Abstract
Emerging infectious diseases are frequently zoonotic, often originating in wildlife, but enteric protozoa are considered relatively minor contributors. Opinions regarding whether pathogenic enteric protozoa may be transmitted between wildlife and humans have been shaped by our investigation tools, and have led to oscillations regarding whether particular species are zoonotic or have host-adapted life cycles.
When the only approach for identifying enteric protozoa was morphology, it was assumed that many enteric protozoa colonized multiple hosts and were probably zoonotic. When molecular tools revealed genetic differences in morphologically identical species colonizing humans and other animals, host specificity seemed more likely. Parasites from animals found to be genetically identical - at the few genes investigated - to morphologically indistinguishable parasites from human hosts, were described as having zoonotic potential. More discriminatory molecular tools have now sub-divided some protozoa again. Meanwhile, some infection events indicate that, circumstances permitting, some “host-specific” protozoa, can actually infect various hosts. These repeated changes in our understanding are linked intrinsically to the investigative tools available.
Here we review how molecular tools have assisted, or sometimes confused, our understanding of the public health threat from nine enteric protozoa and example wildlife hosts (Balantoides coli - wild boar; Blastocystis sp. - wild rodents; Cryptosporidium spp. - wild fish; Encephalitozoon spp. - wild birds; Entamoeba spp. - non-human primates; Enterocytozoon bieneusi - wild cervids; Giardia duodenalis - red foxes; Sarcocystis nesbitti - snakes; Toxoplasma gondii - bobcats).
Molecular tools have provided evidence that some enteric protozoa in wildlife may infect humans, but due to limited discriminatory power, often only the zoonotic potential of the parasite is indicated. Molecular analyses, which should be as discriminatory as possible, are one, but not the only, component of the toolbox for investigating potential public health impacts from pathogenic enteric protozoa in wildlife.
Keywords: Emerging infection, Host specificity, Protozoa, Transmission, Wildlife, Zoonosis
Graphical abstract
Highlights
-
•
Wildlife is a potential reservoir of disease agents that may infect humans.
-
•
The public health threat from enteric protozoa in wildlife is poorly understood.
-
•
Molecular tools may help in understanding this threat, but may also confuse.
-
•
We use nine enteric protozoa-wildlife host examples to review the current position.
-
•
Data accumulate, but more discriminatory tools and other approaches are important.
1. Zoonotic enteric protozoa in wildlife as agents of “emerging” infectious diseases in humans
The potential for wildlife to be a source of infectious diseases in humans was brought into focus in a landmark paper published in 2008, in which the authors estimated that emerging infectious disease (EID) events occurring between 1940 and 2004 were dominated by zoonoses (60.3%), the majority of which (71.8%) originated in wildlife (Jones et al., 2008). These figures, or approximations thereof, have been widely quoted since. The authors of the original article report that the majority of the EID events included in their calculations involve bacteria or rickettsiae (54.3%), but note that in making these estimates they classified every individual drug-resistant microbial strain as a separate pathogen. Although the importance of antimicrobial resistance to global health should be emphasized, this classification may have resulted in the contribution of other pathogen types (virus, protozoa, helminths, etc.) being underestimated; the authors calculated the percentages of EID events caused by other pathogen types to be 25.4% for viral or prion pathogens, 10.7% for protozoa, 6.3% for fungi, and 3.3% for helminths (Jones et al., 2008).
Although wildlife parasitology is of importance in its own right, particularly in consideration of such elements as loss of biodiversity, conservation issues, alterations in land use, impacts of climate change, and the role of invasive species (Thompson and Polley, 2014), it is clear that much of the research in wildlife parasitology is driven by determining whether or not parasitic infections in a wildlife population may serve as a reservoir of diseases that may affect domestic animals or humans (Appelbee et al., 2005). A clear and early example of this was the investigation of beavers for Giardia infection, following an outbreak of waterborne giardiasis in Washington State, USA in 1976, in which Giardia cysts were detected in the raw water and storage reservoirs (Dykes et al., 1980). Three beavers trapped in the watershed area were infected with Giardia, implicating them as a potential source of the outbreak. However, the lack of morphological differences between genetic variants means that, at that time, it was not possible to determine whether the Giardia in the beavers was of the same species as in the patients, or the extent of genetic similarity or difference between the Giardia from the beavers and the infected people. If molecular tools had been available then, it may have been possible to exclude the beavers as the source of the Giardia contaminating the water supply – but what if molecular tools had shown a similar genotype? Would this have indicated that the beavers were the “guilty party”, or would it have simply indicated that humans and some animals in this area were infected with similar genotypes of Giardia? And what of disease potential? Although the infected humans in this 1976 outbreak were obviously symptomatic, it is not evident that the beavers themselves were suffering from clinical disease. Two of the three beavers were dead-trapped, but the third one was live-trapped, and sent, alive, from Camas, Washington to Fort Collins, Colorado for infection studies in beagles (Dykes et al., 1980); given that no comment is made regarding signs in the beaver, it has to be assumed that none were observed.
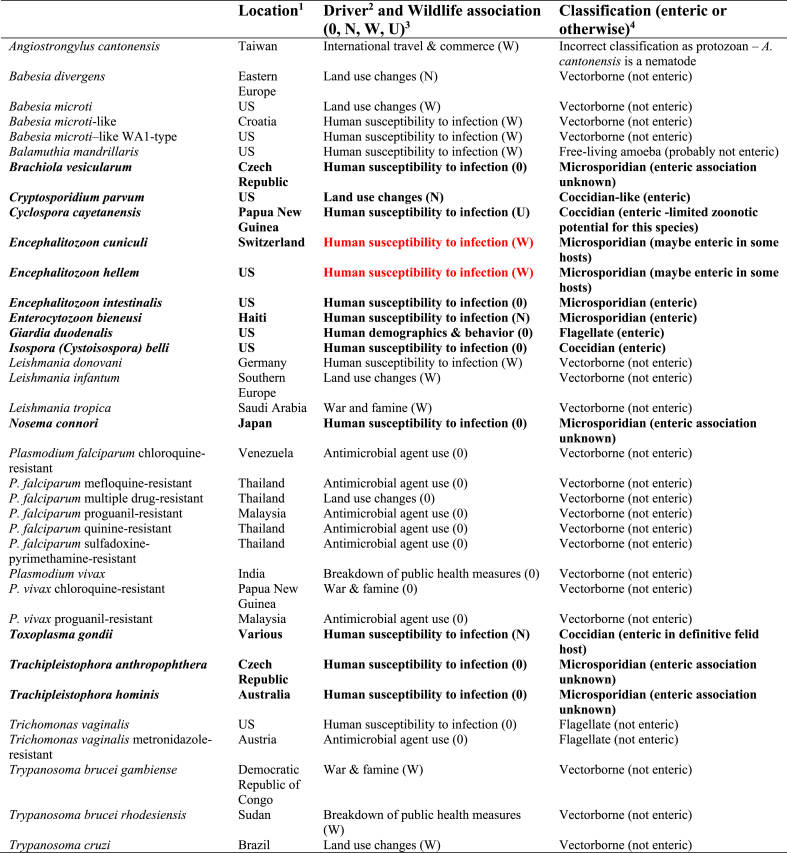
In the database provided as supplementary information to the article considering EIDs in humans (Jones et al., 2008), of the 335 EID events noted to have occurred, 36 were designated as being due to protozoa. Table 1 is extracted from this reference, and gives an overview of the protozoal EID events considered; of the 35 protozoa listed (the nematode Angiostrongylus cantonensis is incorrectly described as a protozoa), a substantial proportion (19/35, 54%) are non-enteric, vectorborne parasites. Of the remaining 16, seven are enteric in some hosts; for a further six, all of which are microsporidia, it is currently unknown whether they may be enteric in some hosts. Of the remaining three, two are not enteric (residing in the urogenital tract), and one (a free-living, opportunistic amoeba) is probably not enteric. Of those that are categorized here as enteric or that their enteric potential for all hosts is unknown (n = 13, highlighted in bold font in Table 1), only two, both microsporidia in the genus Encephalitozoon, are classified as being pathogens of wildlife origin (red font in Table 1).
Table 1.
The 35 protozoa (and one incorrectly classified helminth) responsible for the EID events included in the database, as extracted from Supplementary Table 1 (Jones et al., 2008).
a The location column is based on the location designations used in Table 1 of the Supplementary Material of Jones et al. (2008), in the penultimate column of that table headed “Location”. When a single location is given this is copied verbatim for the country or region heading, regardless of political affiliation. However, more specific pinpointing provided in the original table (such as city, county, or even institution) are not included in the current table. Furthermore, where several countries were noted we have grouped them if feasible (e.g., Eastern Europe for Babesia microti, rather than the various countries listed).
b The drivers written in this column have been copied verbatim from the “Driver” column used in Table 1 of the Supplementary Material of Jones et al. (2008).
c The wildlife association designations used here are based on those used in Table 1 of the Supplementary Material of Jones et al. (2008), specifically, the column in the original table described as “Zoonotic type”. Derived directly from the definitions used for that table (although with a different coding system), with the variable definitions copied verbatim from those provided by Jones et al., (2008), 0 means non-zoonotic (disease emerged via human to human transmission); N means non-wildlife (zoonotic EID event caused by a pathogen with no known wildlife origin); W means wildlife (zoonotic EID event caused by a pathogen with a wildlife origin); and U means unspecified (zoonotic EID event caused by a pathogen with an unknown origin).
d The classifications used in this column are not derived directly from Table 1 of the Supplementary Material of Jones et al. (2008). Instead, for the purposes of the current article, a very brief description indicates whether each protozoa could be considered as enteric.
2. Which enteric protozoan pathogens are zoonotic and what are their wildlife hosts? What information on public health importance has been obtained using molecular tools?
Despite the data from Jones et al. (2008) indicating that enteric protozoan parasites in wildlife are of relatively low public health relevance, we have chosen here to re-visit this topic. In particular, we have focussed upon the greater information obtained in the past decade by the use of molecular tools, and consider whether this extra information has assisted or confused us in determining the extent to which wildlife may act as a reservoir for enteric protozoa that may pose a threat to public health.
The first molecular tools used to address this question involved the use of antibodies and isoenzyme analysis. Currently, however, the most common approaches involve amplification and sequencing of one or more genomic DNA targets, selected to be either more conserved or more variable, depending on the focus of the investigation, followed by the use of phylogenetic analyses, including sequence polymorphism analyses, to determine relationships between the sequences obtained. It is these approaches and their results that are used predominantly in the following parasite-host specific sections.
One of the difficulties that we have in discussing this topic is the terminology. For example, throughout this manuscript we use the term “protozoa” to encompass the group of single-celled eukaryotic parasites under consideration, despite “protozoa” having no real taxonomic meaning. The term “protista” could have been used instead, but, again, the terminology is not founded on phylogenetic relationships, and the debate regarding how microorganisms, particularly eukaryotes, should be most appropriately classified has been a source of debate for centuries (Scamardella, 1999). Here we have chosen to use the term “protozoa” for convenience and to enable simpler comparison with other relevant articles, although some of the parasites covered are no longer considered to fit properly within this terminology. For example, although microsporidia are currently considered to be more related to fungi than other protozoan parasites (in the clade Opisthosporidia), we have chosen to include them here in line with Jones et al. (2008). We also include Blastocystis (which was not mentioned by Jones et al. (2008)), although this organism is now known to belong to the Stramenopiles, a group of organisms that includes, among others, brown algae, diatoms, and oomycetes.
Another terminology issue concerns what we actually mean when we refer to a pathogen as zoonotic. For example, if a pathogen that usually infects only animals is reported on just a single occasion in low numbers from a highly immunocompromised human patient, perhaps as an incidental finding, should it then be considered zoonotic? For the purposes of this document, we have described protozoans in such instances as that as being “potentially zoonotic”; an example of this could be Cryptosporidium suis. The adjective “zoonotic” is only used when we have clear evidence that the protozoa will readily establish in both humans and animals. The concept of zoonanthroponosis, which refers to diseases that are primarily infections of humans, but that have the potential to be naturally transmitted to animals, is also of relevance – but not a major theme of this manuscript.
It is clear that enteric protozoan infections, in which, generally, robust transmission stages are excreted in the faeces, have the ability to contaminate the environment. These may be ingested by another possible host, be that wildlife, domestic animals, or humans, potentially resulting in infection, and possibly disease. However, the extent to which this spillover between groups of potential host species actually occurs is not necessarily clear. Not only does the likelihood of cross-infection between potential host groups depend on the ability of the parasite itself to infect the different hosts, but it also depends on factors relevant to the host (immunity, age, foraging or grazing habits, etc.), and also to the environment where the defecation occurs - whether survival and onwards movement of the transmission stage is favoured, and the likelihood that both host groups use the same environment. It is these interactions between the environment and the health status of people, their domestic animals, and wildlife that together form the basis of the One Health concept.
The likelihood of transmission of an enteric protozoan parasite to a person thus depends not only on whether the parasite has zoonotic potential, but also on a range environmental factors and aspects of the wildlife hosts’ activity, mode of existence, and behaviour. Therefore, rather than list the potential wildlife hosts for each of the relevant protozoan pathogenic parasites considered here, a particular wildlife host has been selected per parasite. An overview of the parasite-wildlife host pairs considered is provided in Table 2. Brief information for each parasite is provided in the table, along with the rationale for the selection of the host species under consideration; that is, particular characteristics of the host that are relevant to transmission of enteric protozoa to people. The protozoan-wildlife hosts are then described in focused vignettes that indicate the current extent of our knowledge regarding zoonotic transmission and public health threat, with particular emphasis on the role of molecular methods in aiding our understanding of the threat to public health encompassed by the particular parasite in that specific wildlife host.
Table 2.
Overview of enteric protozoa (including Microsporidia and Blastocystis) and a potential wildlife host species selected for review in this article, including the reasons for their inclusion.
| Enteric protozoan parasite | Brief description | Wildlife (definitive) hosta | Host characteristics of relevance to transmission of enteric protozoa from wildlife hosts to people |
|---|---|---|---|
| Balantioides coli (Balantidium coli) | Ciliate: relatively large trophozoites inhabit the colon and caecum of the host. Both asexual and sexual replication. | Wild boar | Sus scrofa: most widespread species of wild pig. Distribution from Western Europe to the Far East and Southeast Asia. Introduced populations on all continents except Antarctica. High reproductive rate. Suitable habitats include mixed landscape (agricultural fields and forest). Likely to contaminate environments where humans work, have leisure activities, and produce food. |
| Transmission via cysts in environment. | |||
| Blastocystis sp. | Stramenopiles: exist in several different morphological forms – vacuolar, granular, and amoeboid - that inhabit the intestine. | Rodents | Very diversified mammalian order, living in huge numbers on all continents except Antarctica. Inhabit a wide variety of terrestrial habitats, including man-made environments. Many species are considered pests. Likely to contaminate environments where humans live and produce food. |
| Transmission via cysts in environment. | |||
| Cryptosporidium spp. | Apicomplexan: numerous (>30) species. | Wild fish | Some wild fish species represent not only a food source for humans, but may also inhabit waterways used as drinking water sources or for recreation. Defecation into water favours survival for parasite transmission stages, but also may enable wide dissemination. |
| Sporozoites invade epithelial cells. Epicellular location. | |||
| Both asexual and sexual replication. | |||
| Transmission via oocysts in environment. | |||
| Encephalitozoon spp. | Microsporidian: host cells infected via an extruded polar tubule that injects infective sporoplasm. Multiplication within cells by merogony and schizogony. | Wild birds | With birds living and breeding in nearly all terrestrial habitats and all continents, and some migrating over vast distances, birds provide a mechanism for dissemination of transmission stages of enteric protozoa into all environments, including urban and agricultural, depending on bird species. |
| Spores released by cell bursting; transmission stage in environment. | |||
| Pathogenic Entamoeba spp. | Amoebozoan: trophozoites inhabit large intestine and multiply by binary fission. | Non-human primates (NHP) | The close taxonomic relationship between humans and NHPs facilitates transmission of pathogens between them. Drivers such as urbanization, habitat fragmentation, deforestation, tourism, increase the likelihood of overlap between habitats. |
| Transmission via cysts in environment. | |||
| Enterocytozoon bieneusi | Microsporidian: infects intestinal epithelial cells via injection of sporoplasm through a polar tubule. | Wild cervids | Deer are widely distributed, and indigenous species are found in all continents, except Australia and Antarctica. Some live in sizeable populations. Depending on species, cervids occupy different biomes, from tundra to tropical forest, but mostly inhabit mixed habitats. Adjacent croplands benefit several species, and enable contamination of environments where human food is produced. Further human interaction as many species are important game animals. |
| Multiplication within cells by merogony and schizogony. | |||
| Transmission via spores in environment. | |||
| Giardia duodenalis | Flagellate (Order, Diplomonadida): trophozoites inhabit small intestine and replicate by binary fission. | Red foxes | Vulpes vulpes: widely distributed member of the Carnivora. Occurs throughout the northern hemisphere, adapting rapidly to new environments and food sources. Very successful at adapting to and colonizing urban environments. |
| Transmission via cysts in environment. | |||
| Sarcocystis nesbitti | Apicomplexan with two host (prey, predator) life cycle. Sexual reproduction in intestine of definitive host. | Snakes | Although snakes do not possess specific characteristics that are relevant for transmission of enteric protozoa to people, they are the wildlife species that has been associated with transmission of S. nesbitti to humans. |
| Asexual reproduction in sarcocysts in muscle of intermediate host. | |||
| Oocysts shed in faeces and may lyse, releasing two sporocysts; transmission stages in environment. | |||
| Snakes are indicated as definitive hosts for S. nesbitti. | |||
| Toxoplasma gondii | Apicomplexan with two host (prey, predator) life cycle. Felids are definitive host. | Bobcat | Lynx rufus: although not possessing specific characteristics relevant for transmission of enteric protozoa to people, felids are the only definitive hosts of Toxoplasma. Bobcats range from southern Canada to central Mexico, including most of the USA. Living near agricultural areas and prey population are main drivers for distribution, unrestricted by human populations, provided a suitable habitat is available. |
| Asexual reproduction of bradyzoites in muscles and other tissues of intermediate host. | |||
| Sexual reproduction in intestine of definitive host. | |||
| Oocysts transmission stage in environment. |
The wildlife (definitive) hosts listed here are not the only wildlife hosts for the parasite under consideration, but those selected for consideration for the purpose of this article.
2.1. Balantioides coli (Balantidium coli) in wild boar
Paramecium coli, described by Malmsten (1857) in human samples, was renamed Balantidium coli by Stein (1863) after describing it from pigs. Over the subsequent years, several Balantidium species were described from different wild and domestic mammals and birds based on morphological differences or the host species (Neiva et al., 1914; McDonald, 1922; Hegner, 1934). Alexeieff (1931) proposed transferring B. coli to a new genus, Balantioides, but, until recently, when Pomajbíková et al. (2013) proposed a new genus, Neobalantidium, to accommodate species from mammals, this was not taken into consideration; Chistyakova et al. (2014) consider Neobalantidium to be a junior synonym of Balantioides. As not all authors follow this taxonomic change, different names are used in scientific articles and in genetic databases: veterinarians and researchers not specialized in taxonomic discussions continue to use Balantidium coli, whereas specialists have used Neobalantidium coli and more recently, Balantioides coli. This gradual correction in naming of the parasite may be a source of confusion.
