Abstract
Cystic Echinococcosis (CE) caused by Echinococcus granulosus sensu lato (s.l.) is one of the most important parasitic zoonotic diseases in the world and it represents an important public health and socio-economic concern. In the Mediterranean basin, CE is widespread and it is endemic in Italy, with major prevalence in southern areas. Several studies have investigated CE in domestic pigs, however, such data in wild boars are scant. In the last decades the wild boar population in Italy has increased and this ungulate could play an important role in the spreading of CE in the wild. Here we report on the prevalence and fertility rate of hydatid cysts in wild boars that were shot during two hunting seasons (2016–2017) in the Campania region of southern Italy.
For each animal, a detailed inspection of the carcass and organs (lungs, liver and spleen) was performed and when cysts were found, their number, morphology and fertility were determined by visual and microscopic examination. Cysts were classified morphologically as fertile, sterile, caseous and calcified. Protoscoleces and germinal layers were collected from individual cysts and DNA was extracted to identify different strains/genotypes of E. granulosus s.l.
Out of a total of 2108 wild boars 93 (4.4%) were found positive for CE. Infected animals were 45 males and 48 females, aged between 1 and 8 years. The average number of cysts per wild boar was 1.3 (min 1 - max 13). The total number of cysts collected was 123, of which 118 (95.9%) in the liver, 4 (3.3%) in the lungs and 1 (0.8%) in the spleen. Of all analyzed cysts, 70 (56.9%) were fertile and 53 (43.1%) sterile/acephalous. The presence of fertile cysts in 19.4% of CE-positive animals is noteworthy. Overall, molecular diagnosis showed 19 wild boars infected with the pig strain (G7).
Keywords: Cystic echinococcosis, Wild boar, Genotyping, Drive hunting, Southern Italy
Graphical abstract
Highlights
-
•
A survey on CE on 2.108 wild boars from southern Italy was performed.
-
•
The prevalence of CE was 4.4% with fertility rate of 1.0%.
-
•
Echinococcus granulosus G7 genotype was isolated.
1. Introduction
Cystic Echinococcosis (CE) caused by Echinococcus granulosus sensu lato represents the most important cestode zoonosis in southern Europe due to heavy economic consequences in the public health sector and in livestock industry (Seimenis, 2003). In Italy, CE is widespread in farm animals with local and regional differences. CE has a sporadic diffusion in northern area with a low prevalence (<1%) in ruminants (Manfredi et al., 2011). In central Italy, prevalences of CE were reported in sheep 22.0–47.0% (Garippa, 2006), in cattle 7.3–15.3% and very low value in pigs 0.8% (Garippa and Manfredi, 2009). In southern Italy, endemic and hyperendemic areas of CE occur with high prevalence rates in ruminants: 33.3–75.0% in sheep, 10.4% in cattle and 10.5% in buffaloes (Veneziano et al., 2004; Capuano et al., 2006; Scala et al., 2006; Cringoli et al., 2007; Rinaldi et al., 2008a). Lowest prevalence of infection occurs in horses (<1%) (Varcasia et al., 2008a). CE was also reported in pigs with the prevalence ranging from 9.4 to 11.1% (Garippa et al., 2004; Varcasia et al., 2006).
To date, the Italian scenario shows several species and genotypes circulating in different domestic hosts. In the past, E. granulosus sensu stricto (s.s.) genotypes G1, G2 and G3 were frequently reported in sheep, cattle and buffalo (Busi et al., 2007; Rinaldi et al., 2008b; Deplazes et al., 2017; Kinkar et al., 2017). However, a recent study has now shown that genotype G2 to be a microvariant of G3 (Kinkar et al., 2017). Additionally, in continental Italy genotype G4 (E. equinus) has been described in horses (Varcasia et al., 2008a), G5 (E. ortleppi) in cattle (Casulli et al., 2008), while G7 (the name of the species for G6/G7 is under dispute, see Laurimäe et al., 2018a) was found in pigs of Sardinia island (Varcasia et al., 2006; Laurimäe et al., 2018b).
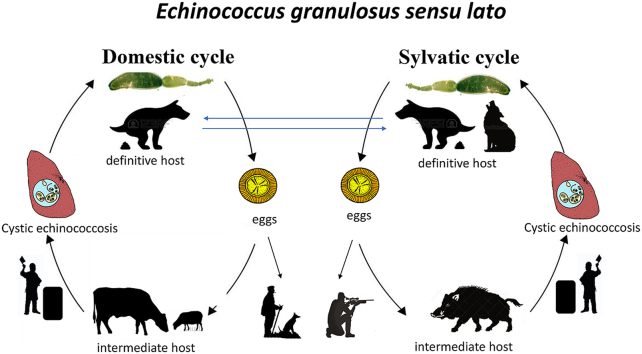
However, the role of wild animals in the transmission of CE is not completely elucidated (Seimenis, 2003). In the last decade wild boar (Sus scrofa) numbers have increased in several European countries, including Italy (Massei et al., 2015) and it has been hypothesized that these ungulates could play an important role in the dissemination of several diseases to livestock and humans (Meng et al., 2009; Fredriksson-Ahomaa, 2018). The role of wild boar as intermediate host of E. granulosus s.l. was reported in Spain (Martin-Hernando et al., 2008), Italy (Di Paolo et al., 2017) and France (Corsica) (Umhang et al., 2014). The data in Italy on the sylvatic cycle by epidemiological investigations on the diffusion of CE are scarce and only two studies have reported E. granulosus s.s. genotypes G1 and G3 in wild boars from non-endemic areas (Di Paolo et al., 2017; Paoletti et al., 2018).
The present study aimed to determine the prevalence and diffusion of CE in wild boars and the fertility of cysts, as well as to characterize the genetic variants of E. granulosus s.l. circulating in endemic areas from southern Italy.
2. Materials and methods
A total of 2108 wild boars hunted in 138 Boar Hunting Areas (BHAs), with a surface of 123,417 ha, from four different provinces (Avellino, Benevento, Caserta and Salerno) of the Campania region, southern Italy (41.488772° N, 15.558892° E) were examined during the hunting seasons in 2016 and 2017.
The sample size was calculated according to Thrusfield (1995) for a theoretically “infinite” population considering the following epidemiological data: expected prevalence of 3.7% for CE in wild boars based on the results of a similar study performed in Sardinian island (Varcasia et al., 2008b); confidence interval (99%) and desired absolute precision (1%).
Fifty-one veterinarians specialized in meat inspection were involved in examining the wild boar carcasses in the field within the regional project named “Piano Emergenza Cinghiali in Campania - PECC 2016–2019”. A detailed form was filled for each wild boar, including hunting area, gender, and age. The boar's age was estimated by the examination of the teeth according to Massei and Toso (1993). Organs of wild boars were transported and carefully examined within 24 h at the Department of Veterinary Medicine and Animal Productions (University of Naples, Italy) for the presence of hydatid cysts by visual inspection, palpation, and serial cuts of the organs. Parasitized organs were examined to determine the number, location, morphotype and viability of the cysts. Cysts were classified morphologically as fertile, sterile, caseous and calcified.
Fertility was assessed at optical microscope (Leica DM 750 HD) observing vitality and motility of protoscoleces, as well as flame cell movements without staining according to Varcasia et al. (2007). Massive infection was defined when more than ten cysts were found in a single animal.
Each cyst was dissected and the germinal layer and the cystic liquid of the hydatid was collected and stored at – 20 °C to the subsequent molecular analysis performed by the Department of Veterinary Medicine (University of Sassari, Italy). DNA was extracted using NucleoSpin Tissue (Macherey-Nagel GmbH & Co. KG, Düren, North Rhine-Westphalia, Germany). Echinococcus strains/genotypes were identified by polymerase chain reaction (PCR) and DNA sequencing.
The first screening of cysts was performed using a PCR/seminested PCR method (Dinkel et al., 2004) with four different PCR reactions in order to discriminate between the G1/G3 genotype cluster from genotypes G5 and G6/7. The sequencing of the mitochondrial genes ND4, ATP6, ND2 and COI as described by Nakao et al. (2000) was performed on the same samples.
First, primers F:COI (5′-TTGAATTTGCCACGTTTGAATGC-3′) and R:COI (5′-GAACCTAACGACATAACATAATGA 3′) were used for the amplification of a fragment of approximately 800 bp corresponding to the partial COI gene, whereas primers F:ND4 (5′-TGGAGTTAGATGGTAAGCGTTGAT-3′) and R:ND2 (5- CAGGAAACTTCATAACAACACTTA-3′) were used to amplify a fragment of approximately 1600 bp corresponding to the ATP6 gene and its flanking region (ND4 and ND2). PCR products were purified using a Nucleospin Gel and PCR Clean Up (Macherey-Nagel GmbH & Co. KG, Düren, North Rhine-Westphalia, Germany) and sent to an external sequencing service (Eurofins Genomics, Germany). Obtained sequences were compared with that on the NCBI database using BLAST (http://www.ncbi.nlm.nih.gov/BLAST/). Statistical analysis was performed using Epi Info v3.5.3. Chi-squared test was used to assess the differences between infections in lungs and livers, and among age and gender infection rates. Odds ratios (OR) were calculated to assess the likelihood of an effect of age and gender on infection rate. A value of P < 0.05 was considered significant.
