Abstract
For a long time, wildlife carnivores have been disregarded for their potential in transmitting zoonotic nematodes. However, human activities and politics (e.g., fragmentation of the environment, land use, recycling in urban settings) have consistently favoured the encroachment of urban areas upon wild environments, ultimately causing alteration of many ecosystems with changes in the composition of the wild fauna and destruction of boundaries between domestic and wild environments. Therefore, the exchange of parasites from wild to domestic carnivores and vice versa have enhanced the public health relevance of wild carnivores and their potential impact in the epidemiology of many zoonotic parasitic diseases. The risk of transmission of zoonotic nematodes from wild carnivores to humans via food, water and soil (e.g., genera Ancylostoma, Baylisascaris, Capillaria, Uncinaria, Strongyloides, Toxocara, Trichinella) or arthropod vectors (e.g., genera Dirofilaria spp., Onchocerca spp., Thelazia spp.) and the emergence, re-emergence or the decreasing trend of selected infections is herein discussed. In addition, the reasons for limited scientific information about some parasites of zoonotic concern have been examined. A correct compromise between conservation of wild carnivores and risk of introduction and spreading of parasites of public health concern is discussed in order to adequately manage the risk of zoonotic nematodes of wild carnivores in line with the ‘One Health’ approach.
Keywords: Parasitic zoonosis, Nematodes, Wild carnivores, Baylisascaris, Capillaria, Dirofilaria, Onchocerca, Thelazia, Toxocara, Trichinella
Graphical abstract
Highlights
-
•
A large variety of wild carnivore nematodes have zoonotic potential.
-
•
Limited epidemiological information is available for nematode zoonoses of wild carnivores.
-
•
Some nematode zoonosis of wild carnivores may display a considerable morbidity.
-
•
A compromise between animal conservation and zoonotic infection is advocated.
-
•
Strict control of exotic carnivore species invading wildlife are urgently needed.
1. Introduction
For a long time, zoonotic diseases transmitted to humans have primarily been attributed to livestock throughout food consumption with a few exceptions such as in the case of rabies and echinococcosis. In addition, the emergence or the re-emergence of zoonotic infections (e.g., West Nile Virus) has emphasized the importance of wildlife in spreading new agents to humans (Polley, 2005). Since the industrial revolution in the mid 18th century, human activities and politics (e.g., industrialization, urbanization, fragmentation of the environment, land use) have consistently favoured at different extent and in almost all latitudes, the encroachment of urban areas upon wild environments. This has ultimately caused imbalance and alteration of many ecosystems with major changes in the composition of the wild fauna and destruction of boundaries between domestic and wild territories (Thompson et al., 2010; Liccioli et al., 2015). As a consequence of the new ecological scenarios, there has been an exchange of parasites mainly from wild to domestic animals but also vice versa (Thompson et al., 2009, 2010). It has been estimated that about 43% of the zoonotic viral, protozoal, bacterial, and fungal human infections naturally originated from carnivore hosts (Cleaveland et al., 2001). This fact enhances the public health relevance of wild carnivores and their potential impact in the epidemiology and transmission of many infectious diseases (Polley, 2005). Parasites shared by wild and domestic carnivores in Europe and their transmission have been reviewed elsewhere, specifically for protozoa and other tick-borne pathogens (Otranto et al., 2015a) as well as helminths and arthropods (Otranto et al., 2015b). Some of these parasites are of zoonotic concern and humans may become infected by hand-to-mouth contact after exposure to a contaminated environment (e.g., Echinococcus spp.) or throughout contaminated food, water (e.g., Giardia), ingestion of intermediate (e.g., Toxoplasma, Angiostrongylus costaricensis) or paratenic hosts (i.e., Toxocara), or direct contact (i.e., Sarcoptes). Another important source of infection is represented by arthropods living in the environment such as ticks and mosquitoes. With the spread of allochthonous carnivore species, such as raccoons (Procyon lotor) or raccoon dogs (Nyctereutes procyonoides), new epidemiological patterns for zoonoses have been introduced to previously non-endemic countries. For example, the raccoon dog originates from eastern Asia and represents an emerging allochthonous species in Europe. Since the successful control of rabies in the 1990s, a westward expansion of this canid has been observed in Europe, and today there are stable, large populations from western Russia and the Baltic States through Poland into eastern Germany with the tendency of dispersion all over Europe (Laurimaa et al., 2016a). The raccoon dog established in large populations in Central and Eastern Europe (e.g., the hunting statistic for raccoon dogs in Germany was around 31,000 for the season 2017/2018; Anonymus, 2019) posing challenges in the control of zoonoses such as rabies, alveolar echinococcosis, trichinellosis (Kauhala and Kowalczyk, 2011) and many others. In fact, the parasitic fauna of European raccoon dogs corresponds to that of the red fox with a minimum of 32 helminth species documented, of which 19 have a certain zoonotic potential (Laurimaa et al., 2016a). On the other hand, the raccoon originating from North America introduced as definitive host the extremely virulent neurotropic ascarid species, Baylisascaris procyonis to Europe and Japan (Bauer, 2013; Mackenstedt et al., 2015). Furthermore, the invasion of urban areas by indigenous carnivores such as foxes in European cities (Deplazes et al., 2004) or coyotes in Northern America (Liccioli et al., 2015) created new infection risks in highly populated environments. Similarly, the resettlement of wolves and golden jackals in Central and Northern Europe opened new pathways of zoonotic transmission, although the zoonotic risk is minimal due to the small population size. In this article, we discuss the risk of zoonotic transmission of nematodes from wild carnivores to humans (Table 1, Table 2) and the emergence, re-emergence or the decreasing trend of selected infections under current and future ecological scenarios.
Table 1.
Vector-borne nematode zoonoses of potential wild carnivore origin: geographical distribution, definitive hosts and ways of transmission.
| Nematode species: zoonosis | Vector species | Geographical distribution | Potential definitive hosts D: domestic W: wild (in bolt species with major transmission) |
|
|---|---|---|---|---|
| Onchocercidae (filarioids) | ||||
| Dirofilaria repens: subcutaneous and ocular dirofilariosis | Aedes, Anopheles, Culex | Europe, Asia, Africa | D: dog, cat W: red fox (Vulpes vulpes), wolf (Canis lupus), coyotes (Canis latrans), least weasel (Mustela nivalis) |
|
| Dirofilaria immitis: mainly pulmonary dirofilariosis of low pathogenicity | Aedes, Anopheles, Culex | Worldwide | D: dog, cat W: wild cat (Felix silvestris), fox, ocelot (Leopardus pardalis), mountain lion (Felis concolor), clouded leopard (Neofelis neburosa), snow leopard (Uncia uncia), Bengal tiger (Panthera tigris), lion (Panthera leo), coyote, jackals (Canis aureus), wolf |
|
| Dirofilaria ursi | Black flies | North America, Japan | W: bear (Ursus americanus, Ursus thibetanus japonicas) | |
| D. tenuis | Aedes, Anopheles, | Canada, USA | W: raccon | |
| Dipetalonema arbuta | Aedes, Taeniorhynchus | Canada, USA | W: porcupine | |
| Dipetalonema sprenti | Mosquitoes | Canada, Oregon, USA | W: beaver | |
| Onchocerca dewittei japonica | Simulium spp. | Japan | W: bear | |
| Onchocerca lupi: ocular onchocercosis | Simulium spp.?? | Europe, USA, Iran | D: dogs, cats W: wolf |
|
| Thelazidae | ||||
| Thelazia callipaeda: ocular thelaziosis | Phortica spp. | China, South East Asia, Europe, USA | D: dogs, cats, rabbits W: fox, wild cat, wolf, beech marten (Martes foina), hare |
|
| T. californiensis: ocular thelaziosis | Fannia canicularis? | UDA, Canada? | D: dogs, cats, rabbits W: coyotes, dogs |
|
Table 2.
Food- water- and soil-borne nematode zoonoses of potential wild carnivore origin: geographical distribution, primary definitive host and ways of transmission.
| Nematode species: zoonosis | Geographical distribution | Potential definitive hosts D: domestic W: wild (in bolt species with major transmission) |
Parasite stage transmitted to humans/way of transmission (L: larva) | |||
|---|---|---|---|---|---|---|
| Ascaridida, Ascarididae (ascarids) | ||||||
| Toxocara canis: visceral, ocular larva migrans | Worldwide | D: dog, feral dog W: red fox (Vulpes vulpes), golden jackal (Canis aureus), wolf (Canis lupus), raccoon dog (Nyctereutes procyonoides), coyotes (Canis latrans) |
L3 (in egg), rarely L3 in paratenic host tissues/per os | |||
| Toxocara cati (Syn T. mystax): Visceral, ocular larva migrans | Worldwide | D: cat, feral cat (Felis catus) W: European wild cat (Felis s. silvestris), Iberian lynx (Lynx pardinus), Eurasian lynx (Lynx lynx), bobcat (Lynx rufus) |
L3 (in egg)/per os | |||
| Baylisascaris procionis: Visceral, ocular and neural larva migrans | North America, Asia, focally Central and East Europ, Japan | D: dog W: Raccoon (Procyon lotor), main host |
L3 (in egg)/per os | |||
| Strongylida, Ancylostomatidae (hookworms) | ||||||
| Uncinaria stenocephala: cutaneous larva migrans | Worldwide | D: dog W: red fox, raccoon dog, golden jackal, wolf |
Free L3/active skin invasion | |||
| Ancylostoma caninum: cutaneous larva migrans, rarely eosinophilic enteritis | Worldwide, predominantly in tropical and subtropical areas | D: dog W: red fox, raccoon dog, golden jackal, wolf, bobcat (Lynx rufus), Canadian lynx (Lynx canadensis) |
Free L3/active skin invasion | |||
| A. ceylanicum: intestinal ancylostomosis | Asia, Australia, South America | D: dog, cat W: dingo (Canis lupus dingo) |
Free L3/active skin invasion | |||
| A. braziliense: cutaneous larva migrans | South and Nord America, Malaysia, Indonesia | D: dog, cat W: bobcat (Lynx rufus), grey fox (Urocyon cinereoargenteus) other |
Free L3/active skin invasion | |||
| Strongylida, Angiostrongylidae | ||||||
| Angiostrongylus costaricensis: abdominal angiostrongylosis | South and North America | D: dog W: white-nosed coati (Nasua narica) |
L3 in slug and snail or in their mucus/per os | |||
| Rhabditida, Strongyloides | ||||||
| Strongyloides stercoralis: Strongyloidosis | Worldwide, predominantly in tropical and subtropical areas | D: dog W: red fox, grey fox (Urocyon cinereoargenteus) |
Free L3/active skin invasion | |||
| Strongyloides procyonis: larva migrans cutanea and a short-lived intestinal infection | USA, Japan | W: raccoon | Free L3/active skin invasion | |||
| Strongylida, Gnathostomatidae | ||||||
| Gnathostoma spinigerum: transient gastrointestinal symptoms, cutaseous, visceral, gnathosomosis, | South East Asia, Central Africa | Wild and domestic canidae, felidae and other carnivores | L3 in fish, frogs, snakes, meet (e.g. wild boar, poultry) or with L1 with coprepodes in water/per os | |||
| Gnathostoma binucleatum: cutaseous, visceral, gnathosomosis | Latin America and USA | D: dog, cat W: wild felids |
L3 in fish … … | |||
| Enoplida, Trichuridae, Trichinellidae | ||||||
| Capillaria (Syn. Eucoleus) aerophila: Rare, pulmonary infection | Europe | W: red fox, raccoon dog, wolf, golden jackal, European wild cat, beech marten (Martes foina), wild felids | L 1 in egg/per os | |||
| Trichinella spp. (e.g. Trichinella britovi, T. nativa, T. nelsoni, T. spiralis, T. pseudospiralis) | Worldwide, | Numerous domestic and wild animals | L1 in meet/per os | |||
2. Vector borne transmitted nematodes
2.1. Dirofilarioses
Amongst zoonotic vector-borne nematodes, the genus Dirofilaria (Spirurida, Onchocercidae) is the best known, primarily because of the worldwide distribution of the two main species, Dirofilaria immitis and Dirofilaria repens, the causative agents of canine heartworm diseases and subcutaneous dirofilariosis, respectively (Table 1). In areas where these filarioids are endemic, specifically in Europe, Asia, Africa and America for D. immitis and Europe for D. repens (Dantas-Torres and Otranto, 2013), they have also been diagnosed in wild carnivores (McCall et al., 2008; Penezić et al., 2014; Ionică et al., 2016). In addition, a further 27 species have been described within the genus Dirofilaria and 15 are of questionable validity (Canestri Trotti et al., 1997). Almost all of the above mentioned species parasitize wild carnivores (e.g., foxes, coyotes, wolves, sea lions, harbour seals, laboratory ferrets, bears, muskrats, raccoons and bobcats), localizing in their subcutaneous tissues (reviewed in Canestri Trotti et al., 1997; Dantas-Torres and Otranto, 2013) with the exception of D. immitis. These filarioids are transmitted by mosquitoes belonging to the genus Culex, Aedes, Ochlerotatus, Anopheles, Armigeres and Mansonia, according to the geographical area, whereas, Aedes vexans, Culex pipiens and Aedes albopictus have been implicated as the most common vectors (Cancrini et al., 2007; Capelli et al., 2013). While biting carnivores, the vector may transmit Dirofilaria spp. third stage larvae also to accidental hosts, such as humans, causing symptoms of various severity according to the localization of migrating larvae into different organs and to the individual immune responsiveness of the patient. In humans, larvae or adult nematodes of D. immitis and D. repens are primary localized in the subcutaneous tissues, but also in pulmonary vessels and in the central nervous system, causing mainly asymptomatic outcome of disease or rarely fatal symptoms (McCall et al., 2008; Genchi et al., 2011; Otranto and Eberhard, 2011). In addition, Dirofilaria ursi of bears, Dirofilaria subdermata of porcupines and Dirofilaria striata of wild cats and bobcats have occasionally been diagnosed in humans (Otranto and Eberhard, 2011). Dirofilaria ursi has furthermore commonly been reported in the American black bear (Ursus americanus Pallas, 1780) across North America, and in the Asiatic black bear (Ursus thibetanus japonicus Cuvier, 1823) in Japan (Uni, 1983; Yokohata et al., 1990; Duffy et al., 1994). The latter mentioned Dirofilaria species, vectored by black flies, may reach a microfilariae prevalence up to 21% in blood from body cavities of hunted bears (Michalski et al., 2010), therefore indicating that a higher prevalence of the infection may occur, if counting the number of non-filaremic animals. For long time, Dirofilaria tenuis, a common parasite of raccoons in the United States, has been considered as a causative agent of human infection (Canestri Trotti et al., 1997). This parasite has been diagnosed in a case of a wrist lesion leading to median nerve compression pathology in a healthy young woman (Ramirez et al., 2013). Certainly, current knowledge about the number of Dirofilaria spp. living in wild carnivores and their zoonotic potential is limited mainly in geographical areas where there is a large variety of wild canids and felids, such as in southern and Central America. For instance, a live nematode surgically extracted from the anterior eye chamber of a patient from Amazon forest in Brazil was morphologically similar, but molecularly distinct, from D. immitis infecting dogs in the same area. This finding suggests the occurrence of a closely related zoonotic Dirofilaria species in wild mammals in Brazil (Otranto et al., 2011a). Nevertheless, most reported zoonotic cases are caused by D. immitis and D. repens, the prevalent species in dog and cat populations, probably because they live in close contact to humans. While both the latter mentioned Dirofilaria spp. develop to adults in domestic dogs and some wild canids, producing abundant and persistent microfilaremia in their blood stream, cats and wild felids are no suitable hosts for these parasites and may develop only low-level, transient microfilaremia (McCall et al., 2008). Dirofilaria immitis does usually not develop to the sexual mature adult stage in humans, whereas circulating microfilariae derived from patent D. repens infections have increasingly been reported in human patients from Europe and Asia (Damle et al., 2014; Genchi and Kramer, 2017; Blaizot et al., 2018). The overall increasing number of human cases of D. repens infection in Europe and, in particular, towards eastern and northern European countries (e.g., up to 1465 human cases in Ukraine from 1996 to 2012; (Sałamatin et al., 2013) has raised the interest of veterinarians and medical physicians working in the field of Public Health about the infection not only in dogs, but also in wild carnivores (Capelli et al., 2018). While in endemic areas frequent chemoprophylactic treatments of domestic dogs reduce the overall prevalence of the infection, wild canids might play a crucial role in the maintenance of the infection. Another reason for the spread of human dirofilarioses caused by D. repens may be the expansion of vector habitats (e.g., more favourable environmental and climate conditions), a less efficient immune reactions to the parasite located in subcutaneous tissues in the human host and the limited diagnostics and treatment approaches in dogs as primary host. Nonetheless, the involvement of wild canids in spreading human dirofilarioses has never been properly investigated, although they could play a major epidemiological role, mainly because they are out of any preventative control strategies. Wild carnivores (Table 1) living in zoological gardens and free ranging ‘safari’ parks were diagnosed with D. immitis at necropsy (McCall et al., 2008; Penezić et al., 2014), however, microfilariae have exclusively been found in a leopard (Panthera pardus pardus) died in a zoological park in a highly endemic area of northern Italy (Mazzariol et al., 2010). In contrast, coyotes (Canis latrans), jackals (Canis aureus), wolves (Canis lupus) and foxes (Vulpes vulpes) living in their natural habitat have been found positive for D. immitis adults at necropsy (reviewed in Otranto et al., 2015b). For example, up to 18.52% of golden jackals from the southern part of Romania have been found positive for D. immitis at the necropsy, suggesting a major role of this animal species in the transmission and the maintenance of natural disease foci of dirofilariosis (Ionică et al., 2016). In addition, the spatial distribution of red foxes and golden jackals infected with D. immitis was similar to that of dogs in the same time period (Bacsadi et al., 2016). Heartworm were also detected in 37.2% of 212 coyotes collected from 28 counties in Florida, US), with a higher prevalence in adults (45.6%) than juveniles (29%) (Aher et al., 2016). Some of the species of wild carnivores mentioned above were also microfilaremic such as in the case of coyotes in the USA and foxes in Italy with a prevalence ranging from 16% to 35% (Miller et al., 2009; Paras et al., 2012) and of 2.3% (Magi et al., 2008), respectively. Microfilariemia indicate the potential for those hosts to serve as a source of infection for mosquitoes. Nonetheless, the high prevalence of D. immitis detected in 9.6% of jackals from Bulgaria (Kirkova et al., 2011), 7.4% from Hungary (Tolnai et al., 2014), and up to 12.7% from Serbia (Penezić et al., 2014), suggests that also these animal species play a role in the maintenance of infection. Similarly, the epidemiological role of foxes needs to be clarified, particularly because of the report of up to 32.3% animals positive for D. immitis in irrigated habitats of Ebro Valley (Spain) (Gortázar et al., 1998). A high prevalence of D. immitis dirofilariosis (i.e., 46%) was recorded in Australian dingoes, but not in sympatric living domestic dogs, suggesting the indirect exchange of parasites between dogs and dingoes (Smout et al., 2018). The evidence of adult D. repens at necropsy (i.e., in the subcutaneous tissue) is more difficult than D. immitis (i.e., in the pulmonary arteries and in heart right ventricle) resulting in fragmentary available data about the distribution of this nematode in wild carnivores. Incidentally, the same applies to all onchocercids species localizing in the subcutaneous tissues of carnivores (e.g. Cercopithifilaria bainae, Cercopithifilaria grassii and O. lupi). The presence of D. repens DNA has recently been detected in spleen samples of red foxes, golden jackals, one grey wolf and one least weasel (Mustela nivalis), suggesting their potential role in the epidemiology of disease (Ionică et al., 2017). Adults of D. repens were furthermore subcutaneously diagnosed in two wolves and a red fox from central Balkan (i.e., Macedonia and Serbia) (Ćirović et al., 2014).
