Abstract
Background
Malignant pleural mesothelioma (MPM) is an aggressive, treatment resistant neoplasm. The current treatment, consisting of antifolate and platinum-based chemotherapy, improves the median overall survival with only 3 months. Adjuvant bevacizumab generates an additional 2 months survival benefit. Checkpoint inhibitors (CI) have shown promising clinical effects in only a minority of patients. A possible reason is that MPM patients have low numbers of tumor-infiltrating CD8+ T-cells. Dendritic cell (DC) therapy can induce an immune response and activate tumor-specific CD8+ T-cells. Allogeneic mesothelioma tumor-lysate loaded DC therapy has proven effective in mice and safe and feasible in humans. We have designed a randomized, phase II/III, multicenter, open-label trial to examine the efficacy of DC therapy in humans with histologically proven MPM.
Methods
In this open-label, multicenter, randomized phase II/III trial patients will be randomized to receive either DC therapy plus best supportive care (BSC) or BSC alone according to the discretion of the local investigator after first line chemotherapy treatment. The primary end point will be overall survival. The secondary endpoints will be safety and tolerability, progression-free survival, overall response rate and quality of life.
Discussion
This phase II/III trial will determine whether DC therapy in patients with MPM is safe and effective as a maintenance treatment and subsequently might be a new treatment option for MPM.
Keywords: Dendritic cell-based therapy, mesothelioma, immunotherapy, clinical trial, phase III
Introduction
Malignant pleural mesothelioma (MPM) is an aggressive malignancy; if left untreated, the overall survival from diagnosis is 6–9 months (1). Because MPM is strongly associated with asbestos exposure (2,3), its incidence should be declining as the use and production of asbestos is banned since the 1980s in western Europe (4-7). In the Netherlands, however, the incidence is currently still not declining (6,8) and environmental exposure from asbestos-containing materials still poses a risk (9). Moreover, countries such as China, Russia and India are still producing large amounts of asbestos, and therefore a future epidemic is to be expected in these nations (4,10).
The current first-line treatment—a combination of antifolate (pemetrexed, raltitrexed) and platinum (cisplatin, carboplatin)-based chemotherapy—has a response rate of around 40%. This combination chemotherapy leads to an overall survival benefit of 3 months compared to single platinum-based chemotherapy; the median overall survival of patients treated with this combination is 12 months (1). Despite many research efforts, no second-line treatment for MPM is registered (11). Only France has approved the addition of bevacizumab to combination chemotherapy as standard treatment for MPM (12). Response rates between 9–25% have been found in phase I/II studies investigating PD-(L)1 checkpoint inhibitors (CIs) treatment in MPM patients (13,14). These CIs reinvigorate anti-tumor T-cell responses by blocking inhibitory signaling of the tumor cells. Therefore, the presence of tumor infiltrating T-cells (TILs) is correlated to the efficacy of and response to treatment with CIs (15,16).
Not surprisingly, high CD8+ T-cell infiltration in MPM is associated with better overall survival.
Therapies that increase the numbers of tumor specific CD8+ T-cells could, enhance the efficacy of CIs (16).
Dendritic cells (DCs) present tumor-associated antigens (TAAs) to the T-cells in the lymph node, thereby inducing proliferation and activation of tumor-specific CD4+ and CD8+ T-cells that will infiltrate the TME.
DC therapy is a cell-based vaccination used to instigate an anti-tumor immune response. DCs are matured and activated ex vivo to enhance their immunogenic function and circumvent tumor immunosuppression. During or before maturation, DCs can be loaded by a wide variety of methods, ranging from synthetic peptides coding for parts of TAAs to whole tumor lysate. When tumor lysate is used, a broad immune response to multiple identified and unidentified TAA will be generated (17). Phase I trials in patients with MPM treated with autologous tumor-lysate loaded DCs show promising clinical responses and a favorable toxicity profile (18). Radiological responses were observed with long lasting, ongoing survival up to 66 months after treatment (18-20). Generating autologous tumor lysate is time consuming and obtaining the required quantity and quality of autologous tumor lysate cannot always be guaranteed (18,20).
