Abstract
Background
Recent studies incorporating dose escalated radiation identified heart dose as a predictor of cardiac toxicity in unresectable lung cancer patients. Whether conventionally dosed radiation impacts cardiac events remains unclear.
Methods
Stage III lung cancer patients undergoing definitive chemoradiation to 60–70 Gy were analyzed. Clinical and dosimetric factors (mean heart dose, heart V5-60 in 5 Gy increments) were analyzed against freedom from ≥ grade 3 cardiac events and overall survival (OS) by log-rank test. Multivariable analysis (MVA) for factors significant on univariate analysis was performed by Cox proportional hazards.
Results
A total of 108 patients were identified. Median follow-up was 18.0 months. One- and two-year OS were 79% and 61%, respectively. On MVA, gross tumor volume (GTV) ≥98.6 cm3 [hazard ratio (HR): 2.11, 95% confidence interval (CI): 1.15–3.93, P=0.02] and female gender (HR: 2.01, 95% CI: 1.09–3.73, P=0.03) predicted for worse survival. Twelve patients (11%) developed ≥ grade 3 cardiac events. One- and two-year freedom from cardiac events (FFCE) was 94% and 84% respectively. On MVA, heart V5 ≥49% predicted for cardiac events (HR: 11.44, 95% CI: 1.31–111.60, P=0.03) while female gender was nearly significant (HR: 3.49, 95% CI: 0.97–16.80, P=0.06). Females presented with similar comorbidity scores, GTVs, and relapse rates but experienced higher heart doses than their male counterparts.
Conclusions
Heart V5 ≥49% predicted for cardiac events after chemoradiation. However, cardiac dosimetry was not associated with survival. Rather, female gender and GTV ≥98.6 cm3 led to worse survival. This study corroborates emerging data that low-dose radiation to the heart impacts cardiac toxicity.
Keywords: Cardiac toxicity, chemoradiation, unresectable lung cancer
Introduction
It is well established that radiation therapy leads to increased risk of heart disease in long-term survivors of Hodgkin’s lymphoma (1-3), breast cancer (4,5), and esophageal cancer (6). In lung cancer patients, the impact of thoracic radiation on cardiac toxicity was historically underappreciated as it was assumed that disease progression and its impact on life expectancy outpaced the development of cardiac events. Radiation Therapy Oncology Group (RTOG) 0617, a landmark study of unresectable stage III non-small lung cancer patients randomized to either standard-dose (60 Gy) or high-dose (74 Gy) chemoradiation with concurrent and consolidative carboplatin and paclitaxel with or without cetuximab, found that high-dose radiation was associated with a lower survival rate than the standard-dose arm and identified that higher cardiac dose (heart V5 and V30) led to worse survival. However, specific heart toxicity outcomes were not tracked and the impact of pre-existing comorbidity on this finding remains unclear (7).
A significant interest in evaluating heart dose and cardiac events has followed. Recent single institution studies show that cardiac dosimetry predicts for cardiac events following thoracic radiation in locally advanced lung cancer patients (8-10). However, some of these studies did not corroborate the finding that heart dose adversely impacts overall survival (OS) (8,9). In addition, patients included in these studies were treated with doses up to 90 Gy or received only hypofractionated radiation (>2 Gy/fraction) on dose escalation protocols.
Due to the limited data in this setting, further validation is necessary to determine the effects of radiation therapy on cardiac events in lung cancer, particularly in the setting of standard radiation doses. We sought to determine if cardiac dose parameters may impact cardiac events following treatment and OS in patients with locally advanced lung cancer undergoing curative intent chemoradiation.
Methods
Patients were retrospectively identified from a database approved by an institutional review board (IRB) at two affiliated centers with unresectable stage IIIA/B (AJCC 7th edition) lung cancer who were treated with curative-intent radiation therapy and chemotherapy between 2010–2016. Waiver of consent was obtained. Exclusion criteria included patients with metastatic disease, omission of concurrent chemotherapy with radiation, surgically resected patients, or those with a prior course of thoracic radiation. Patients receiving altered fractionation, doses over 70 Gy or hypofractionated radiation (>2 Gy/day) were ineligible for this study.
Treatment consisted of platinum-based chemotherapy delivered concurrently with radiation therapy. All patients underwent CT simulation on a 16 slice Brilliance CT scanner (Brilliance CT, Big Bore, Philips Medical Systems, Andover, MA, USA) with 4D CT for radiation planning. Patients were immobilized using standard upper and lower alpha cradles (Smithers Medical Products, North Canton, USA). Respiratory management was utilized for cases in which tumor motion was identified to be ≥1 cm on 4D CT. The gross tumor volume (GTV) was defined as the primary tumor and any regionally involved nodes identified clinically on staging CT (>1 cm) or PET scan, or identified pathologically by endoscopic bronchial ultrasound. An internal target volume (ITV) was generated from review of the 4D CT. A 0.5–1 cm clinical target volume (CTV) expansion was added for microscopic disease extension and an additional 0.5–1 cm planning target volume (PTV) margin was placed to account for setup uncertainty. Radiation therapy was delivered with 6–18 MV photons with either 3D conformal (3D CRT) or intensity-modulated radiation therapy (IMRT). Clinical and demographic information was obtained from electronic medical records. Charlson comorbidity index (CCI) (11) was determined for each patient although the diagnosis of lung cancer was excluded from the index. Patients whose primary tumor or nodes abutted the heart were characterized as having pericardial tumors. Dose-volume histogram data were extracted from the Pinnacle v7.6-10.2 planning system and the following dosimetric parameters were computed: heart V5–60 in 5 Gy increments, mean heart dose, lung V5, lung V20, and mean lung dose. All adverse events were graded by CTCAE Version 4.03. Patients were analyzed for grade 3 or higher cardiac events post-therapy defined as new arrhythmia, structural disease/valvulopathy, myocardial infarction, new or recurrent congestive heart failure, pericarditis or non-malignant pericardial effusion requiring intervention. Lung volume was defined as whole lung excluding GTV and cardiac volumes were recontoured if necessary to comply with RTOG organ at risk definition (12). Institutional planning goals included a whole lung-CTV V20 ≤37%, cord dose of ≤50 Gy, and heart V60 <33%, V45 <66% and V40 <100%.