Distribution of drive hunting areas was obtained with ArcGIS (version 10.3, ESRI, Redlands, CA, USA) and associated with administrative boundaries of Provinces, Regional Parks and National Parks.
For each area the positivity rate was calculated (positive/examined) and subdivided into seven classes with percentages ranging from 0 to 100.
3. Results
An overall CE prevalence of 4.4% (93/2108; CI 95% 3.5–5.3) was found out of a total of 2108 wild boars examined. CE prevalence in the 2016 hunting season was 4.6% (46/997) and 4.2% (47/1111) in 2017 (χ2 = 0.28; P = 0.5983).
A total of 123 hydatids were found. Abundance (number of hydatids/animal sampled) was 0.06 while the average intensity (number of hydatids/positive animal) was 1.3 (range 1–13). Cystic echinococcosis was detected in 0.05% (1/2108) of the spleens, in 0.1% (3/2108) of the lungs, in 4.2% (89/2108) of the livers examined (χ2 = 165.26; P < 0.0001; DF = 2). Of the total hydatids found, 0.8% were in the spleens (1/123), 3.3% in the lungs (4/123) and 95.9% in the liver (118/123; χ2 = 325.54; P < 0.0001; DF = 2).
Hydatid cysts were 56.9% fertile (70/123) and 43.1% sterile (53/123) (χ2 = 4.699; P = 0.030), whereas no caseous/calcified cysts were found. In particular, a fertility of 75.0% was found in the lungs (3/4) and 56.8% in the liver (67/118) (χ2 = 0.04; P = 0.468 Yates corrected), whereas the only hydatid found in the spleen was sterile.
Fertile cysts were found in 19.4% of infected boars (18/93). Fertility rate (calculate as wild boars harbouring fertile hydatids/examined wild boars) was of 0.9% (CI 95% 0.5–1.3).
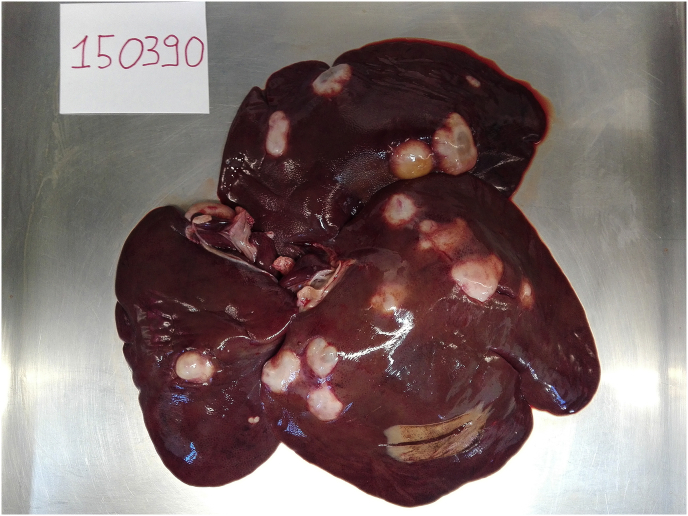
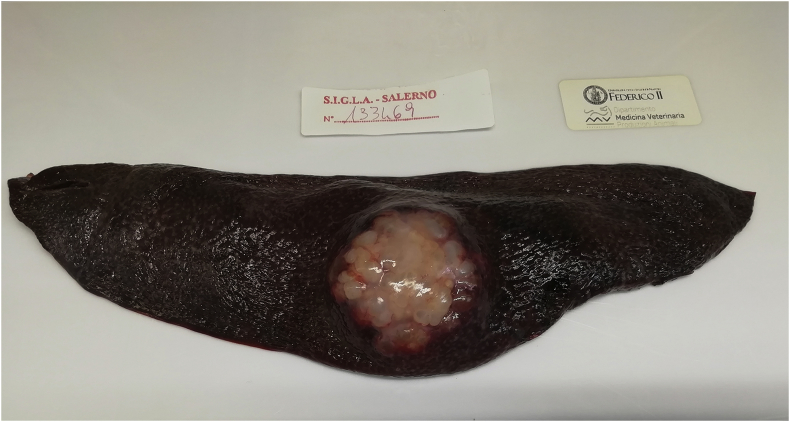
A single animal (1.1% of infected boars) from Sala Consilina municipality (Salerno province) presented a massive infection with 13 hydatid cysts (Fig. 1, Fig. 2). An unusual splenic location was found in a wild boar from Vallo della Lucania municipality (Salerno province) (Fig. 4). No mixed infection (liver, lungs and spleen) was found in examined animals. Details on number, location, typology and viability of recovered hydatid cysts are shown in Table 1 (see Fig. 3).
Fig. 1.
Liver with massive CE infection by Echinococcus granulosussensu stricto.
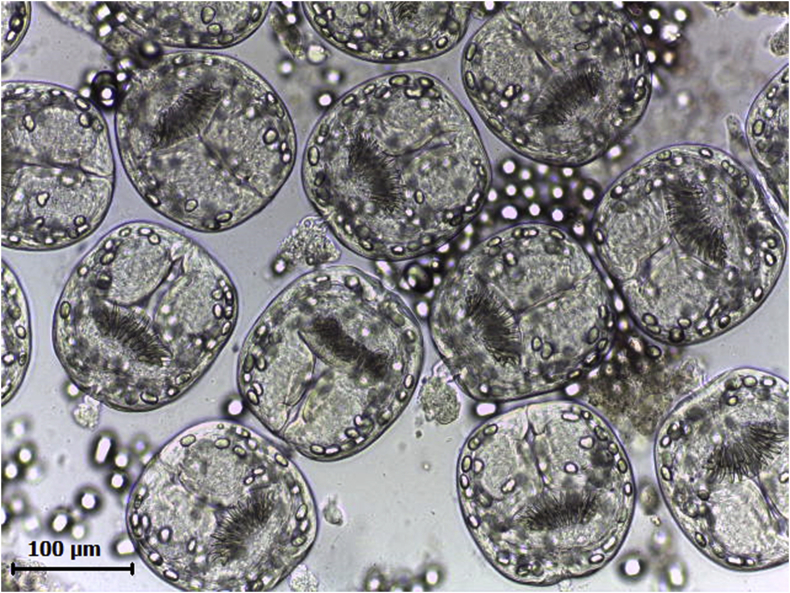
Fig. 2.
Invaginated protoscoleces isolated from a fertile hydatid cyst.
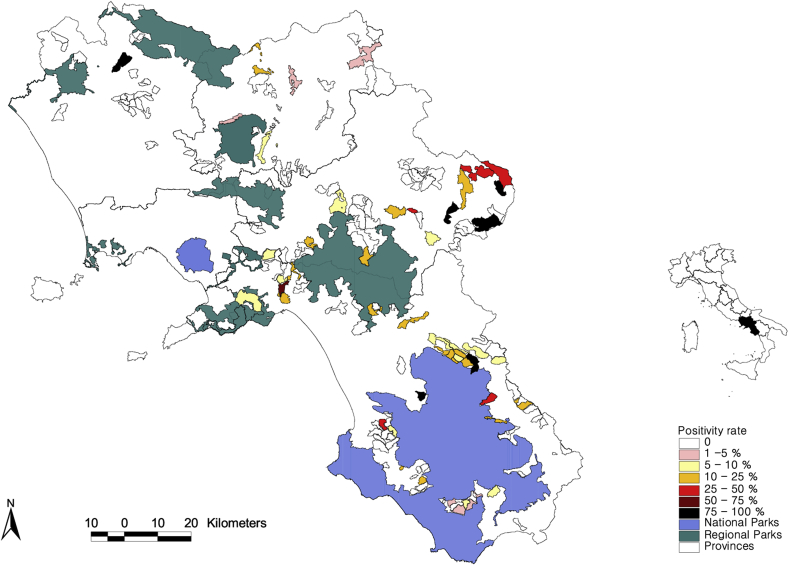
Fig. 4.
Distribution of the 93 positive wild boars in the study area and details of prevalence, provinces, regional and national parks.
Table 1.
Number, location, typology and viability of hydatid cysts recovered from different organs of 2108 examined wild boars.
| Organs | Positive animals | N° hydatid cysts | Typology of hydatid cysts |
Viability of hydatid cysts |
||
|---|---|---|---|---|---|---|
| Unilocular | Pseudo multilocular | Fertile | Sterile/Acephalocyst | |||
| Liver | 89/2108 (4.2%) | 118 | 104 | 14 | 67 | 51 |
| Lungs | 3/2108 (0.1%) | 4 | 4 | 0 | 3 | 1 |
| Spleen | 1/2108 (0.05) | 1 | 0 | 1 | 0 | 1 |
| Overall | 93/2108 (4.4%) | 123 | 108 | 15 | 70 | 53 |
Fig. 3.
