Abstract
In addition to the known prominent role of polyunsaturated (phospho)lipids as structural blocks of biomembranes, there is an emerging understanding of another important function of these molecules as a highly diversified signaling language utilized for intra- and extracellular communications. Technological developments in high-resolution mass spectrometry facilitated the development of a new branch of metabolomics, redox lipidomics. Analysis of lipid peroxidation reactions has already identified specific enzymatic mechanisms responsible for the biosynthesis of several unique signals in response to inflammation and regulated cell death programs. Obtaining comprehensive information about millions of signals encoded by oxidized phospholipids, represented by thousands of interactive reactions and pleiotropic (patho)physiological effects, is a daunting task. However, there is still reasonable hope that significant discoveries, of at least some of the important contributors to the overall overwhelmingly complex network of interactions triggered by inflammation, will lead to the discovery of new small molecule regulators and therapeutic modalities. For example, suppression of the production of AA-derived pro-inflammatory mediators, HXA3 and LTB4, by an iPLA2γ inhibitor, R-BEL, mitigated injury associated with the activation of pro-inflammatory processes in animals exposed to whole-body irradiation. Further, technological developments promise to make redox lipidomics a powerful approach in the arsenal of diagnostic and therapeutic instruments for personalized medicine of inflammatory diseases and conditions.
Keywords: eicosanoids, lipid mediators, lipoxygenase, Oxidized phospholipids, peroxidation, phospholipase A2, phospholipid hydrolysis
“You can’t picture NOTHING, because as soon as you do, it’s SOMETHING”
– Cynthia DeFelice
1 |. EVOLUTIONARY DIVERSITY OF LIPIDS AND POLYUNSATURATED LIPIDS
Phospholipids are amphipathic compounds combining a polar head-group and hydrophobic side chain, usually a fatty acid, in one molecule. This specific feature of their organization, with 2 spatially distinctive polar (water-soluble) and nonpolar (non-water-soluble) moieties, forces them to self-assemble in aqueous environments into closed vesicles surrounded by a highly organized bilayer membrane. This membrane represents a barrier between the inner volume and the outer aqueous spaces of the vesicles. This important type of phospholipid organization is essential for the formation of cells where the plasma membrane lipid bilayer separates the intracellular compartments from the environment. All living organisms have lipid bilayer membranes, and “just as DNA has been described as an ‘eternal molecule’, so lipid membranes could be considered an ‘eternal structure’ as such membranes are the products of preexisting membranes.”1 This principle of vesicular bilayer organization using lipids as the building blocks of biomembranes was essential for the emergence of life and is represented in the primordial bacteria and archaea. These first organisms contained a limited number, a few dozens of fatty acid residues, mostly saturated and mono-unsaturated, which were sufficient for fulfilling their major function of a membrane barrier. Saturated fatty acids (SFA) bearing no double bonds and monounsaturated fatty acids (MUFA) containing only one double bond have the most simple structure among different fatty acids (FA). Because of the high number of different combinations of these fatty acids in membrane phospholipids, there is a large diversity and precision in their structural adaptability that is essential for adjusting to different environmental conditions.
Synthesis of SFA is catalyzed by a multi-enzyme fatty acid synthase complex widely distributed among different organisms. It is represented by 2 main classes using similar catalytic mechanisms: type I systems found in yeast and animals use a single large, multifunctional polypeptide whereas type II systems present in prokaryotes and plants utilize a series of discrete, monofunctional enzymes.2 MUFA are synthesized through 2 major pathways, anaerobic and aerobic. The former is found in many bacteria and generates the double bonds by leaving those created during the biosynthesis of the fatty acid2 while the latter is present in a broad range of living groups (cyanobacteria, higher plants, fungi, invertebrates, and vertebrates) and is catalyzed by the aerobic desaturase Δ9. This enzyme introduces double bonds at the ninth position from the carboxyl end of SFA in a regioselective manner.3,4 Due to the high availability of enzymes involved in the synthesis of SFA as well as MUFA, all organisms can produce SFA and MUFA with aliphatic chains containing 16 or 18 carbon atoms.
The major components of the complex lipid molecules in higher organisms are polyunsaturated fatty acids (PUFA) having chain lengths from 18 carbons or more and at least 2 double bonds.2,5 From an evolutionary point of view, PUFA appeared later than SFA and MUFA. In the absence of oxygen, hydrogen interacts with double bonds of PUFA resulting in the formation of SFA. Therefore, it is unlikely that PUFA would have been in abundance for the 2.5-billion-year period before the Cambrian explosion, when prokaryotic anaerobic metabolism was prevalent.6 Synthesis of PUFA is a highly sophisticated and complicated process fulfilling 2 major goals: (i) elongation of the fatty acid chain to be able to accommodate the increasing numbers of FA with methylene-interrupted segments, and (ii) insertion of the new double bonds. These 2 goals are achieved via a coordinated and specialized network of multi-family enzymes, elongases and desaturases. Lack of certain desaturases and elongases in some organisms makes them incapable of synthesis of the whole spectrum of PUFA. The simplest set of FA is characteristic of archaea, yeast, and bacteria with the exception of several representatives of marine bacteria (mostly from the Shewanella species).7–9 They do not have desaturases necessary for the synthesis of PUFA and can synthesize only SFA and MUFA.
The emergence of PUFA and their integration into phospholipids was associated with a remarkably increased diversity of the lipidome and its subset, the redox lipidome. This was mostly due to the ability to utilize oxygen for the biosynthesis of a huge variety of non-oxygenated and oxygenated PUFA-containing lipids. Relatively conservative estimates indicate that the “aerobic lipidome,” with its oxygenated derivatives, includes more than a million individual species of lipids.10 This remarkable diversity of oxygenated PUFA lipids was accompanied by the gain of new metabolic pathways and functions, in particular, membrane phospholipid signaling. Interestingly, bacterial communities with developed communication features not only contain PUFA lipids but also enzymatic machinery for their oxidation (e.g., lipoxygenases; LOXes).11
2 |. ENZYMATIC AND NONENZYMATIC OXIDATION OF LIPIDS
An oxygen-containing atmosphere created a pro-oxidant environment which dramatically changed the catalytic properties for many metabolic reactions of oxidative metabolism. During the transition from the anaerobic (reductive) to aerobic (oxidizing) conditions, the availability of iron—plentiful in the oceans of the pre-Cambrian period due to its high solubility in the reduced ferrous state (Fe(II))12–14—has changed as a result of its conversion to a poorly soluble ferric (Fe(III) state that precipitated from solution as insoluble complexes).15 Consequently, aerobic organisms that have widely used Fe for catalysis and electron transfer12,13,16 had to face a difficult problem of obtaining sufficient amounts of Fe for their changed metabolic needs in the new aerobic environments.
Iron is crucial for many biological functions including oxygen transport, cell proliferation, and DNA repair. Due to its ability to accept and donate electrons, iron is a highly effective redox catalyst in biological systems. Iron-dependent redox reactions serve many fundamental biological roles such as mitochondrial electron transport, binding, transfer and delivery of oxygen, enzymatic oxidase, and oxygenase processes, including those that are essential for the inflammatory response.17 In spite of this essential need for Fe for major metabolic reactions and cell physiology, free radical reactions, catalyzed by soluble ionic Fe and its small molecule complexes in poorly controlled nonenzymatic reactions, represent a threat to the well-coordinated organization of normal cellular life. From this point of view, the restricted availability of Fe for aerobic organisms has indeed been a key “antioxidant defense.”12,18–21
The products of nonspecific lipid peroxidation may be hydrolyzed to yield free oxygenated fatty acids and lyso-phospholipids.22–27 Among the former, there may be numerous species with the propensities of lipid mediators.28 However, the random character of the peroxidation process precludes the formation of specific lipid mediators dictated by the requirements of the specific stage and context of the inflammatory process. In contrast, recently discovered enzymatic reactions of phospholipid peroxidation occurring in cellular compartments may be considered as a source of context-specific generation of lipid mediators. Examples of these types of reactions are the peroxidation of polyunsaturated CL in mitochondria related to apoptosis and the peroxidation of PE in the endoplasmic reticulum during ferroptosis (see Section 9).
Among the strictly controlled Fe-catalyzed processes is the enzymatically regulated oxidation of free PUFA and PUFA-esterified lipids leading to the highly specific biosynthesis of a large variety of lipid mediators.29 In contrast, H2O2 and lipid hydroperoxy-compounds can be utilized by low molecular weight complexes of Fe as a source of oxidizing equivalents, to generate reactive hydroxyl radicals (HO•) with a very high redox potential (E° (HO•/H2O) = 2.31V). As a result, HO• attacks essentially any organic molecule at a diffusion limited rate.30 Under pro-oxidant conditions with the excessive production and accumulation of H2O2, small molecular Fe-complexes display indiscriminative redox activity and cause massive random lipid peroxidation and generate myriads of primary and secondary products, including oxidatively truncated lipid-derived reactive electrophiles.17,31,32
The integrity of plasma and intracellular membranes is important for normal cell function. Random phospholipid peroxidation results in the accumulation of phospholipid molecules with hydrophilic groups residing on hydrophobic polyunsaturated acyl chains or results in shortened (oxidatively truncated) acyl chains.32,33 These changes decrease the lateral packing of hydrocarbon chains resulting in membrane thinning and the decrease in the lateral ordering of phospholipids.34 The oxidative alterations of membrane phospholipids can dramatically affect membrane properties33,35 resulting in changes in membrane permeability, pore formation, and ultimately membrane rupture.36–38 Uncontrolled lipid peroxidation may lead to significant changes in the structural organization of the membrane lipid bilayer as well as the lipid microenvironment of membrane proteins.22,39,40 One of the earliest manifestations of lipid peroxidation is a dramatic increase in membrane permeability to ions and small molecules resulting in the disruption of ionic gradients and Ca2+ dependent regulation.41 Attempts of the ATPase machinery to reinstate ionic homeostasis result in the nonproductive consumption of ATP and energetic crisis.40,42,43 Increased intracellular concentrations of Ca2+ cause dysregulation of lipid metabolism, largely associated with the activation of Ca2+ dependent phospholipases and accumulation of phospholipid hydrolysis products, free fatty acids, and lyso-phospholipids22–27 that further disrupt the membrane architecture.36–38 Simultaneous accumulation of electrophilic secondary products of lipid peroxidation, so-called oxidatively truncated lipids, may be associated with the modification of essential nucleophilic sites of membrane proteins thus further enhancing oxidative damage. These dramatic consequences of nonspecific lipid peroxidation are associated with the pathogenic oxidative injury of cells and tissues commonly related to chronic inflammation and diseases (e.g., arteriosclerosis, neurodegenerative diseases, renal disorders, and so on; Fig. 1).
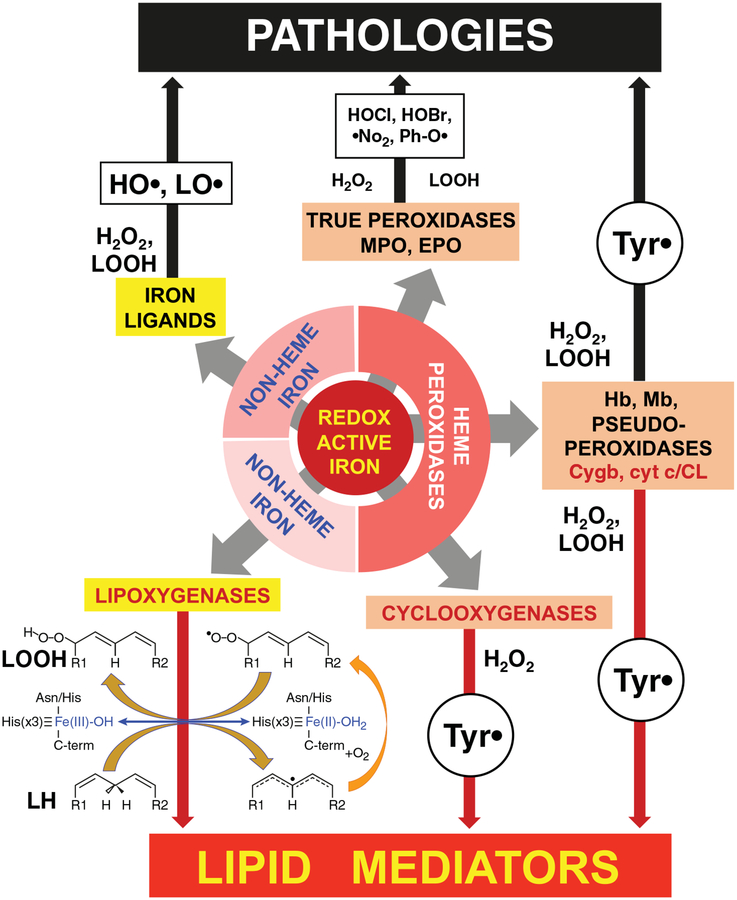
FIGURE 1. Major catalytic pathways for lipid peroxidation by different forms of redox active iron.
Three forms of redox active iron can cause physiologically important lipid oxidation: small molecular complexes of Fe (Fe-Ligands), heme peroxidases, and lipoxygenases. Oxidants produced by various forms of iron and leading to lipid (LH) oxidation. Fe2+ bound to small molecule ligands can reduce H2O2 and lipid hydroperoxides (LOOH) with the formation of highly reactive hydroxyl radicals or alkoxyl radicals, respectively. Peroxidases are divided into 3 groups: true peroxidases, pseudoperoxidases, and cyclooxygenases. MPO and EPO generate highly reactive oxidants (HOCl, HOBr) and free radicals (nitrogen dioxide and phenoxyl radicals) that oxidize proteins and lipids. Pseudoperoxidases (Hb, Mb, Cygb, and cyt c/CL) catalyze lipid oxidation by generating protein-immobilized tyrosyl radicals. Oxidized PUFA-phospholipids (e.g., by Cygb and cyt c/CL complexes) can be hydrolyzed to yield free oxidized PUFA (lipid mediators). COXes are the only peroxidases with the major function of producing lipid mediators (via oxidation of arachidonic acid by the protein-based tyrosyl radicals). LOXes are a family of professional enzymes that generate lipid mediators through site-specific PUFA oxidation. Blue arrow shows changes in Fe-containing catalytic site of LOX during PUFA oxidation. Orange arrows indicate the reactions catalyzed by redox-active Fe in the LOX catalytic site. Yellow arrows show the reaction of Fe-independent addition of oxygen. Random lipid oxidation (black arrows) can cause injury leading to pathologies, while targeted and very specific PUFA and PUFA-phospholipids oxidation (red arrows) leads to the formation of lipid mediators or their precursors
The handling of Fe in the body in a metabolically safe way is achieved by the coordinated work of the enzymes responsible for its redox state and transport. Most of the iron in the body comes from heme degradation in macrophages by heme oxygenase to release ferrous iron. Ferroxidases—hephaestin inside the cells and ceruloplasmin in plasma—convert redox active Fe(II) into inactive Fe(III). In plasma, Fe(III) is bound to transferrin.44 Controlled uptake of Fe via transferrin receptors and its efflux by ferroportin maintain low steady-state concentrations of intracellular Fe.45 Ferritin controls iron in cells allowing for its delivery to specific Fe-protein clients by a series of highly specialized protein chaperons.46 In cells with very high levels of Fe (e.g., macrophages and erythrocytes), specialized proteins like hepcidin, can sequester iron (along with induced H-ferritin), thus serving as an additional endogenous protective mechanism against cell injury and death (e.g., by ferroptosis (see below).47,48
3 |. Fe-PROTEINS AND MECHANISM OF ENZYMATIC OXIDATION
In many enzymes, Fe is integrated in the catalytic site and coordinated by different ligands in such a way that predominantly selective redox reactions take place with effective production of specific products.29 There are many different enzymes in the body that contain iron in the catalytic site that can be categorized in 3 major groups: iron-sulfur proteins; heme-containing proteins; and iron-proteins that are devoid of iron-sulfur clusters or heme.49,50 Among them are Fe-dependent oxygenases, including tryptophan dioxygenase, ferredoxin, and 2-oxoglutarate dioxygenase, iron-sulfur proteins, catalase, and so on. Notably, only the enzymes of the lipoxygenases (LOX) family and heme-containing peroxidases can oxidize lipids under physiologically relevant conditions. LOXes and cyclooxygenases (COXes) are the 2 types of Fe-containing non-heme and heme-proteins whose main function is the oxidation of different “free” PUFA yielding a large variety of pro- and anti-inflammatory lipid mediators—octadecanoids, eicosanoids, docosapentanoids, and docosahexanoids.51,52 Only a small number of epoxy-containing lipid mediators are produced by CYP450.53,54 Notably, COXes do not normally oxidize PUFA-phospholipids.55 Recently, it has been discovered that the peroxidase activity of several hemoproteins (e.g., cytochrome c (cyt c), cytoglobin (Cygb)) may lead to the generation of lipid mediators via direct oxidation of PUFA esterified in phospholipids.56–58 As our emphasis in this hybrid-review is on the oxidation of membrane phospholipids, we will focus on 2 major Fe-proteins that are capable of using PUFA-phospholipids as their substrates—LOXes and cyt c—that will be briefly considered in this section.
