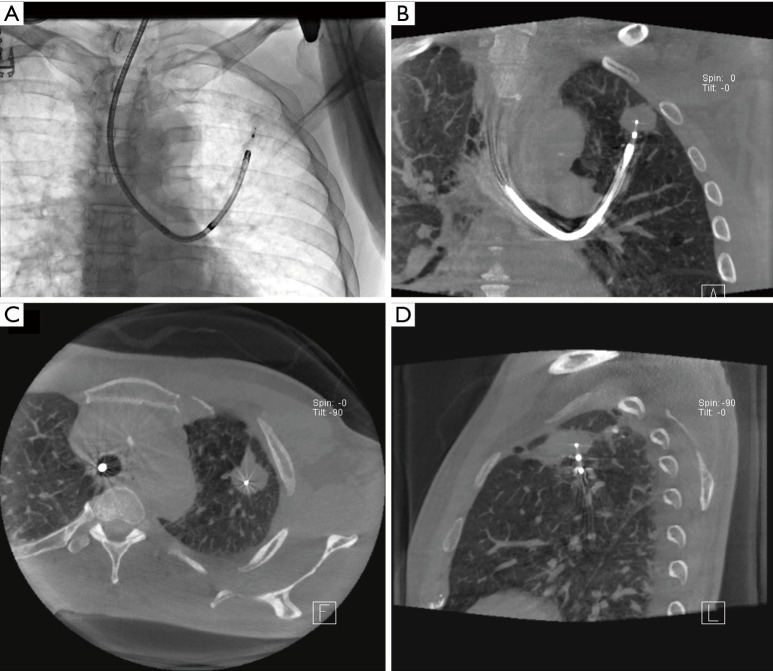
Figure 1.
Cone beam CT-guided ablation of left upper lobe tumor. (A) Fluoroscopy image showing thin scope and ablation catheter reaching tumor; (B,C,D) represent coronal, axial and sagittal CT images demonstrating ablative probe within the lesion.
Figure 2.
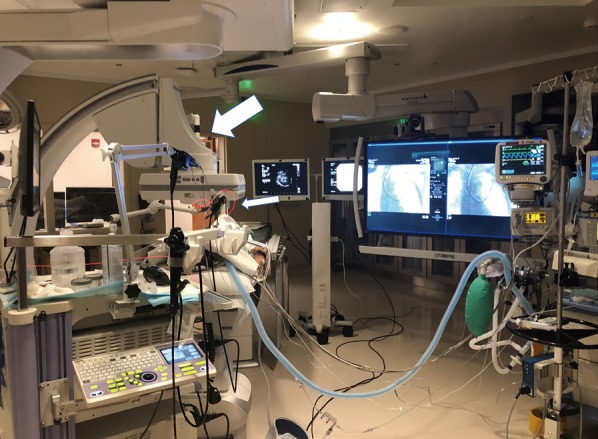
Cone beam CT (CBCT)-guided photodynamic therapy ablation. Large arrow shows CBCT arm around patient’s table. Small arrow demonstrates bronchoscope with laser fiber in place.
As alluded to above, another technical challenge is the proper positioning of the ablative probe relative to the target lesion. With RFA, PDT, and MWA, the ablative probe must penetrate the tumor to be most effective since ablation occurs around the probe (“peri-probe” ablation techniques). To achieve this, then, there must be an airway leading into the tumor and the probe needs to be stiff and sharp enough to penetrate the tumor. When this is not the case, multiple ablations via various nearby airways may be required. On the other hand, with TVA and BLITT, the ablation zones are distal to the catheter tip (“distal ablation” techniques). Thus, tumor penetration is not necessary. A special characteristic of BLITT is that the LDF can be activated and its tip will easily penetrate the airway wall to reach a lesion. This is a potential advantage in cases when there is no airway leading directly to the target. Bronchoscopic transparenchymal access to lung nodules, though mainly investigated for diagnostic purposes, may also play a role in ablation for these tumors without a bronchus sign (48). As described above, the proper location of any ablative probe with regards to the target can only be accurately confirmed with CBCT, which is bound to play a cornerstone role in ablation.