OS was calculated from start date of radiation therapy to death from any cause. Disease-free survival was calculated from radiation start date to date of first disease recurrence or death. Freedom from cardiac events (FFCE) was calculated from start date of radiation therapy to date of grade 3 or higher cardiac event while disease recurrence and deaths unrelated to cardiac events were censored. Univariate analysis (UVA) was performed with log-rank tests. Continuous variables were dichotomized at their median values for survival analyses. The Cox proportional hazards model was used to run multivariable analyses on variables with P values ≤0.20 on UVA. When multiple dose variables were found to be significant on UVA, collinearity was assessed for highly correlated variables and dose variables were excluded from the multivariate model if variance inflation factors >10. OS, disease-free survival, and FFCE were estimated with the Kaplan-Meier method. Categorical variables were compared using Chi-squared tests of independence while t-tests were performed for continuous variables. All analyses were done with JMP version 12 (SAS Institute Inc., Cary, NC, USA, 1989–2007).
Results
A total of 108 patients met inclusion criteria for this analysis. Median follow-up was 18.0 months (range, 3.3–84.6 months). Median OS was 30.7 months. One- and two-year OS for the entire cohort were 79% and 61%, respectively. One- and two-year disease-free survival were 59% and 42%, respectively. Table 1 shows patient clinical and treatment characteristics. Median age was 67 years. A total of 90 (83%) patients presented with non-small cell lung cancer (NSCLC) of whom nearly half (49%) had squamous cell carcinoma while 18 patients (17%) presented with small cell lung cancer. A total of 62 (57%) patients had stage IIIA disease. The Eastern Cooperative Oncology Group (ECOG) performance status was 0, 1 and 2 in 32 (30%), 67 (62%), and 9 (8%) patients, respectively. CCI was <2 in 71 (66%) patients. Pre-existing comorbid heart disease, lung disease and diabetes mellitus were present in 29 (27%), 53 (49%), and 24 (22%) patients, respectively. Of the 29 patients with pre-existing cardiac comorbidities, prior diagnoses included 21 (72%) with coronary artery disease, 8 (28%) with congestive heart failure, 9 (31%) with arrhythmia (including atrial flutter and fibrillation, supraventricular tachycardia, and ventricular tachycardia), 2 (7%) with prior pericardial disease, and 8 (28%) with unspecified or other cardiac disease.
Table 1. Clinical and treatment related factors.
| Factors | N [%] or median (range) | HR (95% CI) | P value |
|---|---|---|---|
| Age, years | 67 [40–85] | 0.86 (0.48–1.52) | 0.60 |
| Gender | |||
| Male | 50 [46] | 1.00 | |
| Female | 58 [54] | 1.58 (0.89–2.86) | 0.12 |
| Race | |||
| White | 57 [53] | 1.00 | |
| Black or African-American | 49 [45] | 1.14 (0.65–2.02) | 0.64 |
| Other | 2 [2] | ||
| Smoking history | |||
| Former smoker | 81 [75] | 1.00 | |
| Non-smoker | 2 [2] | ||
| Current smoker | 25 [23] | 0.87 (0.41–1.68) | 0.68 |
| Pre-existing comorbid disease | |||
| Cardiac | 29 [27] | 1.11 (0.58–2.04) | 0.74 |
| Pulmonary | 53 [49] | 1.04 (0.59–1.83) | 0.89 |
| Diabetes mellitus | 24 [22] | 1.74 (0.88–3.26) | 0.11 |
| AJCC stage | |||
| IIIA | 62 [57] | 1.00 | |
| IIIB | 46 [43] | 1.35 (0.77–2.38) | 0.29 |
| Histology | |||
| Non-small cell lung cancer | 90 [83] | 1.00 | |
| Adenocarcinoma | 40 [44] | ||
| Squamous | 44 [49] | ||
| Other | 6 [7] | ||
| Small cell lung cancer | 18 [17] | 1.21 (0.54–2.41) | 0.62 |
| GTV, cm3 | 98.6 (3.8–891.1) | 1.99 (1.12–3.63) | 0.02 |
| PTV, cm3 | 495.3 (17.2–1388.0) | 1.41 (0.79–2.52) | 0.24 |
| Radiotherapy technique | |||
| 3D CRT | 85 [79] | 1.00 | |
| IMRT | 23 [21] | 0.97 (0.39–2.07) | 0.95 |
| ECOG performance status | |||
| 0 | 32 [30] | 1.00 | |
| 1 | 67 [62] | 1.22 (0.66–2.34) | 0.53 |
| 2 | 9 [8] | 0.75 (0.21–2.09) | 0.61 |
| Charlson comorbidity index | |||
| 0 | 35 [32] | 1.00 | |
| 1 | 36 [33] | 1.36 (0.68–2.78) | 0.39 |
| 2 | 19 [18] | 1.18 (0.47–2.78) | 0.72 |
| 3+ | 18 [17] | 1.01 (0.40–2.37) | 0.98 |
| Radiation dose, Gy | 64 [60–70] | 0.79 (0.44–1.42) | 0.42 |
| Chemotherapy agents | |||
| Carboplatin/paclitaxel | 69 [64] | 1.00 | |
| Cisplatin/etoposide | 23 [21] | 1.99 (0.99–3.83) | 0.90 |
| Platinum/other | 16 [15] | 1.05 (0.46–2.16) | 0.91 |
AJCC, American Joint Committee on Cancer; HR, hazard ratio; CI, confidence interval; GTV, gross tumor volume; PTV, planning target volume; 3D CRT, three-dimensional conformal radiation therapy; IMRT, intensity-modulated radiation therapy; ECOG, Eastern Cooperative Oncology Group; Gy, Gray.
Nearly all (99%) patients underwent diagnostic positron emission tomography (PET) staging. Median GTV and PTV size was 98.6 and 495.3 cm3, respectively. Median radiation dose was 64 Gy (range, 60–70 Gy). Induction chemotherapy was delivered in 20 patients (19%). The most common concurrent chemotherapy regimen was carboplatin/paclitaxel in 69 (64%) patients, followed by cisplatin/etoposide in 23 (21%) patients. Eight-five (79%) patients received 3D CRT while 23 (21%) patients received IMRT. Nearly all patients (98%) underwent treatment with image-guided radiotherapy.
Results of UVA for OS are shown in Tables 2,3. GTV ≥98.6 cm3 was associated with worse survival (P=0.02). Other factors associated with worse survival were lung V5 ≥59.8% (P=0.03) and heart V5 ≥49.4% (P=0.03). Age, performance status, race, current smoking, CCI, cardiac or lung comorbidity were not associated with OS. Female gender (P=0.12) and diabetes (P=0.09) were associated with a non-significant trend towards worse survival. Radiation dose and use of IMRT were not associated with OS. In addition, there were no significant differences in survival between patients with NSCLC vs. small cell lung cancer. On multivariable analysis (Table 4), covariates which were statistically significant for worse survival included larger GTV (HR: 2.11, 95% CI: 1.15–3.93, P=0.02) and female gender (HR: 2.01, 95% CI: 1.09–3.82, P=0.03). Diabetes trended toward significance (P=0.07) while heart V5 and lung V5 were not significant.