LOXes are widely represented in both prokaryotes and eukaryotes and their evolutionary aspects, structural organization, and catalytic mechanisms have been described in several excellent reviews.32,59–62 The family of human LOXes includes 6 members, and only one of them, 15-LOX, represented by 2 tissue-specific isoforms (15LOX-1 and 15LOX-2) can oxidize both free PUFA and PUFA-phospholipids.63 There is a U-shaped PUFA-binding channel where distinct amino acids tightly control PUFA orientation, positioning the selected pentadiene segment juxtaposed to the non-heme iron in the catalytic site.51,60 Dependent on the depth of the substrate binding cleft, LOXes can oxidize free arachidonic acid (AA) at the 5th, 8th, 12th, and 15th carbon, thus designating their nomenclature as 5-, 8-, 12-, and 15-LOX. The iron in the active center has 5 coordination bonds—with nitrogens from 3 conserved histidines, an oxygen from a carboxyl group provided by the C-terminus, and the side chain of asparagine or another histidine as the fifth iron coordinating bond. A hydroxy-group (or water) occupies the sixth Fe coordination bond.59 Site-specific and stereo-selective hydrogen abstraction from the substrate bis-allylic carbon atom by Fe(III)-OH results in the formation of PUFA carbon-centered radical and LOXFe(II)-OH2.64 The substrate radical rearrangement is accompanied by the addition of molecular oxygen delivered via a special channel in the protein. Hydrogen transfer from Fe(II)-OH2 group to the peroxyl-radical converts it into a molecular product, hydroperoxy-PUFA. Simultaneous oxidation of iron to Fe(III)-OH reconstitutes the resting form of the enzyme (Fig. 1). This very tight alignment of “free” PUFA, particularly AA, within the 15-LOX catalytic site affords its very effective oxidation. However, the enzyme has very low activity toward bulkier phospholipid substrates.65,66 This is dramatically changed by interactions of the 15-LOX with a scaffold protein, phosphatidylethanolamine binding protein 1 (PEBP1), that causes several effects.65 Allosteric interaction of PEBP1 provides selectivity toward PUFA-PE (particularly AA-PE) and regio-specificity toward oxidation of AA-PE at the 15th carbon.67 This is particularly important for the production of 15-HpETE-PE acting as a required product in the execution of ferroptosis (vide infra).
Another group of proteins capable of oxidizing (phospho)lipids are peroxidases that utilize H2O2 as a source of oxidizing equivalents (Fig. 1). In peroxidases, heme-iron (in ferric form) is penta-coordinated via interactions, 4 with nitrogens of the tetrapyrrole ring. The fifth ligand on the proximal heme side is a highly conserved imidazole of the histidine residue.68 On the distal side of the heme-moiety, the iron binds water that is replaced by H2O2 upon activation of the enzyme. The structure of the catalytic site enables peroxidases to form highly reactive heme-intermediates with a high oxidizing potential (> 1.0 V). The reduction of one H2O2 molecule is associated with the formation of 2 radical intermediates in the peroxidase cycle. Human peroxidases can be divided into 2 groups: true peroxidases and pseudoperoxidases. The major true peroxidases include COX (or prostaglandin H synthase), myeloperoxidase (MPO), eosinophil peroxidase (EPO), and lactoperoxidase (LPO). COXes are the only group of peroxidases—whose major function is the oxidation of “free” PUFA, particularly AA, to synthesize eicosanoids and other lipid mediators.55 COXes do not directly oxidize PUFA phospholipids.69 MPO, EPO, and LPO can oxidize a number of organic substrates, including lipids, indirectly via the formation of oxidants with strong oxidizing potentials, such as HOCl (1.28V), HOBr(1.13V), •NO2 (1.04V), and phenoxyl radicals (0.94 V). These oxidants can induce lipid peroxidation directly or via the formation of chlorohydrins and lysophospholipids.70
Pseudoperoxidases produce not only active heme-intermediates, but also protein-based tyrosyl radicals (0.94 V) that can directly oxidize lipids.71,72 Several hemo-proteins such as hemoglobin (Hb), myoglobin (Mb), cyt c/cardiolipin (CL) (cyt c/CL) complexes, and Cygb can act as pseudoperoxidases. Peroxidase activity of Mb and Hb may readily lead to lipid peroxidation in the absence of intracellular reductases maintaining these hemoproteins in the catalytically inactive ferrous state.73 Normally, hexa-coordinated Cygb can adopt a penta-coordinated peroxidase competent state due to either oxidation of two Cys residues and formation of the internal disulfide bond or interactions with anionic phospholipids, particularly phosphatidylinositol-phosphates (PIP3 and PIP2).56 Cygb’s peroxidase activity catalyzes the peroxidation of anionic phospholipids.56,58
Due to its specific ability to induce peroxidation of phospholipids, the role and mechanisms of the mitochondrial intermembrane hemoprotein, cyt c, is important in the context of this review. Normally, cyt c shuttles electrons between respiratory complexes III (ubiquinol:cytochrome c reductase) and IV (cytochrome c oxidase). In the depolarized state, a mitochondria-specific phospholipid, CL, transgresses from the inner mitochondrial membrane to the outer mitochondrial membrane, encounters cyt c, and forms a complex in which Fe becomes penta-coordinated due to the weakening of the Fe-Met(80) bond.57 This conversion is associated with the loss of electron-transport activity and gain of the peroxide function of the hemoprotein. The latter displays specific catalytic competence toward peroxidation of PUFA-CLs and several other anionic phospholipids, such as phosphatidylserine (PS) and phosphatidylglycerol (PG).28 The supply of the oxidizing equivalents, H2O2, is provided by the disrupted electron transport leading to the re-direction of the electron flow to O2 to yield O2•- that dismutates to H2O2.74 Initially, the rate constant of the reaction of H2O2 with cyt c/CL heme is very low (k ~ 46 M−1s−1). However, after several catalytic cycles, accumulated CL hydroperoxides react with the cyt c/CL complex much more effectively (103–105 M−1s−1).75 In addition, reactive intermediates formed in this reaction facilitate oxidative modifications of the hemoprotein (carbonylation,76 nitration77–79) that further stimulate the peroxidase activity of the protein.80,81 Of note, these modifications may lead to the loss of specificity of the peroxidase reaction. The peroxidase activity of cyt c/CL complexes is important in the context of the generation of proapoptotic signals and formation of mitochondrial precursors of lipid mediators (vide infra).
5 |. REDOX LIPIDOMICS BASED DECIPHERING OF PUFA-PHOSPHOLIPID OXIDATION
With the above background on evolutionary based biochemistry of lipids and enzymology of lipid oxidation, we can now consider the achievements of redox lipidomics in studies of the mechanisms and pathways as well as identification and quantitative characterization of the role that oxidized (phospho)lipids play in signaling during inflammation and regulated cell death. We immediately need to note that redox lipidomics is just beginning to emerge and there are both conceptual and technological difficulties that research in this area currently faces. Technologically, this analytical work is challenging because the amounts of an oxidatively modified phospholipid of a certain kind usually does not exceed 0.1–1.0% of the respective non-oxidized phospholipid.82 Recent developments in high mass accuracy liquid chromatography-mass spectrometry (LC-MS) platforms, as well as new software packages83–87 have made the detection and analysis of very low abundance lipid oxidation products, such as numerous oxygenated CL species, possible. This means that the low abundance oxidation products have to be detected, identified, and quantitated superimposed on the much larger background of respective non-oxidized species. Given that the stability and metabolic activity of these products is distributed among thousands of oxidizable molecular species combined with the lack of appropriate standards for each of them, makes this an almost unsurmountable task.
Conceptually, one has to bear in mind that in a process as complex as the inflammatory response, the constantly changing cellularity of the inflammatory foci combined with the trans-cellular biosynthesis pathways for a given lipid signal/mediator88 makes the interpretation of the LC-MS based results highly demanding. In addition, there are multiple pathways for the formation, degradation, and storage of these oxidized lipid intermediates. Historically, lipid mediators represented by free oxygenated PUFA were discovered and identified more than 5 decades ago and significant progress has been made in this field (see reviews89–91). However, even for the relatively well-established oxidation products, the questions of their origin, catabolism, and interconversions remain unsolved. A typical example is the products of LOX-catalyzed reactions, as exemplified by leukotriene B4 (LTB4) and hepoxilin A3 (HXA3): they may be formed through a canonical mechanism initiated by one of PLA2 enzymes that attacks AA-containing phospholipids to release AA as a substrate for LOX-catalyzed oxygenation. However, the same products can be obtained via direct attack on the phospholipid by LOX with subsequent hydrolysis of the AAox-phospholipid by another type, Ca2+-independent phospholipase A2, (iPLA2β or pILA2γ) that will specifically release AAox92,93 as a lipid mediator. Moreover, free oxygenated lipid mediators (including LTB4 and HXA3) can be re-esterified into membrane phospholipids by trans-acylases whose specificity and catalytic properties are still insufficiently known. In fact, both “free and esterified” phospholipid forms of LTB4 and HXA3 are simultaneously detectable in different proportions in different types of cells and tissues at any given point in time. With these cautionary notes, we proceed to the description of today’s achievements of redox lipidomics in this area. Because of the paucity of published data, we will, in several cases, utilize our own experimental results in attempts to get better insights into the mechanisms and pathways engaged in the biological responses.
6 |. FREE PUFA AND THEIR OXIDATION PRODUCTS AS PRO-/ANTI-INFLAMMATORY SIGNALS ENZYMATICALLY GENERATEDIN COX-, LOX-, AND CYP450 CATALYZED REACTIONS
Lipid mediators, along with cytokines and chemokines, are produced by immune cells to orchestrate the inflammatory response of the organism to exogenous and endogenous pathogens and damaged cells.89,90,94 Pro-inflammatory AA-derived lipid mediators, such as ω6-AA (20:4ω6), are produced by resident cells in the injured tissue and control the early activation of inflammatory events. The canonical mechanism of this signaling includes hydrolysis of PUFA-phospholipids by phospholipase A2 (PLA2) leading to the release of free AA and its further oxygenation by one of the members of the COX, LOX, and CYP450 families of enzymes.95 COX—prostaglandin endoperoxide H synthases—catalyze the conversion of AA into prostaglandin PGG2 and its subsequent reduction to prostaglandin H2 (PGH2).55 Eicosanoids generated by COX are not metabolically stable and their levels depend not only on their production but also by their degradation by eicosanoid catabolic enzymes. There are 2 forms of COX: COX-1 is constitutively expressed in almost all cells and tissues, while COX-2 is expressed in response to various stimuli such as cytokines and growth factors96,97 and acts at the site of inflammation.98 PGH2 undergoes enzymatic transformation to yield prostaglandins, including PGD2, PGI2, thromboxane A2, PGF2α, and PGE2 that act as mediators and regulate the inflammatory processes through specific receptors.99 Among other eicosanoids, PGE2 reveals a multifaceted role in inflammation. During the initial phase of the inflammatory response, PGE2 exerts a number of pro-inflammatory effects such as the generation of the pro-inflammatory cytokine, interleukin 6 (IL-6)89 and acts as a vasodilator to facilitate the tissue influx of neutrophils, macrophages, and mast cells from the bloodstream leading to swelling and edema.100 At the later stages of the inflammatory response, PGE2 controls a number of mechanisms that lead to the resolution of inflammation and subsequent tissue repair.100,101
The LOX primary oxidation product of AA is hydroperoxy-eicosatetraenoic acid (HpETE), which is reduced to the corresponding alcohol hydroxy-eicosatetraenoic acid (HETE) by glutathione peroxidases. HETE can be further enzymatically converted to a keto derivative, keto-eicosatetraenoic acid (KETE). During the acute phase of inflammation, coordinated action of AA-derived mediators drive the recruitment and activation of granulocytes (e.g., neutrophils), inflammatory macrophages, and lymphocytes to the injury/inflammation site. HXA3 and LTB4 are the 2 major AA-derived neutrophil chemo-attractants102 that initiate and orchestrate the recruitment and effective trans-migration of neutrophils to the site of inflammation.103 They are generated by 12- and 5-LOX initiated pathways, respectively.104 Hepoxilins, 12-hydroperoxy-eicosatetraenoic acid (12-HpETE)-derived epoxy-hydroxy fatty acids are formed in epithelial and endothelial cells as well as in neutrophils103,105 and can also be generated in cytochrome P-450-mediated reactions of stereospecific rearrangement of hydroperoxy fatty acids.106,107 5-LOX activities of neutrophils and macrophages are responsible for the generation of leukotrienes LTB4 and LTD4.108 In addition, 5-LOX is involved in the production of 5-oxo-6,8,11,14-eicosatetraenoic acid (5-oxo-ETE) formed from 5-HETE in reactions driven by microsomal 5-hydroxyeicosanoid dehydrogenase.109 Both LTD4 and 5-oxo-ETE also serve as chemo-attractants for eosinophils.110
Apoptosis of neutrophils and their clearance by phagocytic macrophages sets the stage for the resolution of inflammation and the production of anti-inflammatory lipid mediators.94 This is an active process of cessation of the inflammatory response that is strongly dependent on the production of a series of pro-resolving lipid mediators.94,111 Generally, anti-inflammatory lipid mediators originate from ω3 PUFAs such as docosahexaenoic (DHA, 22:6 ω3) and eicosapentaenoic (EPA, 20:5 ω3) fatty acids. Lipoxins are the derivatives of 20:4 ω6 with anti-inflammatory properties.112 The major function of lipoxins is the stimulation of macrophages to phagocytose and clear apoptotic neutrophils113 as well as to inhibit transmigration of neutrophils to the site of inflammation.114 In contrast to leukotrienes and hepoxilins formed within the same cell, lipoxins are biosynthesized transcellularly with the involvement of epithelial cells, eosinophils, monocytes, and neutrophils with the respective enzymatic reactions accomplished by 15-LOX and 5-LOX115,116 (Fig. 2). 15-HpETE generated by 15-LOX in epithelial cells, monocytes, or eosinophils is released from these cells and taken up by neutrophils, where it is converted to either LXA4 or LXB4 in subsequent reactions catalyzed by 5-LOX and LXA4 or LXB4 hydrolases, respectively. While PGE2 is considered a powerful pro-inflammatory mediator produced by COX-2, it also plays an important role in the resolution of inflammation via inhibition of TNF-α production. In addition, PGE2 induces production of LXA4 and inhibits the formation of LTB4, via the stimulation and inhibition of 15-LOX and 5-LOX activities, respectively.117,118 Moreover, with respect to COX and LOX, AA can be metabolized in endothelial cells by CYP450 to form several HETE and epoxy-eicosatrienoic (EET) acids (5,6-, 8,9-, 11,12-, and 14,15-EET).53,54,119 EETs also have a strong anti-inflammatory action120 by down-regulating the pro-inflammatory cytokine production via inhibition of a transcription factor, NF-kB. EETs are further metabolized to less active dihydroxy-eicosatrienoic acids (DHETs) by soluble or microsomal epoxide hydrolases.121,122
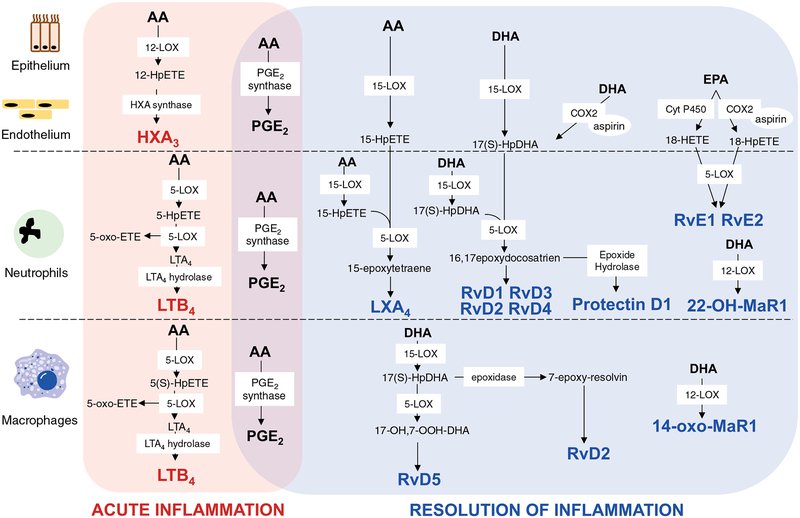
FIGURE 2.