A third obstacle to be addressed is the variability in the ablation zones observed in the aforementioned studies. Ideally, ablation zones would be reproducible, allowing clinicians to better know which tumors are likely to be fully ablated. Proposed explanations for variable ablation results with a given technique include different degrees of secretions, bleeding, atelectasis, or even preexisting pneumonia, particularly in animal models where the latter may not be readily apparent. Another possibility is the ‘heat sink effect’ in different parts of the lung. This term refers to the significant blood flow through the lungs that dissipates the applied heat from thermal ablation; the continuous movement of air provides the same effect (30). Different lung zones have different degrees of perfusion and ventilation and hence the heat sink effect may be dissimilar in these zones. Some experimental methods have been employed to attenuate the heat sink effect, but these are invasive and carry a high risk of complications (49-51).
Finally, there are some technical challenges that are inherent to the individual ablative techniques themselves. As described above, RFA is affected by carbonization of tissue that increases impedance. Cooling of the catheter has led to some improvements but there is room for more. PDT is currently faced with the difficulty of prolonged photosensitivity with the most commonly used photosensitizing agent; further research and development of other agents with shorter half-lives would be useful. RFA and MWA may be somewhat limited in terms of tumor penetration because of the large caliber and blunt tip of their probes. Another issue is undesired destruction of normal lung parenchyma surrounding a given lesion. Maximizing tumor ablation while minimizing collateral damage—particularly in patients with already compromised pulmonary function—is key.
Research challenges
We face multiple challenges when conducting research on this developing technology. One particular challenge is the lack of proper tumor models in large animals. Most animal studies described above utilized normal lung tissue which may react different to any of the ablative techniques when compared to solid tumors. Utilizing animals with spontaneously occurring adenocarcinomas such as in the study of Musani et al. can be of significant difficulty and time consuming.
Due to the lack of understanding of the effects of ablation at the histological and cellular level, and the lack of certainty that our techniques will result in complete tumor kill, our initial human trials should ideally involve ablation followed by surgical resection. This is particularly true for patients with early operable lung cancer who could otherwise be potentially cured with surgery. The latter will not only be the treatment for the patients’ lung tumors, but it will also accurately demonstrate the tumor kill rate and the pathologic effect of ablation on tumor, lung parenchyma and supporting structures. A corollary challenge here is that patients have no direct benefits by participating in such trials since their treatment is surgery, and all we are adding is an increased risk of complications. This may hamper accrual rates and prevent us from enrolling large enough numbers of subjects. In the case of ablate and resect trials, a question that has arisen is that of timing between ablation and resection. A delayed resection will give time for the ablated tissue to decay and look non-viable on histology. It will also give us time to monitor for peri-procedural complications. However, it will postpone surgery with the potential for progression of disease in the interval. On the other hand, early resection (immediate or within 24–48 h) may not confer enough time for all ablated tumor cells to die, and special immunohistochemistry stains may be required to determine tissue viability.
Another potential challenge for some investigators may be their lack of access to a CBCT scanner for their ablative procedures. The lack of use of CBCT for confirmation of ablative probe position may introduce a negative bias in research studies. Ablations may be incomplete not because of failure of a particular ablative technique itself but because of poor positioning of the ablative probe with respect to the target.
Future directions
Aside from the matters needing further refinement as described above, other areas of research may be on the horizon. While not an ablative method per se, a technology that has been proposed for lung cancer is electrochemotherapy (ECT) (30). Applying an electric field to malignant tissue can increase cell membrane permeability to chemicals that would not normally be able to enter a cell, or not to the same degree. This is a process known as electroporation and can be exploited to cause cells to take up chemotherapeutic drugs at much higher levels than otherwise. Initially used for cutaneous and subcutaneous cancers because of the need for electrodes to deliver the electric field, this technology is more recently being adapted for use in internal tumors such as brain cancers and metastases to bone and liver (52,53). Endoscopic ECT has been developed and investigated in canine and murine models of gastrointestinal tumors which exhibited significant responses, even resolution, in these animal models (54). Adoption of this technique for bronchoscopy seems feasible and safe.