2.2. Thelaziosis
From the 16 species of Thelazia eyeworms, which are localized in the conjunctiva, lachrymal ducts and surrounding structures of animal hosts, two species, namely Thelazia callipaeda and Thelazia californiensis Price, 1930 (Spirurida, Thelaziidae) parasitize a wide range of animal hosts including carnivores, lagomorphs and humans. While these nematodes cause mild clinical symptoms in infested animals (e.g., conjunctivitis, epiphora, ocular discharge, keratitis) and rarely severe corneal ulcers and blindness, the zoonotic potential of T. callipaeda is of concern, mainly in rural areas of Southeast Asia (Shen et al., 2006; Otranto and Dutto, 2008) where, according to some molecular investigations, the parasite was originated (Otranto et al., 2005). Scientific information about T. californiensis is limited and uncertain also for the scarce number of reports from Western USA (California) in dogs, cats, coyotes, bears and humans (Doezie et al., 1996). In a unique case, T. californiensis adult nematodes were morphologically and genetically diagnosed in a Swiss patient after a stay in Northern California (Grimm and Deplazes, personal communication). Information about the vector of this eyeworm species is lacking, however, the little house flies Fannia canicularis and Fannia benjamini (Diptera, Fannidae) have been implicated in its transmission (Burnett and Wagner, 1958). Conversely, T. callipaeda lives in the orbital cavities and associated host tissues of dogs and cats as well as a large range of wild carnivore species (e.g., foxes, wolves, beech martens and wild cats; Fig. 1A and B) and lagomorphs (Otranto et al., 2009). This parasite is well known by the scientific community due to its distribution in a large number of geographical areas and to its zoonotic impact. Accordingly, the common name “oriental eyeworm” issuing from the primary occurrence of T. callipaeda in the Far Eastern countries (i.e., Indonesia, Thailand, China, Korea, Myanmar, India, and Japan) (see Anderson, 2000) is obsolete. Indeed, over the last 20 years, this parasite has been identified as an agent of ocular disease in many regions of Europe (Otranto et al., 2013a) primarily in dogs and also in many other animal species, including humans. Since the primary report in Italy, T. callipaeda has rapidly expanded its distribution throughout Europe from western to eastern Portugal, Spain, France, Switzerland, Germany, Bosnia and Herzegovina, Croatia, Romania, Bulgaria, Hungary, Greece and Slovakia (Rossi and Bertaglia, 1989; Otranto et al., 2003; Dorchies et al., 2007; Malacrida et al., 2008; Magnis et al., 2010; Miró et al., 2011; Vieira et al., 2012; Hodžić et al., 2014; Mihalca et al., 2015; Colella et al., 2016; Čabanová et al., 2017; Papadopoulos et al., 2018). The expansion of the parasite across domestic and wild animal species throughout Europe is in accordance with increased numbers of reported human cases (Otranto and Dutto, 2008; Fuentes et al., 2012; Paradzik et al., 2016; Tasic-Otasevic et al., 2016). Nowadays, T. callipaeda is considered in the diagnosis of helminthic infection of human eyes along with the better-known Dirofilaria spp. and, the less known, Onchocerca lupi (Otranto and Eberhard, 2011). Undoubtedly, the relatively short time in which T. callipaeda has been identified in many European countries in the eyes of domestic carnivores clearly indicate that canine thelaziosis is an emerging vector borne helminth. Dogs are supposedly the primary host of this nematode and they can be considered sentinel animals because of the localization of T. callipaeda on the conjunctiva mucosa, which is one of the main anatomical sites diagnosed by practitioners during general clinical examinations. The role of vectors, reservoir hosts and the environment in explaining the emergence of thelaziosis is difficult to assess. For example, foxes (Fig. 1A) are also heavily infected by T. callipaeda with prevalence up to 27.71% in Bosnia Herzegovina (Hodžić et al., 2014; Sargo et al., 2014), 29.38% in Romania (Ionică et al., 2018) and 49.3% in southern Italy (Otranto et al., 2009). In addition, the pan European distribution of the vector Phortica variegata (Drosophilidae, Steganinae) is crucial to explain the spread of T. callipaeda (Máca and Otranto, 2014). Accordingly, thelaziosis has been reported from many areas of Europe identified in a Genetic Algorithm for Rule-Set Prediction model, which was based on ecological field data collected for this lachryphagous drosophilid (Otranto et al., 2006a). The success of this predictive model further indicates the importance of observational field studies, even in the modern concept of veterinary parasitology. Since wild canids (i.e., foxes, beech martens, wolves) and felids (i.e., wild cats) live in forested, meadow hilly areas (e.g., around oak woods, Quercus cerris) and in environments characterized by high relative humidity (e.g. around rivers) and undergrowth, they are exposed to the vector (Otranto et al., 2006a). Therefore, wild animals species may represent proper hosts for T. callipaeda in endemic as well as in non-endemic areas (Mihalca et al., 2016), such as in UK, where merely imported cases of canine thelaziosis have been reported so far (Graham-Brown et al., 2017), although the vector is endemic (Palfreyman et al., 2018). The circulation of eyeworms within different animal species and its recent introduction to Europe is corroborated by the occurrence of a unique genetic haplotype of T. callipaeda amongst all specimens collected from wild and domestic animals (Otranto et al., 2005, and all other subsequent studies). Data above suggest that wild carnivores contribute to the dissemination of infection within their natural territories, which range from 10 to 30 km for foxes (Niewold, 1980; Doncaster and Macdonald, 1991) and up to 800 km for wolves (Mech, 1970). In addition, both canid species are more active during dawn and dusk (Fedriani et al., 1999) when P. variegata is prevalent during summer months (Otranto et al., 2006b).
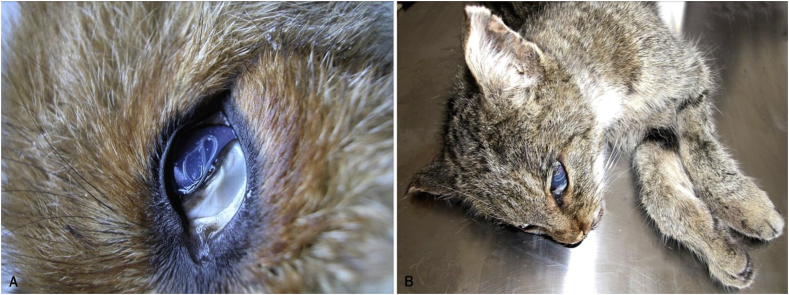
Fig. 1.
Adult specimens of Thelazia callipaeda on the eye of a red fox (A), and a wild cat (B).
2.3. Onchocercosis
The genus Onchocerca (Spirurida, Onchocercidae) encompasses species parasitizing ungulates, with the only exception of Onchocerca volvulus and O. lupi, which primarily parasitize humans and carnivores, respectively (Bain et al., 2013; Lefoulon et al., 2017). The public health impact of O. volvulus in Africa is estimated to about 25 million individuals annually infected in the Middle East in Yemen and Americas (e.g., Mexico, Guatemala, Ecuador, Colombia, Venezuela, and Brazil), making this infection one of the most important neglected tropical diseases (http://www.cdc.gov/parasites/onchocerciasis/gen_info/faqs.html). Conversely, the zoonotic potential of O. lupi has only recently been recognised (Otranto et al., 2011b) and, since then, human cases have been reported in Europe, Middle East regions and US (Mowlavi et al., 2014; Otranto et al., 2015c; Cantey et al., 2016). Since the first report of the parasite in a Caucasian wolf (Canis lupus) from Gruziya (Georgia, former USSR, Rodonaja, 1967), which was the main reason for naming the nematode as O. lupi, the infection has been diagnosed in dogs and cats from Portugal, Spain, Germany, Greece, Hungary, and Romania (Széll et al., 2001; Komnenou et al., 2002; Hermosilla et al., 2005; Faísca et al., 2010; Maia et al., 2015; Tudor et al., 2015; Hodžić et al., 2017), as well as in the U.S. and Canada (Labelle et al., 2011, 2013; Otranto et al., 2015c).
Nonetheless, the largest bulk of above-mentioned reports derives from clinical cases of pet animals presented for surgical removal of nodules, or suffering for conjunctivitis, chemosis, lacrimation and periorbital swelling (Komnenou et al., 2002; Sréter and Széll, 2008). The acute presentation of the infection represents only the tip of the iceberg for the parasite endemicity in canine population, as it was demonstrated by the high prevalence (8.4%) of microfilaremic, but healthy dogs from Greece and Portugal (Otranto et al., 2013b). Therefore, considering the difficulties of diagnosing microfilariae from skin biopsy sediments in necropsied wild animals, data on O. lupi infection in wildlife is inexistent. Nonetheless, the involvement of wild carnivores cannot be ruled out mainly in areas where human, but not canine cases have been reported, such as in Iran (Mowlavi et al., 2014), Tunisia (Otranto et al., 2012) and Turkey (Otranto et al., 2011b). Finally, the lack of data about the insect species acting as vector of this barely known onchocercid, greatly impairs the information about its distribution in wild carnivores and the potential spread of the infection. Up to now, wild-caught Simulium tribulatum have been found molecularly positive for O. lupi and this species has been suggested as putative vector in California (Hassan et al., 2015).
3. Soil-transmitted nematode zoonoses
3.1. Toxocarosis
Ascarids (Ascaridida, Ascarididae) of carnivores are worldwide-distributed, large (10–15 cm in length) intestinal nematodes, some of which have a zoonotic potential (Table 2). Their life cycle is direct but can include paratenic hosts as a source of infection for carnivorous definitive or dead-end hosts. Definitive hosts defecate thick-shelled eggs which embryonate in the environment under suitable conditions (i.e., 28–33 °C in laboratory conditions) within 2–6 weeks to infectious third stage larvae (L3). The biology of Toxocara canis, Toxocara cati and B. procyonis differs significantly in their definitive hosts (Deplazes et al., 2016). Toxocara canis transmission in canids is highly efficient by the in utero infection of the offspring, through the transplacental transmission of reactivated somatic larvae (Schnieder et al., 2011). Furthermore, juvenile and adult foxes can become infected or re-infected by predation of paratenic hosts, mainly rodents and birds, or by ingestion of infectious T. canis eggs (Saeed et al., 2005). The relatively high intestinal infection rate in adult foxes (prevalence between 10 and 40%, see below) in endemic areas suggests a low level of immune protection for intestinal infections and reinfections. Generally, higher prevalence of patent T. canis infections are observed in foxes younger than six months of age (Luty, 2001; Saeed et al., 2006; Reperant et al., 2007), indicating that the transplacental transmission is common. Also the seasonality may play a role in the occurrence of toxocarosis, with a higher prevalence of intestinal T. canis infections in fox cubs during spring (73%) and summer (65%), compared to fall (37%) and winter (42%) (Reperant et al., 2007). The prevalence of patent T. canis infections varies in European foxes between 9 and 65% with data available for Denmark (48–65%, Saeed et al., 2006; Al-Sabi et al., 2014); Switzerland (44% in Geneva and 47% in Zurich, Reperant et al., 2007; Hofer et al., 2000); Austria (48%, Duscher et al., 2014); Italy (9–53%, Magi et al., 2009; Fiocchi et al., 2016); Ireland (20%, Stuart et al., 2013); Lithuania (41%, Bružinskaitė-Schmidhalter et al., 2012); Estonia (30%, Laurimaa et al., 2016b); Poland (11%, Borecka et al., 2013); the Slovak Republic (43%, Miterpáková et al., 2009). Also in other endemic areas high prevalence of T. canis in foxes have been documented (e.g., Australia, New South Wales, up to 35.2%, Ryan, 1976; Japan, 71%, Sato et al., 1999; Iran, 32%, Meshgi et al., 2009; Canada, 32.5% in juveniles, 15.1% in adults, Wapenaar et al., 2013).
Although the biology of T. canis has not been studied in detail in other wild carnivores than those mentioned above, many of them are suitable natural hosts for T. canis. For example, the prevalence of T. canis intestinal infections in raccoon dogs varies according to the geographical areas being 13% in Denmark (Al-Sabi et al., 2014), 18% in Germany (Thieß et al., 2001), 4.7% in Estonia (Laurimaa et al., 2016a), 16% in Poland (Osten-Sacken et al., 2017) and 18% in Lithuania (Bružinskaitė-Schmidhalter et al., 2012). However, when data on T. canis infection is available for foxes and racoon dogs (e.g., Lithuania and Denmark), the prevalence in raccoon dogs is lower as compared to foxes (Bružinskaitė-Schmidhalter et al., 2012; Al-Sabi et al., 2014). The latter observation could be explained by the fact that raccoon dogs decrease their activity during the ‘hibernation’ period in winter and tend to defecate in latrines, which may also reduce their potential to spread parasite eggs.
In golden jackals, prevalence of T. canis intestinal infections was documented in Hungary (20%, Takács et al., 2014) and Iran (27.2%, Vafae Eslahi et al., 2017). Toxocara canis also infects wolves with different prevalence in Italy (17%, Guberti et al., 1993; 33%, Fiocchi et al., 2016), Germany (5%, Bindke et al., 2017; 11%, Lesniak et al., 2017), Belarus (21%, Shimalov and Shimalov, 2000), Estonia (8%, Moks et al., 2006), Poland (6.9%, Borecka et al., 2013) and Spain (6%, Segovia et al., 2003). No wolves infected with T. canis were diagnosed in Sweden (Al-Sabi et al., 2018). In Australien wild dogs (dingoes) the prevalence of T. canis was up to 46% from Wet Tropics region around Cairns, Far North Queensland (Smout et al., 2013), and 27.8% of 18 dingoes from Fraser Island (Queensland) with close ntcoact with human habitation were infected (Mackenstedt et al., 2015). Finally, up to 24% in the coyotes from Southeastern region of Nebraska and Shenandoah area of Iowa in USA (Redman et al., 2016) and, again, to 3.5% in juveniles and 1.1% in adults from Prince Edward Island, Canada (Wapenaar et al., 2013).
