Abstract
Aims/Introduction
Diabetic kidney disease is characterized by increased albuminuria and/or a reduced glomerular filtration rate (GFR). We analyzed secular changes in the prevalence of albuminuria and reduced estimated GFR (eGFR) in Japanese patients with type 2 diabetes, and identified factors associated with these changes.
Materials and Methods
Using 1996, 2001, 2006 and 2014 cohort data from the Japanese serial cross‐sectional studies conducted at Shiga University of Medical Science, secular changes in the prevalence of diabetic kidney disease (albuminuria and/or reduced eGFR), patient characteristics and their associations were analyzed.
Results
The prevalence of microalbuminuria and macroalbuminuria decreased over time, whereas the prevalence of moderately reduced eGFR (30–60 mL/min/1.73 m2) and severely reduced eGFR (<30 mL/min/1.73 m2) increased. Severely reduced eGFR was observed mainly in the patients with macroalbuminuria, regardless of year. Conversely, the prevalence of moderately reduced eGFR increased in the patients without macroalbuminuria. Both macroalbuminuria and moderately reduced eGFR without macroalbuminuria in the 2014 cohort were refractory to the recently recommended intensive therapy. Finally, we showed that obesity accompanied by vascular dysfunction was a risk factor for the development of albuminuria, and that age‐dependent arterial stiffness was associated with reduced eGFR without macroalbuminuria in the 2014 cohort.
Conclusions
During the past 20 years in Japan, the prevalence of albuminuria declined, whereas that of reduced eGFR increased. Additionally, obesity‐ and high age‐related vascular damage seems to be associated with macroalbuminuria and reduced eGFR without macroalbuminuria, respectively.
Keywords: albuminuria, diabetic kidney disease, diabetic nephropathy
Introduction
The natural history of diabetic kidney disease (DKD) has traditionally been described as a high level of albuminuria and subsequent kidney dysfunction leading to end‐stage renal disease (ESRD)1, 2, 3, 4. Thus, therapeutic strategies aimed at reducing albuminuria and subsequently improving renal outcomes have been developed worldwide. These strategies include intensive glycemic control and blood pressure control, particularly using renin–angiotensin system (RAS) inhibitors5, 6, 7, 8, 9, 10. As a result of these changes in DKD management during the past two decades, the number of new‐onset cases of ESRD as a result of DKD has recently become stable, at least in Japan.
An emerging clinical issue has recently arisen from a large cohort study of the National Health and Nutrition Examination Survey in the USA11. This cohort study showed that in patients with DKD from 1988 to 2014, the prevalence of albuminuria declined with improvement in diabetes care, whereas the prevalence of a reduced glomerular filtration rate (GFR) increased11. Furthermore, this trend was evident in the non‐Hispanic white population, but not in Mexican Americans11. Thus, the natural history of DKD might have changed during the past two decades worldwide with the changes in diabetes care. However, such secular changes might be affected by country‐specific factors including race, the etiology of diabetes and diabetes management. Therefore, to further improve renal outcomes in patients with DKD worldwide, we might need to analyze the secular changes in the clinical manifestations of DKD in different countries and identify country‐specific factors associated with these changes.
To this end, the present study was designed to analyze secular changes in the clinical manifestations of DKD in adult Japanese patients with type 2 diabetes, and to identify factors associated with these changes using a Japanese serial cross‐sectional study carried out at Shiga University of Medical Science, Otsu, Japan, from 1996 through 2014.
Methods
Ethics Statement
The protocol and informed consent procedure of the Shiga observational study were approved by the ethics committee of Shiga University of Medical Science, Shiga, Japan (approval no. 2, approval date: 1 February 1996).
Participants and Study Design
We used the clinical data obtained during our observational study in Shiga University of Medical Science from 1996 through 201412, 13. This study was launched in 1996 to assess the patient characteristics associated with the development and progression of diabetic complications. This observational study enrolled patients with type 1 or type 2 diabetes who had agreed with the purpose of the study. The exclusion criteria were an age of <20 years, and apparent infectious and malignant diseases. There were no restrictions on upper age limit, sex, medical history or the severity of diabetic complications.
For the present analysis, we used data from four cohorts at different time points of the original study (1996–2014): patients with type 2 diabetes in 1996 (n = 555), 2001 (n = 420), 2006 (n = 384) and 2014 (n = 326). The goal was to elucidate the secular changes in the clinical backgrounds and DKD manifestations in patients with type 2 diabetes that have occurred during the past two decades. Thus, the present study was carried out to examine the changes in the clinical backgrounds of individual patients in each cohort; it was not a prospective follow‐up study of specific patients.
Patients with diabetes who agreed to participate in the present study and who provided written informed consent were asked to provide a 24‐h urine sample. In addition, blood samples were obtained after an overnight fast and placed in tubes containing ethylenediaminetetraacetic acid, with plasma prepared by centrifuging the blood samples at 1,500 g at 4°C for 15 min. The serum and urine samples were immediately used to measure all laboratory variables at the Shiga University of Medical Science Hospital.
