Abstract
Tigers continue to face unprecedented threats to their existence due to poaching, habitat loss, habitat fragmentation and anthropogenic disturbances. The present study examines the physiological stress response of tigers due to anthropogenic activities including wildlife tourism in Bandhavgarh Tiger Reserve and Kanha Tiger Reserve using faecal glucocorticoid metabolite (fGCM) measurement. We collected a total of 341 faecal samples from both reserves during tourism and non-tourism periods. Data on various anthropogenic disturbances including tourism activities like number of vehicles and visitors were also collected. We ascertained the species identity and sex of all the samples collected using genetic markers. fGCMs were extracted using a previously reported procedure, and fGCM concentrations were subsequently determined using an established enzyme immunoassay. There was no significant difference in overall mean fGCM concentrations between the two tiger reserves, but within each reserve, concentrations were significantly higher in tigers during the tourism period as compared to the non-tourism period. We also found that the number of tourist vehicles and disturbance level significantly correlated with fGCM concentrations. This study further supports the assumption that unbridled tourism associated with high anthropogenic disturbance can be related to perceived stress and consequently may have an impact on the reproductive fitness of tigers and long-term survival of isolated populations.
Keywords: Anthropogenic disturbance, Bandhavgarh Tiger Reserve, faecal glucocorticoid metabolites, Kanha Tiger Reserve, stress, tiger, tourism
Introduction
Large carnivores are among the most threatened species of the world (Ripple et al., 2014), with especially felids experiencing a significant contraction from their historical range (Wolf and Ripple, 2017). They play an important role in maintaining ecological balance as apex predators (Terborgh et al., 2001) and are under severe threat due to habitat fragmentation, habitat loss and isolation, reduction in genetic diversity, prey depletion and poaching (Morell, 2007; Walston et al., 2010). In addition, their biological traits, e.g. solitary life, and large individual home ranges render them vulnerable to threats associated with increasing human population densities (Cardillo et al., 2004).
The tiger (Panthera tigris) is an endangered species that has lost >95% of its global historical home range (Wolf and Ripple, 2017), and its extant population now exists in fragmented habitats across its former area (Ranganathan et al., 2008). Despite steep declines in population size and habitat, the Indian subcontinent remains a key area for tiger conservation as it harbours around 60% of the current global free-roaming tiger population (Mondol et al., 2009). However, tigers continue to suffer from several anthropogenic threats like poaching, habitat loss and fragmentation (Jhala et al. 2008; Ranganathan et al., 2008; Mondol et al., 2013; Goodrich et al., 2015). Consequentially, most of the tiger populations now occur in protected areas (PAs), which are pockets of habitats embedded in human-dominated landscapes and are usually not big enough to hold demographically viable populations by themselves (Ranganathan et al., 2008). Thus, the status of the tiger remains threatened despite of the various conservation efforts. Recent conservation management strategies focus on landscapes including more than one metapopulation (Wikramanayake et al., 2004). The outcome of these efforts has led to the identification of ‘Tiger Conservation Landscapes’, which include interconnected PAs by corridors that could potentially support viable populations (Dinerstein et al., 2007; Sanderson et al., 2010; Joshi et al., 2013).
At present, India harbours over 1.21 billion people (Census of India 2018) and it is projected to even increase to 1.4 billion people by 2022 (World Population Prospects, UN, 2015). The country has still 21% forest cover (Reddy et al., 2013), but only 5% of this land is protected, which largely resides in human-dominated landscapes. Rural households in India depend on locally available resources from the forests for their domestic needs, which include fuelwood, grazing ground for animals and other non-timber forest produce (Hussain et al., 2016). Human presence usually disturbs wildlife, causing animals to focus on people avoidance, thereby potentially reducing reproductive success (Ciuti et al., 2012). In addition, ecotourism in PAs has substantially increased over the last decade (Reed and Merenlender, 2008), and although these activities generate revenue and provision employment for local communities (Buultjens et al., 2005), there have been concerns over the impact of tourism on conservation goals (Ranaweerage et al., 2015). It has been shown that human disturbance, as well as tourism pressures, can act as potential stressors for wildlife, evoking physiological stress and fitness responses (Zwijacz-Kozica et al., 2012; Hadinger et al., 2015; Coetzee and Chown, 2016).
A perceived stressor induces the release of glucocorticoids, which enables the animal to cope and restore homeostasis. A short-term release of glucocorticoids usually enhances fitness benefits via energy mobilization, but chronically elevated glucocorticoid levels can negatively impact many physiological processes including growth, reproductive success, immunosuppression and muscular atrophy (Wingfield et al., 1998; Charbonnel et al., 2008; French et al., 2010; Hadinger et al., 2015). Prolonged anthropogenic disturbance has been shown to increase glucocorticoid levels in many wild species across taxa including amphibians (Janin et al., 2011), reptiles (Knapp et al., 2013), birds (Wasser et al., 1997) and mammals (Creel et al., 2002, 2013; Van Meter et al., 2009). Prolonged stress can directly affect behaviour, fitness and reproductive success (Young et al., 2006; Kumar et al., 2014) and consequently may lead to an overall population decline (Strasser and Heath, 2013). Although reproduction of both sexes can be affected by stress, females tend to be more sensitive in some species, such as Sumatran and Bengal tiger (Barja et al., 2007, 2011; Narayan et al., 2013a, 2013b; Bhattacharjee et al., 2015). Given the challenges mentioned above, it is crucial to monitor the effect of long-term and persistent anthropogenic mediated stressors in iconic and keystone wildlife species like the tiger.
