Abstract
Aim/Introduction
Autoantibodies to the 65 kDa isoform of glutamic acid decarboxylase (GADA) are a valuable diagnostic and predictive marker for type 1 diabetes. Recently, it has been reported that a significant proportion of sera in the commercial RSR radioimmunoassay (RIA) that have tested positive for GADA have then turned negative in RSR enzyme‐linked immunosorbent assay (ELISA) tests in patients with type 1 diabetes. The present study aimed to investigate whether the GADA result discrepancies between RSR‐RIA and RSR‐ELISA are related to autoantibody affinity.
Methods
GADA affinity was measured by a competitive binding experiment using unlabeled recombinant human GAD65 in 12 discordant samples (5 RIA[+]/ELISA[−] and 7 RIA[−]/ELISA[+] sera). Furthermore, the effect of the initial incubation time on the GADA positivity was also examined using the ELISA test.
Results
GADA affinities were >1010 L/mol in two of five RIA(+)/ELISA(−) and all of seven RIA(−)/ELISA(+) sera. After an initial incubation time longer than the recommended 1 h, the GADA titer in three of five RIA(+)/ELISA(−) sera and all RIA(−)/ELISA(+) sera increased 1.6‐ to 100‐fold. However, the titer in 12 GADA‐negative sera from healthy controls remained unchanged after the longer incubation. The increment ratio of GADA titer was positively correlated with GADA affinity (r = 0.991, P < 0.001).
Conclusions
The RSR‐RIA test identifies both high‐ and low‐affinity GADA, whereas the RSR‐ELISA test identifies only high‐affinity GADA. A longer initial incubation time in the RSR‐ELISA test increases the sensitivity of GADA with the same specificity in patients with type 1 diabetes.
Keywords: Affinity, Anti‐glutamic acid decarboxylase antibody, Enzyme‐linked immunosorbent assay
Introduction
Type 1 diabetes is an autoimmune disease characterized by T cell‐mediated destruction of pancreatic β‐cells and the presence of circulating autoantibodies directed against several β‐cell autoantigens1. To date, the expression of anti‐islet autoantibodies is the best phenotypic marker of autoimmune type 1 (type 1A) diabetes1. Among these, autoantibodies to glutamic acid decarboxylase (GADA) are the most valuable tools for diagnosing autoimmune type 1A diabetes, and also for the assessing risk for future development of type 1 diabetes. Under the auspices of the Immunology and Diabetes Society, several workshops have been held to standardize and improve anti‐islet autoantibody assay performance and concordance among laboratories2, 3. Both RSR radioimmunoassay (RIA) and RSR enzyme immunosorbent assay (ELISA) are well established tests for the analysis of GADA2, and are both widely distributed throughout the world as commercial kits. Both of these kits achieved high sensitivity and specificity in the Diabetes Autoantibody Standardization Program or Islet Autoantibody Standardization Program GADA workshop, and the titers of GADA by the ELISA kit closely correlated with those by the RIA kit (r > 0.95). However, recent studies showed that sera from 8 to 15% of GADA‐positive patients with type 1 diabetes showed discrepant results by the two assays4, 5, 6, 7. In the following study, we aimed to evaluate whether the discrepancy of GADA results between RSR‐RIA and RSR‐ELISA is related to autoantibody affinity. Furthermore, the effect of the initial incubation time on the GADA positivity was also examined by the ELISA test.
Methods
Participants
Of 140 serum samples from patients with adult‐onset diabetes (81 type 1 diabetes and 59 type 2 diabetes) who were simultaneously measured for GADA using RSR‐RIA (RiaRSR™ GADAb; RSR Ltd., Cardiff, UK) and RSR‐ELISA (ElisaRSR™ GADAb; RSR Ltd.), 46 (56.8%) and 48 (59.3%) patients with type 1 diabetes were positive for GADA by RIA and ELISA kit, respectively. Furthermore, the GADA titers by the RIA kit correlated with those by the ELISA kit, excluding the patients with RIA‐negative and ELISA‐negative patients (r = 0.913, P < 0.0001; Figure 1), and the regression equation was RSR‐ELISA = 0.539 + 23.3 × RSR‐RIA. A total of 12 discordant samples (5 RIA[+]/ELISA[−] and 7 RIA[−]/ELISA[+] sera) were identified and used for further studies. Details of the patients’ clinical characteristics are shown in Table 1. The RIA(+)/ELISA(−) patients consisted of one case of acute‐onset, one case of fulminant and three cases of slowly progressive type 1 diabetes. The median RSR‐RIA GADA was 4.3 U/mL (range 2.4–25.2 U/mL). In addition, the RIA(−)/ELISA(+) patients consisted of one case of acute‐onset type 1 diabetes and six cases of slowly progressive type 1 diabetes. The median RSR‐ELISA GADA was 12.4 U/mL (range 6.0–36.1 U/mL). Furthermore, sera from 12 healthy controls were also used to study how incubation time affects GADA positivity. The study protocols were approved by the ethics committee of Shin‐Koga Hospital and Okada Clinic, and informed consent was obtained from all participants in accordance with the Declaration of Helsinki. Serum samples were stored at −20°C until use.