Based on the urinary albumin‐to‐creatinine ratio (UACR) at baseline, the patients were classified as having normoalbuminuria (A1 stage; UACR of <30 mg/gCr), microalbuminuria (A2 stage; UACR of 30 to <300 mg/gCr) or macroalbuminuria (A3 stage; UACR of ≥300 mg/gCr). The serum creatinine level was measured by an enzymatic method. The estimated GFR (eGFR) was calculated using the simplified equation proposed by the Japanese Society of Nephrology: eGFR (mL/min/1.73 m2) = 194 × [age (years)]−0.287 × [serum creatinine (mg/dL)]−1.094 × 0.739 (for female patients)14. The hemoglobin A1c (HbA1c) level was evaluated in accordance with the National Glycohemoglobin Standardization Program, based on the recommendations of the Japanese Diabetes Society15. The brachial‐ankle pulse wave velocity (baPWV) was measured with an automatic device (BP‐203RPE; Colin, Komaki, Japan). Data regarding the baPWV in the 1996 cohort were not collected and were thus unavailable. The body mass index (BMI) was calculated as weight in kilograms divided by the square of height in meters (kg/m2). The machines used to measure albuminuria and creatinine changed over time as follows. For measurement of serum creatinine, the Hitachi H736 (Hitachi, Tokyo, Japan), TOSHIBA TBA‐200FR (Toshiba, Tokyo, Japan), JCA‐BM2250 and JCA‐BM6070 (Japan Electron Optics Laboratory, Tokyo, Japan) were used in 1996, 2001, 2006 and 2014, respectively. For measurement of urinary albumin, the Hitachi H712, TOSHIBA TBA‐80FR NEO, JCA‐BM6010 and JCA‐BM6070 were used in 1996, 2001, 2006 and 2014, respectively. However, the compatibility of the results before and after these machine changes was guaranteed.
Statistical Analysis
Clinical data are expressed as the mean ± standard deviation. Differences between two groups and among multiple datasets were analyzed by analysis of variance, followed by Student's t‐test and Tukey's test, respectively. Trend analysis in Table 1 and Figure 5e was carried out with the Jonckheere–Terpstra trend test. All analyses were carried out using the SPSS software package (version 22; SPSS Inc., Chicago, IL, USA), with a two‐sided P‐value of <0.05 considered statistically significant.
Table 1.
Clinical characteristics in different cohorts
| Cohort (year) | 1996 | 2001 | 2006 | 2014 | Trend analysis |
|---|---|---|---|---|---|
| n | 555 | 420 | 384 | 326 | – |
| Age (years) | 58.8 ± 11.8 | 61.5 ± 12.1 | 62.8 ± 11.1 | 66.0 ± 10.8 | P < 0.001 |
| Sex (male/female) | 53.4 / 46.6 | 50.6 / 49.4 | 53.9 / 46.1 | 57.1 / 42.9 | – |
| BMI (kg/m2) | 23.4 ± 3.7 | 23.6 ± 3.52 | 24.3 ± 4.00 | 24.1 ± 4.29 | P = 0.040 |
| Diabetes duration (years) | 15.7 ± 10.9 | 16.6 ± 11.6 | 18.0 ± 10.2 | 17.6 ± 11.0 | P = 0.234 |
| Systolic BP (mmHg) | 135.8 ± 21.7 | 136.9 ± 19.9 | 132.3 ± 16.5 | 138.0 ± 16.6 | P = 0.254 |
| Diastolic BP (mmHg) | 75.7 ± 11.0 | 76.0 ± 10.8 | 72.2 ± 10.2 | 75.1 ± 12.0 | P < 0.07 |
| HbA1c, NGSP (%) | 7.72 ± 1.29 | 7.53 ± 1.04 | 7.72 ± 0.96 | 7.13 ± 1.0 | P < 0.001 |
| Triglyceride (mg/dL) | 118.8 ± 91.2 | 118.3 ± 150.6 | 95.7 ± 51.5 | 105.7 ± 14.2 | P < 0.001 |
| HDL‐C (mg/dL) | 56.6 ± 16.0 | 54.6 ± 15.0 | 57.1 ± 15.5 | 57.6 ± 14.1 | P = 0.215 |
| LDL‐C (mg/dL) | 132.3 ± 37.2 | 126.5 ± 27.0 | 120.1 ± 25.8 | 103.2 ± 27.4 | P < 0.001 |
| UACR (mg/gCr) | 151.6 ± 472.3 | 130.3 ± 443.6 | 111.7 ± 515.3 | 74.6 ± 319.1 | P = 0.100 |
| eGFR (mL/min/1.73 m2) | 85.1 ± 21.7 | 80.0 ± 22.7 | 74.4 ± 21.4 | 75.0 ± 19.4 | P < 0.001 |
| baPWV (cm/s) | NA | 1742.1 ± 418.4 | 1650.0 ± 357.1 | 1669.1 ± 344.6 | P = 0.044 |
| Antidiabetic agents (%) | 83.0 | 100.0 | 98.1 | 89.3 | – |
| Insulin (%) | 34.8 | 37.9 | 45.3 | 35.6 | – |
| Antihypertensive agents (%) | 26.5 | 40.2 | 55.9 | 58.0 | – |
| RAAS inhibitors (%) | 16.9 | 23.8 | 43.0 | 50.9 | – |
| Statins (%) | 1.26 | 26.0 | 39.8 | 51.5 | – |
| Fibrates (%) | 5.41 | 1.43 | 2.08 | 5.52 | – |
Data are expressed as the mean ± standard deviation. Trend analysis was carried out with the Jonckheere–Terpstra trend test. A result was considered statistically significant if the P‐value was <0.05.
