Abstract
Wild mustelids and canids are definitive hosts of Taenia and Versteria spp. while rodents act as natural intermediate hosts. Rarely, larval stages of these parasites can cause serious zoonoses. In Europe, four cases of Taenia martis cysticercosis have been diagnosed in immunocompetent women, and two cases in zoo primates since 2013. In North America, a zoonotic genotype related but distinct from Versteria mustelae has been identified in 2014, which had caused a fatal infection in an orangutan and liver- and disseminated cysticercoses in two severely immune deficient human patients in 2018, respectively. Additionally, we could attribute a historic human case from the USA to this Versteria sp. by reanalysing a published nucleotide sequence. In the last decades, sporadic zoonotic infections by cysticerci of the canid tapeworm Taenia crassiceps have been described (4 in North America, 8 in Europe). Besides, 3 ocular cases from North America and one neural infection from Europe, all in immunocompetent patients, 6 cutaneous infections were described in severely immunocompromised European patients. Correspondingly, besides oral infections with taeniid eggs, accidental subcutaneous oncosphere establishment after egg-contamination of open wounds was suggested, especially in cases with a history of cutaneous injuries at the infection site. Taenia multiceps is mainly transmitted in a domestic cycle. Only five human coenurosis cases are published since 2000. In contrast, T. serialis coenurosis (1 human case since 2000) is primarily transmitted by wild canids. The etiological diagnosis of exotic cysticercoses is challenging. Usually, clinical material does not allow for a morphological identification, and serological tests are not available. These limitations have partly been overcome by molecular tools. Without claiming any dramatic emergence of cysticercoses and coenuroses transmitted by wild carnivores, further sporadic cases of such ‘exotic’ infections have to be expected.
Keywords: Taenia crassiceps, Taenia martis, Taenia serialis, Versteria sp., Mustelids, Wild canids
Graphical abstract
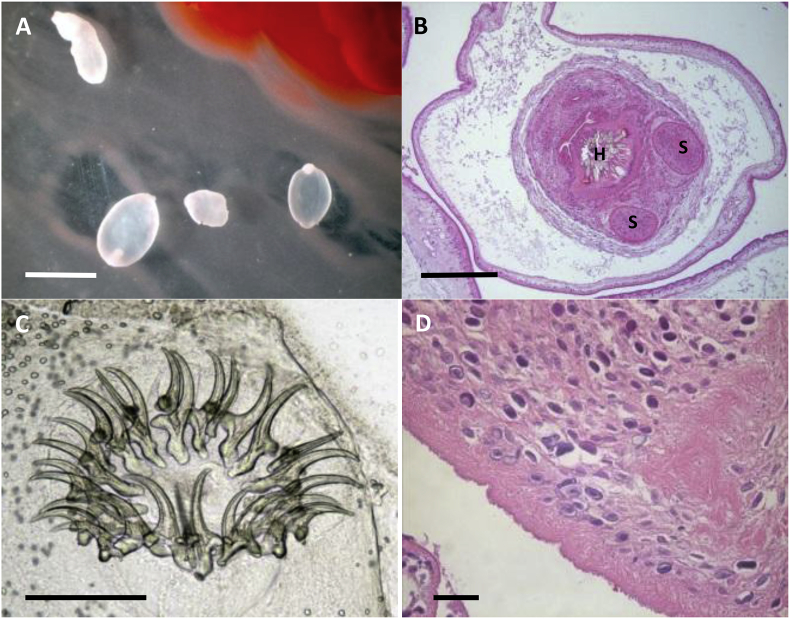
Taenia crassiceps cysticerci from the abdomen of an infected rodent.
Highlights
-
•
Wild canids and mustelids transmit rare but potentially fatal cysticercoses and coenuroses.
-
•
Martens and weasels can rarely transmit dangerous parasitic infections.
-
•
Tapeworm eggs may contaminate wounds and develop locally.
-
•
In North America, the mustelid tapeworm Versteria causes severe human infections.
-
•
Molecular analyses from minute clinical material allows for a specific diagnosis.
1. Introduction
Wildlife is confronted worldwide with expanding urban and agricultural areas and increasing human leisure activities. This development has strong effects on the biodiversity of ecosystems and poses a threat to many species. However, several carnivores and other species may take advantage of urban resources and new habitats, and they are increasing their populations. Furthermore, the stone marten (Martes foina) is known to be adapted to urbanised habitats for centuries (Wereszczuk et al., 2017). More recently, carnivore species thriving in anthropogenic habitats include the red fox (Vulpes vulpes) (Chautan et al., 2000), coyotes (Canis latrans, in Canadian cities) (Liccioli et al., 2015), raccoons (Procyon lotor, in North America and Central Europe) (Mackenstedt et al., 2015) and European badgers (Meles meles, in Europe) (Geiger et al., 2018). Consequently, urbanization influences the transmission of parasites with complex life cycles, which often involve intermediate and definitive host species with very different ecologies (Liccioli et al., 2015; Lafferty, 1999). For example, a distinct increase of human alveolar echinococcosis has been observed in Central Europe, approximately ten years after the establishment of stable urban fox populations (Schweiger et al., 2007). And more recently, a clear increase of angiostrongylosis in dogs has been observed in parallel with a strong increase of the prevalence of this parasite in foxes living in the Swiss Plateau (Gillis-Germitsch and Schnyder, 2018). Furthermore, increasing recreational and sports activities of the urban population, together with their dogs, in agricultural and wildlife areas have increased the transmission risks of parasites between wild and domestic carnivores. This is supported by the observations of many veterinarians reporting increasing numbers of Sarcoptes infestations in dogs after Sarcoptes mange had become more prevalent in foxes in Eastern Switzerland (Deplazes P., unpublished). Trends in the transmission of parasites between wild and domestic carnivores have been reviewed for protozoa and tick-borne agents (Otranto et al., 2015a), as well as for helminths and arthropods (Otranto et al., 2015b).
The causative agents of human alveolar (AE) and cystic echinococcosis (CE) are Echinococcus multilocularis and E. granulosus sensu lato (s.l.), respectively (Kern et al., 2017), while Taenia solium is the etiological agent for cysticercosis (Flisser et al., 2011). Zoonotic transmissions of CE and T. solium cysticercosis mainly involve domestic animals: pigs as the intermediate hosts for T. solium (Flisser et al., 2011), and dogs as the definitive hosts of E. granulosus s.l. (Romig et al., 2017). In contrast, zoonotic transmission of AE is mainly attributed to increased contact zones of the wild definitive hosts (red foxes, coyotes and raccoon dogs) and humans (Romig et al., 2017). Carnivore transmitted taeniid infections other than echinococcosis and T. solium cysticercosis (Table 1) have been sporadically described in immunocompetent (T. martis or T. crassiceps cysticercosis and T. multiceps, T. serialis, T. brauni and T. glomeratus coenuroses) and typically in severely immunocompromised human patients (T. crassiceps and Versteria sp. cysticercoses) (Table 2, Table 3, Table 4), as well as in other primates.
Table 1.
Taenia and Versteria spp. of domestic and wild carnivores with zoonotic potential. Biological characteristics (modified after Deplazes et al., 2016).
| Taenia species | Distribution | Definitive host (rare hosts in parentheses) | Natural intermediate host, common name of larval stage, predilection sites, dead-end hosts |
|---|---|---|---|
| Taenia crassiceps (Zeder 1800) | Northern hemisphere | Fox, wolf, jackal, raccoon dog (dog, cat, wild cat, mustelids) | Rodents, (moles); Cysticercus longicollis; subcutaneous tissue, body cavities. Variety of mammal dead-end hosts, 12 cases described in humans (Table 2). |
| Taenia martis (Zeder, 1803), T. martis americana | Northern hemisphere | Mustelids: Martes, Mustela, Meles, Gulo, Lutra), (fox) | Rodents (voles, murids, red squirrel) cysticercus; body cavities, rarely CNS. Rarely primates as dead-end hosts, recently 4 first cases in humans. |
| Taenia taeniaeformis (Batsch 1786) | Worldwide | Cat, lynx, other felids, (mustelids, fox) | Rodents: Strobilocercus fasciolaris; liver. Rarely in other hosts including a single human case. |
| Taenia multiceps (Leske, 1780) | Worldwide | Dog, red fox, wolf, (hyena, jackal, coyote) | Sheep, goat, cattle, buffalo, yak, other domestic and wild ruminants; Coenurus cerebralis; CNS, connective tissue. 5 case-reports in humans in the last 25 years. Rarely in other primates. |
| Taenia serialis (Gervais, 1847) | Worldwide | Fox, wolf, hyena, coyote, jackal, (dog, cat) | Hare, rabbit, (rodents); Coenurus serialis; subcutaneous and intermuscular tissue. Two case-reports in humans in the last 25 years. Several cases in primates (including abdominal infection). |
| ‘African-type’ coenurosis (Taenia brauni, Taenia glomeratus) | Africa | Dog, fox, jackal, genet | Rodents (swamp rat, porcupine, gerbil), Coenurus brauni/Coenurus glomeratus; no recent report of zoonotic infections, but few historic cases (Supplemental Table 1). |
| Versteria mustelae (Gmelin, 1790) (Syn. Taenia mustelae) | Northern hemisphere | Mustelids | Rodents, cysticercus, liver. No primate cases caused by V. mustelae in Europe and Asia |
| Versteria sp.a(V. mustelae zoonotic genotype) | Northern America | Mustelids: ermine, mink | Rodents (cysticercus), liver. 3 cases of cysticecosis in immunosuppressed human patients, one case in an orangutang (Table 3). |
So far not fully described zoonotic genotype of V. mustelae or new Versteria species.
Table 2.
Cases of Taenia crassiceps cysticercosis in humans.
| Case no.; references | Patient, immune status and case history | Pathological findings | Etiological diagnosis: morphology and DNA analysis (PCRa, sequencing) |
|---|---|---|---|
| Case 1 Shea et al. (1973); Freeman et al. (1973) |
17-year-old immunocompetent Canadian woman, with decreased visual acuity of her right eye since two months. Four months earlier she suffered from a severe generalized erythematosus skin condition. A family dog infected most probabely with T. crassiceps (based on proglottid morphology) lived in intimate association with the patient. |
A large motile cyst with a scolex and several smaller cystic leisons were observed at the posterior pole of her right eye. With a putative diagnosis of a T. crassiceps cysticercosis, cysts were removed by surgical intervention. |
Morphology. Based on clinical material and from the established isolate in mice, T. crassiceps cysticerci were identified by rostellar hook morphology and numbers as well as by the budding proliferation of the cysticerci. DNA analysis. Nd. |
| Case 2 Klinker et al. (1992) |
33-year-old German male, AIDS patient with a Pneumocystis carinii pneumonia and cerebral toxoplasmosis presented a growing paravertebral haematoma. The patient reported a fall around 4 weeks before the first symptoms appeared. | Subcutaneous, paravertebral infiltrate resembling a haematoma which spread over several weeks to cover almost the entire back. A spontaneous rupture of the infiltrate released “whitish spherical masses, 2–3 mm in diameter”. |
Morphology.T. crassiceps diagnosis was confirmed by the number of hooks and the typical budding of the cysticerci. DNA analysis. Nd. |
| Case 3 Chermette et al. (1995) |
33-year-old Frenchman, AIDS patient (stage IV). Suffered around 2 months earlier of a haematoma of the left arm after a fall. | Subcutaneous and muscular tissues invasion of the left arm with extension to the pectoral region. A surgical intervention revealed multiple larval forms. |
Morphology. Ovoid (4 mm × 2 mm), transparent vesicles of different developmental stages, some containing one scolex with 4 suckers and 2 rows of rostellar hooks, some with external buds at the opposite site of the scolex. DNA analysis. Nd. |
| Case 4 Chuck et al. (1997) |
38-year-old USA woman, (immune status not mentioned) with blurred vision in the right eye that had persisted for 4 weeks. The patient played with a 6-month-old German shepherd shortly before the dog passed a tapeworm. | Dilated fundus examination revealed a large, elevated, clear, fluid filled subretinal mass with several oval cystic structures of varying sizes. |
Morphology. One of the cysts contained a mature scolex with characteristic hooks. Budding on the distal part of the cysticerci was indicative for T. crassiceps. DNA analysis. Nd. |
| Case 5 Francois et al. (1998) |
38-year-old Frenchman with severe AIDS. He denied using intravenous drugs. Two months before admission he noticed a rapid progressive swelling in the right arm and forearm. The patient was in close contact with dogs and frequently walked in the forests of Normandy and Jura. | MRI showed a mass, suggesting a soft tissue neoplasm with numerous, heterogenous, invasive cystlike lesions. Surgery revealed lesions “containing a yellowish viscous fluid, hundreds of granules, and cysticerci like small vesicles”. |
Morphology. invaginated scolex with two rows of small and large rostellar hooks, endogenous budding of the cysticerci. DNA analysis. Nd. |
| Case 6 Maillard et al. (1998) |
34-year-old Frenchman, AIDS patient (C3) clinical disease, developed a traumatic haematoma on the left arm after a fall in the countryside. One month later, the swelling spread to the left pectoral region. | US and MRI showed a dissociation of muscular fibres. An incision on the left arm produced “a fluid that contained many transparent, spherical masses, 1–3 mm in diameter”. |
Morphology. Microscopic examination revealed parasites compatible with larvae of T. crassiceps with single or multiple external buds at the opposite side of the scolex. DNA analysis. Nd. |
| Case 7 Heldwein et al. (2006) |
82-year-old German woman, with a history of colon cancer with hemicolectomy and a B cell non-Hodgkin's lymphoma treated with fludarabine phosphate and cyclophosphamide two months before admission. She had also undergone radiotherapy of retrocardiac and iliac lymph nodes. Progressive pain and swelling in the left forearm and back of the left hand had started six weeks earlier after a fall on the hand with soft tissue injury. | US and MRI of the limb showed massive oedema of subcutaneous tissue and in between muscles and tendons and multiple cystic lesions were demonstrated. At fasciotomy jelly-like tissue containing multiple spherical masses with diameters of up to 2 mm, “similar to fish spawn”, was removed from the subcutis, muscles, and tendons. |
Morphology. Cysticerci with an ellipsoid cystic body, a long and retractable neck, and a single scolex with four suckers and two rows of hooklets. Additionally, buds at the posterior end of the larvae were observed. DNA analysis. Nd. |
| Case 8 Goesseringer et al. (2011); Flammer Anikpeh et al. (2014) |
47-year-old Swiss woman, severely immunodeficient (HIV-1 RNA viral load of >4 million copies/mL). Suffered from an injury to her right wrist during her work as a zoo-employee 5 months earlier. Exposed to dogs and foxes. | Swollen and painful right forearm for 2 weeks with clinical presentation and MRI suggestive for a necrotising fasciitis. “Surgical exploration revealed small transparent cystic bodies resembling white caviar”. |
Morphology. Small (4–5 mm) transparent cystic bodies with a retractable neck and a single scolex with four suckers and two rows of hooklets as well as budding were indicative for T. crassiceps. DNA analysis.12S [P6]:“Sequence analysis of a fragment of the small subunit rRNA gene1 permitted species identification” (but this was not substantiated). |
| Case 9 Schmid et al. (2014) |
57-year-old Swiss woman, immunocompetent (serological testing for HIV negative) and other immunological parameters were inconspicuous, presented with swelling and an 8-cm haematoma localized on the right temple. She had no history of a traumatic incident. | Sonography revealeds a hypoechogenic lesion within the temporal muscle. A small amount of whitish material was aspirated. |
Morphology. Smears revealed a putative tapeworm larva and accumulation of eosinophilic granulocytes (Papanicolaou stains). DNA analysis.12S [P6] sequence analysis tentatively identified the parasite as T. crassiceps. nad1 [P3]: Identity 421/421 bp or 100% with T. crassiceps nad1, (GenBank AF216699). |
| Case 10 Ntoukas et al. (2013) |
51-year-old immunocompetent German (Regensburg, southern Germany) woman, was hospitalized with progressive headache, nausea, vomiting and cerebellar ataxia but no further neurologic deficits. She had been living with her dog (not regularly dewormed, with free access to garden and forest) in a rural area for many years. |
Craniotomy revealed subdural and intracerebellar jelly-like tumorous tissue (≈30 × 30 mm). The tumor consisted of multiple spherical masses with diameters of 2–4 mm, which was resected. |
Morphology. Gross morphology and histology revealed typical structures of cestode larvae. DNA analysis.12s [P5]: Identity 99% with T. crassiceps and cox1 [P1]: identity 450/450 bp or 100%, with T. crassiceps. |
| Case 11 (Ronald Neafie, pers. comm.)b |
US Patient (Oregon) without immunosuppression | Site of infection: Eye (subretinal) | No details given |
| Case 12 (Ronald Neafie, pers. comm.)b |
US Patient (Maine) without immunosuppression | Site of infection: Subcutis, shoulder | No details given |
Primer pairs [P] used are given in squared brackets and refer to Table 6.
Cited in Ntoukas et al. (2013).
Table 3.