Intra- and trans-cellular mechanisms for biosynthesis of lipid-derived mediators during acute and resolving stages of inflammation. During the inflammatory response, specific enzymes such as 5-LOX, 15-LOX, and COX-2 are involved in the generation of pro- and anti-inflammatory lipid mediators in different cell types. Pro-inflammatory lipid mediators—HXA3, LTB4, andPGE2—are synthesized intracellularly. Anti-inflammatory lipid mediators are produced via intermediatemetabolites transferred from cell to cell to complete the synthesis. Transcellular biosynthesis is important for the generation of several COX and 5-LOX metabolites such as RvE1 and RvE2 as well as lipid mediators synthesized by several LOX enzymes (LXA3 and resolvins: RvD1, RvD2, RvD3, and RvD4)
DHA- and EPA-derived lipid mediators are the major players generated during the resolving stage of inflammation.94 DHA serves as a precursor of several groups of pro-resolving lipid mediators—D-resolvins (RvD1-RvD4), protectins, and maresins (MaR1) that are detectable in human blood, serum, and exudates.123,124 DHA is present at the inflammatory sites as a “free” fatty acid as well as a fatty acid residue esterified into phospholipids that can be hydrolyzed by PLA2.125,126 Several trans-cellular mechanisms may be involved in the biosynthesis of DHA-derived pro-resolvins (Fig. 2). In epithelial and endothelial cells, DHA can be converted into 17S-hydroperoxy-DHA in a reaction catalyzed by 15-LOX and aspirin acetylated COX-2, respectively. The released product is taken up by neutrophils where it is metabolized by 5-LOX to RvD1, RvD2 and RvD3, RvD4 via epoxide-containing intermediates 7S,8S-epoxy-17S-hydroxy-DHA and 4S,5S-epoxy-17S-hydroxy-DHA, respectively.123,124 CYP450 enzymes can also initiate synthesis of RvD via transformation of DHA to 17S-hydroxy-DHA with the subsequent generation of RvD1 in an aspirin independent manner.127 In addition, neutrophils can convert 17S-hydroperoxy-DHA into a 16(17)-epoxide that can undergo enzymatic ring opening into protectin D1—10,17-dihydroxy-containing metabolite of DHA with 3 conjugated double bonds.123 Alternatively, it can be produced by peripheral blood monocytes and in the brain (known as neuro-protectin D1).128
MaR1, a DHA-derived pro-resolving lipid mediator generated by 12-LOX, regulates responses to bacterial invasion and stimulates the switch of macrophage phenotypes from M1 to M2,129 the phagocytosis of apoptotic neutrophils69,130 and tissue regeneration.129,131 While the main product, 14-oxo-MaR1, is produced predominantly by resident macrophages,130,132,133 22-hydroxy-MaR1 is mainly formed via transcellular mechanisms with involvement of platelets and leukocytes134 (Fig. 2).
Resolvins of the E-series (RvE1 and RvE2) are derived from EPA; they are detectable in plasma and serum.135 These mediators are produced in endothelial cells by aspirin-modified COX-2136,137 or cytochrome P450 enzymes in the absence of aspirin127,136 into 18-HpEPE and 18-HEPE, respectively. These intermediates are taken up by human neutrophils where 5-LOX transforms them into RvE1 and RvE2.138 RvE1 was also found in plasma of healthy people taking aspirin and EPA.139
7 |. PHOSPHOLIPIDS AND THEIR OXIDATION PRODUCTS AS PRO-/ANTI-INFLAMMATORY SIGNALS
In addition to lipid mediators formed from free AA, DHA, and EPA, oxygenated phospholipids play an important role in the regulation of inflammation.140–144 Phospholipids are a class of lipids that consist of a glycerol backbone, a polar head, and 2 acyl chains in the sn-1 and sn-2 positions. The major classes of phospholipids include phosphatidylcholine (PC), phosphatidylethanolamine (PE), PS, phosphatidylinositol (PI), PG, phosphatidic acid (PA), and CL. PUFA, commonly located in the sn-2 position, makes phospholipids susceptible to oxidation.145 Both enzymatic and nonenzymatic pathways may be involved in the generation of oxidized lipids.61 While enzymatic oxidation reactions are selective and specific, that is, preferentially attacking only some but not all classes of phospholipids, their molecular species lead to the formation of regio-specific products.32 In contrast, nonenzymatic oxidation occurs during the excessive accumulation of ROS and also poorly controlled transition metals and their molecular weight complexes.146 In this case, Fenton/Haber-Weiss reactions may be a dominating mechanism leading to random, nonspecific oxidations driven by the higher oxidizability of phospholipids with higher contents of double bonds. Initiated by the generation of carbon-centered radicals and hydroperoxy-derivatives of PUFA-phospholipids, they (PLox) may include hundreds of varying structures from different phospholipid classes. The structure of PLox is determined by the length of the fatty acid, number of double bonds, and oxygen-containing functional groups such as hydroperoxy, hydroxy, epoxy, and keto, aldehydic-positioned on the fatty acid residues.62 Four major receptors—the scavenger receptor CD36,147 TLR members TLR4 and TLR2,148 receptor for platelet-activating factor,96 and G protein-coupled receptors (GPCR)149—have been shown to be involved in the signaling processes by oxidized phospholipids.
PC, particularly palmitoyl(PA)/AA-PC, is a major phospholipid in the plasma membrane and LDLs.10,150 Oxidation of PA/AA-PC results in generation of a wide spectrum of biologically active PC species with oxidatively truncated AA in the sn-2 position.140 Oxidized PC (PCox) are known as inducers of chronic inflammation characteristic of atherosclerosis. Oxidized PC species such as 1-palmitoyl-2-(5,6-epoxyisoprostane E2)-sn-glycero-3-phosphocholine, 1-palmitoyl-2-(5-oxovaleroyl)-sn-glycero-3-phosphoryl-choline (POVPC), and 1-palmitoyl-2-glutaroyl-sn-glycero-3-phosphorylcholine (PGPC) present in the minimally modified (MM)-LDLs were shown to induce endothelial cells to express monocyte-specific adhesion molecules.151,152 Several studies also showed the involvement of exogenously added oxidized phosphatidylcholine in inducing various pro-inflammatory events in mononuclear leukocytes.153,154 Some PCox species have in their sn-2 position a structure similar to platelet-activation factor (PAF), which can be easily hydrolyzed by lipoprotein-associated PLA2(LpPLA2).155 The major PAF-like product, 1-O-hexadecyl-2-(butanoyl or butenoyl)-sn-glycero-3-phosphocholine, has been identified in oxidized LDL156 and oxidized LDL added to cells can activate the PAF receptor to express IL-8 and monocyte binding to endothelial cells.157 A number of PCox derived from the oxidation of LDLs have been detected in blood and plasma at higher levels at the sites of chronic inflammation and in damaged tissues.158 Based on these findings, they have been considered as danger-associated molecular patterns (DAMPs).147,159,160 Accumulation of PCox in atherosclerotic lesions has been linked to the activation of macrophages into a specific Nrf2-dependent phenotype (Mox), distinct from conventional M1 and M2 phenotypes153 by gene expression161 and functional manifestations. Moreover, oxidized PC can also induce NLRP3 inflammasome.162 While these PCox products are commonly ascribed to nonenzymatic ROS-driven oxidation mechanisms, the involvement of enzymatic pathways has not been fully excluded and may represent the subject of future studies.
The complexity of the enzymatic pathways is illustrated by the following examples. Several LOXes can directly oxidize phospholipids to generate hydroperoxy-eicosatetraenoic acid–PEs (HpETE–PEs)163 that serve as substrates for glutathione peroxidase 4 (GHPX4) to form the reduced products—hydroxy-eicosatetraenoic acid–PEs (HETE–PEs).164 The position of the hydroxy groups is correlated with the expression of the specific LOX in cells. Human neutrophils express 5-LOX and generate 5-HETE-PE.165 The major product formed in platelets is 12-HETE-PE,166 while 15-HETE-PE isomers are formed in monocytes and eosinophils.163 Recently, the pro inflammatory roles of PEox were demonstrated.159,167 In addition to endothelial cells and mononuclear leukocytes, exogenously added PLox were also shown to activate neutrophils.168,169 In mice, leukocytes express 12/15-LOX that generates mainly 12-HETE-phospholipids, but also small amounts of 15-HETE-phospholipids. HXA3-PE species are formed in mouse ileum in response to whole body irradiation170 (please see below). In conditions when the activity of GPX4 is suppressed, 15-LOX-derived signals, 15-HpETE-PE,65,171 trigger ferroptosis with pro-inflammatory consequences (vide infra). Up-regulation of GPX4 activity can reduce the production of the 15-HpETE-PE species, hence, stimulating the resolution of the inflammatory response.172 The 15-HpETE and 15-HETE-PEs are also simultaneously detectable in human airway epithelial cells stimulated ex vivo by IL4 or IL13 to Th2 phenotypes.65,173 Similar to PCox, exogenously added isolevuglandins (IsoLG) modified phosphatidylethanolamine can also stimulate NF-κB-driven activation and expression of inflammatory cytokines in macrophages.174
While COXes do not normally attack esterified AA, recently, esterified COX-derived products were detected in cells. COX-2 generates prostaglandin H2 glycerol ester (PGH2-G) via oxidation of 2-arachidonylglycerol or arachidonylethanolamine in macrophages and colon epithelial cells in response to pro-inflammatory stimuli. These glycerol- and ethanolamine-conjugated products can be enzymatically converted to PGD2-G, PGE2-D, PGF2α-G, and PGI2-G.175,176 COX-1–derived, PEox have been found in platelets along with PGE2, PGD2, or dioxolane A3.177,178 The exact sequence of events engaging predominantly direct COX attack on PUFA-phospholipids, or processes of hydrolysis and (re)-esterification of non-oxidized PUFA-phospholipidss and oxidized free PUFA still remain the subject of further studies.
While the presence of oxygenated groups is essential for the biological function of PLox, the polar head structure and intracellular localization of PLox are also important as determinants of inflammatory responses.145 During apoptosis, cytochrome c (cyt c) induced oxidation of CLs in mitochondria results in release of cyt c from mitochondria into the cytosol as a hallmark of apoptosis.57 At the same time, CL also attenuates the inflammatory effects of LPS through a TLR4-dependent pathway.179 In the cytosol, the interaction of cyt c with PUFA-PS in the inner leaflet of plasma membrane can cause PS oxidation that, in turn, facilitates externalization of PSox on the cell surface.180 The oxidized PS serves as a strong recognition signal181 for macrophages that facilitates the engulfment, phagocytosis, and clearance of apoptotic cells (including neutrophils)182 thus initiating the resolution phase of the inflammatory response.
8 |. HYDROLYSIS/RE-ESTERIFICATION OF OXIGENATED LIPIDS AND THEIR POSSIBLE ROLE IN REGULATION OF INFLAMMATION
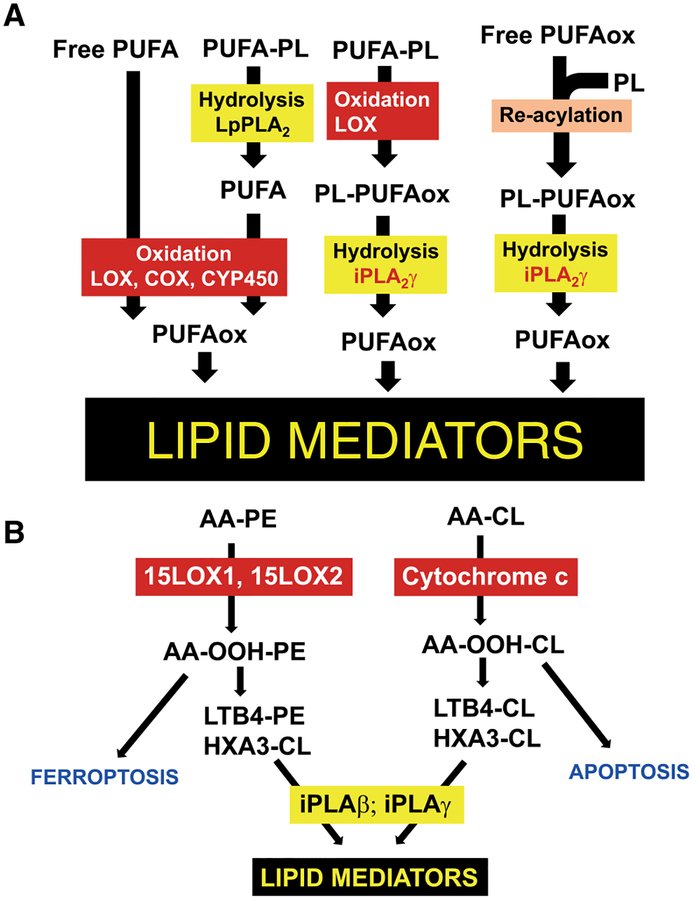
The mutual interconversions of oxidized lipid signals may occur via 3 major pathways183: (i) hydrolysis of non-oxidized phospholipids by PLA2 leading to the release of free PUFAs and their subsequent oxygenation by COX, LOX, and CYP450 driven reactions; (ii) direct enzymatic oxidation of phospholipids and their hydrolysis by PLA2 and release of oxygenated lipid mediators; (iii) re-acylation of free oxygenated PUFA into phospholipids thus forming a pool of esterified lipid mediators and their hydrolysis/release by specific PLA2 dependent on the context of pro-/anti-inflammatory environment (Fig. 3). These different mechanisms may be particularly important for generating the signals at specific time points during the acute and resolving stages of inflammation.
FIGURE 3. Different mechanisms of generating lipid mediators.