Pseudo multilocular hydatid cyst, by Echinococcus granulosussensu stricto, l spleen localization.
We found that 2.1% (45/2108) of males and 2.3% (48/2108) of females were infected. No significant statistical differences between the genders were found (χ2 = 0.09; P = 0.753). Older boars appeared more likely to be infected with CE than younger animals, the rate of infection in wild boars was age dependent (χ2 for trend = 27.89; P < 0.0001). Data about the prevalence according to age of wild boars are reported in Table 2.
Table 2.
Prevalence rates for CE according to age of 2108 examined boars.
| Age classes | Examined boars | Prevalence % | Odds Ratio |
|---|---|---|---|
| Piglet (<1yoa) | 242 | 0.8 | 1.00 |
| Sub-adult (1–2 yoa) | 670 | 1.8 | 2.18 |
| Adult (>2 yoa) | 1196 | 6.6 | 8.48 |
Yo: years old.
Prevalence of infection from the four provinces showed different scenarios, ranging from 0.6% in Caserta Province (CE) to 8.3% in Avellino Province (AV). The difference was statistically significant (χ2 = 21.24; P = 0.00009; DF = 3) and detailed data are reported in Table 3.
Table 3.
Prevalence rates for CE according to provinces of origin of 2108 boars and Hunting season (2016–2017).
| Province | Year | Examined boars | Prevalence % | Statistical comparison between years | Odds Ratio |
|---|---|---|---|---|---|
| Caserta | 2016 | 59 | 0.0 | X2 = 0.09 | |
| 2017 | 108 | 0.9 | P = 0.758a | ||
| Overall | 167 | 0.6 | 1.00 | ||
| Benevento | 2016 | 166 | 3.0 | x2 = 1.32 | |
| 2017 | 220 | 0.9 | P = 0.251a | ||
| Overall | 386 | 1.8 | 3.07 | ||
| Salerno | 2016 | 732 | 5.3 | x2 = 0.43 | |
| 2017 | 595 | 4.5 | P = 0.510 | ||
| Overall | 1327 | 5.0 | 8.69 | ||
| Avellino | 2016 | 40 | 5.0 | x2 = 0.28 | |
| 2017 | 188 | 9.0 | P = 0.599a | ||
| Overall | 228 | 8.3 | 15.9 |
Statistical comparison was performed using chi-square test with Yates correction.
The distribution of the 93 positive animals in the study area and the details of prevalences, provinces, regional and national parks is reported in Figure 5.
The positive areas (BHAs) 49/138 (35.5%) showed an irregular distribution with the exception of two zones in the central-eastern part of the region and to the north of the Cilento National Park. These two areas represent 14% and 25% of the total positive samples, respectively.
The initial screening with PCR/seminested PCR according to Dinkel et al. (2004) carried out in 29 wild boars showed that 6 and 11 wild boars were infected with E. granulosus s.s. and E. granulosus genotypes G6/7, respectively, while 12 samples did not yield a PCR product. The Dinkel protocol did not allow to distinguish among G1 and G3 nor between G6 and G7.
The combined PCR (COI and ATP6 and its flanking region, ND2 and ND4) and DNA sequencing approach revealed the presence of E. granulosus s.s. and genotype G7 in wild boars from the Campania region. Out of a total of 29 boars examined, 6 (20.7%) were E. granulosus s.s. (GenBank accession no.: ND2, COI) and 19 (65.5%) were G7 (GenBank accession no.: ND2, COI) (χ2 = 10.12; P = 0.0014, Yates Correction). Four samples did not yield a PCR product. Out of a total of 19 boars positive to G7, 15 (78.9%) showed fertile hydatids. The method described by Nakao et al. (2000) did not allow confident distinction between genotypes G1 and G3. Details on genotypes found are reported in Table 4.
Table 4.
Genotypes and species of E. granulosus s.l. found in 29 individual cyst from wild boars from southern Italy, according to a first PCR-screening method followed by sequencing of PCR-amplified mtDNA corresponding to ATP6 and flanking regions (ND4 e ND2) and cytochrome c oxidase (COI) genes.
| ID Sample | Organ | Cysts status | Dinkel PCR | COI | ATP6 and flanking region |
|---|---|---|---|---|---|
| 1 | Liver | Fertile | G6/7 | G7 | G7 |
| 2 | Liver | Fertile | FAILED | FAILED | FAILED |
| 3 | Liver | Fertile | G6/7 | G7 | G7 |
| 4 | Liver | Sterile | FAILED | FAILED | FAILED |
| 5 | Liver | Fertile | G6/7 | G7 | G7 |
| 6 | Liver | Fertile | G6/7 | G7 | G7 |
| 7 | Liver | Fertile | G6/7 | G7 | G7 |
| 8 | Liver | Fertile | G6/7 | G7 | G7 |
| 9 | Liver | Fertile | G6/7 | G7 | FAILED |
| 10 | Liver | Sterile | FAILED | FAILED | FAILED |
| 11 | Liver | Fertile | G6/7 | G7 | G7 |
| 12 | Liver | Fertile | G6/7 | G7 | G7 |
| 13 | Liver | Fertile | G6/7 | G7 | G7 |
| 14 | Liver | Sterile | E. granulosus s.s. | FAILED | FAILED |
| 15 | Liver | Fertile | G6/7 | G7 | FAILED |
| 16 | Liver | Fertile | G6/7 | G7 | FAILED |
| 17 | Liver | Fertile | G6/7 | G7 | G7 |
| 18 | Liver | Sterile | FAILED | FAILED | FAILED |
| 19 | Lungs | Fertile | E. granulosus s.s | E. granulosus s.s | FAILED |
| 20 | Lungs | Fertile | E. granulosus s.s. | E. granulosus s.s. | FAILED |
| 21 | Lungs | Fertile | E. granulosus s.s. | E. granulosus s.s. | E. granulosus s.s |
| 22 | Liver | Sterile | G6/7 | G7 | G7 |
| 23 | Liver | Sterile | G6/7 | G7 | G7 |
| 24 | Spleen | Sterile | E. granulosus s.s. | FAILED | FAILED |
| 25 | Liver | Fertile | G6/7 | G7 | G7 |
| 26 | Liver | Fertile | G6/7 | G7 | G7 |
| 27 | Liver | Fertile | G6/7 | G7 | G7 |
| 28 | Liver | Fertile | G6/7 | G7 | G7 |
| 29 | Liver | Fertile | E. granulosus s.s. | E. granulosus s.s. | E. granulosus s.s. |
4. Discussion
The present survey reported genotype G7 in continental Italy, which occurs sympatrically with E. granulosus s.s. in wild boars of southern Italy.
To date, few studies have investigated the occurrence of CE in wild boar populations from different European countries; Onac et al. (2013) reported the presence of G1 and G7 with an overall prevalence of 12.3% from Romania (out of a total of 267 animals), whereas Umhang et al. (2014) reported the presence of G6/G7 from France (Corsica) with a prevalence of 4% on 101 wild boars. G7 has recently been found in wild boars in continental Italy also by Laurimäe et al. (unpublished). Here we demonstrate with a much larger sample size the occurrence of G7 in wild boar in Italy. Out of a total of 29 wild boars genetically analyzed, most of the animals (n = 19; 65.5%) were infected with G7. This finding is in accordance with surveys performed in other European countries, such as Ukraine (Kedra et al., 2000) and Spain (Mwambete et al., 2004), where isolates from wild boars corresponded principally to genotype G7.
In Italy, Varcasia et al. (2008b) reported a prevalence of 3.7% for genotype G1 of 461 wild boars analysed from Sardinia. In central Italy, Di Nicola et al. (2015) revealed a prevalence of 6% among 101 analysed wild boars from Abruzzo and Lazio regions, whereas recently Di Paolo et al. (2017) reported for the first time the genotype G3 in wild boar in Europe. In a recent epidemiological survey, Paoletti et al. (2018) showed a prevalence of 1.0% (8/765) for G1 with a fertility rate of 0.3% from central Italy. In the present study we reported an overall prevalence of 4.4% and a fertility rate of 0.9%, three times higher than that found in central Italy by Paoletti et al. (2018). Regarding cyst locations, of the total hydatids examined, 0.8% were found in the spleen (1/123), 3.3% in the lungs (4/123) and 95.9% in the liver (118/123) in contrast with previous studies showing lung localization in wild boars more frequently and a tropism of G7 for pulmonary parenchyma (Mwambete et al., 2004; Varcasia et al., 2007; Paoletti et al., 2018). In our study, a single wild boar presented a massive infection with 13 hydatids. Such cases of heavy wild boar infestations have been reported also in Spain (Martin-Hernando et al., 2008). Our results suggest that older animals appear more likely parasitized than younger, the age-dependent increment in infection rates has been reported in several studies as a phenomenon probably due to recurrent infections in the same environment (Scala et al., 2006; Otero-Abad and Torgerson, 2013).