Table 2. Univariate analysis of clinical factors for overall survival.
| Factors | HR (95% CI) | P value |
|---|---|---|
| Age (≥67/<67) | 0.86 (0.48–1.52) | 0.60 |
| Gender (female/male) | 1.58 (0.89–2.86) | 0.12 |
| Race (White/non-White) | 1.14 (0.65–2.02) | 0.64 |
| Current smoker (yes/no) | 0.87 (0.41–1.68) | 0.68 |
| Cardiac comorbidity (yes/no) | 1.11 (0.58–2.04) | 0.74 |
| Lung comorbidity (yes/no) | 1.04 (0.59–1.83) | 0.89 |
| Diabetes mellitus (yes/no) | 1.74 (0.11–3.26) | 0.09 |
| Histology (SCLC/NSCLC) | 1.21 (0.54–2.41) | 0.62 |
| GTV (≥98.6 cm3/<98.6 cm3) | 1.99 (1.12–3.63) | 0.02 |
| PTV (≥495.3 cm3/<495.3 cm3) | 1.41 (0.79–2.52) | 0.24 |
| Radiation dose (≥64 Gy/<64 Gy) | 0.79 (0.44–1.42) | 0.42 |
| Radiotherapy technique (IMRT/3D CRT) | 0.97 (0.39–2.07) | 0.95 |
| ECOG performance status (1–2/0) | 1.13 (0.62–2.16) | 0.69 |
| Charlson comorbidity index (≥2/<2) | 0.92 (0.49–1.66) | 0.79 |
SCLC, small cell lung cancer; NSCLC, non-small cell lung cancer; GTV, gross tumor volume; PTV, planning target volume; 3D CRT, three-dimensional conformal radiation therapy; IMRT, intensity-modulated radiation therapy; ECOG, Eastern Cooperative Oncology Group; Gy, gray; HR, hazard ratio; CI, confidence interval.
Table 3. Univariate analysis of dosimetric factors for overall survival.
| Factors | Median (range) | Subcategory | HR (95% CI) | P value |
|---|---|---|---|---|
| MHD (Gy) | 13.1 (5.5–48.7) | ≥13.1 vs. <13.1 | 1.47 (0.83–2.63) | 0.19 |
| MLD (Gy) | 19.1 (8.8–27.6) | ≥19.1 vs. <19.1 | 1.39 (0.79–2.50) | 0.29 |
| Lung V5 (%) | 59.8 (20.6–82.5) | ≥59.8 vs. <59.8 | 1.87 (1.05–3.39) | 0.03 |
| Lung V20 (%) | 32.0 (0–45.3) | ≥32.0 vs. <32.00 | 1.00 (0.56–1.77) | 0.99 |
| Heart V5 (%) | 49.4 (0–100.0) | ≥49.4 vs. <49.4 | 1.90 (1.07–3.45) | 0.03 |
| Heart V10 (%) | 39.4 (0–100.0) | ≥39.4 vs. <39.4 | 1.44 (0.82–2.59) | 0.21 |
| Heart V15 (%) | 31.0 (0–99.8) | ≥31.0 vs. <31.0 | 1.48 (0.84–2.65) | 0.18 |
| Heart V20 (%) | 22.7 (0–99.3) | ≥22.7 vs. <22.7 | 1.26 (0.71–2.24) | 0.43 |
| Heart V25 (%) | 18.3 (0–98.8) | ≥18.3 vs. <18.3 | 1.22 (0.69–2.18) | 0.49 |
| Heart V30 (%) | 15.6 (0–97.8) | ≥15.6 vs. <15.6 | 1.13 (0.64–2.01) | 0.66 |
| Heart V35 (%) | 11.8 (0–76.6) | ≥11.8 vs. <11.8 | 1.30 (0.73–2.31) | 0.37 |
| Heart V40 (%) | 9.1 (0–67.4) | ≥9.1 vs. <9.1 | 1.30 (0.74–2.32) | 0.37 |
| Heart V45 (%) | 6.8 (0–61.3) | ≥6.8 vs. <6.8 | 1.34 (0.76–2.39) | 0.32 |
| Heart V50 (%) | 5.3 (0–48.5) | ≥5.3 vs. <5.3 | 1.38 (0.78–2.47) | 0.27 |
| Heart V55 (%) | 3.5 (0–38.7) | ≥3.5 vs. <3.5 | 1.47 (0.83–2.63) | 0.18 |
| Heart V60 (%) | 1.9 (0–30.7) | ≥1.9 vs. <1.9 | 1.63 (0.92–2.96) | 0.09 |
MHD, mean heart dose; MLD, mean lung dose; HR, hazard ratio; CI, confidence interval.
Table 4. Multivariable analysis for overall survival.
| Factors | Hazard ratio | 95% confidence interval | P value |
|---|---|---|---|
| Female gender | 2.01 | 1.09–3.82 | 0.03 |
| GTV ≥98.6 cm3 | 2.11 | 1.15–3.93 | 0.02 |
| Lung V5 ≥59.8% | 1.55 | 0.72–3.39 | 0.26 |
| Heart V5 ≥49.4% | 1.25 | 0.58–2.73 | 0.56 |
| Diabetes | 1.87 | 0.94–3.54 | 0.07 |
GTV, gross tumor volume.
