Abstract
Mesenchymal stem cells (MSCs) in vivo reside in a complex microenvironment. Changes of both biochemical and biophysical cues in the microenvironment caused by inflammation affect the differentiation behaviors of MSCs. Most studies, however, only focus on either biochemical or biophysical cues, although the synergistic effect of matrix stiffness and inflammatory factors on osteogenic differentiation of MSCs has not been explored yet. Here, we showed that there was a matrix stiffness-dependent modulation in the osteogenic differentiation of human MSCs (hMSCs) with higher matrix stiffness favoring osteogenesis bias. However, when interleukin-1 β (IL-1β) was added, the osteogenic differentiation of hMSCs was suppressed, which was independent of increasing matrix stiffness. Both experimental observations and mathematical modeling confirmed that matrix stiffness and IL-1β could activate the ERK1/2 signaling and contribute to osteogenic differentiation. The p38 signaling activated by IL-1β has a strong role in inhibiting osteoblastic differentiation, thus diminishing the vital effect of ERK1/2 signaling. In addition, sensitivity analysis of the model parameters revealed that activation/deactivation dynamics of sensitive factors (e.g., FAK, ERK, and p38) also played a key role in the synergistic effect of matrix stiffness and IL-1β on the osteogenic differentiation of hMSCs. The outcomes of this study provide new insights into the synergistic effect of biochemical and biophysical microenvironments on regulating MSC differentiation.
Significance
The fate of mesenchymal stem cells in vivo is regulated by combinations of biochemical and biophysical cues in the extracellular microenvironment. Our study demonstrates that the matrix stiffness and inflammatory factor (interleukin-1 β (IL-1β)) have the opposite role in regulating osteogenic differentiation with inflammatory cytokine inhibiting matrix stiffness-induced osteogenic differentiation. Surprisingly, both matrix stiffness and IL-1β can activate the ERK1/2 signaling, contributing to osteogenic differentiation. The p38 signaling activated by IL-1β has a strong role in suppressing OCN expression, thus diminishing the vital effect of ERK1/2 signaling. Our study highlights the negative effects of an inflammatory environment on osteogenic differentiation and may facilitate the development of new strategies to improve the osteoinductive efficacy by mesenchymal stem cell-based therapies in regenerative medicine.
Introduction
Mesenchymal stem cells (MSCs) are capable of differentiating into bone, cartilage, fat, and other stromal cell types, which have found widespread applications in tissue engineering and regenerative medicine (1, 2). For these applications, it is of great importance to regulate MSC behaviors, especially its differentiation bias. MSCs in vivo reside in a complex microenvironment, where both biochemical and biophysical cues in the microenvironment have been found to play important roles in regulating their fate (e.g., differentiation into a specific lineage) (3, 4). The effect of the biochemical cues on stem cell fate has received intensive studies by using growth factors, hormones, small chemicals, etc. (5, 6, 7). Specifically, an inflammatory microenvironment has been found to impair the osteogenic capacity of stem cells (8, 9). In addition to biochemical cues, accumulating evidence has also shown that biophysical cues (e.g., matrix stiffness) play important roles in regulating stem cell fate (10, 11, 12, 13). For instance, in osteogenic media, MSCs did not commit to osteogenesis unless the matrix stiffness increased to a certain threshold (14). This suggests that both matrix stiffness and growth factors contribute to the regulation of MSC differentiation. Besides, a number of subsequent investigations have demonstrated that the cross talk between matrix stiffness and growth factor signaling determines MSC differentiation (15, 16).
In addition to growth factors, inflammatory factors exist in the development of many diseases and are key biochemical stimuli involved in a range of pathological processes. For instance, the capacity of cell differentiation can be affected by inflammatory factors, which affects tissue regeneration and leads to tissue damage (17). Inflammatory cytokines are also the main contributors, which mediate the degradation of extracellular matrix (ECM) in the inflammatory microenvironment (18). Thus, both the inflammatory factors and altered ECM (decreased matrix stiffness) have an impact on MSC behaviors, especially their differentiation, which further affects the regeneration processes of defected tissue. Therefore, improving our knowledge of the synergistic effect of matrix stiffness and inflammatory factors on MSC fate will help realize their potential for tissue regeneration.
The role of matrix stiffness in regulating stem cell differentiation has received intensive study. For instance, it has been reported that matrix stiffness regulates cell osteogenic differentiation through mitogen-activated protein kinase (MAPK) pathway activation (19, 20, 21). Evidence shows that matrix stiffness regulates MSC differentiation through extracellular signal-regulated kinases (ERK) or c-Jun N-terminal kinases activation (19). Besides matrix stiffness, the inflammatory factors have been found to inhibit the capacity of osteogenic differentiation of cells. For instance, interleukin-1 β (IL-1β), as a member of the interleukin-1 family, plays an important role in immune regulation and periodontitis by stimulating the expression of genes related to inflammation and autoimmunity. Similar to matrix stiffness, IL-1β also activates MAPK pathway in inflammatory environments (14, 22). However, in contrast to the positive role of matrix stiffness in osteogenic differentiation, IL-1β has been proved to induce bone loss by activating osteoclastogenesis and inhibiting osteogenic differentiation, leading to a decrease in bone mineral density and bone formation (23, 24). These suggest that matrix stiffness and IL-1β may have opposite effects on osteogenic differentiation, whereas the synergistic effects of matrix stiffness and inflammatory factors on osteogenic differentiation of MSCs remain elusive in view of the cross talk between biochemical and biomechanical cues triggered by the signaling pathways. A broader and deeper understanding of the synergistic effect of matrix stiffness and inflammation on osteogenic differentiation of MSCs is essential to improve the outcome of MSC-based therapies.
Periodontitis is a typical inflammatory disease among the most common oral infectious diseases, especially in adults (25), because of the establishment of a highly pathogenic biofilm that triggers an immune/inflammatory host response. This host response leads to the destruction of supporting periodontal tissue and eventual tooth loss mainly due to the loss of bone tissue in periodontal tissue (25, 26). MSCs in periodontal tissue do not perform tissue repair naturally in diseased periodontal environment (27) for the reasons of the potential inhibition of periodontium formation and regeneration by the special microenvironmental cues in periodontal tissue (e.g., increased inflammation and decreased ECM stiffness). In this study, we constructed substrates with different stiffness and mimicked an inflammatory microenvironment using IL-1β. The synergistic effect was then applied to human MSCs (hMSCs) (PDL-derived). Osteogenic differentiation of hMSCs was then assessed. Signaling pathways (ERK1/2, p38) possibly involved in the regulation of osteogenic differentiation were further explored, followed by mathematical models to help confirm our experimental results and further complement the mechanism. A schematic diagram of the methods for developing this study is depicted in Fig. 1. This study provides a new insight into the synergistic effect of biochemical and biophysical microenvironments on regulating MSC differentiation, which contributes to MSC-based therapies in regenerative medicine.
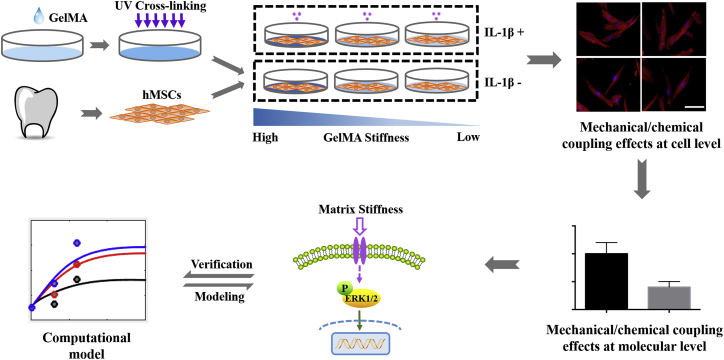
Figure 1.
Schematic of the strategy for studying the synergistic effect of matrix stiffness and inflammatory factors on osteogenic differentiation of human mesenchymal stem cells (hMSCs). hMSCs were acquired from the periodontal ligament. A different concentration of GelMA hydrogel was used to construct a graded stiffness for a two-dimensional cell culture environment. Cells were stimulated w/wo interleukin-1β (IL-1β+/IL-1β−). The effects of combined matrix stiffness and IL-1β on hMSC osteogenic differentiation and the potential modulatory mechanisms were then explored by experiments and followed by the verification of the mathematical model. Scale bars, 100 μm. To see this figure in color, go online.
Materials and Methods
Preparation of gelatin methacryloyl hydrogels
Gelatin methacryloyl (GelMA) macromers were synthesized according to a previously described method (28). Briefly, to obtain a 10 weight percentage (wt %) gelatin aqueous solution, 10 g gelatin (type A, 300 bloom; Sigma-Aldrich, St. Louis, MO) was dissolved in 100 mL of phosphate-buffered saline (PBS) at 60°C under stirring. Then, 8 mL methacrylic anhydride (Sigma-Aldrich) was added into the gelatin solution at a rate of 0.5 mL/min under stirring at 50°C. After a reaction in the dark for 4 h, the products were diluted with fivefold PBS (50°C) and then dialyzed against Milli-Q water for 7 days at 40°C using a dialysis membrane (12–14 kD molecular weight cut-off; Spectrum Laboratory, Cary, NC) to remove salts and excess free methacrylic anhydride. The products were then lyophilized for 2 days to obtain white porous foam.
To obtain GelMA hydrogels with different stiffness, different concentrations of GelMA macromers (10, 12, and 14 wt %) and photoinitiator 2-hydroxy-1-(4-(hydroxyethoxy) phenyl)-2-methyl-1-propanone (Irgacure 2959; Sigma-Aldrich) (1 wt %) were dissolved in PBS at 50°C. The solution was added into a six-well plate (Thermo Fisher Scientific, Waltham, MA), forming a hydrogel substrate with a diameter of ∼35 mm and a thickness of ∼1 mm. The construct was exposed to 365 nm ultraviolet light (CL-1000; Funakoshi, Tokyo, Japan) at a distance of 20 cm for 1 min with an irradiation intensity of 6.9 mW/cm2 (Fig. 1).
The stiffness of GelMA hydrogels was determined by BOSE ELF 3200 dynamic mechanical analyzer (BOSE). Hydrogel samples were incubated in PBS at 37°C for 24 h, and their stiffness was then measured at room temperature. Five samples of each group were measured for the calculation of means and SDs.
Cell culture
hMSCs were isolated and cultured according to the previously published protocols (Fig. 1) (6), and all procedures were approved by the Ethics Committee in Xi’an Jiaotong University. More comprehensively, periodontal membranes were obtained from recently extracted human teeth from five individuals ranging between 12 and 16 years old who were treated at the Stomatology Hospital of Xi’an Jiaotong University. All teeth were extracted for orthodontic reasons and were healthy with no sign of disease. For this study, we used the middle part of the periodontal membrane surrounding the root. To culture primary hMSCs, the periodontal membrane was cut into small pieces and digested with 0.1% type I collagenase (Sigma-Aldrich) for 30 min at 37°C. Then, the cell suspensions were filtered and cultured in Dulbecco minimal essential medium (Gibco, Gaithersburg, MD) supplemented with 20% fetal bovine serum (Gibco), 100 U/mL of penicillin, and 100 mg/mL of streptomycin (Solarbio, Beijing, China) in 5% CO2 at 37°C. After 2 weeks, the cells from tissues were digested by trypsin and single cell-derived colony cultures were obtained using the limiting dilution technique. Multiple colony-derived hMSCs were used in this study after three to four passages. For each experiment, the same passage of hMSCs was used. For hMSC identification, both osteogenic differentiation and adipogenic differentiation abilities were assessed by alizarin red staining and oil red O staining after osteogenic induction and adipogenic induction culture, respectively. And the expression of the surface markers, including STRO-1, CD90, CD146, CD45, and CD34, were detected by the BD FACSCanto flow cytometer (Becton Dickinson, Franklin Lakes, NJ). Cell characterization results were presented in Fig. S1.
