Abstract
Aims/Introduction
The aim of the present study was to examine the associations of pregestational body mass index (BMI) and gestational weight change with birthweight for gestational age in Japanese mothers with gestational diabetes mellitus (GDM).
Materials and Methods
We retrospectively examined the clinical and laboratory characteristics of 101 mothers with GDM (pregestational BMI 24.7 ± 5.8 kg/m2; maternal age at delivery 34.7 ± 5.1 years; gestational age 38.5 ± 1.4 weeks) at a single center from January 2011 to December 2016.
Results
Gestational weight changes were 6.22 ± 5.39 kg, and infant birthweights were 2,987.3 ± 393.6 g. Multivariable analysis showed that, in all mothers, pregestational BMI and gestational weight change were positively associated with infant birthweight (P < 0.001 and P = 0.007, respectively). Pregestational BMI, but not gestational weight change, was positively associated with infant birthweight (P = 0.007) in 31 mothers with GDM who had pregestational BMI ≥25 kg/m2; in 68 mothers with GDM who had pregestational BMI 18.5–24.9 kg/m2, only gestational weight gain was positively associated with infant birthweight (P = 0.039). Two mothers had pregestational BMI <18.5 kg/m2. No statistically significant interactions of pregestational BMI with gestational weight change were found (P = 0.158).
Conclusions
In mothers with GDM, pregestational BMI ≥25 kg/m2 and excessive gestational weight gain were significantly associated with increased infant birthweight. A prospective multicenter clinical study enrolling a larger number of mothers with GDM will be required to verify the effects of adequately controlling pregestational and gestational weights on infant birthweight for gestational age.
Keywords: Birthweight, Gestational diabetes mellitus, Overweight
Introduction
Gestational diabetes mellitus (GDM) frequently causes fetal disorders during pregnancy1. Fetal overgrowth leading to the delivery of infants large for gestational age (LGA) is a well‐known complication of GDM2, 3. LGA can cause birth complications (e.g., cesarean section and shoulder dystocia)4, and LGA infants are more likely to develop obesity5, metabolic syndrome5 or type 2 diabetes mellitus6 in childhood. Apart from GDM, furthermore, maternal obesity, fetal hyperinsulinemia and maternal insulin resistance are known risk factors for the delivery of LGA infants7
Pregestational obesity is a risk factor for GDM and can provoke maternal complications (e.g., pre‐eclampsia and interventions at delivery)8. The Hyperglycemia and Adverse Pregnancy Outcome Study9 showed that obesity is a strong risk factor associated with the delivery of LGA infants and infants with neonatal obesity, and that this association is independent from maternal blood glucose concentrations. Furthermore, excessive gestational weight gain in mothers with GDM can lead to maternal complications (e.g., hypertensive disorders of pregnancy and cesarean section) and is a risk factor for the delivery of LGA infants10.
The new guidelines for gestational weight gain updated by the Institute of Medicine and National Research Council in 200911, which are generally applied to patients with GDM, provide the current standard for the control of bodyweight of patients with GDM. Namely, the guidelines categorize pregestational body mass index (BMI) into four groups (underweight <18.5 kg/m2, normal weight 18.5–24.9 kg/m2, overweight 25.0–29.9 kg/m2 and obese >30.0 kg/m2), to establish the appropriate weight gain range for each category.
As with women who experience excessive gestational weight gain, pregestational overweight/obese patients with GDM are at increased risk for the delivery of LGA infants12, 13. However, the associations of pregestational BMI and gestational weight gain with infant birthweight for gestational age have not been fully elucidated in patients with GDM. The effects of pregestational BMI on infant birthweight potentially differ between Asians and Westerners, because the former, in general, tend to be less obese than the latter. Limited research is available on the adequate range of gestational weight gain in Asian patients with GDM. The objective of the present study was to examine the associations of pregestational BMI, gestational weight change and other indicators with infant birthweight for gestational age in Japanese mothers with GDM.
Methods
Study Design and Population
We carried out a retrospective observational population‐based cohort study of 4,021 pregnant women who had received maternal care and given birth at Saitama Medical University Hospital Saitama, Japan, from January 2011 to December 2016. The institutional review board at our university approved the study.
A total of 205 mothers (43 with pre‐existing diabetes mellitus, 11 with overt diabetes in pregnancy and 151 with GDM) were admitted to the hospital for the strict control of hyperglycemia, and they subsequently delivered their infants. We excluded the following 104 mothers: the aforementioned 43 and 11 mothers with diabetes; 34 mothers who were treated for disorders that are known to affect the mother and fetus during pregnancy (diseases causing hormone metabolism abnormalities of the thyroid, pituitary, adrenal or other endocrine glands); seven mothers who received drugs known to affect the fetus during pregnancy (e.g., psychotropic drugs); and four mothers who had twin pregnancy; among five mothers whose hemoglobin A1c (HbA1c) at weeks 24–28 of pregnancy—an independent variable in the present study—was not determined, one (1.0%) had a miscarriage. Consequently, 101 mothers with GDM were included in the statistical analysis.
