Abstract
Aims/Introduction
The putative tumor suppressor gene, KBTBD11, might play a role in tumorigenesis, and is associated with cellular apoptosis and proliferation in colorectal cancer cells. However, the function of Kbtbd11 during adipogenesis is unknown. The aim of the present study was to investigate the role of Kbtbd11 in the differentiation of 3T3‐L1 preadipocytes.
Materials and Methods
For the fasting–refeeding protocol, mice were subjected to fasting for 24 h, followed by a chow diet for 12 h. Adenovirus infection methods were used to examine the effect of Kbtbd11, and 3T3‐L1 cells were analyzed with Oil Red O staining and real‐time polymerase chain reaction.
Results
The white adipose tissue expression of Kbtbd11 messenger ribonucleic acid (mRNA) was significantly higher in the re‐fed state than in the fasted state. Kbtbd11 mRNA levels were markedly increased in epididymal white adipose tissue of diet‐induced obesity mice compared with those in the mice fed a chow diet. In addition, Kbtbd11 mRNA expression was increased in a differentiation‐dependent manner in 3T3‐L1 cells. Knockdown of Kbtbd11 mRNA through the infection with adenoviral vectors remarkably inhibited triglyceride accumulation and adipocyte differentiation in 3T3‐L1 cells. In contrast, the overexpression of Kbtbd11 promoted the differentiation of 3T3‐L1 adipocytes.
Conclusions
The present findings show that Kbtbd11 expression might be involved in nutritional regulation and is increased in obese adipose tissue. In addition, Kbtbd11 appears to be required for the differentiation of adipocytes in 3T3‐L1 cells. Collectively, these results show a novel link between the expression of Kbtbd11 and fat accumulation, and suggest that Kbtbd11 is a new therapeutic target for obesity.
Keywords: 3T3‐L1 preadipocytes, Differentiation, Kbtbd11
Introduction
Being obese or overweight is associated with the risk of developing type 2 diabetes, atherosclerosis, hyperlipidemia, steatosis and various cancers1, 2, 3. Adipocyte differentiation represents a multistep process involving a cascade of transcription factors for key proteins that induce gene expression and lead to adipocyte development. The 3T3‐L1 cell line is a well‐established preadipose cell line, which is useful for investigating the mechanisms underlying adipocyte proliferation, differentiation and lipid metabolism, as well as the identification of genes that regulate adipocyte physiology4, 5.
Kelch repeat and BTB domain containing 11 (KBTBD11) belongs to the BTB superfamily, which includes the KLHL and KLHDC subfamilies, and has BTB/POZ and Kelch domains. The BTB/POZ domain functions as the domain for protein–protein interaction to enable dimer formation and an interaction with non‐BTB domain‐containing proteins, including the scaffold protein of the E3 ubiquitin ligase complex6, 7. The Kelch domain, an evolutionarily conserved structure, widely found in mammals and insects, usually comprises two to seven repeats of four‐stranded β‐sheet motif forming one blade of the β‐propeller structure7, 8.
A variant allele of KBTBD11, rs11777210, is significantly associated with colorectal cancer cell susceptibility. KBTBD11 expression is significantly decreased in tumor tissues compared with that in adjacent paired normal tissues. Additionally, in colorectal cancer cells, KBTBD11 knockdown inhibits apoptosis and promotes proliferation, whereas KBTBD11 overexpression promotes apoptosis and inhibits cell growth. In tumorigenesis, KBTBD11 is a putative tumor suppressor, the expression of which is regulated by MYC9.
Kbtbd11 might play an important role in cellular differentiation and proliferation in tumor tissues. However, the expression profile and functional significance of Kbtbd11 in adipose tissue is unknown. In the present study, we investigated the expression profile of Kbtbd11 in adipose tissue and its effects in 3T3‐L1 preadipocyte differentiation.
Methods
Animal Experiments
For all experiments, 8‐week‐old male C57BL/6J mice from Japan SLC (Hamamatsu, Japan) were used. The mice were maintained on a standard chow diet. For the fasting–refeeding protocol, the mice were subjected to fasting for 24 h and then fed a chow diet for 12 h. For obtaining diet‐induced obesity (DIO) mice, C57BL/6J mice were fed a high‐fat diet for 4 weeks. Feed ingredients content were as follows: the standard chow diet (CE‐2) comprised 50.3% carbohydrates, 25.4% protein and 4.4% fat. The high‐fat diet (HFD32) comprised 29.4% carbohydrates, 25.5% protein and 32.0% fat (CLEA Japan Inc., Tokyo, Japan). Animal experimental protocols were approved by the animal ethics committee of Jichi Medical University (permit number 17177), and were carried out in accordance with the Use and Care of Experimental Animals Guidelines of the Jichi Medical University Guide for Laboratory Animals.