Osteogenic differentiation was induced by culturing hMSCs in an osteogenic medium, containing 100 nM dexamethasone, 0.28 mM ascorbate-2-phosphate, and 10 mM β-glycerol phosphate (29). Cells were cultured for 2 weeks, with the medium being replaced every 3 days. IL-1β (1 ng/mL; PeproTech, Rocky Hill, NJ) was added into the medium, mimicking the native inflammation (24), and fresh cytokine was added at every medium change. IL-1β was added into the cell culture medium right after seeding the cells onto the surface of the hydrogels. Because cell mechanosensing occurs immediately when cells contact the substrate (30, 31), we assume that the IL-1β and stiffness regulate the cellular signals at the initial time, simultaneously. Osteogenic differentiation was determined by osteocalcin (OCN) and Runx2 expression.
hMSCs behavior characterization
After GelMA was cross-linked by ultraviolet radiation in wells, hMSCs (three to four passages) were seeded on hydrogels at a density of 1 × 105 cells/mL, and the volume of culture medium in each well (six-well plate) was 2 mL. Cell viability was determined by Cell Count Kit-8 (CCK-8; 7Sea Pharmatech, Shanghai, China) with the stimuli of both matrix stiffness and IL-1β. After 3 days of culture, the culture medium was replaced with the medium containing 10% (v/v) CCK-8, and hMSC-laden hydrogels were incubated within the medium at 37°C for 1 h. 100 μL of the reaction solution was taken into a 96-well plate. The optical density of the reaction solution was measured at 450 nm through a microplate reader (Bio-Rad Laboratories, Hercules, CA). Five replicates were measured for each hydrogel substrate. To evaluate cell spreading on different GelMA hydrogels with or without (w/wo) IL-1β treatment after cell seeding, rhodamine-labeled phalloidin (Life Technologies, Carlsbad, CA) and 4′,6-diamidino-2-phenylindole (DAPI) (Sigma-Aldrich) were used to stain F-actin and cell nuclei, respectively. First, hMSC-laden hydrogels were rinsed by PBS three times and were fixed by 4% paraformaldehyde for 15 min. Then, they were permeabilized via 0.1% Triton X-100 for 15 min and subsequently blocked in 5% (w/v) bovine serum albumin (BSA) for 30 min. Next, hMSC-laden hydrogels were incubated orderly in rhodamine-labeled phalloidin solution and DAPI solution at 37°C for 20 and 5 min, respectively. Finally, hMSC-laden hydrogels were imaged using a fluorescence microscope (Olympus, Tokyo, Japan). The cell spreading area was quantified by processing the images via ImageJ software. Briefly, the cell area was first quantified by ImageJ in the unit of pixels and then converted to unit of μm2, according to the relation between scale bar and pixel.
Immunofluorescence staining
hMSCs were seeded onto GelMA hydrogels with a different stiffness and cultured in growth medium (Dulbecco minimal essential medium supplemented with 10% fetal bovine serum) for 1 day. Then, the cells were incubated in osteogenic medium w/wo IL-1β (1 ng/mL) treatment for another 14 days. After washing with PBS, cells were fixed by 4% paraformaldehyde for 20 min, permeabilized in 0.5% Triton X for 10 min, and were finally blocked for 30 min with 5% BSA at room temperature. Then, cells were incubated overnight with anti-OCN antibody and anti-Runx2 antibody (1: 100; Abcam, Cambridge, U.K.) at 4°C. Then, DyLight 488 and DyLight 594 polyclonal (Invitrogen, Carlsbad, CA) were used as a secondary antibody. Nuclei were counterstained with DAPI. The image collection and superimposition were processed by fluorescence microscope (Olympus) and ImageJ, respectively. The experiment was repeated for at least three times. Staining of OCN and Runx2 was quantified in terms of the ratio between the number of OCN- or Runx2-expressing cells to the total number of cells using the ImageJ software.
Real-time PCR
After cell culturing on GelMA hydrogels and incubated in osteogenic medium with or without IL-1β (1 ng/mL) for 14 days, total RNA was isolated from hMSCs using TaKaRa MiniBEST Universal RNA Extraction Kit (TaKaRa Bio, DaLian, China), and the complementary DNAs were synthesized using PrimeScript RT Reagent Kit with genomic DNA Eraser (TaKaRa Bio). Quantitative real-time PCR was performed for 40 cycles at 95°C for 3 s and 60°C for 30 s using a 7500 Fast Real-Time PCR System (Applied Biosystems, Foster City, CA) with the SYBR Premix Ex Taq II Kit (TaKaRa Bio). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as an internal control. All the operations were carried out according to the manufacturer’s instructions. The forward and reverse primers are shown as follows: OCN-forward GTGCAGAGTCCAGCAAAGGT, OCN-reverse TCAGCCAACTCGTCACAGTC, Runx2-forward CACAGAGCAATTAAAGTTAC, Runx2-reverse CTAGGTTTAGAGTCATCAAG, GAPDH-forward GGACCTGACCTGCCGTCTAG, and GAPDH- reverse TAGCCCAGGATGCCCTTGAG.
Real-time PCRs were repeated on at least three independent RNA preparations. The OCN messenger RNA and Runx2 messenger RNA expression values were calculated using the 2−ΔΔCt method (ΔCt = the mean cycle threshold Ct of the target gene – the mean Ct of GAPDH; ΔΔCt = ΔCt of experimental group −ΔCt of control group). The group of cells cultured on low stiffness substrate without IL-1β treatment was used as a control, and other groups were used as experimental groups for gene expression.
Western blot
The phosphorylation levels of ERK1/2 and p38 were evaluated by Western blot. hMSCs were incubated on GelMA hydrogels with or without IL-1β (1 ng/mL) for 0.5, 1, and 2 h, respectively. The incubation times were selected according to literature (32). After treatment, cells were harvested and washed twice with PBS. For Western blotting, total proteins were harvested using the Mammalian Protein Extraction Kit (CoWin BioSciences, Cambridge, MA), according to the manufacturer’s instructions. These protein samples were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred onto preactivated polyvinylidene fluoride membranes. The blotted membranes were blocked with 5% BSA for 1 h at room temperature and incubated overnight with primary antibodies (anti-phospho-ERK1/2 antibody, anti-phospho-p38 antibody, anti-ERK1/2 antibody, and anti-p38 antibody (1:1000; Cell Signaling Technology, Danvers, MA)) at 4°C. After washing with TBST (tris-buffered saline and tween 20), the membranes were incubated with secondary antibodies (1:5000; Cell Signaling Technology) for 1 h at room temperature. Finally, the bands were visualized using the Odyssey infrared imaging system (LI-COR Biosciences, Lincoln, NE). Different MAPK signaling pathways were blocked with the ERK1/2-specific inhibitor U0126 (10 Μm; Abcam) and p38-specific inhibitor SB203580 (10 μM; Abcam) by treating cells at 37°C for 1 h. Band intensity was measured by ImageJ software in term of gray intensity, and the phosphorylation level of p-ERK1/2 and p-p38 were obtained by the ratio between the gray intensity of phosphorylated protein and the corresponding total protein.
Alizarin red staining
After 21 days of osteogenic induction culture, hMSCs were washed with PBS and fixed with 4% paraformaldehyde for ∼15 min. Then, cells were stained with alizarin red S (40 mM; Sigma-Aldrich) solution (pH 4.1) for 10 min to visualize matrix calcium deposition. To further quantify the results of alizarin red staining, we incubated the stained cells in 500 μL of 10% (w/v) cetylpyridinium chloride dissolved in disodium hydrogen phosphate solution at 37°C for 10 min. Finally, the optical density was determined at 570 nm through a microplate reader (Bio-Rad Laboratories).
Mathematical modeling of matrix stiffness and IL-1β-mediated signaling pathway
To investigate the synergistic effects of matrix stiffness and IL-1β on the osteogenic differentiation of hMSCs, we developed a mathematical model composed of a series of ordinary differential equations (ODEs), which can well describe the time-dependent dynamics of the biomolecule interaction system (33, 34). For simplicity, we assumed that the total amount of each protein can be normalized to one in our model. The proteins associated with signaling pathways in our model only contain two states (i.e., the active state and the inactive state). The proposed model contains the following two signaling processes, which affect the osteogenic differentiation of hMSCs (i.e., the OCN activation and expression): 1) mechanical signaling process that involves fibronectin (Fn)/integrin/FAK/ERK cascade (35, 36) 2) and biochemical signaling process that involves IL-1β/IL-1βR/p38 cascade.
First, we used a motor-clutch model to describe the substrate stiffness-dependent FAK activation and obtained the relationship between substrate stiffness and FAK activation rate (34). Then, we used this relationship as an input of the ODE model. In our motor-clutch model, integrin clutches bound to substrate clutches with a rate of kon, forming a force transmission pathway from intracellular cytoskeleton, where contraction arose from inward actin flow, to the extracellular substrate. The unbinding rate of koff,i vary with the force Fi in the connected clutch according to the Bell model:
| (1) |
| (2) |
where is the unloaded unbinding rate, Fb is the characteristic rupture force, xi is the elongation of the connected adhesion-substrate clutch, and ke = kiE/(ki + E) is the effective spring constant of the adhesion-substrate clutch complex. The actin filaments are pulled by nm myosin motors, each capable of exerting a force of Fm resulting in inward actin flow with an unloaded rate of Vu. The effective actin flow rate (Vr) is related to stall force of the ensemble of myosin motors (Fstall = nmFm):
| (3) |
where the traction force Fs transmitted by all connected clutches was:
| (4) |
in which nc is the number of connected clutch bonds. Also, the FAK molecules can be activated by active integrin with a rate of kFon, and the FAK molecule inactivation rate is kFoff. Later, we used the Hill function to fit the simulation data and act as ODE model input:
| (5) |
The motor-clutch model parameters used in the simulation were listed in Table S1.