In a very detailed morphological study, McDonald (1922) proposed that pigs could be infected by two species, B. coli (which could also infect humans) and B. suis (pig-specific). However, there was some controversy about the validity of the second species and finally it was generally accepted as a synonym of B. coli (Awakian, 1937; Levine, 1961, 1985). Nevertheless, even in some recent papers (e.g., Schuster and Ramirez-Avila, 2008; Supriadi et al., 2012; Petrova et al., 2017), B. suis is still used as a name for the species found in pigs. Curiously, although pigs and wild boar are the same species, findings from wild boar are reported as B. coli or Balantidium sp., but not B. suis (e.g., Solaymani-Mohammadi et al., 2004; Mundim et al., 2004; Navarro-González et al., 2013; Yaghoobi et al., 2016).
Balantioides coli is commonly found in both pigs and wild boar (with prevalences ranging up to 100% in domestic pigs, and up to 70% in wild boar; Ponce-Gordo and Jirků-Pomajbíková 2018). Soon after the first description of this parasite, the epidemiological importance of pigs for human infections was noted. Pigs are considered the main reservoir and people living in close contact with them are at greatest risk of becoming infected with this parasite (Ponce-Gordo and Jirků-Pomajbíková, 2018). The epidemiological importance of wild boar is unknown; however, when infected animals congregate in the catchment area of public drinking water sources, then the risk of transmission of their parasites to humans via contaminated water is likely to increase (Hampton et al., 2006). Transmission to humans via contamination of the environment is also likely to be associated with the apparently recent and increasing tendency for wild boar to invade urban areas, mainly searching for feed (Cahill et al., 2012). In Muslim countries, where pig farming is forbidden, wild boar are considered the main reservoirs of B. coli by some authors, but others consider other domestic mammals (camels, donkeys, sheep and goats) as the reservoirs of greatest importance to human health (Ponce-Gordo and Jirků-Pomajbíková, 2018). If parasite identification in human infections is based only on trophozoite and/or cyst morphology, it is not possible to identify the origin of the infections, and thus the most likely transmission routes remain unknown.
Genetic studies on B. coli started around 15 years ago, and currently the only genetic data available are for the nuclear small subunit rRNA gene (SSU-rDNA) and the internal transcribed spacer (ITS1-5.8S rDNA-ITS2) regions; B. coli does not possess mitochondria, and data for other genes have not been published to date. Despite only two ribosomal genes being currently available for comparative work, their analysis is useful for taxonomic studies and interesting results have been obtained. The comparison of SSU-rDNA sequences is a valid tool for taxon differentiation at the genus level or above, and sometimes also at the species level; however, for differentiating between closely related subtypes, analysis of the ITS region, and especially the ITS2 fragment, is considered the best option (Yao et al., 2010; Gou et al., 2012; Han et al., 2013).
The first B. coli sequence published was the SSU-rDNA from a gorilla isolate (Strüder-Kypke et al., 2006), but the first comparison of B. coli DNA isolated from different hosts (pig and ostrich) was made by Ponce-Gordo et al. (2008) by analysing sequences from the SSU-rDNA and ITS regions. In that study, pooled cysts were analysed; SSU-rDNA sequences showed some unresolved ambiguities common to all cyst isolates, and ITS sequences indicated two different sequence variants. To determine the importance of this ITS polymorphism (which could have represented two different species, but with low host specificity), a detailed analysis of individual cysts was made (Ponce-Gordo et al., 2011) and three important results were obtained: (1) the two ITS sequence variants previously identified had clear differences in both the ITS1 and ITS2 regions. (2) Both variants were found within single parasite cells, indicating that the polymorphism was present within one single species. (3) The same ITS sequences of both variants occurred in isolates from human, gorilla, pig and ostrich, indicating that the same species (B. coli) was found in all of them. Pomajbíková et al. (2013) and Da Silva Barbosa et al. (2017) have also found no significant differences between B. coli DNA isolated from non-human primates (NHPs) and wild boar, or NHPs and pigs, respectively. Thus, these genetic data support the presence of a single zoonotic species, B. coli, that infects homeothermic vertebrates and can be transmitted between wild fauna (mainly wild boar), domestic animals (mainly pigs), and humans.
2.2. Blastocystis sp. in wild rodents
Blastocystis sp. has been found in most animal species investigated to date. Its pathogenicity is controversial (Stensvold and Clark, 2016a), although more recent articles describe it as being part of the microbiome, and influencing, or influenced by, the composition of bacterial communities (Nieves-Ramirez et al., 2018; Tito et al., 2018). It is clear that subtyping of Blastocystis isolates is critical for evaluating the relationship between the parasite, gut microbiota profile, and host health. It was long assumed that one species of Blastocystis, Blastocystis hominis, infected humans, and different species of Blastocystis infected other animals. However, genetic analyses have demonstrated that there is no single Blastocystis entity that infects only humans; many (but not all) of the subtypes (ST) identified in animals also infect humans, and currently Blastocystis tends to be identified by the genus name and the ST number.
There are only a few epidemiological studies that investigate the transmission of Blastocystis from rodents, and these mainly focus on trapping and euthanizing the hosts to collect their caeca (Seifollahi et al., 2016; Yoshikawa et al., 2016b; Katsumata et al., 2018), followed by detection using a smear examined by light microscopy or incubation of the sample in Jones’ medium (Katsumata et al., 2018) or agar slant (Yoshikawa et al., 2016b) for three to five days, before microscopy examination of stained or unstained samples. Although these approaches continue to be used (Katsumata et al., 2018), they are being replaced by molecular techniques, not only because Blastocystis can be easily confused with other microorganisms (e.g., Entamoeba) – and thus determining the prevalence of Blastocystis by microscopy or culture methods is likely to result in an inaccurate estimate (Wawrzyniak et al., 2013) - but also because the culture methods may select for particular subtypes (Roberts et al., 2011). PCR and qPCR methods that partially or completely amplify the SSU-rDNA are sufficiently sensitive for both identifying and subtyping Blastocystis (Roberts et al., 2011; Wawrzyniak et al., 2013; Stensvold and Clark, 2016b).
Using a combination of these techniques, in both restricted (Betts et al., 2018; Farah Haziqah et al., 2018) and wider (Cian et al., 2017) sampling regions, has demonstrated that subtypes ST1, ST2, ST4, ST5, ST10, and ST17 predominate in rodents, and, in some cases, mixed infections (of two STs) have been identified (Cian et al., 2017; Betts et al., 2018; Farah Haziqah et al., 2018). Of these subtypes, ST1, ST2, ST4, and ST5 have also been found in humans (and thus have zoonotic potential), whereas ST10 and ST17 have not, to date, been identified in humans, being exclusively found in animals. In human infections, ST3 and ST4 often predominate, but this may vary between locations and circumstances.
Although the different rodent studies using molecular tools have used similar approaches, each used a different set of primers to target the same (5′-end) region of the SSU-rDNA and the (phylogenetic) analyses of the amplified and sequenced regions tend to be inconsistent, sometimes due to the short length of the amplified fragment. For subtyping studies to provide meaningful results, phylogenetic trees should include all 17 subtypes, and be rooted and constructed using both maximum likelihood and Bayesian inference methods (Betts et al., 2018); this has not been the case in all studies.
This means that although some articles have reported on Blastocystis in rodent populations, it is difficult to investigate transmission dynamics, and further studies are important to elucidate the circulation of different Blastocystis subtypes within rodent populations. For example, Betts et al. (2018) identified Blastocystis infections in both wild and captive water voles (Arvicola amphibius), but the subtypes differed, with ST4 dominant in the wild voles and ST1, which was not identified in wild water voles, also present in captive voles. When wild water voles were brought into captivity, ST1 started circulating within this population. How this happened is difficult to determine, although it is tempting to speculate that this could reflect a microbiota-associated effect, related to life in captivity (Betts et al., 2018).
Current molecular methods for the investigation of Blastocystis subtypes in rodents and other wildlife, especially those described in Betts et al. (2018), match those used for investigating Blastocystis subtypes in humans (Alfellani et al., 2013; Yoshikawa et al., 2016a). The potential for zoonotic transmission is indicated by the same ST being found in rodents and humans. However, further studies are needed to determine the extent to which this occurs, whether the single locus evaluation is sufficient to determine genetic identity, and, overall, whether this similarity in Blastocystis subtypes in humans and rodents is of public health significance.
2.3. Cryptosporidium spp. in wild fish
Wild fish represent a source of Cryptosporidium infection for humans. This may be via: (1) consumption of raw or undercooked fish flesh that has been contaminated with oocysts, and (2) consumption of water contaminated with oocysts shed in fish faeces. Despite these two potential routes for transmission, relatively few molecular studies have been conducted on Cryptosporidium in fish and the majority of these have been on farmed or aquarium fish (Murphy et al., 2009; Zanguee et al., 2010; Barugahare et al., 2011; Gibson-Kueh et al., 2011; Morine et al., 2012; Ryan et al., 2015; Yang et al., 2015, 2016; Palermo, 2016; Paparini et al., 2017; Couso-Pérez et al., 2018), with only a handful of studies on wild fish or, particularly, wild fish commonly consumed by people (Alvarez-Pellitero and Sitjà-Bobadilla, 2002, Palenzuela et al., 2010; Reid et al., 2010; Certad et al., 2015) (Table 3).
Table 3.
Cryptosporidium species and genotypes reported in wild fish using molecular tools.
| Species | Host | Marine/Freshwater | Site of Infection | % overall prevalence | GenBank accession number (SSU-rDNA)a | gp60 subtypeb | Reference |
|---|---|---|---|---|---|---|---|
| C. molnari | Gilthead sea bream (Sparus aurata), European sea bass (Dicentrarchus labrax), | Marine | Stomach (and intestine) | 5–25 |
HM243548, HM243550, HQ585890 |
– | Alvarez-Pellitero and Sitjà-Bobadilla, 2002, Palenzuela et al. (2010) |
| C. molnari | Northern pike (Esox lucius) | Freshwater | – | 40 | KP939354 | – | Certad et al. (2015) |
| C. scophthalmi | Turbot (Scophthalmus maximus) | Marine | Intestine | 100 | KR340588 | – | Alvarez-Pellitero et al. (2004) |
| Cryptosporidium Piscine genotype 3 | Mullet (Mugil cephalus) | Marine | Intestine | 2 | GQ925452 | – | Reid et al. (2010) |
| C. scrofarum | School whiting (Sillago vittata) | Marine | – | 4 | – | – | Reid et al. (2010) |
| C. xiaoi | School whiting (Sillago vittata) | Marine | – | 2 | – | – | Reid et al. (2010) |
| C. parvum | School whiting (Sillago vittata) | Marine | Intestine | 2 | – | IIaA18G3R1 | Reid et al. (2010) |
| C. hominis | Mackerel scad (Decapterus macarellus) | Marine | – | 3 | – | IdA15G1 | Koinari et al. (2013) |
| C. parvum | Mackerel scad (Decapterus macarellus) | Marine | – | 7 | – | IIaA15G2R1 | Koinari et al. (2013) |
| C. parvum | Silver barb (Puntius gonionotus) | Freshwater | – | 2 | – | IIaA19G4R1 | Koinari et al. (2013) |
| C. parvum | Arctic char (Salvelinus alpinus), European whitefish (Coregonus lavaretus), European perch (Perca fluviatilis) and roach (Rutilus rutilus) | Freshwater | Intestine | 33–100 | KP939335, KP939337, KP939351 | IIaA15G2R1, IIaA16G2R1, IIaA17G2R1 |
Certad et al. (2015) |
In most cases either only one isolate was identified or only one or no representative sequence was submitted to GenBank.
Corresponding gp60 subtype identified in samples that were positive for either C. hominis or C. parvum at the small subunit rRNA gene (SSU-rDNA).
Currently, three species of Cryptosporidium have been described from fish that are not found in other hosts. These are: (1) Cryptosporidium molnari, which was originally described in wild gilthead sea bream (Sparus aurata) and European sea bass (Dicentrarchus labrax) (Alvarez-Pellitero and Sitjà-Bobadilla, 2002) and was characterized genetically some years later (Palenzuela et al., 2010); (2) Cryptosporidium scophthalmi, described in wild turbot (Psetta maxima syn. Scophthalmus maximus) (Alvarez-Pellitero et al., 2004), and a C. scophthalmi-like strain characterized genetically in 2015 (GenBank accession numbers: KR340588 and KR340589), and (3) Cryptosporidium huwi (previously piscine genotype 1) in a captive guppy (Poecilia reticulata) (Ryan et al., 2015).
Molecular characterisation has also identified piscine genotypes 2–9, two different C. molnari-like genotypes, more than 8 un-named novel genotypes, C. parvum, C. hominis, C. xiaoi, C. scrofarum, and rat genotype III in fish (Murphy et al., 2009; Reid et al., 2010; Zanguee et al., 2010; Barugahare et al., 2011; Morine et al., 2012; Koinari et al., 2013; Certad et al., 2015; Ryan et al., 2015; Yang et al., 2015, 2016; Palermo, 2016; Couso-Pérez et al., 2018). Both C. scrofarum and C. xiaoi have been identified in western school whiting (Sillago vittata) (Reid et al., 2010) and could be of some public health importance; C. scrofarum has been reported in several cases of human cryptosporidiosis (Kváč et al., 2009; Xiao, 2010), and C. xiaoi has been reported in two patients in Ethiopia (Adamu et al., 2014). Cryptosporidium hominis was identified in wild mackerel scad (Decapterus macarellus) in Papua New Guinea (Koinari et al., 2013) and more recently in farmed goldfish (Carassius auratus) in Australia (Palermo, 2016). Cryptosporidium parvum, the species most associated with zoonotic infection, was identified in western school whiting (Reid et al., 2010) and in goldfish (Palermo, 2016) in Australia, in wild-caught mackerel scad and silver barb (Puntius gonionotus) from Papua New Guinea and in cultured Nile tilapia (Oreochromis niloticus) (Koinari et al., 2013). In Lake Geneva, France, C. parvum was detected in Arctic char (Salvelinus alpinus), European whitefish (Coregonus lavaretus), European perch (Perca fluviatilis) and roach (Rutilus rutilus) (Certad et al., 2015). In the latter study, C. parvum was identified at a high prevalence in freshwater fish (13/15, 87%) and C. parvum developmental stages were detected in fish intestines, suggesting that this was infection, rather than simply carriage (Certad et al., 2015).
Only three studies have conducted glycoprotein-60 (gp60) subtyping on Cryptosporidium DNA isolated from wild fish samples, although this tool has become relatively standard for identifying subtypes in potential reservoir species for human infection: (1) C. parvum IIaA18G3R1 was identified in western school whiting from Australia (Reid et al., 2010), (2) C. hominis IdA15G1 in mackerel scad and C. parvum IIaA15G2R1 and IIaA19G4R1 subtypes in mackerel scad and silver barb respectively from Papua New Guinea (Koinari et al., 2013), and (3) in the study in France, C. parvum subtypes IIaA15G2R1, IIaA16G2R1, and IIaA17G2R1 were reported (Certad et al., 2015). These C. parvum subtypes commonly infect both livestock and humans (Xiao, 2010). In the study in Papua New Guinea, the C. hominis detected in the fish could have come from spillback from the human population due to poor sanitation infrastructure, but as parasites were not observed by histology, it was not possible to determine whether this was an actual infection or carriage.
The results presented here show that use of molecular tools has been instrumental in determining the zoonotic potential of Cryptosporidium detected in wild fish. They have demonstrated that although wild fish are infected with apparently host-specific species and genotypes of Cryptosporidium (C. molnari, C. scophthalmi, and piscine genotype 3), there is also the potential that human-infectious species (C. hominis, C. parvum, C. scrofarum, and C. xiaoi) may be identified in samples from wild fish. For C. parvum, at least, the investigations represented true infections, indicating propagation. However, these studies are preliminary and scattered, and further investigations to support these initial findings, preferably with infection studies, are essential in order to better understand the likelihood of wild fish representing a public health risk for transmission of Cryptosporidium.
2.4. Encephalitozoon spp. in wild birds
Birds are often infected by microsporidian parasites in the genus Encephalitozoon (Hinney et al., 2016; Sak et al., 2010). Encephalitozoon spp. have been identified in a wide variety of avian hosts, including in the Orders Anseriformes, Apodiformes, Ciconiiformes, Columbiformes, Falconiformes, Gruiformes, Passeriformes, Podicipediformes, Struthioniformes, and Suliformes, and also in many countries (Hinney et al., 2016). Encephalitozoon intestinalis, which is the most prevalent Encephalitozoon species in humans and also infects various mammalian species (e.g. livestock, dogs, and NHPs), has been reported only sporadically from birds (Pirestani et al., 2013; Galvan-Diaz et al., 2014; Tavalla et al., 2018), whereas E. hellem, which is considered bird-specific, and, to a lesser extent, E. cuniculi, which is considered mammal-specific, have been reported frequently from birds (Hinney et al., 2016).
Most human infections are thought to result from faecal-oral transmission of spores. Encephalitozoon spores have been detected in various water sources (irrigation water, recreational water, drinking water, and wastewater) and food (Dowd et al., 1998; Fournier et al., 2000; Decraene et al., 2012; Kváč et al., 2016). In addition, spores can be aerosolized from disturbed excrement and could be inhaled by hosts as airborne particles (Graczyk et al., 2008). As Encephalitozoon spp. from birds have been shown to be capable of infecting people, both immunocompetent and immunocompromised (Didier and Weiss, 2011), there is a need for better understanding of the role of birds as a reservoir of human microsporidiosis (Table 4).
Table 4.
Zoonotic potential of Encephalitozoon intestinalis, E. cuniculi and E. hellem and their genotyping at various gene targets (Galván et al., 2013; Mathis et al., 1999; Xiao et al., 2001a, Xiao et al., 2001b).
| Species | Genotype |
Zoonotic potential | |||||
|---|---|---|---|---|---|---|---|
| ITSa | SSUb | PTPc | SWPd-1 | IGSe-TH | IGSe-HZ | ||
| E. intestinalis | −f | – | – | NAg | NA | NA | Yes |
| E. cuniculi | I | – | I | Ia, Ib | NA | NA | Yes |
| II | – | II | II | NA | NA | Yes | |
| III | – | III | IIIa, IIIb | NA | NA | Yes | |
| IV | – | NA | NA | NA | NA | Yes | |
| E. hellem | 1A | 1A | 1A | NA | 1 | 1 | Yes |
| 1A | 1A | 1B | NA | 2 | 1 | Yes | |
| 1A | 1C | 1C | NA | 2 | 2 | Yes | |
| 2 | NA | 2A | NA | NA | NA | No | |
| 2 | 2B | 2B | NA | NA | NA | Yes | |
| 3 | NA | 2C | NA | 3 | NA | No | |
| 3 | NA | 2D | NA | NA | NA | Yes | |
The internal transcribed spacer (ITS) of the rRNA gene.