Cells and Adipocyte Differentiation
3T3‐L1 cells were maintained in high‐glucose Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum, and 100 units each of penicillin and streptomycin at 37°C in 5% CO2. For the adipocyte differentiation study, 3T3‐L1 cells were cultured to confluence. After 2 days, the medium was replaced with high‐glucose Dulbecco's modified Eagle's medium containing 5 μg/mL insulin, 1 μmol/L dexamethasone (DEX) and 0.5 mmol/L 3‐isobutyl‐1‐methylxanthine (IBMX). After 48 h, the medium was changed to high‐glucose Dulbecco's modified Eagle's medium containing 5 μg/mL insulin. The medium was renewed every other day.10 For the adenovirus infection study, 3T3‐L1 cells at day 2 before the induction of differentiation were infected with adenovirus, and incubated for 0, 4 and 8 days.
Oil Red O Stain
3T3‐L1 cells were fixed with 10% formalin in phosphate‐buffered saline for 10 min at 37°C and stained with 60% Oil red O solution (Muto pure Chemicals Co., Tokyo, Japan) for 20 min at room temperature. The cells were washed with distilled water, and the retained dye was eluted by 60% isopropanol.
Adenoviral Expression Vectors
Adenoviruses were prepared and amplified with the ViraPower Adenoviral Expression System (Invitrogen, Carlsbad, CA, USA), as previously described.11 Polymerase chain reaction (PCR)‐amplified, mouse Kbtbd11 complementary deoxyribonucleic acid was subcloned into the pENTR Directional TOPO vector (Invitrogen). The short hairpin ribonucleic acids (shRNAs) of Kbtbd11 and LacZ were cloned into BLOCK‐iT U6 entry vector (Invitrogen). The sequence of the shRNA for Kbtbd11 shRNA#1 was as follows: 5′‐cacc GGACATATGTGAAATCTGA ttcaagaga TCAGATTTCACATATGTCC‐3′, and Kbtbd11 shRNA#2 was as follows: cacc GCAAGTAAGTGACATTTAA ttcaagaga TTAAATGTCACTTACTTGC. Inserts of pENTR vectors were transferred into the adenovirus vectors pAd/CMV‐DEST or pAd/PL‐DEST using the Gateway system (Invitrogen). Recombinant adenoviruses were purified by the Adenovirus Purification Miniprep Kit (Cell Biolabs, San Diego, CA, USA) according to the manufacturer's protocol.
Real‐Time PCR
Total RNA was isolated using acid guanidinium thiocyanate–phenol reagent.12 Complementary deoxyribonucleic acid synthesis was carried out using the Verso cDNA Kit (Thermo Scientific, Waltham, MA, USA) with random hexamer primers. Quantitative PCR (qPCR) assays were carried out using the ViiA7 Real‐Time PCR System and KAPA SYBER FAST ROX Low qPCR kit (Kapa Biosystems, Wilmington, MA, USA).12 Relative gene expression levels were quantified by qPCR followed by normalization to the internal control gene 36B4. The following primers were used for this analysis: Kbtbd11 Fwd, 5′‐TCAGCGTTTTCCGCTACCAT‐3′ and Kbtbd11 Rv, 5′‐AACACAACGAAAGGGCTGGA‐3′; Cebpa Fwd, 5′‐GCCATGCCGGGAGAACTCTA‐3′ and Cebpa Rv, 5′‐ GGGCTCTGGAGGTGACTGCT‐3′; Cebpb Fwd, 5′‐GAGCCGCGACAAGGCCAAGA‐3′ and Cebpb Rv, 5′‐GCTCGTTCTCCGCCGTCAGC‐3′; Pparg Fwd, 5′‐ TTCCACTATGGAGTTCATGCTTGT‐3′ and Pparg Rv, 5′‐ TCCGGCAGTTAAGATCACACCTA‐3′; p27 Fwd, 5′‐AGGAGAGCCAGGATGTCAGC‐3′ and p27 Rv, 5′‐ CAGAGTTTGCCTGAGACCCAA‐3′; Cyclin D1 Fwd, 5′‐GTTCATTTCCAACCCACCCTC‐3′ and Cyclin D1 Rv, 5′‐AGAAAGTGCGTTGTGCGGTAG‐3′; Bax Fwd, 5′‐GCTGACATGTTTGCTGATGG‐3′ and Bax Rv, 5′‐ GATCAGCTCGGGCACTTTAG‐3′; Bcl2 Fwd, 5′‐CTGGGATGCCTTTGTGGAAC‐3′ and Bcl2 Rv, 5′‐GAGACAGCCAGGAGAAATCAAAC‐3′; Tnfa Fwd, 5′‐CAGCCGATGGGTTGTACCTT‐3′ and Tnfa Rv, 5′‐ GGGCTCATACCAGGGTTTGA‐3′; Il6 Fwd, 5′‐GAGGATACCACTCCCAACAGACC‐3′ and Il6 Rv, 5′‐ AAGTGCATCATCGTTGTTCATACA‐3′; 36B4 Fwd, 5′‐ATGCAGCAGATCCGCATGT‐3′ and 36B4 Rv, 5′‐TTGCGCATCATGGTGTTCTT‐3′.
