Abstract
Aims/Introduction
Helicobacter pylori infection is associated with insulin resistance and glycemia in non‐diabetes. However, the relationship between H. pylori infection and glycemia in diabetes remains inconclusive. Therefore, we explored the effect of H. pylori infection status and its eradication on glycemic control and antidiabetic therapy in type 2 diabetes patients.
Materials and Methods
A total of 549 diabetes patients were recruited for sequential two‐step approach (immunoglobulin G [IgG] serology followed by 13C‐urea breath test [UBT]) to discriminate “active” (IgG+ and UBT+) from “non‐active” (UBT− or IgG−) H. pylori infection, and “past” (IgG+ but UBT−) from “never/remote” (IgG−) infection. The differences in hemoglobin A1c (A1C) and antidiabetic regimens between groups were compared. In the “active” infection group, the differences in A1C changes between participants with and without 10‐day eradication therapy were compared after 3 months.
Results
Despite no between‐group difference in A1C, the “active” infection group (n = 208) had significantly more prescriptions of oral antidiabetic drug classes (2.1 ± 1.1 vs 1.8 ± 1.1, P = 0.004) and higher percentages of sulfonylurea use (67.3% vs 50.4%, P < 0.001) than the “non‐active” infection group (n = 341). There were no differences in A1C and oral antidiabetic drug classes between “past” (n = 111) and “never/remote” infection groups (n = 230). Compared with the non‐eradication group (n = 99), the eradication group (n = 98) had significant within‐group (−0.17 ± 0.80%, P = 0.036) and between‐group (−0.23 ± 0.10%, P = 0.024) improvements in A1C.
Conclusions
Diabetes patients with active H. pylori infection need higher glycemic treatment intensity to achieve comparable glycemia. Furthermore, H. pylori eradication decreases A1C, and thus improves glycemic control.
Keywords: Diabetes, Glycemic control, Helicobacter eradication
Introduction
Type 2 diabetes mellitus is a growing problem worldwide1. The global number of people with diabetes is projected to rise from 415 million in 2015 to 642 million by 20401. Uncontrolled hyperglycemia causes microvascular and macrovascular complications, which causes adverse effects on the quality of life of patients2 and is an economic burden on healthcare systems3. The pathogenesis of type 2 diabetes is complex and multifaceted, but centered around insulin resistance and impaired pancreatic β‐cell function4. Although some factors associated with insulin resistance are related to genetic mutations, many others are not inherited and probably modifiable5. These modifiable factors include physiological conditions and environmental factors, such as obesity, sedentary lifestyle, chronic inflammation and infections6, 7, 8, 9, and are potential targets to improve glycemic control in type 2 diabetes.
Helicobacter pylori infection is one of the most common chronic infections, and affects approximately 50% of the world's population10. H. pylori infection is associated with increased markers of chronic inflammation, such as tumor necrosis factor‐α11 and C‐reactive protein12, 13, and thus a positive association between H. pylori infection and insulin resistance has been observed in many studies on non‐diabetic individuals14. Therefore, it is plausible that chronic H. pylori infection might predispose individuals to hyperglycemia. Consistent with this notion, several studies on non‐diabetic individuals showed positive associations between H. pylori infection and glycemia15, 16, 17, 18 or metabolic syndrome19, with only a few exceptions20, 21. However, in patients with type 2 diabetes, the association between H. pylori infection and hyperglycemia remains inconclusive. Some studies report higher hemoglobin A1c (A1C) levels in the H. pylori‐infected individuals22, 23, but some others do not24, 25, 26, 27, 28, 29. Such an apparent discrepancy between diabetes and non‐diabetes might be due to the methodological differences in diagnosing H. pylori infection, and thus fail to differentiate active from past H. pylori infection30, 31. Furthermore, currently available studies lack consideration of the effects of background antidiabetic medications, which might mitigate the consequences of H. pylori infection with regard to glycemia. Therefore, to investigate the glycemic impact of H. pylori infection on diabetes, the present study used a two‐step diagnostic approach with the aim of investigating the effects of active H. pylori infection and background antidiabetic therapy on glycemic control in a cross‐sectional diabetes cohort. Furthermore, the changes in A1C level after eradication of active H. pylori infection were examined in an interventional subcohort.
Methods
Participants
This study was approved by the institutional review board of National Cheng Kung University Hospital (NCKUH B‐ER‐102‐081), and all eligible participants signed informed consent forms before participation. All patients with type 2 diabetes aged 20–80 years visiting the endocrinology outpatient clinic of NCKUH from June 2013 to January 2014 were screened. The diagnosis of type 2 diabetes was based on the 2010 American Diabetes Association criteria32. Individuals with the following conditions or diseases were excluded: (i) type 1 diabetes mellitus; (ii) having a previous history of H. pylori eradication or major gastrointestinal surgery, or any symptoms suggestive of active peptic ulcer disease; (iii) acute ischemic heart event, cerebrovascular accident or pancreatitis; (iv) acute infection, such as pneumonia, urinary tract infection, soft tissue infection or cellulitis, or sepsis; (v) current use of drugs that affect the carbohydrate metabolism, such as corticosteroids, thiazides, sympathomimetic agents and atypical antipsychotic drugs; (vi) receiving proton pump inhibitor treatment; (vii) pregnancy; and (viii) any other major diseases, including generalized inflammation or advanced malignant diseases contraindicating this study.
Cross‐Sectional Study Design
A two‐step diagnostic approach was used to diagnose active H. pylori infection. First, all patients recruited were screened for H. pylori infection by the serology test for H. pylori immunoglobulin (IgG) antibody (HEL‐p TEST™ II; AMRAD Biotech, Perth, WA, Australia; with sensitivity and specificity as 96.9% and 90.4%, respectively33). A serum level of H. pylori IgG antibody ≥8 (U/mL) was defined as a positive result and <8 as a negative result. Next, those who had positive serology results had their current infection status further confirmed using the 13C‐urea breath test (UBT) applied in our previous study34. A UBT value of >3.5‰ was defined as active H. pylori infection (UBT+), and ≤3.5‰ as past H. pylori infection (UBT−). The schematic flow chart of the present study's design is shown in Figure 1.