Cases of Taenia martis, Versteria sp. (zoonotic genotype) and non-specified cysticercoses in humans.
| Taeniid species, cases, citations | Patient, immune status and case history | Pathological findings | Etiological diagnosis: morphology and or DNA analysis (PCRa), sequencing) |
|---|---|---|---|
|
Taenia martis Case 1, Eberwein et al. (2013) |
43-year-old immunocompetent German woman (Freiburg im Breisgau), with mobile subretinal tumor with adjacent intraretinal and subhyaloid bleeding. | The removed cyst (after 8 days of albendazole/dexamethason therapy) showed the characteristic macroscopic and histologic features of a cysticercus bladder wall. | DNA Analysis. cox1 [P1], nd1 [P4], 12S [P8]; all sequences showed highest identity with T. martis (99%–100%). |
|
T. martis Case 2, Brunet et al. (2015) |
44-year-old immunocompetent French woman (Alsace), with suspected meningoencephalitis. | Thick-walled parasitic cyst with dense fibrosis and intense mononuclear inflammation contained dense fluid consisting of thick bright eosinophilic ribbons of membranous tissue and calcareous corpuscles. | DNA analysis. cox1 [P1], (396/396 bp or 100% with T. martis EU544557), nd1 [P3] (488/188 bp or 100% with EU544607) and 12S [P6] (263/265 bp or 99.2% with JX415820). |
|
T. martis Case 3, Koch et al. (2016) |
70-year-old immunocompetent woman from northern Germany; visual acuity of her left eye dropped significantly. | Intraocular inflammation and vitreous haemorrhage without fundus view in the affected eye. On US, a retinal detachment was disclosed and at surgery a moving larval parasite was extracted from the eye. |
Morphology. Macroscopically, the 3 mm long semi-translucent parasite had the appearance of a cestode larva. Tissue sections showed a characteristic tapeworm tegument. DNA analysis. Identities for cox1 [P1], nd1 [p4], 12S [P8] were 100%, 99%, and 99%, respectively with T. martis sequences. |
|
T. martis Case 4, Rudelius et al. (2017) |
36-year-old immunocompetent woman (Germany); recurring, asymptomatic ascites, which progressed slightly over a period of 8 weeks | Histopathology assessment revealed a tumor mass with central necrosis and aggregates of epitheloid cells with intermingled multinucleated giant cells of Langhans type in the periphery. |
Morphology. A larva could be detected with suckers, a two-layered tegmentum and calcified corpuscles. DNA analysis. 12S [P5]: Identitiy 99% with T. martis (ENA LT837855). |
|
Versteria sp. (zoonotic genotype) Case 1, Connor et al. (1976); Olson et al. (2003) |
58-year-old man from Pennsylvania (USA) with Hodgkin disease died after repeated courses of chemotherapy and radiotherapy. | Disseminated cyst like structures throughout the viscera, blood vessels, lymph nodes, and subcutaneous tissues were observed. |
Morphology. Not conclusive. DNA analysis. Our recent re-evaluation of the 183-bp sequence of the 18S rRNA gene including the V2 region published by Olson et al. (2003) (GenBank AY193876), showed an identity of 95% (172/180 bp, 3 gaps) with the corresponding sequence of V. mustelae (GenBank AB731633), and the sequence had a 99.7% identity with the amplified sequence of the same gene of patient 2 (GenBank MK641670). |
|
Versteria sp. (zoonotic genotype) Case 2, Barkati et al. (2018) |
53-year-old female from rural New Brunswick (Atlantic Canada) with a 3-day history of fever, productive cough, myalgia, malaise, and anorexia. Her past medical history included an obstructive nephropathy necessitating a kidney transplant with immunosuppression with tacrolimus, mycophenolate mofetil, and prednisone. | “The patient presented with severe pulmonary and systemic symptoms”. CT revealed mixed alveolar opacities and “a large heterogeneous central hepatic lesion (19.3 × 15 × 8.7 cm) abutting the middle hepatic and left portal veins, with multiple satellite nodules”. |
Morphology. Histopathology revealed cestode larvae with parts of a rostellum and 2 refractile hooklets without species diagnosis. DNA analysis. Needle biopsies of the liver and formalin-fixed paraffin- embedded liver tissue were used for DNA analyses. 12S [P6]: Identity 98% with a sequence from a Versteria sp. isolate (GenBank KF303341) from a captive orangutan. |
|
Versteria sp. (zoonotic genotype) Case 3, Lehman et al. (2018) |
68-year-old North American woman with hypogammaglobulinemia and previously treated lymphoma presented with fever and abdominal pain. | CT revealed numerous nodules in the lung, eye, brain, and liver. An open liver biopsy revealed “numerous nodular lesions and a mass made up of multifocal coalescing cystic lesions”. Histopathology revealed 3-layered lesions a bladder wall and calcareous corpuscles in a matrix of granulomatous tissue inflammation with areas of necrosis. |
Morphology. Suggestive of metacestodes DNA analysis. cox1 [primer not specified]: Identity 98% (of 129 bp) with a sequence of Versteria sp. (GenBank KT223034). |
|
Taenia sp. Ocular cysticercosis, Mougeot et al. (1996) |
14-year-old (probably immunocompetent) man from Auvergne (Central France), was presented with acute ocular pain, haemorrhage of the conjunctiva and fever. The boy grew up in a rural environment with a pet dog and vegetable garden. | A “parasite“ was observed in the internal chamber and surgically resected. |
Morphology. A 6 mm long cysticercus was diagnosed by parasitologists, but the morphology did not allow species identification. DNA analysis. Nd. |
|
Taenia sp. Ocular cysticercosis, Arocker-Mettinger et al. (1992) |
15-year-old, Austrian, immune-competent woman presented with an iridocyclitis in the right eye. She had close contact to the young family dog. | A living parasite stage could be isolated from the anterior chamber. |
Morphology. Contractable parasite stage of 2 mm length without characteristic structures such as a scolex. A putative T. crassiceps diagnosis was based on antibody reactions against T. crassiceps larval antigens in Western blotsb DNA analysis. Nd. |
Nd: Not done or no data given.
Primer pairs used are given in squared brackets and refer to Table 6.
Analysis could not consider T. martis and other rare cysticercoses.
Table 4.
Taenia spp. and Vesrsteria sp. cysticercoses and Taenia spp. coenuroses in non-human primates.
| Taenia sp., case no., citation | Primate species, patient, origin, and case history | Pathological findings | Etiological diagnosis: morphology and DNA analysis (PCR, sequencing)a |
|---|---|---|---|
|
Taenia crassiceps Case 1, Baer and Scheidegger (1946) |
Hamadryas baboon (Papio hamadryas) “no individual data available”, lived in a group in the Zoo Basel, Switzerland, together with a couple of foxes.The animal was necropsied after a history of tetraplegia. | Necropsy. Larval stages subcutaneously and in the smooth muscles, penetrating to the retroperitoneal cavity, with parasite infiltration into the spinal cord. |
Morphology. Diagnosis based on number (n = 30–35) and size (large 182–200, small 138 < 150 μm) of rostellar hooks and budding of the cysticerci. DNA analysis. Nd. |
|
T. crassiceps Case 2, Dyer and Greve (1998) |
Adult female black lemur (Eulemur macaco macaco) with a clinical history of lethargy, anorexia, and depression of a week's duration was submitted for necropsy. The animal was housed outdoors in a group. | Necropsy. Fluctuant swelling measuring 10 cm by 6 cm on the left back. A well-defined cystic structure contained hundreds of ellipsoidal to spherical cysts of 1–4 mm in diameter. Infiltration of both the peritoneal and pleural cavities by large numbers of cysticerci were found replacing approximately 90% of the left lung. |
Morphology. Cysticerci with calcareous corpuscles, a scolex with a rostellum with hooks, spinous tegument, and exogenous budding. DNA analysis. Nd. |
|
T. crassiceps Case 3, Young et al. (2000) |
6-year-old female red ruffed lemur (Varecia variegata rubra) with 2 days history of lethargy and anorexia was presented with a large, fluctuant subcutaneous swelling extending from the dorsal aspect of the skull to the ventral cervical region. | Radiographs of the cervical region showed the mass containing discrete areas of mineralization. Surgical exploration “revealed a multiloculated mass with each individual cyst-like structure containing hundreds of bead-like nodules (<1 mm)”. |
Morphology. A wet-prep of the nodules revealed cestode larvae. Histopathology. A single mass was described as granulomatous cellulitis with intralesional larval cestodes. DNA analysis. Nd. |
|
T. crassiceps Case 4, Luzón et al. (2010) |
5-year-old male, ring-tailed lemur (Lemur catta) in the Madrid Zoo-Aquarium, Spain, presented a large swelling in its abdomen (15 cm in length × 10 cm in width × 4 cm in height) and was anesthetized for a complete medical evaluation. | US image of the swelling suggested a septated subcutaneous mass. Exploratory surgery revealed an irregular fibrous cystic structure, containing numerous small transparent vesicles (ca. 3 mm in diameter) and after the peritoneal cavity was opened more vesicles were extirpated. |
Morphology. Based on the scolex and hooks and budding was observed. DNA analysis. cox1 [P1] and nd1 [P3]: Identities 99–100% with corresponding published T. crassiceps sequences. |
|
T. crassiceps Case 5, Alić et al. (2017) |
15-year-old female ring-tailed lemur (L. catta) from Sarajevo Zoo, Bosnia and Herzegovinawas was presented after sudden death | Necropsy. “large multicystic structure, subdivided with fibrous septa and filled with numerous translucent, oval to ellipsoid bladder-like cysts, almost completely replacing right lung lobe “. |
Morphology. Cysticerci with single inverted scolex, a rostelum with two rows of hooks and exogenous buds. DNA analysis. cox1 [P2]: Identity 880/880bp, 100% with T. crassiceps (GenBank AF216699, AB033411). |
|
T. crassiceps Case 6, Bleyer et al. (2018) |
28-year-old female, captive-born Nilgiri langur (Semnopithecus johnii) from a German zoo, with a right ankle joint age-related myxosarcoma developed an edematous swelling of the left thigh and also suffered from lethargy and anorexia. Animal was euthanized because of poor general condition. | Necropsy. Skeletal muscle of the left thigh had been replaced by a multilocular cystic mass containing numerous sand-grain–sized whitish structures; similar cysts were also present in the lung and the myocardium. |
Morphology. Based on hook morphology. DNA analysis. cox1 [P1]: Identities 98–100% (450 bp) with T. crassiceps sequences. |
|
Taenia serialis Case 1, Schwartz (1927) |
Gelada baboon (Theropithecus gelada) from the National Zoological Park at Washington, D. C, with a large subcutaneous tumor in the right thoracic region. | Necropsy. Large subcutaneous tumor (1.5 kg). The majority of the mature cysts contained several hundred scoleces. |
Morphology based on larval hooks and of adult worms from experimentally infected dogs. DNA analysis. Nd. |
|
T. serialis Case 2, Sandground (1937) |
Juvenile (<2 years) spotted-nose monkey (Cercopithecus nictitans) imported from the West African Coast to the USA as a pet animal. Slight torticollis for 3–4 weeks, soft swelling at the back of the head. Died. | Necropsy.Many discolored bodies (1–2 cm) attached to mesentery. Scalp with bone erosion and protrusion of a mass of translucent, white cysts. |
Morphology. Microscopical examination corresponds to Coenurus serialis. DNA analysis. Nd. |
|
T. serialis Case 3, Elek and Finkelstein (1939) |
4-year-old gelada baboon (T. gelada), borne in captivity in Germany and imported to USA (zoological park). Spastic paralysis in right lower extremity before death. | Necropsy. Multiple subcutaneous nodules in upper and lower extremities (1–12 cm), and a large intra-abdominal cystic mass infiltrating underlying muscles. |
Morphology. Diagnosis of cysts (no microscopic details provided). DNA analysis. Nd. |
|
T. serialis Case 4, Clark (1969) |
Male gelada baboon (T. gelada), transported between two zoological parks in the USA. Nodular enlargement on right thigh. Euthanized because surgery was not successful. | Necropsy. Subcutaneous nodular enlargement (11–25 cm) with small ulcerations. |
Morphology. Diagnosis of cysts (no microscopic details provided). Infection of 2 dogs was not successful. DNA analysis. Nd. |
|
T. serialis Case 5, Schneider-Crease et al. (2013) |
13-year-old male, wild Ethiopian gelada baboon (T. gelada) from a national park. Tissue was extracted from a protuberant coenurus on the left ventral forelimb. | Small to large swellings in various parts of the animal's body. |
Morphology. Not reported. DNA analysis.Its2 [P9] and 12S [P5]: 99% and 99% identity with T. serialis sequences. |
|
T. multiceps (probably misdiagnosed) Leith and Satterfield (1974) |
6-year-old female gelada baboon (T. gelada) from a zoological park in the USA. Animal, which was imported from Ethiopia with 2 years, presented multiple subcutaenous cysts. | Necropsy. Multilocular easily ruptured cystic masses in the left masseter and temporal muscle region. Further subcutaneous cysts in the scapular region extending into the abdomen. |
Morphology. Size, number, and shape of hooklets provided by the author, but inconclusive according to Table 5. DNA analysis. Nd. |
|
T. braunib Railliet and Marullaz (1919) |
Rhesus monkey (Macaca mulatta), kept in France but unknown origin, infected with Leishmania tropica, died in experiment. | Necropsy. Abdominal tumor at left perineum (2.7–5 cm). |
Morphology. Microscopical measurements performed.b DNA analysis. Nd. |
|
Taenia brauni Fain (1956) |
Monkey (Cercopithecus mitis doggetti), Rwanda | Cysts in brain (parietal lobe; 2 cm), heart (apex; 1 cm), and parotid gland |
Morphology. Performed but no details provided by the author. DNA analysis. Nd. |
|
Taenia sp. coenurosis Lau et al. (1973) |
Whitehanded gibbon (Hylobates lar) at zoological park in the USA. Left eye progressing gradually for 3–4 weeks. Retrobulbar mass at radiography. Euthanasia. | Cyst behind the left eyeball. |
Morphology. Size and shape analysis of hooklets inconclusive. DNA analysis. Nd. |
|
Taenia martis De Liberato et al. (2014) |
18-year-old male ring-tailed lemur (L. catta), immunocompetent, lives in a zoo in Rome (Italy). Apathy, loss of appetite, abdominal distension and diarrhoea were observed 10 days before death. |
Severe exudative fibrinous-purulent peritonitis with numerous adhesions between the abdominal wall and the bowel loops. After intestine removal, two free and viable, 4 cm long, whitish, leaf-like parasitic forms were pinpointed. |
Morphology. Macroscopic examination of the two parasites allowed their identification as larval stages of cestodes. DNA analysis. cox1 [P1]: Identity 98% with T. martis sequences. |
|
T. martis Brunet et al. (2014) |
3-years-old subadult mail tonkean macaque (Macaca tonkeana), immunocompetent, born and raised at Strasbourg University Centre of Primatology, France. | Abdominal mass (±10 cm × 5 cm) was detected at palpation without clinical signs. |
Morphology. Cysticerci and rostellar hooks were most close to the T. martis description. DNA analysis. cox1 [P1]: Identity 382/383 bp or 99.7% with T. martis sequence (GenBank AB731758). nd1 [P3]: Identity 438/439 or 99.8% with EU544606. |
|
Versteria sp. Goldberg et al. (2014) |
Juvenile (sex?) Bornean orangutan (Pongo pygmaeus), captive born in Colorado, USA, (no indications of immunosuppression). Loss of appetite and intermittent, moist cough; became increasingly lethargic and was found dead after 2 days. | Necropsy. Diffuse hemorrhages in the lungs, splenomegaly, a pale mottled liver, and thoracic and pericardial effusions. Histopathology of the liver revealed cystic structures containing eukaryotic parasite cells. Cause of death: “acute respiratory distress due to disseminated infection with an unknown parasite”. |
Morphology. Not conclusive. DNA analysis. Deep sequencing identified a Taenia spp. 12S [P7] and cox1/nd1 [P1/P3] phylogenetic trees placed the organisme within Versteria sp. (Cestoda: Taeniidae). |
|
Taenia hydatigena Hobbs et al. (2003) |
5-year-old male rhesus macaque (M. mulatta), born and raised in a primate colony in China and imported 15 months earlier to the Oregon National Primate Research Centre. An abdominal mass was discovered during routine physical examination. | Necropsy. A pale yellow cyst attached to the greater omentum containing 500 ml of flocculent yellow fluid. |
Morphology. Rostellar hooks, the large size of the cysticercus, and its location within the peritoneal cavity were consistent with the diagnosis of a Taenia hydatigena cysticercosis. DNA analysis. Nd. |
|
T. hydatigena Tsubota et al. (2009) |
5-years-old male long-tailed macaque (Macaca fascicularis), born and raised in a primate research colony in China in a toxicity study in Osaka (Japan). The monkey showed no clinical signs and was sacrificed at the end of a dosing experiment. |
Necropsy. A yellow cyst filled with more than 100 ml of pale yellow fluid was found in the abdominal cavity. |
Morphology. A cysticercus with a well developed scolex was found in the cyst. DNA analysis. nd1 [P3]: Identities of 96.7%–98.5% with T. hydatigena sequences. |
|
Taenia sp. Wolff et al. (1989) |
Captive-born 1-year-old male red ruffed lemur (V. variegata rubra) with unremarkable routine quarantine examination with radiography (zoo, USA). | Necropsy. Extrapleural calcified larval cestode in the left ventro-caudal thorax and pulmonary nodule with a cysticercus in the left dorso-caudal lung. |
Morphology. C ysticercus with armed scolex; species identification not done. DNA analysis. Nd. |
Nd: Not done or no data given.
Primer pairs used are given in squared brackets and refer to Table 6.
Diagnosed as Multiceps ramosus or M. lemuris by the author, which are considered to be a synonym for T. brauni (Loos-Frank, 2000).
Primates are dead-end intermediate hosts for canid-transmitted taeniid species but seem to be susceptible to some degree to the development of metacestodes (larval stages) after accidental infection with eggs. The epidemiology of these rare zoonoses is not well understood. Their apparently low incidences in humans might be attributed to a very low infection pressure or to a relatively high resistance of humans against these parasites. For example, most cases of subcutaneous T. crassiceps cysticercosis as well as all cases of fatal Versteria sp. infections have been described in severely immunodeficient patients (Table 2). Interestingly, humans have been considered highly resistant against AE, which would explain the relatively low incidences in areas with high environmental contamination with E. multilocularis eggs (Gottstein et al., 2015). However, in patients with impaired immunological status due to AIDS (Sailer et al., 1997), cancer and drug-based immunosuppression (Chauchet et al., 2014), severe progression of AE may occur (Vuitton et al., 2015).
Based on a relatively high number of cases in relation to the small population sizes, non-human primates in zoos seem to be highly susceptible for metacestode infections. Therefore, these primates might be considered as sentinels for the zoonotic potential of cestode parasite species. Indeed, mesocestoides infections have been detected in dead-end hosts such as zoo primates (Hubbard et al., 1993; Tokiwa et al., 2014; Montalbano Di Filippo et al., 2018) and in humans (Fuentes et al., 2004). However, how humans and primates get infected is not clear, and the taxonomy of Mesocestoides spp. from domestic and wild carnivores is under investigation (Varcasia et al., 2015a). Furthermore, E. equinus (G4), initially not considered to be zoonotic, was long known to be highly prevalent in horses as intermediate and in foxhounds as definitive hosts in Great Britain. In 2012, CE caused by E. equinus was identified in lemurs (Varecia rubra, V. variegatarubra) living in a British zoo (Boufana et al., 2012; Denk et al., 2016) and in 2018, the first human CE case caused by E. equinus has been discovered in Uzbekistan (Timbur et al., 2018). Other examples of T. crassiceps, T. martis, and Versteria sp., T. multiceps and T. serialis infections in humans and other primates will be documented in more detail in this review (Table 2, Table 3, Table 4 and supplemental material).
Genetic analyses of specified gene sequences allow for a reliable species differentiation, even if only small amounts of parasite tissue are available. The detection of T. martis and Versteria sp. cysticercosis in humans and other primates can especially be attributed to the new molecular diagnostic strategies, and probably does not represent an emergence of these zoonoses.
