Abstract
Background: Lycorine, a natural alkaloid, has been indicated to have various physiological effects, including a potential effect against cancer. However, the anticancer effect of lycorine on osteosarcoma (OS) and the detailed molecular mechanisms involved remain unclear.
Purpose: The purpose of this study was to examine the effect of lycorine on human OS and elucidated it underlying mechanisms
Materials and methods: In vitro assays, OS cells were treated with lycorine at various concentrations. Then the cell proliferation, colony formation, cell cycle distribution, apoptosis, migration and invasion were assayed to detect the anticancer effect of lycorine on OS cell lines. Western blotting analysis was used to verify the expression of related proteins. In addition, the mouse xenograft model was performed to evaluate lycorine’s therapeutic potential on OS in vivo.
Results: The in vitro results demonstrated that lycorine induced apoptosis and cell cycle arrest and suppressed the migration and invasion by suppressing constitutive signal transducers and activators of transcription 3 (STAT3) activation through enhancing the expression of SH2 domain-containing phosphatase 1 (SHP-1) and downregulating the expression of STAT3 target proteins. Moreover, our mouse xenograft model revealed that lycorine inhibited the tumor growth in vivo.
Conclusion: These results demonstrated that the anti-OS effects of lycorine were at least partly due to the suppression of the Janus kinase 2/signal transducers and activators of transcription 3 (JAK2)/STAT3 pathway. Taken together, these results indicate that lycorine possesses the potential to be a promising candidate in clinical therapy for human OS in the future.
Keywords: lycorine, osteosarcoma, anticancer, SHP-1, JAK2/STAT3
Introduction
Osteosarcoma (OS) is the most common primary malignant bone tumour in children and adolescents.1 In recent decades, although the combination of neoadjuvant therapy and aggressive surgical resection has dramatically improved the five-year survival rate of OS patients, the prognosis of those with recurrence and distant metastasis remains unsatisfactory.2,3 Therefore, there is an urgent need to develop more effective therapeutic strategies for patients with OS.
Signal transducers and activators of transcription 3 (STAT3), a latent transcription factor, plays an important role in the transcriptional activation of apoptosis and cell cycle progression.4 STAT3 can be activated by the activation of non-receptor tyrosine kinases such as Janus kinase (JAK2); and upon activation, STAT3 undergoes phosphorylation-induced homodimerization and translocates into the nucleus where it regulates its target genes.5,6 Accumulating evidence indicatesthat the JAK2/STAT3 pathway is constitutively activated in various malignant tumours7–9 and exerts an essential role in various biological processes including proliferation, apoptosis, angiogenesis, migration, invasion and metastasis.10,11 Increasing evidence from previous studies indicates that the inhibition of the JAK2/STAT3 signalling pathway is a promising therapeutic target for cancer therapy.12,13 Moreover, there is even research suggesting that the JAK2/STAT3 pathway plays an essential role in OS formation.14,15 Therefore, suppressing the JAK2/STAT3 pathway might be a potential therapeutic strategy for OS.
Lycorine, an active alkaloid extracted from genera in the Amaryllidaceae, has shown various biological effects, such as antiviral,16 antiinflammation,17 antimalarial,18 antibacterial19 and antitumour effects.20 In recent years, the potential antitumour properties of lycorine have received increasing attention. Previous studies have revealed that lycorine has certain inhibitory effects in various malignant tumours, such as breast cancer,21 prostate cancer,22 multiple myeloma23 and hepatocellular carcinoma.24 However, until now, the anticancer effect of lycorine on OS has not been reported and the defined molecular mechanisms remain unclear.
In the present study, we aimed to investigate the anticancer effect of lycorine on OS cell lines in vitro and in vivo. Furthermore, we explored the underlying mechanisms by which lycorine exerts it antitumor activity against OS cells, which was through the suppression of the JAK2/STAT3 pathway. Our findings indicated that lycorine might possess attractive advantages as a promising and effective candidate in clinical therapy for human OS in the future.
Materials and methods
Reagents and antibodies
Lycorine (purity >98%), purchased from Solarbio (Beijing, China), was dissolved in dimethylsulfoxide (DMSO) (Sigma) and stored at −20 °C. Cisplatin was purchased from Qilu Pharmaceutical Co., Ltd (Jinan, Shandong, People’s Republic of China). Primary antibodies against p-JAK2, JAK2, p-STAT3, STAT3, SHP1, Cyclin D1, CDK4, CDK2, and GAPDH were purchased from Cell Signaling Technology (Beverly, MA, USA). Antibodies against E-cadherin, N-cadherin, MMP-9, MMP-2, Bcl-2, and Bax were purchased from Abcam (Cambridge, UK).
Cell culture
Human OS cell lines, MNNG/HOS, U2OS, MG63 and Saos-2, were obtained from the Cell Bank of the Type Culture Collection of Chinese Academy of Sciences (Shanghai, China). The MNNG/HOS and MG63 cells were cultured and maintained in Eagle’s minimum essential medium (Thermo Fisher Scientific) containing 10% foetal bovine serum (FBS) (Gibco; Thermo Fisher Scientific), while the U2OS and Saos-2 cells were cultured in RPMI 1640 medium (Thermo Fisher Scientific) supplemented with 15% FBS (Gibco; Thermo Fisher Scientific), containing 100 units/ml penicillin and 100 μg/ml streptomycin. All cells were maintained at 37 °C in a humidified incubator with 5% CO2.
CCK8 assay
Cell counting kit 8 (CCK-8; Dojindo, Kyushu Island, Japan) was used to measure cell viability according to the manufacturer’s protocol. Briefly, 3×103 cells were seeded in each well of a 96-well microplate and cultured overnight. The cells were then treated with various concentrations of lycorine (0, 0.5, 1, 2, 5, 10, 20, and 50 μΜ). Following treatment with lycorine for 24, 48, and 72 h, the culture medium was replaced with 100 μl of thecorresponding culture medium containing 10% CCK-8 solution and incubated at 37 °C for 2 h. The absorbance was read at a wavelength of 450 nm in a microplate reader (Biotek, Winooski, VT, USA).