Figure 1.
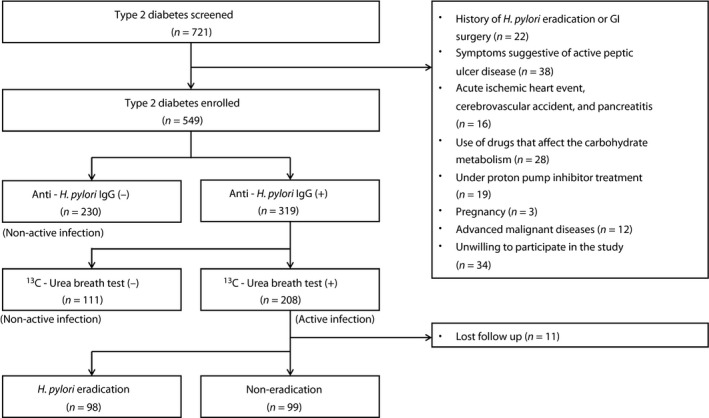
Study flow chart. GI, gastrointestinal; IgG, immunoglobulin G.
After an overnight 12‐h fast, all participants received a blood test including fasting plasma glucose, A1C, renal function (creatinine), liver enzyme (alanine aminotransferase) and lipid profiles (including total cholesterol, high‐density lipoprotein cholesterol, low‐density lipoprotein cholesterol and triglyceride).
Wearing light indoor clothes, each participant's anthropometric data, including body height (to the nearest 0.1 cm) and weight (to the nearest 0.1 kg) were measured. Body mass index (in kg/m2) was calculated as weight (in kg) divided by height (in m) squared. For the blood pressure measurement, participants were resting in a supine position in a quiet ambience, and measurements were then obtained. Hypertension is defined as a systolic blood pressure ≥140 mmHg, a diastolic blood pressure ≥90 mmHg or being treated with antihypertensive agents. In addition, a comprehensive medication history of antihypertensive drugs, antidiabetic regimens and lipid‐lowering agents were reviewed and recorded by an investigator who was blind to the patients’ past history or biochemical results to reduce potential bias. For antidiabetic regimens, the use of insulin and different classes of oral antidiabetic agents, including metformin, sulfonylurea, glinide, thiazolidinedione, α‐glucosidase inhibitor and dipeptidyl peptidase‐4 inhibitor, were recorded.
Interventional Study Design
All patients with UBT+ were invited to receive a 10‐day treatment for H. pylori eradication. Before eradication therapy, we carried out an esophagogastroduodenoscopy examination to rule out the possibility of gastric cancer, because H. pylori infection increases the risk of developing gastric cancer35. The final decision of receiving examination and treatment (“H. pylori eradication” group) or not (“non‐eradication” group) was up to the patients themselves after a comprehensive explanation of the procedures. Patients in the eradication group were prospectively randomized into two therapeutic groups, namely, a clarithromycin‐based sequential group and a levofloxacin‐based concomitant group. The former group received a 10‐day regimen, including pantoprazole 40 mg and amoxicillin 1000 mg twice daily for the first 5 days, followed by pantoprazole 40 mg, clarithromycin 500 mg and metronidazole 500 mg twice daily for another 5 days. In the latter group, the 10‐day regimen included pantoprazole 40 mg, and amoxicillin 1000 mg, metronidazole 500 mg twice daily, as well as levofloxacin 500 mg once daily36. All patients receiving 10‐day treatment for H. pylori eradication repeated UBT 6–8 weeks after therapy. A change from UBT+ to UBT− after therapy was defined as successful H. pylori eradication. Furthermore, A1C levels of all participants with UBT+ were followed up 3 months later without change of the antidiabetic regimens during this 3‐month period.
Statistical Analysis
Data were analyzed with the Windows version of the Statistical Package for the Social Sciences (SPSS version 21.0; SPSS, Chicago, IL, USA). Continuous variables were expressed as the means ± standard deviation and categorical variables as percentages. Participants with a negative H. pylori IgG antibody were defined as “never/remote infection,” and those with a positive H. pylori IgG antibody, but negative UBT, were designated as “past infection.” Furthermore, “never/remote infection” and “past infection” were collectively classified as “non‐active infection,” and “active infection” indicated individuals with their H. pylori IgG antibody and UBT both positive. The differences of continuous variables between “non‐active infection” and “active infection” groups, “never/remote infection” and “past infection” groups, as well as between “H. pylori eradication” and “non‐eradication” groups, were compared using Student's t‐tests. The χ2‐tests were used to analyze the differences of categorical variables between groups. All statistical tests were two‐sided, and a P‐value <0.05 was considered statistically significant.
Results
A total of 549 type 2 diabetes patients, including 269 women and 280 men, were enrolled in the present study. The prevalence rates of H. pylori infection defined by anti‐H. pylori IgG and UBT were 58.1% and 37.9%, respectively.
Cross‐Sectional Comparisons Between “Active” and “Non‐Active” Infection Groups
The clinical variables for “active” infection participants (n = 208) and “non‐active” infection participants (n = 341) were compared, as shown in Table 1. There were no differences in A1C (7.68 ± 1.38 vs 7.65 ± 1.49%, P = 0.829) and fasting plasma glucose (7.8 ± 2.5 vs 7.8 ± 2.6 mmol/L, P = 0.935) between the “active” and “non‐active” infection groups.
Table 1.