In the last few decades, because of reintroductions and improved management (Apollonio et al., 2010), the numbers of wild ungulates have increased all over Europe and have been the main determinants of range expansion and population growth of the wolf (Chapron et al., 2014; Galaverni et al., 2015; Hindrikson et al., 2017). It is well recognised that abundance of wild boars in the diet of wolves should lead to decreased predation on livestock (Meriggi et al., 2011) and that, in similar ecological conditions, even wolf–dog hybrids have the same food preferences as wolves, with the tendency to feed mainly on wild ungulates (Bassi et al., 2017).
Recently, from the Apennine regions of northern Italy, E. granulosus s.s. G1 and G3 were identified in wolf populations (Canis lupus italicus) with a prevalence of 5.6% (Gori et al., 2015) and 5.5% (Poglayen et al., 2017) respectively, emphasizing the importance of wild species as possible indicators for CE environmental spreading.
In this scenario, the typical behaviour of wolves, characterized by long-distance dispersal and wide home ranges and often with overlapping ranges of sheep and wild boar could promote the parasite spread, according to Gori et al. (2015). Wild boars, which are characterized by extreme adaptability in different habitats, wide geographical distribution and high reproductive rates (Massei et al., 2015), could represent the sentinel animal of CE circulation in domestic animals. In rural Mediterranean areas, other important factors promoting the diffusion of CE infection are represented by cultural and economic background of dog owner's, their scant knowledge about parasite transmission and the poor deworming practices; in particular, hunting dogs may have access to the location where wild animals are slaughtered and feed on raw viscera (Otero-Abad and Torgerson, 2013) leading the parasite to establish a spill-over between wild and domestic animals.
Recently, a report by EFSA (ENETWILD Consortium et al., 2018) on spatial distribution and density of wild boar population showed a significant presence of this ungulate in Campania region. Here, during the hunting season, the high number of carcasses discarded on the ground could represent a critical source of infection for wild carnivores (wolves) and hunting dogs. In this regard, it is likely that the presence of a semi-domestic life cycle of E. granulosus s.l. in which hunting dogs, feeding on raw infected offal of wild boars (or other intermediate hosts), may contaminate the environment through their faeces containing parasite eggs, ingested by wild boars, according to Paoletti et al. (2018). Feeding dogs with raw viscera and organs of wild boar is a very common practice among hunters as a reward for hunting dogs. In this epidemiological scenario, many hunters have the habit to unfit extra-label use of macrocyclic lactones (mainly ivermectin) on their dogs giving rise to cestode infections (Piantedosi et al., 2017). It should be emphasized that the role of hunting dogs which could represent a crucial potential risk of infection to human, considering that domestic dogs have been long identified as the main infection for humans, according to Otero-Abad and Torgerson (2013). In Italy, human CE represents a severe public health concern due to an annual incidence of 1.6/105 inhabitants, with over 1000 cases per year (Brundu et al., 2014), each of them resulting in a mean of 0.97 DALYs (Disability Adjusted Life Years) (Torgerson et al., 2015). The highest incidence rate of CE was observed in Sardinia (6.8/105 inhabitants), where home slaughtering and poor deworming practices are very common according to Varcasia et al. (2011), followed by the southern Italian regions with an average incidence of 1.9/105 inhabitants (Brundu et al., 2014).
5. Conclusion
This survey reports the presence of E. granulosus sensu lato genotype G7 circulating in wild boar population in southern Italy. Elucidations on the role of genotype G7 in the epidemiology of CE in Italy and its zoonotic potential requires further surveys. The presence of G7 in Italian wild boars has important implications for the implementation of hydatid control programs which include regular cestocidal treatments of dogs, also considering the shorter maturation period of G7 compared to G1 in dogs (Thompson and McManus, 2002). The coprological examination of samples of dogs and wolves from the same hunting areas employed by wild boars are needed to clarify the transmission dynamics scenario of CE trough a sylvatic and domestic cycles.
The current study shows the need of a constant surveillance program in hunting wild boars.
Hunters should be trained on hunting hygiene in order to avoid the dispersion of CE into the environment through organs and viscera of hunted boars; in this regard it is essential that hunters have hunting lodges for the collection and inspection of carcasses.
It is very important to use dogs to track blood for the recovery of wounded animals to avoid that the abandoned wild boar carcasses becoming a source of infection for definitive host carnivores, as well as the treatment of hunting dogs with molecules acting on tapeworms.
These aspects could be crucial elements for the reduction of this principal zoonotic disease and to ensure food safety. A multi-sectoral framework should be promoted involving all stakeholders working in public health, veterinary services and hunting associations in the view of a “One Health” approach.
Ethical statement
Animals included in the present study were examined during post mortem mandatory inspection visit by official veterinaries according to a specific agreement with Government of Campania Region, UOD Piano Emergenza Cinghiali (PECC, 2016–2019).
Conflicts of interest
The authors declare that they have no conflicts of interest.
Acknowledgements
The study was supported by the grant from Regione Campania UOD Prevenzione e Sanità Pubblica Veterinaria, Piano Emergenza Cinghiali (PECC 2016–2019), by the grant from Ministry of Health of the Italian Republic (IZS ME 04/18 RC) and by the institutional research funding (IUT20-32) from the Estonian Ministry of Education and Research.
The authors wish to thank Prof. Giuseppe Cringoli for the critical comments and for the revision of the manuscript.
The authors are grateful to all 51 veterinary doctors involved in the project for their kind collaboration in the collection of samples used in this survey: Aniello Amato (ASL Salerno) Pasquale Apicella (ASL Salerno), Antonio Apuzzo, Gennaro Barra, Domenico Benedetto, Luca Borrelli, Francesco Buono, Pasquale Buonocore, Giovanni Calicchio (ASL Salerno), Roberto Carbone (ASL Salerno), Luigi Cardinale (ASL Avellino), Danila Carlucci (ASL Benevento), Francesco Celano, Angelo Coletta, Domenico Crocetta, Giovanni Costantino (ASL Salerno), Carmela D'Acierno (CRIUV), Vincenzo D'Amato (ASL Avellino), Francesco Antonio D'Orilia (ASL Salerno), Luca De Blasio, Luigi De Luca Bossa (ASL Benevento), Giovanni De Lucia (ASL Salerno), Lucio De Maria (ASL Caserta), Francesco Di Domenico, Vittorio Di Matteo, Giuseppe Fornino (ASL Salerno), Antonio Gargiulo (CRIUV), Gianni Gesa, Eduardo Grieco (ASL Salerno), Simone Iovino, Piero Matonte, Giulio Marino (ASL Salerno), Giuseppe Mollo, Luigi Morena (ASL Salerno), Benedetto Neola, Laura Pacifico, Simone Palmieri, Gerardo Paraggio (ASL Salerno), Antonio Parisi, Diego Piantedosi, Massimiliano Boris Pietraggi (ASL Caserta), Laura Pitaro (CRIUV), Domenico Rufrano, Pasquale Russo, Valerio Toscano (CRIUV), Andrea Santillo, Giampiero Sepe, Pasquale Silvestri, Antonio Raffaele, Giovanni Russo (ASL Salerno), Alessio Vitale.
References
- Apollonio M., Andersen R., Putman R. Cambridge University Press; Cambridge: 2010. European Ungulates and Their Management in the Twenty-First Century. [Google Scholar]; Apollonio, M., Andersen, R., Putman, R., 2010. European ungulates and their management in the twenty-first century. Cambridge University Press, Cambridge.
- Bassi E., Canu A., Firmo I., Mattioli L., Scandura M., Apollonio M. Trophic overlap between wolves and free-ranging wolf × dog hybrids in the Apennine Mountains, Italy. Glob. Ecol. Conserv. 2017;9:39–49. [Google Scholar]; Bassi, E., Canu, A., Firmo, I., Mattioli, L., Scandura, M., Apollonio, M., 2017. Trophic overlap between wolves and free-ranging wolf × dog hybrids in the Apennine Mountains, Italy. Glob. Ecol. Conserv. 9, 39-49.