(A) In cells and tissues, lipid mediators are produced via the enzymatic modification of free polyunsaturated fatty acids (PUFA). Hydrolysis of non-oxidized phospholipids by phospholipase A2 (PLA2) results in release of free PUFA followed by oxygenation in cyclooxygenase (COX), lipoxygenase (LOX), and cytochrome p450 (CYP450) driven reactions. Phospholipids can be directly oxidized in enzymatic reactions catalyzed by LOX with subsequent hydrolysis of oxidized phospholipids by PLA2 yielding oxygenated lipid mediators. Re-acylation of free oxygenated PUFA into phospholipids results in generation of a pool of esterified lipid mediators and their hydrolysis by specific PLA2 that leads to the release of lipid mediators dependent on the context of pro-/anti-inflammatory environment. (B) Enzymatic oxidation of CL by cyt c in mitochondria and PE by 15-LOX in cellular membranes yields pro-apoptotic and pro-ferroptotic signals in cells and tissues, respectively. These oxygenated phospholipids can be enzymatically converted to oxygenated species containing LTB4 and HXA3 in the sn-2 position. LTB4 and HXA3 can be released from CL and PE in reactions catalyzed by iPLA2γ and iPLA2β and serve as pro-inflammatory lipid mediators
Conventionally, eicosanoids are produced via the enzymatic modification of AA after its release from phospholipids by phospholipase A2 (PLA2).89 There are several types of PLA2 that govern the release of AA.89 Cytosolic calcium-dependent PLA2 (cPLA2) and secreted PLA2 (sPLA2) are activated in response to exogenous stimuli and they are responsible for the release of PUFA in macrophages.184 In contrast, oxygenated phospholipids serve as a source of lipid mediators and can be hydrolyzed by specialized phospholipases A2 such as lipoprotein lipase A2 or PAF hydrolase and calcium independent iPLA2γ or iPLA2β that catalytically hydrolyze preferentially oxygenated fatty acid residues.25,185 High levels of expression of iPLA2γ, mainly localized in mitochondria,186 is commonly associated with the accumulation of pro-inflammatory oxygenated octadecanoids and eicosanoids caused by the dysregulation of mitochondrial Ca2+ mechanisms.187,188 These mechanisms may be particularly important in mitochondria where hydrolysis of CLox by iPLA2γ,93 yields a diversified series of pro-inflammatory and pro-resolving lipids mediators.28 Specific features of this utilization of mitochondria as a source of CLox-derived lipid mediators needs further detailed analysis in the context of intracellular inflammatory responses, CL externalization, and CL-dependent assembly of inflammasomes (NLRP3) and CLox induced apoptosis.57,189
Finally, oxygenated PUFA can be taken up by immune cells and incorporated into phospholipids.167 In neutrophils, 15-HETE is predominantly incorporated into PI while 5-HETE preferably esterifies PC or triglycerides.190 15-HETE can be taken up by the murine resident macrophages and incorporated into PI, PC, and PE.191 12-HETE is integrated into PC and PI in human peripheral blood mononuclear cells.192 In neutrophils and macrophages, esterified HETEs can act as a pool of “stored” eicosanoids, which can be released from these cells upon activation. Activation of neutrophils results in the deacylation of 15-HETE-PI and release of 15-HETE that acts as a signal suppressing LTB4 generation.193 Similarly, EETs are incorporated into the sn-2 position of cell phospholipids, mainly PC and PI, through a coenzyme A-dependent pathway.194–196 PUFA epoxides are found in plasma LDLs, likely after being released by lipoprotein lipase A2.197
9 |. METABOLIC INTERCONVERSIONS OF LIPID MEDIATORS AND THEIR CONTROL OF THE RECRUITMENT OF INNATE IMMUNE CELLS AND OUTCOME OF THE INJURY IN VIVO
9.1 |. Whole body irradiation
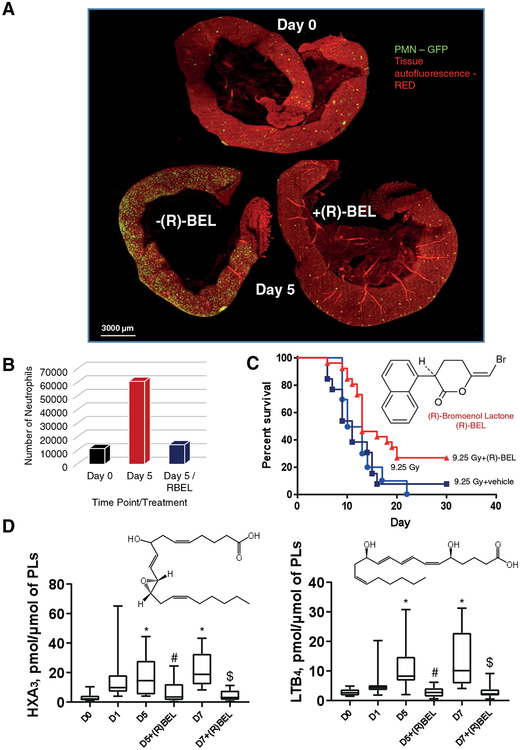
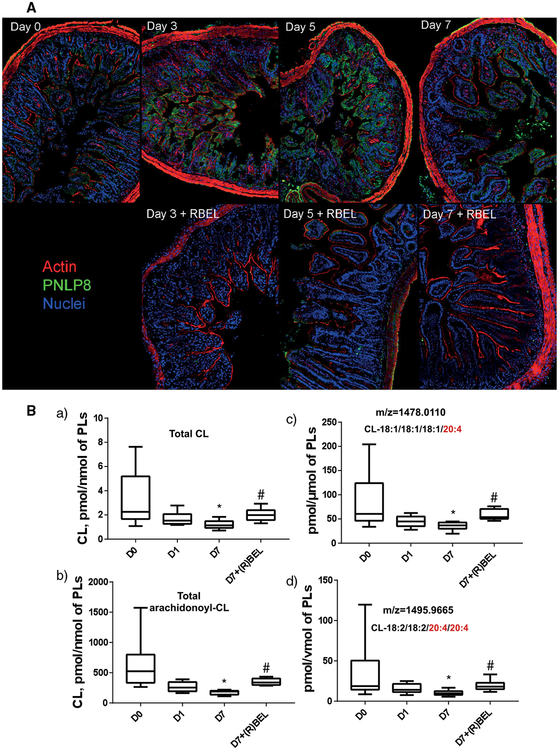
Although known as an acute radiation syndrome, whole body irradiation (WBI) represents an inflammatory disease that includes a series of responses and initiates the chain of pathogenic events where the primary reactive intermediates of water radiolysis create predominantly a pro-oxidant environment and trigger—directly and indirectly—programs of massive death in radiosensitive cell populations, such as the gut epithelium and bone marrow hematopoietic cells.198,199 Different types of signals are triggered by WBI. The irradiation damage of rapidly proliferating crypt intestinal epithelial cells (Figs. 4 and 5) and bone marrow hematopoietic cells initiates the recruitment of inflammatory cells such as neutrophils (Fig. 4A and B) and macrophages to the site of injury. The processes are mainly coordinated by a variety of cytokines and lipid mediators. The activated innate immune cells, particularly neutrophils, generate and release massive amounts of prooxidants thus creating a hostile microenvironment and causing collateral damage to the intestinal epithelium (Fig. 4A and B). As a marker of an ongoing active inflammatory response, the highly increased levels of iPLA2γ are readily detectable in the intestinal epithelial cells of mice after WBI (Fig. 5A). iPLA2γ can hydrolyze non-oxidized as well as peroxidized phospholipids.93 In line with this, iPLA2γ expression was accompanied by the accumulation of free AA and AA-derived pro-inflammatory lipid mediators, HXA3 and LTB4 (Fig. 4D). Assuming that this poorly controlled recruitment of neutrophils may be involved in the pathogenesis of radiation damage through breach of the epithelial barrier, penetration of bacteria followed by bacteremia, multiple organ failure, and sepsis, a specific and irreversible inhibitor of iPLA2γ, (R)-BEL, was chosen as a treatment strategy. Notably, (R)-BEL was able to markedly decrease the level of the iPLA2γ expression and block accumulation of pro-inflammatory mediators, HXA3 and LTB4 (Fig. 4D). Importantly, this treatment of irradiated mice with (R)-BEL afforded a strong protection of the intestinal epithelium and preserved its integrity (Fig. 5A). Combined with marked radiomitigative effects of (R)-BEL, these results suggest that reactions of hydrolysis of PUFA phospholipids and their oxidation products catalyzed by iPLA2γ have indeed been involved in the chain of pathogenic events leading to WBI induced epithelial damage. In contrast, the changes in the levels of PGE2 and LXA4 were not sensitive to (R)-BEL, suggesting the involvement of other PLA2, for instance cytosolic Ca2+-dependent PLA2, (cPLA2) and secreted PLA2 (sPLA2),200 in the pathway that is engaged in the generation of these lipid mediators. It has been demonstrated that iPLA2γ is the major enzyme responsible for the release of oxidized acyl chains from CL.93 In addition, CL is exclusively localized in mitochondria and the subcellular localization of iPLA2γ is limited to mitochondrial membranes.186 Furthermore, we recently demonstrated that the oxidation of polyunsaturated CLs in mitochondria and the accumulation of their hydrolysis products including oxygenated arachidonic acids are activated in vivo after acute tissue injury.28 Thus, we suggested that CL and oxCL may be involved in the inflammatory response induced by WBI. Indeed, the analysis of lipids revealed a significantly lower level of CL, mainly species containing AA, in the ileum of irradiated mice that was restored in mice treated with (R)-BEL (Fig. 5B). Thus, lipid mediators originating from CL in mitochondria are also possible participants and potential drivers of the inflammatory responses. Moreover, suppression of their formation by a specific inhibitor of iPLA2γ, (R)-BEL, is highly protective against WBI-induced injury associated with excessive activation of pro-inflammatory processes, crypt damage (Fig. 5A), and mouse death (Fig. 4C).
FIGURE 4. iPLA2γ inhibitor, R-BEL, mitigates against WBI-induced injury associated with activation of pro-inflammatory processes.
In (R)-BEL treated animals, the intestine returns to near normal (including the number of neutrophils) (A). Shown are all 3 intestines together (A) and the number of neutrophils quantified in each intestine (B). Each intestine was cut to a similar length. (R)-BEL is highly mitigative against the injury induced by whole-body irradiation (9.25 Gy) (C). WBI-induced generation of AA-derived pro-inflammatory mediators HXA3 and LTB4 is mitigated by (R)-BEL (D). R-BEL (6 mg/kg body weight) was introduced to mice (i.p.) on day 1 after WBI (9.5 Gy). Data are mean ± SD, N = 10–12, *P < 0.05 vs non-irradiated mice (D0), #P < 0.05 vs irradiated mice (D5), $P < 0.05 vs irradiated mice (D7), Kruskal–Wallis test
FIGURE 5. R-BEL mitigates against WBI-induced expression of iPLA2γ (PNLP8).
(A) (R)-BEL treatment decreased PNLP8 expression (green) and mitigated epithelial barrier disruption, as indicated by the continuity of the actin layer (red) particularly at the apex of the crypt (A). Notes: aside from the decreases in PNLP8 with R-BEL, one can also see the protection of the epithelial layer in the continuity of the actin (red) signal surrounding the crypts, which is clearly disrupted/discontinuous at days 3, 5, and 7 after radiation. (B) The levels of total CL (a), total arachidonoyl-CL (b), and arachidonoyl-CL molecular species (c and d) in the ileum of mice. R-BEL (6 mg/kg body weight) was introduced to mice (i.p.) on day 1 after WBI (9.5 Gy). Data are mean ± SD, N = 10–12, *P < 0.05 vs non-irradiated mice (D0), #P < 0.05 versus irradiated mice (D7), Kruskal–Wallis test
9.2 |. Inhalation exposure to single walled carbon nanotubes
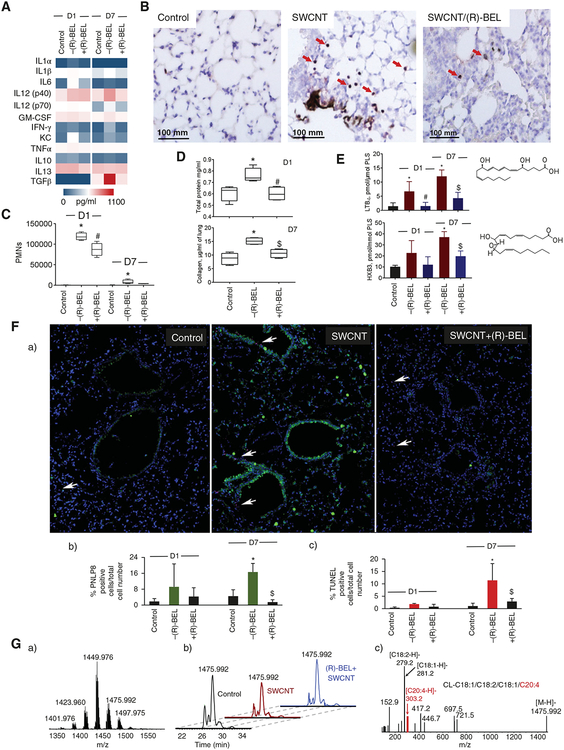
Inhalation of single walled carbon nanotubes (SWCNT) leads to a robust inflammation culminating in the early onset of pulmonary fibrosis and accumulation of oxygenated phospholipids.201,202 It includes the early arrival of neutrophils followed by the accumulation of macrophages and the emergence of enhanced positivity for collagen staining (Fig. 6). In terms of cytokines, a sharp spike in IL-1 α, IL-1β, IL-6, IL-12p40, IL-12p70, GM-CSF, INF-γ, KC, and TNF-α was substituted by the slower accumulation of anti-inflammatory cytokines (IL10, IL13, and TGF-β; Fig. 6A). Notably, this inflammatory response is also associated with the increased level of iPLA2γ (Fig. 6F) and accumulation of pro-inflammatory lipid mediators, LTB4 and HXB3 (Fig. 6E), in the lungs of exposed mice. Redox lipidomics analysis revealed that exposure to SWCNT results in changes in the content of AA-containing CL molecular species in the lung. (Fig. 6G). As this effect could be caused by CL oxidation and subsequent hydrolysis of the oxygenated CL species, we employed (R)-BEL to explore its possible protective effects (Fig. 6G). The iPLA2γ inhibitor dramatically suppressed the levels iPLA2γ in the lung (Fig. 6F) and markedly “softened” the accumulation of: (i) neutrophils (Fig. 6B and C), (ii) cytokines (Fig. 6A), and (iii) collagen accumulation (Fig. 6D). It also protected CL against hydrolysis thus preventing the release of AA and AA-derived pro-inflammatory lipid mediators. While this work provided unequivocal evidence for the involvement and essentiality of iPLA2γ in inflammatory responses, the demands for small molecule regulators as potential therapeutic modalities require exploration of specific pharmacological approaches. Overall, our phospholipidomics and redox lipidomics assessments illustrate the potential of these novel technological approaches in deciphering the leading features of pathogenesis and choosing new and effective therapeutic strategies
FIGURE 6. Effect of iPLA2γ inhibitor, (R)-BEL, on SWCNT-induced pulmonary inflammation.
(A) Production of pro- and anti-inflammatory cytokines in BAL of mice. Measurements were performed using Bio-Plex Pro Mouse Cytokine 23-Plex Immunoassay kit, composed of a combination of pro- and anti-inflammatory cytokines. (B) Representative images of lung sections from mice on day 1 post exposure to SWCNT or R-BEL alone, SWCNT/R-BEL together and control group (neutrophils indicated by arrows). (C) Average neutrophil counts per whole lung sections from 3 mice per group. (D) Tissue damage and fibrogenic response in BAL of mice. Pulmonary tissue damage was measured as protein in BAL of mice on day 1 after exposure to SWCNT (40 μg/mouse; a). Fibrogenic response was assessed on day 7 post exposure to SWCNT (40 μg/mouse) by the levels of collagen measured in the lung of mice (b). (E) Production of pro-inflammatory lipid mediators, LTB4 (a), and HXB3 (b) in lung of C57B6J mice. Structural formulas of LTB4 and HXB3 (inserts). (F) (R)-BEL treatment reversed the increases in PNLP8 expression (green) and cell death (TUNEL(magenta) positive nuclei, arrows) induced by SWCNT exposure in mouse lungs (a), % PNLP8 positive cells relative to total cell number (b), % TUNEL positive cells relative to total cell number (c). (G) MS spectrum of CL obtained from control mouse lung (a). Base peak chromatograms of CL molecular species with m/z 1475.992 from lung of control mouse and mouse exposed to SWCNT in the absence and in the presence of (R)-BEL on day 1 after treatment (b). MS2 spectrum of CL molecular species with m/z 1475.992 containing arachidonic acid (C20:4), CL-C18:1/C18:2/C18:1/C20:4, obtained from control mouse lung (c). All data are mean ± SD (n = 4–5 mice per group). *P < 0.05 versus control (non-exposed mice). #P < 0.05 versus –(R)-BEL at D1 (SWCNT exposed mice without (R)-BEL treatment), $P < 0.05 versus –(R)-BEL at D7 (SWCNT exposed mice without (R)-BEL treatment), one way ANOVA
10 |. PROGRAMS OF CELL DEATH IN THE CONTEXT OF INFLAMMATION
There are 2 important aspects of lipid mediators in conjunction with the execution of regulated cell death programs. The first one is related to the direct participation of oxygenated phospholipids in the realization of the specific types of programs. The second is associated with pro-/anti-inflammatory signals generated by dying cells dependent on the type of program. Oxidized lipids have gained a reputation as regulatory molecules and participants in several cell death signaling pathways. Knowledge in the field of cell death has greatly increased during the past 25 years that led to its better understanding at the biochemical, genetic, and immunological levels. Apoptosis is the first cell death modality that was described as a regulated (programmed) form of cell death. Cells undergoing apoptosis show typical, well-defined morphological changes, including plasma membrane bleb-bing, chromatin condensation, and formation of apoptotic bodies,203 which are efficiently cleared by phagocytes.204 At the biochemical level, apoptosis has been characterized by activation of a subfamily of cysteine proteases (known as caspases), nuclear fragmentation, and inter-nucleosomal DNA cleavage.205,206 The major caspase activation pathway is the cyt c-initiated pathway during which a variety of apoptotic stimuli cause cyt c release from mitochondria into the cytosol, leading to the induction of a series of biochemical reactions that culminate in caspase activation via its binding to apoptosis protease-activating factor Apaf1, and the formation of apoptsomes.207,208 Early in apoptosis, CL undergoes oxidation catalyzed by a cardiolipin-specific peroxidase activity of cyt c.57 The peroxidase function of cyt c requires its direct physical interaction with CL. Since CL displays transmembrane distribution asymmetry and it is normally residing almost exclusively in the IMM, binding of cyt c to CL depends on the availability of the latter in the outer leaflet of the IMM.209 Of note, pro-apoptotic CL oxidation is not random and has a specificity unrelated to the pattern of CL polyunsaturation.28,209 Importantly, CL oxidation is highly specific for the execution of apoptosis but not for other types of cell death modalities.
Apoptotic cells are efficiently cleared via efferocytosis by non-professional and professional phagocytes, thereby leading to the generation of anti-inflammatory or tolerogenic responses that are crucial for normal tissue homeostasis.210,211 However, under certain conditions or disease states, apoptotic cells can release DAMPs,212,213 which can modulate the immune system. It has been shown that cardiolipin, normally residing in the inner mitochondrial membrane, can be released into the extracellular milieu as a mitochondrial DAMP.214 CL blocks IL10-production causing persistent inflammation during bacterial pneumonia.215 Moreover, it has been shown that the oxidation state of CL may contribute to the modulation of inflammation.216
For many years apoptosis was contrasted to necrotic cell death, a passive process lacking underlying signaling events (i.e., often named as accidental necrosis) and occurring under extreme physicochemical conditions. However, in the course of the rapid advance in the field, recent new concepts arose, which placed necrosis in a new context of regulated cell death modalities. It is now obvious that regulated necrosis217 is an umbrella term for multiple types of regulated necrotic cell death modalities including necroptosis and ferroptosis.218 At the biochemical level, necroptosis is transduced by the kinase activities of receptor interacting protein kinase-1 (RIPK1), RIPK3, and the activation (i.e., phosphorylation and oligomerization) of mixed lineage kinase domain-like (MLKL) leading to the translocation of MLKL to lipid rafts in the plasma membrane.219 MLKL executes necroptosis by acting at the plasma membrane, through interactions with phosphatidylinositol phosphate (PIP)220,221 thereby inducing membrane destabilization and pore formation.222–224 Recently, it has been found that in addition to PIP, other lipids might be involved in necroptosis. Very long chain fatty acids have been shown to accumulate during necroptosis.225 In this study, the authors showed that the knockdown of fatty acid synthase and elongation of very long chain fatty acid protein 1 and 7 prevented necroptotic cell death. While this study suggested that lipids are involved in necroptosis, the precise molecular mechanisms and involvement of other classes of phospholipids and their oxidation products in necroptosis is still limited, and many more interesting and challenging findings are expected.
