Abstract
Aims/Introduction
Insulin‐like growth factor‐1 (IGF‐1) regulates mitochondrial function, oxidative stress, inflammation, stellate cells and insulin sensitivity in the liver, and it might be associated with liver fibrosis from non‐alcoholic steatohepatitis. In contrast, type 2 diabetes mellitus is closely associated with the progression from non‐alcoholic fatty liver to non‐alcoholic steatohepatitis and cirrhosis, so careful evaluation of liver fibrosis is required for patients with type 2 diabetes mellitus. Therefore, we examined the relationship between IGF‐1 and liver fibrosis markers in type 2 diabetes patients without obvious alcoholic consumption and determined whether IGF‐1 is associated with fibrosis of non‐alcoholic fatty liver disease.
Materials and Methods
We selected 415 patients with type 2 diabetes without obvious alcohol consumption, who were admitted to Uwajima City Hospital between May 2013 and December 2016. We collected and analyzed clinical data to determine correlations between IGF‐1 or IGF‐1 standard deviation score and fibrosis‐4 index or 7S domain of type IV collagen.
Results
Multiple linear regression analysis showed that the fibrosis‐4 index was inversely correlated with IGF‐1 and IGF‐1 standard deviation score. Furthermore, the 7S domain of type IV collagen was also inversely correlated with IGF‐1 and IGF‐1 standard deviation score.
Conclusions
IGF‐1 was inversely correlated with liver fibrosis markers in type 2 diabetes mellitus patients without obvious alcoholic consumption. Measuring serum IGF‐1 levels might help clinicians to identify type 2 diabetes mellitus patients with advanced non‐alcoholic steatohepatitis.
Keywords: Insulin‐like growth factor‐1, Non‐alcoholic fatty liver disease, Type 2 diabetes mellitus
Introduction
Liver fibrosis is one of the most important factors of liver disease progression1, 2, and advanced liver fibrosis is associated with hepatocellular carcinoma and liver failure. In non‐alcoholic fatty liver disease (NAFLD), similarly, advanced fibrosis is reported to be a predictor of poor prognosis of liver‐related diseases and cardiovascular diseases3, 4, 5, 6, it is therefore desirable to identify high‐risk groups for advanced liver diseases among patients, easily.
Type 2 diabetes mellitus is frequently complicated with NAFLD. The prevalence of NAFLD in Japanese patients depends on blood glucose level; 27% in the subgroup with normal fasting glucose, 43% in impaired glucose and 62% in newly diagnosed diabetes7. Additionally, the presence of diabetes in NAFLD patients is a significant predictor of moderate‐to‐severe fibrosis8, 9, and paired biopsies showed that the prevalence of diabetes in NAFLD patients with fibrosis progression was higher than in those with non‐progressed NAFLD10. Therefore, type 2 diabetes mellitus not only often complicates NAFLD, but is also closely associated with the progression from non‐alcoholic fatty liver (NAFL) to non‐alcoholic steatohepatitis (NASH) and cirrhosis, and careful evaluation of advanced NASH is required for patients with type 2 diabetes mellitus. In contrast, insulin‐like growth factor‐1 (IGF‐1) levels are also associated with glucose intolerance11, 12, 13, and glucose intolerance might affect the levels of IGF‐1.
IGF‐1 is a peptide growth factor and is predominantly secreted from hepatocytes14. IGF‐1 plays important roles, including stimulating cell growth and proliferation, inhibiting programmed cell death, and affecting various tissues15, 16, 17, 18. In the liver, IGF‐1 regulates mitochondrial function, oxidative stress, inflammation and insulin sensitivity19, 20, 21, 22. Previous studies have shown that low levels of IGF‐1 are observed in patients with various chronic liver diseases. In NAFLD, IGF‐1 levels were associated with liver fibrosis markers and the histological fibrosis of NAFLD23, 24. However, it is unknown whether IGF‐1 correlates with liver fibrosis and liver fibrosis markers among type 2 diabetes mellitus patients, although type 2 diabetes mellitus has a high risk of advanced NASH and affects serum IGF‐1 levels25.
In the present study, we examined the relationship between IGF‐1, including IGF‐1 standard deviation scores (SDS), and liver fibrosis. We evaluated liver fibrosis by the fibrosis‐4 (FIB‐4) index (which is a non‐invasive panel used to predict the fibrosis stage of several liver diseases)26, 27 and 7S domain of type IV collagen (IV‐7S) in type 2 diabetes mellitus patients without obvious alcoholic consumption.
Methods
The present study, which utilized a cross‐sectional and partially longitudinal study design, included 415 patients who were aged at least 20 years, had been diagnosed with type 2 diabetes mellitus, and were admitted to Uwajima City Hospital for diabetes treatment between May 2013 and December 2016. Patients who met the following criteria were excluded: daily consumption of ≥30 or ≥20 g of alcohol for men and women; the presence of other liver diseases (e.g., viral or autoimmune hepatitis, primary biliary cirrhosis, metabolic, or genetic liver disease); a cancer diagnosis; or a history of corticosteroids drug use. Of 415, 160 patients had available data from 3 years after their first hospitalization, and these patients were included in the longitudinal observational analysis.
Participants’ height and bodyweight at the time of hospitalization were measured without wearing shoes or outer clothing and were used to calculate body mass index (BMI). Data on the patients’ exercise and smoking habits were obtained using a questionnaire. The administration of statins and oral hypoglycemic agents, such as glimepiride, metformin, α‐glucosidase inhibitors, glinides, pioglitazone, dipeptidyl peptidase‐4 inhibitors, glucagon‐like peptide‐1 receptor agonists (GLP‐1 RAs) and sodium–glucose cotransporter 2 inhibitor (SGLT2i) was determined from medical records. The participants’ visceral fat areas were determined from computed tomography images at the navel level with a SYNAPSE VINCENT volume analyzer (FUJIFILM Corporation, Tokyo, Japan).