ACR, urinary albumin creatinine ratio; baPWV, brachial‐ankle pulse wave velocity; BMI, body mass index; BP, blood pressure, eGFR, estimated glomerular filtration rate; HDL‐C, high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol; NA, not applicable; RAAS, renin–angiotensin–aldosterone system.
Figure 5.
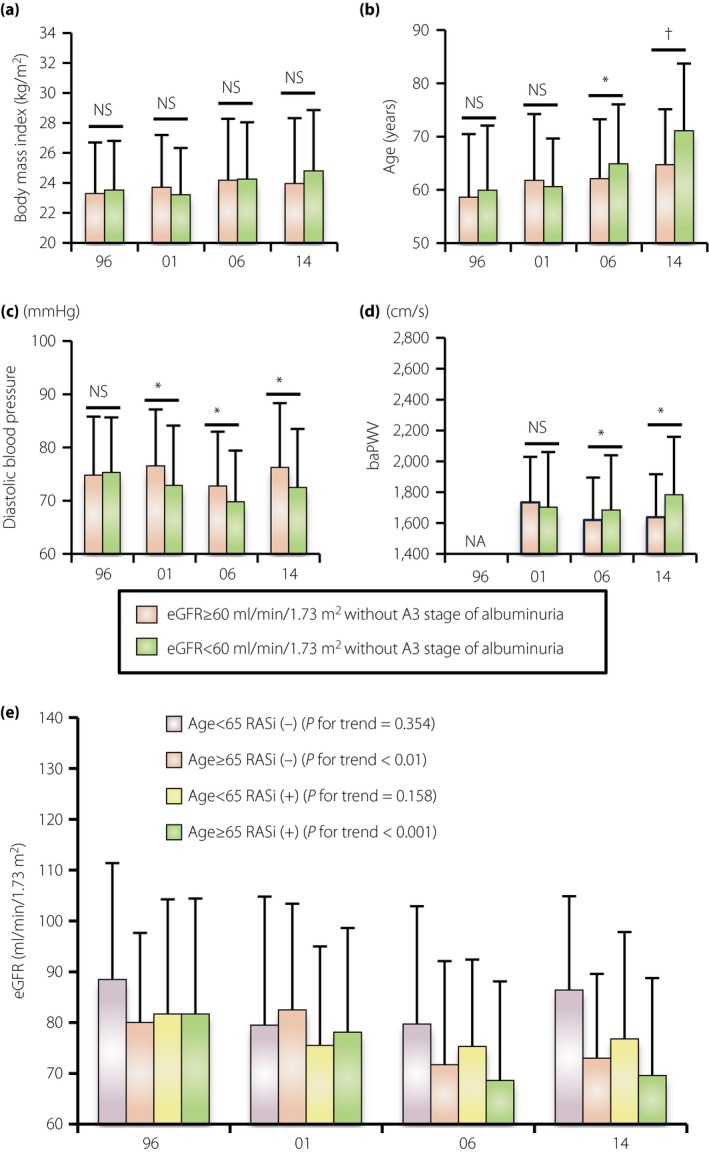
Secular changes in the clinical parameters in different estimated glomerular filtration rate (eGFR) categories. (a) Body mass index, (b) age, (c) diastolic blood pressure (d) and brachial‐ankle pulse wave velocity (baPWV) in the different eGFR categories without macroalbuminuria. (e) Mean eGFR values in four different cohorts, which were divided by age and renin–angiotensin system (RAS) use among the patients with a reduced GFR without macroalbuminuria (trend analysis was carried out with the Jonckheere–Terpstra trend test. P‐value of <0.05 considered statistically significant). Data are the mean ± standard deviation. *P < 0.05, † P < 0.01. NS, not statistically significant.