The corresponding ODE equations of above signaling pathways were then developed according to the mass-action principle. The integrin could be activated by Fn and then clustered into integrin clusters:
| (6) |
| (7) |
where the k1f and k1r are the integrin activation and deactivation rates (min−1); [CFn], [Cintegrin-on], and [Cintegrin-off] are the concentrations of Fn, active integrin molecules, and inactive integrin molecules, respectively. The stiffness-dependent FAK activation and FAK autophosphorylation can be expressed as follows:
| (8) |
| (9) |
| (10) |
where the k2f-S and k2f are the stiffness-mediated FAK activation and autophosphorylation rates (min−1), k2r is the deactivation rate (min−1), E is the value of matrix stiffness, [CFAK-on] and [CFAK-off] are the concentrations of active and inactive FAK molecules, and K1 is the fitting parameter. The FAK-mediated ERK1/2 activation and, importantly, IL-1β receptor (IL-1βR)-dependent ERK1/2 activation can be expressed as follows:
| (11) |
| (12) |
| (13) |
| (14) |
where the k3f and k3f-B are the FAK-mediated ERK1/2 activation and autophosphorylation rates (min−1), k3f-I is the IL-1βR-mediated activation rate (min−1), k3r is the deactivation rate (min−1), and [CERK-on] and [CERK-off] are the concentrations of active and inactive ERK1/2 molecules. The binding and activation of IL-1βR with IL-1β on the membrane can be expressed as follows:
| (15) |
| (16) |
where the k4f and k4r are the IL-1β binding and unbinding rates (min−1), and [CIL], [CILR-on], and [CILR-off] are the concentrations of IL-1β, active, and inactive IL-1βR molecules. The p38 activation and deactivation processes can be expressed as follows:
| (17) |
| (18) |
| (19) |
where the k5f and k5f-B are the IL-1β-mediated and -based p38 activation rates (min−1), k5r is the p38 dephosphorylation rate (min−1), and [Cp38-on] and [Cp38-off] are the concentrations of active and inactive p38 molecules. Finally, we assumed that the EKR1/2 and p38 are the main regulators of OCN production. Activation of ERK can promote OCN expression, whereas p38 inhibits OCN expression, and for simplicity, this process can be expressed as follows:
| (20) |
| (21) |
where the k6f and k6r are the OCN activation and deactivation rates (min−1) and [COCN] is the concentration of OCN molecules. The MAPK phosphatase (MKP) activation and deactivation can be described as follows:
| (22) |
| (23) |
| (24) |
where k7f_B and k7f are MKP-based activation and p38-mediated MKP activation rates, and k7r is the deactivation rate. The MATLAB (The MathWorks, Natick, MA) optimization function lsqnonlin was used to find the parameters that can best fit to experimental data.
Sensitivity analysis of the model parameters
The sensitivity value S of the level of OCN to a parameter p is defined in Eq. 25, where p is the base parameter value, and Cocn is the level of OCN:
| (25) |
The S value is equivalent to the slope of Cocn versus p on a log-log plot and represents the fold-change in the level of OCN resulting from a fold change in a parameter value. The S value was calculated by plotting Cocn at 0.1-, 0.3-, 1-, 3-, and 10-fold changes of the base parameter on a log-log scale. A line was fitted to the data points with the slope of the line taken to be the S value.
Statistical analysis
The data were analyzed by one-way analysis of variance with SPSS13.0. The values were expressed as the mean ± SD. p < 0.05 was considered statistically significant.
Results
Characterizations of the GelMA hydrogel
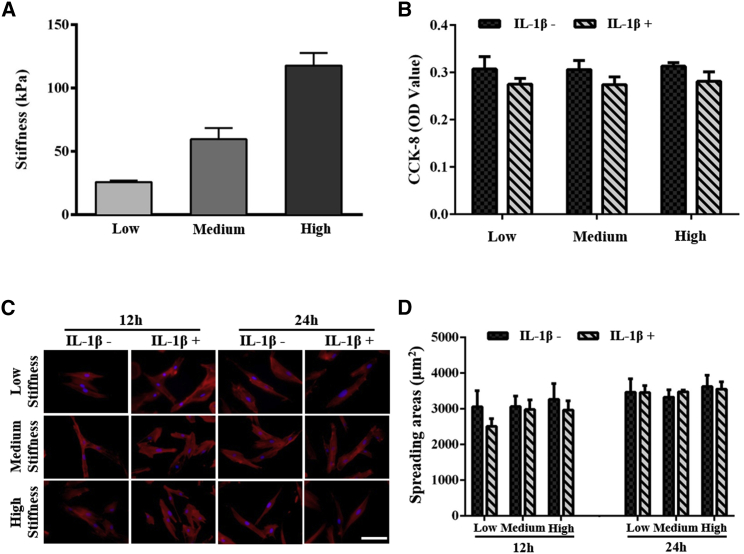
To simulate the matrix stiffness under normal and inflammatory conditions, we prepared GelMA hydrogels with three different stiffness by tuning the concentration of GelMA macromers (i.e., 10, 12, and 14%). By increasing the macromere concentration, we obtained GelMA hydrogel with a matrix stiffness of 25.75 ± 1.21, 59.71 ± 8.87, and 117.82 ± 9.83 kPa, respectively (Fig. 2 A). The range of the matrix stiffness mimics native PDL stiffness from being healthy to inflammatory.
Figure 2.
Characterization of the mechanical property of GelMA hydrogel and the hMSC behaviors on different stiffness of GelMA hydrogels w/wo IL-1β treatment. (A) Shown are GelMA hydrogels with high, medium, and low stiffness. (B) CCK-8 quantitative analysis of hMSCs viability on day 3 is shown. (C) Shown are F-actin/DAPI fluorescence images of hMSCs spreading after 12 and 24 h of culture. Scale bars, 100 μm. (D) Shown is the quantification of the staining of cell spreading area after 12 and 24 h of culture by the ImageJ software. ∗ indicates p < 0.05. To see this figure in color, go online.
To investigate the rate of cell survival, we assessed the synergistic effect of matrix stiffness and inflammatory cues (i.e., IL-1β here) on hMSC viability on day 3 (Fig. 2 B). We observed that there was no significant difference in cell viability on GelMA hydrogels with different stiffness and w/wo IL-1β treatment. By using immunofluorescence staining of F-actin, we characterized cell spreading on GelMA hydrogels with different stiffness and w/wo IL-1β treatment (Fig. 2 C). We observed that hMSCs in each group spread completely at 12 h, showing a long spindle morphology. Quantification of the spreading areas showed no significant difference in cell spreading between groups w/wo IL-1β treatment (Fig. 2 D).
The effect of matrix stiffness and IL-1β on hMSCs osteogenic differentiation
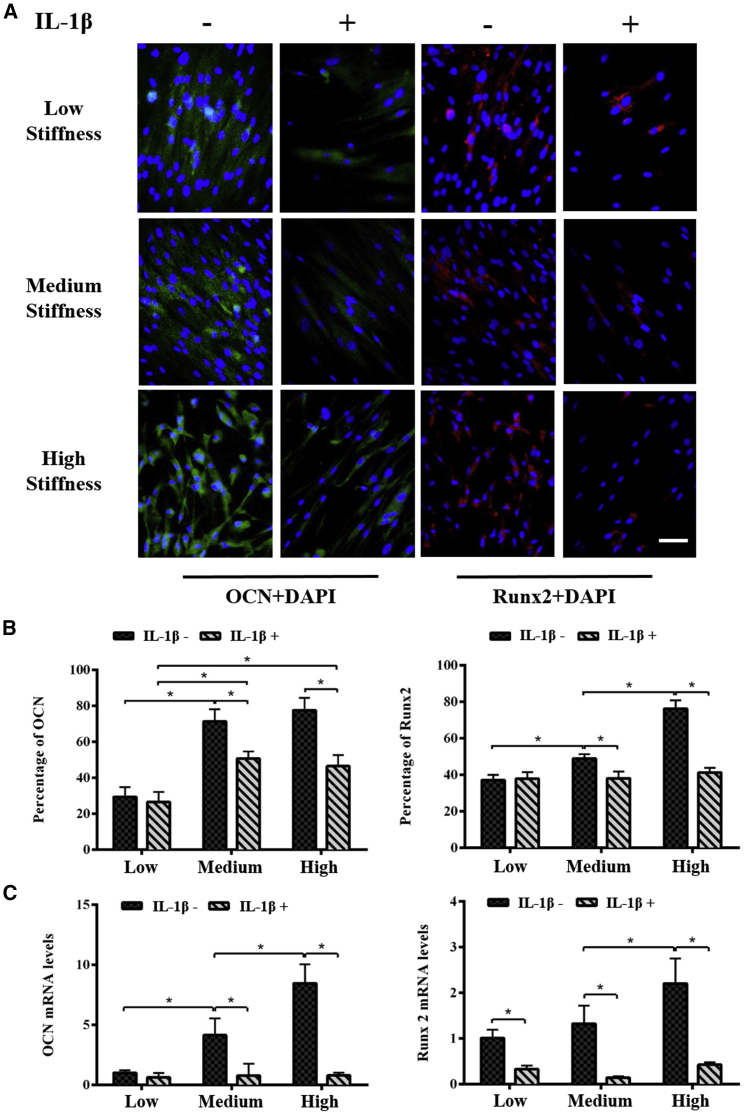
To investigate the synergistic effect of matrix stiffness and IL-1β on osteogenic differentiation of hMSCs, we first assessed the osteogenesis proteins (e.g., OCN and Runx2) expression of hMSCs cultured for 14 days in osteoinduction medium (Fig. 3). We found that the expressions of both OCN and Runx2 were significantly enhanced with an increasing matrix stiffness in all groups, whereas the addition of IL-1β resulted in decreased OCN and Runx2 expression as compared with those without IL-1β treatment in the groups with medium and high matrix stiffness (Fig. 3 A). We further quantified the OCN and Runx2 protein expression, which confirms the positive regulation of matrix stiffness and negative regulation of IL-1β on osteogenic differentiation of hMSCs (Fig. 3 B). To further confirm the synergistic effect, we then analyzed the expressions of OCN and Runx2 genes. Similar to the protein expressions, we also observed upregulated OCN and Runx2 genes with an increasing matrix stiffness and the downregulated OCN and Runx2 genes with IL-1β treatment (Fig. 3 C). The alizarin red staining results confirm both real-time PCR and immunofluorescence staining results (Fig. S2).
Figure 3.
Osteogenic differentiation of hMSCs cultured on different stiffness of GelMA hydrogels w/wo IL-1β treatment for 14 days. (A) Shown is the immunofluorescence staining of OCN (green) and Runx2 (red) in hMSCs. Expressions of both OCN and Runx2 were enhanced with increasing matrix stiffness, whereas they were inhibited with IL-1β treatment. Scale bars, 50 μm. (B) Shown is the quantification of the staining of OCN and Runx2 using the ImageJ software. (C) Real-time PCR was performed to analyze the expression of OCN and Runx2. OCN and Runx2 genes were upregulated with increasing matrix stiffness and then downregulated with IL-1β treatment. ∗ indicates p < 0.05. To see this figure in color, go online.