Statistical Analysis
Experimental studies were carried out in triplicates or greater. Statistical significance was tested using the unpaired two‐tailed Student's t‐test. Data are shown as mean ± standard error of the mean. Differences were considered to be significant at P < 0.05.
Results
Kbtbd11 mRNA Expression in Epididymal White Adipose Tissue
We first examined the expression pattern of Kbtbd11 in epididymal white adipose tissue (eWAT) of C57BL/6J mice in the fasted and re‐fed states. In the fasted state, Kbtbd11 mRNA expression was low, but was profoundly promoted by re‐feeding in the eWAT of C57BL/6J mice (Figure 1a). Next, we compared the expression of Kbtbd11 levels in the eWAT of obese mice. In DIO mice, Kbtbd11 mRNA was elevated >10‐fold (Figure 1b). These results show that Kbtbd11 expression levels might be dependent on triglyceride accumulation in WAT.
Figure 1.
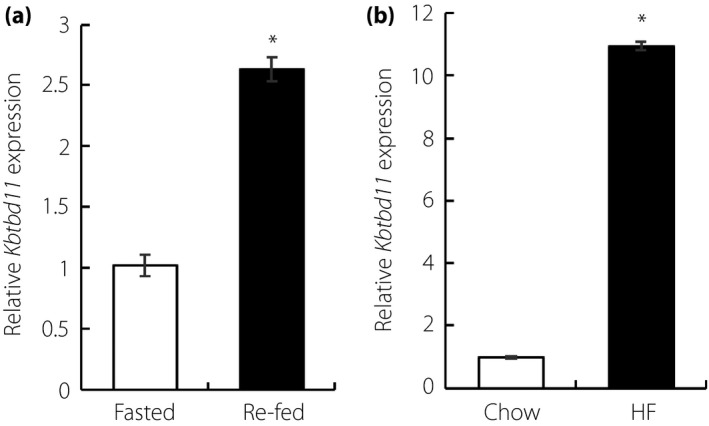
Kbtbd11 expression levels in epididymal white adipose tissue. (a) The expression of Kbtbd11 in epididymal white adipose tissue of C57BL/6J mice; mice were fasted for 24 h or fasted for 24 h/re‐fed for 12 h; n = 3 per group, *P < 0.01 versus fasted. (b) The expression of Kbtbd11 in epididymal white adipose tissue of diet‐induced obesity mice; diet‐induced obesity mice were fed a high‐fat diet for 1 month. The mice were fasted for 24 h; n = 3 per group, *P < 0.01 versus chow.
Kbtbd11 Expression During 3T3‐L1 Adipose Differentiation
To elucidate whether Kbtbd11 plays a role in adipose differentiation, we examined the expression of Kbtbd11 in 3T3‐L1 cell differentiation using qPCR methods. 3T3‐L1 cells were induced to differentiate using a cocktail mix that included DEX, IBMX and insulin. RNA was extracted from cells at day 0, 2, 4, 8 and 10 after the induction of adipocyte differentiation. Kbtbd11 expression increased by fourfold between day 0 and day 4. Furthermore, during 3T3‐L1 cell differentiation, Kbtbd11 levels subsequently increased by 18‐fold compared with those on day 0 (Figure 2). Figure 2 shows the expression levels of CCAAT/enhancer‐binding protein alpha (Cebpa) and peroxisome proliferator‐activated receptor gamma (Pparg), which are the transcription factors of adipogenic differentiation induced during adipose differentiation. Cebpb, the key early regulator of adipogenesis, was also induced. These results suggested that an increase in Kbtbd11 mRNA expression might be related to lipid accumulation and adipose differentiation.
Figure 2.