5-Ethynyl-2-deoxyuridine (EdU) assay
MNNG/HOS and U2OS cells were seeded in 96-well plates at a concentration of 3×103 cells per hole and treated with various concentrations of Lycorine for48 h. Then 100 μl of 50 μM EdU (Cell Light EdUDNA Imaging Kit; Guangzhou RiboBio, China) was added to each well, and the cells were cultured in the medium for an additional 2 h. The cells were then stained as previously described.25 Finally, the cells were observed under fluorescence microscopy and images were taken. EdU positive cells were calculated with the following formula: (EdU add-in cells/Hoechst stained cells) × 100%.26
Colony formation assay
The anchorage-independent ability of OS cells was evaluated by the clone formation assay. Cells were trypsinized and seeded in six-well plates at a density of 500 cells per well. Then the cells were treated with lycorine at the indicated concentrations for 48 h, and, the medium was subsequently changed with fresh medium. After being cultured for 10 days, the cells were washed and fixed with 4% paraformaldehyde and stained with crystal violet (Sigma-Aldrich, Steinheim, Germany). The colonies that consisted of at least 50 cells were counted and analysed manually.
Apoptosis analysis by flow cytometry
Cells were seeded in six-well plates at a density of 1×105 cells per well and then treated with Lycorine at different concentrations for 24 h. After lycorine treatment, the cells were harvested and washed twice with ice cold phosphate-buffered saline (PBS) buffer. Then the cells were stained with Annexin V-phycoerythrin (PE)/7-amino actinomycin (7-AAD) dual staining (Nanjing Keygen Biotech, Nanjing, China) according to the manufacturer’s protocol. After incubation in the dark for 20 min at room temperature, the apoptosis rates of the cells were analysed and calculated by flow cytometry (Becton Dickinson, Franklin Lakes, New Jersey, USA).
Cell cycle analysis by flow cytometry
In brief, the cells were seeded in six-well plates at a density of 1×105 cells per well and then treated with lycorine at different concentrations for 48 h. After lycorine treatment, the cells were collected, washed twice with ice cold PBS buffer and fixed with 75% ethyl alcohol at 4 °C overnight. To detect the cell cycle distribution, the cells were stained with 50 μg/ml propidium iodide (PI) in the presence of 20 μg/ml RNase A (Beyotime Biotechnology) in the dark for 20 min at room temperature and then analysed by flow cytometry.
Wound healing assay
In brief, 3×105 cells were seeded in six-well plates in culture medium until cells were grown to confluence. Wounds were created by a sterile 200 μμl pipette tip and the unattached cells were gently washed twice with PBS. The cells were further cultured with 1% FBS complete medium containing various concentrations of lycorine for 24 h Imagesof the wounds were taken at 0 and 24 h post-treatment to visualize wound healing. The percentage of wound closure (original width - Width at 24 h after scraping/original width) was calculated.27
Transwell migration assay
To further investigate the inhibitory effects of lycorine on the migration of MNNG/HOS and U2OS cells, we performed the Transwell migration assay. In brief, 5×104 cells in 200 µl of serum-free medium were pre-treated with various concentrations of lycorine and seeded into the upper part of each Transwell chamber (Corning Costar, Rochester, NY, USA), while 600 μl of medium with 20% FBS was added to the lower chamber. After 24 h of incubation, the nonmigratory cells were removed from the upper chamber surface by using cotton swabs. Then the migrated cells were fixed with 4% paraformaldehyde and stained with crystal violet. The number of the migratory cells was observed and counted from five randomly selected fields under a light microscope.
Invasion assay
An invasion assay was used to evaluate the effect of lycorine on the invasion of MNNG/HOS and U2OS cells. The Transwell chambers were pre-coated with 70 μl of Matrigel (BD Biosciences, San Jose, CA, USA) overnight. Then, the cells treated with lycorine as described in the Transwell migration assay and placed in the upper part of each chamber, while 20% FBS-medium was added in the lower chamber. After 24 h of incubation for MNNG/HOS cells and 48 h of incubation for U2OS cells, the non-invaded cells were removed with cotton swabs and the invaded cells were then fixed with 4% paraformaldehyde and stained with crystal violet. The number of invasive cells were observed and counted from five randomly selected fields under a light microscope.
Western blotting analysis
Cells were treated with the indicated concentrations of lycorine for 48 h, and then lysed in RIPA buffer (Thermo Fisher Scientific) containing protease inhibitors and phosphatase inhibitors. The lysates were then centrifuged at 12,000 rpm for 15 min at 4 °C and the supernatant was carefully collected. The protein concentrations were measured by using the BCA Protein Assay Kit (Beyotime Biotechnology Co. Ltd). Equal amounts of lysates (20 µg) were electrophoresed by sodium dodecyl-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto polyvinylidene difluoride (PVDF) membranes (Millipore, Billerica, MA). The membranes were then blocked with 5% non-fat milk in Tris-buffered saline plus Tween-20 (TBST) buffer for 1 h at room temperature and incubated with the respective primary antibodies at 4 °C overnight. After being washed three times with TBST buffer, the membranes were incubated with horseradish peroxidase-conjugated secondary antibody for 1 h at room temperature. Subsequently, the membranes were washed three times with TBST buffer and detected by using electrochemiluminescence detection reagent (EMD Millipore) according to the manufacturer’s instructions
SHP-1 phosphatase activity
In brief, after 48 h of treatment with lycorine, cell protein extracts were incubated with anti-SHP-1 antibody in immunoprecipitation buffer overnight. SHP-1 activity was detected subsequently by using the RediPlate 96 EnzChek Tyrosine Phosphatase Assay Kit (R-22067) according to the manufacturer’s instructions.
