Summary
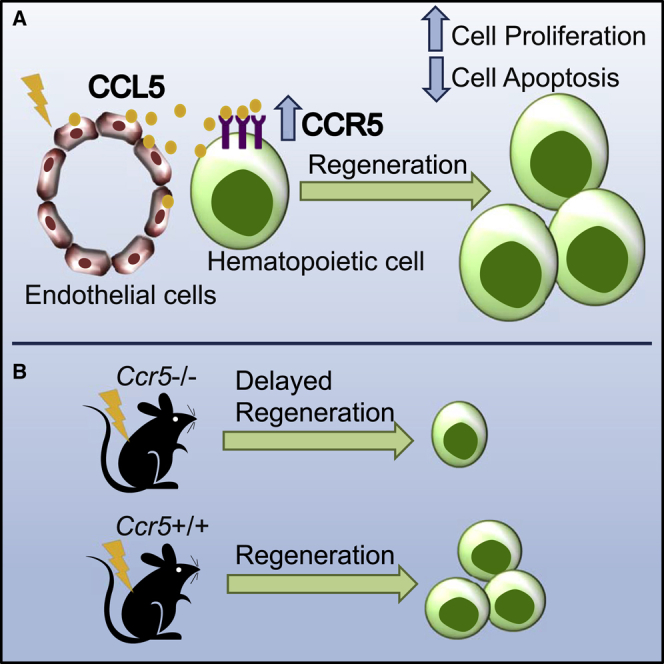
Hematopoietic stem and progenitor cells (HSPCs) depend on regulatory cytokines from the marrow microenvironment. From an unbiased cytokine screen of murine marrow supernatants, we identified C-C motif chemokine ligand 5 (CCL5) as an endothelial cell-secreted hematopoietic growth factor. Following treatment with CCL5, hematopoietic regeneration is accelerated and survival is prolonged after radiation. In mice with deletion of Ccr5, hematopoietic regeneration is delayed compared to control mice. Deletion of Ccr5 specifically in hematopoietic cells was sufficient to delay regeneration, while the deletion of Ccr5 in stromal/endothelial cells was not. Mechanistically, CCL5 promotes hematopoietic cell cycling and cell survival. Like murine hematopoietic cells, human hematopoietic cells (cord blood, healthy marrow, and peripheral blood) increase CCR5 expression after radiation exposure to promote cell survival. These data establish that CCL5 and CCR5 signaling play critical roles in hematopoietic regeneration and could serve as therapeutic targets to shorten the duration of myelosuppression.
Keywords: hematopoietic stem cell, endothelial cell, CCL5, CCR5, RANTES, regeneration, hematopoietic growth factor, hematopoietic cytokine, hematopoietic microenvironment, vascular niche
Graphical Abstract
Highlights
-
•
CCL5 and CCR5 expression in the bone marrow is increased following radiation
-
•
CCL5 promotes cell cycling and survival after irradiation
-
•
CCR5 is necessary for hematopoietic regeneration
-
•
CCL5 can shorten the duration of myelosuppression following total body radiation
CC-chemokine receptor 5 (CCR5) may be best known as a co-receptor for HIV, but its role in hematopoiesis is incompletely defined. Using a combination of transplantation studies, genetic murine models, and human specimens, Doan and colleagues demonstrate that CCL5/CCR5 signaling promotes hematopoietic regeneration in response to myelosuppressive radiation injury.
Introduction
Hematopoietic stem cells (HSCs) reside adjacent to bone marrow sinusoidal vessels (Kiel et al., 2005) and rely on extrinsic factors from endothelial cells for both HSC self-renewal and regeneration (Butler et al., 2010, Ding et al., 2012, Himburg et al., 2010, Mendelson and Frenette, 2014). Identification of these factors can produce targeted therapies for stem cell expansion for hematopoietic transplantation or accelerate hematopoietic regeneration following myeloablation. These endothelial cell-derived growth factors include stem cell factor, notch ligands, pleiotrophin, and epidermal growth factor (EGF), among others (Ding et al., 2012, Doan et al., 2013a, Himburg et al., 2010, Hooper et al., 2009, Kobayashi et al., 2010). EGF was identified as a growth factor from an 84-target cytokine array that demonstrated increased levels of EGF in the marrow supernatants of mice with deletions of proapoptotic genes Bak and Bax in Tie2+ cells and prolonged survival following lethal dose total body irradiation (TBI) compared with littermate control mice with lower levels of EGF (Doan et al., 2013a, Doan et al., 2013b). From this same array, we discovered that C-C motif chemokine ligand 5 (CCL5) was increased by 11-fold in mice with more intact HSCs and endothelial cell (EC) vasculature after TBI compared with littermate controls, suggesting that CCL5 could play a role in hematopoietic regeneration following radiation injury (Doan et al., 2013b).
Certain chemokines are required for HSC maintenance and retention in the marrow (Petit et al., 2002, Sugiyama et al., 2006). For example, constitutive deletion of the chemokine (C-X-C motif) ligand 12 (Cxcl12) results in depletion of HSCs (Sugiyama et al., 2006, Tzeng et al., 2011). Through systematic deletion of Cxcl12 in a cell-specific manner, HSCs were shown to rely on a perivascular niche (Ding and Morrison, 2013). Whether other chemokines, such as CCL5, could modulate hematopoietic function is not completely defined. CCL5 is increased in the marrow microenvironment with aging, which is associated with bias in myeloid cell production in aged mice (Ergen et al., 2012). Deficiency of CCL5 results in skewing of myeloid-to-lymphocyte cell ratios resulting in an increase in T cells, lymphoid-biased HSCs, and a corresponding decrease in myeloid progenitor cells in aged mice (Ergen et al., 2012). Further, CCL5 promotes angiogenesis via two distinct mechanisms, either by direct signaling on ECs or by increasing vascular endothelial growth factor (Liu et al., 2015, Sax et al., 2016). When Ccr5, the cognate receptor for CCL5, is genetically deleted, macrophage trafficking is impaired (Kuziel et al., 2003). These studies suggest that CCL5 and CCR5 signaling can modulate hematopoiesis under homeostatic conditions.
While CCL5 has been studied in the context of aging and hematopoiesis in steady state, the role of CCL5 signaling in hematopoietic regeneration is unknown. We set out to define whether CCL5 and CCR5 could contribute to hematopoietic regeneration following myelosuppressive injury with ionizing radiation. We have identified CCL5 as a pharmacologic agent that could accelerate hematopoietic regeneration, which bears therapeutic implications for the treatment of acute radiation sickness or for chemotherapy-induced myelosuppression.
Results
CCL5 and CCR5 Expression Are Increased following Irradiation
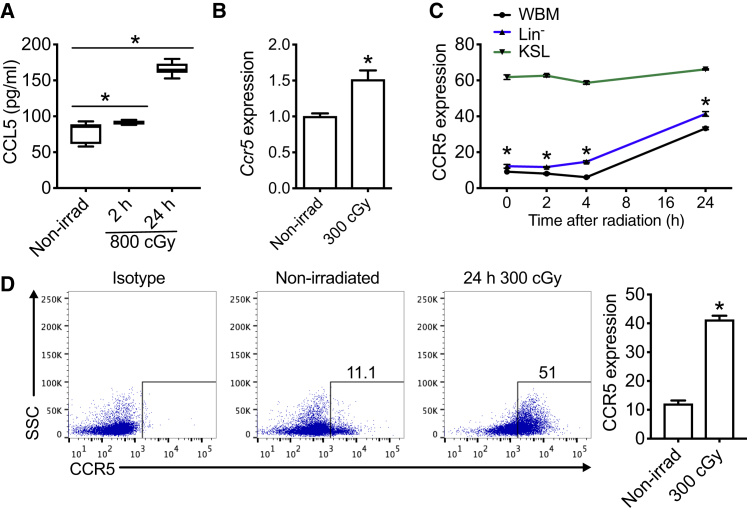
Mice with deletion of Bax in Tie2+ ECs displayed both prolonged survival following lethal dose TBI and increased levels of CCL5 in marrow supernatants compared with littermate control mice (Doan et al., 2013b). We sought to confirm that primary C57BL/6 marrow ECs elaborate CCL5. At steady state, CCL5 is detected in the conditioned media from cultures of ECs (Figure 1A). Following irradiation, levels of CCL5 increase as early as 2 h and continue to rise by 2-fold at 24 h compared with nonirradiated cultures (Figure 1A). Its receptor CCR5 on hematopoietic cells is also inducible and responsive to irradiation. At 2 h following 300 cGy, cKit+Sca-1+Lin− (KSL) cells had a 1.5-fold increase in Ccr5 mRNA expression compared with nonirradiated cells (Figure 1B). There was an enrichment of Ccr5 expression in KSL cells after irradiation in comparison with bone marrow (BM) lineage-negative (Lin−) cells (Figure S1A). Of hematopoietic cell subsets, KSL cells display the highest levels of CCR5 protein expression compared with either whole bone marrow (WBM) or Lin− cells (Figure 1C). Lin− cells display increased CCR5 as early as 2 h following 300 cGy (Figures 1C, 1D, and S1B) and remained elevated at least until day 7 (Figure S1C). These data demonstrate that CCR5 expression is enriched in hematopoietic progenitor cell subsets compared with more differentiated WBM cells.