In felids, T. cati (syn. Toxocara mystax) is mainly transmitted by predation of paratenic hosts or ingestion of infectious eggs. Infection with large numbers of T. cati eggs during the last third of gravidity in female cats results in the trans-mammary transmission of infective larvae to the kittens (Coati et al., 2004), however, the epidemiological significance of this way of transmission for wild felids is not confirmed (Fig. 2). In Europe, T. cati is highly prevalent in the domestic cat populations that have free access to the environment and to potentially infected paratenic hosts (Giannelli et al., 2017). Furthermore, large populations of feral or stray cats represent the main reservoir for the maintenance of the T. cati life cycle. A variety of wild felids are susceptible to T. cati intestinal infections, with a report in an Iberian lynx (Lynx pardinus) in Spain, and very high prevalences in Eurasian lynxes (Lynx lynx) in Finland (98%, Deksne et al., 2013) or in Poland (7 of 11 animals examined, Kołodziej-Sobocińska et al., 2018), or bobcats (Lynx rufus) in USA, Midwest (70%, Hiestand et al., 2014). Furthermore, this parasite was also endemic in wild cats in Sicily Island (southern Italy) with 43.8% of 121 cat scats positive for T. cati eggs (Napoli et al., 2016). In addition, T. cati was the most prevalent ascarid found in the Siberian tiger (Panthera tigris altaica), although the epidemiological role of this animal species is unknown (Moskvina et al., 2018).
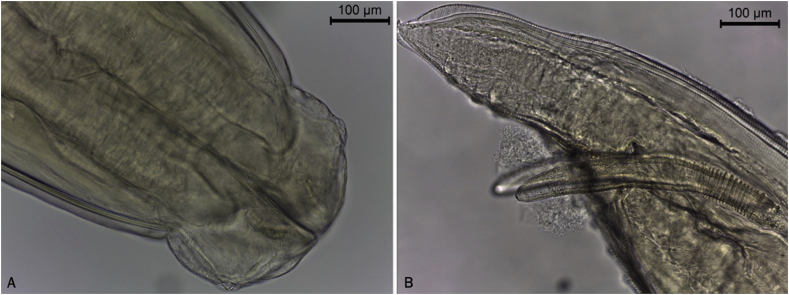
Fig. 2.
Anterior edge of Toxocara cati from the gut of a wild cat. The short and wide cervical algae give the typical appearance of an arrow.
Human Toxocara infections are predominantly acquired from ingestion of embryonated eggs by hand-to-mouth contact after activities in contaminated environments such as sandpits, parks or other places where carnivores have defecated. The transmission has been also hypothetically linked to water or foodborne sources. The actual importance of wildlife contributing to environmental contamination with ascarid eggs is still unknown. Since the 1940th in Great Britain and, later on, in the periurban areas throughout Europe, a steady increase in populations of foxes has been reported (Scott et al., 2014). These highly reproductive fox populations present annually a new highly susceptible generation of cubs with high T. canis worm burdens, contributing significantly to the environmental contamination with eggs. Within the fox home ranges, which can be much smaller in urban areas (Deplazes et al., 2004), a complex pattern of spatial faeces distribution can be observed with significantly higher density both on the borders of ploughed fields and on road verges (Guislain et al., 2007). Furthermore, marking of territories along roads, pavements and feeding places in private gardens may contribute to the environmental contamination with zoonotic helminths (e.g. Toxocara and Echinococcus eggs). Based on the complex wild animal ecology, we can assume that foxes may play a major role in the maintenance of the wildlife cycle of T. canis and strengthening a continuous transmission to humans and pet dogs. Similarly, a study of host density of sympatric cats, dogs and foxes in the city of Bristol (UK) in relationship to the environmental contamination with Toxocara eggs revealed that dog puppies “dominate total egg output”, although “foxes could take over as the primary source of eggs environmental contamination” (Morgan et al., 2013). However, the authors further discussed that the relative contribution to the egg contamination of dogs, cats and foxes might differ among specific locations dependant on animal densities and their access to the public. Furthermore, involvement of large feral or stray domestic dogs and cat populations might again have a high epidemiological impact in the contamination with eggs of Toxocara spp. (Otranto et al., 2017). Although Toxocara eggs have been found in the hair coat of dogs and foxes, the importance of the direct transmission to human has been discussed controversially in the past as, in most of the cases, eggs were not fully larvated (Deplazes et al., 2011; Merigueti et al., 2017). Furthermore, an experimental study confirms that T. canis eggs did not fully embryonate on the coat of dog puppies but during the same observation time in their surrounding (Nagy et al., 2011). Epidemiological studies further confirm that pet ownership was not associated with increased human seroprevalence for toxocarosis (Lee et al., 2014). Finally, humans could also become infected by eating row tissues of T. canis paratenic host as experimentally documented for raw liver or other viscera of pigs or chickens (Taira et al., 2004). The relative contribution of wild carnivores in such a rather “exotic” way of transmission might be strongly related to certain cultural settings, as it was discussed for eating habits in East Asia (e.g. South Korea and Japan) through ingestion of raw beef, lamb, chicken or ostrich liver (Akao and Ohta, 2007).
Clinically toxocarosis is categorised as a visceral, neurological and ocular larva migrans syndrome that can affect millions of children and adolescent populations worldwide. Many reviews claim this disease to be neglected, under-reported or under-diagnosed (Deplazes et al., 2011; Lee et al., 2014; Chen et al., 2018). Asymptomatic and self-limiting infections are probably frequent based on high serological prevalence often associated with eosinophilia. Series of clinical cases or serological studies estimate burdens of disease in diverse geographic regions worldwide, but especially in impoverished communities. A comprehensive literature review focussing on clinical cases (Chen et al., 2018) documented worldwide a total of 823 cases of ocular toxocarosis (i.e., 282 in Europe, 317 in Asia, five in Australia, 218 in Latin America, and few from other areas), but interestingly, the highest number has been reported in Japan and Korea, France, Brazil and the USA. In 99 cases, neurotoxocarosis was diagnosed (32 in Europe, 32 in Asia, 20 in Americas and few from other areas) and up to 247 visceral larva migrans cases documented. Despite the worldwide distribution of T. canis in dog and fox populations, cases are not equally distributed in the endemic areas indicating differences in clinical and diagnostic management of patients. For example reports of human cases in Austria are rare, but Auer (2011) estimated for this country several hundred undiagnosed overt cases per year.
3.2. Baylisascariosis
Baylisascaris procyonis (Fig. 3) is an ascarid endemic in North America. Main definitive hosts of B. procyonis are raccoons (Procyon lotor) and related procionids, however, also in dogs patent infections can develop (Kazacos, 2001). Definitive hosts acquire the infection by ingesting larvated eggs or by predation of paratenic hosts, which often display neurological disease. Raccoons originating from North America have been imported into Europe (i.e., Germany, Russia and Poland) and Japan many decades ago (Bauer, 2013; Mackenstedt et al., 2015). In Germany, it is believed that several independent introductions of the raccoon occurred, which then widespread throughout the country (Fischer et al., 2015; Osten-Sacken et al., 2018), representing the largest population outside America with an impressive hunting statistic of about 172,000 individuals for the season 2017/2018 (Anonymus, 2019). It has been estimated that up to 700 raccoons found per square mile live in the U.S. (https://study.com/academy/answer/how-many-raccoons-are-there-in-the-world.html).
Fig. 3.
Baylisascaris procionis anterior end with typical lips (A), and male posterior end with spicules (B) and papillae (F). Scale bars = 220 μm (A), 600 μm (B), 900 μm (C), 200 μ (D, E), 100 μm (F).
Baylisascaris infections have been documented in around 70% of raccoons in Central Germany (Bauer, 2013), even in the intestine of 24 raccoons (75%) originating from the urban area of Leipzig (Rentería-Solís et al., 2018), and in the faeces of animals from a zoo in Denmark (Al-Sabi et al., 2015) and Poland (Karamon et al., 2014). In addition, B. procyonis, Toxocara and Toxascaris eggs have been recently documented in the intestine of raccoons illegally imported to Norway (Davidson et al., 2013). Furthermore, B. procyonis was found in captive raccoons in Japan (Page, 2013) and in 50% of those examined in Costa Rica (Baldi et al., 2016). Similar as for Toxocara spp., human infections with B. procyonis eggs occurs via ingestion of larvated eggs in contaminated areas, mainly represented by the animal's defecation sites and latrines (Page et al., 1999). Baylisascaris procyonis causes visceral and neurological larva migrans, which can particularly be severe because of the increased growth of the larvae compared to Toxocara spp. during its migration (reviewed by Kazacos, 2001). Human cases of baylisascariosis are relatively rare; however, many cases show severe clinical complications. Since 1973, at least 28 cases of human baylisacariosis have been reported in the USA (Gavin et al., 2005; Sircar et al., 2016) and most of them occurred in children. So far, only a single human baylisascariosis case outside America has been reported in Germany in a patient presented with unilateral neuroretinitis syndrome, who had purchased a raccoon from a local zoo (Küchle et al., 1993). Similar to toxocarosis, epidemiological investigations suggest that subclinical infections with B. procyonis may occur in human patients. Relatively high seroprevalence in the population have been found in endemic areas; for example in Chicago, 8% of 389 children without a history of disease (Brinkman et al., 2003) or healthy individuals was found seropositive after exposure to raccoons or their faeces (Conraths et al., 1996). Finally, experimental evidence in Mongolian jirds (Sato et al., 2004) supports the zoonotic potential of Baylisascaris transfuga, a parasite for example found frequently in brown bears (Ursus arctos) in Slovakia, therefore, the zoonotic role of this species should be further investigated (Štrkolcová et al., 2018).
3.3. Strongyloides
Strongyloides stercoralis (Rhabditida: Strongyloididae) is soil-transmitted species with zoonotic potential (Thamsborg et al., 2017), it occurs in humans, other primates and dogs mainly in tropical and subtropical areas (Bisoffi et al., 2013; Paradies et al., 2017). However, the parasite is also present in Europe, but data on the epidemiology of canine strongyloidosis is missing (Iatta et al., 2018). Strongyloides spp. have been sporadically diagnosed in wild carnivores and were mostly allocated to S. stercoralis (Thamsborg et al., 2017), however, ata based on morphological identification of larvae from environmental samples without genetic analyses have to be critically interpreted. A Strongyloides spp. prevalence of 0.7% was recorded in foxes from the Netherlands (Borgsteede, 1984), the Slovak Republic (1.6%, Miterpáková et al., 2009). A Strongyloides sp. has also been documented in grey foxes (Urocyon cinereoargenteus Schreber, 1775) in dry tropical highlands of central Mexico (Hernández-Camacho et al., 2011). Even if S. stercoralis may cause a serious disease especially for chronically infected and immunocompromised hosts (Bisoffi et al., 2013), sporadic infections in wild carnivores or in domestic dogs have so far not been associated as a major zoonotic risk for humans. Strongyloides procyonis was reported for the first time in Louisiana and was demonstrated to cause experimental creeping eruption and a short-lived intestinal infection in inoculated human volunteers (Little, 1965). Although the zoonotic role has never been demonstrated in naturally infected human patients, the parasitic females of S. procyonis were identified in 66 (28.3%) of 233 raccoons collected in Japan, indicating the potential zoonotic risk in particular environments (Sato et al., 2006).
3.4. Hookworm infections
Hookworms (Strongylida, Ancylostomatidae) display a high pathogenic potential due to their blood-feeding behaviour. A recent systematic review of the literature on hookworms demonstrated that at least 68 hookworm species parasitize the gastrointestinal tract of wildlife mammals, black bears, red foxes, and bobcats harboured the highest diversity of hookworm species (Seguel and Gottdenker, 2017). The parasites have been studied mainly in red foxes and coyotes, probably because they are commonly hunted/culled (Seguel and Gottdenker, 2017). The occurrence and the prevalence of hookworm species depend on peculiarities in local humidity, temperature and host population density, which induce a dynamic scenario in their infections of animal-human-wildlife. For example, Ancylostoma caninum has worldwide distribution mainly in tropical and subtropical areas in domestic dogs, but it occurs also in wild canids and felids but infects humans as cutaneous larva migrans (CLM) and rarely intestinally causing eosinophilic enteritis (Bowman, 2011; Mclaughlin et al., 1993). Ancylostoma braziliense occurs in dogs, cats and various wild animals, and in humans as CLM (Bowman, 2011). Ancylostoma ceylanicum intestinal infections have been mainly documented in humans and dogs in Asia (Traub, 2013) and in wild dingoes in Northern Australia (Smout et al., 2013, 2018; Mackenstedt et al., 2015). Domestic cats and wild felids are usually infected by Ancylostoma tubaeforme worldwide, but its zoonotic potential has so far not been documented (Bowman, 2011). In bobcats from the USA, three species: A. tubaeforme, A. caninum and A. braziliense have been documented (Hiestand et al., 2014). Finally, Uncinaria stenocephala is worldwide distributed in dogs and wild canids, but rarely in cats and wild felids (Deplazes et al., 2016). The life cycle of the hookworm species is direct, with intestinal females excreting non-larvated, thin-shelled eggs with the faeces. Under suitable environmental conditions, L1 hatch from the eggs and moult to second stage larvae (L2) and L3 within 5–8 days, however, the development can be delayed at temperatures below 15 °C. Cutaneous and peroral infections with free-living L3 of A. caninum have been described for definitive, paratenic or dead-end hosts. Furthermore, in definitive hosts, transmission of parasitic L3 with milk or the ingestion of paratenic hosts (e.g., rodents) is possible (Deplazes et al., 2016). Uncinaria stenocephala transmission occurs predominantly by ingestion of L3 from the environment or paratenic hosts; however, L3 of this species can also penetrate the skin (Gibbs, 1961). In foxes, high prevalence of U. stenocephala infections have been reported all over Europe: Denmark 60–86% (rev. Al-Sabi et al., 2014), Spain 58–71% (Criado-Fornelio et al., 2000; Segovia et al., 2004), Switzerland 64–78% (Hofer et al., 2000; Reperant et al., 2007), Ireland 38% (Stuart et al., 2013), the United Kingdom 68% (Richards et al., 1995), Lithuania 99% (Bružinskaitė-Schmidhalter et al., 2012), Poland 19% (Borecka et al., 2009), the Slovak Republic 7% (Miterpáková et al., 2009), the Netherlands 60% (Borgsteede, 1984), Croatia 26% (Rajković-Janje et al., 2002), France 68% (Petavy and Deblock, 1980), Portugal 77% (Eira et al., 2006), Italy 39–75% (Di Cerbo et al., 2008; Magi et al., 2009; Fiocchi et al., 2016) and Slovenia 59% (Vergles Rataj et al., 2013). Ancylostoma caninum is worldwide endemic especially in canids, in Europe mainly in Southern and Southeastern regions, however, this species has also been described in foxes in central Europe up to Denmark. Interestingly, this species has not been documented in several studies in foxes from Italy, Slovenia and Portugal (Eira et al., 2006; Magi et al., 2009; Vergles Rataj et al., 2013) where, conversely, high prevalence with U. stenocephala was found. A low A. caninum prevalence (1.4%) has been found in France (Auvergne) as compared with U. stenocephala (68%) (Petavy and Deblock, 1980), comparably, in Italy (Emilia-Romagna) prevalence of 1.8% for A. caninum and 75.4% for U. stenocephala were reported (Fiocchi et al., 2016). Similarly, the prevalence of U. stenocephala (68.6%) in Denmark was higher that A. caninum (0.6%) in 1040 foxes (Saeed et al., 2006). Accordingly, a very high prevalence of U. stenocephala (68%) was also detected in 843 rural and urban red foxes from the UK without indication of occurrence of A. caninum (Richards et al., 1995). In Tunisia, one of 9 foxes was infected with A. caninum, 4 with U. stenocephala (Lahmar et al., 2014). Investigations in Australian red foxes (n = 930), New South Wales found prevalence for U. stenocephala and A. caninum of 30.6% and 7.5%, respectively (Ryan, 1976). Conversely, in Iran prevalence in foxes reached up to 5.4% for A. caninum and up to 8.1% for U. stenocephala (Meshgi et al., 2009) and in the Slovak Republic the average prevalence of U. stenocephala (i.e., 6.9%) was lower than A. caninum (i.e., 18.1%) in 1198 foxes. Finally, the highest prevalence of A. caninum (up to 32%) was determined in areas with a high proportion of irrigated soil (Miterpáková et al., 2009). A single study reported the prevalence of A. caninum in foxes (12%) from Spain without records of U. stenocephala (Lledó et al., 2015), though identification data have not been discussed.