Results
Secular Changes in Clinical Characteristics
Although the sex ratio and the average duration of diabetes were not different among the four cohorts, the mean age and the average BMI of the patients with type 2 diabetes in our cohorts significantly increased over time (Table 1). More than 80% and 35% of the patients underwent treatment with any antidiabetic agents and insulin, respectively, regardless of year (Table 1). The mean HbA1c level was >7.5% until 2006, but it significantly decreased to 7.13% ± 1.0% in the 2014 cohort. The rate of use of antihypertensive agents, including RAS inhibitors, increased over time, but the mean systolic blood pressure was not different among all cohorts (Figure 1b). With respect to lipid control, the rate of use of statins dramatically increased, and the mean low‐density lipoprotein cholesterol (LDL‐C) level accordingly decreased from 132.3 ± 37.2 to 103.2 ± 27.4 mg/dL (Table 1). The trend of fibrate use was inconsistent in our serial cohorts. As a result, the mean UACR tended to decrease from 151.6 ± 472.3 to 74.6 ± 379.1 mg/gCr, whereas the eGFR significantly decreased from 85.1 ± 21.7 to 75.0 ± 19.4 mL/min/1.73 m2 from 1996 to 2014 (Table 1).
Figure 1.
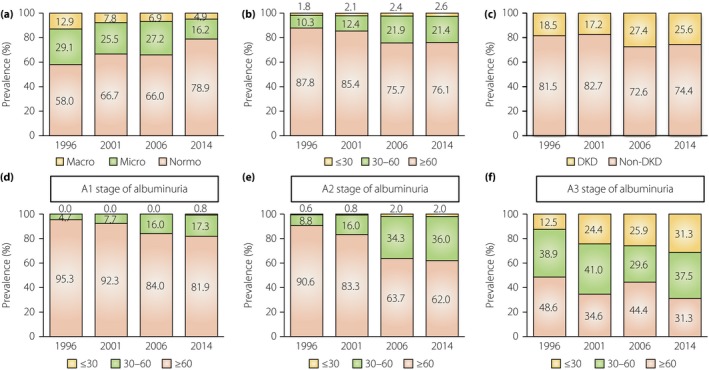
Secular changes in the prevalence of albuminuria and reduced estimated glomerlular filtration rate (eGFR). (a) The prevalence of albuminuria in the 1996, 2001, 2006 and 2014 cohorts. (b) The prevalence of a reduced eGFR in the indicated cohorts. (c) The prevalence of diabetic kidney disease (DKD), defined by albuminuria and/or a reduced eGFR (≤60 mL/min/1.73 m2) in the indicated cohorts. (d–f) The prevalence of a reduced eGFR in the stages of (d) normoalbuminuria, (e) microalbuminuria and (f) macroalbuminuria.
Changes in the Prevalence of Albuminuria and Reduced eGFR in Patients with DKD
In the 1996 cohort, the prevalence of albuminuria (albuminuria stage A2 plus A3) was 42.0% (Figure 1a). In accordance with changes in DKD management aimed at reducing albuminuria, the prevalence of albuminuria decreased to 21.1% in the 2014 cohort (Figure 1a). In contrast, the prevalence of both severe renal dysfunction (eGFR of <30 mL/min/1.73 m2) and mild renal dysfunction (eGFR of 30–60 mL/min/1.73 m2) increased over time (Figure 1b). As a result, the total number of patients with DKD defined by the appearance of albuminuria and/or a reduced eGFR gradually increased (Figure 1c), despite the fact that the prevalence of albuminuria decreased during this time.
Next, the prevalence of a reduced eGFR in different stages of albuminuria was analyzed. The prevalence of severe renal dysfunction (eGFR of <30 mL/min/1.73 m2) was observed mainly in the A3 stage; dysfunction was not evident in either the A1 or A2 stage (Figure 1d–f). Interestingly, a secular increase in the prevalence of a reduced eGFR (<60 mL/min/1.73 m2) was observed in diabetes patients with the A1 and A2 stage (Figure 1d,e). Thus, our serial cohort study showed that the current A3 stage of albuminuria and the time‐dependent increase in the prevalence of a reduced eGFR without the A3 stage of albuminuria in patients with diabetes are possible emerging clinical issues to be resolved in future.
Changes in Clinical Characteristics of Patients with Type 2 diabetes with Different Stages of Albuminuria
In our cohorts, the rate of use of diabetic agents was not different among the three categories of albuminuria in all cohorts, and the rate of use of insulin analogs decreased in the A3 stage, possibly with improvements in the oral antidiabetic agents used for patients with renal dysfunction (Figure 2a). The rate of use of an antihypertensive agent and a RAS inhibitor was higher in the A3 stage in the 1996 and 2001 cohorts. Additionally, the rate of use increased over time in patients in the A1 and A2 stage, and a RAS inhibitor was finally used in approximately half of patients in 2014 regardless of the albuminuria stage (Figure 2b). Statin use increased in all albuminuria stages over time (Figure 2c).
Figure 2.