Figure 1.
CCL5 and CCR5 Expression Are Increased following Ionizing Irradiation
(A) ELISA of CCL5 expression from C57BL/6 ECs at 0 (Non-irrad), 2, and 24 h following 800 cGy irradiation. n = 6–8 per group, ∗p = 0.03 and p < 0.0001 for 2 and 24 h compared with nonirradiated ECs, respectively.
(B) Ccr5 mRNA expression of C57BL/6 KSL cells at 2 h following 300 cGy compared with nonirradiated KSL cells. Data are normalized to nonirradiated control samples and GAPDH. n = 8 per group, ∗p = 0.002.
(C) CCR5 protein expression by flow cytometric analysis of whole bone marrow (WBM), Lin−, and KSL cells following 300 cGy over time compared with nonirradiated control mice (0 h). n = 3–5 per group per time point, ∗p < 0.0001 for times 0, 2, 4, and 24 h.
(D) Representative flow cytometric analysis and quantification of CCR5 expression in BM Lin− cells at 24 h following 300 cGy compared with nonirradiated control mice. n = 5 per group per time point, ∗p < 0.0001. SSC, side scatter. Data are shown as means ± SEM. Student's t test (two-tailed with unequal variance) was applied to the datasets except in (C) where a one-way ANOVA analysis was applied.
CCL5 Treatment Promotes Hematopoietic Regeneration In Vitro
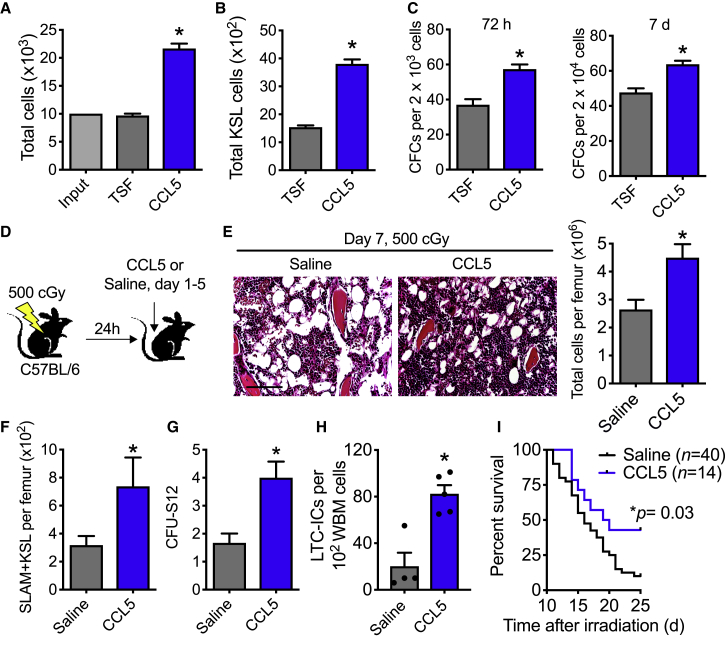
Without irradiation injury, competitive transplantation of KSL cells treated with CCL5 does not increase long-term hematopoietic stem content compared with control cultures (Figures S2A–S2C). Yet, when irradiated (300 cGy) C57BL/6 KSL cells were cultured with CCL5, they demonstrated greater than a 2-fold total cell expansion and increased total KSL cells compared with control cultures with cytokines alone (thrombopoietin, stem cell factor, Flt-3 ligand [TSF], Figures 2A and 2B). There was a corresponding increase in recovery of colony-forming units (CFUs) in CCL5-treated cultures compared with control cultures (Figure 2C). To determine whether CCL5 could increase irradiated HSC content following exposure in vitro, we performed competitive transplantation assays with donor irradiated CD34− KSL cells that had been treated with either CCL5 or TSF alone (Figures S2D–S2F). No differences in donor engraftment were detected in the peripheral or BM of recipient mice (Figures S2E and S2F). These data suggest that in vitro pharmacologic treatment with CCL5 might not alter HSC content, but could increase lineage-committed cells in vitro.
Figure 2.
CCL5 Accelerates Hematopoietic Regeneration following Ionizing Radiation
(A) Total cells following 300 cGy and culture with 30 ng/mL CCL5 + TSF compared with TSF alone at 72 h. n = 3 per group, ∗p = 0.0002.
(B) Total KSL cells at 72 h after 300 cGy in culture with TSF + CCL5 compared with TSF alone. n = 3 per group, ∗p = 0.0002.
(C) Left, CFCs of KSL and progeny at 72 h and right, CFCs at 7 days following 300 cGy and culture with CCL5 + TSF or TSF alone. n = 3 per group, ∗p = 0.008 and p = 0.007 for 72 h and 7 days, respectively.
(D) Schematic diagram of study. C57BL/6 mice were exposed to 500 cGy. 24 h after irradiation, mice were injected subcutaneously with 0.1 μg/g body weight CCL5 or saline from day 1 to 5. On day 7, marrow was collected for hematopoietic assays.
(E) Left, H&E staining of marrow at day 7. Scale bar, 100 μm. Right, bone marrow cell counts per femur. n = 11–18 per group, ∗p = 0.004.
(F) Total SLAM + KSL cells per femur. n = 5–10 per group, ∗p = 0.03.
(G) CFU-S12 on day 7 following 500 cGy and treatment with CCL5 or saline. n = 3 per group, ∗p = 0.03.
(H) Levels of LTC-ICs on day 7 following 500 cGy and treatment with CCL5 or saline. n = 4–5 per group. ∗p = 0.002.
(I) Survival following 700 cGy TBI and subcutaneous injections given 3 doses of 0.1 μg/g body weight CCL5 or saline per week from days 1 to 15. n = 14–40 mice per group. ∗p = 0.03 by log rank analysis. Four of 40 mice were alive in the saline-treated group compared with 6 of 14 mice alive in the CCL5-treated group at day 25. The median survival for saline-treated and CCL5-treated mice are 16 and 19.5 days, respectively. Data are shown as means ± SEM. Student's t test (two-tailed with unequal variance) was applied to the data except for survival analysis where log rank analysis was applied.
CCL5 Promotes HSPC Regeneration In Vivo and Prolongs Survival
To determine whether CCL5 promotes hematopoietic regeneration in vivo, we measured HSPC cells in C57BL/6 mice following subcutaneous injection with 0.1 μg/g body weight CCL5 or saline control beginning 24 h after 500 cGy TBI, which is a sublethal and highly myelosuppressive radiation dose (Figure 2D) (Himburg et al., 2017, Himburg et al., 2018). On day 7 following irradiation, CCL5-treated mice displayed preserved BM cellularity, increased total BM cells, increased CD150+ CD48− KSL (SLAM + KSL) cells (Figure S3), and colony-forming unit spleen (CFU-S12) (Figures 2E–2G). Using these same experimental conditions, we performed limiting dilutions of long-term culture-initiating cell (LTC-IC) assays. Irradiated mice treated with CCL5 had a 4-fold increase in LTC-IC cells compared with saline-treated mice (Figures 2H and S4A). Following competitive transplantation with WBM from either saline-treated or CCL5-treated donor mice, no differences in long-term HSC content were detected in recipient mice in either the peripheral blood or BM (Figures S4B–S4D). When mice were exposed to 700 cGy, which is a lethal dose of irradiation (LD60-90/30) (Gluzman-Poltorak et al., 2014a, Gluzman-Poltorak et al., 2014b), and then treated with CCL5, CCL5-treated mice displayed a modest increase in marrow cellularity and colony-forming cells (CFCs), but not in CFU-S12 or LTC-ICs at this radiation dose (Figures S5A–S5D). Based on these data, CCL5 can accelerate hematopoietic progenitor cell regeneration in vivo, with the greatest benefit to hematopoietic recovery following moderate, myelosuppressive doses of irradiation.
To determine whether CCL5 contributes to prolong survival following TBI, we treated C57BL/6 mice with 3 doses per week from day 1 to 15 with CCL5 or saline (total 7 doses) following 700 cGy. Ten percent (4 of 40) of saline-treated mice survived past day 25 compared with 43% (6 of 14) of CCL5-treated mice (Figure 2I). These results demonstrate that CCL5 administration prolongs survival after lethal dose TBI.