Mouse xenograft model
Animal experiments were approved by the Institutional Animal Care and Use Committee (IACUC) at Tongji Medical College, Huazhong University of Science and Technology (IACUC Number: S818). Four-week-old female BALB/c nude mice were purchased from Beijing HFK Bioscience Co. Ltd. and housed in a standard animal laboratory throughout the experimental duration. All procedures for the mouse experiments were performed in accordance with the Guidelines for the Care and Use of Laboratory Animals of the National Institutes of Health. In brief, 1×107 MNNG/HOS cells suspended in 100 μl of cold PBS were subcutaneously injected into the right axilla of nude mice. Mice with similar tumour volumes were randomly divided into 4 groups (n=3 per group) when the tumour could be palpated subcutaneously (at 4 days after inoculation), and then treated with lycorine (10 mg/kg every 2 days or 20 mg/kg every 2 days), or DMSO (negative control) or cisplatin (positive control, 2 mg/kg twice a week). The body weights and tumour sizes were measured every 2 days to observe the growth of thetumours. Ten days after treatment, the mice were sacrificed, and the tumours and major organs (heart, liver, spleen, lung, and kidney) were removed for subsequent use in immunohistochemical experiments.
Histopathology and immunohistochemistry
Tumour tissue specimens and the major organs of mice were fixed with 4% paraformaldehyde and embedded in paraffin and then sectioned into 4-μm sections. Standard immunohistochemical staining was conducted as described previously.28 The slides were incubated at 4 °C overnight with antibodies against Ki-67 (1:100 dilution) and washed three times with PBS. The slides were then incubated with the second antibody for 30 min at room temperature. Immunoreactivity was visualized by using the Vectastain Elite DAB Kit (Vector Laboratories, Burlingame, CA, USA) according to the manufacturer’s instructions. Images were obtained using a microscope.
Statistical analysis
The data are expressed as the mean ± standard deviation (s.d.) from at least three independent experiments, with triplicate samples for each individual treatment or dosage. The results were statistically analysed using one-way ANOVA coupled with Student’s t-test, which were analysed using GraphPad Prism version 6.01 for Windows. A P-value <0.05 was considered statistically significant.
Results
Lycorine inhibits the proliferation and colony formation of OS cells
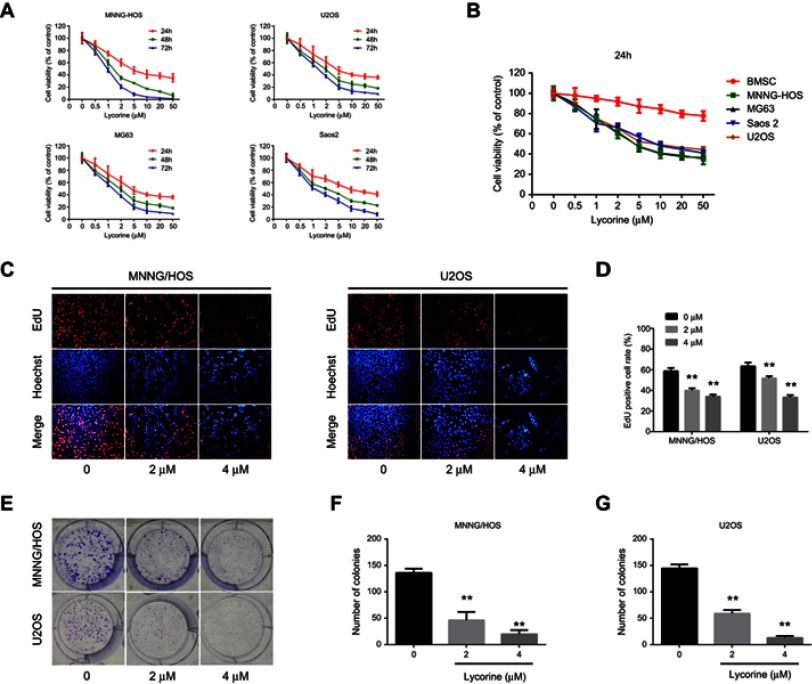
To investigate the effect of lycorine on the growth of OS cells, MNNG/HOS, U2OS, MG63 and Saos-2, were exposed to various concentrations of lycorine for 24, 48 or 72 h, and then the cell viabilities were analysed by CCK-8 assays. The results indicated that lycorine suppressed the proliferation of OS cells in a concentration- and time-dependent manner (Figure 1A). However, the normal human cells (bone marrow stromal cells (BMSCs)) showed strong resistance to lycorine (Figure 1B), which indicated that lycorine selectively inhibited the growth of OS cells but had less cytotoxicity on normal human cells. Additionally, the results of the EdU assay demonstrated that the number of EdU-positive MNNG/HOS and U2OS cells significantly decreased with increasing concentrations of lycorine (Figure 1C and D). Colony formation assays were performed to evaluate the potential role of lycorine in the tumourigenesis of OS cells. As shown in Figure 1E–G, both the size and the number of colonies were significantly decreased in a concentration dependent manner after lycorine treatment.
Figure 1.
Effect of lycorine on the proliferation and colony formation of OS cells.
Notes: (A) The anti-proliferative effect of lycorine on OS cell lines was determined by CCK8 assay. Cells were treated with different concentrations of lycorine for 24, 48, and 72 h. (B) Comparison of the effect of lycorine on human bone marrow stromal cells with that on OS cells for 24 h. (C) EdU assay detecting cell proliferation. EdU assays were performed 48 h after treatment with lycorine at various concentrations. (D) EdU-positive cells were caculated with the following formula: (EdU add-in cells/Hoechst stained cells) ×100%. (E) Representative images of the colony formation assays of MNNG/HOS and U2OS cells are shown. (F, G) The colonies that consisted of at least 50 cells were counted. Data are presented as the mean ± SD of three independent experiments. **P<0.001 vs control.
Abbreviations: OS, osteosarcoma; EdU, 5-ethynyl-2-deoxyuridine.