Diagnosis of GDM
According to the criteria of the International Association of Diabetes and Pregnancy Study Groups14, GDM was diagnosed if one or more values reached or exceeded the following thresholds in a 75‐g oral glucose tolerance test: 92 mg/dL (5.1 mmol/L) for fasting plasma glucose; 180 mg/dL (10.0 mmol/L) for 1‐h plasma glucose (1‐h PG); and 153 mg/dL (8.5 mmol/L) for 2‐h PG.
Study Outcomes and Measures
Mothers with GDM were assessed for the following independent variables: (i) maternal age at delivery; (i) gestational age; (iii) height measured at the first visit to the hospital; (iv) pregestational bodyweight—measured at home or during a medical checkup at work within 1 month before pregnancy—and self‐reported during history taking at the first visit to the hospital—and resulting pregestational BMI; and (v) height and bodyweight—measured just before entry into the labor room within 24 h before delivery—and resulting BMI at delivery. Gestational weight changes were calculated by deducting pregestational weight from maternal weight at delivery. The total daily dose of insulin at delivery was recorded in 90 mothers who were receiving insulin therapy. In a total of 101 mothers, GDM was diagnosed by carrying out a 75‐g oral glucose tolerance test that each mother underwent once only at weeks 11–15 (n = 25) or 24–28 (n = 76) of pregnancy.
Serum HbA1c was determined at weeks 24–28 of pregnancy (n = 101) to strengthen the diagnosis. Plasma glycated albumin concentrations were measured in 35 mothers only. We also obtained information on history of cesarean section, number of premature births, infant birthweight, infant birthweight for gestational age and infant anomalies (Table 1).
Table 1.
Clinical, obstetric, and laboratory characteristics of mothers and infants
| Indicators | |
|---|---|
| Number of mothers analyzed | 101 |
| Age at delivery, years | 34.7 ± 5.1 |
| Height, cm | 157.4 ± 5.8 |
| Pregestational weight, kg | 61.3 ± 16.0 |
| Pregestational BMI, kg/m2 | 24.7 ± 5.8 |
| Underweight: <18.5, % (n) | 2.0 (2) |
| Normal: 18.5–24.9, % (n) | 67.3 (68) |
| Overweight: 25.0–29.9, % (n) | 17.8 (18) |
| Obese: ≥30.0, % (n) | 12.8 (13) |
| Maternal weight at delivery, kg | 67.5 ± 14.3 |
| Maternal BMI at delivery, kg/m2 | 27.2 ± 5.1 |
| Gestational weight change, kg | 6.22 ± 5.39 |
| HbA1c measured at weeks 24 to 28, % | 5.3 ± 0.4 |
| HbA1c measured at weeks 24 to 28, mmol/mol | 34.3 ± 4.5 |
| Glycated albumin at diagnosis of GDM*, % | 13.4 ± 1.2 |
| FPG in 75‐g OGTT, mg/dL | 84.7 ± 13.3 |
| 1‐h PG in 75‐g OGTT, mg/dL | 187.1 ± 28.3 |
| 2‐h PG in 75‐g OGTT, mg/dL | 163.4 ± 26.2 |
| Gestational age, week | 38.5 ± 1.4 |
| Insulin therapy, % (n) | 85.1 (86) |
| Total daily dose of insulin at delivery, U | 18.9 ± 19.6 |
| Smoking during pregnancy, % (n) | 0 (0) |
| Cesarean section, % (n) | 22.8 (23) |
| Premature births, % (n) | 5.9 (6) |
| Hydramnios, % (n) | 1.0 (1) |
| Oligoamnios, % (n) | 2.0 (2) |
| Parity | |
| Gravida 1, % (n) | 54.5 (42) |
| Gravida 2, % (n) | 31.2 (24) |
| Gravida 3, % (n) | 14.3 (11) |
| Infant birthweight, g | 2987.3 ± 393.6 |
| Birth weight percentile | 0.57 ± 0.27 |
| Z‐score for birthweight | 0.25 ± 0.94 |
| Low‐birth‐weight infant, % (n) | 11.9 (12) |
| Excessively large infant, % (n) | 1.0 (1) |
| Infant anomalies†, % (n) | 1.0 (1) |
| Type of delivery | |
| Transvaginal, % (n) | 69.3 (70) |
| Caesarean, % (n) | 22.8 (23) |
| Vacuum extraction, % (n) | 2.9 (3) |
| Forceps delivery, % (n) | 4.0 (4) |
| Induced labour, % (n) | 1.0 (1) |
| Infant male sex, % (n) | 57.4 (58) |
| Infant birthweight for gestational age‡ | |
| AGA, % (n) | 81.1 (82) |
| LGA, % (n) | 14.9 (15) |
| SGA, % (n) | 4.0 (4) |
BMI, body mass index; HbA1c, hemoglobin A1c; FPG, fasting plasma glucose; OGTT, oral glucose tolerance test; AGA, appropriate for gestational age; SGA, small for gestational age; LGA, large for gestational age. Values are expressed as mean ± standard deviation * n = 37. †Right hydronephrosis of the fetus. ‡AGA, LGA, and SGA were defined as having birthweight in the 10th to 90th, >90th, and <10th percentiles for Japanese infants, respectively.