The small subunit rRNA gene (SSU-rDNA).
The polar tube protein (PTP).
The spore wall protein 1 (SWP-1).
The intergenic spacers of the ribosomal genes.
Not differentiated into genotypes.
NA: sequence of the gene is not available.
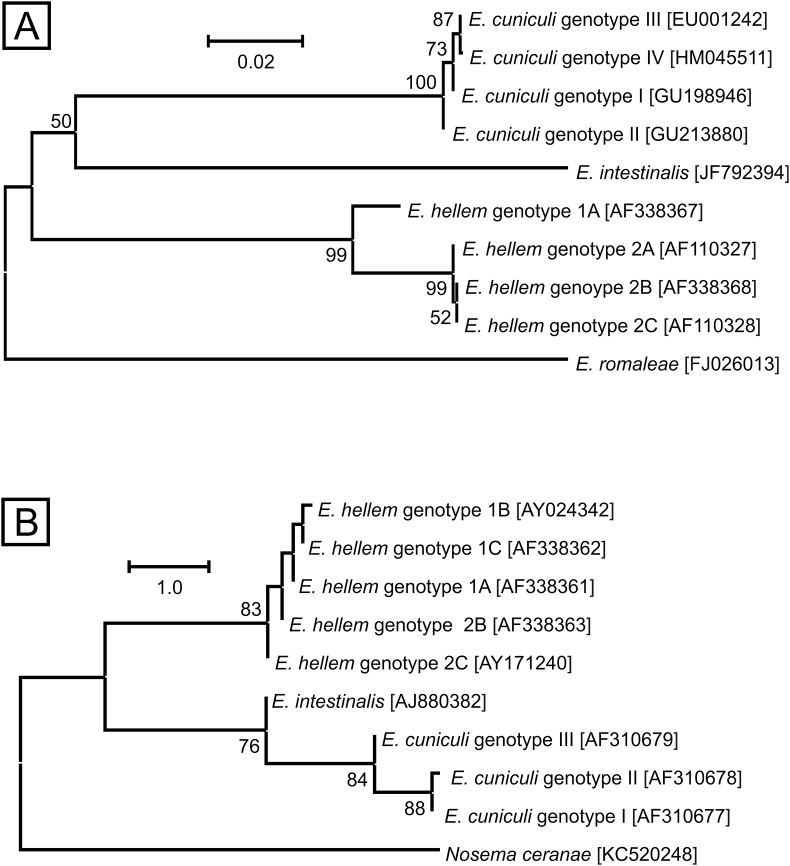
Genotyping has proven useful for high-throughput sample screening and, despite a limited number of molecular markers, has revealed some important details about heterogeneity among and within Encephalitozoon species. Most studies have targeted the ITS region of ribosomal RNA genes (ITS, Fig. 1A) (Hinney et al., 2016). Less common markers include the polar tube protein (PTP; Fig. 1B), SSU-rDNA, intergenic spacers of ribosomal genes, (IGS) IGS-TH and IGS-HZ, and the spore wall protein (SWP) gene (Mathis et al., 1999; Xiao et al., 2001a,b; Haro et al., 2003). Sequence analysis of these markers has distinguished four E. cuniculi genotypes (I, II, III, and IV) and seven E. hellem genotypes (1A, 1B, 1C, 2A, 2B, 2C, and 2D) (Mathis et al., 1999; Xiao et al., 2001a, Xiao et al., 2001b; Haro et al., 2003; Galván et al., 2013, Table 4). Encephalitozoon cuniculi genotypes I, II and III have also been differentiated by fragment size analysis of the Spo11 gene (Selman et al., 2013).
Fig. 1.
Phylogenetic relationships among genotypes of Encephalitozoon cuniculi, Encephalitozoon hellem and Encephalitozoon intestinalis based on: A) partial sequences of internal transcribed spacer gene (ITS), and B) partial sequences of polar tube protein gene (PTP). Numbers at the nodes represent the bootstrap values gaining more than 50% support based on 1000 replications. Phylogenetic trees were inferred by the Neighbour-Joining method with the A) Jukes-Cantor and B) Kimura 2-parameter models in MEGA6 software.
Although several studies have been conducted, genetic heterogeneity in the ITS and the PTP genes has not yet been observed in E. intestinalis (Didier et al., 1996; del Aguila et al., 1998; Rinder et al., 1999; Sobottka et al., 1999; Liguory et al., 2000; Xiao et al., 2001b). Genotypic variation of E. intestinalis, genotypes I and II, has been demonstrated by Galván et al. (2013) at the M2A, M3 M5, M7A, and M8 loci, but this approach has not been applied to broader surveys. As almost all Encephalitozoon genotypes can be distinguished by sequencing at the ITS and PTP loci, these are most commonly used as they enable comparison of results among studies, and these loci can be recommended for routine investigations.
A major constraint to investigations of whether birds are a relevant source of human Encephalitozoon infection is that spore shedding is generally intermittent and therefore difficult to detect; the limit of detection may also be reached at lower levels of shedding. Extensive sampling is therefore required to determine whether an animal is infected; for example, Sak et al. (2010) screened nine budgerigars (Melopsittacus undulatus) naturally infected with E. hellem genotypes 1A and 2C (previously known as genotype 3) and E. cuniculi genotype III for spore shedding. Although the cumulative prevalence of Encephalitozoon spp. was 100%, daily prevalence ranged from 0 to 67%, with a mean of 27%. Such intermittent shedding of spores has also been reported for infections in humans, rodents, and horses (Sak et al., 2011a, 2017; Wagnerová et al., 2013), suggesting that, without repeated sampling, the prevalence of Encephalitozoon spp. in host populations could be underestimated.
A second major limitation to investigations of whether birds are a relevant source of human Encephalitozoon infection is due to the broad host range, which may limit the value of genotyping for identifying sources of human infection and means that molecular results must be supported by traditional epidemiological investigations. For example, E. intestinalis and all E. cuniculi genotypes infect several mammals and birds (Hinney et al., 2016). In contrast, E. hellem almost exclusively infects birds, and genotypes show no differences in host specificity. Nevertheless, genotyping should be an integral component of epidemiological surveillance and genetic data should be interpreted together with the case report and not separately. As an example, Haro et al. (2003, 2005) demonstrated that E. hellem genotype 1A from human immunodeficiency virus-positive patients was apparently the identical genotype to that of E. hellem identified in urban pigeons in a park (using a PCR amplifying a gene fragment of 208 bp, including the ITS region), and, based on this, suggested that these birds could be considered a potential source of human infection. However, direct evidence of human infection through contact with pigeons was lacking. Kašičková et al. (2009) and Sak et al. (2011b) later demonstrated that this genotype infected other avian and mammalian hosts, and therefore, in the absence of traditional epidemiological associations, pigeons were not necessarily the source of the infection. Use of further genetic markers may strengthen or weaken an association, but traditional epidemiological investigations cannot usually be replaced by molecular techniques at this time for confirming zoonotic transmission routes.
2.5. Pathogenic Entamoeba spp. in non-human primates (NHPs)
In the early 1900s, many species of Entamoeba were described in NHPs. By light microscopy, these were, for the most part, indistinguishable from the species found in humans. In his great work of 1919, “The Amoebae Living in Man”, Dobell concluded on p. 133 that “it is by no means impossible that the amoebae are really identical” to E. histolytica and E. coli (the only Entamoeba species he recognized as colonising the human gut), and that “there is as yet no proof that monkeys harbour Entamoebae in any way different from those of man”. Subsequently, based on his own life-cycle studies and experimental infection results, he concluded that the species were indeed the same.
This understanding of Entamoeba species in NHPs persisted unchallenged for many decades. Implicit in this view is that NHPs are a potential reservoir for E. histolytica and therefore for an important disease of humans. Supporting his conclusion were reports of dysentery (e.g., Eichhorn and Gallagher, 1916) and liver abscesses (e.g., Castellani, 1908) in NHPs that, based on both morphology and histology, were apparently caused by E. histolytica.
Molecular tools arrived with the use of antibodies and isoenzyme analyses in the 1970s. Almost immediately both approaches identified that there were two distinct groups within E. histolytica, one linked to cases of disease (named pathogenic zymodemes) and one linked to asymptomatic infections (non-pathogenic zymodemes). Application of isoenzyme analyses to isolates from captive (e.g., Smith and Meerovitch, 1985) and wild (Jackson et al., 1990) NHPs initially indicated that all their E. histolytica belonged to non-pathogenic zymodemes. The accumulation of DNA data – restriction fragment length polymorphisms, Southern blots, gene sequences - and other information, eventually led to non-pathogenic zymodemes being reclassified as a distinct species of Entamoeba, E. dispar (Diamond and Clark, 1993); it was concluded that NHPs mostly harboured E. dispar.
Thus, use of molecular tools initially led to a switch from viewing NHPs as a potential reservoir of E. histolytica to viewing them as carrying primarily non-pathogenic Entamoeba species. The use of DNA-based tools for detection and differentiation of Entamoeba species spread rapidly, initially with the use of PCR alone and subsequently with PCR combined with DNA amplicon sequencing. The PCR target was primarily SSU-rDNA, but the divergence between E. histolytica and E. dispar is such that many genes are suitable targets for species-specific PCR tests, if desired.
One of the applications of these tools was screening of primates imported for medical research in order to identify those carrying pathogens, including E. histolytica. This led to the discovery that E. histolytica was not the (only) pathogenic Entamoeba in NHPs (Suzuki et al., 2007; Tachibana et al., 2007; Takano et al., 2007). Initially described as a variant of E. histolytica, this Entamoeba species is now generally referred to as E. nuttalli – the name originally given to the species responsible for liver abscesses in macaques by Castellani in 1908. Pathogenic in both primates and rodent models, E. nuttalli is closely related to, but definitely distinct from, E. histolytica. However, most tools that had been developed at that time to differentiate E. histolytica from E. dispar did not differentiate E. histolytica from E. nuttalli. The SSU-rDNA sequences of E. histolytica and E. nuttalli differ by less than 1%, compared with over 2% divergence between E. histolytica and E. dispar, and, as a result, many previously designed PCR primers annealed to sequences that were identical in E. histolytica and E. nuttalli. Species-specific primers for E. nuttalli SSU-rDNA were developed quickly (Tachibana et al., 2007). Gene sequences, such as those encoding chitinase and the serine-rich protein (Takano et al., 2007; Tachibana et al., 2007), and isoenzymes (Suzuki et al., 2007; Tachibana et al., 2007) also differentiate between E. histolytica and E. nuttalli, as do the tRNA-linked non-coding short-tandem-repeats that can be used for genotyping of all three species (e.g., Guan et al., 2016; Feng et al., 2018). In no sequence datasets do the two species overlap.
It seemed likely, therefore, that reports of E. histolytica carriage and disease in NHPs attributed to E. histolytica were actually due to E. nuttalli. In addition, because isoenzyme analyses can differentiate between E. histolytica and E. nuttalli, the absence of the E. nuttalli ‘variant’ pattern among the thousands of human samples that were analysed in the 1980s (Sargeaunt, 1987) suggested that humans are not hosts for E. nuttalli. Thus, a host specificity seemed clear; E. histolytica was infective to humans and E. nuttalli infected NHPs – neither species was zoonotic.
This simple view did not last long; some true E. histolytica infections were reported in NHPs (Verweij et al., 2003; Rivera et al., 2010) and then infection of a zookeeper with E. nuttalli was reported, indicating that humans could be susceptible to colonisation if exposed (Levecke et al., 2015). No cases of invasive disease in humans attributed to E. nuttalli have been reported to date. Most recently, using high throughput sequencing, populations of humans and wild gorillas living in a nature reserve in Cameroon were both found to be carrying E. histolytica and E. nuttalli and E. dispar (Vlčková et al., 2018). The patterns of transmission in this location remain unclear, but this finding does demonstrate unequivocally that humans and great apes can be colonised by both E. histolytica and E. nuttalli.
Over time, our perception of the potential for NHPs to be reservoirs for E. histolytica has shifted several times, from microscopy indicating that NHPs were reservoirs of E. histolytica, to isoenzymes indicating that they were not, to NHPs carrying a pathogen - but not E. histolytica - to the current view, which is, we suspect, “it depends”. Where contact exists between NHPs and humans, the potential for E. histolytica to be transmitted between the two host groups is real. However, the circumstances in Cameroon are not likely to be common and even if, technically, NHPs can be a reservoir for human infection with E. histolytica, it seems unlikely that they are a major source of the parasites responsible for invasive amoebiasis in humans. Molecular tools may assist in clarifying the species of Entamoeba present in NHPs, but as yet they cannot fully clarify the public health threat that they pose.
2.6. Enterocytozoon bieneusi in wild cervids
Among the 1300–1500 formally described species (within 187 genera) of microsporidia (Wang et al., 2018), 14 species infect humans, and, of these, Enterocytozoon bieneusi is the most common and reported to be responsible for more than 90% of human cases of microsporidiosis (Matos et al., 2012).
Although light microscopy of stained clinical smears is commonly used for diagnosis of microsporidia infections in humans and animals (Zhao et al., 2017), the small spore size and lack of definitive staining characteristics mean that detection of E. bieneusi by this technique is difficult (Li et al., 2016). As genotype identification cannot be assessed by microscopy, many of the investigations of E. bieneusi infections in cervids (and other hosts) use molecular tools. Distinguishing between different genotypes of E. bieneusi to date has usually been based on analysis of the ITS region, amplified by nested PCR. The occurrence of both single-nucleotide polymorphisms (SNPs) and short polymorphic insertions or deletions in these sequences is usually used to identify E. bieneusi ITS genotypes (Buckholt et al., 2002), and based on nucleotide divergence within the ITS gene fragment, over 300 E. bieneusi genotypes have been defined in humans and animals (Wang et al., 2018).
Two pairs of primers (EBITS3 and EBITS4, and EBITS1 and EBITS2.4) have mostly been used to amplify a 390-base pair (bp) fragment of the rDNA (containing 76 bp of the 3 ′-end of SSU-rDNA, 243 bp of the ITS, and 71 bp of 5′-region of the large subunit (LSU)-rDNA) for studies on E. bieneusi in cervids (Buckholt et al., 2002). However, another study used the alternative primers (MSP-1 and MSP-2B, and MSP-3 and MSP-4B) to amplify a 535 bp sequence, which also includes the 243 bp ITS gene sequence (Zhang et al., 2018).
Based on ITS sequence identification, an average prevalence of E. bieneusi in cervids of 18.4% (415/2251) has been reported from the combined results of 15 studies, the majority of which have been conducted in China and have included at least 8 different cervid species (Table 5). Among the studies conducted, genotype BEB6 predominates, having been identified in 40.3% (170/422) of the known genotypes and in 10 of the studies (Table 5).
Table 5.
The presence and distributions of different genotypes of Enterocytozoon bieneusi in cervids.
| Species | Collection sites | % prevalence (positive/total) | ITS genotype (no.) | Potentially zoonotic genotypes | Reference |
|---|---|---|---|---|---|
| Sika deer (Cervus nippon)b | China: Heilongjiang and Jilin | 33 (28/86) | BEB6 (20), HLJD-I (1), HLJD-II (1), HLJD-III (1), HLJD-IV (1), HLJD-V (4) | HLJD-II, HLJD-III | Zhao et al. (2014) |
| Red deer (Cervus elaphus)c | China: Heilongjiang | 20 (1/5) | HLJD-V (1) | – | Zhao et al. (2014) |
| Pere David's deer (Elaphurus davidianus)d | China: Henan | 34 (16/47) | Type IV (4), EbpC (4), EbpA (4), BEB6 (2), COS-I (1), COS-II (1) | type IV, EbpC, EbpA | Zhang et al. (2015) |
| Wild reindeers (Rangifer tarandus)d | China: Northeast forest region of Great Hinggan Mountains | 17 (21/125) | Peru6 (6), CHN-RD1 (12), CHN-RD2 (1), CHN-RD3 (1), CHN-RD4 (1) | Peru6, CHN-RD1, CHN-RD2, CHN-RD3, CHN-RD4 | Liu et al. (2015) |
| Sika deer (Cervus nippon)b | China: Jilin | 7 (23/326) | J (11), BEB6 (4), EbpC (1), KIN-1 (1), CHN-DC1 (1), JLD-1 (2), JLD-2 (2), JLD-3 (1) | CHN-DC-1, KIN-1, EbpC, JLD-2, JLD-3 | Zhang et al. (2016) |
| Hog deer (Axis porcinus)c | China: Sichuan | 75 (3/4) | BEB6 (2), CHS9 (1) | – | Li et al. (2016) |
| Red deer (Cervus elaphus)c | China: Sichuan | 25 (1/4) | BEB6 (1) | – | Li et al. (2016) |
| Sika deer (Cervus nippon)c | China: Sichuan | 50 (2/4) | BEB6 (1), SC03 (1) | SC03 | Li et al. (2016) |
| Red deer (Cervus elaphus)d | China: Heilongjiang and Jilin | 8 (8/104) | BEB6 (7), HLJD-VI (1) | HLJD-VI | Zhao et al. (2017) |
| Siberian roe deer (Capreolus pygargus)d | China: Heilongjiang and Jilin | 11 (2/18) | BEB6 (2) | – | Zhao et al. (2017) |
| Sika deer (Cervus nippon)b | China: Henan and Jilin | 36 (215/599) | BEB6 (129), HLJD-I (18), EbpC (3), HLJD-IV (2), COS-I (1), EbpA (1), D (1), JLD-I (7), JLD-II (5), HND-I (4), JLD-III (2), HND-II (1), JLD-IV (3), JLD-V (2), JLD-VI (5), HND-III (1), JLD-VII (1), JLD-VIII (16), JLD-IX (1), JLD-X (1), HND-IV (1), JLD-XI (2), JLD-XII (1), JLD-XIV (7) | D, HND-I, EbpC, HND-II, JLD-I, JLD-II, JLD-III, JLD-IV, JLD-V, JLD-VI, EbpA | Huang et al. (2017) |
| Red deer (Cervus elaphus)b | China: Henan and Jilin | 38 (6/16) | BEB6 (2), JLD-IV (3), JLD-XIII (1) | JLD-IV | Huang et al. (2017) |
| Musk deer (Moschus berezovskii)e | China: Sichuan | 17 (38/223) | SC03 (38) | SC03 | Song et al. (2018) |
| White-tailed deer (Odocoileus virginianus)f | USA: Maryland | 33 (26/80) | WL4 (11), I (7), J (1), LW1 (1), DeerEb1-DeerEb13 (one each) | – | Santín and Fayer, 2015 |
| Wild deerf | Australia: Melbourne | 4 (25/610)a | D (3), J (1), Type IV (1), MWC_d1 (19), MWC_d2 (1) | D, Type IV, MWC_d1, MWC_d2 | Zhang et al. (2018) |
| Total | China, USA, Australia | 18% (415/2251) | Potentially zoonotic genotypes are in bold font: BEB6 (170), SC03 (39), MWC_d1 (19), HLJD-I (19), JLD-VIII (16), J (13), CHN-RD1 (12), WL4 (11), EbpC (8), I (7), JLD-XIV (7), JLD-I (7), JLD-IV (6), Peru6 (6), JLD-II (5), JLD-VI (5), HLJD-V (5), Type IV (5), EbpA (5), D (4), HND-I (4), HLJD-IV (3), JLD-1 (2), JLD-2 (2), JLD-III (2), JLD-XI (2), JLD-V (2), COS-I (2), COS-II (1), KIN-1 (1), HLJD-II (1), HLJD-III (1), HLJD-VI (1), CHN-DC1 (1), CHN-RD2 (1), CHN-RD3 (1), CHN-RD4 (1), JLD-3 (1), JLD-VII (1), JLD-IX (1), JLD-X (1), JLD-XII (1), JLD-XIII (1), CHS9 (1), HND-II (1), HND-III (1), HND-IV (1), LW1 (1), MWC_d2 (1), DeerEb1-DeerEb13 (one each) |
||
Mixed infections.