Lycorine induces apoptosis in MNNG/HOS and U2OS cells
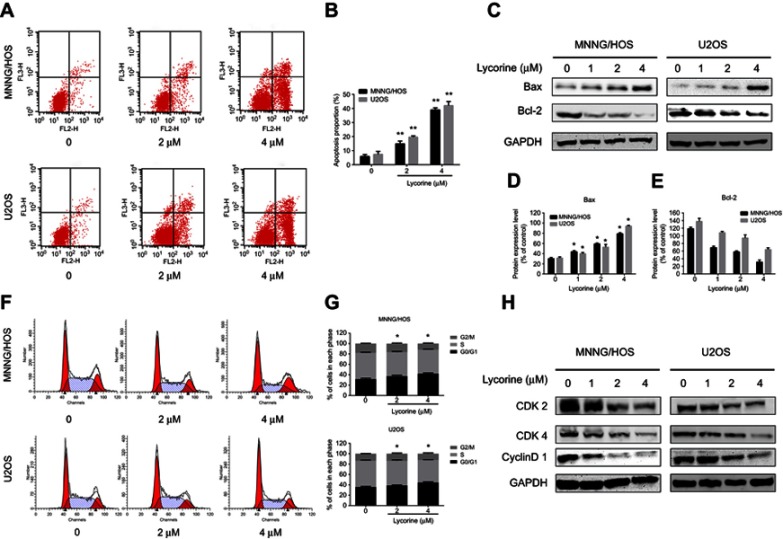
To investigate whether the anti-proliferation effect induced by lycorine was associated with apoptosis, flow cytometry analyses with Annexin V-PE/7-AAD dual staining was performed. The results indicated that the number of apoptotic cells was increased in a dose dependent manner after treatment with lycorine for 24 h (Figure 2A and B). To further explore the apoptotic proteins involved in lycorine-induced cell death, the expression of the related proteins was investigated by Western blot analysis. After treatment with Lycorine for48 h, the expression of the anti-apoptotic protein Bcl-2 decreased while the expression of the apoptotic protein Bax increased in a concentration-dependent manner (Figure 2C–E).
Figure 2.
Lycorine induces apoptosis and cell cycle arrest in MNNG/HOS and U2OS cells.
Notes: (A) MNNG/HOS and U2OS cells treated with lycorine were stained with Annexin V-PE/7-AAD and analysed by flow cytometry. (B) The proportion of apoptotic MNNG/HOS and U2OS cells. (C-E) MNNG/HOS and U2OS cells were treated with Lycorine for 48 h. The expressions expression levels of cell apoptosis-related proteins were measured by Western blot. And the Bcl-2 protein expression levels in both cells were statistically analysed. (F, G) Flow cytometric analysis of the percentage of cells treated with Lycorine in various phases of the cell cycle. (H) The expressions of cell cycle-regulated proteins were measured by Western blot. Data are presented as the mean ± SD of three independent experiments. *P<0.05 vs control, **P<0.001 vs control.
Abbreviation: Annexin V-PE/7-AAD, Annexin V-phycoerythrin/7-amino actinomycin.
Lycorine induces cell cycle arrest in MNNG/HOS and U2OS cells
To validate whether lycorine inhibited cell proliferation by inducing cell cycle arrest, the cell cycle distribution of MNNG/HOS and U2OS cells was analysed after treatment with lycorine. As shown in Figure 2F and G, lycorine induced the accumulation of cells in the G0/G1 phase, accompanied by decreased cell numbers in the G2/M and S phases in both HOS and U2OS cells. To elucidate the underlying mechanisms, the cell cycle-regulatory proteins were examined using Western blot analysis. The results showed that lycorine downregulated the expression of CDK2, CDK4 and Cyclin D1 (Figure 2H). All these data indicated that the G0/G1 phase arrest induced by lycorine was, at least partly, due to alterations in the expression of cell cycle-related proteins.
Lycorine inhibits migration and invasion in MNNG/HOS and U2OS cells
To determine the role of lycorine in the migration and invasion of MNNG/HOS and U2OS cells, wound healing assay was performed. The results of indicated that the cell migration abilities of MNNG/HOS and U2OS cells were significantly decreased after treatment with lycorine for 24 h (Figure 3A–D). The results of Transwell migration assays similarly showed that lycorine significantly inhibited the migration of MNNG/HOS and U2OS cells (Figure 3E and F). Moreover, Transwell assays were used to examine the effect of lycorine on invasive abilities. The results revealed that the migration and invasion abilities of MNNG/HOS and U2OS cells were dramatically inhibited after lycorine treatment (Figure 3G and H). Furthermore, the tumour metastasis-related protein levels were detected by Western blotting. As shown in Figure 3I, lycorine reduced the expression levels of N-cadherin, MMP2 and MMP9 while enhancing the expression of E-cadherin in a dose-dependent manner in MNNG/HOS and U2OS cells.
Figure 3.
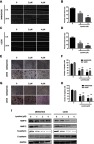
Effects of lycorine on the migration and invasion of MNNG/HOS and U2OS cells.
Notes: (A, B) Micrographs of wound healing assays for MNNG/HOS and U2OS cells treated with Lycorine. Images were obtained at 0 and 24 h (×100 magnification). The percentage of wound closure (original width –width at 24 h after scraping/original width) was calculated. (E, F) A Transwell assay was used to detect the migration of MNNG/HOS and U2OS cells. The number of migratory cells was observed and counted by using a light microscope (×100 magnification). (G, H) A Transwell assay was used to detect the invasion of MNNG/HOS and U2OS cells. The number of the invasive cells was observed and counted by using a light microscope (×100 magnification). (I) The tumour metastasis-related protein levels were detected by Western blotting. Data are presented as the mean ± SD of three independent experiments **P<0.001 vs control.
