Abstract
Oxidative stress contributes to endothelial dysfunction, a key step in cardiovascular disease development. Ageing-related vascular dysfunction involves defective antioxidant response. Nuclear factor erythroid 2-like-2 (Nrf2), orchestrates cellular response to oxidative stress. We evaluated the impact of Nrf2-activation on endothelium-dependent and H2O2-mediated vasodilations in: aorta (RA), mesenteric artery (RMA), coronary artery (RCA) and corpus cavernosum (RCC) from ageing rats and in human penile arteries (HPRA) and corpus cavernosum (HCC) from erectile dysfunction (ED) patients. Relaxant responses were evaluated in organ chambers and wire myographs. Nrf2 content and heme oxygenase-1 (HO-1) were determined by ELISA. Superoxide and Nrf2 were detected by immunofluorescence. Pharmacological activation of Nrf2 with sulforaphane (SFN) improved NO- and endothelium-derived hyperpolarizing factor-mediated endothelium-dependent vasodilation and H2O2-induced relaxation in vascular beds from aging rats. SFN-induced effects were associated with increased Nrf2 (RMA, RCA) and reduced superoxide detection in RCA. Improvement of vascular function was confirmed in HPRA and HCC from ED patients and mimicked by another Nrf2 activator, oltipraz. Nrf2 increase and superoxide reduction together with HO-1 increase by Nrf2 activation was evidenced in HCC from ED patients. PDE5 inhibitor-induced relaxations of HPRA and HCC from ED patients were enhanced by SFN. Nrf2 short-term pharmacological activation attenuates age-related impairment of endothelium-dependent and reactive oxygen species (ROS)-induced vasodilation in different rat and human vascular territories by upregulation of Nrf2-related signaling and decreased oxidative stress. In ED patients target tissues, Nrf2 potentiates the functional effect of ED conventional pharmacological therapy suggesting potential therapeutic implication.
Keywords: Oxidative stress, Endothelial dysfunction, Nrf2, Ageing, Human vasculature, Erectile dysfunction
Abbreviations
- ACh
acetylcholine
- ADMA
asymmetric dimethyl arginine
- CVD
cardiovascular disease
- DAPI
diamidino-2-phenylindole
- DHE
dihydroethidium
- DMSO
dimethyl sulfoxide
- ED
erectile dysfunction
- EDHF
endothelium-derived hyperpolarizing factor
- HCC
human corpus cavernosum
- HO-1
heme oxygenase-1
- H2O2
hydrogen peroxide
- HPRA
human penile resistance arteries
- 5-HT
serotonin
- INDO
indomethacin
- KHS
Krebs-Henseleit solution
- l-NAME
L-nitro-arginine methyl ester
- NE
norepinephrine
- Nrf2
nuclear factor erythroid 2-like 2
- OLT
oltipraz
- PDE5
Type 5 phosphodiesterase
- PE
phenylephrine
- RA
rat aorta
- RCA
rat coronary arteries
- RCC
rat corpus cavernosum
- RMA
rat mesenteric arteries
- SFN
sulforaphane
- SNP
sodium nitroprusside
- TAD
tadalafil
1. Introduction
Vascular dysfunction and, specifically, endothelial dysfunction represents a key step in the development of cardiovascular disease (CVD) [1]. Oxidative stress has been proposed as an outstanding mechanism in endothelial dysfunction associated with different pathological situations and ageing [[2], [3], [4]]. Moreover, manifestations of CVD are related to the presence of oxidative stress [5]. Imbalance between reactive oxygen species (ROS) generation and antioxidant defense systems progressively leads to endothelial dysfunction, vascular damage and CVD [[6], [7], [8]]. In this sense, antioxidant therapies have been proposed to prevent endothelial dysfunction and, hence, CVD [9].
Similarly to some pathological situations, ageing contributes to the progressive decline of endothelial function and is considered the main risk factor for CVD. In this sense, the ageing process is associated with reduction of the endothelium-dependent vasodilation, both in the micro and the macrovasculature derived from animal models [[10], [11], [12]] and humans [[13], [14], [15]]. A progressive impact of ageing on endothelial function asynchronously affecting different vascular territories in the rat has been recently reported, pointing to coronary vascular bed as especially vulnerable to ageing process [16]. Although the mechanisms responsible for ageing-related vascular dysfunction have not been completely elucidated, inflammation and oxidative stress outstand as most probable candidate processes leading to vascular impairment in ageing [2,3,17]. The maintenance of a correct function of the vascular bed seems to be an essential determinant of healthy ageing [18]. Supporting the role of endothelial function in determining ageing outcome, exceptional longevity in humans has been reported to be associated with the expression of the variant of a protein promoting endothelial function and repair [19] and asymmetric dimethyl arginine (ADMA) has been shown to be associated to functional impairment in older people [20].
Vascular impairment is a determinant for the development of erectile dysfunction (ED) [21] and ageing represents the main risk factor for this condition [22,23]. Although age-related co-morbidities could influence the increase of ED prevalence with ageing [[21], [22], [23]], the impact of ageing process on penile vascular structures plays a key role by itself in the development of ED [24,25]. In fact, as for CVD, ED has been shown to be related to functional outcomes in older people [26].
Adequate antioxidant response is essential for tissue homeostasis and function. However, in some pathological situations and ageing, systems responsible for antioxidant response are impaired. This is the case for nuclear factor erythroid 2-like 2 (NFE2L2), commonly known as Nrf2, which orchestrates cellular response to oxidative stress and plays an important role in protecting from endothelial injuries, preserving cardiovascular function, and regulating the ageing process [[27], [28], [29]]. In fact, Nrf2 signaling has been proposed to play an outstanding role in the decline of adaptive homeostasis in ageing [30]. Although ROS play hormetic roles by facilitating physiological responses in different systems [29,31], a chronic elevation of ROS levels, as it is the case in ageing or diabetes, hampers the physiological responses induced by an acute generation of ROS [3]. In fact, a progressive decline in the ability of hydrogen peroxide (H2O2) to cause relaxation of different rat vessels has been detected during ageing [16]. Despite an increase in superoxide production, vasculature from aged animals displays a decrease in Nrf2 activity [32]. The importance of Nrf2 activity on human ageing outcome is supported by the recent evidence showing that low expression of NFE2L2 gene and some of its target genes in peripheral blood is related to increased risk for frailty in community-dwelling older people [33].
Some evidences point to endothelial protective effects by Nrf2 in cell culture approaches and vessels from chronically treated animals [28,29,[34], [35], [36]]. In fact, deletion of Nrf2 gene in rats results in endothelial dysfunction, oxidant stress and microvascular alterations [37]. Furthermore, polymorphisms of the gene for Nrf2 have been associated with impaired vasodilator responses in humans [38]. However, the short-term effects of pharmacological activation of Nrf2 on endothelial function in ageing as well as the functional impact of Nrf2 activation on endothelial function in human vasculature are unknown. The aim of the present work was to determine the impact of short-term Nrf2 activation on endothelium-dependent and H2O2-mediated vasodilations in different vascular beds, with different endothelium functional characteristics: aorta, mesenteric artery, coronary artery and corpus cavernosum from ageing rats, as well as to analyze the effects of Nrf2 activation on functional and molecular response in human penile arteries and corpus cavernosum from patients with erectile dysfunction (ED) that is representative of human endothelial dysfunction.
2. Methods
2.1. Experimental animals
Animal studies were performed in accordance with the Declaration of Helsinki and with the Guide for the Care and Use of Laboratory Animals, as adopted and promulgated by National Institutes of Health, following European regulations and were approved by the Ethics Committees for Animal Experimentation of the Hospital Universitario de Getafe and the Hospital Universitario Ramón y Cajal, as well as by regional authorities (PROEX 005/15). Three months old (young, n = 26) and 20 months old (aged, n = 37) male Sprague-Dawley rats were obtained from the Animal Facilities of the Hospital Universitario de Getafe. Rats were bred and let to age in the facilities. Animals were maintained in 12 h light/dark cycles with free access to food and water until experimental procedures. Rats were weighed, anesthetized with diazepam (5 mg/kg) and ketamine (90 mg/kg) and exsanguinated by carotid arteries section. Always under deep anaesthesia, exsanguination caused the death of the animals. Right after blood extraction, heart, thoracic aorta, omentum (for isolation of mesenteric small vessels), and penis were carefully excised for functional evaluations. Experiments with rats from both ages were intercalated to avoid possible sequence-dependent bias.
2.2. Functional evaluation of rat aortic segments
The thoracic aorta was carefully excised, cleaned of surrounding fat and connective tissue and placed in a Petri dish with Krebs-Henseleit solution (KHS) at 4 °C. Composition of KHS was (in mM): NaCl 119, KCl 4.6, CaCl2 2.5, MgCl2 1.2, NaHCO3 24.9, glucose 11, KH2PO4 1.2 and EDTA 0.027. Aortae were cut into 4 to 5 mm-long cylindrical segments. For circular isometric tension recording, each vascular cylinder was set up in an organ bath containing KHS at 37 °C continuously bubbled with 95% O2/5% CO2 mixture, which gave a pH of 7.4, according to the method described elsewhere [16,39,40]. Tension was continuously recorded in a data acquisition system (MP100A BIOPAC System, Santa Barbara, CA, USA). To assess vessel viability, preparations were then exposed to 75 mM K+ and the contractile response was measured. Aortic segments were contracted with norepinephrine (NE, 10–30 nM; 80% of K+-induced contraction, approximately) and, when a stable plateau was reached, increasing concentrations of acetylcholine (ACh, 0.01–10 μM), H2O2 (0.1 μM–0.1 mM) or sodium nitroprusside (SNP, 1 nM to 1 μM) were added and vasodilatory responses were determined. Each preparation was exposed to just one of these pharmacological agents. This also applies for the other vascular preparations. Sulforaphane (SFN, 10 μM) or the vehicle (0.1% DMSO), were added 60 min before the concentration-response curves started.
2.3. Vascular reactivity of rat mesenteric arteries
Second to third order branches of mesenteric arterial tree (lumen diameter 200–400 μm) were obtained from omentum specimens and dissected by carefully removing the adhering fat tissue. Arterial ring segments (~2 mm long) were subsequently mounted on microvascular wire myographs (Danish MyoTechnology, Aarhus, Denmark) for circular isometric tension recordings, as previously described [16,39,40]. The vessels were allowed to equilibrate for 30 min in KHS continuously bubbled with 95% O2/5% CO2 mixture to maintain a pH of 7.4. The passive tension and internal circumference of vascular segments when relaxed in situ under a transmural pressure of 100 mmHg (L100), were determined. The arteries were then set to an internal circumference equivalent to 90% of L100, at which the force development is close to maximal. To assess vessel viability, preparations were then exposed to 125 mM K+ (KKHS, equimolar substitution of NaCl for KCl in KHS) and the contractile response was measured. After a washout and stabilization period, rat arteries were contracted with 1–3 μM NE (80% of KKHS-induced contraction, approximately) and relaxation responses were evaluated by cumulative additions of ACh (1 nM–10 μM), H2O2 (1 μM–1 mM), or SNP (1 nM–100 μM) to the chambers. In some indicated experiments, KCl (25–50 mM) was used instead of NE for contracting vascular preparations. SFN (10 μM) or the vehicle was added 60 min before the concentration-response curves started while L-nitro-arginine methyl ester (l-NAME, 100 μM) and indomethacin (INDO, 10 μM) were added 30 min before the concentration-response curves started. Where indicated, endothelium was removed by repeatedly passing a human hair through the lumen of mounted arterial segments. After that, viability of the arterial preparation and success in endothelium removal were confirmed by exposure to KKHS and 10 μM ACh, respectively.
2.4. Functional evaluation of rat coronary arteries
Left and right coronary arteries (average diameter: 351 ± 7 μm) were isolated from excised hearts and cleaned from surrounding cardiac tissue as previously described [16]. Segments of coronary arteries (~2 mm long) were set in wire myographs for isometric tension recording in the same way as above described for mesenteric arteries. For relaxation experiments, coronary arterial segments were contracted with 1–3 μM serotonin (5-HT; 80% of 125 mM K+ (KKHS)-induced contraction, approximately) and relaxation responses were evaluated by cumulative additions of ACh (1 nM–10 μM), H2O2 (1 μM–1 mM), or SNP (1 nM–10 μM) to the chambers. In some set of experiments the effects of the Nrf2 inhibitor, trigonelline (TRIG, 30 μM), were evaluated on SFN-induced effects in this vascular bed.