Infant birthweight is dependent on gestational age, infant sex and parity. We calculated the percentiles of infant birthweight for gestational age with reference to infant birthweights according to anthropometric charts for Japanese infants15. The calculated percentiles of infant birthweight for gestational age were classified into the following three groups: small for gestational age (SGA), appropriate for gestational age (AGA) and LGA. SGA, AGA and LGA were defined as infant birthweight <10th, 10–90th and >90th percentiles for Japanese infants, respectively. Infant birthweight for gestational age was assessed using the z‐scores according to the least mean squares method proposed by Cole16.
Statistical Analysis
We carried out multiple linear regression analysis using the z‐scores for infant birthweight for gestational age (infant birthweight z‐scores) as the dependent variable. In addition, the following independent variables were used as continuous variables: pregestational BMI, gestational weight change, maternal age at delivery, HbA1c concentrations at weeks 24–28 of pregnancy and total daily dose of insulin at delivery.
Published guidelines11 have established four categories of pregestational BMI and have defined the recommended weight gain for each category, advocating less weight gain for women who are overweight/obese before pregnancy than for women who have pregestational normal BMI. To further examine the effects of gestational weight gain on infant birthweight for gestational age with reference to these guidelines, we carried out multiple linear regression analyses separately for two maternal subgroups: the normal maternal BMI subgroup that consisted of mothers who had pregestational normal BMI; and the maternal overweight/obesity subgroup that consisted of pregestational overweight/obese mothers with GDM (Table 2). In model 1 of Table 2, gestational weight change was analyzed as a continuous variable.
Table 2.
Multiple linear regression analysis on the z‐scores for infant birthweight for gestational age
| Estimated value (95% CI) | P‐value | |
|---|---|---|
| Model 1: included gestational weight change as a continuous variable | ||
| Study population* (n = 101) | ||
| Pregestational BMI | 0.08 (0.05 to 0.11) | <0.001 |
| Gestational weight change as a continuous variable | 0.05 (0.01 to 0.09) | 0.007 |
| Maternal age at delivery | −0.03 (−0.06 to 0.01) | 0.144 |
| HbA1c at weeks 24–28 of pregnancy | −0.11 (−0.54 to 0.33) | 0.630 |
| Total daily dose of insulin at delivery | 0.003 (−0.01 to 0.01) | 0.522 |
| Normal maternal BMI subgroup† (n = 68) | ||
| Pregestational BMI | 0.05 (−0.06 to 0.17) | 0.351 |
| Gestational weight change as a continuous variable | 0.06 (0.003 to 0.12) | 0.039 |
| Maternal age at delivery | −0.01 (−0.06 to 0.03) | 0.570 |
| HbA1c at weeks 24–28 of pregnancy | −0.37 (−0.98 to 0.24) | 0.227 |
| Total daily dose of insulin at delivery | 0.01 (−0.01 to 0.02) | 0.282 |
| Maternal overweight/obesity subgroup‡ (n = 31) | ||
| Pregestational BMI | 0.09 (0.03 to 0.16) | 0.007 |
| Gestational weight change as a continuous variable | 0.04 (−0.01 to 0.10) | 0.128 |
| Maternal age at delivery | −0.04 (−0.10 to 0.03) | 0.279 |
| HbA1c at weeks 24–28 of pregnancy | 0.09 (−0.77 to 0.95) | 0.826 |
| Total daily dose of insulin at delivery | −0.01 (−0.03 to 0.02) | 0.504 |
| Maternal underweight subgroup§ (n = 2) | NAǁ | NAǁ |
| Model 2¶: included gestational weight change as a binary variable (≥16 kg vs <16 kg) | ||
| Normal maternal BMI subgroup (n = 68) | ||
| Gestational weight gain | ||
| <16 kg (n = 66) | Reference | |
| ≥16 kg (n = 2) | 1.29 (0.02 to 2.57) | 0.047 |