Lycorine inhibits the JAK2/STAT3 signalling pathway
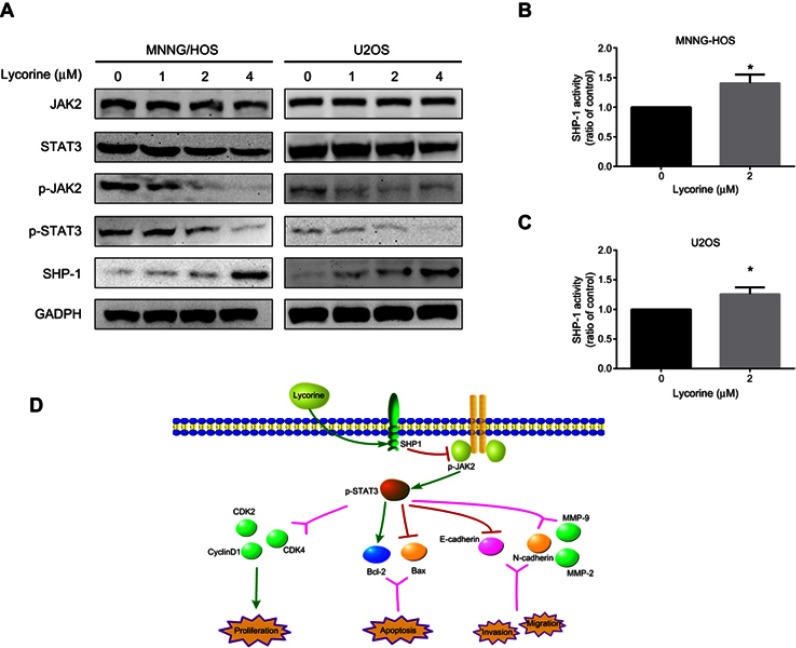
The aforementioned experiments revealed that lycorine exhibits inhibitory effects on variety of important biological programmers, including cell proliferation, cell cycle progression, migration, invasion and apoptosis. To further elucidate the underlying mechanisms of the antitumour activity exerted against OS cells by lycorine, the expression levels of the phosphorylated forms of JAK2 and STAT3 following exposure to lycorine were investigated. The results demonstrated that lycorine decreased the constitutive phosphorylation of JAK2 and STAT3 in a dose-dependent manner without affecting their total protein levels. In addition, the expression level of SHP-1 (negative STAT3 regulator) was upregulated by lycorine (Figure 4A).
Figure 4.
Lycorine inhibits the JAK2/STAT3 signaling pathway by enhancing SHP-1 expression.
Notes: (A) MNNG/HOS and U2OS cells were treated with various concentrations of lycorine for 48 h and then the cell lysates were extracted for Western blot analysis by using antibodies specific to JAK2, p-JAK2, STAT3, p-STAT3, and SHP-1. (B, C) SHP-1 activity was measured in the cells 24 h after treatment with or without lycorine. (D) A schematic illustration of the hypothesized mechanism for the anti-OS activity of lycorine. Data are presented as the mean ± SD of three independent experiments. **P<0.001 vs control.
Abbreviations: JAK2, Janus kinase 2; STAT3, signal transducers and activators of transcription 3; SHP-1, SH2 domain-containing phosphatase 1.
Lycorine increased SHP-1 activity
Accumulating evidence has demonstrated that SHP-1 is highly expressed in cancer cells and plays an essential role in the growth, survival and metastasis of cancer cells during cancer progression.29,30 Therefore, we determined the effect of lycorine on SHP-1 activity in OS cells. Our results revealed that lycorine significantly increased SHP-1 activity in MNNG/HOS and U2OS cells (Figure 4B and C). In addition, the schematic illustration in Figure 4D shows the mechanisms by which lycorine inhibits OS growth and motility.
Lycorine inhibits the growth of OS in vivo
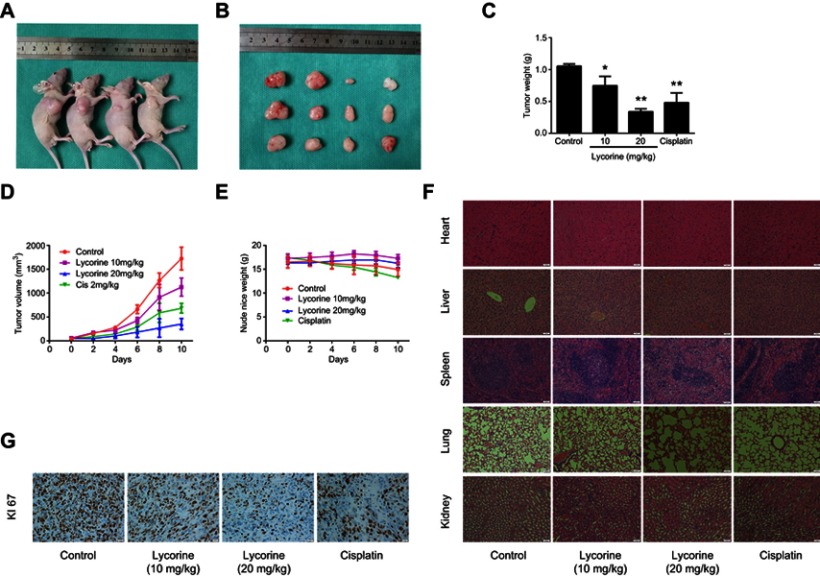
To evaluate the antitumour effect of lycorine in vivo, a xenograft model was established by inoculating MNNG/HOS cells into the right axilla of nude mice. After 10 days of drug administration, the tumour growth was efficiently inhibited by lycorine at doses of 20 mg/kg compared with the tumour growth of the negative control mice (Figure 5A and B). The tumour weight and volume in the lycorine-injected group were reduced in a dose-dependent manner compared with those of negative control group (Figure 5C and D). No obvious change in body weight in the lycorine-injected group or in negative control group was observed during the treatment period. However, the average body weight of the mice in the cisplatin-injected group tended to decrease (Figure 5E). To assess the potential toxicity of lycorine on normal tissues, HE staining of major organs collected at the end of the experiment was performed; the results showed no obvious pathological changes compared with those in the control group (Figure 5F). Then, we performed immunohistochemical assays to evaluate the expression of Ki-67, which is considered a marker of proliferation. The results demonstrated that compared with that in the control group, the number of Ki-67-positive tumour cells was significantly decreased by Lycorine at doses of 20 mg/kg (Figure 5G). All the results demonstrated that lycorine could ameliorate tumour growth with low toxicity in vivo.
Figure 5.
Lycorine inhibits OS xenograft growth in vivo.