Farmed deer.
Zoo deer.
Forest farmed.
Deer breeding center.
Wild deer.
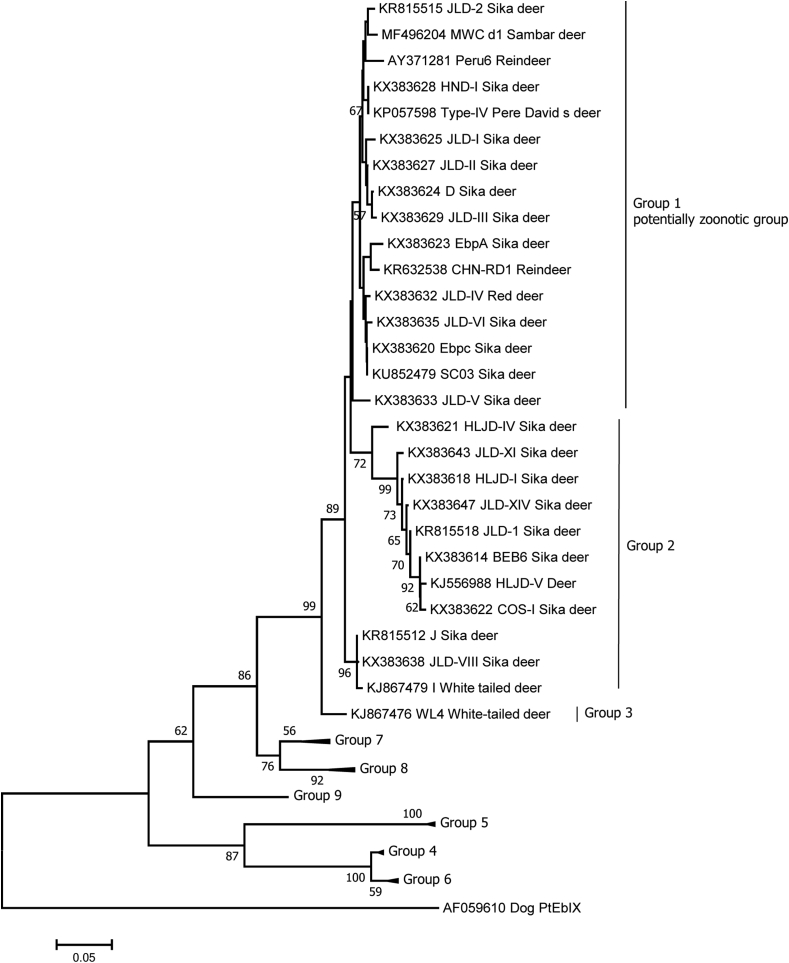
In such studies, zoonotic potential is assessed by phylogenetic analysis of the ITS sequences, with those isolates of E. bieneusi that cluster with known zoonotic isolates being considered to be potentially zoonotic (Fig. 2). Overall, 62 different E. bieneusi genotypes have been identified in cervids using ITS sequence-polymorphism analysis (Table 5); of these, around 44% (27/62) clustered into the group with zoonotic potential, thereby raising public health concerns (Santín and Fayer, 2009). Although a genotype (BEB6) that is not in the zoonotic potential group appears to predominate in cervids, the second most prevalent genotype SC03 (9.2%; 39/422), reported from two studies, clusters in the zoonotic potential group. However, most of the genotypes (54.8%; 34/62) were only identified in a single specimen.
Fig. 2.
Phylogenetic relationships of the Enterocytozoon bieneusi genotypes identified in cervids. The phylogenetic tree was inferred with a neighbour-joining analysis of the E. bieneusi ITS sequences, based on distances calculated with the Kimura two-parameter model. Bootstrap values > 50% from 1,000 replicates are shown on the nodes. The E. bieneusi-ITS genotypes detected in cervids (more than one isolate) are shown, and those isolates that clustered into the same clade as those considered to be zoonotic are considered to be in Group 1, the “potentially zoonotic group”.
Multilocus sequence typing (MLST) analysis, with high-resolution and targeting three microsatellites (MS1, MS3 and MS7) and one minisatellite (MS4), has been widely used for investigating multilocus genotypes (MLG) of E. bieneusi in humans and animals (Feng et al., 2011; Wang et al., 2018). For cervids, this MLST approach has been used in studies including E. bieneusi DNA isolated from samples from red deer, hog deer, sika deer, and musk deer (Li et al., 2016; Song et al., 2018). In one study, strains of the same ITS genotype (BEB6) were identified as including two different MLGs (Li et al., 2016), and in another study DNA of the potentially zoonotic ITS genotype, SC03, was reported to include two different microsatellite MS3 types (Song et al., 2018). The significance of the higher resolution of the MLST tool regarding the zoonotic potential of E. bieneusi in cervids is yet to be clarified. However, these data suggest that use of a single genetic locus to determine whether two isolates are similar enough to be considered identical may be insufficient, and may result in strains being incorrectly classified as indicating a particular transmission source, whereas additional genetic information may provide a more nuanced and accurate picture. Thus, although molecular tools have indicated the potential for Enterocytozoon spp. from cervids to pose a threat to public health, as tools become more discriminatory, our current understanding may require revision.
2.7. Giardia duodenalis in red foxes
Giardia duodenalis, often described as being a ubiquitous protozoan of major global public health significance, is commonly found in a range of host species, including humans, domestic animals, and wildlife (Feng and Xiao, 2011). However, although considerable molecular data on G. duodenalis isolated from samples from various host species have been accumulated, interpreting these data, regarding whether they represent zoonotic potential, is not entirely straightforward; even nomenclature remains controversial, with some authors referring to genetic clusters as Assemblages A to H (with some sub-assemblages within these larger groups), whereas others report the assemblages as representing distinct species (Feng and Xiao, 2011; Thompson and Ash, 2016). Indeed, the lack of consistent systems for characterising and naming strains from different host species has led to some authors claiming “new” sub-assemblage groups, even when this is based on only a few minor SNPs compared with a previously reported genotype, and without any other defining epidemiological traits (Ye et al., 2012).
Nevertheless, it is clear that different assemblages exhibit different patterns of infection; both Assemblages A and B have wide host ranges, infecting humans and a variety of animal species, whereas Assemblages C to H are considered to be more host specific, infecting predominantly canids (C and D), bovids and suids (E), felids (F), rodents (G), and pinnipeds (H) (Feng and Xiao, 2011). Based on this nomenclature and division, it is common to refer to Giardia cysts belonging to Assemblages A or B as having zoonotic potential. However, this is not clear-cut, as some sub-Assemblage groups apparently are not zoonotic (e.g., AIII; Sprong et al., 2009), and there are an increasing number of reports of assemblages other than A and B found in humans (Cacciò et al., 2018).
Various characteristics of the red fox (see Table 2), mean that this species is of particular interest as a reservoir of infections of importance to public health. Nevertheless, although red foxes have been commonly reported to excrete Giardia cysts, the role that they play, if any, in the zoonotic transmission of G. duodenalis is unclear (Onac et al., 2015; Debenham et al., 2017; Mateo et al., 2017). Dogs are in the same family (Canidae) as foxes but have a much closer relationship and contact with humans, and thus a greater potential to share pathogens. However, dogs tend not to be considered an important source of zoonotic transmission, being largely infected with canid-specific Assemblages C and D (Ballweber et al., 2010).
Molecular studies seeking to investigate the potential role of red foxes as a reservoir of G. duodenalis of public health importance, have primarily focused on using conventional PCR to amplify sequences within various genetic loci (including SSU-rDNA and ITS1 and ITS2, and targets in glutamate dehydrogenase (gdh), triosephosphate isomerase (tpi), and β-giardin (bg) genes) to determine the assemblages most commonly occurring (Hamnes et al., 2007; McCarthy et al., 2007; Beck et al., 2011; Onac et al., 2015; Debenham et al., 2017; Mateo et al., 2017). For sub-assemblage genotyping, SSU-rDNA has insufficient genetic variation, thus tpi, bg, and gdh, and, to a lesser extent, ITS1 and ITS2 genetic targets are used (Stojecki et al., 2015; Debenham et al., 2017; Mateo et al., 2017).
In general, however, amplification of these sequences of G. duodenalis from red fox samples has a poor success rate, compared with G. duodenalis from humans and other animals, such as ruminants. Among those studies in which PCR was performed on samples already confirmed to contain Giardia cysts by immunofluorescent antibody microscopy (IFA), poor amplification success was observed across all five of the common gene loci (Table 6). In G. duodenalis isolated from samples from other host species, SSU-rDNA appears to provides the greatest amplification success, probably at least in part due to it being found in multiple gene copies, and is therefore often used for detection (Thompson and Ash, 2016). However, in these studies on Giardia from fox samples, even at this locus, good amplification was usually not observed. Similar limitations of PCR have been reported by Onac et al. (2015), who obtained positive amplification in 10 out of 217 fox samples at the bg gene, but only three of these gave positive results for G. duodenalis when sequenced, and by Mateo et al. (2017) who obtained DNA amplification in seven out of 87 fox samples at the SSU-rDNA, but none of these resulted in amplification by qPCR at the gdh gene.
Table 6.
Amplification of Giardia duodenalis DNA by PCR at different gene loci for isolates from red fox samples already confirmed to contain Giardia cysts by immunofluorescent antibody microscopy.
| Country | % prevalence by IFAa (number positive/samples examined) | Amplification at genes targeted for PCR (successful amplification/isolates tested) |
Assemblages reported | Reference | ||||
|---|---|---|---|---|---|---|---|---|
| SSU-rDNAb | BGc | TPId | GDHe | ITS1 & ITS2f | ||||
| Croatia | 5 (3/66) | 1/3 | NAg | 0/3 | NAg | 0/3 | A | Beck et al. (2011) |
| Norway | 5 (13/269) | NAg | 1/3 | NAg | 7/12 | NAg | A and B | Hamnes et al. (2007) |
| Poland | 19 (4/21) | NAg | 0/21h | NAg | NAg | NAg | – | Stojecki et al. (2015) |
| Sweden | 45 (46/104) | 0/14 | 0/14 | 4/14 | 0/14 | NAg | B | Debenham et al. (2017) |
Immunofluorescent antibody microscopy.
Small subunit rRNA gene.
β-giardin.
Triosephosphate isomerase.
Glutamate dehydrogenase.
Internal transcribed spacer loci 1 and 2.
Not attempted.
PCR was performed on all samples, not only those positive by IFA.
Due to their close relationship with domestic dogs, it may be expected that red foxes would be primarily infected with G. duodenalis Assemblages C or D (reported to be specific to canid hosts; Thompson and Ash, 2016), and the most frequently detected assemblages in dogs (Ballweber et al., 2010). However, this does not appear to be the case, with the majority of Giardia DNA isolated from red fox samples, and for which characterisation has been successful, being found to be Assemblage A or B (Hamnes et al., 2007; McCarthy et al., 2007; Beck et al., 2011; Onac et al., 2015). Additionally, Ng et al. (2011) amplified Giardia SSU-rDNA in 32% (6/19) of fox samples, and sequence results revealed the presence of Assemblage D in only two samples, but A and/or E in four samples. Given that foxes coexist with domestic dogs infected with Assemblages C and D, and are known to eat rodents, as well as both wild and domestic ungulates, these findings are both unexpected and interesting and may suggest that foxes play a greater role in the zoonotic transmission of this parasite than dogs. However, most of these results are based on sequences obtained from amplification of a single gene locus, and thus should be interpreted with caution.
Despite some results having been obtained, the generally poor success of these molecular tools in investigating Giardia isolated from the red fox samples (and, to some extent, from other canids also) is interesting when compared with the much better results obtained using the same tools on Giardia collected from human or domestic livestock samples. One reason for this poor amplification success could be due to collection variables; samples collected from wildlife often require extended storage and this could impact cyst or DNA integrity. In addition, the sample populations are usually non-selected apparently healthy foxes, rather than symptomatic individuals suffering from giardiasis, which is the case in many human studies and sometimes for studies in domestic livestock. However, given similar limitations of PCR are seen in other canids then a more host-specific explanation should be considered; for example, the faecal matrix of canids may contain inhibitors that are not accounted for using traditional DNA extraction or PCR amplification techniques (Stojecki et al., 2015; Sommer et al., 2015).
Overall, although molecular tools have suggested that red foxes should not be dismissed as potential reservoirs of Giardia of public health relevance, the apparently poor sensitivity of conventional PCR tools for amplifying gene sequences from Giardia DNA obtained from red foxes has greatly limited our ability to reach a meaningful conclusion. The most widely used molecular tools for Giardia investigations remain of limited value for both detection and characterisation of Giardia from red foxes. Until these issues are addressed and resolved, our understanding of the role of this widespread predator as a reservoir of zoonotic G. duodenalis will remain limited.
2.8. Sarcocystis nesbitti in snakes
Between the years 1993 and 2014, several outbreaks of extra-intestinal or invasive muscle sarcocystosis occurred among travellers returning from central Malaysia and Malaysian islands. Until then, reports on muscle sarcocystosis in humans had been relatively rare, although an unexplained accumulation of reports from South-East Asia, especially from Malaysia, had been noticed (Beaver et al., 1979; Kan and Pathmanathan, 1991; Wong and Pathmanathan, 1992; Fayer et al., 2015). Differences in tissue cyst morphology between cases had indicated that various Sarcocystis species were involved and probably humans were aberrant, dead-end hosts (Beaver et al., 1979; Fayer et al., 2015). Most of the case descriptions from Malaysia were incidental biopsy findings, with the exception of an outbreak affecting seven members of a 15-man U.S. military team, for which a positive muscle biopsy was also reported in one of the cases (Arness et al., 1999).
The most recent outbreaks were large, involving more than 100 persons, and initiated a more through diagnostic investigation and follow-up of patients (Von Sonnenburg et al., 2012; Abubakar et al., 2013; Esposito et al., 2014; Italiano et al., 2014; Tappe et al., 2014). One of the characteristics observed in affected humans was a biphasic course of the disease starting about two weeks after return from Malaysia, often with fever, frequently myalgia, fatigue, and headache, and, less often, arthralgia. After a period of remission, a second phase started around six weeks after return, this time myalgia was the dominating symptom, followed by fever and fatigue and less often arthralgia and headache (Esposito et al., 2014).
Biopsies were taken from the patients involved in the more recent outbreaks on the Malaysian islands of Tioman and Pankor, but sarcocysts were identified by histology in only a few patients (Abubakar et al., 2013; Esposito et al., 2014; Italiano et al., 2014). However, using PCR primers targeting part of the SSU-rDNA locus and originally developed to amplify Sarcocystis DNA from ruminants, amplicons were obtained. Sequencing revealed them to be identical to an SSU-rDNA sequence obtained from sarcocysts from a crab-eating macaque (Macaca fascicularis) in China that had been suspected to be infected with Sarcocystis nesbitti (Yang et al., 2005; Tian et al., 2012). Sarcocystis nesbitti had first been described from a rhesus macaque (Macaca mulatta) from Northern India (Mandour, 1969). As material from this initial description was not available for morphological and molecular confirmation that Mandour (1969) and Yang et al. (2005) were actually describing the same species, the Sarcocystis found in these infected patients were referred to as S. nesbitti-like by others (Dubey, 2015).
Phylogenetic analyses (Tian et al., 2012; Abubakar et al., 2013) of sequences from S. nesbitti-like parasites from M. fascicularis grouped this species close to Sarcocystis singaporensis (Jäkel et al., 2001) and Sarcocystis atheridis (Šlapeta et al., 2003), both of which infect snakes. Based on this and further phylogenetic analyses, it appeared very likely that sporocysts derived from the faeces of infected snakes may have been the source of the Malaysian outbreaks of extra-intestinal sarcocystosis (Lau et al., 2013, 2014), and that people may have become infected by oral uptake of these sporocysts in, for example, contaminated water or on fresh produce consumed raw (Lau et al., 2014; Fayer et al., 2015). Other reptiles, such as monitors, have also been discussed as potential definitive hosts and the source of the human infections (Tappe et al., 2013). Sarcocystis nesbitti-like sequences were also identified among DNA isolated from faecal samples from wild reticulated python (Braghammerus reticulatus) and monocled cobra (Naja kaouthia) captured in Malaysia (Lau et al., 2013) and in sediment samples from tank and river water collected at Tioman Island, Malaysia (Shahari et al., 2016). The suggested life cycle at that time included monkeys (Macaca mulatta, Macaca fascicularis, Cercocebus atys, and Papio papio) as natural intermediate hosts with snakes (cobra and python) as definitive hosts (Lau et al., 2013, 2014). Humans were regarded as aberrant, dead-end intermediate hosts (Dubey, 2015); their susceptibility may result from the close phylogenetic relationship with NHPs, the natural intermediate hosts.
Although molecular tools assisted diagnosis of this sarcocystosis outbreak, their diagnostic sensitivity seems to be low. The first signs of invasive sarcocystosis in people were generally non-specific (fever, headache, myalgia) and similar to those seen during other infectious diseases, including parasitoses such as toxoplasmosis and trichinellosis. Whether circulating parasites or parasite DNA could be detected in blood of early-stage patients is unknown, and more sensitive diagnostic methods are necessary. During the second phase of invasive sarcocystosis, patients developed myositis but obtaining positive biopsies nevertheless remains challenging. Sarcocysts were only observed in 6/14 patients in the Malaysian outbreak cohort, despite intensive searches including examination of more than 60 sections from a single muscle biopsy (Esposito et al., 2014). It has been suggested that the chance of a positive finding may increase should biopsy material be collected from sites where magnetic resonance imaging suggests myositis (Italiano et al., 2014) or sites where muscles are swollen, painful, or show signs of inflammation (Fayer et al., 2015).