Matrix stiffness and IL-1β regulate osteogenic differentiation of hMSCs via ERK1/2 and p38 signaling
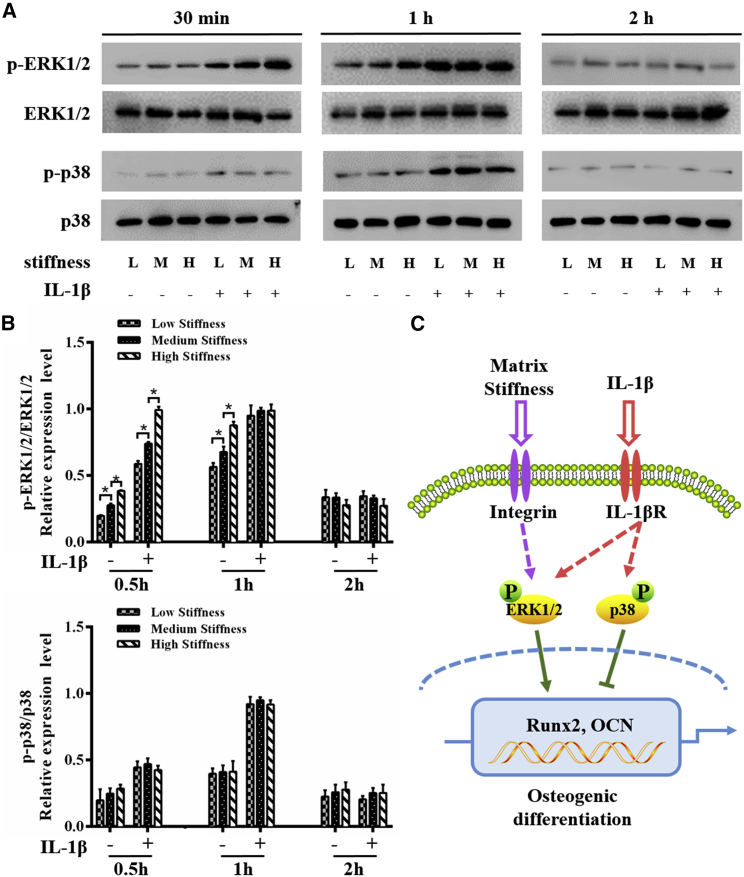
According to previous studies, matrix stiffness-driven MSC osteogenesis is associated with a high phosphorylation level of ERK (37, 38), whereas IL-1β inhibits osteogenic differentiation of stem cells by activating p38 (32). To determine how ERK1/2 and p38 signaling are involved in osteogenic differentiation of hMSCs upon the synergistic effect of matrix stiffness and IL-1β, we tested the phosphorylation level of ERK1/2 and p38 in the cells cultured on hydrogel substrates with a different stiffness and w/wo IL-1β treatment for 0.5, 1, and 2 h, respectively. We then analyzed the time-dependent changes in ERK1/2 phosphorylation and p38 phosphorylation (Fig. 4 A). Quantitative analysis of ERK1/2 and p38 phosphorylation showed a significant difference in the two groups (Fig. 4 B). It is noted that when cells were stimulated with matrix stiffness alone, ERK1/2 phosphorylation was significantly increased along with increasing matrix stiffness. And the activation of ERK1/2 was strengthened at 0.5 and 1 h after IL-1β treatment. As the incubation time increased to 2 h, the ERK1/2 phosphorylation decreased to a low level in all groups. Quite different from ERK1/2 phosphorylation, we found that the activation of p38 was time independent in groups without IL-1β treatment (0.5, 1, and 2 h), whereas IL-1β treatment effectively enhances the phosphorylation level of p38 with the phosphorylation level peaking at 1 h post-treatment. Besides, quantitative analysis of the Western blot bands shows that the phosphorylation level of p38 generally stayed at a low level at a different time without IL-1β treatment. These results indicate that matrix stiffness alone activates ERK1/2 but does not affect the p38 signaling, whereas IL-1β treatment activates both ERK1/2 and p38 signaling.
Figure 4.
Phosphorylation of ERK1/2 and p38 signaling molecules during the osteogenic differentiation of hMSCs w/wo IL-1β (1 ng/mL) treatment. (A) The levels of phosphorylated ERK1/2 and p38 at 0.5, 1, and 2 h were examined in whole-cell lysates via Western blotting. Matrix stiffness alone activates ERK1/2 but does not affect the p38 signaling, whereas IL-1β treatment activates both ERK1/2 and p38 signaling. (B) The average ratios of phosphorylated (p)-ERK1/2/ERK1/2 and p-p38/p38 were quantified by the ImageJ software based on the analysis of the gray band intensity. (C) Shown is a schematic of the effects of matrix stiffness and IL-1β on the osteogenesis of hMSCs via ERK1/2 and p38 signaling pathways. ∗ indicates p < 0.05. To see this figure in color, go online.
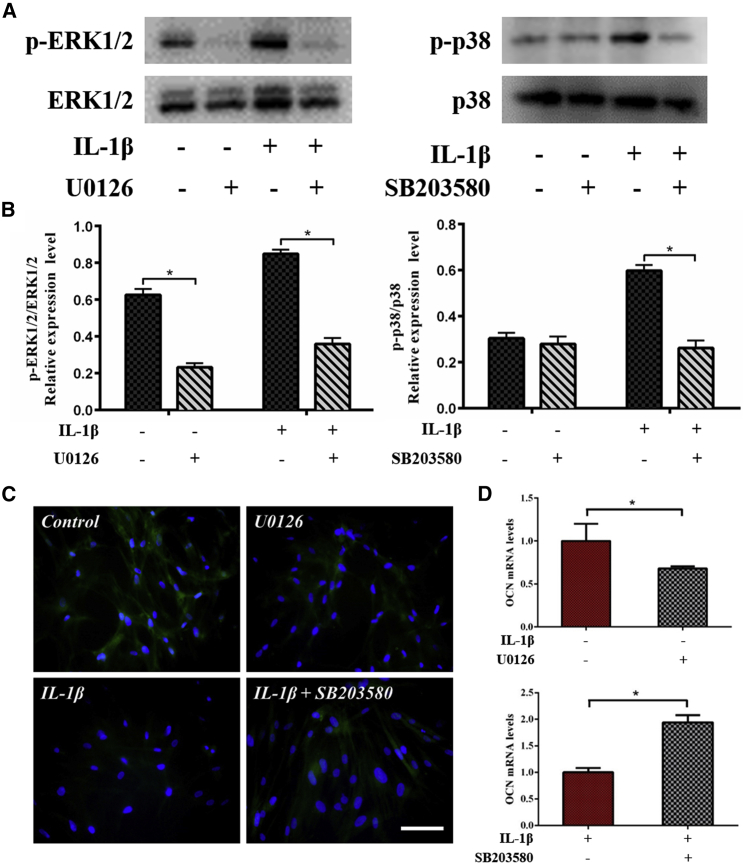
Based on the experimental observations of osteogenic differentiation (Fig. 3) and ERK1/2 and p38 phosphorylation (Fig. 4), we hypothesize that matrix stiffness probably promotes osteogenic differentiation of hMSCs through ERK1/2 signaling, whereas IL-1β inhibits it by activating p38 signaling. Besides, IL-1β treatment could also activate ERK1/2, but the inhibition of p38 plays a major role during this process as schematically shown in Fig. 4 C. To further clarify the role of ERK1/2 and p38 in osteogenic differentiation upon the synergistic effect of matrix stiffness and IL-1β treatment, we next blocked different MAPK signaling pathways with ERK1/2 (U0126) or p38 (SB203580) inhibitors. We found that phosphorylation levels of ERK1/2 and p38 after 1 h were effectively reduced with the treatment of inhibitors (Fig. 5, A and B). The immunofluorescence results show that blocking ERK1/2 decreases the expression level of OCN protein, whereas blocking p38 increases the expression level of OCN protein (Fig. 5 C). These results confirm that ERK1/2 has a positive role, whereas p38 has a negative role in osteogenic differentiation of hMSCs. The reverse transcription PCR results further support these observations (Fig. 5 D), showing that blocking ERK1/2 decreases and blocking p38 increases OCN expression at 14 days.
Figure 5.
The effects of ERK1/2 and p38 inhibitors on the osteogenic differentiation of hMSCs. (A) Shown is the Western blot analysis of the levels of phosphorylated ERK1/2 and p38 at 1 h. (B) The average ratios of phosphorylated (p)-ERK1/2/ERK1/2 and p-p38/p38 were calculated by the ImageJ software, showing that levels of phosphorylated ERK1/2 and p38 are effectively reduced with treatment of inhibitors. (C) Immunofluorescence staining of OCN (green) in hMSCs shows blocking ERK1/2 decreased OCN protein expression levels, whereas blocking p38 increased OCN protein expression levels. (D) Real-time PCR was performed with hMSCs and shows the same results with immunofluorescence staining. hMSCs were incubated on the high stiffness substrate in osteogenic differentiation medium with/without IL-1β (1 ng/mL) along with an inhibitor of ERK1/2 (U0126) or p38 (SB203580). Control: IL-1β−, U0126−; U0126: IL-1β−, U0126+; IL-1β: IL-1β+, SB203580−; IL-1β+; SB203580: IL-1β+, SB203580+. ∗ indicates p < 0.05. Scale bars, 100 μm. To see this figure in color, go online.
Mathematical modeling of stiffness and IL-1β-mediated signaling pathway
To investigate the mechanism underlying the synergistic effect, we developed a mathematical model using a set of ODEs to describe the coupled ERK1/2 and p38 signaling dynamical processes (Fig. 6 A). The phosphorylation level of FAK is associated with matrix stiffness, that is, a higher level of FAK phosphorylation can be found in cells when cultured on the stiffer substrate (39), thus promoting ERK activation, resulting in upregulated OCN expression (40). Hence, the effect of matrix stiffness was coupled into the mathematical model through a matrix stiffness-dependent FAK activation rate. However, the level of ERK is not only regulated by FAK phosphorylation but also regulated by IL-1β receptors on the membrane, which are activated by IL-1β (41). Thus, the ERK is an important cross talk biomolecule in the synergistic effect of matrix stiffness and IL-1β on the osteogenic differentiation of hMSCs.
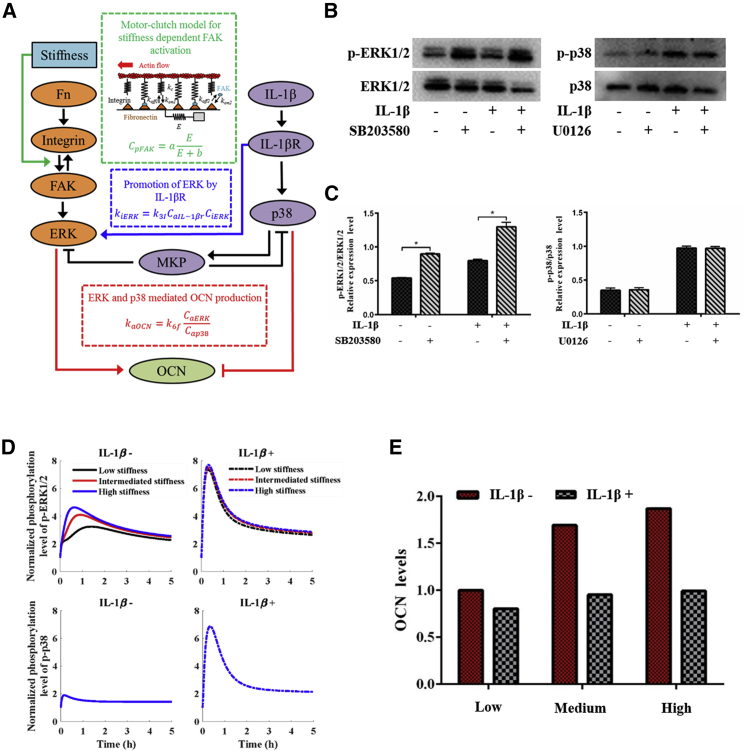
Figure 6.