Notes: MNNG/HOS cells were injected into the right axilla of nude mice. Four days after tumour inoculation, the mice were randomly divided into four groups and then treated with lycorine (10 mg/kg every 2 days or 20 mg/kg every 2 days), DMSO (negative control) or cisplatin (positive control). (A, B) An image of the nude mice xenograft tumours was taken after the lycorine treatment for 10 days. (C) Tumour weights were measured after ten days of lycorine administration. (D, E) Tumour volumes and nude mice weights were measured and calculated every 2 days. (F) H&E staining indicated that no major organ(heart, liver, spleen, lung, and kidney) toxicities were observed (×200 magnification). (G) Immunohistochemical analysis of Ki-67 to indicate cell proliferation in tumour tissues (×500 magnification). Data are presented as the mean ± SD of three independent experiments. *P<0.05 vs control, **P<0.001 vs control.
Discussion
OS is the most commom primary malignant bone tumour with high mortality. In the recent decades, the 5-year survival of patients with OS has improved, but relapse and metastasis are the main causes for the failure of surgical operation in treating OS. Moreover, the current anticancer chemotherapies for OS remain unsatisfactory because of the obvious side-effects and toxicity of systemic chemotherapy. Therefore, novel therapeutic approaches for patients with OS remain urgently needed. Increasing evidence has suggested that traditional Chinese medicine has its unique advantages in cancer treatment. In the present study, we explored the anticancer effects of lycorine on OS and revealed its underlying mechanisms. Our results demonstrated that the inhibitory effect of lycorine on OS cells may be through the suppression of the JAK2/STAT3 pathway.
The dysregulation of cell cycle progression is believed to play an essential role in tumourigenesis.31,32 A previous study has investigated whether lycorine could surppress the proliferation of the human chronic myelocytic leukaemia cell line K562 through G0/G1 phase arrest.33 Consistently, our experiment demonstrated that lycorine caused G0/1 phase arrest and significantly reduced the expression levels of cyclin D1, CDK2 and CDK4 in MNNG/HOS and U2OS cells in a dose-dependent manner.
Apoptosis plays a vital role in the development and homeostasis and the aberrant of apoptotic signaling may induce malignant tumours.34 Bcl-2 family proteins, which consist of the pro-apoptotic proteins BH3-interacting domain death agonist, Bax, and Bak and the anti-apoptotic proteins Bcl-2 and Bcl-xL, exert a significant role in the mitochondria-dependent apoptotic pathway.35,36 Bax can promote apoptotic factors and enhance apoptosis, while Bcl-2 exerts precisely the opposite effects to inhibit apoptosis.37 The present study demonstrated that the apoptotic rates of MNNG/HOS and U2OS cells were both increased after lycorine treatment. Moreover, the results of Western blotting showed that lycorine decreased the expression of the anti-apoptotic protein Bcl-2 while increasing the expression level of the apoptotic protein Bax in a concentration-dependent manner.
Epithelial-mesenchymal transition (EMT) is considered to exert a crucial role in the initiation of the metastatic progression of tumour cells.38 Previous studies have demonstrated that EMT is correlated with the progression of various types of cancer.39 Moreover, other studies have suggested that EMT may be an essential step for the metastasis of OS due to its mesenchymal origin.40,41 To determine the effect of lycorine on EMT of OS, EMT-associated markers were detected. Our results revealed that lycorine significantly inhibited the migration and invasion abilities of MNNG/HOS and U2OS cells. The Western blotting results showed that lycorine increased the expression of E-cadherin while decreasing the expression of N-cadherin. In addition, MMPs also exert an essential role in tumourinvasion and metastasis by degrading the extracellular matrix.42 Therefore, it is possible to suppress the metastasis of tumour cells by inhibiting the MMP activity.43 Our results showed that the protein levels of MMP-2 and MMP-9 were reduced by lycorine in MNNG/HOS and U2OS cells.
Accumulating evidence indicates that the JAK2/STAT3 signal transduction pathway is involved in the progression of various malignant tumours.7–9 Previous studies have demonstrated that lycorine dramatically inhibited the growth and metastasis of breast cancer and hormone-refractory prostate cancer through inhibiting the JAK2/STAT3 pathway.21,22 Therefore, we further investigated whether the anti-OS effect of lycorine occurs through inhibiting the JAK2/STAT3 signalling pathway. Our results demonstrated that lycorine significantly reduced the phosphorylation of JAK2 and STAT3 in a dose-dependent manner in OS cells. SHP1, a non-receptor protein tyrosine phosphatase, is considered to be crucial for the regulation of cytokine/protein tyrosine kinase-mediated signaling.44,45 Increasing evidence has suggested that the expression of SHP-1 is down-regulated in various of cancers.46,47 In the present study, the expression level of SHP-1 was upregulated, and the activity of SHP-1 was enhanced in MNNG/HOS and U2OS cells after treatment with lycorine. Moreover, lycorine downregulated the expression of STAT3 target proteins, such as the invasion-related proteins (MMP-2 and MMP-9) and anti-apoptotic proteins (Bcl-2).
Conclusion
Conclusively, our results demonstrated for the first time thatthe anti-osteosarcoma effects of Lycorine were at least partly due to the suppression of the JAK2/STAT3 pathway. . In addition, our study revealed that lycorine exerted potent antitumour activity with low levels of toxicity in vivo. These findings indicate that lycorine might possess attractive advantages as a promising and effective candidate in clinical therapy for human OS in the future.
Acknowledgments
This study was supported by Grants 2016YFC1100100 from The National Key Research and Development Program of China, Grants 91649204 from Major Research Plan of National Natural Science Foundation of China.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Disclosure
The authors declare that they have no conflict of interest in this work.