Despite molecular tools having been key in clarification of the outbreaks of sarcocystosis in Malaysia, the full identity and life cycle of S. nesbitti is still a matter of discussion. A recent publication reported a finding of S. nesbitti-like SSU-rDNA in an Australian scrub python (Simalia amethistina, sampled at the Cape York Peninsula, Queensland); as NHPs, which were regarded as the natural intermediate hosts of S. nesbitti are not found in Australia, such a finding is unexpected (Wassermann et al., 2017). Australian scrub pythons prey preferentially on birds and mammals, including rodents and wallabies, and therefore it was hypothesized that S. nesbitti might actually have a snake-rodent life-cycle, with primates and humans as aberrant or – due to low intermediate host-specificity – alternative hosts (Wassermann et al., 2017). Thus, it is possible that the true natural intermediate hosts of the S. nesbitti-like parasites that caused the outbreaks in Malaysia may not yet have been identified.
Another complication is that the suitability of the SSU-rDNA locus to differentiate between Sarcocystis spp. is questionable (Gjerde, 2013; Poulsen and Stensvold, 2014; Dubey, 2015), and some Sarcocystis species that are phylogenetically closely related, are actually biologically distinct from each other (e.g., Sarcocystis neurona, Sarcocystis falcatula; Dubey, 2015). Thus, although the Sarcocystis species in NHPs, snakes, and humans have very similar SSU-rDNA sequences, it is possible that they belong to different species, with their own distinct life cycles and host ranges.
Based on research on Sarcocystis species in domestic and wild ruminants, the ITS1 locus and parts of the LSU-rDNA gene may also be valuable targets for further characterizations (Gjerde, 2016). Furthermore, sequencing the partial mitochondrial cytochrome c oxidase subunit I gene (cox1) may offer a useful possibility for distinguishing between even closely related Sarcocystis species (Gjerde, 2013, 2016). However, cox1-based tools may be of limited suitability for investigations of crude clinical or environmental samples in which parasite DNA is not enriched. Although the analytical sensitivity is not yet characterized, it is likely to be much lower than those targeting the SSU-rDNA locus. The ideal molecular targets for resolving the S. nesbitti question would be species-specific targets including highly repetitive gene elements, similar to those developed for Toxoplasma gondii (Burg et al., 1989; Homan et al., 2000). However, with the limited availability of reference material, the identification of such repetitive elements currently seems out of reach.
In conclusion, although SSU-rDNA sequencing was extremely helpful in identifying potential sources and routes of transmission, and obviously aided in the establishment of interventions to reduce the likelihood of further human infections, the heading in one of the early reviews is valid still “Unsolved mysteries: The source of infection …” (Tappe et al., 2013).
2.9. Toxoplasma gondii in bobcats
Wild felids are important in the sylvatic cycle of Toxoplasma gondii. In a study of T. gondii infections in feral domestic and wild felids in California, USA, T. gondii DNA was detected in 49 of 166 feral cats (Felis catus), 10 of 73 mountain lions (Puma concolor), and 11 of 27 bobcats (Lynx rufus) (VanWormer et al., 2014). The researchers concluded that wild cats that come in contact with feral and domestic cycles may introduce atypical genotypes to domestic cats and thereby facilitate the transmission of potentially more virulent genotypes to humans, domestic animals and wildlife (VanWormer et al., 2014). In that study, bobcats, with >40% seroprevalence, seemed to be more likely to be seropositive, and thus, presumably, more exposed to T. gondii infection than the other wild or feral cats investigated. This subject is of more than simply academic interest, because atypical genotypes circulating in wildlife have been found recently in humans in the USA (Pomares et al., 2018). In addition, atypical genotypes were identified in cases of fatal human toxoplasmosis in French Guiana (Carme et al., 2009).
In North America, the bobcat is a common wild felid and could play an important role in the transmission of T. gondii. There are millions of bobcats in the USA (Roberts and Crimmins, 2010) and their T. gondii seroprevalence tends to be high. It was shown previously that 71% (15/21) of bobcats from northern California (Riemann et al., 1975), 52% (30/58) from the US and Mexico (Kikuchi et al., 2004), 83% (109/131) from Pennsylvania (Mucker et al., 2006), and 100% (35/35) from Mississippi (Verma et al., 2017), were seropositive for T. gondii. These data are not dissimilar to those of domestic cats in the USA, ranging from 34 to 100% depending on cat type, age, and habitat (Dubey and Jones, 2008); felids existing entirely on a prey diet are more likely to be infected than domestic cats that are fed only canned or dried food. Given the high infection rates, bobcats are probably responsible for a very high (>65%) T. gondii infection rate in deer in USA. In 2004, unfrozen samples (blood, heart, tongue, and faeces) were collected from 35 free ranging wild bobcats from Mississippi, USA. Toxoplasma gondii antibodies were detected in serum by the modified agglutination test (1:≥200) in all 35 bobcats. Hearts from all bobcats were bioassayed in mice and viable T. gondii was isolated from 21; these strains were further propagated in cell culture. Additionally, DNA was extracted from digests of tongues and hearts of all 35 bobcats; T. gondii DNA was detected in tissues of all 35 bobcats. Genetic characterisation of DNA from cell culture-derived isolates was performed by multiplex PCR using 10 PCR-RFLP markers. Results showed that ToxoDB genotype #5 predominated (in 18 isolates) with a few other genotypes (#24 in two isolates, and #2 in one isolate). The genotype #5 (also known as clonal type 12) is a major genotype in wildlife in North America (Dubey et al., 2011; Khan et al., 2011). From the above 35 bobcats, PCR-DNA sequencing at two polymorphic marker loci, GRA6 and GRA7, detected mixed strains co-infecting the tissues of bobcats; most possessing Type II ToxoDB genotype #1 or #3 alleles at GRA7 versus Type X (type 12, ToxoDB genotype #5) alleles at GRA6 (Verma et al., 2017). These results suggest that individual bobcats have been exposed to more than one parasite strain during their life time. Together with high seroprevalence, the data suggest that these bobcats were exposed and contributed to a high level of contamination of their environment with T. gondii.
Genetic typing of T. gondii strains has evolved since the seminal study of Howe and Sibley (1995) who first used six multilocus PCR-RFLP markers to study genetic diversity. This method is simple, cost-effective, and, as no special equipment is needed, it is cheaper than MLST typing. Later, a multiplex multilocus PCR-RFLP method was developed using 10 markers (Su et al., 2010). This method improved resolution in distinguishing different T. gondii strains, and as it is a nested PCR, it is more sensitive. This effort generated an integrated database to reveal global T. gondii diversity (Su et al., 2012), distribution of genotypes world-wide (Shwab et al., 2014), and partition of genotypes into a spatial gradient and animal species in North America (Jiang et al., 2018). Surprisingly, the phylogenetic network generated from the PCR-RFLP data is similar to that obtained from whole genome sequence data (Lorenzi et al., 2016).
There is very little variation in SSU-rDNA of T. gondii, so it is not a useful marker for genotyping (strain identification). However, due to the high copy number of this gene, it is an excellent marker for identification of T. gondii infection. Currently 10 PCR-RFLP markers, including SAG1, SAG2, SAG3, BTUB, GRA6, c22-8, c29-2, L358, PK1, and Apico, are used for genotyping. Among these markers, c22-8, c29-2, and L358 are non-coding polymorphic DNA sequences, Apico is an intron sequence in the apicoplast, and the others are polymorphic genes.
Toxoplasma gondii genotype data from humans is very limited due to the limited access to human tissue for isolation and characterisation of the parasite. Indeed, >90% of existing genotype data on T. gondii is derived from animal sources. However, the accepted view is that humans can be infected by all sub-types. With regards to the interplay with bobcats, human infection may arise indirectly from bradyzoites in undercooked meat from domestic or game animals that have themselves been infected via oocysts originating from the faeces of bobcats, or directly from oocysts in contaminated water, soil, or fresh produce consumed raw. It should also be noted that bobcat meat is exported for human consumption, and may also be eaten by hunters.
The virulence of different T. gondii strains is also of relevance. Experimental studies have confirmed that some genotypes are lethal to mice, whereas others are non-lethal, and that the virulence phenotype is linked to polymorphic virulence genes ROP18 and ROP5 (Shwab et al., 2016). Although epidemiological data are clear that some T. gondii genotypes are more virulent to humans than others, we do not have sufficient information at present to know whether particular genotypes associated with particular wildlife species are more or less likely to be virulent in humans. This is clearly an area that could be usefully explored using the molecular tools available.
3. Conclusions
The number of wildlife species that may be hosts to enteric protozoan parasites of potential zoonotic importance is huge. However, regardless of whether a particular protozoan can infect both humans and a particular wildlife species, the public health importance is determined not only by that possibility, but also by the likelihood of transmission occurring, along with the severity of disease from any infections that may arise. Rather than consider each enteric protozoan of potential zoonotic importance and consider how molecular tools have contributed to our understanding of different wildlife species as reservoir hosts, we have decided to use selected parasite-host pairs to illustrate some of the progress and frustrations in this field.
Although these different example vignettes make it clear that there is no simple answer, it is also obvious that the use of molecular tools for different protozoa in different wildlife hosts has enabled improved understanding of the complexity of the situation. These examples also highlight the challenges of applying molecular tools to unravel this complexity and to gain a better understanding of the extent to which enteric protozoa in wildlife may present a threat to public health. For each of our selected parasite-wildlife host pairs, we summarize in Table 7 three of the major advances and challenges associated with the use of molecular tools to investigate zoonotic potential.
Table 7.
Overview of advances and challenges associated with the use of molecular tools for understanding the public health threat from enteric protozoan parasites in specific wildlife host species.
| Enteric protozoan parasite | Wildlife host | Use of molecular tools for understanding the public health threat from enteric protozoa in wildlife |
|
|---|---|---|---|
| Main advances made | Challenges and remaining hurdles | ||
| Balantioides coli (Balantidium coli) | Wild boar | 1. Resolution of the taxonomic position of the genus. | 1. Changes in taxonomical status over time means that the current genus name still not used widely. |
| 2. Evidence that there is only a single species. | 2. Published genetic data currently not extensive for comparative studies; currently only a couple of genetic markers have been used. | ||
| 3. Confirmation that the species is zoonotic. | 3. Within-cyst sequence polymorphisms may create confusion. | ||
| 4. The importance of wild boar (and other wildlife species) as a reservoir for human infection remains unresolved despite improved knowledge of zoonotic potential. | |||
| Blastocystis sp. | Rodents | 1. Greater understanding of the phylogeny of the genus. | 1. The interaction between different STs and the host microbiome is not yet understood. |
| 2. Improved understanding of subtypes (STs) and their host specificity. | 2. Phylogenetic studies sometimes inconsistent; GenBank sequences that are not Blastocystis. | ||
| 3. ST discrimination based on SSU-rDNA. | 3. The importance of rodents (and other wildlife species) as a reservoir for human infection remains unresolved despite knowledge of zoonotic potential for some STs. | ||
| Cryptosporidium spp. | Wild fish | 1. Greater understanding of the phylogeny of the genus. | 1. Differentiation between true infections of fish and carriage not always clear. |
| 2. Considerable data accrued on Cryptosporidium species (and some on subtypes) found in some fish species, although most studies on wild or aquarium fish. | 2. Many of the studies are ad hoc and preliminary, without epidemiology-based planning. | ||
| 3. Understanding that wild fish may harbour both zoonotic species and fish-specific species. | 3. The importance of wild fish (and other wildlife species) as a reservoir for human infection remains unresolved, despite knowledge of zoonotic potential for some Cryptosporidium species. | ||
| Encephalitozoon spp. | Wild birds | 1. Greater understanding of the heterogeneity within and between species. | 1. Spore shedding intermittent, and therefore extensive sampling required. |
| 2. Considerable data accrued on Encephalitozoon species and subtypes at different genes, enabling comparisons. | 2. Broad host range can limit the value of genotyping for determining zoonotic potential. | ||
| 3. Genetic loci recommended for routine investigations | 3. The importance of wild birds (and other wildlife species) as a reservoir for human infection remains unresolved, despite knowledge of zoonotic potential for some species and genotypes. | ||
| Pathogenic Entamoeba spp. | Non-human primates (NHPs) | 1. Understanding that different species of morphologically identical Entamoeba, with different pathogenicity, infect humans. | 1. Although SSU-rDNA sequence divergence between E. histolytica and E. dispar is over 2%, it is below 1% for E. histolytica and E. nuttalli, so primer design is crucial. |
| 2. Identification of Entamoeba nuttalli in NHPs. | 2. Predictive markers for invasiveness are yet to be identified. | ||
| 3. Realisation that, should suitable circumstances arise, humans and NHPs may be infected by the same Entamoeba species. | 3. The importance of NHPs as a reservoir for human infection remains unresolved, despite an improved understanding of zoonotic potential for some species. | ||
| Enterocytozoon bieneusi | Wild cervids | 1. Greater understanding of the heterogeneity within the E. bieneusi species. | 1. Identical subtypes at one gene may differ when multilocus genotyping is applied. |
| 2. Phylogenetic analysis can be used to determine potentially zoonotic isolates. | 2. The significance of results from higher resolution tools is yet to be understood, and more discriminatory tools may alter our understanding. | ||
| 3. Cervids may harbour zoonotic genotypes. | 3. The importance of cervids as a reservoir for human infection remains unresolved, despite an improved understanding of zoonotic potential for some isolates. | ||
| Giardia duodenalis | Red foxes | 1. Understanding that foxes may harbour zoonotic assemblages. | 1. Amplification of DNA from Giardia isolates in fox faeces often unsuccessful. |
| 2. Dogs and red foxes appear not to harbour the same assemblages. | 2. Low resolution from SSU-rDNA. | ||
| 3. Understanding that PCR may not provide most sensitive identification method for some isolates or isolates in some matrices. | 3. The importance of red foxes as a reservoir for human infection remains unresolved, despite an improved understanding of the zoonotic potential for some isolates. | ||
| Sarcocystis nesbitti | Snakes | 1. Phylogenetic analyses used to indicate potential definitive hosts. | 1. Lack of accessible material hinders development of appropriate tools, with high sensitivity and sufficient resolution. |
| 2. Potential lifecycles indicated by detection of particular DNA sequences. | 2. Natural intermediate hosts still not necessarily identified. | ||
| 3. Identification of potential transmission routes enabling planning of appropriate interventions. | 3. Although snakes may be a reservoir for human infection, their importance in transmission seems to be unclear; the severity of infection means that even infrequent infections or outbreaks may be considered of public health importance. | ||
| Toxoplasma gondii | Bobcat | 1. Greater understanding of the heterogeneity within the T. gondii species. | 1. Lack of accessible material from human infections means that our knowledge of the strains of greatest public health significance is limited. |
| 2. Several useful PCR-RFLP markers have been identified. | 2. Identification of markers for pathogenicity will assist in determining those strains of greatest virulence. | ||
| 3. Global database developed from PCR-RFLP markers supported by whole genome sequencing data. | 3. Although bobcats are a reservoir for human infection, their importance remains unresolved; information on the virulence of isolates associated with bobcats would be useful. | ||
Among the commonalities in the advances, are the increasing accumulation of data and sequences and an improved understanding of the phylogeny, interrelationships, and differences between species (although a related challenge for all is that some of the main sequence data resources are not curated, and contain errors). Among the commonalities of the challenges are that the DNA from protozoan transmission stages in faecal samples or environmental samples is not always easily accessible for amplification, and may be in small quantities or be of low quality. This encourages researchers to use multicopy genes, such as SSU-rDNA, as targets for amplification. However, as these are well conserved, they do not necessarily provide the necessary resolution to determine whether two very similar sequences are actually derived from the same genotype or even the same species. As molecular tools become more sophisticated, enabling easier and faster comparison of greater stretches of the genome, up to whole genome sequencing, we may find that different strains of similar parasites that we previously combined in the same sub-type due to being very similar or even identical at one genetic locus, are actually very different at other loci. The extent and meaning of these similarities and differences will require careful interpretation; even strains that appear different may still utilize the same spectrum of hosts.
In conclusion, molecular tools have provided us with more information about the possibility that several enteric protozoa from wildlife may infect humans, resulting in cases or outbreaks of diseases occurring that have not necessarily been included in important articles on this subject. The usefulness of these tools to indicate plausible transmission sources and routes for specific cases or outbreaks has been well proven. Nevertheless, this is not the end of the story, and molecular tools often only indicate the possibility that a particular protozoan parasite may be zoonotic, not that it is. Nor do these tools necessarily indicate the likelihood of transmission occurring or the impact, both of which are relevant when assessing a threat. Well-designed traditional epidemiological studies, experimental studies to confirm reservoir hosts, and identification of markers for virulence are all important approaches that should not be overlooked for ensuring that the results obtained using molecular tools are useful and of relevance when considering potential public health impacts.
Declarations of interest
None.