A total of 12 (11%) grade 3 or higher cardiac events developed at a median of 8.5 months (range, 2.8–40.2 months), leading to a 1- and 2-year FFCE rate of 94% and 84%, respectively. Four (33%) of these patients had pre-existing heart disease. Of these four patients, three patients had a prior diagnosis of coronary artery disease, 1 of whom also had co-existing congestive heart failure. The fourth patient had a prior diagnosis of diastolic congestive heart failure. Ten patients developed tachyarrhythmias of whom 3 developed arrhythmias in the setting of malignant pericardial effusions. Two of these 10 patients also experienced congestive heart failure or exacerbations. One patient developed heart failure secondary to diastolic dysfunction alone and another patient developed a large non-malignant pericardial effusion requiring pericardiocentesis. Tables 5,6 show results of UVA for FFCE. Dosimetric variables significant for cardiac events include heart V5, V10, V15, and mean heart dose while heart V25 trended toward significance. Heart V5 <49.4% vs. >49.4% was most significant and was associated with a 2-year FFCE rate of 97% vs. 67%, P=0.005, respectively (Figure 1). Age, race, GTV, CCI, cardiac or lung disease, diabetes, current smoking, performance status, and use of IMRT were not significant for cardiac events while female gender trended towards statistical significance (2-year FFCE: 96% vs. 71%, P=0.06). There was also no statistically significant difference in cardiac events between those who relapsed vs. those who did not (2-year FFCE: 85% vs. 83%, P=0.67). Among the 53 (49%) patients who did not relapse, mean heart dose, heart V5, V10, V15, V25 remained significant for the development of cardiac events (data not shown). On multivariable analysis for FFCE, heart V5 and mean heart dose were among the significant cardiac metrics entered, along with gender, given the clinical relevance of these variables across multiple studies. In addition, heart V10, V15, V25 were found to be collinear (data not shown) and therefore excluded from the analysis to avoid overfitting. Only heart V5 (HR: 11.44, 95% CI: 1.31–111.60, P=0.03) was predictive of cardiac events, while gender (HR: 3.49, 95% CI: 0.97–16.80, P=0.06) trended toward significance (Table 7). A total of 25 (23%) patients were found to have non-malignant pericardial effusions, classified as trace/minimal (18%), moderate (5%) or large/severe (1%). Only one patient required pericardiocentesis for non-malignant effusion. No cardiac variables were associated with the risk of pericardial effusion. Ultimately, the development of a cardiac event led to worse OS compared to those who did not experience a cardiac event (2-year OS: 38% vs. 64%, P=0.01).
Table 5. Univariate analysis of clinical factors for freedom from cardiac events.
| Factors | HR (95% CI) | P value |
|---|---|---|
| Age (≥67/<67 years) | 0.94 (0.29–3.01) | 0.92 |
| Gender (female/male) | 3.39 (1.00–15.42) | 0.05 |
| Race (White/non-White) | 0.75 (0.22–2.35) | 0.62 |
| Current smoker (yes/no) | 1.23 (0.39–4.15) | 0.73 |
| Cardiac comorbidity (yes/no) | 1.44 (0.38–4.62) | 0.56 |
| Lung comorbidity (yes/no) | 1.04 (0.33–3.33) | 0.95 |
| Diabetes mellitus (yes/no) | 0.80 (0.12–3.06) | 0.77 |
| Histology (SCLC/NSCLC) | 0.52 (0.03–2.72) | 0.50 |
| GTV (≥98.6 cm3/<98.6 cm3) | 1.88 (0.60–6.38) | 0.28 |
| PTV (≥495.3 cm3/<495.3 cm3) | 1.67 (0.53–5.65) | 0.38 |
| Radiotherapy technique (IMRT/3D CRT) | 1.84 (0.40–6.51) | 0.40 |
| ECOG performance status (1–2/0) | 1.53 (0.45–6.92) | 0.52 |
| Charlson comorbidity index (≥2/<2) | 0.58 (0.13–1.96) | 0.40 |
GTV, gross tumor volume; PTV, planning target volume; 3D CRT, three-dimensional conformal radiation therapy; IMRT, intensity-modulated radiation therapy; ECOG, Eastern Cooperative Oncology Group; Gy, gray.
Table 6. Univariate analysis of dosimetric factors for freedom from cardiac events.
| Factor | Median (range) | Subcategory | HR (95% CI) | P value |
|---|---|---|---|---|
| MHD (Gy) | 13.1 (5.5–48.7) | ≥13.1 vs. <13.1 | 3.59 (1.06–16.30) | 0.04 |
| Heart V5 (%) | 49.4 (0–100) | ≥49.4 vs. <49.4 | 6.69 (1.74–43.80) | 0.005 |
| Heart V10 (%) | 39.4 (0–100.0) | ≥39.4 vs. <39.4 | 6.13 (1.61–40.00) | 0.008 |
| Heart V15 (%) | 31.0 (0–99.8) | ≥31.0 vs. <31.0 | 6.08 (1.59–39.74) | 0.009 |
| Heart V20 (%) | 22.7 (0–99.3) | ≥22.7 vs. <22.7 | 2.23 (0.70–8.40) | 0.18 |
| Heart V25 (%) | 18.3 (0–98.8) | ≥18.3 vs. <18.3 | 3.26 (0.97–14.73) | 0.06 |
| Heart V30 (%) | 15.6 (0–97.8) | ≥15.6 vs. <15.6 | 2.16 (0.68–8.14) | 0.20 |
| Heart V35 (%) | 11.8 (0–76.6) | ≥11.8 vs. <11.8 | 2.29 (0.72–8.63) | 0.17 |
| Heart V40 (%) | 9.1 (0–67.4) | ≥9.1 vs. <9.1 | 2.29 (0.72–8.63) | 0.17 |
| Heart V45 (%) | 6.8 (0–61.3) | ≥6.8 vs. <6.88 | 1.50 (0.48–5.10) | 0.48 |
| Heart V50 (%) | 5.3 (0–48.5) | ≥5.3 vs. <5.3 | 1.57 (0.50–5.33) | 0.44 |
| Heart V55 (%) | 3.5 (0–38.7) | ≥3.5 vs. <3.5 | 1.67 (0.53–5.66) | 0.38 |
| Heart V60 (%) | 1.9 (0–30.7) | ≥1.9 vs. <1.9 | 1.58 (0.50–5.34) | 0.43 |
MHD, mean heart dose; CI, confidence interval.
Figure 1.
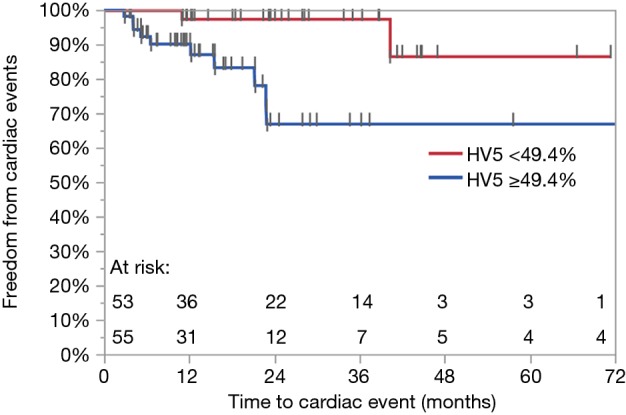
Kaplan-Meier freedom from cardiac event curve for Heart V5. P=0.005.