Comparisons of baseline characteristics between participants with “active” and “non‐active” Helicobacter pylori infection
| Non‐active infection | Active infection | P‐value | |
|---|---|---|---|
| n | 341 | 208 | |
| Age (years) | 60.2 ± 11.7 | 61.9 ± 9.6 | 0.054 |
| Male (%) | 50.4 | 51.9 | 0.792 |
| BMI (kg/m2) | 26.5 ± 4.7 | 27.1 ± 4.7 | 0.128 |
| Diabetes duration (years) | 11.4 ± 8.0 | 11.2 ± 7.4 | 0.754 |
| Hypertension (%) | 62.1 | 61.4 | 0.928 |
| Statins (%) | 70.4 | 70.7 | 1.000 |
| eGFR (mL/min/1.73 m2) | 70.8 ± 21.7 | 70.0 ± 20.9 | 0.642 |
| ALT (U/L) | 33.2 ± 39.3 | 31.6 ± 31.4 | 0.562 |
| Total cholesterol (mmol/L) | 4.0 ± 0.9 | 4.1 ± 0.9 | 0.197 |
| HDL‐C (mmol/L) | 1.4 ± 0.4 | 1.3 ± 0.4 | 0.144 |
| LDL‐C (mmol/L) | 2.4 ± 0.8 | 2.5 ± 0.8 | 0.120 |
| Triglyceride (mmol/L) | 1.5 ± 1.1 | 1.6 ± 0.9 | 0.483 |
| Fasting plasma glucose (mmol/L) | 7.8 ± 2.6 | 7.8 ± 2.5 | 0.935 |
| A1C (%) | 7.65 ± 1.49 | 7.68 ± 1.38 | 0.829 |
| A1C (mmol/mol) | 60.1 ± 16.3 | 60.4 ± 15.1 | 0.826 |
| Antidiabetic medications | |||
| Insuin (%) | 35.5 | 33.2 | 0.644 |
| Insulin dose/day (Unit) | 38.7 ± 24.4 | 37.0 ± 28.1 | 0.659 |
| Insulin dose/kg/day (Unit) | 0.56 ± 0.32 | 0.52 ± 0.39 | 0.497 |
| OAD classes | 1.8 ± 1.1 | 2.1 ± 1.1 | 0.004 |
| No. participants (percentage) | |||
| 0 class of OAD | 32 (9.4) | 18 (8.7) | * |
| 1 class of OAD | 108 (31.7) | 42 (20.2) | * |
| 2 classes of OAD | 107 (31.4) | 67 (32.2) | * |
| 3 classes of OAD | 75 (22.0) | 65 (31.3) | * |
| 4 classes of OAD | 19 (5.6) | 15 (7.2) | * |
| 5 classes of OAD | 0 (0) | 1 (0.5) | * |
| OAD (%) | 90.6 | 91.3 | 0.879 |
| Metformin (%) | 79.4 | 83.7 | 0.262 |
| Sulfonylureas (%) | 50.4 | 67.3 | <0.001 |
| Glinides (%) | 1.8 | 0.5 | 0.259 |
| Thiazolidinediones (%) | 18.5 | 24.0 | 0.129 |
| AGIs (%) | 10.3 | 11.1 | 0.776 |
| DPP‐4is (%) | 22.6 | 23.1 | 0.917 |
Data are expressed as the mean ± standard deviation or percentage. * P‐value by 2 × 6 χ2‐test <0.05. Active infection: participants with both positive Helicobacter pylori immunoglobulin G antibody and UBT. Non‐active infection: participants with a negative H. pylori immunoglobulin G antibody or “a positive H. pylori immunoglobulin G antibody, but negative urea breath test.” A1C, hemoglobin A1c; AGIs, alpha‐glucosidase inhibitors; ALT, alanine transaminase; BMI, body mass index; DPP‐4is, dipeptidyl peptidase‐4 inhibitors; eGFR, estimated glomerular filtration rate; HDL‐C, high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol; OAD, oral antidiabetic drug.
The percentages of insulin users between “active” infection and “non‐active” infection participants were similar (33.2 vs 35.5%, P = 0.644). Among the insulin users, there were no differences between “active” and “non‐active” infection groups in daily insulin dose (37.0 ± 28.1 vs 38.7 ± 24.4 units, P = 0.659) or insulin dose per kilogram per day (0.52 ± 0.39 vs 0.56 ± 0.32 units, P = 0.497). However, the “active” infection participants were treated with more classes of oral antidiabetic drugs (OADs) than the “non‐active” infection participants (2.1 ± 1.1 vs 1.8 ± 1.1, P = 0.004). A higher percentage of patients in the “active” infection group took two or more, and three or more classes of OAD than in the “non‐active” infection group (71.2 vs 58.9%, P = 0.005; 38.9 vs 27.6%, P = 0.006, respectively). Specifically, there was a significantly higher percentage of sulfonylureas use in the “active” infection group than “non‐active” infection group (67.3 vs 50.4%, P < 0.001). The use of other classes of OAD showed similar percentages between groups.
Cross‐Sectional Comparisons Between “Never/Remote Infection” and “Past Infection” Groups
There were no differences between “never/remote infection” patients (n = 230) and “past infection” patients (n = 111), in A1C level (7.64 ± 1.49 vs 7.66 ± 1.50%, P = 0.890), percentage of insulin users (36.5 vs 33.3%, P = 0.629), classes of OAD use (1.8 ± 1.0 vs 1.9 ± 1.1, P = 0.430) and percentage of sulfonylureas use (48.7 vs 54.1%, P = 0.358; Table 2).
Table 2.