On the morning after admission, blood pressure was determined using an automated sphygmomanometer after a 15‐min resting period, and blood samples were collected after a 12‐h fast to evaluate the levels of fasting plasma glucose (FPG), hemoglobin A1c (HbA1c), total cholesterol, triglycerides (TG), high‐density (HDL‐C) and low‐density lipoprotein cholesterol, aspartate (AST) and alanine aminotransferase (ALT), γ‐glutamyl transpeptidase (γ‐GTP), growth hormone (GH), and IGF‐1, as well as platelet count. Serum levels of GH and IGF‐1 were measured with Elecsys hGH (Roche Diagnostics K.K., Tokyo, Japan) and IGF‐1 assay “Daiichi” (Fujirebio Inc., Tokyo, Japan), respectively. A METABO Rhythm (Eli Lilly Japan K.K., Kobe, Japan) was used to determine the IGF‐1 SDS (i.e., IGF‐1 level corrected for age and sex)28. The IV‐7S levels were determined in 176 patients. The diagnosis of type 2 diabetes mellitus was based on The Japan Diabetes Society criteria29. The FIB‐4 index was calculated as follows: age (years) × AST [U/L] / (platelets [109/L] × )27. Advanced liver fibrosis was defined when the FIB‐4 index was >3.2526. Additionally, for those participants in the longitudinal study, FIB‐4 index was examined in outpatients using serum after a 12‐h fast 3 years after the initial hospitalization.
The protocol for this study was approved by the institutional review board of Uwajima City Hospital (Approval ID no. 163‐91, University Hospital Medical Information Network ID: UMIN000021519). This study conformed to the ethical guidelines of the 1983 revision of the Declaration of Helsinki (original publication: 1975). All participants provided written informed consent before participating.
Statistical analysis
To evaluate the correlation between FIB‐4 or IV‐7S and each clinical variable, Spearman's correlation coefficients were used. If the P‐value was ≤0.2, the factor was chosen as an independent variable. Mann–Whitney's test was used to compare liver fibrosis markers, IGF‐1 and IGF‐1 SDS between groups medicated with each agent or not. Each medication was selected as an independent factor if the medication had significant associations with FIB‐4 index or IV‐7S. Additionally, it was also used to compare the differences of IGF‐1 and IGF‐1 SDS in the patients with liver fibrosis and without fibrosis of different sexes. Multiple linear regression analysis was then carried out to obtain the association between the FIB‐4 index or IV‐7S and IGF‐1 or IGF‐1 SDS. On analysis of the FIB‐4 index, model 1 was adjusted for the following factors: sex, BMI, FPG, HbA1c, VFA, SBP, TG, HDL‐C, γ‐GTP and GH. Model 2 was adjusted for factors that were found to be an independent variable in univariate analyses, such as BMI, FPG, HbA1c, VFA, TG, HDL‐C, γ‐GTP, glimepiride, glinides and statins. Similarly, on analysis of IV‐7S, model 1 was adjusted for the following factors: age, BMI, FPG, HbA1c, VFA, SBP, TG, HDL‐C, AST, ALT, γ‐GTP, platelets and GH. Model 2 was adjusted for factors that were found to be an independent variable in univariate analyses, such as age, VFA, AST, ALT, γ‐GTP and platelets. The Steel–Dwass test was used to examine the FIB‐4 index of each quartile based on IGF‐1 and IGF‐1 SDS among the different sexes. All statistical analyses were calculated using JMP version 12.0 software (SAS Institute Japan Inc., Tokyo, Japan). P‐values <0.05 were considered significant.
Results
Baseline characteristics
Table 1 summarizes the baseline characteristics of the participants. The mean age, BMI and HbA1c were 61.9 years, 25.8 kg/m2 and 9.8%, respectively, and the mean liver enzymes were mildly high (Table 1). The administration rates of oral hypoglycemic agents and statins were 58.8 and 38.1%, respectively. The FIB‐4 index and the IV‐7S were 1.6 ± 1.1 and 4.0 ± 1.1 ng/mL, respectively. Patients taking glimepiride, glinides and statins had higher FIB‐4 indices than those who did not take these drugs (Table S1). However, there was no significant difference on IV‐7S between patients who were taking hypoglycemic agents compared with those who were not (Table S1). The mean IGF‐1 was 118.6 ng/mL and the mean IGF‐1 SDS was −0.54 (Table 1). On IGF‐1 and IGF‐1 SDS, there was no significant difference between patients who were taking hypoglycemic agents compared with those who were not (Table S2). In sex‐specific comparisons, the mean age of men was significantly lower than that of women. In addition, the current smoker ratio, the mean levels of HDL‐C, γ‐GTP and IGF‐1, and platelet counts were significantly different between men and women (Table 1).
Table 1.
Baseline characteristics of patients (n = 415)
| Characteristics | Total (n = 415) | Men (n = 248) | Women (n = 167) | P‐value | |||
|---|---|---|---|---|---|---|---|
| Mean ± SD | Participants (%) | Mean ± SD | Participants (%) | Mean ± SD | Participants (%) | ||