- Brundu D., Piseddu T., Stegel G., Masu G., Ledda S., Masala G. Retrospective study of human cystic echinococcosis in Italy based on the analysis of hospital discharge records between 2001 and 2012. Acta Trop. 2014;140:91–96. doi: 10.1016/j.actatropica.2014.08.011. [DOI] [PubMed] [Google Scholar]; Brundu, D., Piseddu, T., Stegel, G., Masu, G., Ledda, S., Masala, G., 2014. Retrospective study of human cystic echinococcosis in Italy based on the analysis of hospital discharge records between 2001 and 2012. Acta Trop. 140, 91-96. [DOI] [PubMed]
- Busi M., Snabel V., Varcasia A., Garippa G., Perrone V., De Liberato C., D'Amelio S. Genetic variation within and between G1 and G3 genotypes of Echinococcus granulosus in Italy revealed by multilocus DNA sequencing. Vet. Parasitol. 2007;150:75–83. doi: 10.1016/j.vetpar.2007.09.003. [DOI] [PubMed] [Google Scholar]; Busi, M., Snabel, V., Varcasia, A., Garippa, G., Perrone, V., De Liberato, C., D’Amelio, S., 2007. Genetic variation within and between G1 and G3 genotypes of Echinococcus granulosus in Italy revealed by multilocus DNA sequencing. Vet. Parasitol. 150, 75-83. [DOI] [PubMed]
- Capuano F., Rinaldi L., Maurelli M.P., Perugini A.G., Veneziano V., Garippa G., Genchi C., Musella V., Cringoli G. Cystic echinococcosis in water buffaloes: epidemiological survey and molecular evidence of ovine (G1) and buffalo (G3) strains. Vet. Parasitol. 2006;137:262–268. doi: 10.1016/j.vetpar.2006.01.016. [DOI] [PubMed] [Google Scholar]; Capuano, F., Rinaldi, L., Maurelli, M.P., Perugini, A.G., Veneziano, V., Garippa, G., Genchi, C., Musella, V., Cringoli, G., 2006. Cystic echinococcosis in water buffaloes: epidemiological survey and molecular evidence of ovine (G1) and buffalo (G3) strains. Vet. Parasitol. 137, 262-268. [DOI] [PubMed]
- Casulli A., Manfredi M.T., La Rosa G., Cerbo A.R., Genchi C., Pozio E. Echinococcus ortleppi and E. granulosus G1, G2 and G3 genotypes in Italian bovines. Vet. Parasitol. 2008;155:168–172. doi: 10.1016/j.vetpar.2008.04.004. [DOI] [PubMed] [Google Scholar]; Casulli, A., Manfredi, M.T., La Rosa, G., Cerbo, A.R., Genchi, C., Pozio, E., 2008. Echinococcus ortleppi and E. granulosus G1, G2 and G3 genotypes in Italian bovines. Vet. Parasitol. 155, 168-172. [DOI] [PubMed]
- Chapron G., Kaczensky P., Linnel J.D.C., Von Arx M., Huber D., Andrèn H., Lòpez-Bao J.V., Adamec M., Alvares F., Anders O., Balčiauskas L., Balys V., Bedö P., Bego F., Blanco J.C., Breitenmoser U., Brøseth H., Bufka L., Bunikyte R., Ciucci P., Dutsov A., Engleder T., Fuxjäger C., Groff C., Holmala K., Hoxha B., Iliopoulos Y., Ionescu O., Jeremić J., Jerina K., Kluth G., Knauer F., Kojola I., Kos I., Krofel M., Kubala J., Kunovac S., Kusak J., Kutal M., Liberg O., Majić A., Männil P., Mertzanis Y., Myslayek R.W., Nowak S., Odden J., Ozolins J., Palomero G., Paunović M., Persson J., Potočnik H., Quenette P.Y., Rauer G., Reinhardt I., Rigg R., Ryser A., Salvatori V., Skrbinšek T., Stojanov A., Swenson J.E., Szemethy L., Trajçe A., Tsingarska- Sedefcheva E., Váňa M., Veeroja R., Wabakken P., Wölfl M., Wölfl S., Zimmermann F., Zlatanova D., Boitani L. Recovery of large carnivores in Europe's modern human-dominated landscapes. Science. 2014;346:1517–1519. doi: 10.1126/science.1257553. [DOI] [PubMed] [Google Scholar]; Chapron, G., Kaczensky, P., Linnel, J.D.C., Von Arx, M., Huber, D., Andren, H., Lopez-Bao, J.V., Adamec, M., Alvares, F., Anders, O., Balčiauskas, L., Balys, V., Bedo, P., Bego, F., Blanco, J.C., Breitenmoser, U., Broseth, H., Bufka, L., Bunikyte, R., Ciucci, P., Dutsov, A., Engleder, T., Fuxjager, C., Groff, C., Holmala, K., Hoxha, B., Iliopoulos, Y., Ionescu, O., Jeremić, J., Jerina, K., Kluth, G., Knauer, F., Kojola, I., Kos, I., Krofel, M., Kubala, J., Kunovac, S., Kusak, J., Kutal, M., Liberg, O., Majić, A., Mannil, P., Mertzanis, Y., Myslayek, R.W., Nowak, S., Odden, J., Ozolins, J., Palomero, G., Paunović, M., Persson, J., Potočnik, H., Quenette, P.Y., Rauer, G., Reinhardt, I., Rigg, R., Ryser, A., Salvatori, V., Skrbinšek, T., Stojanov, A., Swenson, J.E., Szemethy, L., Trajçe, A., Tsingarska- Sedefcheva, E., Vaňa, M., Veeroja, R., Wabakken, P., Wolfl, M., Wolfl, S., Zimmermann, F., Zlatanova, D., Boitani, L., 2014. Recovery of large carnivores in Europe’s modern human-dominated landscapes. Science 346, 1517-1519. [DOI] [PubMed]
- Cringoli G., Rinaldi L., Musella V., Veneziano V., Maurelli M.P., Di Pietro F., Frisiello M., Di Pietro S. Geo-referencing livestock farms as tool for studying cystic echinococcosis epidemiology in cattle and water buffaloes from southern Italy. Geospat. Health. 2007;2:105–111. doi: 10.4081/gh.2007.259. [DOI] [PubMed] [Google Scholar]; Cringoli, G., Rinaldi, L., Musella, V., Veneziano, V., Maurelli, M.P., Di Pietro, F., Frisiello, M., Di Pietro, S., 2007. Geo-referencing livestock farms as tool for studying cystic echinococcosis epidemiology in cattle and water buffaloes from southern Italy. Geospat. Health 2, 105-111. [DOI] [PubMed]
- ENETWILD Consortium. Croft S., Smith G.C., Acevedo P., Vicente J. EFSA supporting publication; 2018. Wild Boar in Focus: Review of Existing Models on Spatial Distribution and Density of Wild Boar: Report Containing the Review of Models, and Proposal for Next Steps. 2018: EN- 1490. 44. [DOI] [Google Scholar]; ENETWILD Consortium, Croft, S., Smith, G.C., Acevedo, P., Vicente, J., 2018. Wild boar in focus: Review of existing models on spatial distribution and density of wild boar: Report containing the review of models, and proposal for next steps. EFSA supporting publication 2018: EN- 1490. 44 pp. https://doi.org/10.2903/sp.efsa.2018.EN-1490
- Deplazes P., Rinaldi L., Alvarez Rojas C.A., Torgerson P.R., Harandi M.F., Romig T., Antolova D., Schurer J.M., Lahmar S., Cringoli G. Global distribution of alveolar and cystic echinococcosis. Adv. Parasitol. 2017;95:315–493. doi: 10.1016/bs.apar.2016.11.001. [DOI] [PubMed] [Google Scholar]; Deplazes, P., Rinaldi, L., Alvarez Rojas, C.A., Torgerson, P.R., Harandi, M.F., Romig, T., Antolova, D., Schurer, J.M., Lahmar, S., Cringoli, G., 2017. Global Distribution of Alveolar and Cystic Echinococcosis. Adv. Parasitol. 95, 315-493. [DOI] [PubMed]
- Di Nicola U., Scacchia M., Marruchella G. Pathological and serological findings in wild boars (Sus scrofa) from gran sasso and monti della Laga national park (Central Italy) Large Anim. Rev. 2015;21:167–171. [Google Scholar]; Di Nicola, U., Scacchia, M., Marruchella, G., 2015. Pathological and serological findings in wild boars (Sus scrofa) from Gran Sasso and Monti della Laga National Park (Central Italy). Large Animal Review 21, 167-171.