This study examined the relationship between anthropogenic disturbance and physiological stress levels in two tiger populations in central India by assessing faecal glucocorticoid metabolite (fGCM) concentrations of individual tigers and the status of anthropogenic disturbance of the related reserves during tourism and non-tourism periods. We also examined the differential sensitivity of male and female tigers with reference to anthropogenic disturbance. We hypothesized that individuals in areas under high tourism pressure and proximity to human settlements would perceive more stress reflected in higher fGCM concentrations, with higher stress steroid level found in females compared to males.
Materials and methods
Study sites
Both study sites, Bandhavgarh Tiger Reserve (BTR) and Kanha Tiger Reserve (KTR) (Figs 1 and 2, respectively), are situated in Madhya Pradesh state of central India, which is regarded as a global priority tiger conservation landscape (Sanderson et al., 2010). Both parks have a similar habitat of primarily tropical moist deciduous sal (Shorea robusta) forests. The surrounding landscape is a matrix of forest and human land use habitats. The average rainfall is 1173 mm, most of which occurs between July and October (Sankar et al., 2013).
Figure 1.
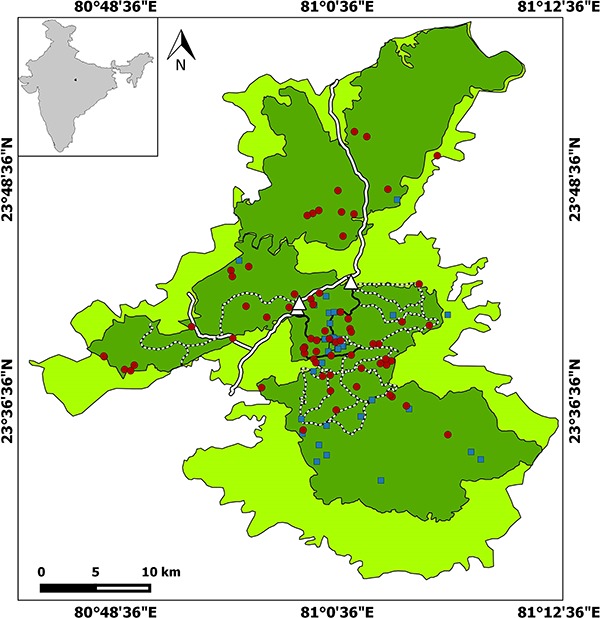
Map showing BTR sample locations and tourist routes (solid black line, ≥51 vehicles per day; dotted line, <50 vehicles per day; white thick line, state highway road). Red round represents samples collected during tourism period, while the blue square represents sample collected during the non-tourism period. Dark green colour in the map is core zone while florescent colour is buffer zone. The white triangle is the entry point to the park.
Figure 2.
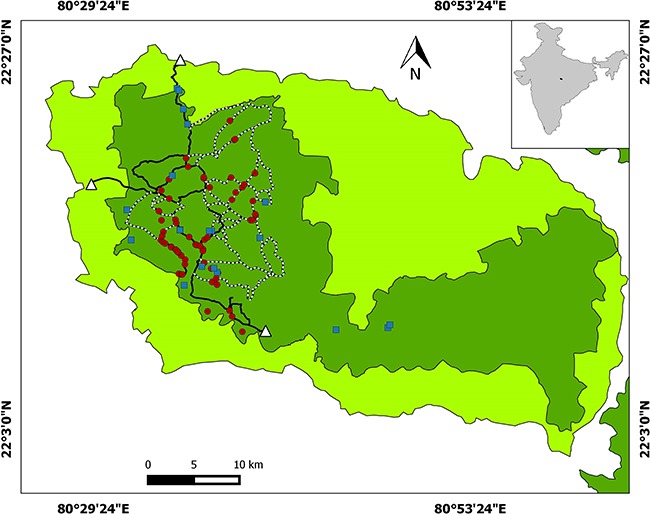
Map showing KTR sample locations and tourist routes (solid line, ≥51 vehicles per day; dotted line, < 50 vehicles per day). Red round represents samples collected during tourism period while the blue square represents sample collected during the non-tourism period. Dark green colour in the map is core zone while fluorescent colour is buffer zone. The white triangle is the entry point to the park.
The multi-use buffer zone of KTR spans around 1134 km2 where human habitation and other anthropogenic activities like cattle grazing are allowed. The buffer zone surrounds a 917 km2 core area of where no anthropogenic activity is permitted except tourism. The reserve consists of two conservation units: Kanha National Park and Phen Wildlife Sanctuary. The core zone of Kanha is inhabited by more than 8000 humans and approximately 7000 cattle. The buffer zone is experiencing further strong anthropogenic pressure by supporting around 129 300 people and more than 85 000 cattle (Miller et al., 2015).
The BTR lies on the north-eastern border of the Madhya Pradesh state and is situated north of KTR. The BTR consists of two PAs: Bandhavgarh National Park and Panphata Wildlife Sanctuary, with a total area of 1598 km2 (716 km2 of core area surrounded by 820 km2 buffer area). There are 15 villages located inside BTR, harbouring a human population of over 60 000 with more than 110 000 livestock (Sankar et al., 2013).