| Pregestational BMI | 0.01 (−0.10 to 0.12) | 0.864 |
| Maternal age at delivery | −0.02 (−0.06 to 0.03) | 0.448 |
| HbA1c at weeks 24–28 of pregnancy | −0.14 (−0.71 to 0.43) | 0.622 |
| Total daily dose of insulin at delivery | 0.004 (−0.01 to 0.02) | 0.431 |
| Model 3** included gestational weight change as a binary variable (≥12 kg vs <12 kg) | ||
| Normal maternal BMI subgroup (n = 68) | ||
| Gestational weight gain | ||
| ≤12 kg (n = 61) | Reference | |
| >12 kg (n = 7) | 0.86 (0.15 to 1.57) | 0.018 |
| Pregestational BMI | 0.02 (−0.10 to 0.14) | 0.689 |
| Maternal age at delivery | −0.02 (−0.07 to 0.03) | 0.375 |
| HbA1c at weeks 24–28 of pregnancy | −0.11 (−0.70 to 0.47) | 0.695 |
| Total daily dose of insulin at delivery | 0.01 (−0.01 to 0.02) | 0.336 |
CI, confidence interval; BMI, body mass index; HbA1c, hemoglobin A1c; GDM, gestational diabetes mellitus; NA, not applicable. All the models were adjusted for maternal age at delivery, HbA1c at weeks 24–28 of pregnancy, and total daily dose of insulin at delivery. * Includes pregestational BMI and gestational weight changes in the study population that consisted of 31, 68, and 2 mothers with GDM who had a pregestational BMI ≥25 kg/m2, 18.5–24.9 kg/m2, and <18.5 kg/m2. †Includes pregestational BMI and gestational weight changes in the subgroup of pregestational normal BMI (18.5–24.9 kg/m2) mothers with GDM. ‡ Includes pregestational BMI, gestational weight changes in the subgroup of pregestational overweight/obese (BMI ≥25 kg/m2) mothers with GDM. §Includes pregestational underweight (BMI <18.5 kg/m2) mothers with GDM. ǁDue to a small sample size. ¶Includes gestational weight gains (<16 kg and ≥16 kg) and pregestational BMI in the subgroup of pregestational normal BMI (18.5–24.9 kg/m2) mothers with GDM. **Includes gestational weight gains (≤12 kg and >12 kg) and pregestational BMI in the subgroup of pregestational normal BMI (18.5–24.9 kg/m2) mothers with GDM.
Furthermore, we included the dichotomous—rather than continuous—variable of gestational weight gain in the multiple linear regression model as described below (models 2 and 3 in Table 2). The referenced guidelines11 define maternal weight gain ≥16 kg during pregnancy as excessive gestational weight gain for pregestational normal BMI women. To examine differences in the infant birthweight z‐scores according to the presence or absence of excessive gestational weight gain in the normal maternal BMI subgroup, therefore, we dichotomized the gain into ≥16 kg versus <16 kg (model 2 in Table 2). Subsequently, a similar analysis was carried out with reference to the Japanese guidelines17 on gestational weight gain. In these guidelines, weight gain >12 kg is defined as excessive gestational weight gain17 with reference to the recommended gestational weight gain when intended to deliver AGA infants in Japan. For this reason, we dichotomized weight gain into >12 kg versus ≤12 kg for mothers with GDM in the normal maternal BMI subgroup and included the dichotomous—rather than continuous—variable of gestational weight gain in the multiple linear regression model (model 3 in Table 2).
Single regression analysis was carried out to examine the correlation between pregestational BMI and gestational weight change (Figure 1). The χ2‐test of differences in the delivery rates of LGA and AGA infants was carried out between the normal maternal BMI subgroup and the maternal overweight/obesity subgroup.
Figure 1.
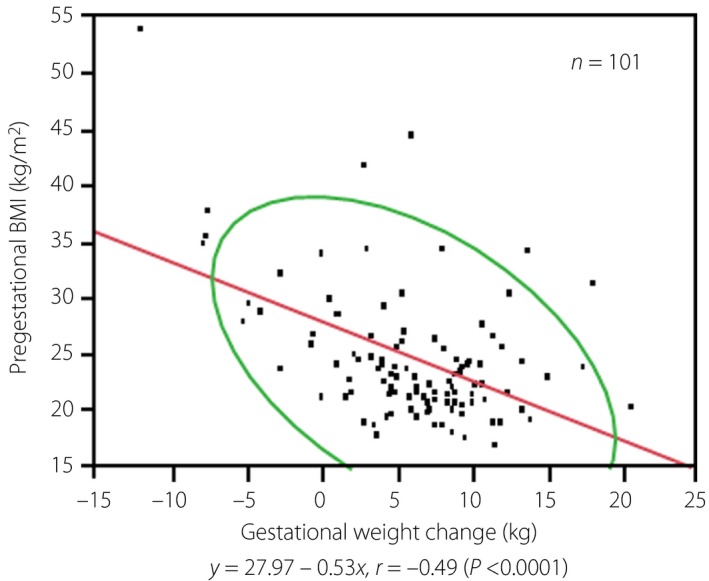
Correlation diagram of pregestational body mass index (BMI) and gestational weight change in mothers with gestational diabetes mellitus. Red line, regression line; green line, 95% probability ellipse.