- Di Paolo A., Piseddu T., Sebastianelli M., Manuali E., Corneli S., Paniccià M., Papa P., Viali S., Mazzone P. Detection of Echinococcus granulosus G3 in a wild boar (Sus scrofa) in Central Italy using PCR and sequencing. J. Wildl. Dis. 2017;53(2):399–401. doi: 10.7589/2015-12-344. [DOI] [PubMed] [Google Scholar]; Di Paolo, A., Piseddu, T., Sebastianelli, M., Manuali, E., Corneli, S., Paniccia, M., Papa, P., Viali, S., Mazzone, P., 2017. Detection of Echinococcus granulosus G3 in a Wild Boar (Sus scrofa) in Central Italy using PCR and Sequencing. J. Wildl. Dis. 53(2), 399-401. [DOI] [PubMed]
- Dinkel A., Njoroge E.M., Zimmermann A., Walz M., Zeyhle E., Elmahdi I.E., Mackenstedt U., Romig T. A PCR system for detection of species and genotypes of the Echinococcus granulosus-complex, with reference to the epidemiological situation in eastern Africa. Int. J. Parasitol. 2004;34:645–653. doi: 10.1016/j.ijpara.2003.12.013. [DOI] [PubMed] [Google Scholar]; Dinkel, A., Njoroge, E.M., Zimmermann, A., Walz, M., Zeyhle, E., Elmahdi, I.E., Mackenstedt, U., Romig, T., 2004. A PCR system for detection of species and genotypes of the Echinococcus granulosus-complex, with reference to the epidemiological situation in eastern Africa. Int. J. Parasitol. 34, 645-653. [DOI] [PubMed]
- Fredriksson-Ahomaa M. Wild boar: a reservoir of foodborne zoonoses. Foodb. Pathog. Dis. 2018 doi: 10.1089/fpd.2018.2512. [DOI] [PubMed] [Google Scholar]; Fredriksson-Ahomaa, M., 2018. Wild boar: a reservoir of foodborne zoonoses. Foodb. Pathog. Dis.. https://doi.org/10.1089/fpd.2018.2512 [DOI] [PubMed]
- Galaverni M., Caniglia R., Fabbri E., Milanesi P., Randi E. One, no one, or one thousand: how many wolves are there currently in Italy? Mamm. Res. 2015;61:13–24. [Google Scholar]; Galaverni, M., Caniglia, R., Fabbri, E., Milanesi, P., Randi, E., 2015. One, no one, or one thousand: how many wolves are there currently in Italy? Mamm. Res. 61, 13-24.
- Garippa G. Updates on cystic echinococcosis (CE) in Italy. Parassitologia. 2006;48:57–59. [PubMed] [Google Scholar]; Garippa, G., 2006. Updates on cystic echinococcosis (CE) in Italy. Parassitologia 48, 57-59. [PubMed]
- Garippa G., Manfredi M.T. Cystic echinococcosis in Europe and in Italy. Vet. Res. Commun. 2009;33(Suppl. 1):S35–S39. doi: 10.1007/s11259-009-9245-0. [DOI] [PubMed] [Google Scholar]; Garippa, G., Manfredi, M.T., 2009. Cystic echinococcosis in Europe and in Italy. Vet. Res. Commun. 33(suppl. 1), S35-S39. [DOI] [PubMed]
- Garippa G., Battelli G., Cringoli G., Giangaspero A., Giannetto S., Manfredi M.T. Animal echinococcosis in Italy: epidemiological update. Parassitologia. 2004;46:33–38. [PubMed] [Google Scholar]; Garippa, G., Battelli, G., Cringoli, G., Giangaspero, A., Giannetto, S., Manfredi, M.T., 2004. Animal echinococcosis in Italy: epidemiological update. Parassitologia 46, 33-38. [PubMed]
- Gori F., Armua-Fernandez M.T., Milanesi P., Serafini M., Magi M., Deplazes P., Macchioni F. The occurrence of taeniids of wolves in Liguria (northern Italy) Int. J. Parasitol. Parasit. Wildl. 2015;4:252–255. doi: 10.1016/j.ijppaw.2015.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]; Gori, F., Armua-Fernandez, M.T., Milanesi, P., Serafini, M., Magi, M., Deplazes, P., Macchioni, F., 2015. The occurrence of taeniids of wolves in Liguria (northern Italy). Int. J. Parasitol. Parasit. Wildl. 4, 252-255. [DOI] [PMC free article] [PubMed]
- Hindrikson M., Remm J., Pilot M., Godinho R., Stronen A.V., Baltrunaite L., Czarnomska S.D., Leonard J.A., Randi E., Nowak C., Akesson M., Lopez-Bao J.V., Alvares F., Llaneza L., Echegaray J., Vila C., Ozolins J., Rungis D., Aspi J., Paule L., Skrbinšek T., Saarma U. Wolf population genetics in Europe: a systematic review, meta-analysis and suggestions for conservation and management. Biol. Rev. 2017;92:1601–1629. doi: 10.1111/brv.12298. [DOI] [PubMed] [Google Scholar]; Hindrikson, M., Remm, J., Pilot, M., Godinho, R., Stronen, A.V., Baltrunaite, L., Czarnomska, S.D., Leonard, J.A., Randi, E., Nowak, C., Akesson, M., Lopez-Bao, J.V., Alvares, F., Llaneza, L., Echegaray, J., Vila, C., Ozolins, J., Rungis, D., Aspi, J., Paule, L., Skrbinšek, T., Saarma, U., 2017. Wolf population genetics in Europe: a systematic review, meta-analysis and suggestions for conservation and management. Biol. Rev. 92, 1601-1629. [DOI] [PubMed]
- Kedra A.H., Tkach V.V., Swiderski Z.P., Pawlowski Z., Emets A., Pawlowski J. Molecular characterisation of Echinococcus granulosus from a wild boar. Acta Parasitol. 2000;45(2):121–122. [Google Scholar]; Kedra, A.H., Tkach, V.V., Swiderski, Z.P., Pawlowski, Z., Emets, A., Pawlowski, J., 2000. Molecular characterisation of Echinococcus granulosus from a wild boar. Acta Parasitol. 45(2), 121-122.
- Kinkar L., Laurimäe T., Sharbatkhori M., Mirhendi H., Kia E.B., Ponce-Gordo F., Andresiuk V., Simsek S., Lavikainen A., Irshadullah M., Umhang G., Oudni-M’rad M., Acosta-Jamett G., Rehbein S., Saarma U. New mitogenome and nuclear evidence on the phylogeny and taxonomy of the highly zoonotic tapeworm Echinococcus granulosus sensu stricto. Infect. Genet. Evol. 2017;52:52–58. doi: 10.1016/j.meegid.2017.04.023. [DOI] [PubMed] [Google Scholar]; Kinkar, L., Laurimae, T., Sharbatkhori, M., Mirhendi, H., Kia, E.B., Ponce-Gordo, F., Andresiuk, V., Simsek, S., Lavikainen, A., Irshadullah, M., Umhang, G., Oudni-M’rad, M., Acosta-Jamett, G., Rehbein, S., Saarma, U., 2017. New mitogenome and nuclear evidence on the phylogeny and taxonomy of the highly zoonotic tapeworm Echinococcus granulosus sensu stricto. Infect. Genet. Evol. 52, 52-58. [DOI] [PubMed]
- Laurimäe T., Kinkar L., Moks E., Romig T., Omer R.A., Casulli A., Umhang G., Bagrade G., Irshadullah M., Sharbatkhori M., Mirhendi H., Ponce-Gordo F., Soriano S.V., Varcasia A., Rostami-Nejad M., Andresiuk V., Saarma U. Molecular phylogeny based on six nuclear genes suggests that Echinococcus granulosus sensu lato genotypes G6/G7 and G8/G10 can be regarded as two distinct species. Parasitology. 2018;145:1929–1937. doi: 10.1017/S0031182018000719. [DOI] [PubMed] [Google Scholar]; Laurimae, T., Kinkar, L., Moks, E., Romig, T., Omer, R.A., Casulli, A., Umhang, G., Bagrade, G., Irshadullah, M., Sharbatkhori, M., Mirhendi, H., Ponce-Gordo, F., Soriano, S.V., Varcasia, A., Rostami-Nejad, M., Andresiuk, V., Saarma, U., 2018a. Molecular phylogeny based on six nuclear genes suggests that Echinococcus granulosus sensu lato genotypes G6/G7 and G8/G10 can be regarded as two distinct species. Parasitology 145, 1929-1937. [DOI] [PubMed]
- Laurimäe T., Kinkar L., Romig T., Omer R.A., Casulli A., Umhang G., Gasser R.B., Jabbar A., Sharbatkhori M., Mirhendi H., Ponce-Gordo F., Lazzarini L.E., Soriano S.V., Varcasia A., Rostami-Nejad M., Andresiuk V., Maravilla P., Gonzalez L.M., Dybicz M., Gawor J., Šarkunas M., Šnabel V., Kuzmina T., Saarma U. The benefits of analysing complete mitochondrial genomes: deep insights into the phylogeny and population structure of Echinococcus granulosus sensu lato genotypes G6 and G7. Infect. Genet. Evol. 2018;64:85–94. doi: 10.1016/j.meegid.2018.06.016. [DOI] [PubMed] [Google Scholar]; Laurimae, T., Kinkar, L., Romig, T., Omer, R.A., Casulli, A., Umhang, G., Gasser, R.B., Jabbar, A., Sharbatkhori, M., Mirhendi, H., Ponce-Gordo, F., Lazzarini, L.E., Soriano, S.V., Varcasia, A., Rostami-Nejad, M., Andresiuk, V., Maravilla, P., Gonzalez, L.M., Dybicz, M., Gawor, J., Šarkunas, M., Šnabel, V., Kuzmina, T., Saarma, U., 2018b. The benefits of analysing complete mitochondrial genomes: Deep insights into the phylogeny and population structure of Echinococcus granulosus sensu lato genotypes G6 and G7. Infect. Genet. Evol. 64, 85-94. [DOI] [PubMed]
- Manfredi M.T., Di Cerbo A.R., Zanzani S., Moriggia A., Fattori D., Siboni A., Bonazza V., Filice C., Brunetti E. Prevalence of echinococcosis in humans, livestock and dogs in northern Italy. Helminthologia. 2011;48:59–66. [Google Scholar]; Manfredi, M.T., Di Cerbo, A.R., Zanzani, S., Moriggia, A., Fattori, D., Siboni, A., Bonazza, V., Filice, C., Brunetti, E., 2011. Prevalence of echinococcosis in humans, livestock and dogs in northern Italy. Helminthologia. 48, 59-66.