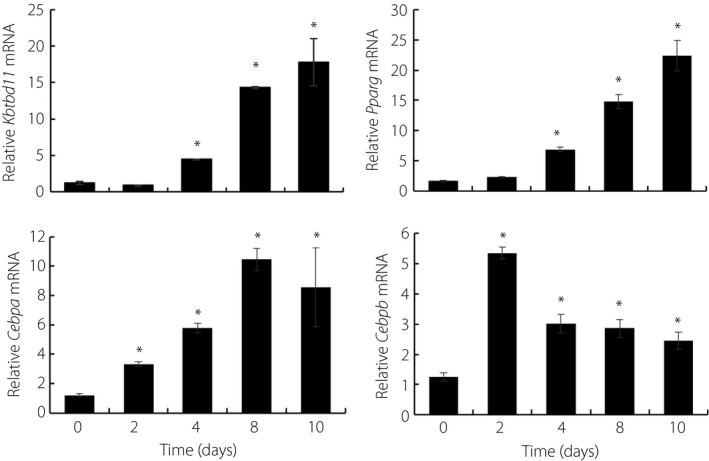
Kbtbd11 messenger ribonucleic acid (mRNA) expression in differentiating 3T3‐L1 cells. Total RNA was extracted from 3T3‐L1 cells at day 0, 2, 4, 8 and 10 after the induction of differentiation by treatment with an adipogenic cocktail; n = 3 per group, *P < 0.01 versus day 0.
Expression of Kbtbd11 During Early 3T3‐L1 Adipose Differentiation
3T3‐L1 cells undergo differentiation to adipocytes in response to a cocktail of adipogenic stimuli containing insulin; the synthetic glucocorticoid, DEX; and the phosphodiesterase inhibitor, IBMX.13 As Kbtbd11 mRNA expression increased in the re‐fed eWAT and during 3T3‐L1 adipose differentiation, the presence of individual adipogenic stimuli in the short‐term might have any influence on Kbtbd11 mRNA expression in 3T3‐L1 preadipocytes. To elucidate the Kbtbd11 expression pattern in individual adipogenic factors, 3T3‐L1 preadipocytes were collected at 0, 1, 4 and 8 h after the addition of one of the following: insulin, DEX and IBMX. Total RNA was then isolated from cells and subjected to qPCR to evaluate Kbtbd11 mRNA levels. Kbtbd11 mRNA levels increased rapidly by insulin, and were upregulated threefold at early times from 2 to 4 h after the addition of DEX (Figure 3a,b). After the addition of IBMX, Kbtbd11 mRNA gradually, but significantly, increased from 4 h to 8 h. The levels of Cebpa and Cebpb, the inducers of adipocyte differentiation, also rapidly increased in preadipocytes by adding individual drugs. These results suggested that Kbtbd11 transcripts, increased rapidly by the adipogenic cocktail, were involved in 3T3‐L1 adipocyte differentiation.
Figure 3.
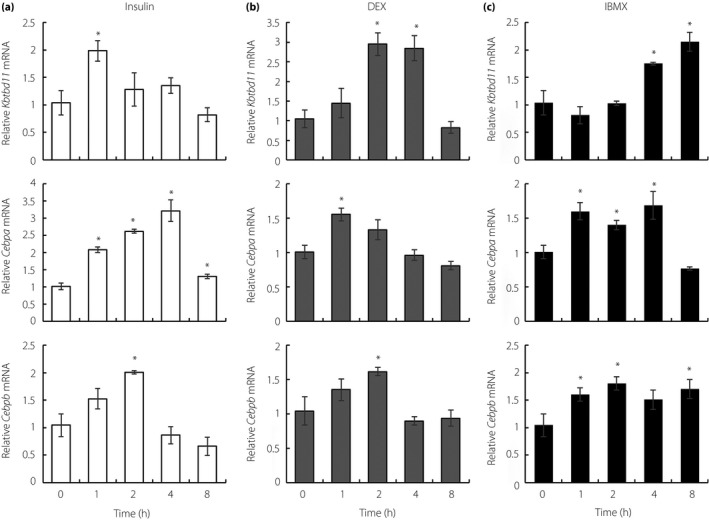
Kbtbd11 messenger ribonucleic acid (mRNA) expression in response to adipogenic stimuli at early time points. 3T3‐L1 preadipocytes were cultured to confluence, serum‐starved for 12 h and treated with (a) 5 μg/mL insulin, (b) 2.5 μmol/L dexamethasone (DEX) or (c) 200 μmol/L 3‐isobutyl‐1‐methylxanthine (IBMX) for the indicated times; n = 3 per group, *P < 0.01 versus 0 h.