Table 7. Multivariable analysis for freedom from cardiac events.
| Factor | Hazard ratio | 95% confidence interval | P value |
|---|---|---|---|
| Female gender | 3.49 | 0.97–16.80 | 0.06 |
| Heart V5 ≥49.4% | 11.44 | 1.31–111.60 | 0.03 |
| MHD ≥13.1 Gy | 0.49 | 0.09–4.28 | 0.49 |
MHD, mean heart dose.
On further analysis of gender differences, women and men presented with similar CCI scores and rates of heart and lung disease; however, women presented with lower rates of diabetes (14% vs. 32%, P=0.02) than men. Women also presented with similar GTVs, similar rates of relapse and were not more likely to experience treatment breaks or longer treatment lengths. However, women were more likely to have received higher mean heart doses and overall heart doses (V20–55) and experienced a higher trend in the presentation of pericardial tumors (64% vs. 46%, P=0.06) than men (Table S1).
Table S1. Clinical and dosimetric variable associations with gender.
| Characteristic | All patients | Female | Male | P value | |||||
|---|---|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | ||||
| Total No. of patients | 108 | 100.0 | 58 | 54 | 50 | 46 | – | ||
| Age | 0.17 | ||||||||
| <67 years | 53 | 49 | 32 | 55 | 21 | 42 | |||
| ≥67 years | 55 | 51 | 26 | 45 | 29 | 58 | |||
| Race | 0.53 | ||||||||
| Caucasian | 57 | 53 | 29 | 50 | 28 | 56 | |||
| Other | 51 | 47 | 29 | 50 | 22 | 44 | |||
| Smoking status | 0.79 | ||||||||
| Current smoker | 25 | 23 | 14 | 24 | 11 | 22 | |||
| Non-current smoker | 83 | 77 | 44 | 76 | 39 | 78 | |||
| ECOG PS | 0.36 | ||||||||
| 0 | 32 | 30 | 15 | 26 | 17 | 34 | |||
| 1–2 | 76 | 70 | 43 | 74 | 33 | 66 | |||
| CCI | 0.24 | ||||||||
| 0–1 | 71 | 66 | 41 | 71 | 30 | 60 | |||
| 2+ | 37 | 34 | 17 | 29 | 20 | 40 | |||
| Pre-existing diabetes | 0.02 | ||||||||
| Yes | 24 | 22 | 8 | 14 | 16 | 32 | |||
| No | 84 | 78 | 50 | 86 | 34 | 68 | |||
| Pre-existing cardiac disease | 0.49 | ||||||||
| Yes | 29 | 27 | 14 | 24 | 15 | 30 | |||
| No | 79 | 73 | 44 | 76 | 35 | 70 | |||
| Pre-existing lung disease | 0.55 | ||||||||
| Yes | 53 | 49 | 30 | 52 | 23 | 46 | |||
| No | 55 | 51 | 28 | 48 | 27 | 54 | |||
| Relapse | 0.86 | ||||||||
| Yes | 55 | 51 | 30 | 52 | 25 | 50 | |||
| No | 53 | 49 | 28 | 48 | 25 | 50 | |||
| Gross tumor volume | 0.08 | ||||||||
| <98.6 cm3 | 55 | 51 | 34 | 59 | 21 | 42 | |||
| ≥98.6 cm3 | 53 | 49 | 24 | 41 | 29 | 58 | |||
| Tumor location | 0.06 | ||||||||
| Pericardial | 60 | 56 | 37 | 64 | 23 | 46 | |||
| Non-pericardial | 48 | 44 | 21 | 36 | 27 | 54 | |||
| Treatment length | 0.61 | ||||||||
| <45 days | 49 | 45 | 25 | 43 | 24 | 48 | |||
| ≥45 days | 59 | 55 | 33 | 57 | 26 | 52 | |||
| Treatment break | 0.77 | ||||||||
| Yes | 14 | 13 | 7 | 12 | 7 | 14 | |||
| No | 94 | 87 | 51 | 88 | 43 | 86 | |||
| Mean heart dose | 0.01 | ||||||||
| <13.1 Gy | 53 | 49 | 22 | 38 | 31 | 62 | |||
| ≥13.1 Gy | 55 | 51 | 36 | 62 | 19 | 38 | |||
| Heart V5 | 0.18 | ||||||||
| <49.4% | 53 | 49 | 25 | 43 | 28 | 56 | |||
| ≥49.4% | 55 | 51 | 33 | 57 | 22 | 44 | |||
| Heart V10 | 0.18 | ||||||||
| <39.4% | 53 | 49 | 25 | 43 | 28 | 56 | |||
| ≥39.4% | 55 | 51 | 33 | 57 | 22 | 44 | |||
| Heart V15 | 0.08 | ||||||||
| <31.0% | 53 | 49 | 24 | 41 | 29 | 58 | |||
| ≥31.0% | 55 | 51 | 34 | 59 | 21 | 42 | |||
| Heart V20 | 0.01 | ||||||||
| <22.7% | 53 | 49 | 22 | 38 | 31 | 62 | |||
| ≥22.7% | 55 | 51 | 36 | 62 | 19 | 38 | |||
| Heart V25 | 0.01 | ||||||||
| <18.3% | 53 | 49 | 22 | 38 | 31 | 62 | |||
| ≥18.3% | 55 | 51 | 36 | 62 | 19 | 38 | |||
| Heart V30 | 0.03 | ||||||||
| <15.6% | 53 | 49 | 23 | 40 | 30 | 60 | |||
| ≥15.6% | 55 | 51 | 35 | 60 | 20 | 40 | |||
| Heart V35 | 0.01 | ||||||||
| <11.8% | 53 | 49 | 22 | 38 | 31 | 62 | |||
| ≥11.8% | 55 | 51 | 36 | 62 | 19 | 38 | |||
| Heart V40 | 0.01 | ||||||||
| <9.1% | 53 | 49 | 22 | 38 | 31 | 62 | |||
| ≥9.1% | 55 | 51 | 36 | 62 | 19 | 38 | |||
| Heart V45 | 0.03 | ||||||||
| <6.8% | 53 | 49 | 23 | 40 | 30 | 60 | |||
| ≥6.8% | 55 | 51 | 35 | 60 | 20 | 40 | |||
| Heart V50 | 0.03 | ||||||||
| <5.3% | 53 | 49 | 23 | 40 | 30 | 60 | |||
| ≥5.3% | 55 | 51 | 35 | 60 | 20 | 40 | |||
| Heart V55 | |||||||||
| <3.5% | 54 | 50 | 24 | 41 | 30 | 60 | 0.05 | ||
| ≥3.5% | 54 | 50 | 34 | 59 | 20 | 40 | |||
| Heart V60 | 0.25 | ||||||||
| <1.9% | 54 | 50 | 26 | 45 | 28 | 56 | |||
| ≥1.9% | 54 | 50 | 32 | 55 | 22 | 44 | |||
| Mean lung dose | 0.57 | ||||||||
| <19.1 Gy | 55 | 51 | 31 | 53 | 24 | 48 | |||
| ≥19.1 Gy | 53 | 49 | 27 | 47 | 26 | 52 | |||
| Lung V5 | 0.34 | ||||||||
| <59.8% | 55 | 51 | 32 | 55 | 23 | 46 | |||
| ≥59.8% | 53 | 49 | 26 | 45 | 27 | 54 | |||
| Lung V20 | 0.84 | ||||||||
| <32.0% | 55 | 51 | 29 | 50 | 26 | 52 | |||
| ≥32.0% | 53 | 49 | 29 | 50 | 24 | 48 | |||
ECOG PS, Eastern Cooperative Oncology Group performance status; CCI, Charlson comorbidity index.