Comparisons of baseline characteristics between “never/remote infection” and “past infection” groups
| Never/remote infection | Past infection | P‐value | |
|---|---|---|---|
| n | 230 | 111 | |
| Age (years) | 59.0 ± 12.2 | 62.5 ± 10.1 | 0.005 |
| Male (%) | 52.2 | 46.8 | 0.419 |
| BMI (kg/m2) | 26.4 ± 4.6 | 26.5 ± 4.9 | 0.904 |
| Diabetes duration (years) | 11.0 ± 7.8 | 12.2 ± 8.3 | 0.217 |
| Hypertension (%) | 61.6 | 63.1 | 0.813 |
| Statins (%) | 68.3 | 74.8 | 0.255 |
| eGFR (mL/min/1.73 m2) | 71.7 ± 22.0 | 69.1 ± 21.1 | 0.296 |
| ALT (U/L) | 31.7 ± 26.1 | 38.5 ± 58.8 | 0.137 |
| Total cholesterol (mmol/L) | 4.1 ± 0.9 | 3.9 ± 0.8 | 0.210 |
| HDL‐C (mmol/L) | 1.4 ± 0.4 | 1.4 ± 0.3 | 0.989 |
| LDL‐C (mmol/L) | 2.4 ± 0.8 | 2.4 ± 0.8 | 0.741 |
| Triglyceride (mmol/L) | 1.6 ± 1.2 | 1.4 ± 0.8 | 0.094 |
| Fasting plasma glucose (mmol/L) | 7.8 ± 2.7 | 8.0 ± 2.4 | 0.475 |
| A1C (%) | 7.64 ± 1.49 | 7.66 ± 1.50 | 0.890 |
| A1C (mmol/mol) | 60.0 ± 16.4 | 60.2 ± 16.3 | 0.905 |
| Antidiabetic medications | |||
| Insulin (%) | 36.5 | 33.3 | 0.629 |
| Insulin dose/day (Unit) | 39.9 ± 25.2 | 36.0 ± 22.5 | 0.411 |
| Insulin dose/kg/day (Unit) | 0.58 ± 0.34 | 0.51 ± 0.27 | 0.265 |
| OAD classes | 1.8 ± 1.0 | 1.9 ± 1.1 | 0.430 |
| No. participants (percentage) | |||
| 0 class of OAD | 20 (8.7) | 12 (10.8) | * |
| 1 class of OAD | 78 (33.9) | 30 (27.0) | * |
| 2 classes of OAD | 75 (32.6) | 32 (28.8) | * |
| 3 classes of OAD | 43 (18.7) | 32 (28.8) | * |
| 4 classes of OAD | 14 (6.1) | 5 (4.5) | * |
| 5 classes of OAD | 0 (0) | 0 (0) | * |
| OAD (%) | 91.3 | 89.2 | 0.555 |
| Metformin (%) | 81.7 | 74.8 | 0.154 |
| Sulfonylureas (%) | 48.7 | 54.1 | 0.358 |
| Glinides (%) | 1.3 | 2.7 | 0.403 |
| Thiazolidinediones (%) | 17.8 | 20.0 | 0.656 |
| AGIs (%) | 10.4 | 9.9 | 1.000 |
| DPP‐4is (%) | 20.0 | 27.9 | 0.128 |
Data are expressed as the mean ± standard deviation or percentage. * P‐value by 2 × 6 χ2‐test: not significant. Never/remote infection: participants with a negative Helicobacter pylori immunoglobulin G antibody. Past infection: participants with a positive H. pylori immunoglobulin G antibody, but negative urea breath test. A1C, hemoglobin A1c; AGIs, alpha‐glucosidase inhibitors; ALT, alanine transaminase; BMI, body mass index; DPP‐4is, dipeptidyl peptidase‐4 inhibitors; eGFR, estimated glomerular filtration rate; HDL‐C, high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol; OAD, oral antidiabetic drug.
Interventional Study with H. pylori Eradication
Among the 208 patients of “active” infection group, we prospectively followed 197 patients successfully, including 98 with and 99 without H. pylori eradication. Each enrolled patient, either with or without H. pylori eradication, was followed up with regard to the paired A1C levels, one at enrollment and the other 3 months later. The baseline characteristics between “H. pylori eradication” and “non‐eradication” groups were comparable, including age, sex, body mass index, diabetes duration, percentage of hypertension and statin use, renal function, alanine transaminase, and lipid profiles (Table 3). The fasting plasma glucose at baseline (8.0 ± 2.2 vs 7.3 ± 2.2 mmol/L, P = 0.019) was higher in the “H. pylori eradication” group than the “non‐eradication” group. However, there was no difference in A1C between the “H. pylori eradication” and “non‐eradication” groups (7.50 ± 1.24 vs 7.54 ± 1.40%, P = 0.844).
Table 3.