Raccoon dogs seem to be highly susceptible to U. stenocephala with a prevalence of 99% in 85 necropsied animals in Lithuania, 65% in 74 animals in Germany (Thieß et al., 2001; Bružinskaitė-Schmidhalter et al., 2012). Both A. caninum and U. stenocephala were detected in golden jackals, in Hungary with prevalence of 45% and 40%, in Tunisia with 10% and 68%, respectively (Lahmar et al., 2014). Wolves are susceptible to both hookworm species in Italy (Guberti et al., 1993) and Poland (Borecka et al., 2013). Based on the hookworm prevalence recorded in European foxes, this species contributes significantly to the transmission of U. stenocephala whereas, foxes are not as frequently infected with A. caninum and therefore, based on the limited data available, the contribution of foxes to A. caninum transmission cannot be conclusively determined. However, in particular epidemiological situations, wild canids such as wild dogs (dingoes and dingo hybrids) may contribute to Ancylostoma spp. transmission. High prevalence of A. caninum (up to 100%) was found in dogs and sympatric dingoes in an Aboriginal community in the Wet Tropics of Australia, with A. ceylanicum being diagnosed in around 11% of the same canid population (Smout et al., 2013, 2018; Mackenstedt et al., 2015). Furthermore, coyotes are highly susceptible for A. caninum and U. stenocephala infections (Seguel and Gottdenker, 2017) and, based on the invasion and increase of populations of this animal species in urban areas in North America (Liccioli et al., 2015), they may represent a risk factor for the occurrence of hookworms and other helminths, such as Echinococcus multilocularis. Based on their relative low population sizes, other large canids (e.g., wolf, jackal) and also wild felids play probably an insignificant role in the transmission of hookworms. However, in one study, documenting high prevalence of hookworms in bobcats throughout the state of Florida (A. tubaeforme, 11%; A. caninum, 18%; A. braziliense, 19%; Ancylostoma pluridentatum, 29%) Mclaughlin et al. (1993) suggested that this wild felid could serve as a zoonotic reservoir of hookworms.
The migration of hookworm (Ancylostoma, Uncinaria) larvae through the human skin may result in CLM, also known as ‘creeping eruptions’ (Bowman et al., 2010), though the zoonotic role of Uncinaria spp. is unclear (Bowman et al., 2010). However, in the UK, CLM was attributed to U. stenocephala because A. caninum is not endemic (Beattie and Fleming, 2002). Cases of CLM have been reported from several European countries (e.g., the UK, Germany, Italy and Serbia) with four cases described from Germany, whereas some of the patients spent time in a shelter together with domestic and wild animals during the flooding of the river Elbe (reviewed by Bowman et al., 2010). In any case, all patients had a history of visiting lakesides and riverbanks around urban areas (Klose et al., 1996), where wildlife species were prevalent, potentially contributing to the contamination of such environments. In addition, dog-owners used to walk in those areas, therefore favouring the transmission of hookworms among wildlife and dogs (and vice versa) and, ultimately to humans (Smith et al., 2014).
3.5. Respiratory capillariosis
Capillaria aerophila (syn. Eucoleus aerophilus) (Enoplida, Capillaridae) is a lungworm of wild and domestic carnivores, but it has also rarely been described in humans. Eggs are excreted with the faeces of the definitive hosts, and the infective L1 develops inside the eggs within 40–60 days (Conboy, 2009). Infections occur by ingestion of larvated eggs; but facultative or transport hosts, such as earthworms can be involved in the transmission (Otranto et al., 2015b). Prevalence in European red foxes are usually high; reaching 97% in Lithuania (Bružinskaitė-Schmidhalter et al., 2012); 88% in Norway (Davidson et al., 2006); 74.1% in Denmark (Saeed et al., 2006); 76.2% in Poland (Karamon et al., 2018); 67–75% in Germany (Schug et al., 2018); 66% in Hungary (Sréter et al., 2003); 49% in Serbia (Ilić et al., 2016); 46.8% in The Netherlands (Borgsteede, 1984) and 41.8% in Italy (Magi et al., 2015). Capillaria aerophila has been also reported in European wildcat, wolf, jackal, beech marten (Martes foina) and raccoon dog with prevalence rates ranging from 5% (jackals), 33.3% (wild cats), and 32% in raccoon dogs (rev. Bružinskaitė-Schmidhalter et al., 2012; Otranto et al., 2015b). These epidemiological data strongly support the hypothesis, that wild carnivores act as main definitive hosts for the C. aerophila transmission. Respiratory C. aerophila infections in humans can mimic the clinical and radiographic findings of pulmonary neoplasia, with bronchitis, cough, mucoid sputum, haemoptysis, fever, dyspnoea and eosinophilia (Lalosevic et al., 2008). Human cases have been described in Ukraine, Russia, Morocco, Iran and France (reviewed in Di Cesare et al., 2012). The last documented case in humans was in Serbia, where the prevalence of infection in foxes is high (84%) (Lalosevic et al., 2008, 2013).
4. Other nematodes
4.1. Trichinellosis
Within the genus Trichinella (Trichuroidea, Trichinellidae), 12 species and additional several genotypes are recognised with a diverse host range, geographical distribution, ecology and histopathological features (encapsulated or not encapsulated). Trichinella spp. have a peculiar life cycle which completes in a single host and transmission occurs through the ingestion of infected meat containing larvae (Gottstein et al., 2009). Trichinella spp. are amongst the most prevalent parasites of predatory and scavenger wild carnivores (Pozio and Zarlenga, 2013). Zoonotic transmission has been documented for most Trichinella spp. with the exception of Trichinella papuae and Trichinella zimbabwensis. Trichinella britovi is the most prevalent species among wild carnivores as well as wild boars (the main source of human infection) in Europe, Western Asia, North and Western Africa (Gottstein et al., 2009). In humans, the clinical course of the disease is characterized by an enteral (i.e., adult worms in the intestinal mucosa) and a parenteral (i.e., larvae invading the host muscles) phase (Gottstein et al., 2009). The parenteral phase may occur with high degree of severity and it is characterized by fever, myalgia, periorbital swelling and eosinophilia. The ocular signs (i.e., periorbital oedema, conjunctivitis, macular and retinal haemorrhage) appear after about three weeks from the infection and they are caused by both the effect of migrating larvae and the allergic reactions (Otranto and Eberhard, 2011). The diagnosis in humans is based on clinical signs, corroborated by the anamnesis of enteral syndromes linked to history of ingesting raw or inadequately cooked meat and by serologic confirmatory tests.
The diagnosis of trichinellosis in animals is based on the evidence of larvae in muscles at meat inspections. Muscle distribution of Trichinella spp. larvae in production animals and wildlife can differ significantly. Experimental studies indicate that larvae of different Trichinella spp. establish in high numbers in foxes (Fig. 4), localizing primarily in the tongue, extremities and diaphragm (encapsulating species), and in the diaphragm (non-encapsulating species) (Kapel et al., 2005).
Fig. 4.
Encapsulated larvae of Trichinella britovi in the striated muscular tissue of a fox.
Importantly, refined diagnostic methods may increase the sensitivity in diagnosing Trichinella infection in muscles of foxes, for example by artificial digestion of frozen muscles, combined with larva isolation by a sequential sieving method (SSM). Using the SSM, dead Trichinella larvae were detected in one frozen muscle sample from a red fox (0.067 larvae per gram) during a survey from the Netherlands (n = 369), suggesting a decrease in Trichinella spp. prevalence in Dutch red foxes from 3.9% to 0.27%, within fifteen years (Franssen et al., 2014). In 47 trichinellosis outbreaks from 1995 to 2002 (i.e., 864 human cases) in Russia, the consumption of pork meat was implicated in only 35.8% of the cases during 1998–2002, compared to 80% in 1995–1996 (Ozeretskovskaya et al., 2005). The remaining cases were due to ingestion of bear (Ursus arctos) (39.5%), badger (Meles meles) (10.6%), or even dog (11.9%) meat. Interestingly, clinically severe cases of disease derived from the consumption of pork (7.7%) and bear (7.9%) meat with a significantly higher number of moderate cases from pork than from bear meat (Ozeretskovskaya et al., 2005). Due to the habits of the aboriginal Siberian population of feeding bear meat the incidence of clinically severe cases of trichinellosis is high in those populations (Ozeretskovskaya et al., 2005). A great success in controlling the infection in humans has been achieved in the USA and Europe by determine ad hoc regulations on food inspection and safety as well as feeding practices in pigs, which has reduced the perpetuation of Trichinella spp. domestic cycle. Conversely, pigs continue to be responsible for human infection in many countries and wild game meat represents a global threat to the perpetuation of trichinellosis when meat is ingested without proper cooking. Indeed, a range of wild animals can harbour infection with Trichinella larvae maintaining the sylvatic cycle (Hurníková et al., 2007). Due to their high population level, red foxes and raccoon dogs are the more suitable reservoir of the parasite in Europe. A prevalence of Trichinella up to 21.5% and a mean larval density of 10.5 larvae per gram were recorded at the muscle digestion method of 121 foxes from Romania (Imre et al., 2015). Of the Trichinella larvae identified, 96% were T. britovi and only 4% were Trichinella spiralis, suggesting a major role of the fox as a reservoir of T. britovi (sylvatic cycle), but minor importance in the epidemiology of T. spiralis in the domestic cycle (Imre et al., 2015). Due to their small population densities, other wild carnivores such as martens, badgers, bears, lynxes, wild cats, wolves, etc. play probably a secondary role in the transmission of Trichinella spp. to humans. Ways of transmission from wild carnivores to humans can occur with undercooked meet (bear, rarely other species) (Rostami et al., 2017) or by illegal use of wild carnivore meet for meat products (Gottstein et al., 2009). Carnivores are at the end of the food chain and therefore probably often dead-end hosts. However, transmission from carnivores can occur by predation (foxes or raccoon dogs hunted by larger carnivores), by scavenging (e.g., wild boars) but also rarely by free ranging pigs on dead animals, such as road kills or carcasses left in the environment by hunters (Duscher et al., 2014; Gottstein et al., 2009. Trichinellosis outbreaks due to T. britovi and Trichinella murrelli have been identified to be horsemeat-related. Horses can become accidentally infected by grazing in pastures contaminated with infected small animal and rodent carcasses or through hay contaminated with pieces of rodents (Bruschi and Dupouy-Camet, 2014), but also illegal protein sources, including wild animal meet, have been claimed to be source of infections of horses used for meat production (Gottstein et al., 2009).
4.2. Angiostrongylosis
Angiostrongylus costaricensis (Strongylida, Angiostrongylidae) is a nematode species causing severe or fatal zoonosis in North and South America. Besides the wide range of rodents (i.e., cotton rat Sigmodon hispidus) as most important definitive hosts, white-nosed coati (Nasua narica) and the domestic dog can act as potential definitive hosts excreting L1 with their faces (Alfaro-Alarcón et al., 2016). Definitive hosts and humans may become infected ingesting L3 in raw or undercooked intermediate hosts (slug and snails) or with vegetables contaminated with mucus of them containing L3 (Spratt, 2015). Around 200 human cases of human angiostrongylosis have been described mainly in children (Romero-Alegría et al., 2014). However, based on serological studies, the number of human cases might be much higher (e.g., 600 cases in Costa Rica per year), though human cases of A. costaricensis have also been reported from Africa (Zaire) and Europe.
4.3. Gnathostomosis
Gnathostoma spinigerum Owen 1836, is the most widespread zoonotic species in South East Asia, China, Japan, the Indian subcontinent and Central Africa. Definitive hosts with gastric nodules are domestic cats and dogs, but also various wild felids feeding on mammals, reptiles, amphibians and aquatic prey have been occasionally diagnosed with gastric nodules (Eshwaran et al., 2003; Shrivastav et al., 2011; Boo Liat, 1976). Gnathostoma binucleatum has been reported from northern Latin America and occasionally the USA, with dogs, cats, wild felids as definitive hosts.
Humans acquire the infection mainly from L3 in raw fish, but also from raw frogs, snakes, wild boar, poultry, but possible also from L1 copepods in water. The larvae migrate to the skin and subcutaneous tissue causing the typical migratory swellings (cutaneous disease in the majority of cases), occasionally the larvae may penetrate into deeper tissues (viscera, the lungs, eyes, ears, gastrointestinal and genitourinary systems and rarely, but often fatally, the Central Nervous System (Herman and Chiodini, 2009). Gnathostoma spinigerum infections have been recognised as an emerging cause of eosinophilic encephalomeningitis (Diaz, 2010) and ocular infections (Lenka et al., 2016). Cutaneous gnathostomosis developes within an incubation time of weeks up to 12 months as larva migrans in cutaneous nodules. The importance of the wild carnivores (e.g., felids) in the zoonotic transmission or as reservoir of Gnathostoma spp. is not well elucidated in the literature; however, probably the so far findings in rather exotic and rare felid species do not support an important zoonotic contribution of wildlife carnivores.
5. Conclusions
For long time, wildlife carnivores have been disregarded for their potential in transmitting zoonotic nematodes. Nonetheless, increasing numbers of case reports suggest their role in the maintenance and spread of such infections due to a complex range of interactions favouring their contact with humans. Amongst others, factors are linked to i) human habits (e.g., increased outdoor activities such as camping and tracking in forested or wild environments), ii) environmental encroachment in urban areas (such as the tropical forest in the peripheries of the Brazilian towns) and, iii) the altered ecology of wild animals (e.g., fox or coyotes populations in urban settings). On the other hand, improved diagnostic strategies including DNA analyses of clinical material enabled the specific diagnosis of even exotic parasitic infections. In addition, insect vectors have no boundaries in spreading zoonotic nematode infections to domestic or wild carnivore reservoirs, as long as they find suitable ecological conditions. Due to the fact that a large number of zoonotic helminths are shared among wild and domestic carnivores, the actual importance of wildlife contributing to environmental contamination is difficult to assess. This is the case of foxes in shedding ascarid eggs. In addition, the limited scientific information about some parasites, the limited access to biological samples or the correct diagnostic tools may also impair a clear picture of the infection in animals and their zoonotic risks. This is substantiated by the fact that knowledge about the occurrence of zoonotic nematodes amongst wild carnivores mostly relies on necropsy studies, which not always offer the most appropriate samples for diagnosing some parasites (e.g., microfilariae from skin biopsy sediments for O. lupi). Since urban and rural/forested ecosystems are often without frontiers and inter-related, the study of zoonotic parasites in wild carnivores requires a multifaceted approach whit the involvement of biologists, epidemiologists, ecologists and veterinary and medical microbiologists and parasitologists. Such an approach could contribute to a better understanding regarding the population dynamics of carnivore hosts and their parasites in continuously changing ecosystems. For example, the introduction or invasion of exotic carnivore species in a given habitat may increase the transmission of zoonotic nematodes. It is the case of raccoon dogs and raccoons. Since their introduction into Europe, the populations of these animal species well adapted and increased in Europe and they showed to be suitable hosts for almost all the zoonotic nematodes herein reported (Kauhala and Kowalczyk, 2011; García et al., 2012). The procyonids have caused the introduction and spread of “exotic” parasites, such as B. procyonis in Central European countries, with a potential risk of spill-over to other autochthonous species. These facts have never been considered as a concern into international or national legislation on the conservation of biodiversity (92/43/EEC), which should require a population control of invading animal species in order to reduce the risk of spreading exotic parasites. Indeed, with the exception of rabies in foxes, no other zoonotic infectious agents of wild carnivores are considered in the international legislation aiming to protect human health. This might also be due to the fact that in the EU Member States, wildlife management and conservation authorities are not responsible for animal health but National Veterinary Services being in charge for health and food safety in domestic animals. Therefore, the impact of diseases on wildlife population dynamics is often underestimated and assessed through research projects or local surveys. Conversely, wildlife diseases are included in the international reporting system for animal health of the World Organization for Animal Health (OIE) (World Animal Health Information Database–WHAIS, available at: http://www.oie.int/animal-health-in-the-world/the-world-animal-health-information-system/the-oie-data-system/). Finally, taking into account the complex wildlife/domestic animals/humans/parasites relationships, a correct compromise between conservation of wild carnivores and risk of introduction and spreading of parasites of public health concern is requested in order to correctly manage the risk of zoonotic nematodes of wild carnivores in line with the ‘One Health’ approach to scientific research in these areas.
Acknowledgements
Authors thank Deborha Joekel (Institute of Parasitology, University of Zürich Switzerland) for her critical reading of the manuscript, Linh Nguyen (Dipartimento di Medicina Veterinaria, Università degli Studi di Bari, Italy) for references formatting. Riccardo Paolo Lia (Dipartimento di Medicina Veterinaria, Università degli Studi di Bari, Italy) for figure preparation and Mario Santoro and Antonio Varcasia for providing some figures.