| Age (years) | 61.9 ± 12.6 | 60.3 ± 12.0 | 64.3 ± 13.1 | <0.0002 | |||
| BMI (kg/m2) | 25.8 ± 4.8 | 25.6 ± 4.7 | 26.1 ± 4.9 | 0.37 | |||
| FPG (mg/dL) | 164.3 ± 53.2 | 164.2 ± 54.2 | 164.6 ± 51.7 | 0.88 | |||
| HbA1c (%) | 9.8 ± 2.1 | 9.9 ± 2.2 | 9.8 ± 1.9 | 0.80 | |||
| Regular exercise | 63 (15.2) | 52 (21.0) | 11 (6.6) | 0.049 | |||
| Current smoker | 62 (14.9) | 57 (23.0) | 5 (3.0) | <0.0001 | |||
| Glimepiride | 127 (30.6) | 70 (28.2) | 57 (34.1) | 0.23 | |||
| Metformin | 75 (18.1) | 43 (17.3) | 32 (19.2) | 0.70 | |||
| α‐GI | 61 (14.7) | 36 (14.5) | 25 (15.0) | 0.89 | |||
| Glinides | 6 (1.4) | 4 (1.6) | 2 (1.1) | 1.00 | |||
| DPP4i | 153 (36.9) | 83 (33.5) | 70 (41.9) | 0.10 | |||
| Pioglitazone | 23 (5.5) | 17 (6.9) | 6 (3.6) | 0.19 | |||
| SGLT2i | 4 (0.1) | 3 (1.2) | 1 (0.6) | 0.65 | |||
| GLP‐1 RA | 2 (0.5) | 0 (0.0) | 2 (1.2) | 0.16 | |||
| Statins | 158 (38.1) | 90 (36.2) | 68 (40.7) | 0.41 | |||
| VFA (cm2) | 142.6 ± 68.2 | 149.2 ± 75.9 | 133.8 ± 55.3 | 0.12 | |||
| SBP (mmHg) | 129.9 ± 20.2 | 128.3 ± 19.1 | 132.2 ± 21.5 | 0.20 | |||
| DBP (mmHg) | 75.4 ± 13.2 | 75.4 ± 13.2 | 75.3 ± 13.1 | 0.76 | |||
| T‐chol (mg/dL) | 189.6 ± 45.7 | 186.7 ± 45.1 | 193.9 ± 46.3 | 0.11 | |||
| TG (mg/dL) | 165.9 ± 136.3 | 172.6 ± 152.8 | 156.0 ± 107.1 | 0.11 | |||
| HDL‐C (mg/dL) | 49.9 ± 14.0 | 47.4 ± 13.3 | 53.6 ± 14.2 | <0.0001 | |||
| LDL‐C (mg/dL) | 118.3 ± 40.5 | 117.0 ± 40.1 | 120.3 ± 41.0 | 0.44 | |||
| AST (U/L) | 27.8 ± 18.8 | 27.3 ± 18.1 | 28.6 ± 19.8 | 0.52 | |||
| ALT (U/L) | 32.3 ± 27.3 | 31.6 ± 23.5 | 33.2 ± 32.1 | 0.76 | |||
| γ‐GTP (IU/L) | 57.7 ± 92.9 | 68.3 ± 113.6 | 42.2 ± 44.5 | <0.0001 | |||
| Platelets (×104/μL) | 22.7 ± 7.7 | 21.6 ± 7.3 | 24.2 ± 8.0 | 0.0001 | |||
| FIB‐4 index | 1.60 ± 1.08 | 1.64 ± 1.19 | 1.54 ± 0.91 | 0.96 | |||
| IV‐7S (ng/mL) | 4.04 ± 1.12 | 4.02 ± 1.11 | 4.08 ± 1.14 | 0.81 | |||
| GH (ng/mL) | 0.55 ± 1.36 | 0.64 ± 1.56 | 0.43 ± 0.99 | 0.39 | |||
| IGF‐1 (ng/mL) | 118.6 ± 50.2 | 128.2 ± 52.9 | 104.3 ± 42.3 | <0.0001 | |||
| IGF‐1 SDS | −0.54 ± 1.42 | −0.48 ± 1.46 | −0.6 ± 1.36 | 0.34 | |||
Data were expressed as mean ± standard deviation or frequency (percentage). ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; DBP, diastolic blood pressure; DPP4i, dipeptidyl peptidase 4 inhibitors; FIB‐4, fibrosis‐4; FPG, fasting plasma glucose; GH, growth hormone; GLP‐1 RA, glucagon like peptide‐1 receptor agonists; HbA1c, hemoglobin A1c; HDL‐C, high‐density lipoprotein cholesterol; IGF‐1, insulin‐like growth factor‐1; IV‐7S, 7S domain of type IV collagen; LDL‐C, low‐density lipoprotein cholesterol; SBP, systolic blood pressure; SD, standard deviation; SDS, standard deviation score; SGLT2i, sodium glucose cotransporter 2 inhibitors; T‐chol, total cholesterol; TG, triglyceride; VFA, visceral fat area; α‐GI, α‐glucosidase inhibitors; γ‐GTP, gamma glutamyl transpeptidase.
Clinical variables correlated with FIB‐4 index and IV‐7S
A positive correlation was observed between the FIB‐4 index and the serum levels of γ‐GTP, and an inverse correlation was observed between FIB‐4 index and BMI, FPG, HbA1c, serum levels of IGF‐1, and IGF‐1 SDS (Table 2, Figure 1a). In contrast, IV‐7S showed a positive correlation with age, VFA, FIB‐4 index and serum levels of AST, ALT and γ‐GTP; and an inverse correlation with platelets, IGF‐1 and IGF‐1 SDS (Table 2; Figure 1b). In men, the FIB‐4 index and IV‐7S were inversely correlated with IGF‐1 SDS (r = −0.33, P < 0.0001 and r = −0.43, P < 0.0001, respectively; Figure S1a,b). In contrast, only IV‐7S was inversely correlated with IGF‐1 SDS (r = −0.41, P = 0.01) in women (Figure S1c,d).
Table 2.
Correlations between each liver fibrotic marker and clinical variables
| FIB‐4 index (n = 415) | IV‐7S (n = 176) | |||
|---|---|---|---|---|
| r | P‐value | r | P‐value | |
| Age | NA | NA | 0.22 | 0.004 |
| BMI | −0.10 | 0.045 | 0.09 | 0.24 |
| FPG | −0.14 | 0.007 | −0.08 | 0.32 |
| HbA1c | −0.15 | 0.003 | −0.006 | 0.94 |
| VFA | 0.10 | 0.06 | 0.16 | 0.04 |
| SBP | −0.04 | 0.39 | −0.06 | 0.44 |
| TG | −0.07 | 0.19 | −0.10 | 0.21 |
| HDL‐C | 0.07 | 0.13 | 0.03 | 0.72 |
| AST | NA | NA | 0.37 | <0.0001 |
| ALT | NA | NA | 0.21 | 0.006 |
| γ‐GTP | 0.34 | <0.0001 | 0.35 | <0.0001 |
| Platelets | NA | NA | −0.28 | 0.0001 |
| FIB‐4 index | NA | NA | 0.48 | <0.0001 |
| GH | 0.03 | 0.57 | 0.09 | 0.21 |
| IGF‐1 | −0.38 | <0.0001 | −0.43 | <0.0001 |
| IGF‐1 SDS | −0.26 | <0.0001 | −0.43 | <0.0001 |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; FIB‐4, fibrosis‐4; FPG, fasting plasma glucose; GH, growth hormone; HbA1c, hemoglobin A1c; HDL‐C, high‐density lipoprotein cholesterol; IGF‐1, insulin‐like growth factor‐1; IV‐7S, 7S domain of type IV collagen; NA, not applicable; SBP, systolic blood pressure; SDS, standard deviation score; TG, triglyceride; VFA, visceral fat area; γ‐GTP, gamma glutamyl transpeptidase.
Figure 1.