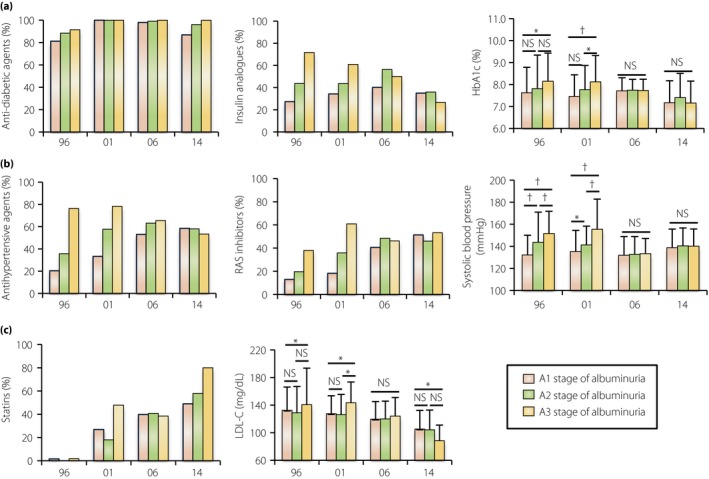
Changes in the clinical characteristics in the type 2 diabetes patients with different stages of albuminuria. (a) The use rate of any antidiabetic agents and insulin analogs, and hemoglobin A1c (HbA1c) value in the different albuminuria stages in the 1996, 2001, 2006 and 2014 cohorts. (b) The use rate of any antihypertensive agents and renin–angiotensin system (RAS) inhibitors, and systolic blood pressure in the different cohorts. (c) The use rate of statins and low‐density lipoprotein cholesterol (LDL‐C) value in the different cohorts. Data are the mean ± standard deviation. *P < 0.05, † P < 0.01. NS, not statistically significant.
Higher levels of HbA1c, systolic blood pressure and LDL‐C, which are established risk factors for albuminuria development, were actually associated with a higher prevalence of the A3 stage of albuminuria in the 1996 and 2001 cohorts (Figure 2a–c). However, in accordance with the improvements in diabetes care, the levels of HbA1c, systolic blood pressure, and LDL‐C in the A2 and A3 stages gradually improved over time, finally reaching almost the same values as the A1 stage in the 2006 and 2014 cohorts (Figure 2a–c). Thus, the current A3 stage of albuminuria seems to be resistant to the established intensive treatment.
Changes in Clinical Characteristics in Patients with Type 2 diabetes with Different Categories of eGFR Decline in the A1 and A2 Stages of Albuminuria
In all cohorts, the rate of use of antidiabetic agents, insulin analogs, antihypertensive agents, RAS inhibitors, and statins did not differ between patients with a preserved eGFR and those with a reduced eGFR in the A1 and A2 stages (Figure 3a–c). Furthermore, the levels of HbA1c, systolic blood pressure and LDL‐C were also not different between the two categories of eGFR in each year's cohort (Figure 3a–c). Thus, a reduced eGFR without the A3 stage of albuminuria also seems to be refractory to the currently recommended intensive treatment.
Figure 3.
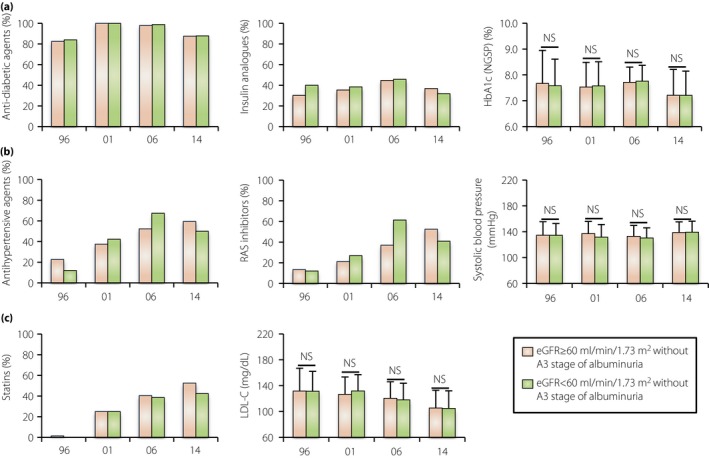
Changes in the clinical characteristics of patients with type 2 diabetes with different categories of estimated glomerular filtration rate (eGFR) decline without macroalbuminuria. (a) The rate of use of any anti‐diabetic agents and insulin analogs and the hemoglobin A1c (HbA1c) level in the different eGFR stages in the 1996, 2001, 2006 and 2014 cohorts. (b) The rate of use of any antihypertensive agents and renin–angiotensin system (RAS) inhibitors and the systolic blood pressure in the different cohorts. (c) The rate of use of statins and the low‐density lipoprotein cholesterol (LDL‐C) level in the different cohorts. Data are the mean ± standard deviation. NS, not statistically significant.
Identification of Clinical Characteristics Associated with the Current A3 Stage of Albuminuria and Reduced eGFR Without A3 Stage of Albuminuria
Finally, additional clinical parameters, such as BMI, age, diastolic blood pressure and baPWV, were analyzed to identify risk factors for the current A3 stage of albuminuria and reduced eGFR without the A3 stage of albuminuria.
Similar to the result of systolic blood pressure (Figure 2b), higher diastolic blood pressure was associated with the A3 stage of albuminuria in the previous cohorts, but not in the recent cohorts (Figure 4a). In contrast, we found that the prevalence of the A3 stage of albuminuria was associated with a high BMI and high baPWV in the recent cohorts (Figure 4b,c). Age was not associated with albuminuria stage progression regardless of cohort (Figure 4d).