References
- Aher A.M., Caudill D., Caudill G., Butryn R.D., Wolf D., Fox M., Blake D.P., Cunningham M.W. Prevalence, genetic analyses, and risk factors associated with heartworm (Dirofilaria immitis) in wild coyotes (Canis latrans) from Florida, USA. J. Wildl. Dis. 2016;52:785–792. doi: 10.7589/2015-09-223. [DOI] [PubMed] [Google Scholar]
- Akao N., Ohta N. Toxocariasis in Japan. Parasitol. Int. 2007;56:87–93. doi: 10.1016/j.parint.2007.01.009. [DOI] [PubMed] [Google Scholar]
- Al-Sabi M.N.S., Halasa T., Kapel C.M.O. Infections with cardiopulmonary and intestinal helminths and sarcoptic mange in red foxes from two different localities in Denmark. Acta Parasitol. 2014;59:98–107. doi: 10.2478/s11686-014-0214-6. [DOI] [PubMed] [Google Scholar]
- Al-Sabi M.N.S., Chriél M., Hansen M.S., Enemark H.L. Baylisascaris procyonis in wild raccoons (Procyon lotor) in Denmark. Vet. Parasitol. Reg. Stud. Reports. 2015;1–2:55–58. doi: 10.1016/j.vprsr.2016.03.001. [DOI] [PubMed] [Google Scholar]
- Al-Sabi M.N.S., Rääf L., Osterman-Lind E., Uhlhorn H., Kapel C.M.O. Gastrointestinal helminths of gray wolves (Canis lupus lupus) from Sweden. Parasitol. Res. 2018;117:1891–1898. doi: 10.1007/s00436-018-5881-z. [DOI] [PubMed] [Google Scholar]
- Alfaro-Alarcón A., Veneziano V., Galiero G., Cerrone A., Gutierrez N., Chinchilla A., Annoscia G., Colella V., Dantas-Torres F., Otranto D., Santoro M. First report of a naturally patent infection of Angiostrongylus costaricensis in a dog. Vet. Parasitol. 2016;212:431–434. doi: 10.1016/j.vetpar.2015.08.016. [DOI] [PubMed] [Google Scholar]
- Anderson R.C. CABI Publishing; Wallingford: 2000. Nematode Parasites of Vertebrates. Their Development and Transmission. [Google Scholar]
- Anonymus . Deutscher Jagdverband; 2019. Jahresjagdstrecke Bundesrepublik Deutschland.https://www.jagdverband.de Daten und Fakten, Jagdstatistik. [Google Scholar]
- Auer H. Jagdbares wild als parasitäre Infektionsquelle für den Menschen in Österreich. Wien. Tierarztl. Monatsschr. 2011;98:245–250. [Google Scholar]
- Bacsadi Á., Papp A., Szeredi L., Tóth G., Nemes C., Imre V., Tolnai Z., Széll Z., Sréter T. Retrospective study on the distribution of Dirofilaria immitis in dogs in Hungary. Vet. Parasitol. 2016;220:83–86. doi: 10.1016/j.vetpar.2016.03.001. [DOI] [PubMed] [Google Scholar]
- Bain O., Mutafchiev Y., Junker K. Order Spirurida. Nematoda. In: Schmidt-Rhaesa A., editor. vol. 2. 2013. pp. 661–732. (Handbook of Zoology). Nematoda. De Gruyter, Berlin. [Google Scholar]
- Baldi M., Alvarado G., Smith S., Santoro M., Bolaños N., Jiménez C., Hutter S.E., Walzer C. Baylisascaris procyonis parasites in raccoons, Costa Rica, 2014. Emerg. Infect. Dis. 2016;22:1502–1503. doi: 10.3201/eid2208.151627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer C. Baylisascariosis- infections of animals and humans with ‘unusual’ roundworms. Vet. Parasitol. 2013;193:404–412. doi: 10.1016/j.vetpar.2012.12.036. [DOI] [PubMed] [Google Scholar]
- Beattie P.E., Fleming C.J. Cutaneous larva migrans in the west coast of Scotland. Clin. Exp. Dermatol. 2002;27:248–249. doi: 10.1046/j.1365-2230.2002.09852.x. [DOI] [PubMed] [Google Scholar]
- Bindke J.D., Springer A., Böer M., Strube C. Helminth fauna in captive European gray wolves (Canis lupus lupus) in Germany. Front. Vet. Sci. 2017;22:4–228. doi: 10.3389/fvets.2017.00228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisoffi Z., Buonfrate D., Montresor A., Requena-Mendez A., Munoz J., Krolewiecki A.J., Albonico M. Strongyloides stercoralis: a plea for action. PLoS Neglected Trop. Dis. 2013;7:7–10. doi: 10.1371/journal.pntd.0002214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaizot R., Receveur M.C., Millet P., Otranto D., Malvy D.J.M. Systemic infection with Dirofilaria repens in Southwestern France. Ann. Intern. Med. 2018;168:228–229. doi: 10.7326/L17-0426. [DOI] [PubMed] [Google Scholar]
- Boo Liat L. Gnathostoma spinigerum Owen, 1836 (Nematoda: Gnathostomidae) from a civet cat, Prionodon linsang Hardwick, with reference to its dietary habits. Southeast Asian J. Trop. Med. Publ. Health. 1976;7:530–533. [PubMed] [Google Scholar]
- Borecka A., Gawor J., Malczewska M., Malczewski A. Prevalence of zoonotic helminth parasites of the small intestine in red foxes from central Poland. Med. Weter. 2009;65:33–35. [Google Scholar]
- Borecka A., Gawor J., Zieba F.A. A survey of intestinal helminths in wild carnivores from the Tatra National Park, southern Poland. Ann. Parasitol. 2013;59:169–172. [PubMed] [Google Scholar]
- Borgsteede F.H. Helminth parasites of wild foxes (Vulpes vulpes L.) in The Netherlands. Z. Parasitenkd. 1984;70:281–285. doi: 10.1007/BF00927813. [DOI] [PubMed] [Google Scholar]
- Bowman D.D. Zoonotic hookworm infections. In: Palmer S.R., Soulsby L., Torgerson P., Brown D.W.G., editors. Oxford Textbook of Zoonoses. second ed. Oxford University Press; Oxford: 2011. pp. 767–786. [Google Scholar]
- Bowman D.D., Montgomery S.P., Zajac A.M., Eberhard M.L., Kazacos K.R. Hookworms of dogs and cats as agents of cutaneous larva migrans. Trends Parasitol. 2010;26:162–167. doi: 10.1016/j.pt.2010.01.005. [DOI] [PubMed] [Google Scholar]
- Brinkman W.B., Kazacos K.R., Gavin P.J., Binns H.J., Robichaud J.D., O'Gorman M., Shulman S.T. Proc. 2003 Ann. Meet. Pediatr. Acad. Soc., Seattle (Abstr. no. 1872) 2003. Seroprevalence of Baylisascaris procyonis (raccoon roundworm) in Chicago area children. [Google Scholar]
- Bruschi F., Dupouy-Camet J. Trichinellosis. In: Bruschi F., editor. Helminth Infections and Their Impact on Global Public Health. Springer-Verlag Wien; 2014. pp. 229–273. [Google Scholar]
- Bružinskaitė-Schmidhalter R., Šarkūnas M., Malakauskas A., Mathis A., Torgerson P.R., Deplazes P. Helminths of red foxes (Vulpes vulpes) and raccoon dogs (Nyctereutes procyonoides) in Lithuania. Parasitology. 2012;139:120–127. doi: 10.1017/S0031182011001715. [DOI] [PubMed] [Google Scholar]
- Burnett H.S., Wagner E.D. Two new definitive hosts for the eye worm, Thelazia californiensis Price 1930. J. Parasitol. 1958;44:502. [Google Scholar]
- Čabanová V., Kocák P., Víchová B., Miterpáková M. First autochthonous cases of canine thelaziosis in Slovakia: a new affected area in Central Europe. Parasites Vectors. 2017;10:179. doi: 10.1186/s13071-017-2128-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cancrini G., Scaramozzino P., Gabrielli S., Paolo M. Di, Toma L., Romi R. Aedes albopictus and Culex pipiens implicated as natural vectors of Dirofilaria repens in Central Italy: Table 1. J. Med. Entomol. 2007;44:1064–1066. doi: 10.1603/0022-2585(2007)44[1064:aaacpi]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Canestri Trotti G., Pampiglione S., Rivasi F. The species of the genus Dirofilaria, Railliet & Henry, 1911. Parassitologia. 1997;39:369–374. [PubMed] [Google Scholar]
- Cantey P.T., Eberhard M., Weeks J., Swoboda S., Ostovar G.A. Letter to the Editor: Onchocerca lupi infection. J. Neurosurg. Pediatr. 2016;17:118–119. doi: 10.3171/2015.6.PEDS15344. [DOI] [PubMed] [Google Scholar]
- Capelli G., Frangipane di Regalbono A., Simonato G., Cassini R., Cazzin S., Cancrini G., Otranto D., Pietrobelli M. Risk of canine and human exposure to Dirofilaria immitis infected mosquitoes in endemic areas of Italy. Parasites Vectors. 2013;6:60. doi: 10.1186/1756-3305-6-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capelli G., Genchi C., Baneth G., Bourdeau P., Brianti E., Cardoso L., Danesi P., Fuehrer H.P., Giannelli A., Ionică A.M., Maia C., Modrý D., Montarsi F., Krücken J., Papadopoulos E., Petrić D., Pfeffer M., Savić S., Otranto D., Poppert S., Silaghi C. Recent advances on Dirofilaria repens in dogs and humans in Europe. Parasites Vectors. 2018;11(1):663. doi: 10.1186/s13071-018-3205-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Liu Q., Liu G.H., Zheng W.B., Hong S.J., Sugiyama H., Zhu X.Q., Elsheikha H.M. Toxocariasis: a silent threat with a progressive public health impact. Infect. Dis. Poverty. 2018;7:59. doi: 10.1186/s40249-018-0437-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ćirović D., Penezić A., Pavlović I., Kulišić Z., Ćosić N., Burazerović J., Maletić V. First records of Dirofilaria repens in wild canids from the region of Central Balkan. Acta Vet. Hung. 2014;62:481–488. doi: 10.1556/AVet.2014.021. [DOI] [PubMed] [Google Scholar]
- Cleaveland S., Laurenson M.K., Taylor L.H. Diseases of humans and their domestic mammals: pathogen characteristics, host range and the risk of emergence. Philos. Trans. R. Soc. B Biol. Sci. 2001;356:991–999. doi: 10.1098/rstb.2001.0889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coati N., Schnieder T., Epe C. Vertical transmission of Toxocara cati Schrank 1788 (Anisakidae) in the cat. Parasitol. Res. 2004;92:142–146. doi: 10.1007/s00436-003-1019-y. [DOI] [PubMed] [Google Scholar]
- Colella V., Kirkova Z., Fok E., Mihalca A.D., Tasic-Otasevic S., Hodzic A., Dantas-Torres F., Otranto D. Increase in eyeworm infections in eastern Europe. Emerg. Infect. Dis. 2016;22:1513–1515. doi: 10.3201/eid2208.160792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conboy G. Helminth parasites of the canine and feline respiratory tract. Vet. Clin. North. Am. Small. Anim. Pract. 2009;39:1109–1126. doi: 10.1016/j.cvsm.2009.06.006. [DOI] [PubMed] [Google Scholar]
- Conraths F.J., Bauer C., Cseke J., Laube H. Arbeitsplatzbedingte Infektionen des Menschen mit dem Waschbarspulwurm Baylisascaris procyonis. Arbeitsmed. Sozialmed. Umweltmed. 1996;31:13–17. [Google Scholar]
- Criado-Fornelio A., Gutierrez-Garcia L., Rodriguez-Caabeiro F., Reus-Garcia E., Roldan-Soriano M.A., Diaz-Sanchez M.A. A parasitological survey of wild red foxes (Vulpes vulpes) from the province of Guadalajara, Spain. Vet. Parasitol. 2000;92:245–251. doi: 10.1016/s0304-4017(00)00329-0. [DOI] [PubMed] [Google Scholar]
- Damle A.S., Iravane Bajaj J.A., Khaparkhuntikar M.N., Maher G.T., Patil R.V. Microfilaria in human subcutaneous dirofilariasis: a case report. J. Clin. Diagn. Res. 2014;8:113–114. doi: 10.7860/JCDR/2013/6886.4123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantas-Torres F., Otranto D. Dirofilariosis in the Americas: a more virulent Dirofilaria immitis? Parasites Vectors. 2013;6:288. doi: 10.1186/1756-3305-6-288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson R.K., Gjerde B., Vikøren T., Lillehaug A., Handeland K. Prevalence of Trichinella larvae and extra-intestinal nematodes in Norwegian red foxes (Vulpes vulpes) Vet. Parasitol. 2006;136:307–316. doi: 10.1016/j.vetpar.2005.11.015. [DOI] [PubMed] [Google Scholar]
- Davidson R.K., Øines Ø., Hamnesf I.S., Schulze J.E. Illegal wildlife imports more than just animals - Baylisascaris procyonis in raccoons (Procyon lotor) in Norway. J. Wildl. Dis. 2013;49:986–990. doi: 10.7589/2012-06-154. [DOI] [PubMed] [Google Scholar]
- Deksne G., Laakkonen J., Näreaho A., Jokelainen P., Holmala K., Kojola I., Sukura A. Endoparasites of the Eurasian Lynx (Lynx lynx) in Finland. J. Parasitol. 2013;99:229–234. doi: 10.1645/GE-3161.1. [DOI] [PubMed] [Google Scholar]
- Deplazes P., Hegglin D., Gloor S., Romig T. Wilderness in the city: the urbanization of Echinococcus multilocularis. Trends Parasitol. 2004;20:77–84. doi: 10.1016/j.pt.2003.11.011. [DOI] [PubMed] [Google Scholar]
- Deplazes P., van Knapen F., Schweiger A., Overgaauw P.A.M. Role of pet dogs and cats in the transmission of helminthic zoonoses in Europe, with a focus on echinococcosis and toxocarosis. Vet. Parasitol. 2011;182:41–53. doi: 10.1016/j.vetpar.2011.07.014. [DOI] [PubMed] [Google Scholar]
- Deplazes P., Eckert J., Mathis A., von Samson-Himmelstjerna G., Zahner H. Wageningen Academic Publishers; Wageningen: 2016. Parasitology in Veterinary Medicine. [Google Scholar]
- Di Cerbo A.R., Manfredi M.T., Bregoli M., Ferro Milone N., Cova M. Wild carnivores as source of zoonotic helminths in north-eastern Italy. Helminthologia. 2008;45:13–19. [Google Scholar]
- Di Cesare A., Castagna G., Otranto D., Meloni S., Milillo P., Latrofa M.S., Paoletti B., Bartolini R., Traversa D. Molecular detection of Capillaria aerophila, an agent of canine and feline pulmonary capillariosis. J. Clin. Microbiol. 2012;5:1958–1963. doi: 10.1128/JCM.00103-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz J.H. Recently re-emerging helminthic infections causing eosinophilic meningoencephalitis: neuroangiostrongyliasis, baylisascariasis and gnathostomiasis. J. Neuroparasitol. 2010;1:1–14. [Google Scholar]
- Doezie A.M., Lucius R.W., Aldeen W., Hale D.V., Smith D.R., Mamalis N. Thelazia californiensis conjunctival infestation. Ophthalmic Lit. 1996;49:225. [PubMed] [Google Scholar]
- Doncaster C.P., Macdonald D.W. Ecology and ranging behaviour of red foxes (Vulpes vulpes) in the city of Oxford. Hystrix. 1991;3:11–20. [Google Scholar]
- Dorchies P., Chaudieu G., Siméon L.A., Cazalot G., Cantacessi C., Otranto D. First reports of autochthonous eyeworm infection by Thelazia callipaeda (Spirurida, Thelaziidae) in dogs and cat from France. Vet. Parasitol. 2007;149:294–297. doi: 10.1016/j.vetpar.2007.08.005. [DOI] [PubMed] [Google Scholar]
- Duffy M.S., Greaves T.A., Burt M.D.B. Helminths of the black bear, Ursus americanus, in new Brunswick. J. Parasitol. 1994;80:478. [PubMed] [Google Scholar]
- Duscher G.G., Leschnik M., Fuehrer H.P., Joachim A. Wildlife reservoirs for vector-borne canine, feline and zoonotic infections in Austria. Int. J. Parasitol. Parasites Wildl. 2014;4:88–96. doi: 10.1016/j.ijppaw.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eira C., Vingada J., Torres J., Miquel J. The helminth community of the red fox, Vulpes vulpes, in dunas de Mira (Portugal) and its effect on host condition. Wildl. Biol. Pract. 2006;2:26–36. [Google Scholar]
- Eshwaran K.R., Ravindran R., Pillai K.M. Parasitic infection of some wild animals at Thakkady in Kerala. Zoo’s Print J. 2003;18:1030. [Google Scholar]
- Faísca P., Morales-Hojas R., Alves M., Gomes J., Botelho M., Melo M., Xufre A. A case of canine ocular onchocercosis in Portugal. Vet. Ophthalmol. 2010;13:117–121. doi: 10.1111/j.1463-5224.2010.00763.x. [DOI] [PubMed] [Google Scholar]
- Fedriani J.M., Palomares F., Delibes M. Niche relations among three sympatric Mediterranean carnivores. Oecologia. 1999;121:138–148. doi: 10.1007/s004420050915. [DOI] [PubMed] [Google Scholar]