Mathematical modeling of stiffness and IL-1β-mediated signaling pathway. (A) Shown is a schematic of the mathematical model using a set of ordinary differential equations (ODEs). (B) Western blot analysis of the levels of phosphorylated ERK1/2 (with p38 inhibitor SB203850) and p38 (with ERK1/2 inhibitor U0126). (C) The average ratios of phosphorylated (p)-ERK1/2/ERK1/2 and p-p38/p38 were calculated by the ImageJ software showing levels of phosphorylated ERK1/2 rise significantly with the treatment of p38 inhibitors, whereas p38 doesn’t change significantly with the treatment of ERK1/2 inhibitors. (D) Simulated results of ERK1/2 and p38 phosphorylation and dephosphorylation are shown. (E) OCN level in the hMSCs is predicted by the mathematical model. To see this figure in color, go online.
The interaction between ERK and p38 has been demonstrated in literature (41). The cross-reactivity experiment confirms the observation by showing that the phosphorylation level of ERK1/2 rises significantly compared to the group without the p38 inhibitor, whereas a negligible change in the phosphorylation level of p38 has been identified as compared with the group without ERK1/2 inhibitor (Fig. 6, B and C). Thus, the interaction between ERK and p38 was incorporated in our model. With the model, we simulated the process in which hMSCs perceive matrix stiffness and IL-1β and then influence osteogenic differentiation. We first obtained the optimized parameters of the mathematical model based on our experimental data of the phosphorylation levels of ERK and p38 (Fig. 4, A and B). The modeling results show that the IL-1β inhibits the osteogenic differentiation of hMSCs and suppresses the stiffness-dependent mechanosensing of hMSCs. The acceptable agreement between the simulation results (Fig. 6 D) and Western blot results (Fig. 4, A and B) confirms that ERK rather than p38 has the ability to sense the matrix stiffness (i.e., the stiffness can promote ERK activation). The IL-1β can improve the phosphorylation levels of both ERK and p38, which has an antagonistic effect on the OCN activation and expression. We further tested our mathematical model by comparing the predicted OCN level (Fig. 6 E) with our experimental result (Fig. 3 B). We found that the modeling result is basically consistent with the experimental result, further confirming the rationality and reliability of our modified mathematical model.
FAK, ERK, and p38 activation/deactivation dynamics regulate the synergistic effect of stiffness and IL-1β on the osteogenic differentiation of hMSCs
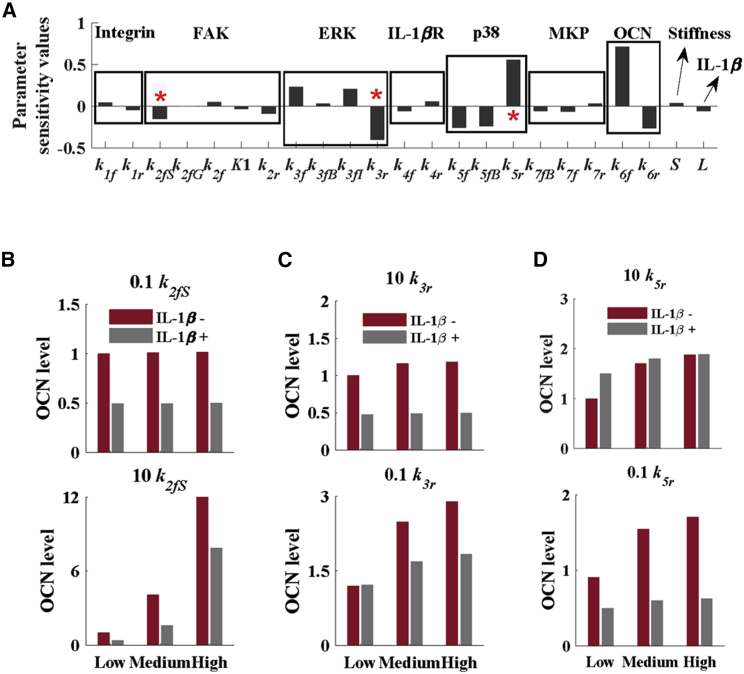
To evaluate the roles of intracellular signaling dynamics for both matrix stiffness and IL-1β-mediated osteogenic differentiation in hMSCs, we performed parameter sensitivity analysis using the proposed mathematical model. We divided the parameters into the following five categories according to the key biomolecules involved in the OCN expression: 1) activation and deactivation of integrin and IL-1β receptors on the membrane, 2) stiffness-mediated FAK mechanosensing, 3) activation and deactivation of the MAPK (p38 and ERK) signaling pathways in the cytoplasm, 4) the levels of input factors (e.g., stiffness and IL-1β), and 5) the OCN production. Later, we evaluated the effects of FAK, p38, and ERK on the osteogenic differentiation of hMSCs. The sensitivity analysis of parameters shows that the FAK activation rate (k2fS), ERK deactivation rate (k3r), and p38 deactivation rate (k5r) played major roles in affecting the synergistic effect of matrix stiffness and IL-1β on the osteogenic differentiation of hMSCs (Fig. 7 A). We changed the values of these parameters to investigate their roles in the osteogenic differentiation of hMSCs. We used 10k2fs and 0.1k2fs to represent the increase and decrease of FAK activation rates, respectively (Fig. 7 B), 10k3r and 0.1k3r to represent the increase and decrease of ERK inactivation rates, respectively (Fig. 7 C), and 10k5r and 0.1k5r to represent the increase and decrease of p38 inactivation rates, respectively (Fig. 7 D). We observed that the decrease of the FAK activation rate could totally abolish the trend of osteogenic differentiation of hMSCs on a stiffer substrate with IL-1β treatment, whereas an increase of FAK activation rate could increase osteogenic differentiation of hMSCs on various stiffness substrates with and without IL-1β (Fig. 7 B). The decrease of the ERK deactivation rate can decrease the hMSC stiffness sensitivity on the different substrates with or without IL-1β (Fig. 7 C). Finally, we investigated the effect of the p38 deactivation rate on the osteogenic differentiation of hMSCs. We found that the decrease of p38 activation rate could increase the level of OCN in the cells on various stiffness substrates treated with IL-1β as compared with those without IL-1β treatment (Fig. 7 D).
Figure 7.
Sensitivity analysis of the model parameters on the osteogenic differentiation of hMSCs. (A) Sensitivity values of OCN, p38, IL-1βR, ERK, FAK, MKP, and integrin. Parameter sensitivity analysis shows that the FAK activation rate and the ERK and p38 deactivation rates can effectively influence the OCN production. The effects of (B) FAK, (C) ERK, and (D) p38 on the osteogenic differentiation of hMSCs with different stiffness are shown. To see this figure in color, go online.
Discussion
Many diseases, such as inflammation, will result in changes in the cell microenvironment (biochemical and biophysical cues). For instance, the periodontal ligament (PDL) is a special soft connective tissue located between the tooth root and alveolar bone. The stiffness of the PDL ranges from tens to hundreds kPa (42, 43, 44, 45), which is significantly decreased because of the degradation (e.g., collagen degradation) of PDL tissue during periodontitis (46). Such inflammation-induced changes in the microenvironment have been found to affect the osteogenic differentiation of stem cells, which plays an important role in the bone repair (14, 47). The impact of either matrix stiffness or inflammatory factors upon osteogenic differentiation of MSCs has been well described; here, we provide the, to our knowledge, first demonstration of the synergistic effect of matrix stiffness and inflammatory factors on osteogenic differentiation of hMSCs. We found that there was matrix stiffness-dependent modulation in the osteogenic of hMSCs with a higher matrix stiffness favoring osteogenesis bias, which confirms existing studies (10, 12). However, when IL-1β was added, osteogenesis of hMSCs was suppressed, although matrix stiffness increased. We then found that the cross talk between ERK1/2 and p38 signaling pathways probably participated in the regulation of osteogenic differentiation.
The mechanism of matrix stiffness-induced stem cell osteogenic differentiation has been intensively studied (37, 38). The stem cell fate has been found to be affected by a complex and interconnected network of cross talk between multiple signals triggered by matrix stiffness (48). When cultured in osteogenic medium with diverse stiffness, MSCs show an increased ERK activity on a stiffer matrix (38). It has been reported that ERK1/2 plays a key role in the osteogenic differentiation of stem cells (49). In our study, we found that matrix stiffness promoted hMSC osteogenesis by activating ERK1/2, which was confirmed by existing studies that attributed matrix stiffness-driven MSC osteogenesis to a high phosphorylation level of ERK1/2 (37, 38). After the blocking of ERK1/2 by U0126, we found a decreased expression of OCN protein, indicating that ERK1/2 has a positive role in the osteogenesis of hMSCs. However, the opposite viewpoint with the role of ERK1/2 in the osteogenic differentiation of cells has also been reported (50). This may be because of different types of cells.
Periodontitis originates from bacterially derived factors and antigens, causing a local inflammatory reaction and the release of a large number of proinflammatory cytokines. IL-1β is a representative proinflammatory cytokine involved in periodontitis and has a strong relationship with periodontal tissue destruction (51). We found that IL-1β reduced osteogenic differentiation of cells even on substrate with a high stiffness. When the matrix stiffness and IL-1β acted simultaneously, the osteogenic differentiation of hMSCs was significantly suppressed and did not have the tendency to increase with an increasing matrix stiffness. This phenomenon is novel, to our knowledge, and may help to explain the fact that damaged alveolar bone tissue is difficult to repair under an inflammatory condition. We then explored the mechanism of IL-1β on suppressing osteogenic differentiation of hMSCs. We confirmed that IL-1β could activate both p38 and ERK1/2 signaling in hMSCs. This is in accordance with previous studies that p38 and ERK1/2 signaling could be activated by inflammatory cytokines, including IL-1β (32). Notably, the presence of IL-1β was found to strongly diminish matrix stiffness-induced OCN activity through the activation of p38 signaling. After the blocking of p38 by SB203580, the expression of OCN protein increased significantly as compared with the group in which only IL-1β was added. These results show that when ERK1/2 and p38 signaling are both activated, osteogenic differentiation of hMSCs is reduced significantly. These results indicate that the inhibitory effect of p38 signaling plays a more significant role than the promoting effect of ERK1/2 signaling during osteogenic differentiation.
The mathematical model by a set of ODEs helps to further investigate the mechanisms underlying the synergistic effect of matrix stiffness and IL-1β on mediating the osteogenic differentiation of hMSCs. The proposed mathematical model is mainly based on two signaling processes, with the former describing Fn/integrin/FAK/ERK cascade and the latter describing IL-1β/IL-1βR/p38 cascade. Cells sense matrix stiffness through integrins, a type of transmembrane proteins that transduce forces from the ECM to intracellular structures (40). The β1 subunits of integrins activate focal adhesion kinase (FAK) in a stiffness-dependent manner (52), and β3 subunits are activated by Fn and transmit mechanical signals into cells (53). Both β1 and β3 integrins have been shown to possess mechanosensing capability and are involved in outside-in signaling to intracellular kinases like FAK (52, 53). The osteogenic differentiation of hMSCs could be regulated through the integrin-mediated FAK/ERK signaling pathway (54). Besides, as discussed before, the osteogenic differentiation of hMSCs could also be regulated through the IL-1β-mediated p38 signaling pathway. Based on these results, we developed a series of ODEs to simulate the entire signaling pathway. The simulation results (e.g., OCN level) confirmed our experimental observations, indicating the correctness of the proposed pathways associated with the mechanisms of stiffness and IL-1β-mediated osteogenic differentiation of hMSCs.