Furthermore, the zoonotic potential of several other Taenia spp. (e.g. T. ovis, T. hydatigena or T. taeniaeformis) has been claimed in textbooks based on few observations. However, for T. hydatigena, two well documented cases of Cysticercus tenuicollis abdominal cysticercosis with large cysts have been described in non-human primates (Table 4). As an example, intestinal T. taeniaeformis infections are mostly found in felids, rarely also in red foxes (Table 1). Historically, a human intestinal infection with a Taenia sp. named T. infantis has been reported in a 5-year-old boy from Buenos Aires by Bacigalupo (1922). However, Joyeux and Baer (1929) later showed that T. infantis was a synonym of T. taeniaeformis. Furthermore, the infections of a 55 years old woman and several children from Japan were attributed to T. taeniaeformis (cited in Sterba and Barus, 1976). The intermediate host stage of T. taeniaeformis, Strobilocercus fasciolaris, infects a large range of rodents and rabbits (Table 1), but there is at least one well documented record of T. taeniaeformis metacestode infection of the liver in a 77-year-old man diagnosed at post-mortem examination in 1974 (Sterba and Barus, 1976). Interestingly, Sterba and Barus (1976) mentioned a Strobilocercus infection in a gibbon (Hylobates leuciscus). However, considering the worldwide distribution of T. taeniaeformis, we can conclude that this species has a neglectable zoonotic potential and should not be listed as a zoonotic agent.
Similarly, coenuroses caused by T. multiceps, T. serialis, T. brauni, and T. glomeratus have been described as serious zoonoses for decades (Supplemental Table 1). For T. multiceps, the parasite lifecycle is mainly maintained in a domestic cycle including dogs and farm ruminants (Abera et al., 2016). Nevertheless, wild carnivores such as foxes and wolves have been demonstrated to be responsible for the dissemination of tapeworm eggs (Varcasia et al., 2015b; Otranto et al., 2015b). In contrast, for T. serialis, the cycle is mainly based on wildlife with wild canids and herbivores (primarily lagomorphs) as natural hosts. In Africa, two more Taenia spp. of wildlife origin have been described causing coenurosis with zoonotic potential (T. brauni and T. glomeratus) (Morel, 1959), to which we refer as ‘African-type coenurosis’ in this review. However, based on their appearance, these species might be variants/strains of T. serialis (no molecular data is available). Over the last century, several cases of coenurosis have been described in humans and other primates. Clincially, T. multiceps coenurosis in humans caused severe central nervous symptoms and ended fatally in 42% of the collected case reports (Supplemental Table 1). In contrast, coenurosis by T. serialis and the ‘African-type’ coenuroses have a tropism to subcutaneous and muscle tissues, and the clinical outcome is favorable. Taking account of the high prevalence of these parasites globally and the low number of cases published, the zoonotic risk of coenuroses has to be considered minimal, irrespective of potential severe consequences.
In this review we focus on non-T. solium cysticercoses and on coenuroses that are transmitted through wild carnivores (mustelids, canids, and exceptionally felids), exemplify the habitual infection biology of involved species, refer to their zoonotic potential and discuss “state-of-the-art” diagnostic strategies. The herein reported information was retrieved from full texts by comprehensive database searches (PubMed and Google scholar). Extensive screening of reference lists for historical and missing publications was accomplished.
2. Mustelid-transmitted cysticercoses
2.1. Taenia martis cysticercosis
Taenia martis is a large tapeworm of around 20 cm in length that develops in the small intestine of wild carnivores, mainly Mustelidae. In North America, T. martis americana has been described in Martes americanum (Hoberg et al., 1990). In Europe, it was described in the stone marten (Martes foina) and in the pine marten (M. martes). However, other mustelids and rarely canids and felids might also act as definitive hosts (Table 1). Taenia martis is transmitted in a wild-animal cycle between mustelid carnivores and their pray, including rodents and other small mammals as intermediate hosts. In the intermediate hosts the larval form (cysticercus) reaches a size of 6–32 mm (more details see diagnostic section). It mainly develops in the pleural and peritoneal cavity as a pseudo segmented larva, not surrounded by a fibrous capsule, and without asexual multiplication (Schuster and Benitz, 1992).
In Europe, T. martis has been observed in martens in Italy (Millan et al., 2001), Belgium (Mathy et al., 2009) Poland (Kornaś et al., 2013) and Belarus where it has also been detected in the European polecats (Shimalov, 2010). In Southwestern Germany, T. martis was found in 1 (0.03%) of 3573 red foxes, in 2 (2.2%) of 84 badgers (Meles meles) and in 17 (37%) of 47 stone martens (Loos-Frank and Zeyhle, 1982). In southwestern Yakutia (Sibiria) between 1981 and 1987, T. martis was found in 28% of 272 and 30% of 1548 Martes zibellina, respectively (Sedalischev and Odnokurtsev, 2011; Odnokurtsev and Sedalischev, 2011).
Taenia martis cysticercosis in rodents seems to occur all over Europe but focally in variable prevalence. As an example in Eastern Switzerland, T. martis larval stages (cysticerci) were detected in 43 (10.5%) of 411 Apodemus flavicollis, in 100 (7.8%) of 1276 A. sylvaticus and in 294 (24.3%) of 1211 Myodes (syn. Clethrionomys) glareolus, but not in 894 Arvicola terrestris, 347 Microtus arvalis and 250 M. agrestis of the same area (Schaerer, 1987). In contrast, in Western Switzerland (Geneva) T. martis was found in 2 of 99 A. flavicollis, but not in 466 A. terrestris, 58 M. glareolus and 35 M. arvalis (Reperant et al., 2009). Comparable low prevalence of T. martis were found in Berlin, Germany (1.4% of 59 M. glareolus, 78 Apodemus agrarius and 82 A. flavicollis) (Krücken et al., 2017), and in Western France in nutria (Myocastor coypus) (Umhang et al., 2013). In Germany, muskrats (Ondatra zibethicus) were highly infected with T. martis cysticerci (Loos-Frank and Zeyhle, 1981), similar to the Limburg region in the Netherlands (18.6% of 526 muskrats), but in contrast to animals from Groningen (n = 1200) with a prevalence of merely 0.7% (Borgsteede et al., 2003). From Ireland, Loxton et al. (2017) reported a prevalence of T. martis of 0.77% (CI: 0·16–2·24) in wood mice (A. sylvaticus). Reports from northern Spain document the occurrence of T. martis in 0.7–1.0% of 376 M. glareolus (Ribas et al., 2009). In Serbia 4.1% of 588 M. glareolus were infected with T. martis (Bjelić-Čabrilo et al., 2011). In Belarus, the red-backed vole, the striped field mouse and the yellow-necked mouse are hosts of this helminth (Shimalov, 2010), and recently it was documented there as a parasite of red squirrels (Sciurus vulgaris Linnaeus, 1758) (Shimalov, 2016).
To our knowledge, four cases of human T. martis cysticercosis have been reported in immunocompetent women (Table 3). Interestingly, three of these patients originated from Alsace (France) and Western Germany, while the remaining fourth case was described in northern Germany. In these cases, cysticercosis manifested itself as a solitary lesion in the brain, in the peritoneum and in two cases in the eye. Furthermore, two cases in non-human primates, one originating from Alsace, the other from a zoo in Rome (Italy), presented as abdominal T. martis-cysticercosis (Table 4). Finally, in a 14-year-old Frenchman and a 15-year-old Austrian woman, ocular cysticercoses caused by juvenile taeniid larvae could not be morphologically specified (Table 3). Similarly, a cysticercosis with fully developed cysticerci isolated from the abdomen of a lemur from a zoo in the USA in 1989 was diagnosed as Taenia sp. cysticercosis (Table 4).
Routes of human infections can only be speculated about. Two patients were recreational gardeners and could have been exposed to marten faeces during such activities. Extensive recreational hiking in the Alps was reported from another patient but without obvious contact to wild animals. All four patients could successfully be treated by surgery and anthelminthics.
Interestingly, all T. martis-cysticercosis patients described so far originated from Europe, and no data are available on the zoonotic potential of T. martis americana. Furthemore, all four human T. martis cases were documented in 36, 43, 44 and 70 years old women, though the number of cases is too small to speculate on a gender association. However, the host's endocrine system can have an impact on the susceptibility to cestode infections. For example, castration of male and pregnancy in female pigs significantly increased the prevalence of naturally acquired T. solium cysticercosis (Morales et al., 2002), female mice are more susceptible to experimental infections with T. crassiceps than males (Morales-Montor et al., 2002), and female rabbits are more frequently infected with T. pisiformis metacestodes than males (Domínguez-Roldan et al., 2018).
2.2. Versteria mustelae and Versteria sp. cysticercosis
Versteria mustelae (new genus created by Nakao et al., 2013; syn. Taenia mustelae) is an up to 10 cm long tapeworm of mustelids with a wide range of rodents as intermediate hosts, bearing in the liver the rather small, ovoid, 0.4–2.0 mm large cysticerci containing many calcerous bodies (Freeman, 1956; Slais, 1973). In Europe, weasel (Mustela sp.), stone marten (Martes foina) and pine marten (M. martes), and in the USA American pine marten (Martes americana), ermine (Mustela ermine) and mink (Neovison vison) have been described as definitive hosts (Lee et al., 2016, Hoberg et al., 1990). Between 1981 and 1987, V. mustelae was identified in 2.9% of 272 Martes zibellina in Southwestern Yakutia (Sibiria) (Sedalischev and Odnokurtsev, 2011).
A variety of rodents have been identified as intermediate hosts of V. mustelae in Europe and Asia. In eastern Switzerland, V. mustelae was fond in very low prevalences in 0.1% of 1276 A. sylvaticus, in 0.1% of 1211 M. glareolus, in 0.3% of 347 M. arvalis and in 0.4% of 250 M. agrestis, but neither in 411 A. flavicollis nor in 894 A. terrestris (Schaerer, 1987). In Zealand (Denmark), 14 of 46 M. glareolus were infected with V. mustelae (Tenora et al., 1991), and this species was found in 9.3% of 172 M. glareolus trapped in rural forest habitats and in 0.4% of 231 animals from urban forests and parks but not in 41 and 129 A. flavicollis, respectively (Al-Sabi et al., 2015). In this study, prevalences were determined by molecular analyses with considerably higher sensitivities as compared to morphological identification. Interestingly, the authors stated that “several poorly developed cysts without specific morphology were observed”. In Sweden, V. mustelae was diagnosed by PCR in 13% of 56 A. amphibius and in 14% of 187 M. agrestis from field habitats, and in 8.4% of 655 M. glareolus, but not in 79 A. flavicollis and 206 A. sylvaticus from forest habitats (Miller et al., 2017). In Finland M. glareolus, M. rutilius, M. rufocanus, M. agrestis, M. oeconomus and A. flavicollis have been identified as intermediate hosts of V. mustelae (syn. Taenia tenuicollis) (Tenora et al., 1983). In South-Central Finland, cysts of V. mustelae were detected in 9% of 34 M. agrestis and in 27% of 117 C. glareolus in the liver in a habitat with Mustela nivalis and M. erminea as main definitive hosts (Soveri et al., 2000).
In France (Jura), V. mustelae was more frequently observed in M. glareolus (24% of 349) than in Pitymys subterraneus (5% of 75), M. agrestis (9% of 47), M. arvalis (1% of 2520) and Apodemus sp. (1% of 230) (Le Pesteur et al., 1992). In Spain, V. mustelae was reported in P. duodecimcostatus, P. lusitanicus, P. pyrenaicus, M. agrestis, M. arvalis, M. cabrerae and M. glareolus (Feliu et al., 1997). Ribas et al. (2009) documented the occurrence of T. tenuicollis (former syn. of V. mustelae, not to be confused with Cysticercus tenuicollis of T. hydatigena) in 6.7–12.6% of 376 M. glareolus caught in northern Spain.
Genetic investigations revealed that a complex of species, genotypes and genetic lineages exist within the genus Versteria in North America (Lee et al., 2016). The authors suggested the occurrence of a “western lineage” (present in Colorado, Oregon, and Idaho, and the Nordwest Territories (NWT) of Canada) and a “northern continental lineage” (presentin Wisconsin and the NWT), with sympatry in the NWT. This northern continental lineage clusters with V. mustelae from Eurasia (Finland and Siberia). The western lineage responsible for fatal infections of an orangutan and a human patient (Case 2, Table 2) also infects muskrats (Ondatra zibethicus) in Idaho, USA, and the NWT.
So far, V. mustelae genetically related to the European isolates, have not been found in primates, including humans all over the northern hemisphere. On the other hand, in North America, a “zoonotic” lineage named Versteria sp. responsible for a fatal infection of an orangutan (Table 5) and in 2018 of infections in two human patients (Table 3) seems to be genetically closely related, but distinct from V. mustelae. In this review we refer to this genotype as Versteria sp. or North American zoonotic V. mustelae variant as used by Goldberg et al., 2014; Lee et al., 2016. Furthermore, based on the description of a patient from Pennsylvania (USA) with Hodgkin disease and an undefined helminthic infection (Connor et al., 1976), Olson et al. (2003) published a 18S sequence (GenBank: AY193876) without significant homology to other sequences in the database at the time of publication. We reanalysed this sequence in 2019 and identified a homology of 95% with V. mustelae (GenBank: AB731633) of European origin. Moreover, we recently sequenced the corresponding 18S region obtained by molecular analysis of clinical material of a female patient from Atlantic Canada with a Versteria sp. infection (published by Barkati et al., 2018, Table 3), and detected a homology of 99.7% with the GenBank entry (AY193876) of the Pennsylvanian patient mentioned above. Therefore, we conclude that the patient originating from Pennsylvania (USA) (Connor et al., 1976) represents the first documented case of human Vesteria sp. cysticercosis (patient 1, Table 3).
Table 5.
Taenia and Versteria spp. of domestic and wild carnivores with zoonotic potential. Morphological characteristics (modified after Loos-Frank, 2000).
| Species | Larval type and name | Size | Asexual replication | Number of scoleces | Number of hooks | Length of large hooks (μm) |
|---|---|---|---|---|---|---|
| T. crassiceps | Cysticercus (Cysticercus longicollis) | 2–8 mm | Yes | One | 30–34 | 178–195 |
| T. martis | Cysticercus | 6–32 mm | No | One | 28–30 | 183–218 |
| T. taeniaeformis | Strobilocercus (Strobilocercus fasciolaris) | up to several cm | No | One | 24–52 | 300–530 |
| T. multiceps | Coenurus (Coenurus cerebralis) | up to several cm | No | Many | 24–32 | 157–177 |
| T. serialis | Coenurus (Coenurus serialis) | 12–34 mm | No | Many | 28–34 | 145–170 |
| T. brauni | Coenurus (Coenurus brauni) | >1 cma | No | Many | 22–30 | 85–160 |
| T. glomeratusb | Coenurus (Coenurus glomeratus) | 5.5–27.2 mm (up to 121 mm) | No | Many | 18–34 | 90–110b |
| Versteria (Syn. Taenia) mustelae | Cysticercus | 0.4–2 mm | Yesc | One | 30–74 | 14–38 |
Cysts from an ape in various parts of the body (Fain, 1956).
According to Turner and Leiper (1919); Clapham (1940a).
Observed with genetically not characterized North American isolates (Freeman, 1956).
Interestingly, North American V. mustelae isolates from Minnesota (USA) and from Algonquin Park (Canada) are capable of asexual multiplication in the intermediate host (Freeman, 1956), however it is unclear whether V. mustelae or the zoonotic Versteria sp. have been investigated. This phenomenon is distinct from the budding multiplication of T. crassiceps, and could be responsible for the systemic and invasive infections in heavily immunocompromised human patients. However, further investigations with genetically defined isolates are needed to elucidate the fascinating biology of Versteria spp. Furthermore, based on the small, not fully developed Versteria sp. cysticerci in the liver in immunocompromised patients, this infection must be considered in the differential diagnosis of alveolar echinococcosis as proposed by Barkati et al. (2018).
3. Canid-transmitted cysticercosis and coenurosis
3.1. Taenia crassiceps cysticercosis
Taenia crassiceps is a relatively large but harmless tapeworm, 10–22 cm in length, inhabiting the intestines of carnivore definitive hosts, and it is widly distributed in the northern hemisphere (Table 1). In rodents, which are the natural intermediate hosts for the parasite, the larval (metacestode) form of T. crassiceps has a particular asexual reproduction by budding both exogenously and endogenously (Baer and Scheidegger, 1946, Freeman, 1962; Slais, 1973). Exogenous budding at the abscolex pole can produce 1–6 daughter cysticerci, which can bud off or remain attached and form a scolex of their own. This continuous and uncontrolled proliferation leads to massive infections, most frequently involving the subcutis, and both pleural and peritoneal cavities, causing death of the intermediate host within several months, or serious pathological implications in a large variety of dead-end hosts, including humans and other primates. Sporadic cases of cysticercosis caused by T. crassiceps have been documented in humans (Table 2), non-human primates (Tabel 4), but also rarely in e.g. domestic dogs (Ballweber, 2009; Beugnet et al., 2009; Chermette et al., 1993; Hoberg et al., 1999), a cat (Wunschmann et al., 2003), red foxes (Konjević et al., 2016; Whipp et al., 2017) and a chinchilla (Chincilla lanigera) (Basso et al., 2014).
Natural intestinal infections with T. crassiceps have been described in the northern hemisphere mainly in the red fox (V. vulpes), but also in several other canids. A study in New Brunswick and Nova Scotia in eastern Canada in red foxes reported prevalences of 50% for T. crassiceps (Smith, 1978). T. crassiceps was also reported from Greenland (Andreassen et al., 2017), from Svalbard island (Stien et al., 2010), and from China, with 2 of 27 red foxes, and 1 of 9 Tibetan sand foxes found infected (Li et al., 2013).
In European red foxes T. crassiceps is widely distributed with prevalence varying between 4.3 and 29%, and 7.6% in foxes living in the city of Zurich, Switzerland (Hofer et al., 2000). Similar prevalence ranges have been described for Germany (17.7–28.5%, Ballek et al., 1992; Loos-Frank and Zeyhle, 1982; Pfeiffer et al., 1997; Welzel et al., 1995), France (15.9–29%, Pétavy and Deblock, 1980; Pétavy et al., 1990), Spain (4.3–23%, Alvarez et al., 1995; Segovia et al., 2004), and Lithuania (26.4%, Bruzinskaite-Schmidhalter et al., 2012). In Russia, 19% of 68 hunted red foxes were infected with T. crassiceps in central Yakutia (Sedalischev and Odnokurtsev, 2013), and 49% of 247 red foxes and 7% of 43 corsac foxes (V. corsac) of Omsk Oblast (Siberia) between 2000 and 2004 (Bukova, 2006).
Taenia crassiceps was also detected in wolves in Canada, Europe and Russia (Abuladze, 1970; Craig and Craig, 2005). In Latvia 9% of 34 (Bagrade et al., 2009), and in North-West Caucasia 2.8% of 36 wolves were infected with T. crassiceps (Itin et al., 2018). Freeman (1961) detected T. crassiceps in 1.7% of 58 Canadian wolves and in one of 6 coyote-dog hybrids, but not in 68 coyotes.