Figure 1.
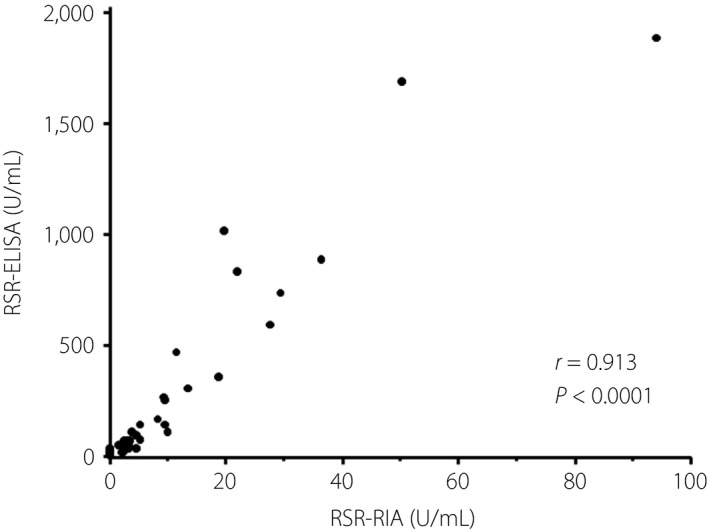
Correlation between the titer of glutamic acid decarboxylase antibody by RSR radioimmunoassay (RSR‐RIA) and RSR enzyme‐linked immunosorbent assay (RSR‐ELISA). Autoantibody‐positive sera whose titers are within assay range were used in this analysis (n = 42).
Table 1.
Clinical characteristics
| Category | ID | Sex | Age at onset (years) | Type | Duration (years) | GADA‐RIA (U/mL) | GADA‐ELISA (U/mL) | IA‐2A‐RIA | ZnT8A‐ELISA | TPOA | TgA |
|---|---|---|---|---|---|---|---|---|---|---|---|
| GADA‐RIA(+)/ELISA(−) | SK8 | F | 32 | Acute | 35 | 2.4† | 1.8 | Negative | Negative | Positive | Positive |
| SK13 | F | 31 | Fulminant | 10 | 4.1† | 1.3 | Negative | Negative | Negative | Negative | |
| SK15 | M | 47 | SPIDDM | 12 | 25.2† | 1.6 | Negative | Negative | Negative | Negative | |
| OK78 | F | 40 | SPIDDM | 23 | 8.1† | 1.6 | Negative | Negative | Negative | Negative | |
| OK30 | F | 48 | SPIDDM | 14 | 4.3† | 1.3 | Negative | Negative | Negative | Negative | |
| GADA‐RIA(−)/ELISA(+) | SK1 | M | 39 | SPIDDM | 12 | 0.0 | 8.8† | Negative | Negative | Negative | Negative |
| SK18 | F | 57 | Acute | 19 | 1.4 | 55.4† | Negative | Negative | Positive | Negative | |
| SK25 | M | 50 | SPIDDM | 11 | 0.0 | 37.1† | Negative | Negative | Negative | Negative | |
| SK27 | M | 29 | SPIDDM | 36 | 0.0 | 10.7† | Negative | Negative | Negative | Negative | |
| SK30 | M | 86 | SPIDDM | 3 | 0.0 | 11.9† | Positive | Positive | Positive | Negative | |
| SK5 | F | 39 | SPIDDM | 26 | 0.0 | 11.7† | Negative | Negative | Negative | Negative | |
| OK61 | F | 30 | SPIDDM | 39 | 0.0 | 23.0† | Negative | Negative | Negative | Negative |
Autoantibody positive. Acute, acute‐onset type 1 diabetes; Fulminant, fulminant type 1 diabetes; GADA, glutamic acid decarboxylase antibody; IA‐2A, insulinoma‐associated antigen‐2 antibody; SPIDDM, slowly progressive type 1 diabetes; TgA, thyroglobulin antibody; TPOA, thyroid peroxidase antibody; ZnT8A, zinc transporter‐8 antibody.
