Abstract
Allergic bronchopulmonary aspergillosis (ABPA) is an immunologically mediated disease characterized by a hypersensitivity reaction to fungal colonization by Aspergillus. Hydropneumothoraces and bronchopleural fistulae are rare occurrences in patients with ABPA. However, the diagnosis of ABPA is important to consider, as it is easily treatable with specific therapy. We report an unusual case of a patient with ABPA who presented to us with hydropneumothorax with bronchopleural fistula.
Keywords: Hydropneumothorax, Allergic bronchopulmonary aspergillosis, Pulmonary aspergillosis, Secondary spontaneous pneumothorax
1. Introduction
Allergic bronchopulmonary aspergillosis (ABPA) is an immunologically mediated disease characterized by a hypersensitivity reaction to respiratory fungal colonization by Aspergillus fumigatus [1]. Bronchial asthma and cystic fibrosis account for the majority of patients who develop ABPA. Patients with ABPA typically present with recurrent asthma exacerbations, fever, malaise, and occasionally hemoptysis [1]. The diagnosis of ABPA is ascertained by fulfillment of the diagnostic criteria proposed by the International Society for Human and Animal Mycology (ISHAM). Per requirements of the criteria, all diagnosed patients must have a predisposing disorder (bronchial asthma or cystic fibrosis), immediate cutaneous hypersensitivity to Aspergillus antigens (or alternatively elevated Aspergillus specific IgE antibody levels) and elevated total IgE levels (>1000 IU/mL). Additionally, laboratory testing must demonstrate two of the following: presence of serum precipitins (IgG antibodies to Aspergillus), radiographic pulmonary infiltrates consistent with ABPA or peripheral blood eosinophilia (>500 cells/μL) [2]. Current guidelines recommend screening all patients with newly diagnosed asthma for ABPA with Aspergillus specific IgE levels [2].
ABPA, like bronchial asthma, follows a natural course marked by exacerbations and remissions with a gradual but progressive decline in lung function. The management of ABPA comprises of anti-inflammatory therapy with systemic glucocorticoids to suppress the aberrant immune response and anti-fungal agents to reduce the fungal load in the airway [3]. While controlled trials to ascertain the dose of glucocorticoids remain wanting, a regimen of 0.5 mg/kg prednisolone for two weeks followed by a gradual taper over six to eight weeks is presently recommended [2]. Antifungal therapy has found utility in severe and steroid dependent ABPA with current guidelines recommending itraconazole therapy with therapeutic drug monitoring for a period of 16 weeks [4].
We report the unusual case of a girl who presented to us with hydropneumothorax in the background of fever and respiratory complaints and was diagnosed with ABPA.
2. Case
Our patient is a fifteen-year old girl who had been symptomatic for a period of four years. She developed complaints of recurrent nasal block with frontal headache at eleven years of age. This was diagnosed and managed as allergic rhinosinusitis with topical medications on which she improved. One year following the onset of these symptoms, she developed complaints of dyspnea on exertion and non-productive cough. She was successfully treated with inhaled bronchodilator medication with a diagnosis of bronchial asthma. Her symptoms recurred twice in the following years, responding to inhaled steroids.
She presented to us with a two-month history of fever, shortness of breath, and cough with partial response to bronchodilators. She had developed left sided chest pain one week prior to presentation. She had been evaluated at another hospital before presenting to us. Based on preliminary evaluation suggestive of hydropneumothorax in a region of high endemicity, the patient was presumptively diagnosed to have pulmonary tuberculosis and initiated on anti-tuberculous therapy (ATT) at this center. Further evaluation, if any, performed at the outside center is not available to the authors.
On examination on the day of admission (day 0), the patient was in respiratory distress with respiratory system examination significant for a right-sided tracheal shift, and reduced chest expansion and air entry over the entire left hemithorax. Chest radiography at the time demonstrated a left sided hydropneumothorax. She thus underwent emergent intercostal drain (ICD) placement.