A value of P < 0.05 was considered statistically significant. All statistical analyses were carried out using JMP software, version 9.0 (SAS Institute, Cary, NC, USA).
Results
Study Population
The clinical, obstetric and laboratory characteristics of 101 mothers with GDM are shown in Table 1. Multiple linear regression analysis was carried out in these 101 mothers. The mean of pregestational BMI, categorized as underweight (2.0%), normal (67.3%), overweight (17.8%) and obese (12.9%), as per the referenced guidelines11, was 24.7 ± 5.8. Gestational age was 38.5 ± 1.4 weeks. Maternal age and BMI at delivery were 34.7 ± 5.1 years and 27.2 ± 5.1, respectively. The mean of gestational weight change was 6.22 ± 5.39 kg. The mean of plasma glycated albumin was 13.4 ± 1.2%. Cesarean section was carried out in 23 mothers (22.8%) for the following reasons: seven had undergone cesarean delivery before the onset of the present study, two had placenta previa and four had breech presentation. The birthweight of infants was 2,987.3 ± 393.6 g; only one excessively large infant (1.0%) was born. The numbers of infants according to birthweight percentiles for gestational age were 82 (81.1%) AGA, 15 (14.9%) LGA and four (4.0%) SGA infants.
All mothers in the normal maternal BMI subgroup (n = 68) experienced gestational weight gains (7.56 ± 3.79 kg); 91.2% (62), 5.9% (4) and 2.9% (2) of them gave birth to 62 AGA, four LGA and two SGA infants, respectively. In contrast, the maternal overweight/obesity subgroup (n = 31) showed gestational weight changes (3.12 ± 7.08 kg); 67.7% (21) and 32.3% (10) of them experienced weight gain and weight loss, respectively; 64.5% (20) and 35.5% (11) of them gave birth to 20 AGA infants and 11 LGA infants including one excessively large infant, respectively.
In addition, we examined the association between pregestational BMI and gestational weight change in mothers with GDM. Consequently, pregestational BMI showed a significant inverse correlation (r = −0.49; P < 0.0001) with gestational weight change (Figure 1). In particular, mothers with a pregestational BMI less than approximately 35 showed a greater weight gain in proportion to gestational weight change.
Multiple Linear Regression Analysis on the Infant Birthweight Z‐Scores
We carried out multiple linear regression analysis to examine the associations between dependent and independent variables after adjustment for maternal age at delivery, HbA1c at weeks 24–28 of pregnancy and total daily dose of insulin at delivery (Table 2). In the entire study population, greater pregestational BMI was significantly associated with higher infant birthweight z‐scores (a 1‐unit increment in pregestational BMI was associated with a 0.08‐increment in the infant birthweight z‐score; P < 0.001; model 1 in Table 2). Furthermore, greater gestational weight change was significantly associated with higher infant birthweight z‐scores (a 1‐kg increment in gestational weight was associated with a 0.05‐increment in the infant birthweight z‐score; P = 0.007; model 1 in Table 2). Statistical analyses of the normal maternal BMI and maternal overweight/obesity subgroups showed no statistically significant interactions of pregestational BMI with gestational weight change (P = 0.158), probably because of the small sample size of the present study. Nevertheless, we consider it reasonable to use these subgroups because of their formation in consideration of the categories as defined in the referenced guidelines11. Subsequently, we analyzed the association between gestational weight change and the infant birthweight z‐scores in the normal maternal BMI subgroup and the maternal overweight/obesity subgroup. In the normal maternal BMI subgroup, greater gestational weight gain was significantly associated with the higher infant birthweight z‐scores (a 1‐kg increment in gestational weight was associated with a 0.06‐increment in the infant birthweight z‐score; P = 0.039; model 1 in Table 2). In the maternal overweight/obesity subgroup, greater pregestational BMI was significantly associated with higher infant birthweight z‐scores (a 1‐unit increment in BMI was associated with a 0.09‐increment in the infant birthweight z‐score; P = 0.007; model 1 in Table 2).
Association of Gestational Weight Change with Infant Birthweight for Gestational Age in the Normal Maternal BMI Subgroup
Because a statistically significant association was found between gestational weight change and the infant birthweight z‐scores in the normal maternal BMI subgroup (model 1 in Table 2), we decided to examine differences in the infant birthweight z‐scores according to the presence or absence of excessive gestational weight gain in the subgroup. With reference to excessive gestational weight gain, as defined by the guideline used in the present study11, we compared mothers who had gestational weight gains ≥16 kg and <16 kg, respectively. The comparisons showed that the infant birthweight z‐scores were significantly higher (P = 0.047) in mothers who had gestational weight gain ≥16 kg than in their counterparts who had gestational weight gain <16 kg (model 2 in Table 2).
Based on the recommendations in Japan for gestational weight gain17, we also compared mothers who had weight gains >12 kg and ≤12 kg. The infant birthweight z‐scores were significantly higher (P = 0.018) in mothers who had weight gain >12 kg than in their counterparts who had weight gain ≤12 kg (model 3 in Table 2).