Islet Autoantibody Measurement
RSR‐RIA GADA and insulinoma‐associated antigen‐2 antibody (IA‐2A) were determined by liquid‐phase RIA using 125I‐labeled recombinant human GAD65 and IA‐2 as a tracer reagent, respectively8, 9. Briefly, serum sample and 125I‐labeled GAD65 or IA‐2 were incubated in a tube and immune complexes were adsorbed onto solid‐phase protein A. Assay buffer was then added and after centrifugation, supernatants were aspirated and the radioactivity of the sediment was counted in a γ‐counter. Results were read from a calibration curve constructed in the same run with the calibrators, and expressed in U/mL. RSR‐ELISA GADA and ZnT8A were determined using bivalent ELISA using biotinylated GAD65 and ZnT8, respectively, as previously described10, 11. Those are based on the sandwich type principle. Briefly, serum sample and unlabeled recombinant GAD65 or ZnT8 molecules coated onto the ELISA plate were incubated in the well. After washing the wells, biotinylated GAD65 or ZnT8 was added to each well, unbound biotinylated antigens were then removed by washing. Streptavidin–peroxidase conjugate was added, and the absorbance of the plate wells was read at 405 and 450 nm using an ELISA plate reader after the addition of tetramethylbenzidine. Results were read from a calibration curve constructed in the same run with the calibrators, and expressed in U/mL. The cut‐off value for the RSR‐RIA GADA was 1.5 U/mL, 5.0 U/mL for the RSR‐ELISA GADA, 0.4 U/mL for the IA‐2A and 10 U/mL for ZnT8A, respectively. The lower detection limits for the RSR‐RIA GADA and RSR‐ELISA GADA were 0.11 and 0.57 U/mL, respectively. The intra‐ and interassay coefficients of variation for the RSR‐RIA GADA were 3.6–3.7% and 5.5–6.9%, respectively, whereas those for the RSR‐ELISA GADA were 3.5–8.5% and 5.2–6.4%, respectively (taken from the manufacturer's data sheets of the two kits; RSR Ltd.).
Thyroid Autoantibody Measurement
Autoantibodies to thyroid peroxidase and thyroglobulin were determined by electrochemiluminescence immunoassay using Roche ECLusys Anti‐Tg and Anti‐TPO (Roche Diagnostics GmbH, Mannheim, Germany). The cut‐off value for thyroid peroxidase A and thyroglobulin A was 16 and 28 IU/mL, respectively.
GADA Affinity Measurement
GADA affinity was measured by competitive binding experiments with unlabeled recombinant human GAD65. The assay format was identical to the RSR‐RIA and RSR‐ELISA. Serum (100 μL) was incubated with seven different concentrations of unlabeled GAD65 (10 μL; RSR Ltd.) varying from 1.9 × 10−5 to 1.9 × 10−8 mol/L for 1 h at room temperature before GADA measurements. Half‐maximal inhibitory concentration (IC50) and Kd values were calculated by non‐linear regression analysis using SigmaPlot version 14.0 software (Systat Software Inc., San Jose, CA, USA). Kd values were determined using the dissociation constants in GADA‐positive patients and ligand concentration for each assay12, 13. GADA affinity was expressed as reciprocal Kd value (L/mol). Subsequently, displacement curves were computed from the U/mL for each competition reaction with a one‐site binding model.
Evaluation of Initial Incubation Time in RSR‐ELISA
According to the RSR‐ELISA GADA protocol from RSR Ltd., it is recommended that the patients’ sera, calibrators and controls be incubated with recombinant GAD65 that has been coated onto ELISA plate wells and kept for 1 h at room temperature in the first incubation step. To evaluate whether a longer initial incubation time affected the GADA positivity in discordant samples and healthy controls, the initial incubation time of sera in the ELISA plate wells were extended to 2, 4 and 24 h before GADA measurements. Each assay included calibrators in the kit, and 5.0 U/mL was used as the cut‐off value.
Statistical Analysis
Results are expressed as mean ± standard deviation or median (range). Differences in non‐parametric data were tested using the Mann–Whitney U‐test. The correlation between autoantibody affinity and the increment ratio was analyzed using Spearman's rank correlation test. A P‐value <0.05 was considered statistically significant. Statistical analysis was carried out using StatView statistical software (version 5.0; SAS Institute, Cary, NC, USA).