CCR5 Is Necessary for Hematopoietic Regeneration
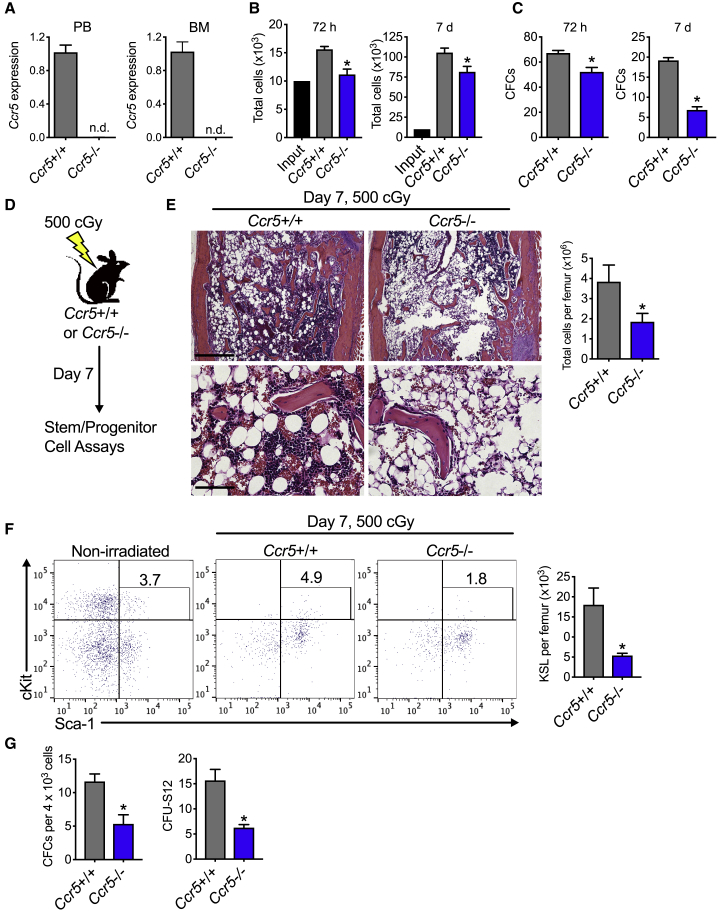
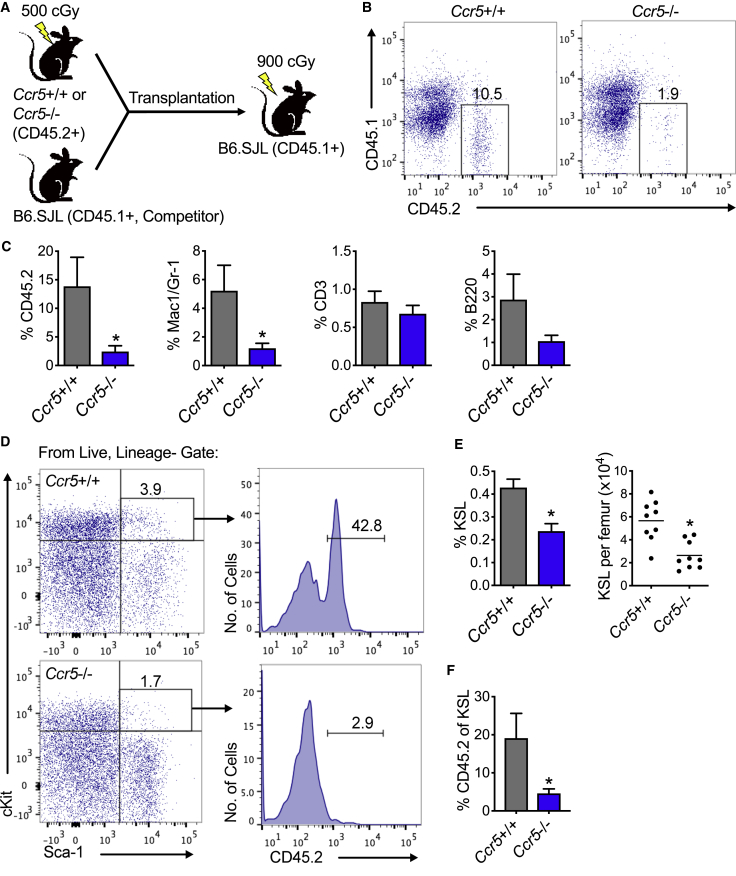
Since CCL5 can activate CCR5 as well as CCR1 and CCR3, we sought to determine whether CCR5 signaling was necessary for hematopoietic regeneration by using mice that are deficient in Ccr5 (Ccr5−/− mice) (Kuziel et al., 2003). We confirmed that Ccr5 mRNA expression is not detected in either the peripheral blood or BM of Ccr5−/− mice (Figure 3A). At steady state, Ccr5−/− mice display no differences in complete blood counts, BM cellularity, KSL cells, SLAM + KSL cells, or CFCs, compared with Ccr5+/+ mice (Figures S6A–S6F). Following 300 cGy irradiation, KSL cells from Ccr5−/− displayed decreased total cell expansion and CFCs compared with KSL cells from Ccr5+/+ mice at both 72 h and day 7 in culture (Figures 3B and 3C). When these mice are exposed to 500 cGy TBI, Ccr5-deficient mice display decreased marrow cellularity compared with Ccr5+/+ mice (Figures 3D and 3E). At day 7 following 500 cGy, total KSL cells, CFCs, and CFU-S12 were decreased by 3.3-, 2.2-, and 2.5-fold, respectively, in Ccr5-deficient mice compared with control mice that retained Ccr5 (Figures 3F and 3G). To measure long-term HSC content, we performed competitive transplantation assays on day 7 following 500 cGy TBI (Figure 4A). At 16 weeks following transplantation, recipients of Ccr5−/− donor cells displayed a 5.8-fold decrease in total donor marrow engraftment compared with recipients of Ccr5+/+ donor cells (Figures 4B and 4C). While no differences were detected in engraftment of either B or T cells, there was a 4.3-fold decrease in engraftment of donor myeloid cells (Mac1/Gr-1+ cells, Figure 4C). The percentage of KSL cells was decreased in recipients of donor cells from Ccr5-deficient mice compared with recipients of Ccr5+/+ mice (Figures 4D and 4E). Finally, we found that total donor engraftment corresponded with donor chimerism within the marrow KSL cells for each group, in which there was a 4.2-fold decrease in recipients of Ccr5-deficient donor cells compared with recipients of Ccr5+/+ mice (Figures 4D and 4F). These data demonstrate that CCR5 signaling could be necessary for hematopoietic regeneration, especially in the myeloid compartment, and that its deficiency results in delayed hematopoietic recovery following sublethal TBI.
Figure 3.
CCR5 Is Necessary for Hematopoietic Regeneration In Vitro and In Vivo
(A) mRNA expression of Ccr5 in peripheral blood (PB) or bone marrow (BM) in Ccr5−/− compared with Ccr5+/+ control mice. n = 6–30 per group. n.d., not detected. Data are normalized to Ccr5+/+ control samples and GAPDH.
(B) Total cells at 72 h or day 7 of 300-cGy irradiated BM KSL cells from Ccr5−/− or Ccr5+/+ mice. n = 5–6 per group, ∗p = 0.004 and p = 0.03 for 72 h and day 7, respectively.
(C) CFCs per 2 × 103 KSL and progeny cells at 72 h or day 7. n = 6 per group, ∗p = 0.004 and p < 0.0001 for 72 h and day 7, respectively.
(D) Schematic diagram of study. Ccr5−/− or control Ccr5+/+ mice were exposed to 500 cGy. Hematopoietic assays were performed on day 7 postirradiation.
(E) H&E-stained femurs from Ccr5−/− mice at day 7 following 500 cGy compared with Ccr5+/+ mice. Scale bars, 500 μm (top) and 100 μm (bottom). Quantification of total cells per femur. n = 6–9 per group, ∗p = 0.03.
(F) Left, representative flow cytometric analysis from Lin− gate of KSL cells on day 7 from Ccr5−/− and Ccr5+/+ mice irradiated with 500 cGy. Nonirradiated wild-type control is shown to left. Right, quantification of KSL cells per femur. n = 8–9 per group, ∗p = 0.01.
(G) Levels of CFCs and CFU-S12 on day 7 following 500 cGy of Ccr5−/− and Ccr5+/+ mice. n = 3–12 per group. ∗p = 0.002 and p = 0.005 for CFCs and CFU-S12, respectively. Data are shown as means ± SEM. Student's t test (two-tailed with unequal variance) was applied to the data.
Figure 4.
CCR5 Is Necessary for Hematopoietic Regeneration
(A) Schematic diagram of irradiation of Ccr5+/+ or Ccr5−/− mice and transplantation of 106 donor cells and 2 × 105 competing host marrow cells into lethally irradiated B6.SJL recipient mice.
(B) Representative flow cytometric analysis of total donor bone marrow engraftment at 16 weeks posttransplantation.
(C) Percentage of total donor CD45.2+ cell, myeloid (Mac1/Gr-1), T cell (CD3), and B cell (B220) engraftment in the bone marrow of CD45.1 recipient mice at 16 weeks following transplantation. n = 9 per group, ∗p = 0.04 for total engraftment and Mac1/Gr-1, respectively.
(D) Representative flow cytometric analysis of CD45.2+ donor cells within bone marrow KSL cells at 16 weeks.
(E) Quantification of percentage KSL and total KSL cells per femur. n = 9 per group, ∗p = 0.002 and p = 0.0007 for percentage KSL cells and total KSL cells, respectively.
(F) The mean percentages of CD45.2 donor cells within bone marrow KSL cells. n = 9 per group, ∗p = 0.04. Data are shown as means ± SEM. Student's t test (two-tailed with unequal variance) was applied to the data.