Comparison of the Delivery Rates of LGA Versus AGA Infants Between Mothers with Normal BMI and Overweight/Obesity
Because a statistically significant association was found between pregestational BMI and the infant birthweight z‐scores in the maternal overweight/obesity subgroup (model 1 in Table 2), we carried out the χ2‐test on the delivery rates of LGA and AGA infants between the normal maternal BMI subgroup and the maternal overweight/obesity subgroup. The test showed that the delivery rate of LGA infants was significantly higher (P = 0.001) in the maternal overweight/obesity subgroup than in the normal maternal BMI subgroup. The proportions of mothers who delivered LGA and AGA infants were 35.5% (n = 11) and 64.5% (n = 20) in the maternal overweight/obesity subgroup, respectively, in comparison with 5.9% (n = 4) and 91.2% (n = 62), respectively, in the normal maternal BMI subgroup (data not shown in tables). Just four SGA infants were born in the present study, which hampered the statistical analysis.
Discussion
A very limited number of studies of Japanese women have examined the effects of pregestational BMI and gestational weight gain on infant birthweight for gestational age12, 13. To the best of our knowledge, we are the first to report a statistically significant positive association of pregestational BMI and gestational weight gain with infant birthweight for gestational age in mothers with GDM.
Overweight or obese women before and during pregnancy are at increased risk for GDM, and should reduce maternal BMI and maternal weight gain to avoid the risk18. Secondary analysis in the Hyperglycemia and Adverse Pregnancy Outcome Study carried out by Catalano et al.19 showed that overweight and obesity are associated with the delivery of heavy‐for‐date infants, independently of GDM, and that GDM and obesity synergistically lead to the delivery of heavy‐for‐date infants. Considering the results of the Hyperglycemia and Adverse Pregnancy Outcome Study and the present study, overweight or obese women who desire to become pregnant cannot avoid the increased risk of delivering LGA infants unless they adhere to the strict control of pregestational BMI to achieve an appropriate weight before becoming pregnant.
A meta‐analysis including 33 clinical studies on the effects of gestational weight gain on infant birthweight for gestational age in patients with GDM, only three of which were carried out among Asian, non‐Japanese patients with GDM10, showed that excessive gestational weight gain is a risk factor for delivering LGA infants; the present data are compatible with this finding. However, the adequate range of gestational weight gain remains to be determined, and the data of Asian patients available to date are limited. In Japan, therefore, gestational weight is generally controlled in accordance with recommended weight gain as described in published guidelines, with excessive gestational weight gain defined as gains of ≥16 kg11 and >12 kg17. In the present study, the infant birthweight z‐scores were significantly higher in mothers with GDM who had gestational weight gains ≥16 kg and >12 kg than in their respective counterparts who had gestational weight gains <16 kg and ≤12 kg (models 2 and 3 in Table 2); these findings support the abovementioned categorizations11, 17.
Regarding the mechanism by which pregestational overweight/obesity and excessive gestational weight gain exert an effect on infant birthweight for gestational age among patients with GDM, lipids including free fatty acids and triglycerides are considered to become excessive due to maternal dyslipidemia, which might occur in overweight or obese mothers, thereby causing an increase in fetal bodyweight7, 20. Fetal growth can be influenced by elevated lipid levels in pregnant women who were overweight or obese before pregnancy rather than by plasma glucose concentrations that start to increase in the latter half of pregnancy13. Therefore, the adequate control of pregestational BMI and gestational weight gain is considered necessary for the prevention of fetal overgrowth. Additionally, Tyrrell et al.21 recently reported the potential causality of genetically elevated maternal BMI with the greater birthweight of offspring. Hence, the potential influence of maternal genetic susceptibility to obesity on an increase in infant birthweight for gestational age might lead to a positive association between pregestational BMI and infant birthweight for gestational age in the present study.
The present study had the following strengths. First, we successfully determined the clinical and laboratory characteristics required for the appropriate care of mothers with GDM who received dietary counseling, as well as the strict control of hyperglycemia and bodyweight in accordance with the Diagnostic and Therapeutic Manual of Diabetic Pregnancy17 at our institution. Due to the control, mothers with GDM in the present study showed smaller and greater gestational weight gains in proportion to greater and smaller pregestational BMIs, respectively (Figure 1). Namely, this inverse correlation between these variables indicates the successful weight control of many mothers with GDM. Second, we excluded mothers with underlying disease that might disturb the achievement of the objective of the present study (e.g., metabolic and endocrine disorders). These strengths allowed us to precisely assess complications of GDM by reducing confounding factors. According to a 2003–2009 nationwide survey on the incidence of infant complications owing to GDM in 1,774 Japanese patients with GDM22, the incidences of excessively large and heavy‐for‐date infants were 2.6% and 21.5%, respectively. However, the incidences of such complications were lower in the present study. These facts indicate that the clinical care of GDM patients at our hospital is equivalent to or better than standard maternal care for GDM in Japan. Therefore, we consider that the present study reflects current real‐world clinical practice in Japan.