Results
Difference in the GADA Affinity Detected by RSR‐RIA and RSR‐ELISA
The GADA‐competitive binding curves for five RIA(+)/ELISA(−) sera are shown in Figure 2a. GADA affinities ranged from 4.6 × 108 to 1.7 × 1011 L/mol (Figure 3). Two of five RIA(+)/ELISA(−) sera showed GADA affinity >1010 L/mol. Regarding the RIA(−)/ELISA(+) sera, almost all of the sera inhibited >80% binding of GADA to biotin‐GAD65 by 1.9 × 10−8 mol/L recombinant GAD65 protein (Figure 2b), and all sera showing GADA affinity >1010 L/mol ranged from 3.1 × 1010 to 1.0 × 1012 L/mol (Figure 3). GADA affinities were not related to the subtype of type 1 diabetes, GADA titers, the presence of other islet autoantibodies or the presence of thyroid autoimmunity.
Figure 2.
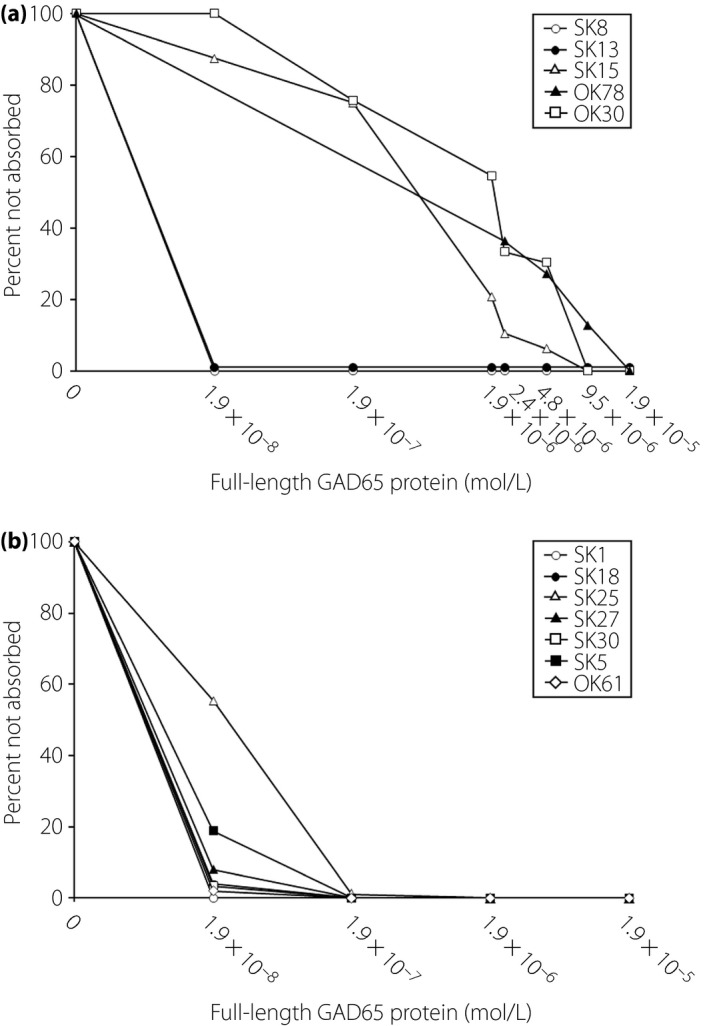
Glutamic acid decarboxylase antibody‐competitive binding curve for (a) radioimmunoassay (RIA)(+)/enzyme‐linked immunosorbent assay (ELISA)(−) sera and for (b) RIA(−)/ELISA(+) sera.
Figure 3.
