Abstract
Carotid ultrasonography is a non‐invasive, simple and inexpensive modality to assess the severity of atherosclerosis. This article reviews related articles, summarizes the rationale for the application of carotid ultrasonography in clinical practice, and addresses the features and the limitations of carotid ultrasonography in cardiovascular risk prediction. Numerous large studies have confirmed that various carotid ultrasound measures, such as carotid intima‐media thickness, the presence or absence of carotid plaque, plaque number and plaque area, can be independent predictors of cardiovascular diseases in individuals with and without diabetes mellitus. Furthermore, many studies showed that the use of carotid intima‐media thickness (especially maximum intima‐media thickness, including plaque thickness) and/or carotid plaque in addition to traditional risk factors significantly improved the prediction of the occurrence of cardiovascular diseases, while controversy remains. Several studies showed that the progression of carotid intima‐media thickness also can be a surrogate end‐point of cardiovascular events. However, the accumulated evidence has not been sufficient. Further study with sufficient power should be carried out. As plaque disruption, which plays a crucial role in the pathogenesis of cardiovascular events, is dependent on the content of lipid in the atheroma and the thickness of the fibrous cap, tissue characterization of a plaque might be useful for determining its fragility. Interestingly, recent studies have shown that ultrasonic tissue characterization of carotid lesions could improve the prediction ability of future cardiovascular diseases. Thus, carotid ultrasonography is a useful modality for better clinical practice of atherosclerosis in patients with diabetes.
Keywords: Carotid ultrasound, Diabetes mellitus, Intima‐media thickness
Patients with diabetes mellitus are at a high risk of atherosclerotic cardiovascular (CV) diseases (CVDs), such as cerebrovascular diseases, coronary artery disease (CAD), peripheral arterial disease and other vascular diseases. CVDs are major causes of mortality in patients with diabetes mellitus, and significantly impair their quality of life. In addition, once a CVD has developed, patients with diabetes mellitus have worse outcome than non‐diabetic patients. Therefore, early identification of individuals at high‐risk for CVD and subsequent intervention are required.
As the development of atherosclerotic CVDs involves several traditional risk factors, such as sex, aging, hyperglycemia, dyslipidemia, hypertension, obesity, family history and smoking, assessment of CV risk based on traditional risk factors is recommended for identifying individuals with high risk for CVD. However, this approach showed only moderate performance in validation studies1, 2, 3.
In contrast, sophisticated cardiac imaging modalities (e.g., myocardial perfusion scintigraphy, coronary computed tomography angiography and coronary angiography) can determine the presence and severity of CAD with a high degree of sensitivity and specificity. However, it is difficult to use these modalities as a screening tool, because of their potential of significant adverse effects, technical difficulty and high cost. The same is applicable to other CVDs. Therefore, non‐invasive and inexpensive indices of subclinical and silent atherosclerosis with more than moderate predictive ability are required.
Carotid ultrasonography is one of the probable candidates. Numerous large studies have confirmed that various carotid ultrasound measures, such as carotid intima‐media thickness (CIMT), the presence/absence of carotid plaque, plaque number and plaque area, can be predictors of CVD.
The present article reviews related articles, summarizes the rationale for the application of carotid ultrasonography in clinical practice, and addresses the features and the limitations of carotid ultrasonography in CV risk prediction.
Rationale for Using Carotid Ultrasonography for CV Risk Assessment
Compared with other methods, carotid ultrasonography has several advantages: (i) it can be carried out in a reproducible manner, because of its simple, non‐invasive and inexpensive nature; (ii) it can be carried out with equipment often already available; and (iii) it is not focused on the arterial lumen, but on the arterial wall, which is the real target of atherosclerosis.
Carotid ultrasonography allows clinicians to visualize the carotid wall and lumen surfaces, measure hemodynamic parameters, and thus, quantify the severity of atherosclerosis. Various extents of atherosclerotic changes, including intima‐media thickening, plaque formation, stenosis and occlusion in carotid arteries, can be identified. In particular, CIMT measured with B‐mode ultrasound is related well with that obtained by pathological measurement, and is confirmed to be a quantitative and reproducible measure of carotid atherosclerosis4. In addition, numerous studies have shown that CIMT is associated with both CVD and its risk factors5, 6. Thus, CIMT is one of the best indices for the detection of “early‐stage” atherosclerosis, which is located between CV risk factors and “hard” clinical CVD.
Furthermore, a recent study investigated whether providing ultrasound‐based pictorial information about subclinical carotid atherosclerosis to both physicians and patients improves CV risk scores. Notably, the pictorial presentation of silent atherosclerosis by carotid ultrasonography significantly improved the CV risk scores at the 1‐year follow up7.
Standard Method for Carotid Ultrasonography
Although the detailed guidelines vary by country, and universally standardized methods of carotid ultrasonography have not been established, major guidelines are almost consistent regarding the appropriate means of carrying out ultrasonic scans for the evaluation of CIMT and carotid plaques.
In general, the extracranial common carotid arteries (CCA), the carotid bulbs (Bul or Bif) and the internal carotid arteries (ICA) are scanned bilaterally in more than three different longitudinal projections, as well as transverse projections.
In B‐mode, the carotid wall is visualized as three layers: a hyperechoic layer, a hypoechoic layer and another hyperechoic layer. The two layers closer to the vascular lumen are defined as the “intima‐media complex,” and the thickness of the intima‐media complex is defined as the CIMT. The intima‐media complex on the far wall of the carotid artery is defined as the distance from the leading edge of the lumen‐intima interface to the leading edge of the media‐adventitia interface (Figure 1). The CCA “far wall” IMT measurement is validated as accurately representing the true biological thickness of the arterial far wall. In contrast, the CCA “near wall” IMT measurement is at higher risk of a measurement error, as the echogenicity of the adventitial layer could affect the visualization of the adventitial–medial boundary. However, CIMT should also be measured in the near wall, as it is not rare for atherosclerotic changes to be located in the near wall alone.
Figure 1.
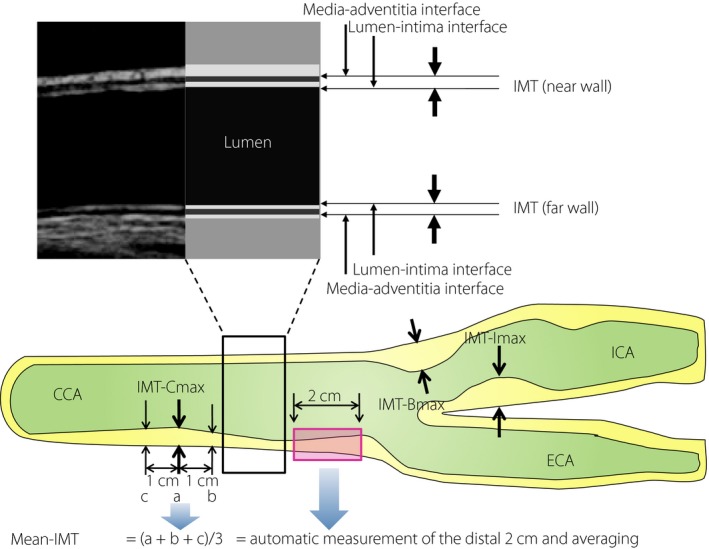
Definitions of carotid ultrasound measures. The intima‐media thickness (IMT) is a double‐line pattern on the near and far walls of the carotid arteries when visualized by ultrasound. It is shown by two parallel lines that delineate the leading edges of two anatomical boundaries, the lumen‐intima and media‐adventitia interfaces. The Japan Academy of Neurosonology recommends: (i) measuring carotid IMT at the common carotid artery (IMT‐Cmax), carotid sinus or the bifurcation of the common carotid artery (IMT‐Bmax), and internal carotid artery (IMT‐Imax) as the thickness at the thickest point, including plaque; (ii) recording the highest value among the three carotid IMT measurements as the maximum carotid IMT (max‐IMT); (iii) calculating the mean carotid IMT (mean‐IMT) as the mean value of the IMT values at the thickest point in the common carotid artery, and 1 cm distal and proximal from the thickest point (= (a + b + c) / 3). In the clinical studies using CIMT as an end‐point, automatic measurement of multiple points of the far wall of the distal 1 or 2 cm of each common carotid artery (CCA) using automated digital edge‐detection software is essential. ECA, external carotid artery; ICA, internal carotid artery.
As the differences among individuals and annual changes are small, a high degree of accuracy and good reproducibility is critical in CIMT measurement. Therefore, training of sonographers and strict adherence to scanning protocol are critical. Recently, ultrasound devices equipped with automatic IMT measuring software have become widely used to reduce the inter‐examiner error, as well as the examination time8. In particular, in the clinical studies using CIMT as an end‐point, automatic measurement using automated digital edge‐detection software is essential (Table 1).
Table 1.
Basic points of attention in carotid ultrasonography and comparisons of predictive ability for cardiovascular disease among carotid ultrasound measures
| Predictive ability for CVD | ||
|---|---|---|
| Higher | Lower | |
| Mean or max | Max | Mean |
| Plaque or CIMT | Plaque | CIMT |
| Plaque‐incorporated CIMT or not | Plaque‐incorporated CIMT | Plaque non‐incorporated CIMT |
| Whole carotid tree or CCA only | Whole carotid tree | CCA only |
B‐mode ultrasonography of the carotid artery should be carried out using an ultrasound machine equipped with a linear probe with a center frequency of ≥7.5 MHz. (ii) Scanning should be carried out bilaterally in more than three different longitudinal projections, as well as transverse projections. As compared with the near wall, the far wall IMT measurement has a lower risk of systematic measurement error. The images should be acquired during the final part of the diastolic phase. Training of sonographers and strict adherence to scanning protocol are critical. Automatic intima‐media thickness measurement using automated digital edge‐detection software reduces the inter‐examiner error. CCA, common carotid artery; CIMT, carotid intima‐media thickness; CVD, cardiovascular disease.
Definitions of Carotid Ultrasound Measures
Although CIMT is roughly defined as the distance between the lumen‐intima and media‐adventitia interfaces of a carotid segment, it should be noted that the detailed definitions of CIMT varied among individual studies. For example, some studies measured only one side of the neck, whereas others investigated bilaterally. Some measured CIMT in the CCA segment only, but others included multiple segments (CCA, Bul and ICA). There are also inconsistencies in the type of CIMT measurements (e.g., mean‐IMT or max‐IMT, etc.), definition of plaque and whether plaques were included in the CIMT measurements. Such variability in the assessment of CIMT makes it difficult to compare studies or to combine the results from different studies, as each of these CIMT measures might reflect different phenotypes.