In compliance with the guidelines for diet therapy published by the Ministry of Health, Labor and Welfare of Japan23, mothers received thorough guidance for dietary intake as follows: mothers with BMI ≥25, ideal bodyweight (height in meters squared × 22) × 30 kcal/day; mothers with BMI <25 require the energy intake of an additional 50–450 kcal. Hence, gestational weight gain should differ between mothers who had pregestational BMI ≥25 and <25. The number of mothers who experienced gestational weight loss was obviously greater in mothers who had pregestational BMI ≥25 than in mothers with lower BMI. Therefore, it was difficult to precisely investigate differences in the effects of gestational weight gain on infant birthweight for gestational age between these two subpopulations of mothers. We analyzed pregestational overweight and obese mothers together, because current diet therapy in Japan considers the ranges of overweight/obesity (BMI ≥25 kg/m2) in one combined category; therefore, there is no difference in dietary therapeutic strategy for overweight and obese patients with GDM in Japan.
The present study included some limitations. First, the number of mothers with GDM was as small as 101 at a single center. Therefore, our study involves the issue of generalizability and the number of complications that might be caused by GDM (e.g., excessively large infant, congenital anomaly, hypoglycemia, respiratory distress in the infant, miscarriage, premature labor, shoulder dystocia and cesarean section) was limited. Consequently, we could not statistically analyze these complications. Second, glycated albumin—an indicator for the control of blood glucose during pregnancy that is considered more useful than HbA1c24—was determined in a small portion of mothers (34.7%, 35/101). Third, two biases cannot be ruled out: (i) selection bias might be present, because pregnant women with severe obesity are prone to being hospitalized for the management of high‐risk fetuses; and (ii) information bias might have occurred, because pregestational BMI was self‐reported. Olson, et al. reported that 35% of pregnant women in the obese BMI group self‐reported lower pregestational weight25. Such an underreporting of pregestational weight might have affected our estimates. Fourth, we could not investigate confounding factors, such as food intake, physical activity and socioeconomic factors. None of the included mothers smoked (Table 1); therefore, we did not adjust for smoking in the multivariable analysis. In the future, we will carry out a prospective study to examine insulin resistance and confounding factors that were not investigated in the present study. Fifth, pregestational overweight and obesity, as well as excessive gestational weight gain, are potential risk factors for GDM18, 19. Therefore, caution should be exercised in interpreting the clinical findings from the present study that investigated mothers with GDM alone. Sixth, the present study involves a statistical model limitation: the correlation between pregestational BMI and the infant birthweight z‐scores is potentially non‐linear, although we assumed their linearity.
In conclusion, pregestational BMI ≥25 and excessive gestational weight gain in mothers with GDM were significantly associated with increased infant birthweight for gestational age. A prospective multicenter clinical study enrolling a larger number of mothers with GDM will be required to verify the effects of adequately controlling pregestational and gestational weights on infant birthweight for gestational age.
Disclosure
The authors declare no conflict of interest.
Acknowledgments
The authors express their gratitude Kazuyuki Inoue MD and Taki Okajima MD for their role in the data collection. We also thank Satoshi Sakima MD for his valuable discussion regarding the manuscript.
J Diabetes Investig 2019; 10: 1075–1082
References
- 1. Moyer VA; U.S. Preventive Services Task Force . Screening for gestational diabetes mellitus: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2014; 160: 414–420. [DOI] [PubMed] [Google Scholar]
- 2. HAPO Study Cooperative Research Group ; Metzger BE, Lowe LP, Dyer AR, et al Hyperglycemia and adverse pregnancy outcomes. N Engl J Med 2008; 358:1991–2002. [DOI] [PubMed] [Google Scholar]
- 3. Weissmann‐Brenner A, Simchen MJ, Zilberberg E, et al Maternal and neonatal outcomes of large for gestational age pregnancies. Acta Obstet Gynecol Sc 2012; 91: 844–849. [DOI] [PubMed] [Google Scholar]
- 4. Khambalia AZ, Algert CS, Bowen JR, et al Long‐term outcomes for large for gestational age infants born at term. J Paediatr Child Health 2017; 53: 876–881. [DOI] [PubMed] [Google Scholar]