Necroptotic cells can also phagocytized by APCs, and can release DAMPs and cytokines/chemokines and thus are often pro-inflammatory or immunogenic.226–228 This becomes especially interesting as a potential alternative treatment strategy to overcome apoptosis resistance, which is often one of the hallmarks of cancer.229 However, information on the role of phospholipids and their oxidation products as immune modulators in necroptosis is limited and therefore more work is required to identify their role.
In contrast to apoptosis and necroptosis, ferroptosis is an iron-dependent form of cell death, which is characterized by antioxidant system dysfunction that leads to a lethal lipid peroxidation.230 Ferroptosis can be induced by conditions that either inhibit glutathione biosynthesis or the glutathione-dependent antioxidant enzyme glutathione peroxidase 4 (GPX4). GPX4 is a key enzyme that modifies potentially toxic lipid hydroperoxides (L-OOH) to nontoxic lipid alcohols (L-OH).231 It has been shown by using global redox phospholipidomics that several molecular species of hydroperoxyeicosatetraenoyl-phosphatidyl-ethanolamines (sn2-15-HpETE-PE) are the death signals and are involved in the execution of ferroptosis171,232 suggesting the participation of specific enzymatic mechanisms. Important in the context of this review, esterified sn2-15-HpETE-PE—but not free 15-HpETE—was shown to trigger ferroptosis in 15-LOX-deficient cells171,233 thus emphasizing the specific role of PE oxidation products in the execution of the ferroptotic program. Of considerable note is that the loss of function of ACSL4 and LPCAT3 gene products leads to the depletion of the phospholipid substrates for lipid peroxidation and inhibition of ferroptosis.171,234,235 It is important to mention that ferroptotic cell death can be modulated by nonheme, iron-containing enzymes such as LOXs.171,236 Ferroptosis is executed via oxygenation of polyunsaturated PE by 15-LOX.171,232 Indeed, inhibitors of lipoxygenases (a relatively selective 12/15-LOX inhibitor, baicalein, and the pan-LOX inhibitor nordihydroguaiaretic acid) protected acute lymphoblastic leukemia cells from ferroptosis.237 Also, the knockdown of 15-LOX significantly decreased, whereas exogenous overexpression of 15-LOX enhanced, ferroptotic cell death238 suggesting that ferroptotic cell death is modulated by LOX-catalyzed lipid hydroper-oxide. Experiments with exogenously added bacterial 15-LOX, (from P. aeruginosa) directly proved its sufficiency to trigger ferroptosis in co-incubated recipient HBE cells.233 It has been discovered that a scaffold protein inhibitor of protein kinase cascades, PE binding protein-1 (PEBP1) complexes with 2 15-LOX isoforms, 15-LOX-1 and 15-LOX-2, and changes their substrate competence from free ETE to generate sn2–15-HpETE-PE.65 However, until recently it was not clear how the enzymatic complex selects sn2-15-HpETE-PE among many oxidizable membrane PUFA phospholipids. Several factors determine the selective and specific production of peroxidation sn2-15-HpETE-PE by 15-LOX/PEBP1 complexes in ferroptosis. These include a higher enzyme reactivity toward hexagonally organized ETE-PE, allosteric modification of the enzyme in the 15-LOX-2/PEBP1 complex, and relative prevalence of ETE-PE species versus other oxidizable molecular species of PE and other phospholipids.67 This study underlines the role of enzymatic versus random stochastic free radical reactions in ferroptosis. Thus, 2 types of cell death programs—apoptosis and ferroptosis—are executed with the required selective and specific enzymatic peroxidation of oxidizable PUFA residues esterified into phospholipids, CL and PE, respectively.
Suppression of ferroptosis by small molecules (e.g., ferrostatin-1) can be beneficial to reduce cellular and tissue damage and thus inflammation (i.e., necroinflammation) in several preclinical animal disease models.239–241 For example, preventing ferroptosis in the model of nephrotoxic folic acid-induced acute kidney injury significantly decreased signs of inflammation such as IL-33 levels and the infiltration of F4/80+ macrophages.242 Moreover, chemically induced up-regulation of GPX-4 activity can reduce the production of inflammatory mediators and promote inflammation resolution pointing for a promising therapeutic strategy in lipid –peroxidation-mediated diseases.172
11 |. TIME-DEPENDENT ALTERNATING CASCADES OF LIPID SIGNALING AND CELL DEATH
While detailed redox lipidomics analysis performed on cell systems identified the phospholipid peroxidation products associated with the execution of specific death programs, in vivo acute injury and chronic degenerative processes are characterized by simultaneous death programs occurring in different types of cells along with pro-/anti-inflammatory responses. As a result, overlapping patterns of (phospho)lipid oxidation are typically found in these conditions.82,243–247
11.1 |. Traumatic brain injury
Traumatic brain injury (TBI)-induced damage triggers a complex and time coordinated series of cellular events. The brain is rich in PUFA containing phospholipids, enabling the production of a wide range of lipid mediators to regulate intra- and intercellular communications.248 The earlier response after the mechanical impact is marked with the surge of oxidized free fatty acids due to Ca2+ influx and activation of Ca2+-dependent phospholipases249 as well as LOXes and COXes.65 Very early after the injury, the levels of both pro- and anti-inflammatory mediators are elevated. However, pro inflammatory signals such as 9-HODE, PGF2α, 5-HETE overpower the anti-inflammatory signals,250 leading to the pro-inflammatory environment. Among the early pro-inflammatory events, are microglia activation,251 blood-brain barrier (BBB) damage,252 and neutrophil infiltration.253 Redox lipidomics studies documented the occurrence of typical oxidized apoptosis biomarkers, such as oxygenated CL species followed the appearance of ferroptotic markers, hydroperoxyarachidonoyl-PE. Notably, both the anti-apoptotic electron scavenger, XJB-125 and an anti-ferroptotic lipoxygenase inhibitor, baicalein, attenuated the damage by inhibiting the formation of the ferroptotic phospholipid peroxidation products.57,254 Notably, at later stages of TBI, peroxidation of yet another phospholipid, phosphatidylserine, takes place.255 This PSox plays an essential role in efferocytosis, macrophage-dependent phagocytosis of apoptotic cells.181 During this stage of the TBI process, higher contents of anti-inflammatory oxidized free fatty acids are observed suggesting a switch to an anti-inflammatory environment.250
In spite of the complexity and overlap of the numerous aberrant mechanisms and pathways triggered by disease conditions, the high resolution power of redox-lipidomics allows, at least in some cases, the ability to detect characteristic products of phospholipid peroxidation in disease conditions in vivo. While still limited in number, there are several examples of successful applications of redox lipidomics that have detected oxygenated phospholipid biomarkers of ferroptosis in several tissues. These include kidney epithelium in animals and humans,65,171 pulmonary airway epithelium in cystic fibrosis, and lower respiratory infections and asthma.65,233
11.2 |. Utilization of lipid signaling mechanisms of regulated cell death pathways by bacterial pathogens
Bacterial pathogens have contrived numerous strategies to target and divert host lipids as well as lipid driven signaling pathways for their benefit. Some pathogens including Mycobacterium tuberculosis, Salmonella typhimurium, Helicobacter pylori, and Legionella pneumophila, after their interaction with the host, integrate host lipids like cholesterol, phosphatidylcholine, or sphingolipids into their membranes to ease the process of internalization and escape host immune surveillance, phagocytosis, and T cell activation.256,257 On the other hand, pathogens like Pseudomonas aeruginosa, in addition to utilizing a host lipid integration strategy, produce different types of virulence factors: (i) toxins secreted and delivered into the host’s cytoplasm by endocytosis or the formation of pores; and (ii) effectors delivered directly into the host cell by highly regulated needle-like delivery systems (type III and type IV secretion systems) or secreted as vesicles (outer membrane vesicles, OMV), which interact and fuse with lipid rafts in the host’s plasma membrane.258 To disseminate and to evade the host’s immune system, Pseudomonas aeruginosa utilizes secreted virulence factors (pyocyanin, exotoxin A, Protease) and type-III secretion system (T3SS) injected effectors (exoenzymes- ExoS, T, U, Y) to induce apoptosis in target cells (neutrophils, macrophages, epithelial/fibroblast cells) by generating ROS, activating the mitochondrial pathway and caspase 3.257 Importantly, both in vitro (macrophages, neutrophils, epithelial cells) and in vivo (lungs, cornea, and burn wounds) systems suggest that the ability of Pseudomonas aeruginosa to induce apoptosis is mediated by the multicellular population of Pseudomonas aeruginosa and not by a single bacterial cell.259 In fact, a recent report suggests that Pseudomonas aeruginosa quorum-sensing auto inducer N-(3-oxo-dodecanoyl) homoserine lactone triggers apoptotic cell death in lymphocytes by the interaction and dissolution of the host’s lipid domains.260 On the other hand, under conditions promoting biofilm growth, Pseudomonas aeruginosa has been shown to secrete OMVs containing virulence factors including a lipoxygenase (pLoxA), which induces ferroptotic cell death in host HBE cells.52 pLoxA interacts with and utilizes host PUFA-PE and their metabolizing enzymes (ACSL4, LPCAT3) to generate ferroptotic death signals sn-2-15-HpETE-PE in HBE cells. These death signals propagate to neighboring cells in a cell-autonomous fashion thereby enhancing colonization. Interestingly, sn-2-15-HpETE-PE signals were also detected in cystic fibrosis (CF) lung tissue samples infected with Pseudomonas aeruginosa but not in CF without Pseudomonas aeruginosa infection.233
12 |. CONCLUDING REMARKS AND FUTURE PERSPECTIVES
Lipidomics and its newer branch, redox lipidomics, are making their first timid steps toward identification and characterization of the complete picture of remarkably diversified lipid signaling in response to injurious pro-inflammatory insults. In spite of the new technological advancements in MS, the enormous diversity of oxygenated species of phospholipids complicates their detailed quantitative analysis. Yet, not only has there been significant success in their structural characterization but also MS-based high-resolution imaging of lipids at the cellular and subcellular level has become a reality. With the understanding that obtaining comprehensive information about thousands of interactive reactions and pleiotropic (patho)physiological effects is a daunting task, there is still reasonable hope that significant discoveries of at least some of the important contributors to the overall overwhelmingly complex network of interactions will lead to the discovery of new small molecule regulators and therapeutic modalities. Further technological developments promise to make redox lipidomics a powerful approach in the arsenal of diagnostic and therapeutic instruments for personalized medicine.
13 |. MATERIALS AND METHODS
13.1 |. Reagents
All reagents were purchased from Fisher Scientific (Pittsburgh, PA) unless otherwise indicated. SWCNT were purchased from CNI Inc. (San Francisco, CA); Hematoxylin QS, BLOXALL endogenous peroxidase, and alkaline phosphatase blocking solution were purchased from Vector Laboratories (Burlingame, CA); Hoechst from Sigma–Aldrich (St. Louis, MO), TUNEL was purchased from Roche (Indianapolis, IN); Lactate Dehydrogenase reagent set from Pointe Scientific (Canton, MI); Sircol Collagen Assay kit from Accurate Chemical and Scientific Corporation Inc. (Westbury, NY).
13.2 |. Mice
C57BL/6NHsd female mice and C57BL/6J female mice (7–8 weeks) were from Jackson Laboratories (Bar Harbor, ME). All experimental procedures were conducted in accordance with the guidelines and policy set forth by the Institute of Laboratory Animal Resources, National Research Council and approved by the NIOSH Institutional Animal Care and Use Committee (IACUC, protocol number 16-AS-M-014) and the protocols established by the Institutional Animal Care and Use Committee of the University of Pittsburgh (IACUC, protocol number 18022000).
13.3 |. Whole body irradiation
Groups of control C57BL/6NHsd female mice were irradiated with 9.5 Gy using a J. L. Shepherd Mark 1 Model 68 cesium irradiator at a dose rate of 80 cGy/min as described previously.244,245 Mice received the Ca2+-independent iPLA2 inhibitor (R)-BEL (6E-(bromoethylene)tetrahydro-3R-(1-naphthalenyl)-2H-pyran-2-one, (R)-bromoenol lactone) at a dose of 6 mg/kg body weight by i.p. 24 h after WBI. Mice were euthanized 1, 2, 5, and 7 days later by CO2 inhalation.
13.4 |. SWCNT aspiration and exposure to (R)-BEL
Mouse pharyngeal aspiration was used for SWCNT administration.261 SWCNT (CNI Inc.) were produced by the high pressure CO (HiPco) disproportionation technique, employing CO in a continuous-flow gas phase as the carbon feedstock and Fe(CO)5 as the iron-containing catalyst precursor.262 Purified SWCNT were prepared by acid treatment to remove metal contaminates.263 The mean diameter and surface areas of SWCNT were 1–4 nm and 1040 m2/g, respectively. After anesthesia using a mixture of ketamine (Phoenix) and xylazine (Phoenix) (62.5 and 2.5 mg/kg subcutaneous in the abdominal area), the mouse was placed on a board in a near vertical position and the animal’s tongue was extended with lined forceps. A suspension of SWCNT (40 μg/mouse) was placed posterior in the throat and the tongue held until the suspension was aspirated into the lungs. Mice were administered R-BEL (6 mg/kg) via intraperitoneal injection on day 0. The mice in the 7 days post exposure group continued to receive R-BEL injections every other day (day 2, 4, 6), resulting in 4 injections. Mice were sacrificed with intraperitoneal injection of sodium pentobarbital (>100 mg/kg) and exsanguinated. At each time point (1 and 7 days) the samples were collected for inflammation, pulmonary damage, and fibrogenesis.
13.5 |. Cytokines/chemokines, cell differentials, and tissue damage
The trachea was cannulated with a blunted 22 gauge needle, and BAL was performed using cold sterile PBS at a volume of 0.9 mL for first lavage (kept separate) and 1.0 mL for subsequent lavages. Approximately 5 mL of BAL fluid per mouse was collected in sterile centrifuge tubes. Pooled BAL cells for each individual mouse were washed in PBS by alternate centrifugation (800 × g, 10 min, 4°C). Cells were resus-pended in PBS and total cells were determined using a Multisizer 3 Coulter Counter (Coulter Multisizer 3, Beckman Coulter Life Sciences). Following the cytospin, slides with BAL cells were stained with HEMA 3 system (ThermoFisher). Cytospin slides were analyzed for cell differentials by light microscopy and evaluated using the Olympus Cell Sens Dimension software (Tokyo, Japan). At least 350 cells per slide were counted for each sample. The activity of lactate dehydrogenase (LDH) in BAL fluid was assayed using a Synergy H1 Hybrid Reader (BioTek). The reduction of nicotinamide adenine dinucleotide in the presence of lactate to pyruvate using a lactate dehydrogenase reagent set (Pointe Scientific) was monitored at 340 nm. Measurement of total protein in the BAL fluid was performed using a modified Bradford assay according to the manufacturer’s instructions (Bio-Rad) with BSA as the standard control. The levels of cytokines, chemokines, and growth factors in BAL fluids were measured using a 23-Plex mouse cytokine assay kit and TGF-β 3-plex assays (Bio Rad) employing a Bio-Plex 200 System (Bio-Rad). The concentrations were calculated using Bio-Plex Manager 6.1 software from standard curves.
13.6 |. Immunohistochemistry
Lung tissues were harvested and inflation fixed in situ with 4% paraformaldehyde at constant pressure of 10 cm H2O for 10 min with the chest cavity open. Coronal sections were cut from the lungs, embedded in paraffin, and sectioned at a thickness of 5 μm with an HM 320 rotary microtome (Carl Zeiss). Lung sections were deparaffinized and used for immunohistochemistry. Slides were incubated with BLOXALL Endogenous Peroxidase and Alkaline Phosphatase Blocking Solution (Vector Laboratories) for 10 min at room temperature to block endogenous peroxidase activity. The assay was performed with the ImmPRESS Polymer Detection system (Vector Laboratories). ImmPACT NovaRED (Vector Laboratories) was applied as the peroxidase substrate. Slides were counterstained with Hematoxylin QS (Vector Laboratories) to visualize nuclei with a blue-violet color. Anti-neutrophil mAb (NIMP-R14, Abcam), highly specific for murine Ly-6G and Ly-6C, was used as a primary Ab. Images were taken using an Invitrogen EVOS FL Auto 2 Cell Imaging Systems (ThermoFisher). Neutrophils were counted per whole lung section from 3 mice per experimental group.