Furthermore, in some indicated experiments, KCl (25–50 mM) was used instead of 5-HT for contracting vascular preparations. There were no differences in vascular responses between right and left coronary arteries (data not shown). SFN (3–10 μM), oltipraz (OLT, 30 μM) or the vehicle were added 60 min before the concentration-response curves started while l-NAME (100 μM) and INDO (10 μM) were added 30 min before the concentration-response curves started. Where indicated, endothelium was removed by repeatedly passing a human hair through the lumen of mounted arterial segments. After that, viability of the arterial preparation and success in endothelium removal were confirmed by exposure to KKHS and 10 μM ACh, respectively.
2.5. Functional evaluation of rat corpus cavernosum tissues
Two strips of corpus cavernosum from each penis were carefully dissected through respective longitudinal incisions along the tunica albuginea. As previously described, strips of rat corpus cavernosum were mounted on force transducers in 8 ml organ baths (37 °C) containing KHS continuously bubbled with 95% O2/5% CO2 mixture and submitted to 0.3 g of resting tension [16,41,42]. After 60 min equilibration period, tissues were exposed to 75 mM K+ to assess vessel viability and contraction was measured. For relaxation experiments, strips were contracted with 1–3 μM phenylephrine (PE; 80% of K+-induced contraction, approximately) and relaxation responses were evaluated by cumulative additions of carbachol (CCh, 1 nM to 10 μM), H2O2 (1 μM–1 mM), or SNP (1 nM–10 μM) to the chambers. SFN (3–10 μM), OLT (30 μM), or the vehicle was added 60 min before the concentration-response curves started.
2.6. Human tissues
Human corpus cavernosum specimens were obtained from men with ED who gave written informed consent at the time of penile prosthesis implantation. Protocols and consent forms signed by ED patients were approved by the Ethic Committees at the hospitals where the tissues were collected: Hospital Santo Antonio, Porto, in Portugal (2015.210(174-DEFI/156-CES) and Hospital Puerta de Hierro Majadahonda, Madrid, in Spain (Acta 288/27-05-13). Tissues were maintained at 4-6 °C in M − 400 solution (composition per 100 ml: mannitol, 4.19 g; KH2PO4, 0.205 g; K2HPO4·3H2O, 0.97 g; KCl, 0.112 g; NaHCO2, 0.084 g; pH 7.4) until their use, which ranged between 16 and 24 h from extraction [39,41,[43], [44], [45]]. Clinical characteristics from ED patients are depicted in Table 1. Cavernosal tissue was also obtained from twelve organ donors without erectile dysfunction (No ED) with no reported history of ED at the time of organ collection for transplantation at Hospital Doce de Octubre, Madrid, Spain, after obtaining written informed consent by their relatives (Ethics Approval procedure 16/045). Tissues from No ED were handled exactly as described for those from ED patients. No ED were free of known cardiovascular risk factors and were 36.2 ± 3.6 years old in average.
Table 1.
Characteristics of patients with erectile dysfunction (ED) from whom the tissues were collected.
| n | 59 |
|---|---|
| Age (years) | 62.0 ± 1.0 |
| Hypertension (%) | 26 (44.1) |
| Type 1 diabetes (%) | 3 (5.1) |
| Type 2 diabetes (%) | 18 (30.5) |
| Dislipidemia (%) | 19 (32.2) |
| Smoking habit (%) | 9 (15.2) |
| Obesity (%) | 4 (6.8) |
| Cardiovascular disease (%) | 12 (20.3) |
| Pulmonary disease (%) | 8 (13.6) |
Numerical variable (age) is expressed as mean ± SEM. n indicates number of patients.
2.7. Functional evaluation of human corpus cavernosum
Strips of cavernosal tissue (3 × 3 × 7 mm) were immersed in 8 ml organ chambers containing KHS maintained at 37 °C and aerated with 95% O2/5% CO2, pH 7.4, for isometric tension recording as previously described [39,41,[43], [44], [45]]. Each tissue strip was incrementally stretched to optimal isometric tension, as determined by maximal contractile response to 1 μM PE. The preparations were then exposed to 125 mM K+ (KKHS) and the contractile response was measured. Strips were contracted with 1–3 μM PE (80% of KKHS-induced contraction, approximately), and relaxation response was evaluated by cumulative additions of ACh (1 nM–10 μM), H2O2 (1 μM–1 mM), SNP (1 nM–10 μM), or tadalafil (TAD, 1 nM to 10 μM) to the chambers. SFN (10 μM), OLT (30 μM) or the vehicle was added 60 min before the concentration-response curves started.
2.8. Functional evaluation of human penile resistance arteries
Small penile arteries — helicine arteries (lumen diameter 150 μm–400 μm), which are the terminal branches of deep penile arteries — were dissected by carefully removing the adhering trabecular tissue. Then arterial ring segments (2-mm long) were mounted on microvascular wire myographs for circular isometric tension recordings, as previously described [39,44,46]. The arteries were then set to an internal circumference equivalent to 90% in the same way as described above for rat mesenteric and coronary arteries. The preparations were then exposed to 125 mM K+ (KKHS) and the contractile response was measured. The arteries were contracted with 1–3 μM NE (80% of KKHS-induced contraction, approximately), and relaxation response was evaluated by cumulative additions of ACh (1 nM–10 μM), H2O2 (1 μM–1 mM), SNP (1 nM–100 μM), or tadalafil (TAD, 1 nM to 100 μM) to the chambers. SFN (3–10 μM), OLT (30 μM) or the vehicle were added 60 min before the concentration-response curves started while l-NAME (100 μM) and INDO (10 μM) were added 30 min before the concentration-response curves started.
Since the development of a stable contractile tone is a key aspect for adequate evaluation of vascular relaxation responses, the selection of the different agonists to induce the contractile tone for each specific vascular bed is based on the previous experience with all of the vascular territories evaluated in the present study [16,[39], [40], [41], [42], [43], [44], [45], [46]]. In this sense, both α-adrenergic-induced- (NE and PE) and serotonin-induced contractions seems to involve common intracellular signalling for contraction of vascular smooth muscle [47]. In the same sense, CCh was used instead of ACh (both have the same pharmacological activity on muscarinic receptors but CCh is resistant to acetylcholinesterase activity) because our previous experience showed that this muscarinic agonist displayed more consistent relaxations in this tissue [41,42].
2.9. Determination of Nrf2 content in rat mesenteric arteries
Isolated rat mesenteric arteries specimens, free from adherent connective tissue and fat, were incubated for 1 h with SFN (10 μM) or vehicle in KHS oxygenated with 95% O2/5% CO2 at 37 °C. The last 10 min, H2O2 (300 μM) was further added to the organ bath chambers. Subsequently, tissues were removed, frozen in liquid N2 and stored at −80 °C until assay determination. Frozen tissues were homogenized in PBS with MagNa Laser electric homogenizer (Roche Diagnostics GmbH, Mannheim, Germany). Total protein concentration was determined by BCA method (Thermo Scientific, Rockford, IL, USA) and Nrf2 concentration was assessed by using a colorimetric ELISA kit (Cloud-Clone Corporation, Houston, TX, USA) in accordance with the manufacturer's instructions. Nrf2 levels evaluated in rat mesenteric artery homogenates were normalized to the protein content of each sample. All samples were assessed in duplicate.
2.10. Detection of Nrf2 in human and rat vascular tissue
Rat coronary arteries and human corpus cavernosum specimens were incubated for 1 h with SFN (10 μM), OLT (30 μM) (only human corpus cavernosum) or vehicle and additionally with H2O2 (300 μM) for further 10 min in KHS oxygenated with 95% O2/5% CO2 at 37 °C. Untreated HCC strips from No ED subjects and ED patients were used for comparison of basal Nrf2 levels in these two populations. Tissues were removed, immersed in saccharose (30% w/v), embedded in OCT and stored at −80 °C until immunofluorescence assay. OCT blocks were cut in a cryostat and mounted on polylysine-coated glass slides. 6 μm-thick sections were blocked for 30 min with PBS containing 2% BSA and then incubated with rabbit antibodies against Nrf2 (1:100 dilution; Abcam, Cambridge, UK, cat# ab31163) overnight at 4 °C. After washout in PBS plus 0.05% Triton X-100, the sections were incubated with a secondary Alexa Fluor 488-conjugated goat anti-rabbit antibody (dilution 1:250; Life Technologies, Alcobendas, Spain) and with 300 nM diamidino-2-phenylindole (DAPI, Life Technologies) to counterstain nuclei for 1 h at room temperature. Sections were mounted and viewed by fluorescence microscopy (Olympus BX51, Japan). Controls without primary antibodies showed no unspecific reactivity (data not shown). Five random images from each specimen were captured and area displaying fluorescence was quantified and normalized by total nuclei number by using Image J software (McBiophotonics Image J, NIH, Bethesda, Maryland). An average value for each specimen was obtained. The treatment corresponding to each specimen was blinded for the investigator capturing and quantifying immunofluorescence images.
2.11. Detection of superoxide anion generation
Rat coronary arteries and human corpus cavernosum specimens were treated, processed and stored exactly as described for Nrf2 detection. Untreated human corpus cavernosum strips from No ED subjects and ED patients were used for comparison of basal superoxide levels in these two populations. In situ production of superoxide anion was measured by means of the fluorescent dye dihydroethidium (DHE) as described previously [48]. Briefly, 6 μm-thick sections of rat coronary arteries and human corpus cavernosum were incubated with DHE (4 μM; Invitrogen, Life Technologies Corporation, Eugene, USA) for 30 min at 37 °C in a humidified chamber protected from light. In the presence of superoxide anion, DHE is oxidized to ethidium that yields bright red fluorescence. After washing with PBS plus 0.05% Triton X-100, sections were mounted and visualized by fluorescence microscopy. The percentage of nuclei showing positive red signal with respect to total nuclei (counterstained with DAPI as above described) was determined with Image J imaging software.
2.12. Determination of heme oxygenase-1 (HO-1) content in human corpus cavernosum
Human corpus cavernosum tissue specimens were incubated for 1 h with SFN (10 μM), OLT (30 μM) or vehicle and for the last 10 min additionally with H2O2 (300 μM) in KHS oxygenated with 95% O2/5% CO2 at 37 °C. Tissues were removed, snap frozen in liquid N2 and stored at −80 °C until determinations. At that moment, frozen tissues were homogenized and disrupted in 2 ml centrifuge tubes containing PBS and 3 mm (mean diameter) stainless steel beads with the TissueLyser LT (Qiagen, Hilden, Germany) following manufacturer instructions. The homogenates were centrifuged at 10000 g for 5 min. One aliquot of the supernatant was used for protein concentration measurement by BCA method while HO-1 content was determined by using a commercial kit from R&D Systems (Minneapolis, MN, USA).
2.13. Statistical analysis
pEC50 is defined as the –log M of the concentration required to obtain 50% of maximal relaxation. For comparison of complete concentration-response curves a two-factors analysis of variance (ANOVA) test was applied by means of StatView software for Apple computers (SAS, Cary, NC). This statistical test compares concentration-response curves in its entirety, including all concentrations in the analysis. All other data were compared by t-test or by one-factor ANOVA followed by Student-Newman-Keuls test for multiple comparisons using GraphPad InStat (San Diego, CA, USA).