Comparisons of baseline characteristics between the “Helicobacter pylori eradication” and “non‐eradication” groups
| Eradication | Non‐eradication | P‐value | |
|---|---|---|---|
| n | 98 | 99 | |
| Age (years) | 61.9 ± 9.7 | 62.5 ± 9.6 | 0.649 |
| Male (%) | 54.1 | 49.5 | 0.569 |
| BMI (kg/m2) | 27.0 ± 5.3 | 27.1 ± 4.3 | 0.933 |
| Diabetes duration (years) | 11.0 ± 6.9 | 11.2 ± 7.7 | 0.879 |
| Hypertension (%) | 68.4 | 57.1 | 0.139 |
| Statins (%) | 72.4 | 67.7 | 0.534 |
| eGFR (mL/min/1.73 m2) | 70.7 ± 21.9 | 68.7 ± 20.2 | 0.509 |
| ALT (U/L) | 34.6 ± 43.1 | 28.0 ± 13.8 | 0.147 |
| Total cholesterol (mmol/L) | 4.1 ± 0.9 | 4.1 ± 0.8 | 0.853 |
| HDL‐C (mmol/L) | 1.3 ± 0.4 | 1.3 ± 0.4 | 0.967 |
| LDL‐C (mmol/L) | 2.5 ± 0.8 | 2.5 ± 0.8 | 0.602 |
| Triglyceride (mmol/L) | 1.6 ± 0.8 | 1.7 ± 1.1 | 0.404 |
| Fasting plasma glucose (mmol/L) | 8.0 ± 2.2 | 7.3 ± 2.2 | 0.019 |
| A1C (%) | 7.50 ± 1.24 | 7.54 ± 1.40 | 0.844 |
| A1C (mmol/mol) | 58.5 ± 13.6 | 58.9 ± 15.4 | 0.817 |
| Antidiabetic medications | |||
| Insulin (%) | 33.7 | 31.3 | 0.763 |
| Insulin dose/day (Unit) | 37.0 ± 27.5 | 36.7 ± 29.3 | 0.974 |
| Insulin dose/kg/day (Unit) | 0.51 ± 0.42 | 0.54 ± 0.37 | 0.780 |
| OAD classes | 2.0 ± 1.2 | 2.2 ± 1.0 | 0.327 |
| No. participants (percentage) | |||
| 0 class of OAD | 12 (12.2) | 4 (4.0) | * |
| 1 class of OAD | 21 (21.4) | 20 (20.2) | * |
| 2 classes of OAD | 26 (26.5) | 38 (38.4) | * |
| 3 classes of OAD | 32 (32.7) | 29 (29.3) | * |
| 4 classes of OAD | 6 (6.1) | 8 (8.1) | * |
| 5 classes of OAD | 1 (1.0) | 0 (0) | * |
| OAD (%) | 87.8 | 96.0 | 0.040 |
| Metformin (%) | 79.6 | 87.9 | 0.126 |
| Sulfonylureas (%) | 63.3 | 71.7 | 0.226 |
| Glinides (%) | 1.0 | 0 | 0.497 |
| Thiazolidinediones (%) | 24.5 | 24.2 | 1.000 |
| AGIs (%) | 10.2 | 11.1 | 1.000 |
| DPP4is (%) | 24.5 | 22.2 | 0.739 |
Data are expressed as the mean ± standard deviation or percentage. * P‐value by 2 × 6 χ2‐test: not significant. A1C, hemoglobin A1c; AGIs, alpha‐glucosidase inhibitors; ALT, alanine transaminase; BMI, body mass index; DPP‐4is, dipeptidyl peptidase‐4 inhibitors; eGFR, estimated glomerular filtration rate; HDL‐C, high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol; OAD, oral anti‐diabetes drug.
As for antidiabetic regimens, at baseline, there was a lower percentage (87.8 vs 96.0%, P = 0.04) of patients receiving one or more class of OAD in the “H. pylori eradication” group than in the “non‐eradication” group. Otherwise, no differences in antidiabetic regimens were noted between groups, including the percentage of insulin users, insulin dose, the number of OAD classes used and the percentages of use for each class of OAD (Table 3).
After 3 months of follow up, without changing antidiabetic regimens during this period, the A1C level decreased in the “H. pylori eradication” group (from 7.50 ± 1.24 to 7.33 ± 1.14%, P = 0.036), but remained unchanged in the “non‐eradication” group (from 7.54 ± 1.40 to 7.60 ± 1.38%, P = 0.345). The between‐group difference of changes in A1C level was statistically significant (−0.23 ± 0.10%, P = 0.024; Figure 2). It is noted that the change in A1C level is independently associated with H. pylori eradication therapy after adjustment for age, sex, body mass index, diabetic duration, blood biochemistry and statin use (P = 0.019). Furthermore, we did not adjust the lipid‐lowering agents and antihypertensive drugs during this 3‐month period. The bodyweight, lipid profile and blood pressure data of all participants with UBT+ were collected and compared. There were no differences in bodyweight, lipid profiles and blood pressure at baseline and after 3 months in either the “H. pylori eradication” or “non‐eradication” group. The between‐group differences of changes in bodyweight, lipid profiles and blood pressure during 3 months were statistically insignificant (Table S1).
Figure 2.
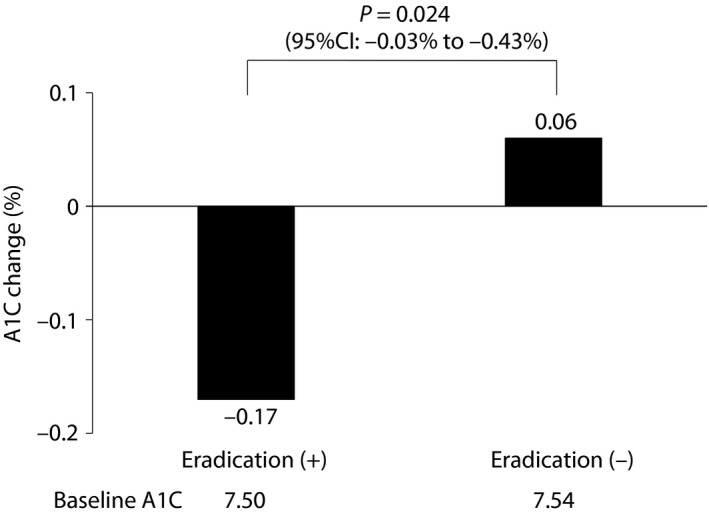
The comparisons of hemoglobin A1c (A1C) changes from baseline to 3 months after between the “Helicobacter pylori eradication” and “non‐eradication” groups. CI, confidence interval.
In the “H. pylori eradication” group, 92 patients had successful H. pylori eradication, while six patients still had positive UBT result 3 months later. The eradication rate was thus 93.9%, which was comparable with that in previous studies37, 38, 39.
Discussion
To the best of our knowledge, this is the first study to comprehensively evaluate the glycemic effect of H. pylori infection in real‐world patients with diabetes. We found that diabetes patients with asymptomatic active H. pylori infection had a comparable level of glycemia to those without active infection, but at the expense of a higher intensity of antidiabetic therapy, particularly sulfonylureas. In addition, eradication of active H. pylori infection resulted in a significant improvement in glycemic control in diabetes patients.