In particular, whether the plaque lesion is included in CIMT measurement or not is critical, as the pathophysiology of the development of carotid plaque and that of CIMT are somehow different. Plaque, a localized elevated lesion into the vascular lumen, is an atherosclerotic lesion that consists of a collection of vascular smooth muscle cells and inflammatory cells often accompanied with intracellular and extracellular lipid accumulation. In contrast, increased IMT could reflect both the progression of atherosclerotic process and non‐atherosclerotic compensatory enlargement of the artery.
Generally, guidelines on carotid ultrasonography published in Japan9, 10, 11, 12 and the USA13 recommend that plaque lesions should be included in the measurement of IMT, whereas those in Europe14 recommend that plaque lesions should not be included (Table 2).
Table 2.
Differences in the definitions of carotid ultrasound measures among the Japanese, American and European guidelines
| Definition of carotid plaque | Definitions of mean‐IMT | Definitions of max‐IMT | Inclusion of plaque in CIMT | |
|---|---|---|---|---|
| The Japan Society of Ultrasonics in Medicine (JSUM)9, 10 | Localized elevated lesions with maximum thickness of >1 mm, having a point of inflection on the surface of the intima‐media complex are defined as “plaques.” In cases of vascular remodeling, the term “plaques” may be used, irrespective of the presence/absence of elevation of the lesion into the vascular lumen. | An average of readings at two or more points of measurement performed on the right and left common carotid artery, excluding the bulbus | Measurements in the observation‐possible areas of the CCA, Bul and ICA, and plaque lesion are included in max‐IMT measurement. | Included |
| The Japan Academy of Neurosonology (JAN)11, 12 | All wall hyperplasias at a thickness of ≥1.1 mm | The mean value of the IMT values at the thickest point in the common carotid artery and 1 cm distal and proximal from the thickest point | Measurements in the observation‐possible areas of the CCA, Bul and ICA, and plaque lesion are included in max‐IMT measurement. | Included |
| The American Society of Echocardiography13 | Focal wall thickening that is at least 50% greater than that of the surrounding vessel wall or as a focal region with CIMT >1.5 mm that protrudes into the lumen that is distinct from the adjacent boundary | Average values of far wall of the distal 1 cm of each CCA mean‐mean; values from the far walls of the right and left CCAs (average of segmental mean CIMT values) | Regional maximum measurement along the distal 1‐cm region of each CCA (mean‐maximum; average of segmental maximum CIMT values) | Included |
| The Mannheim Carotid Intima‐Media Thickness Consensus14 | A focal structure that encroaches into the arterial lumen of at least 0.5 mm or 50% of the surrounding IMT value, or shows a thickness >1.5 mm as measured from the intima‐lumen interface to the media‐adventitia interface | Not clearly defined (however, it is recommended that IMT should preferably be measured on the far wall of the CCA at least 5 mm below its end). | Not clearly defined (however, it is recommended that IMT should preferably be measured on the far wall of the CCA at least 5 mm below its end). | Not included |
Bul, bulbs; CCA, common carotid artery; CIMT, carotid intima‐media thickness; ICA, internal carotid artery; IMT, intima‐media thickness.
Associations Between Carotid Ultrasound Measures and CVD
Coronary artery disease
Despite the above‐mentioned inconsistencies in the definitions and assessment methods, many studies have shown that carotid ultrasound measures, such as CIMT (whether plaque thickness was incorporated in measurements) and carotid plaque‐related indices, are associated with the presence or severity of coronary atherosclerosis15, 16, 17 and myocardial ischemia18, 19, and the presence and/or past history of CAD20, 21.
Furthermore, recent studies using c‐statistic (i.e., area under the receiver operating characteristic curve) have shown that carotid ultrasound measures represent a more than moderate predictive ability for the presence of CAD22, 23, 24, 25, 26.
Cerebrovascular disease
Carotid atherosclerotic plaques are one of the major direct sources of cerebral emboli, and carotid stenosis is closely related to cerebral ischemic events27, 28, 29 and silent cerebral infarction30, 31. There is also a close association between cerebrovascular disease and more early‐stage atherosclerotic changes observed in carotid arteries, such as increased IMT32, as they share common CV risk factors underlying both processes.
Peripheral arterial disease
High CIMT has a significant association with the presence33 and development of peripheral arterial disease34.
CIMT as an Independent Predictor of CV Events
As shown in Table 3, many longitudinal studies have demonstrated that CIMT and plaque are independent predictors of CV events35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50. A meta‐analysis of some of these studies also confirmed that CIMT is an independent predictor of CV events51.
Table 3.
Relative risk of myocardial infarction, stroke and cardiovascular disease associated with carotid intima‐media thickness in major prospective studies
| Study | Year | Sample number | Sex (male, %) | Age (years) | Follow‐up period | Outcome events | Ultrasound parameters | Plaques | Relative risk (95% CI) |
|---|---|---|---|---|---|---|---|---|---|
| KIHD35 | 1991 | 1,288 | 100 | 42–60 | 1 | MI | Mean‐IMT (CCA) | Not specified | 2.17 (0.70–6.74) [IMT ≥1 vs <1 mm] |
| Plaque | ― | 4.15 (1.51–11.47) [small plaque] | |||||||
| Plaque | ― | 6.71 (1.33–33.91) [stenotic plaque] | |||||||
| ARIC36, 37 | 1997 | 5,552 | 100 | 54.3 | 5.2 | MI | Mean‐IMT (overall) | Included | 1.85 (1.28–2.69) [>1 mm, yes vs no]‡ |
| 7,289 | 0 | 53.7 | 5.2 | MI | Mean‐IMT (overall) | Included | 5.07 (3.08–8.36) [>1 mm, yes vs no]‡ | ||
| 2000 | 6,349 | 100 | 54.5 | 7.2 | Stroke | Mean‐IMT (overall) | Included | 1.98 (1.24–3.15) [>1 mm, yes vs no]‡ | |
| 7,865 | 0 | 53.8 | 7.2 | Stroke | Mean‐IMT (overall) | Included | 3.31 (1.88–5.81) [>1 mm, yes vs no]‡ | ||
| Rotterdam38, 39, 40 | 1997 | 1,373 | 64 | 71 | 2.7 | MI | Mean‐IMT (CCA) | Not specified | 1.43 (1.16–1.78) [per 1 SD (0.16 mm)]† |
| Stroke | Mean‐IMT (CCA) | Not specified | 1.41 (1.25–1.82) [per 1 SD (0.16 mm)]† | ||||||
| 2003 | 5,479 | 38.1 | 69.3 | 6.1 | Stroke | Max‐IMT (CCA, average) | Not specified | 1.28 (1.15–1.44) [per 1 SD]§ | |
| Plaque | ― | 1.15 (1.07–1.24) [severe plaque]§ | |||||||
| 2004 | 6,389 | 38.1 | 69.3 | 7–10 | MI | Max‐IMT (CCA, average) | Not specified | 1.95 (1.19–3.19) [highest quartile]§ | |
| Plaque | ― | 1.83 (1.27–2.62) [severe plaque]§ | |||||||
| CHS41, 42 | 1999 | 4,476 | 38.8 | 72.5 | 6.2 | MI | Max‐IMT (CCA) | Included | 3.17 (1.96–5.12) [highest quintile]† |
| Stroke | Max‐IMT (CCA) | Included | 2.76 (1.80–4.24) [highest quintile]† | ||||||
| 2007 | 5,020 | 39.8 | 72.6 | 11 | CVD | Composite‐IMT (overall) | Included | 1.84 (1.54–2.20) [highest tertile] | |
| Plaque | ― | 1.38 (1.14–1.67) [high risk plaque] | |||||||
| MDCS43 | 2005 | 5,163 | 41 | 46–68 | 7.0 | MI | Mean‐IMT (CCA, right) | Included | 2.05 (1.22–3.43) [highest tertile]† |
| Stroke | Mean‐IMT (CCA, right) | Included | 3.00 (1.57–3.75) [highest tertile]† | ||||||
| CAPS44 | 2006 | 5,056 | 49 | 19–90 | 4.2 | MI | Mean‐IMT (CCA) | Not specified | 1.18 (1.08–1.28) [per 1 SD]† |
| Mean‐IMT (Bif) | Not specified | 1.24 (1.13–1.36) [per 1 SD]† | |||||||
| Mean‐IMT (ICA) | Not specified | 1.11 (1.01–1.36) [per 1 SD]† | |||||||
| Stroke | Mean‐IMT (CCA) | Not specified | 1.16 (1.03–1.32) [per 1 SD]† | ||||||
| Mean‐IMT (Bif) | Not specified | 1.21 (1.05–1.40) [per 1 SD]† | |||||||
| Mean‐IMT (ICA) | Not specified | 1.17 (1.03–1.33) [per 1 SD]† | |||||||
| Tromsø Study45, 46 | 2007 | 6,226 | 56 | 25–84 | 5.4 | MI | Mean‐IMT (overall) | Included | 1.73 (0.98–3.06) [highest quartile] men, 2.86 |
| (1.07–7.65) [highest quartile] women§ | |||||||||
| 2011 | 6,584 | 53 | 25–84 | 9.6 | Ischemic stroke | Mean‐IMT (overall) | Included | 1.08 (0.95–1.22) [per 1 SD] men, 1.24 | |
| (1.05–1.48) [per 1 SD] women§ | |||||||||
| Plaque area | ― | 1.23 (1.09–1.38) [per 1 SD] men, 1.19 | |||||||
| (1.01–1.41) [per 1 SD] women§ | |||||||||
| Framingham Offspring Study47 | 2011 | 2,965 | 44.7 | 58 | 7.2 | CVD | Mean‐IMT (CCA) | Excluded | 1.13 (1.02–1.24) [per 1 SD]§ |
| Mean‐IMT (ICA) | Excluded | 1.21 (1.13–1.29) [per 1 SD]§ | |||||||
| Yoshida, et al.48 | 2012 | 783 (T2DM) | ― | 30–75 | 7.2 | CVD | Mean‐IMT (CCA) | Included | 2.39 (1.19–4.81) [per 1 SD]† |
| MESA49 | 2013 | 6,562 | 47.4 | 61.1 | 7.8 | CVD | Max‐IMT (ICA) | Excluded | 1.21 (1.13–1.30) [per mm]§ |
| Max‐IMT (ICA) > 1.5 mm | Excluded | 1.48 (1.21–1.80) [per mm]§ | |||||||
| Katakami, et al.50 | 2018 | 3,263 (T2DM) | 65.5 | 60.9 | 6.8 | CVD | Mean‐IMT (CCA) (n = 3260) | Included | 1.08 (1.05–1.11) [per 0.1 mm] |
| Max‐IMT (CCA) (n = 2243) | Included | 1.07 (1.04–1.10) [per 0.1 mm] | |||||||
| Max‐IMT (overall) (n = 540) | Included | 1.08 (1.05–1.11) [per 0.1 mm] |
†Age and sex adjusted. ‡Age and race adjusted. §Traditional risk factors adjusted. ARIC, Atherosclerosis Risk in Communities; Bif, bifurcation; CAPS, Carotid Atherosclerosis Progression Study; CCA, common carotid artery; CHS, Cardiovascular Health Study; CI, confidence interval; CVD, cardiovascular disease; ICA, internal carotid artery; IMT, intima‐media thickness; KIHD, Kuopio Ischemic Heart Disease Study; MDCS, Malmo Diet and Cancer Study; MESA, Multi‐Ethnic Study of Atherosclerosis; MI, myocardial infarction; SD, standard deviation; T2DM, type 2 diabetes mellitus.