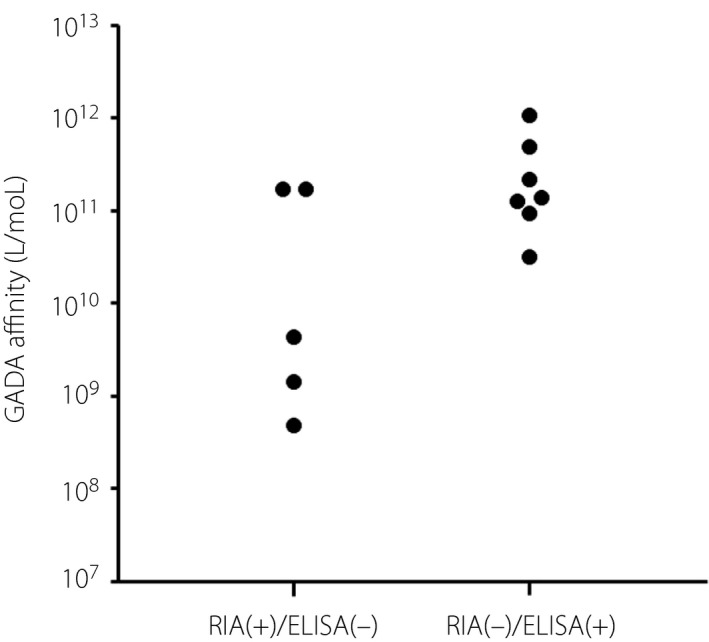
Glutamic acid decarboxylase antibody (GADA) affinities in radioimmunoassay (RIA) (+)/enzyme‐linked immunosorbent assay (ELISA)(−) and RIA(−)/ELISA(+) sera.
Longer Initial Incubation Time of RSR‐ELISA Changes GADA‐Negative to Positive in Higher‐Affinity Sera
To address the reason why higher GADA affinity sera (>1010 L/mol) showed negative results in the RSR‐ELISA, five RIA(+)/ELISA(−) and three RIA(−)/ELISA(+) sera that had sufficient serum available were used in this experiment. The GADA ELISA assays were carried out according to the manufacturer's instructions, except that the initial incubation time of sera in the ELISA plate wells was extended to 2, 4 and 24 h. As shown in Figure 4a, the longer incubation time increased the GADA titers from 1.6‐ to 100‐fold in three of five RIA(+)/ELISA(−) sera, and in all RIA(−)/ELISA(+) sera compared with the recommended incubation time (1 h). The aforementioned three of five GADA‐negative sera (SK8, SK13 and OK30) turned positive after the longer initial incubation time. The titer of GADA plateaued after a 2‐h incubation period, and remained essentially unchanged for the remainder of the incubation period. In contrast, the GADA titer in sera from 12 GADA‐negative healthy controls remained unchanged, even after the longer initial incubation (Figure 4b). Then, the correlation between the GADA affinity and the increment ratio of GADA titer of the 2‐h incubation period compared with the 1‐h incubation period was examined. As shown in Figure 5, the increment ratio of GADA titer was significantly correlated with GADA affinity (r = 0.991, P < 0.0001).
Figure 4.
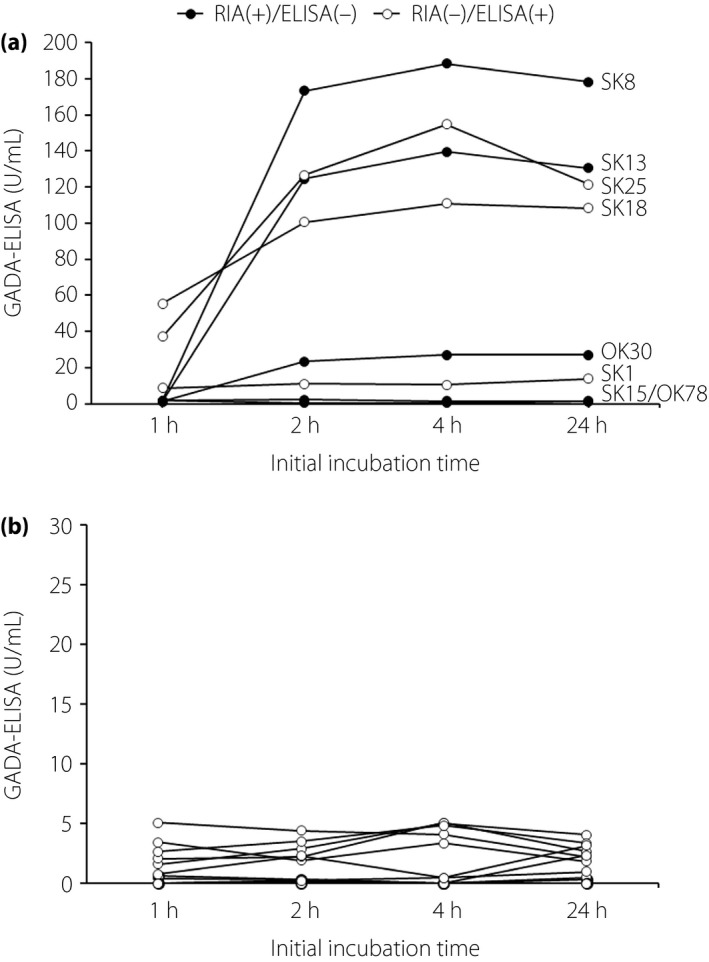
Relationship between the initial incubation time and Glutamic acid decarboxylase antibody (GADA)‐enzyme‐linked immunosorbent assay (ELISA) levels in discordant samples of (a) type 1 diabetes patients and (b) in healthy controls. RIA, radioimmunoassay.