Of 17 Hungarian golden jackals (Canis aureus L., 1758), T. crassiceps has been found in 40% (Takács et al., 2014) and infections were recorded in 0.6% of 179 wolves in northern Italy (Gori et al., 2015). The raccoon dog (Nyctereutes procyonoides) seems to be another suitable host of T. crassiceps: it was identified in 6 of 72 animals from the Republic of Belarus (Subbotin, 2009), and it was found in 3.5% of 85 raccoon dogs in Lithuania (Bruzinskaite-Schmidhalter et al., 2012).
However, not only canids seem to be susceptible to T. crassiceps intestinal infections. Raccoons (Procyon lotor) were infected with T. crassiceps as reported from the North-Western Caucasus with a prevalence of 24% in 42 animals (Itin et al., 2018). Schuster et al. (1993) identified T. crassiceps tapeworms in 2 of 25 wild cats (Felis silvestris) and Loos-Frank and Zeyhle (1982) found infections in 6% of 47 stone martens (Martes foina) in Germany.
Finally, in several human patients with T. crassiceps cysticercosis, close contacts to domestic dogs were assumed as source of infection (cases 1, 4, 5, 8, 10; Table 2). However, the epidemiological importance of domestic carnivores is probably overestimated. Umhang et al. (2014) reported 5 of 817 (0.6%) dog faecal samples from eastern France to be positive for T. crassiceps eggs. Dyachenko et al. (2008) detected T. crassiceps eggs in 7 of 17,894 (0.04%) dog samples in Germany and other European countries, but in none of 9064 cat faeces. However, in an older study in southwest Germany intestinal infections with T. crassiceps were also observed in 1% of 387 stray cats supplied for rabies examination, some had been shot by private hunters and some were road kills (Loos-Frank and Zeyhle, 1982).
As intermediate hosts in northern America, the muskrat (Ondatra zibethicus), the common vole (Microtus arvalis), the eastern chipmunk (Tamias striatus), the deer mouse (Peromyscus maniculatus), the meadow vole (Microtus pennsylvanicus), but also the woodchuck (Marmota monax), and lemmings (Dicrostonyx groenlandicus richardsonii, Lemmus trimucronatus trimucronatus) were identified (Leiby and Whittaker, 1966; Albert et al., 1972). In Europe T. crassiceps cysticercosis seems to occur focally in variable prevalence in rodents. In Switzerland, T. crassiceps cysticerci were detected in 2 (0.22%) of 894 Arvicola terrestris, in 3 of 347 (0.86%) Microtus arvalis, and in 1 (0.4%) of 250 M. agrestis, but not in 411 Apodemus flavicollis, 1276 A. sylvaticus and 1211 Myodes (Syn. Clethrionomy) glareolus of the same area in eastern Switzerland (Schaerer, 1987). These very low prevalence of T. crassiceps, as well as very low prevalence of E. multilocularis (0.11%) in A. terrestris, and the absence in the other species mentioned above, were probably associated with the significantly reduced fox population during this time, which was attributed to the rabies epidemic and the corresponding control measurements. Later studies reported higher T. crassiceps prevalence, e.g., in 2.0% of 889 A. terrestris, whereas none were detected in neither 83 M. glareolus nor 154 Apodemus sp. (Stieger et al., 2002), while another study found T. crassiceps in 1.9% of 856 A. terrestris (Burlet et al., 2011) in the Zurich Area (Switzerland). In the Geneva area (Switzerland) Reperant et al. (2009) detected T. crassiceps cysticerci in 2.6% of 466 A. terrestris, in 2.9% of 35 M. arvalis, and in 1.0% of 99 A. flavicollis, but not in 58 M. glareolus. Interestingly, no T. crassiceps or T. taeniaeformis were identified in a study in Berlin (Germany), including 77 A. flavicollis, 25 A. sylvaticus, 72 A. agrarius, 56 M. glareolus, and 10 Microtus sp. (Krücken et al., 2017). Finaly, in Japan T. crassiceps was found in three of 46 M. montebelli but not in 187 Apodemus speciosus (Ihama et al., 2000).
Interestingly, no reports on the occurrence of T. crassiceps were found from Scandinavia, Ireland and the UK. No T. crassiceps infections were detected in 197 foxes from Wales (Jones and Walters, 1992a), in 843 foxes from Southern England (Richards et al., 1995), and in 366 foxes from Northern Ireland (Ross and Fairley, 1969). Furthermore, in this paper the intestinal helminths in foxes in the UK were reviewed and T. crassiceps was not mentioned in several older studies. Further, no T. crassiceps findings are mentioned in rodent investigations in Zealand, Denmark (Al-Sabi et al., 2015; Tenora et al., 1991), in 1702 rodents investigated in Sweden (Miller et al., 2016), nor in high numbers of rodents investigated in Finland (Tenora et al., 1983; Soveri et al., 2000), and Ireland (Loxton et al., 2017).
Because of the high T. crassiceps prevalence in red foxes and their high population densities, the fox seems to be responsible for the perpetuation of the parasite cycle, as well as for the contamination of the environment with eggs in Central Europe. A similar situation can be observed with E. multilocularis (Hegglin and Deplazes, 2013). However, only a few studies have documented environmental contamination with T. crassiceps eggs. For example, Hauser et al. (2015) identified T. crassiceps eggs through DNA analyses in 5 (8.6%) of 58 fox faecal samples, and in one (0.2%) of 402 dog samples collected during the course of one year in 14 different grassland areas in the canton of Zurich, Switzerland. Furthermore, T. crassiceps eggs have been identified in the washing water of one of 141 samples of food, which consisted each of around 40 heads of lettuce, as well as various vegetables and fruits (Federer et al., 2016).
A number of well documented cases of T. crassiceps cysticercoses have been published in humans and other primates (Table 2, Table 4). Most of the cases, which have all been published in the last 30 years, originated from Central Europe (Germany, Switzerland, and France). Most cases of humans involving subcutis and muscles have been associated with underlying immunosuppression (cases 2, 3, 5–8; Table 2), except case 9, where a subcutaneous infection was associated with a haematoma localized on the right temple in an immunocompetent Swiss patient, and case 12 where a subcutaneous infection on the shoulder of a patient from USA was documented (Table 2). In contrast, an intercerebellar (case 10) or intraocular infections (cases 1, 4, and 11; Table 2) were not associated with an impaired immune system.
Surprisingly, in all cases, the infection started uni-focally and progressed by infiltration of the surrounding tissues, especially in the non-ocular/neural cases. Due to the systemic spread of the activated oncospheres in the blood circulation after oral egg uptake we would expect simultaneous multifocal infections as described for T. solium (especially in severely immunocompromised patients) and for T. serialis, where in some cases multiple lesions were observed in individuals without any indication of immuno-suppression. Interestingly, among the 7 cases of subcutaneous T. crassiceps cysticercosis (Table 2), 5 had a history of precedent injuries associated with the later development of cysticercosis. In cases 2, 3, 6, 7 a haematoma after a fall was reported at the site of subsequent cysticercosis development. In case 8, the patient remembered, that 5 months before the swelling on the same arm started, an injury to her right wrist occurred during her work as a zoo-employee.
Generally, humans acquire taeniid infection by oral ingestion of infective eggs. The most probable route of transmission after contact with taeniid eggs in the contaminated environment is the hand-to-mouth route. Hypothetically, transmission has also been linked to water or food-borne sources (vegetables/fruit/berries), but any source attribution is uncertain (Alvarez-Rojas et al., 2018). Taeniid eggs can be dispersed from carnivore faeces with water or by adhering to objects (e.g. shoes and tyres). For example E. multilocularis eggs have been found on the hair coat of foxes (Nagy et al., 2011), suggesting a variety of potential infection routes to humans.
There is experimental evidence that taeniid eggs can hatch and develop further without the gastric passage. In sheep it was demonstrated that intra-tracheal E. granulosus egg inoculation was followed by cystic echinococcosis development in the lungs (Thompson, 1995). Furthermore, embryophore-free (based on sodium hypochlorite treatment) but not enzymatically activated E. multilocularis oncospheres caused local alveolar echinococcosis after subcutaneous injection in a mouse model designed for the documentation of egg viability (Federer et al., 2015). The same method using 1000 T. crassiceps eggs resulted in subcuataneous cysticercosis (confirmed by PCR/sequencing as described in Trachsel et al., 2007) in 2 of 3 inoculated BALB/c mice (Joekel D. and Deplazes P., unpublished data). Therefore, taeniid eggs accidently contaminating cutaneous injuries might locally hatch and further develop to larval stages. A human case of subcutaneous alveolar echinococcosis (Tschudi and Ammann, 1988) associated with a cutaneous injury, and a subcutaneous cystic echinococcosis in the popliteal fossa at the site of a previous wasp sting (Battyany et al., 2010), have been documented.
3.2. Coenuroses
3.2.1. Introduction
Coenurosis results from infection with metacestodes of Taenia multiceps, T. serialis, T. brauni and T. glomeratus. Canids and rarely other carnivores are the definitive hosts harbouring adult tapeworms in the intestine (Table 1). Tapeworms mature within 15–42 days, with 3–4 proglottids shed daily containing more than 35,000 eggs per proglottid (Willis and Herbert, 1984). For T. multiceps it was shown that most eggs are released already from the proglottis in the intestine of the final host (Herbert et al., 1984; Scala and Varcasia, 2006). Each of the described tapeworm species usually infects a specific range of intermediate hosts (Table 1). After spreading of the oncosphere through the blood system, a vesicle-shaped cyst develops as coenurus, containing a few to several hundred protoscolices (Lescano and Zunt, 2013; Willis and Herbert, 1984).
Classically, only T. multiceps was considered to cause CNS, spinal cord, and eye infections, while T. serialis, T. brauni and T. glomeratus distribute to soft tissue/subcutaneous connective tissue, the musculo-skeletal system, and visceral organs (Lescano and Zunt, 2013). However, a morphologically similar and genetically identical strain (based on mitochondrial genes) of T. multiceps (referred to as T. gaigeri or T. multiceps gaigeri in goats and T. skrjabini in sheep) was shown to cause non-cerebral coenurosis in goats and to a lesser extent in sheep (Christodoulopoulos et al., 2013; Schuster et al., 2010; Verster, 1969). However, for none of these strains the zoonotic potential has been documented.
Humans may be infected as dead-end hosts, with the cystic larvae usually developing in the CNS, eye, or subcutaneous or intramuscular tissues. Between 1994 and 2018, 7 cases of coenurosis in humans have been described, mainly in individuals with close contact to canids (Supplemental Table 1). Human coenurosis is much less common than cystic echinococcosis, though both causing agents have the same definitive hosts. Cases have been reported predominantly from African countries, and only a few cases have been documented in South- and North America and Europe. Patients with coenurosis usually have a space-occupying lesion caused by a single cyst of 2–6 cm in diameter. However, cysts up to 10 cm in diameter have been reported.
3.2.2. Taenia multiceps coenurosis
Taenia multiceps (formerly Multiceps multiceps) is a large tapeworm with variable size between 20 and 120 cm. Taenia multiceps coenurosis (sometimes called gid, staggers, or sturdy) has been documented in scattered foci all over the world, with the exception of Australia and New Zealand (Scala and Varcasia, 2006). In North America, however, the last case of T. multiceps gid has been reported in the thirties and the disease is considered absent, despite one supposed human case in 1982 (Becklund, 1970; Ing et al., 1998; Schellhas and Norris, 1985).
The parasite life cycle is predominantly maintained by domestic and stray dogs, when they have access to residuals from improper discarded slaughtered meat, or roaming of stray dogs around small ruminant farms and early access to animals that died in the field (Abera et al., 2016; Vasileiou et al., 2015). Global transport of domestic dogs (travel and trading) represents yet another risk for the dissemination of taeniid eggs. Correspondingly, in Switzerland where only rare and sporadic cases are noticed, an outbreak of coenurosis in a dairy sheep flock has been reported, with approx. 10% of 140 sheep presenting clinical signs. The source of infection was a single livestock guardian dog imported from Italy (Schweizer et al., 2006).
The importance of wild carnivores for the distribution of taeniid eggs is valid where wild canids (e.g. wolves and foxes) have access to animals that have died in the field infected with T. multiceps. The role of wild carnivores was neglected as it was assumed that they have no access to sheep brains protected by the thick skull. However, firstly, the presence of cysts is associated with thinner skull bones, thus allowing access, even for foxes (Scala and Varcasia, 2006). Secondly, infection trials have demonstrated that foxes can develop gravid proglottis and infective eggs (Varcasia et al., 2015b). Correspondingly, in various regions where T. multiceps coenurosis is endemic in sheep, tapeworms have been reported from fox species, with 1–28.2% positive results from necropsied foxes in Russia (summarised in Varcasia et al., 2015b), 0–5% in Germany (Ballek et al., 1992; Loos-Frank and Zeyhle, 1982; Welzel et al., 1995), 2% in Peru (Moro et al., 1998), 3.8% in Jordan (El-Shehabi et al., 1999), 4.8–8.2% in Iran (Dalimi et al., 2006; Nabavi et al., 2014), and 6% in Tibetan sand foxes in China (Li et al., 2013). An extensive survey on fox hounds and foxes in Wales revealed 0.46%–1.7% dogs infected with T. multiceps but none of the foxes (Jones and Walters, 1992a, 1992b). However, the foxes had other Taenia species, including T. serialis which is responsible for coenurosis in lagomorphs in the UK (see below). In addition, earlier publications demonstrate the presence of T. multiceps in foxes in Bulgaria, Czech Republic, Italy, Poland and Switzerland (summarised in Loos-Frank and Zeyhle, 1982).
The wolf is a known definitive host for T. multiceps and T. serialis (Craig and Craig, 2005; Otranto et al., 2015b). Data are available for European grey wolf populations with prevalences of 0.5% in Croatia (Hermosilla et al., 2017), 3.9% in Serbia (Cirovic et al., 2015a), 0–9% in Italy (Gori et al., 2015; Guberti et al., 1993; Paoletti et al., 2017), 27% in Estonia (Moks et al., 2006), 28–29.8% in Spain (Segovia et al., 2001, 2003), and up to 47.1% in Latvia (Bagrade et al., 2009). Nabavi et al. (2014) found 1 of 4 wolves from Iran positive for T. multiceps. Interestingly, golden jackals (C. aureus) from Italy as well as from the western part of Iran were negative for T. multiceps and T. serialis (Dalimi et al., 2006; Nabavi et al., 2014; Paoletti et al., 2017). In Serbia, however, T. multiceps was found in 1.6% of golden jackals , another wild carnivore of eastern Europe, the Middle East and Asia (Cirovic et al., 2015b). These tapeworm species were absent in 80 brown bears in a T. multiceps/T. serialis endemic region in central Italy (Paoletti et al., 2017). Henke et al. (2002) described T. multiceps in coyotes in Texas but this report needs to be considered with care as it could be a morphological miss-diagnosis of T. serials, and generally T. multiceps is assumed to be abset in North America.
Metacestode infections (coenurosis) have also been reported from dead-end hosts (including dogs, horses, humans and other primates). Taenia multiceps coenurosis in humans and other primates represents a serious clinical condition. Supplemental Table 1 summarizes 45 cases of human T. multiceps-coenurosis, which were from Africa (n = 21), Europe (n = 17), Asia (n = 2), South America (n = 1), USA (n = 3) and the Middle East (Israel; n = 1). Where specified, the age of the patients was between 1 and 55 years (median: 33; SD: 17.0), showing that all age groups are potentially affected. Similar to infections in ruminants, the predominant infected tissue was the brain (n = 35/44), followed by intra-/periocular infections (n = 8/44) and rarely infections in the spinal cord (n = 1/44). Correspondingly, the infection was often fatal (42%) or ended with the loss of vision after eye removal (15%). Symptoms like partial paresis or paraplegia remained (12%), and only one third of the patients recovered partially or completely after surgery. However, in spite of the severity of the outcome of an infection in humans, coenurosis represents a rather minor risk for public health, given the relatively high infection pressure demonstrated by the wide occurrence and high prevalence of T. multiceps in canids but only 5 published cases in the past 25 years. Furthermore, there are no indications of immunodeficiency in these cases. Similarly, T. multiceps is rarely reported in other primates.
3.2.3. Taenis serialis coenurosis
The adult T. serialis tapeworm has a size of 20–72 cm and is morphologically very similar to T. multiceps but can be differentiated by hook number, size, and shape (Table 5). The life cycle of T. serialis includs canids as final hosts and hares and rabbits (rarely rodents) as intermediate host (Table 1), with Coenurus serialis in subcutaneous and muscle tissues, and occasionally in abdominal cavities (Pfaffenberger and Valencia, 1988; Verster, 1969). Taenia serialis is prevalent in North America, Europe and Africa. However, there are also recent reports of T. serialis in rabbits from Iran and China (Moshiri et al., 2018; Zhang et al., 2018). Furthermore, PCR confirmed T. serialis eggs in the faeces from 2 out of 1425 dogs from rural Australia (Jenkins et al., 2014). Hence, it is not surprising that this parasite has been occasionally found in Australian ring-tailed possums (Pseudocheirus peregrinus) and in kangaroos (Macropus fulginosus) (Dunsmore and Howkins, 1968; Hough, 2000).
In contrast to T. multiceps with a predominantly domestic life cycle, wildlife plays a more prominent role in T. serialis. Surveys in the USA showed high prevalence throughout the nation, e.g. 12% (429 dogs) in Arizona and New Mexico and overall 3–29% in definitive hosts (Ing et al., 1998). With regard to intermediate hosts, 46% of 35 black-tailed jack rabbits (Lepus californicus) and 19% of a local rodent population were infected with T. serialis (Pfaffenberger and Valencia, 1988; Schantz et al., 1977). Henke et al. (2002) reported on T. multiceps in coyotes (C. latrans) from Texas but a misdiagnosis with T. serialis is probable in the absence of molecular species confirmation. Correspondingly, a recent study from Edmonton (Canada) found T. serialis in 13% out of 23 urban coyote carcasses (Luong et al., 2018). Interestingly, T. serialis was also found in arctic foxes (Vulpes lagopus) from north-east Greenland, demonstrating the widespread occurrence of this parasite in wild carnivores and the importance of a wildlife-cycle (Andreassen et al., 2017). In Europe, T. serialis occurs in regions where lagomorphs are present. In the UK, 0.3–0.6% of hunting dogs and 0.5% of 197 foxes from the same region have been found positive for T. serialis, respectively (Jones and Walters, 1992a, 1992b). Interestingly, T. multiceps was absent in the same fox population, despite being in a sheep raising region with observed cases of coenurosis. In Northern Ireland, up to 4% of the local fox population was infected with T. serialis (Ross and Fairley, 1969). On the European mainland, this cestode is rarely reported in foxes. Correspondingly, by examining large numbers of foxes from Germany, 0.15–2.3% of foxes were positive (Loos-Frank and Zeyhle, 1982; Pfeiffer et al., 1997; Welzel et al., 1995). In wolves, however, reported infection rates were 2.1% of 47 and 8% of 50 wolves in Spain (Segovia et al., 2001, 2003), 5.9% of 68 wolves in Portugal (Guerra et al., 2013), and 1% of 102 wolves in Serbia (Cirovic et al., 2015a). Hence, wolf migration might be a risk for the dissemination of T. serialis from the southern and eastern parts of Europe to new areas. In this sense, T. serialis was found in 1.1% of 447 golden jackals from Serbia, a species migrating into Central Europe (Cirovic et al., 2015b). A survey in Germany could not find adult T. serialis in 84 badgers, 47 stone marten, and 387 cats (Loos-Frank and Zeyhle, 1982).