Similarly, regarding diabetes patients, it has been reported that CIMT is a predictor of the future development of non‐fatal CAD52. The PROG‐IMT Study Group carried out a comprehensive meta‐analysis of the data from 3,902 patients with type 2 diabetes mellitus from 21 population‐based cohorts. They concluded that the hazard ratio (HR) of CVD events was 1.22 (95% confidence interval [CI] 1.12–1.33) per standard deviation difference in mean CCA‐IMT and 1.23 (95% CI 1.08–1.40) per standard deviation difference in maximum (max) CCA‐IMT, after adjustment for traditional risk factors. Although there was no statistically significant difference, the HR in patients with diabetes mellitus was a little higher than in people without diabetes mellitus (1.22 vs 1.15), suggesting that the association between CIMT and CVD might be more evident in individuals with diabetes mellitus53. More recently, a combined analysis of data obtained in five longitudinal studies that included 3,263 patients with diabetes, but without CVD, also confirmed that the mean CCA‐IMT (HR 1.08 for every 0.1‐mm increment, 95% CI 1.05–1.11, P < 0.001), CCA‐max‐IMT (HR 1.07 for every 0.1‐mm increment, 95% CI 1.04–1.10, P < 0.001) and max‐IMT (max‐IMT in the CCA, Bul and ICA segments; HR 1.08 for every 0.1‐mm increment, 95% CI 1.05–1.11, P < 0.001) at baseline could be a predictor for the development of CVD (CAD, cerebrovascular disease or peripheral arterial disease) in asymptomatic patients with type 2 diabetes mellitus, even after adjustment for traditional risk factors50.
Contribution of CIMT to CV Risk Prediction
Controversy remains regarding the contribution of CIMT to CV risk prediction over and above the traditional risk factors.
In the Multi‐Ethnic Study of Atherosclerosis, CCA‐IMT did not add significant predictive information for CAD or stroke over and above the Framingham risk score (FRS) alone (c‐statistics; 0.78 for FRS plus CIMT vs 0.77 for FRS alone)54. Similarly, in the Carotid Atherosclerosis Progression Study, CIMT did not improve the risk classification of study participants when added to the FRS55. Furthermore, a summary of systematic reviews56 and another meta‐analysis57 concluded that the addition of CIMT did not add clinically useful information to the standard prediction models. Under the influence of these findings, the 2013 ACC/AHA Cardiovascular Risk Guidelines58 and the 2016 European Guidelines on CVD prevention in clinical practice59 questioned the contribution of CIMT to risk assessment over and above the conventional risk scores.
In contrast, in participants of the Framingham Offspring Study cohort, max‐IMT and the presence of plaque in the ICA significantly improved the classification of risk of CVD over and above the FRS (increase in the c‐statistic of 0.009, 95% CI 0.003–0.016). Similarly, in the Atherosclerosis Risk in Communities study, Nambi et al.60 reported that CAD risk prediction can be improved by the addition of “CIMT + plaque” information to traditional risk factors. Baldassarre et al.61 also showed that CIMT improved the prediction of CV events. Furthermore, based on a systematic review of the previous studies, Peters et al.62 reported that the additional predictive value estimated by the increase in c‐statistic ranged from 0.00 to 0.03 for CIMT and from 0.01 to 0.05 for carotid plaques. They also reported that net reclassification improvement ranged from −1.4% to 12% for CIMT and 8% to 11% for carotid plaques.
The predictive performance of CIMT has been also evaluated in individuals with diabetes. Yoshida et al.48 reported that the combination of traditional risk factors and CIMT improved the prediction of CAD in patients with diabetes mellitus. More recently, a combined analysis of data obtained from longitudinal studies showed that the assessment of CIMT, such as CCA‐mean‐IMT, CCA‐max‐IMT and max‐IMT, in addition to traditional risk factors significantly improved the prediction of the occurrence of CVD in diabetes patients without apparent CVD50. It was also reported that CIMT improved the prediction of CV events in dyslipidemic patients63.
What Kind of Carotid Ultrasound Parameters Should Be Measured?
Comparisons of predictive ability for cardiovascular disease among carotid ultrasound measures are summarized as Table 1.
Plaque or CIMT
Although carotid plaque and CIMT are correlated with each other, they show different patterns of association with traditional risk factors and atherosclerotic diseases64. Therefore, plaque assessment in addition to CIMT should be considered in CV risk prediction. Several articles have shown that the assessment of carotid plaque (e.g., presence/absence of plaque, plaque thickness, plaque area or plaque score) is more useful than CIMT for predicting future CVD22, 65, 66. Furthermore, considering not only the largest identified plaque, but also the total plaque burden in both carotid arteries might further improve risk estimation67.
CIMT including plaque or CIMT not including plaque
According to a cohort study carried out in European countries, when plaques are incorporated into CIMT measurements, the predictive power of CIMT is greater compared with the information derived from plaques (regardless of the definition used) alone or from CIMT measured in plaque‐free areas alone61.
Which segment should be measured?
CIMT in Bul and ICA segments might reflect atherosclerosis more accurately than those obtained exclusively on the CCA, as Bul and ICA are more influenced by flow turbulence than CCA, and plaques are preferentially localized at Bul and ICA. Indeed, it has been reported that ICA‐IMT and composite variables derived from measurements that included Bul and ICA segments are more accurate predictors of CV events than CCA‐IMT24, 47, 61, 68. Similar results were observed in individuals with diabetes mellitus: it was shown that max‐IMT (=maximum value in the CCA, Bul and ICA segments) was more predictive than the mean IMT of the CCA for coronary artery stenosis. The c‐statistic for max‐IMT was significantly higher than that of the mean IMT (0.73 [95% CI 0.67–0.79] vs 0.64 [95% CI 0.58–0.72], P = 0.031)23.
Ultrasonic Tissue Characteristics of Carotid Lesions and CVDs
Assessment of plaque morphology with conventional B‐mode ultrasound
Atherothrombotic disease, including acute coronary syndrome, is caused by a disruption of “unstable” plaque, which consists mainly of high lipid content, inflammatory infiltration and neovascular vessels, and is covered by a thin cap. Therefore, it would be important to assess the stability of plaques to specify patients at high‐risk for atherothrombotic diseases.
Generally, the assessment of carotid plaques is carried out based on the following: (i) echogenicity; (ii) heterogeneity; and (iii) structure (Figure 2). Typically, carotid plaques are classified into hypoechoic (echolucent), isoechoic or hyperechoic (echodense) plaques, and then subclassified into heterogeneous or homogeneous plaques. It is considered that atheroma and hematoma appear as hypoechoic lesions, fibrosis appears as isoechoic lesions and calcification appears as hyperechoic lesions (Figure 3). Echolucent carotid plaques have been reported to predict future strokes69. It is also believed that heterogenous plaques with a complex echo pattern are more vulnerable to rupture than homogenous plaques. The surface morphology of the plaque is classified as smooth, irregular or ulcerated, and it has been reported that the risk of stroke is higher in individuals with ulcerated or irregular plaques70. However, assessment of tissue morphology using the above‐mentioned approach is subjective and qualitative.
Figure 2.
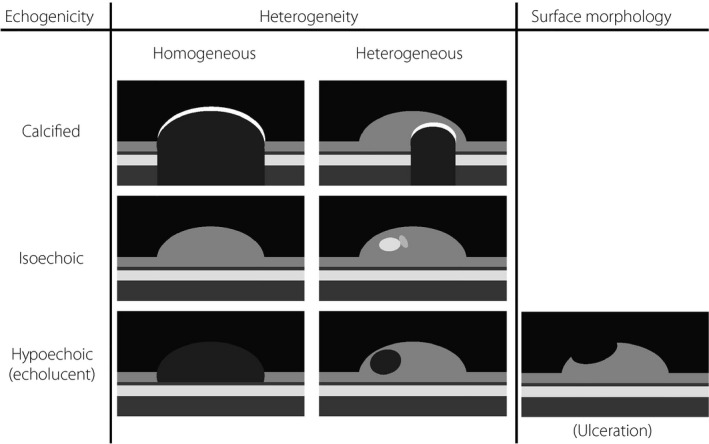
Assessment of plaque morphology with conventional B‐mode ultrasound. Generally, the assessment of carotid plaques are carried out based on the following: (i) echogenicity; (ii) heterogeneity; and (iii) structure. Typically, carotid plaques are classified into hypoechoic (echolucent), isoechoic or hyperechoic (echodense) plaques, and then subclassified into heterogeneous or homogeneous plaques. Plaque surface morphology is classified as smooth, irregular or ulcerated.
Figure 3.
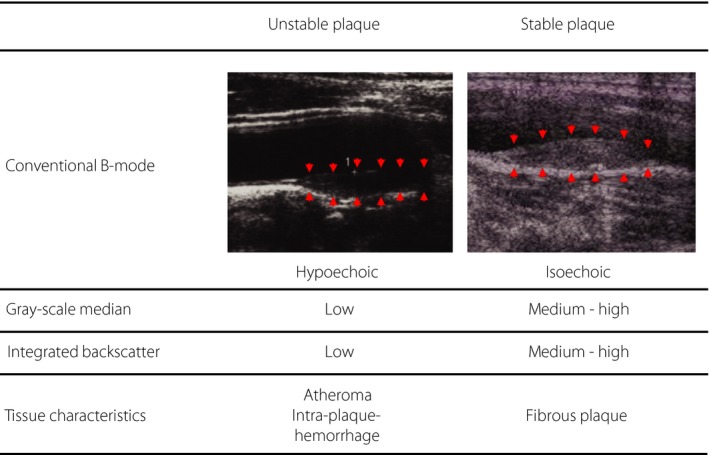
Association between pathological characteristics and ultrasonic tissue characteristics of carotid lesions. Unstable plaques, which consist mainly of foam cells and/or neovascular vessels, appear hypoechoic in conventional B‐mode ultrasound imaging and show low gray‐scale median (GSM) values and low integrated backscatter values. In contrast, stable plaques, which consist mainly of fibrous tissue and calcific components, appear hyperechoic and show medium (or relatively high) gray‐scale median values and medium (or relatively high) integrated backscatter values.