To create practically applicable and potent tumor lysate, our group has developed an allogeneic tumor lysate source. Five cell lines have been developed by selecting and culturing tumor cells from pleural effusions of patients with MPM. These proprietary cell lines (21) were selected based on the patients’ distinctive tumor-profiles. The lysate of these cell lines (PheraLys) can be used to load autologous monocyte-derived DCs during culture for vaccination therapy (MesoPher). Aerts et al. showed that allogeneic tumor-lysate loaded DC therapy in mice was as effective as autologous tumor-lysate loaded DC therapy (21). In a dose evaluation study in patients with MPM, allogeneic tumor-lysate loaded DC therapy (MesoPher) was proven to be safe and feasible. Grade 3/4 toxicities or serious adverse events (SAE) were not observed (21).
On the basis of these results, we designed an international, randomized phase II/III trial to evaluate the efficacy of autologous DCs loaded with allogenic tumor lysate (MesoPher) in MPM patients after first line treatment with chemotherapy.
Methods
Study design and treatment
The proposed DENdritic cell Immunotherapy for Mesothelioma (DENIM) trial is an open-label, multicenter, randomized phase II/III trial comparing overall survival (OS) in patients with MPM—either treated with DC therapy with MesoPher and best supportive care (BSC) or BSC alone. OS is determined as the time from randomization until death.
The study will be conducted according to the principles of the Declaration of Helsinki and has been approved by the independent ethics committees of participating countries.
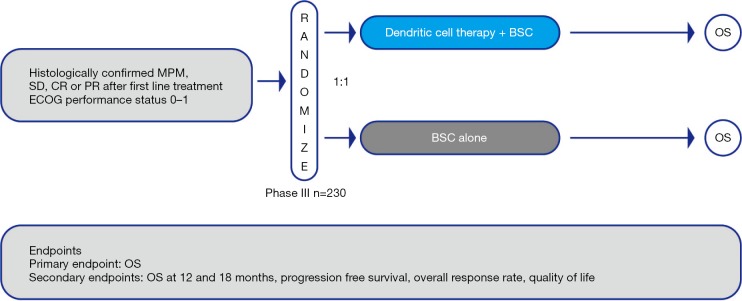
Patients with stable disease or response after first line chemotherapy will be randomized on a 1:1 basis to receive either MesoPher and BSC (study arm A) or BSC alone (study arm B) (Figure 1). The randomization will be stratified by histology (epithelial vs. other) and study center.
Figure 1.
Study design for the phase III DENIM trial. MPM, malignant pleural mesothelioma; SD, stable disease; PR, partial response; CR, complete response; BSC, best supportive care; OS, overall survival.
Patients in study arm A will receive a maximum of 5 doses of MesoPher (days 1, 15, 29, and weeks 18 and 30). Treatment will start between 9 and 13 weeks after the last cycle of chemotherapy. At each time point, a total of 25×106 DCs will be injected. Two-thirds of the DCs will be administered intravenously and one-third will be injected intradermally. Treatment will be continued if no disease progression, unmanageable toxicity or withdrawal is reported. Patients in study arm B will receive BSC according to the discretion of the local investigator and institutional standards. Upon disease progression, second line treatment according to institutional policy is allowed. The use of CIs is allowed as second line treatment.