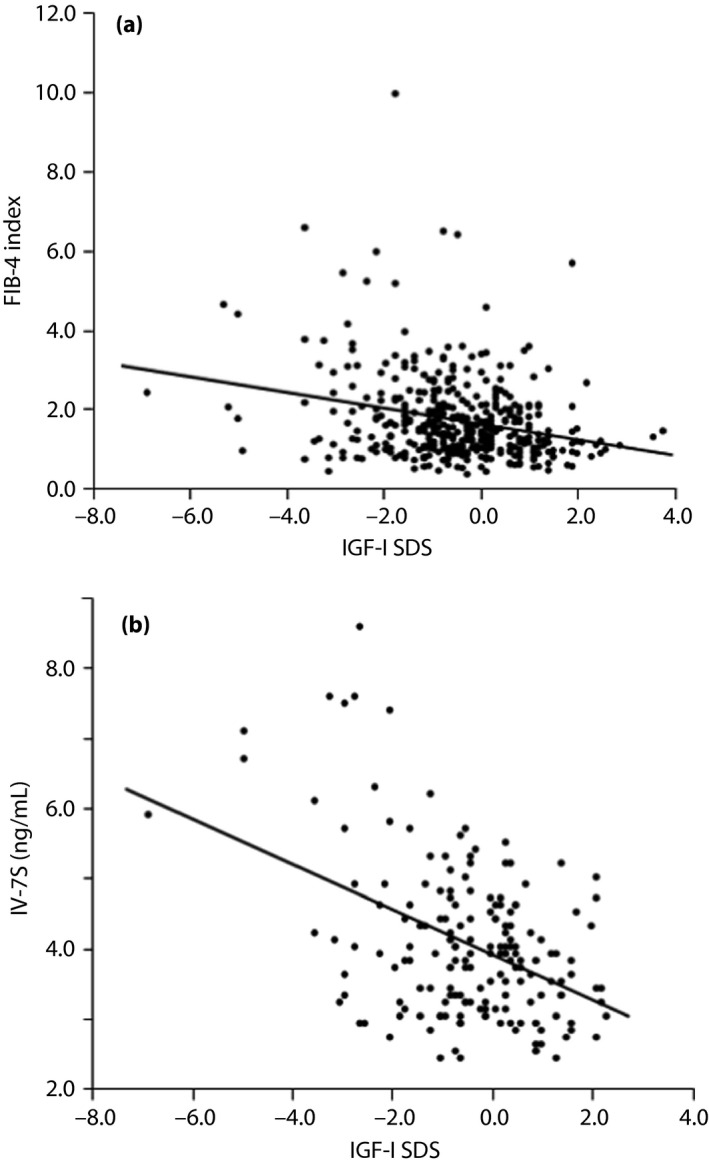
| (a) Correlation between insulin‐like growth factor‐1 (IGF‐1) standard deviation scores (SDS) and the fibrosis‐4 (FIB‐4) index (n = 415). IGF‐1 SDS was inversely correlated with the FIB‐4 index (r −0.26, P < 0.0001). (b) Correlation between IGF‐1 SDS and 7S domain of type IV collagen (IV‐7S; n = 176). IGF‐1 SDS was inversely correlated with IV‐7S (r −0.43, P < 0.0001)
Correlation between FIB‐4 index and IGF‐1
The results of the multiple linear regression analysis with FIB‐4 index as the dependent variable are shown in Tables 3, S3 and S4. Model 1 showed that the FIB‐4 index was inversely correlated with IGF‐1 (β −0.32, B −0.007, 95% confidence interval [CI] −0.009, −0.005, P < 0.0001) and IGF‐1 SDS (β −0.22, B −0.17, 95% CI −0.24, −0.09, P < 0.0001). Similarly, model 2 showed that the FIB‐4 index was inversely correlated with IGF‐1 (β −0.29, B −0.006, 95% CI −0.009, −0.004, P < 0.0001) and IGF‐1 SDS (β −0.22, B −0.17, 95% CI −0.25, −0.10, P < 0.0001).
Table 3.
Results of the multiple linear regression analysis of the fibrosis‐4index and 7S domain of type IV collagen (n = 176) and the insulin‐like growth factor‐1 and insulin‐like growth factor‐1 standard deviation score
| Age‐adjusted | Model 1 | Model 2 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β | B | 95% CI (Low, High) | P value | β | B | 95% CI (Low, High) | P value | β | B | 95% CI (Low, High) | P value | |
| FIB‐4 index | ||||||||||||
| n = 415 | ||||||||||||
| IGF‐1 | NA | NA | NA | NA | −0.32 | −0.007 | −0.009, −0.005 | <0.0001 | −0.29 | −0.006 | −0.009, −0.004 | <0.0001 |
| IGF‐1 SDS | NA | NA | NA | NA | −0.22 | −0.17 | −0.24, −0.09 | <0.0001 | −0.22 | −0.17 | −0.25, −0.10 | <0.0001 |
| IV‐7S | ||||||||||||
| n = 176 | ||||||||||||
| IGF‐1 | −0.40 | −0.009 | −0.01, −0.006 | <0.0001 | −0.24 | −0.006 | −0.009, −0.002 | 0.004 | −0.27 | −0.007 | −0.01, −0.003 | 0.0004 |
| IGF‐1 SDS | −0.44 | −0.34 | −0.44, −0.24 | <0.0001 | −0.27 | −0.20 | −0.31, −0.09 | 0.0004 | −0.30 | −0.23 | −0.34, −0.13 | <0.0001 |
The 95% confidence intervals (CI) were expressed as (Low, High). Fibrosis‐4 (FIB‐4) index: model 1 was adjusted for the following factors: sex, body mass index (BMI), fasting plasma glucose (FPG), hemoglobin A1c (HbA1c), visceral fat area (VFA), systolic blood pressure (SBP), triglyceride (TG), high‐density lipoprotein cholesterol (HDL‐C), gamma glutamyl transpeptidase (γ‐GTP) and growth hormone (GH). Model 2 was adjusted for factors that were found to be an independent variable in univariate analyses, such as BMI, FPG, HbA1c, VFA, TG, HDL‐C, γ‐GTP, glimepiride, glinides and statins. IV‐7S: Model 1 was adjusted for the following factors: age, sex, BMI, FPG, HbA1c, VFA, SBP, TG, HDL‐C, AST, ALT, γ‐GTP, platelets and GH. Model 2 was adjusted for factors that were found to be an independent variable in univariate analyses, such as age, VFA, AST, ALT, γ‐GTP and platelets. ALT, alanine aminotransferase; AST, aspartate aminotransferase; B, partial regression coefficient; IGF‐1, insulin‐like growth factor‐1; IV‐7S, 7S domain of type IV collagen.
Correlation between IV‐7S and IGF‐1
The results of the multiple linear regression analysis with IV‐7S as the dependent variable are shown in Tables 3, S5 and S6. Age‐adjusted analysis showed that IV‐7S was inversely correlated with IGF‐1 (β −0.4, B −0.009, 95% CI −0.01, −0.006, P < 0.0001) and IGF‐1 SDS (β −0.44, B −0.34, 95% CI −0.44, −0.24, P < 0.0001). Model 1 showed that IV‐7S was inversely correlated with IGF‐1 (β −0.24, B −0.006, 95% CI −0.009, −0.002, P = 0.004) and IGF‐1 SDS (β −0.27, B −0.20, 95% CI −0.31, −0.09, P = 0.0004). Similarly, model 2 showed that IV‐7S was inversely correlated with IGF‐1 (β −0.27, B −0.007, 95% CI −0.01, −0.003, P = 0.0004) and IGF‐1 SDS (β −0.30, B −0.23, 95% CI −0.34, −0.13, P < 0.0001).