- Fiocchi A., Gustinelli A., Gelmini L., Rugna G., Renzi M., Fontana M.C., Poglayen G. Helminth parasites of the red fox Vulpes vulpes (L., 1758) and the wolf Canis lupus italicus Altobello, 1921 in Emilia-Romagna, Italy. Ital. J. Zool. 2016;83:503–513. [Google Scholar]
- Fischer M.L., Hochkirch A., Eddergott M., Schulze C., Anheyer-Behmenburg H.E., Lang J., Michler F.-U., Hohmann U., Ansorge H., Hoffmann L., Klein R., Franz A. Historical invasion records can be misleading: genetic evidence for multiple introductions of invasive raccoons (Procyon lotor) in Germany. PLoS One. 2015;10:e0125441. doi: 10.1371/journal.pone.0125441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franssen F., Deksne G., Esíte Z., Havelaar A., Swart A., van der Giessen J. Trend analysis of Trichinella in a red fox population from a low endemic area using a validated artificial digestion and sequential sieving technique. Vet. Res. 2014;45:120. doi: 10.1186/s13567-014-0120-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuentes I., Montes I., Saugar J.M., Latrofa S., Gárate T., Otranto D. Thelaziosis in humans, a zoonotic infection, Spain, 2011. Emerg. Infect. Dis. 2012;18:2073–2075. doi: 10.3201/eid1812.120472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García J.T., García F.J., Alda F., Gonazáles J.L., Aramburu M.J., Cortés Y., Prieto B., Perez M., Herrera J., García-Román L. Recent invasion and status of the raccoon (Procyon lotor) in Spain. Biol. Invasions. 2012;14:1305–1310. [Google Scholar]
- Gavin P.J., Kazacos K.R., Shulman S.T. Baylisascariasis. Clin. Microbiol. Rev. 2005;18:703–718. doi: 10.1128/CMR.18.4.703-718.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genchi C., Kramer L. Subcutaneous dirofilariosis (Dirofilaria repens): an infection spreading throughout the old world. Parasites Vectors. 2017;10:517. doi: 10.1186/s13071-017-2434-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genchi C., Kramer L.H., Rivasi F. Dirofilarial infections in Europe. Vector Borne Zoonotic Dis. 2011;11:1307–1317. doi: 10.1089/vbz.2010.0247. [DOI] [PubMed] [Google Scholar]
- Giannelli A., Capelli G., Joachim A., Hinney B., Losson B., Kirkova Z., René-Martellet M., Papadopoulos E., Farkas R., Napoli E., Brianti E., Tamponi C., Varcasia A., Margarida Alho A., Madeira de Carvalho L., Cardoso L., Maia C., Mircean V., Mihalca A.D., Miró G., Schnyder M., Cantacessi C., Colella V., Cavalera M.A., Latrofa M.S., Annoscia G., Knaus M., Halos L., Beugnet F., Otranto D. Lungworms and gastrointestinal parasites of domestic cats: a European perspective. Int. J. Parasitol. 2017;47:517–528. doi: 10.1016/j.ijpara.2017.02.003. [DOI] [PubMed] [Google Scholar]
- Gibbs H.C. Studies on the life cycle and developmental morphology of Dochmoides stenocephala (Railliet 1884) (Ancylostomidae: Nematoda) Can. J. Zool. 1961;39:325–348. [Google Scholar]
- Gortázar C., Villafuerte R., Lucientres J., Fernández-de-Luco D. Habitat related differences in helminth parasites of red foxes in the Ebro valley. Vet. Parasitol. 1998;80:75–81. doi: 10.1016/s0304-4017(98)00192-7. [DOI] [PubMed] [Google Scholar]
- Gottstein B., Pozio E., Nöckler K. Epidemiology, diagnosis, treatment, and control of trichinellosis. Clin. Microbiol. Rev. 2009;22:127–145. doi: 10.1128/CMR.00026-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham-Brown J., Gilmore P., Colella V., Moss L., Dixon C., Andrews M., Arbeid P., Barber J., Timofte D., McGarry J., Otranto D., Williams D. Three cases of imported eyeworm infection in dogs: a new threat for the United Kingdom. Vet. Rec. 2017;181:346. doi: 10.1136/vr.104378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guberti V., Stancampiano L., Francisci F. Intestinal helminth parasite community in wolves (Canis lupus) in Italy. Parassitologia. 1993;35:59–65. [PubMed] [Google Scholar]
- Guislain M.H., Raoul F., Poulle M.L., Giraudoux P. Fox faeces and vole distribution on a local range: ecological data in a parasitological perspective for Echinococcus multilocularis. Parasite. 2007;14:299–308. doi: 10.1051/parasite/2007144299. [DOI] [PubMed] [Google Scholar]
- Hassan H.K., Bolcen S., Kubofcik J., Nutman T.B., Eberhard M.L., Middleton K., Wekesa J.W., Ruedas G., Nelson K.J., Dubielzig R. Isolation of Onchocerca lupi in dogs and black flies, California, USA. Emerg. Infect. Dis. 2015;21:789–796. doi: 10.3201/eid2105.142011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman J.S., Chiodini P.L. Gnathostomiasis, another emerging imported disease. Clin. Microbial. Rev. 2009;22:484–492. doi: 10.1128/CMR.00003-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermosilla C., Hetzel U., Bausch M., Grübl J., Bauer C. First autochthonous case of canine ocular onchocercosis in Germany. Vet. Rec. 2005;156:450–452. doi: 10.1136/vr.156.14.450. [DOI] [PubMed] [Google Scholar]
- Hernández-Camacho N., Pineda-López R., López-González C.A., Jones R.W. Nematodes parasites of the gray fox (Urocyon cinereoargenteus Schreber, 1775) in the seasonally dry tropical highlands of central Mexico. Parasitol. Res. 2011;108:1425–1429. doi: 10.1007/s00436-010-2191-5. [DOI] [PubMed] [Google Scholar]
- Hiestand S.J., Nielsen C.K., Jiménez F.A. Epizootic and zoonotic helminths of the bobcat (Lynx rufus) in Illinois and a comparison of its helminth component communities across the American Midwest. Parasite. 2014;21:4. doi: 10.1051/parasite/2014005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodžić A., Latrofa M.S., Annoscia G., Alić A., Beck R., Lia R.P., Dantas-Torres F., Otranto D. The spread of zoonotic Thelazia callipaeda in the Balkan area. Parasites Vectors. 2014;30:352. doi: 10.1186/1756-3305-7-352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodžić A., Hinney B., König S., Naucke T., Duscher G., Joachim A. A case of ocular infection with Onchocerca lupi in a dog from Germany. Transbound. Emerg. Dis. 2017;65:214–216. doi: 10.1111/tbed.12715. [DOI] [PubMed] [Google Scholar]
- Hofer S., Gloor S., Müller U., Mathis A., Hegglin D., Deplazes P. High prevalence of Echinococcus multilocularis in urban red foxes (Vulpes vulpes) and voles (Arvicola terrestris) in the city of Zürich, Switzerland. Parasitology. 2000;120:135–142. doi: 10.1017/s0031182099005351. [DOI] [PubMed] [Google Scholar]
- Hurníková Z., Chovancová B., Bartková D., Dubinský P. The role of wild carnivores in the maintenance of trichinellosis in the Tatras National Park, Slovakia. Helminthologia. 2007;44:18. [Google Scholar]
- Iatta R., Buonfrate D., Paradies P., Cavalera M.A., Capogna A., Iarussi F., Šlapeta J., Giorli G., Trerotoli P., Bisoffi Z., Otranto D. Occurrence, diagnosis and follow-up of canine strongyloidiosis in naturally infected shelter dogs. Parasitology. 2018;30:1–7. doi: 10.1017/S0031182018001312. [DOI] [PubMed] [Google Scholar]
- Ilić T., Becskei Z., Tasić A., Stepanović P., Radisavljević K., Đurić B., Dimitrijević S. Red foxes (Vulpes vulpes) as reservoirs of respiratory capillariosis in Serbia. J. Vet. Res. 2016;60:153–157. [Google Scholar]
- Imre K., Pozio E., Tonanzi D., Sala C., Ilie M.S., Imre M., Morar A. The red fox (Vulpes vulpes) plays a minor role in the epidemiology of the domestic cycle of Trichinella in Romania. Vet. Parasitol. 2015;212:448–450. doi: 10.1016/j.vetpar.2015.06.032. [DOI] [PubMed] [Google Scholar]
- Ionică A.M., Matei I.A., D'Amico G., Daskalaki A.A., Juránková J., Ionescu D.T., Mihalca A.D., Modrý D., Gherman C.M. Role of golden jackals (Canis aureus) as natural reservoirs of Dirofilaria spp. in Romania. Parasites Vectors. 2016;9:240. doi: 10.1186/s13071-016-1524-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ionică A.M., Matei I.A., D'Amico G., Ababii J., Daskalaki A.A., Sándor A.D., Enache D.V., Gherman C.M., Mihalca A.D. Filarioid infections in wild carnivores: a multispecies survey in Romania. Parasites Vectors. 2017;10:332. doi: 10.1186/s13071-017-2269-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ionică A.M., Deak G., Matei I.A., D'Amico G., Cotuţiu V.D., Gherman C.M., Mihalca A.D. Thelazia callipaeda, an endemic parasite of red foxes (Vulpes vulpes) in Western Romania. J. Wildl. Dis. 2018;54:829–833. doi: 10.7589/2017-10-251. [DOI] [PubMed] [Google Scholar]
- Kapel C.M., Webster P., Gamble H.R. Muscle distribution of sylvatic and domestic Trichinella larvae in production animals and wildlife. Vet. Parasitol. 2005;132:101–105. doi: 10.1016/j.vetpar.2005.05.036. [DOI] [PubMed] [Google Scholar]
- Karamon J., Kochanowski M., Cencek T., Bartoszewicz M., Kusyk P. Gastrointestinal helminths of raccoons (Procyon lotor) in western Poland (Lubuskie province) - with particular regard to Baylisascaris procyonis. Bull. Vet. Inst. Pulawy. 2014;58:547–552. [Google Scholar]
- Karamon J., Dąbrowska J., Kochanowski M., Samorek-Pieróg M., Sroka J., Różycki M., Cencek T. Prevalence of intestinal helminths of red foxes (Vulpes vulpes) in central Europe (Poland): a significant zoonotic threat. Parasites Vectors. 2018;11:436. doi: 10.1186/s13071-018-3021-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauhala K., Kowalczyk R. Invasion of the raccoon dog Nyctereutes procyonoides in Europe history of colonization, features behind its success, and threats to native fauna. Curr. Zool. 2011;57:584–598. doi: 10.1093/czoolo/57.5.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazacos K.R. Baylisascaris procyonis and related species. In: Samuel W.M., Pybus M.J., Kocan A.A., editors. Parasitic Diseases of Wild Mammals. Iowa State University Press; Iowa: 2001. pp. 301–341. [Google Scholar]
- Kirkova Z., Raychev E., Georgieva D. Studies on feeding habits and parasitological status of red fox, golden jackal, wild cat and stone marten in Sredna Gora, Bulgaria. J. Life Sci. 2011;5:264–270. [Google Scholar]
- Klose C., Mravak S., Geb M., Bienzle U., Meyer C.G. Autochthonous cutaneous larva migrans in Germany. Trop. Med. Int. Health. 1996;1:503–504. doi: 10.1046/j.1365-3156.1996.d01-86.x. [DOI] [PubMed] [Google Scholar]
- Komnenou A., Eberhard M.L., Kaldrymidou E., Tsalie E., Dessiris A. Subconjunctival filariasis due to Onchocerca sp. in dogs: report of 23 cases in Greece. Vet. Ophthalmol. 2002;5:119–126. doi: 10.1046/j.1463-5224.2002.00235.x. [DOI] [PubMed] [Google Scholar]
- Kołodziej-Sobocińska M., Yakovlev Y., Schmidt K., Hurníková Z., Ruczyńska I., Bednarski M., Tokarska M. Update of the helminth fauna in Eurasian lynx (Lynx lynx) in Poland. Parasitol. Res. 2018;117:2613–2621. doi: 10.1007/s00436-018-5953-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Küchle M., Knorr H.L., Medenblik-Frysch S., Weber A., Bauer C., Naumann G.O. Diffuse unilateral subacute neuroretinitis syndrome in a German most likely caused by the raccoon roundworm: Baylisascaris procyonis. Graefes Arch. Clin. Exp. Ophthalmol. 1993;231:48–51. doi: 10.1007/BF01681701. [DOI] [PubMed] [Google Scholar]
- Labelle A.L., Daniels J.B., Dix M., Labelle P. Onchocerca lupi causing ocular disease in two cats. Vet. Ophthalmol. 2011;14:105–110. doi: 10.1111/j.1463-5224.2011.00911.x. [DOI] [PubMed] [Google Scholar]
- Labelle A.L., Maddox C.W., Daniels J.B., Lanka S., Eggett T.E., Dubielzig R.R., Labelle P. Canine ocular onchocercosis in the United States is associated with Onchocerca lupi. Vet. Parasitol. 2013;193:297–301. doi: 10.1016/j.vetpar.2012.12.002. [DOI] [PubMed] [Google Scholar]
- Lahmar S., Boufana B., Ben Boubaker S., Landolsi F. Intestinal helminths of golden jackals and red foxes from Tunisia. Vet. Parasitol. 2014;204:297–303. doi: 10.1016/j.vetpar.2014.05.038. [DOI] [PubMed] [Google Scholar]
- Lalosevic D., Lalosevic V., Klem I., Stanojev-Jovanovic D., Pozio E. Pulmonary capillariasis miming bronchial carcinoma. Am. J. Trop. Med. Hyg. 2008;78:14–16. [PubMed] [Google Scholar]
- Lalosevic V., Lalosevic D., Capo I., Simin V., Galfi A., Traversa D. High infection rate of zoonotic Eucoleus aerophilus infection in foxes from Serbia. Parasite. 2013;20:3. doi: 10.1051/parasite/2012003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurimaa L., Süld K., Davison J., Moks E., Valdmann H., Saarma U. Alien species and their zoonotic parasites in native and introduced ranges: the raccoon dog example. Vet. Parasitol. 2016;219:24–33. doi: 10.1016/j.vetpar.2016.01.020. [DOI] [PubMed] [Google Scholar]
- Laurimaa L., Moks E., Soe E., Valdmann H., Saarma U. Echinococcus multilocularis and other zoonotic parasites in red foxes in Estonia. Parasitology. 2016;143:1450–1458. doi: 10.1017/S0031182016001013. [DOI] [PubMed] [Google Scholar]
- Lee R.M., Moore L.B., Bottazzi M.E., Hotez P.J. Toxocariasis in North America: a systematic review. PLoS Neglected Trop. Dis. 2014;8:e3116. doi: 10.1371/journal.pntd.0003116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefoulon E., Giannelli A., Makepeace B.L., Mutafchiev Y., Townson S., Uni S., Verocai G.G., Otranto D., Martin C. Whence river blindness? The domestication of mammals and host-parasite co-evolution in the nematode genus Onchocerca. Int. J. Parasitol. 2017;47:457–470. doi: 10.1016/j.ijpara.2016.12.009. [DOI] [PubMed] [Google Scholar]
- Lenka D.R., Johns J., Gopi J., Chandy G., Narayanan P.M., Kalarikkal D.C., Ravindran R. Occurrence of gnathostoma spinigerum in a leopard cat from Wayanad wildlife Sanctuary, Kerala. J. Parasit. Dis. 2016;40:555–557. doi: 10.1007/s12639-014-0502-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesniak I., Heckmann I., Heitlinger E., Szentiks C.A., Nowak C., Harms V., Jarausch A., Reinhardt I., Kluth G., Hofer H., Krone O. Population expansion and individual age affect endoparasite richness and diversity in a recolonising large carnivore population. Nat. Sci. Rep. 2017;7:41730. doi: 10.1038/srep41730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liccioli S., Giraudoux P., Deplazes P., Massolo A. Wilderness in the 'city' revisited: different urbes shape transmission of Echinococcus multilocularis by altering predator and prey communities. Trends Parasitol. 2015;31:297–305. doi: 10.1016/j.pt.2015.04.007. [DOI] [PubMed] [Google Scholar]
- Little M.D. Dermatitis in a human volunteer infected with Strongyloides of nutria and raccoon. Am. J. Trop. Med. Hyg. 1965;14:1007–1009. doi: 10.4269/ajtmh.1965.14.1007. [DOI] [PubMed] [Google Scholar]