In view of a clinical presentation of fever with hydropneumothorax in an endemic region, ATT was continued initially. On further evaluation, the patient was found to have peripheral blood eosinophilia, raised total serum IgE, presence of serum precipitins to aspergillus and elevated aspergillus specific IgE (Table 1). Pleural fluid demonstrated an exudative pattern with lymphocyte predominance, a low adenosine de-aminase level and negative microbiological workup for tuberculosis and other bacterial/fungal infections. Computed tomography (CT) scanning demonstrated a left sided hydropneumothorax with collapse of the underlying lung and central bronchiectatic changes (Fig. 1). Bronchoscopy and a broncho-alveolar lavage (BAL) were undertaken to rule out a possible infective cause. BAL fluid analysis demonstrated negative tubercular, bacterial and fungal cultures and malignant cytology. A diagnosis of ABPA was established per the criteria suggested by the (ISHAM) [2]. Important alternative diagnosis considered for the hydropneumothorax included tuberculosis that was excluded based on negative laboratory and microbiological tests and a locally complicated bacterial pneumonia that was considered unlikely based on the chronicity of symptoms and background history.
Table 1.
Diagnostic evaluation for allergic bronchopulmonary aspergillosis.
| Laboratory Investigation | Result |
|---|---|
| Absolute Eosinophil Count | 1540/μL |
| Asperg llus-specific Precipitins | Positive (By Immunodiffusion) |
| Serum Total IgE level | 19969 IU/mL |
| Aspergillus-Specific IgE | 13.7 kU/L |
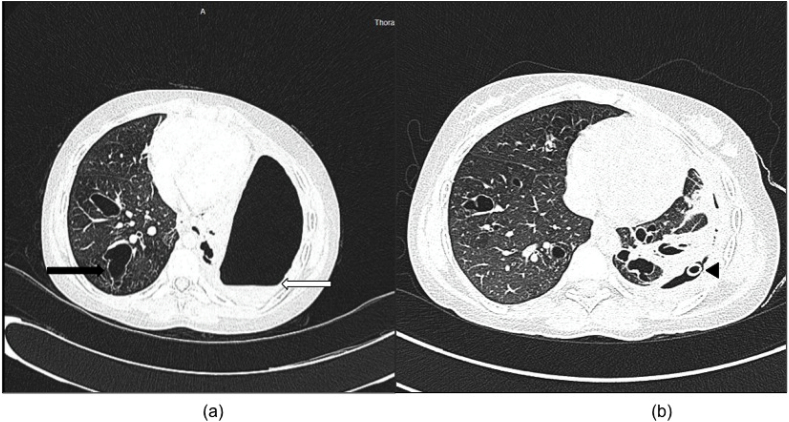
Fig. 1.
(a) High-Resolution Computed Tomography scan of the chest demonstrating a left sided hydropneumothorax with an air fluid level (white arrow) with collapse of the underlying lung. The right lung shows central bronchiectatic changes (black arrow). (b) Partial re-expansion of the left lung was noted post-tube thoracostomy (black arrowhead) with bilateral central bronchiectasis.
ATT was discontinued after exclusion of an etiological diagnosis of tuberculosis and therapy initiated with steroids (prednisolone at an initial dose of 30 mg/d) and itraconazole (200 mg twice daily). Subsequent chest radiographs demonstrated only partial lung re-expansion and negative suction was placed on the ICD. However, the lung failed to re-expand and a persistent air leak was detected. (Cerfolio Grade 2, E). She underwent a repeat CT scan that demonstrated a bronchopleural fistula (BPF).
Numerous bronchoscopic techniques for BPF closure have been described in literature. We attempted a bronchoscopic closure with doxycycline and autologous blood patch instillation (day 8), which was unsuccessful [5]. Subsequent chest radiographs failed to show complete re-expansion and the air leak persisted. She was considered for video-assisted thoracoscopic (VATS) closure of the BPF; however, she was not a surgical candidate in view of bilateral underlying lung disease and poor general condition. A multi-disciplinary team comprising of physicians, surgeons, pulmonologist and family members favored a conservative approach to surgical management. The air leak gradually diminished, having resolved completely by the 15th day post tube thoracostomy. Chest radiographs demonstrated gradual re-expansion of the left lung. The ICD was removed and there was no respiratory distress or pneumothorax during observation in hospital.
The patient was discharged on steroids and anti-fungal therapy. Prednisolone was continued at a dose of 30 mg/d till day 14 followed by a gradual taper. She was successfully tapered off steroids after a period of 13 weeks (day 90). Itraconazole was continued for the recommended duration (day 120). She was noted to improve further over the course of outpatient follow up with a gradual resolution of fever and cough.
3. Discussion
Hydropneumothorax is defined as the abnormal presence of air and fluid in the pleural space. A recent cohort study [6] conducted in an Indian tertiary care center evaluated fifty-seven patients with non-traumatic hydropneumothorax. Tuberculosis was found to be the commonest etiology, followed by bacterial infections and pleural or pulmonary malignancies.