Figure 5.
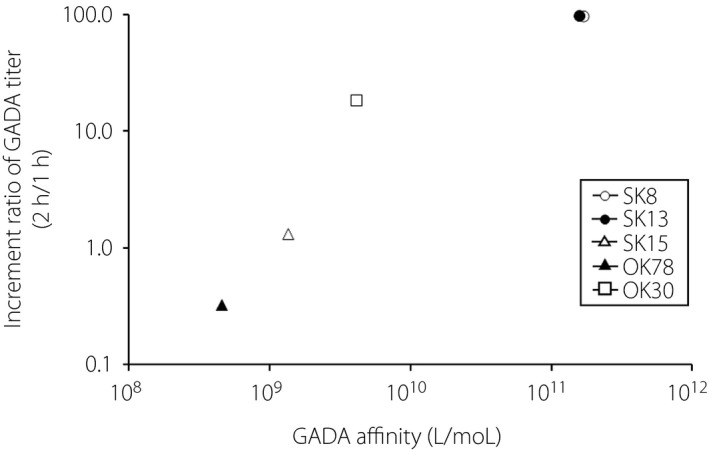
Increment ratio of glutamic acid decarboxylase antibody (GADA) titer of 2‐h incubation compared with 1‐h incubation against GADA affinities.
Discussion
In the present study, we showed that: (i) a difference in the GADA affinity was detected by RSR‐RIA and RSR‐ELISA; and (ii) there was an improvement in the GADA measurement by RSR‐ELISA assay under the modified assay condition. It has previously been reported that high‐affinity islet autoantibodies can be used to predict who might develop diabetes in populations with an increased risk of type 1 diabetes14, 15, 16. It has also been reported that the measurement of autoantibody affinity therefore distinguishes between disease‐relevant and non‐disease‐relevant antibodies. Furthermore, in patients with latent autoimmune diabetes in adults (also known as slowly progressive type 1 diabetes), it was shown that patients with low‐affinity GADA have a prolonged preservation of residual β‐cell function and are therefore at a lower risk of requiring insulin therapy17. It is therefore worth determining disease‐specific autoantibody affinities for the prediction of the development or progression of autoimmune diabetes.
Recent studies have reported that the degree of agreement between RSR‐RIA and RSR‐ELISA is poorer in patients with slowly progressive type 1 diabetes than in patients with acute‐onset and fulminant type 1 diabetes. Also, 25–30% of GADA‐positive slowly progressive type 1 diabetes patients originally diagnosed by RIA have later been found to be negative by ELISA6, 7. Furthermore, it is suggested that slowly progressive type 1 diabetes patients with RIA(+)/ELISA(−) GADA are at lower risk of progressing to an insulin‐dependent state6. These discordant results between the two assays might be related to the epitope specificity of the two assays, because the GAD65 molecules used in these two kits are different; a truncated GAD65 lacking amino acids 2–45 in the N‐terminal region in the RIA kit and a full‐length recombinant protein in the ELISA kit. Another possible reason for a GADA discordant result might be associated with the different washing stringency between the two assays. The RSR‐RIA kit uses a single wash step before counting the radioactivity, in contrast to the three wash steps in the RSR‐ELISA kit9, 11. However, both RIA(+)/ELISA(−) and RIA(−)/ELISA(+) sera can compete with native GAD65 protein, and thus are not non‐specific reactions. Anti‐thyroid autoimmunity and the duration of type 1 diabetes are not associated with the discordant results between the two assays (Table 1; Figure S1). In the present study, it was found that GADA, detected only by RSR‐ELISA kits, were high‐affinity antibodies, whereas RSR‐RIA kits detected both low‐ and high‐affinity antibodies. In a previous study comparing these two kits, it was reported that patients with low GADA titers detected by RIA kit were negative by ELISA kit because of the cut‐off difference4. As the GADA titer by RIA kit is approximately one‐twentieth of that by the ELISA kit, the cut‐off value for RSR‐ELISA (5.0 U/mL) is equivalent to 0.25 U/mL by the RIA kit, which is below the cut‐off value (1.5 U/mL) for RSR‐RIA. Therefore, the negative result by the RIA kit in our seven ELISA(+) sera might be due to low GADA titers, even though they have high affinity antibodies. It is therefore possible that cases of low‐affinity GADA that have been detected only by the RIA kit are not associated with destructive insulitis in slowly progressive type 1 diabetes patients. These hypotheses need to be proved in the future.