References
- Abubakar S., Teoh B.T., Sam S.S., Chang L.Y., Johari J., Hooi P.S., Lakhbeer-Singh H.K., Italiano C.M., Omar S.F., Wong K.T., Ramli N., Tan C.T. Outbreak of human infection with Sarcocystis nesbitti, Malaysia, 2012. Emerg. Infect. Dis. 2013;19:1989–1991. doi: 10.3201/eid1912.120530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamu H., Petros B., Zhang G., Kassa H., Amer S., Ye J., Feng Y., Xiao L. Distribution and clinical manifestations of Cryptosporidium species and subtypes in HIV/AIDS patients in Ethiopia. PLoS. Negl. Trop. Dis. 2014;8 doi: 10.1371/journal.pntd.0002831. e2831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexeieff A. Sur quelques particularités de structure de Balantioides (nom. nov.) coli (Malmsten) C.R. Séances Soc. Biol. Ses. Fil. 1931;107:210–211. [Google Scholar]
- Alfellani M.A., Taner-Mulla D., Jacob A.S., Imeede C.A., Yoshikawa H., Stensvold C.R., Clark C.G. Genetic diversity of Blastocystis in livestock and zoo animals. Protist. 2013;164:497–509. doi: 10.1016/j.protis.2013.05.003. [DOI] [PubMed] [Google Scholar]
- Alvarez-Pellitero P., Quiroga M.I., Sitjà-Bobadilla A., Redondo M.J., Palenzuela O., Padrós F., Vázquez S., Nieto J.M. Cryptosporidium scophthalmi n. sp. (Apicomplexa: Cryptosporidiidae) from cultured turbot Scophthalmus maximus. Light and electron microscope description and histopathological study. Dis. Aquat. Org. 2004;62:133–145. doi: 10.3354/dao062133. [DOI] [PubMed] [Google Scholar]
- Alvarez-Pellitero P., Sitjà-Bobadilla A. Cryptosporidium molnari n. sp. (Apicomplexa: Cryptosporidiidae) infecting two marine fish species, Sparus aurata L. and Dicentrarchus labrax L. Int. J. Parasitol. 2002;32:1007–1021. doi: 10.1016/s0020-7519(02)00058-9. [DOI] [PubMed] [Google Scholar]
- Appelbee A.J., Thompson R.C., Olson M.E. Giardia and Cryptosporidium in mammalian wildlife--current status and future needs. Trends Parasitol. 2005;21:370–376. doi: 10.1016/j.pt.2005.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arness M.K., Brown J.D., Dubey J.P., Neafie R.C., Granstrom D.E. An outbreak of acute eosinophilic myositis attributed to human Sarcocystis parasitism. Am. J. Trop. Med. Hyg. 1999;61:548–553. doi: 10.4269/ajtmh.1999.61.548. [DOI] [PubMed] [Google Scholar]
- Awakian A. Studies on the intestinal protozoa of rats. II. Rats as carriers of Balantidium. Trans. R. Soc. Trop. Med. Hyg. 1937;31:93–98. [Google Scholar]
- Ballweber L.R., Xiao L., Bowman D.D., Kahn G., Cama V.A. Giardiasis in dogs and cats: update on epidemiology and public health significance. Trends Parasitol. 2010;26:180–189. doi: 10.1016/j.pt.2010.02.005. [DOI] [PubMed] [Google Scholar]
- Barugahare R., Dennis M.M., Becker J.A., Šlapeta J. Detection of Cryptosporidium molnari oocysts from fish by fluorescent-antibody staining assays for Cryptosporidium spp. affecting humans. Appl. Environ. Microbiol. 2011;77:1878–1880. doi: 10.1128/AEM.02691-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaver P.C., Gadgil K., Morera P. Sarcocystis in man: a review and report of five cases. Am. J. Trop. Med. Hyg. 1979;28:819–844. [PubMed] [Google Scholar]
- Beck R., Sprong H., Lucinger S., Pozio E., Cacciò S.M. A large survey of Croatian wild mammals for Giardia duodenalis reveals a low prevalence and limited zoonotic potential. Vector Borne Zoonotic Dis. 2011;11:1049–1055. doi: 10.1089/vbz.2010.0113. [DOI] [PubMed] [Google Scholar]
- Betts E.L., Gentekaki E., Thomasz A., Breakell V., Carpenter A.I., Tsaousis A.D. Genetic diversity of Blastocystis in non-primate animals. Parasitology. 2018;145:1228–1234. doi: 10.1017/S0031182017002347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckholt M.A., Lee J.H., Tzipori S. Prevalence of Enterocytozoon bieneusi in swine: an 18-month survey at a slaughterhouse in Massachusetts. Appl. Environ. Microbiol. 2002;68:2595–2599. doi: 10.1128/AEM.68.5.2595-2599.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burg J.L., Grover C.M., Pouletty P., Boothroyd J.C. Direct and sensitive detection of a pathogenic protozoan, Toxoplasma gondii, by polymerase chain reaction. J. Clin. Microbiol. 1989;27:1787–1792. doi: 10.1128/jcm.27.8.1787-1792.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacciò S.M., Lalle M., Svärd S.G. Host specificity in the Giardia duodenalis species comlex. Infect. Genet. Evol. 2018;66:335–345. doi: 10.1016/j.meegid.2017.12.001. [DOI] [PubMed] [Google Scholar]
- Cahill S., Llimona F., Cabañeros L., Calomardo F. Characteristics of wild boar (Sus scrofa) habituation to urban areas in the Collserola Natural Park (Barcelona) and comparison with other locations. Anim. Biodivers. Conserv. 2012;35:221–233. [Google Scholar]
- Carme B., Demar M., Ajzenberg D., Dardé M.L. Severe acquired toxoplasmosis caused by wild cycle of Toxoplasma gondii, French Guiana. Emerg. Infect. Dis. 2009;15:656–658. doi: 10.3201/eid1504.081306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellani A. Note on a liver abscess of amoebic origin in a monkey. Parasitology. 1908;1:101–102. [Google Scholar]
- Certad G., Dupouy-Camet J., Gantois N., Hammouma-Ghelboun O., Pottier M., Guyot K., Benamrouz S., Osman M., Delaire B., Creusy C., Viscogliosi E., Dei-Cas E., Aliouat-Denis C.M., Follet J. Identification of Cryptosporidium species in fish from Lake Geneva (lac Leman) in France. PLoS One. 2015;10 doi: 10.1371/journal.pone.0133047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chistyakova L.V., Kostygov A.Y., Kornilova O.A., Yurchenko V. Reisolation and redescription of Balantidium duodenis, Stein, 1867 (Litostomatea, Trichostomatia) Parasitol. Res. 2014;113:4207–4215. doi: 10.1007/s00436-014-4096-1. [DOI] [PubMed] [Google Scholar]
- Cian A., El Safadi D., Osman M., Moriniere R., Gantois N., Benamrouz-Vanneste S., Delgado-Viscogliosi P., Guyot K., Li L.L., Monchy S., Noel C., Poirier P., Nourrisson C., Wawrzyniak I., Delbac F., Bosc S., Chabe M., Petit T., Certad G., Viscogliosi E. Molecular epidemiology of Blastocystis sp. in various animal groups from two French zoos and evaluation of potential zoonotic risk. PLoS One. 2017;12 doi: 10.1371/journal.pone.0169659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couso-Pérez S., Ares-Mazás E., Gómez-Couso H. Identification of a novel piscine Cryptosporidium genotype and Cryptosporidium parvum in cultured rainbow trout (Oncorhynchus mykiss) Parasitol. Res. 2018;117:2987–2996. doi: 10.1007/s00436-018-5995-3. [DOI] [PubMed] [Google Scholar]
- Da Silva Barbosa A., Ponce-Gordo F., Verdan Dib L., Antunes Uchôa C.M., Pereira Bastos O.M., Pissinatti A., Reis Amedoeira M.R. First molecular characterization of Balantioides coli (Malmsten, 1857) isolates maintained in vitro culture and from feces of captive animals, Rio de Janeiro, Brazil. Vet. Parasitol. Reg. Stud. Reports. 2017;10:102–113. doi: 10.1016/j.vprsr.2017.08.014. [DOI] [PubMed] [Google Scholar]
- Debenham J.J., Landuyt H., Troell K., Tysnes K., Robertson L.J. Occurrence of Giardia in Swedish red foxes (Vulpes vulpes) J. Wildl. Dis. 2017;53:649–652. doi: 10.7589/2017-01-002. [DOI] [PubMed] [Google Scholar]
- Decraene V., Lebbad M., Botero-Kleiven S., Gustavsson A.M., Lofdahl M. First reported foodborne outbreak associated with microsporidia, Sweden, October 2009. Epidemiol. Infect. 2012;140:519–527. doi: 10.1017/S095026881100077X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Aguila C., Croppo G.P., Moura H., Da Silva A.J., Leitch G.J., Moss D.M., Wallace S., Slemenda S.B., Pieniazek N.J., Visvesvara G.S. Ultrastructure, immunofluorescence, western blot, and PCR analysis of eight isolates of Encephalitozoon (Septata) intestinalis established in culture from sputum and urine samples and duodenal aspirates of five patients with AIDS. J. Clin. Microbiol. 1998;36:1201–1208. doi: 10.1128/jcm.36.5.1201-1208.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond L.S., Clark C.G. A redescription of Entamoeba histolytica Schaudinn, 1903 (emended Walker, 1911) separating it from Entamoeba dispar Brumpt, 1925. J. Eukaryot. Microbiol. 1993;40:340–344. doi: 10.1111/j.1550-7408.1993.tb04926.x. [DOI] [PubMed] [Google Scholar]
- Didier E.S., Rogers L.B., Orenstein J.M., Baker M.D., Vossbrinck C.R., Van Gool T., Hartskeerl R., Soave R., Beaudet L.M. Characterization of Encephalitozoon (Septata) intestinalis isolates cultured from nasal mucosa and bronchoalveolar lavage fluids of two AIDS patients. J. Eukaryot. Microbiol. 1996;43:34–43. doi: 10.1111/j.1550-7408.1996.tb02470.x. [DOI] [PubMed] [Google Scholar]
- Didier E.S., Weiss L.M. Microsporidiosis: not just in AIDS patients. Curr. Opin. Infect. Dis. 2011;24:490–495. doi: 10.1097/QCO.0b013e32834aa152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobell C. J. Bale, Sons, and Danielson; London, UK: 1919. The Amoebae Living in Man. A Zoological Monograph. [Google Scholar]
- Dowd S.E., Gerba C.P., Pepper I.L. Confirmation of the human-pathogenic microsporidia Enterocytozoon bieneusi, Encephalitozoon intestinalis, and Vittaforma corneae in water. Appl. Environ. Microbiol. 1998;64:3332–3335. doi: 10.1128/aem.64.9.3332-3335.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubey J.P. Foodborne and waterborne zoonotic sarcocystosis. Food Water. Parasitol. 2015;1:2–11. [Google Scholar]
- Dubey J.P., Jones J.L. Toxoplasma gondii infection in humans and animals in the United States. Int. J. Parasitol. 2008;38:1257–1278. doi: 10.1016/j.ijpara.2008.03.007. [DOI] [PubMed] [Google Scholar]
- Dubey J.P., Velmurugan G.V., Rajendran C., Yabsley M.J., Thomas N.J., Beckmen K.B., Sinnett D., Ruid D., Hart J., Fair P.A., McFee W.E., Shearn-Bochsler V., Kwok O.C.H., Ferreira L.R., Choudhary S., Faria E.B., Zhou H., Felix T.A., Su C. Genetic characterisation of Toxoplasma gondii in wildlife from North America revealed widespread and high prevalence of the fourth clonal type. Int. J. Parasitol. 2011;41:1139–1147. doi: 10.1016/j.ijpara.2011.06.005. [DOI] [PubMed] [Google Scholar]
- Dykes A.C., Juranek D.D., Lorenz R.A., Sinclair S., Jakubowski W., Davies R. Municipal waterborne giardiasis: an epidemilogic investigation. Beavers implicated as a possible reservoir. Ann. Intern. Med. 1980;92:165–170. doi: 10.7326/0003-4819-92-2-165. [DOI] [PubMed] [Google Scholar]
- Eichhorn A., Gallagher B. Spontaneous amebic dysentery in monkeys. J. Infect. Dis. 1916;19:395–407. [Google Scholar]
- Esposito D.H., Stich A., Epelboin L., Malvy D., Han P.V., Bottieau E., da Silva A., Zanger P., Slesak G., van Genderen P.J., Rosenthal B.M., Cramer J.P., Visser L.G., Munoz J., Drew C.P., Goldsmith C.S., Steiner F., Wagner N., Grobusch M.P., Plier D.A., Tappe D., Sotir M.J., Brown C., Brunette G.W., Fayer R., von Sonnenburg F., Neumayr A., Kozarsky P.E., Tioman Island Sarcocystosis Investigation Team Acute muscular sarcocystosis: an international investigation among ill travelers returning from Tioman Island, Malaysia, 2011-2012. Clin. Infect. Dis. 2014;59:1401–1410. doi: 10.1093/cid/ciu622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farah Haziqah M.T., Mohd Zain S.N., Chandrawathani P., Premaalatha B., Mohd Khairul Nizam M.K., Arutchelvan R., Suresh K. Genetic diversity of rodent Blastocystis sp from peninsular Malaysia. Trop. Biomed. 2018;25:586–592. [PubMed] [Google Scholar]
- Fayer R., Esposito D.H., Dubey J.P. Human infections with Sarcocystis species. Clin. Microbiol. Rev. 2015;28:295–311. doi: 10.1128/CMR.00113-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng M., Pandey K., Yanagi T., Wang T., Putaporntip C., Jongwutiwes S., Cheng X., Sherchand J.B., Pandey B.D., Tachibana H. Prevalence and genotypic diversity of Entamoeba species in inhabitants in Kathmandu, Nepal. Parasitol. Res. 2018;117:2467–2472. doi: 10.1007/s00436-018-5935-2. [DOI] [PubMed] [Google Scholar]
- Feng Y., Li N., Dearen T., Lobo M.L., Matos O., Cama V., Xiao L. Development of a multilocus sequence typing tool for high-resolution genotyping of Enterocytozoon bieneusi. Appl. Environ. Microbiol. 2011;77:4822–4828. doi: 10.1128/AEM.02803-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y., Xiao L. Zoonotic potential and molecular epidemiology of Giardia species and giardiasis. Clin. Microbiol. Rev. 2011;24:110–140. doi: 10.1128/CMR.00033-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier S., Liguory O., Santillana-Hayat M., Guillot E., Sarfati C., Dumoutier N., Molina J., Derouin F. Detection of microsporidia in surface water: a one year follow-up study. FEMS Immunol. Med. Microbiol. 2000;29:95–100. doi: 10.1111/j.1574-695X.2000.tb01510.x. [DOI] [PubMed] [Google Scholar]
- Galván A., Magnet A., Izquierdo F., Fenoy S., Henriques-Gi,l N., del Aguila C. Variability in minimal genomes: analysis of tandem repeats in the microsporidia Encephalitozoon intestinalis. Infect. Genet. Evol. 2013;20:26–33. doi: 10.1016/j.meegid.2013.07.024. [DOI] [PubMed] [Google Scholar]
- Gibson-Kueh S., Yang R., Thuy N.T., Jones J.B., Nicholls P.K., Ryan U. The molecular characterization of an Eimeria and Cryptosporidium detected in Asian seabass (Lates calcarifer) cultured in Vietnam. Vet. Parasitol. 2011;181:91–96. doi: 10.1016/j.vetpar.2011.05.004. [DOI] [PubMed] [Google Scholar]
- Gjerde B. Phylogenetic relationships among Sarcocystis species in cervids, cattle and sheep inferred from the mitochondrial cytochrome c oxidase subunit I gene. Int. J. Parasitol. 2013;43:579–591. doi: 10.1016/j.ijpara.2013.02.004. [DOI] [PubMed] [Google Scholar]
- Gjerde B. Molecular characterisation of Sarcocystis bovifelis, Sarcocystis bovini n. sp., Sarcocystis hirsuta and Sarcocystis cruzi from cattle (Bos taurus) and Sarcocystis sinensis from water buffaloes (Bubalus bubalis) Parasitol. Res. 2016;115:1473–1492. doi: 10.1007/s00436-015-4881-5. [DOI] [PubMed] [Google Scholar]
- Gou H., Guan G., Liu A., Ma M., Xu Z., Liu Z., Ren Q., Li Y., Yang J., Chen Z., Yin H., Luo J. A DNA barcode for Piroplasmea. Acta Trop. 2012;124:92–97. doi: 10.1016/j.actatropica.2012.07.001. [DOI] [PubMed] [Google Scholar]
- Graczyk T.K., Majewska A.C., Schwab K.J. The role of birds in dissemination of human waterborne enteropathogens. Trends Parasitol. 2008;24:55–59. doi: 10.1016/j.pt.2007.10.007. [DOI] [PubMed] [Google Scholar]
- Guan Y., Feng M., Cai J., Min X., Zhou X., Xu Q., Tan N., Cheng X., Tachibana H. Comparative analysis of genotypic diversity in Entamoeba nuttalli isolates from Tibetan macaques and rhesus macaques in China. Infect. Genet. Evol. 2016;38:126–131. doi: 10.1016/j.meegid.2015.12.014. [DOI] [PubMed] [Google Scholar]
- Hamnes I.S., Gjerde B.K., Forberg T., Robertson L.J. Occurrence of Giardia and Cryptosporidium in Norwegian red foxes (Vulpes vulpes) Vet. Parasitol. 2007;143:347–353. doi: 10.1016/j.vetpar.2006.08.032. [DOI] [PubMed] [Google Scholar]
- Hampton J., Spencer P.B.S., Elliot A.D., Thompson R.C.A. Prevalence of zoonotic pathogens from feral pigs in major public drinking water catchments in Western Australia. EcoHealth. 2006;3:103–108. [Google Scholar]
- Han J., Zhu Y., Chen X., Liao B., Yao H., Song J., Chen S., Meng F. The short ITS2 sequence serves as an efficient taxonomic sequence tag in comparison with the full-length ITS. BioMed Res. Int. 2013;2013:741476. doi: 10.1155/2013/741476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haro M., Del Aguila C., Fenoy S., Henriques-Gil N. Intraspecies genotype variability of the microsporidian parasite Encephalitozoon hellem. J. Clin. Microbiol. 2003;41:4166–4171. doi: 10.1128/JCM.41.9.4166-4171.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haro M., Izquierdo F., Henriques-Gil N., Andrés I., Alonso F., Fenoy S., del Aguila C. First detection and genotyping of human-associated microsporidia in pigeons from urban parks. Appl. Environ. Microbiol. 2005;71:3153–3157. doi: 10.1128/AEM.71.6.3153-3157.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegner H. Specificity in the genus Balantidium based on size and shape of body and macronucleus, with descriptions of six new species. Am. J. Epidemiol. 1934;19:38–67. [Google Scholar]
- Hinney B., Sak B., Joachim A., Kváč M. More than a rabbit's tale - Encephalitozoon spp. in wild mammals and birds. Int. J. Parasitol. Parasites. Wildl. 2016;5:76–87. doi: 10.1016/j.ijppaw.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homan W.L., Vercammen M., De Braekeleer J., Verschueren H. Identification of a 200- to 300-fold repetitive 529 bp DNA fragment in Toxoplasma gondii, and its use for diagnostic and quantitative PCR. Int. J. Parasitol. 2000;30:69–75. doi: 10.1016/s0020-7519(99)00170-8. [DOI] [PubMed] [Google Scholar]
- Howe D.K., Sibley L.D. Toxoplasma gondii comprises three clonal lineages: correlation of parasite genotype with human disease. J. Infect. Dis. 1995;172:1561–1566. doi: 10.1093/infdis/172.6.1561. [DOI] [PubMed] [Google Scholar]