Both reserves, KTR and BTR, support large tiger populations of over 60 individuals each (Dutta et al., 2016), which make them major tiger tourism spots attracting huge numbers of tourists each year. We acquired data from the forest department on the number of tourists and related vehicles entering the park (permission from the Principal Chief Conservator of Forests, Madhya Pradesh letter Reference No. 7616, dated 12 October 2014). The forest department monitors the number of tourists and vehicles at all entry points and permits a maximum of 50 vehicles per day (morning and evening sessions combined) from each entry point. We estimated the mean number of people visited and the mean number of vehicle entered into the parks based on the data obtained from Forest department.
Faecal sample collection
Samples were collected from both reserves between January and March (tourism period) as well as in September (non-tourism period) in 2015. We surveyed pre-existing forest roads and trails to collect faecal material, and only fresh samples, ~1 day old (based on the outline shape, moisture content, smell and insect activity), were collected. Collected faecal samples were split into two portions, for hormone analysis and DNA profiling, respectively. For DNA profiling, samples were sprayed with ethanol and dried using a hot air blower on the same day of collection and then stored with silica beads in zip lock bags. For hormone analysis, the collected samples were partially extracted in the field and then transported to the laboratory (Council for Scientific and Industrial Research—Centre for Cellular and Molecular Biology) for further processing. Each road/trail was sampled only once in 3 days to avoid collection of faecal samples from the same individual and to maximize area coverage. Geographic location using GPS and other information such as signs of the presence of livestock and villagers, woodcutting and lopping and tourist vehicle per day on the particular trail/road were recorded for each sample.
DNA extraction
Faecal material was dried overnight in a hot air oven at 50°C to remove any moisture. DNA extraction was carried out in separate, pre-PCR (Polymerase chain reaction) laboratory space, in a set of n = 11 samples along with an extraction control to monitor the risk of contamination. Faecal surface material was taken for isolation of DNA. Genomic DNA was subsequently extracted using a Qiagen stool kit following the manufacturer’s protocol. The extracted DNA was stored in elution buffer, and DNA quantification was conducted using a NanoDrop spectrometer.
Species confirmation and identification of the sexes
Three tiger-specific mitochondrial markers have been used to ascertain the species identity of collected faecal material. Two tiger-specific primers (Tig490 and Tig509) amplify short fragments from two regions of the NADH5 sub-unit (Mukherjee et al., 2007) and one primer (TIF/TIR) amplifying a short region of the mitochondrial cytochrome b gene were used for species identification (Bhagavatula and Singh, 2006).
Sex was identified using two sets of markers previously designed and standardized for felids (Pilgrim et al., 2005). The two primers amplify the zinc finger (Znf) and amylogenin (Amg) domain on the sex chromosomes, respectively. PCRs were carried out using the protocol as described in the study by Pilgrim et al. (2005). The total reaction volume was 15 μl with 1× BSA, 1× PCR buffer, 0.25 mM of each dNTP, 2.5 mM MgCl2, 0.25 μM each of forward and reverse primers and 0.75 units of Taq polymerase (ExTaq HS DNA polymerase, Takara Bio Inc.). All PCR reactions were carried out with multiple negative controls. Pre- and post-PCR work was carried out at separate places. Visualization of PCR products was done on a 2% agarose gel.
Faecal steroid extraction and analysis
Faecal steroid extraction and fGCM quantification was carried out using earlier described procedures (Umapathy et al., 2013; Bhattacharjee et al., 2015). About 0.2 g of dried and pulverized faecal material was boiled in 5 ml of 90% of ethanol for 20 min. After centrifugation at 500 g for 10 min, the supernatant was decanted and the pellet re-suspended in 5 ml of 90% ethanol, vortexed for 1 min and again centrifuged to recover the supernatant. Both supernatants were combined, dried in an oven at 40°C, re-suspended in 1 ml of absolute methanol by vortexing for 1 min and then stored at −20°C until further processing.
We determined fGCM concentrations in faecal extracts using a cortisol enzyme immunoassay (EIA) (Dr Coralie Munro, University of California, Davis). The cortisol EIA has shown to provide reliable information on adrenocortical function in tigers (Bhattacharjee et al., 2015). The assay was carried out as previously described in Kumar et al. (2014) and Bhattacharjee et al. (2015). Serial dilution of pooled faecal extracts of tigers gave parallel displacement curves to the respective standard curves of the cortisol assay. Assay sensitivity at 90% binding was 0.195 ng/g dried faecal matter. Intra- and inter-assay coefficients of variation determined by repeated measurements of quality controls were 4.25% and 7.49%, respectively.
Estimating anthropogenic disturbance
To estimate the anthropogenic disturbance due to tourism, we obtained information from the forest department about the number of vehicles entering the park daily. In both reserves, there are multiple routes and entry points for tourist vehicles. Sample locations were characterized as high, moderate, less and no disturbance based on the presence of livestock and villagers, wood cutting and lopping and vehicular movements (Bhattacharjee et al., 2015). In BTR, the number samples collected were 40 (from no or less disturbance area), 19 (low disturbance), 35 (moderate disturbance) and 20 (high disturbance), while in KTR, the number samples were 21, 8, 45 and 17 from no or less, low, moderate and high disturbance areas, respectively.