13.7 |. Lung collagen measurements
Total lung collagen content was determined by quantifying total soluble collagen using the Sircol Collagen Assay kit (Accurate Chemical and Scientific Corporation). Briefly, lungs were homogenized in 0.7 mL of 0.5 M acetic acid containing pepsin (Accurate Chemical and Scientific Corporation) with a 1:10 ratio of pepsin: tissue wet weight. Each sample was stirred vigorously for 24 h at 4°C, centrifuged, and 200 μL of supernatant was assayed according to the manufacturer’s instructions.
13.8 |. Immunostaining and confocal microscopy
Tissues were fixed in 2% paraformaldehyde at 4°C for 1–2 h and cryo-sectioned. The 7 micron sections were affixed to slides and permeabilized with 0.1% Triton X-100 in PBS+ 0.5% BSA (PBB) for 15 min. Tissue sections were then blocked with 5% donkey serum for 45 min and incubated for 2 h at room temperature with the primary antibodies PNLP8 (ThermoFisher, PA5–54151), TUNEL (Roche, 03333566001), and for 1 h with Alexa 488 and streptavidin 488 (ThermoFisher, A21206 and S11223, respectively). Sections were counterstained with Hoechst (Sigma B2883) 1 mg/100 mL dH20 and phalloidin (ThermoFisher, R415), mounted using Gelvatol, and high resolution (60 × 1.43NA) large area montages (5 × 5 fields) were collected using a Nikon A1 confocal equipped with GAsP detectors. Data acquisition and analysis was performed using NIS Elements software (Nikon Inc).
13.9 |. Tissue clearing and ribbon scanning confocal microscopy
Tissues were fixed overnight in 4% paraformaldehyde at 4°C and then washed in PBS containing 0.02% sodium azide. Large segments of duodenum were then optically cleared using the CUBIC R1/R2 method (PMID 30005145, 24746791) for 1 week at 37°C. The tissues were then scanned using the RS-G4 ribbon scanning confocal (Caliber ID, Rochester NY) fitted with a 20×/1.00 Glyc (CFI90 20XC, Nikon), correction collar set to 1.44. Linear interpolation of 488 nm laser excitation (iChrome-MLE-LFA, Toptica) was set between 10% and 20% power, top to bottom of z-stack. Emission was detected using a 520/40 band-pass filter, PMT settings were HV, 85; offset, 5. Voxels were measured (0.495 × 0.495 × 12.2 μm). Each sample required approximately 24 h of total acquisition time. Imagery was collected at a 16 bit pixel depth and comprised approximately 1 TB per segment. Cells were quantified using the spot count function in Imaris Surpass using the same parameters for all groups. The spots were used as a 3D mask to color each of the cells differently (green) from the surrounding autofluorescent tissue (red).
13.10 |. LC-MS analysis of cardiolipin
Cardiolipin was extracted by solid phase extraction as described264 and molecular species of cardiolipin were analyzed by LC-ESI-MS/MS as previously described.28,265 MS analysis of cardiolipin molecular species was performed on an Orbitrap™ Fusion™ Lumos™ mass spectrometer (ThermoFisher). Cardiolipins were separated on a C18 column Luna (2) 3 μm, 100 Å, 150 × 1 mm (Phenomenex) at a flow rate of 0.050 mL/min on a Dionex Ultimate 3000 HPLC system. The column was eluted using gradient of solvent system consisting of mobile phase A (acetonitrile/water/triethylamine, 45/5/2.5 v/v) and B (2-propanol/water/trimethylamine, 45/5/2.5 v/v). Both mobile phases contained 5 mM acetic acid and 0.01% formic acid. The resolution was set up at 140,000 that corresponds to 5 ppm in m/z measurement error. m/z Values for CLs and their oxidation species are presented to 4 decimal points. TMCL (Avanti Polar Lipids) was used as an internal standard. Minimum 3 technical replicates for each sample were run to evaluate reproducibility.
13.11 |. LC-MS analysis of lipid mediators
LTB4 and HXA3 were analyzed by LC-MS using a Dionex Ultimate™ 3000 HPLC system coupled on-line to Q-Exactive hybrid quadrupole-orbitrap mass spectrometer (ThermoFisher Scientific, San Jose, CA) using a C18 column (Accliam PepMap RSLC, 300 μm 15 cm, Thermo Scientific). Gradient solvents A: methanol (20%)/water (80%) (v/v) and B: methanol (90%)/water (10%) (v/v), both containing 5 mM ammonium acetate, were used. The column was eluted at a flow rate of 12 μL/min using a linear gradient from 30% solvent B to 95% solvent B over 70 min, held at 95% B from 70 to 80 min followed by a return to initial conditions by 83 min and re-equilibration for an additional 7 min. Spectra were acquired in negative ion mode. The scan range for MS analysis was 150–600 m/z with a maximum injection time of 100 ms using 1 microscan and a resolution of 140,000. An isolation window of 1.0 Da was set for the MS and MS2 scans with an inclusion list of 102 potential oxidized and non-oxidized fatty acyl products. Capillary spray voltage was set at 2.6 kV, and capillary temperature was 250°C. The S-lens Rf level was set to 60. Analytical data were acquired and analyzed using Xcalibur software. Minimum 3 technical replicates for each sample were run to evaluate reproducibility.
13.12 |. Statistical analysis
Statistical analyses were performed by one way ANOVA for normally distributed samples and by Kruskal-Wallis test for the data that were not normally distributed. The statistical significance of differences was set at P < 0.05. All the statistical analyses were performed using Graph-Pad Prism software. In the box and whisker plots, the lines in the boxes represent the first quartile, median, and third quartile values. The whiskers represent the minimum and maximum values. In bar graphs, the values are represented as mean ± SD. The number of samples used in each group was denoted in the figure legends.
ACKNOWLEDGMENTS
This work was supported by NIH (U19AI068021, HL114453, NS076511, NS061817, and CA165065) and by Russian academic excellence project “5–100.”
Abbreviations:
- 5-oxo-ETE
5-oxo-6,8,11,14-eicosatetraenoic acid
- AA
arachidonic acid
- ACSL4
acyl-CoA synthase 4
- CL
cardiolipin
- COX
cyclooxygenase
- cPLA2
cytosolic calcium-dependent PLA2
- Cygb
cytoglobin
- cyt c
cytochrome c
- DAMPs
danger associated molecular patterns
- DHA
docosahexaenoic acid
- EET
epoxy-eicosatrienoic
- EPA
eicosapentaenoic acid
- FA
fatty acids
- GPX4
glutathione peroxidase 4
- HETE
hydroxy-eicosatetraenoic acid
- HETE–PE
hydroxy-eicosatetraenoyl–phosphatidylethanolamine
- HOBr
hypobromite
- HOCl
hypochlorite
- HpETE
hydroperoxy-eicosatetraenoic acid
- HpETE–PE
hydroperoxy-eicosatetraenoyl–phosphatidylethanolamine
- HXA3
hepoxilin A3
- iPLA2
Ca2+-independent phospholipase A2
- KETE
keto-eicosatetraenoic acid
- LC-MS
liquid chromatography-mass spectrometry
- LOX
lipoxygenase
- LPCAT3
lysophosphatidylcholine-acyl-transferase
- LpPLA2
lipoprotein-associated PLA2
- LTB4
leukotriene B4
- MaR1
maresins
- MLKL
mixed lineage kinase domain-like
- MM-LDL
minimally modified-low density lipoproteins
- MUFA
monounsaturated fatty acids
- PA
phosphatidic acid
- PC
phosphatidylcholine
- PE
phosphatidylethanolamine
- PEBP1
phosphatidylethanolamine binding protein 1
- PG
phosphatidylglycerol
- PGH2
prostaglandin H2
- PGPC
1-palmitoyl-2-glutaroylsn-glycero-3-phosphorylcholine
- PI
phosphatidylinositol
- PLA2
phospholipase A2
- POVPC
1-palmitoyl-2-(5,6-epoxyisoprostane E2)-sn-glycero-3-phosphocholine, 1-palmitoyl-2-(5-oxovaleroyl)-sn-glycero-3-phosphoryl-choline
- PS
phosphatidylserine
- PUFA
polyunsaturated fatty acid
- RIPK1
(R)-E-6-(Bromoethylene)tetrahydro-3-(1-naphthyl)-2H-pyran-2-one
- (R)-BEL
receptor interacting protein kinase-1
- ROS
reactive oxygen species
- RvD1-RvD4
resolvins
- SFA
Saturated fatty acids
- sPLA2
secreted PLA2
Footnotes
DISCLOSURE
The authors declare no conflict of interest. The content and conclusions of this publication are those of the authors and do not necessarily reflect the views or policies of the National Institute for Occupational Safety and Health, Centers for Disease Control and Prevention, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. government. The authors declare no competing financial interest.
REFERENCES
- 1.Hulbert AJ, Kelly MA, Abbott SK. Polyunsaturated fats, membrane lipids and animal longevity. J Comp Physiol B. 2014;184:149–166. [DOI] [PubMed] [Google Scholar]
- 2.Castro LF, Tocher DR, Monroig O. Long-chain polyunsaturated fatty acid biosynthesis in chordates: insights into the evolution of Fads and Elovl gene repertoire. Prog Lipid Res. 2016;62:25–40. [DOI] [PubMed] [Google Scholar]
- 3.Nakamura MT, Nara TY. Structure, function, and dietary regulation of delta6, delta5, and delta9 desaturases. Annu Rev Nutr. 2004;24: 345–376. [DOI] [PubMed] [Google Scholar]
- 4.Meesapyodsuk D, Qiu X. The front-end desaturase: structure, function, evolution and biotechnological use. Lipids. 2012;47:227–237. [DOI] [PubMed] [Google Scholar]
- 5.Hulbert AJ, Turner N, Storlien LH, Else PL. Dietary fats and membrane function: implications for metabolism and disease. Biol Rev Camb Philos Soc. 2005;80:155–169. [DOI] [PubMed] [Google Scholar]
- 6.Crawford MA, Broadhurst CL, Cunnane S, et al. Nutritional armor in evolution: docosahexaenoic acid as a determinant of neural, evolution and hominid brain development. Mil Med. 2014;179:61–75. [DOI] [PubMed] [Google Scholar]
- 7.Russell NJ, Nichols DS. Polyunsaturated fatty acids in marine bacteria–a dogma rewritten. Microbiology. 1999;145(Pt 4): 767–779. [DOI] [PubMed] [Google Scholar]
- 8.Siliakus MF, van der Oost J, Kengen SWM. Adaptations of archaeal and bacterial membranes to variations in temperature, pH and pressure. Extremophiles. 2017;21:651–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dailey FE, McGraw JE, Jensen BJ, et al. The microbiota of freshwater fish and freshwater niches contain omega-3 fatty acid-producing Shewanella species. Appl Environ Microbiol. 2016;82:218–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Quehenberger O, Armando AM, Brown AH, et al. Lipidomics reveals a remarkable diversity of lipids in human plasma. J Lipid Res. 2010;51:3299–3305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.An JU, Hong SH, Oh DK. Regiospecificity of a novel bacterial lipoxygenase from Myxococcus xanthus for polyunsaturated fatty acids. Biochim Biophys Acta Mol Cell Biol Lipids. 2018;1863:823–833. [DOI] [PubMed] [Google Scholar]
- 12.Halliwell B, Gutteridge JMC. Free Radicals in Biology and Medicine. 5th ed Oxford, UK: Clarendon Press; 2015. [Google Scholar]
- 13.Fischer WW, Hemp J, Valentine JS. How did life survive Earth’s great oxygenation? Curr Opin Chem Biol. 2016;31:166–178. [DOI] [PubMed] [Google Scholar]
- 14.Lane N Oxygen—the molecule that made the world. Oxford Landmark Science. 2016. [Google Scholar]
- 15.Gutteridge JMC, Halliwell B. Mini-review: oxidative stress, redox stress or redox success? Biochem Biophys Res Commun. 2018;502:183–186. [DOI] [PubMed] [Google Scholar]
- 16.Hosseinzadeh P, Lu Y. Design and fine-tuning redox potentials of metalloproteins involved in electron transfer in bioenergetics. Biochim Biophys Acta. 2016;1857:557–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Valko M, Jomova K, Rhodes CJ, Kuca K, Musilek K. Redox- and non-redox-metal-induced formation of free radicals and their role in human disease. Arch Toxicol. 2016;90:1–37. [DOI] [PubMed] [Google Scholar]
- 18.Halliwell B, Gutteridge JM. Role of iron in oxygen radical reactions. Methods Enzymol. 1984;105:47–56. [DOI] [PubMed] [Google Scholar]
- 19.Halliwell B, Gutteridge JM. Oxygen toxicity, oxygen radicals, transition metals and disease. Biochem J. 1984;219:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Halliwell B, Gutteridge JM. Oxygen free radicals and iron in relation to biology and medicine: some problems and concepts. Arch Biochem Biophys. 1986;246:501–514. [DOI] [PubMed] [Google Scholar]
- 21.Halliwell B, Gutteridge JM. The antioxidants of human extracellular fluids. Arch Biochem Biophys. 1990;280:1–8. [DOI] [PubMed] [Google Scholar]
- 22.Bochkov V, Gesslbauer B, Mauerhofer C, Philippova M, Erne P, Oskolkova OV. Pleiotropic effects of oxidized phospholipids. Free Radic Biol Med. 2017;111:6–24. [DOI] [PubMed] [Google Scholar]
- 23.Abe A, Hiraoka M, Ohguro H, Tesmer JJ, Shayman JA. Preferential hydrolysis of truncated oxidized glycerophospholipids by lysosomal phospholipase A2. J Lipid Res. 2017;58:339–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tselepis AD. Oxidized phospholipids and lipoprotein-associated phospholipase A(2) as important determinants of Lp(a) functionality and pathophysiological role. J Biomed Res. 2018;32:13–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tyurin VA, Yanamala N, Tyurina YY, Klein-Seetharaman J, Macphee CH, Kagan VE. Specificity of lipoprotein-associated phospholipase A(2) toward oxidized phosphatidylserines: liquid chromatography-electrospray ionization mass spectrometry characterization of products and computer modeling of interactions. Biochemistry. 2012;51:9736–9750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vankuijk FJGM, Sevanian A, Handelman GJ, Dratz EA. A new role for phospholipase-A2—protection of membranes from lipidperoxidation damage. Trends Biochem Sci. 1987;12:31–34. [Google Scholar]
- 27.Salgo MG, Corongiu FP, Sevanian A. Enhanced interfacial catalysis and hydrolytic specificity of phospholipase A2 toward peroxidized phosphatidylcholine vesicles. Arch Biochem Biophys. 1993;304: 123–132. [DOI] [PubMed] [Google Scholar]
- 28.Tyurina YY, Poloyac SM, Tyurin VA, et al. A mitochondrial pathway for biosynthesis of lipid mediators. Nat Chem. 2014;6:542–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schneider C, Pratt DA, Porter NA, Brash AR. Control of oxygenation in lipoxygenase and cyclooxygenase catalysis. Chem Biol. 2007;14:473–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Imlay JA. Pathways of oxidative damage. Annu Rev Microbiol. 2003;57:395–418. [DOI] [PubMed] [Google Scholar]
- 31.Jomova K, Vondrakova D, Lawson M, Valko M. Metals, oxidative stress and neurodegenerative disorders. Mol Cell Biochem. 2010;345:91–104. [DOI] [PubMed] [Google Scholar]
- 32.Reis A, Spickett CM. Chemistry of phospholipid oxidation. Biochim Biophys Acta. 2012;1818:2374–2387. [DOI] [PubMed] [Google Scholar]
- 33.Rosa R, Spinozzi F, Itri R. Hydroperoxide and carboxyl groups preferential location in oxidized biomembranes experimentally determined by small angle X-ray scattering: implications in membrane structure. Biochim Biophys Acta Biomembr. 2018;1860:2299–2307. [DOI] [PubMed] [Google Scholar]
- 34.Makky A, Tanaka M. Impact of lipid oxidization on biophysical properties of model cell membranes. J Phys Chem B. 2015;119:5857–5863. [DOI] [PubMed] [Google Scholar]
- 35.Parra-Ortiz E, Browning KL, Damgaard LSE, et al. Effects of oxidation on the physicochemical properties of polyunsaturated lipid membranes. J Colloid Interface Sci. 2019;538:404–419. [DOI] [PubMed] [Google Scholar]
- 36.Heuvingh J, Bonneau S. Asymmetric oxidation of giant vesicles triggers curvature-associated shape transition and permeabilization. Biophys J. 2009;97:2904–2912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Runas KA, Malmstadt N. Low levels of lipid oxidation radically increase the passive permeability of lipid bilayers. Soft Matter. 2015;11:499–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Caetano W, Haddad PS, Itri R, et al. Photo-induced destruction of giant vesicles in methylene blue solutions. Langmuir. 2007;23: 1307–1314. [DOI] [PubMed] [Google Scholar]
- 39.Vahaheikkila M, Peltomaa T, Rog T, Vazdar M, Poyry S, Vattulainen I. How cardiolipin peroxidation alters the properties of the inner mitochondrial membrane. Chem Phys Lipids. 2018;214:15–23. [DOI] [PubMed] [Google Scholar]
- 40.Stark G Functional consequences of oxidative membrane damage. J Membr Biol. 2005;205:1–16. [DOI] [PubMed] [Google Scholar]
- 41.Kagan VE. Lipid Peroxidation in Biomembranes. Boca Raton, FL: CRC Press; 1988. [Google Scholar]
- 42.Ahuja RP, Borchman D, Dean WL, et al. Effect of oxidation on Ca2+-ATPase activity and membrane lipids in lens epithelial microsomes. Free Radic Biol Med. 1999;27:177–185. [DOI] [PubMed] [Google Scholar]