- Lledó L., Giménez-Pardo C., Saz J.V., Serrano J.L. Wild red foxes (Vulpes vulpes) as sentinels of parasitic diseases in the province of Soria, Northern Spain. Vector Borne Zoonotic Dis. 2015;15:743–749. doi: 10.1089/vbz.2014.1766. [DOI] [PubMed] [Google Scholar]
- Luty T. Prevalence of species of Toxocara in dogs, cats and red foxes from the Poznan region, Poland. J. Helminthol. 2001;75:153–156. [PubMed] [Google Scholar]
- Máca J., Otranto D. Drosophilidae feeding on animals and the inherent mystery of their parasitism. Parasites Vectors. 2014;7:516. doi: 10.1186/s13071-014-0516-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenstedt U., Jenkins D., Romig T. The role of wildlife in the transmission of parasitic zoonoses in peri-urban and urban areas. Int. J. Parasitol. Parasites. Wildl. 2015;4:71–79. doi: 10.1016/j.ijppaw.2015.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magi M., Calderini P., Gabrielli S., Dell'Omodarme M., Macchioni M., Prati M.C., Cancrini G. Vulpes vulpes: a possible wild reservoir for zoonotic filariae. Vector Borne Zoonotic Dis. 2008;8:249–252. doi: 10.1089/vbz.2007.0207. [DOI] [PubMed] [Google Scholar]
- Magi M., Macchioni F., Dell'Omodarme M., Prati M.C., Calderini P., Gabrielli S., Iori A., Cancrini G. Endoparasites of red fox (Vulpes vulpes) in central Italy. J. Wildl. Dis. 2009;45:881–885. doi: 10.7589/0090-3558-45.3.881. [DOI] [PubMed] [Google Scholar]
- Magi M., Guardone L., Prati M.C., Mignone W., Macchioni F. Extraintestinal nematodes of the red fox Vulpes vulpes in north-west Italy. J. Helminthol. 2015;89:506–511. doi: 10.1017/S0022149X1400025X. [DOI] [PubMed] [Google Scholar]
- Magnis J., Naucke T.J., Mathis A., Deplazes P., Schnyder M. Local transmission of the eye worm Thelazia callipaeda in southern Germany. Parasitol. Res. 2010;106:715–717. doi: 10.1007/s00436-009-1678-4. [DOI] [PubMed] [Google Scholar]
- Maia C., Annoscia G., Latrofa M.S., Pereira A., Giannelli A., Pedroso L., Otranto D. Onchocerca lupi nematode in cat, Portugal. Emerg. Infect. Dis. 2015;21:2252–2254. doi: 10.3201/eid2112.150061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malacrida F., Hegglin D., Bacciarini L., Otranto D., Nägeli F., Nägeli C., Bernasconi C., Scheu U., Balli A., Marenco M., Togni L., Deplazes P., Schnyder M. Emergence of canine ocular thelaziosis caused by Thelazia callipaeda in southern Switzerland. Vet. Parasitol. 2008;157:321–327. doi: 10.1016/j.vetpar.2008.07.029. [DOI] [PubMed] [Google Scholar]
- Mazzariol S., Cassini R., Voltan L., Aresu L., Frangipane di Regalbono A. Heartworm (Dirofilaria immitis) infection in a leopard (Panthera pardus pardus) housed in a zoological park in north-eastern Italy. Parasites Vectors. 2010;3:25. doi: 10.1186/1756-3305-3-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCall J.W., Genchi C., Kramer L.H., Guerrero J., Venco L. Heartworm disease in animals and humans. Adv. Parasitol. 2008;66:193–285. doi: 10.1016/S0065-308X(08)00204-2. [DOI] [PubMed] [Google Scholar]
- Mclaughlin G.S., Obstbaum M., Forrester D.J., Roelke M.E., Brady J.R. Hookworms of bobcats (Felis rufus) from Florida. J. Helminthol. Soc. Washingt. 1993;60:10–13. [Google Scholar]
- Mech L.D. The Natural History Press; Garden City: 1970. The Wolf: the Ecology and Behavior of an Endangered Species. [Google Scholar]
- Merigueti Y.F.F.B., Santarém V.A., Ramires L.M., da Silveira Batista A., da Costa Beserra L.V., Nuci A.L., de Paula Esposte T.M. Protective and risk factors associated with the presence of Toxocara spp. eggs in dog hair. Vet. Parasitol. 2017;244:39–43. doi: 10.1016/j.vetpar.2017.07.020. [DOI] [PubMed] [Google Scholar]
- Meshgi B., Eslami A., Bahonar A.R., Kharrazian-Moghadam M., Gerami-Sadeghian A. Prevalence of parasitic infections in the red fox (Vulpes vulpes) and golden Jackal (Canis aureus) in Iran. Iran. J. Vet. Res. 2009;10:4. [Google Scholar]
- Michalski M.L., Bain O., Fischer K., Fischer P.U., Kumar S., Foster J.M. Identification and phylogenetic analysis of Dirofilaria ursi (Nematoda: Filarioidea) from Wisconsin black bears (Ursus americanus) and its Wolbachia endosymbiont. J. Parasitol. 2010;96:412–419. doi: 10.1645/GE-2208.1. [DOI] [PubMed] [Google Scholar]
- Mihalca A.D., D'Amico G., Scurtu I., Chirila R., Matei I.A., Ionica A.M. Further spreading of canine oriental eyeworm in Europe: first report of Thelazia callipaeda in Romania. Parasites Vectors. 2015;8:48. doi: 10.1186/s13071-015-0663-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihalca A.D., Ionică A.M., D'Amico G., Daskalaki A.A., Deak G., Matei I.A., Șimonca V., Iordache D., Modrý D., Gherman C.M. Thelazia callipaeda in wild carnivores from Romania: new host and geographical records. Parasites Vectors. 2016;9:350. doi: 10.1186/s13071-016-1628-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller D.L., Schrecengost J., Merrill A., Kilgo J., Ray H.S., Miller K.V., Baldwin C.A. Hematology, parasitology, and serology of free-ranging coyotes (Canis latrans) from South Carolina. J. Wildl. Dis. 2009;45:863–869. doi: 10.7589/0090-3558-45.3.863. [DOI] [PubMed] [Google Scholar]
- Miró G., Montoya A., Hernández L., Dado D., Vázquez M.V., Benito M., Villagrasa M., Brianti E., Otranto D. Thelazia callipaeda infection in dogs: a new parasite for Spain. Parasites Vectors. 2011;4:148. doi: 10.1186/1756-3305-4-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miterpáková M., Hurníková Z., Antolová D., Dubinský P. Endoparasites of red fox (Vulpes vulpes) in the Slovak Republic with the emphasis on zoonotic species Echinococcus multilocularis and Trichinella spp. Helminthologia. 2009;46:73–79. [Google Scholar]
- Moks E., Jõgisalu I., Saarma U., Talvik H., Järvis T., Valdmann H. Helminthologic survey of the wolf (Canis lupus) in Estonia, with an emphasis on Echinococcus granulosus. J. Wildl. Dis. 2006;42:359–365. doi: 10.7589/0090-3558-42.2.359. [DOI] [PubMed] [Google Scholar]
- Morgan E.R., Azam D., Pegler K. Quantifying sources of environmental contamination with Toxocara spp. eggs. Vet. Parasitol. 2013;15:390–397. doi: 10.1016/j.vetpar.2012.12.034. [DOI] [PubMed] [Google Scholar]
- Moskvina T.V., Schelkanov M.Y., Begun M.A. Endoparasites of the Siberian tiger (Panthera tigris altaica) Integr. Zool. 2018;13:507–516. doi: 10.1111/1749-4877.12342. [DOI] [PubMed] [Google Scholar]
- Mowlavi G., Farzbod F., Kheirkhah A., Mobedi I., Bowman D.D., Naddaf S.R. Human ocular onchocerciasis caused by Onchocerca lupi (Spirurida, Onchocercidae) in Iran. J. Helminthol. 2014;88:250–255. doi: 10.1017/S0022149X13000060. [DOI] [PubMed] [Google Scholar]
- Nagy A., Ziadinov I., Schweiger A., Schnyder M., Deplazes P. Hair coat contamination with zoonotic helminth eggs of farm and pet dogs and foxes. Berl. Münchener Tierärztliche Wochenschr. 2011;124:503–511. [PubMed] [Google Scholar]
- Napoli E., Anile S., Arrabito C., Scornavacca D., Mazzamuto M.V., Gaglio G., Otranto D., Giannetto S., Brianti E. Survey on parasitic infections in wildcat (Felis silvestris silvestris Schreber, 1777) by scat collection. Parasitol. Res. 2016;115:255–261. doi: 10.1007/s00436-015-4742-2. [DOI] [PubMed] [Google Scholar]
- Niewold F.J.J. Aspects of the social structure of red fox populations: a summary. In: Zimen E., editor. Biogeographica Vol. 18 - the Red Fox. Dr. W. Junk B. V. Publishers; The Hague: 1980. pp. 185–193. [Google Scholar]
- Osten-Sacken N., Słodkowicz-Kowalska A., Pacoń J., Skrzypczak Ł., Werner A. Intestinal and external parasites of raccoon dogs (Nyctereutes procyonoides) in western Poland. Ann. Parasitol. 2017;63:37–44. doi: 10.17420/ap6301.83. [DOI] [PubMed] [Google Scholar]
- Osten-Sacken N., Heddergott M., Schleimer A., Anheyer-Behmenburg H.E., Runge M., Horsburgh G.J., Camp L., Nadler S.A., Frantz A.C. Similar yet different: co-analysis of the genetic diversity and structure of an invasive nematode parasite and its invasive mammalian host. Int. J. Ritol. 2018;48:233–243. doi: 10.1016/j.ijpara.2017.08.013. [DOI] [PubMed] [Google Scholar]
- Otranto D., Dutto M. Human thelaziasis, Europe. Emerg. Infect. Dis. 2008;14:647–649. doi: 10.3201/eid1404.071205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otranto D., Eberhard M.L. Zoonotic helminths affecting the human eye. Parasites Vectors. 2011;4:41. doi: 10.1186/1756-3305-4-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otranto D., Ferroglio E., Lia R., Traversa D., Rossi L. Current status and epidemiological observations of Thelazia callipaeda (Spirurida, Thelaziidae) in dogs, cats and foxes in Italy: a “coincidence” or a parasitic disease of the Old Continent? Vet. Parasitol. 2003;116:315–325. doi: 10.1016/j.vetpar.2003.07.022. [DOI] [PubMed] [Google Scholar]
- Otranto D., Testini G., Deluca F., Hu M., Shamsi S., Gasser R.B. Analysis of genetic variability within Thelazia callipaeda (Nematoda: Thelazioidea) from Europe and Asia by sequencing and mutation scanning of mitochondrial cytochrome c oxidase subunit 1. Mol. Cell. Probes. 2005;19:306–313. doi: 10.1016/j.mcp.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Otranto D., Brianti E., Cantacessi C., Lia R.P., Máca J. Morphology, ecology and biological niches of the zoophilic fruitfly, Thelazia callipaeda. Med. Vet. Entomol. 2006;20:358–364. doi: 10.1111/j.1365-2915.2006.00643.x. [DOI] [PubMed] [Google Scholar]
- Otranto D., Cantacessi C., Testini G., Lia R.P. Phortica variegata as an intermediate host of Thelazia callipaeda under natural conditions: evidence for pathogen transmission by a male arthropod vector. Int. J. Parasitol. 2006;36:1167–1173. doi: 10.1016/j.ijpara.2006.06.006. [DOI] [PubMed] [Google Scholar]
- Otranto D., Dantas-Torres F., Mallia E., DiGeronimo P.M., Brianti E., Testini G., Traversa D., Lia R.P. Thelazia callipaeda (Spirurida, Thelaziidae) in wild animals: report of new host species and ecological implications. Vet. Parasitol. 2009;166:262–267. doi: 10.1016/j.vetpar.2009.08.027. [DOI] [PubMed] [Google Scholar]
- Otranto D., Diniz D.G., Dantas-Torres F., Casiraghi M., de Almeida I.N., de Almeida L.N., dos Santos J.N., Furtado A.P., de Almeida Sobrinho E.F., Bain O. Human intraocular filariasis caused by Dirofilaria sp. nematode, Brazil. Emerg. Infect. Dis. 2011;17:863–866. doi: 10.3201/eid1705.100916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otranto D., Sakru N., Testini G., Gürlü V.P., Yakar K., Lia R.P., Dantas-Torres F., Bain O. Case report: first evidence of human zoonotic infection by Onchocerca lupi (Spirurida, Onchocercidae) Am. J. Trop. Med. Hyg. 2011;84:55–58. doi: 10.4269/ajtmh.2011.10-0465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otranto D., Dantas-Torres F., Cebeci Z., Yeniad B., Buyukbabani N., Boral O.B., Gustinelli A., Mounir T., Mutafchiev Y., Bain O. Human ocular filariasis: further evidence on the zoonotic role of Onchocerca lupi. Parasites Vectors. 2012;5:84. doi: 10.1186/1756-3305-5-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otranto D., Dantas-Torres F., Brianti E., Traversa D., Petrić D., Genchi C., Capelli G. Vector-borne helminths of dogs and humans in Europe. Parasites Vectors. 2013;6:16. doi: 10.1186/1756-3305-6-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otranto D., Dantas-Torres F., Giannelli A., Latrofa M.S., Papadopoulos E., Cardoso L., Cortes H. Zoonotic Onchocerca lupi infection in dogs, Greece and Portugal, 2011-2012. Emerg. Infect. Dis. 2013;19:2000–2003. doi: 10.3201/eid1912.130264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otranto D., Cantacessi C., Pfeffer M., Dantas-Torres F., Brianti E., Deplazes P., Genchi C., Guberti V., Capelli G. The role of wild canids and felids in spreading parasites to dogs and cats in Europe. Part I: Protozoa and tick-borne agents. Vet. Parasitol. 2015;213:12–23. doi: 10.1016/j.vetpar.2015.04.022. [DOI] [PubMed] [Google Scholar]
- Otranto D., Cantacessi C., Dantas-Torres F., Brianti E., Pfeffer M., Genchi C., Guberti V., Capelli G., Deplazes P. The role of wild canids and felids in spreading parasites to dogs and cats in Europe. Part II: helminths and arthropods. Vet. Parasitol. 2015;213:24–37. doi: 10.1016/j.vetpar.2015.04.020. [DOI] [PubMed] [Google Scholar]
- Otranto D., Giannelli A., Latrofa M.S., Dantas-Torres F., Trumble N.S., Chavkin M., Kennard G., Eberhard M.L., Bowman D.D. Canine infections with Onchocerca lupi nematodes, United States, 2011-2014. Emerg. Infect. Dis. 2015;21:868–871. doi: 10.3201/eid2105.141812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otranto D., Dantas-Torres F., Mihalca A.D., Traub R.J., Lappin M., Baneth G. Zoonotic parasites of sheltered and stray dogs in the era of the global economic and political crisis. Trends Parasitol. 2017;33:813–825. doi: 10.1016/j.pt.2017.05.013. [DOI] [PubMed] [Google Scholar]
- Ozeretskovskaya N.N., Mikhailova L.G., Sabgaida T.P., Dovgalev A.S. New trends and clinical patterns of human trichinellosis in Russia at the beginning of the XXI century. Vet. Parasitol. 2005;132:167–171. doi: 10.1016/j.vetpar.2005.05.056. [DOI] [PubMed] [Google Scholar]
- Page L.K. Parasites and the conservation of small populations: the case of Baylisascaris procyonis. Int. J. Parasitol. Parasites Wildl. 2013;2:203–210. doi: 10.1016/j.ijppaw.2013.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page L.K., Swihart R.K., Kazacos K.R. Implications of raccoon latrines in the epizootiology of Baylisascariasis. J. Wildl. Dis. 1999;35:474–480. doi: 10.7589/0090-3558-35.3.474. [DOI] [PubMed] [Google Scholar]
- Palfreyman J., Graham-Brown J., Caminade C., Gilmore P., Otranto D., Williams D.J.L. Predicting the distribution of Phortica variegata and potential for Thelazia callipaeda transmission in Europe and the United Kingdom. Parasites Vectors. 2018;11:72. doi: 10.1186/s13071-018-2842-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadopoulos E., Komnenou A., Thomas A., Ioannidou E., Colella V., Otranto D. Spreading of Thelazia callipaeda in Greece. Transbound. Emerg. Dis. 2018;65:248–252. doi: 10.1111/tbed.12626. [DOI] [PubMed] [Google Scholar]
- Paradies P., Iarussi F., Sasanelli M., Capogna A., Lia R.P., Zucca D., Greco B., Cantacessi C., Otranto D. Occurrence of strongyloidiasis in privately owned and sheltered dogs: clinical presentation and treatment outcome. Parasites Vectors. 2017;10:345. doi: 10.1186/s13071-017-2275-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paradzik M.T., Samardzic K., Zivicnjak T., Martinkovic F., Janjetovic Z., Miletic-Medved M. Thelazia callipaeda - first human case of thelaziosis in Croatia. Wien Klin. Wochenschr. 2016;128:221–223. doi: 10.1007/s00508-015-0889-1. [DOI] [PubMed] [Google Scholar]