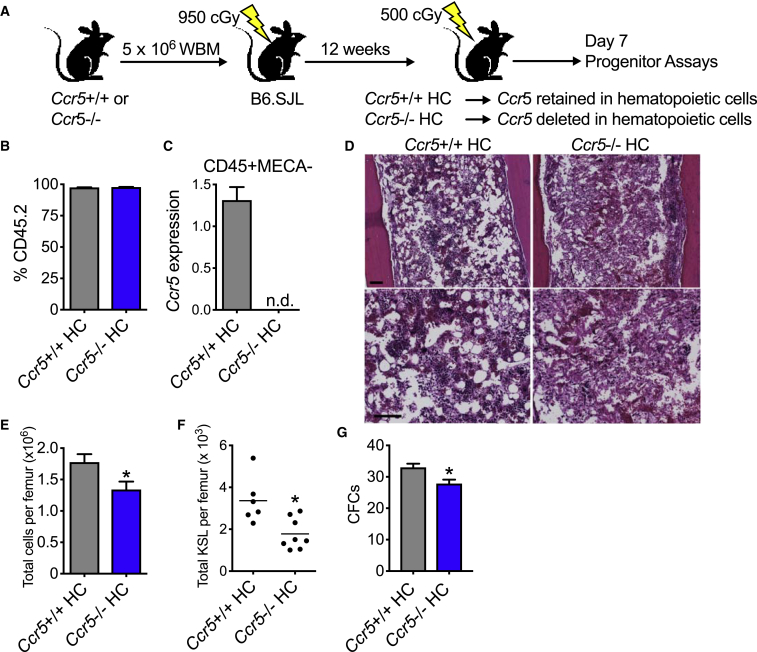
Deletion of Ccr5 in Hematopoietic Cells Is Sufficient to Delay Hematopoietic Regeneration
CCR5 is expressed on a number of cell subsets including hematopoietic cells and nonhematopoietic cells including ECs and fibroblasts (Rottman et al., 1997). We sought to isolate the effect of Ccr5 deficiency to hematopoietic cells. Using established hematopoietic transplantation models in which hematopoietic cells with desired genetic mutations are transplanted into wild-type recipient animals (Doan et al., 2013b, Shao et al., 2010), we generated chimeric mice with deletion of Ccr5 in hematopoietic cells only (Ccr5−/− hematopoietic cell [HC]) along with control mice that were recipients of Ccr5+/+ marrow (Ccr5+/+ HC, Figure 5A). At 12 weeks following transplantation, we confirmed full donor chimerism in both groups (Figure 5B). Using fluorescence-activated cell sorting analysis, we isolated hematopoietic cells that were CD45+ (a pan-hematopoietic cell marker) and cells that were negative for mouse endothelial cell antigen (MECA-32), an endothelial cell marker (Kopp et al., 2005). We detected Ccr5 mRNA expression in the hematopoietic cells of Ccr5+/+ HC mice, but not in Ccr5−/− mice (Figure 5C). At day 7 following 500 cGy TBI, Ccr5−/− HC mice display decreased marrow cellularity and increased hemorrhage (Figures 5D and 5E). There was also a corresponding decrease in KSL content and committed progenitors (Figures 5F and 5G). In competitive repopulating assays at day 7 following 500 cGy, Ccr5−/− HC and Ccr5+/+ HC had comparable levels of HSC function both in the peripheral blood and marrow at 16 weeks posttransplantation (Figures S7A–S7C). Since the hematopoietic response to radiation injury of chimeric mice with deletion of Ccr5 in hematopoietic cells only was attenuated compared with mice with constitutive deletion of Ccr5, these results demonstrate that CCR5 deficiency, specifically in hematopoietic cells, delays the hematopoietic response to radiation injury.
Figure 5.
Deletion of Ccr5 in Hematopoietic Cells Delays Hematopoietic Regeneration
(A) Schematic diagram of isolation of Ccr5 deficiency to the hematopoietic compartment. B6.SJL (CD45.1) recipient mice were irradiated with 950 cGy and then transplanted with 5 × 106 WBM cells from Ccr5+/+ or Ccr5−/− mice. At 12 weeks posttransplantation, donor chimerism was measured. Hematopoietic progenitor assays were performed on day 7 following 500 cGy TBI.
(B) Levels of peripheral blood donor engraftment by flow cytometric analysis in Ccr5+/+ HC or Ccr5−/− HC mice. n = 15 per group. Levels of mean engraftment were >97%.
(C) RT-PCR of Ccr5 mRNA expression of hematopoietic cells that are CD45+ and negative for mouse endothelial cell antigen (MECA). n.d., not detected. n = 3 per group. Data are normalized to Ccr5+/+ HC and GAPDH.
(D) H&E staining of femurs on day 7 following 500 cGy TBI. Scale bars, 100 μm.
(E–G) Bone marrow cell counts (E), total KSL cells per femur (F), and CFCs (G) per 4 × 104 WBM cells in Ccr5+/+ HC and Ccr5−/− HC at day 7 following 500 cGy TBI. n = 6–8 per group. ∗p = 0.03, ∗p = 0.007, and ∗p = 0.01 for total cells per femur, total KSL cells per femur, and CFCs, respectively. Data are shown as means ± SEM. Student's t test (two-tailed with unequal variance) was applied to the data.
To determine whether CCR5 in ECs and other cells within the microenvironment could contribute to hematopoietic regeneration (Rottman et al., 1997), we isolated the deletion of Ccr5 to the marrow microenvironment by transplanting wild-type hematopoietic cells (B6.SJL and CD45.1) into Ccr5+/+ or Ccr5−/− recipient mice (CD45.2, Figure S7D). At 12 weeks posttransplantation, mice displayed full donor engraftment (Figure S7E). At day 7 following 500 cGy, these chimeric mice display no differences in total cell counts or in CFC content (Figure S7F). These data demonstrate that Ccr5 expression in the marrow microenvironment could be dispensable for the hematopoietic response following ionizing irradiation.
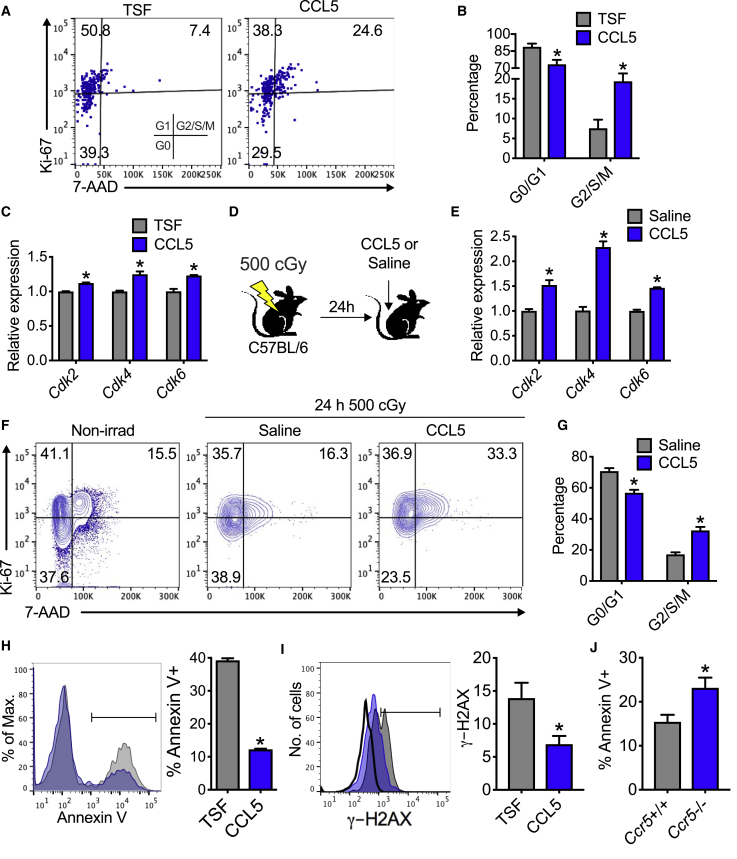
CCL5 Increases Cell Cycling and Cell Survival after Irradiation
Since CCL5 can induce cell cycling in certain cancer systems (Zhao et al., 2015), we sought to determine whether CCL5 could promote cell cycling following irradiation. When KSL cells are irradiated and then cultured with CCL5, there is a 2.6-fold increase in cells in G2/S/M phase compared with cultures with TSF alone (Figures 6A and 6B). Since cyclin-dependent kinases (Cdks) regulate cell cycle (Lim and Kaldis, 2013), we measured the levels of Cdk2, Cdk4, and Cdk6 in KSL cells (Figure 6C). At 72 h following 300 cGy, KSL cells cultured with CCL5 displayed increased mRNA expression of these Cdks (Figure 6C). Moreover, irradiated C57BL/6 mice treated with CCL5 display increased Cdk2, Cdk4, and Cdk6 compared with saline-treated mice (Figures 6D and 6E). Likewise, KSL cells from irradiated C57BL/6 mice display a 1.9-fold increase in G2/S/M cells by flow cytometric analysis with CCL5 treatment compared with saline controls (Figures 6F and 6G). These data suggest that both in vitro and in vivo treatment with CCL5 increases hematopoietic progenitor cell cycling following irradiation.