- Huang J., Zhang Z., Yang Y., Wang R., Zhao J., Jian F., Ning C., Zhang L. New genotypes of Enterocytozoon bieneusi isolated from sika deer and red deer in China. Front. Microbiol. 2017;8:879. doi: 10.3389/fmicb.2017.00879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Italiano C.M., Wong K.T., AbuBakar S., Lau Y.L., Ramli N., Syed Omar S.F., Kahar Bador M., Tan C.T. Sarcocystis nesbitti causes acute, relapsing febrile myositis with a high attack rate: description of a large outbreak of muscular sarcocystosis in Pangkor Island, Malaysia, 2012. PLoS Negl. Trop. Dis. 2014;8 doi: 10.1371/journal.pntd.0002876. e2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson T.F., Sargeaunt P.G., Visser P.S., Gathiram V., Suparsad S., Anderson C.B. Entamoeba histolytica: naturally occurring infections in baboons. Arch. Invest. Med. 1990;21:153–156. [PubMed] [Google Scholar]
- Jäkel T., Scharpfenecker M., Jitrawang P., Rückle J., Kliemt D., Mackenstedt U., Hongnark S., Khoprasert Y. Reduction of transmission stages concomitant with increased host immune responses to hypervirulent Sarcocystis singaporensis, and natural selection for intermediate virulence. Int. J. Parasitol. 2001;31:1639–1647. doi: 10.1016/s0020-7519(01)00289-2. [DOI] [PubMed] [Google Scholar]
- Jiang T., Shwab E.K., Martin R.M., Gerhold R.W., Rosenthal B.M., Dubey J.P., Su C. A partition of Toxoplasma gondii genotypes across spatial gradients and among host species, and decreased parasite diversity towards areas of human settlement in North America. Int. J. Parasitol. 2018;48:611–619. doi: 10.1016/j.ijpara.2018.01.008. [DOI] [PubMed] [Google Scholar]
- Jones K.E., Patel N.G., Levy M.A., Storeygard A., Balk D., Gittleman J.L., Daszak P. Global trends in emerging infectious diseases. Nature. 2008;451:990–993. doi: 10.1038/nature06536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kan S.P., Pathmanathan R. Review of sarcocystosis in Malaysia. Southeast Asian J. Trop. Med. Publ. Health. 1991;22(Suppl. l):129–134. [PubMed] [Google Scholar]
- Kašičková D., Sak B., Kváč M., Ditrich O. Sources of potentially infectious human microsporidia: molecular characterisation of microsporidia isolates from exotic birds in the Czech Republic, prevalence study and importance of birds in epidemiology of the human microsporidial infections. Vet. Parasitol. 2009;165:125–230. doi: 10.1016/j.vetpar.2009.06.033. [DOI] [PubMed] [Google Scholar]
- Katsumata M., Yoshikawa H., Tokoro M., Mizuno T., Nagamoto T., Hendarto J., Asih P.B.S., Rozi I.E., Kimata I., Takami K., Syafruddin D. Molecular phylogeny of Blastocystis isolates from wild rodents captured in Indonesia and Japan. Parasitol. Res. 2018;117:2841–2846. doi: 10.1007/s00436-018-5973-9. [DOI] [PubMed] [Google Scholar]
- Khan A., Dubey J.P., Su C., Ajioka J.W., Rosenthal B.M., Sibley L.D. Genetic analyses of atypical Toxoplasma gondii strains reveal a fourth clonal lineage in North America. Int. J. Parasitol. 2011;41:645–655. doi: 10.1016/j.ijpara.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi Y., Chomel B.B., Kasten R.W., Martenson J.S., Swift P.K., O'Brien S.J. Seroprevalence of Toxoplasma gondii in American free-ranging or captive pumas (Felis concolor) and bobcats (Lynx rufus) Vet. Parasitol. 2004;120:1–9. doi: 10.1016/j.vetpar.2004.01.002. [DOI] [PubMed] [Google Scholar]
- Koinari M., Karl S., Ng-Hublin J., Lymbery A.J., Ryan U.M. Identification of novel and zoonotic Cryptosporidium species in fish from Papua New Guinea. Vet. Parasitol. 2013;198:1–9. doi: 10.1016/j.vetpar.2013.08.031. [DOI] [PubMed] [Google Scholar]
- Kváč M., Květoňová D., Sak B., Ditrich O. Cryptosporidium pig genotype II in immunocompetent man. Emerg. Infect. Dis. 2009;15:982–983. doi: 10.3201/eid1506.07621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kváč M., Tomanová V., Samková E., Koubová J., Kotková M., Hlásková L., McEvoy J., Sak B. Encephalitozoon cuniculi in raw cow's milk remains infectious after pasteurization. Foodb. Pathog. Dis. 2016;13:77–79. doi: 10.1089/fpd.2015.2036. [DOI] [PubMed] [Google Scholar]
- Lau Y.L., Chang P.Y., Subramaniam V., Ng Y.H., Mahmud R., Ahmad A.F., Fong M.Y. Genetic assemblage of Sarcocystis spp. in Malaysian snakes. Parasit. Vectors. 2013;6:257. doi: 10.1186/1756-3305-6-257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau Y.L., Chang P.Y., Tan C.T., Fong M.Y., Mahmud R., Wong K.T. Sarcocystis nesbitti infection in human skeletal muscle: possible transmission from snakes. Am. J. Trop. Med. Hyg. 2014;90:361–364. doi: 10.4269/ajtmh.12-0678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levecke B., Dorny P., Vercammen F., Visser L.G., Van Esbroeck M., Vercruysse J., Verweij J.J. Transmission of Entamoeba nuttalli and Trichuris trichiura from nonhuman primates to humans. Emerg. Infect. Dis. 2015;21:1871–1872. doi: 10.3201/eid2110.141456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine N.D. The ciliates. In: Levine N.D., editor. Protozoan Parasites of Domestic Animals and of Man. Burgess Publishing Company; Minneapolis: 1961. pp. 347–376. [Google Scholar]
- Levine N.D. Ciliophora. In: Levine N.D., editor. Veterinary Protozoology. Iowa State University Press; Ames: 1985. pp. 334–364. [Google Scholar]
- Li W., Deng L., Yu X., Zhong Z., Wang Q., Liu X., Niu L., Xie N., Deng J., Lei S., Wang L., Gong C., Zhou Z., Hu Y., Fu H., Xu H., Geng Y., Peng G. Multilocus genotypes and broad host-range of Enterocytozoon bieneusi in captive wildlife at zoological gardens in China. Parasit. Vectors. 2016;9:395. doi: 10.1186/s13071-016-1668-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liguory O., Fournier S., Sarfati C., Derouin F., Molina J.M. Genetic homology among thirteen Encephalitozoon intestinalis isolates obtained from human immunodeficiency virus-infected patients with intestinal microsporidiosis. J. Clin. Microbiol. 2000;38:2389–2391. doi: 10.1128/jcm.38.6.2389-2391.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W., Nie C., Zhang L., Wang R., Liu A., Zhao W., Li H. First detection and genotyping of Enterocytozoon bieneusi in reindeers (Rangifer tarandus): a zoonotic potential of ITS genotypes. Parasit. Vectors. 2015;8:526. doi: 10.1186/s13071-015-1155-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzi H., Khan A., Behnke M.S., Namasivayam S., Swapna L.S., Hadjithomas M., Karamycheva S., Pinney D., Brunk B.P., Ajioka J.W., Ajzenberg D., Boothroyd J.C., Boyle J.P., Dardé M.L., Diaz-Miranda M.A., Dubey J.P., Fritz H.M., Gennari S.M., Gregory B.D., Kim K., Saeij J.P., Su C., White M.W., Zhu X.Q., Howe D.K., Rosenthal B.M., Grigg M.E., Parkinson J., Liu L., Kissinger J.C., Roos D.S., Sibley L.D. Local admixture of amplified and diversified secreted pathogenesis determinants shapes mosaic Toxoplasma gondii genomes. Nat. Commun. 2016;7:e10147. doi: 10.1038/ncomms10147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malmsten P.H. Infusorien als intestinal-thiere beim menschen. Arch. Pathol. Anat. Physiol. Klin. Med. 1857;12:902–909. [Google Scholar]
- Mandour A.M. Sarcocystis nesbitti n. sp. from the rhesus monkey. J. Protozool. 1969;16:353–354. doi: 10.1111/j.1550-7408.1969.tb02281.x. [DOI] [PubMed] [Google Scholar]
- Mateo M., de Mingo M.H., de Lucio A., Morales L., Balseiro A., Espí A., Barral M., Lima Barbero J.F., Habela M., Fernández-García J.L., Bernal R.C., Köster P.C., Cardona G.A., Carmena D. Occurrence and molecular genotyping of Giardia duodenalis and Cryptosporidium spp. in wild mesocarnivores in Spain. Vet. Parasitol. 2017;235:86–93. doi: 10.1016/j.vetpar.2017.01.016. [DOI] [PubMed] [Google Scholar]
- Mathis A., Tanner I., Weber R., Deplazes P. Genetic and phenotypic intraspecific variation in the microsporidian Encephalitozoon hellem. Int. J. Parasitol. 1999;29:767–770. doi: 10.1016/s0020-7519(99)00025-9. [DOI] [PubMed] [Google Scholar]
- Matos O., Lobo M.L., Xiao L. Epidemiology of Enterocytozoon bieneusi infection in humans. J. Parasitol. Res. 2012;2012:981424. doi: 10.1155/2012/981424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy S., Ng J., Gordon C., Miller R., Wyber A., Ryan U.M. Prevalence of Cryptosporidium and Giardia species in animals in irrigation catchments in the southwest of Australia. Exp. Parasitol. 2007;118:596–599. doi: 10.1016/j.exppara.2007.10.014. [DOI] [PubMed] [Google Scholar]
- McDonald J.D. vol 20. University of California Publications in Zoology; 1922. pp. 243–300. (On Balantidium coli (Malmsten) and Balantidium suis (sp. nov.) with an Account of Their Neuromotor Apparatus). [Google Scholar]
- Morine M., Yang R., Ng J., Kueh S., Lymbery A.J., Ryan U.M. Additional novel Cryptosporidium genotypes in ornamental fishes. Vet. Parasitol. 2012;190:578–582. doi: 10.1016/j.vetpar.2012.06.036. [DOI] [PubMed] [Google Scholar]
- Mucker E.M., Dubey J.P., Lovallo M.J., Humphreys J.G. Seroprevalence of antibodies to Toxoplasma gondii in the Pennsylvania bobcat (Lynx rufus rufus) J. Wildl. Dis. 2006;42:188–191. doi: 10.7589/0090-3558-42.1.188. [DOI] [PubMed] [Google Scholar]
- Mundim M.J.S., Mundim A.V., Santos A.L.Q., Cabral D.D., Faria E.S.M., Moraes F.M. Helmintos e protozoários em fezes de javalis (Sus scrofa scrofa) criados em cautiverio. Arq. Bras. Med. Vet. Zootec. 2004;56:792–795. [Google Scholar]
- Murphy B.G., Bradway D., Walsh T., Sanders G.E., Snekvik K. Gastric cryptosporidiosis in freshwater angelfish (Pterophyllum scalare) J. Vet. Diagn. Invest. 2009;21:722–727. doi: 10.1177/104063870902100523. [DOI] [PubMed] [Google Scholar]
- Navarro-González N., Fernández-Llario P., Pérez-Martín J.E., Mentaberre G., López-Martín J.M., Lavín S., Serrano E. Supplemental feeding drives endoparasite infection in wild boar in Western Spain. Vet. Parasitol. 2013;196:114–123. doi: 10.1016/j.vetpar.2013.02.019. [DOI] [PubMed] [Google Scholar]
- Neiva A., Marques Da Cunha A., Travassos L. Contribuçôes parazitolojicas I. Mem. Inst. Oswaldo Cruz. 1914;6:180–191. [Google Scholar]
- Ng J., Yang R., Whiffin V., Cox P., Ryan U. Identification of zoonotic Cryptosporidium and Giardia genotypes infecting animals in Sydney's water catchments. Exp. Parasitol. 2011;128:138–144. doi: 10.1016/j.exppara.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Nieves-Ramirez M.E., Partida-Rodriguez O., Laforest-Lapointe I., Reynolds L.A., Brown E.M., Valdez-Salazar A., Moran-Silva P., Rojas-Velazquez L., Morien E., Parfrey L.W., Jin M., Walter J., Torres J., Arrieta M.C., Ximenez-Garcia C., Finlay B.B. Asymptomatic intestinal colonization with protist Blastocystis is strongly associated with distinct microbiome ecological patterns. mSystems. 2018;3 doi: 10.1128/mSystems.00007-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onac D., Oltean M., Mircean V., Jarca A., Cozma V. Occurrence of Giardia duodenalis zoonotic assemblages in red foxes from Romania. Sci. Parasitol. 2015;16:177–180. [Google Scholar]
- Palenzuela O., Alvarez-Pellitero P., Sitjà-Bobadilla A. Molecular characterization of Cryptosporidium molnari reveals a distinct piscine clade. Appl. Environ. Microbiol. 2010;76:7646–7649. doi: 10.1128/AEM.01335-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palermo C. Murdoch University; 2016. Cryptosporidium in Fish: Morphological and Molecular Characterisation.http://researchrepository.murdoch.edu.au/id/eprint/35248/ Honours thesis. [Google Scholar]
- Paparini A., Yang R.C., Chen L.D., Tong K.S., Gibson-Kueh S., Lymbery A., Ryan U.M. Cryptosporidium in fish: alternative sequencing approaches and analyses at multiple loci to resolve mixed infections. Parasitology. 2017;144:1811–1820. doi: 10.1017/S0031182017001214. [DOI] [PubMed] [Google Scholar]
- Petrova M.S., Belova L.M., Gavrilova N.A., Loginova O.A., Shiryaeva V.A. Medications for treatment of pigs with mono- and mixed parasitic infections. Bulg. J. Vet. Med. 2017;20:359–361. [Google Scholar]
- Pirestani M., Sadraei J., Forouzandeh M. Molecular characterization and genotyping of human related microsporidia in free-ranging and captive pigeons of Tehran. Iran. Infect. Genet. Evol. 2013;20:495–499. [PubMed] [Google Scholar]
- Pomajbíková K., Oborník M., Horák A., Petrzelková K.J., Grim J.N., Levecke B., Todd A., Mulama M., Kiyang J., Modrý D. Novel insights into the genetic diversity of Balantidium and Balantidium-like cyst-forming ciliates. PLoS Negl. Trop. Dis. 2013;7:e2140. doi: 10.1371/journal.pntd.0002140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomares C., Devillard S., Holmes T.H., Olariu T.R., Press C.J., Ramirez R., Talucod J., Estran R., Su C., Dubey J.P., Ajzenberg D., Montoya J.G. Genetic characterization of Toxoplasma gondii DNA samples isolated from humans living in North America: an unexpected high prevalence of atypical genotypes. J. Infect. Dis. 2018;218:1783–1791. doi: 10.1093/infdis/jiy375. [DOI] [PubMed] [Google Scholar]
- Ponce-Gordo F., Jiménez-Ruiz E., Martínez-Díaz R.A. Tentative identification of Balantidium from ostriches (Struthio camelus) as Balantidium coli-like by analysis of polymorphic DNA. Vet. Parasitol. 2008;157:41–49. doi: 10.1016/j.vetpar.2008.06.024. [DOI] [PubMed] [Google Scholar]
- Ponce-Gordo F., Fonseca-Salamanca F., Martínez-Díaz R.A. Genetic heterogeneity in internal transcribed spacer genes of Balantidium coli (Litostomatea, Ciliophora) Protist. 2011;162:774–794. doi: 10.1016/j.protis.2011.06.008. [DOI] [PubMed] [Google Scholar]
- Ponce-Gordo F., Jirků-Pomajbíková K. Balantidium coli. In: Rose J.B., Jiménez-Cisneros B., editors. Global Water Pathogens Project. Michigan State University; E. Lansing, MI: 2018. p. 7. In Fayer, R. and Jakubowski, W. (Eds) Part 3. Protists. UNESCO. [Google Scholar]
- Poulsen C.S., Stensvold C.R. Current status of epidemiology and diagnosis of human sarcocystosis. J. Clin. Microbiol. 2014;52:3524–3530. doi: 10.1128/JCM.00955-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid A., Lymbery A., Ng J., Tweedle S., Ryan U. Identification of novel and zoonotic Cryptosporidium species in marine fish. Vet. Parasitol. 2010;168:190–195. doi: 10.1016/j.vetpar.2009.11.015. [DOI] [PubMed] [Google Scholar]
- Riemann H.P., Howarth J.A., Ruppanner R., Franti C.E., Behymer D.E. Toxoplasma antibodies among bobcats and other carnivores of Northern California. J. Wildl. Dis. 1975;11:272–276. doi: 10.7589/0090-3558-11.2.272. [DOI] [PubMed] [Google Scholar]
- Rinder H., Katzwinkel-Wladarsch S., Thomschke A., Loscher T. Strain differentiation in microsporidia. Tokai J. Exp. Clin. Med. 1999;23:433–437. [PubMed] [Google Scholar]
- Rivera W.L., Yason J.A., Adao D.E. Entamoeba histolytica and E. dispar infections in captive macaques (Macaca fascicularis) in the Philippines. Primates. 2010;51:69–74. doi: 10.1007/s10329-009-0174-x. [DOI] [PubMed] [Google Scholar]
- Roberts N.M., Crimmins S.M. Bobcat population status and management in North America: evidence of large-scale population increase. J. Fish Wildlife Manag. 2010;1:169–174. [Google Scholar]
- Roberts T., Barratt J., Harkness J., Ellis J., Stark D. Comparison of microscopy, culture, and conventional polymerase chain reaction for detection of Blastocystis sp. in clinical stool samples. Am. J. Trop. Med. Hyg. 2011;84:308–312. doi: 10.4269/ajtmh.2011.10-0447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan U., Paparini A., Tong K.S., Yang R.C., Gibson-Kueh S., O'Hara A., Lymbery A., Xiao L.H. Cryptosporidium huwi n. sp (Apicomplexa: Eimeriidae) from the guppy (Poecilia reticulata) Exp. Parasitol. 2015;150:31–35. doi: 10.1016/j.exppara.2015.01.009. [DOI] [PubMed] [Google Scholar]
- Sak B., Kašičková D., Kváč M., Květoňová D., Ditrich O. Microsporidia in exotic birds: intermittent spore excretion of Encephalitozoon spp. in naturally infected budgerigars (Melopsittacus undulatus) Vet. Parasitol. 2010;168:196–200. doi: 10.1016/j.vetpar.2009.11.012. [DOI] [PubMed] [Google Scholar]
- Sak B., Kotková M., Hlásková L., Kváč M. Limited effect of adaptive immune response to control encephalitozoonosis. Parasite. Immunol. 2017;39 doi: 10.1111/pim.12496. [DOI] [PubMed] [Google Scholar]
- Sak B., Kváč M., Kučerová Z., Květoňová D., Saková K. Latent microsporidial infection in immunocompetent individuals - a longitudinal study. PLoS Negl. Trop. Dis. 2011;5:e1162. doi: 10.1371/journal.pntd.0001162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sak B., Kváč M., Květoňová D., Albrecht T., Piálek J. The first report on natural Enterocytozoon bieneusi and Encephalitozoon spp. infections in wild east-European house mice (Mus musculus musculus) and west-European house mice (M. m. domesticus) in a hybrid zone across the Czech Republic-Germany border. Vet. Parasitol. 2011;178:246–250. doi: 10.1016/j.vetpar.2010.12.044. [DOI] [PubMed] [Google Scholar]
- Santín M., Fayer R. Enterocytozoon bieneusi genotype nomenclature based on the internal transcribed spacer sequence: a consensus. J. Eukaryot. Microbiol. 2009;56:34–38. doi: 10.1111/j.1550-7408.2008.00380.x. [DOI] [PubMed] [Google Scholar]