Eligibility criteria
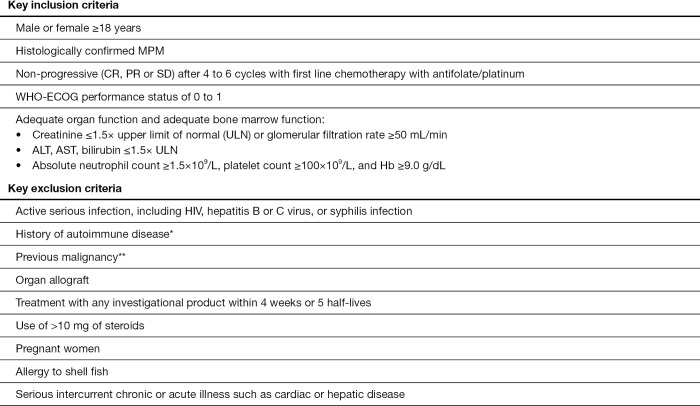
Main inclusion and exclusion criteria are listed in Figure 2. Informed consent must be given. Patients who used to receive bevacizumab will be eligible if bevacizumab was stopped due to non-tolerance before or by the end of the chemotherapy period. Disease progression will be monitored with CT-scans at screening, 6 weeks after the first vaccination and then approximately every 12 weeks. Disease status will be assessed with the modified Response Evaluation Criteria in Solid Tumors (RECIST) criteria version 1.0 (22).
Figure 2.
Key in and exclusion criteria for the phase III DENIM trial. *, except for diabetes mellitus type 1; **, except adequately treated basal cell or squamous cell skin cancer, superficial or in-situ cancer of the bladder or other cancer for which the subject has been disease-free for at least 3 years. MPM, malignant pleural mesothelioma; CR, complete response; PR, partial response; SD, stable disease; HIV, human immunodeficiency virus.
Study end points
The primary end point is OS. Secondary end points are overall survival rate at 18 months after randomization, progression free survival (PFS), overall response rate and duration of response according to modified RECIST criteria (22). The change in quality of life (QoL) will be assessed with the European Organization for Research and Treatment of Cancer (EORTC) Quality of Life Questionnaire (QLQ)-C30; its lung cancer (LC)-specific module EORTC QLQ-LC13 will be used to evaluate QoL. Therapy-induced changes of the blood immune profile, and immune responses towards MesoPher and KLH will be investigated in blood, serum, skin biopsies and tumor biopsies.
Statistical design
All efficacy analyses will be done on the full analysis set, which includes all patients randomized on an intention-to-treat basis.
For the sample size estimation, a median survival time of 21 months after randomization for study arm A and 12 months for study arm B are hypothesized, corresponding to a hazard ratio of 0.5714 for exponential survival times. The total study duration will be 36 months with an expected accrual time of 24 months. A dropout rate of 0.5% per month is anticipated. With these assumptions, a sample size of 115 subjects per treatment group will provide 90% power to test the null hypothesis that the true HR =1 vs. the alternative that the true HR =0.5174 with a 2-sided type I error rate of 5%. The expected number of deaths at the end of the study is 134.
The stratified log-rank test will be used for the primary comparison of treatment groups, with histology and study center as strata. Kaplan-Meier curves will be provided to determine median survival times.
An interim analysis is planned when 50 deaths have occurred, expectedly 18 months after the study start, when approximately 173 patients should have been enrolled.
An overall false-positive rate of 5% level for the key secondary efficacy end points will be maintained; in that no significance of key secondary endpoints will be claimed unless the primary statistical analysis is significant at the 5% level.
Quality of life will be compared between study arms using a mixed-effect model repeated measures (MMRM) including treatment group, histology and study center as fixed effects.
Immunomonitoring data will be summarized and categorized per patient to show continuous immunological data per patient. Furthermore, this data will be correlated to clinical categorical data (e.g., responders, non-responders) using appropriate statistical analysis depending on the distribution and data type.