Figure 6.
CCL5 Promotes Cell Cycling and Cell Survival after Irradiation
(A) Left, representative flow cytometric analysis of KSL cells in G0 (Ki67−7-AAD−), G1 (Ki67+7-AAD−), and G2/S/M (Ki67+7-AAD+) phase at 72 h following 300 cGy in culture with CCL5 + TSF (CCL5) compared with TSF alone.
(B) Cell-cycle analysis of KSL cells at 72 h following 300 cGy in culture with CCL5 + TSF (CCL5) compared with TSF alone. n = 7–8 per group, ∗p = 0.009 and p = 0.005 for G0/G1 and G2/S/M, respectively.
(C) RT-PCR analysis of Cdk2, Cdk4, and Cdk6 mRNA expression from KSL cells at 72 h following 300 cGy and treatment with TSF or 30 ng/mL CCL5. n = 3 per group, ∗p = 0.0002, p = 0.004, and p = 0.004 for Cdk2, Cdk4, and Cdk6, respectively. Data are normalized to GAPDH and TSF for each Cdk.
(D) Schematic diagram of study design for Figures 6E–6G. At 24 h after 500 cGy TBI, mice were injected with saline or 0.1 μg/g body weight of CCL5 and analyzed at 2 h postinjection.
(E) RT-PCR analysis of Cdk2, Cdk4, and Cdk6 of BM Lin− cells. n = 4 per group, ∗p = 0.003, p < 0.0001, and p < 0.0001 for Cdk2, Cdk4, and Cdk6, respectively. Data are normalized to GAPDH and saline for each Cdk.
(F) Representative flow cytometric Ki67/7-AAD cell-cycle analysis from KSL cells following 500 cGy TBI and treatment with CCL5 or saline. Nonirradiated control is shown for comparison.
(G) Levels of G0/G1 and G2/S/M of KSL cells from irradiated CCL5- or saline-treated C57BL/6 mice. n = 5 per group, ∗p < 0.001 for G0/G1 and G2/S/M.
(H) Left, representative flow cytometric analysis of annexin V+ KSL cells and progeny at 72 h following 300 cGy in culture with CCL5 (blue line) compared with TSF alone (gray line). Right, quantification of total annexin V+ cells day 7 following 300 cGy and treatment with CCL5 or TSF alone. n = 3 per group, ∗p < 0.0001.
(I) Representative flow cytometric analysis and quantification of γ-H2AX in KSL and progeny following 300 cGy irradiation and 24 h culture with CCL5 (blue) or TSF alone (gray). Isotype is shown as black curve. n = 6 per group, ∗p = 0.02.
(J) Flow cytometric analysis of annexin V+ from KSL cells and progeny of Ccr5+/+ and Ccr5−/− mice at 72 h following 300 cGy. n = 6 per group, ∗p = 0.02. Data are shown as means ± SEM. Student's t test (two-tailed with unequal variance) was applied to the data.
To determine whether CCL5 could improve cell survival after irradiation, we performed flow cytometric analysis for annexin V+. Irradiated KSL cells in culture with CCL5 demonstrated significantly less annexin V+ staining compared with cultures with TSF alone (Figure 6H). Since irradiation can introduce DNA breaks (Kao et al., 2005), leading to cell death if not repaired, we measured γ-H2AX to quantify these DNA breaks (Figure 6I). With CCL5, irradiated KSL cells have 50% less DNA breaks compared with cultures with TSF alone (Figure 6I). In the absence of CCR5 signaling, KSL cells from Ccr5-deficient mice displayed increase levels of annexin V+ staining (Figure 6J). These data demonstrate that CCL5/CCR5 signaling ameliorate radiation-induced HSPC cell death in part via reduction of DNA breaks.
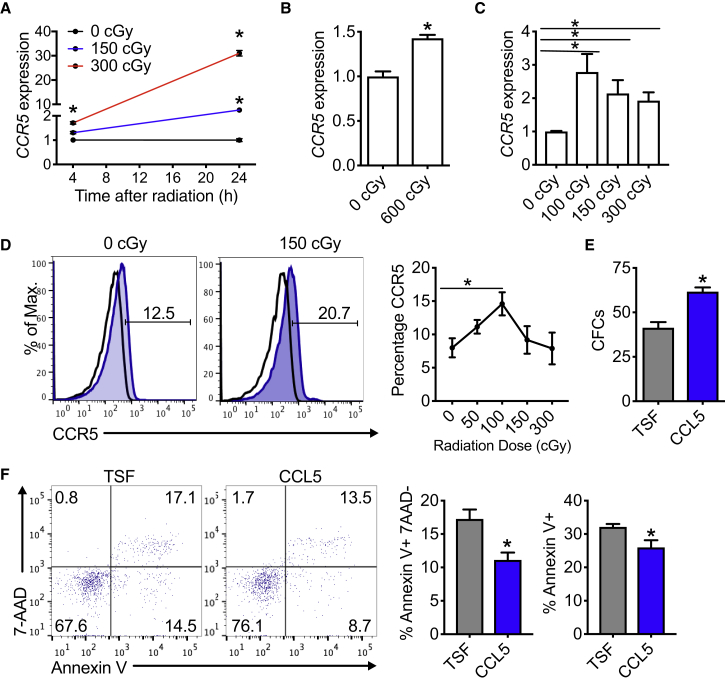
CCR5 Is Inducible in Human HSPCs following Irradiation
Since CCR5 expression is inducible following irradiation in murine HSPCs, we sought to determine whether CCR5 expression was translationally relevant to human hematopoietic cells. Following irradiation of cord blood mononuclear cells, CCR5 expression was increased proportionately to the levels of irradiation at both early (4 h) and later time points (24 h) (Figure 7A). At 24 h following irradiation, a 150-cGy dose resulted in a 2.5-fold increase in CCR5 expression. Strikingly, a 300-cGy dose resulted in a 30-fold increase in CCR5 (Figure 7A). Radiation exposure to human CD34+ marrow also increased CCR5 expression (Figure 7B). Likewise, CCR5 expression was inducible in peripheral blood of a group of healthy human donors with irradiation doses as low as 100 cGy (Figure 7C). Using flow cytometric analysis, CCR5 was also increased following irradiation in the peripheral blood of healthy donors (Figure 7D). Culture of human CD34+ BM with CCL5 increased the levels of CFUs compared with cultures with cytokines alone (Figure 7E). Consistent with the increase in CFUs, cultured human CD34+ BM with CCL5 displayed decreased apoptotic cell death compared with cultures with cytokines alone (Figure 7F). These data suggest that treatment with recombinant CCL5 could increase hematopoietic progenitor cells and ameliorate radiation-induced cell death. Since CCL5 promoted cell survival in murine cultures following irradiation, we demonstrate that CCL5 provided a survival benefit for human CD34+ BM cells after irradiation. These data suggest that irradiated human hematopoietic cells increase CCR5 expression, which, in part, could contribute to cell survival following radiation injury.
Figure 7.
CCR5 Expression Is Induced in Human Hematopoietic Stem/Progenitor Cells to Promote Cell Survival following Irradiation Injury
(A) CCR5 mRNA expression by RT-PCR of cord blood mononuclear cells at 4 and 24 h following either 150 or 300 cGy. Data are shown relative to 0 cGy for each time point. n = 3 per group. ∗p = 0.03 and p = 0.002 for 150 and 300 cGy versus 0 cGy at 4 h, respectively. ∗p = 0.0004 and p < 0.0001 for 150 and 300 cGy versus 0 cGy at 24 h, respectively.
(B) CCR5 mRNA expression of human bone marrow CD34+ cells at 24 h following irradiation. n = 3 per group. ∗p = 0.003.
(C) CCR5 mRNA expression of human peripheral blood from healthy donors at 16 h following irradiation. n = 14–15 per group. ∗p = 0.004, p = 0.008, and p = 0.001 for 100, 150, and 300 cGy versus 0 cGy, respectively.
(D) Representative flow cytometric analysis and quantification of CCR5 protein expression in human peripheral blood from healthy donors at 16 h following irradiation. n = 6–10 per group. ∗p = 0.02 for 100 versus 0 cGy.
(E) CFCs from cord blood CD34+ cells following 7 days in culture with 30 ng/mL CCL5 + TSF compared with TSF alone. n = 3 per group. ∗p = 0.007.
(F) Left, representative flow cytometric analysis of annexin V, 7-AAD stained human CD34+ bone marrow cells following 48 h in culture with 30 ng/mL CCL5 + TSF or TSF alone. Middle, levels of annexin V+, 7-AAD− cells; right, levels of annexin V+ cells at 48 h following culture with specified conditions. n = 4 per group. ∗p = 0.01 and p = 0.04, respectively. Data are shown as means ± SEM. Student's t test (two-tailed with unequal variance) was applied to the data.