Data analysis
fGCM concentrations are given as ng/g faecal dry weight (DW) with respective hormone values being presented as mean and standard error. Since our data were not normally distributed, we used non-parametric tests for analyses. Mann–Whitney U test (M–W test) was used to test for differences in fGCM concentrations between the two seasons (tourism, October–June; non-tourism, July–September) as well as the two reserves (BTR and KTR). A generalized linear model (GLM) was used to examine variations in fGCM concentrations with reference to various factors (sex, season, anthropogenic disturbance level and location—buffer and core) as the explanatory variables, which included both continuous and categorical data, and the response variable was continuous data. Pearson correlation was used to examine the relationship between mean number of vehicles traversed per day and fGCM concentration of tigers in that location. A data analysis was carried out using SPSS ver 17.1.
Results
Tourists and vehicles
In total, 244 179 people visited both BTR (106 535) and KTR (137 644) during the 9 months of tourism season (October 2014–June 2015), with an average of 395 people/day in BTR and 509 people/day in KTR. To travel inside the parks, 51 695 vehicles have been used in total (BTR, 23 011; KTR, 28 684) during the study with an average of 85 and 106 vehicles/day in BTR and KTR, respectively.
Sample collection and DNA profiling
We collected 341 suspected tiger faecal samples in total, of which 206 samples (BTR, 114; KTR, 92) were identified to be samples from tigers. Of the 114 samples collected from BTR, 91 (41 from males; 50 from females) were collected between January and March (tourism period) and 23 (11 from males; 12 from females) samples during September 2015 (non-tourism period). In KTR, 75 (43 from males; 32 from females) samples were collected between January and March) and 17 (13 from males; 4 from females) samples during September 2015.
GCM concentration in faecal samples
Overall mean fGCM concentrations of tigers roaming at BTR (51.45 ± 4.75 ng/g DW) and KTR (56.46 ± 6.6 ng/g DW) were not significantly different (M–W test, n = 206, P = 0.87).
We found significantly higher fGCM concentrations in tigers at BTR during the tourism period (56.47 ± 5.81 ng/g DW, n = 91) compared to the non-tourism period (32.69 ± 2.76 ng/g DW, n = 23; M–W test, n = 114, P = 0.001; Fig. 3a). Similarly, fGCM concentrations showed a positive correlation with the number of vehicles visited per day during the tourism period (r = 0.34; P = 0.001; n = 91). There were no significant differences in fGCM concentrations between the sexes during tourism and non-tourism period (M–W test, n = 91, P = 0.15 and n = 23, P = 0.56, respectively; Fig. 4a). GLM results showed that fGCM concentrations are significantly influenced by tourism season (F1 = 4.710; P = 0.032), number of vehicles (F4 = 3.97; P = 0.010) and disturbance level (F3 = 6.62; P = 0.0001; Fig. 5). Sex and sample location (core and buffer) did not influence fGCM concentrations determined during the study period (GLM F1 = 0.13; P = 0.60 and F1 = 0.033; P = 0.75, respectively).
Figure 3.
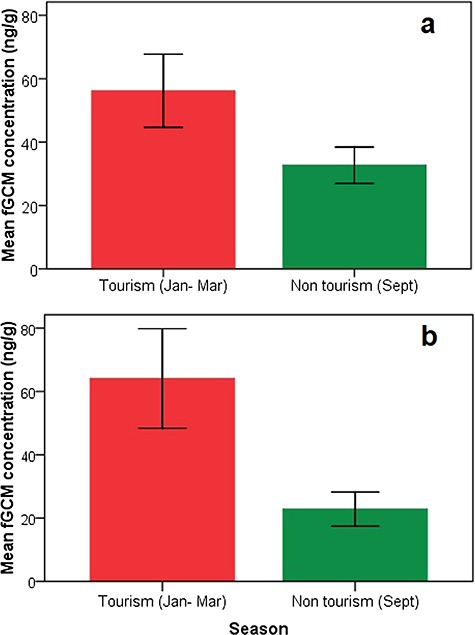
(a and b) Mean (±SEM) fGCM concentrations in tigers during tourism period (January–March) and non-tourism (September) in BTR and KTR
Figure 4.
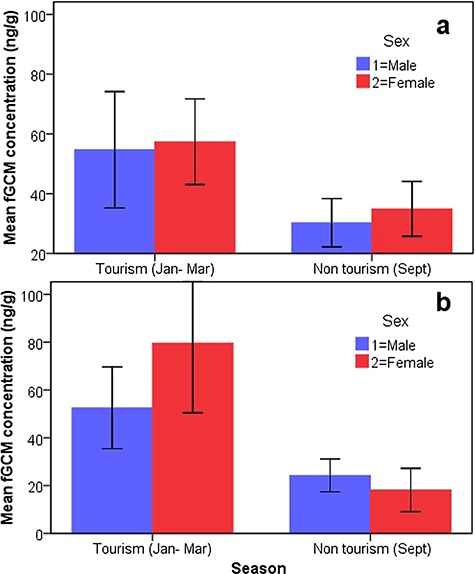
(a and b) Mean (±SEM) fGCM concentrations between tourism and non-tourism seasons among male and female in BTR (a) and KTR (b)
Figure 5.