- 43.Castilho RF, Carvalho-Alves PC, Vercesi AE, Ferreira ST. Oxidative damage to sarcoplasmic reticulum Ca(2+)-pump induced by Fe2+/H2O2/ascorbate is not mediated by lipid peroxidation or thiol oxidation and leads to protein fragmentation. Mol Cell Biochem. 1996;159:105–114. [DOI] [PubMed] [Google Scholar]
- 44.Frazer DM, Anderson GJ. The regulation of iron transport. Biofactors. 2014;40:206–214. [DOI] [PubMed] [Google Scholar]
- 45.Bonaccorsi di Patti MC, Cutone A, Polticelli F, et al. The ferroportinceruloplasmin system and the mammalian iron homeostasis machine: regulatory pathways and the role of lactoferrin. Biometals. 2018;31:399–414. [DOI] [PubMed] [Google Scholar]
- 46.Stoyanovsky DA, Tyurina YY, Shrivastava I, et al. Iron catalysis of lipid peroxidation in ferroptosis: regulated enzymatic or random free radical reaction? Free Radic Biol Med. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Swaminathan S Iron homeostasis pathways as therapeutic targets in acute kidney injury. Nephron. 2018;140:156–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Meynard D, Babitt JL, Lin HY. The liver: conductor of systemic iron balance. Blood. 2014;123:168–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Todorovic S, Teixeira M. Resonance Raman spectroscopy of Fe-S proteins and their redox properties. J Biol Inorg Chem. 2018;23: 647–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rouault TA, Maio N. Biogenesis and functions of mammalian iron-sulfur proteins in the regulation of iron homeostasis and pivotal metabolic pathways. J Biol Chem. 2017;292:12744–12753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xu S, Mueser TC, Marnett LJ. Crystal structure of 12-lipoxygenase catalytic-domain-inhibitor complex identifies a substrate-binding channel for catalysis. Structure. 2012;20:1490–1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ding XZ, Hennig R, Adrian TE. Lipoxygenase and cyclooxygenase metabolism: new insights in treatment and chemoprevention of pancreatic cancer. Mol Cancer. 2003;2:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Spector AA. Arachidonic acid cytochrome P450 epoxygenase pathway. J Lipid Res. 2009;50(Suppl):S52–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gilroy DW, Edin ML, De Maeyer RP, et al. CYP450-derived oxylipins mediate inflammatory resolution. Proc Natl Acad Sci USA. 2016;113:E3240–3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Marnett LJ. Cyclooxygenase mechanisms. Curr Opin Chem Biol. 2000;4:545–552. [DOI] [PubMed] [Google Scholar]
- 56.Tejero J, Kapralov AA, Baumgartner MP, et al. Peroxidase activation of cytoglobin by anionic phospholipids: mechanisms and consequences. Biochim Biophys Acta. 2016;1861:391–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kagan VE, Tyurin VA, Jiang J, et al. Cytochrome c acts as a cardiolipin oxygenase required for release of proapoptotic factors. Nat Chem Biol. 2005;1:223–232. [DOI] [PubMed] [Google Scholar]
- 58.Reeder BJ, Svistunenko DA, Wilson MT. Lipid binding to cytoglobin leads to a change in haem co-ordination: a role for cytoglobin in lipid signalling of oxidative stress. Biochem J. 2011;434:483–492. [DOI] [PubMed] [Google Scholar]
- 59.Gaffney BJ. Connecting lipoxygenase function to structure by electron paramagnetic resonance. Acc Chem Res. 2014;47:3588–3595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Newcomer ME, Brash AR. The structural basis for specificity in lipoxygenase catalysis. Protein Sci. 2015;24:298–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bochkov VN, Oskolkova OV, Birukov KG, Levonen AL, Binder CJ, Stockl J. Generation and biological activities of oxidized phospholipids. Antioxid Redox Signal. 2010;12:1009–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Thomas CP, O’Donnell VB. Oxidized phospholipid signaling in immune cells. Curr Opin Pharmacol. 2012;12:471–477. [DOI] [PubMed] [Google Scholar]
- 63.Kagan VE, Mao G, Qu F, et al. Oxidized arachidonic and adrenic PEs navigate cells to ferroptosis. Nat Chem Biol. 2017;13:81–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Suardiaz R, Masgrau L, Lluch JM, Gonzalez-Lafont A. An insight into the regiospecificity of linoleic acid peroxidation catalyzed by mammalian 15-lipoxygenases. J Phys Chem B. 2013;117:3747–3754. [DOI] [PubMed] [Google Scholar]
- 65.Wenzel SE, Tyurina YY, Zhao J, et al. PEBP1 wardens ferroptosis by enabling lipoxygenase generation of lipid death signals. Cell. 2017;171:628–641 e26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kuhn H, Banthiya S, van Leyen K. Mammalian lipoxygenases and their biological relevance. Biochim Biophys Acta. 2015;1851:308–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Anthonymuthu TS, Kenny EM, Shrivastava IH, et al. Empowerment of 15-lipoxygenase catalytic competence in selective oxidation of membrane ETE-PE to ferroptotic death signals, HpETE-PE. J Am Chem Soc. 2018;140:17835–17839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Furtmuller PG, Zederbauer M, Jantschko W, et al. Active site structure and catalytic mechanisms of human peroxidases. Arch Biochem Biophys. 2006;445:199–213. [DOI] [PubMed] [Google Scholar]
- 69.Wang CW, Colas RA, Dalli J, et al. Maresin 1 biosynthesis and proresolving anti-infective functions with human-localized aggressive periodontitis leukocytes. Infect Immun. 2015;84:658–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Davies MJ, Hawkins CL, Pattison DI, Rees MD. Mammalian heme peroxidases: from molecular mechanisms to health implications. Antioxid Redox Signal. 2008;10:1199–1234. [DOI] [PubMed] [Google Scholar]
- 71.Reeder BJ, Grey M, Silaghi-Dumitrescu RL, et al. Tyrosine residues as redox cofactors in human hemoglobin: implications for engineering nontoxic blood substitutes. J Biol Chem. 2008;283:30780–30787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vlasova II. Peroxidase activity of human hemoproteins: keeping the fire under control. Molecules. 2018:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Reeder BJ. Redox and peroxidase activities of the hemoglobin superfamily: relevance to health and disease. Antioxid Redox Signal. 2017;26:763–776. [DOI] [PubMed] [Google Scholar]
- 74.Buettner GR, Ng CF, Wang M, Rodgers VG, Schafer FQ. A new paradigm: manganese superoxide dismutase influences the production of H2O2 in cells and thereby their biological state. Free Radic Biol Med. 2006;41:1338–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Belikova NA, Tyurina YY, Borisenko G, et al. Heterolytic reduction of fatty acid hydroperoxides by cytochrome c/cardiolipin complexes: antioxidant function in mitochondria. J Am Chem Soc. 2009;131:11288–11289. [DOI] [PubMed] [Google Scholar]
- 76.Matamoros MA, Kim A, Penuelas M, et al. Protein carbonylation and glycation in legume nodules. Plant Physiol. 2018;177:1510–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cassina AM, Hodara R, Souza JM, et al. Cytochrome c nitration by peroxynitrite. J Biol Chem. 2000;275:21409–21415. [DOI] [PubMed] [Google Scholar]
- 78.Quijano C, Hernandez-Saavedra D, Castro L, McCord JM, Freeman BA, Radi R. Reaction of peroxynitrite with Mn-superoxide dismutase. Role of the metal center in decomposition kinetics and nitration. J Biol Chem. 2001;276:11631–11638. [DOI] [PubMed] [Google Scholar]
- 79.Radi R Nitric oxide, oxidants, and protein tyrosine nitration. Proc Natl Acad Sci USA. 2004;101:4003–4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Alvarez-Paggi D, Hannibal L, Castro MA, et al. Multifunctional cytochrome c: learning new tricks from an old dog. Chem Rev. 2017;117:13382–13460. [DOI] [PubMed] [Google Scholar]
- 81.Barayeu U, Lange M, Mendez L, et al. Cytochrome c auto-catalyzed carbonylation in the presence of hydrogen peroxide and cardiolipins. J Biol Chem. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bayir H, Tyurin VA, Tyurina YY, et al. Selective early cardiolipin peroxidation after traumatic brain injury: an oxidative lipidomics analysis. Ann Neurol. 2007;62:154–169. [DOI] [PubMed] [Google Scholar]
- 83.Koelmel JP, Kroeger NM, Ulmer CZ, et al. LipidMatch: an automated workflow for rule-based lipid identification using untargeted high-resolution tandem mass spectrometry data. BMC Bioinformatics. 2017;18:331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ulmer CZ, Koelmel JP, Ragland JM, Garrett TJ, Bowden JA. Lipid-Pioneer: a comprehensive user-generated exact mass template for lipidomics. J Am Soc Mass Spectrom. 2017;28:562–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.O’Connor A, Brasher CJ, Slatter DA, et al. LipidFinder: a computational workflow for discovery of lipids identifies eicosanoidphosphoinositides in platelets. JCI Insight. 2017;2:e91634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ni Z, Angelidou G, Hoffmann R, Fedorova M. LPPtiger software for lipidome-specific prediction and identification of oxidized phospholipids from LC-MS datasets. Sci Rep. 2017;7:15138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Goracci L, Tortorella S, Tiberi P, et al. Lipostar, a comprehensive platform-neutral cheminformatics tool for lipidomics. Anal Chem. 2017;89:6257–6264. [DOI] [PubMed] [Google Scholar]
- 88.Folco G, Murphy RC. Eicosanoid transcellular biosynthesis: from cell-cell interactions to in vivo tissue responses. Pharmacol Rev. 2006;58:375–388. [DOI] [PubMed] [Google Scholar]
- 89.Dennis EA, Norris PC. Eicosanoid storm in infection and inflammation. Nat Rev Immunol. 2015;15:511–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Levy BD, Vachier I, Serhan CN. Resolution of inflammation in asthma. Clin Chest Med. 2012;33:559–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Serhan CN, Chiang N, Dalli J. New pro-resolving n-3 mediators bridge resolution of infectious inflammation to tissue regeneration. Mol Aspects Med. 2018;64:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhao Z, Zhang X, Zhao C, et al. Protection of pancreatic beta-cells by group VIA phospholipase A(2)-mediated repair of mitochondrial membrane peroxidation. Endocrinology. 2010;151:3038–3048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Liu GY, Moon SH, Jenkins CM, et al. The phospholipase iPLA2gamma is a major mediator releasing oxidized aliphatic chains from cardiolipin, integrating mitochondrial bioenergetics and signaling. J Biol Chem. 2017;292:10672–10684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Serhan CN. Pro-resolving lipid mediators are leads for resolution physiology. Nature. 2014;510:92–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Dennis EA. Liberating chiral lipid mediators, inflammatory enzymes, and LIPID MAPS from biological grease. J Biol Chem. 2016;291:24431–24448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Smith WL, Garavito RM, DeWitt DL. Prostaglandin endoperoxide H synthases (cyclooxygenases)-1 and −2. J Biol Chem. 1996;271: 33157–33160. [DOI] [PubMed] [Google Scholar]
- 97.Morita I Distinct functions of COX-1 and COX-2. Prostaglandins Other Lipid Mediat. 2002;68–69:165–175. [DOI] [PubMed] [Google Scholar]
- 98.Crofford LJ. COX-1 and COX-2 tissue expression: implications and predictions. J Rheumatol Suppl. 1997;49:15–19. [PubMed] [Google Scholar]
- 99.Tilley SL, Coffman TM, Koller BH. Mixed messages: modulation of inflammation and immune responses by prostaglandins and thromboxanes. J Clin Invest. 2001;108:15–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Nakanishi M, Rosenberg DW. Multifaceted roles of PGE2 in inflammation and cancer. Semin Immunopathol. 2013;35:123–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ho ATV, Palla AR, Blake MR, et al. Prostaglandin E2 is essential for efficacious skeletal muscle stem-cell function, augmenting regeneration and strength. Proc Natl Acad Sci USA. 2017;114:6675–6684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mrsny RJ, Gewirtz AT, Siccardi D, et al. Identification of hepoxilin A3 in inflammatory events: a required role in neutrophil migration across intestinal epithelia. Proc Natl Acad Sci USA. 2004;101: 7421–7426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Pazos MA, Pirzai W, Yonker LM, Morisseau C, Gronert K, Hurley BP. Distinct cellular sources of hepoxilin A3 and leukotriene B4 are used to coordinate bacterial-induced neutrophil transepithelial migration. J Immunol. 2015;194:1304–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lewis RA, Austen KF, Soberman RJ. Leukotrienes and other products of the 5-lipoxygenase pathway. Biochemistry and relation to pathobiology in human diseases. N Engl J Med. 1990;323:645–655. [DOI] [PubMed] [Google Scholar]
- 105.Tamang DL, Pirzai W, Priebe GP, et al. Hepoxilin A(3) facilitates neutrophilic breach of lipoxygenase-expressing airway epithelial barriers. J Immunol. 2012;189:4960–4969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Song WC, Baertschi SW, Boeglin WE, Harris TM, Brash AR. Formation of epoxyalcohols by a purified allene oxide synthase. Implications for the mechanism of allene oxide synthesis. J Biol Chem. 1993;268:6293–6298. [PubMed] [Google Scholar]
- 107.Song WC, Funk CD, Brash AR. Molecular cloning of an allene oxide synthase: a cytochrome P450 specialized for the metabolism of fatty acid hydroperoxides. Proc Natl Acad Sci USA. 1993;90:8519–8523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Radmark O, Werz O, Steinhilber D, Samuelsson B. 5-Lipoxygenase, a key enzyme for leukotriene biosynthesis in health and disease. Biochim Biophys Acta. 2015;1851:331–339. [DOI] [PubMed] [Google Scholar]
- 109.Powell WS, Rokach J. Biochemistry, biology and chemistry of the 5-lipoxygenase product 5-oxo-ETE. Prog Lipid Res. 2005;44:154–183. [DOI] [PubMed] [Google Scholar]
- 110.Chourey S, Ye Q, Reddy CN, et al. In vivo alpha-hydroxylation of a 2-alkylindole antagonist of the OXE receptor for the eosinophil chemoattractant 5-oxo-6,8,11,14-eicosatetraenoic acid in monkeys. Biochem Pharmacol. 2017;138:107–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Serhan CN. Discovery of specialized pro-resolving mediators marks the dawn of resolution physiology and pharmacology. Mol Aspects Med. 2017;58:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Levy BD. Lipoxins and lipoxin analogs in asthma. Prostaglandins Leukot Essent Fatty Acids. 2005;73:231–237. [DOI] [PubMed] [Google Scholar]
- 113.Godson C, Mitchell S, Harvey K, Petasis NA, Hogg N, Brady HR. Cutting edge: lipoxins rapidly stimulate nonphlogistic phagocytosis of apoptotic neutrophils by monocyte-derived macrophages. J Immunol. 2000;164:1663–1667. [DOI] [PubMed] [Google Scholar]
- 114.Fierro IM, Colgan SP, Bernasconi G, et al. Lipoxin A4 and aspirin-triggered 15-epi-lipoxin A4 inhibit human neutrophil migration: comparisons between synthetic 15 epimers in chemotaxis and trans-migration with microvessel endothelial cells and epithelial cells. J Immunol. 2003;170:2688–2694. [DOI] [PubMed] [Google Scholar]
- 115.Lehmann C, Homann J, Ball AK, et al. Lipoxin and resolvin biosynthesis is dependent on 5-lipoxygenase activating protein. FASEB J. 2015;29:5029–5043. [DOI] [PubMed] [Google Scholar]
- 116.Green AR, Freedman C, Tena J, et al. 5 S,15 S-Dihydroperoxyeicosatetraenoic acid (5,15-diHpETE) as a lipoxin intermediate: reactivity and kinetics with human leukocyte 5-lipoxygenase, platelet 12-lipoxygenase, and reticulocyte 15-lipoxygenase-1. Biochemistry. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Levy BD, Clish CB, Schmidt B, Gronert K, Serhan CN. Lipid mediator class switching during acute inflammation: signals in resolution. Nat Immunol. 2001;2:612–619. [DOI] [PubMed] [Google Scholar]
