Abstract
Background:
No studies of the prevalence of one of the most common movement disorders, essential tremor (ET), have been undertaken in the Faroe Islands. Given the potential for founder effects in the Islands, and the highly genetic nature of ET, the Faroe Islands provide a particularly interesting setting in which to study the prevalence of ET.
Objective:
To estimate the prevalence of ET and study its characteristics.
Methods:
We used a two-phase, population-based design, screening 1,328 randomly-selected Faroese individuals aged ≥40 years. A subsample of 282 individuals who had returned the spirals and questionnaire was selected to participate in an in-person clinical evaluation. Tremor was systematically quantified by a senior movement disorder neurologist with particular specialization in tremor using a reliable and valid clinical rating scale followed by the application of rigorous diagnostic criteria used by tremor investigators internationally.
Results:
The overall crude prevalence was 2.9%. The age-adjusted prevalence was 3.1%. There was an age-associated rise in prevalence; by age ≥70, prevalence reached 4.80%. Twenty-six of 27 (96.2%) were previously undiagnosed.
Conclusions:
This is the first population based-study of the prevalence of ET in the Faroe Islands. The estimated prevalence was similar to studies using the same or comparable methodologies.
Keywords: essential tremor, prevalence, two-phase population-based design, Faroe Islands
1. Introduction
Essential tremor (ET) is among the most common neurological diseases and the most common tremor disorder. Estimates of the prevalence of ET have been derived from diverse population settings in Africa, Europe, Asia and the Americas. In a meta-analysis, the prevalence was estimated to be 4.6% in the population age ≥ 65 years[1], and this increased with age, and especially with advanced age. Establishing a precise national prevalence estimate is important as knowledge about prevalence is of crucial importance to inform national policy development, planning and costs of health services. Additionally, subgroup differences in prevalence can offer initial clues about the existence of environmental or underlying biological factors that might be of etiological or mechanistic importance. Finally is it important for investigators to be able to confidently identify and count cases before they can study them further[1].
The hallmark feature of ET is kinetic tremor, which may be associated with varying degrees of functional disability[2–4]. The etiology of ET is complex; both genetic and environmental factors are likely contributors[5–9].
The Faroe Islands are located in the North Atlantic Ocean, between Norway and Iceland and are inhabited by 49,121 individuals of whom 24,154 are age 40 or older (January 1, 2016). Due to the isolated geographic location and homogenous population[10], the Faroe Islands have been the focus of epidemiological investigations of a number of neurological disorders. The prevalence of several movement disorders, Parkinson’s disease (PD)[11, 12] and primary focal dystonia[13], are high while multiple sclerosis[14, 15] and amyotrophic lateral sclerosis (ALS)[16] are comparable to other European countries. Yet curiously, no studies of prevalence of one of the most common neurological disorders, ET, have been undertaken in the Faroe Islands. Given the potential for founder effects in the Islands, and the highly genetic nature of ET[17], the Faroe Islands provide a particularly interesting setting in which to study the prevalence of ET. Thus, our aim was to estimate the prevalence of ET in a population-based sample in the Faroe Islands and to study the characteristics of ET in that population.
2. Methods
2.1. Study Population and Sampling Frame
We used a two-phase, population-based study design. The study was undertaken between August 2016 and December 2017. From the 24,154 individuals aged ≥40 years living in the Faroe Islands, the names and addresses of 4,798 individuals aged ≥40 were obtained from the Faroese Population Registry. These individuals were selected based on six randomly-selected birthdates (10th, 16th, 17th, 18th, 22th, and 30th). From this group, all 1,155 individuals aged ≥70 years were selected into the screening group, while the remaining 1,845 were selected by random sampling using SPSS. Thus, the screening group comprised 3,000 Faroese individuals aged ≥40 years (Fig. 1).
Fig. 1.
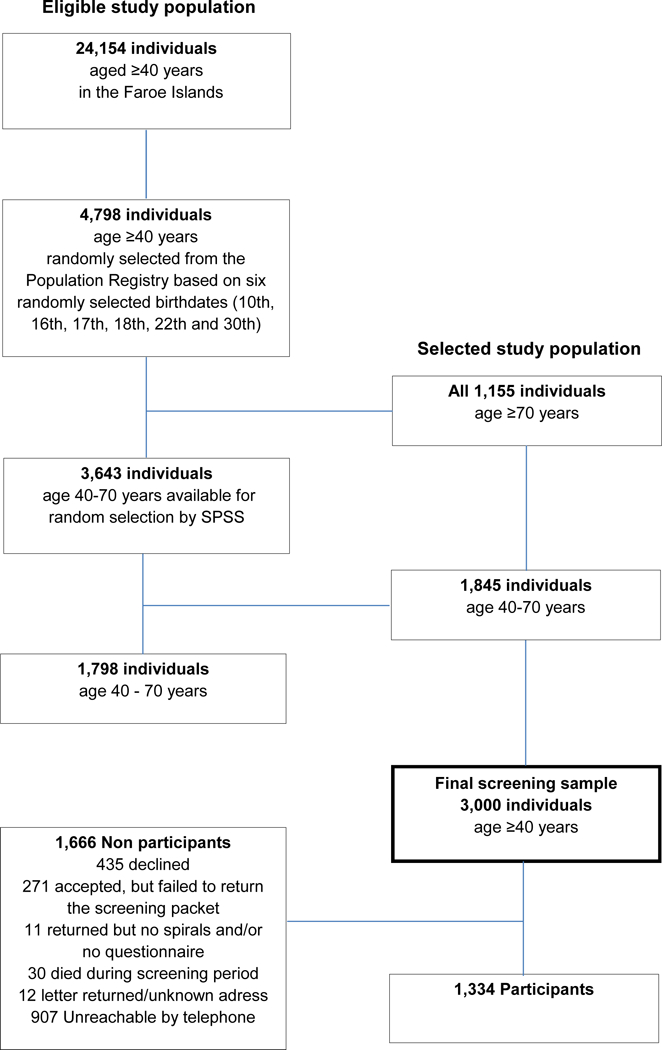
Selection of screening populations from 24,154 individuals, age 40 and older. 3,000 were selected for the final screening sample, of whom 1334 participated.