The simulation results show that IL-1β can abolish the stiffness-mechanosensing behaviors of hMSCs and further inhibit the OCN expression, that is, the osteogenic differentiation of hMSCs. We demonstrated that p38 has a strong inhibition of OCN production although ERK promotes the OCN production. Furthermore, the mathematical model provides a powerful tool to screen the key chemical reactions that can regulate the stiffness-sensitivity of hMSCs treated w/wo IL-1β. To achieve this goal, we performed the parameter sensitivity analysis to determine which biochemical reaction rate is most sensitive to regulate the stiffness sensitivity of hMSCs. The parameter sensitivity analysis shows that the increase of FAK activation can significantly recover the stiffness sensitivity of hMSCs even with IL-1β treatment. These results show that FAK is a key factor molecule that can significantly influence the cellular mechanosensing. However, the parameter sensitivity analysis also shows that the ERK deactivation rate (k3r) and the p38 deactivation rate (k5r) can affect the OCN level significantly. For example, a decrease of the ERK deactivation rate (0.1 k3r) can increase the OCN level w/wo IL-1β. The increase of the p38 deactivation rate (10 k5r) could increase the level of OCN in the cells on various stiffness substrates treated with IL-1β as compared with those without IL-1β treatment. Besides, changes in p38 and ERK dynamics did not change the tendency in mechanosensing of hMSCs to matrix stiffness. Therefore, our modeling results provide mechanistic insights into the synergistic effects of mechanochemical interactions, thus paving the way for a better design of medical treatments to regulate the osteogenic differentiation of hMSCs in vivo.
In clinical (e.g., periodontitis, a chronic inflammation in periodontal tissue that may lead to the loss of alveolar bone), elevated inflammatory cytokines and decreased matrix stiffness co-exist in the tissues (e.g., PDL). Therefore, we treated hMSCs with matrix stiffness and IL-1β simultaneously to mimic the clinical situation and investigated the exact role of p38 and ERK1/2 signaling in vitro. Our observations suggest that IL-1β inhibits matrix stiffness-induced OCN expression and activation. p38 is the key signaling pathway that is required for this inhibitory effect, although ERK1/2 could be activated by both IL-1β and matrix stiffness. This antagonistic effect suggests that when hMSCs are treated with matrix stiffness alone, matrix stiffness activates ERK1/2 signaling acting as a noncanonical pathway and has a weak inductive role in the upregulation of OCN expression and activation. However, the addition of IL-1β causes strong p38 signaling activation, which downregulates OCN expression. These data indicate that the inflammatory environment inhibits the osteogenic differentiation of hMSCs, and high matrix stiffness has a negligible effect on the promotion of osteogenesis in an inflammatory environment.
In summary, our results demonstrate that the matrix stiffness and IL-1β have the opposite roles in regulating osteogenic differentiation in clinical condition (e.g., periodontitis), whereby inflammatory cytokine inhibits matrix stiffness-induced osteogenic differentiation. This result provides a possible interpretation for the low alveolar bone regeneration under clinical conditions. Besides, both matrix stiffness and IL-1β can activate the ERK1/2 signaling, contributing to osteogenic differentiation. The p38 signaling activated by IL-1β has a strong role in suppressing OCN expression and activation, thus diminishing the vital effect of ERK1/2 signaling. Our study highlights the negative effects of an inflammatory environment on osteogenic differentiation and may facilitate the development of new strategies to improve the osteoinductive efficacy by MSC-based therapies and to enhance bone formation in clinics.
Author Contributions
M.L. and F.X. designed the study, in consultation with W.W. B.C. performed experiments, modeling, collected, and analyzed data. W.W., B.C., C.Z., and Y.M. prepared the manuscript. All authors read and commented on the manuscript.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (11772253, 11522219, and 11532009), the Natural Science Foundation of Shaanxi Province (2018JM1007), the Opening Project of Key Laboratory of Shaanxi Province for Craniofacial Precision Medicine Research (2016LHM-KFKT007), and the Fundamental Research Funds for the Central Universities (2016qngz03).
Editor: Cynthia Reinhart-King.
Footnotes
Wanting Wan and Bo Cheng contributed equally to this work.
Supporting Material can be found online at https://doi.org/10.1016/j.bpj.2019.05.019.
Supporting Material
References
- 1.Squillaro T., Peluso G., Galderisi U. Clinical trials with mesenchymal stem cells: an update. Cell Transplant. 2016;25:829–848. doi: 10.3727/096368915X689622. [DOI] [PubMed] [Google Scholar]; Squillaro, T., G. Peluso, and U. Galderisi. 2016. Clinical trials with mesenchymal stem cells: an update. Cell Transplant. 25:829-848. [DOI] [PubMed]
- 2.Pittenger M.F., Mackay A.M., Marshak D.R. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]; Pittenger, M. F., A. M. Mackay, …, D. R. Marshak. 1999. Multilineage potential of adult human mesenchymal stem cells. Science. 284:143-147. [DOI] [PubMed]
- 3.Ding J., Ghali O., Magne D. TNF-α and IL-1β inhibit RUNX2 and collagen expression but increase alkaline phosphatase activity and mineralization in human mesenchymal stem cells. Life Sci. 2009;84:499–504. doi: 10.1016/j.lfs.2009.01.013. [DOI] [PubMed] [Google Scholar]; Ding, J., O. Ghali, …, D. Magne. 2009. TNF-α and IL-1β inhibit RUNX2 and collagen expression but increase alkaline phosphatase activity and mineralization in human mesenchymal stem cells. Life Sci. 84:499-504. [DOI] [PubMed]
- 4.Higuchi A., Ling Q.-D., Umezawa A. Physical cues of cell culture materials lead the direction of differentiation lineages of pluripotent stem cells. J. Mater. Chem. B. 2015;3:8032–8058. doi: 10.1039/c5tb01276g. [DOI] [PubMed] [Google Scholar]; Higuchi, A., Q.-D. Ling, …, A. Umezawa. 2015. Physical cues of cell culture materials lead the direction of differentiation lineages of pluripotent stem cells. J. Mater. Chem. B. 3:8032-8058. [DOI] [PubMed]
- 5.Yao H., Miura Y., Maekawa T. Parathyroid hormone enhances hematopoietic expansion via upregulation of cadherin-11 in bone marrow mesenchymal stromal cells. Stem Cells. 2014;32:2245–2255. doi: 10.1002/stem.1701. [DOI] [PubMed] [Google Scholar]; Yao, H., Y. Miura, …, T. Maekawa. 2014. Parathyroid hormone enhances hematopoietic expansion via upregulation of cadherin-11 in bone marrow mesenchymal stromal cells. Stem Cells. 32:2245-2255. [DOI] [PubMed]
- 6.Wang L., Wu F., Jin Z. Long noncoding RNA related to periodontitis interacts with miR-182 to upregulate osteogenic differentiation in periodontal mesenchymal stem cells of periodontitis patients. Cell Death Dis. 2016;7:e2327. doi: 10.1038/cddis.2016.125. [DOI] [PMC free article] [PubMed] [Google Scholar]; Wang, L., F. Wu, …, Z. Jin. 2016. Long noncoding RNA related to periodontitis interacts with miR-182 to upregulate osteogenic differentiation in periodontal mesenchymal stem cells of periodontitis patients. Cell Death Dis. 7:e2327. [DOI] [PMC free article] [PubMed]
- 7.Houshmand B., Behnia H., Khojasteh A. Osteoblastic differentiation of human stem cells derived from bone marrow and periodontal ligament under the effect of enamel matrix derivative and transforming growth factor-beta. Int. J. Oral Maxillofac. Implants. 2013;28:e440–e450. doi: 10.11607/jomi.te24. [DOI] [PubMed] [Google Scholar]; Houshmand, B., H. Behnia, …, A. Khojasteh. 2013. Osteoblastic differentiation of human stem cells derived from bone marrow and periodontal ligament under the effect of enamel matrix derivative and transforming growth factor-beta. Int. J. Oral Maxillofac. Implants. 28:e440-e450. [DOI] [PubMed]
- 8.Liu W., Konermann A., Jin Y. Canonical Wnt signaling differently modulates osteogenic differentiation of mesenchymal stem cells derived from bone marrow and from periodontal ligament under inflammatory conditions. Biochim. Biophys. Acta. 2014;1840:1125–1134. doi: 10.1016/j.bbagen.2013.11.003. [DOI] [PubMed] [Google Scholar]; Liu, W., A. Konermann, …, Y. Jin. 2014. Canonical Wnt signaling differently modulates osteogenic differentiation of mesenchymal stem cells derived from bone marrow and from periodontal ligament under inflammatory conditions. Biochim. Biophys. Acta. 1840:1125-1134. [DOI] [PubMed]
- 9.Wang N., Zhou Z., Yu X. TNF-α-induced NF-κB activation upregulates microRNA-150-3p and inhibits osteogenesis of mesenchymal stem cells by targeting β-catenin. Open Biol. 2016;6:150258. doi: 10.1098/rsob.150258. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]; Wang, N., Z. Zhou, …, X. Yu. 2016. TNF-α-induced NF-κB activation upregulates microRNA-150-3p and inhibits osteogenesis of mesenchymal stem cells by targeting β-catenin. Open Biol. 6:150258. [DOI] [PMC free article] [PubMed] [Retracted]
- 10.Trappmann B., Gautrot J.E., Huck W.T. Extracellular-matrix tethering regulates stem-cell fate. Nat. Mater. 2012;11:642–649. doi: 10.1038/nmat3339. [DOI] [PubMed] [Google Scholar]; Trappmann, B., J. E. Gautrot, …, W. T. Huck. 2012. Extracellular-matrix tethering regulates stem-cell fate. Nat. Mater. 11:642-649. [DOI] [PubMed]
- 11.Teixeira A.I., Ilkhanizadeh S., Hermanson O. The promotion of neuronal maturation on soft substrates. Biomaterials. 2009;30:4567–4572. doi: 10.1016/j.biomaterials.2009.05.013. [DOI] [PubMed] [Google Scholar]; Teixeira, A. I., S. Ilkhanizadeh, …, O. Hermanson. 2009. The promotion of neuronal maturation on soft substrates. Biomaterials. 30:4567-4572. [DOI] [PubMed]