Discussion
In an unbiased screen of cytokines from marrow supernatants, we identified CCL5 to be elevated in mice with deletion of proapoptotic proteins in Tie2+ cells compared with littermate control mice at 6 h following lethal dose TBI (Doan et al., 2013b). CCL5 differs from other inflammatory cytokines in that it has been reported to be elevated 3–5 days following T cell activation, with its later expression being associated with maintaining inflammation (Krensky and Ahn, 2007, Schall et al., 1988, Song et al., 2000). In the setting of ionizing radiation, CCL5 expression could be quickly induced in ECs within 2 h, and this expression increases at 24 h. Likewise, CCR5 is also responsive to radiation and increases expression on hematopoietic progenitor cells. Since both CCL5 and CCR5 increase following irradiation, this finding suggests that CCR5 signaling could be functional in the early hematopoietic response to radiation.
CCL5 has previously been shown to be a proinflammatory cytokine that accumulates in the aging marrow (Ergen et al., 2012). Either exposure to CCL5 or enforced expression of CCL5 resulted in lineage skewing to favor production of myeloid cells and a corresponding decrease in lymphocyte production. In our competitive transplantation studies, in which recipient mice receive donor cells from irradiated, CCL5-treated mice, these recipient mice did not demonstrate an increase in myeloid cells compared with control recipients. Notably, these studies were performed in younger mice (<12 weeks of age) at the time of transplantation compared with mice used in studies by Ergen et al. It is possible that the myeloid bias seen in the aging marrow requires additional cytokines that are altered in the elderly marrow (>70 weeks of age) or that the dosing of CCL5 in this current study was insufficient to reproduce lineage skewing.
Competitive transplantation studies with HSCs with either CCL5-deficient or CCL5-replete cells did not demonstrate differences in the levels of total engraftment (Ergen et al., 2012). Since ligands for CCR5 include CCL3 and CCL4, it is possible that these ligands could provide functional redundancy for CCR5 signaling when CCL5 alone is depleted (Lee et al., 2018, Menten et al., 2002). For these reasons, we sought to genetically abolish CCR5 signaling by using Ccr5-deficient mice. In loss-of-function studies, irradiated Ccr5-deficient mice display delayed HSC regeneration compared with Ccr5-replete mice. Since CCR5 is expressed on both hematopoietic and ECs (Rottman et al., 1997), we employed transplantation studies to isolate the deletion of Ccr5 to either hematopoietic cells or to the marrow microenvironment (i.e., ECs). We found that deletion of Ccr5 in hematopoietic cells delayed hematopoietic regeneration compared with control mice. Since the deletion of Ccr5 in nonhematopoietic cells did not alter hematopoietic regeneration, these findings suggest that Ccr5 deletion in ECs could be dispensable.
To make these findings in murine systems translationally relevant, we demonstrate that CCR5 expression is also inducible following irradiation in three human HC populations (cord blood, marrow, and peripheral blood). CCR5 expression increases with time (i.e., CCR5 expression is more highly expressed at 24 h compared with 4 h). Interestingly, CCR5 increases at lower doses of irradiation, although its expression is not sustained at higher doses, possibly due to increased necrotic cell death and degraded mRNA. This proportionate increase in CCR5 expression after irradiation in human hematopoietic cell populations, particularly in the peripheral blood, suggests that CCR5 could be used as a marker or dosimeter for accidental radiation exposures.
The discovery that people who bear homozygous mutations of CCR5 are resistant to HIV infection, and that those with heterozygous deletion of CCR5 have slower progression of HIV once infected, provided the rationale to investigate CCR5 in HIV (Huang et al., 1996, Liu et al., 1996). More recently, an HIV-infected patient who received an allogeneic HSC transplant for treatment of Hodgkin's lymphoma with donor cells bearing a homozygous mutation for CCR5 achieved HIV remission while off antiretroviral therapy (Gupta et al., 2019). CCR5 antagonists, such as maraviroc, are being developed to modulate the immune system both in infection and following HSC transplantation. Moreover, maraviroc is a small-molecule inhibitor that selectively and reversibly binds CCR5 (Gulick et al., 2008). Since CCR5 is a co-receptor for HIV, CCR5 antagonists can impair HIV infection and modulate the host immune system (Dean et al., 1996, Huang et al., 1996). In a study with over 1,000 patients, differences in complete blood counts or myelosuppression were not noted between treatment with maraviroc and placebo groups (Gulick et al., 2008). Since CCL5 could contribute to alloreactivity following hematopoietic cell transplantation through recruitment of lymphocytes (Choi et al., 2007), blockade of CCL1, CCL3, and CCL5 signaling with maraviroc was effective to prevent the onset of acute graft-versus-host disease (Moy et al., 2017, Reshef et al., 2012). In this phase I/II study to determine whether maraviroc could prevent GVHD following allogeneic transplantation, no impact on the levels of hematopoietic engraftment or toxicity was observed (Reshef et al., 2012). It is important to note that this study used reduced-intensity preparative regimens, which were performed without radiation. Like these clinical findings using maraviroc for CCR5 blockade, our data demonstrate no differences in hematopoietic profiles in mice with deletion of Ccr5 compared with control mice in the absence of irradiation. Whether CCR5 blockade following preparative regimens that include radiotherapy would be deleterious to hematopoiesis and engraftment was not investigated in these clinical studies. For these reasons, CCL5 could be employed cautiously to accelerate hematopoietic regeneration in recipients of HSC transplantation.
Whether CCL5 cooperates with other chemokines, such as CXCL12 or other growth factors, for hematopoietic regeneration is still unknown. Future studies are required to determine whether CCL5 could accelerate hematopoietic regeneration following other mechanisms of myelosuppressive stress such as chemotherapy. In summary, our data demonstrate that CCR5 signaling is a necessary pathway for hematopoietic regeneration. These data demonstrate that CCL5 could be used pharmacologically following either therapeutic or accidental radiation injury.
Experimental Procedures
Animals
Eight- to 12-week-old C57BL/6 (CD 45.2+), B6.SJL (CD 45.1+), and Ccr5−/− mice were purchased from Jackson Laboratory (Bar Harbor, ME). To isolate the deletion of Ccr5 to the hematopoietic compartment, Ccr5+/+ HC and Ccr5−/− HC mice were generated by transplanting 5 × 106 WBM from Ccr5+/+ or Ccr5−/− mice by tail vein injection into lethally irradiated (950 cGy) recipient B6.SJL mice. To isolate Ccr5 deletion to nonhematopoietic cells in the marrow, Ccr5+/+ EC or Ccr5−/− EC mice were generated by transplanting 5 × 106 WBM from B6.SJL mice into Ccr5+/+ or Ccr5−/− recipient mice. Donor chimerism was confirmed by flow cytometric analysis of percentage CD45.2+ or CD45.1+ cells (BD Biosciences; 560695, 553775). Biologic variables such as age, sex, and preirradiation weight were matched for each study. Duke University Animal Care and Use Committee approved all animal studies.
Measurements of CCL5 and CCR5 Expression
CCL5 was measured in conditioned media from cultures of C57BL/6 BM ECs using an ELISA detection kit according to the manufacturer’s specifications (R&D Systems; MMR00). Ccr5 mRNA expression analyses (Thermo Fisher Scientific; Mm01963251_s1) were performed on peripheral blood or BM subsets as indicated in the figure legends. Ccr5 expression was calculated using delta-delta CT analysis with normalization to GAPDH (Thermo Fisher Scientific; Mm99999915_g1) as described previously (Livak and Schmittgen, 2001). For flow cytometric analysis of CCR5, C57BL/6 mice were irradiated with 300 cGy TBI and were sacrificed at specified time points. Cells from femurs were collected by flushing into Iscove's Modification of DMEM (Corning; 10-016-cv) with 10% fetal bovine serum (HyClone, GE Healthcare Bio-Sciences; SH30071.03), and 1% penicillin-streptomycin (Gibco; 15140-122). Red blood cells were depleted with ammonium-chloride-potassium (ACK) lysing buffer (Lonza; 10-548E). WBM cells were stained with anti-CCR5 antibody (BioLegend; 313712), antilineage antibody APC (BD; 558074), anti-cKit PE (BD; 553355), anti-Sca-1 APC-Cy7 (BD; 560654), and 7-AAD (BD; 559925). Isotype controls were included for all analyses.
To measure Ccr5 expression in CD45+ MECA-marrow cells, cells from femurs were isolated with fluorescence-activated cell sorting, as described previously (Doan et al., 2013b), and then analyzed for Ccr5 expression as noted above.
Hematopoietic Progenitor Cell Assays, Histologic Analyses, and Survival Study
Complete blood counts were quantified on a HemaVet 950 (Drew Scientific, Dallas, TX). BM collection, KSL, SLAM + KSL cells, and CFC methylcellulose assays were performed using published methods (Doan et al., 2013a). H&E staining of femurs were performed by the Duke Research Immunohistology Lab. For CFU-S12 analysis, 2 × 105 WBM cells were injected into 900-cGy irradiated C57BL/6 mice. At day 12 postinjection, colonies were counted independently by two investigators. LTC-IC limiting dilution analyses were performed as described previously (Piryani et al., 2018). For competitive transplantation assays, donor mice were exposed to 500 cGy TBI and then sacrificed at 7 days postirradiation. Cell doses of 106 WBM donor cells and 2 × 105 nonirradiated host marrow were transplanted by single tail vein injection into 900-cGy irradiated recipient mice. Donor engraftment was measured in the BM at 12 weeks posttransplantation using flow cytometric analysis using published methods (Piryani et al., 2018).