Tissue characterization of carotid plaques using gray‐scale median
Recent resolution enhancement of images obtained by B‐mode and computer‐assisted image processing has enabled semiquantitative evaluation of the echogenicity of carotid plaques. The gray‐scale median (GSM) of the frequency distribution of gray values of the pixels within the plaque is used as an index of echogenicity71. As the measurement of GSM values requires neither specific software nor a specific ultrasonic diagnostic equipment, it can be a practical approach (Figure 3).
To investigate whether non‐invasive ultrasonic tissue characterization of carotid plaque using GSM can predict future CVD, we carried out a prospective study of 287 diabetes patients with carotid plaque, but free from CVD, and showed that both the presence of low GSM plaques and plaque thickness were independent predictors of future occurrence of CV events, even after adjusting for traditional risk factors. The addition of plaque thickness to FRS significantly increased the c‐statistic (from 0.60 [95% CI 0.49–0.70] to 0.73 [95% CI 0.63–0.82], P < 0.05). Furthermore, the addition of the information about plaque echogenicity (i.e., presence or absence of low GSM plaque) to the FRS and plaque thickness further and significantly increased the c‐statistic (from 0.73 [95% CI 0.63–0.82] to 0.82 [95% CI 0.75–0.88], P < 0.05), suggesting that ultrasonic tissue characterization of carotid plaque by the GSM method can enhance the risk prediction of CV events in asymptomatic patients with diabetes mellitus 72.
Tissue characterization of carotid plaques using integrated backscatter ultrasound imaging
As B‐mode carotid ultrasonography is intended to delineate the edge of carotid plaque tissue, raw signals reflected from the tissue are processed with complex algorithms, such as omission of weak signals and derivation. Therefore, the resultant images have lost detailed information on the plaque tissue characteristics, and do not reflect small differences in reflection strength caused by difference in tissue morphology. Recent advancements in devices and image processing techniques have developed novel ultrasonic techniques that reflect small differences in reflection strength by analyses of ultrasonic signals before image processing. Backscatter signals from a reflector that is much smaller as compared with the wavelengths used in regular ultrasonic devices include scattered waves from inside the structure. In the integrated backscatter (IBS) approach, these backscatter signals are integrated over time. As this approach could quantify reflection of the ultrasound waves in a relatively wide range of 60 dB, it is effective in detecting small differences in echo strength and enables detailed quantitative analysis of lumen morphology.
Urbani et al.73 measured the IBS index in 15 individuals who were scheduled to have carotid thromboendarterectomy, and compared the value with pathological findings after surgery. In that study, they showed that the IBS index in atheroma, thrombi and intraplaque hemorrhage lesions was lower, but that the index in calcified sites was higher than in fibrous sites. Comparison of the ultrasound findings with pathological findings obtained from 12 individuals who had in vivo ultrasound examination of the carotid and femoral arteries in life, and ex vivo ultrasound examination after autopsy also showed similar results74. These findings suggested that the tissue characteristics of the carotid arterial wall could be estimated with the IBS index of the carotid wall (Figure 3).
Takiuchi et al.75 have reported that the IBS value of the carotid intima‐media complex was lower in individuals with at least two coronary risk factors and patients who had myocardial infarction within the past 3 months than in individuals with fewer coronary risk factors. In our study of patients with type 2 diabetes mellitus76, the IBS value of the carotid artery was significantly lower in individuals with a past history of acute coronary syndrome or atherothrombotic cerebral infarction within 6 months than in those with no apparent atherosclerotic disease, suggesting that tissue characterization using the IBS would be utilized in the risk assessment of future CVD. In our prospective study of 85 asymptomatic individuals with type 2 diabetes mellitus77, the risk of developing CV events was significantly elevated in individuals with low calibrated IBS values of the thickest point at baseline as compared with higher values, indicating that the IBS value of the carotid wall is an independent risk factor for future CV events. The c‐statistic for the prediction of future CV events was 0.63 when using the FRS, and increased significantly to 0.83 when using the FRS, IMT and IBS. These results show that the IBS method is useful for prediction of future CV events.
The calibrated IBS values in the carotid artery wall were significantly lower in individuals with than without untreated hyperlipidemia, and the improvement in the calibrated IBS during statin treatment was larger in individuals with a larger reduction in serum low‐density lipoprotein cholesterol levels78. The calibrated IBS values of the carotid artery wall were also lower in individuals with a larger number of risk factors for metabolic syndrome79.
Further clinical data are necessary to establish the IBS approach as a new method of assessment of atherosclerotic lesions.
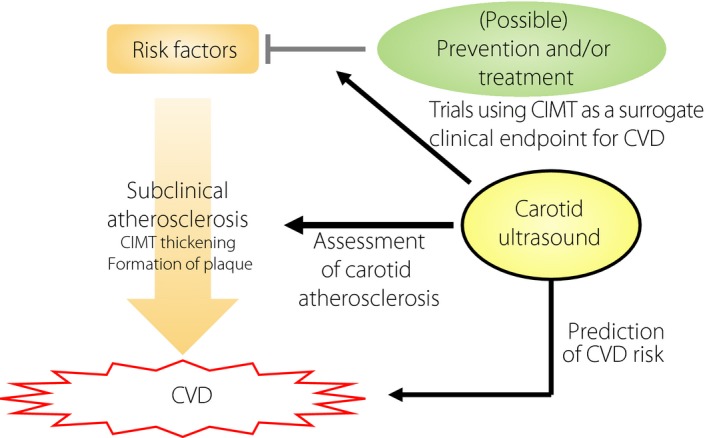
The above shows that the addition of ultrasonic tissue characteristics of carotid lesions, together with CIMT, to traditional risk factors improves the prediction ability of future CVD (Figure 4).
Figure 4.
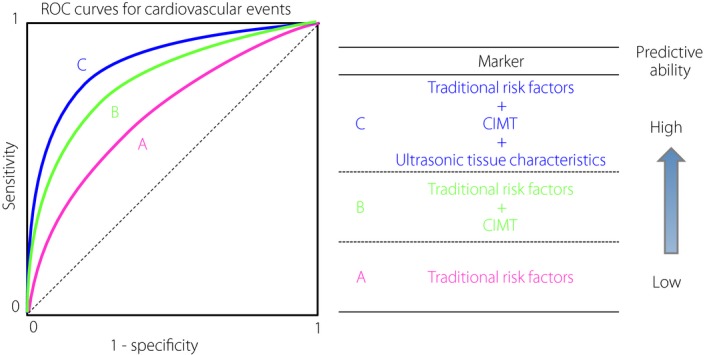
Ultrasonic tissue characterization of carotid lesions for the prediction of future cardiovascular events. The addition of information about ultrasonic tissue characteristics of carotid lesions assessed by the gray‐scale median of the frequency distribution of gray values of the pixels within the plaque or integrated backscatter ultrasound imaging, together with carotid intima‐media thickness (CIMT), to traditional risk factors significantly and substantially improves the prediction ability of future cardiovascular events. ROC, receiver operating characteristic.
Progression of CIMT and Risk of CVD: Potential as a Surrogate Outcome
As CIMT is a quantitative and repeatable measure that can be assessed non‐invasively, change over time in CIMT is a promising candidate for a surrogate outcome for CV events in clinical trials. Indeed, many clinical trials for antidyslipidemic and antidiabetic agents have used the CIMT as a surrogate clinical end‐point for CV events. However, sufficient evidence has not been accumulated regarding whether the progression of CIMT reflects an increased risk of subsequent CV events.
In considering this theme, the following reports will probably be useful references. In 146 men who previously had undergone coronary artery bypass graft surgery and completed the 2‐year Cholesterol Lowering Atherosclerosis Study80, the risk for CAD or coronary death was increased in individuals with a greater progression of CIMT. Sabeti et al.81 observed 1,065 individuals with carotid stenosis for 3.2 years, and found that individuals with progression in carotid stenosis had a significantly higher risk for CV events compared with those without it. More recently, in an epidemiological study of CVD based on a random sampling of more than 15,000 participants in Japan, Kokubo et al. showed that the multivariate‐adjusted HRs for CVD and stroke were 2.80 (95% CI 1.54–5.11) and 2.30 (95% CI 1.14–4.63) in the fourth quartile, respectively, as compared with the first quartile of 5‐year progression of max‐IMT82. These findings show that the progression of CIMT and carotid stenosis can be used as a surrogate endpoint of CV events.
There have been several meta‐analyses of clinical studies on the association between the development of CVD and the CIMT progression. In an early meta‐analysis of seven placebo‐controlled trials of statins that evaluated both CIMT outcomes and CV events, Espeland et al.83 concluded that the effect of statins on CIMT progression and CV events was qualitatively similar. Based on a meta‐analysis of 28 randomized clinical trials evaluating various CV therapies in 15,598 patients, Goldberger et al.84 showed that less progression in CIMT was associated with a lower risk for myocardial infarction.
In contrast, another meta‐analysis of 41 randomized clinical trials (18,307 patients, mean follow‐up period 2.4 years) showed that the regression or slowed progression of CIMT did not reflect a reduction in CV events85. The PROG‐IMT Study Group86 carried out an individual participant data meta‐analysis of the general population studies that measured CIMT at least twice, and followed up participants for myocardial infarction, stroke or death (16 studies, 36,984 participants, median follow‐up period 4 years, 2,028 combined end‐points). As a result, there was no significant association between the CIMT progression and the development of combined end‐points.
Based on a meta‐analysis of 3,902 adults with type 2 diabetes mellitus from population‐based cohorts, the PROG‐IMT Study Group also reported that the HR per standard deviation difference in the annual progression of mean CCA‐IMT over a mean time of 3.6 years was 0.99 (95% CI 0.91–1.08), and concluded that there was no significant association between CIMT progression and future event risk53. In contrast, a combined analysis of five longitudinal studies in which CIMT was evaluated in a total of 1,881 Japanese patients with diabetes following a standardized protocol showed that that the increase in the CCA‐mean‐IMT during the observation period was a significant prognostic factor for CVD (HR 2.37 for every 0.1‐mm/year increment, 95% CI 1.63–3.47, P < 0.001). Interestingly, the increments in the CCA‐mean‐IMT remained prognostic factors for CVD even after adjusting for traditional risk factors (HR 1.77 for every 0.1‐mm/year increment, 95% CI 1.18–2.66, P = 0.006)50.