- 12.Jiang P., Mao Z., Gao C. Combinational effect of matrix elasticity and alendronate density on differentiation of rat mesenchymal stem cells. Acta Biomater. 2015;19:76–84. doi: 10.1016/j.actbio.2015.03.018. [DOI] [PubMed] [Google Scholar]; Jiang, P., Z. Mao, and C. Gao. 2015. Combinational effect of matrix elasticity and alendronate density on differentiation of rat mesenchymal stem cells. Acta Biomater. 19:76-84. [DOI] [PubMed]
- 13.Choi Y.S., Vincent L.G., Engler A.J. Mechanical derivation of functional myotubes from adipose-derived stem cells. Biomaterials. 2012;33:2482–2491. doi: 10.1016/j.biomaterials.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]; Choi, Y. S., L. G. Vincent, …, A. J. Engler. 2012. Mechanical derivation of functional myotubes from adipose-derived stem cells. Biomaterials. 33:2482-2491. [DOI] [PMC free article] [PubMed]
- 14.Engler A.J., Sen S., Discher D.E. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]; Engler, A. J., S. Sen, …, D. E. Discher. 2006. Matrix elasticity directs stem cell lineage specification. Cell. 126:677-689. [DOI] [PubMed]
- 15.Cipitria A., Salmeron-Sanchez M. Mechanotransduction and growth factor signalling to engineer cellular microenvironments. Adv. Healthc. Mater. 2017 doi: 10.1002/adhm.201700052. Published online May 8, 2017. 10.1002/adhm.201700052. [DOI] [PubMed] [Google Scholar]; Cipitria, A., and M. Salmeron-Sanchez. 2017. Mechanotransduction and growth factor signalling to engineer cellular microenvironments. Adv. Healthc. Mater. Published online May 8, 2017. 10.1002/adhm.201700052. [DOI] [PubMed]
- 16.Comoglio P.M., Boccaccio C., Trusolino L. Interactions between growth factor receptors and adhesion molecules: breaking the rules. Curr. Opin. Cell Biol. 2003;15:565–571. doi: 10.1016/s0955-0674(03)00096-6. [DOI] [PubMed] [Google Scholar]; Comoglio, P. M., C. Boccaccio, and L. Trusolino. 2003. Interactions between growth factor receptors and adhesion molecules: breaking the rules. Curr. Opin. Cell Biol. 15:565-571. [DOI] [PubMed]
- 17.Maldonado M., Nam J. The role of changes in extracellular matrix of cartilage in the presence of inflammation on the pathology of osteoarthritis. BioMed Res. Int. 2013;2013:284873. doi: 10.1155/2013/284873. [DOI] [PMC free article] [PubMed] [Google Scholar]; Maldonado, M., and J. Nam. 2013. The role of changes in extracellular matrix of cartilage in the presence of inflammation on the pathology of osteoarthritis. BioMed Res. Int. 2013:284873. [DOI] [PMC free article] [PubMed]
- 18.Bonnans C., Chou J., Werb Z. Remodelling the extracellular matrix in development and disease. Nat. Rev. Mol. Cell Biol. 2014;15:786–801. doi: 10.1038/nrm3904. [DOI] [PMC free article] [PubMed] [Google Scholar]; Bonnans, C., J. Chou, and Z. Werb. 2014. Remodelling the extracellular matrix in development and disease. Nat. Rev. Mol. Cell Biol. 15:786-801. [DOI] [PMC free article] [PubMed]
- 19.Kyriakis J.M., Avruch J. Mammalian MAPK signal transduction pathways activated by stress and inflammation: a 10-year update. Physiol. Rev. 2012;92:689–737. doi: 10.1152/physrev.00028.2011. [DOI] [PubMed] [Google Scholar]; Kyriakis, J. M., and J. Avruch. 2012. Mammalian MAPK signal transduction pathways activated by stress and inflammation: a 10-year update. Physiol. Rev. 92:689-737. [DOI] [PubMed]
- 20.Lee J., Abdeen A.A., Kilian K.A. Rewiring mesenchymal stem cell lineage specification by switching the biophysical microenvironment. Sci. Rep. 2014;4:5188. doi: 10.1038/srep05188. [DOI] [PMC free article] [PubMed] [Google Scholar]; Lee, J., A. A. Abdeen, and K. A. Kilian. 2014. Rewiring mesenchymal stem cell lineage specification by switching the biophysical microenvironment. Sci. Rep. 4:5188. [DOI] [PMC free article] [PubMed]
- 21.Liu N., Shi S., Jin Y. High levels of β-catenin signaling reduce osteogenic differentiation of stem cells in inflammatory microenvironments through inhibition of the noncanonical Wnt pathway. J. Bone Miner. Res. 2011;26:2082–2095. doi: 10.1002/jbmr.440. [DOI] [PubMed] [Google Scholar]; Liu, N., S. Shi, …, Y. Jin. 2011. High levels of β-catenin signaling reduce osteogenic differentiation of stem cells in inflammatory microenvironments through inhibition of the noncanonical Wnt pathway. J. Bone Miner. Res. 26:2082-2095. [DOI] [PubMed]
- 22.Mao C.Y., Wang Y.G., Lu E.Y. Double-edged-sword effect of IL-1β on the osteogenesis of periodontal ligament stem cells via crosstalk between the NF-κB, MAPK and BMP/Smad signaling pathways. Cell Death Dis. 2016;7:e2296. doi: 10.1038/cddis.2016.204. [DOI] [PMC free article] [PubMed] [Google Scholar]; Mao, C. Y., Y. G. Wang, …, E. Y. Lu. 2016. Double-edged-sword effect of IL-1β on the osteogenesis of periodontal ligament stem cells via crosstalk between the NF-κB, MAPK and BMP/Smad signaling pathways. Cell Death Dis. 7:e2296. [DOI] [PMC free article] [PubMed]
- 23.Lacey D.C., Simmons P.J., Hamilton J.A. Proinflammatory cytokines inhibit osteogenic differentiation from stem cells: implications for bone repair during inflammation. Osteoarthritis Cartilage. 2009;17:735–742. doi: 10.1016/j.joca.2008.11.011. [DOI] [PubMed] [Google Scholar]; Lacey, D. C., P. J. Simmons, …, J. A. Hamilton. 2009. Proinflammatory cytokines inhibit osteogenic differentiation from stem cells: implications for bone repair during inflammation. Osteoarthritis Cartilage. 17:735-742. [DOI] [PubMed]
- 24.Nokhbehsaim M., Deschner B., Deschner J. Interactions of regenerative, inflammatory and biomechanical signals on bone morphogenetic protein-2 in periodontal ligament cells. J. Periodontal Res. 2011;46:374–381. doi: 10.1111/j.1600-0765.2011.01357.x. [DOI] [PubMed] [Google Scholar]; Nokhbehsaim, M., B. Deschner, …, J. Deschner. 2011. Interactions of regenerative, inflammatory and biomechanical signals on bone morphogenetic protein-2 in periodontal ligament cells. J. Periodontal Res. 46:374-381. [DOI] [PubMed]
- 25.Pihlstrom B.L., Michalowicz B.S., Johnson N.W. Periodontal diseases. Lancet. 2005;366:1809–1820. doi: 10.1016/S0140-6736(05)67728-8. [DOI] [PubMed] [Google Scholar]; Pihlstrom, B. L., B. S. Michalowicz, and N. W. Johnson. 2005. Periodontal diseases. Lancet. 366:1809-1820. [DOI] [PubMed]
- 26.Eke P.I., Thornton-Evans G., Genco R. Advances in surveillance of periodontitis: the centers for disease control and prevention periodontal disease surveillance project. J. Periodontol. 2012;83:1337–1342. doi: 10.1902/jop.2012.110676. [DOI] [PMC free article] [PubMed] [Google Scholar]; Eke, P. I., G. Thornton-Evans, …, R. Genco. 2012. Advances in surveillance of periodontitis: the centers for disease control and prevention periodontal disease surveillance project. J. Periodontol. 83:1337-1342. [DOI] [PMC free article] [PubMed]
- 27.Chen F.M., Sun H.H., Yu Q. Stem cell-delivery therapeutics for periodontal tissue regeneration. Biomaterials. 2012;33:6320–6344. doi: 10.1016/j.biomaterials.2012.05.048. [DOI] [PubMed] [Google Scholar]; Chen, F. M., H. H. Sun, …, Q. Yu. 2012. Stem cell-delivery therapeutics for periodontal tissue regeneration. Biomaterials. 33:6320-6344. [DOI] [PubMed]
- 28.Li X., Chen S., Chen G. 3D culture of chondrocytes in gelatin hydrogels with different stiffness. Polymers (Basel) 2016;8:269. doi: 10.3390/polym8080269. [DOI] [PMC free article] [PubMed] [Google Scholar]; Li, X., S. Chen, …, G. Chen. 2016. 3D culture of chondrocytes in gelatin hydrogels with different stiffness. Polymers (Basel). 8:269. [DOI] [PMC free article] [PubMed]
- 29.Ma Y., Ji Y., Lin M. Bioprinting-based PDLSC-ECM screening for in vivo repair of alveolar bone defect using cell-laden, injectable and photocrosslinkable hydrogels. ACS Biomater. Sci. Eng. 2017;3:3534–3545. doi: 10.1021/acsbiomaterials.7b00601. [DOI] [PubMed] [Google Scholar]; Ma, Y., Y. Ji, …, M. Lin. 2017. Bioprinting-based PDLSC-ECM screening for in vivo repair of alveolar bone defect using cell-laden, injectable and photocrosslinkable hydrogels. ACS Biomater. Sci. Eng. 3:3534-3545. [DOI] [PubMed]
- 30.Changede R., Sheetz M. Integrin and cadherin clusters: a robust way to organize adhesions for cell mechanics. BioEssays. 2017;39:1–12. doi: 10.1002/bies.201600123. [DOI] [PubMed] [Google Scholar]; Changede, R., and M. Sheetz. 2017. Integrin and cadherin clusters: a robust way to organize adhesions for cell mechanics. BioEssays. 39:1-12. [DOI] [PubMed]
- 31.Strohmeyer N., Bharadwaj M., Müller D.J. Fibronectin-bound α5β1 integrins sense load and signal to reinforce adhesion in less than a second. Nat. Mater. 2017;16:1262–1270. doi: 10.1038/nmat5023. [DOI] [PubMed] [Google Scholar]; Strohmeyer, N., M. Bharadwaj, …, D. J. Muller. 2017. Fibronectin-bound α5β1 integrins sense load and signal to reinforce adhesion in less than a second. Nat. Mater. 16:1262-1270. [DOI] [PubMed]
- 32.Huang R.L., Yuan Y., Li Q. Opposing TNF-α/IL-1β- and BMP-2-activated MAPK signaling pathways converge on Runx2 to regulate BMP-2-induced osteoblastic differentiation. Cell Death Dis. 2014;5:e1187. doi: 10.1038/cddis.2014.101. [DOI] [PMC free article] [PubMed] [Google Scholar]; Huang, R. L., Y. Yuan, …, Q. Li. 2014. Opposing TNF-α/IL-1β- and BMP-2-activated MAPK signaling pathways converge on Runx2 to regulate BMP-2-induced osteoblastic differentiation. Cell Death Dis. 5:e1187. [DOI] [PMC free article] [PubMed]
- 33.Cheng B., Lin M., Xu F. Cellular mechanosensing of the biophysical microenvironment: a review of mathematical models of biophysical regulation of cell responses. Phys. Life Rev. 2017;22–23:88–119. doi: 10.1016/j.plrev.2017.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]; Cheng, B., M. Lin, …, F. Xu. 2017. Cellular mechanosensing of the biophysical microenvironment: a review of mathematical models of biophysical regulation of cell responses. Phys. Life Rev. 22-23:88-119. [DOI] [PMC free article] [PubMed]
- 34.Cheng B., Lin M., Xu F. An integrated stochastic model of matrix-stiffness-dependent filopodial dynamics. Biophys. J. 2016;111:2051–2061. doi: 10.1016/j.bpj.2016.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]; Cheng, B., M. Lin, …, F. Xu. 2016. An integrated stochastic model of matrix-stiffness-dependent filopodial dynamics. Biophys. J. 111:2051-2061. [DOI] [PMC free article] [PubMed]