Rare cases of coenurosis in cats caused by T. serialis, but not by T. multiceps have been reported (Hayes and Creighton, 1978; Huss et al., 1994; Orioles et al., 2014; Slocombe et al., 1989; Smith et al., 1988). Correspondingly, T. serialis-coenurosis represents a zoonosis with 11 case reports found in literature (Supplemental Table 1). The predominant location of the coenuri was subcutaneously or intramuscularly on the trunk of the patient. While cerebral infections due to T. serialis have been reported in sheep (and cats), there are no such reported cases in humans. The lesions were presented as palpable swellings, which were usually painless. Where indicated, all patients recovered after surgical removal of the lesion.
Several cases of T. serialis coenurosis in non-human primates have been reported, in contrast to T. multiceps with only one discribed case with uncertain diagnosis (Table 4). Unlike in humans, cerebral, intra-abdominal, intramuscular and subcutaneous infections have been observed. Interestingly, in a study between 1971 and 1972 in an Ethiopian national park, metacestodes of T. serialis have been identified morphologically in large visible subcutaneous swellings in 9.2% of 92 gelada baboons. In a follow-up study (1974–1975) observing the same animals, 10.6% were infected (Dunbar, 1980). Forty years later (2011), 4.8% of 291 other gelada baboons living in the same park had visible swellings, and in some of them, T. serialis was molecularly confirmed (Schneider-Crease et al., 2013). Unlike in other intermediate hosts, these visible asymmetric masses can grow very large in baboons (>16 × 20 cm) and may show ulcerations, disturbing the animals obtrusively (Schneider-Crease et al., 2013; Schwartz, 1927). Potentially, these large lesions can develop over long time periods because of a long life span and a low predation rate of the infected animals. In fact, only 0.9% of juvenile baboons presented visible swellings, as compared to 9.9% of adults (Schneider-Crease et al., 2013). In spite of an endemic infection of primates, there were no human case-reports from Ethiopia, relativizing the zoonotic risk of T. serialis. Noteworthy, in contrast to e.g. T. crassiceps cysticercosis with single subcutaenous locations, many cases of T. serialis-coenurosis show multiple lesions, indicating a peroral infection and systemic parasite dissemination.
3.2.4. ‘African-type’ coenuroses
Reported cases of soft-tissue coenuroses in Africa are considered to be caused by T. brauni and T. glomeratus (syn. T. glomerata). Taenia brauni is a tropical tapeworm of Eastern and Northern Africa, Rwanda and the Democratic Republic of Congo, with a wild animal life cycle, including dog, fox, jackal and genet as definitive hosts and rodents such as the swamp rat, porcupine and gerbil as intermediate hosts (Collomb et al., 2007; Fain, 1956; Vanderick et al., 1964). Coenuri in the brain only have been reported from rats (Rattus r. rattus) and non-human primates (Fain, 1956). Taenia glomeratus, which can be distinguished from T. serialis by careful morphological examination (Clapham, 1940a, 1940b), has only been reported from Nigeria and the Democratic Republic of Congo (Morel, 1959; Turner and Leiper, 1919).
In humans, 27 cases of T. brauni and one case of T. glomeratus have been reported from Rwanda, Uganda, Nigeria, and Congo, based on morphological identification and geographical association (Supplemental Table 1). There is only one report in a non-human primate (Cercopithecus mitis doggetti) from Rwanda with cysts in the brain, heart and parotid gland (Fain, 1956).
Overall, only limited information is available on these cestode species and any recent data is missing. It has to be considered that these species may be strains of T. serialis, based on the shared host range, tissue tropism, and clinical signs (Loos-Frank, 2000; Morel, 1959). However, there is no genetic data on T. brauni and T. glomeratus, and only a small number of published sequences for T. multiceps and T. serialis, which impair epidemiological studies (Collomb et al., 2007).
4. Diagnosis of cysticercosis and coenurosis
The tumor-like pathology of metacestode development in primates as dead-end hosts represents a challenge for the etiological diagnosis, which is mainly achieved only after necropsy, surgery or invasive diagnostic interventions. Serological tests have not been developed or validated with the exception of T. solium cysticercosis. The morphological characteristics of metacestodes, especially size and form of the scolex hooks, allowed highly probable diagnoses on species level (Verster, 1969; Slais, 1973; Loos-Frank, 2000). However, incomplete or damaged larval stages without or with only a few scolex hooks might be present in clinical diagnostic material. Furthermore, their development in dead-end hosts might be atypical and lead to altered morphological characteristics. For example, Bamrungphol et al. (1972) described a juvenile strobilate tapeworm found in the spinal cord of a young man from Thailand, who had suffered from paraplegia for several months. The recovered worm (76 mm long with 300 segments) had a scolex with four muscular suckers and a rostellum with few remaining, morphologically highly variable hooks. The authors concluded that “the immature state of the worm and the loss of many of the hooks from the scolex make its specific identification impossible”. However, they suggested that this strobilate form of a tapeworm found in extra-intestinal tissue likely belongs to the family Taeniidae.
Among the parasites discussed, three different metacestodes types exist, cysticercus, strobilocercus and coenurus (Table 5). These stages consist of a fluid-filled bladder of various sizes and forms, with a single (in the case of cysticerci or strobilocerci) or multiple (in the case of coenurus) invaginated scolex or scoleces. At the anterior end, scoleces carry a rostellum, a retractable muscular organ, that is equipped with four suckers and each a row of characteristic large and somewhat smaller hooks. Taenia crassiceps cysticerci are able to multiply locally and to produce daughter cysticerci by an exo- or endogenous budding process (see chapter 3.1). Cysticerci are whitish, translucent, oval bladders of 0.4 to more than 30 mm in diameter, depending on the species. At one pole, a clearly visible, white, non-translucent knob-like structure is present: the invaginated scolex. Daughter cysts usually develop at the opposite pole. More challenging is the morphological diagnosis of Versteria sp. cysticercosis, as this currently not well described species proliferates in the liver without many precise morphological characteristics (see chapter 2.2) and so far only 3 cases have been identified in humans based on molecular analyses (Table 3). The strobilocercus of T. taeniaeformis lives encapsulated in spherical cysts in the liver of its intermediate host. Isolated strobilocerci appear to be segmented and resemble adult tapeworms. However, the body has an unsegmented, flattened tube-like form, may reach several centimeters in length and carries a single scolex (Table 5). Coenuri are translucent, fluid-filled sacks of up to several centimeters in size that contain many scoleces or even clusters of scoleces (Verster, 1969; Loos-Frank, 2000; Deplazes et al., 2016).
Characteristic structures that allow a tentative morphological identification include calcareous corpuscules, microtriches and scolex hooks. In HE stained histological sections, calcareous corpuscules appear as small (5–15 μm) oval structures of a whorled appearance in the parenchyma of cestodes. The corpuscules are diagnostic for cestodes and are especially prominent in larval stages. They contain calcium carbonate whose role is not understood. The bladder wall may be smooth or have a warty appearance. Microvilli-like structures (microtriches) are present on the outer surface of the larval stages (Orihel and Ash, 1995). As shown for T. solium, they appear early in the development from the oncosphere to the cysticercus (Fig. 1) (Chile et al., 2016).
Fig. 1.
Taenia crassiceps metacestodes (case 8, Table 2). A. Cysticerci isolated at surgery (wet preparation, scale bar 5 mm). B. HE stained section of an invaginated scolex with suckers (S) and hooks (H) (scale bar 500 μm). C. Large and small hooks, calcareous corpuscules (wet preparation, scale bar 200 μm). D. Calcareous corpuscules in the parenchyma and microvilli like structures (microtriches) on the outer surface of a metacestode wall (HE stain, scale bar 20 μm).
For a morphological species identification, number, form and size of the scolex hooks are important. However, reported size ranges of hooks are quite broad and do not allow for a definite species identification (Table 5). Moreover, many measurements refer to hooks of adults and some differences between adult and larval hooks and differences between the development in natural and in accidental and dead-end hosts may exist.
Today, identification of larval cestodes in clinical material is mainly based on the analyses of the nucleotide sequences of the mitochondrial 12S rRNA, ND1 (NADH dehydrogenase 1), and COI (cytochrome c oxidase subunit 1) genes or the nuclear ITS2 (ribosomal internal transcribed spacer 2) sequence (Bowles et al., 1992; Bowles and McManus, 1993; von Nickisch-Rosenegk et al., 1999; Trachsel et al., 2007) (Table 6). The same technique and in particular COI sequences have been broadly used for phylogenetic analyses (Sharma et al., 2016). Comparison of sequence data to published sequences in databases allow for a most reliable identification of cestodes, even if only small amounts of larval material is available, and this approach should be accepted as gold standard. However, sequence data especially of T. brauni and T. glomeratus are completely lacking, hindering the molecular identification in these cases. Therefore, efforts to produce and provide such data preferably based on morphologically characterized specimens should be intensified.
Table 6.
Genetic targets for the PCR-based identification of cestode parasites.
| Symbol (bold) and aliases | Primer designations | Primer sequence (5‘-3‘) and approximate amplicon size | Reference and primer code [in squared brackets] as referred to in Table 2, Table 3, Table 4, Table 5 |
|---|---|---|---|
| Target gene: Mitochondrial cytochrome c oxidase subunit 1 | |||
|
mt-CO1 cox1, COX1, COI |
JB3 JB4.5 |
5'-TTT TTT GGG CAT CCT GAG GTT TAT-3' 5'-TAA AGA AAG AAC ATA ATG AAA ATG-3' 529 bp |
Bowles et al. (1992). [P1] |
| F/COI R/COI |
5'-TTG AAT TTG CCA CGT TTG AAT GC-3' 5'-GAA CCT AAC GAC ATA ACA TAA TGA-3' 880 bp |
Nakao et al. (2000). [P2] | |
| Target gene: Mitochondrial NADH:ubiquinone oxidoreductase core subunit 1 | |||
|
mt-ND1 nd1, ND1, NAD1 |
JB11 JB12 |
5'-AGA TTC GTA AGG GGC CTA ATA-3' 5'-ACC ACT AAC TAA TTC ACT TTC-3' 529 bp |
Bowles and McManus (1993). [P3] |
| NAD1-FF NAD1-RR |
5'-ATT GGK TTA TTT CAG AGT TTT TCT GAT TTA-3' 5'-CTC MCC ATA ATC AAA TGG ACT ACG-3' 394 bp |
Eberwein et al. (2013). [P4] | |
| Target gene: Mitochondrial 12S rRNA | |||
| mt-RNR1 12S |
60.for 375.rev |
5'-TTA AGA TAT ATG TGG TAC AGG ATT AGA TAC CC-3' 5'-AAC CGA GGG TGA CGG GCG GTG TGT ACC-3' 357 bp |
von Nickisch-Rosenegk et al. (1999). [P5] |
| Cest3 Cest5 |
5'-YGA YTC TTT TTA GGG GAA GGT GTG-3' 5'-GCG GTG TGT ACM TGA GCT AAA C-3' 267 bp |
Trachsel et al. (2007). [P6] | |
| CES12SF CES12SR |
5'-AGG GGA TAG GAC ACA GTG CCA GC-3' 5'-CGG TGT GTA CMT GAG YTA AAC-3' 480 bp |
Goldberg et al. (2014). [P7] | |
| 12S Taenia FF 12S Taenia RR |
5'-CAC AGT GCC AGC ATC YGC GGT-3' 5'-GAG GGT GAC GGG CGG TGT GTA C-3' 426 bp |
Eberwein et al. (2013). [P8] | |
| Target gene: Ribosomal Internal Transcribed Spacer | |||
| ITS2its2 | NC-2 NC-6 |
5'-TTA GTT TCT TTT CCT CCG CT-3' 5'-ATC GAC ATC TTG AAC GCA CAT TGC-3' 600-700 bp |
Gasser and Chilton (1995). [P9] |
Acknowledgments
The authors thank Teivi Laurimae and Alexander Mathis for their critical revision of the manuscript, Cristian Alvarez-Rojas for sequence analysis of a clinical sample (Institute of Parasitology, University of Zürich, Switzerland) and Bruno Gottstein (Institute of Parasitology, University of Bern, Switzerland) for supplying DNA of a clinical case.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijppaw.2019.03.013.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Abera S., Wubit T., Nejash A. Cerebral coenurosis in small ruminants: a review. J. Anim. Sci. Adv. 2016;6:1595–1608. [Google Scholar]
- Abuladze K.I. Taeniata of animals and man and diseases caused by them. In: Skrjabin K.I., editor. vol. IV. Israel Program for Scientific Translations; Jerusalem: 1970. pp. 62–67. (Essentials of Cestodology). [Google Scholar]
- Al-Sabi M.N.S., Jensen P.M., Christensen M.U., Kapel C.M.O. Morphological and molecular analyses of larval taeniid species in small mammals from contrasting habitats in Denmark. J. Helminthol. 2015;89:112–117. doi: 10.1017/S0022149X13000680. [DOI] [PubMed] [Google Scholar]
- Albert T.F., Schueler R.L., Panuska J.A., Ingling A.L. Tapeworm larvae (Taenia crassiceps) in woodchucks. J. Am. Vet. Med. Assoc. 1972;161:648–651. [PubMed] [Google Scholar]
- Alić A., Hodžić A., Škapur V., Alić S., Prašović S., Duscher G.G. Fatal pulmonary cysticercosis caused by Cysticercus longicollis in a captive ring-tailed lemur (Lemur catta) Vet. Parasitol. 2017;241:1–4. doi: 10.1016/j.vetpar.2017.05.004. [DOI] [PubMed] [Google Scholar]
- Alvarez M.F., Iglesias R., Garcia J., Paniagua E., Sanmartin M.L. Intestinal helminths of the red fox (Vulpes vulpes L.) in Galicia (Northwest Spain) Wiad. Parazytol. 1995;41:429–442. [PubMed] [Google Scholar]
- Alvarez-Rojas C.A., Mathis A., Deplazes P. Assessing the contamination of food and the environment with Taenia and Echinococcus eggs and their zoonotic transmission. Curr. Clin. Micro. Rpt. 2018;5:154–163. [Google Scholar]
- Andreassen P.N.S., Schmidt N.M., Kapel C.M.O., Christensen M.U., Sittler B., Gilg O., Enemark H.L., Al-Sabi M.N.S. Gastrointestinal parasites of two populations of Arctic foxes (Vulpes lagopus) from north-east Greenland. Polar Res. 2017;36:13. [Google Scholar]
- Arocker-Mettinger E., Huber-Spitzy V., Auer H., Grabner G., Stur M. Taenia crassiceps in the anterior chamber of the human eye. A case report. Klin. Monatsbl. Augenheilkd. 1992;201:34–37. doi: 10.1055/s-2008-1045865. [DOI] [PubMed] [Google Scholar]
- Bacigalupo J. Sobre una nueva especie de Taenia, Taenia infantis. Semana Med. 1922;26:726. [Google Scholar]
- Baer G.J., Scheidegger S. Un cas interessant de tetraplegie d'origine parasitaire. Schweiz. Z. Path. Bakt. 1946;9:61–66. [PubMed] [Google Scholar]
- Bagrade G., Kirjusina M., Vismanis K., Ozolins J. Helminth parasites of the wolf Canis lupus from Latvia. J. Helminthol. 2009;83:63–68. doi: 10.1017/S0022149X08123860. [DOI] [PubMed] [Google Scholar]
- Ballek D., Takla M., Ising-Volmer S., Stoye M. The helminth fauna of red foxes (Vulpes vulpes Linnaeus 1758) in north Hesse and east Westphalia 1. Cestodes. Dtsch. Tierarztl. Wochenschr. 1992;99:362–365. [PubMed] [Google Scholar]
- Ballweber L.R. Taenia crassiceps subcutaneous cysticercosis in an adult dog. Vet. Rec. 2009;165:693–694. [PubMed] [Google Scholar]
- Bamrungphol V., Little M.D., Virayavejakul A., Sujtanond M., Chaiyaporn V. Juvenile strobilate tapeworm from the human spinal cord. Report of a case. Am. J. Trop. Med. Hyg. 1972;21:435–439. doi: 10.4269/ajtmh.1972.21.435. [DOI] [PubMed] [Google Scholar]