Figure 4.
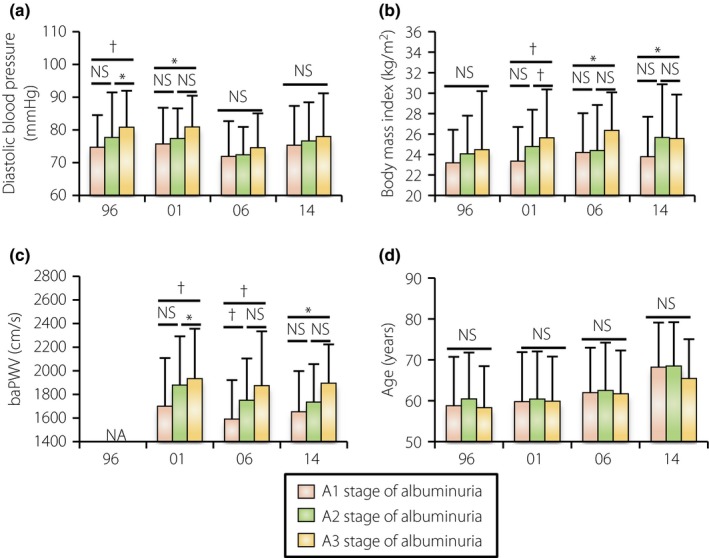
Secular changes in the clinical parameters in different albuminuria stages. (a) Diastolic blood pressure, (b) body mass index, (c) brachial‐ankle pulse wave velocity (baPWV) and (d) age in the different albuminuria stages in the 1996, 2001, 2006 and 2014 cohorts. Data are the mean ± standard deviation. *P < 0.05, † P < 0.01. NS, not statistically significant.
In the analysis of a reduced eGFR without the A3 stage of albuminuria, BMI was not associated with a reduced eGFR, but high age was associated with reduced eGFR in the 2006 and 2014 cohorts (Figure 5a,b). This age‐dependent change was accompanied by lower diastolic blood pressure and high baPWV (Figure 5c,d). Finally, we analyzed the association between age and use of RAS inhibitors in the analysis of a reduced eGFR without the A3 stage of albuminuria. RAS inhibitor use tended to decrease eGFR in both the younger and older adult cohorts (Figure 5e). Furthermore, a trend analysis showed a time‐dependent decrease in eGFR in the patients with a reduced eGFR without the A3 stage of albuminuria in the cohort of higher age (>65 years) regardless of RAS inhibitor use (Figure 5e).
Discussion
Along with the development of diabetes care aimed at reducing albuminuria, a decreased prevalence of albuminuria and increased prevalence of a reduced eGFR are being recognized as characteristics of the current trend in DKD manifestations11. However, these changes might be affected by country‐specific factors, and the trend in Japan has not been fully elucidated. In the present analysis, we showed that this trend is also present in Japanese patients with type 2 diabetes.
In our cohort, severe renal dysfunction was observed mainly in patients with the A3 stage of albuminuria, and less frequently in those with the A1 and A2 stages, suggesting that the A3 stage of albuminuria might be more closely associated with future development of ESRD than other stages of albuminuria. In our 1996 cohort, poor control of HbA1c, systolic blood pressure and LDL‐C were associated with a higher prevalence of albuminuria. However, throughout the development of DKD care during the past two decades, no significant differences have occurred in these clinical parameters regardless of albuminuria stage. Thus, the current A3 stage of albuminuria may be refractory to the currently established intensive therapy.
The reasons for the current A3 stage of albuminuria should be discussed. One possibility is that the intensive therapy in the patients with type 2 diabetes in the present cohort was still insufficient. The Japan Diabetes Optimal Integrated Treatment study for three major risk factors of cardiovascular diseases showed that more intensified diabetic therapy further improved renal outcomes in Japanese patients with diabetes in whom the HbA1c, systolic blood pressure and LDL‐C levels were <7.0%, <130 mmHg and <90 mg/dL, respectively16. All of these parameters in our cohort were still higher than those achieved in the intensive group of the Japan Diabetes Optimal Integrated Treatment study for three major risk factors of cardiovascular diseases study. Actually, in our cohorts, obesity along with very high baPWV was associated with the prevalence of the A3 stage of albuminuria regardless of year. Thus, earlier and more intensified therapy, including lifestyle interventions, might be effective even in our cohorts. The next possibility is that this A3 stage of albuminuria is already irreversible or mediated by other etiologies that cannot be cured by the current intensive care. The final possibility is that this A3 stage of albuminuria is due to another type of glomerular disease. We must continue to search for the reason behind this A3 stage of albuminuria in both clinical and basic studies to further improve renal outcomes in patients with type 2 diabetes.