Knockdown and overexpression of Kbtbd11 mRNA are reciprocally involved in mitotic clonal expansion (MCE) and triglyceride accumulation during 3T3‐L1 adipocyte differentiation
To investigate the role of Kbtbd11 in adipocyte differentiation, including in the early stage, we examined the effects of Kbtbd11 knockdown on 3T3‐L1 differentiation. Adenoviral infection of either of the two independent Kbtbd11 shRNA constructs decreased Kbtbd11 mRNA expression levels by up to approximately 60% compared with that of the LacZ‐specific short hairpin ribonucleic acid (shLacZ) control (Figure 4a). On day 8, after inducing differentiation, the shLacZ control cells showed abundant lipid droplets (observed with Oil Red O staining) (Figure 4b), and a significant increase in the expression of Cebpa, Cebpb and Pparg during the middle (day 4) and late stages (day 8) of 3T3‐L1 adipogenesis. In addition, in the early stage, the mRNA expression of Cebpb was upregulated, reaching a peak at 2 h after the induction of differentiation. Furthermore, the expression of Cyclin D1, acting on MCE, rapidly increased in preadipocytes after the addition of the adipogenic cocktail. Meanwhile, the expression of p27, a key regulator of cell cycle progression, decreased (Figure 4c). In contrast, lipid accumulation and adipocyte differentiation markers (Cebpa, Cebpb and Pparg) were significantly decreased in Kbtbd11‐knockdown cells during the middle and late stages of 3T3‐L1 adipogenesis (Figure 4b,c). In the early stage, Cebpb and Cyclin D1 were significantly decreased and p27 was increased in Kbtbd11‐knockdown cells (Figure 4c). In Kbtbd11‐overexpressing 3T3‐L1 cells, Kbtbd11 mRNA expression increased by 40‐fold (Figure 4d), which promoted lipid accumulation (Figure 4e). Adipocyte differentiation markers were significantly increased in the Kbtbd11‐overexpressing 3T3‐L1 cells on days 4 and 8 after the induction of differentiation. In the early stage, Cebpb and Cyclin D1 were significantly increased, and p27 was decreased in Kbtbd11‐overexpressing cells (Figure 4f). These results suggest that Kbtbd11 plays an important role in MCE, and is involved in triglyceride accumulation and adipocyte differentiation.
Figure 4.
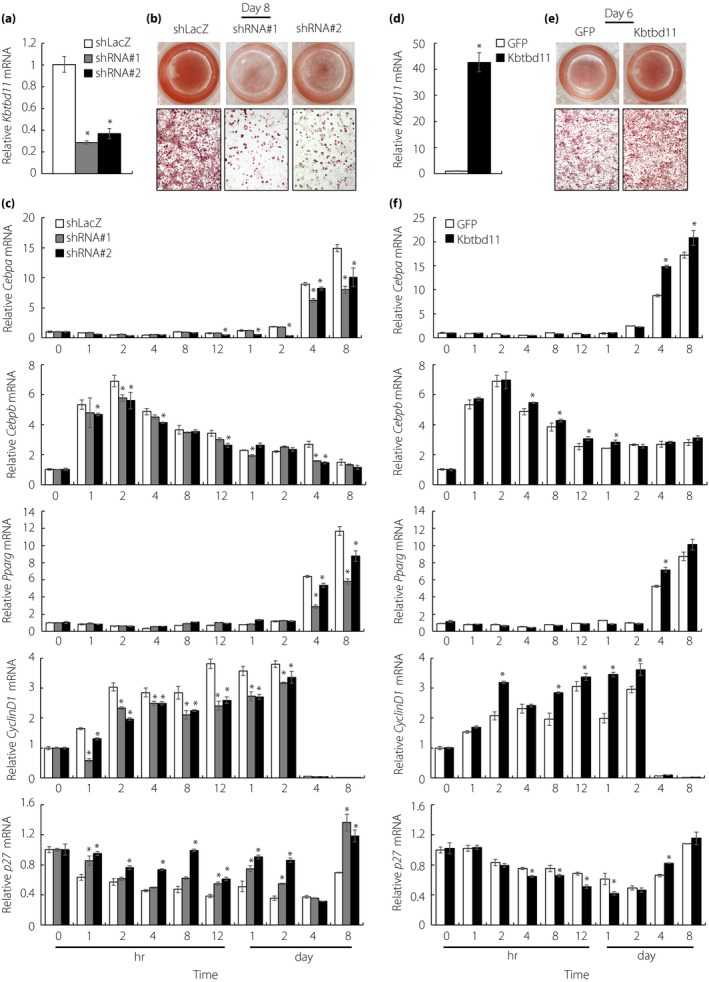