3. Results
3.1. Nrf2 activation recovers ageing-related endothelial dysfunction in rats
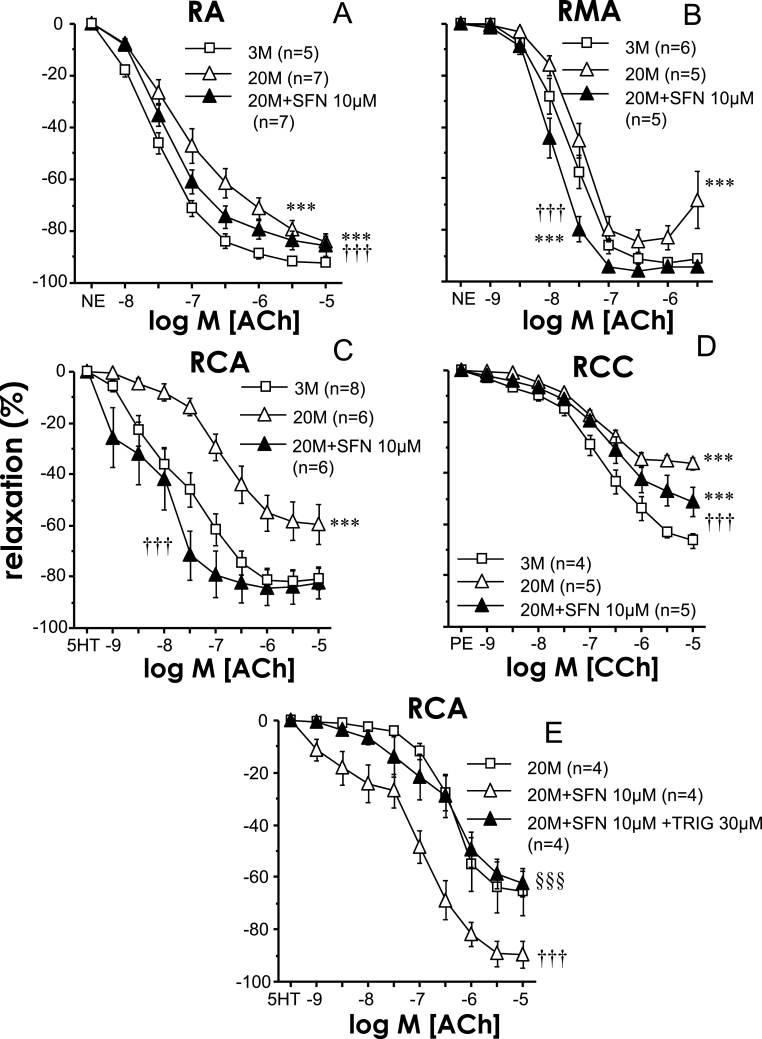
Ageing caused a generalized impairment of endothelial relaxation in the different vessels studied in the rat, as demonstrated by the significant reduction in ACh- or CCh-induced relaxation in aortae (pEC50 for ACh 6.72 ± 0.19 vs 7.34 ± 0.08 for 20 M and 3 M, respectively, p < 0.05), mesenteric arteries (pEC50 for ACh 7.41 ± 0.10 vs 7.59 ± 0.08 for 20 M and 3 M, respectively, p < 0.05), coronary arteries (pEC50 for ACh 5.79 ± 0.33 vs 7.36 ± 0.29, for 20 M and 3 M, respectively, p < 0.01) or corpus cavernosum (pEC50 for CCh 4.55 ± 0.01 vs 6.18 ± 0.19 for 20 M and 3 M, respectively, p < 0.05) from 20 months old rats when compared with responses obtained in vessels from 3 months old rats (Fig. 1). Pharmacological activation of Nrf2 with SFN (10 μM) for 1 h improved endothelium-dependent relaxation in all vascular tissues from ageing rats. In rat mesenteric (pEC50 for ACh 7.89 ± 0.07, p < 0.05 vs 20 M without SFN) and coronary arteries (pEC50 for ACh 8.02 ± 0.37, p < 0.001 vs 20 M without SFN) endothelial impairment was completely reversed by SFN, while in aorta (pEC50 for ACh 7.24 ± 0.08, p < 0.05 vs 20 M without SFN) and corpus cavernosum (pEC50 for CCh 5.28 ± 0.36 p < 0.05 vs 20 M without SFN) it was partially reversed (Fig. 1). In coronary arteries from old animals, the reduction in SFN concentration to 3 μM resulted in a significant potentiation of endothelial vasodilation but of lower magnitude than 10 μM concentration (+1.09 vs. +2.23 log units of pEC50 increment for Ach, p = 0.048), suggesting a concentration-dependent effect by SFN. Given the clear potentiation of ACh-induced responses driven by SFN in coronary arteries from aged rats, the effects of the Nrf2 inhibitor, trigonelline (TRIG, 30 μM), were evaluated on SFN-induced effects in this vascular bed. Inhibition of Nrf2 with TRIG prevented the potentiation of endothelium-dependent relaxations caused by SFN (10 μM) in coronary arteries from aged rats (Fig. 1E). Treatment with SFN (10 μM) failed to significantly modify ACh/CCh-induced relaxations in all the above mentioned vascular beds derived from young (3 months old) rats (Supplementary Table 1).
Fig. 1.
Sulforaphane improves endothelium-dependent vasodilation in aorta (RA), mesenteric arteries (RMA), coronary arteries (RCA), and corpus cavernosum (RCC) from aged rats. Endothelium-dependent relaxation to acetylcholine (ACh) in RA (A) and RMA (B) precontracted with norepinephrine (NE) and in RCA (C) precontracted with serotonin (5-HT), and to carbachol (CCh) in RCC (D) precontracted with phenylephrine (PE) obtained from young (three months old, 3 M) and aged (20 months old, 20 M) rats and effects of sulforaphane (SFN, 10 μM) or vehicle (0.1% DMSO) on such responses in aged rats. Panel E shows the effects of the Nrf2 inhibitor, trigonelline (TRIG, 30 μM) on SFN-induced potentiation of endothelial vasodilations in RCA from aged rats (20 M). Data are expressed as mean ± SEM of the percentage of maximal relaxation induced by papaverine (0.1 mM) at the end of the experiment. n indicates the number of animals. ***p < 0.001 vs. data obtained in 3 M rats, †††p < 0.001 vs. 20 M rats and §§§p < 0.001 vs. 20 M + SFN by a two-factors ANOVA test.
An additional Nrf2 activator, oltipraz (OLT), was evaluated on endothelium-dependent relaxation in coronary arteries and corpus cavernosum of aged rats, where a complete and partial recovery of endothelial function by SFN was achieved, respectively. Sixty minutes incubation with OLT (30 μM) significantly improved endothelium dependent relaxations in coronary arteries (pEC50 for ACh 5.81 ± 0.23 vs. 6.93 ± 0.21 for vehicle and OLT, respectively, n = 7, p < 0.05) and in corpus cavernosum (pEC50 for CCh 5.51 ± 0.28 vs. 6.84 ± 0.22 for vehicle and OLT, respectively, n = 4, p < 0.05).
3.2. Improvement of H2O2-induced responses by Nrf2 activation in vessels from aged rats
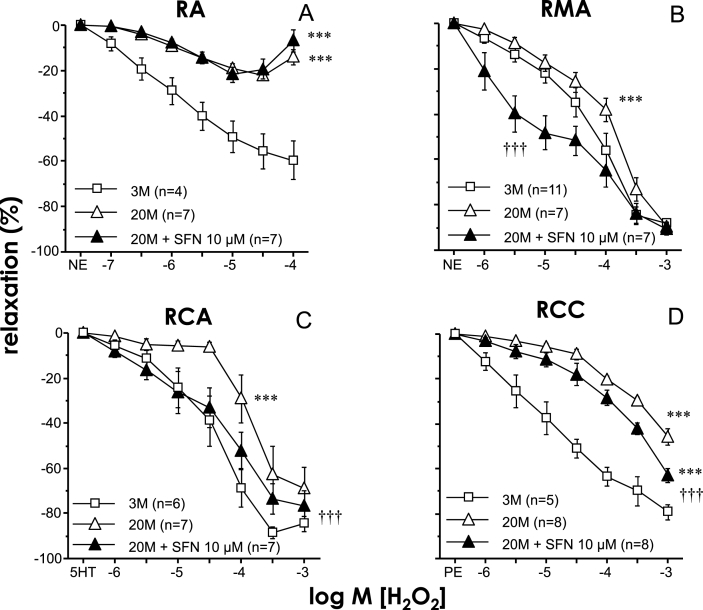
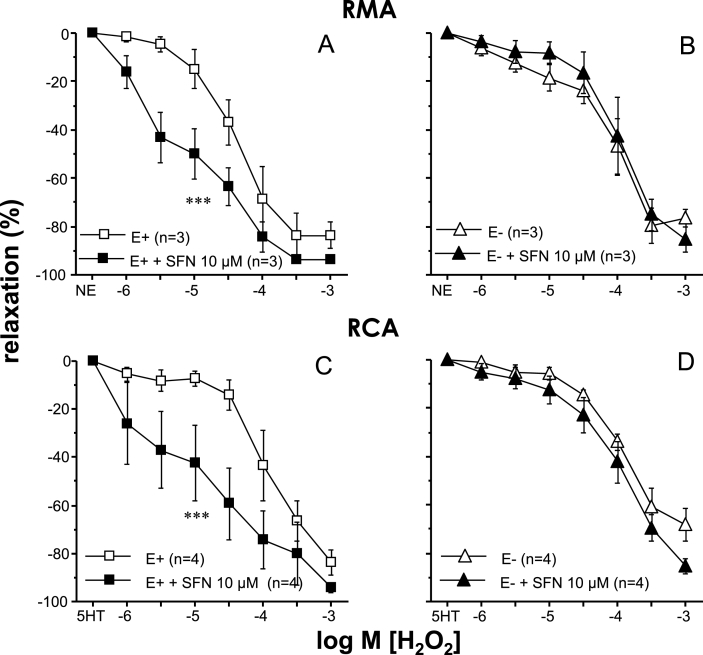
Acute exposure to H2O2 promoted vascular relaxation in all vascular beds studied while these relaxant responses were significantly reduced in vessels from aged rats (Fig. 2). Treatment with SFN (10 μM) resulted in improved relaxations to H2O2 in mesenteric and coronary arteries and in corpus cavernosum from aged rats but failed to increase these responses in aortae from old rats (Fig. 2). Endothelium was removed in some mesenteric and coronary artery segments from old rats to evaluate the influence of the endothelium on SFN-induced effects. Endothelial denudation caused a reduction in vasodilatory capacity of H2O2 in these arteries and abolished the potentiating effects of SFN (Fig. 3). Treatment with SFN (10 μM) did not induce significant modifications of H2O2-induced relaxations in all the above mentioned vascular beds from young rats (Supplementary Table 1).
Fig. 2.
Sulforaphane improves hydrogen peroxide (H2O2)-induced vasodilation in mesenteric arteries (RMA), coronary arteries (RCA), and corpus cavernosum (RCC) from aged rats. Relaxation induced by H2O2 in RA (A) and RMA (B) precontracted with norepinephrine (NE), in RCA (C) precontracted with serotonin (5-HT), and in RCC (D) precontracted with phenylephrine (PE) obtained from young (three months old, 3 M) and aged (20 months old, 20 M) rats and effects of sulforaphane (SFN, 10 μM) or vehicle (0.1% DMSO) on such responses in aged rats. Data are expressed as mean ± SEM of the percentage of maximal relaxation induced by papaverine (0.1 mM) at the end of the experiment. n indicates the number of animals. ***p < 0.001 vs. data obtained in 3 M rats, †††p < 0.001 vs. 20 M rats by two-factors ANOVA test.
Fig. 3.
Relevance of endothelium in sulforaphane-induced effects on H2O2-induced relaxations. Relaxation induced by H2O2 in mesenteric arteries (RMA, A-B) precontracted with norepinephrine (NE) and in coronary arteries (RCA, C-D) precontracted with serotonin (5-HT) obtained from old (20 months old, 20 M) rats. The influence of adding sulforaphane (SFN, 10 μM) or vehicle (0.1% DMSO) on such responses in intact arteries (E+) (A, C) and in those where endothelium was mechanically removed (E−) (B, D) is shown. Data are expressed as mean ± SEM of the percentage of maximal relaxation induced by papaverine (0.1 mM). n indicates the number of animals. ***p < 0.001 by two-factors ANOVA test vs E+.