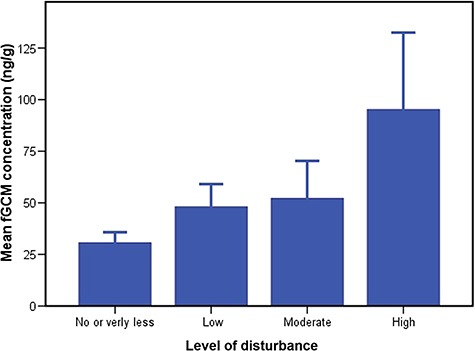
Mean (±SEM) fGCM concentrations in tigers with reference to disturbance level including high vehicular movement in BTR
Similarly, we found significantly higher fGCM concentrations in tigers at KTR during the tourism period (64.09 ± 7.88 ng/g DW; n = 92) compared to the non-tourism period (22.82 ± 2.54 ng/g DW; n = 17; M–W test, n = 109, P = 0.001; Fig. 3b). For the tourism as well as non-tourism period, no significant differences in fGCM concentrations were found between the sexes (M–W test, n = 92, P = 0.24 and n = 17, P = 0.29, respectively; Fig. 4b). A GLM analysis showed that fGCM concentrations are significantly influenced by tourism (F1 = 10.07; P = 0.001) but not by sex (F1 = 0.130; P = 0.27), disturbance level (F3 = 0.011; P = 0.57), number of vehicles (F3 = 0.09; P = 0.62) or location (F1 = 0.044; P = 0.85).
Discussion
Understanding the impact of anthropogenic stressors on tiger populations can provide valuable information for optimizing conservation and management strategies. Our study showed that wildlife tourism can cause distinct physiological stress in tigers in PAs. A significant positive correlation was observed between fGCM concentrations and the number of vehicles visiting BTR. These results are concordant with results of other studies on various wildlife species. Bhattacharjee et al. (2015) demonstrated that reintroduced tigers show high fGCM levels when challenged by anthropogenic disturbance such as traffic, human encounters and manned livestock. Other researchers have demonstrated that the use of snowmobiles increased fGCM levels in elk (Cervus elephus) and wolves (Canis lupus) (Creel et al., 2002). Similarly, increasing fGCM concentrations have been found in relation to anthropogenic disturbance, e.g. for African lions (Panthera leo) roaming within a human-dominated buffer zone (Creel et al., 2013), spotted hyenas (Crocuta crocuta) occurring in disturbed areas of a National Reserve (Van Meter et al., 2009) or free-roaming European pine martens (Martens martens) occurring near tourist areas in a natural park (Barja et al., 2007).
Perception of prolonged stress is known to affect survival and reproduction by influencing the immune system and increase susceptibility to diseases (Munck et al., 1984, Arlettaz et al. 2007). One of our previous studies has demonstrated that recently introduced tigers failed to reproduce effectively presumably due to high levels of stress caused by high anthropogenic disturbance (Bhattacharjee et al., 2015). Although some individuals might adjust to the presence of humans, the overall pattern of increased fGCM concentrations found in this study clearly indicate that tourism can elevate physiological stress in tigers, which may affect the reproductive potential on a population level. Although we cannot exclude the possibility of a potential impact of reproductive state or age on fGCM output, our study did not find any significant difference in fGCM concentrations between the sexes. Thus, our findings are more likely related to the anthropogenic disturbances described rather than potential sex- or reproductive status-biassed stress (Creel et al., 2013; Narayan et al., 2013a; Webster et al., 2018).
Current guidelines from the National Tiger Conservation Authority (NTCA) limit tourism activities to 20% of the core area and restricting vehicle access to 40 cars per day in an Indian reserve (NTCA management plan 2010). Although we were unable to exactly estimate the percentage of core area used for tourism at our study sites; it seems often impacted beyond the recommended 20% of the core area in BTR (see Fig. 1), and the number of vehicles entering BTR also exceeds the recommended number according to a respective management document (National Tiger Conservation Authority, 2010). Similarly, KTR management has permitted an average of 106 vehicles (officially recorded) per day against the 40 car per day recommended by the NTCA. Furthermore, recommended distances between vehicles is not often followed during a tiger sighting, which leads to an over-crowding of vehicles around the animal (pers. obs.). This behaviour might directly affect the territorial and mating behaviour of tigers, resulting in an overall lower reproductive success due to increased stress e.g. for tigers (Bhattacharjee et al., 2015) and wild cats (Piñeiro et al., 2012). Since carnivores occur in low densities, changes in reproductive success and survival rate of especially adult females can severely affect the sustainability of isolated populations (Knight and Eberhardt, 1985; Smith and Mcdougal, 1991; Kerley et al., 2002). Overall, such disturbance can have severe implications on the survival of wildlife populations, especially of tigers, which are facing the multi-dimensional threat to their existence in an increasing human-modified landscape.
We demonstrate that tourism and thus anthropogenic disturbance are correlated with fGCM concentrations of tigers in both monitored reserves. Although both reserves experience a similar tourism pressure, the stronger correlation found in BTR might be attributed to the comparatively higher number of human settlements and cattle densities in and around the reserve. Since the tigers at BTR are genetically less connected to other populations as the ones at KTR (Yumnam et al., 2014; Thatte et al., 2018), conservation efforts should even focus on the BTR population. However, as our study only provides a snapshot of the effects of anthropogenic disturbance on tiger, long-term, individual-based studies with greater spatial and temporal sampling would be crucial to better understand the adverse effects of anthropogenic stressors on the physiology of this keystone species.