A secular increase in the prevalence of a reduced eGFR without the A3 stage of albuminuria was also observed from 1996 through 2014 in Japan. In Figure 5e, the use of RAS inhibitors actually decreased eGFR in any cohorts, suggesting that the introduction of a RAS inhibitor might be involved in the cause behind a reduced eGFR without the A3 stage of albuminuria. Furthermore, we identified high age, lower diastolic blood pressure and high baPWV in the 2014 cohort as factors associated with a reduced eGFR without the A3 stage of albuminuria, which was not observed in the 1996 and 2001 cohorts. Additionally, a secular decrease in eGFR in the elderly population, but not in the young population, was observed regardless of RAS inhibitor use. Thus, the background of patients with a reduced eGFR without the A3 stage of albuminuria seems to have been modified by the development of DKD care including RAS inhibitors and increased age over time, and is totally different from that of patients with albuminuria development. This might mean the therapeutic approach should be different between them.
Although we observed the secular change in DKD manifestations during the past two decades, in a large Japanese cohort of the JDDM study, there was no significant increase in the prevalence of a reduced eGFR between the 2004 and 2014 cohorts17. This suggests that the changes in DKD manifestations can be observed only when comparing the cohorts before and after 2000, when multifactorial intensive therapy including the use of RAS inhibitors began to be applied for treatment of classic diabetic nephropathy in the clinical setting. These results suggest that data from a previous cohort (before 2000) are unreasonable to identify the current risk factors associated with each DKD manifestation, because a risk identified using an old cohort should have been modified by the recent changes in diabetes care.
In the National Health and Nutrition Examination Survey cohort, the authors reported that earlier onset of obesity in the younger generation and a subsequent longer diabetes duration, but not high age, might be related to the increased prevalence of a reduced eGFR decline without the A3 stage of albuminuria11. In the present study, however, high age, lower diastolic blood pressure and higher baPWV, which might be explained by age‐dependent aortic stiffness, were identified as possible risk factors for DKD manifestation in our 2014 cohort. Thus, the trend in DKD manifestation is almost identical worldwide, but the clinical characteristics behind it are likely to be modified by country‐specific factors. Obesity‐related insulin resistance and age‐dependent decreases in the insulin secretion capacity largely contribute to the increasing numbers of patients with type 2 diabetes in the USA and Japan, respectively. This difference in the etiology of type 2 diabetes might affect the identified factors associated with a reduced eGFR without the A3 stage of albuminuria.
In the present study of Japanese patients with type 2 diabetes, arterial stiffness as measured by the baPWV might have been associated with albuminuria and a low eGFR. This finding is consistent with the results of previously published data showing that a high baPWV was associated with both the development of albuminuria and a decline in the eGFR without albuminuria in Korean patients with type 2 diabetes18. However, in this previous study, the baPWV was not identified as an independent risk factor for a decline in the eGFR without albuminuria after adjusting for significant clinical variables, such as BMI, duration of diabetes, systolic BP, pulse pressure, heart rate, smoking, alcohol consumption, HbA1c level, high‐density lipoprotein cholesterol level, C‐reactive protein level, insulin treatment and RAS inhibitor use. These results might suggest that multifactorial intensive intervention is currently the best treatment available to prevent a decline in the eGFR in patients with diabetes, although whether a causal relationship exists between arterial stiffness and an eGFR decline without albuminuria is still unclear.
The present study had some limitations, including the small sample size, single‐center cohort and selection bias for the recruited patients. This study did not uncover the effect of a novel class of antidiabetic agents, such as DPP‐4 inhibitors, GLP‐1 analogs and SGLT2 inhibitors, on diabetes care and DKD manifestations in our cohorts. Additionally, in the National Health and Nutrition Examination Survey cohort, mortality was high in the diabetes patients with a reduced eGFR without the A3 stage of albuminuria19. However, the present study did not reveal the patients’ health prognosis, including mortality and the risk for developing ESRD in patients with the current A3 stage of albuminuria or a reduced eGFR without the A3 stage of albuminuria. Therefore, a prospective follow‐up study is required to address these factors in Japan.
Increased albuminuria has been recognized as a strong predictive factor associated with future renal dysfunction leading to ESRD in patients with classic diabetic nephropathy1, 2. Thus, therapeutic approaches aimed at reducing albuminuria have been rapidly developed mainly during the past two decades5, 6, 7, 8, 9, 10, 16. As a result, the prevalence of albuminuria and new‐onset ESRD has been decreasing, at least in Japan5, 12, 16. Notably, this therapeutic approach is essential and effective. However, given the results of the present study, now might be the time to discuss whether this strategy is actually sufficient to completely combat DKD in future. We must identify a strategy in addition to the current intensive therapy to further improve renal outcomes in patients with DKD based on the current situation of DKD manifestation.
Disclosure
The authors declare no conflict of interest.
Acknowledgments
We are grateful to Dr Ryuichi Kikkawa, Dr Hideki Hidaka, Dr Masahiko Terada, Dr Keiko Kondo, Ms Yumiko Omura, and all members of the Maegawa Laboratory for their scientific input and contributions to the Shiga Prospective Observational Follow‐up Study. We also thank Edanz Group for editing a draft of this manuscript.