References
- 1.Miao J, Wu S, Peng Z, Tania M, Zhang C. MicroRNAs in osteosarcoma: diagnostic and therapeutic aspects. Tumour Biol. 2013;34(4):2093–2098. doi: 10.1007/s13277-013-0940-7 [DOI] [PubMed] [Google Scholar]
- 2.Hameed M, Dorfman H. Primary malignant bone tumors–recent developments. Semin Diagn Pathol. 2011;28(1):86–101. [DOI] [PubMed] [Google Scholar]
- 3.Mirabello L, Troisi RJ, Savage SA. Osteosarcoma incidence and survival rates from 1973 to 2004: data from the surveillance, epidemiology, and end results program. Cancer. 2009;115(7):1531–1543. doi: 10.1002/cncr.24121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yu H, Pardoll D, Jove R. STATs in cancer inflammation and immunity: a leading role for STAT3. Nat Rev Cancer. 2009;9(11):798–809. doi: 10.1038/nrc2734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hemmann U, Gerhartz C, Heesel B, et al. Differential activation of acute phase response factor/Stat3 and Stat1 via the cytoplasmic domain of the interleukin 6 signal transducer gp130. II. Src homology SH2 domains define the specificity of stat factor activation. J Biol Chem. 1996;271(22):12999–13007. [DOI] [PubMed] [Google Scholar]
- 6.Jarnicki A, Putoczki T, Ernst M. Stat3: linking inflammation to epithelial cancer - more than a “gut” feeling? Cell Div. 2010;5:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nam S, Xie J, Perkins A, et al. Novel synthetic derivatives of the natural product berbamine inhibit Jak2/Stat3 signaling and induce apoptosis of human melanoma cells. Mol Oncol. 2012;6(5):484–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wei R, Yang Q, Han B, et al. microRNA-375 inhibits colorectal cancer cells proliferation by downregulating JAK2/STAT3 and MAP3K8/ERK signaling pathways. Oncotarget. 2017;8(10):16633–16641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang N, Han F, Cui H, et al. Matrine suppresses proliferation and induces apoptosis in human cholangiocarcinoma cells through suppression of JAK2/STAT3 signaling. Pharmacol Rep. 2015;67(2):388–393. [DOI] [PubMed] [Google Scholar]
- 10.Bromberg J. Stat proteins and oncogenesis. J Clin Invest. 2002;109(9):1139–1142. doi: 10.1172/JCI15617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Avalle L, Pensa S, Regis G, Novelli F, Poli V. STAT1 and STAT3 in tumorigenesis: a matter of balance. JAKSTAT. 2012;1(2):65–72. doi: 10.4161/jkst.20045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park KR, Yun HM, Quang TH, et al. 4-Methoxydalbergione suppresses growth and induces apoptosis in human osteosarcoma cells in vitro and in vivo xenograft model through down-regulation of the JAK2/STAT3 pathway. Oncotarget. 2016;7(6):6960–6971. doi: 10.18632/oncotarget.6873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Um HJ, Min KJ, Kim DE, Kwon TK. Withaferin A inhibits JAK/STAT3 signaling and induces apoptosis of human renal carcinoma Caki cells. Biochem Biophys Res Commun. 2012;427(1):24–29. doi: 10.1016/j.bbrc.2012.08.133 [DOI] [PubMed] [Google Scholar]
- 14.Yan J, Wang Q, Zou K, et al. Inhibition of the JAK2/STAT3 signaling pathway exerts a therapeutic effect on osteosarcoma. Mol Med Rep. 2015;12(1):498–502. doi: 10.3892/mmr.2015.3439 [DOI] [PubMed] [Google Scholar]
- 15.Peng HY, Cheng YC, Hsu YM, et al. MPT0B098, a microtubule inhibitor, suppresses JAK2/STAT3 signaling pathway through modulation of SOCS3 stability in oral squamous cell carcinoma. PLoS One. 2016;11(7):e0158440. doi: 10.1371/journal.pone.0158440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hwang YC, Chu JJ, Yang PL, Chen W, Yates MV. Rapid identification of inhibitors that interfere with poliovirus replication using a cell-based assay. Antiviral Res. 2008;77(3):232–236. doi: 10.1016/j.antiviral.2007.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mikami M, Kitahara M, Kitano M, et al. Suppressive activity of lycoricidinol (narciclasine) against cytotoxicity of neutrophil-derived calprotectin, and its suppressive effect on rat adjuvant arthritis model. Biol Pharm Bull. 1999;22(7):674–678. [DOI] [PubMed] [Google Scholar]
- 18.Sener B, Orhan I, Satayavivad J. Antimalarial activity screening of some alkaloids and the plant extracts from Amaryllidaceae. Phytother Res. 2003;17(10):1220–1223. doi: 10.1002/ptr.1346 [DOI] [PubMed] [Google Scholar]
- 19.Locarek M, Novakova J, Kloucek P, et al. Antifungal and antibacterial activity of extracts and alkaloids of selected amaryllidaceae species. Nat Prod Commun. 2015;10(9):1537–1540. [PubMed] [Google Scholar]
- 20.Nair JJ, van Staden J. Cytotoxicity studies of lycorine alkaloids of the Amaryllidaceae. Nat Prod Commun. 2014;9(8):1193–1210. [PubMed] [Google Scholar]
- 21.Wang J, Xu J, Xing G. Lycorine inhibits the growth and metastasis of breast cancer through the blockage of STAT3 signaling pathway. Acta Biochim Biophys Sin (Shanghai). 2017;49(9):771–779. doi: 10.1093/abbs/gmx076 [DOI] [PubMed] [Google Scholar]
- 22.Hu M, Peng S, He Y, et al. Lycorine is a novel inhibitor of the growth and metastasis of hormone-refractory prostate cancer. Oncotarget. 2015;6(17):15348–15361. doi: 10.18632/oncotarget.3610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roy M, Liang L, Xiao X, et al. Lycorine downregulates HMGB1 to inhibit autophagy and enhances bortezomib activity in multiple myeloma. Theranostics. 2016;6(12):2209–2224. doi: 10.7150/thno.15584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yu H, Qiu Y, Pang X, et al. Lycorine promotes autophagy and apoptosis via TCRP1/Akt/mTOR axis inactivation in human hepatocellular carcinoma. Mol Cancer Ther. 2017;16(12):2711–2723. doi: 10.1158/1535-7163.MCT-17-0498 [DOI] [PubMed] [Google Scholar]