Rates of grade 2 or higher pneumonitis events were observed in 30 (28%) patients. Grade 2, 3, 4 and 5 pneumonitis were present in 23 (21%), 4 (4%), 1 (1%) and 2 (2%) patients, respectively. On UVA for median lung V5, V20 and mean lung dose, lung V20 <32% resulted in a trend towards a decrease in grade 2+ pneumonitis (20% vs. 35%, P=0.07). Gender, age, race, comorbid lung disease, use of IMRT, and GTV did not increase the risk of grade 2 or higher pulmonary toxicity; however current smoking was protective against grade 2+ pulmonary toxicity (4% vs. 35%, P=0.0006) (Table S2). The development of grade 3 or higher, but not grade 2 or higher, pneumonitis was associated with a non-significant decrement in OS (2-year OS: 38% vs. 63%, P=0.06).
Table S2. Clinical and dosimetric variable associations with grade 2+ lung toxicities.
| Characteristic | All patients | Pneumonitis grade <2 | Pneumonitis grade ≥2 | P value | |||||
|---|---|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | ||||
| Total No. of patients | 108 | 100.0 | 78 | 72 | 30 | 28 | – | ||
| Age | 0.24 | ||||||||
| <67 years | 53 | 49 | 41 | 77 | 12 | 23 | |||
| ≥67 years | 55 | 51 | 37 | 67 | 18 | 33 | |||
| Gender | 0.63 | ||||||||
| Female | 58 | 54 | 43 | 74 | 15 | 26 | |||
| Male | 50 | 46 | 35 | 70 | 15 | 30 | |||
| Race | 0.22 | ||||||||
| Caucasian | 57 | 53 | 44 | 77 | 13 | 23 | |||
| Other | 51 | 47 | 34 | 67 | 17 | 33 | |||
| Smoking status | 0.0006 | ||||||||
| Current smoker | 25 | 23 | 24 | 96 | 1 | 4 | |||
| Non-current smoker | 83 | 77 | 54 | 65 | 29 | 35 | |||
| Radiotherapy technique | 0.41 | ||||||||
| 3D CRT | 85 | 79 | 63 | 74 | 22 | 26 | |||
| IMRT | 23 | 21 | 15 | 65 | 8 | 35 | |||
| Pre-existing lung disease | 0.16 | ||||||||
| Yes | 53 | 49 | 35 | 66 | 18 | 34 | |||
| No | 55 | 51 | 43 | 78 | 12 | 22 | |||
| Gross tumor volume | 0.91 | ||||||||
| <98.6 cm3 | 55 | 51 | 40 | 73 | 15 | 27 | |||
| ≥98.6 cm3 | 53 | 49 | 38 | 72 | 15 | 28 | |||
| Mean lung dose | 0.16 | ||||||||
| <19.1 Gy | 55 | 51 | 43 | 78 | 12 | 22 | |||
| ≥19.1 Gy | 53 | 49 | 35 | 66 | 18 | 34 | |||
| Lung V5 | 0.16 | ||||||||
| <59.8% | 55 | 51 | 43 | 78 | 12 | 22 | |||
| ≥59.8% | 53 | 49 | 35 | 66 | 18 | 34 | |||
| Lung V20 | 0.07 | ||||||||
| <32.0% | 54 | 50 | 44 | 81 | 11 | 20 | |||
| ≥32.0% | 54 | 50 | 34 | 63 | 19 | 35 | |||
3D CRT, three-dimensional conformal radiation therapy; IMRT, intensity-modulated radiation therapy; Gy, gray.
Discussion
Our findings suggest that while heart dose parameters are associated with cardiac events following radiation therapy, they are not independently associated with survival. While this finding conflicts with RTOG 0617 (7), it is consistent with several reports showing that the OS is driven by GTV and disease progression rather than cardiac dose (9,13). In a secondary analysis of the ESPATUE study (13) which randomized 161 patients with operable stage III NSCLC to resection versus continuation of chemoradiation following neoadjuvant chemoradiation, heart dose was not predictive of survival although cardiac events were not specifically assessed. Patients in the ESPATUE study were acknowledged to be healthier than those in RTOG 0617 given eligibility for operability which may account for the differences. It is possible that the relatively small cohorts in the current study and other negative studies are insufficiently powered to detect the influence of cardiac dose on survival despite the observation that cardiac events themselves predict for a higher risk of death (8,9,13).
An important finding was that crude grade 3 or higher cardiac events occurred in 11% of patients at a median follow-up of 18 months. This rate is similar to that reported by Dess et al. (9), who observed a 2-year cumulative incidence rate of 11% and by Wang et al. (8), who reported a 2-year event rate of 10% in patients with unresectable lung cancer treated with definitive chemoradiation. In addition, these studies also found that higher heart or substructure V5 predicted for cardiac events. This metric has also been validated in studies with patients with lymphoma or breast cancer (5,14). In a validation study to assess the findings set forth by Darby et al. (4), who found that patients with breast cancer treated with adjuvant radiation experience a relative increase in ischemic heart disease by 7.4%/Gy in mean heart dose with “no apparent threshold”, left ventricular V5 appeared to be a better predictor of acute coronary events than mean heart dose (5). Potential mechanisms for cardiac injury from low-dose radiation include endothelial damage and atherosclerosis; however, the impact of low-dose radiation on other known sequela from radiation such as myocardial injury, conduction or perfusion related abnormalities is unclear (15). Finally, grade 1 or higher non-malignant pericardial effusions occurred in 23% of patients with only 1% requiring intervention. This relatively low grade 3+ pericardial effusion rate is similar to that (2.6%) reported by one recent series in which a prospectively followed cohort of unresectable lung cancer patients received proton beam or IMRT chemoradiation to 60–74 Gy (16).