Analysis of FIB‐4 index grouped by quartiles of IGF‐1 and IGF‐SDS on different sexes
The results of analysis of the FIB‐4 index grouped by quartiles of IGF‐1 and IGF‐1 SDS are shown in Figure 2. The average levels of quartile 1 of IGF‐1 were significantly lower than that of quartile 4 in both sexes (Figure 2a,c). In contrast, the average levels of quartile 1 of IGF‐1 SDS in men were significantly higher than that of quartile 4, but there was no significant difference in women (Figure 2b,d).
Figure 2.
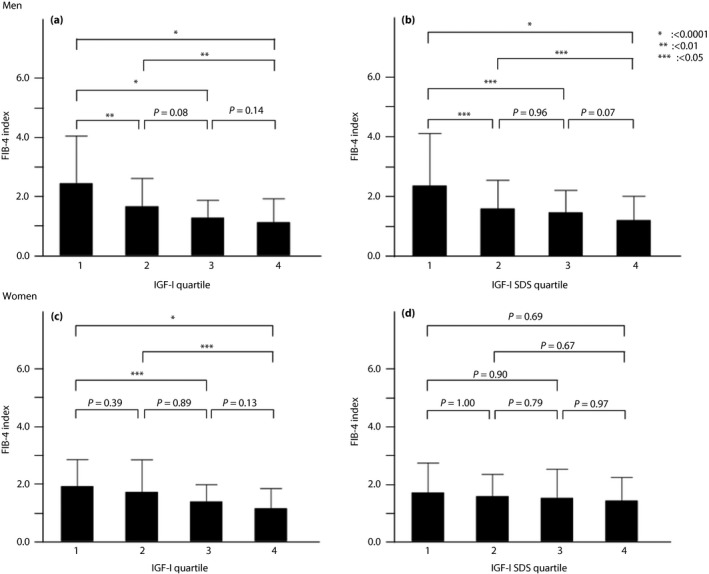
| (a) Analysis of the fibrosis‐4 (FIB‐4) index grouped by quartiles of insulin‐like growth factor‐1 (IGF‐1) in men. (b) Analysis of FIB‐4 index grouped by quartiles of IGF‐1 standard deviation scores (SDS) in men. (c) Analysis of FIB‐4 index grouped by quartiles of IGF‐1 in women. (d) Analysis of FIB‐4 index grouped by quartiles of IGF‐1 SDS in women.
Analysis of the levels of IGF‐1 and IGF‐1 SDS in patients with versus without advanced liver fibrosis of different sexes
Table S7 shows the results of the analysis of the levels of IGF‐1 and IGF‐1 SDS in patients with and without advanced liver fibrosis in different sexes. In men, the levels of IGF‐1 and IGF‐1 SDS with advanced liver fibrosis were significantly lower than that of the patients without advanced liver fibrosis. In women, the levels of IGF‐1 SDS were not significantly different between those patients with advanced liver fibrosis compared with those without advanced liver fibrosis, although the levels of IGF‐1 in patients with advanced liver fibrosis were significantly lower than that of those patients without advanced liver fibrosis.
Longitudinal observational analysis between high IGF‐1 level group versus low IGF‐1 level group
Table 4 shows the results of the longitudinal observational analysis between the high IGF‐1 level group versus low IGF‐1 level group. The cut‐off value of IGF‐1 was defined as 96.0 ng/mL for advanced liver fibrosis (FIB‐4 index >3.25; Table S8). The change of the FIB‐4 index for 3 years in the patients in the low IGF‐1 level group was significantly worse compared with the patients in the high IGF‐1 level group (P = 0.049).
Table 4.
Differences in the fibrosis‐4 index 3 years after hospitalization between two groups divided by insulin‐like growth factor‐1 cut‐off levels
| IGF‐1 (ng/mL) | Low IGF‐1 level group (<96.0) | High IGF‐1 level group (≥96.0) | P‐value |
|---|---|---|---|
| n | 59 | 101 | |
| ⊿FIB‐4 | 0.27 ± 1.31 | −0.03 ± 0.06 | 0.049 |
The ⊿ fibrosis‐4 (FIB‐4) was calculated as follows: FIB‐4 index (3 years) – FIB‐4 index (original).
Discussion
In the present study, we examined the relationship between IGF‐1 and liver fibrosis markers in type 2 diabetes mellitus patients without obvious alcoholic consumption, and showed that IGF‐1 was inversely correlated with FIB‐4 index and IV‐7S. This association remained significant after adjusting for potential confounders. Additionally, the liver fibrosis marker of the patients with low IGF‐1 levels was worse than that of patients with high IGF‐1 levels after 3 years.
Previously, several studies have shown the relationship between IGF‐1 and liver fibrosis marker and histological liver fibrosis in NAFLD patients. Hribal et al.23 showed that plasma IGF‐1 levels inversely correlated with NAFLD fibrosis scores in 221 NAFLD patients, diagnosed by ultrasonography and with at least one cardiometabolic risk factor. Furthermore, they showed that the liver IGF‐1 messenger ribonucleic acid levels were inversely associated with the fibrosis stage in 50 biopsy‐proven NAFLD patients. Ichikawa et al. examined the plasma IGF‐1 levels in 52 NAFLD patients, and reported that plasma low IGF‐1 levels were associated with stage 2–3 by multivariate logistic regression analysis (stage 0–1 vs stage 2–3)24. Garcia‐Galiano et al.30 analyzed 36 NAFLD patients with morbid obesity, and identified IGF‐1 <110 ng/mL as an independent predictor of NASH (NAFLD activity score 5–8) by the multivariate regression analysis. Additionally, Colak et al.31 collected the serum samples of 92 NAFLD patients, and showed IGF‐1 levels were significantly decreased in patients with moderate‐to‐severe fibrosis (stage 2–3) compared with patients with no or mild fibrosis (stage 0–1). However, these findings were not determined with IGF‐1 levels adjusted by age. In contrast, Sumida et al.26 investigated the SDS of IGF‐1 by age and sex in 199 NAFLD patients, and showed that the IGF‐1 SDS decreased significantly with increasing lobular inflammation and fibrosis, and showed in the multivariate logistic regression analysis the significant association between the IGF‐1 SDS values and the severity of NAFLD (NASH vs NAFL or fibrosis stage 0–2 vs 3–4) after adjusting for age, sex and insulin resistance. Additionally, Dichtel et al.32 examined 21 controls with no steatosis, lobular inflammation or fibrosis and 121 NAFLD patients, and showed that IGF‐1 was lower in individuals with lobular inflammation, hepatocyte ballooning, higher fibrosis stages (stage 2–4 vs 0–1) and NASH; all factors examined remained significant after controlling for age, BMI and the diagnosis of diabetes. However, these studies did not examine the relationship between IGF‐1 and liver fibrosis among type 2 diabetes mellitus, which are high‐risk groups of advanced liver disease; hence, this was our primary outcome measure. Previous reports showed that glucose intolerance is associated with low levels of IGF‐1, and insulin secretion is important for the maintenance of IGF‐1 level11, 12, 13. Therefore, progression of liver fibrosis in diabetes mellitus patients might be affected by both diabetes itself and low levels of IGF‐1, and proceed further.