- 25.Yang L, Liu ZM, Rao YW, Cui SQ, Wang H, Jia XJ. Downregulation of microRNA-586 inhibits proliferation, invasion and metastasis and promotes apoptosis in human osteosarcoma U2-OS cell line. Cytogenet Genome Res. 2015;146(4):268–278. doi: 10.1159/000441073 [DOI] [PubMed] [Google Scholar]
- 26.Feng S, Cong S, Zhang X, et al. MicroRNA-192 targeting retinoblastoma 1 inhibits cell proliferation and induces cell apoptosis in lung cancer cells. Nucleic Acids Res. 2011;39(15):6669–6678. doi: 10.1093/nar/gkr232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qing X, Shao Z, Lv X, et al. Anticancer effect of (S)-crizotinib on osteosarcoma cells by targeting MTH1 and activating reactive oxygen species. Anticancer Drugs. 2018;29(4):341–352. doi: 10.1097/CAD.0000000000000602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang K, Chen Y, Zhang R, et al. Honokiol induces apoptosis and autophagy via the ROS/ERK1/2 signaling pathway in human osteosarcoma cells in vitro and in vivo. Cell Death Dis. 2018;9(2):157. doi: 10.1038/s41419-018-1111-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen KF, Su JC, Liu CY, et al. A novel obatoclax derivative, SC-2001, induces apoptosis in hepatocellular carcinoma cells through SHP-1-dependent STAT3 inactivation. Cancer Lett. 2012;321(1):27–35. doi: 10.1016/j.canlet.2012.03.023 [DOI] [PubMed] [Google Scholar]
- 30.Li R, Hu Z, Sun SY, et al. Niclosamide overcomes acquired resistance to erlotinib through suppression of STAT3 in non-small cell lung cancer. Mol Cancer Ther. 2013;12(10):2200–2212. doi: 10.1158/1535-7163.MCT-13-0095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu W, McArthur G. Cell cycle regulation and melanoma. Curr Oncol Rep. 2016;18(6):34. doi: 10.1007/s11912-016-0524-y [DOI] [PubMed] [Google Scholar]
- 32.Hartwell LH, Kastan MB. Cell cycle control and cancer. Science. 1994;266(5192):1821–1828. [DOI] [PubMed] [Google Scholar]
- 33.Li L, Dai HJ, Ye M, et al. Lycorine induces cell-cycle arrest in the G0/G1 phase in K562 cells via HDAC inhibition. Cancer Cell Int. 2012;12(1):49. doi: 10.1186/1475-2867-12-49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mahoney JA, Rosen A. Apoptosis and autoimmunity. Curr Opin Immunol. 2005;17(6):583–588. doi: 10.1016/j.coi.2005.09.018 [DOI] [PubMed] [Google Scholar]
- 35.Gross A, McDonnell JM, Korsmeyer SJ. BCL-2 family members and the mitochondria in apoptosis. Genes Dev. 1999;13(15):1899–1911. doi: 10.1101/gad.13.15.1899 [DOI] [PubMed] [Google Scholar]
- 36.Reed JC. Apoptosis-regulating proteins as targets for drug discovery. Trends Mol Med. 2001;7(7):314–319. [DOI] [PubMed] [Google Scholar]
- 37.Yang SH, Chien CM, Lu MC, Lin YH, Hu XW, Lin SR. Up-regulation of Bax and endonuclease G, and down-modulation of Bcl-XL involved in cardiotoxin III-induced apoptosis in K562 cells. Exp Mol Med. 2006;38(4):435–444. doi: 10.1038/emm.2006.51 [DOI] [PubMed] [Google Scholar]
- 38.Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139(5):871–890. doi: 10.1016/j.cell.2009.11.007 [DOI] [PubMed] [Google Scholar]
- 39.Tian L, Shen D, Li X, et al. Ginsenoside Rg3 inhibits epithelial-mesenchymal transition (EMT) and invasion of lung cancer by down-regulating FUT4. Oncotarget. 2016;7(2):1619–1632. doi: 10.18632/oncotarget.6451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang G, Yuan J, Li K. EMT transcription factors: implication in osteosarcoma. Med Oncol. 2013;30(4):697. [DOI] [PubMed] [Google Scholar]
- 41.Niinaka Y, Harada K, Fujimuro M, et al. Silencing of autocrine motility factor induces mesenchymal-to-epithelial transition and suppression of osteosarcoma pulmonary metastasis. Cancer Res. 2010;70(22):9483–9493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yun HM, Park KR, Quang TH, et al. 4-parvifuran inhibits metastatic and invasive actions through the JAK2/STAT3 pathway in osteosarcoma cells. Arch Pharm Res. 2017;40(5):601–609. [DOI] [PubMed] [Google Scholar]
- 43.Okada N, Ishida H, Murata N, Hashimoto D, Seyama Y, Kubota S. Matrix metalloproteinase-2 and −9 in bile as a marker of liver metastasis in colorectal cancer. Biochem Biophys Res Commun. 2001;288(1):212–216. [DOI] [PubMed] [Google Scholar]
- 44.Bhattacharya R, Kwon J, Wang E, Mukherjee P, Mukhopadhyay D. Src homology 2 (SH2) domain containing protein tyrosine phosphatase-1 (SHP-1) dephosphorylates VEGF Receptor-2 and attenuates endothelial DNA synthesis, but not migration*. J Mol Signal. 2008;3:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fan LC, Teng HW, Shiau CW, et al. Pharmacological targeting SHP-1-STAT3 signaling is a promising therapeutic approach for the treatment of colorectal cancer. Neoplasia. 2015;17(9):687–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wu C, Sun M, Liu L, Zhou GW. The function of the protein tyrosine phosphatase SHP-1 in cancer. Gene. 2003;306:1–12. [DOI] [PubMed] [Google Scholar]
- 47.Liu CY, Su JC, Huang TT, et al. Sorafenib analogue SC-60 induces apoptosis through the SHP-1/STAT3 pathway and enhances docetaxel cytotoxicity in triple-negative breast cancer cells. Mol Oncol. 2017;11(3):266–279. [DOI] [PMC free article] [PubMed] [Google Scholar]