It should be noted that there are several limitations in the analysis of the pooled data. First, CIMT is measured in many different ways with different approaches, and this variability might have influenced the adequate detection of significant findings. Second, as hard CV outcomes are considered to be typically apparent only after longer treatment, inclusion of many short‐term follow‐up trials could lead to incorrect conclusions about the association between CIMT and hard CV outcomes. Third, as the associations derived from meta‐regression analysis are observational, it could underestimate the real relationships derived from large, randomized, definitive controlled clinical trials87. Therefore, further study with sufficient power should be carried out to evaluate whether CIMT can be used as a surrogate end‐point of CVD.
Diabetes and CIMT
Diabetes promotes atherosclerosis
Individuals with diabetes mellitus and those with borderline glucose tolerance show higher CIMT than individuals with normal glucose tolerance19, 88, 89. A meta‐analysis of 23 studies including 24,111 participants with and without glucose intolerance showed that the patients with diabetes mellitus and individuals with impaired glucose tolerance had greater CIMT compared with the controls by 0.13 mm (95% CI 0.12–0.14 mm) and 0.04 mm (95% CI 0.014–0.071 mm), respectively89.
In a meta‐analysis of eight interventional trails in individuals with type 2 diabetes mellitus that assessed the effect of interventions on CIMT progression, the overall weighed rate of change in mean‐IMT among untreated type 2 diabetes mellitus patients was 0.034 mm/year (95% CI 0.029–0.039)90. As the annual increase in CIMT in healthy individuals has been reported to be 0.007–0.008 mm, CIMT thickening is supposed to be accelerated in individuals with poor‐controlled diabetes.
Reduction of CIMT Progression by Antidiabetic Treatment
It has been reported that CIMT correlates with chronic hyperglycemia. The aforementioned meta‐analysis of eight clinical studies90 showed a close correlation between the averaged HbA1c and CIMT progression during the follow‐up periods. This finding indicates that CIMT progression is attenuated when HbA1c, an index of chronic hyperglycemia, is lowered by antidiabetic treatment.
Insulin resistance plays a critical role in the pathogenesis of atherosclerosis. Therefore, it would be compatible that antidiabetic agents that improve insulin resistance, such as metformin91, 92 and pioglitazone93, 94, 95, were more effective in attenuating CIMT progression than sulfonylureas.
Temelkova‐Kurktschiev et al.96 reported that 2‐h post‐challenge plasma glucose and maximal plasma glucose levels during an oral glucose tolerance test were more closely associated with CIMT than fasting plasma glucose levels. Similarly, Esposito et al.97 reported that CIMT progression was greater in patients with larger incremental glucose peaks. These findings indicate that postprandial hyperglycemia plays an important role in CIMT progression. Actually, it has been shown that α‐glucosidase inhibitors98 and glinides99, 100 that ameliorate postprandial hyperglycemia can attenuate CIMT progression.
Experimental studies have shown that dipeptidyl peptidase‐4 (DPP‐4) inhibitors exert pleiotropic anti‐atherosclerotic effects in GLP‐1‐dependent and ‐independent manners. However, randomized clinical trials to evaluate the long‐term effect on major CV events of adding DPP‐4 inhibitors to usual care in patients with type 2 diabetes mellitus and CVD (e.g., the Examination of Cardiovascular Outcomes with Alogliptin versus Standard of Care (EXAMINE), the Saxagliptin Assessment of Vascular Outcomes Recorded in Patients with Diabetes Mellitus ‐Thrombolysis in Myocardial Infarction 53 trial (SAVOR‐TIMI53) and the Trial Evaluating Cardiovascular Outcomes with Sitagliptin (TECOS)) showed that the addition of DPP‐4 inhibitors to usual care did not have a significant influence on the rates of major adverse CV events in these populations. Interestingly, the Study of Preventive Effects of Alogliptin on Diabetic Atherosclerosis trial, an RCT in 341 patients with type 2 diabetes mellitus free of apparent CVD, showed that a DPP‐4 inhibitor, alogliptin, significantly attenuated the CIMT progression compared with the conventional treatment101. Similarly, the results of the Sitagliptin Preventive study of Intima‐media thickness Evaluation trial showed that another DPP‐4 inhibitor, sitagliptin, also attenuated the CIMT progression in insulin‐treated type 2 diabetes mellitus patients free of apparent CVD compared with conventional treatment102. However, in the Program of Vascular Evaluation under Glucose Control by DPP‐4 Inhibitor (PROLOGUE) study, in which type 2 diabetes mellitus patients both with and without previous CVD were enrolled, the addition of sitagliptin to the usual care failed to attenuate the CIMT progression103. Interestingly, the post‐hoc analysis of the PROLOGUE study showed significant inhibitory effects of sitagliptin on the mean and maximum internal CIMT in the primary prevention subgroup104. These findings suggest a favorable effect of DPP‐4 inhibitor treatment on CIMT in patients with type 2 diabetes mellitus but without apparent CVD.
Sodium–glucose cotransporter 2 (SGLT2) inhibitors, which enhance urinary glucose excretion, improve glycemic control with a low risk of hypoglycemia and ameliorate a variety of CV risk factors. The Empagliflozin Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients‐Removing Excess Glucose (EMPA‐REG) OUTCOME and the Canagliflozin Cardiovascular Assessment Study (CANVAS) Program, randomized clinical trials to evaluate the long‐term effect on major CV events of adding SGLT2 inhibitors to the usual care in diabetes patients with high risk for CVD, showed that the addition of SGLT2 inhibitors to usual care reduced the risk of major adverse CV events in these populations. However, the preventive effects of SGLT2 inhibitors on the CIMT progression remain unclear, whereas one small‐scale single‐arm study reported that 52 weeks of ipragliflozin treatment did not change CIMT. Ongoing RCTs, such as The Study of Using Tofogliflozin for Possible better Intervention against Atherosclerosis for type 2 diabetes patients (UTOPIA)105 and the Prevention of atherosclerosis by SGLT2 inhibitor; multicenter, randomized controlled study (PROTECT)106, are expected to show the preventive effect of SGLT2 inhibitors on CIMT progression.
Conclusion
Carotid ultrasonography is now widely used as a major marker of atherosclerosis (Table 4). Carotid ultrasound measures, including CIMT and carotid plaque, are useful markers of the progression of atherosclerosis throughout the body, and can be independent predictors of CV events in the general population and patients with diabetes. However, whether CIMT provides additional prognostic information over and above the traditional risk factors remains controversial. This confusion would not be irrelevant to the lack of a standardized and univocally accepted protocol for measurement of CIMT. Establishment of universally standardized methods is required. Notably, recent studies have shown that ultrasonic tissue characterization of carotid lesions using novel approaches (e.g., GSM, IBS) improves the prediction ability of future CV events. Thus, carotid ultrasonography is a useful tool for better clinical practice of atherosclerosis in patients with diabetes (Figure 5).
Table 4.
Characteristics of major functional/morphological markers of atherosclerosis
| Carotid IMT | Carotid plaque | Coronary artery calcium | FMD | PWV | ABI | |
|---|---|---|---|---|---|---|
| Predictive ability | Moderate | Good | Pretty good | Moderate | Moderate | Good |
| Safety | Very safe | Very safe | Relatively safe | Safe | Very safe | Very safe |
| Convenience | Convenient | Convenient | Complicated | Complicated | Very convenient | Convenient |
| Reproducibility | Good | Good | Good | Relatively good | Relatively good | Good |
| Cost | Low | Low | High | Low | Low | Low |
| ACC/AHA Guideline comments† |
III No benefit Level B |
(None) |
IIb Level B |
(None) | (None) |
IIb Level B |
| European Guideline comments‡ |
Class III Level A |
Class IIb Level B |
Class IIb Level B |
(None) | (None) |
Class IIb Level B |
†The 2013 American College of Cardiology (ACC)/American Heart Association (AHA) Cardiovascular Risk Guidelines: Classification of recommendation – I: Benefit >>> Risk. Procedure/treatment should be performed/administered. IIa: Benefit >> Risk. It is reasonable to perform the procedure/administer treatment. IIb: Benefit ≥ Risk. Procedure/treatment might be considered. III No benefit: Not helpful. III Harm: Excess cost without benefit or harmful. Level of evidence: A: Data derived from multiple randomized clinical trials or meta‐analyses. B: Data derived from a single randomized clinical trial or non‐randomized studies. C: Only consensus opinion of experts, case studies or standard of care. ‡The 2016 European Guidelines on cardiovascular disease prevention in clinical practice: Classes of recommendations – I: Evidence and/or general agreement that a given treatment or procedure is beneficial, useful or effective. II: Conflicting evidence and/or a divergence of opinion about the usefulness/efficacy of the given treatment or procedures. IIa: Weight of evidence/opinion is in favor of usefulness/efficacy. IIb: Usefulness/efficacy is less well established by evidence/opinion. III: Evidence or general agreement that the given treatment or procedure is not useful/effective, and in some cases might be harmful. Level of evidence: A: Data derived from multiple randomized clinical trials or meta‐analyses. B: Data derived from a single randomized clinical trial or large non‐randomized studies. C: Consensus of opinion of experts and/or small studies, retrospective studies, registries. ABI, ankle‐brachial index; FMD, flow‐mediated vasodilation; IMT, intima‐media thickness; PWV, pulse wave velocity.
Figure 5.
Carotid ultrasonography as a useful modality for clinical practice of atherosclerosis in patients with diabetes. Carotid ultrasound measures, including carotid intima‐media thickness (CIMT) and carotid plaque, are useful markers of the progression of atherosclerosis throughout the body, and can be independent predictors of cardiovascular events. Although sufficient evidence has not been accumulated, change over time in CIMT is a good candidate for a surrogate outcome for cardiovascular events in clinical trials. CVD, cardiovascular disease.