The finding that GTV is an independent prognostic factor for OS has been shown by multiple studies in patients with lung cancer (17-19). Higher GTV leads to increased disease progression and worse survival. Our study cohort presented with similar GTVs and PTVs (median of 99 and 495 cm3, respectively) to those of RTOG 0617 (median GTV and PTV size were 75–110 and ~500 cm3, respectively). Despite increasing GTV being significant for worse survival on UVA in RTOG 0617, this specific factor was not assessed in a multivariable model. Rather, PTV volume was marginally significant for the radiation endpoint (7).
The finding that gender was associated with worse survival and a non-significant trend toward higher rates of cardiac events was unexpected. We did not observe differences in baseline characteristics between females and males with the exception of lower rates of pre-existing diabetes in women (Table S1). Total treatment duration and relapse rates were also similar between women and men. However, women received significantly higher mean heart and overall heart doses. This finding may be attributed to tumor location but regardless underscores the link between cardiac doses and cardiac events despite a comparable, if not better, comorbid background in women versus men in this study. Limited data suggest that females may be more sensitive to treatment related cardiac injury. This finding has been observed in at least one study of 102 patients treated with chemoradiation for esophageal cancer in which heart V20, V30, V40 and female gender were predictive of cardiac toxicity (20). Aside from radiation, gender differences in cardiac injury from anthracycline based chemotherapy is also well documented in childhood survivors of lymphoma (21,22).
In addition, we found that grade 3 pneumonitis, present in 6.5% of the cohort, was associated with higher risk of death which is similar to other studies (23,24). We did not observe an association between whole lung V5 and pulmonary toxicity but did see a non-significant trend towards higher lung V20 and development of grade 2+ pneumonitis. This finding is similar to that of RTOG 0617 in which higher lung V20, but not lung V5, was associated with heightened risk of (grade 3+) pneumonitis (25). Current smoking was found to be associated with decreased risk of development of grade 2+ pneumonitis. Similar findings of the protective effects of current smoking on radiation pneumonitis have been reported in other studies, and one hypothesis is that there is a diminished inflammatory response in smokers vs. non-smokers (26). This finding could be used to improve the accuracy of predictive models for determining patients at risk of developing radiation pneumonitis (27). We could not evaluate the impact of prior versus no smoking on outcomes as 98% of our study cohort had a history of tobacco use.
This study has a number of limitations. First, the analysis included a relatively small patient cohort which led to limited statistical power. The cohort included 17% of patients with small cell lung cancer who received conventional daily radiation therapy. Such patients historically present with similar median survival (23–30 months) and 2-year OS rates (46–53%) after chemoradiation to those of NSCLC patients (28,29). In fact, we observed no difference in survival between these subgroups. We acknowledge that the inclusion of these patients results in heterogeneity in the patient sample and may have impacted study results owing to different outcomes and therapies. Additional limitations of this study include the retrospective data collection and limited follow-up which led to potential underestimation of adverse events. This study also included 79% of patients who received 3D CRT. The use of 3D CRT has been found to be associated with higher heart doses than IMRT in patients with locally advanced NSCLC and therefore may be less likely to be utilized (25). However, we observed no difference in OS or FFCE in these patients. The cause of cardiac events could not be characterized as events related to general decline, disease progression, salvage therapy, pre-existing medical comorbidities or radiation therapy-induced injury. Nonetheless, the study population received relatively homogeneous radiation doses (60–70 Gy) and treatment parameters as all patients eligible for this analysis received concurrent chemoradiation. This study provides further validation to the growing evidence that cardiac dose predicts early cardiac events in this patient population.
Conclusions
Heart V5 ≥49% predicted for cardiac events after chemoradiation. However, cardiac dosimetry was not associated with survival. Rather, female gender and GTV ≥98.6 cm3 led to worse survival. This study corroborates emerging data that low dose radiation to the heart impacts cardiac toxicity.
Acknowledgments
None.
Ethical Statement: Patients were retrospectively identified from a database approved by an institutional review board (IRB) at two affiliated centers. Waiver of consent was obtained. The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Bhakta N, Liu Q, Yeo F, et al. Cumulative burden of cardiovascular morbidity in paediatric, adolescent, and young adult survivors of Hodgkin's lymphoma: an analysis from the St Jude Lifetime Cohort Study. Lancet Oncol 2016;17:1325-34. 10.1016/S1470-2045(16)30215-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hull MC, Morris CG, Pepine CJ, et al. Valvular dysfunction and carotid, subclavian, and coronary artery disease in survivors of hodgkin lymphoma treated with radiation therapy. JAMA 2003;290:2831-7. 10.1001/jama.290.21.2831 [DOI] [PubMed] [Google Scholar]
- 3.van Nimwegen FA, Ntentas G, Darby SC, et al. Risk of heart failure in survivors of Hodgkin lymphoma: effects of cardiac exposure to radiation and anthracyclines. Blood 2017;129:2257-65. 10.1182/blood-2016-09-740332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Darby SC, Ewertz M, McGale P, et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med 2013;368:987-98. 10.1056/NEJMoa1209825 [DOI] [PubMed] [Google Scholar]