Another way in which bronchoscopic ablation may overlap with medical oncology in the future is by applying concepts from immunotherapy. Thermal ablation causes release of a host of antigens which can trigger recruitment, differentiation, and activation of a variety of immune cells, including B-cells, T-cells, antigen-presenting cells and natural killer cells (15). Downstream immune cascades can then produce tumor-specific immunity that could theoretically eradicate residual tumor cells that survived ablation. In an attempt to augment this specific immune response, Chen et al. recently developed nanoparticles that contain an immune-activating Toll-like receptor-7 (TLR-7) agonist as well as a near-infrared (NIF) dye that causes the nanoparticle to release its contents upon exposure to near-infrared laser, known as photothermal therapy (PTT) (55). In various in vitro and in vivo mouse tumor models, after PTT, not only was tumor-specific immunity enhanced, but tumor responses were robust. This even occurred not only at the ablation site but at sites of metastasis. The authors postulated that endoscopy-based delivery of near-infrared laser would be needed and feasible in order to reach tumors deeper in the body.
In the same vein as immune activation, it has been suggested that cryoablation induces a greater post-ablation immune response than heat therapies (56). Indeed, cryotherapy may be a candidate for bronchoscopic ablation in its own right but certainly in conjunction with the immune-activating technologies described above. Cryoablation has already been in use for percutaneous ablation of lesions and it is soon to be delivered by bronchoscopy for ablation of these peripheral tumors (55).
Conclusions
The near and distant future is likely to see an increase in the detection of peripheral pulmonary nodules and, along with that, early stage lung cancers. A significant portion of this is likely to be in non-surgical candidates given our aging population. Bronchoscopic ablation may be a therapeutic alternative with several advantages over currently available therapies for these medically inoperable patients. Various ablative techniques have been proposed, but most data originate in animal studies thus far. Nonetheless, there are multiple original human studies underway worldwide. These will hopefully help us address the various challenges facing this exciting new technology, allowing it to ultimately be brought into clinical practice.
Acknowledgments
None.
Footnotes
Conflicts of Interest: RF Casal has received research grants from Siemens and Concordia, and he is a paid consultant for Olympus. BF Sabath has no conflicts of interest to declare.
References
- 1.Moyer VA. Screening for lung cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2014;160:330-8. [DOI] [PubMed] [Google Scholar]
- 2.Detterbeck FC, Mazzone PJ, Naidich DP, et al. Screening for lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e78S-92S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jaklitsch MT, Jacobson FL, Austin JH, et al. The American Association for Thoracic Surgery guidelines for lung cancer screening using low-dose computed tomography scans for lung cancer survivors and other high-risk groups. J Thorac Cardiovasc Surg 2012;144:33-8. 10.1016/j.jtcvs.2012.05.060 [DOI] [PubMed] [Google Scholar]
- 4.Wender R, Fontham ET, Barrera E, Jr, et al. American Cancer Society lung cancer screening guidelines. CA Cancer J Clin 2013;63:107-17. 10.3322/caac.21172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wiener RS, Gould MK, Arenberg DA, et al. An official American Thoracic Society/American College of Chest Physicians policy statement: implementation of low-dose computed tomography lung cancer screening programs in clinical practice. Am J Respir Crit Care Med 2015;192:881-91. 10.1164/rccm.201508-1671ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wood DE, Eapen GA, Ettinger DS, et al. Lung cancer screening. J Natl Compr Canc Netw 2012;10:240-65. 10.6004/jnccn.2012.0022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gould MK, Tang T, Liu IL, et al. Recent Trends in the Identification of Incidental Pulmonary Nodules. Am J Respir Crit Care Med 2015;192:1208-14. 10.1164/rccm.201505-0990OC [DOI] [PubMed] [Google Scholar]