J Diabetes Investig 2019; 10: 1032–1040
References
- 1. Eijkelkamp WB, Zhang Z, Remuzzi G, et al Albuminuria is a target for renoprotective therapy independent from blood pressure in patients with type 2 diabetic nephropathy: post hoc analysis from the Reduction of Endpoints in NIDDM with the Angiotensin II Antagonist Losartan (RENAAL) trial. J Am Soc Nephrol 2007; 18: 1540–1546. [DOI] [PubMed] [Google Scholar]
- 2. Katayama S, Moriya T, Tanaka S, et al Low transition rate from normo‐ and low microalbuminuria to proteinuria in Japanese type 2 diabetic individuals: the Japan Diabetes Complications Study (JDCS). Diabetologia 2011; 54: 1025–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Moriya T, Tanaka S, Kawasaki R, et al Diabetic retinopathy and microalbuminuria can predict macroalbuminuria and renal function decline in Japanese type 2 diabetic patients: Japan Diabetes Complications Study. Diabetes Care 2013; 36: 2803–2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wada T, Haneda M, Furuichi K, et al Clinical impact of albuminuria and glomerular filtration rate on renal and cardiovascular events, and all‐cause mortality in Japanese patients with type 2 diabetes. Clin Exp Nephrol 2014; 18: 613–620. [DOI] [PubMed] [Google Scholar]
- 5. Yokoyama H, Araki S, Honjo J, et al Association between remission of macroalbuminuria and preservation of renal function in patients with type 2 diabetes with overt proteinuria. Diabetes Care 2013; 36: 3227–3233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Group AC, Patel A, MacMahon S, et al Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008; 358: 2560–2572. [DOI] [PubMed] [Google Scholar]
- 7. Ismail‐Beigi F, Craven T, Banerji MA, et al Effect of intensive treatment of hyperglycaemia on microvascular outcomes in type 2 diabetes: an analysis of the ACCORD randomised trial. Lancet 2010; 376: 419–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ohkubo Y, Kishikawa H, Araki E, et al Intensive insulin therapy prevents the progression of diabetic microvascular complications in Japanese patients with non‐insulin‐dependent diabetes mellitus: a randomized prospective 6‐year study. Diabetes Res Clin Pract 1995; 28: 103–117. [DOI] [PubMed] [Google Scholar]
- 9. Araki S, Haneda M, Koya D, et al Reduction in microalbuminuria as an integrated indicator for renal and cardiovascular risk reduction in patients with type 2 diabetes. Diabetes 2007; 56: 1727–1730. [DOI] [PubMed] [Google Scholar]
- 10. Diabetes C, Complications Trial Research G , Nathan DM, Genuth S, et al The effect of intensive treatment of diabetes on the development and progression of long‐term complications in insulin‐dependent diabetes mellitus. N Engl J Med 1993; 329: 977–986. [DOI] [PubMed] [Google Scholar]
- 11. Afkarian M, Zelnick LR, Hall YN, et al Clinical Manifestations of Kidney Disease Among US Adults With Diabetes, 1988‐2014. JAMA 2016; 316: 602–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Araki S, Haneda M, Koya D, et al Predictive effects of urinary liver‐type fatty acid‐binding protein for deteriorating renal function and incidence of cardiovascular disease in type 2 diabetic patients without advanced nephropathy. Diabetes Care 2013; 36: 1248–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kume S, Araki S, Ono N, et al Predictive properties of plasma amino acid profile for cardiovascular disease in patients with type 2 diabetes. PLoS ONE 2014; 9: e101219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Matsuo S, Imai E, Horio M, et al Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis 2009; 53: 982–992. [DOI] [PubMed] [Google Scholar]
- 15. Committee of the Japan Diabetes Society on the Diagnostic Criteria of Diabetes M , Seino Y, Nanjo K, et al Report of the committee on the classification and diagnostic criteria of diabetes mellitus. J Diabetes Investig 2010; 1: 212–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ueki K, Sasako T, Okazaki Y, et al Effect of an intensified multifactorial intervention on cardiovascular outcomes and mortality in type 2 diabetes (J‐DOIT3): an open‐label, randomised controlled trial. Lancet Diabetes Endocrinol 2017; 5: 951–964. [DOI] [PubMed] [Google Scholar]
- 17. Yokoyama H, Araki SI, Kawai K, et al Declining trends of diabetic nephropathy, retinopathy and neuropathy with improving diabetes care indicators in Japanese patients with type 2 and type 1 diabetes (JDDM 46). BMJ Open Diabetes Res Care 2018; 6: e000521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kim JH, Kim SS, Kim IJ, et al Arterial stiffness is more associated with albuminuria than decreased glomerular filtration rate in patients with type 2 diabetes mellitus: the REBOUND study. J Diabetes Res 2017; 2017: 7047909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kramer H, Boucher RE, Leehey D, et al Increasing mortality in adults with diabetes and low estimated glomerular filtration rate in the absence of albuminuria. Diabetes Care 2018; 41: 775–781. [DOI] [PMC free article] [PubMed] [Google Scholar]