In assessing the relationship between H. pylori infection and glycemia, the method used for diagnosing H. pylori infection status is critical. Current diagnostic methods of H. pylori infection can be divided into two types: invasive tests (histology examination, rapid urease test, and culture) and non‐invasive tests (UBT, stool antigen test and serum or urine anti‐H. pylori IgG)31. With the aid of gastrointestinal endoscopy, a positive result from the invasive tests confirms the presence of active H. pylori infection. As for non‐invasive tests, while positive UBT and stool antigen tests also indicate an active infection status, serum or urine anti‐H. pylori IgG are just markers of exposure to H. pylori. Therefore, an IgG test alone cannot exactly indicate whether active infection is present. It is thus not surprising that when using serum20 or urine21 IgG assay as the diagnostic method, no relationship between H. pylori infection and glycemia exists in studies of non‐diabetic individuals20, 21. In contrast, a positive relationship between H. pylori infection and glycemia15, 16, 17, 18 or metabolic syndrome19 is noted in mainly15, 16, 17, 18 or exclusively19 non‐diabetic individuals, when applying diagnostic tests that verify active H. pylori infection. Thus, it is evident that active or inactive infection affects the relationship between H. pylori infection and glycemia. Accordingly, tests with high diagnostic accuracy should be used to define the active infection of H. pylori, and thus its exact impact on glycemia control can be more clearly assessed. The IgG kit used in the present study has been confirmed to have high diagnostic accuracy (96.9% sensitivity and 90.4% specificity; positive/negative predictive value: 94.9%/94.0%) in a Taiwanese population33. Therefore, we believe the impact of false positive or false negative IgG results on the findings of the present study is quite small. Meanwhile, although UBT+ might result from the existence of other urease‐producing bacteria in the oral cavity or in the stomach, the clinical relevance is also very slight40. Thus, the present study design confidently ensured the active H. pylori infection status using a two‐step diagnostic approach. After large‐scale screening by the serum anti‐H. pylori IgG, all the patients with positive results had their active infection status further confirmed by UBT.
Despite the clear positive relationship between active H. pylori infection and glycemia in non‐diabetic individuals, the effect of H. pylori infection on the A1C level in the diabetic patients remains inconclusive22, 23, 24, 25, 26, 27, 28, 29. Most studies using diagnostic methods other than IgG testing report no relationship26, 27, 28, 29, except for some that show higher A1C levels in the infected diabetes patients22, 23. Similarly, we also found no differences in glycemic control (in terms of A1C) between diabetes patients with “active” infection and “non‐active” infection. We postulate that the discrepancy between non‐diabetic and diabetic individuals might be due to the lack of consideration of the effects of background antidiabetic medications. In fact, in a glycemic targeted diabetes care system, the intensity of glycemic therapy significantly influences the adequacy and consequence of glycemic control. However, none of the previous studies of type 2 diabetes patients analyzed the regimen of antidiabetic medications, and then took their effects on A1C into consideration while interpreting the glycemic effects of H. pylori infection. Therefore, we analyzed the background antidiabetic regimens in the current study.
We found that the “active” infection group had a significantly higher intensity of glycemic treatment than the “non‐active” infection group. This suggests that the comparable A1C level in type 2 diabetes patients with active H. pylori infection was actually achieved by a higher intensity of glycemic treatment. Specifically, there was a significantly higher percentage of sulfonylureas use in the “active” infection group than “non‐active” infection group. In both groups, a similar percentage (~80%) of patients were prescribed with metformin as the first‐line therapy. After that, it is highly recommended that the second‐line therapy should be selected based on patient‐specific considerations. In real‐world practice, sulfonylureas are the most commonly considered add‐on therapy for patients whose glycemia cannot be adequately controlled by metformin41, 42. This might be why sulfonylurea was the only class of OAD that had a higher percentage of use in the “active” infection group than in the “non‐active” infection group.
Our finding that active H. pylori infection was associated with a higher intensity of glycemic treatment was further supported by the interventional study. Without changing the antidiabetic regimens, the A1C level decreased in the “H. pylori eradication” group. These data supported the view that H. pylori eradication can provide additional glycemic benefit to current antidiabetic therapy.
In the present study, approximately 70% of the participants were taking statin. Although statin therapy has been found to be associated with new‐onset diabetes in non‐diabetic patients43, whether statin therapy affects A1C levels in diabetes patients remains inconclusive44. Additionally, we further carried out a multiple regression analysis and found that the change in A1C level is independently associated with H. pylori eradication therapy, even after adjustment for statin use (P = 0.019). Therefore, we believe that statin therapy did not affect the findings of the present study.
Interestingly, we also found no differences in classes of OAD and percentages of sulfonylureas use between the “never/remote infection” and “past infection” groups. This again showed that it is only the “active” infection, not “past” infection, that is associated with worse glycemic control in diabetes patients.
The findings of the present study raise an important issue with regard to whether to treat asymptomatic active H. pylori infection in diabetes patients or not. During the past few years, more prescriptions and thus higher expenditures of antidiabetic drugs have been required for diabetes patients45, which places a huge economic burden on healthcare systems3. Given the beneficial effects of improving glycemia, thus reducing microvascular/macrovascular complications and medical costs of antidiabetic agents, as well as the very high response rate of H. pylori eradication36 and its low‐cost treatment, it is promising to advocate the eradication of H. pylori from the viewpoint of medical economics. However, more long‐term large‐scale studies are required to validate the exact costs and benefits.
There were some limitations in this work, as follows. First, the initial part of this work was a cross‐sectional design, which thus does not allow for causal inference between the higher intensity of glycemic treatment and active H. pylori infection. However, the subsequent interventional study to eradicate active H. pylori infection resulted in improved glycemic control, which provides strong support to our speculation. Second, as patients with a negative serum IgG result did not receive further UBT, a false negative result might thereby lead to a misclassification bias, although the possibility seems slight. Third, the design of our interventional study, although prospective, was not randomized. According to medical ethics, all patients with active H. pylori infection were invited to receive an esophagogastroduodenoscopy examination followed by medical treatment for H. pylori eradication. Ultimately, 98 patients decided to receive esophagogastroduodenoscopy examination followed by H. pylori eradication (“H. pylori eradication” group), whereas 99 patients refused (“non‐eradication” group). Despite non‐randomization, the baseline characteristics between “H. pylori eradication” and “non‐eradication” groups were still comparable, which adds strength to the results.