In aged rats, treatment with another Nrf2 activator (OLT, 30 μM) produced a significant potentiation of H2O2-induced relaxations in coronary arteries (pEC50 for H2O2 3.68 ± 0.10 vs. 4.07 ± 0.14 for vehicle and OLT, respectively, n = 7, p < 0.05) and in corpus cavernosum (pEC50 for H2O2 2.99 ± 0.32 vs. 4.52 ± 0.38 for vehicle and OLT, respectively, n = 5, p < 0.05).
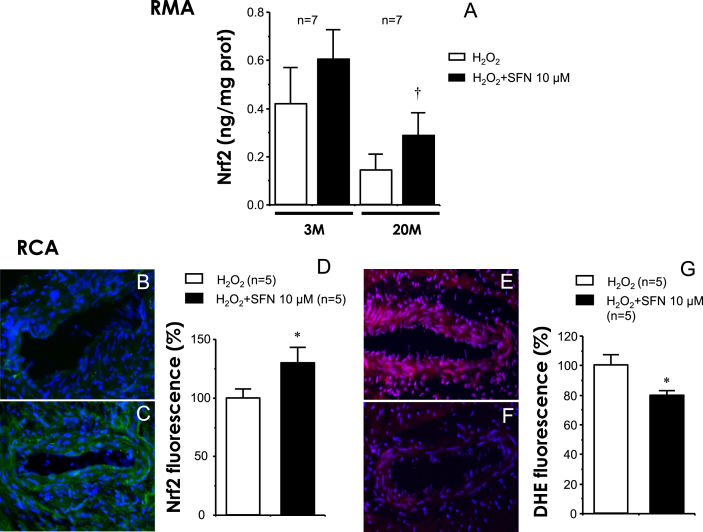
Treatment with SFN increases Nrf2 content in rat mesenteric and coronary arteries, while reduces superoxide in coronary arteries exposed to H2O2
In rat mesenteric arteries, where Nrf2 activation exerted a clear functional effect, the treatment with SFN (10 μM) for 60 min resulted in an increase in Nrf2 protein content in arteries exposed to H2O2 in aged rats (p = 0.047) but not in young rats (p = 0.381) (Fig. 4A). In fact, although not reaching statistical significance (p = 0.058), a reduction in Nrf2 protein content after exposure to H2O2 was observed in mesenteric arteries from aged rats with respect to young animals (Fig. 4A).
Fig. 4.
Sulforaphane increases Nrf2 content and decreases oxidative stress in arteries from aged rats. Panel A shows the effect of sulforaphane (SFN 10 μM) on Nrf2 content in mesenteric arteries (RMA) from young (three months old, 3 M) and old (20 months old, 20 M) rats exposed to H2O2. Data are expressed as mean ± SEM of ng of Nrf2 per mg of tissue protein. n indicates the number of animals. †p < 0.05 vs. H2O2 alone by paired t-test. Panels B and C show representative images (x200) of Nrf2 detection by immunofluorescence (green) in RCA from aged (20 M) rats treated with vehicle (0.1% DMSO) (B) or SFN 10 μM (C) for 60 min and then exposed to H2O2 (300 μM) for 10 min. Panels E and F show representative images (x200) of dihydroethidium (DHE) detection (red) in RCA from 20 M rats treated vehicle (E) or SFN (F) as above described. Counterstaining of nuclei with DAPI in blue is merged into all images. Panels D and G show quantification of Nrf2 and DHE-induced fluorescence, respectively, relative to number of nuclei. Data are expressed as mean ± SEM of the percentage with respect to H2O2 alone. n indicates the number of animals. *p < 0.05 vs. H2O2 by Student t-test. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
In coronary arteries derived from aged rats, where a functional effect by Nrf2 activators was also clearly observed, the increase in Nrf2 protein expression by SFN was confirmed by immunofluorescence detection since the availability of tissue amount of coronary arteries is markedly reduced with respect to mesenteric arteries. Treatment with SFN (10 μM) for 60 min enhanced the Nrf2 detection in coronary arteries from aged rats exposed to H2O2 (Fig. 4B and C) and that effect reached statistical significance after quantification of fluorescence signal (Fig. 4D).
The oxidative stress status was evaluated by detecting superoxide content with DHE probe in coronary arteries from aged rats exposed to H2O2. In these arteries, a significant reduction in superoxide production was detected after treatment for 60 min with SFN (10 μM) (Fig. 4E–G).
Nrf2 activation improves endothelial and ROS-induced relaxation in human dysfunctional vascular tissue.
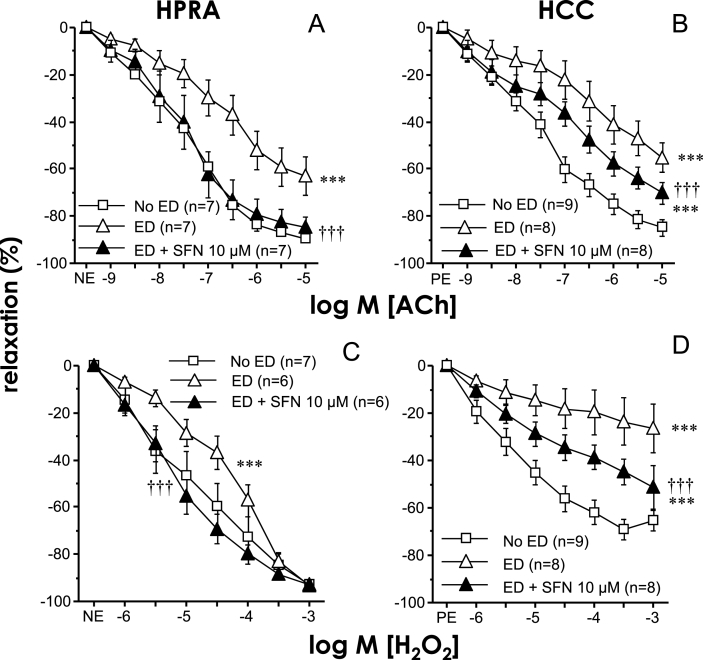
As shown in Table 1, patients with ED from whom the cavernosal and penile arterial tissues were collected presented a significant number of cardiovascular risk factors including advanced age in many cases (age range was 46–80 years). We have previously demonstrated that these tissues are dysfunctional in terms of endothelial responses [[43], [44], [45]]. In fact, a significant impairment of both ACh- and H2O2-induced relaxations in penile resistance arteries (pEC50 for ACh 5.82 ± 0.38 vs 7.17 ± 0.17 for ED and No ED, respectively, p < 0.05) as well as in corpus cavernosum strips (pEC50 for ACh 5.18 ± 0.28 vs 7.25 ± 0.25 for ED and No ED, respectively, p < 0.05) from ED patients was here confirmed when compared to tissues from No ED without history of ED (Fig. 5). Treatment of penile resistance arteries from ED patients for 60 min with SFN (10 μM) resulted in a significant potentiation of endothelial vasodilation (Fig. 5A) leading to complete recovery of ACh-induced relaxations (pEC50 for ACh 7.21 ± 0.32, p < 0.05 vs ED without SFN). This potentiating effect by SFN (10 μM) on endothelial relaxations was also observed in corpus cavernosum but it was not associated with a complete recovery of such responses (pEC50 for ACh 6.34 ± 0.37, p < 0.05 vs ED without SFN) (Fig. 5B). A significant potentiation of H2O2-induced relaxations was also produced by treating penile resistance arteries or corpus cavernosum with the Nrf2 activator SFN (10 μM). In the same way as for ACh-induced responses, the impairment of H2O2-induced responses in penile tissues from ED patients was completely reversed by SFN in penile arteries while a partial reversion was produced in corpus cavernosum (Fig. 5C and D). The enhancement in ACh-induced relaxations in penile tissues from ED patients was reproduced with the additional Nrf2 activator, OLT (30 μM) (pEC50 for ACh 5.35 ± 0.56 vs. 7.21 ± 0.39, p < 0.05, in penile resistance arteries, and 5.06 ± 0.27 vs. 6.27 ± 0.36, p < 0.05, in corpus cavernosum, for vehicle and OLT treated tissues, respectively). Similarly, OLT (30 μM) was able to significantly improve relaxations driven by H2O2 in tissues from ED patients (pEC50 for H2O2 4.34 ± 0.20 vs. 5.12 ± 0.24, p < 0.05, in penile resistance arteries, and 3.67 ± 0.61 vs. 5.06 ± 0.18, p < 0.05, in corpus cavernosum, for vehicle and OLT treated tissues, respectively).
Fig. 5.
Sulforaphane improves endothelium-dependent and H2O2-induced relaxations in human vascular tissue. Relaxation induced by acetylcholine (ACh, A-B) and H2O2 (C–D) in human penile resistance arteries (HPRA, A, C) and human corpus cavernosum (HCC, B, D) from patients with erectile dysfunction (ED) and from organ donors without erectile dysfunction (No ED). The influence of adding sulforaphane (SFN, 10 μM) on such responses in HPRA and HCC from ED patients is shown. Data are expressed as mean ± SEM of the percentage of maximal relaxation induced by papaverine (0.1 mM). n indicates the number of patients. ***p < 0.001 vs. No ED and ††† vs. ED by two-factors ANOVA test.
3.3. Mild effect of Nrf2 activation on endothelium-independent relaxations
In contrast to the generalized positive effect observed by Nrf2 activation on endothelial relaxation in all evaluated vascular preparations, its impact on relaxations induced by directly acting on smooth muscle with a nitric oxide (NO) analogue was limited. Of all rat and human vascular tissues evaluated, SFN (10 μM) slightly but significantly potentiated SNP-induced vasodilations in coronary arteries from aged rats and penile resistance arteries from ED patients (Supplementary Fig. 1).
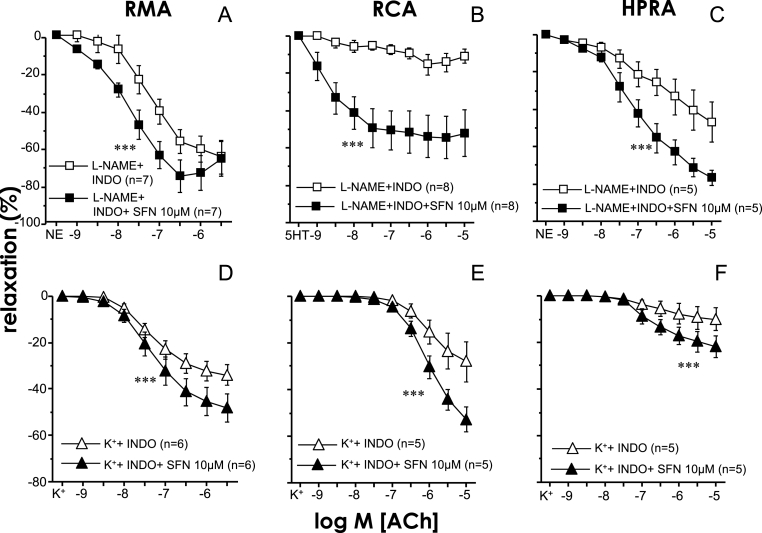
Nrf2 activation enhances both NO and EDHF components of endothelium-dependent vasodilation in rat and human arteries.
When the endothelium-derived hyperpolarizing factor (EDHF) component of endothelial vasodilation was isolated in the presence of the NO synthase (NOS) inhibitor, l-NAME (100 μM) and the COX inhibitor, indomethacin (10 μM), the treatment with SFN (10 μM) was able to significantly increase these EDHF-mediated vasodilations in rat mesenteric and coronary arteries as well as in human penile resistance arteries (Fig. 6A–C). In addition, when vessels were preincubated with indomethacin and a high concentration of K+ (25–50 mM) to isolate the NO component of endothelial vasodilation, SFN (10 μM) was also able to significantly enhance the NO-mediated vasodilations in both the animal and human arterial preparations (Fig. 6D–F).
Fig. 6.