- Barkati S., Gottstein B., Müller N., Sheitoyan-Pesant C., Metrakos P., Chen T., Garceau R., Libman M.D., Ndao M., Yansouni C.P. First human case of metacestode infection caused by Versteria sp. in a kidney transplant recipient. Clin. Infect. Dis. 2018;68:680–683. doi: 10.1093/cid/ciy602. [DOI] [PubMed] [Google Scholar]
- Basso W., Rütten M., Deplazes P., Grimm F. Generalized Taenia crassiceps cysticercosis in a chinchilla (Chinchilla lanigera) Vet. Parasitol. 2014;199:116–120. doi: 10.1016/j.vetpar.2013.09.023. [DOI] [PubMed] [Google Scholar]
- Battyany I., Andrea L., Nagy K.K. Subcutaneous hydatid cyst in the popliteal fossa at the site of a previous wasp sting. Diagn. Interv. Radiol. 2010;17:163–165. doi: 10.4261/1305-3825.DIR.2933-09.1. [DOI] [PubMed] [Google Scholar]
- Becklund W.W. Current knowledge of the gid bladder worm, Coenurus cerebralis (=Taenia multiceps), in North American domestic sheep, Ovis aries. Proc. Helm. Soc. Wash. 1970;37:200–203. [Google Scholar]
- Beugnet F., Delpon B., Gevrey J., Souchere T. Note á propos d’un cas de cysticercose sous-cultanèe chez un chien. Rev. Med. Vet-Toulouse. 2009;147:227–232. [Google Scholar]
- Bjelić-Čabrilo O., Kostic D., Popovic E., Lujic J. Helminth fauna of the bank vole Myodes glareolus (rodentia, arvicolinae) on the territory of fruska gora mountain (Serbia)-a potential source of zoonoses. Bulg. J. Agric. Sci. 2011;17:829–836. [Google Scholar]
- Bleyer M., Risch T., Roos C., Kaup F.J., Mätz-Rensing K. Taenia crassiceps cysticercosis in a nilgiri langur (Semnpithecus johnii) J. Zoo Wildl. Med. 2018;49:501–504. doi: 10.1638/2017-0156.1. [DOI] [PubMed] [Google Scholar]
- Borgsteede F.H., Tibben J.H., van der Giessen J.W. The musk rat (Ondatra zibethicus) as intermediate host of cestodes in The Netherlands. Vet. Parasitol. 2003;117:29–36. doi: 10.1016/j.vetpar.2003.07.015. [DOI] [PubMed] [Google Scholar]
- Boufana B., Stidworthy M.F., Bell S., Chantrey J., Masters N., Unwin S., Wood R., Lawrence R.P., Potter A., McGarry J., Redrobe S., Killick R., Foster A.P., Mitchell S., Greenwood A.G., Sako Y., Nakao M., Ito A., Wyatt K., Lord B., Craig P.S. Echinococcus and Taenia spp. from captive mammals in the United Kingdom. Vet. Parasitol. 2012;190:95–103. doi: 10.1016/j.vetpar.2012.05.023. [DOI] [PubMed] [Google Scholar]
- Bowles J., McManus D.P. NADH dehydrogenase 1 gene sequences compared for species and strains of the genus Echinococcus. Int. J. Parasitol. 1993;23:969–972. doi: 10.1016/0020-7519(93)90065-7. [DOI] [PubMed] [Google Scholar]
- Bowles J., Blair D., McManus D.P. Genetic variants within the genus Echinococcus identified by mitochondrial DNA sequencing. Mol. Biochem. Parasitol. 1992;54:165–174. doi: 10.1016/0166-6851(92)90109-w. [DOI] [PubMed] [Google Scholar]
- Brunet J., Pesson B., Chermette R., Regnard P., Grimm F., Deplazes P., Ferreira X., Sabou M., Pfaff A.W., Abou-Bacar A., Candolfi E. First case of peritoneal cysticercosis in a non-human primate host (Macaca tonkeana) due to Taenia martis. Parasites Vectors. 2014;7:422. doi: 10.1186/1756-3305-7-422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunet J., Benoilid A., Kremer S., Dalvit C., Lefebvre N., Hansmann Y., Chenard M.P., Mathieu B., Grimm F., Deplazes P., Pfaff A.W., Abou-Bacar A., Marescaux C., Candolfi E. First case of human cerebral Taenia martis cysticercosis. J. Clin. Microbiol. 2015;53:2756–2759. doi: 10.1128/JCM.01033-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruzinskaite-Schmidhalter R., Sarkunas M., Malakauskas A., Mathis A., Torgerson P.R., Deplazes P. Helminths of red foxes (Vulpes vulpes) and raccoon dogs (Nyctereutes procyonoides) in Lithuania. Parasitology. 2012;139:120–127. doi: 10.1017/S0031182011001715. [DOI] [PubMed] [Google Scholar]
- Bukova A.M. Cestodes of wild carnivorous animals in the Omsk region. Scientific Bulletin Omskiy nauchnyy vestnik. 2006;7:163–165. [Google Scholar]
- Burlet P., Deplazes P., Hegglin D. Age, season and spatio-temporal factors affecting the prevalence of Echinococcus multilocularis and Taenia taeniaeformis in Arvicola terrestris. Parasites Vectors. 2011;4:1–9. doi: 10.1186/1756-3305-4-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauchet A., Grenouillet F., Knapp J., Richou C., Delabrousse E., Dentan C., Millon L., Di Martino V., Contreras R., Deconinck E., Blagosklonov O., Vuitton D.A., Bresson-Hadni S., Franc Echino Network Increased incidence and characteristics of alveolar echinococcosis in patients with immunosuppression-associated conditions. Clin. Infect. Dis. 2014;59:1095–1104. doi: 10.1093/cid/ciu520. [DOI] [PubMed] [Google Scholar]
- Chautan M., Pontier D., Artois M. Role of rabies in recent demographic changes in Red Fox (Vulpes vulpes) populations in Europe. Mammalia. 2000;64:391–410. [Google Scholar]
- Chermette R., Bussieras J., Mialot M., Raynal P.C. Subcutaneous Taenia crassiceps cysticercosis in a dog. J. Am. Vet. Med. Assoc. 1993;203:263–265. [PubMed] [Google Scholar]
- Chermette R., Bussiéras J., Marionneau J., Boyer E., Roubin C., Prophette B., Maillard H., Fabiani B. Invasive cysticercosis due to Taenia crassiceps in an AIDS patient. Bull. Acad. Natl. Med. 1995;179:777–780. [PubMed] [Google Scholar]
- Chile N., Clark T., Arana Y., Ortega Y.R., Palma S., Mejia A., Angulo N., Kosek J.C., Kosek M., Gomez-Puerta L.A., Garcia H.H., Gavidia C.M., Gilman R.H., Verastegui M., Cysticercosis Working Group in Peru In vitro study of Taenia solium postoncospheral form. PLoS Neglected Trop. Dis. 2016;10:e0004396. doi: 10.1371/journal.pntd.0004396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christodoulopoulos G., Kassab A., Theodoropoulos G. Occurrence of non-cerebral coenurosis in sheep. J. Helminthol. 2013;87:125–127. doi: 10.1017/S0022149X1100085X. [DOI] [PubMed] [Google Scholar]
- Chuck R.S., Olk R.J., Weil G.J., Akduman L., Benenson I.L., Smith M.E., Kaplan H.J. Surgical removal of a subretinal proliferating cysticercus of Taeniaeformis crassiceps. Arch. Ophthalmol. 1997;115:562–563. doi: 10.1001/archopht.1997.01100150564028. [DOI] [PubMed] [Google Scholar]
- Cirovic D., Pavlovic I., Penezic A. Intestinal helminth parasites of the grey wolf (Canis lupus L.) in Serbia. Acta Vet. Hung. 2015;63:189–198. doi: 10.1556/AVet.2015.016. [DOI] [PubMed] [Google Scholar]
- Cirovic D., Pavlovic I., Penezic A., Kulisic Z., Selakovic S. Levels of infection of intestinal helminth species in the golden jackal Canis aureus from Serbia. J. Helminthol. 2015;89:28–33. doi: 10.1017/S0022149X13000552. [DOI] [PubMed] [Google Scholar]
- Clapham P.A. Studies on coenurus glomeratus. J. Helminthol. 1940;18:45–52. [Google Scholar]
- Clapham P.A. Further studies on coenurus glomeratus. J. Helminthol. 1940;18:95–102. [Google Scholar]
- Clark J.D. Coenurosis in a gelada baboon (Theropithecus gelada) J. Am. Vet. Med. Assoc. 1969;155:1258–1263. [PubMed] [Google Scholar]
- Collomb J., Machouart M., Biava M.F., Brizion M., Montagne K., Plenat F., Fortier B. Contribution of NADH dehydrogenase subunit I and cytochrome C oxidase subunit I sequences toward identifying a case of human coenuriasis in France. J. Parasitol. 2007;93:934–937. doi: 10.1645/GE-1160R.1. [DOI] [PubMed] [Google Scholar]
- Connor D.H., Sparks A.K., Strano A.J., Neafie R.C., Juvelier B. Disseminated parasitosis in an immunosuppressed patient: possibly a mutated sparganum. Arch. Pathol. Lab Med. 1976;100:65–68. [PubMed] [Google Scholar]
- Craig H.L., Craig P.S. Helminth parasites of wolves (Canis lupus): a species list and an analysis of published prevalence studies in Nearctic and Palaearctic populations. J. Helminthol. 2005;79:95–103. doi: 10.1079/joh2005282. [DOI] [PubMed] [Google Scholar]
- Dalimi A., Sattari A., Motamedi G. A study on intestinal helminthes of dogs, foxes and jackals in the western part of Iran. Vet. Parasitol. 2006;142:129–133. doi: 10.1016/j.vetpar.2006.06.024. [DOI] [PubMed] [Google Scholar]
- De Liberato C., Berrilli F., Meoli R., Friedrich K.G., Di Cerbo P., Cocumelli C., Eleni C. Fatal infection with Taenia martis metacestodes in a ring-tailed lemur (Lemur catta) living in an Italian zoological garden. Parasitol. Int. 2014;63:695–697. doi: 10.1016/j.parint.2014.05.008. [DOI] [PubMed] [Google Scholar]
- Denk D., Boufana B., Masters N.J., Stidworthy M.F. Fatal echinococcosis in three lemurs in the United Kingdom--A case series. Vet. Parasitol. 2016;218:10–14. doi: 10.1016/j.vetpar.2015.12.033. [DOI] [PubMed] [Google Scholar]
- Deplazes P., Eckert J., Mathis A., von Samson-Himmelstjerna G., Zahner H. Wageningen Academic Publishers; Wageningen, The Netherlands: 2016. Parasitology in Veterinary Medicine. [Google Scholar]
- Domínguez-Roldan R., Pérez-Martínez M., Rosetti M.F., Arias-Hernández D., Bernal-Fernández G., Flores-Pérez F.I., Hallal-Calleros C. High frequency of Taenia pisiformis metacestodes and high sex-associated susceptibility to cysticercosis in naturally infected wild rabbits. Parasitol. Res. 2018;117:2201–2206. doi: 10.1007/s00436-018-5907-6. [DOI] [PubMed] [Google Scholar]
- Dunbar R.I.M. Demographic and life history variables of a population of gelada baboons (Theropithecus gelada) J. Anim. Ecol. 1980;49:485–506. [Google Scholar]
- Dunsmore J.D., Howkins A.B. Coenurus serialis in a grey kangaroo. Aust. Vet. J. 1968;30:465. [Google Scholar]
- Dyachenko V., Pantchev N., Gawlowska S., Vrhovec M.G., Bauer C. Echinococcus multilocularis infections in domestic dogs and cats from Germany and other European countries. Vet. Parasitol. 2008;157:244–253. doi: 10.1016/j.vetpar.2008.07.030. [DOI] [PubMed] [Google Scholar]
- Dyer N.W., Greve J.H. Severe Cysticercus longicollis cysticercosis in a black lemur (Eulemur macaco macaco) J. Vet. Diagn. Investig. 1998;10:362–364. doi: 10.1177/104063879801000410. [DOI] [PubMed] [Google Scholar]
- Eberwein P., Haeupler A., Kuepper F., Wagner D., Kern W.V., Muntau B., Racz P., Agostini H., Poppert S. Human infection with marten tapeworm. Emerg. Infect. Dis. 2013;19:1152–1154. doi: 10.3201/eid1907.121114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Shehabi F.S., Abdel-Hafez S.K., Kamhawi S.A. Prevalence of intestinal helminths of dogs and foxes from Jordan. Parasitol. Res. 1999;85:928–934. doi: 10.1007/s004360050660. [DOI] [PubMed] [Google Scholar]
- Elek S.R., Finkelstein L.E. Multiceps serialis infestation in a baboon. Report of a case exhibiting connective tissue cystic masses. Zoologica. 1939;24:323–328. [Google Scholar]
- Fain A. Coenurus of Taenia brauni Setti parasitic in man and animals from the Belgian Congo and Ruanda-Urundi. Nature. 1956;178:1353. doi: 10.1038/1781353a0. [DOI] [PubMed] [Google Scholar]
- Federer K., Armua-Fernandez M.T., Hoby S., Wenker C., Deplazes P. In vivo viability of Echinococcus multilocularis eggs in a rodent model after different thermo-treatments. Exp. Parasitol. 2015;154:14–19. doi: 10.1016/j.exppara.2015.03.016. [DOI] [PubMed] [Google Scholar]
- Federer K., Armua-Fernandez M.T., Gori F., Hoby S., Wenker C., Deplazes P. Detection of taeniid (Taenia spp., Echinococcus spp.) eggs contaminating vegetables and fruits sold in European markets and the risk for metacestode infections in captive primates. Int. J. Parasitol. Parasites Wildl. 2016;5:249–253. doi: 10.1016/j.ijppaw.2016.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feliu C., Renaud F., Catzeflis F., Hugot J.-P., Durand P., Morand S. A comparative analysis of parasite species richness of Iberian rodents. Parasitology. 1997;115:453–466. doi: 10.1017/s0031182097001479. [DOI] [PubMed] [Google Scholar]
- Flammer Anikpeh Y., Grimm F., Lindenblatt N., Zinkernagel A. It isn't always caviar. BMJ Case Rep. 2014 doi: 10.1136/bcr-2013-200078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flisser A., Craig P.S., Ito A. Cysticercosis and taeniosis: Taenia solium, Taenia saginata and Taenia asiatica. In: Palmer S.R., Soulsby L., Torgerson P.R., Brown D.W.G., editors. Oxford Textbook of Zoonoses Biology, Clinical Practice, and Public Health Control. Oxford University Press; 2011. pp. 625–649. [Google Scholar]
- Francois A., Favennec L., Cambon-Michot C., Gueit I., Biga N., Tron F., Brasseur P., Hemet J. Taenia crassiceps invasive cysticercosis: a new human pathogen in acquired immunodeficiency syndrome? Am. J. Surg. Pathol. 1998;22:488–492. doi: 10.1097/00000478-199804000-00015. [DOI] [PubMed] [Google Scholar]
- Freeman R.S. Life history studies on Taenia mustelae Gmelin, 1790, and the taxonomy of certain taenioid cestodes from mustelidae. Can. J. Zool. 1956;34:219–242. [Google Scholar]
- Freeman R.S. Cestodes of wolves, coyotes and coyote–dog hybrids in Ontario. Can. J. Zool. 1961;39:527–532. [Google Scholar]
- Freeman R.S. Studies on the biology of Taenia crassiceps (Zeder, 1800) Rudolphi, 1810 (Cestoda) Can. J. Zool. 1962;40:969–990. [Google Scholar]
- Freeman R.S., Fallis A.M., Shea M., Maberley A.L., Walters J. Intraocular Taenia crassiceps (Cestoda). II. The parasite. Am. J. Trop. Med. Hyg. 1973;22:493–495. doi: 10.4269/ajtmh.1973.22.493. [DOI] [PubMed] [Google Scholar]
- Fuentes M.V., Sáez S., Trelis M., Galán-Puchades M.T., Esteban J.G. The helminth community of the wood mouse, Apodemus sylvaticus, in the Sierra Espuna, Murcia, Spain. J. Helminthol. 2004;78:219–223. doi: 10.1079/joh2003226. [DOI] [PubMed] [Google Scholar]
- Gasser R.B., Chilton N.B. Characterisation of taeniid cestode species by PCR-RFLP of ITS2 ribosomal DNA. Acta Trop. 1995;59:31–40. doi: 10.1016/0001-706x(94)00085-f. [DOI] [PubMed] [Google Scholar]
- Geiger M., Taucher A.T., Gloor G., Hegglin D., Bontadina B. In the footsteps of city foxes: evidence for a rise of urban badger populations in Switzerland. Hystrix it. J. Mammal. 2018;29:236–238. [Google Scholar]
- Gillis-Germitsch N., Schnyder M. Proceedings of the 6th European Dirofilaria and Angiostrongylus Days, Belgrade, Serbia. 2018. The spread of Angiostrongylus vasorum in the Swiss fox population in the last 30 years. [Google Scholar]
- Goesseringer N., Lindenblatt N., Mihic-Probst D., Grimm F., Giovanoli P. Taenia crassiceps upper limb fasciitis in a patient with untreated AIDS and chronic hepatitis C infection - the role of surgical debridement. J. Plast. Reconstr. Aesthet. Surg. 2011;64:174–176. doi: 10.1016/j.bjps.2011.02.011. [DOI] [PubMed] [Google Scholar]
- Goldberg T.L., Gendron-Fitzpatrick A., Deering K.M., Wallace R.S., Clyde V.L., Lauck M. Fatal metacestode infection in Bornean orangutan caused by unknown Versteria species. Emerg. Infect. Dis. 2014;20:109–113. doi: 10.3201/eid2001.131191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gori F., Armua-Fernandez M.T., Milanesi P., Serafini M., Magi M., Deplazes P., Macchioni F. The occurrence of taeniids of wolves in Liguria (northern Italy) Int. J. Parasitol. Parasites Wildl. 2015;4:252–255. doi: 10.1016/j.ijppaw.2015.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottstein B., Stojkovic M., Vuitton D.A., Millon L., Marcinkute A., Deplazes P. Threat of alveolar echinococcosis to public health–a challenge for Europe. Trends Parasitol. 2015;31:407–412. doi: 10.1016/j.pt.2015.06.001. [DOI] [PubMed] [Google Scholar]
- Guberti V., Stancampiano L., Francisci F. Intestinal helminth parasite community in wolves (Canis lupus) in Italy. Parassitologia. 1993;35:59–65. [PubMed] [Google Scholar]
- Guerra D., Armua-Fernandez M.T., Silva M., Bravo I., Santos N., Deplazes P., Carvalho L.M. Taeniid species of the Iberian wolf (Canis lupus signatus) in Portugal with special focus on Echinococcus spp. Int. J. Parasitol. Parasites Wildl. 2013;2:50–53. doi: 10.1016/j.ijppaw.2012.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser M., Basso W., Deplazes P. Dog and fox faecal contamination of farmland. Schweiz. Arch. Tierheilkd. 2015;157:449–455. doi: 10.17236/sat00030. [DOI] [PubMed] [Google Scholar]