The relationships between IGF‐1 and liver fibrosis markers in type 2 diabetes mellitus patients without obvious alcoholic consumption in the present study might be associated with several potential mechanisms. The IGF‐1 level regulates mitochondrial functions and oxidative stress, and corrects inflammation molecules. Perez et al.33 showed that IGF‐1 administration exerted a mitochondrial protection (which resulted in the reduced apoptosis and increased adenosine triphosphate production), as well as improved liver dysfunction and fibrosis in a rat cirrhotic model. Sanz et al.34 reported that the overexpression of IGF‐1 in hepatic stellate cells, restricted hepatic stellate cells activation, attenuated fibrosis and accelerated liver regeneration in a cirrhotic model. Furthermore, IGF‐1 stimulated the production of hepatocyte growth factor, which is a mitogen for hepatocytes, and suppressed the fibrosis in a cirrhotic model35. Therefore, the low IGF‐1 levels might be a risk factor for the progression of liver fibrosis.
In contrast, antidiabetic agents affect the pathophysiology of NASH. Pioglitazone, GLP‐1RA and SGLT2i are expected to improve inflammation and fibrosis of NASH35, 36, 37, 38. However, in the present study, the relationship between liver fibrosis markers and antidiabetic agents, such as GLP‐1RA and SGLT2i, were not significant. Similarly, antidiabetic agents did not affect the IGF‐1 level. In a small sample size and short duration study, glimepiride treatment for 6 weeks for patients with type 1 diabetes with multiple insulin injection therapy increased serum levels of IGF‐139. An experimental study also showed that sitagliptin significantly increased the plasma IGF‐1 level in rabbits40. The reasons for why there are differences between the present results and previous studies might be because the number of patients treated with GLP‐1RA and SGLT2i was small.
Sex differences were observed in the analysis of IGF‐1 SDS. In women, the levels of IGF‐1 with liver fibrosis were significantly lower than that of without liver fibrosis. However, the levels of IGF‐1 SDS were not significant between those with liver fibrosis and without liver fibrosis. These results might be affected by estrogen. In women, as they grow older, estrogen decreases as well as IGF‐ I. The decrease of estrogen is associated with the progression and severity of NAFLD41, 42. Therefore, the decrease of not only IGF‐ I, but also estrogen, might affect the present results.
The present study had a few limitations. First, we did not carry out a liver biopsy, which is a gold standard examination for liver fibrosis. However liver biopsy is an invasive examination for patients and expensive, and a several studies used surrogate markers for predicting the degree of hepatic fibrosis instead of liver biopsy43, 44. Second, as patients were selected from a single center, selection bias might have occurred. Third, IV‐7S data were not available for all participants. However, it is important to add the fibrosis marker in addition to the FIB‐4 index to prove that the relationship between IGF‐1 and fibrosis is more certain; the present study showed a relationship between IV‐7S and IGF‐1. Fourth, we did not measure the estrogen level in women. Therefore, we could not research the effect of estrogen to liver fibrosis. Finally, this was a cross‐sectional study, and causal relationships between IGF‐1 levels and liver fibrosis could not be established in this analysis. Therefore, further prospective studies are required.
Despite the above‐mentioned limitations, the present study provided several notable results. In particular, our findings showed that low serum IGF‐1 levels are a possible risk factor for liver fibrosis in type 2 diabetes mellitus patients. This result might help clinicians to identify type 2 diabetes mellitus patients with advanced NASH by measuring serum IGF‐1 levels. Therefore, we might need to pay attention to IGF‐1 in type 2 diabetes mellitus patients.
Disclosure
The authors declare no conflict of interest.
Supporting information
Table S1 | Association between liver fibrosis markers and each medication.
Table S2 | Association of insulin‐like growth factor‐1, insulin‐like growth factor‐1 standard deviation score and each medication.
Table S3 | Results of the multiple linear regression analysis of the fibrosis‐4 index and insulin‐like growth factor‐1.
Table S4 | Results of the multiple linear regression analysis of fibrosis‐4 index and insulin‐like growth factor‐1 standard deviation score.
Table S5 | Results of the multiple linear regression analysis of 7S domain of type IV collagen and insulin‐like growth factor‐1 (n = 176).
Table S6 | Results of the multiple linear regression analysis of 7S domain of type IV collagen and insulin‐like growth factor‐1 standard deviation score (n = 176).
Table S7 | Analysis of insulin‐like growth factor‐1 levels and insulin‐like growth factor‐1 standard deviation score in patients with and without advanced liver fibrosis of different sexes.
Table S8 | Accuracy of insulin‐like growth factor‐1 for each fibrosis marker.
Figure S1 | (a) The correlation between insulin‐like growth factor‐1 (IGF‐1) standard deviation score (SDS) and fibrosis‐4 (FIB‐4) index in men (n = 248). IGF‐1 SDS was significantly correlated with the FIB‐4 index (r −0.33, P < 0.0001). (b) The correlation between IGF‐1 SDS and 7S domain of type IV collagen (IV‐7S) in men (n = 140). IGF‐1 SDS was significantly correlated with IV‐7S (r −0.43, P < 0.0001). (c) Correlation between IGF‐1 SDS and FIB‐4 index in women (n = 167). IGF‐1 SDS was not significantly correlated with the FIB‐4 index (r −0.12, P = 0.13). (d) The correlation between IGF‐1 SDS and IV‐7S in women (n = 36). IGF‐1 SDS was significantly correlated with IV‐7S (r −0.41, P = 0.01).