2.2. First Phase Screening
In the first screening phase (September 2016 - December 2016), these 3,000 individuals received an invitation letter to participate in a study of lifestyle, diet and neurological conditions. The invitation letter included a screening package that comprised questionnaires and a request for hand-drawn spirals, and a return stamped envelope. The questionnaires included five demographic questions, one question regarding smoking status, one question about years since most recent hospitalization, one question about handedness, and seven screening questions for tremor (e.g., “do you often have shaking or tremor that you cańt control”, “has a doctor diagnosed you as having familial tremor or benign essential tremor”, “does your head often shake uncontrollably”).
The letter included instructions on how to draw two Archimedes spirals with each hand and four blank sheets of paper. As described in prior population-based settings [18–20], screenees were instructed to draw each spiral freely on a blank, standard 8.5 × 11-inch sheet of paper using a ballpoint pen, while seated at a table. They were instructed to center the paper at right angles horizontally directly in front of them and start at the center of the page, without lifting their pen. Prior to drawing the spirals, the participants were required to answer five questions about their use of caffeinated coffee, tea, and soda, cigarette smoking and the use of an asthma inhalator on that day, as each may produce or exacerbate tremor[20].
After one month, a reminder telephone call was made to individuals who had not returned their screening package. A total of 1,334 (44.5%) individuals returned the completed screening package. Of the remaining 1,666 individuals 435 (26.1%) declined participation, 271 (16.3%) accepted participation but failed to return the screening packet,11 (0.7%) returned the screening packet with no spiral drawings and/or no questionnaire, 30 (1.8%) died during the screening period, 12 (0.7%) letters were returned stating unknown address and 907 (54.4%) were unreachable by telephone (e.g. not at home/no answer or telephone number was not available) (Fig. 1).
Tremor on each spiral was rated by a senior movement disorders neurologist (E.D.L) who used an ordinal clinical rating scale (0 – 3.0), which included ratings of 0 (none), 0.5 (very mild), 1.0 (mild), 1.5 (mild-to-moderate), 2.0 (moderate), and 3.0 (severe), as used in prior epidemiological studies[21]. Based on data from the questionnaire and spiral scores, the participants were stratified into four groups: (1) those with a high likelihood of having ET (e.g., spiral ratings ≥1.5 on one or more dominant hand spirals or having been diagnosed previously with ET or having endorsed “head tremor” on the questionnaire), (2) those with an intermediate likelihood of having ET (e.g., both non-dominant hand spiral ratings ≥1.5), (3) those with a low-intermediate likelihood of having ET (e.g., a single non-dominant arm spiral rating ≥1.5) and (4) those with a low likelihood of having ET (e.g., all spiral ratings ≤1). Hand dominance was a factor in this stratification scheme because in conditions such as enhanced physiological tremor, tremor occurs to a greater extent in the non-dominant than dominant hand[22]. Hence, mild or mild-to-moderate non-dominant hand tremor could be the result of enhanced physiological tremor than ET.
2.3. Second Phase
In the second phase, a subsample of 282 individuals who had returned the spirals and questionnaire was invited to participate in an in-person clinical evaluation at the Department of Occupational Medicine and Public Health or in their own homes. All 85 individuals in group 1 were invited. Also, a randomly-selected subsample of 197 individuals in group 2 (n=97), group 3 (n=32) or group 4 (n=68) were also invited. A total of 227 (80.5%) individuals accepted and completed the in-person clinical evaluation, while 54 declined participation and one person had died. The participation rate was 78% (group 1), 85% (group 2), 84% (group 3), and 76% (group 4) (Fig. 2).
Fig. 2.
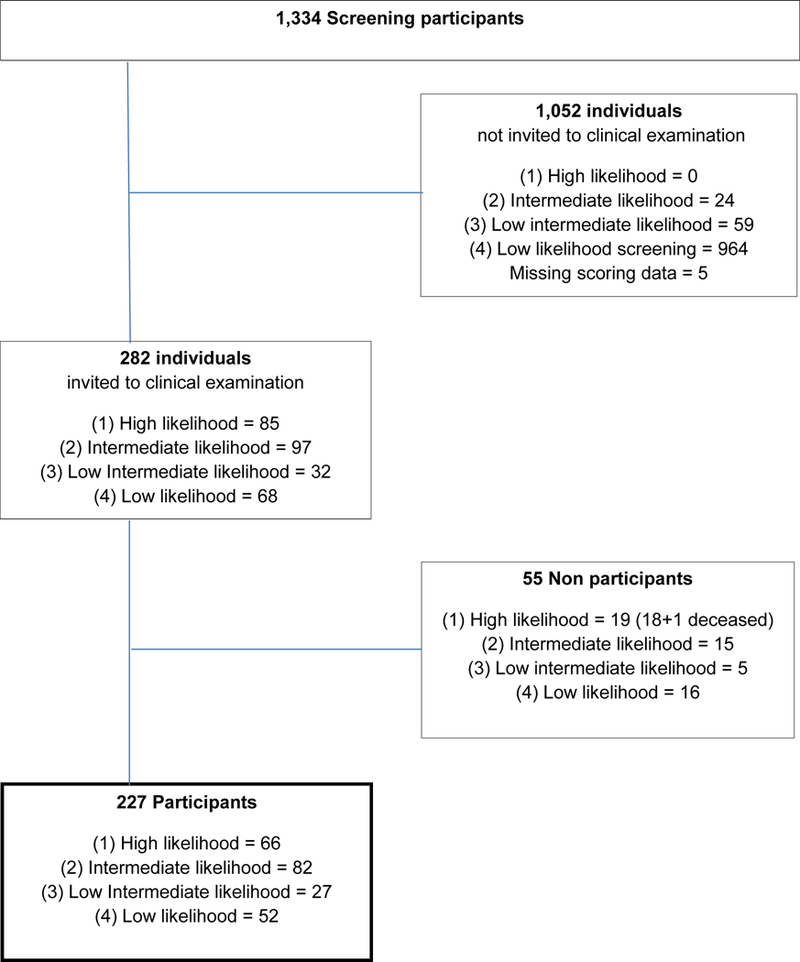
Selection of individuals for in-person clinical evaluation from 1334 screenees, age 40 and older, of whom 227 participated.