Disclosure
N Katakami is a staff member of the endowed chair (the Department of Metabolism and Atherosclerosis) donated by Kowa Pharmaceutical Co. Ltd., has received research funds from MSD, and lecture fees from Arkray Co. Ltd., Astellas Pharma Inc., AstraZeneca K.K., Boehringer Ingelheim, Daiichi Sankyo Inc., Dainippon Sumitomo Pharma Co., Eli Lilly, Kissei Pharmaceutical Co., Kowa Pharmaceutical Co., Kyowa Hakko Kirin Co. Ltd., Mitsubishi Tanabe Pharma Co., MSD K.K., Novartis Pharma K.K., Novo Nordisk Pharma, Ono Pharmaceutical Co., Otsuka Pharmaceutical Co., Taisho Toyama Pharmaceutical Co., Ltd., Takeda Pharmaceutical Co., Sanofi‐Aventis and Shionogi & Co. T Matsuoka has been assigned to Associate Professor (Department of Metabolic Medicine); has received research funds from Daiichi Sankyo Inc., Ono Pharmaceutical Co, Eli Lilly, Novo Nordisk Pharma and Takeda Pharmaceutical Co.; and lecture fees from MSD, Astellas Pharma Inc., Boehringer Ingelheim, Daiichi Sankyo Inc., Dainippon Sumitomo Pharma Co., Eli Lilly, Kissei Pharmaceutical Co., Kowa Pharmaceutical Co., Mitsubishi Tanabe Pharma Co., Novartis Pharma K.K., Novo Nordisk Pharma, Ono Pharmaceutical Co., Taisho Toyama Pharmaceutical Co., Ltd., Sanwa Kagaku Kenkyusho Co. and Sanofi Co. I Shimomura received lecture fees from Astellas Pharma Inc., AstraZeneca K.K., MSD K.K., Ono Pharmaceutical Co., Kyowa Hakko Kirin Co., Kowa Pharmaceutical Co., Sanofi K.K., Sanwa Kagaku Kenkyusho Co., Daiichi Sankyo Co., Takeda Pharma K.K., Mitsubishi Tanabe Pharma Co., Teijin Pharma, Eli Lilly Japan K.K., Nippon Boehringer Ingelheim Co., Novartis Pharma K.K., Novo Nordisk Pharma, Bayer Yakuhin, Pfizer Japan Inc., Bristol‐Myers K.K., Mochida Pharmaceutical Co., Shionogi & Co. and Taisho Toyama Pharmaceutical Co.; clinical commissioned/joint research grants from Mochida Pharmaceutical Co., Kowa Pharmaceutical Co. and ROHTO Pharmaceutical Co.; and research funds from Astellas Pharma Inc., AstraZeneca K.K., MSD K.K, Ono Pharmaceutical Co., Kissei Pharmaceutical Co., Kyowa Hakko Kirin Co., Sanofi K.K., Shionogi & Co., Daiichi Sankyo Co., Dainippon Sumitomo Pharma Co., Takeda Pharma K.K., Mitsubishi Tanabe Pharma Co., Teijin Pharma, Novartis Pharma K.K., Novo Nordisk Pharma, Mochida Pharmaceutical Co., Eli Lilly Japan K.K, Kowa Co., Ltd. and Kowa Pharmaceutical Co.
J Diabetes Investig 2019; 10: 883–898
References
- 1. van Dieren S, Peelen LM, Nöthlings U, et al External validation of the UK Prospective Diabetes Study (UKPDS) risk engine in patients with type 2 diabetes. Diabetologia 2011; 54: 264–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Simmons RK, Coleman RL, Price HC, et al Performance of the UK Prospective Diabetes Study Risk Engine and the Framingham Risk Equations in Estimating Cardiovascular Disease in the EPIC‐ Norfolk Cohort. Diabetes Care 2009; 32: 708–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Stephens JW, Ambler G, Vallance P, et al Cardiovascular risk and diabetes. Are themethods of risk prediction satisfactory?. Eur J Cardiovasc Prev Rehabil 2004; 11: 521–528. [DOI] [PubMed] [Google Scholar]
- 4. Pignoli P, Tremoli E, Poli A, et al Intimal plus medial thickness of the arterial wall: a direct measurement with ultrasound imaging. Circulation 1986; 74: 1399–1406. [DOI] [PubMed] [Google Scholar]
- 5. Huang LC, Lin RT, Chen CF, et al Predictors of carotid intima‐media thickness and plaque progression in a Chinese population. J Atheroscler Thromb 2016; 23: 940–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wada S, Koga M, Toyoda K, et al Associated with intima‐media complex thickness of the common carotid artery in Japanese noncardioembolic stroke patients with hyperlipidemia: the J‐STARS Echo study. J Atheroscler Thromb 2017; 25: 359–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Näslund U, Ng N, Lundgren A, et al Visualization of asymptomatic atherosclerotic disease for optimum cardiovascular prevention (VIPVIZA): a pragmatic, open‐label, randomised controlled trial. Lancet 2018; 393: 133–142. [DOI] [PubMed] [Google Scholar]
- 8. Gepner AD, Korcarz CE, Aeschlimann SE, et al Validation of a carotid intima‐media thickness border detection program for use in an office setting. J Am Soc Echocardiogr 2006; 19: 223–228. [DOI] [PubMed] [Google Scholar]
- 9. Terminology and Diagnostic Criteria Committee , Japan Society of Ultrasonics in Medicine , Subcommittee for Preparing Guidelines for Ultrasound Diagnosis of Carotid Artery . Standard method for ultrasound evaluation of carotid artery lesions. J Med Ultrason 2009; 36: 511–518. [Google Scholar]
- 10. Subcommittee for Guidelines for Ultrasound Diagnosis of Carotid Artery . Standard method for ultrasound evaluation of carotid artery lesions 2017. The Japan Society of Ultrasonics in Medicine; Available from http://www.jsum.or.jp/committee/diagnostic/pdf/jsum0515_guideline.pdf (Japanese) [Google Scholar]
- 11. Joint Committee with the Guidelines Subcommittee of the Japan Academy of Neurosonology for Ultrasonic Assessment of Carotid Artery Disease and the Subcommittee for Research into Methods of Screening Atherosclerotic Lesions . Guidelines for ultrasonic assessment of carotid artery disease: preliminary report. Neurosonology 2002; 15: 20–33 (Japanese). [Google Scholar]
- 12. The Joint Committee of “The Japan Academy of Neurosonology” and “The Japan Society of Embolus Detection and Treatment” on Guideline for Nuerosonology . Guidelines for carotid ultrasound examination. Neurosonology 2006; 19: 49–69(Japanese) [Google Scholar]
- 13. Stein JH, Korcarz CE, Hurst RT, et al Use of carotid ultrasound to identify subclinical vascular disease and evaluate cardiovascular disease risk: a consensus statement from the American Society of Echocardiography Carotid Intima‐Media Thickness Task Force. Endorsed by the Society for Vascular Medicine. J Am Soc Echocardiogr 2008; 21: 93–111. [DOI] [PubMed] [Google Scholar]
- 14. Touboul PJ, Hennerici MG, Meairs S, et al Mannheim carotid intima‐media thickness consensus (2004–2006–2011): an update on behalf of the advisory board of the 3rd and 4th watching the risk symposium 13th and 15th European Stroke Conferences, Mannheim, Germany, 2004, and Brussels, Belgium, 2006. Cerebrovasc Dis 2012; 34: 290–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wofford JL, Kahl FR, Howard GR, et al Relation of extent of extracranial carotid artery atherosclerosis as measured by B‐mode ultrasound to the extent of coronary atherosclerosis. Arterioscler Thromb 1991; 11: 1786–1794. [DOI] [PubMed] [Google Scholar]
- 16. Hulthe J, Wikstrand J, Emanuelsson H, et al Atherosclerotic changes in the carotid artery bulb as measured by B‐mode ultrasound are associated with the extent of coronary atherosclerosis. Stroke 1997; 28: 1189–1194. [DOI] [PubMed] [Google Scholar]
- 17. Kasami R, Kaneto H, Katakami N, et al Relationship between carotid intima‐media thickness and the presence and extent of coronary stenosis in type 2 diabetic patients with carotid atherosclerosis but without history of coronary artery disease. Diabetes Care 2011; 34: 468–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nagai Y, Metter EJ, Earley CJ, et al Increased carotid artery intimal‐medial thickness in asymptomatic older subjects with exercise‐induced myocardial ischemia. Circulation 1998; 98: 1504–1509. [DOI] [PubMed] [Google Scholar]
- 19. Yamasaki Y, Kwamori R, Matsushima H, et al Asymptomatic hyperglycaemia is associated with increased intimal plus medial thickness of the carotid artery. Diabetologia 1995; 38: 585–591. [DOI] [PubMed] [Google Scholar]
- 20. Mitstuhashi N, Onuma T, Kubo S, et al Coronary artery disease and carotid artery intima‐media thickness in Japanese type 2 diabetic patients. Diabetes Care 2002; 25: 1308–1312. [DOI] [PubMed] [Google Scholar]
- 21. O'Leary DH, Polak JF, Kronmal RA, et al Distribution and correlates of sonographically detected carotid artery disease in the Cardiovascular Health Study. The CHS Collaborative Research Group. Stroke 1992; 23: 1752–1760. [DOI] [PubMed] [Google Scholar]
- 22. Inaba Y, Chen JA, Bergmann SR. Carotid plaque, compared with carotid intima‐media thickness, more accurately predicts coronary artery disease events: a meta‐analysis. Atherosclerosis 2012; 220: 128–133. [DOI] [PubMed] [Google Scholar]
- 23. Irie Y, Katakami N, Kaneto H, et al Maximum carotid intima‐media thickness improves the prediction ability of coronary artery stenosis in type 2 diabetic patients without history of coronary artery disease. Atherosclerosis 2012; 221: 438–444. [DOI] [PubMed] [Google Scholar]
- 24. Fujihara K, Suzuki H, Sato A, et al Carotid artery plaque and LDL‐to‐HDL cholesterol ratio predict atherosclerotic status in coronary arteries in asymptomatic patients with type 2 diabetes mellitus. J Atheroscler Thromb 2013; 20: 452–464. [DOI] [PubMed] [Google Scholar]
- 25. Irie Y, Katakami N, Kaneto H, et al The utility of carotid ultrasonography in identifying severe coronary artery disease in asymptomatic type 2 diabetes patients without history of coronary artery disease. Diabetes Care 2013; 36: 1327–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hirai K, Imamura S, Hirai A, et al Risk factors and utility of maximum carotid intima‐media thickness as a surrogate marker for coronary artery stenosis. Ther Clin Risk Manag 2018; 14: 1407–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Flaherty ML, Kissela B, Khoury JC, et al Carotid artery stenosis as a cause of stroke. Neuroepidemiology 2013; 40: 36–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Petty GW, Brown RD, Whisnant JP, et al Ischemic stroke subtypes: a population‐based study of incidence and risk factors. Stroke 1999; 30: 2513–2516. [DOI] [PubMed] [Google Scholar]