Acknowledgments
We thank Hisashi Yamashita for the visceral fat area measurements.
J Diabetes Investig 2019; 10: 1083–1091
Clinical Trial Registry
University Hospital Medical Information Network
UMIN000021519
References
- 1. Matteoni CA, Younossi ZM, Gramlich T, et al Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology 1999; 116: 1413–1419. [DOI] [PubMed] [Google Scholar]
- 2. Brunt EM, Janney CG, Di Bisceglie AM, et al Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. Am J Gastroenterol 1999; 94: 2467–2474. [DOI] [PubMed] [Google Scholar]
- 3. Musso G, Gambino R, Cassader M, et al Meta‐analysis: natural history of non‐alcoholic fatty liver disease (NAFLD) and diagnostic accuracy of non‐invasive tests for liver disease severity. Ann Med 2011; 43: 617–649. [DOI] [PubMed] [Google Scholar]
- 4. Rafiq N, Bai C, Fang Y, et al Long‐term follow‐up of patients with nonalcoholic fatty liver. Clin Gastroenterol Hepatol 2009; 7: 234–238. [DOI] [PubMed] [Google Scholar]
- 5. Ekstedt M, Franzen LE, Mathiesen UL, et al Long‐term follow‐up of patients with NAFLD and elevated liver enzymes. Hepatology 2006; 44: 865–873. [DOI] [PubMed] [Google Scholar]
- 6. Adams LA, Lymp JF, St Sauver J, et al The natural history of nonalcoholic fatty liver disease: a population‐based cohort study. Gastroenterology 2005; 129: 113–121. [DOI] [PubMed] [Google Scholar]
- 7. Jimba S, Nakagami T, Takahashi M, et al Prevalence of non‐alcoholic fatty liver disease and its association with impaired glucose metabolism in Japanese adults. Diabet Med 2005; 22: 1141–1145. [DOI] [PubMed] [Google Scholar]
- 8. Hossain N, Afendy A, Stepanova M, et al Independent predictors of fibrosis in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol 2009; 7: 1224–1229, 1229 e1221‐1222. [DOI] [PubMed] [Google Scholar]
- 9. Angulo P, Keach JC, Batts KP, et al Independent predictors of liver fibrosis in patients with nonalcoholic steatohepatitis. Hepatology 1999; 30: 1356–1362. [DOI] [PubMed] [Google Scholar]
- 10. McPherson S, Hardy T, Henderson E, et al Evidence of NAFLD progression from steatosis to fibrosing‐steatohepatitis using paired biopsies: implications for prognosis and clinical management. J Hepatol 2015; 62: 1148–1155. [DOI] [PubMed] [Google Scholar]
- 11. Oberg D, Salemyr J, Ortqvist E, et al A longitudinal study of serum insulin‐like growth factor‐I levels over 6 years in a large cohort of children and adolescents with type 1 diabetes mellitus: a marker reflecting diabetic retinopathy. Pediatr Diabetes 2018; 19: 972–978. [DOI] [PubMed] [Google Scholar]
- 12. Gutefeldt K, Hedman CA, Thyberg ISM, et al Dysregulated growth hormone‐insulin‐like growth factor‐1 axis in adult type 1 diabetes with long duration. Clin Endocrinol 2018; 89: 424–430. [DOI] [PubMed] [Google Scholar]
- 13. Sandhu MS, Heald AH, Gibson JM, et al Circulating concentrations of insulin‐like growth factor‐I and development of glucose intolerance: a prospective observational study. Lancet 2002; 359: 1740–1745. [DOI] [PubMed] [Google Scholar]
- 14. Yakar S, Rosen CJ, Beamer WG, et al Circulating levels of IGF‐1 directly regulate bone growth and density. J Clin Invest 2002; 110: 771–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ali AT, Hochfeld WE, Myburgh R, et al Adipocyte and adipogenesis. Eur J Cell Biol 2013; 92: 229–236. [DOI] [PubMed] [Google Scholar]
- 16. Clemmons DR. Role of IGF‐I in skeletal muscle mass maintenance. Trends Endocrinol Metab 2009; 20: 349–356. [DOI] [PubMed] [Google Scholar]
- 17. Simpson HL, Jackson NC, Shojaee‐Moradie F, et al Insulin‐like growth factor I has a direct effect on glucose and protein metabolism, but no effect on lipid metabolism in type 1 diabetes. J Clin Endocrinol Metab 2004; 89: 425–432. [DOI] [PubMed] [Google Scholar]
- 18. Holt RI, Simpson HL, Sonksen PH. The role of the growth hormone‐insulin‐like growth factor axis in glucose homeostasis. Diabet Med 2003; 20: 3–15. [DOI] [PubMed] [Google Scholar]
- 19. Takahashi Y. Essential roles of growth hormone (GH) and insulin‐like growth factor‐I (IGF‐I) in the liver. Endocr J 2012; 59: 955–962. [DOI] [PubMed] [Google Scholar]
- 20. Hao CN, Geng YJ, Li F, et al Insulin‐like growth factor‐1 receptor activation prevents hydrogen peroxide‐induced oxidative stress, mitochondrial dysfunction and apoptosis. Apoptosis 2011; 16: 1118–1127. [DOI] [PubMed] [Google Scholar]
- 21. Puche JE, Garcia‐Fernandez M, Muntane J, et al Low doses of insulin‐like growth factor‐I induce mitochondrial protection in aging rats. Endocrinology 2008; 149: 2620–2627. [DOI] [PubMed] [Google Scholar]
- 22. Takahashi Y. The role of growth hormone and insulin‐like growth factor‐I in the liver. Int J Mol Sci 2017; 18: 1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hribal ML, Procopio T, Petta S, et al Insulin‐like growth factor‐I, inflammatory proteins, and fibrosis in subjects with nonalcoholic fatty liver disease. J Clin Endocrinol Metab 2013; 98: E304–E308. [DOI] [PubMed] [Google Scholar]
- 24. Ichikawa T, Nakao K, Hamasaki K, et al Role of growth hormone, insulin‐like growth factor 1 and insulin‐like growth factor‐binding protein 3 in development of non‐alcoholic fatty liver disease. Hepatol Int 2007; 1: 287–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kim MS, Lee DY. Insulin‐like growth factor (IGF)‐I and IGF binding proteins axis in diabetes mellitus. Ann Pediatr Endocrinol Metab 2015; 20: 69–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sumida Y, Yoneda M, Hyogo H, et al Validation of the FIB4 index in a Japanese nonalcoholic fatty liver disease population. BMC Gastroenterol 2012; 12: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sterling RK, Lissen E, Clumeck N, et al Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology 2006; 43: 1317–1325. [DOI] [PubMed] [Google Scholar]