Our literature search yielded three case reports of pneumothorax in ABPA. Ricketti et al. were the first to describe a case of secondary spontaneous pneumothorax (SSP) in a patient with ABPA. They hypothesized that the SSP was a consequence of underlying bullous lung disease and that this finding is rare in ABPA because of the central distribution of the disease [7]. Judson et al. have reported a case of SSP associated with BPF in a patient with ABPA that is the only previous case with the finding of BPF. They have suggested that the formation of the subpleural cystic spaces may be secondary to ball valve obstruction of the small airways by mucus [8]. Pneumothorax is a common complication of lung disease in cystic fibrosis, which contributes significantly to the morbidity and mortality of these patients. ABPA, which is also known to occur commonly in patients with cystic fibrosis, has been found to be associated with an increased risk of pneumothorax in this population [9]. To the best of our knowledge, this is the first case reported of hydropneumothorax in a patient with ABPA.
While SSP due to ABPA with asthma is managed with tube thoracostomy and treatment of the underlying disease, prolonged air leak is a challenging problem. Current guidelines recommend surgical intervention in patients with prolonged air leak (lasting more than 5–7 days) [10]. VATS is the preferred technique for the management of these cases. The goals of treatment in patients presenting with acute symptoms of ABPA include control of acute symptoms, prevention of future exacerbations and preservation of lung function. The mainstay of therapy is oral corticosteroids [1]. Mounting evidence supports the added benefit of anti-fungal agents in patients with severe or steroid-dependent ABPA [3,4].
Conflict of interest
The authors have no conflicts of interest to declare.
References
- 1.Greenberger P.A. Allergic bronchopulmonary aspergillosis. J. Allergy Clin. Immunol. 2002;110(5):685–692. doi: 10.1067/mai.2002.130179. [DOI] [PubMed] [Google Scholar]
- 2.Agarwal R., Chakrabarti A., Shah A., Gupta D., Meis J.F., Guleria R. Allergic bronchopulmonary aspergillosis: review of literature and proposal of new diagnostic and classification criteria. Clin. Exp. Allergy. 2013;43(8):850–873. doi: 10.1111/cea.12141. [DOI] [PubMed] [Google Scholar]
- 3.Wark P.A., Hensley M.J., Saltos N., Boyle M.J., Toneguzzi R.C., Epid G.D. Anti-inflammatory effect of itraconazole in stable allergic bronchopulmonary aspergillosis: a randomized controlled trial. J. Allergy Clin. Immunol. 2003;111:952–957. doi: 10.1067/mai.2003.1388. [DOI] [PubMed] [Google Scholar]
- 4.Denning D.W., Van Wye J.E., Lewiston N.J., Stevens D.A. Adjunctive therapy of allergic bronchopulmonary aspergillosis with itraconazole. Chest. 1991;100:813–819. doi: 10.1378/chest.100.3.813. [DOI] [PubMed] [Google Scholar]
- 5.Sarkar P., Chandak T., Shah R., Talwar A. Diagnosis and management bronchopleural fistula. Indian J. Chest Dis. Allied Sci. 2010;52(2):97–104. [PubMed] [Google Scholar]
- 6.Kasargod V., Awad N.T. Clinical profile, etiology, and management of hydropneumothorax: an Indian experience. Lung India. 2016;33(3):278–280. doi: 10.4103/0970-2113.180804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ricketti A.J., Greenberger P.A., Glassroth J. Spontaneous pneumothorax in allergic bronchopulmonary aspergillosis. Arch. Intern. Med. 1984;144(1):151–152. [PubMed] [Google Scholar]
- 8.Judson M.A., Marshall C., Beale G., Holt J.B. Pneumothorax and bronchopleural fistula during treatment of allergic bronchopulmonary aspergillosis. South. Med. J. 1993;86(9):1061–1063. doi: 10.1097/00007611-199309000-00019. [DOI] [PubMed] [Google Scholar]
- 9.Flume P.A., Strange C., Ye X., Ebeling M., Hulsey T., Clark L.L. Pneumothorax in cystic fibrosis. Chest. 2005;128(2):720–728. doi: 10.1378/chest.128.2.720. [DOI] [PubMed] [Google Scholar]
- 10.Davies H.E., Davies R.J., Davies C.W., BTS Pleural Disease Guideline Group Management of pleural infection in adults: British thoracic society pleural disease Guideline2010. Thorax. 2010;65:ii41–53. doi: 10.1136/thx.2010.137000. Suppl 2. [DOI] [PubMed] [Google Scholar]