- 35.Serrels B., Frame M.C. FAK and talin: who is taking whom to the integrin engagement party? J. Cell Biol. 2012;196:185–187. doi: 10.1083/jcb.201112128. [DOI] [PMC free article] [PubMed] [Google Scholar]; Serrels, B., and M. C. Frame. 2012. FAK and talin: who is taking whom to the integrin engagement party? J. Cell Biol. 196:185-187. [DOI] [PMC free article] [PubMed]
- 36.Sun Z., Guo S.S., Fässler R. Integrin-mediated mechanotransduction. J. Cell Biol. 2016;215:445–456. doi: 10.1083/jcb.201609037. [DOI] [PMC free article] [PubMed] [Google Scholar]; Sun, Z., S. S. Guo, and R. Fassler. 2016. Integrin-mediated mechanotransduction. J. Cell Biol. 215:445-456. [DOI] [PMC free article] [PubMed]
- 37.Xue R., Li J.Y., Chien S. Effects of matrix elasticity and cell density on human mesenchymal stem cells differentiation. J. Orthop. Res. 2013;31:1360–1365. doi: 10.1002/jor.22374. [DOI] [PubMed] [Google Scholar]; Xue, R., J. Y. Li, …, S. Chien. 2013. Effects of matrix elasticity and cell density on human mesenchymal stem cells differentiation. J. Orthop. Res. 31:1360-1365. [DOI] [PubMed]
- 38.Shih Y.R., Tseng K.F., Lee O.K. Matrix stiffness regulation of integrin-mediated mechanotransduction during osteogenic differentiation of human mesenchymal stem cells. J. Bone Miner. Res. 2011;26:730–738. doi: 10.1002/jbmr.278. [DOI] [PubMed] [Google Scholar]; Shih, Y. R., K. F. Tseng, …, O. K. Lee. 2011. Matrix stiffness regulation of integrin-mediated mechanotransduction during osteogenic differentiation of human mesenchymal stem cells. J. Bone Miner. Res. 26:730-738. [DOI] [PubMed]
- 39.Provenzano P.P., Inman D.R., Keely P.J. Matrix density-induced mechanoregulation of breast cell phenotype, signaling and gene expression through a FAK-ERK linkage. Oncogene. 2009;28:4326–4343. doi: 10.1038/onc.2009.299. [DOI] [PMC free article] [PubMed] [Google Scholar]; Provenzano, P. P., D. R. Inman, …, P. J. Keely. 2009. Matrix density-induced mechanoregulation of breast cell phenotype, signaling and gene expression through a FAK-ERK linkage. Oncogene. 28:4326-4343. [DOI] [PMC free article] [PubMed]
- 40.Schroer A.K., Ryzhova L.M., Merryman W.D. Network modeling approach to predict myofibroblast differentiation. Cell. Mol. Bioeng. 2014;7:446–459. doi: 10.1007/s12195-014-0344-9. [DOI] [PMC free article] [PubMed] [Google Scholar]; Schroer, A. K., L. M. Ryzhova, and W. D. Merryman. 2014. Network modeling approach to predict myofibroblast differentiation. Cell. Mol. Bioeng. 7:446-459. [DOI] [PMC free article] [PubMed]
- 41.Kulawik A., Engesser R., Bode J.G. IL-1β-induced and p38MAPK-dependent activation of the mitogen-activated protein kinase-activated protein kinase 2 (MK2) in hepatocytes: signal transduction with robust and concentration-independent signal amplification. J. Biol. Chem. 2017;292:6291–6302. doi: 10.1074/jbc.M117.775023. [DOI] [PMC free article] [PubMed] [Google Scholar]; Kulawik, A., R. Engesser, …, J. G. Bode. 2017. IL-1β-induced and p38MAPK-dependent activation of the mitogen-activated protein kinase-activated protein kinase 2 (MK2) in hepatocytes: signal transduction with robust and concentration-independent signal amplification. J. Biol. Chem. 292:6291-6302. [DOI] [PMC free article] [PubMed]
- 42.Pietrzak G., Curnier A., Belser U. A nonlinear elastic model of the periodontal ligament and its numerical calibration for the study of tooth mobility. Comput. Methods Biomech. Biomed. Engin. 2002;5:91–100. doi: 10.1080/10255840290032117. [DOI] [PubMed] [Google Scholar]; Pietrzak, G., A. Curnier, …, U. Belser. 2002. A nonlinear elastic model of the periodontal ligament and its numerical calibration for the study of tooth mobility. Comput. Methods Biomech. Biomed. Engin. 5:91-100. [DOI] [PubMed]
- 43.Poppe M., Bourauel C., Jäger A. Determination of the elasticity parameters of the human periodontal ligament and the location of the center of resistance of single-rooted teeth a study of autopsy specimens and their conversion into finite element models. J. Orofac. Orthop. 2002;63:358–370. doi: 10.1007/s00056-002-0067-8. [DOI] [PubMed] [Google Scholar]; Poppe, M., C. Bourauel, and A. Jager. 2002. Determination of the elasticity parameters of the human periodontal ligament and the location of the center of resistance of single-rooted teeth a study of autopsy specimens and their conversion into finite element models. J. Orofac. Orthop. 63:358-370. [DOI] [PubMed]
- 44.Xia Z., Jiang F., Chen J. Estimation of periodontal ligament’s equivalent mechanical parameters for finite element modeling. Am. J. Orthod. Dentofacial Orthop. 2013;143:486–491. doi: 10.1016/j.ajodo.2012.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]; Xia, Z., F. Jiang, and J. Chen. 2013. Estimation of periodontal ligament’s equivalent mechanical parameters for finite element modeling. Am. J. Orthod. Dentofacial Orthop. 143:486-491. [DOI] [PMC free article] [PubMed]
- 45.Keilig L., Drolshagen M., Bourauel C. In vivo measurements and numerical analysis of the biomechanical characteristics of the human periodontal ligament. Ann. Anat. 2016;206:80–88. doi: 10.1016/j.aanat.2015.08.004. [DOI] [PubMed] [Google Scholar]; Keilig, L., M. Drolshagen, …, C. Bourauel. 2016. In vivo measurements and numerical analysis of the biomechanical characteristics of the human periodontal ligament. Ann. Anat. 206:80-88. [DOI] [PubMed]
- 46.Ujiie Y., Shimada A., Fukae M. Degradation of noncollagenous components by neutrophil elastase reduces the mechanical strength of rat periodontal ligament. J. Periodontal Res. 2008;43:22–31. doi: 10.1111/j.1600-0765.2007.00990.x. [DOI] [PubMed] [Google Scholar]; Ujiie, Y., A. Shimada, …, M. Fukae. 2008. Degradation of noncollagenous components by neutrophil elastase reduces the mechanical strength of rat periodontal ligament. J. Periodontal Res. 43:22-31. [DOI] [PubMed]
- 47.Kong X., Liu Y., Jin Y. GSK3β is a checkpoint for TNF-α-mediated impaired osteogenic differentiation of mesenchymal stem cells in inflammatory microenvironments. Biochim. Biophys. Acta. 2013;1830:5119–5129. doi: 10.1016/j.bbagen.2013.07.027. [DOI] [PubMed] [Google Scholar]; Kong, X., Y. Liu, …, Y. Jin. 2013. GSK3β is a checkpoint for TNF-α-mediated impaired osteogenic differentiation of mesenchymal stem cells in inflammatory microenvironments. Biochim. Biophys. Acta. 1830:5119-5129. [DOI] [PubMed]
- 48.Lv H., Li L., Li Y. Mechanism of regulation of stem cell differentiation by matrix stiffness. Stem Cell Res. Ther. 2015;6:103. doi: 10.1186/s13287-015-0083-4. [DOI] [PMC free article] [PubMed] [Google Scholar]; Lv, H., L. Li, …, Y. Li. 2015. Mechanism of regulation of stem cell differentiation by matrix stiffness. Stem Cell Res. Ther. 6:103. [DOI] [PMC free article] [PubMed]
- 49.Yamauchi N., Taguchi Y., Umeda M. High-power, red-light-emitting diode irradiation enhances proliferation, osteogenic differentiation, and mineralization of human periodontal ligament stem cells via ERK signaling pathway. J. Periodontol. 2018;89:351–360. doi: 10.1002/JPER.17-0365. [DOI] [PubMed] [Google Scholar]; Yamauchi, N., Y. Taguchi, …, M. Umeda. 2018. High-power, red-light-emitting diode irradiation enhances proliferation, osteogenic differentiation, and mineralization of human periodontal ligament stem cells via ERK signaling pathway. J. Periodontol. 89:351-360. [DOI] [PubMed]
- 50.Higuchi C., Myoui A., Itoh K. Continuous inhibition of MAPK signaling promotes the early osteoblastic differentiation and mineralization of the extracellular matrix. J. Bone Miner. Res. 2002;17:1785–1794. doi: 10.1359/jbmr.2002.17.10.1785. [DOI] [PubMed] [Google Scholar]; Higuchi, C., A. Myoui, …, K. Itoh. 2002. Continuous inhibition of MAPK signaling promotes the early osteoblastic differentiation and mineralization of the extracellular matrix. J. Bone Miner. Res. 17:1785-1794. [DOI] [PubMed]
- 51.Chaudhari A.U., Byakod G.N., Karhadkar V.M. Correlation of levels of interleukin-1β in gingival crevicular fluid to the clinical parameters of chronic periodontitis. J. Contemp. Dent. Pract. 2011;12:52–59. doi: 10.5005/jp-journals-10024-1010. [DOI] [PubMed] [Google Scholar]; Chaudhari, A. U., G. N. Byakod, …, V. M. Karhadkar. 2011. Correlation of levels of interleukin-1β in gingival crevicular fluid to the clinical parameters of chronic periodontitis. J. Contemp. Dent. Pract. 12:52-59. [DOI] [PubMed]
- 52.Friedland J.C., Lee M.H., Boettiger D. Mechanically activated integrin switch controls alpha5beta1 function. Science. 2009;323:642–644. doi: 10.1126/science.1168441. [DOI] [PubMed] [Google Scholar]; Friedland, J. C., M. H. Lee, and D. Boettiger. 2009. Mechanically activated integrin switch controls alpha5beta1 function. Science. 323:642-644. [DOI] [PubMed]
- 53.Roca-Cusachs P., Gauthier N.C., Sheetz M.P. Clustering of alpha(5)beta(1) integrins determines adhesion strength whereas alpha(v)beta(3) and talin enable mechanotransduction. Proc. Natl. Acad. Sci. USA. 2009;106:16245–16250. doi: 10.1073/pnas.0902818106. [DOI] [PMC free article] [PubMed] [Google Scholar]; Roca-Cusachs, P., N. C. Gauthier, …, M. P. Sheetz. 2009. Clustering of alpha(5)beta(1) integrins determines adhesion strength whereas alpha(v)beta(3) and talin enable mechanotransduction. Proc. Natl. Acad. Sci. USA. 106:16245-16250. [DOI] [PMC free article] [PubMed]
- 54.Tang B., Zhuang J., Weng W. Harnessing cell dynamic responses on magnetoelectric nanocomposite films to promote osteogenic differentiation. ACS Appl. Mater. Interfaces. 2018;10:7841–7851. doi: 10.1021/acsami.7b19385. [DOI] [PubMed] [Google Scholar]; Tang, B., J. Zhuang, …, W. Weng. 2018. Harnessing cell dynamic responses on magnetoelectric nanocomposite films to promote osteogenic differentiation. ACS Appl. Mater. Interfaces. 10:7841-7851. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.