For the survival study, C57BL/6 mice were exposed to 700 cGy TBI. Twenty-four hours later, mice received subcutaneous injections of either 0.1 μg/g body weight of CCL5 (R&D Systems; 478-MR-050) in 200 μL volume or 1× sterile PBS. Injections were administered three times per week until day 15 for a total of 7 doses. Animals were monitored for survival or were sacrificed for humane endpoints according to approved animal protocols.
HSC Cycling and Cell Survival Assays
RT-PCR for Cdk2, Cdk4, and Cdk6 was performed on irradiated KSL cells or BM Lin− cells as specified in the figure legends (Thermo Fisher Scientific; Mm00443947_m1, Mm00726334_s1, Mm01311342_m1, respectively). Expression was calculated using delta-delta CT analysis with normalization to GAPDH and control groups. For in vitro cell-cycle analysis by flow cytometry, KSL-labeled cells were treated with Fix Buffer I (BD; 557870), Perm Buffer III (BD; 558050), Ki67-FITC (BD; 556026), and 7-AAD (BD; 559925) according to the manufacturer’s specifications (BD). For in vivo cell-cycle analysis, C57BL/6 mice were irradiated with 500 cGy. At 24 h, mice were injected subcutaneously with saline or 0.1 μg/g body weight of CCL5. At 2 h postinjection, marrow cells were collected, depleted of red blood cells, and enriched for Lin− cells using a lineage cell depletion kit and LS columns according to the manufacturer's specifications (Miltenyi Biotech; 130-090-858, 130-042-401). Cells were labeled for KSL, Ki67/7-AAD, and analyzed by flow cytometry as described above.
For cell death analysis, KSL cells were irradiated with 300 cGy and then cultured for 72 h or 7 days in 30 ng/mL CCL5 in 20 ng/mL thrombopoietin (R&D Systems; 488-TO-200), 125 ng/mL stem cell factor (R&D Systems; 455-MC-500), and 50 ng/mL Flt-3 ligand (R&D Systems; 427-FL-025) (TSF) (Doan et al., 2013a) compared with TSF alone. KSL cells and progeny were stained with annexin V FITC and 7-AAD according to the manufacturer's specifications (BD; 556419). For analysis for expression of γ-H2AX, 300-cGy irradiated KSL cells were cultured for 24 h with 30 ng/mL CCL5 + TSF or TSF alone. Cells were fixed in Fix Buffer I, permeabilized in Phosflow Perm Buffer III, and labeled with anti-γ-H2AX antibody (BD; 562377).
Human Specimens and Assays
De-identified cord blood was obtained from the Carolinas Cord Blood Bank (Durham, NC). De-identified human BM was obtained from healthy marrow donors. Peripheral blood was obtained from healthy donors. Male and female subjects were included. Samples were collected from subjects older than 18 years of age. Samples were collected in heparinized tubes, irradiated at specified doses, and cultured without additional cytokine or media supplementation at 37°C. At 16 h, red blood cells were depleted with ACK lysing buffer, and labeled with anti-CCR5 antibody (BioLegend). RNA from human specimens was isolated using RNeasy Kit (QIAGEN; 74104) according to the manufacturer's specifications. Cell survival analysis was performed as noted above. Written and informed consent was obtained from donors. The Duke Institutional Review Board approved all studies.
Statistical Analyses and Image Capture
Data are shown as means ± SEM. Student's t test (two-tailed with unequal variance), or one-way ANOVA analyses were used as specified in the figure legends. Survival analysis was performed using a log rank test. Analyses were performed using GraphPad Prism (v.8.0, La Jolla, CA).
Images for immunohistochemical analysis were obtained with Zeiss AxioImager Z2 and Axiocam 506.
Author Contributions
S.O.P., A.Y.F.K., and U.T.V. performed the experiments, analyzed the data, and reviewed the paper. N.J.C. provided samples of human peripheral blood, discussed the experiments, and reviewed the paper. P.L.D. conceived of the study, designed the experiments, performed the experiments, analyzed the data, and wrote the paper. The authors declare no competing interests.
Acknowledgments
We thank Benny J. Chen, MD (Duke University) for scientific discussion. We thank Evelyna G. Kliassov and Hee Su Park for technical support. This work was funded by the National Cancer Institute of the NIH under Award Number K08CA184552 (to P.L.D.), Duke Strong-Start Physician-Scientist Award (to P.L.D.), and the Duke Cancer Institute (to P.L.D.).
Published: May 30, 2019
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.stemcr.2019.04.023.
Supplemental Information
References
- Butler J.M., Nolan D.J., Vertes E.L., Varnum-Finney B., Kobayashi H., Hooper A.T., Seandel M., Shido K., White I.A., Kobayashi M. Endothelial cells are essential for the self-renewal and repopulation of Notch-dependent hematopoietic stem cells. Cell Stem Cell. 2010;6:251–264. doi: 10.1016/j.stem.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi S.W., Hildebrandt G.C., Olkiewicz K.M., Hanauer D.A., Chaudhary M.N., Silva I.A., Rogers C.E., Deurloo D.T., Fisher J.M., Liu C. CCR1/CCL5 (RANTES) receptor-ligand interactions modulate allogeneic T-cell responses and graft-versus-host disease following stem-cell transplantation. Blood. 2007;110:3447–3455. doi: 10.1182/blood-2007-05-087403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean M., Carrington M., Winkler C., Huttley G.A., Smith M.W., Allikmets R., Goedert J.J., Buchbinder S.P., Vittinghoff E., Gomperts E. Genetic restriction of HIV-1 infection and progression to AIDS by a deletion allele of the CKR5 structural gene. Hemophilia Growth and Development Study, Multicenter AIDS Cohort Study, Multicenter Hemophilia Cohort Study, San Francisco City Cohort, ALIVE Study. Science. 1996;273:1856–1862. doi: 10.1126/science.273.5283.1856. [DOI] [PubMed] [Google Scholar]
- Ding L., Morrison S.J. Haematopoietic stem cells and early lymphoid progenitors occupy distinct bone marrow niches. Nature. 2013;495:231–235. doi: 10.1038/nature11885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding L., Saunders T.L., Enikolopov G., Morrison S.J. Endothelial and perivascular cells maintain haematopoietic stem cells. Nature. 2012;481:457–462. doi: 10.1038/nature10783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doan P.L., Himburg H.A., Helms K., Russell J.L., Fixsen E., Quarmyne M., Harris J.R., Deoliviera D., Sullivan J.M., Chao N.J. Epidermal growth factor regulates hematopoietic regeneration after radiation injury. Nat. Med. 2013;19:295–304. doi: 10.1038/nm.3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doan P.L., Russell J.L., Himburg H.A., Helms K., Harris J.R., Lucas J., Holshausen K.C., Meadows S.K., Daher P., Jeffords L.B. Tie2(+) bone marrow endothelial cells regulate hematopoietic stem cell regeneration following radiation injury. Stem Cells. 2013;31:327–337. doi: 10.1002/stem.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ergen A.V., Boles N.C., Goodell M.A. Rantes/CCL5 influences hematopoietic stem cell subtypes and causes myeloid skewing. Blood. 2012;119:2500–2509. doi: 10.1182/blood-2011-11-391730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluzman-Poltorak Z., Mendonca S.R., Vainstein V., Kha H., Basile L.A. Randomized comparison of single dose of recombinant human IL-12 versus placebo for restoration of hematopoiesis and improved survival in rhesus monkeys exposed to lethal radiation. J. Hematol. Oncol. 2014;7:31. doi: 10.1186/1756-8722-7-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluzman-Poltorak Z., Vainstein V., Basile L.A. Recombinant interleukin-12, but not granulocyte-colony stimulating factor, improves survival in lethally irradiated nonhuman primates in the absence of supportive care: evidence for the development of a frontline radiation medical countermeasure. Am. J. Hematol. 2014;89:868–873. doi: 10.1002/ajh.23770. [DOI] [PubMed] [Google Scholar]