Our management recommendations include strict regulation of vehicular traffic and number of tourist vehicle, shifting of artificial waterholes away from tourist roads and reducing other anthropogenic disturbance, including relocation of villages from the core area of a tiger reserve.
Acknowledgements
We thank Madhya Pradesh Forest Department for permissions to conduct research and Dr Sankar and his team of Wildlife Institute of India for local logistics help during sample collection in BTR. Conceived and designed the study: G.U., A.G. and S.N.; data and field sample collections: A.T., S.K., V.K. and M.R.; laboratory analysis: S.K., A.T. and V.K.; data analysis: G.U., A.T. and V.K.; wrote manuscript: G.U., A.T. and A.G.
Ethical approval and permits
Our study does not involve any experiments with live animals, and all (faecal) samples were collected non-invasively; consequently, no ethical clearance is required. However, we have obtained necessary permission to enter the forest, collect data and faecal samples from Forest Department of Madhya Pradesh (Ref. no. 2351 dated 14 April 2013).
Funding
The study was supported by the International Collaboration Division, Department of Science and Technology, Government of India (Ref. No. INT/RUS/RFBR/P-245 to G.U), Council for Scientific and Industrial Research (to G.U) and Russian Foundation for Basic Research (Ref. No. 16-54-45017 to S.N.).
References
- Arlettaz R, Patthey P, Baltic M, Leu T, Schaub M, Palme R, Jenni-Eiermann S (2007) Spreading free-riding snow sports represent a novel serious threat for wildlife. Proc Biol Sci 274: 1219–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barja I, Silván G, Martínez-Fernández L, Illera JC (2011) Physiological stress responses, fecal marking behavior, and reproduction in wild European pine martens (Martes martes). J Chem Ecol 37: 253–259. [DOI] [PubMed] [Google Scholar]
- Barja I, Silván G, Rosellini S, Piñeiro A, González-Gil A, Camacho L, Illera JC (2007) Stress physiological responses to tourist pressure in a wild population of European pine marten. J Steroid Biochem Mol Biol 104: 136–142. [DOI] [PubMed] [Google Scholar]
- Bhagavatula J, Singh L (2006) Genotyping faecal samples of Bengal tiger Panthera tigris tigris for population estimation: a pilot study. BMC Genet 7: 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharjee S, Kumar V, Chandrasekhar M, Malviya M, Ganswindt A, Ramesh K, Sankar K, Umapathy G (2015) Glucocorticoid stress responses of reintroduced tigers in relation to anthropogenic disturbance in Sariska Tiger Reserve in India. PLoS One 10: e0127626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buultjens J, Ratnayake I, Gnanapala A, Aslam M (2005) Tourism and its implications for management in Ruhuna National Park (Yala), Sri Lanka. Tour Manag 26:733–742. [Google Scholar]
- Cardillo M, Purvis A, Sechrest W, Gittleman JL, Bielby J, Mace GM (2004) Human population density and extinction risk in the world’s carnivores. PLoS Biol 2: e197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Census of India Website: Office of the Registrar General and Census Commissioner, India (2018) http://www.censusindia.gov.in/2011-prov-results/paper2-vol2/data_files/India2/Table_1_PR_Districts_TRU.pdf (last visited on 12 April 2019).
- Charbonnel N, Chaval Y, Berthier K, Deter J, Morand S, Palme R, Cosson J-F (2008) Stress and demographic decline: a potential effect mediated by impairment of reproduction and immune function in cyclic vole populations. Physiol Biochem Zool 81: 63–73. [DOI] [PubMed] [Google Scholar]
- Ciuti S, Northrup JM, Muhly TB, Simi S, Musiani M, Pitt JA, Boyce MS (2012) Effects of humans on behaviour of wildlife exceed those of natural predators in a landscape of fear. PLoS One 7: e50611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coetzee BWT, Chown SL (2016) A meta-analysis of human disturbance impacts on Antarctic wildlife. Biol Rev Camb Philos Soc 91: 578–596. [DOI] [PubMed] [Google Scholar]
- Creel S, Christianson D, Schuette P (2013) Glucocorticoid stress responses of lions in relationship to group composition, human land use, and proximity to people. Conserv Physiol 1: doi: 10.1093/conphys/cot021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creel S, Fox JE, Hardy A, Sands J, Garrott B, Peterson RO (2002) Snowmobile activity and glucocorticoid stress responses in wolves and elk. Conserv Biol 16: 809–814. [Google Scholar]
- Dinerstein E, et al. (2007) The fate of wild tigers. BioScience 57: 508–514. [Google Scholar]
- Dutta T, Sharma S, McRae BH, Roy PS, DeFries R (2016) Connecting the dots: mapping habitat connectivity for tigers in central India. Reg Environ Change 16: 53–67. [Google Scholar]
- French SS, DeNardo DF, Greives TJ, Strand CR, Demas GE (2010) Human disturbance alters endocrine and immune responses in the Galapagos marine iguana (Amblyrhynchus cristatus). Horm Behav 58: 792–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodrich J, Lynam A, Miquelle D, Wibisono H, Kawanishi K, Pattanavibool A, Htun S, Tempa T, Karki J, Jhala Y et al. (2015) Panthera tigris. The IUCN Red List of Threatened Species 2015: e.T15955A50659951. https://www.iucnredlist.org/species/15955/50659951.