- 29. Schneider AT, Kissela B, Woo D, et al Ischemic stroke subtypes: a population‐based study of incidence rates among blacks and whites. Stroke 2004; 35: 1552–1556. [DOI] [PubMed] [Google Scholar]
- 30. Hougaku H, Matsumoto M, Handa N, et al Asymptomatic carotid lesions and silent cerebral infarction. Stroke 1994; 25: 566–570. [DOI] [PubMed] [Google Scholar]
- 31. Finn C, Giambrone AE, Gialdini G, et al The association between carotid artery atherosclerosis and silent brain infarction: a systematic review and meta‐analysis. J Stroke Cerebrovasc Dis 2017; 26: 1594–1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Burke GL, Evans GW, Riley WA, et al Arterial wall thickness is associated with prevalent cardiovascular disease in middle‐aged adults. The Atherosclerosis Risk in Communities (ARIC) Study. Stroke 1995; 26: 386–391. [DOI] [PubMed] [Google Scholar]
- 33. Allan PL, Mowbray PL, Lee AJ, et al Relationship between carotid intima‐media thickness and symptomatic and asymptomatic peripheral arterial disease. The Edinburgh Artery Study. Stroke 1997; 28: 348–353. [DOI] [PubMed] [Google Scholar]
- 34. Wattanakit K, Folsom AR, Selvin E, et al Risk factors for peripheral arterial disease incidence in persons with diabetes: the Atherosclerosis Risk in Communities (ARIC) Study. Atherosclerosis 2005; 180: 389–397. [DOI] [PubMed] [Google Scholar]
- 35. Salonen JT, Salonen R. Ultrasonographically assessed carotid morphology and the risk of coronary heart disease. Atheroscler Thromb 1991; 11: 1245–1249. [DOI] [PubMed] [Google Scholar]
- 36. Chambless LE, Heiss G, Folsom AR, et al Association of coronary heart disease incidence with carotid arterial wall thickness and major risk factors: the Atherosclerosis Risk in Communities (ARIC) Study, 1987–1993. Am J Epidemiol 1997; 146: 483–494. [DOI] [PubMed] [Google Scholar]
- 37. Chambless LE, Folsom AR, Clegg LX, et al Carotid wall thickness is predictive of incident clinical stroke: the Atherosclerosis Risk in Communities (ARIC) study. Am J Epidemiol 2000; 151: 478–487. [DOI] [PubMed] [Google Scholar]
- 38. Bots ML, Hoes AW, Koudstaal PJ, et al Common carotid intima‐media thickness and risk of stroke and myocardial infarction: the Rotterdam Study. Circulation 1997; 96: 1432–1437. [DOI] [PubMed] [Google Scholar]
- 39. Hollander M, Hak AE, Koudstaal PJ, et al Comparison between measures of atherosclerosis and risk of stroke: the Rotterdam Study. Stroke 2003; 34: 2367–2372. [DOI] [PubMed] [Google Scholar]
- 40. van der Meer IM, Bots ML, Hofman A, et al Predictive value of noninvasive measures of atherosclerosis for incident myocardial infarction: the Rotterdam Study. Circulation 2004; 109: 1089–1094. [DOI] [PubMed] [Google Scholar]
- 41. O'Leary DH, Polak JF, Kronmal RA, et al Carotid‐artery intima and media thickness as a risk factor for myocardial infarction and stroke in older adults. Cardiovascular Health Study Collaborative Research Group. N Engl J Med 1999; 340: 14–22. [DOI] [PubMed] [Google Scholar]
- 42. Cao JJ, Arnold AM, Manolio TA, et al Association of carotid artery intima‐media thickness, plaques, and C‐reactive protein with future cardiovascular disease and all‐cause mortality: the Cardiovascular Health Study. Circulation 2007; 116: 32–38. [DOI] [PubMed] [Google Scholar]
- 43. Rosvall M, Janzon L, Berglund G, et al Incidence of stroke is related to carotid IMT even in the absence of plaque. Atherosclerosis 2005; 179: 325–331. [DOI] [PubMed] [Google Scholar]
- 44. Lorenz MW, von Kegler S, Steinmetz H, et al Carotid intima‐media thickening indicates a higher vascular risk across a wide age range: prospective data from the Carotid Atherosclerosis Progression Study (CAPS). Stroke 2006; 37: 87–92. [DOI] [PubMed] [Google Scholar]
- 45. Johnsen SH, Mathiesen EB, Joakimsen O, et al Carotid atherosclerosis is a stronger predictor of myocardial infarction in women than in men: a 6‐year follow‐up study of 6226 persons: the Tromsø Study. Stroke 2007; 38: 2873–2880. [DOI] [PubMed] [Google Scholar]
- 46. Mathiesen EB, Johnsen SH, Wilsgaard T, et al Carotid plaque area and intima‐media thickness in prediction of first‐ever ischemic stroke: a 10‐year follow‐up of 6584 men and women: the Tromsø Study. Stroke 2011; 42: 972–978. [DOI] [PubMed] [Google Scholar]
- 47. Polak JF, Pencina MJ, Pencina KM, et al Carotid‐wall intima‐media thickness and cardiovascular events. N Engl J Med 2011; 365: 213–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Yoshida M, Mita T, Yamamoto R, et al Combination of the Framingham risk score and carotid intima‐media thickness improves the prediction of cardiovascular events in patients with type 2 diabetes. Diabetes Care 2012; 35: 178–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Polak JF, Szklo M, Kronmal RA, et al The value of carotid artery plaque and intima‐media thickness for incident cardiovascular disease: the multi‐ethnic study of atherosclerosis. J Am Heart Assoc 2013; 2: e000087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Katakami N, Mita T, Gosho M, et al Clinical utility of carotid ultrasonography in the prediction of cardiovascular events in patients with diabetes ‐ a combined analysis of data obtained in five longitudinal studies. J Atheroscler Thromb 2018; 25: 1053–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Lorenz MW, Markus HS, Bots ML, et al Prediction of clinical cardiovascular events with carotid intima‐media thickness: a systematic review and meta‐analysis. Circulation 2007; 115: 459–467. [DOI] [PubMed] [Google Scholar]
- 52. Yamasaki Y, Kodama M, Nishizawa H, et al Carotid intima‐media thickness in Japanese type 2 diabetic subjects: predictors of progression and relationship with incident coronary heart disease. Diabetes Care 2000; 23: 1310–1315. [DOI] [PubMed] [Google Scholar]
- 53. Lorenz MW, Price JF, Robertson C, et al Carotid intima‐media thickness progression and risk of vascular events in people with diabetes: results from the PROG‐IMT collaboration. Diabetes Care 2015; 38: 1921–1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Yeboah J, McClelland RL, Polonsky TS, et al Comparison of novel risk markers for improvement in cardiovascular risk assessment in intermediate risk individuals. JAMA 2012; 308: 788–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Lorenz MW, Schaefer C, Steinmetz H, et al Is carotid intima media thickness useful for individual prediction of cardiovascular risk? Ten‐year results from the Carotid Atherosclerosis Progression Study (CAPS). Eur Heart J 2010; 31: 2041–2048. [DOI] [PubMed] [Google Scholar]
- 56. Helfand M, Buckley DI, Freeman M, et al Emerging risk factors for coronary heart disease: a summary of systematic reviews conducted for the U.S. Preventive Services Task Force. Ann Intern Med 2009; 151: 496–507. [DOI] [PubMed] [Google Scholar]
- 57. Den Ruijter HM, Peters SA, Anderson TJ, et al Common carotid intima‐media thickness measurements in cardiovascular risk prediction: a meta‐analysis. JAMA 2012; 308: 796–803. [DOI] [PubMed] [Google Scholar]
- 58. Goff DC Jr, Lloyd‐Jones DM, Bennett G, et al 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014; 129: S49–S73. [DOI] [PubMed] [Google Scholar]
- 59. Piepoli MF, Hoes AW, Agewall S, et al 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts) Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Atherosclerosis 2016;252:207–274. [DOI] [PubMed] [Google Scholar]
- 60. Nambi V, Chambless L, He M, et al Common carotid artery intima‐media thickness is as good as carotid intima‐media thickness of all carotid artery segments in improving prediction of coronary heart disease risk in the Atherosclerosis Risk in Communities (ARIC) study. Eur Heart J 2012; 33: 183–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Baldassarre D, Hamsten A, Veglia F, et al Measurements of carotid intima‐media thickness and of interadventitia common carotid diameter improve prediction of cardiovascular events: results of the IMPROVE (Carotid Intima Media Thickness [IMT] and IMT‐Progression as Predictors of Vascular Events in a High Risk European Population) study. J Am Coll Cardiol 2012; 60: 1489–1499. [DOI] [PubMed] [Google Scholar]
- 62. Peters SA, den Ruijter HM, Bots ML, et al Improvements in risk stratification for the occurrence of cardiovascular disease by imaging subclinical atherosclerosis: a systematic review. Heart 2012; 98: 177–184. [DOI] [PubMed] [Google Scholar]
- 63. Baldassarre D, Amato M, Pustina L, et al Measurement of carotid artery intima‐media thickness in dyslipidemic patients increases the power of traditional risk factors to predict cardiovascular events. Atherosclerosis 2007; 191: 403–408. [DOI] [PubMed] [Google Scholar]
- 64. Ebrahim S, Papacosta O, Whincup P, et al Carotid plaque, intima media thickness, cardiovascular risk factors, and prevalent cardiovascular disease in men and women: the British Regional Heart Study. Stroke 1999; 30: 841–850. [DOI] [PubMed] [Google Scholar]
- 65. Störk S, van den Beld AW, von Schacky C, et al Carotid artery plaque burden, stiffness, and mortality risk in elderly men: a prospective, population‐based cohort study. Circulation 2004; 110: 344–348. [DOI] [PubMed] [Google Scholar]
- 66. Yoon HJ, Kim KH, Park H, et al Carotid plaque rather than intima‐media thickness as a predictor of recurrent vascular events in patients with acute ischemic stroke. Cardiovasc Ultrasound 2017; 15: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Spence JD. Technology Insight: ultrasound measurement of carotid plaque–patient management, genetic research, and therapy evaluation. Nat Clin Pract Neurol 2006; 2: 611–619. [DOI] [PubMed] [Google Scholar]