- Hayes M.A., Creighton S.R. A coenurus in the brain of a cat. Can. Vet. J. 1978;19:341–343. [PMC free article] [PubMed] [Google Scholar]
- Hegglin D., Deplazes P. Control of Echinococcus multilocularis: strategies, feasibility and cost-benefit analyses. Int. J. Parasitol. 2013;43:327–337. doi: 10.1016/j.ijpara.2012.11.013. [DOI] [PubMed] [Google Scholar]
- Heldwein K., Biedermann H.G., Hamperl W.D., Bretzel G., Loscher T., Laregina D., Frosch M., Buttner D.W., Tappe D. Subcutaneous Taenia crassiceps infection in a patient with non-Hodgkin’s lymphoma. Am. J. Trop. Med. Hyg. 2006;75:108–111. [PubMed] [Google Scholar]
- Henke S.E., Pence D.B., Bryant F.C. Effect of short-term coyote removal on populations of coyote helminths. J. Wildl. Dis. 2002;38:54–67. doi: 10.7589/0090-3558-38.1.54. [DOI] [PubMed] [Google Scholar]
- Herbert I.V., Edwards G.T., Willis J.M. Some host factors which influence the epidemiology of Taenia multiceps infections in sheep. Ann. Trop. Med. Parasitol. 1984;78:243–248. doi: 10.1080/00034983.1984.11811809. [DOI] [PubMed] [Google Scholar]
- Hermosilla C., Kleinertz S., Silva L.M., Hirzmann J., Huber D., Kusak J., Taubert A. Protozoan and helminth parasite fauna of free-living Croatian wild wolves (Canis lupus) analyzed by scat collection. Vet. Parasitol. 2017;233:14–19. doi: 10.1016/j.vetpar.2016.11.011. [DOI] [PubMed] [Google Scholar]
- Hobbs T.R., Colgin L.M.A., Maginnis G.M., Lewis A.D. Abdominal cysticercosis in a rhesus macaque (Macaca mulata) Comp. Med. 2003;53:545–547. [PubMed] [Google Scholar]
- Hoberg E.P., Ebinger W., Render J.A. Fatal cysticercosis by Taenia crassiceps (Cyclophyllidea: Taeniidae) in a presumed immunocompromised canine host. J. Parasitol. 1999;85:1174–1178. [PubMed] [Google Scholar]
- Hofer S., Gloor S., Müller U., Mathis A., Hegglin D., Deplazes P. High prevalence of Echinococcus multilocularis in urban red foxes (Vulpes vulpes) and voles (Arvicola terrestris) in the city of Zurich, Switzerland. Parasitology. 2000;120:135–142. doi: 10.1017/s0031182099005351. [DOI] [PubMed] [Google Scholar]
- Hough I. Subcutaneous larval Taenia serialis in a ring-tailed possum (Pseudocheirus peregrinus) Aust. Vet. J. 2000;78 doi: 10.1111/j.1751-0813.2000.tb11860.x. 468-468. [DOI] [PubMed] [Google Scholar]
- Hubbard G.B., Gardiner C.H., Bellini S., Ehler W.J., Conn D.B., King M.M. Mesocestoides infection in captive olive baboons (Papio cynocephalus anubis) Lab. Anim. Sci. 1993;43:625–627. [PubMed] [Google Scholar]
- Huss B.T., Miller M.A., Corwin R.M., Hoberg E.P., O'Brien D.P. Fatal cerebral coenurosis in a cat. J. Am. Vet. Med. Assoc. 1994;205:69–71. [PubMed] [Google Scholar]
- Ihama Y., Sato H., Makino Y., Kamiya H. Two Taenia species found in Japan, with new distribution record of Taenia polyacantha Leuckart, 1856 (Cestoda: Taeniidae) Parasitol. Int. 2000;48:303–306. doi: 10.1016/s1383-5769(99)00030-6. [DOI] [PubMed] [Google Scholar]
- Ing M.B., Schantz P.M., Turner J.A. Human coenurosis in North America: case reports and review. Clin. Infect. Dis. 1998;27:519–523. doi: 10.1086/514716. [DOI] [PubMed] [Google Scholar]
- Itin G.S., Kravchenko V.M., Shantiz A.Y. Characteristics of helminth infections of american mink (Mustela vison), raccoon (Procyon lotor) and wolf (Canis lupus) on the territor the North-Western Caucasus. Sci.J. KubSAU. 2018;136 [Google Scholar]
- Jenkins D.J., Lievaart J.J., Boufana B., Lett W.S., Bradshaw H., Armua-Fernandez M.T. Echinococcus granulosus and other intestinal helminths: current status of prevalence and management in rural dogs of eastern Australia. Aust. Vet. J. 2014;92:292–298. doi: 10.1111/avj.12218. [DOI] [PubMed] [Google Scholar]
- Jones A., Walters T.M. A survey of taeniid cestodes in farm dogs in mid-Wales. Ann. Trop. Med. Parasitol. 1992;86:137–142. doi: 10.1080/00034983.1992.11812643. [DOI] [PubMed] [Google Scholar]
- Jones A., Walters T.M. The cestodes of foxhounds and foxes in Powys, mid-Wales. Ann. Trop. Med. Parasitol. 1992;86:143–150. doi: 10.1080/00034983.1992.11812644. [DOI] [PubMed] [Google Scholar]
- Joyeux C., Baer J.G. Les cestodes rares de l'homme. Bull. Soc. Path. Exot. 1929;22:114–136. [Google Scholar]
- Kern P., Menezes da Silva A., Akhan O., Müllhaupt B., Vizcaychipi K.A., Budke C., Vuitton D.A. The Echinococcoses: diagnosis, clinical management and Burden of disease. Adv. Parasitol. 2017;96:259–369. doi: 10.1016/bs.apar.2016.09.006. [DOI] [PubMed] [Google Scholar]
- Klinker H., Tintelnot K., Joeres R., Muller J., Gross U., Schmidt- Rotte H., Landwehr P., Richter E. Taenia crassiceps infection in AIDS. Dtsch. Med. Wochenschr. 1992;117:133–138. doi: 10.1055/s-2008-1062291. [DOI] [PubMed] [Google Scholar]
- Koch T., Schoen C., Muntau B., Addo M., Ostertag H., Wiechens B., Tappe D. Molecular diagnosis of human Taenia martis eye infection. Am. J. Trop. Med. Hyg. 2016;94:1055–1057. doi: 10.4269/ajtmh.15-0881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konjević D., Živičnjak T., Kurilj A.G., Sindičić M., Martinković F., Jan D.S. When things go wrong: cysticercus longicollis in an adult wild red fox (Vulpes vulpes) Parasitol. Res. 2016;115:1345–1348. doi: 10.1007/s00436-015-4894-0. [DOI] [PubMed] [Google Scholar]
- Kornaś S., Wierzbowska I.A., Górski P., Okarma H. Occurrence of internal parasites in stone martens (Martes foina) from Cracow and suburbs. Ann. Parasitol. 2013;59:203–205. [PubMed] [Google Scholar]
- Krücken J., Blümke J., Maaz D., Demeler J., Ramünke S., Antolová D., Schaper R., von Samson-Himmelstjerna G. Small rodents as paratenic or intermediate hosts of carnivore parasites in Berlin, Germany. PLoS One. 2017;12:e0172829. doi: 10.1371/journal.pone.0172829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafferty K.D. The evolution of trophic transmission. Parasitol. Today. 1999;15:111–115. doi: 10.1016/s0169-4758(99)01397-6. [DOI] [PubMed] [Google Scholar]
- Lau D.T., Casey W.J., Jones M.D. Coenurosis in a whitehanded gibbon. J. Am. Vet. Med. Assoc. 1973;163:633–635. [PubMed] [Google Scholar]
- Le Pesteur M.H., Giraudoux P., Delattre P., Damange J.P., Quéré J.P. Spatiotemporal distribution of four species of cestodes in a landscape of mid-altitude mountains (Jura, France) Ann. Parasitol. Hum. Comp. 1992;67:155–160. [Google Scholar]
- Lee L.M., Wallace R.S., Clyde V.L., Gendron-Fitzpatrick A., Sibley S.D., Stuchin M., Lauck M., O'Connor D.H., Nakao M., Lavikainen A., Hoberg E.P., Goldberg T.L. Definitive hosts of Versteria tapeworms (Cestoda: Taeniidae) causing fatal infection in North America. Emerg. Infect. Dis. 2016;22:707–710. doi: 10.3201/eid2204.151446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehman B.D.O., Leal S., Procop G.W., O'Connell E.M., Nash T., Jones S., Braunthal S., Cruise M., Mukhopadhyay S., Banzon J. Proceedings of the IDWeek 2018, Washington, USA. 2018. Disseminated metacestode infection due to an unknown Versteria species. [Google Scholar]
- Leiby P.D., Whittaker F.H. Occurrence of Taenia crassiceps in the conterminous United States. J. Parasitol. 1966;52:786. [Google Scholar]
- Leith J.D., Satterfield W.C. Coenurus cysts of Taenia multiceps in a baboon, Theropithecus gelada. J. Zoo Anim. Med. 1974;5:32–34. [Google Scholar]
- Lescano A.G., Zunt J. Other cestodes: sparganosis, coenurosis and Taenia crassiceps cysticercosis. Handb. Clin. Neurol. 2013;114:335–345. doi: 10.1016/B978-0-444-53490-3.00027-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Guo Z., Duo H., Fu Y., Peng M., Shen X., Tsukada H., Irie T., Nasu T., Horii Y., Nonaka N. Survey on helminths in the small intestine of wild foxes in Qinghai, China. J. Vet. Med. Sci. 2013;75:1329–1333. doi: 10.1292/jvms.13-0187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liccioli S., Bialowas C., Ruckstuhl K.E., Massolo A. Feeding ecology informs parasite epidemiology: prey selection modulates encounter rate with Echinococcus multilocularis in urban coyotes. PLoS One. 2015;10 doi: 10.1371/journal.pone.0121646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loos-Frank B. An up-date of Verster's (1969) 'Taxonomic revision of the genus Taenia Linnaeus' (Cestoda) in table format. Syst. Parasitol. 2000;45:155–183. doi: 10.1023/a:1006219625792. [DOI] [PubMed] [Google Scholar]
- Loos-Frank B., Zeyhle E. The intestinal helminths of the red fox and some other carnivores in southwest Germany. Z. Parasitenkd. 1982;67:99–113. doi: 10.1007/BF00929518. [DOI] [PubMed] [Google Scholar]
- Loos-Frank B., Zeyhle E. Zur Parasitierung von 3603 Rotfüchsen in Württemberg. Z. Jagdwiss. 1981;27:258–266. [Google Scholar]
- Loxton K.C., Lawton C., Stafford P., Holland C.V. Parasite dynamics in an invaded ecosystem: helminth communities of native wood mice are impacted by the invasive bank vole. Parasitology. 2017;144:1476–1489. doi: 10.1017/S0031182017000981. [DOI] [PubMed] [Google Scholar]
- Luong L.T., Chambers J.L., Moizis A., Stock T.M., St Clair C.C. Helminth parasites and zoonotic risk associated with urban coyotes (Canis latrans) in Alberta, Canada. J. Helminthol. 2018:1–5. doi: 10.1017/S0022149X1800113X. [DOI] [PubMed] [Google Scholar]
- Luzón M., de la Fuente-Lopez C., Martinez-Nevado E., Fernandez-Moran J., Ponce-Gordo F. Taenia crassiceps cysticercosis in a ring-tailed lemur (Lemur catta) J. Zoo Wildl. Med. 2010;41:327–330. doi: 10.1638/2009-0062R.1. [DOI] [PubMed] [Google Scholar]
- Mackenstedt U., Jenkins D., Romig T. The role of wildlife in the transmission of parasitic zoonoses in peri-urban and urban areas. Int. J. Parasitol. Parasites. Wildl. 2015;4:71–79. doi: 10.1016/j.ijppaw.2015.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maillard H., Marionneau J., Prophette B., Boyer E., Celerier P. Taenia crassiceps cysticercosis and AIDS. AIDS. 1998;20:1551–1552. doi: 10.1097/00002030-199812000-00019. [DOI] [PubMed] [Google Scholar]
- Mathy A., Hanosset R., Adant S., Losson B. The carriage of larval Echinococcus multilocularis and other cestodes by the musk rat (Ondatra zibethicus) along the Ourthe River and its tributaries (Belgium) J. Wildl. Dis. 2009;45:279–287. doi: 10.7589/0090-3558-45.2.279. [DOI] [PubMed] [Google Scholar]
- Millan J., Real C.E., Ferroglio E. Helminth parasites in stone martens (Martes foina) from Italy. Z. Jagdwiss. 2001;47:229–231. [Google Scholar]
- Miller A.L., Olsson G.E., Walburg M.R., Sollenberg S., Skarin M., Ley C., Wahlström H., Höglund J. First identification of Echinococcus multilocularis in rodent intermediate hosts in Sweden. Int. J. Parasitol. Parasites Wildl. 2016;5:56–63. doi: 10.1016/j.ijppaw.2016.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A.L., Olsson G.E., Sollenberg S., Walburg M.R., Skarin M., Höglund J. Transmission ecology of taeniid larval cestodes in rodents in Sweden, a low endemic area for Echinococcus multilocularis. Parasitology. 2017;144:1041–1051. doi: 10.1017/S0031182017000257. [DOI] [PubMed] [Google Scholar]
- Moks E., Jogisalu I., Saarma U., Talvik H., Jarvis T., Valdmann H. Helminthologic survey of the wolf (Canis lupus) in Estonia, with an emphasis on Echinococcus granulosus. J. Wildl. Dis. 2006;42:359–365. doi: 10.7589/0090-3558-42.2.359. [DOI] [PubMed] [Google Scholar]
- Montalbano Di Filippo M., Meoli R., Cavallero S., Eleni C., De Liberato C., Berrilli F. Molecular identification of Mesocestoides sp. metacestodes in a captive gold-handed tamarin (Saguinus midas) Infect. Genet. Evol. 2018;65:399–405. doi: 10.1016/j.meegid.2018.08.008. [DOI] [PubMed] [Google Scholar]
- Morales J., Velasco T., Tovar V., Fragoso G., Fleury A., Beltrán C., Villalobos N., Aluja A., Rodarte L.F., Sciutto E., Larralde C. Castration and pregnancy of rural pigs significantly increase the prevalence of naturally acquired Taenia solium cysticercosis. Vet. Parasitol. 2002;108:41–48. doi: 10.1016/s0304-4017(02)00168-1. [DOI] [PubMed] [Google Scholar]
- Morales-Montor J., Baig S., Hallal-Calleros C., Damian R.T. Taenia crassiceps: androgen reconstitution of the host leads to protection during cysticercosis. Exp. Parasitol. 2002;100:209–216. doi: 10.1016/s0014-4894(02)00028-0. [DOI] [PubMed] [Google Scholar]
- Morel P. Les helminthes des animaux domestiques de l'Afrique occidentale. Rev. Elev. Med. Vet. Pay. 1959;12:153–174. [Google Scholar]
- Moro P.L., Ballarta J., Gilman R.H., Leguia G., Rojas M., Montes G. Intestinal parasites of the grey fox (Pseudalopex culpaeus) in the central Peruvian Andes. J. Helminthol. 1998;72:87–89. doi: 10.1017/s0022149x00001048. [DOI] [PubMed] [Google Scholar]
- Moshiri A., Shamsian S., Berenji F., Jadidoleslami A., Moghaddas E. Coenurus serialis in northeastern Iran: a probable danger to human. Int. J. Infect. 2018;5 [Google Scholar]
- Mougeot G., Cambon M., Dimeglio V., Menerath J.M. Intraocular cestode larva in a 14-year-old boy in Auvergne. Presse Med. 1996;25:1168. [PubMed] [Google Scholar]
- Nabavi R., Manouchehri Naeini K., Zebardast N., Hashemi H. Epidemiological study of gastrointestinal helminthes of canids in chaharmahal and bakhtiari province of Iran. Iran. J. Parasitol. 2014;9:276–281. [PMC free article] [PubMed] [Google Scholar]
- Nagy A., Ziadinov I., Schweiger A., Schnyder M., Deplazes P. Hair coat contamination with zoonotic helminth eggs of farm and pet dogs and foxes. Berl. Münchener Tierärztliche Wochenschr. 2011;124:503–511. [PubMed] [Google Scholar]
- Nakao M., Sako Y., Yokoyama N., Fukunaga M., Ito A. Mitochondrial genetic code in cestodes. Mol. Biochem. Parasitol. 2000;111:415–424. doi: 10.1016/s0166-6851(00)00334-0. [DOI] [PubMed] [Google Scholar]
- Nakao M., Lavikainen A., Iwaki T., Haukisalmi V., Konyaev S., Oku Y. Molecular phylogeny of the genus Taenia (Cestoda: Taeniidae): proposals for the resurrection of Hydatigera Lamarck, 1816 and the creation of a new genus Versteria. Int. J. Parasitol. 2013;43:427–437. doi: 10.1016/j.ijpara.2012.11.014. [DOI] [PubMed] [Google Scholar]
- Ntoukas V., Tappe D., Pfütze D., Simon M., Holzmann T. Cerebellar cysticercosis caused by larval Taenia crassiceps tapeworm in immunocompetent woman, Germany. Emerg. Infect. Dis. 2013;19:2008–2011. doi: 10.3201/eid1912.130284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odnokurtsev V.A., Sedalischev V.T. Helminthes fauna of sable (Martes zibellina Linnaeus, 1758) in Yakutia. Tomsk State Univ. J.Biol. 2011;2:22–34. [Google Scholar]
- Olson P.D., Yoder K., Fajardo L.-G.L.F., Marty A.M., van de Pas S., Olivier C., Relman D.A. Lethal invasive cestodiasis in immunosuppressed patients. J. Infect. Dis. 2003;187:1962–1966. doi: 10.1086/375357. [DOI] [PubMed] [Google Scholar]
- Orihel T.C., Ash L.R. ASCP Press; Chicago: 1995. Parasites in Human Tissues. [Google Scholar]
- Orioles M., Beltran E., Stewart J., Boufana B., Holloway A. Cerebral coenuosis in a cat. Vet. Rec. Case Rep. 2014;2 [Google Scholar]
- Otranto D., Cantacessi C., Pfeffer M., Dantas-Torres F., Brianti E., Deplazes P., Genchi C., Guberti V., Capelli G. The role of wild canids and felids in spreading parasites to dogs and cats in Europe. Part I: Protozoa and tick-borne agents. Vet. Parasitol. 2015;213:12–23. doi: 10.1016/j.vetpar.2015.04.022. [DOI] [PubMed] [Google Scholar]
- Otranto D., Cantacessi C., Dantas-Torres F., Brianti E., Pfeffer M., Genchi C., Guberti V., Capelli G., Deplazes P. The role of wild canids and felids in spreading parasites to dogs and cats in Europe. Part II: helminths and arthropods. Vet. Parasitol. 2015;213:24–37. doi: 10.1016/j.vetpar.2015.04.020. [DOI] [PubMed] [Google Scholar]
- Paoletti B., Iorio R., Traversa D., Di Francesco C.E., Gentile L., Angelucci S., Amicucci C., Bartolini R., Marangi M., Di Cesare A. Helminth infections in faecal samples of Apennine wolf (Canis lupus italicus) and Marsican brown bear (Ursus arctos marsicanus) in two protected national parks of central Italy. Ann. Parasitol. 2017;63:205–212. doi: 10.17420/ap6303.107. [DOI] [PubMed] [Google Scholar]