Sulforaphane improves both endothelium-derived hyperpolarizing factor (EDHF)- and NO-mediated components of endothelial vasodilation in arteries from aged rats and human patients. Effects of sulforaphane (SFN, 10 μM) or the vehicle (0.1% DMSO) on relaxation induced by acetylcholine (ACh) in mesenteric arteries (RMA) (A, D) and coronary arteries (RCA) (B, E) from aged (20 M) rats and in human penile resistance arteries (HPRA) (C, F) from patients with erectile dysfunction (ED). Upper panels show ACh-induced relaxations in the presence of NG-nitro l-arginine methyl ester (l-NAME, 100 μM) and indomethacin (INDO, 10 μM) to isolate the EDHF component of endothelial vasodilation. Lower panels show ACh-induced relaxations in arteries contracted with KCl (25–50 mM) in the presence of INDO to isolate the NO component of endothelial vasodilation. Data are expressed as mean ± SEM of the percentage of maximal relaxation induced by papaverine (0.1 mM). n indicates the number of animals (A, B, D, E) or patients (C, F). ***p < 0.001 vs. l-NAME + INDO or vs K++ INDO by two-factors ANOVA test.
Treatment with SFN or OLT effectively increases Nrf2 protein content, decreases superoxide production, and augments HO-1 protein content in human cavernosal tissue.
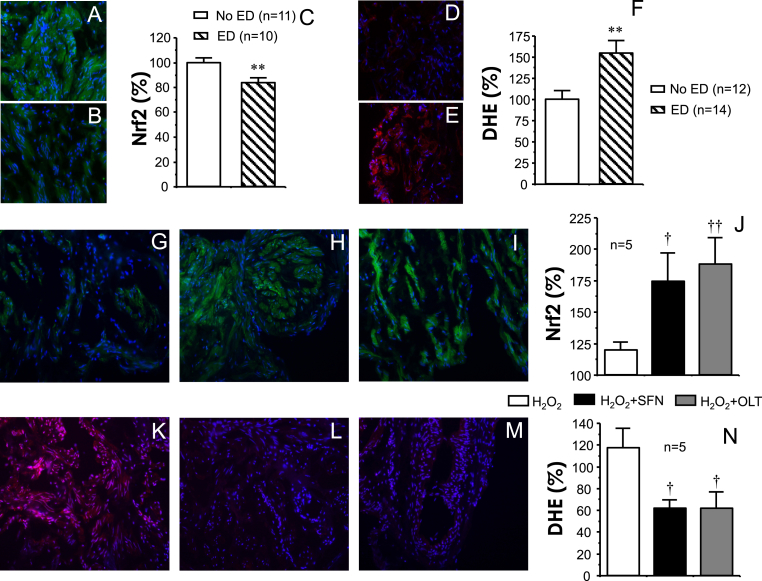
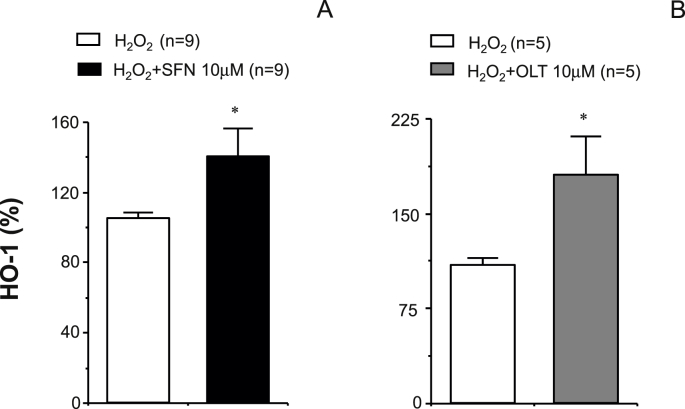
The content of Nrf2 detected by immunofluorescence in human cavernosal tissues was significantly reduced in tissues from ED patients when compared to No ED (Fig. 7A–C). In contrast, the amount of superoxide anion detected by the fluorescent probe, DHE, was significantly increased in corpus cavernosum from ED patients (Fig. 7D–F). Nrf2 content in cavernosal tissue from ED patients exposed to ROS (H2O2, 300 μM) significantly augmented after treatment with the Nrf2 activators, SFN (10 μM) or OLT (30 μM) (Fig. 7G–J). This increase in Nrf2 content was paralleled by a significant decrease in the amount of superoxide anion detected by the fluorescent probe, DHE, in human cavernosal tissues exposed to H2O2 (300 μM) after adding SFN (10 μM) or OLT (30 μM) (Fig. 7K-N). In line with the above, the treatment with SFN (10 μM) or OLT (30 μM) resulted in a significant up-regulation of the content of the Nrf2 target protein, HO-1, in human cavernosal tissues exposed to H2O2 (300 μM) (Fig. 8).
Fig. 7.
Nrf2 activators increase Nrf2 content, decrease oxidative stress content in vascular tissues from patients with erectile dysfunction (ED). Representative images (x200) of immunodetection of Nrf2 (green) (A, B) and on superoxide content detected by dihydroethidium (DHE)-induced fluorescence (red) (D, E) in corpus cavernosum from organ donors without erectile dysfunction (No ED) (A, D) and from patients with erectile dysfunction (ED) (B, E). Nuclei staining with DAPI (blue) are merged in all images. Reduced Nrf2 content and increased DHE staining in ED is confirmed by fluorescence quantification relative to number of nuclei (C and F, respectively). Data are expressed as mean ± SEM of the percentage of the average value obtained in No ED. n indicates the number of patients. **p < 0.01 vs. No ED by Student t-test. Other panels show representative images (x200) of the effects of treatment with vehicle (0.1% DMSO) (G, K), sulforaphane (SFN 10 μM) (H, L), or oltipraz (OLT, 30 μM) (I, M) on Nrf2 immunodetection (G–I) and DHE-induced fluorescence (K–M) in cavernosal tissue from patients with ED. Tissues were treated with SFN, OLT or vehicle for 60 min and then exposed to H2O2 (300 μM) for 10 min. Panels J and N shows quantification of Nrf2 and DHE fluorescence, respectively, relative to number of nuclei. Data are expressed as mean ± SEM of the percentage of the value obtained in control conditions (without H2O2). n indicates the number of patients. †p < 0.05, ††p < 0.01 vs. H2O2 by one-factor ANOVA followed by Student-Newmann-Keuls test. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Fig. 8.
Nrf2 activation increases heme oxygenase expression in human vascular tissues from ED patients. Panels A and B show the effects of treatment with vehicle (0.1% DMSO), sulforaphane (SFN, 10 μM) (A) or oltipraz (OLT, 10 μM) (B) on heme oxygenase-1 (HO-1) content determined by ELISA in cavernosal tissue from patients with ED. Tissues were treated with SFN, OLT or vehicle for 60 min and then exposed to H2O2 (300 μM) for 10 min. Data are expressed as mean ± SEM of the percentage of the value obtained in control conditions (without H2O2). n indicates the number of patients. *p < 0.05 vs. H2O2 by Student t-test.
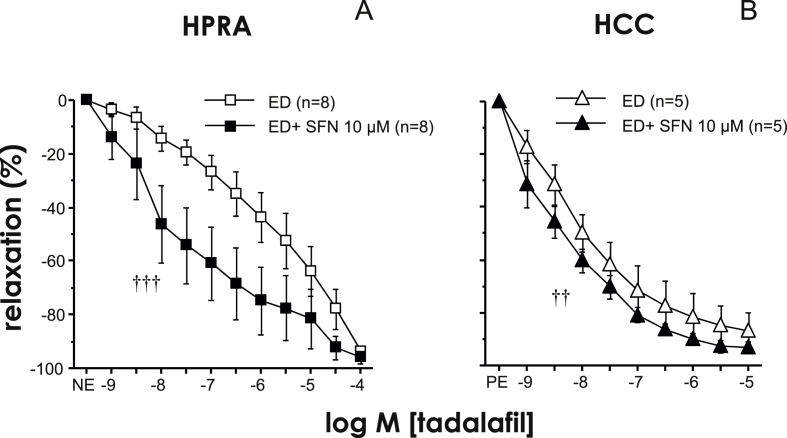
3.4. Relaxant capacity of tadalafil in human penile tissues is enhanced by SFN
PDE5 inhibitors represent the first line therapy in the management of ED. The PDE5 inhibitor, tadalafil, caused relaxation of penile resistance arteries and corpus cavernosum from patients with ED. The relaxant capacity of tadalafil was significantly potentiated by the treatment with SFN (10 μM) in both human penile resistance arteries and corpus cavernosum (Fig. 9).
Fig. 9.
Nrf2 activation enhances relaxant capacity of the type 5 phosphodiesterase inhibitor, tadalafil, in human vascular tissues from ED patients. Effects of the treatment with SFN (10 μM) or vehicle on relaxation induced by tadalafil in human penile resistance arteries (HPRA) (A) and human's corpus cavernosum (HCC) (B) from patients with ED. Data are expressed as mean ± SEM of the percentage of maximal relaxation induced by papaverine (0.1 mM). n indicates the number of patients. ††p < 0.01, †††p < 0.001 vs. ED by two-factors ANOVA test.
4. Discussion
We provide evidence showing that short-term Nrf2 activation improves vascular endothelial function in situations associated with endothelial dysfunction. This functional observation is demonstrated in four different animal vascular beds. This multiple evaluation does not merely represent a repetition of experiments to increase confirmation strength. The different evaluated vessels represent specific vascular territories with structural and functional differences and a distinguishable sensitivity to the impact of aging [16]. In fact this approach helps to appraise a novel integrative view of the effect of short-term activation of Nrf2 on vasculature. Furthermore, we demonstrate for the first time the functional improvement driven by short-term Nrf2 activation in human pathological vascular tissues. The positive functional effects driven by pharmacological activation of Nrf2 are further supported by different biological determinations confirming Nrf2 activation and antioxidant response associated increment.
Injury due to excessive oxidative stress importantly contributes to vascular alterations associated with ageing [2,13]. Moreover, a defective Nrf2-mediated antioxidant response to metabolic and oxidant injuries has been detected in aortae from aged rats, suggesting that age-related Nrf2 dysfunction increases sensitivity and vulnerability of aged vasculature to oxidative stress-induced damage [32]. Our results point to a decisive beneficial impact on vascular function in ageing by the recovery of Nrf2-mediated response in aged vessels. This idea is supported by the clear improvement of endothelial vasodilation driven by the Nrf2 activator, SFN, in vessels from aged rats. SFN is a well established Nrf2 activator that probably exerts such effect through modification of cysteine sensor(s) of Keap1 that disables its substrate adaptor function. Consequently, Nrf2 is not degraded, Keap1 is not regenerated and Nrf2 accumulates and initiates transcription of target genes [49]. It is important to note that the positive effect exerted by SFN is not restricted to a unique vascular territory since four very different vascular beds from aging animals were evaluated and a significant improvement of endothelial relaxation was exerted by SFN in all of them. However, macrovascular structures seems to be more reluctant to the effects of Nrf2 activation as suggested by the only partial recovery obtained in aorta and corpus cavernosum from aged rats. Interestingly, the marked improvement of endothelial vasodilation in aged vessels was not observed in the same vascular beds from young rats, suggesting that SFN-induced improvement could be related to a recovery of defective Nrf2-mediated response in aged vessels while this is not produced when a preserved Nrf2-mediated response exists. The effects driven by SFN are likely mediated by its Nrf2-enhancing activity since the presence of the Nrf2 inhibitor, trigonelline [50], prevented SFN-induced potentiation of endothelial vasodilation in aged vessels. Supporting this concept, the treatment with a different Nrf2 activator, oltipraz [51,52], resulted in similar improvement of endothelium-dependent relaxation in coronary artery and corpus cavernosum from aged animals, two representative vascular beds of complete and partial recovery of endothelial responses by SFN.
In addition to a very well-known decline in endothelium-dependent relaxation, it has been recently demonstrated that relaxation induced by exposure to H2O2 also decline with ageing in different vascular beds [16]. This situation agrees with the idea that free radicals could exert a hormetic effect generating an adaptive response that may be hampered in conditions associated with excessive oxidative stress like ageing [3,53]. We have confirmed defective H2O2-induced relaxation in aorta, mesenteric artery, coronary artery and corpus cavernosum from aged rats while SFN was able to improve such responses in all vascular beds except for aorta. This enhancement of H2O2-induced vasodilations in aged vessels by SFN likely involves an endothelial effect since de-endothelialization of mesenteric and coronary arteries from aged rats precludes the SFN-induced potentiation of vasodilations mediated by H2O2 in these vessels.