The in-person clinical evaluation was conducted by a trained nurse (E.H.E.) and included 1) anthropometric measures (body weight [kilograms] and height [cm]), 2) a questionnaire encompassing demographic data and data on medication usage, smoking habits, ethanol intake, tremor, and family history of tremor, and 3) a detailed videotaped tremor examination. The videotaped tremor examination included (1) an ET-specific metric comprised of one test for postural tremor and five for kinetic tremor (e.g., pouring, drinking) performed with each arm (12 tests total, with total tremor score = 0 – 36)[23], (2) the motor portion of the Unified Parkinson’s Disease Rating Scale (UPDRS)[24] excluding an assessment of rigidity, and (3) an assessment of dystonia.
2.4. Diagnosis of ET and Other Neurological Conditions
ET diagnoses were assigned by E.D.L. by review of questionnaire data and the videotaped neurological examination and based on published diagnostic criteria (moderate or greater amplitude kinetic tremor during three or more activities or a head tremor in the absence of PD or another known cause [e.g., medication-induced tremor, tremor from hyperthyroidism])[25]. These diagnostic criteria for ET were developed for a population-based genetic study and based on data from approximately 2,000 normal (non-diseased controls)[25] the criteria carefully specify the specific examination maneuvers during which tremor should be present and the severity of tremor that should be evident during these maneuvers to distinguish normal from ET. These criteria have been shown to be both reliable[26] and valid[27], and have been used by tremor investigators in the United States and internationally[28–39]. As in prior reports, borderline tremor was a diagnosis assigned to individuals who did not fully meet strict diagnostic criteria for ET (defined above) but were nonetheless considered by E.D.L. to have clinical features that aligned them more with ET than normal[23, 40]. The diagnosis of dystonia was confirmed using published diagnostic criteria[41], as was the diagnosis of PD[42].
2.5. Statistical Analyses
Differences between the ET and non-ET group were tested with Student’s t-test for continuous variables and when evaluating categorical variables, chi square test or Fischer’s exact test was used. Body mass index (BMI) was calculated by dividing weight [kilograms] by squared height [meters].
Based on the number of ET cases diagnosed clinically, we standardized the proportions in each stratification group to the screening population using a direct standardization method and calculated the prevalence stratified by gender and age group: 40–49 years, 50–59 years, 60–69 years and ≥70 years.
To estimate the overall prevalence in the Faroe Islands, the prevalence estimates from the screening group were adjusted by age and gender with regard to the Faroese population aged ≥ 40 years using a direct standardization method. Statistical analyses were performed using SPSS version 24.0.
3. Results
3.1. Characteristics of Screened Population
A total of 1,334 individuals ≥40 years old were screened for ET (Fig 1). We excluded six individuals with incomplete data. Hence, the current analysis focused on data from 1,328 participants (Table 1).
Table 1.