- Pétavy A.F., Deblock S. Helminths of the common fox (Vulpes vulpes L.) from the massif central (France) Ann. Parasitol. Hum. Comp. 1980;55:379–391. [PubMed] [Google Scholar]
- Pétavy A.F., Deblock S., Prost C. Epidemiology of alveolar echinococcosis in France. 1. Intestinal helminths in the red fox (Vulpes vulpes L.) from Haute-Savoie. Ann. Parasitol. Hum. Comp. 1990;65:22–27. doi: 10.1051/parasite/1990651022. [DOI] [PubMed] [Google Scholar]
- Pfaffenberger G.S., Valencia V.B. Helminths of sympatric black-tailed jack rabbits (Lepus californicus) and desert cottontails (Sylvilagus audubonii) from the high plains of eastern New Mexico. J. Wildl. Dis. 1988;24:375–377. doi: 10.7589/0090-3558-24.2.375. [DOI] [PubMed] [Google Scholar]
- Pfeiffer F., Kuschfeldt S., Stoye M. Helminth fauna of the red fox (Vulpes vulpes LINNE 1758) in south Sachsen-Anhalt -1: Cestodes. Dtsch. Tierarztl. Wochenschr. 1997;104:445–448. [PubMed] [Google Scholar]
- Railliet A., Marullaz M. Sur un Cénure nouveau du Bonnet chinois (Macacus sinicus) B. Soc. Pathol. Exot. 1919;12:223–228. [Google Scholar]
- Reperant L.A., Hegglin D., Tanner I., Fischer C., Deplazes P. Rodents as shared indicators for zoonotic parasites of carnivores in urban environments. Parasitology. 2009;136:329–337. doi: 10.1017/S0031182008005428. [DOI] [PubMed] [Google Scholar]
- Ribas A., Torre I., Feliu C., Arrizabalaga A., Casanova J.C. Helminth communities of the bank vole Myodes glareolus in two populations: Montsenyu natural park (NE Spain) and Pi Natural reserve (French Pyrenees) Rev. Ibero-Latinoam. Parasitol. Int. 2009;1:73–89. [Google Scholar]
- Richards D.T., Harris S., Lewis J.W. Epidemiological studies on intestinal helminth parasites of rural and urban red foxes (Vulpes vulpes) in the United Kingdom. Vet. Parasitol. 1995;59:39–51. doi: 10.1016/0304-4017(94)00736-v. [DOI] [PubMed] [Google Scholar]
- Romig T., Deplazes P., Jenkins D., Giraudoux P., Massolo A., Craig P.S., Wassermann M., Takahashi K., De La Rue M. Ecology and life cycle patterns of Echinococcus species. Adv. Parasitol. 2017;95:213–314. doi: 10.1016/bs.apar.2016.11.002. [DOI] [PubMed] [Google Scholar]
- Ross J.G., Fairley J.S. Studies of disease in the red fox (Vulpes vulpes) in northern Ireland. J. Zool. 1969;157:375–381. [Google Scholar]
- Rudelius M., Brehm K., Poelcher M., Spinner C., Rosenwald A., da Costa C.P. First case of human peritoneal cysticercosis mimicking peritoneal carcinosis: necessity of laparoscopy and histologic assessment for the correct diagnosis. JMM Case Rep. 2017;4 doi: 10.1099/jmmcr.0.005097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sailer M., Soelder B., Allerberger F., Zaknun D., Feichtinger H., Gottstein B. Alveolar echinococcosis of the liver in a six-year-old girl with acquired immunodeficiency syndrome. J. Pediatr. 1997;130:320–323. doi: 10.1016/s0022-3476(97)70364-0. [DOI] [PubMed] [Google Scholar]
- Sandground J.H. On a coenurus from the brain of a monkey. J. Parasitol. 1937;23:482–490. [Google Scholar]
- Scala A., Varcasia A. Updates on morphobiology, epidemiology and molecular characterization of coenurosis in sheep. Parassitologia. 2006;48:61–63. [PubMed] [Google Scholar]
- Schaerer O. University of Zurich; Zurich, Switzerland: 1987. Die Metacestoden der Kleinsäuger (Insectivora und Rodentia) und ihre Wirtsarten, Verbreitung und Häufigkeit im Kanton Thurgau (Schweiz) Dissertation. [Google Scholar]
- Schantz P.M., Alstine C.V., Blacksheep A., Sinclair S. Prevalence of Echinococcus granulosus and other cestodes in dogs on the Navajo reservation in Arizona and New Mexico. Am. J. Vet. Res. 1977;38:669–670. [PubMed] [Google Scholar]
- Schellhas K.P., Norris G.A. Disseminated human subarachnoid coenurosis: computed tomographic appearance. Am. J. Neuroradiol. 1985;6:638–640. [PMC free article] [PubMed] [Google Scholar]
- Schmid S., Grimm F., Huber M., Beck B., Custer P., Bode B. Taenia crassiceps infection - an unusual presentation of a tapeworm diagnosed by FNA cytology and PCR. Cytopathology. 2014;25:340–341. doi: 10.1111/cyt.12092. [DOI] [PubMed] [Google Scholar]
- Schneider-Crease I.A., Snyder-Mackler N., Jarvey J.C., Bergman T.J. Molecular identification of Taenia serialis coenurosis in a wild Ethiopian gelada (Theropithecus gelada) Vet. Parasitol. 2013;198:240–243. doi: 10.1016/j.vetpar.2013.08.015. [DOI] [PubMed] [Google Scholar]
- Schuster R., Benitz R. On the development of Taenia martis (Zeder, 1803) in the intermediate host. Helminthologia. 1992;29:13–18. [Google Scholar]
- Schuster R., Heidecke D., Schierhorn K. Contribution to the parasite fauna of local hosts. On the endoparasitic fauna of Felis silvestris. Appl. Parasitol. 1993;34:113–120. [PubMed] [Google Scholar]
- Schuster R.K., Sivakumar S., Wieckowsky T. Non-cerebral coenurosis in goats. Parasitol. Res. 2010;107:721–726. doi: 10.1007/s00436-010-1919-6. [DOI] [PubMed] [Google Scholar]
- Schwartz B. A subcutaneous tumor in a primate caused by tapeworm larvae experimentally reared to maturity in dogs. J. Agric. Res. 1927;35:471–480. [Google Scholar]
- Schweiger A., Ammann R.W., Candinas D., Clavien P.A., Eckert J., Gottstein B., Halkic N., Muellhaupt B., Prinz B.M., Reichen J., Tarr P.E., Torgerson P.R., Deplazes P. Human alveolar echinococcosis after fox population increase, Switzerland. Emerg. Infect. Dis. 2007;13:878–882. doi: 10.3201/eid1306.061074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweizer G., Grunenfelder F., Sydler T., Rademacher N., Braun U., Deplazes P. Imported coenurosis in sheep. Schweiz. Arch. Tierheilkd. 2006;148:490–499. doi: 10.1024/0036-7281.148.9.490. [DOI] [PubMed] [Google Scholar]
- Sedalischev V.T., Odnokurtsev V.A. Ecology of sable (Martes zibellina l., 1758) of southwestern Yakutia. Vestnik okhotovedeniya. 2011;8 [Google Scholar]
- Sedalischev V.T., Odnokurtsev V.A. vol. 3. Izvestia of Samara Scientific Center of the Russian Academy of Sciences; 2013. (On Ecology of Red Fox (Vulpes vulpes L. 1785) in Yakutia). [Google Scholar]
- Segovia J.M., Torres J., Miquel J., Llaneza L., Feliu C. Helminths in the wolf, Canis lupus, from north-western Spain. J. Helminthol. 2001;75:183–192. [PubMed] [Google Scholar]
- Segovia J.M., Guerrero R., Torres J., Miquel J., Feliu C. Ecological analyses of the intestinal helminth communities of the wolf, Canis lupus, in Spain. Folia Parasitol. 2003;50:231–236. doi: 10.14411/fp.2003.041. [DOI] [PubMed] [Google Scholar]
- Segovia J.M., Torres J., Miquel J. Helminth parasites of the red fox (Vulpes vulpes L., 1758) in the Iberian Peninsula: an ecological study. Acta Parasitol. 2004;49:67–79. [Google Scholar]
- Sharma S., Lyngdoh D., Roy B., Tandon V. Molecular phylogeny of Cyclophyllidea (Cestoda: Eucestoda): an in-silico analysis based on mtCOI gene. Parasitol. Res. 2016;115:3329–3335. doi: 10.1007/s00436-016-5092-4. [DOI] [PubMed] [Google Scholar]
- Shea M., Maberley A.L., Walters J., Freeman R.S., Fallis A.M. Intraocular Taenia crassiceps (Cestoda) Trans. Am. Acad. Ophthalmol. Otolaryngol. 1973;77:778–783. [PubMed] [Google Scholar]
- Shimalov V.V. Taenia martis (Cestoda, Taeniidae) from vertebrates in the Republic of Belarus. Parazitologiya. 2010;44:435–440. [PubMed] [Google Scholar]
- Shimalov V.V. New data about the helminth fauna of the red squirrel (Sciurus vulgaris Linnaeus, 1758) in Belorussian Polesie. J. Parasit. Dis. 2016;40:1620–1621. doi: 10.1007/s12639-015-0668-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slais J. Functional morphology of cestode larvae. Adv. Parasitol. 1973;11:395–480. [PubMed] [Google Scholar]
- Slocombe R.F., Arundel J.H., Labuc R., Doyle M.K. Cerebral coenuriasis in a domestic cat. Aust. Vet. J. 1989;66:92–93. doi: 10.1111/j.1751-0813.1989.tb09753.x. [DOI] [PubMed] [Google Scholar]
- Smith H.J. Parasites of red foxes in new Brunswick and Nova Scotia. J. Wildl. Dis. 1978;14:366–370. doi: 10.7589/0090-3558-14.3.366. [DOI] [PubMed] [Google Scholar]
- Smith M.C., Bailey C.S., Baker N., Kock N. Cerebral coenurosis in a cat. J. Am. Vet. Med. Assoc. 1988;192:82–84. [PubMed] [Google Scholar]
- Soveri T., Henttonen H., Rudbäck E., Schildt R., Tanskanen R., Husu-Kallio J., Haukisalmi V., Sukura A., Laakkonen J. Disease patterns in field and bank vole populations during a cyclicdecline in central Finland. Comp. Immunol. Microbiol. Infect. Dis. 2000;23:73–89. doi: 10.1016/s0147-9571(99)00057-0. [DOI] [PubMed] [Google Scholar]
- Sterba J., Barus V. First record of Strobilocercus fasciolaris (Taeniidae-larvae) in man. Folia Parasitol. 1976;23:221–226. [PubMed] [Google Scholar]
- Stieger C., Hegglin D., Schwarzenbach G., Mathis A., Deplazes P. Spatial and temporal aspects of urban transmission of Echinococcus multilocularis. Parasitology. 2002;124:631–640. doi: 10.1017/s0031182002001749. [DOI] [PubMed] [Google Scholar]
- Stien A., Voutilainen L., Haukisalmi V., Fuglei E., Mørk T., Yoccoz N.G., Ims R.A., Henttonen H. Intestinal parasites of the Arctic fox in relation to the abundance and distribution of intermediate hosts. Parasitol. 2010;137:149–157. doi: 10.1017/S0031182009990953. [DOI] [PubMed] [Google Scholar]
- Subbotin A.M. Analysis of helmintocenosis structure in raccoon dog (Nyctereutes procyonoides) in the Republic of Belarus. Sci. Bull. V.S.U. 2009;1 [Google Scholar]
- Takács A., Szabó L., Juhász L., Takács A.A., Lanszki J., Takács P.T., Heltai M. Data on the parasitological status of golden jackal (Canis aureus L., 1758) in Hungary. Acta Vet. Hung. 2014;62:33–41. doi: 10.1556/AVet.2013.058. [DOI] [PubMed] [Google Scholar]
- Tenora F., Henttonen H., Haukisalmi V. On helminths of rodents in Finland. Ann. Zool. Fenn. 1983;20:37–45. [Google Scholar]
- Tenora F., Andreassen J., Hindsbo O., Lodal J. Helminths of small rodents in Denmark. Helminthologia. 1991;28:151–154. [Google Scholar]
- Thompson R.C.A. Biology and systematics of Echinococcus multilocularis. In: Thompson R.C.A., Lymbery A.J., editors. Echinococcus and Hydatid Disease. CAB International; 1995. pp. 1–50. [Google Scholar]
- Timbur M., Suvonkulov U., Zafar S., Olesya A., Kim H.-J., Yong T., Lee G.-J., Park G.-M., Shin M.-H., Yu H.S. Proceedings of the 14th International Congress of Parasitology, Daegu, Korea. 2018. Genotypic and phylogenetic characteristics of Echinococcus granulosus sensu lato in Uzbekstan. [Google Scholar]
- Tokiwa T., Taira K., Yamazaki M., Kashimura A., Une Y. The first report of peritoneal tetrathyridiosis in squirrel monkey (Saimiri sciureus) Parasitol. Int. 2014;63:705–707. doi: 10.1016/j.parint.2014.06.005. [DOI] [PubMed] [Google Scholar]
- Trachsel D., Deplazes P., Mathis A. Identification of taeniid eggs in the faeces from carnivores based on multiplex PCR using targets in mitochondrial DNA. Parasitology. 2007;134:911–920. doi: 10.1017/S0031182007002235. [DOI] [PubMed] [Google Scholar]
- Tschudi K., Ammann R. Recurrent chest wall abscess. Result of a probable percutaneous infection with Echinococcus multilocularis following a dormouse bite. Schweiz. Med. Wschr. 1988;118:1011–1015. [PubMed] [Google Scholar]
- Tsubota K., Nakatsuji S., Matsumoto M., Fujihira S., Yoshizawa K., Okazaki Y., Murakami Y., Anagawa A., Oku Y., Oishi Y. Abdominal cysticercosis in a cynomolgus monkey. Vet. Parasitol. 2009;161:339–341. doi: 10.1016/j.vetpar.2009.01.024. [DOI] [PubMed] [Google Scholar]
- Turner M., Leiper R.T. On the occurrence of Coenurus glomeratus in man in West Africa. Trans. Roy. Soc. Trop. Med. Hyg. 1919;13:23–24. [Google Scholar]
- Umhang G., Richomme C., Boucher J.M., Guedon G., Boué F. Nutrias and muskrats as bioindicators for the presence of Echinococcus multilocularis in new endemic areas. Vet. Parasitol. 2013;197:283–287. doi: 10.1016/j.vetpar.2013.05.003. [DOI] [PubMed] [Google Scholar]
- Umhang G., Comte S., Raton V., Hormaz V., Boucher J.M., Favier S., Combes B., Boué F. Echinococcus multilocularis infections in dogs from urban and periurban areas in France. Parasitol. Res. 2014;113:2219–2222. doi: 10.1007/s00436-014-3875-z. [DOI] [PubMed] [Google Scholar]
- Vanderick F., Fain A., Langi S., Vanbalen H. 2 new cases of human coenurosis caused by Taenia brauni in Rwanda, with orbital localization of the Coenurus. Ann. Soc. Belges. Med. Trop. Parasitol. Mycol. 1964;44:1077–1079. [PubMed] [Google Scholar]
- Varcasia A., Sanna D., Casu M., Lahmar S., Dessì G., Pipia A.P., Tamponi C., Gaglio G., Hrčková G., Otranto D., Scala A. Species delimitation based on mtDNA genes suggests the occurrence of new species of Mesocestoides in the Mediterranean region. Parasites Vectors. 2015;11:619. doi: 10.1186/s13071-018-3185-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varcasia A., Tamponi C., Tosciri G., Pipia A.P., Dore F., Schuster R.K., Kandil O.M., Manunta M.L., Scala A. Is the red fox (Vulpes vulpes) a competent definitive host for Taenia multiceps? Parasites Vectors. 2015;8:491. doi: 10.1186/s13071-015-1096-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasileiou N.G., Fthenakis G.C., Papadopoulos E. Dissemination of parasites by animal movements in small ruminant farms. Vet. Parasitol. 2015;213:56–60. doi: 10.1016/j.vetpar.2015.04.031. [DOI] [PubMed] [Google Scholar]
- Verster A. A taxonomic revision of the genus Taenia Linnaeus, 1758 s.str. Onderstepport J. Vet. Res. 1969;36:3–58. [PubMed] [Google Scholar]
- von Nickisch-Rosenegk M., Lucius R., Loos-Frank B. Contributions to the phylogeny of the Cyclophyllidea (Cestoda) inferred from mitochondrial 12S rDNA. J. Mol. Evol. 1999;48:586–596. doi: 10.1007/pl00006501. [DOI] [PubMed] [Google Scholar]
- Vuitton L., Bresson-Hadni S., Millon L. Clinical epidemiology of human AE in Europe. Vet. Parasitol. 2015;213:110–120. doi: 10.1016/j.vetpar.2015.07.036. [DOI] [PubMed] [Google Scholar]
- Welzel A., Steinbach G., von Keyserlingk M., Stoye M. Zur Helminthenfauna des Rotfuchses (Vulpes vulpes L.) in Südniedersachsen. Z. Jagdwiss. 1995;41:100–109. [Google Scholar]
- Wereszczuk A., Leblois R., Zalewski A. Genetic diversity and structure related to expansion history and habitat isolation: stone marten populating rural-urban habitats. BMC Ecol. 2017;17:46. doi: 10.1186/s12898-017-0156-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whipp C.J., Daoust P.Y., Conboy G., Gelens H. Abdominal cysticercosis in a red fox (Vulpes vulpes) J. Wildl. Dis. 2017;53:197–199. doi: 10.7589/2016-03-058. [DOI] [PubMed] [Google Scholar]
- Willis J.M., Herbert I.V. Some factors affecting the eggs of Taenia multiceps: their transmission onto pasture and their viability. Ann. Trop. Med. Parasitol. 1984;78:236–242. doi: 10.1080/00034983.1984.11811808. [DOI] [PubMed] [Google Scholar]
- Wolff M., Bush M., Montali R., Gardiner C.H. Cysticercus pneumonitis and pleuritis in a red-ruffed lemur. J. Zoo Wildl. Med. 1989;20:383–385. [Google Scholar]
- Wunschmann A., Garlie V., Averbeck G., Kurtz H., Hoberg E.P. Cerebral cysticercosis by Taenia crassiceps in a domestic cat. J. Vet. Diagn. Investig. 2003;15:484–488. doi: 10.1177/104063870301500517. [DOI] [PubMed] [Google Scholar]
- Young L.A., Morris P.J., Keener L., Gardiner C.H., Stalis I.H., Bicknese E., Sutherland-Smith M., Janssen D.L. Proceedings of the AAZV and IAAAM Joint Conference, New Orleans, USA. 2000. Subcutaneous Taenia crassiceps cysticercosis in a red ruffed lemur (Varecia variegata rubra) pp. 251–252. [Google Scholar]
- Zhang X.Y., Jian Y.N., Ma L.Q., Li X.P., Karanis P. A case of coenurosis in a wild rabbit (Lepus sinensis) caused by Taenia serialis metacestode in Qinghai Tibetan plateau Aarea, China. Kor. J. Parasitol. 2018;56:195–198. doi: 10.3347/kjp.2018.56.2.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.