The effects of knockdown and overexpression of Kbtbd11 on 3T3‐L1 cellular differentiation. (a) The expression of Kbtbd11 messenger ribonucleic acid (mRNA) in Kbtbd11 knockdown 3T3‐L1 cells at day 8. Kbtbd11 knockdown adenovirus particles were used with either of the two independent Kbtbd11 short hairpin ribonucleic acid (shRNA) constructs (shRNA#1 and shRNA#2); n = 3 per group, *P < 0.01 versus LacZ‐specific short hairpin ribonucleic acid (shLacZ); (b) triglyceride accumulation in 3T3‐L1 cells on day 8, visualized using Oil Red O staining; (c) the mRNA levels in 3T3‐L1 cells expressing each shRNA at various time points after inducing differentiation; n = 3 per group, *P < 0.01 versus shLacZ; (d) the expression of Kbtbd11 mRNA in Kbtbd11‐overexpressing 3T3‐L1 cells at day 8. Cells were infected with adenoviral vectors for expressing green fluorescent protein (GFP) or mouse Kbtbd11; n = 3 per group, *P < 0.01 versus GFP; (e) triglyceride accumulation in 3T3‐L1 cells on day 6 was detected using Oil Red O staining. (f) Relative mRNA levels in each group of 3T3‐L1 cells at various time points after inducing differentiation; n = 3 per group, *P < 0.01 versus GFP.
Effects of Kbtbd11 Knockdown on Mature 3T3‐L1 Adipocytes
To examine the role of Kbtbd11 in mature adipocytes, we investigated effects of Kbtbd11 knockdown on mature 3T3‐L1 adipocytes. On day 8 after the induction of differentiation, mature 3T3‐L1 adipocytes were infected with adenoviral vectors for expressing shLacZ control or Kbtbd11 shRNA. Kbtbd11 adenoviral shRNA decreased Kbtbd11 mRNA expression levels by up to approximately 60% compared with the shLacZ control (Figure 5b). The number of lipid droplets of mature 3T3‐L1 adipocytes was not different between the shLacZ control and Kbtbd11 knockdown (Figure 5a). mRNA expression analyses of adipocytes (Pparg and aP2) and inflammation markers (Tnfa and Il6), as well as lipogenic (Fasn) and proapoptotic genes (Bax and Bcl2), were carried out using qPCR. mRNA levels of these markers and genes in adipocytes were not different compared with those in shLacZ control, suggesting that Kbtbd11 might only play a role in the early stage of adipogenesis.
Figure 5.
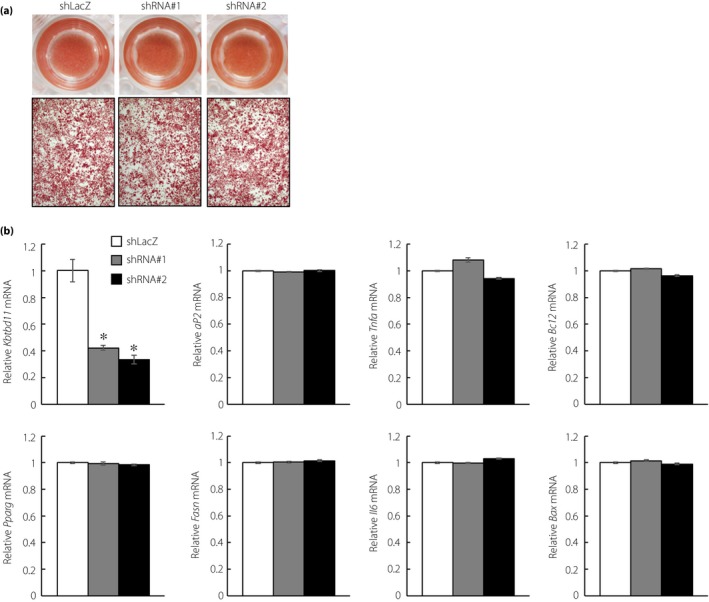
Effects of knockdown of Kbtbd11 on mature 3T3‐L1 adipocytes. (a) Triglyceride accumulation in Kbtbd11‐knockdown mature 3T3‐L1 adipocytes at 48 h after either of the two independent Kbtbd11 short hairpin ribonucleic acid (shRNA) adenoviral infections (shRNA#1 and shRNA#2) visualized using Oil Red O staining; (b) RNA was harvested at 48 h after adenoviral infection, and expression levels of Kbtbd11, adipocytes (Pparg and aP2) and inflammation markers (Tnfa and Il6), and lipogenic (Fasn) and proapoptotic genes (Bax and Bcl2) were measured using quantitative polymerase chain reaction; n = 3 per group, *P < 0.01 versus shLacZ.