- 8.Howington JA, Blum MG, Chang AC, et al. Treatment of stage I and II non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e278S-313S. [DOI] [PubMed] [Google Scholar]
- 9.Cornwell LD, Echeverria AE, Samuelian J, et al. Video-assisted thoracoscopic lobectomy is associated with greater recurrence-free survival than stereotactic body radiotherapy for clinical stage I lung cancer. J Thorac Cardiovasc Surg 2018;155:395-402. 10.1016/j.jtcvs.2017.07.065 [DOI] [PubMed] [Google Scholar]
- 10.Stokes WA, Rusthoven CG. Surgery vs. SBRT in retrospective analyses: confounding by operability is the elephant in the room. J Thorac Dis 2018;10:S2007-10. 10.21037/jtd.2018.05.40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Samson P, Keogan K, Crabtree T, et al. Interpreting survival data from clinical trials of surgery versus stereotactic body radiation therapy in operable Stage I non-small cell lung cancer patients. Lung Cancer 2017;103:6-10. 10.1016/j.lungcan.2016.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bryant AK, Mundt RC, Sandhu AP, et al. Stereotactic Body Radiation Therapy Versus Surgery for Early Lung Cancer Among US Veterans. Ann Thorac Surg 2018;105:425-31. 10.1016/j.athoracsur.2017.07.048 [DOI] [PubMed] [Google Scholar]
- 13.Smith BD, Jiang J, Chang JY, et al. Cost-effectiveness of stereotactic radiation, sublobar resection, and lobectomy for early non-small cell lung cancers in older adults. J Geriatr Oncol 2015;6:324-31. 10.1016/j.jgo.2015.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lievens Y, Obyn C, Mertens AS, et al. Stereotactic body radiotherapy for lung cancer: how much does it really cost? J Thorac Oncol 2015;10:454-61. 10.1097/JTO.0000000000000421 [DOI] [PubMed] [Google Scholar]
- 15.Palussière J, Catena V, Buy X. Percutaneous thermal ablation of lung tumors - Radiofrequency, microwave and cryotherapy: Where are we going? Diagn Interv Imaging 2017;98:619-25. 10.1016/j.diii.2017.07.003 [DOI] [PubMed] [Google Scholar]
- 16.Hiraki T, Mimura H, Gobara H, et al. Two cases of needle-tract seeding after percutaneous radiofrequency ablation for lung cancer. J Vasc Interv Radiol 2009;20:415-8. 10.1016/j.jvir.2008.12.411 [DOI] [PubMed] [Google Scholar]
- 17.Sakurai J, Hiraki T, Mukai T, et al. Intractable pneumothorax due to bronchopleural fistula after radiofrequency ablation of lung tumors. J Vasc Interv Radiol 2007;18:141-5. 10.1016/j.jvir.2006.10.011 [DOI] [PubMed] [Google Scholar]
- 18.Hiraki T, Gobara H, Mimura H, et al. Brachial nerve injury caused by percutaneous radiofrequency ablation of apical lung cancer: a report of four cases. J Vasc Interv Radiol 2010;21:1129-33. 10.1016/j.jvir.2010.03.007 [DOI] [PubMed] [Google Scholar]
- 19.Matsui Y, Hiraki T, Gobara H, et al. Phrenic nerve injury after radiofrequency ablation of lung tumors: retrospective evaluation of the incidence and risk factors. J Vasc Interv Radiol 2012;23:780-5. 10.1016/j.jvir.2012.02.014 [DOI] [PubMed] [Google Scholar]
- 20.Le TX, Andrews RT. Thermal osteonecrosis of the rib after radiofrequency ablation in the thorax. J Vasc Interv Radiol 2008;19:940-4. 10.1016/j.jvir.2008.02.017 [DOI] [PubMed] [Google Scholar]
- 21.Kodama H, Yamakado K, Murashima S, et al. Intractable bronchopleural fistula caused by radiofrequency ablation: endoscopic bronchial occlusion with silicone embolic material. Br J Radiol 2009;82:e225-7. 10.1259/bjr/23975691 [DOI] [PubMed] [Google Scholar]
- 22.Yamakado K, Akeboshi M, Nakatsuka A, et al. Tumor seeding following lung radiofrequency ablation: a case report. Cardiovasc Intervent Radiol 2005;28:530-2. 10.1007/s00270-004-0246-7 [DOI] [PubMed] [Google Scholar]