- Paras K.L., Little S.E., Reichard M.V., Reiskind M.H. Detection of Dirofilaria immitis and Ehrlichia species in coyotes (Canis latrans), from rural Oklahoma and Texas. Vector Borne Zoonotic Dis. 2012;12:619–621. doi: 10.1089/vbz.2011.0815. [DOI] [PubMed] [Google Scholar]
- Penezić A., Selaković S., Pavlović I., Ćirović D. First findings and prevalence of adult heartworms (Dirofilaria immitis) in wild carnivores from Serbia. Parasitol. Res. 2014;113:3281–3285. doi: 10.1007/s00436-014-3991-9. [DOI] [PubMed] [Google Scholar]
- Petavy A.F., Deblock S. Helminthes du Renard commun (Vulpes vulpes L.) dans la région du Massif Central (France) Ann. Parasitol. Hum. Comp. 1980;55:379–391. [PubMed] [Google Scholar]
- Polley L. Navigating parasite webs and parasite flow: emerging and re-emerging parasitic zoonoses of wildlife origin. Int. J. Parasitol. 2005;35:1279–1294. doi: 10.1016/j.ijpara.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Pozio E., Zarlenga D.S. New pieces of the Trichinella puzzle. Int. J. Parasitol. 2013;43:983–997. doi: 10.1016/j.ijpara.2013.05.010. [DOI] [PubMed] [Google Scholar]
- Rajković-Janje R., Marinculić A., Bosnić S., Benić M., Vinković B., Mihaljević Ž. Prevalence and seasonal distribution of helminth parasites in red foxes (Vulpes vulpes) from the Zagreb County (Croatia) Z. Jagdwiss. 2002;48:151–160. [Google Scholar]
- Ramirez J.M., Ramirez M.A., Essilfie A., Taylor C.E., Stearns H.C., Mollano A. Roundworm-associated median nerve compression: a case report. Iowa Orthop. J. 2013;33:225–227. [PMC free article] [PubMed] [Google Scholar]
- Redman W.K., Bryant J.E., Ahmad G. Gastrointestinal helminths of coyotes (Canis latrans) from Southeast Nebraska and Shenandoah area of Iowa. Vet. World. 2016;9:970–975. doi: 10.14202/vetworld.2016.970-975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rentería-Solís Z., Birka S., Schmäschke R., Król N., Obiegala A. First detection of Baylisascaris procyonis in wild raccoons (Procyon lotor) from Leipzig, Saxony, eastern Germany. Parasitol. Res. 2018;117:3289–3292. doi: 10.1007/s00436-018-5988-2. [DOI] [PubMed] [Google Scholar]
- Reperant L.A., Hegglin D., Fischer C., Kohler L., Weber J.M., Deplazes P. Influence of urbanization on the epidemiology of intestinal helminths of the red fox (Vulpes vulpes) in Geneva, Switzerland. Parasitol. Res. 2007;101:605–611. doi: 10.1007/s00436-007-0520-0. [DOI] [PubMed] [Google Scholar]
- Richards D.T., Harris S., Lewis J.W. Epidemiological studies on intestinal helminth parasites of rural and urban red foxes (Vulpes vulpes) in the United Kingdom. Vet. Parasitol. 1995;59:39–51. doi: 10.1016/0304-4017(94)00736-v. [DOI] [PubMed] [Google Scholar]
- Rodonaja T.E. A new species of nematode, Onchocerca lupi n. sp., from Canis lupus cubanensis. Bull. Acad. Sci. Georgian SSR. 1967;45:715–719. [Google Scholar]
- Romero-Alegría A., Belhassen-García M., Velasco-Tirado V., Garcia-Mingo A., Alvela-Suárez L., Pardo-Lledias J., Sánchez M.C. Angiostrongylus costaricensis: systematic review of case reports. Adv. Infect. Dis. 2014;4:36–41. [Google Scholar]
- Rossi L., Bertaglia P.P. Presence of Thelazia callipaeda Railliet & Henry, 1910, in Piedmont, Italy. Parassitologia. 1989;31:167–172. [PubMed] [Google Scholar]
- Rostami A., Gamble H.R., Dupouy-Camet J., Khazan H., Bruschi F. Meat sources of infection for outbreaks of human trichinellosis. Food Microbiol. 2017;64:65–71. doi: 10.1016/j.fm.2016.12.012. [DOI] [PubMed] [Google Scholar]
- Ryan G.E. Helminth parasites of the fox (Vulpes vulpes) in New South Wales. Aust. Vet. J. 1976;52:126–131. doi: 10.1111/j.1751-0813.1976.tb05445.x. [DOI] [PubMed] [Google Scholar]
- Saeed I., Taira K., Kapel C.M.O. Toxocara canis in experimentally infected silver and arctic foxes. Parasitol. Res. 2005;97:160–166. doi: 10.1007/s00436-005-1414-7. [DOI] [PubMed] [Google Scholar]
- Saeed I., Maddox-Hyttel C., Monrad J., Kapel C.M.O. Helminths of red foxes (Vulpes vulpes) in Denmark. Vet. Parasitol. 2006;139:168–179. doi: 10.1016/j.vetpar.2006.02.015. [DOI] [PubMed] [Google Scholar]
- Sargo R., Loureiro F., Catarino A.L., Valente J., Silva F., Cardoso L., Otranto D., Maia C. First report of Thelazia callipaeda in red foxes (Vulpes vulpes) from Portugal. J. Zoo Wildl. Med. 2014;45:458–460. doi: 10.1638/2013-0294R.1. [DOI] [PubMed] [Google Scholar]
- Sato H., Inaba T., Ihama Y., Kamiya H. Parasitological survey on wild carnivora in north-western Tohoku, Japan. J. Vet. Med. Sci. 1999;61:1023–1026. doi: 10.1292/jvms.61.1023. [DOI] [PubMed] [Google Scholar]
- Sato H., Matsuo K., Osanai A., Kamiya H., Akao N., Owaki S., Furuoka H. Larva migrans by Baylisascaris transfuga: fatal neurological diseases in Mongolian jirds, but not in mice. J. Parasitol. 2004;90:774–781. doi: 10.1645/GE-3330. [DOI] [PubMed] [Google Scholar]
- Sato H., Suzuki K., Osanai A., Kamiya H., Furuoka H. Identification and characterization of the threadworm, Strongyloides procyonis, from feral raccoons (Procyon lotor) in Japan. J. Parasitol. 2006;92:63–68. doi: 10.1645/GE-623R.1. [DOI] [PubMed] [Google Scholar]
- Sałamatin R., Pavlikovska T., Sagach O., Nikolayenko S., Kornyushin V., Kharchenko V., Masny A., Cielecka D., Konieczna-Sałamatin J., Conn D., Golab E. Human dirofilariasis due to Dirofilaria repens in Ukraine, an emergent zoonosis: epidemiological report of 1465 cases. Acta Parasitol. 2013;58:592–598. doi: 10.2478/s11686-013-0187-x. [DOI] [PubMed] [Google Scholar]
- Schnieder T., Laabs E.M., Welz C. Larval development of Toxocara canis in dogs. Vet. Parasitol. 2011;10:193–206. doi: 10.1016/j.vetpar.2010.10.027. [DOI] [PubMed] [Google Scholar]
- Schug K., Krämer F., Schaper R., Hirzmann J., Failing K., Hermosilla C., Taubert A. Prevalence survey on lungworm (Angiostrongylus vasorum, Crenosoma vulpis, Eucoleus aerophilus) infections of wild red foxes (Vulpes vulpes) in central Germany. Parasites Vectors. 2018;11:85. doi: 10.1186/s13071-018-2672-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott D.M., Berg M.J., Tolhurst B.A., Chauvenet A.L.M., Smith G.C., Neaves K., Lochhead J., Baker P.J. Changes in the distribution of red foxes (Vulpes vulpes) in urban areas in Great Britain: findings and limitations of media-driven nationwide survey. PLoS One. 2014;9 doi: 10.1371/journal.pone.0099059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segovia J.M., Guerrero J., Torres J., Miquel J., Feliu C. Ecological analyses of the intestinal helminth communities of the wolf, Canis lupus, in Spain. Folia Parasitol. 2003;50:231–236. doi: 10.14411/fp.2003.041. [DOI] [PubMed] [Google Scholar]
- Segovia J.M., Torres J., Miquel J. Helminth parasites of the red fox (Vulpes vulpes L., 1758) in the Iberian Peninsula: an ecological study. Acta Parasitol. 2004;49:67–79. [Google Scholar]
- Seguel M., Gottdenker N. The diversity and impact of hookworm infections in wildlife. Int. J. Parasitol. Parasites Wildl. 2017;6:177–194. doi: 10.1016/j.ijppaw.2017.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen J., Gasser R.B., Chu D., Wang Z., Yuan X., Cantacessi C., Otranto D. Human thelaziosis-a neglected parasitic disease of the eye. J. Parasitol. 2006;92:872–875. doi: 10.1645/GE-823R.1. [DOI] [PubMed] [Google Scholar]
- Shimalov V.V., Shimalov V.T. Helminth fauna of the wolf (Canis lupus Linnaeus, 1758) in Belorussian Polesie. Parasitol. Res. 2000;86:163–164. doi: 10.1007/s004360050026. [DOI] [PubMed] [Google Scholar]
- Shrivastav A.B., Singh K.P., Bhat M.A., Mishra A. Occurrence of Gnathostoma spinigerum in free range tigress. J. Parasit. Dis. 2011;35:75–76. doi: 10.1007/s12639-011-0029-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sircar A.D., Abanyie F., Blumberg D., Chin-Hong P., Coulter K.S., Cunningham D., Huskins W.C., Langelier C., Reid M., Scott B.J., Shirley D.A., Babik J.M., Belova A., Sapp S.G.H., McAuliffe I., Rivera H.N., Yabsley M.J., Montgomery S.P. Raccoon roundworm infection associated with central nervous system disease and ocular disease-six states, 2013–2016. Morb. Mortal. Wkly. Rep. 2016;65:930–933. doi: 10.15585/mmwr.mm6535a2. [DOI] [PubMed] [Google Scholar]
- Smith A.F., Semeniuk C.A., Kutz S.J., Massolo A. Dog-walking behaviours affect gastrointestinal parasitism in park-attending dogs. Parasites Vectors. 2014;7:429. doi: 10.1186/1756-3305-7-429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smout F.A., Thompson R.C., Skerratt L.F. First report of Ancylostoma ceylanicum in wild canids. Int. J. Parasitol. Parasites Wildl. 2013;2:173–177. doi: 10.1016/j.ijppaw.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smout F.A., Skerratt L.F., Johnson C.N., Butler J.R.A., Congdon B.C. Zoonotic helminth diseases in dogs and dingoes utilising shared resources in an australian aboriginal community. Trop. Med. Infect. Dis. 2018;3:110. doi: 10.3390/tropicalmed3040110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spratt D.M. Species of Angiostrongylus (Nematoda: Metastrongyloidea) in wildlife: a review. Int. J. Parasitol. Parasites Wildl. 2015;4:178–189. doi: 10.1016/j.ijppaw.2015.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sréter T., Széll Z. Onchocercosis: a newly recognized disease in dogs. Vet. Parasitol. 2008;151:1–13. doi: 10.1016/j.vetpar.2007.09.008. [DOI] [PubMed] [Google Scholar]
- Sréter T., Széll Z., Marucci G., Pozio E., Varga I. Extraintestinal nematode infections of red foxes (Vulpes vulpes) in Hungary. Vet. Parasitol. 2003;115:329–334. doi: 10.1016/s0304-4017(03)00217-6. [DOI] [PubMed] [Google Scholar]
- Štrkolcová G., Goldová M., Šnábel V., Špakulová M., Orosová T., Halán M., Mojžišová J. A frequent roundworm Baylisascaris transfuga in overpopulated brown bears (Ursus arctos) in Slovakia: a problem worthy of attention. Acta Parasitol. 2018;63:167–174. doi: 10.1515/ap-2018-0019. [DOI] [PubMed] [Google Scholar]
- Stuart P., Golden O., Zintl A., de Waal T., Mulcahy G., McCarthy E., Lawton C. A coprological survey of parasites of wild carnivores in Ireland. Parasitol. Res. 2013;112:3587–3593. doi: 10.1007/s00436-013-3544-7. [DOI] [PubMed] [Google Scholar]
- Széll Z., Erdélyi I., Sréter T., Albert M., Varga I. Canine ocular onchocercosis in Hungary. Vet. Parasitol. 2001;97:245–251. doi: 10.1016/s0304-4017(01)00397-1. [DOI] [PubMed] [Google Scholar]
- Taira K., Saeed I., Permin A., Kapel C.M.O. Zoonotic risk of Toxocara canis through consumption of pig or poultry viscera. Vet. Parasitol. 2004;121:115–124. doi: 10.1016/j.vetpar.2004.01.018. [DOI] [PubMed] [Google Scholar]
- Takács A., Szabó L., Juhász L., Takács A.A., Lanszki J., Takács P.T., Heltai M. Data on the parasitological status of golden jackal (Canis aureus L., 1758) in Hungary. Acta Vet. Hung. 2014;62:33–41. doi: 10.1556/AVet.2013.058. [DOI] [PubMed] [Google Scholar]
- Tasic-Otasevic S., Gabrielli S., Trenkic-Bozinovic M., Petrovic A., Gajic B., Colella V., Momčilović S., Cancrini G., Otranto D. Eyeworm infections in dogs and in a human patient in Serbia: a One Health approach is needed. Comp. Immunol. Microbiol. Infect. Dis. 2016;45:20–22. doi: 10.1016/j.cimid.2016.01.003. [DOI] [PubMed] [Google Scholar]
- Thamsborg S.M., Ketzis J., Horii Y., Matthews J.B. Strongyloides spp. infections of veterinary importance. Parasitology. 2017;144:274–284. doi: 10.1017/S0031182016001116. [DOI] [PubMed] [Google Scholar]
- Thieß A., Schuster R., Nöckler K., Mix H. Helminthenfunde beimeinheimischen Marderhund Nyctereutes procyonoides (gray, 1834) Berl. Münchener Tierärztliche Wochenschr. 2001;114:273–276. [PubMed] [Google Scholar]
- Thompson A.R.C., Kutz S., Smith A. Parasite zoonoses and wildlife: emerging issues. Int. J. Environ. Res. Publ. Health. 2009;6:678–693. doi: 10.3390/ijerph6020678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson R.C.A., Lymbery A.J., Smith A. Parasites, emerging disease and wildlife conservation. Int. J. Parasitol. 2010;40:1163–1170. doi: 10.1016/j.ijpara.2010.04.009. [DOI] [PubMed] [Google Scholar]
- Tolnai Z., Széll Z., Sproch Á., Szeredi L., Sréter T. Dirofilaria immitis: an emerging parasite in dogs, red foxes and golden jackals in Hungary. Vet. Parasitol. 2014;203:339–342. doi: 10.1016/j.vetpar.2014.04.004. [DOI] [PubMed] [Google Scholar]
- Traub R.J. Ancylostoma ceylanicum, a re-emerging but neglected parasitic zoonosis. J. Parasitol. 2013;43:1009–1015. doi: 10.1016/j.ijpara.2013.07.006. [DOI] [PubMed] [Google Scholar]
- Tudor P., Turcitu M., Mateescu C., Dantas-Torres F., Tudor N., Bărbuceanu F., Ciuca L., Burcoveanu I., Acatrinei D., Rinaldi L., Mateescu R., Bădicu A., Ionașcu I., Otranto D. Zoonotic ocular onchocercosis caused by Onchocerca lupi in dogs in Romania. Parasitol. Res. 2015;115:859–862. doi: 10.1007/s00436-015-4816-1. [DOI] [PubMed] [Google Scholar]
- Uni S. Filarial parasites from the black bear of Japan. Ann. Parasitol. Hum. Comp. 1983;58:71–84. doi: 10.1051/parasite/1983581071. [DOI] [PubMed] [Google Scholar]
- Vafae Eslahi A., Kia E.B., Mobedi I., Sharifdini M., Badri M., Mowlavi G. Road killed carnivores illustrate the status of zoonotic helminthes in caspian sea littoral of Iran. Iran. J. Parasitol. 2017;12:230–235. [PMC free article] [PubMed] [Google Scholar]
- Vergles Rataj A., Posedi J., Zele D., Vengušt G. Intestinal parasites of the red fox (Vulpes vulpes) in Slovenia. Acta Vet. Hung. 2013;61:454–462. doi: 10.1556/AVet.2013.029. [DOI] [PubMed] [Google Scholar]
- Vieira L., Rodrigues F.T., Costa Á., Diz-Lopes D., Machado J., Coutinho T., Tuna J., Latrofa M., Cardoso L., Otranto D. First report of canine ocular thelaziosis by Thelazia callipaeda in Portugal. Parasites Vectors. 2012;5:124. doi: 10.1186/1756-3305-5-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wapenaar W., Barkema H.W., O'Handley R. Fecal shedding of Toxocara canis and other parasites in foxes and coyotes on Prince Edward Island, Canada. J. Wildl. Dis. 2013;49:394–397. doi: 10.7589/2012-04-113. [DOI] [PubMed] [Google Scholar]
- Yokohata Y., Fujita O., Kamiya M., Fujita T., Kaneko K., Ohbayashi M. Parasites from the Asiatic black bear (Ursus thibetanus) on Kyushu Island, Japan. J. Wildl. Dis. 1990;26:137–138. doi: 10.7589/0090-3558-26.1.137. [DOI] [PubMed] [Google Scholar]