All discordant samples, except one, used in the present study were obtained from long‐standing patients with type 1 diabetes. As it has been reported that GADA affinity remained relatively constant for >10 years in GADA‐positive non‐diabetic schoolchildren18, the duration of type 1 diabetes might not affect GADA affinity. However, it needs to be confirmed whether GADA affinity remains constant after the onset of type 1 diabetes using follow‐up samples.One limitation of the current study was that it was a cross‐sectional study with a relatively small sample size, and did not have data on insulin secretory capacity. Furthermore, the present study lacks the GADA affinity data in RIA(+)/ELISA(+) samples. These findings should therefore be validated in larger independent studies including RIA(+)/ELISA(+) sera.
Of note, the present study also showed that the initial incubation time affects the sensitivity of the RSR‐ELISA kit. A 2‐h incubation of sera with coated GAD65 showed a 1.6‐ to 100‐fold increase of GADA titer when compared with 1‐h incubation time recommended by RSR Ltd. In addition to this, 60% of the GADA‐negative sera became positive under the longer incubation period, and the increment ratio of GADA titer showed a strong correlation with the GADA affinity. At this time, the reasons for an improvement in the GADA measurement by RSR‐ELISA assay caused by the longer initial incubation time remain unclear, although, it is apparent that some disease‐relevant antibodies might be ignored by the current RSR‐ELISA kit. An example of this is in cases of a single subtype of type 1 diabetes that might not be included in these results, as those patients whose GADA turned positive by modified ELISA protocol consisted of all three subtypes of type 1 diabetes; that is, acute‐onset, fulminant and slowly progressive type 1 diabetes. It has been reported that the GADA titer in RIA(+)/ELISA(−) sera is lower than in RIA(+)/ELISA(+) sera6, 7. In general, high‐affinity antibodies bind a greater amount of antigen in a shorter period of time than low‐affinity antibodies. However, titers of polyclonal antibodies depend not only on their affinities, but also on their concentrations. Therefore, the kinetics of an antigen‐antibody reaction or antigenic epitopes might differ between these two types of sera. Further studies are required to examine the prevalence of GADA‐negative patients whose GADA turn positive in RSR‐ELISA tests with a longer initial incubation time, and also to examine whether GADA titer increases even in high‐titer GADA sera.
In conclusion, the present study showed that the RSR‐RIA test identifies both high‐ and low‐affinity GADA, whereas the RSR‐ELISA test only identifies high‐affinity GADA. Furthermore, longer initial incubation time with the RSR‐ELISA kit increased the sensitivity of GADA with the same specificity in patients with type 1 diabetes. Further investigation using a larger number of samples is required to confirm these findings.
Disclosure
The authors declare no conflict of interest.
Supporting information
Figure S1 | Effect of duration of type 1 diabetes on the prevalence of glutamic acid decarboxylase antibody measured by RSR radioimmunoassay and RSR enzyme‐linked immunosorbent assay.
Acknowledgments
We thank Dr T Fukui, Dr A Fukase and Dr Y Mori (Showa University) for providing healthy control sera and carrying out assays. The competitive binding experiments and the evaluation of the initial incubation time in RSR‐ELISA were carried out in the Department of Laboratory Medicine, Cosmic Corporation Co., Ltd. (Tokyo, Japan). This research did not receive any specific grant from funding agencies in the public, commercial or not‐for‐profit sectors.