Again, the treatment with an additional Nrf2 activator, OLT, paralleled the effects produced by SFN on H2O2-induced relaxations in aged vessels, providing additional support to the role of Nrf2 in the improvement of these defective responses. The key participation of Nrf2 activation in the observed effects is reinforced by the fact that at the same concentration and the same treatment time as in functional studies, where an improvement of H2O2-induced vasodilations was observed, SFN caused a significant increase in Nrf2 content in mesenteric and coronary arteries and reduced superoxide anion content in coronary arteries from aged rats.
Although animal model provides key information, moreover when different vascular territories are evaluated, validation of the phenomena in human vascular tissue represents an important added value to the outcome of the study. In this sense, we have used human tissue from patients with ED. Ageing represents the main risk factor for ED [22,23]. However, ED patients from our sample display, in addition to advanced age, other cardiovascular risk factors and diseases. This fact, although precluding the isolation of the effect of ageing, makes the sample closer to the real situation that depicts an aging condition associated with the presence of this type of comorbidities. Like in vascular ageing, oxidative stress is considered to play a role in ED pathophysiology. In fact, antioxidant therapy has been shown to improve erectile function in animal models of diabetic ED [54] and in aged rats [55]. In addition, ED shares with vascular ageing the presence of endothelial dysfunction, which has been proven in key human penile vascular tissues, corpus cavernosum and penile resistance arteries [43,45]. In these human vascular structures, the exposure to Nrf2 activators results in increased endothelium-dependent and H2O2-induced relaxations confirming that the vascular function improvement driven by Nrf2 activation is also applicable to human vasculature. In addition to display impaired vascular functional responses with respect to young OD, cavernosal tissue from ED patients manifests reduced Nrf2 protein content and increased superoxide content. Concomitantly with functional improvement, the activity of SFN and OLT in human penile tissue was accompanied by an increase in Nrf2 and a decrease in superoxide detection, further suggesting that the beneficial effects on vascular function exerted by these compounds are mediated by the antioxidant response orchestrated by Nrf2. However, Nrf2 is a transcription factor that exerts its antioxidant activity by upregulating the expression of some effector enzymes, including, among others, HO-1 [28] that has been related to preservation of endothelium-dependent vasodilation [56,57]. Moreover, induction of HO-1 has been reported to enhance erectile signaling in corpus cavernosum from aged rats [58] and to improve erectile responses in vivo in diabetic rats [59]. Demonstration of HO-1 upregulation by SFN and OLT in human corpus cavernosum further supports that vascular function improvement by these activators is attributable to an increased transcriptional activity of Nrf2 leading to antioxidant response. Taken together, the evidences provided with an additional established Nrf2 activator (oltipraz), the prevention by a known Nrf2 inhibitor (trigonelline) and the associated enhancement of Nrf2 signaling in different vascular preparations make hard to consider that Nrf2 has not a key role in the effects driven by short-term exposure to SFN in the present study. However, Nrf2-independent mechanisms in vascular protective effects by SFN proposed by other investigators [60,61] prevent us to completely discard that Nrf2-independent effects could additionally contribute to the effects driven by SFN on the recovery on vascular function in aged rats and patients with ED.
Nrf2 activation has been reported to promote an antioxidant, anti-inflammatory, and anti-atherogenic profile in cultured endothelial cells and vasculature from animals chronically treated [28,29,[34], [35], [36]]. The decreased flow-mediated vasodilation (FMD) observed in young smokers, which was related to reduced glutathione concentrations, together with the fact that serum from smokers reduced Nrf2 and HO-1 in cultured endothelial cells led Pasini and collaborators to propose that repression of Nrf2 would be a mechanism potentially involved in the impairment of FMD in humans [62]. These indirect evidences would be in agreement with the here demonstrated ability of short-term Nrf2 activation to improve endothelial vasodilatory function in human vascular tissue from patients with endothelial dysfunction.
On the other hand, endothelial vasodilation involves distinct mediators in different vascular beds highlighting the importance of evaluating various vascular territories. Interestingly, the improvement of endothelial vasodilation by Nrf2 was more marked in rat mesenteric and coronary arteries, and in human penile resistance arteries where an important contribution of EDHF to their endothelium-dependent vasodilations in addition to NO has been proven [[63], [64], [65]]. Thus, it was worthy to ascertain the mediator(s) of endothelial vasodilation sensitive to the action of Nrf2 activators. A bidirectional relationship between NO and Nrf2 has been previously proposed, suggesting activation of Nrf2 by NO [66] but also a positive effect of Nrf2 activation on eNOS/NO pathway [67]. This concept is supported by the significant potentiation of NO-mediated endothelial vasodilation in arteries from aged rats and vascular tissues from ED patients and would be supported by studies proposing that Nrf2 activation augments NO bioactivity by reducing ROS content and increasing availability of eNOS cofactor tetrahydrobiopterine [68,69]. In contrast, we did not find information on the interaction between Nrf2 and EDHF. Nrf2 activation even more clearly potentiated the EDHF-mediated component of endothelial relaxation than NO in the arteries from aged rats or from patients with ED proposing that Nrf2 activation also improves this component of vasodilation in conditions associated with endothelial dysfunction and could explain the better response of microvasculature to Nrf2 activators with respect to rat aorta and corpus cavernosum or human corpus cavernosum. EDHF-mediated vasodilations in rat mesenteric and coronary arteries, and human penile resistance arteries involve activation of calcium-activated potassium channels (KCa) [46,70,71]. In this sense, Nrf2 activation has been shown to up-regulate large conductance KCa in endothelial cells and coronary arteries from diabetic rats [72,73].
Although most evidences point to an endothelium-dependent effect by Nrf2 activators, we have detected a potentiation of SNP-induced relaxations in rat coronary arteries from aged rats and in penile arteries from patients with ED. Thus, a potential effect on vascular smooth muscle by Nrf2 activation under situations associated to oxidative stress cannot be completely discarded. In this sense, although it is rather speculative, we do not discard that the potentiation of SNP-induced relaxations by SFN in rat coronary arteries and human penile resistance arteries could be related to down-regulation of Rho-kinase activity in smooth muscle by Nrf2 activation since increased Rho-kinase activity has been suggested to contribute to vascular dysfunction associated to oxidative stress [74,75] and Nrf2 activation ameliorates arterial dysfunction in diabetic animals through modulation of Rho-kinase activity in vascular smooth muscle [76].
PDE5 inhibitors represent the first line oral therapy for ED patients [77,78]. However, a significant proportion of ED patients is resistant to PDE5 inhibition [79]. The ability of SFN treatment to potentiate relaxations induced by the PDE5 inhibitor, tadalafil, in human penile arteries and corpus cavernosum strips suggests that Nrf2 activation could be a potential strategy to enhance the therapeutic effect of PDE5 inhibitors. If this hypothesis is confirmed, it would expectedly have therapeutic relevance in the treatment of ED patients resistant to oral PDE5 inhibitors at first instance.
In conclusion, we have demonstrated that short-term pharmacological activation of Nrf2 attenuates age-related impairment of endothelium-dependent and ROS-induced vasodilation in multiple vascular territories of the rat with different functional characteristics. This functional observation is supported by vascular upregulation of Nrf2-related signaling and decreased oxidative stress and confirmed for the first time in human vasculature from patients with endothelial dysfunction representing a potential target for therapeutic intervention. Nrf2 activation improves both NO- and EDHF-mediated vasodilation and potentiates the functional effect of the conventional pharmacological therapy for ED, PDE5 inhibitors, in human target tissues, further suggesting potential therapeutic implications in ED.
Acknowledgements
The research leading to these results has received funding from the European Union's Seventh Framework Programme (FP7/2007–2013) under grant agreement number 305483, FRAILOMIC Project, by grants from the Ministry of Economy and Competitiveness and co-financed by FEDER funds (Instituto de Salud Carlos III, PI15/01160, PI15/00674, PI15/01969 and CIBERFES (CB16/10/00464), Spanish Government.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.redox.2019.101271.
Author contributions
Conception and design of the experiments: J.A., and L.R.M.
Collection, analysis and interpretation of data: J.A., M.E.A, A.S.O., A.F., A.S–F., J.R–O, J.I. M-S., J.M. L. F., and L.R.M.
Drafting the article: J.A., M.E.A., and L.R.M.
All authors were responsible for final content and read and approved the final manuscript.
Conflicts of interest
None.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Steyers C.M., 3rd, Miller F.J., Jr. Endothelial dysfunction in chronic inflammatory diseases. Int. J. Mol. Sci. 2014;15:11324–11349. doi: 10.3390/ijms150711324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.El Assar M., Angulo J., Rodríguez-Mañas L. Oxidative stress and vascular inflammation in aging. Free Radic. Biol. Med. 2013;65:380–401. doi: 10.1016/j.freeradbiomed.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 3.El Assar M., Angulo J., Rodríguez-Mañas L. Diabetes and ageing-induced vascular inflammation. J. Physiol. 2016;594:2125–2146. doi: 10.1113/JP270841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Incalza M.A., D'Oria R., Natalicchio A., Perrini S., Laviola L., Giorgino F. Oxidative stress and reactive oxygen species in endothelial dysfunction associated with cardiovascular and metabolic diseases. Vasc. Pharmacol. 2018;100:1–19. doi: 10.1016/j.vph.2017.05.005. [DOI] [PubMed] [Google Scholar]
- 5.Münzel T., Camici G.G., Maack C., Bonetti N.R., Fuster V., Kovacic J.C. Impact of oxidative stress on the heart and vasculature: part 2 of a 3-part series. J. Am. Coll. Cardiol. 2017;70:212–229. doi: 10.1016/j.jacc.2017.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maccarrone M., Ulivi L., Giannini N., Montano V., Ghiadoni L., Bruno R.M., Bonuccelli U., Mancuso M. Endothelium and oxidative stress: the Pandora's box of cerebral (and non-only) small vessel disease? Curr. Mol. Med. 2017;17:169–180. doi: 10.2174/1566524017666170822114739. [DOI] [PubMed] [Google Scholar]