- Hadinger U, Haymerle A, Knauer F, Schwarzenberger F, Walzer C (2015) Faecal cortisol metabolites to assess stress in wildlife: evaluation of a field method in free-ranging chamois. Methods Ecol Evol 6: 1349–1357. [Google Scholar]
- Hussain A, Dasgupta S, Bargali HS (2016) Fuelwood consumption patterns by semi-nomadic pastoralist community and its implication on conservation of Corbett Tiger Reserve, India. Energy Ecol Environ 1: 49–59. [Google Scholar]
- Janin A, Léna J-P, Joly P (2011) Beyond occurrence: body condition and stress hormone as integrative indicators of habitat availability and fragmentation in the common toad. Biol Conserv 144: 1008–1016. [Google Scholar]
- Jhala YV, Gopal R, Qureshi Q (2008) Status of the tigers, co-predators, and prey in India. Dehradun: National Tiger Conservation Authority, government of India, New Delhi and wildlife Institute of India. https://projecttiger.nic.in/WriteReadData/PublicationFile/Tiger_Status_oct_2010.pdf (last visited on 27/12/2018).
- Joshi A, Vaidyanathan S, Mondol S, Edgaonkar A, Ramakrishnan U (2013) Connectivity of tiger (Panthera tigris) populations in the human-influenced forest mosaic of central India. PLOS ONE 8: e77980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerley LL, Goodrich JM, Miquelle DG, Smirnov EN, Quigley HB, Hornocker MG (2002) Effects of roads and human disturbance on Amur tigers. Conserv Biol 16: 97–108. [DOI] [PubMed] [Google Scholar]
- Knapp CR, Hines KN, Zachariah TT, Perez-Heydrich C, Iverson JB, Buckner SD, Halach SC, Lattin CR, Romero LM (2013) Physiological effects of tourism and associated food provisioning in an endangered iguana. Conserv Physiol 1: doi: 10.1093/conphys/cot032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight RR, Eberhardt LL (1985) Population dynamics of Yellowstone grizzly bears. Ecology 66: 12. [Google Scholar]
- Kumar V, Palugulla Reddy V, Kokkiligadda A, Shivaji S, Umapathy G (2014) Non-invasive assessment of reproductive status and stress in captive Asian elephants in three south Indian zoos. Gen Comp Endocrinol 201: 37–44. [DOI] [PubMed] [Google Scholar]
- Miller JRB, Jhala YV, Jena J, Schmitz OJ (2015) Landscape-scale accessibility of livestock to tigers: implications of spatial grain for modeling predation risk to mitigate human–carnivore conflict. Ecol Evol 5: 1354–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondol S, Bruford MW, Ramakrishnan U (2013) Demographic loss, genetic structure and the conservation implications for Indian tigers. Proc Biol Sci 280. doi: 10.1098/rspb.2013.0496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondol S, Karanth KU, Kumar NS, Gopalaswamy AM, Andheria A, Ramakrishnan U (2009) Evaluation of non-invasive genetic sampling methods for estimating tiger population size. Biol Conserv 142: 2350–2360. [Google Scholar]
- Morell V. (2007) Wildlife biology. Can the wild tiger survive? Science 317: 1312–1314. [DOI] [PubMed] [Google Scholar]
- Mukherjee N, Mondol S, Andheria A, Ramakrishnan U (2007) Rapid multiplex PCR based species identification of wild tigers using non-invasive samples. Conserv Genet 8: 1465–1470. [Google Scholar]
- Munck A, Guyre PM, Holbrook NJ (1984) Physiological functions of glucocorticoids in stress and their relation to pharmacological actions. Endocr Rev 5: 25–44. [DOI] [PubMed] [Google Scholar]
- Narayan EJ, Parnell T, Clark G, Martin-Vegue P, Mucci A, Hero J-M (2013a) Faecal cortisol metabolites in Bengal (Panthera tigris tigris) and Sumatran tigers (Panthera tigris sumatrae). Gen Comp Endocrinol 194: 318–325. [DOI] [PubMed] [Google Scholar]
- Narayan EJ, Webster K, Nicolson V, Mucci A, Hero J-M (2013b) Non-invasive evaluation of physiological stress in an iconic Australian marsupial: the koala (Phascolarctos cinereus). Gen Comp Endocrinol 187: 39–47. [DOI] [PubMed] [Google Scholar]
- National Tiger Conservation Authority (2010) Compendium of guidelines/advisories/gazetted notifications. National Tiger Conservation Authority. https://projecttiger.nic.in/WriteReadData/PublicationFile/Compendium(2).pdf (last visited on 27 December 2018)
- Pilgrim KL, Mckelvey KS, Riddle AE, Schwartz MK (2005) Felid sex identification based on noninvasive genetic samples. Mol Ecol Notes 5: 60–61. [Google Scholar]
- Piñeiro A, Barja I, Silván G, Illera JC (2012) Effects of tourist pressure and reproduction on physiological stress response in wildcats: management implications for species conservation. Wildl Res 39: 532–539. [Google Scholar]
- Ranaweerage E, Ranjeewa ADG, Sugimoto K (2015) Tourism-induced disturbance of wildlife in protected areas: a case study of free ranging elephants in Sri Lanka. Glob Ecol Conserv 4: 625–631. [Google Scholar]