Discussion
Most patients with MPM respond poorly to treatment and current first-line treatment only provides a moderate increase in overall survival. There is currently no maintenance or second line therapy for MPM. DC therapy could be a new treatment option. Preclinical data regarding DC therapy in mesothelioma mouse models are encouraging and clinical data from a phase I/II dose evaluation study with MesoPher have indicated its safety and feasibility in MPM patients. The DENIM study is designed to examine the efficacy of DC therapy in MPM patients who received first-line treatment with combination chemotherapy. If the primary end point is met, MesoPher might be a new treatment option for patients with MPM. Study enrollment began in May 2018; accrual will take 2 years and the total study time is expected to be 3 years.
Acknowledgements
The authors would like to thank the research nurses and site staff of the department of pulmonary medicine from the Erasmus MC, University Hospital Antwerp, Centre Hospitalier Régional Universitaire de Lille, University of Leicester, Università Politecnica delle Marche - Ospedali Riuniti di Ancona, the NKI(dutch cancer institute) and the apheresis unit of the hematology department of the Erasmus MC for their support. We would like to thank Rob Meijer and Ilona Enninga from Amphera BV. for their major input in making this trial possible.
Funding: This project has received funding from Amphera (sponsor) and the European Union’s Horizon 2020 research and innovation programme under grant agreement No. 668769.
Ethical Statement: The study protocol and informed consent documents were approved by the ethical committees of the participating institutions, and informed consent was obtained from all patients.
Footnotes
Conflicts of Interest: JGJV Aerts reports receiving commercial research grants from Amphera and Roche, holds ownership interest (including patents) in Amphera BV, and is a consultant/advisory board member for Amphera, Boehringer Ingelheim, Bristol-Myers Squibb, Eli-Lilly, MSD, Takeda, Bayer en Astra-Zeneca and Roche. R. Cornelissen reports consulting for Roche, MSD, Boehringer Ingelheim and receiving speakers fee from BMS, Roche, Pfizer, Boehringer Ingelheim, Novartis. P. Baas reports receiving advisorships from Merck and BMS. The other authors have no conflicts of interest to declare.
References
- 1.Vogelzang NJ, Rusthoven JJ, Symanowski J, et al. Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol 2003;21:2636-44. 10.1200/JCO.2003.11.136 [DOI] [PubMed] [Google Scholar]
- 2.Baas P, Fennell D, Kerr KM, et al. Malignant pleural mesothelioma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2015;26 Suppl 5:v31-9. 10.1093/annonc/mdv199 [DOI] [PubMed] [Google Scholar]
- 3.Delgermaa V, Takahashi K, Park E-K, et al. Global mesothelioma deaths reported to the World Health Organization between 1994 and 2008. Bull World Health Organ 2011;89:716-24. 10.2471/BLT.11.086678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bianchi C, Bianchi T. Malignant Mesothelioma: Global Incidence and Relationship with Asbestos. Ind Health 2007;45:379-87. 10.2486/indhealth.45.379 [DOI] [PubMed] [Google Scholar]
- 5.Peto J, Decarli A, Vecchia C, et al. The European mesothelioma epidemic. Br J Cancer 1999;79:666-72. 10.1038/sj.bjc.6690105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burdorf A. Sterfte aan maligne mesothelioom in Nederland: huidige trends en toekomstige verwachting. Sterfte aan maligne mesothelioom in Nederland: huidige trends en toekomstige verwachting 0100. Available online: https://www.asbestslachtoffers.nl/docs/Burdorf%20A%20(18)%20Sterfte%20aan%20maligne%20mesothelioom%20in%20Nederland%20hudige%20trends%20en%20toekomstige%20verwachting%20%20maart%202018.pdf