- Santín M., Fayer R. Enterocytozoon bieneusi, Giardia, and Cryptosporidium infecting white-tailed deer. J. Eukaryot. Microbiol. 2015;62:34–43. doi: 10.1111/jeu.12155. [DOI] [PubMed] [Google Scholar]
- Sargeaunt P.G. The reliability of Entamoeba histolytica zymodemes in clinical diagnosis. Parasitol. Today. 1987;3:40–43. doi: 10.1016/0169-4758(87)90211-0. [DOI] [PubMed] [Google Scholar]
- Scamardella J.M. Not plants or animals: a brief history of the origin of Kingdoms Protozoa, Protista and Protoctista. Int. Microbiol. 1999;2:207–216. [PubMed] [Google Scholar]
- Schuster F.L., Ramirez-Avila L. Current world status of Balantidium coli. Clin. Microbiol. Rev. 2008;28:626–638. doi: 10.1128/CMR.00021-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifollahi Z., Sarkari B., Motazedian M.H., Asgari Q., Ranjbar M.J., Abdolahi Khabisi S. Protozoan parasites of rodents and their zoonotic significance in Boyer-Ahmad District, Southwestern Iran. Vet. Med. Int. 2016;2016:3263868. doi: 10.1155/2016/3263868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selman M., Sak B., Kváč M., Farinelli L., Weiss L.M., Corradi N. Extremely reduced levels of heterozygosity in the vertebrate pathogen Encephalitozoon cuniculi. Eukaryot. Cell. 2013;12:496–502. doi: 10.1128/EC.00307-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahari S., Tengku-Idris T.I., Fong M.Y., Lau Y.L. Molecular evidence of Sarcocystis nesbitti in water samples of Tioman Island, Malaysia. Parasit. Vectors. 2016;9:598. doi: 10.1186/s13071-016-1883-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shwab E.K., Jiang T., Pena H.F.J., Gennari S.M., Dubey J.P., Su C. The ROP18 and ROP5 gene allele types are highly predictive of virulence in mice across globally distributed strains of Toxoplasma gondii. Int. J. Parasitol. 2016;46:141–146. doi: 10.1016/j.ijpara.2015.10.005. [DOI] [PubMed] [Google Scholar]
- Shwab E.K., Zhu X.Q., Majumdar D., Pena H.F.J., Gennari S.M., Dubey J.P., Su C. Geographical patterns of Toxoplasma gondii genetic diversity revealed by multilocus PCR-RFLP genotyping. Parasitology. 2014;141:453–461. doi: 10.1017/S0031182013001844. [DOI] [PubMed] [Google Scholar]
- Šlapeta J.R., Modrý D., Votýpka J., Jirků M., Lukeš J., Koudela B. Evolutionary relationships among cyst-forming coccidia Sarcocystis spp. (Alveolata: Apicomplexa: Coccidea) in endemic African tree vipers and perspective for evolution of heteroxenous life cycle. Mol. Phylogenet. Evol. 2003;27:464–475. doi: 10.1016/s1055-7903(03)00018-6. [DOI] [PubMed] [Google Scholar]
- Smith J.M., Meerovitch E. Primates as a source of Entamoeba histolytica, their zymodeme status and zoonotic potential. J. Parasitol. 1985;71:751–756. [PubMed] [Google Scholar]
- Sobottka I., Albrecht H., Visvesvara G.S., Pieniazek N.J., Deplazes P., Schwartz D.A., Laufs R., Elsner H.A. Inter- and intra-species karyotype variations among microsporidia of the genus Encephalitozoon as determined by pulsed field gel electrophoresis. Scand. J. Infect. Dis. 1999;31:555–558. doi: 10.1080/00365549950164427. [DOI] [PubMed] [Google Scholar]
- Solaymani-Mohammadi S., Rezaian M., Hooshyar H., Mowlavi G.R., Babaei Z., Anwar M.A. Intestinal protozoa in wild boars (Sus scrofa) in Western Iran. J. Wildl. Dis. 2004;40:801–803. doi: 10.7589/0090-3558-40.4.801. [DOI] [PubMed] [Google Scholar]
- Sommer M.F., Beck R., Ionita M., Stefanovska J., Vasic A., Zdravkovic N., Hamel D., Rehbein S., Knaus M., Mitrea I.L., Shukullari E., Kirkova Z., Rapti D., Capari B., Silaghi C. Multilocus sequence typing of canine Giardia duodenalis from South Eastern European countries. Parasitol. Res. 2015;114:2165–2174. doi: 10.1007/s00436-015-4405-3. [DOI] [PubMed] [Google Scholar]
- Song Y., Li W., Liu H., Zhong Z., Luo Y., Wei Y., Fu W., Ren Z., Zhou Z., Deng L., Cheng J., Peng G. First report of Giardia duodenalis and Enterocytozoon bieneusi in forest musk deer (Moschus berezovskii) in China. Parasit. Vectors. 2018;11:204. doi: 10.1186/s13071-018-2681-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprong H., Cacciò S.M., van der Giessen J.W., ZOOPNET Network, Partners Identification of zoonotic genotypes of Giardia duodenalis. Plos Negl. Trop. Dis. 2009;3:e558. doi: 10.1371/journal.pntd.0000558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein F. 1863. Über das Paramaecium (?) coli, Malmst. Amtlicher Bericht über die Sieben und Dreissigste Versammlung Deutscher Naturforscher und Aerzte in Karlsbad in September 1862; p. 165. [Google Scholar]
- Stensvold C.R., Clark C.G. Current status of Blastocystis: a personal view. Parasitol. Int. 2016;65:763–771. doi: 10.1016/j.parint.2016.05.015. [DOI] [PubMed] [Google Scholar]
- Stensvold C.R., Clark C.G. Molecular identification and subtype analysis of Blastocystis. Curr. Protoc. Microbiol. 2016;43 doi: 10.1002/cpmc.17. 20A.2.1-20A.2.10. [DOI] [PubMed] [Google Scholar]
- Stojecki K., Sroka J., Cacciò S.M., Cencek T., Dutkiewicz J., Kusyk P. Prevalence and molecular typing of Giardia duodenalis in wildlife from eastern Poland. Folia Parasitol. (Praha) 2015;62 doi: 10.14411/fp.2015.042. [DOI] [PubMed] [Google Scholar]
- Strüder-Kypke M.C., Wright A.-D.G., Foissner W., Chatzinotas A., Lynn D.H. Molecular phylogeny of litostome ciliates (Ciliophora, Litostomatea) with emphasis on free-living haptorian genera. Protist. 2006;157:261–278. doi: 10.1016/j.protis.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Su C., Asis A.K., Peng Z., Majumdar D., Ajzenberg D., Darde M.L., Zhu X.Q., Ajioka J.W., Rosenthal B.M., Dubey J.P., Sibley L.D. Globally diverse Toxoplasma gondii isolates comprise six major clades originating from a small number of distinct ancestral lineages. Proc. Natl. Acad. Sci. U.S.A. 2012;109:5844–5849. doi: 10.1073/pnas.1203190109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su C., Shwab E.K., Zhou P., Zhu X.Q., Dubey J.P. Moving towards an integrated approach to molecular detection and identification of Toxoplasma gondii. Parasitology. 2010;137:1–11. doi: 10.1017/S0031182009991065. [DOI] [PubMed] [Google Scholar]
- Supriadi, Fitria W.A., Nurcahyo R.W. Balantidium sp. infection in faeces samples of orangutan (Pongo pygmaeus) from Care center and Tanjung putting national park area, central Borneo. BIOMEDICH. 2012;1:47–52. [Google Scholar]
- Suzuki J., Kobayashi S., Murata R., Yanagawa Y., Takeuchi T. Profiles of a pathogenic Entamoeba histolytica-like variant with variations in the nucleotide sequence of the small subunit ribosomal RNA isolated from a primate (De Brazza's guenon) J. Zoo Wildl. Med. 2007;38:471–474. doi: 10.1638/2006-0068.1. [DOI] [PubMed] [Google Scholar]
- Tachibana H., Yanagi T., Pandey K., Cheng X.J., Kobayashi S., Sherchand J.B., Kanbara H. An Entamoeba sp. strain isolated from rhesus monkey is virulent but genetically different from Entamoeba histolytica. Mol. Biochem. Parasitol. 2007;153:107–114. doi: 10.1016/j.molbiopara.2007.02.006. [DOI] [PubMed] [Google Scholar]
- Takano J., Narita T., Tachibana H., Terao K., Fujimoto K. Comparison of Entamoeba histolytica DNA isolated from a cynomolgus monkey with human isolates. Parasitol. Res. 2007;101:539–546. doi: 10.1007/s00436-007-0510-2. [DOI] [PubMed] [Google Scholar]
- Tappe D., Abdullah S., Heo C.C., Kannan Kutty M., Latif B. Human and animal invasive muscular sarcocystosis in Malaysia - recent cases, review and hypotheses. Trop. Biomed. 2013;30:355–366. [PubMed] [Google Scholar]
- Tappe D., Stich A., Langeheinecke A., von Sonnenburg F., Muntau B., Schafer J., Slesak G. Suspected new wave of muscular sarcocystosis in travellers returning from Tioman Island, Malaysia, May 2014. Euro Surveill. 2014;19:20816. doi: 10.2807/1560-7917.es2014.19.21.20816. [DOI] [PubMed] [Google Scholar]
- Tavalla M., Mardani-Kateki M., Abdizadeh R., Soltani S., Saki J. Molecular diagnosis of potentially human pathogenic Enterocytozoon bieneusi and Encephalitozoon species in exotic birds in Southwestern Iran. J. Infect. Public. Health. 2018;11:192–196. doi: 10.1016/j.jiph.2017.07.028. [DOI] [PubMed] [Google Scholar]
- Thompson R.C.A., Ash A. Molecular epidemiology of Giardia and Cryptosporidium infections. Infect. Genet. Evol. 2016;40:315–323. doi: 10.1016/j.meegid.2015.09.028. [DOI] [PubMed] [Google Scholar]
- Thompson R.C., Polley L. Parasitology and one health. Int. J. Parasitol. Parasites Wildl. 2014;3:A1–A2. doi: 10.1016/j.ijppaw.2014.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian M., Chen Y., Wu L., Rosenthal B.M., Liu X., He Y., Dunams D.B., Cui L., Yang Z. Phylogenetic analysis of Sarcocystis nesbitti (Coccidia: Sarcocystidae) suggests a snake as its probable definitive host. Vet. Parasitol. 2012;183:373–376. doi: 10.1016/j.vetpar.2011.07.034. [DOI] [PubMed] [Google Scholar]
- Tito R.Y., Chaffron S., Caenepeel C., Lima-Mendez G., Wang J., Vieira-Silva S., Falony G., Hildebrand F., Darzi Y., Rymenans L., Verspecht C., Bork P., Vermeire S., Joossens M., Raes J. Gut In press; 2018. Population-level Analysis of Blastocystis Subtype Prevalence and Variation in the Human Gut Microbiota. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanWormer E., Miller M.A., Conrad P.A., Grigg M.E., Rejmanek D., Carpenter T.E., Mazet J.A.K. Using molecular epidemiology to track Toxoplasma gondii from terrestrial carnivores to marine hosts: implications for public health and conservation. PLoS Negl. Trop. Dis. 2014;8:e2852. doi: 10.1371/journal.pntd.0002852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma S.K., Sweeny A.R., Lovallo M.J., Calero-Bernal R., Kwok O.C.H., Jiang T., Su C., Grigg M.E., Dubey J.P. Seroprevalence, isolation and co-infection of multiple Toxoplasma gondii strains in individual bobcats (Lynx rufus) from Mississippi, USA. Int. J. Parasitol. 2017;47:297–303. doi: 10.1016/j.ijpara.2016.12.007. [DOI] [PubMed] [Google Scholar]
- Verweij J.J., Vermeer J., Brienen E.A., Blotkamp C., Laeijendecker D., van Lieshout L., Polderman A.M. Entamoeba histolytica infections in captive primates. Parasitol. Res. 2003;90:100–103. doi: 10.1007/s00436-002-0808-z. [DOI] [PubMed] [Google Scholar]
- Vlčková K., Kreisinger J., Pafčo B., Čížková D., Tagg N., Hehl A.B., Modrý D. Diversity of Entamoeba spp. in African great apes and humans: an insight from Illumina MiSeq high-throughput sequencing. Int. J. Parasitol. 2018;48:519–530. doi: 10.1016/j.ijpara.2017.11.008. [DOI] [PubMed] [Google Scholar]
- Von Sonnenburg F., Cramer J.P., Freedman D.O., Plier D.A., Esposito D.H., Sotir M.J., Lankau E.W. Acute muscular sarcocystosis among returning travelers – Tioman Island, Malaysia, 2011. MMWR Morb. Mortal. Wkly. Rep. 2012;61:2. [PubMed] [Google Scholar]
- Wagnerová P., Sak B., Květoňová D., Maršálek M., Langrová I., Kváč M. Humoral immune response and spreading of Encephalitozoon cuniculi infection in experimentally infected ponies. Vet. Parasitol. 2013;197:1–6. doi: 10.1016/j.vetpar.2013.05.007. [DOI] [PubMed] [Google Scholar]
- Wang S.S., Wang R.J., Fan X.C., Liu T.L., Zhang L.X., Zhao G.H. Prevalence and genotypes of Enterocytozoon bieneusi in China. Acta Trop. 2018;183:142–152. doi: 10.1016/j.actatropica.2018.04.017. [DOI] [PubMed] [Google Scholar]
- Wassermann M., Raisch L., Lyons J.A., Natusch D.J.D., Richter S., Wirth M., Preeprem P., Khoprasert Y., Ginting S., Mackenstedt U., Jakel T. Examination of Sarcocystis spp. of giant snakes from Australia and Southeast Asia confirms presence of a known pathogen - Sarcocystis nesbitti. PLoS One. 2017;12 doi: 10.1371/journal.pone.0187984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wawrzyniak I., Poirier P., Viscogliosi E., Dionigia M., Texier C., Delbac F., Alaoui H.E. Blastocystis, an unrecognized parasite: an overview of pathogenesis and diagnosis. Ther. Adv. Infect. Dis. 2013;1:167–178. doi: 10.1177/2049936113504754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong K.T., Pathmanathan R. High prevalence of human skeletal-muscle sarcocystosis in South-East Asia. Trans. R. Soc. Trop. Med. Hyg. 1992;86:631–632. doi: 10.1016/0035-9203(92)90161-5. [DOI] [PubMed] [Google Scholar]
- Xiao L. Molecular epidemiology of cryptosporidiosis: an update. Exp. Parasitol. 2010;124:80–89. doi: 10.1016/j.exppara.2009.03.018. [DOI] [PubMed] [Google Scholar]
- Xiao L., Li L., Moura H., Sulaiman I., Lal A.A., Gatti S., Scaglia M., Didier E.S., Visvesvara G.S. Genotyping Encephalitozoon hellem isolates by analysis of the polar tube protein gene. J. Clin. Microbiol. 2001;39:2191–2196. doi: 10.1128/JCM.39.6.2191-2196.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao L., Li L., Visvesvara G.S., Moura H., Didier E.S., Lal A.A. Genotyping Encephalitozoon cuniculi by multilocus analyses of genes with repetitive sequences. J. Clin. Microbiol. 2001;39:2248–2253. doi: 10.1128/JCM.39.6.2248-2253.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaghoobi K., Sarkari B., Mansouri M., Motazedian M.H. Zoonotic intestinal protozoan of the wild boars, Sus scrofa, in Persian Gulf's coastal area (Bushehr province), Southwestern Iran. Vet. World. 2016;9:1047–1050. doi: 10.14202/vetworld.2016.1047-1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang R.C., Dorrestein G.M., Ryan U. Molecular characterisation of a disseminated Cryptosporidium infection in a Koi carp (Cyprinus carpio) Vet. Parasitol. 2016;226:53–56. doi: 10.1016/j.vetpar.2016.06.027. [DOI] [PubMed] [Google Scholar]
- Yang R.C., Palermo C., Chen L.D., Edwards A., Paparini A., Tong K.S., Gibson-Kueh S., Lymbery A., Ryan U. Genetic diversity of Cryptosporidium in fish at the 18S and actin loci and high levels of mixed infections. Vet. Parasitol. 2015;214:255–263. doi: 10.1016/j.vetpar.2015.10.013. [DOI] [PubMed] [Google Scholar]
- Yang Z.Q., Wei C.G., Zen J.S., Song J.L., Zuo Y.X., He Y.S., Zhang H.F., Attwood S.W., Chen X.W., Yang G.C., Zhou X., Quan X., Li C.Y., Han D., Liu A.W., Lin P. A taxonomic re-appraisal of Sarcocystis nesbitti (Protozoa: Sarcocystidae) from the monkey Macaca fascicularis in Yunnan, PR China. Parasitol. Int. 2005;54:75–81. doi: 10.1016/j.parint.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Yao H., Song J., Liu C., Luo K., Han H., Li Y., Pang X., Xu H., Zhu Y., Xiao P., Chen S. Use of ITS2 region as the universal DNA barcode for plants and animals. PLoS One. 2010;5:e13102. doi: 10.1371/journal.pone.0013102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye J., Xiao L., Ma J., Guo M., Liu L., Feng Y. Anthroponotic enteric parasites in monkeys in public park, China. Emerg. Infect. Dis. 2012;18:1640–1643. doi: 10.3201/eid1810.120653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikawa H., Koyama Y., Tsuchiya E., Takami K. Blastocystis phylogeny among various isolates from humans to insects. Parasitol. Int. 2016;65:750–759. doi: 10.1016/j.parint.2016.04.004. [DOI] [PubMed] [Google Scholar]
- Yoshikawa H., Tokoro M., Nagamoto T., Arayama S., Asih P.B., Rozi I.E., Syafruddin D. Molecular survey of Blastocystis sp. from humans and associated animals in an Indonesian community with poor hygiene. Parasitol. Int. 2016;65:780–784. doi: 10.1016/j.parint.2016.03.010. [DOI] [PubMed] [Google Scholar]
- Zanguee N., Lymbery J.A., Lau J., Suzuki A., Yang R., Ng J., Ryan U. Identification of novel Cryptosporidium species in aquarium fish. Vet. Parasitol. 2010;174:43–48. doi: 10.1016/j.vetpar.2010.08.006. [DOI] [PubMed] [Google Scholar]
- Zhang X.X., Cong W., Liu G.H., Ni X.T., Ma J.G., Zheng W.B., Zhao Q., Zhu X.Q. Prevalence and genotypes of Enterocytozoon bieneusi in sika deer in Jilin province, Northeastern China. Acta Parasitol. 2016;61:382–388. doi: 10.1515/ap-2016-0050. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Koehler A.V., Wang T., Haydon S.R., Gasser R.B. First detection and genetic characterisation of Enterocytozoon bieneusi in wild deer in Melbourne's water catchments in Australia. Parasit. Vectors. 2018;11:2. doi: 10.1186/s13071-017-2577-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z., Huang J., Karim M.R., Zhao J., Dong H., Ai W., Li F., Zhang L., Wang R. Zoonotic Enterocytozoon bieneusi genotypes in pere david's deer (Elaphurus davidianus) in Henan, China. Exp. Parasitol. 2015;155:46–48. doi: 10.1016/j.exppara.2015.05.008. [DOI] [PubMed] [Google Scholar]
- Zhao W., Wang J., Yang Z., Liu A. Dominance of the Enterocytozoon bieneusi genotype BEB6 in red deer (Cervus elaphus) and Siberian roe deer (Capreolus pygargus) in China and a brief literature review. Parasite. 2017;24:54. doi: 10.1051/parasite/2017056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao W., Zhang W., Wang R., Liu W., Liu A., Yang D., Yang F., Karim M.R., Zhang L. Enterocytozoon bieneusi in sika deer (Cervus nippon) and red deer (Cervus elaphus): deer specificity and zoonotic potential of ITS genotypes. Parasitol. Res. 2014;113:4243–4250. doi: 10.1007/s00436-014-4100-9. [DOI] [PubMed] [Google Scholar]