- 5.van den Bogaard VA, Ta BD, van der Schaaf A, et al. Validation and Modification of a Prediction Model for Acute Cardiac Events in Patients With Breast Cancer Treated With Radiotherapy Based on Three-Dimensional Dose Distributions to Cardiac Substructures. J Clin Oncol 2017;35:1171-8. 10.1200/JCO.2016.69.8480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beukema JC, van Luijk P, Widder J, et al. Is cardiac toxicity a relevant issue in the radiation treatment of esophageal cancer? Radiother Oncol 2015;114:85-90. 10.1016/j.radonc.2014.11.037 [DOI] [PubMed] [Google Scholar]
- 7.Bradley JD, Paulus R, Komaki R, et al. Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): a randomised, two-by-two factorial phase 3 study. Lancet Oncol 2015;16:187-99. 10.1016/S1470-2045(14)71207-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang K, Eblan MJ, Deal AM, et al. Cardiac Toxicity After Radiotherapy for Stage III Non–Small-Cell Lung Cancer: Pooled Analysis of Dose-Escalation Trials Delivering 70 to 90 Gy. J Clin Oncol 2017;35:1387-94. 10.1200/JCO.2016.70.0229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dess RT, Sun Y, Matuszak MM, et al. Cardiac Events After Radiation Therapy: Combined Analysis of Prospective Multicenter Trials for Locally Advanced Non–Small-Cell Lung Cancer. J Clin Oncol 2017;35:1395-402. 10.1200/JCO.2016.71.6142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Speirs CK, DeWees TA, Rehman S, et al. Heart Dose Is an Independent Dosimetric Predictor of Overall Survival in Locally Advanced Non-Small Cell Lung Cancer. J Thorac Oncol 2017;12:293-301. 10.1016/j.jtho.2016.09.134 [DOI] [PubMed] [Google Scholar]
- 11.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373-83. 10.1016/0021-9681(87)90171-8 [DOI] [PubMed] [Google Scholar]
- 12.Contouring Atlases [Internet]. [cited 2017 Sep 21]. Available online: https://www.rtog.org/CoreLab/ContouringAtlases.aspx
- 13.Guberina M, Eberhardt W, Stuschke M, et al. Heart dose exposure as prognostic marker after radiotherapy for resectable stage IIIA/B non-small-cell lung cancer: secondary analysis of a randomized trial. Ann Oncol 2017;28:1084-9. 10.1093/annonc/mdx069 [DOI] [PubMed] [Google Scholar]
- 14.Hahn E, Jiang H, Ng A, et al. Late Cardiac Toxicity After Mediastinal Radiation Therapy for Hodgkin Lymphoma: Contributions of Coronary Artery and Whole Heart Dose-Volume Variables to Risk Prediction. Int J Radiat Oncol Biol Phys 2017;98:1116-23. 10.1016/j.ijrobp.2017.03.026 [DOI] [PubMed] [Google Scholar]
- 15.Taunk NK, Haffty BG, Kostis JB, et al. Radiation-induced heart disease: pathologic abnormalities and putative mechanisms. Front Oncol 2015;5:39. 10.3389/fonc.2015.00039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ning MS, Tang L, Gomez DR, et al. Incidence and Predictors of Pericardial Effusion After Chemoradiation Therapy for Locally Advanced Non-Small Cell Lung Cancer. Int J Radiat Oncol Biol Phys 2017;99:70-9. 10.1016/j.ijrobp.2017.05.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tucker SL, Liu A, Gomez D, et al. Impact of heart and lung dose on early survival in patients with non-small cell lung cancer treated with chemoradiation. Radiother Oncol 2016;119:495-500. 10.1016/j.radonc.2016.04.025 [DOI] [PubMed] [Google Scholar]
- 18.Warner A, Dahele M, Hu B, et al. Factors Associated with Early Mortality in Patients Treated With Concurrent Chemoradiation Therapy for Locally Advanced Non-Small Cell Lung Cancer. Int J Radiat Oncol Biol Phys 2016;94:612-20. 10.1016/j.ijrobp.2015.11.030 [DOI] [PubMed] [Google Scholar]
- 19.Bradley JD, Ieumwananonthachai N, Purdy JA, et al. Gross tumor volume, critical prognostic factor in patients treated with three-dimensional conformal radiation therapy for non-small-cell lung carcinoma. Int J Radiat Oncol Biol Phys 2002;52:49-57. 10.1016/S0360-3016(01)01772-2 [DOI] [PubMed] [Google Scholar]
- 20.Konski A, Li T, Christensen M, et al. Symptomatic cardiac toxicity is predicted by dosimetric and patient factors rather than changes in 18F-FDG PET determination of myocardial activity after chemoradiotherapy for esophageal cancer. Radiother Oncol 2012;104:72-7. 10.1016/j.radonc.2012.04.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Silber JH, Jakacki RI, Larsen RL, et al. Increased risk of cardiac dysfunction after anthracyclines in girls. Med Pediatr Oncol 1993;21:477-9. 10.1002/mpo.2950210704 [DOI] [PubMed] [Google Scholar]
- 22.Lipshultz SE, Lipsitz SR, Mone SM, et al. Female sex and higher drug dose as risk factors for late cardiotoxic effects of doxorubicin therapy for childhood cancer. N Engl J Med 1995;332:1738-43. 10.1056/NEJM199506293322602 [DOI] [PubMed] [Google Scholar]
- 23.Inoue A, Kunitoh H, Sekine I, et al. Radiation pneumonitis in lung cancer patients: a retrospective study of risk factors and the long-term prognosis. Int J Radiat Oncol Biol Phys 2001;49:649-55. 10.1016/S0360-3016(00)00783-5 [DOI] [PubMed] [Google Scholar]
- 24.Wang JY, Chen KY, Wang JT, et al. Outcome and prognostic factors for patients with non-small-cell lung cancer and severe radiation pneumonitis. Int J Radiat Oncol Biol Phys 2002;54:735-41. 10.1016/S0360-3016(02)02994-2 [DOI] [PubMed] [Google Scholar]
- 25.Chun SG, Hu C, Choy H, et al. Impact of Intensity-Modulated Radiation Therapy Technique for Locally Advanced Non–Small-Cell Lung Cancer: A Secondary Analysis of the NRG Oncology RTOG 0617 Randomized Clinical Trial. J Clin Oncol 2017;35:56-62. 10.1200/JCO.2016.69.1378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bjermer L, Franzén L, Littbrand B, et al. Effects of Smoking and Irradiated Volume on Inflammatory Response in the Lung of Irradiated Breast Cancer Patients Evaluated with Bronchoalveolar Lavage. Cancer Res 1990;50:2027-30. [PubMed] [Google Scholar]
- 27.Mörth C, Kafantaris I, Castegren M, et al. Validation and optimization of a predictive model for radiation pneumonitis in patients with lung cancer. Oncol Lett 2016;12:1144-8. 10.3892/ol.2016.4678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Turrisi AT, Kim K, Blum R, et al. Twice-Daily Compared with Once-Daily Thoracic Radiotherapy in Limited Small-Cell Lung Cancer Treated Concurrently with Cisplatin and Etoposide. N Engl J Med 1999;340:265-71. 10.1056/NEJM199901283400403 [DOI] [PubMed] [Google Scholar]
- 29.Faivre-Finn C, Snee M, Ashcroft L, et al. Concurrent once-daily versus twice-daily chemoradiotherapy in patients with limited-stage small-cell lung cancer (CONVERT): an open-label, phase 3, randomised, superiority trial. Lancet Oncol 2017;18:1116-25. 10.1016/S1470-2045(17)30318-2 [DOI] [PMC free article] [PubMed] [Google Scholar]