J Diabetes Investig 2019; 10: 990–996
References
- 1. Kawasaki E, Gill RG, Eisenbarth GS. Type 1 diabetes mellitus In: Eisenbarth GS. (ed). Molecular Mechanisms of Endocrine and Organ Specific Autoimmunity. Austin, TX: R.G. Landes Company, 1999; 149–182. [Google Scholar]
- 2. Törn C, Mueller PW, Schlosser M, et al Diabetes Antibody Standardization Program: evaluation of assays for autoantibodies to glutamic acid decarboxylase and islet antigen‐2. Diabetologia 2008; 51: 846–852. [DOI] [PubMed] [Google Scholar]
- 3. Bingley PJ, Bonifacio E, Mueller PW. Diabetes Antibody Standardization Program: first assay proficiency evaluation. Diabetes 2003; 52: 1128–1136. [DOI] [PubMed] [Google Scholar]
- 4. Oikawa Y, Tanaka M, Horie I, et al A study on the correlation between GAD antibody titers measured by ELISA kit and RIA kit. Jpn J Med Pharm Sci 2015; 72: 1551–1560. (in Japanese). [Google Scholar]
- 5. Kawasaki E, Miwa M, Taknaka M. Basic and clinical evaluation of ELISA assay kits (Cosmic) for GADAb and IA‐2Ab. Jpn J Med Pharm Sci 2011; 66: 345–352. (in Japanese). [Google Scholar]
- 6. Oikawa Y, Tanaka H, Uchida J, et al Slowly progressive insulin‐dependent (type 1) diabetes positive for anti‐GAD antibody ELISA test may be strongly associated with a future insulin‐dependent state. Endocr J 2017; 64: 163–170. [DOI] [PubMed] [Google Scholar]
- 7. Murata T, Tsuzaki K, Nirengi S, et al Diagnostic accuracy of the anti‐glutamic acid decarboxylase antibody in type 1 diabetes mellitus: comparison between radioimmunoassay and enzyme‐linked immunosorbent assay. J Diabetes Investig 2017; 8: 475–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sera Y, Kawasaki E, Abiru N, et al Autoantibodies to multiple islet autoantigens in patients with abrupt onset type 1 diabetes and diabetes diagnosed with urinary glucose screening. J Autoimmun 1999; 13: 257–265. [DOI] [PubMed] [Google Scholar]
- 9. Masuda M, Powell M, Chen S, et al Autoantibodies to IA‐2 in insulin‐dependent diabetes mellitus. Measurements with a new immunoprecipitation assay. Clin Chim Acta 2000; 291: 53–66. [DOI] [PubMed] [Google Scholar]
- 10. Kawasaki E, Tanaka M, Miwa M, et al Novel enzyme‐linked immunosorbent assay for bivalent ZnT8 autoantibodies. Acta Diabetol 2014; 51: 429–434. [DOI] [PubMed] [Google Scholar]
- 11. Brooking H, Ananieva‐Jordanova R, Arnold C, et al A sensitive non‐isotopic assay for GAD65 autoantibodies. Clin Chim Acta 2003; 331: 55–59. [DOI] [PubMed] [Google Scholar]
- 12. Orosz F, Ovadi J. A simple method for the determination of dissociation constants by displacement ELISA. J Immunol Methods 2002; 270: 155–162. [DOI] [PubMed] [Google Scholar]
- 13. Coco G, Chen S, Powell M, et al Analysis of the GAD65‐GAD65 autoantibody interaction. Clin Chim Acta 2008; 391: 51–59. [DOI] [PubMed] [Google Scholar]
- 14. Krause S, Chmiel R, Bonifacio E, et al IA‐2 autoantibody affinity in children at risk for type 1 diabetes. Clin Immunol 2012; 145: 224–229. [DOI] [PubMed] [Google Scholar]
- 15. Mayr A, Schlosser M, Grober N, et al GAD autoantibody affinity and epitope specificity identify distinct immunization profiles in children at risk for type 1 diabetes. Diabetes 2007; 56: 1527–1533. [DOI] [PubMed] [Google Scholar]
- 16. Achenbach P, Koczwara K, Knopff A, et al Mature high‐affinity immune responses to (pro)insulin anticipate the autoimmune cascade that leads to type 1 diabetes. J Clin Invest 2004; 114: 589–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Krause S, Landherr U, Agardh CD, et al GAD autoantibody affinity in adult patients with latent autoimmune diabetes, the study participants of a GAD65 vaccination trial. Diabetes Care 2014; 37: 1675–1680. [DOI] [PubMed] [Google Scholar]
- 18. Bender C, Schlosser M, Christen U, et al GAD autoantibody affinity in schoolchildren from the general population. Diabetologia 2014; 57: 1911–1918. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 | Effect of duration of type 1 diabetes on the prevalence of glutamic acid decarboxylase antibody measured by RSR radioimmunoassay and RSR enzyme‐linked immunosorbent assay.