Discussion
In the present study, we identified Kbtbd11 as a newly discovered adipogenesis‐related gene. At first, we examined the expression of Kbtbd11 mRNA in nutritional regulation and obese adipose tissue using qPCR analysis. Kbtbd11 was significantly increased in re‐fed eWAT compared with that in fasted eWAT, and was strongly induced in eWAT of DIO mice compared with that in the mice fed a chow diet (Figure 1b). We hypothesized that the elevation of Kbtbd11 in eWAT of obese mice might be associated with the accumulation of triglyceride in adipocytes. Next, we investigated whether Kbtbd11 was induced in 3T3‐L1 cells during adipose differentiation. The present results showed that Kbtbd11 expression was induced during 3T3‐L1 adipocyte differentiation (Figure 2). These findings, demonstrating that Kbtbd11 mRNA expression levels in 3T3‐L1 adipocytes show differentiation‐dependent expression, support our hypothesis.
As Kbtbd11 mRNA expression increased in the re‐fed eWAT, and during 3T3‐L1 adipose differentiation, we further examined whether Kbtbd11 transcription is regulated by insulin, DEX and IBMX individually. The present results showed that the expression of Kbtbd11 was rapidly increased by insulin (Figure 3a), suggesting that Kbtbd11 is indeed regulated by nutritional regulation. The differentiation of 3T3‐L1 preadipocytes into adipocytes was accompanied by a transient induction of Cebpb expression in response to treatment with IBMX and DEX, respectively. In addition, Cebpb led to the upregulation of the expression of adipogenic factors, such as Cebpa and Pparg, which appears to perform an important function in adipogenesis by controlling the late‐stage differentiated phenotype.14 After the addition of DEX and IBMX, respectively, in 3T3‐L1 preadipocytes, Kbtbd11 was upregulated later than the expression of Cebpa and Cebpb (Figure 3b,c). These data suggested that Kbtbd11 is regulated by adipogenic factors and related to adipose differentiation process.
Additional convincing evidence came from our results showing a close relationship between Kbtbd11 and adipocyte differentiation in 3T3‐L1 cells. In Kbtbd11 knockdown cells, differentiation of 3T3‐L1 adipocytes was markedly inhibited, with an accompanying decrease in the expression of Cebpa and Pparg (Figure 4b,c). Conversely, Kbtbd11‐overexpressing 3T3‐L1 cells upregulated the expression of Cebpa, Cebpb and Pparg. Together, these results suggested that Kbtbd11 regulates the differentiation of 3T3‐L1 adipocytes.
After hormonal induction, growth‐arrested 3T3‐L1 preadipocytes synchronously re‐enter the cell cycle for one to two rounds of cell division, a phenomenon known as MCE, which is one of the important events occurring at the early stage during 3T3‐L1 adipocyte differentiation. Cyclin D1 functions as a key sensor for mitogenic stimuli and promotes cell cycle progression from the G1 to S phase.15 In contrast, p27, a cyclin‐dependent kinase inhibitor, is a key negative regulator of the cell cycle during progression from the G1 to S phase.16 The balance of the cyclin D1‐p27 control system might play an important role in MCE. After MCE, CEBPB activates Cebpa and Pparg, which then transcriptionally activate genes that give rise to the adipocyte phenotype. These are accompanied by the early events of the cell cycle and initiation of a transcriptional cascade, which results in terminal adipogenic differentiation.17 Because KBTBD11 is associated with cellular differentiation and proliferation in tumor tissues,9 Kbtbd11 might play an important role in MCE of 3T3‐L1 differentiating adipocytes. Although KBTBD11 knockdown promotes proliferation in colorectal cancer cells, Kbtbd11 knockdown inhibits MCE and 3T3‐L1 differentiating adipocytes. The role of Kbtbd11 in 3T3‐L1 adipogenesis might be different from that in cellular differentiation and proliferation during tumorigenesis. Furthermore, Kbtbd11 knockdown inhibited adipogenesis, but only before MCE (not mature 3T3‐L1 adipocytes), and Kbtbd11 overexpression induced MCE, leading to the expression of Cebpa and Pparg (Figure 4c,f).
In conclusion, the present study shows that Kbtbd11 expression is involved in nutritional regulation and is increased in obese adipose tissue. Kbtbd11 is a regulator of 3T3‐L1 adipose differentiation that acts in the early stages of adipogenesis. These data showed a novel link between the expression of Kbtbd11 and fat accumulation, suggesting that Kbtbd11 could represent a new therapeutic target in obesity. However, further research is required to elucidate the physiological functions of Kbtbd11. KBTBD11 transgenic mice have not yet been reported, but would be an important biological tool in understanding the molecular mechanism(s) of adipogenesis, including adipocyte differentiation.