- Martin-Hernando M.P., Gonzalez L.M., Ruiz-Fons F., Garate T., Gortazar C. Massive presence of Echinococcus granulosus (Cestoda, Taeniidae) cysts in a wild boar (Sus scrofa) from Spain. Parasitol. Res. 2008;103:705–707. doi: 10.1007/s00436-008-0989-1. [DOI] [PubMed] [Google Scholar]; Martin-Hernando, M.P., Gonzalez, L.M., Ruiz-Fons, F., Garate, T., Gortazar, C., 2008. Massive presence of Echinococcus granulosus (Cestoda, Taeniidae) cysts in a wild boar (Sus scrofa) from Spain. Parasitol. Res. 103, 705-707. [DOI] [PubMed]
- Massei G., Toso S. Istituto Nazionale per la Fauna Selvatica; 1993. Biologia e gestione del cinghiale; pp. 1–75. [Google Scholar]; Massei, G., Toso, S., 1993. Biologia e gestione del cinghiale. Istituto Nazionale per la Fauna Selvatica, pp. 1-75.
- Massei G., Kindberg J., Licoppe A., Gačić D., Šprem N., Kamler J., Baubet E., Hohmann U., Monaco A., Ozoliņš J., Cellina S., Podgόrski T., Fonseca C., Markov N., Pokorny B., Rosell C., Náhlikq A. Wild boar populations up, numbers of hunters down? A review of trends and implications for Europe. Pest Manag. Sci. 2015;71:492–500. doi: 10.1002/ps.3965. [DOI] [PubMed] [Google Scholar]; Massei, G., Kindberg, J., Licoppe, A., Gačić, D., Šprem, N., Kamler, J., Baubet, E., Hohmann, U., Monaco, A., Ozoliņš, J., Cellina, S., Podgόrski, T., Fonseca, C., Markov, N., Pokorny, B., Rosell, C., Nahlikq, A., 2015. Wild boar populations up, numbers of hunters down? A review of trends and implications for Europe. Pest Manag. Sci. 71, 492-500. [DOI] [PubMed]
- Meng X., Lindsay D., Sriranganathan N. Wild boars as sources for infectious diseases in livestock and humans. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2009;364:2697–2707. doi: 10.1098/rstb.2009.0086. [DOI] [PMC free article] [PubMed] [Google Scholar]; Meng, X., Lindsay, D., Sriranganathan N., 2009. Wild boars as sources for infectious diseases in livestock and humans. Philos. Trans. R. Soc. Lond. B Biol. Sci. 364, 2697-2707. [DOI] [PMC free article] [PubMed]
- Meriggi A., Brangi A., Schenone L., Signorelli D., Milanesi P. Changes of wolf (Canis lupus) diet in Italy in relation to the increase of wild ungulate abundance. Ethol. Ecol. Evol. 2011;23:195–210. [Google Scholar]; Meriggi, A., Brangi, A., Schenone, L., Signorelli, D., Milanesi, P., 2011. Changes of wolf (Canis lupus) diet in Italy in relation to the increase of wild ungulate abundance. Ethol. Ecol. Evol. 23, 195-210.
- Mwambete K.D., Ponce-Gordo F., Cuesta-Bandera C. Genetic identification and host range of the Spanish strains of Echinococcus granulosus. Acta Trop. 2004;91:87–93. doi: 10.1016/j.actatropica.2004.04.001. [DOI] [PubMed] [Google Scholar]; Mwambete, K.D., Ponce-Gordo, F., Cuesta-Bandera, C., 2004. Genetic identification and host range of the Spanish strains of Echinococcus granulosus. Acta Trop. 91, 87-93. [DOI] [PubMed]
- Nakao M., Sako Y., Yokoyama N., Fukunaga M., Ito A. Mitochondrial genetic code in cestodes. Mol. Biochem. Parasitol. 2000;111(2):415–424. doi: 10.1016/s0166-6851(00)00334-0. [DOI] [PubMed] [Google Scholar]; Nakao, M., Sako, Y., Yokoyama, N., Fukunaga, M., Ito, A., 2000. Mitochondrial genetic code in cestodes. Mol. Biochem. Parasitol. 111 (2), 415-424. [DOI] [PubMed]
- Onac D., Gyorke A., Oltean M., Gavrea R., Cozma V. First detection of Echinococcus granulosus G1 and G7 in wild boars (Sus scrofa) and red deer (Cervus elaphus) in Romania using PCR and PCR-RFLP techniques. Vet. Parasitol. 2013;193:289–291. doi: 10.1016/j.vetpar.2012.11.044. [DOI] [PubMed] [Google Scholar]; Onac, D., Gyorke, A., Oltean, M., Gavrea, R., Cozma, V., 2013. First detection of Echinococcus granulosus G1 and G7 in wild boars (Sus scrofa) and red deer (Cervus elaphus) in Romania using PCR and PCR-RFLP techniques. Vet. Parasitol. 193, 289-291. [DOI] [PubMed]
- Otero-Abad B., Torgerson P.R. A systematic review of the epidemiology of echinococcosis in domestic and wild animals. PLoS Neglected Trop. Dis. 2013;7(6):e2249. doi: 10.1371/journal.pntd.0002249. [DOI] [PMC free article] [PubMed] [Google Scholar]; Otero-Abad, B., Torgerson, P.R., 2013. A systematic review of the epidemiology of echinococcosis in domestic and wild animals. PLoS Neglected Trop. Dis. 7(6), e2249. https//doi.org/10.1371/journal.pntd.0002249 [DOI] [PMC free article] [PubMed]
- Paoletti B., Della Salda L., Di Cesare A., Iorio R., Vergara1 A., Fava C., Olivastri A., Dessì G., Scala A., Varcasia A. Epidemiological survey on cystic echinococcosis in wild boar from Central Italy. Parasitol. Res. 2018 doi: 10.1007/s00436-018-6112-3. [DOI] [PubMed] [Google Scholar]; Paoletti, B., Della Salda, L., Di Cesare, A., Iorio, R., Vergara1, A., Fava, C., Olivastri, A., Dessi, G., Scala, A., Varcasia, A., 2018. Epidemiological survey on cystic echinococcosis in wild boar from Central Italy. Parasitol. Res. https://doi.org/10.1007/s00436-018-6112-3 [DOI] [PubMed]
- Piantedosi D., Neola B.,, D'Alessio N., Di Prisco F., Santoro M., Pacifico L., Sgroi G., Auletta L., Buch J., Chandrashekar R., Breitschwerdt E.B., Veneziano V. Seroprevalence and risk factors associated with Ehrlichia canis, Anaplasma spp., Borrelia burgdorferi sensu lato and D. immitis in hunting dogs from southern Italy. Parasitol. Res. 2017;116:2651–2660. doi: 10.1007/s00436-017-5574-z. [DOI] [PubMed] [Google Scholar]; Piantedosi, D., Neola, B, D'Alessio, N., Di Prisco, F., Santoro, M., Pacifico, L., Sgroi, G., Auletta, L., Buch, J., Chandrashekar, R., Breitschwerdt, E.B., Veneziano, V., (2017). Seroprevalence and risk factors associated with Ehrlichia canis, Anaplasma spp., Borrelia burgdorferi sensu lato and D. immitis in hunting dogs from southern Italy. Parasitol. Res. 116, 2651-2660. [DOI] [PubMed]
- Poglayen G., Varcasia A., Pipia A.P., Tamponi C., Parigi M., Marchesi B., Morandi B., Benfenati V., Scala A. Retrospective study on cystic echinococcosis in cattle of Italy. J. Infect. Dev. Ctries. 2017;11:11719–11726. doi: 10.3855/jidc.9433. [DOI] [PubMed] [Google Scholar]; Poglayen, G., Varcasia, A., Pipia, A.P., Tamponi, C., Parigi, M., Marchesi, B., Morandi, B., Benfenati, V., Scala, A., 2017. Retrospective study on Cystic Echinococcosis in cattle of Italy. J. Infect. Dev. Ctries. 11, 11719-11726. [DOI] [PubMed]
- Rinaldi L., Maurelli M.P., Veneziano V., Capuano F., Perugini A.G., Cringoli S. The role of cattle in the epidemiology of Echinococcus granulosus in an endemic area of southern Italy. Parasitol. Res. 2008;103:175–179. doi: 10.1007/s00436-008-0948-x. [DOI] [PubMed] [Google Scholar]; Rinaldi, L., Maurelli, M.P., Veneziano, V., Capuano, F., Perugini, A.G., Cringoli, S., 2008a. The role of cattle in the epidemiology of Echinococcus granulosus in an endemic area of southern Italy. Parasitol. Res. 103, 175-179. [DOI] [PubMed]
- Rinaldi L., Maurelli M.P., Capuano F., Perugini A.G., Veneziano V., Cringoli S. Molecular update on cystic echinococcosis in cattle and water buffaloes of southern Italy. Zoonoses Public Health. 2008;55:119–123. doi: 10.1111/j.1863-2378.2007.01101.x. [DOI] [PubMed] [Google Scholar]; Rinaldi, L., Maurelli, M.P., Capuano, F., Perugini, A.G., Veneziano, V., Cringoli, S., 2008b. Molecular update on cystic echinococcosis in cattle and water buffaloes of southern Italy. Zoonoses Public Health 55, 119-123. [DOI] [PubMed]
- Scala A., Varcasia A., Pipia A.P., Pilo C., Garippa G. First molecular isolation of Echinococcus granulosus horse strain (G4) in Sardinia (Italy) Parassitologia. 2006;48:344. [Google Scholar]; Scala, A., Varcasia, A., Pipia, A.P., Pilo, C., Garippa, G., 2006. First molecular isolation of Echinococcus granulosus horse strain (G4) in Sardinia (Italy). Parassitologia 48, 344.