Taken together, we found no difference in glycemic level between type 2 diabetes patients with and without active H. pylori infection. However, the comparable glycemia in patients with active H. pylori infection was actually achieved at the cost of a higher intensity of glycemic treatment. Meanwhile, eradication of active H. pylori infection in these patients resulted in improvement of glycemic control. Further validation of this H. pylori test‐and‐treat strategy will be promising to improve the glycemic control in asymptomatic active H. pylori‐infected diabetes patients.
Disclosure
The authors declare no conflict of interest.
Supporting information
Table S1 ¦ Between‐group differences of changes in bodyweight, lipid levels and blood pressure over 3 months.
Acknowledgments
The work was supported by grants from the Landmark Project to Promote the Innovation & Competitiveness of Clinical Trials by the Excellent Clinical Trial and Research Center in National Cheng Kung University Hospital, Ministry of Health and Welfare, Taiwan (MOHW105‐TDU‐B‐211‐133016). We thank Ms Ching‐Chun Chuang for her invaluable assistance throughout this research and acknowledge the unrestricted grant from the Chen Jie Chen Scholarship Foundation to Horng‐Yih Ou MD, PhD. Additionally, we are grateful to Dr Sheng‐Hsiang Lin for providing the statistical consulting services from the Biostatistics Consulting Center, Clinical Medicine Research Center, National Cheng Kung University Hospital.
J Diabetes Investig 2019; 10: 1092–1101
References
- 1. Ogurtsova K, da Rocha Fernandes JD, Huang Y, et al IDF Diabetes Atlas: global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res Clin Pract 2017; 128: 40–50. [DOI] [PubMed] [Google Scholar]
- 2. Rubin RR, Peyrot M. Quality of life and diabetes. Diabetes Metab Res Rev 1999; 15: 205–218. [DOI] [PubMed] [Google Scholar]
- 3. Liebl A, Khunti K, Orozco‐Beltran D, et al Health economic evaluation of type 2 diabetes mellitus: a clinical practice focused review. Clin Med Insights Endocrinol Diabetes 2015; 8: 13–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mahler RJ, Adler ML. Clinical review 102: Type 2 diabetes mellitus: update on diagnosis, pathophysiology, and treatment. J Clin Endocrinol Metab 1999; 84: 1165–1171. [DOI] [PubMed] [Google Scholar]
- 5. Tritos NA, Mantzoros CS. Clinical review 97: syndromes of severe insulin resistance. J Clin Endocrinol Metab 1998; 83: 3025–3030. [DOI] [PubMed] [Google Scholar]
- 6. Wilcox G. Insulin and insulin resistance. Clin Biochem Rev 2005; 26: 19–39. [PMC free article] [PubMed] [Google Scholar]
- 7. Kahn BB, Flier JS. Obesity and insulin resistance. J Clin Invest 2000; 106: 473–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest 2006; 116: 1793–1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Milner KL, van der Poorten D, Trenell M, et al Chronic hepatitis C is associated with peripheral rather than hepatic insulin resistance. Gastroenterology 2010; 138: 932–941 e931–933. [DOI] [PubMed] [Google Scholar]
- 10. Brown LM. Helicobacter pylori: epidemiology and routes of transmission. Epidemiol Rev 2000; 22: 283–297. [DOI] [PubMed] [Google Scholar]
- 11. Perri F, Clemente R, Festa V, et al Serum tumour necrosis factor‐alpha is increased in patients with Helicobacter pylori infection and CagA antibodies. Ital J Gastroenterol Hepatol 1999; 31: 290–294. [PubMed] [Google Scholar]
- 12. Oshima T, Ozono R, Yano Y, et al Association of Helicobacter pylori infection with systemic inflammation and endothelial dysfunction in healthy male subjects. J Am Coll Cardiol 2005; 45: 1219–1222. [DOI] [PubMed] [Google Scholar]
- 13. Jackson L, Britton J, Lewis SA, et al A population‐based epidemiologic study of Helicobacter pylori infection and its association with systemic inflammation. Helicobacter 2009; 14: 108–113. [DOI] [PubMed] [Google Scholar]
- 14. Polyzos SA, Kountouras J, Zavos C, et al The association between Helicobacter pylori infection and insulin resistance: a systematic review. Helicobacter 2011; 16: 79–88. [DOI] [PubMed] [Google Scholar]
- 15. Yang W, Xuan C. Influence of Helicobacter pylori infection on metabolic syndrome in Old Chinese People. Gastroenterol Res Pract 2016; 2016: 6951264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chen Y, Blaser MJ. Association between gastric Helicobacter pylori colonization and glycated hemoglobin levels. J Infect Dis 2012; 205: 1195–1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hsieh MC, Wang SS, Hsieh YT, et al Helicobacter pylori infection associated with high HbA1c and type 2 diabetes. Eur J Clin Invest 2013; 43: 949–956. [DOI] [PubMed] [Google Scholar]
- 18. Han X, Li Y, Wang J, et al Helicobacter pylori infection is associated with type 2 diabetes among a middle‐ and old‐age Chinese population. Diabetes Metab Res Rev 2016; 32: 95–101. [DOI] [PubMed] [Google Scholar]
- 19. Shin DW, Kwon HT, Kang JM, et al Association between metabolic syndrome and Helicobacter pylori infection diagnosed by histologic status and serological status. J Clin Gastroenterol 2012; 46: 840–845. [DOI] [PubMed] [Google Scholar]