- 7.Varghese J.F., Patel R., Yadav U.C.S. Novel insights in the metabolic syndrome-induced oxidative stress and inflammation-mediated atherosclerosis. Curr. Cardiol. Rev. 2018;14:4–14. doi: 10.2174/1573403X13666171009112250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grochowski C., Litak J., Kamieniak P., Maciejewski R. Oxidative stress in cerebral small vessel disease. Role of reactive species. Free Radic. Res. 2018;52:1–13. doi: 10.1080/10715762.2017.1402304. [DOI] [PubMed] [Google Scholar]
- 9.Varadharaj S., Kelly O.J., Khayat R.N., Kumar P.S., Ahmed N., Zweier J.L. Role of dietary antioxidants in the preservation of vascular function and the modulation of health and disease. Front Cardiovasc Med. 2017;4:64. doi: 10.3389/fcvm.2017.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lakatta E.G., Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part I: aging arteries: a "set up" for vascular disease. Circulation. 2003;107:139–146. doi: 10.1161/01.cir.0000048892.83521.58. [DOI] [PubMed] [Google Scholar]
- 11.Dal-Ros S., Bronner C., Auger C., Schini-Kerth V.B. Red wine polyphenols improve an established aging-related endothelial dysfunction in the mesenteric artery of middle-aged rats: role of oxidative stress. Biochem. Biophys. Res. Commun. 2012;419:381–387. doi: 10.1016/j.bbrc.2012.02.031. [DOI] [PubMed] [Google Scholar]
- 12.Gano L.B., Donato A.J., Pasha H.M., Hearon C.M., Jr., Sindler A.L., Seals D.R. The SIRT1 activator SRT1720 reverses vascular endothelial dysfunction, excessive superoxide production, and inflammation with aging in mice. Am. J. Physiol. Heart Circ. Physiol. 2014;307:H1754–H1763. doi: 10.1152/ajpheart.00377.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rodríguez-Mañas L., El Assar M., Vallejo S., López-Dóriga P., Solís J., Petidier R., Montes M., Nevado J., Castro M., Gómez-Guerrero C., Peiró C., Sánchez-Ferrer C.F. Endothelial dysfunction in aged humans is related with oxidative stress and vascular inflammation. Aging Cell. 2009;8:226–238. doi: 10.1111/j.1474-9726.2009.00466.x. [DOI] [PubMed] [Google Scholar]
- 14.Angulo J., Vallejo S., El Assar M., García-Septiem J., Sánchez-Ferrer C.F., Rodríguez-Mañas L. Age-related differences in the effects of α and γ peroxisome proliferator-activated receptor subtype agonists on endothelial vasodilation in human microvessels. Exp. Gerontol. 2012;47:734–740. doi: 10.1016/j.exger.2012.06.014. [DOI] [PubMed] [Google Scholar]
- 15.Walker A.E., Kaplon R.E., Pierce G.L., Nowlan M.J., Seals D.R. Prevention of age-related endothelial dysfunction by habitual aerobic exercise in healthy humans: possible role of nuclear factor kappaB. Clin. Sci. (Lond.) 2014;127:645–654. doi: 10.1042/CS20140030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.El Assar M., Fernández A., Sánchez-Ferrer A., Angulo J., Rodríguez-Mañas L. Multivessel analysis of progressive vascular aging in the rat: asynchronous vulnerability among vascular territories. Mech. Ageing Dev. 2018;173:39–49. doi: 10.1016/j.mad.2018.03.012. [DOI] [PubMed] [Google Scholar]
- 17.Wadley A.J., Veldhuijzen van Zanten J.J., Aldred S. The interactions of oxidative stress and inflammation with vascular dysfunction in ageing: the vascular health triad. Age (Dordr) 2013;35:705–718. doi: 10.1007/s11357-012-9402-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Virdis A., Ghiadoni L., Giannarelli C., Taddei S. Endothelial dysfunction and vascular disease in later life. Maturitas. 2010;67:20–24. doi: 10.1016/j.maturitas.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 19.Villa F., Carrizzo A., Spinelli C.C., Ferrario A., Malovini A., Maciąg A., Damato A., Auricchio A., Spinetti G., Sangalli E., Dang Z., Madonna M., Ambrosio M., Sitia L., Bigini P., Calì G., Schreiber S., Perls T., Fucile S., Mulas F., Nebel A., Bellazzi R., Madeddu P., Vecchione C., Puca A.A. Genetic analysis reveals a longevity-associated protein modulating endothelial function and angiogenesis. Circ. Res. 2015;117:333–345. doi: 10.1161/CIRCRESAHA.117.305875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alonso-Bouzón C., Carcaillon L., García-García F.J., Amor-Andrés M.S., El Assar M., Rodríguez-Mañas L. Association between endothelial dysfunction and frailty: the toledo study for healthy aging. Age (Dordr) 2014;36:495–505. doi: 10.1007/s11357-013-9576-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gratzke C., Angulo J., Chitaley K., Dai Y.T., Kim N.N., Paick J.S., Simonsen U., Uckert S., Wespes E., Andersson K.E., Lue T.F., Stief C.G. Anatomy, physiology, and pathophysiology of erectile dysfunction. J. Sex. Med. 2010;7:445–475. doi: 10.1111/j.1743-6109.2009.01624.x. [DOI] [PubMed] [Google Scholar]
- 22.Feldman H.A., Goldstein I., Hatzichristou D.G., Krane R.J., McKinlay J.B. Impotence and its medical and psychosocial correlates: results of the Massachusetts Male Aging Study. J. Urol. 1994;151:54–61. doi: 10.1016/s0022-5347(17)34871-1. [DOI] [PubMed] [Google Scholar]
- 23.Martin-Morales A., Sanchez-Cruz J.J., Saenz de Tejada I., Rodriguez-Vela L., Jimenez-Cruz J.F., Burgos-Rodriguez R. Prevalence and independent risk factors for erectile dysfunction in Spain: results of the Epidemiologia de la Disfuncion Erectil Masculina Study. J. Urol. 2001;166:569–574. doi: 10.1016/s0022-5347(05)65986-1. [DOI] [PubMed] [Google Scholar]
- 24.Kaya E., Sikka S.C., Kadowitz P.J., Gur S. Aging and sexual health: getting to the problem. Aging Male. 2017;20:65–80. doi: 10.1080/13685538.2017.1295435. [DOI] [PubMed] [Google Scholar]
- 25.Echeverri Tirado L.C., Ferrer J.E., Herrera A.M. Aging and erectile dysfunction. Sex Med Rev. 2016;4:63–73. doi: 10.1016/j.sxmr.2015.10.011. [DOI] [PubMed] [Google Scholar]
- 26.ChungHS, Shin M.H., Park K. Association between hand-grip strength and erectile dysfunction in older men. Aging Male. 2018;21:225–230. doi: 10.1080/13685538.2017.1412423. [DOI] [PubMed] [Google Scholar]
- 27.Lewis K.N., Mele J., Hayes J.D., Buffestein R. Nrf2, a guardian of healthspan and gatekeeper of species longevity. Integr. Comp. Biol. 2010;50:829–843. doi: 10.1093/icb/icq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen B., Lu Y., Chen Y., Cheng J. The role of Nrf2 in oxidative stress-induced endothelial injuries. J. Endocrinol. 2015;225:R83–R99. doi: 10.1530/JOE-14-0662. [DOI] [PubMed] [Google Scholar]
- 29.Satta S., Mahmoud A.M., Wilkinson F.L., Alexander M.Y., White S.J. The role of Nrf2 in cardiovascular function and disease. Oxid Med Cell Longev. 2017;2017:9237263. doi: 10.1155/2017/9237263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pomatto L.C.D., Davies K.J.A. The role of declining adaptive homeostasis in ageing. J. Physiol. 2017;595:7275–7309. doi: 10.1113/JP275072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Powers S.K., Duarte J., Kavazis A.N., Talbert E.E. Reactive oxygen species are signalling molecules for skeletal muscle adaptation. Exp. Physiol. 2010;95:1–9. doi: 10.1113/expphysiol.2009.050526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ungvari Z., Bailey-Downs L., Sosnowska D., Gautam T., Koncz P., Losonczy G., Ballabh P., de Cabo R., Sonntag W.E., Csiszar A. Vascular oxidative stress in aging: a homeostatic failure due to dysregulation of NRF2-mediated antioxidant response. Am. J. Physiol. Heart Circ. Physiol. 2011;301:H363–H372. doi: 10.1152/ajpheart.01134.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.El Assar M., Angulo J., Carnicero J.A., Walter S., García-García F.J., López-Hernández E., Sánchez-Puelles J.M., Rodríguez-Mañas L. Frailty is associated with lower expression of genes involved in cellular response to stress: results from the toledo study for healthy aging. J. Am. Med. Dir. Assoc. 2017;18:734. doi: 10.1016/j.jamda.2017.04.019. [DOI] [PubMed] [Google Scholar]
- 34.Dai G., Vaughn S., Zhang Y., Wang E.T., Garcia-Cardena G., Gimbrone M.A., Jr. Biochemical forces in atherosclerosis-resistant vascular regions regulate endothelial redox balance via phosphoinositol 3-kinase/Akt-dependent activation of Nrf2. Circ. Res. 2007;101:723–733. doi: 10.1161/CIRCRESAHA.107.152942. [DOI] [PubMed] [Google Scholar]
- 35.Zakkar M., Van der Heiden K., Luong L.A., Chaudhury H., Cuhlmann S., Hamdulay S.S., Krams R., Edirisinghe I., Rahman I., Carlsen H., Haskard D.O., Mason J.C., Evans P.C. Activation of Nrf2 in endothelial cells protects arteries from exhibiting a proinflammatory state. Arterioscler. Thromb. Vasc. Biol. 2009;29:1851–1857. doi: 10.1161/ATVBAHA.109.193375. [DOI] [PubMed] [Google Scholar]
- 36.Shehatou G.S.G., Suddek G.M. Sulforaphane attenuates the development of atherosclerosis and improves endothelial dysfunction in hypercholesterolemic rabbits. Exp. Biol. Med. 2016;241:426–436. doi: 10.1177/1535370215609695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Priestley J.R.C., Kautenburg K.E., Casati M.C., Endres B.T., Geurts A.M., Lombard J.H. The NRF2 knockout rat: a new animal model to study endothelial dysfunction, oxidant stress, and microvascular rarefaction. Am. J. Physiol. Heart Circ. Physiol. 2016;310:H478–H487. doi: 10.1152/ajpheart.00586.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marczak E.D., Marzec J., Zeldin D.C., Kleeberger S.R., Brown N.J., Pretorius M., Lee C.R. Polymorphisms in the transcription factor NRF2 and forearm vasodilator responses in humans. Pharmacogenetics Genom. 2012;22:620–628. doi: 10.1097/FPC.0b013e32835516e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.El Assar M., Sánchez-Puelles J.M., Royo I., López-Hernández E., Sánchez-Ferrer A., Aceña J.L., Rodríguez-Mañas L., Angulo J. FM19G11 reverses endothelial dysfunction in rat and human arteries through stimulation of the PI3K/Akt/eNOS pathway, independently of mTOR/HIF-1α activation. Br. J. Pharmacol. 2015;172:1277–1291. doi: 10.1111/bph.12993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.El Assar M., Angulo J., Santos-Ruiz M., Ruiz de Adana J.C., Pindado M.L., Sánchez-Ferrer A., Hernández A., Rodríguez-Mañas L. Asymmetric dimethylarginine (ADMA) elevation and arginase up-regulation contribute to endothelial dysfunction related to insulin resistance in rats and morbidly obese humans. J. Physiol. 2016;594:3045–3060. doi: 10.1113/JP271836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Martínez-Salamanca J.I., La Fuente J.M., Martínez-Salamanca E., Fernández A., Pepe-Cardoso A.J., Louro N., Carballido J., Angulo J. α1A-adrenergic receptor antagonism improves erectile and cavernosal responses in rats with cavernous nerve injury and enhances neurogenic responses in human corpus cavernosum from patients with erectile dysfunction secondary to radical prostatectomy. J. Sex. Med. 2016;13:1844–1857. doi: 10.1016/j.jsxm.2016.10.005. [DOI] [PubMed] [Google Scholar]
- 42.Martínez-Salamanca J.I., Zurita M., Costa C., Martínez-Salamanca E., Fernández A., Castela A., Vaquero J., Carballido J., Angulo J. Dual strategy with oral phosphodiesterase type 5 inhibition and intracavernosal implantation of mesenchymal stem cells is superior to individual approaches in the recovery of erectile and cavernosal functions after cavernous nerve injury in rats. J. Sex. Med. 2016;13:1–11. doi: 10.1016/j.jsxm.2015.12.001. [DOI] [PubMed] [Google Scholar]