Demographic characteristics of 1,328 study participants
| Age in years, mean ± SD (median, range) | 61.7 ± 13.0 (61.0, 40 – 98) |
| Female gender, n (%) | 703 (52.9) |
| Education, n (%) | |
| Less than high school graduate | 389 (29.3) |
| High school graduate | 87 (6.6) |
| Some college but not a college graduate | 352 (26.5) |
| College graduate | 380 (28.6) |
| Masters or doctorate degree | 81 (6.1) |
| Missing data | 39 (2.9) |
| Marital status, n (%) | |
| Married or remarried | 937 (70.6) |
| Widowed | 135 (10.2) |
| Never married | 122 (9.2) |
| Divorced or separated | 90 (6.8) |
| Other | 43 (3.2) |
| Missing data | 1 (0.1) |
| Place (i.e., Island) of residence, n (%) | |
| Suðurstreymoy | 568 (42.8) |
| Eysturoy | 304 (22.9) |
| Norðoyggjar | 153 (11.5) |
| Suðuroy | 128 (9.6) |
| Vágoy | 78 (5.9) |
| Norðstreymoy | 57 (4.3) |
| Sandoy | 40 (3.0) |
| Current cigarette smoker, n (%) | 265 (20.0) |
| Handedness, n (%) | |
| Right | 944 (71.1) |
| Left | 86 (6.5) |
| Ambidextrous, but mostly right | 186 (14.0) |
| Ambidextrous, but mostly left | 42 (3.2) |
| Totally ambidextrous | 11 (0.8) |
| Reported “Don’t know” | 4 (0.3) |
| Missing | 55 (4.1) |
| Cups of caffeinated coffee on day of screening, mean ± SD (median, range) | 1.9 ± 2.0 (1.0, 0 – 12) |
| Cups of caffeinated tea on day of screening, mean ± SD (median, range) | 1.5 ± 1.7 (1.0, 0 – 10) |
| Cups of caffeinated soda on day of screening, mean ± SD (median, range) | 0.1 ± 0.6 (0.0, 0 – 10) |
| Smoked cigarettes on day of screening, n (%) | 224 (16.9) |
|
Number of cigarettes smoked on day of screening, mean ± SD (median, range) | |
| All participants | 1.2 ± 3.5 (0.0, 0– 30) |
| 224 participants who smoked on day of screening | 7.0 ± 5.6 (5.0, 1 – 30) |
| Used an asthma inhaler on the day of screening, n (%) | 74 (5.6) |
| Hours since used asthma inhaler*, mean ± SD (median, range) | 5.5 ± 3.8 (5.0, 0.5 – 16) |
| Spiral score, mean ± SD (median, range) | |
| Right hand spiral 1 score | 0.61 ± 0.38 (0.5, 0.0 – 2.0) |
| Right hand spiral 2 score | 0.59 ± 0.38 (0.5, 0.0 – 2.0) |
| Right hand mean spiral score | 0.60 ± 0.36 (0.5, 0.0 – 2.0) |
| Left hand spiral 1 score | 0.87 ± 0.41 (1.0, 0.0 – 2.0 ) |
| Left hand spiral 2 score | 0.91 ± 0.40 (1.0, 0.0 – 2.0) |
| Left hand mean spiral score | 0.89 ± 0.38 (1.0, 0.0 – 2.0) |
| Total (4 spirals) mean spiral score | 0.74 ± 0.33 (0.75, 0.0 – 2.0) |
SD=standard deviation
69 participants
A total of 227 subjects participated in the in-person clinical evaluation. A diagnosis of ET was assigned to 27 of 227 individuals (11.9%): 15 out of 66 (22.7%) individuals in group 1, 11 of 82 (13.4%) individuals in group 2, 1 of 27 (3.7%) individuals in group 3, and 0 of 52 (0.0%) individuals in group 4. The ET and non-ET groups were comparable with respect to all variables except four variables from the screening and clinical exam questionnaires regarding tremor (Table 2). The ET group reported significantly more tremor than the control group. A total of 15 participants (55.6%) in the ET group responded positively to the question regarding presence of tremor, with a mean age of 47.4 years (median 58, range 12 – 75) (n=9) when the tremor first was noticed; six of fifteen did not recalled the age. Eight of fifteen (53%) stated that the tremor was embarrassing, 6 (40%) answered “no”, and one (7%) did not know.
Table 2.
Comparison of 227 subjects with ET and without ET
| ET group | Non-ET group | p value | |
|---|---|---|---|
| Number, n | 27 | 200 | |
| Age in years, mean ± SD | 66.5 ± 11.7 | 62.2 ± 12.3 | 0.09 |
| Female gender, n (%) | 10 (37.0) | 93 (46.5) | 0.35 |
| Marital Status, n (%) | |||
| Married or remarried | 13 (48.1) | 138 (69.0) | 0.14* |
| Widowed | 6 (22.2) | 22 (11.0) | |
| Never married | 5 (18.5) | 22 (11.0) | |
| Divorced or separate | 2 (7.4) | 14 (7.0) | |
| Other | 1 (3.7) | 4 (2.0) | |
| Education (%) | |||
| Less than high school | 8 (29.6) | 54 (27.0) | 0.34* |
| High school graduate | 2 (7.4) | 7 (3.5) | |
| Some college but not a college graduate | 8 (29.6) | 63 (31.5) | |
| College graduate | 5 (18.5) | 58 (29.0) | |
| Masters or doctorate degree | 2 (7.4) | 14 (7.0) | |
| Missing data | 2 (7.4) | 4 (2.0) | |
| Place (i.e., Island) of residence, n (%) | |||
| Suðurstreymoy | 10 (37.0) | 106 (53.0) | 0.35* |
| Eysturoy | 4 (14.8) | 28 (14.0) | |
| Norðoyggjar | 5 (18.5) | 27 (13.5) | |
| Suðuroy | 5 (18.5) | 15 (7.5) | |
| Vágoy | 2 (7.4) | 10 (5.0) | |
| Norðstreymoy | 1 (3.7) | 7 (3.5) | |
| Sandoy | 0 (0.0) | 7 (3.5) | |
| Current cigarette smoker, n (%) | 9 (33.3) | 40 (20.0) | 0.11 |
| BMI, mean ± SD | 28.0 ± 3.8 | 28.4 ± 4.6 | 0.66 |
| Yes to question “do you drink alcohol”, n (%) | 18 (66.7) | 134 (67.0) | 0.97 |
| Yes to question “do you have a tremor”, n (%) | 15 (55.6) | 31 (15.5) | <0.0001 |
| Yes to question “does anyone in your family have shaking/tremor”, n (%) | 13 (48.1) | 66 (33.0) | 0.23* |
| Yes to question “do you often have shaking or tremor that you cańt control”, n (%) | 8 (29.6) | 25 (12.5) | 0.08* |
| Yes to question “do other people often tell you that you have a tremor”, n (%) | 9 (33.3) | 8 (4.0) | <0.0001* |
| Yes to question “has a doctor diagnosed you as having familial tremor or benign essential tremor”, n (%) | 1 (3.7) | 4 (2.0) | 0.19* |
| Yes to question “do you often have shaking or tremor in your hands or arms that you cańt control”, n (%) | 0.013* | ||
| Yes, right arm | 1 (3.7) | 8 (4.0) | |
| Yes, left arm | 0 (0.0) | 2 (1.0) | |
| Yes, both arms | 7 (25.9) | 12 (6.0) | |
| Yes to question “does your head often shake uncontrollably”, n (%) | 1 (3.7) | 6 (3.0) | 0.15* |
| Yes to question “does your voice almost always tremble when you talk”, n (%) | 0 (0.0) | 1 (0.5) | 1.0* |
| Yes to question “does your hand usually tremble when you hold a pen or write your name”, n (%) | 11 (40.7) | 14 (7.0) | <0.0001* |
P <0.05; T-test for continuous variables and chi square or Fischer’s exact test for categorical variables
SD=standard deviation
Fisheŕs exact test
3.2. Clinical characteristics of persons with ET
Of the 27 participants with ET, head and/or jaw tremor was present in 12 individuals (44.4%) while none had voice tremor. The majority were right handed. Mean score for all four spirals for the ET group was 1.42 ± 0.27 (Table 3) and there was a significantly difference between right and left hand mean spiral score (p<0.01). Of note, same significantly difference was observed in the screening group.