- Gulick R.M., Lalezari J., Goodrich J., Clumeck N., DeJesus E., Horban A., Nadler J., Clotet B., Karlsson A., Wohlfeiler M. Maraviroc for previously treated patients with R5 HIV-1 infection. N. Engl. J. Med. 2008;359:1429–1441. doi: 10.1056/NEJMoa0803152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta R.K., Abdul-Jawad S., McCoy L.E., Mok H.P., Peppa D., Salgado M., Martinez-Picado J., Nijhuis M., Wensing A.M.J., Lee H. HIV-1 remission following CCR5Delta32/Delta32 haematopoietic stem-cell transplantation. Nature. 2019;568:244–248. doi: 10.1038/s41586-019-1027-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himburg H.A., Doan P.L., Quarmyne M., Yan X., Sasine J., Zhao L., Hancock G.V., Kan J., Pohl K.A., Tran E. Dickkopf-1 promotes hematopoietic regeneration via direct and niche-mediated mechanisms. Nat. Med. 2017;23:91–99. doi: 10.1038/nm.4251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himburg H.A., Muramoto G.G., Daher P., Meadows S.K., Russell J.L., Doan P., Chi J.T., Salter A.B., Lento W.E., Reya T. Pleiotrophin regulates the expansion and regeneration of hematopoietic stem cells. Nat. Med. 2010;16:475–482. doi: 10.1038/nm.2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himburg H.A., Termini C.M., Schlussel L., Kan J., Li M., Zhao L., Fang T., Sasine J.P., Chang V.Y., Chute J.P. Distinct bone marrow sources of pleiotrophin control hematopoietic stem cell maintenance and regeneration. Cell Stem Cell. 2018;23:370–381.e5. doi: 10.1016/j.stem.2018.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper A.T., Butler J.M., Nolan D.J., Kranz A., Iida K., Kobayashi M., Kopp H.G., Shido K., Petit I., Yanger K. Engraftment and reconstitution of hematopoiesis is dependent on VEGFR2-mediated regeneration of sinusoidal endothelial cells. Cell Stem Cell. 2009;4:263–274. doi: 10.1016/j.stem.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y., Paxton W.A., Wolinsky S.M., Neumann A.U., Zhang L., He T., Kang S., Ceradini D., Jin Z., Yazdanbakhsh K. The role of a mutant CCR5 allele in HIV-1 transmission and disease progression. Nat. Med. 1996;2:1240–1243. doi: 10.1038/nm1196-1240. [DOI] [PubMed] [Google Scholar]
- Kao J., Rosenstein B.S., Peters S., Milano M.T., Kron S.J. Cellular response to DNA damage. Ann. N. Y. Acad. Sci. 2005;1066:243–258. doi: 10.1196/annals.1363.012. [DOI] [PubMed] [Google Scholar]
- Kiel M.J., Yilmaz O.H., Iwashita T., Terhorst C., Morrison S.J. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 2005;121:1109–1121. doi: 10.1016/j.cell.2005.05.026. [DOI] [PubMed] [Google Scholar]
- Kobayashi H., Butler J.M., O'Donnell R., Kobayashi M., Ding B.S., Bonner B., Chiu V.K., Nolan D.J., Shido K., Benjamin L. Angiocrine factors from Akt-activated endothelial cells balance self-renewal and differentiation of haematopoietic stem cells. Nat. Cell Biol. 2010;12:1046–1056. doi: 10.1038/ncb2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopp H.G., Avecilla S.T., Hooper A.T., Shmelkov S.V., Ramos C.A., Zhang F., Rafii S. Tie2 activation contributes to hemangiogenic regeneration after myelosuppression. Blood. 2005;106:505–513. doi: 10.1182/blood-2004-11-4269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krensky A.M., Ahn Y.T. Mechanisms of disease: regulation of RANTES (CCL5) in renal disease. Nat. Clin. Pract. Nephrol. 2007;3:164–170. doi: 10.1038/ncpneph0418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuziel W.A., Dawson T.C., Quinones M., Garavito E., Chenaux G., Ahuja S.S., Reddick R.L., Maeda N. CCR5 deficiency is not protective in the early stages of atherogenesis in apoE knockout mice. Atherosclerosis. 2003;167:25–32. doi: 10.1016/s0021-9150(02)00382-9. [DOI] [PubMed] [Google Scholar]
- Lee D., Shin K.J., Kim D.W., Yoon K.A., Choi Y.J., Lee B.N.R., Cho J.Y. CCL4 enhances preosteoclast migration and its receptor CCR5 downregulation by RANKL promotes osteoclastogenesis. Cell Death Dis. 2018;9:495. doi: 10.1038/s41419-018-0562-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim S., Kaldis P. Cdks, cyclins and CKIs: roles beyond cell cycle regulation. Development. 2013;140:3079–3093. doi: 10.1242/dev.091744. [DOI] [PubMed] [Google Scholar]
- Liu G.T., Huang Y.L., Tzeng H.E., Tsai C.H., Wang S.W., Tang C.H. CCL5 promotes vascular endothelial growth factor expression and induces angiogenesis by down-regulating miR-199a in human chondrosarcoma cells. Cancer Lett. 2015;357:476–487. doi: 10.1016/j.canlet.2014.11.015. [DOI] [PubMed] [Google Scholar]
- Liu R., Paxton W.A., Choe S., Ceradini D., Martin S.R., Horuk R., MacDonald M.E., Stuhlmann H., Koup R.A., Landau N.R. Homozygous defect in HIV-1 coreceptor accounts for resistance of some multiply-exposed individuals to HIV-1 infection. Cell. 1996;86:367–377. doi: 10.1016/s0092-8674(00)80110-5. [DOI] [PubMed] [Google Scholar]
- Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Mendelson A., Frenette P.S. Hematopoietic stem cell niche maintenance during homeostasis and regeneration. Nat. Med. 2014;20:833–846. doi: 10.1038/nm.3647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menten P., Wuyts A., Van Damme J. Macrophage inflammatory protein-1. Cytokine Growth Factor Rev. 2002;13:455–481. doi: 10.1016/s1359-6101(02)00045-x. [DOI] [PubMed] [Google Scholar]
- Moy R.H., Huffman A.P., Richman L.P., Crisalli L., Wang X.K., Hoxie J.A., Mick R., Emerson S.G., Zhang Y., Vonderheide R.H. Clinical and immunologic impact of CCR5 blockade in graft-versus-host disease prophylaxis. Blood. 2017;129:906–916. doi: 10.1182/blood-2016-08-735076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petit I., Szyper-Kravitz M., Nagler A., Lahav M., Peled A., Habler L., Ponomaryov T., Taichman R.S., Arenzana-Seisdedos F., Fujii N. G-CSF induces stem cell mobilization by decreasing bone marrow SDF-1 and up-regulating CXCR4. Nat. Immunol. 2002;3:687–694. doi: 10.1038/ni813. [DOI] [PubMed] [Google Scholar]
- Piryani S.O., Kam A.Y.F., Kliassov E.G., Chen B.J., Spector N.L., Chute J.P., Hsu D.S., Chao N.J., Doan P.L. Epidermal growth factor and granulocyte colony stimulating factor signaling are synergistic for hematopoietic regeneration. Stem Cells. 2018;36:252–264. doi: 10.1002/stem.2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reshef R., Luger S.M., Hexner E.O., Loren A.W., Frey N.V., Nasta S.D., Goldstein S.C., Stadtmauer E.A., Smith J., Bailey S. Blockade of lymphocyte chemotaxis in visceral graft-versus-host disease. N. Engl. J. Med. 2012;367:135–145. doi: 10.1056/NEJMoa1201248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottman J.B., Ganley K.P., Williams K., Wu L., Mackay C.R., Ringler D.J. Cellular localization of the chemokine receptor CCR5. Correlation to cellular targets of HIV-1 infection. Am. J. Pathol. 1997;151:1341–1351. [PMC free article] [PubMed] [Google Scholar]
- Sax M.J., Gasch C., Athota V.R., Freeman R., Rasighaemi P., Westcott D.E., Day C.J., Nikolic I., Elsworth B., Wei M. Cancer cell CCL5 mediates bone marrow independent angiogenesis in breast cancer. Oncotarget. 2016;7:85437–85449. doi: 10.18632/oncotarget.13387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schall T.J., Jongstra J., Dyer B.J., Jorgensen J., Clayberger C., Davis M.M., Krensky A.M. A human T cell-specific molecule is a member of a new gene family. J. Immunol. 1988;141:1018–1025. [PubMed] [Google Scholar]
- Shao L., Sun Y., Zhang Z., Feng W., Gao Y., Cai Z., Wang Z.Z., Look A.T., Wu W.S. Deletion of proapoptotic Puma selectively protects hematopoietic stem and progenitor cells against high-dose radiation. Blood. 2010;115:4707–4714. doi: 10.1182/blood-2009-10-248872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song A., Nikolcheva T., Krensky A.M. Transcriptional regulation of RANTES expression in T lymphocytes. Immunol. Rev. 2000;177:236–245. doi: 10.1034/j.1600-065x.2000.17610.x. [DOI] [PubMed] [Google Scholar]
- Sugiyama T., Kohara H., Noda M., Nagasawa T. Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity. 2006;25:977–988. doi: 10.1016/j.immuni.2006.10.016. [DOI] [PubMed] [Google Scholar]
- Tzeng Y.S., Li H., Kang Y.L., Chen W.C., Cheng W.C., Lai D.M. Loss of Cxcl12/Sdf-1 in adult mice decreases the quiescent state of hematopoietic stem/progenitor cells and alters the pattern of hematopoietic regeneration after myelosuppression. Blood. 2011;117:429–439. doi: 10.1182/blood-2010-01-266833. [DOI] [PubMed] [Google Scholar]
- Zhao L., Wang Y., Xue Y., Lv W., Zhang Y., He S. Critical roles of chemokine receptor CCR5 in regulating glioblastoma proliferation and invasion. Acta Biochim. Biophys. Sin. 2015;47:890–898. doi: 10.1093/abbs/gmv095. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.