- Seimenis A. Overview of the epidemiological situation on echinococcosis in the Mediterranean region. Acta Trop. 2003;85:191–195. doi: 10.1016/s0001-706x(02)00272-3. [DOI] [PubMed] [Google Scholar]; Seimenis, A., 2003. Overview of the epidemiological situation on echinococcosis in the Mediterranean region. Acta Trop. 85, 191-195. [DOI] [PubMed]
- Thompson R.C.A., McManus D.P. Towards a taxonomic revision of the genus Echinococcus. Trends Parasitol. 2002;18:452–457. doi: 10.1016/s1471-4922(02)02358-9. [DOI] [PubMed] [Google Scholar]; Thompson, R.C.A., McManus, D.P., 2002. Towards a taxonomic revision of the genus Echinococcus. Trends Parasitol. 18, 452-457. [DOI] [PubMed]
- Thrusfield M. In: Veterinary Epidemiology. Royal School of Veterinary Studies, University of Edinburgh. third ed. Thrusfield M., editor. Blackwell Scientific Ltd; Oxford: 1995. pp. 178–198. [Google Scholar]; Thrusfield, M., 1995. In: Thrusfield, M. (third Ed.), Veterinary Epidemiology. Royal School of Veterinary Studies, University of Edinburgh, Oxford: Blackwell Scientific Ltd, pp. 178-198.
- Torgerson P.R., Devleesschauwer B., Praet N., Speybroeck N., Willingham A.L., Kasuga F., Rokni M.B., Zhou X.N., Fevre E.M., Sripa B., Gargouri N., Furst T., Budke C.M., Carabin H., Kirk M.D., Angulo F.J., Havelaar A., de Silva N. World health organization estimates of the global and regional disease burden of 11 foodborne parasitic diseases, 2010: a data synthesis. PLoS Med. 2015;12 doi: 10.1371/journal.pmed.1001920. [DOI] [PMC free article] [PubMed] [Google Scholar]; Torgerson, P.R., Devleesschauwer, B., Praet, N., Speybroeck, N., Willingham, A.L., Kasuga, F., Rokni, M.B., Zhou, X.N., Fevre, E.M., Sripa, B., Gargouri, N., Furst, T., Budke, C.M., Carabin, H., Kirk, M.D., Angulo, F.J., Havelaar, A., de Silva, N., 2015. World health organization estimates of the global and regional disease burden of 11 foodborne parasitic diseases, 2010: a data synthesis. PLoS Med.. 12, e1001920. https://doi.org/10.1371/journal.pmed.1001920 [DOI] [PMC free article] [PubMed]
- Umhang G., Richomme C., Hormaz V., Boucher J.M., Boue F. Pigs and wild boar in Corsica harbor Echinococcus canadensis G6/7 at levels of concern for public health and local economy. Acta Trop. 2014;133:64–68. doi: 10.1016/j.actatropica.2014.02.005. [DOI] [PubMed] [Google Scholar]; Umhang, G., Richomme, C., Hormaz, V., Boucher, J.M., Boue, F., 2014. Pigs and wild boar in Corsica harbor Echinococcus canadensis G6/7 at levels of concern for public health and local economy. Acta Trop. 133, 64-68. [DOI] [PubMed]
- Varcasia A., Canu S., Lightowlers M.W., Scala A., Garippa G. Molecular characterization of Echinococcus granulosus strains in Sardinia. Parasitol. Res. 2006;98:273–277. doi: 10.1007/s00436-005-0059-x. [DOI] [PubMed] [Google Scholar]; Varcasia, A., Canu, S., Lightowlers, M.W., Scala, A., Garippa, G., 2006. Molecular characterization of Echinococcus granulosus strains in Sardinia. Parasitol. Res. 98, 273-277. [DOI] [PubMed]
- Varcasia A., Canu S., Kogkos A., Pipia A.P., Scala A., Garippa G., Seimenis A. Molecular characterization of Echinococcus granulosus in sheep and goats of Peloponnesus, Greece. Parasitol. Res. 2007;101:1135–1139. doi: 10.1007/s00436-007-0568-x. [DOI] [PubMed] [Google Scholar]; Varcasia, A., Canu, S., Kogkos, A., Pipia, A.P., Scala, A., Garippa, G., Seimenis, A., 2007. Molecular characterization of Echinococcus granulosus in sheep and goats of Peloponnesus, Greece. Parasitol. Res. 101, 1135-1139. [DOI] [PubMed]
- Varcasia A., Garippa G., Pipia A.P., Scala A., Brianti E., Giannetto S., Battelli G., Poglayen G., Micagni G. Cystic echinococcosis in equids in Italy. Parasitol. Res. 2008;102:815–818. doi: 10.1007/s00436-007-0862-7. [DOI] [PubMed] [Google Scholar]; Varcasia, A., Garippa, G., Pipia, A.P., Scala, A., Brianti, E., Giannetto, S., Battelli, G., Poglayen, G., Micagni, G., 2008a. Cystic echinococcosis in equids in Italy. Parasitol. Res. 102, 815-818. [DOI] [PubMed]
- Varcasia A., Piseddu T., Pipia A.P., Schianchi G., Marongiu A., Petruzzi V., Scala A., Garippa G. Epidemiological and biomolecular updates on cystic echinococcosis in pigs and wild boars of Sardinia (Italy) Lucr. Stiintifice Med. Veterinara. 2008;41:385–387. [Google Scholar]; Varcasia, A., Piseddu, T., Pipia, A.P., Schianchi, G., Marongiu, A., Petruzzi, V., Scala, A., Garippa, G., 2008b. Epidemiological and biomolecular updates on cystic echinococcosis in pigs and wild boars of Sardinia (Italy). Lucr. Stiintifice Med. Veterinara 41, 385-387.
- Varcasia A., Tanda B., Giobbe M., Solinas C., Pipia A.P., Malgor R., Carmona C., Garippa G., Scala A. Cystic echinococcosis in Sardinia: farmers' knowledge and dog infection in sheep farms. Vet. Parasitol. 2011;181:335–340. doi: 10.1016/j.vetpar.2011.05.006. [DOI] [PubMed] [Google Scholar]; Varcasia, A., Tanda, B., Giobbe, M., Solinas, C., Pipia, A.P., Malgor, R., Carmona, C., Garippa, G., Scala, A., 2011. Cystic echinococcosis in Sardinia: farmers’ knowledge and dog infection in sheep farms. Vet. Parasitol. 181, 335-340. [DOI] [PubMed]
- Veneziano V., Rinaldi L., Apicella G., Garippa G., Cringoli G. Cystic echinococcosis in the Campania region (southern Italy) Parassitologia. 2004;46:449–451. [PubMed] [Google Scholar]; Veneziano, V., Rinaldi, L., Apicella, G., Garippa, G., Cringoli, G., 2004. Cystic echinococcosis in the Campania region (southern Italy). Parassitologia 46, 449-451. [PubMed]