Disclosure
The authors declare no conflict of interest.
Acknowledgments
This study was supported by grants‐in‐aid (15K19523, 17K16153 and 17K09864) from Japan Society for the Promotion of Science (JSPS); a MEXT‐supported program for the strategic research foundation at private universities (2013–2017); and Jichi Medical University young investigator award. Ms Kayo Nagashima and Ms Yukiko Ohashi for their excellent technical assistance.
J Diabetes Investig 2019; 10: 925–932
References
- 1. Gallagher EJ, LeRoith D. Obesity and diabetes: the increased risk of cancer and cancer‐related mortality. Physiol Rev 2015; 95: 727–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hui E, Xu A, Bo Yang H, et al Obesity as the common soil of non‐alcoholic fatty liver disease and diabetes: role of adipokines. J Diabetes Investig 2013; 4: 413–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Vucenik I, Stains JP. Obesity and cancer risk: evidence, mechanisms, and recommendations. Ann N Y Acad Sci 2012; 1271: 37–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ruiz‐Ojeda FJ, Ruperez AI, Gomez‐Llorente C, et al Cell models and their application for studying adipogenic differentiation in relation to obesity: a review. Int J Mol Sci 2016; 17: 1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhu HJ, Ding HH, Deng JY, et al Inhibition of preadipocyte differentiation and adipogenesis by zinc‐alpha2‐glycoprotein treatment in 3T3‐l1 cells. J Diabetes Investig 2013; 4: 252–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Canning P, Cooper CD, Krojer T, et al Structural basis for Cul3 protein assembly with the BTB‐Kelch family of E3 ubiquitin ligases. J Biol Chem 2013; 288: 7803–7814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gong W, Gohla RM, Bowlin KM, et al Kelch repeat and BTB domain containing protein 5 (Kbtbd5) regulates skeletal muscle myogenesis through the e2f1‐dp1 complex. J Biol Chem 2015; 290: 15350–15361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dhanoa BS, Cogliati T, Satish AG, et al Update on the Kelch‐like (KLHL) gene family. Hum Genomics 2013; 7: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gong J, Tian J, Lou J, et al A polymorphic MYC response element in KBTBD11 influences colorectal cancer risk, especially in interaction with a MYC regulated SNP rs6983267. Ann Oncol 2017; 29: 632–639. [DOI] [PubMed] [Google Scholar]
- 10. Inoue N, Yahagi N, Yamamoto T, et al Cyclin‐dependent kinase inhibitor, p21WAF1/CIP1, is involved in adipocyte differentiation and hypertrophy, linking to obesity, and insulin resistance. J Biol Chem 2008; 283: 21220–21229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Watanabe K, Watson E, Cremona ML, et al ILDR2: an endoplasmic reticulum resident molecule mediating hepatic lipid homeostasis. PLoS One 2013; 8: e67234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Watanabe K, Nakayama K, Ohta S, et al ZNF70, a novel ILDR2‐interacting protein, contributes to the regulation of HES1 gene expression. Biochem Biophys Res Commun 2016; 477: 712–716. [DOI] [PubMed] [Google Scholar]
- 13. Pantoja C, Huff JT, Yamamoto KR. Glucocorticoid signaling defines a novel commitment state during adipogenesis in vitro. Mol Biol Cell 2008; 19: 4032–4041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wu Z, Bucher NL, Farmer SR. Induction of peroxisome proliferator‐activated receptor gamma during the conversion of 3T3 fibroblasts into adipocytes is mediated by C/EBPbeta, C/EBPdelta, and glucocorticoids. Mol Cell Biol 1996; 16: 4128–4136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hishida T, Naito K, Osada S, et al Crucial roles of d‐type cyclins in the early stage of adipocyte differentiation. Biochem Biophys Res Commun 2008; 370: 289–294. [DOI] [PubMed] [Google Scholar]
- 16. Ferguson BS, Nam H, Morrison RF. Curcumin inhibits 3t3‐l1 preadipocyte proliferation by mechanisms involving post‐transcriptional p27 regulation. Biochem Biophysics Rep 2016; 5: 16–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Merkestein M, Laber S, McMurray F, et al Fto influences adipogenesis by regulating mitotic clonal expansion. Nature Commun 2015; 6: 6792. [DOI] [PMC free article] [PubMed] [Google Scholar]