Table 3.
Clinical characteristics of 27 participants with essential tremor (ET)
| Presence* of head or jaw tremor, n (%) | |
| Present in head | 2 (7.4) |
| Present in jaw | 7 (25.9) |
| Present in both | 3 (11.1) |
| Presence* of voice tremor, n (%) | 0 (0.0) |
| Handedness, n (%) | |
| Right | 19 (70.4) |
| Ambidextrous, but mostly right | 5 (18.5) |
| Missing data | 3 (11.1) |
| Spiral score, Mean ± SD (median, range) | |
| Right hand spiral 1 score | 1.20 ± 0.42 (1.0, 0.5 – 2.0) |
| Right hand spiral 2 score | 1.26 ± 0.40 (1.0, 0.5 – 2.0) |
| Right hand mean spiral score | 1.23 ± 0.39 (1.25, 0.5 – 2.0) |
| Left hand spiral 1 score | 1.60 ± 0.32 (1.5, 1.0 – 2.0) |
| Left hand spiral 2 score | 1.65 ± 0.27 (1.5, 1.0 – 2.0) |
| Left hand mean spiral score | 1.63 ± 0.26 (1.5, 1.0 – 2.0) |
| Total (4 spirals) mean spiral score | 1.42 ± 0.27 (1.38, 0.88 – 2.0) |
SD=standard deviation
On videotaped neurological examination
3.3. Prevalence
A total of 27 of 227 individuals were assessed to have ET - the crude overall prevalence = 2.93% (Table 4). The prevalence of ET increased with age (p = 0.01) and was more common in men than women; however, the gender difference was not statistically significant (p = 0.5).
Table 4.
Prevalence estimates of essential tremor (ET) stratified by age and gender
| Prevalence (%) 95% confidence interval | |
|---|---|
| Crude prevalence | 2.93 (2.16 – 3.99) |
| Age group, years | |
| 40–49 | 0.74 (0.18 – 2.32) |
| 50–59 | 2.62 (1.30 – 4.98) |
| 60–69 | 3.56 (1.93 – 6.78) |
| ≥70 | 4.80 (3.19 – 7.15) |
| Age adjusted | 3.11 (2.28 – 4.16) |
| Age–standardized* | 2.84 (2.64 – 3.06) |
| Gender | |
| Male | 3.79 (2.59 – 5.65) |
| Female | 2.10 (1.30 – 3.49) |
| Gender adjusted | 2.90 (2.09 – 3.90) |
| Gender-standardized* | 2.96 (2.76 – 3.19) |
Standardized to Faroese Population ≥ 40 years
4. Discussion
Prior epidemiological work has established high prevalence of several movement disorders (i.e., PD[43] and primary focal dystonia[13]) in the Faroe Islands, providing an impetus to study the most common adult-onset movement disorder, ET. Also, given the potential for founder effects in the Islands, and the highly genetic nature of ET, the Faroe Islands provide a particularly interesting setting in which to study the prevalence of ET. Nonetheless, the prevalence of ET in this study was similar to that of other studies, as discussed below.
The overall prevalence was 2.9% while the age-adjusted prevalence was 3.1%. In our study, the study subjects were screened first and then examined based on a positive response to a screening questionnaire and spiral drawing. However, in our selection of indviduals for clinical examinations we did invite subjects from all four stratification groups, i.e. also individuals not expected to have ET based on screening. This allowed us to calculate an estimated prevalence that approximated a situation in which all subjects had been examined. Comparing the Faroese prevalence with (1) population-based studies using either the same procedure (examination in a sub-set with extrapolation of findings up to the full sample) or a similar procedure (examining all) and (2) reporting the prevalence from age 40 and older, our prevalence estimate is higher than in a Italian study[44], which reported a crude prevalence of 0.8 but lower than a Turkish study[28] reporting a crude prevalence of 4% and a study from Finland[45] reporting a prevalence of 5.6%.
We observed the same age-associated rise in prevalence as other studies, which bolsters the notion that age is a risk factor for ET. By age ≥70, prevalence reached 4.80%. Further, our data indicate the slight male predominance, although the difference was not statistically significant. A meta-analysis of 28 population-based prevalence studies reported that while the majority of studies have not revealed a gender difference, a sizable minority (more than one‐third) showed a statistically significant gender difference, with nearly all of those showing a higher prevalence among men than women [1].