- 23.Tsushima K, Koizumi T, Tanabe T, et al. Bronchoscopy-guided radiofrequency ablation as a potential novel therapeutic tool. Eur Respir J 2007;29:1193-200. 10.1183/09031936.00111306 [DOI] [PubMed] [Google Scholar]
- 24.Ferguson J, Egressy K, Schefelker R, et al. Bronchoscopically-guided microwave ablation in the lung. Chest 2013;144:87A 10.1378/chest.170535923392731 [DOI] [Google Scholar]
- 25.Henne E, Ferguson JS, Mest R, et al. Thermal Vapor Ablation for Lung Lesions in a Porcine Model. Respiration 2015;90:146-54. 10.1159/000433558 [DOI] [PubMed] [Google Scholar]
- 26.Musani AI, Veir JK, Huang Z, et al. Photodynamic therapy via navigational bronchoscopy for peripheral lung cancer in dogs. Lasers Surg Med 2018;50:483-90. 10.1002/lsm.22781 [DOI] [PubMed] [Google Scholar]
- 27.Casal RF, Walsh G, McArthur M, et al. Bronchoscopic Laser Interstitial Thermal Therapy: An Experimental Study in Normal Porcine Lung Parenchyma. J Bronchology Interv Pulmonol 2018;25:322-9. 10.1097/LBR.0000000000000501 [DOI] [PubMed] [Google Scholar]
- 28.Tanabe T, Koizumi T, Tsushima K, et al. Comparative study of three different catheters for CT imaging-bronchoscopy-guided radiofrequency ablation as a potential and novel interventional therapy for lung cancer. Chest 2010;137:890-7. 10.1378/chest.09-1065 [DOI] [PubMed] [Google Scholar]
- 29.Koizumi T, Tsushima K, Tanabe T, et al. Bronchoscopy-Guided Cooled Radiofrequency Ablation as a Novel Intervention Therapy for Peripheral Lung Cancer. Respiration 2015;90:47-55. 10.1159/000430825 [DOI] [PubMed] [Google Scholar]
- 30.Jahangeer S, Forde P, Soden D, et al. Review of current thermal ablation treatment for lung cancer and the potential of electrochemotherapy as a means for treatment of lung tumours. Cancer Treat Rev 2013;39:862-71. 10.1016/j.ctrv.2013.03.007 [DOI] [PubMed] [Google Scholar]
- 31.Casal RF, Tam AL, Eapen GA. Radiofrequency ablation of lung tumors. Clin Chest Med 2010;31:151-63. 10.1016/j.ccm.2009.08.021 [DOI] [PubMed] [Google Scholar]
- 32.Gazelle GS, Goldberg SN, Solbiati L, et al. Tumor ablation with radio-frequency energy. Radiology 2000;217:633-46. 10.1148/radiology.217.3.r00dc26633 [DOI] [PubMed] [Google Scholar]
- 33.Goldberg SN, Gazelle GS, Halpern EF, et al. Radiofrequency tissue ablation: importance of local temperature along the electrode tip exposure in determining lesion shape and size. Acad Radiol 1996;3:212-8. 10.1016/S1076-6332(96)80443-0 [DOI] [PubMed] [Google Scholar]
- 34.Reddy C. Photodynamic therapy. In: Ernst A, Herth F, editors. Principles and Practice of Interventional Pulmonology. 1st ed. New York, NY: Springer, 2013: 377-85. [Google Scholar]
- 35.Akopov A, Rusanov A, Gerasin A, et al. Preoperative endobronchial photodynamic therapy improves resectability in initially irresectable (inoperable) locally advanced non small cell lung cancer. Photodiagnosis Photodyn Ther 2014;11:259-64. 10.1016/j.pdpdt.2014.03.011 [DOI] [PubMed] [Google Scholar]
- 36.Vogl TJ, Nour-Eldin NA, Albrecht MH, et al. Thermal Ablation of Lung Tumors: Focus on Microwave Ablation. Rofo 2017;189:828-43. 10.1055/s-0043-109010 [DOI] [PubMed] [Google Scholar]
- 37.Thompson SM, Schmitz JJ, Thompson RH, et al. Introduction of Microwave Ablation Into a Renal Ablation Practice: Valuable Lessons Learned. AJR Am J Roentgenol 2018;211:1381-9. [DOI] [PubMed] [Google Scholar]
- 38.Dou JP, Liang P, Yu J. Microwave ablation for liver tumors. Abdom Radiol (NY) 2016;41:650-8. 10.1007/s00261-016-0662-6 [DOI] [PubMed] [Google Scholar]
- 39.Herth FJ, Ernst A, Baker KM, et al. Characterization of outcomes 1 year after endoscopic thermal vapor ablation for patients with heterogeneous emphysema. Int J Chron Obstruct Pulmon Dis 2012;7:397-405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Snell GI, Hopkins P, Westall G, et al. A feasibility and safety study of bronchoscopic thermal vapor ablation: a novel emphysema therapy. Ann Thorac Surg 2009;88:1993-8. 10.1016/j.athoracsur.2009.06.038 [DOI] [PubMed] [Google Scholar]