- 5. Boney CM, Verma A, Tucker R, et al Metabolic syndrome in childhood: association with birth weight, maternal obesity, and gestational diabetes mellitus. Pediatrics 2005; 115: e290–e296. [DOI] [PubMed] [Google Scholar]
- 6. Sugihara S, Sasaki N, Amemiya S, et al Analysis of weight at birth and at diagnosis of childhood‐onset type 2 diabetes mellitus in Japan. Pediatr Diabetes 2008; 9(4 Pt 1): 285–290. [DOI] [PubMed] [Google Scholar]
- 7. Catalano PM, Hauguel‐De Mouzon S. Is it time to revisit the Pedersen hypothesis in the face of the obesity epidemic? Am J Obstet Gynecol 2011; 204: 479–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Vinturache A, Moledina N, McDonald S, et al Pre‐pregnancy Body Mass Index (BMI) and delivery outcomes in a Canadian population. BMC Pregnancy Childbirth 2014; 14: 422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. HAPO Study Cooperative Research Group . Hyperglycaemia and Adverse Pregnancy Outcome (HAPO) Study: associations with maternal body mass index. BJOG 2010; 117: 575–584. [DOI] [PubMed] [Google Scholar]
- 10. Viecceli C, Remonti LR, Hirakata VN, et al Weight gain adequacy and pregnancy outcomes in gestational diabetes: a meta‐analysis. Obes Rev 2017; 18: 567–580. [DOI] [PubMed] [Google Scholar]
- 11. Rasmussen KM, Catalano PM, Yaktine AL. New guidelines for weight gain during pregnancy: what obstetrician/gynecologists should know. Curr Opin Obstet Gynecol 2009; 21: 521–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Takimoto H, Sugiyama T, Nozue M, et al Maternal antenatal body mass index gains as predictors of large‐for‐gestational‐age infants and cesarean deliveries in Japanese singleton pregnancies. J Obstet Gynaecol Res 2011; 37: 553–562. [DOI] [PubMed] [Google Scholar]
- 13. Iwama N, Sugiyama T, Metoki H, et al; JAGS Group . Maternal body mass index is a better indicator of large‐for‐gestational‐age infants compared with a 75‐g oral glucose tolerance test in early pregnancy: the JAGS trial. Diabetes Res Clin Pract 2017; 132: 10–18. [DOI] [PubMed] [Google Scholar]
- 14. Minakami H, Maeda T, Fujii T, et al Guidelines for obstetrical practice in Japan: Japan Society of Obstetrics and Gynecology (JSOG) and Japan Association of Obstetricians and Gynecologists (JAOG) 2014 edition. J Obstet Gynaecol Res 2014; 40: 1469–1499. [DOI] [PubMed] [Google Scholar]
- 15. Itabashi K, Miura F, Uehara R, et al New Japanese neonatal anthropometric charts for gestational age at birth. Pediatr Int 2014; 56: 702e708. [DOI] [PubMed] [Google Scholar]
- 16. Cole TJ. The LMS method for constructing normalized growth standards. Eur J Clin Nutr 1990; 44: 45e60. [PubMed] [Google Scholar]
- 17. The Japanese Society of Diabetes and Pregnancy (ed). Diagnostic and Therapeutic Manual of Diabetic Pregnancy. 1st edn Tokyo: Medical View, 2015. (Japanese). [Google Scholar]
- 18. Chu SY, Callaghan WM, Kim SY, et al Maternal obesity and risk of gestational diabetes mellitus. Diabetes Care 2007; 30: 2070–2076. [DOI] [PubMed] [Google Scholar]
- 19. Catalano PM, McIntyre HD, Cruickshank JK, et al; HAPO Study Cooperative Research Group . The hyperglycemia and adverse pregnancy outcome study: associations of GDM and obesity with pregnancy outcomes. Diabetes Care 2012; 35: 780–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schaefer‐Graf UM, Graf K, Kulbacka I, et al Maternal lipids as strong determinants of fetal environment and growth in pregnancies with gestational diabetes mellitus. Diabetes Care 2008; 31: 1858–1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tyrrell J, Richmond RC, Palmer TM, et al Genetic evidence for causal relationships between maternal obesity‐related traits and birth weight. JAMA 2016; 315: 1129–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sugiyama T; The Japanese Society of Diabetes and Pregnancy . Special study and project report. Diabetes & Pregnancy 2013; 13: 34–38. (Japanese). [Google Scholar]
- 23. Reference material: pregnant and breast‐feeding women In: Hishida A, Sasaki S. (eds). Dietary Reference Intakes for Japanese, 2015, Tokyo: Dai‐ichi Shuppan, 2014; 345–353 (Japanese). [Google Scholar]
- 24. Hiramatsu Y, Shimizu I, Omori Y, et al; JGA (Japan Glycated Albumin) Study Group . Determination of reference intervals of glycated albumin and hemoglobin A1c in healthy pregnant Japanese women and analysis of their time courses and influencing factors during pregnancy. Endocr J 2012; 59: 145–151. [DOI] [PubMed] [Google Scholar]
- 25. Olson CM, Strawderman MS. Modifiable behavioral factors in a biopsychosocial model predict inadequate and excessive gestational weight gain. J Am Diet Assoc 2003; 103: 48–54. [DOI] [PubMed] [Google Scholar]