Of the 27 with ET, only one was previously diagnosed. Thus, our data indicate that Faroese do not seek medical attention for their tremor or the tremor may be mild enough so that their treating physicians do not make the diagnosis when they present for other complaints. These results are in line with other epidemiological studies showing that 80 −100% of ET cases were previously un-diagnosed[1].
The prevalence of PD in the Faroe Islands is high[43], as is the prevalence of certain forms of dystonia[13]. However, the current data indicate that the prevalence of ET, another involuntary movement, is comparable to that of other populations. This is of particular interest given the potential for founder effects in the Islands and the highly genetic nature of ET. It raises questions as to whether ET is less of a genetic disorder than often presumed and/or whether complex genetics play a larger role than traditionally thought. These issues have been discussed elswhere[46–48].
This study has considerable strengths. First, the sample size in this population based study is large, with data on 1,328 individuals, which corresponds to 5.5% of the entire Faroese population ≥40 years. More important, however, is that, tremor was (1) systematically quantified (2) by a senior movement disorder neurologist with particular specialization in tremor (3) using a reliable and valid clinical rating scale (4) followed by the application of rigorous diagnostic criteria used by tremor investigators in the United States and internationally[28–39].
There are also limitations. First, some of the screenees who were deemed to have a low-intermediate or low likelihood of ET and who were therefore not seen in person, may have had ET. This is unlikely as our data showed that no individuals in group 4 (relatively normal spirals) had ET and virtually none in group 3 (mild tremor on spirals) had ET. Second, data on medication use were limited in the screening process, and therefore, we were not able to fully account for the effects of medication on the expression of hand tremor. This is not likely to have been important either; individuals with medication-induced tremors would have been carefully separated from ET during the videotaped examination. Third, the number of ET cases was limited; hence, some comparisons between the ET and non-ET group did not yield significant differences despite the expectation that they would (e.g., does anyone in your family have shaking or tremor? [Table 2]). Fourth, the study is undertaken within the context of a very homogeneous population. Thus, the results of this work may not generalize to largely mixed populations.
Participation bias could also be a potential problem. However, we carefully worded the screening letter and did not mention ET but rather described the study as one of lifestyle, diet and neurological conditions. Thus, it is unlikely that people with ET were more likely to participate in the screening. A total of 1,334 (44.5%) individuals returned the completed screening package. Participants who returned the packages were on average five years younger and were 6% more likely to be female than non-participants. The prevalence of ET increased with age; hence, it is possible that we underestimated the overall prevalence by under-ascertaining older individuals. This would not have affected our estimates of age-stratified prevalence, however. In the second phase with the clinical examinations, the highest number of decliners (22%) were in group 1, which were the individuals assessed to have high likelihood of having ET. Thus, a bias towards lower participation of those most likely to have ET may also have led to lower estimate.
In summary, in this large, population-based sample of 1,328 individuals, we estimate the age-adjusted prevalence to be 3.1%, which is similar to studies using the same or comparable methodologies.
Acknowledgement:
The authors thank the participants of the study for their support.
Funding: This work was supported by NIH grants R01 NS039422 and NS094607 (Dr. Louis).
Footnotes
Statements of Etics: All study procedures were approved by the local ethical review committee of the Faroe Islands and by the Institutional Review Board at Yale University, with participation on a voluntary basis and signed informed consent obtained from each enrollee.
Conflicts of Interest: There are no conflicts of interest or competing financial interest.
References:
- [1].Louis ED and Ferreira JJ, “How common is the most common adult movement disorder? Update on the worldwide prevalence of essential tremor,” Mov Disord, vol. 25, pp. 534–41, April 15 2010. [DOI] [PubMed] [Google Scholar]
- [2].Louis ED, Barnes L, Albert SM, Cote L, Schneier FR, Pullman SL, et al. , “Correlates of functional disability in essential tremor,” Mov Disord, vol. 16, pp. 914–20, September 2001. [DOI] [PubMed] [Google Scholar]
- [3].Bain PG, Mally J, Gresty M, and Findley LJ, “Assessing the impact of essential tremor on upper limb function,” J Neurol, vol. 241, pp. 54–61, November 1993. [DOI] [PubMed] [Google Scholar]