- 28. Isojima T, Shimatsu A, Yokoya S, et al Standardized centile curves and reference intervals of serum insulin‐like growth factor‐I (IGF‐I) levels in a normal Japanese population using the LMS method. Endocr J 2012; 59: 771–780. [DOI] [PubMed] [Google Scholar]
- 29. Committee of the Japan Diabetes Society on the Diagnostic Criteria of Diabetes Mellitus , Seino Y, Nanjo K, et al Report of the committee on the classification and diagnostic criteria of diabetes mellitus. J Diabetes Investig 2010; 1: 212–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Garcia‐Galiano D, Sanchez‐Garrido MA, Espejo I, et al IL‐6 and IGF‐1 are independent prognostic factors of liver steatosis and non‐alcoholic steatohepatitis in morbidly obese patients. Obes Surg 2007; 17: 493–503. [DOI] [PubMed] [Google Scholar]
- 31. Colak Y, Senates E, Ozturk O, et al Serum concentrations of human insulin‐like growth factor‐1 and levels of insulin‐like growth factor‐binding protein‐5 in patients with nonalcoholic fatty liver disease: association with liver histology. Eur J Gastroenterol Hepatol 2012; 24: 255–261. [DOI] [PubMed] [Google Scholar]
- 32. Dichtel LE, Corey KE, Misdraji J, et al The association between IGF‐1 levels and the histologic severity of nonalcoholic fatty liver disease. Clin Transl Gastroenterol 2017; 8: e217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Perez R, Garcia‐Fernandez M, Diaz‐Sanchez M, et al Mitochondrial protection by low doses of insulin‐like growth factor‐ I in experimental cirrhosis. World J Gastroenterol 2008; 14: 2731–2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sanz S, Pucilowska JB, Liu S, et al Expression of insulin‐like growth factor I by activated hepatic stellate cells reduces fibrogenesis and enhances regeneration after liver injury. Gut 2005; 54: 134–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Matsuda Y, Matsumoto K, Yamada A, et al Preventive and therapeutic effects in rats of hepatocyte growth factor infusion on liver fibrosis/cirrhosis. Hepatology 1997; 26: 81–89. [DOI] [PubMed] [Google Scholar]
- 36. Takeda A, Irahara A, Nakano A, et al The improvement of the hepatic histological findings in a patient with non‐alcoholic steatohepatitis with type 2 diabetes after the administration of the sodium‐glucose cotransporter 2 inhibitor ipragliflozin. Intern Med 2017; 56: 2739–2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Akuta N, Watanabe C, Kawamura Y, et al Effects of a sodium‐glucose cotransporter 2 inhibitor in nonalcoholic fatty liver disease complicated by diabetes mellitus: Preliminary prospective study based on serial liver biopsies. Hepatol Commun 2017; 1: 46–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Belfort R, Harrison SA, Brown K, et al A placebo‐controlled trial of pioglitazone in subjects with nonalcoholic steatohepatitis. N Engl J Med 2006; 355: 2297–2307. [DOI] [PubMed] [Google Scholar]
- 39. Wudy SA, Hogel J, Dollinger B, et al Glimepiride treatment and IGF‐I in adolescents with type 1 diabetes: a prospective, randomized, double‐blind, placebo‐controlled study. Diabetes Care 2003; 26: 1312–1313. [DOI] [PubMed] [Google Scholar]
- 40. Choi B, Lee S, Kim SM, et al Dipeptidyl peptidase‐4 induces aortic valve calcification by inhibiting insulin‐like growth factor‐1 signaling in valvular interstitial cells. Circulation 2017; 135: 1935–1950. [DOI] [PubMed] [Google Scholar]
- 41. Brady CW. Liver disease in menopause. World J Gastroenterol 2015; 21: 7613–7620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kamada Y, Kiso S, Yoshida Y, et al Estrogen deficiency worsens steatohepatitis in mice fed high‐fat and high‐cholesterol diet. Am J Physiol Gastrointest Liver Physiol 2011; 301: G1031–G1043. [DOI] [PubMed] [Google Scholar]
- 43. Singh A, Le P, Lopez R, et al The utility of noninvasive scores in assessing the prevalence of nonalcoholic fatty liver disease and advanced fibrosis in type 1 diabetic patients. Hepatol Int 2018; 12: 37–43. [DOI] [PubMed] [Google Scholar]
- 44. Giorda CB, Forlani G, Manti R, et al Trend over time in hepatic fibrosis score in a cohort of type 2 diabetes patients. Diabetes Res Clin Pract 2018; 135: 65–72. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 | Association between liver fibrosis markers and each medication.
Table S2 | Association of insulin‐like growth factor‐1, insulin‐like growth factor‐1 standard deviation score and each medication.
Table S3 | Results of the multiple linear regression analysis of the fibrosis‐4 index and insulin‐like growth factor‐1.
Table S4 | Results of the multiple linear regression analysis of fibrosis‐4 index and insulin‐like growth factor‐1 standard deviation score.
Table S5 | Results of the multiple linear regression analysis of 7S domain of type IV collagen and insulin‐like growth factor‐1 (n = 176).
Table S6 | Results of the multiple linear regression analysis of 7S domain of type IV collagen and insulin‐like growth factor‐1 standard deviation score (n = 176).
Table S7 | Analysis of insulin‐like growth factor‐1 levels and insulin‐like growth factor‐1 standard deviation score in patients with and without advanced liver fibrosis of different sexes.
Table S8 | Accuracy of insulin‐like growth factor‐1 for each fibrosis marker.
Figure S1 | (a) The correlation between insulin‐like growth factor‐1 (IGF‐1) standard deviation score (SDS) and fibrosis‐4 (FIB‐4) index in men (n = 248). IGF‐1 SDS was significantly correlated with the FIB‐4 index (r −0.33, P < 0.0001). (b) The correlation between IGF‐1 SDS and 7S domain of type IV collagen (IV‐7S) in men (n = 140). IGF‐1 SDS was significantly correlated with IV‐7S (r −0.43, P < 0.0001). (c) Correlation between IGF‐1 SDS and FIB‐4 index in women (n = 167). IGF‐1 SDS was not significantly correlated with the FIB‐4 index (r −0.12, P = 0.13). (d) The correlation between IGF‐1 SDS and IV‐7S in women (n = 36). IGF‐1 SDS was significantly correlated with IV‐7S (r −0.41, P = 0.01).