- 43.Angulo J., González-Corrochano R., Cuevas P., Fernández A., La Fuente J.M., Rolo F., Allona A., Sáenz de Tejada I. Diabetes exacerbates the functional deficiency of NO/cGMP pathway associated with erectile dysfunction in human corpus cavernosum and penile arteries. J. Sex. Med. 2010;7:758–768. doi: 10.1111/j.1743-6109.2009.01587.x. [DOI] [PubMed] [Google Scholar]
- 44.Martínez-Salamanca J.I., La Fuente J.M., Cardoso J., Fernández A., Cuevas P., Wright H.M., Angulo J. Nebivolol potentiates the efficacy of PDE5 inhibitors to relax corpus cavernosum and penile arteries from diabetic patients by enhancing the NO/cGMP pathway. J. Sex. Med. 2014;11:1182–1192. doi: 10.1111/jsm.12477. [DOI] [PubMed] [Google Scholar]
- 45.Martínez-Salamanca J.I., La Fuente J.M., Fernández A., Martínez-Salamanca E., Pepe-Cardoso A.J., Carballido J., Angulo J. Nitrergic function is lost but endothelial function is preserved in the corpus cavernosum and penile resistance arteries of men after radical prostatectomy. J. Sex. Med. 2015;12:590–599. doi: 10.1111/jsm.12801. [DOI] [PubMed] [Google Scholar]
- 46.González-Corrochano R., La Fuente J., Cuevas P., Fernández A., Chen M., Sáenz de Tejada I., Angulo J. Ca2+-activated K+ channel (KCa) stimulation improves relaxant capacity of PDE5 inhibitors in human penile arteries and recovers the reduced efficacy of PDE5 inhibition in diabetic erectile dysfunction. Br. J. Pharmacol. 2013;169:449–461. doi: 10.1111/bph.12143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Guo Z., Su W., Ma Z., Smith G.M., Gong M.C. Ca2+-independent phospholipase A2 is required for agonist-induced Ca2+ sensitization in vascular smooth muscle. J. Biol. Chem. 2003;278:1856–1863. doi: 10.1074/jbc.M211075200. [DOI] [PubMed] [Google Scholar]
- 48.El Assar M., Ruiz de Adana J.C., Angulo J., Pindado Martínez M.L., Hernández Matías A., Rodríguez-Mañas L. Preserved endothelial function in human obesity in the absence of insulin resistance. J. Transl. Med. 2013;1:263. doi: 10.1186/1479-5876-11-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dinkova-Kostova A.T., Fahey J.W., Kostov R.V. KEAP1 and done? Targeting the NRF2 pathway with sulforaphane. Trends Food Sci. Technol. 2017;69:257–269. doi: 10.1016/j.tifs.2017.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhu J., Wang H., Chen F., Fu J., Xu Y., Hou Y., Kou H.H., Zhai C., Nelson M.B., Zhang Q., Andersen M.E., Pi J. An overview of chemical inhibitors of the Nrf2-ARE signaling pathway and their potential applications in cancer therapy. Free Radic. Biol. Med. 2016;99:544–556. doi: 10.1016/j.freeradbiomed.2016.09.010. [DOI] [PubMed] [Google Scholar]
- 51.Eba S., Hoshikawa Y., Moriguchi T., Mitsuishi Y., Satoh H., Ishida K., Watanabe T., Shimizu T., Shimokawa H., Okada Y., Yamamoto M., Kondo T. The nuclear factor erythroid 2-related factor 2 activator oltipraz attenuates chronic hypoxia-induced cardiopulmonary alterations in mice. Am. J. Respir. Cell Mol. Biol. 2013;49:324–333. doi: 10.1165/rcmb.2011-0396OC. [DOI] [PubMed] [Google Scholar]
- 52.Zhao S., Ghosh A., Lo C.S., Chenier I., Scholey J.W., Filep J.G., Ingelfinger J.R., Zhang S.L., Chan J.S.D. Nrf2 deficiency upregulates intrarenal angiotensin-converting enzyme-2 and angiotensin 1-7 receptor expression and attenuates hypertension and nephropathy in diabetic mice. Endocrinology. 2018;159:836–852. doi: 10.1210/en.2017-00752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jackson M.J., McArdle A. Role of reactive oxygen species in age-related neuromuscular deficits. J. Physiol. 2016;594:1979–1988. doi: 10.1113/JP270564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Angulo J., Peiró C., Cuevas P., Gabancho S., Fernández A., González-Corrochano R., La Fuente J.M., Baron A.D., Chen K.S., Sáenz de Tejada I. The novel antioxidant, AC3056 (2,6-di-t-butyl-4-((dimethyl-4-methoxyphenylsilyl)methyloxy)phenol), reverses erectile dysfunction in diabetic rats and improves NO-mediated responses in penile tissue from diabetic men. J. Sex. Med. 2009;6:373–387. doi: 10.1111/j.1743-6109.2008.01088.x. [DOI] [PubMed] [Google Scholar]
- 55.Bivalacqua T.J., Armstrong J.S., Biggerstaff J., Abdel-Mageed A.B., Kadowitz P.J., Hellstrom W.J., Champion H.C. Gene transfer of extracellular SOD to the penis reduces O2-* and improves erectile function in aged rats. Am. J. Physiol. Heart Circ. Physiol. 2003;284:H1408–H1421. doi: 10.1152/ajpheart.00770.2002. [DOI] [PubMed] [Google Scholar]
- 56.El-Bassossy H.M., Hassan N., Zakaria M.N. Heme oxygenase-1 alleviates vascular complications associated with metabolic syndrome: effect on endothelial dependent relaxation and NO production. Chem. Biol. Interact. 2014;223:109–115. doi: 10.1016/j.cbi.2014.09.014. [DOI] [PubMed] [Google Scholar]
- 57.Wenzel P., Rossmann H., Müller C., Kossmann S., Oelze M., Schulz A., Arnold N., Simsek C., Lagrange J., Klemz R., Schönfelder T., Brandt M., Karbach S.H., Knorr M., Finger S., Neukirch C., Häuser F., Beutel M.E., Kröller-Schön S., Schulz E., Schnabel R.B., Lackner K., Wild P.S., Zeller T., Daiber A., Blankenberg S., Münzel T. Heme oxygenase-1 suppresses a pro-inflammatory phenotype in monocytes and determines endothelial function and arterial hypertension in mice and humans. Eur. Heart J. 2015;36:3437–3446. doi: 10.1093/eurheartj/ehv544. [DOI] [PubMed] [Google Scholar]
- 58.Abdel Aziz M.T., Mostafa T., Atta H., Mahfouz S., Wassef M., Fouad H., Kamel M., Rashed L., Sabry D., Mouhamed O. Effect of HO-1 cDNA-liposome complex transfer on erectile signaling of aged rats. Andrologia. 2009;41:176–183. doi: 10.1111/j.1439-0272.2008.00911.x. [DOI] [PubMed] [Google Scholar]
- 59.Abdel Aziz M.T., El Asmer M.F., Mostafa T., Atta H., Mahfouz S., Fouad H., Rashed L., Sabry D., Hassouna A., Abdel Aziz A.T., Senbel A., Demery A. Effects of losartan, HO-1 inducers or HO-1 inhibitors on erectile signaling in diabetic rats. J. Sex. Med. 2009;6:3254–3264. doi: 10.1111/j.1743-6109.2009.01517.x. [DOI] [PubMed] [Google Scholar]
- 60.Holloway P.M., Gillespie S., Becker F., Vital S.A., Nguyen V., Alexander J.S., Evans P.C., Gavins F.N.E. Sulforaphane induces neurovascular protection against a systemic inflammatory challenge via both Nrf2-dependent and independent pathways. Vasc. Pharmacol. 2016;85:29–38. doi: 10.1016/j.vph.2016.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shawky N.M., Segar L. Sulforaphane inhibits platelet-derived growth factor-induced vascular smooth muscle cell proliferation by targeting mTOR/p70S6kinase signaling independent of Nrf2 activation. Pharmacol. Res. 2017;119:251–264. doi: 10.1016/j.phrs.2017.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pasini A.F., Albiero A., Stranieri C., Cominacini M., Pasini A., Mozzini C., Vallerio P., Cominacini L., Garbin U. Serum oxidative-stress induced repression of Nrf2 and GSH depletion: a mechanism potentially involved in endotelial dysfunction of young smokers. PLoS One. 2012;7 doi: 10.1371/journal.pone.0030291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gschwend S., Pinto-Sietsma S.J., Buikema H., Pinto Y.M., van Gilst W.H., Schulz A., de Zeeuw D., Kreutz R. Impaired coronary endothelial function in a rat model of spontaneous albuminuria. Kidney Int. 2002;62:181–191. doi: 10.1046/j.1523-1755.2002.00431.x. [DOI] [PubMed] [Google Scholar]
- 64.Leo C.H., Hart J.L., Woodman O.L. Impairment of both nitric oxide-mediated and EDHF-type relaxation in small mesenteric arteries from rats with streptozotocin-induced diabetes. Br. J. Pharmacol. 2011;162:365–377. doi: 10.1111/j.1476-5381.2010.01023.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Angulo J., Cuevas P., Fernández A., Gabancho S., Videla S., Sáenz de Tejada I. Calcium dobesilate potentiates endothelium-derived hyperpolarizing factor-mediated relaxation of human penile resistance arteries. Br. J. Pharmacol. 2003;139:854–862. doi: 10.1038/sj.bjp.0705293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Buckley B.J., Marshall Z.M., Whorton A.R. Nitric oxide stimulates Nrf2 translocation in vascular endothelium. Biochem. Biophys. Res. Commun. 2003;307:973–979. doi: 10.1016/s0006-291x(03)01308-1. [DOI] [PubMed] [Google Scholar]
- 67.Hwang S.M., Lee Y.J., Yoon J.J., Lee S.M., Kim J.S., Kang D.G., Lee H.S. Prunella vulgaris suppresses HG-induced vascular inflammation via Nrf2/HO-1/eNOS activation. Int. J. Mol. Sci. 2012;13:1258–1268. doi: 10.3390/ijms13011258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Heiss E.H., Schachner D., Werner E.R., Dirsch V.M. Active NF-E2-related factor (Nrf2) contributes to keep endothelial NO synthase (eNOS) in the coupled state: role of reactive oxygen species (ROS), eNOS, and heme oxygenase (HO-1) levels. J. Biol. Chem. 2009;284:31579–31586. doi: 10.1074/jbc.M109.009175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Xia N., Förstermann U., Li H. Resveratrol and endothelial nitric oxide. Molecules. 2014;19:16102–16121. doi: 10.3390/molecules191016102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gschwend S., Henning R.H., de Zeeuw D., Buikema H. Coronary myogenic constriction antagonizes EDHF-mediated dilation: role of KCa channels. Hypertension. 2003;41:912–918. doi: 10.1161/01.HYP.0000063883.83470.7B. [DOI] [PubMed] [Google Scholar]
- 71.Dora K.A., Gallagher N.T., McNeish A., Garland C.J. Modulation of endothelial cell KCa3.1 channels during endothelium-derived hyperpolarizing factor signaling in mesenteric resistance arteries. Circ. Res. 2008;102:1247–1255. doi: 10.1161/CIRCRESAHA.108.172379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Li Y., Wang X.L., Sun X., Chai Q., Li J., Thompson B., Shen W.K., Lu T., Lee H.C. Regulation of vascular large-conductance calcium-activated potassium channels by Nrf2 signalling. Diabetes Vasc. Dis. Res. 2017;14:353–362. doi: 10.1177/1479164117703903. [DOI] [PubMed] [Google Scholar]
- 73.Lu T., Sun X., Li Y., Chai Q., Wang X.L., Lee H.C. Role of Nrf2 signaling in the regulation of vascular BK channel β1 subunit expression and BK channel function in high-fat diet-induced diabetic mice. Diabetes. 2017;66:2681–2690. doi: 10.2337/db17-0181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Knock G.A., Ward J.P. Redox regulation of protein kinases as a modulator of vascular function. Antioxidants Redox Signal. 2011;15:1531–1547. doi: 10.1089/ars.2010.3614. [DOI] [PubMed] [Google Scholar]
- 75.Denniss S.G., Jeffery A.J., Rush J.W. RhoA-Rho kinase signaling mediates endothelium- and endoperoxide-dependent contractile activities characteristic of hypertensive vascular dysfunction. Am. J. Physiol. Heart Circ. Physiol. 2010;298:H1391–H1405. doi: 10.1152/ajpheart.01233.2009. [DOI] [PubMed] [Google Scholar]
- 76.Alves-Lopes R., Neves K.B., Montezano A.C., Harvey A., Carneiro F.S., Touyz R.M., Tostes R.C. Internal pudendal artery dysfunction in diabetes mellitus is mediated by NOX1-derived ROS-, Nrf2-, and Rho kinase-dependent mechanisms. Hypertension. 2016;68:1056–1064. doi: 10.1161/HYPERTENSIONAHA.116.07518. [DOI] [PubMed] [Google Scholar]
- 77.Shamloul R., Ghanem H. Erectile dysfunction. Lancet. 2013;381:153–165. doi: 10.1016/S0140-6736(12)60520-0. [DOI] [PubMed] [Google Scholar]
- 78.Andersson K.E. PDE5 inhibitors – pharmacotherapy and clinical applications 20 years after sildenafil discovery. Br. J. Pharmacol. 2018;175:2554–2565. doi: 10.1111/bph.14205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hatzimouratidis K., Hatzichristou D. Phosphodiesterase type 5 inhibitors: the day after. Eur. Urol. 2007;51:75–88. doi: 10.1016/j.eururo.2006.07.020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.