- [4].Heroux ME, Parisi SL, Larocerie-Salgado J, and Norman KE, “Upper-extremity disability in essential tremor,” Arch Phys Med Rehabil, vol. 87, pp. 661–70, May 2006. [DOI] [PubMed] [Google Scholar]
- [5].Louis ED, Ford B, Frucht S, Barnes LF, M XT, and Ottman R, “Risk of tremor and impairment from tremor in relatives of patients with essential tremor: a community-based family study,” Ann Neurol, vol. 49, pp. 761–9, June 2001. [DOI] [PubMed] [Google Scholar]
- [6].Louis ED, “Environmental epidemiology of essential tremor,” Neuroepidemiology, vol. 31, pp. 139–49, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Clark LN and Louis ED, “Essential tremor,” Handb Clin Neurol, vol. 147, pp. 229–239, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Jimenez-Jimenez FJ, de Toledo-Heras M, Alonso-Navarro H, Ayuso-Peralta L, Arevalo-Serrano J, Ballesteros-Barranco A, et al. , “Environmental risk factors for essential tremor,” Eur Neurol, vol. 58, pp. 106–13, 2007. [DOI] [PubMed] [Google Scholar]
- [9].Tanner CM, Goldman SM, Lyons KE, Aston DA, Tetrud JW, Welsh MD, et al. , “Essential tremor in twins: an assessment of genetic vs environmental determinants of etiology,” Neurology, vol. 57, pp. 1389–91, October 23 2001. [DOI] [PubMed] [Google Scholar]
- [10].Als TD, Jorgensen TH, Borglum AD, Petersen PA, Mors O, and Wang AG, “Highly discrepant proportions of female and male Scandinavian and British Isles ancestry within the isolated population of the Faroe Islands,” Eur J Hum Genet, vol. 14, pp. 497–504, April 2006. [DOI] [PubMed] [Google Scholar]
- [11].Petersen MS, Bech S, Nosova E, Aasly J, and Farrer MJ, “Familial aggregation of Parkinson’s disease in the Faroe Islands,” Mov Disord, vol. 30, pp. 538–44, April 2015. [DOI] [PubMed] [Google Scholar]
- [12].Petersen MS, Guella I, Bech S, Gustavsson E, and Farrer MJ, “Parkinson’s disease, genetic variability and the Faroe Islands,” Parkinsonism Relat Disord, vol. 21, pp. 75–8, January 2015. [DOI] [PubMed] [Google Scholar]
- [13].Joensen P, “High prevalence of primary focal dystonia in the Faroe Islands,” Acta Neurol Scand, vol. 133, pp. 55–60, January 2016. [DOI] [PubMed] [Google Scholar]
- [14].Binzer S, Imrell K, Binzer M, Kyvik KO, Hillert J, and Stenager E, “High inbreeding in the Faroe Islands does not appear to constitute a risk factor for multiple sclerosis,” Mult Scler, vol. 21, pp. 996–1002, July 2015. [DOI] [PubMed] [Google Scholar]
- [15].Joensen P, “Multiple sclerosis: variation of incidence of onset over time in the Faroe Islands,” Mult Scler, vol. 17, pp. 241–4, February 2011. [DOI] [PubMed] [Google Scholar]
- [16].Joensen P, “Incidence of amyotrophic lateral sclerosis in the Faroe Islands,” Acta Neurol Scand, vol. 126, pp. 62–6, July 2012. [DOI] [PubMed] [Google Scholar]
- [17].Louis ED and Ottman R, “How familial is familial tremor? The genetic epidemiology of essential tremor,” Neurology, vol. 46, pp. 1200–5, May 1996. [DOI] [PubMed] [Google Scholar]
- [18].Louis ED, Hafeman D, Parvez F, Alcalay RN, Islam T, Siddique AB, et al. , “Prevalence of essential tremor in Araihazar, Bangladesh: a population-based study,” Neuroepidemiology, vol. 36, pp. 71–6, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Louis ED, Hafeman D, Parvez F, Liu X, Alcalay RN, Islam T, et al. , “Tremor severity and age: a cross-sectional, population-based study of 2,524 young and midlife normal adults,” Mov Disord, vol. 26, pp. 1515–20, July 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Hafeman D, Ahsan H, Islam T, and Louis E, “Betel quid: Its tremor-producing effects in residents of Araihazar, Bangladesh,” Mov Disord, vol. 21, pp. 567–71, April 2006. [DOI] [PubMed] [Google Scholar]
- [21].Louis ED, Zhao Q, Meng H, and Ding D, “Screening for action tremor in epidemiological field surveys: assessing the reliability of a semi-quantitative, visual, template-based scale for rating hand-drawn spirals,” Tremor Other Hyperkinet Mov (N Y), vol. 2, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Louis ED, Ford B, Pullman S, and Baron K, “How normal is ‘normal’? Mild tremor in a multiethnic cohort of normal subjects,” Arch Neurol, vol. 55, pp. 222–7, February 1998. [DOI] [PubMed] [Google Scholar]
- [23].Louis ED, Ottman R, and Clark LN, “Clinical classification of borderline cases in the family study of essential tremor: an analysis of phenotypic features,” Tremor Other Hyperkinet Mov (N Y), vol. 4, p.220, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Goetz CG, Fahn S, Martinez-Martin P, Poewe W, Sampaio C, Stebbins GT, et al. , “Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): Process, format, and clinimetric testing plan,” Mov Disord, vol. 22, pp. 41–7, January 2007. [DOI] [PubMed] [Google Scholar]
- [25].Louis ED, Ottman R, Ford B, Pullman S, Martinez M, Fahn S, et al. , “The Washington Heights-Inwood Genetic Study of Essential Tremor: methodologic issues in essential-tremor research,” Neuroepidemiology, vol. 16, pp. 124–33, 1997. [DOI] [PubMed] [Google Scholar]
- [26].Louis ED, Ford B, and Bismuth B, “Reliability between two observers using a protocol for diagnosing essential tremor,” Mov Disord, vol. 13, pp. 287–93, March 1998. [DOI] [PubMed] [Google Scholar]