- Ranganathan J, Chan KMA, Karanth KU, Smith JL (2008) Where can tigers persist in the future? A landscape-scale, density-based population model for the Indian subcontinent. Biol Conserv 141: 67–77. [Google Scholar]
- Reddy CS, Sreelekshmi S, Jha CS, Dadhwal VK (2013) National assessment of forest fragmentation in India: landscape indices as measures of the effects of fragmentation and forest cover change. Ecol Eng 60: 453–464. [Google Scholar]
- Reed SE, Merenlender AM (2008) Quiet, nonconsumptive recreation reduces protected area effectiveness. Conserv Lett 1: 146–154. [Google Scholar]
- Ripple WJ, et al. (2014) Status and ecological effects of the world’s largest carnivores. Science 343: 1241484. [DOI] [PubMed] [Google Scholar]
- Sanderson EW, et al. (2010) Setting priorities for tiger conservation In Tigers of the World. Academic press, Massachusetts, USA, pp. 143–161. [Google Scholar]
- Sankar K, Pabla HS, Patil CK, Nigam P, Qureshi Q, Navaneethan B, Manjreakar M, Virkar PS, Mondal K (2013) Home range, habitat use and food habits of re-introduced gaur (Bos gaurus gaurus) in Bandhavgarh Tiger Reserve, central India. Trop Conserv Sci 6: 50–69. [Google Scholar]
- Smith JLD, Mcdougal C (1991) The contribution of variance in lifetime reproduction to effective population size in tigers. Conserv Biol 5: 484–490. [Google Scholar]
- Strasser EH, Heath JA (2013) Reproductive failure of a human-tolerant species, the American kestrel, is associated with stress and human disturbance. J Appl Ecol 50: 912–919. [Google Scholar]
- Terborgh J, et al. (2001) Ecological meltdown in predator-free forest fragments. Science 294: 1923–1926. [DOI] [PubMed] [Google Scholar]
- Thatte P, Joshi A, Vaidyanathan S, Landguth E, Ramakrishnan U (2018) Maintaining tiger connectivity and minimizing extinction into the next century: insights from landscape genetics and spatially-explicit simulations. Biol Conserv 218: 181–191. [Google Scholar]
- Umapathy G, Kumar V, Wasimuddin KM, Shivaji S (2013) Detection of pregnancy and fertility status in big cats using an enzyme immunoassay based on 5α-pregnan-3α-ol-20-one. Gen Comp Endocrinol 180: 33–38. [DOI] [PubMed] [Google Scholar]
- Van Meter PE, French JA, Dloniak SM, Watts HE, Kolowski JM, Holekamp KE (2009) Fecal glucocorticoids reflect socio-ecological and anthropogenic stressors in the lives of wild spotted hyenas. Horm Behav 55: 329–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walston J, et al. (2010) Bringing the tiger back from the brink—the six percent solution. PLoS Biol 8: e1000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasser SK, Bevis K, King G, Hanson E (1997) Noninvasive physiological measures of disturbance in the northern spotted owl. Conserv Biol 11: 1019–1022. [Google Scholar]
- Webster AB, Burroughs RBJ, Laver P, Ganswindt A (2018) Non-invasive assessment of adrenocortical activity as a measure of stress in leopards (Panthera pardus). Afr Zool 53: 53–60. [Google Scholar]
- Wikramanayake E, McKnight M, Dinerstein E, Joshi A, Gurung B, Smith D (2004) Designing a conservation landscape for tigers in human-dominated environments. Conserv Biol 18: 839–844. [Google Scholar]
- Wingfield JC, Maney DL, Breuner CW, Jacobs JD, Lynn S, Ramenofsky M, Richardson RD (1998) Ecological bases of hormone–behavior interactions: the “emergency life history stage”. Am Zool 38: 191–206. [Google Scholar]
- Wolf C, Ripple WJ (2017) Range contractions of the world’s large carnivores. R Soc Open Sci 4: 170052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Population Prospects, UN (2015) http://www.un.org/en/development/desa/publications/world-population-prospects-2015-revision.html(last visited on 22 December 2018).
- Young AJ, Carlson AA, Monfort SL, Russell AF, Bennett NC, Clutton-Brock T (2006) Stress and the suppression of subordinate reproduction in cooperatively breeding meerkats. Proc Natl Acad Sci USA 103: 12005–12010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yumnam B, Jhala YV, Qureshi Q, Maldonado JE, Gopal R, Saini S, Srinivas Y, Fleischer RC (2014) Prioritizing Tiger conservation through landscape genetics and habitat linkages. PLoS One 9: e111207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwijacz-Kozica T, Selva N, Barja I, Silván G, Martínez-Fernández L, Illera JC, Jodłowski M (2012) Concentration of fecal cortisol metabolites in chamois in relation to tourist pressure in Tatra National Park (South Poland). Acta Theriol (Warsz) 58: 215–222. [Google Scholar]