- 68. Polak JF, Pencina MJ, Meisner A, et al Associations of carotid artery intima‐media thickness (IMT) with risk factors and prevalent cardiovascular disease: comparison of mean common carotid artery IMT with maximum internal carotid artery IMT. J Ultrasound Med 2010; 29: 1759–1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Gronholdt ML, Nordestgaard BG, Schroeder TV, et al Ultrasonic echolucent carotid plaques predict future strokes. Circulation 2001; 104: 68. [DOI] [PubMed] [Google Scholar]
- 70. Troyer A, Saloner D, Pan XM, et al Major carotid plaque surface irregularities correlate with neurologic symptoms. J Vasc Surg 2002; 35: 741. [DOI] [PubMed] [Google Scholar]
- 71. Falkowski A, Kaczmarczyk M, Cieszanowski A, et al Computer‐assisted characterisation of a carotid plaque. Med Sci Monit 2004; 10: 67–70. [PubMed] [Google Scholar]
- 72. Irie Y, Katakami N, Kaneto H, et al The utility of ultrasonic tissue characterization of carotid plaque in the prediction of cardiovascular events in diabetic patients. Atherosclerosis 2013; 230: 399–405. [DOI] [PubMed] [Google Scholar]
- 73. Urbani MP, Picano E, Parenti G, et al In vivo radiofrequency‐based ultrasonic tissue characterization of the atherosclerotic plaque. Atherosclerosis 1993; 24: 1507–1512. [DOI] [PubMed] [Google Scholar]
- 74. Kawasaki M, Takatsu H, Noda T, et al Noninvasive quantitative tissue characterization and two‐dimensional color‐coded map of human atherosclerotic lesions using ultrasound integrated backscatter: comparison between histology and integrated backscatter images. J Am Coll Cardiol 2001; 38: 486–492. [DOI] [PubMed] [Google Scholar]
- 75. Takiuchi S, Rakugi H, Honda K, et al Quantitative ultrasonic tissue characterization can identify high‐risk atherosclerotic alteration in human carotid arteries. Circulation 2000; 102: 766. [DOI] [PubMed] [Google Scholar]
- 76. Katakami N, Yamasaki Y, Kosugi K, et al Tissue characterization identifies subjects with high risk of cardiovascular diseases. Diabetes Res Clin Pract 2004; 63: 93–102. [DOI] [PubMed] [Google Scholar]
- 77. Katakami N, Takahara M, Kaneto H, et al Ultrasonic tissue characterization of carotid plaque improves the prediction of cardiovascular events in diabetic patients: a pilot study. Diabetes Care 2012; 35: 2640–2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Katakami N, Sakamoto K, Kaneto H, et al Lipid‐lowering with atorvastatin improves tissue characteristics of carotid wall. Atherosclerosis 2005; 183: 369–371. [DOI] [PubMed] [Google Scholar]
- 79. Katakami N, Kaneto H, Matsuhisa M, et al Clustering of several cardiovascular risk factors affects tissue characteristics of the carotid artery. Atherosclerosis 2008; 198: 208–213. [DOI] [PubMed] [Google Scholar]
- 80. Hodis HN, Mack WJ, Labree L, et al The role of carotid arterial intima‐media thickness in predicting clinical coronary events. Ann Intern Med 1998; 128: 262–269. [DOI] [PubMed] [Google Scholar]
- 81. Sabeti S, Schlager O, Exner M, et al Progression of carotid stenosis detected by duplex ultrasonography predicts adverse outcomes in cardiovascular high‐risk patients. Stroke 2007; 38: 2887–2894. [DOI] [PubMed] [Google Scholar]
- 82. Kokubo Y, Watanabe M, Higashiyama A, et al Impact of intima‐media thickness progression in the common carotid arteries on the risk of incident cardiovascular disease in the Suita study. J Am Heart Assoc 2018; 7: pii: e007720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Espeland MA, O'Leary DH, Terry JG, et al Carotid intimal‐media thickness as a surrogate for cardiovascular disease events in trials of HMG‐CoA reductase inhibitors. Curr Control Trials Cardiovasc Med 2005; 6: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Goldberger ZD, Valle JA, Dandekar VK, et al Are changes in carotid intima‐media thickness related to risk of nonfatal myocardial infarction? A critical review and meta‐regression analysis. Am Heart J 2010; 160: 701–714. [DOI] [PubMed] [Google Scholar]
- 85. Costanzo P, Perrone‐Filardi P, Vassallo E, et al Does carotid intima‐media thickness regression predict reduction of cardiovascular events? A meta‐analysis of 41 randomized trials. J Am Coll Cardiol 2010; 56: 2006–2020. [DOI] [PubMed] [Google Scholar]
- 86. Lorenz MW, Polak JF, Kavousi M, et al Carotid intima‐media thickness progression to predict cardiovascular events in the general population (the PROG‐IMT collaborative project): a meta‐analysis of individual participant data. Lancet 2012; 379: 2053–2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. LeLorier J, Grégoire G, Benhaddad A, et al Discrepancies between meta‐analyses and subsequent large randomized, controlled trials. N Engl J Med 1997; 337: 536–542. [DOI] [PubMed] [Google Scholar]
- 88. Wagenknecht LE, D'Agostino RB, Haffner SM, et al Impaired glucose tolerance, type 2 diabetes, and carotid wall thickness. The insulin resistance atherosclerosis study. Diabetes Care 1998; 21: 1812–1818. [DOI] [PubMed] [Google Scholar]
- 89. Brohall G, Oden A, Fagerberg B. Carotid artery intima‐media thickness in patients with Type 2 diabetes mellitus and impaired glucose tolerance: a systematic review. Diabet Med 2005; 23: 609–616. [DOI] [PubMed] [Google Scholar]
- 90. Yokoyama H, Katakami N, Yamasaki Y. Recent advances of intervention to inhibit progression of carotid intima‐media thickness in patients with type 2 diabetes mellitus. Stroke 2006; 37: 2420–2427. [DOI] [PubMed] [Google Scholar]
- 91. Katakami N, Yamasaki Y, Hayaishi‐Okano R, et al Metformin or gliclazide, rather than glibenclamide, attenuate progression of carotid intima‐media thickness in subjects with type 2 diabetes. Diabetologia 2004; 47: 1906–1913. [DOI] [PubMed] [Google Scholar]
- 92. Matsumoto K, Sera Y, Abe Y, et al Metformin attenuates progression of carotid arterial wall thickness in patients with type 2 diabetes. Diabetes Res Clin Pract 2004; 64: 225–228. [DOI] [PubMed] [Google Scholar]
- 93. Koshiyama H, Shimono D, Kuwamura N, et al Inhibitory effect of pioglitazone on carotid arterial wall thickness in type 2 diabetes. J Clin Endocrinol Metab 2001; 86: 3452–3456. [DOI] [PubMed] [Google Scholar]
- 94. Davidson M, Meyer PM, Haffner S, et al Increased high‐density lipoprotein cholesterol predicts the pioglitazone‐mediated reduction of carotid intima‐media thickness progression in patients with type 2 diabetes mellitus. Circulation 2008; 117: 2123–2130. [DOI] [PubMed] [Google Scholar]
- 95. Yamasaki Y, Katakami N, Furukado S, et al Long term effects of pioglitazone on carotid atherosclerosis in Japanese patients with type 2 diabetes without a recent history of macrovascular morbidity. J Atheroscler Thromb 2010; 17: 1132–1140. [DOI] [PubMed] [Google Scholar]
- 96. Temelkova‐Kurktschiev TS, Koehler C, Henkel E, et al Postchallenge plasma glucose and glycemic spikes are more strongly associated with atherosclerosis than fasting glucose or HbA1c level. Diabetes Care 2000; 23: 1830–1834. [DOI] [PubMed] [Google Scholar]
- 97. Esposito K, Ciotla M, Carleo D, et al Post‐meal glucose peaks at home associate with carotid intima‐media thickness in type 2 diabetes. J Clin Endocrinol Metab 2008; 93: 1345–1350. [DOI] [PubMed] [Google Scholar]
- 98. Yamasaki Y, Katakami N, Hayaishi‐Okano R, et al Alpha‐Glucosidase inhibitor reduces the progression of carotid intima‐media thickness. Diabetes Res Clin Pract 2005; 67: 204–210. [DOI] [PubMed] [Google Scholar]
- 99. Esposito K, Giugliano D, Nappo F, et al Regression of carotid atherosclerosis by control of postprandial hyperglycemia in type 2 diabetes mellitus. Circulation 2004; 110: 214–219. [DOI] [PubMed] [Google Scholar]
- 100. Mita T, Watada H, Shimizu T, et al Nateglinide reduces carotid intima‐media thickening in type 2 diabetic patients under good glycemic control. Arterioscler Thromb Vasc Biol 2007; 27: 2456–2462. [DOI] [PubMed] [Google Scholar]
- 101. Mita T, Katakami N, Yoshii H, et al Alogliptin, a dipeptidyl peptidase‐4 inhibitor, prevents the progression of carotid atherosclerosis in patients with type 2 diabetes mellitus: the study of preventive effects of alogliptin on diabetic atherosclerosis (SPEAD‐A). Diabetes Care 2016; 39: 139–148. [DOI] [PubMed] [Google Scholar]
- 102. Mita T, Katakami N, Shiraiwa T, et al Sitagliptin attenuates the progression of carotid intima‐media thickening in insulin‐treated patients with type 2 diabetes mellitus: the Sitagliptin Preventive study of Intima‐media thickness Evaluation (SPIKE): a randomized controlled trial. Diabetes Care 2016; 39: 455–464. [DOI] [PubMed] [Google Scholar]
- 103. Oyama J, Murohara T, Kitakaze M, et al The effect of sitagliptin on carotid artery atherosclerosis in type 2 diabetes: the PROLOGUE randomized controlled trial. PLoS Med 2016; 13: e1002051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Tanaka A, Yoshida H, Nanasato M, et al Sitagliptin on carotid intima‐media thickness in type 2 diabetes patients receiving primary or secondary prevention of cardiovascular disease: a subgroup analysis of the PROLOGUE study. Int J Cardiol 2018; 271: 331–335. [DOI] [PubMed] [Google Scholar]
- 105. Katakami N, Mita T, Yoshii H, et al Rationale, design, and baseline characteristics of the UTOPIA trial for preventing diabetic atherosclerosis using an SGLT2 inhibitor: a prospective, randomized, open‐label. Parallel‐group Comparative study. Diabetes Ther 2017; 8: 999–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Tanaka A, Murohara T, Taguchi I, et al Rationale and design of a multicenter randomized controlled study to evaluate the preventive effect of ipragliflozin on carotid atherosclerosis: the PROTECT study. Cardiovasc Diabetol 2016; 15: 133. [DOI] [PMC free article] [PubMed] [Google Scholar]