- [27].Louis ED and Pullman SL, “Comparison of clinical vs. electrophysiological methods of diagnosing of essential tremor,” Mov Disord, vol. 16, pp. 668–73, July 2001. [DOI] [PubMed] [Google Scholar]
- [28].Dogu O, Sevim S, Camdeviren H, Sasmaz T, Bugdayci R, Aral M, et al. , “Prevalence of essential tremor: door-to-door neurologic exams in Mersin Province, Turkey,” Neurology, vol. 61, pp. 1804–6, December 23 2003. [DOI] [PubMed] [Google Scholar]
- [29].Inzelberg R, Mazarib A, Masarwa M, Abuful A, Strugatsky R, and Friedland RF, “Essential tremor prevalence is low in Arabic villages in Israel: door-to-door neurological examinations,” J Neurol, vol. 253, pp. 1557–60, December 2006. [DOI] [PubMed] [Google Scholar]
- [30].Gasparini M, Bonifati V, Fabrizio E, Fabbrini G, Brusa L, Lenzi GL, et al. , “Frontal lobe dysfunction in essential tremor: a preliminary study,” J Neurol, vol. 248, pp. 399–402, May 2001. [DOI] [PubMed] [Google Scholar]
- [31].Farrer M, Gwinn-Hardy K, Muenter M, DeVrieze FW, Crook R, Perez-Tur J, et al. , “A chromosome 4p haplotype segregating with Parkinson’s disease and postural tremor,” Hum Mol Genet, vol. 8, pp. 81–5., 1999. [DOI] [PubMed] [Google Scholar]
- [32].Dogu O, Sevim S, Louis ED, Kaleagasi H, and Aral M, “Reduced body mass index in patients with essential tremor: a population-based study in the province of Mersin, Turkey,” Arch Neurol, vol. 61, pp386–9, March 2004. [DOI] [PubMed] [Google Scholar]
- [33].Gatto EM, Roca MC, Raina G, and Micheli F, “Low doses of topiramate are effective in essential tremor: a report of three cases,” Clin Neuropharmacol, vol. 26, pp. 294–6, Nov-Dec 2003. [DOI] [PubMed] [Google Scholar]
- [34].Benito-Leon J, Alvarez-Linera J, Hernandez-Tamames JA, Alonso-Navarro H, Jimenez-Jimenez FJ, and Louis ED, “Brain structural changes in essential tremor: voxel-based morphometry at 3-Tesla,” J Neurol Sci, vol. 287, pp. 138–42, December 15 2009. [DOI] [PubMed] [Google Scholar]
- [35].Obwegeser AA, Uitti RJ, Turk MF, Strongosky AJ, and Wharen RE, “Thalamic stimulation for the treatment of midline tremors in essential tremor patients,” Neurology, vol. 54, pp. 2342–4, June 27 2000. [DOI] [PubMed] [Google Scholar]
- [36].Putzke JD, Uitti RJ, Obwegeser AA, Wszolek ZK, and Wharen RE, “Bilateral thalamic deep brain stimulation: midline tremor control,” J Neurol Neurosurg Psychiatry, vol. 76, pp. 684–90, May 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Seijo-Martinez M, Del Rio MC, Alvarez JR, Prado RS, Salgado ET, Esquete JP, et al. , “Prevalence of Essential Tremor on Arosa Island, Spain: a Community-based, Door-to-Door Survey,” Tremor Other Hyperkinet Mov (N Y), vol. 3, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Sur H, Ilhan S, Erdogan H, Ozturk E, Tasdemir M, and Boru UT, “Prevalence of essential tremor: a door-to-door survey in Sile, Istanbul, Turkey,” Parkinsonism Relat Disord, vol. 15, pp. 101–4, February 2009. [DOI] [PubMed] [Google Scholar]
- [39].Ozel L, Demir R, Ozdemir G, Ozyildirim E, Avsar U, Ulvi H, et al. , “Investigation of the prevalence of essential tremor in individuals aged 18–60 in Erzurum,” Acta Neurol Belg, vol. 113, pp. 127–31, June 2013. [DOI] [PubMed] [Google Scholar]
- [40].Louis ED, Hernandez N, Sebastian AA, Clark LN, and Ottman R, “Validity of probands’ reports and self-reports of essential tremor: Data from a large family study in North America,” J Neurol Sci, vol. 393, pp. 45–50, October 15 2018. [DOI] [PubMed] [Google Scholar]
- [41].Fahn S, “Concept and classification of dystonia,” Adv Neurol, vol. 50, pp. 1–8, 1988. [PubMed] [Google Scholar]
- [42].Hughes AJ, Ben-Shlomo Y, Daniel SE, and Lees AJ, “What features improve the accuracy of clinical diagnosis in Parkinson’s disease: a clinicopathologic study. 1992,” Neurology, vol. 57, pp. S34–8, November 2001. [PubMed] [Google Scholar]
- [43].Wermuth L, Bech S, Petersen MS, Joensen P, Weihe P, and Grandjean P, “Prevalence and incidence of Parkinson’s disease in The Faroe Islands,” Acta Neurol Scand, vol. 118, pp. 126–31, August 2008. [DOI] [PubMed] [Google Scholar]
- [44].Mancini ML, Stracci F, Tambasco N, Sarchielli P, Rossi A, and Calabresi P, “Prevalence of essential tremor in the territory of Lake Trasimeno, Italy: results of a population-based study,” Mov Disord, vol. 22, pp. 540–5, March 15 2007. [DOI] [PubMed] [Google Scholar]
- [45].Rautakorpi I, Takala J, Marttila RJ, Sievers K, and Rinne UK, “Essential tremor in a Finnish population,” Acta Neurol Scand, vol. 66, pp. 58–67, July 1982. [DOI] [PubMed] [Google Scholar]
- [46].Clark LN and Louis ED, “Challenges in essential tremor genetics,” Rev Neurol (Paris), vol. 171, pp. 466–74, Jun-Jul 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Testa CM, “Key issues in essential tremor genetics research: Where are we now and how can we move forward?,” Tremor Other Hyperkinet Mov (N Y), vol. 3, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Ma S, Davis TL, Blair MA, Fang JY, Bradford Y, Haines JL, et al. , “Familial essential tremor with apparent autosomal dominant inheritance: should we also consider other inheritance modes?,” Mov Disord, vol. 21, pp. 1368–74, September 2006. [DOI] [PubMed] [Google Scholar]