- 20. Malamug LR, Karnchanasorn R, Samoa R, et al The role of Helicobacter pylori seropositivity in insulin sensitivity, beta cell function, and abnormal glucose tolerance. Scientifica 2014; 2014: 870165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tamura T, Morita E, Kawai S, et al No association between Helicobacter pylori infection and diabetes mellitus among a general Japanese population: a cross‐sectional study. Springerplus 2015; 4: 602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bajaj S, Rekwal L, Misra SP, et al Association of Helicobacter pylori infection with type 2 diabetes. Indian J Endocrinol Metab 2014; 18: 694–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fernandini‐Paredes GG, Mezones‐Holguin E, Vargas‐Gonzales R, et al In patients with type 2 diabetes mellitus, are glycosylated hemoglobin levels higher for those with Helicobacter pylori infection than those without infection? Clin Infect Dis 2008; 47: 144–146. [DOI] [PubMed] [Google Scholar]
- 24. Vafaeimanesh J, Bagherzadeh M, Mirzaei A, et al Effect of Helicobacter pylori on metabolic syndrome parameters in diabetic patients. Gastroenterol Hepatol Bed Bench 2016; 9: S36–S41. [PMC free article] [PubMed] [Google Scholar]
- 25. Senmaru T, Fukui M, Kuroda M, et al Serum pepsinogen I/II ratio is correlated with albuminuria in patients with type 2 diabetes. Endocr J 2013; 60: 161–166. [DOI] [PubMed] [Google Scholar]
- 26. Tanriverdi O. Association of Helicobacter pylori infection with microalbuminuria in type 2 diabetic patients. Turk J Gastroenterol 2011; 22: 569–574. [DOI] [PubMed] [Google Scholar]
- 27. Demir M, Gokturk HS, Ozturk NA, et al Helicobacter pylori prevalence in diabetes mellitus patients with dyspeptic symptoms and its relationship to glycemic control and late complications. Dig Dis Sci 2008; 53: 2646–2649. [DOI] [PubMed] [Google Scholar]
- 28. Gulcelik NE, Kaya E, Demirbas B, et al Helicobacter pylori prevalence in diabetic patients and its relationship with dyspepsia and autonomic neuropathy. J Endocrinol Invest 2005; 28: 214–217. [DOI] [PubMed] [Google Scholar]
- 29. Ko GT, Chan FK, Chan WB, et al Helicobacter pylori infection in Chinese subjects with type 2 diabetes. Endocr Res 2001; 27: 171–177. [DOI] [PubMed] [Google Scholar]
- 30. Testerman TL, Morris J. Beyond the stomach: an updated view of Helicobacter pylori pathogenesis, diagnosis, and treatment. World J Gastroenterol 2014; 20: 12781–12808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ricci C, Holton J, Vaira D. Diagnosis of Helicobacter pylori: invasive and non‐invasive tests. Best Pract Res Clin Gastroenterol 2007; 21: 299–313. [DOI] [PubMed] [Google Scholar]
- 32. American Diabetes Association . Standards of medical care in diabetes–2010. Diabetes Care 2010;33 (Suppl 1): S11–S61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hung HH, Chen TS, Lin HC. Immunoglobulin G antibody against Helicobacter pylori is an accurate test for atrophic gastritis. J Chin Med Assoc 2010; 73: 355–359. [DOI] [PubMed] [Google Scholar]
- 34. Yang YJ, Sheu BS. Probiotics‐containing yogurts suppress Helicobacter pylori load and modify immune response and intestinal microbiota in the Helicobacter pylori‐infected children. Helicobacter 2012; 17: 297–304. [DOI] [PubMed] [Google Scholar]
- 35. Sokic‐Milutinovic A, Alempijevic T, Milosavljevic T. Role of Helicobacter pylori infection in gastric carcinogenesis: current knowledge and future directions. World J Gastroenterol 2015; 21: 11654–11672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yang YJ, Wu CT, Ou HY, et al Ten days of levofloxacin‐containing concomitant therapy can achieve effective Helicobacter pylori eradication in patients with type 2 diabetes. Ann Med 2017; 49: 479–486. [DOI] [PubMed] [Google Scholar]
- 37. Chen KY, Lin TJ, Lin CL, et al Hybrid vs sequential therapy for eradication of Helicobacter pylori in Taiwan: a prospective randomized trial. World J Gastroenterol 2015; 21: 10435–10442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Liou JM, Chen CC, Chang CY, et al Sequential therapy for 10 days versus triple therapy for 14 days in the eradication of Helicobacter pylori in the community and hospital populations: a randomised trial. Gut 2016; 65: 1784–1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Liou JM, Chen CC, Chen MJ, et al Sequential versus triple therapy for the first‐line treatment of Helicobacter pylori: a multicentre, open‐label, randomised trial. Lancet 2013; 381: 205–213. [DOI] [PubMed] [Google Scholar]
- 40. Gisbert JP, Pajares JM. Review article: 13C‐urea breath test in the diagnosis of Helicobacter pylori infection – a critical review. Aliment Pharmacol Ther 2004; 20: 1001–1017. [DOI] [PubMed] [Google Scholar]
- 41. Chang CH, Jiang YD, Chung CH, et al National trends in anti‐diabetic treatment in Taiwan, 2000‐2009. J Formos Med Assoc 2012; 111: 617–624. [DOI] [PubMed] [Google Scholar]
- 42. Sharma M, Nazareth I, Petersen I. Trends in incidence, prevalence and prescribing in type 2 diabetes mellitus between 2000 and 2013 in primary care: a retrospective cohort study. BMJ Open 2016; 6: e010210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sattar N, Preiss D, Murray HM, et al Statins and risk of incident diabetes: a collaborative meta‐analysis of randomised statin trials. Lancet 2010; 375: 735–742. [DOI] [PubMed] [Google Scholar]
- 44. Jacobson TA. NLA Task Force on Statin Safety–2014 update. J Clin Lipidol 2014; 8: S1–S4. [DOI] [PubMed] [Google Scholar]
- 45. Turner LW, Nartey D, Stafford RS, et al Ambulatory treatment of type 2 diabetes in the U.S., 1997‐2012. Diabetes Care 2014; 37: 985–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 ¦ Between‐group differences of changes in bodyweight, lipid levels and blood pressure over 3 months.