- 41.Ferguson JS, Henne E. Bronchoscopically Delivered Thermal Vapor Ablation of Human Lung Lesions. J Bronchology Interv Pulmonol 2018. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Khemasuwan D, Mehta AC, Wang KP. Past, present, and future of endobronchial laser photoresection. J Thorac Dis 2015;7:S380-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fielding DI, Buonaccorsi G, Hanby A, et al. Interstitial laser photocoagulation of normal lung parenchyma in rats. Thorax 1998;53:692-7. 10.1136/thx.53.8.692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mudambi L, Ost DE. Advanced bronchoscopic techniques for the diagnosis of peripheral pulmonary lesions. Curr Opin Pulm Med 2016;22:309-18. 10.1097/MCP.0000000000000284 [DOI] [PubMed] [Google Scholar]
- 45.Rojas-Solano JR, Ugalde-Gamboa L, Machuzak M. Robotic Bronchoscopy for Diagnosis of Suspected Lung Cancer: A Feasibility Study. J Bronchology Interv Pulmonol 2018;25:168-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pritchett MA, Schampaert S, de Groot JAH, et al. Cone-Beam CT With Augmented Fluoroscopy Combined With Electromagnetic Navigation Bronchoscopy for Biopsy of Pulmonary Nodules. J Bronchology Interv Pulmonol 2018;25:274-82. 10.1097/LBR.0000000000000536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Orth RC, Wallace MJ, Kuo MD, et al. C-arm cone-beam CT: general principles and technical considerations for use in interventional radiology. J Vasc Interv Radiol 2008;19:814-20. 10.1016/j.jvir.2008.02.002 [DOI] [PubMed] [Google Scholar]
- 48.Herth FJ, Eberhardt R, Sterman D, et al. Bronchoscopic transparenchymal nodule access (BTPNA): first in human trial of a novel procedure for sampling solitary pulmonary nodules. Thorax 2015;70:326-32. 10.1136/thoraxjnl-2014-206211 [DOI] [PubMed] [Google Scholar]
- 49.Hiraki T, Gobara H, Sakurai J, et al. Radiofrequency ablation of normal lungs after pulmonary artery embolization with use of degradable starch microspheres: results in a porcine model. J Vasc Interv Radiol 2006;17:1991-8. 10.1097/01.RVI.0000251152.12254.AC [DOI] [PubMed] [Google Scholar]
- 50.Anai H, Uchida BT, Pavcnik D, et al. Effects of blood flow and/or ventilation restriction on radiofrequency coagulation size in the lung: an experimental study in swine. Cardiovasc Intervent Radiol 2006;29:838-45. 10.1007/s00270-005-0217-7 [DOI] [PubMed] [Google Scholar]
- 51.Oshima F, Yamakado K, Akeboshi M, et al. Lung radiofrequency ablation with and without bronchial occlusion: experimental study in porcine lungs. J Vasc Interv Radiol 2004;15:1451-6. 10.1097/01.RVI.0000140640.87399.58 [DOI] [PubMed] [Google Scholar]
- 52.Linnert M, Iversen HK, Gehl J. Multiple brain metastases - current management and perspectives for treatment with electrochemotherapy. Radiol Oncol 2012;46:271-8. 10.2478/v10019-012-0042-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Miklavčič D, Serša G, Brecelj E, et al. Electrochemotherapy: technological advancements for efficient electroporation-based treatment of internal tumors. Med Biol Eng Comput 2012;50:1213-25. 10.1007/s11517-012-0991-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Forde PF, Sadadcharam M, Bourke MG, et al. Preclinical evaluation of an endoscopic electroporation system. Endoscopy 2016;48:477-83. 10.1055/s-0042-101343 [DOI] [PubMed] [Google Scholar]
- 55.Chen Q, Xu L, Liang C, et al. Photothermal therapy with immune-adjuvant nanoparticles together with checkpoint blockade for effective cancer immunotherapy. Nat Commun 2016;7:13193. 10.1038/ncomms13193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mehta A, Oklu R, Sheth RA. Thermal Ablative Therapies and Immune Checkpoint Modulation: Can Locoregional Approaches Effect a Systemic Response? Gastroenterol Res Pract 2016;2016:9251375. 10.1155/2016/9251375 [DOI] [PMC free article] [PubMed] [Google Scholar]