- 7.Hodgson JT, McElvenny DM, Darnton AJ, et al. The expected burden of mesothelioma mortality in Great Britain from 2002 to 2050. Br J Cancer 2005;92:587-93. 10.1038/sj.bjc.6602307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ahmed M, Flannery A, Mujammil I, et al. Variation in incidence trends of malignant pleural mesothelioma in Europe. Eur Respir J 2018. doi: 10.1183/13993003.02384-2017 [DOI] [PubMed] [Google Scholar]
- 9.Yap TA, Aerts JG, Popat S, et al. Novel insights into mesothelioma biology and implications for therapy. Nat Rev Cancer 2017;17:475-88. 10.1038/nrc.2017.42 [DOI] [PubMed] [Google Scholar]
- 10.Scherpereel A, Astoul P, Baas P, et al. Guidelines of the European Respiratory Society and the European Society of Thoracic Surgeons for the management of malignant pleural mesothelioma. Zhongguo Fei Ai Za Zhi 2010;13:C23-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zauderer MG, Kass SL, Woo K, et al. Vinorelbine and gemcitabine as second- or third-line therapy for malignant pleural mesothelioma. Lung Cancer 2014;84:271-4. 10.1016/j.lungcan.2014.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brosseau S, Assoun S, Naltet C, et al. A review of bevacizumab in the treatment of malignant pleural mesothelioma. Future Oncol 2017;13:2537-46. 10.2217/fon-2017-0307 [DOI] [PubMed] [Google Scholar]
- 13.Lievense LA, Sterman DH, Cornelissen R, et al. Checkpoint Blockade in Lung Cancer and Mesothelioma. Am J Respir Crit Care Med 2017;196:274-82. 10.1164/rccm.201608-1755CI [DOI] [PubMed] [Google Scholar]
- 14.Dumoulin DW, Aerts JG, Cornelissen R. Is immunotherapy a viable option in treating mesothelioma?. Future Oncol 2017;13:1747-50. 10.2217/fon-2017-0234 [DOI] [PubMed] [Google Scholar]
- 15.Tumeh PC, Harview CL, Yearley JH, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 2014;515:568-71. 10.1038/nature13954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aerts JG, Hegmans JP. Tumor-Specific Cytotoxic T Cells Are Crucial for Efficacy of Immunomodulatory Antibodies in Patients with Lung Cancer. Cancer Res 2013;73:2381-8. 10.1158/0008-5472.CAN-12-3932 [DOI] [PubMed] [Google Scholar]
- 17.Buonaguro L, Petrizzo A, Tornesello ML, et al. Translating tumor antigens into cancer vaccines. Clin Vaccine Immunol 2011;18:23-34. 10.1128/CVI.00286-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hegmans JP, Veltman JD, Lambers ME, et al. Consolidative Dendritic Cell-based Immunotherapy Elicits Cytotoxicity against Malignant Mesothelioma. Am J Respir Crit Care Med 2010;181:1383-90. 10.1164/rccm.200909-1465OC [DOI] [PubMed] [Google Scholar]
- 19.Cornelissen R, Hegmans JP, Maat AP, et al. Extended Tumor Control after Dendritic Cell Vaccination with Low-Dose Cyclophosphamide as Adjuvant Treatment in Patients with Malignant Pleural Mesothelioma. Am J Respir Crit Care Med 2016;193:1023-31. 10.1164/rccm.201508-1573OC [DOI] [PubMed] [Google Scholar]
- 20.Hegmans JP, Hemmes A, Aerts JG, et al. Immunotherapy of Murine Malignant Mesothelioma Using Tumor Lysate-pulsed Dendritic Cells. Am J Respir Crit Care Med 2005;171:1168-77. 10.1164/rccm.200501-057OC [DOI] [PubMed] [Google Scholar]
- 21.Aerts JG, Goeje P, Cornelissen R, et al. Autologous dendritic cells pulsed with allogeneic tumor cell lysate in mesothelioma: From mouse to human. Clin Cancer Res 2018;24:766-76. 10.1158/1078-0432.CCR-17-2522 [DOI] [PubMed] [Google Scholar]
- 22.Byrne MJ, Nowak AK. Modified RECIST criteria for assessment of response in malignant pleural mesothelioma. Ann Oncol 2004;15:257-60. 10.1093/annonc/mdh059 [DOI] [PubMed] [Google Scholar]