- 118.Chan MM, Moore AR. Resolution of inflammation in murine autoimmune arthritis is disrupted by cyclooxygenase-2 inhibition and restored by prostaglandin E2-mediated lipoxin A4 production. J Immunol. 2010;184:6418–6426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Schmelzer KR, Inceoglu B, Kubala L, et al. Enhancement of antinociception by coadministration of nonsteroidal anti-inflammatory drugs and soluble epoxide hydrolase inhibitors. Proc Natl Acad Sci USA. 2006;103:13646–13651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Inceoglu B, Jinks SL, Schmelzer KR, Waite T, Kim IH, Hammock BD. Inhibition of soluble epoxide hydrolase reduces LPS-induced thermal hyperalgesia and mechanical allodynia in a rat model of inflammatory pain. Life Sci. 2006;79:2311–2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Inceoglu B, Schmelzer KR, Morisseau C, Jinks SL, Hammock BD. Soluble epoxide hydrolase inhibition reveals novel biological functions of epoxyeicosatrienoic acids (EETs). Prostaglandins Other Lipid Mediat. 2007;82:42–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Fang X, Weintraub NL, McCaw RB, et al. Effect of soluble epoxide hydrolase inhibition on epoxyeicosatrienoic acid metabolism in human blood vessels. Am J Physiol Heart Circ Physiol. 2004;287:H2412–20. [DOI] [PubMed] [Google Scholar]
- 123.Hong S, Gronert K, Devchand PR, Moussignac RL, Serhan CN. Novel docosatrienes and 17S-resolvins generated from docosahexaenoic acid in murine brain, human blood, and glial cells. Autacoids in anti-inflammation. J Biol Chem. 2003;278:14677–14687. [DOI] [PubMed] [Google Scholar]
- 124.Serhan CN, Hong S, Gronert K, et al. Resolvins: a family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter proinflammation signals. J Exp Med. 2002;196:1025–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Murakami M, Taketomi Y, Sato H, Yamamoto K. Secreted phospholipase A2 revisited. J Biochem. 2011;150:233–255. [DOI] [PubMed] [Google Scholar]
- 126.Yamamoto K, Isogai Y, Sato H, Taketomi Y, Murakami M. Secreted phospholipase A2, lipoprotein hydrolysis, and atherosclerosis: integration with lipidomics. Anal Bioanal Chem. 2011;400:1829–1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Serhan CN, Clish CB, Brannon J, Colgan SP, Gronert K, Chiang N. Anti-microinflammatory lipid signals generated from dietary N-3 fatty acids via cyclooxygenase-2 and transcellular processing: a novel mechanism for NSAID and N-3 PUFA therapeutic actions. J Physiol Pharmacol. 2000;51:643–654. [PubMed] [Google Scholar]
- 128.Mukherjee PK, Marcheselli VL, Serhan CN, Bazan NG. Neuroprotectin D1: a docosahexaenoic acid-derived docosatriene protects human retinal pigment epithelial cells from oxidative stress. Proc Natl Acad Sci USA. 2004;101:8491–8496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Dalli J, Zhu M, Vlasenko NA, et al. The novel 13S,14S-epoxy-maresin is converted by human macrophages to maresin 1 (MaR1), inhibits leukotriene A4 hydrolase (LTA4H), and shifts macrophage phenotype. FASEB J. 2013;27:2573–2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Colas RA, Dalli J, Chiang N, et al. Identification and actions of the maresin 1 metabolome in infectious inflammation. J Immunol. 2016;197:4444–4452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Dalli J, Sanger JM, Rodriguez AR, Chiang N, Spur BW, Serhan CN. Identification and actions of a novel third maresin conjugate in tissue regeneration: MCTR3. PLoS One. 2016;11:e0149319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Serhan CN, Yang R, Martinod K, et al. Maresins: novel macrophage mediators with potent antiinflammatory and proresolving actions. J Exp Med. 2009;206:15–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Deng B, Wang CW, Arnardottir HH, et al. Maresin biosynthesis and identification of maresin 2, a new anti-inflammatory and pro-resolving mediator from human macrophages. PLoS One. 2014;9:e102362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Abdulnour RE, Dalli J, Colby JK, et al. Maresin 1 biosynthesis during platelet-neutrophil interactions is organ-protective. Proc Natl Acad Sci USA. 2014;111:16526–16531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Norris PC, Skulas-Ray AC, Riley I, et al. Identification of specialized pro-resolving mediator clusters from healthy adults after intravenous low-dose endotoxin and omega-3 supplementation: a methodological validation. Sci Rep. 2018;8:18050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Serhan CN, Clish CB, Brannon J, Colgan SP, Chiang N, Gronert K. Novel functional sets of lipid-derived mediators with antiinflammatory actions generated from omega-3 fatty acids via cyclooxygenase 2-nonsteroidal antiinflammatory drugs and transcellular processing. J Exp Med. 2000;192:1197–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ho KJ, Spite M, Owens CD, et al. Aspirin-triggered lipoxin and resolvin E1 modulate vascular smooth muscle phenotype and correlate with peripheral atherosclerosis. Am J Pathol. 2010;177: 2116–2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Tjonahen E, Oh SF, Siegelman J, et al. Resolvin E2: identification and anti-inflammatory actions: pivotal role of human 5-lipoxygenase in resolvin E series biosynthesis. Chem Biol. 2006;13:1193–1202. [DOI] [PubMed] [Google Scholar]
- 139.Arita M, Bianchini F, Aliberti J, et al. Stereochemical assignment, anti-inflammatory properties, and receptor for the omega-3 lipid mediator resolvin E1. J Exp Med. 2005;201:713–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Fu P, Birukov KG. Oxidized phospholipids in control of inflammation and endothelial barrier. Transl Res. 2009;153:166–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Ashraf MZ, Kar NS, Podrez EA. Oxidized phospholipids: biomarker for cardiovascular diseases. Int J Biochem Cell Biol. 2009;41: 1241–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Maskrey BH, Megson IL, Whitfield PD, Rossi AG. Mechanisms of resolution of inflammation: a focus on cardiovascular disease. Arterioscler Thromb Vasc Biol. 2011;31:1001–1006. [DOI] [PubMed] [Google Scholar]
- 143.Salomon RG. Structural identification and cardiovascular activities of oxidized phospholipids. Circ Res. 2012;111:930–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Serbulea V, DeWeese D, Leitinger N. The effect of oxidized phospholipids on phenotypic polarization and function of macrophages. Free Radic Biol Med. 2017;111:156–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Fruhwirth GO. Loidl A and Hermetter A. Oxidized phospholipids: from molecular properties to disease. Biochim Biophys Acta. 2007;1772:718–736. [DOI] [PubMed] [Google Scholar]
- 146.Repetto MG, Ferrarotti NF, Boveris A. The involvement of transition metal ions on iron-dependent lipid peroxidation. Arch Toxicol. 2010;84:255–262. [DOI] [PubMed] [Google Scholar]
- 147.Hazen SL. Oxidized phospholipids as endogenous pattern recognition ligands in innate immunity. J Biol Chem. 2008;283:15527–15531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Erridge C, Kennedy S, Spickett CM, Webb DJ. Oxidized phospholipid inhibition of toll-like receptor (TLR) signaling is restricted to TLR2 and TLR4: roles for CD14, LPS-binding protein, and MD2 as targets for specificity of inhibition. J Biol Chem. 2008;283: 24748–24759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Parhami F, Fang ZT, Yang B, Fogelman AM, Berliner JA. Stimulation of Gs and inhibition of Gi protein functions by minimally oxidized LDL. Arterioscler Thromb Vasc Biol. 1995;15:2019–2024. [DOI] [PubMed] [Google Scholar]
- 150.van Meer G, Voelker DR, Feigenson GW. Membrane lipids: where they are and how they behave. Nat Rev Mol Cell Biol. 2008;9:112–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Watson AD, Subbanagounder G, Welsbie DS, et al. Structural identification of a novel pro-inflammatory epoxyisoprostane phospholipid in mildly oxidized low density lipoprotein. J Biol Chem. 1999;274:24787–24798. [DOI] [PubMed] [Google Scholar]
- 152.Leitinger N, Tyner TR, Oslund L, et al. Structurally similar oxidized phospholipids differentially regulate endothelial binding of monocytes and neutrophils. Proc Natl Acad Sci USA. 1999;96:12010–12015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Kadl A, Meher AK, Sharma PR, et al. Identification of a novel macrophage phenotype that develops in response to atherogenic phospholipids via Nrf2. Circ Res. 2010;107:737–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Adamson S, Leitinger N. Phenotypic modulation of macrophages in response to plaque lipids. Curr Opin Lipidol. 2011;22:335–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Goncalves I, Edsfeldt A, Ko NY, et al. Evidence supporting a key role of Lp-PLA2-generated lysophosphatidylcholine in human atherosclerotic plaque inflammation. Arterioscler Thromb Vasc Biol. 2012;32:1505–1512. [DOI] [PubMed] [Google Scholar]
- 156.Marathe GK, Davies SS, Harrison KA, et al. Inflammatory platelet-activating factor-like phospholipids in oxidized low density lipoproteins are fragmented alkyl phosphatidylcholines. J Biol Chem. 1999;274:28395–28404. [DOI] [PubMed] [Google Scholar]
- 157.Beaudeux JL, Said T, Ninio E, et al. Activation of PAF receptor by oxidised LDL in human monocytes stimulates chemokine releases but not urokinase-type plasminogen activator expression. Clin Chim Acta. 2004;344:163–171. [DOI] [PubMed] [Google Scholar]
- 158.Tsimikas S, Aikawa M, Miller FJ, et al. Increased plasma oxidized phospholipid:apolipoprotein B-100 ratio with concomitant depletion of oxidized phospholipids from atherosclerotic lesions after dietary lipid-lowering: a potential biomarker of early atherosclerosis regression. Arterioscler Thromb Vasc Biol. 2007;27:175–181. [DOI] [PubMed] [Google Scholar]
- 159.Miller YI, Choi SH, Wiesner P, et al. Oxidation-specific epitopes are danger-associated molecular patterns recognized by pattern recognition receptors of innate immunity. Circ Res. 2011;108:235–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Huber J, Vales A, Mitulovic G, et al. Oxidized membrane vesicles and blebs from apoptotic cells contain biologically active oxidized phospholipids that induce monocyte-endothelial interactions. Arterioscler Thromb Vasc Biol. 2002;22:101–107. [DOI] [PubMed] [Google Scholar]
- 161.Nguyen T, Nioi P, Pickett CB. The Nrf2-antioxidant response element signaling pathway and its activation by oxidative stress. J Biol Chem. 2009;284:13291–13295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Yeon SH, Yang G, Lee HE, Lee JY. Oxidized phosphatidylcholine induces the activation of NLRP3 inflammasome in macrophages. J Leukoc Biol. 2017;101:205–215. [DOI] [PubMed] [Google Scholar]
- 163.O’Donnell VB, Murphy RC. New families of bioactive oxidized phospholipids generated by immune cells: identification and signaling actions. Blood. 2012;120:1985–1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Maiorino M, Conrad M, Ursini F. GPx4, lipid peroxidation, and cell death: discoveries, rediscoveries, and open issues. Antioxid Redox Signal. 2018;29:61–74. [DOI] [PubMed] [Google Scholar]
- 165.Clark SR, Guy CJ, Scurr MJ, et al. Esterified eicosanoids are acutely generated by 5-lipoxygenase in primary human neutrophils and in human and murine infection. Blood. 2011;117:2033–2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Maskrey BH, Bermudez-Fajardo A, Morgan AH, et al. Activated platelets and monocytes generate four hydroxyphosphatidylethanolamines via lipoxygenase. J Biol Chem. 2007;282: 20151–20163. [DOI] [PubMed] [Google Scholar]
- 167.Hammond VJ, O’Donnell VB. Esterified eicosanoids: generation, characterization and function. Biochim Biophys Acta. 2012;1818:2403–2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Smiley PL, Stremler KE, Prescott SM, Zimmerman GA, McIntyre TM. Oxidatively fragmented phosphatidylcholines activate human neutrophils through the receptor for platelet-activating factor. J Biol Chem. 1991;266:11104–11110. [PubMed] [Google Scholar]
- 169.Latchoumycandane C, Nagy LE, McIntyre TM. Myeloperoxidase formation of PAF receptor ligands induces PAF receptor-dependent kidney injury during ethanol consumption. Free Radic Biol Med. 2015;86:179–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Tyurina YY, Shrivastava I, Tyurin VA, et al. “Only a life lived for others is worth living”: redox signaling by oxygenated phospholipids in cell fate decisions. Antioxid Redox Signal. 2018;29:1333–1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Doll S, Proneth B, Tyurina YY, et al. ACSL4 dictates ferroptosis sensitivity by shaping cellular lipid composition. Nat Chem Biol. 2017;13:91–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Li C, Deng X, Xie X, Liu Y, Friedmann Angeli JP, Lai L. Activation of glutathione peroxidase 4 as a novel anti-inflammatory strategy. Front Pharmacol. 2018;9:1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Zhao J, Maskrey B, Balzar S, et al. Interleukin-13-induced MUC5AC is regulated by 15-lipoxygenase 1 pathway in human bronchial epithelial cells. Am J Respir Crit Care Med. 2009;179:782–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Guo L, Chen Z, Amarnath V, et al. Isolevuglandin-type lipid aldehydes induce the inflammatory response of macrophages by modifying phosphatidylethanolamines and activating the receptor for advanced glycation endproducts. Antioxid Redox Signal. 2015;22: 1633–1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Kozak KR, Marnett LJ. Oxidative metabolism of endocannabinoids. Prostaglandins Leukot Essent Fatty Acids. 2002;66:211–220. [DOI] [PubMed] [Google Scholar]
- 176.Kozak KR, Crews BC, Morrow JD, et al. Metabolism of the endocannabinoids, 2-arachidonylglycerol and anandamide, into prostaglandin, thromboxane, and prostacyclin glycerol esters and ethanolamides. J Biol Chem. 2002;277:44877–44885. [DOI] [PubMed] [Google Scholar]
- 177.Aldrovandi M, Hammond VJ, Podmore H, et al. Human platelets generate phospholipid-esterified prostaglandins via cyclooxygenase-1 that are inhibited by low dose aspirin supplementation. J Lipid Res. 2013;54:3085–3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Aldrovandi M, Hinz C, Lauder SN, et al. DioxolaneA3-phosphatidylethanolamines are generated by human platelets and stimulate neutrophil integrin expression. Redox Biol. 2017;11: 663–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Balasubramanian K, Maeda A, Lee JS, et al. Dichotomous roles for externalized cardiolipin in extracellular signaling: promotion of phagocytosis and attenuation of innate immunity. Sci Signal. 2015;8:ra95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Kagan VE, Gleiss B, Tyurina YY, et al. A role for oxidative stress in apoptosis: oxidation and externalization of phosphatidylserine is required for macrophage clearance of cells undergoing Fas-mediated apoptosis. J Immunol. 2002;169:487–499. [DOI] [PubMed] [Google Scholar]
- 181.Greenberg ME, Sun M, Zhang R, Febbraio M, Silverstein R, Hazen SL. Oxidized phosphatidylserine-CD36 interactions play an essential role in macrophage-dependent phagocytosis of apoptotic cells. J Exp Med. 2006;203:2613–2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Kagan VE, Borisenko GG, Serinkan BF, et al. Appetizing rancidity of apoptotic cells for macrophages: oxidation, externalization, and recognition of phosphatidylserine. Am J Physiol Lung Cell Mol Physiol. 2003;285:L1–17. [DOI] [PubMed] [Google Scholar]
- 183.O’Donnell VB, Aldrovandi M, Murphy RC, Kronke G. Enzymatically oxidized phospholipids assume center stage as essential regulators of innate immunity and cell death. Sci Signal. 2019;12. [DOI] [PubMed] [Google Scholar]
- 184.Burke JE, Dennis EA. Phospholipase A2 structure/function, mechanism, and signaling. J Lipid Res. 2009;50(Suppl):S237–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Huber J, Furnkranz A, Bochkov VN, et al. Specific monocyte adhesion to endothelial cells induced by oxidized phospholipids involves activation of cPLA2 and lipoxygenase. J Lipid Res. 2006;47:1054–1062. [DOI] [PubMed] [Google Scholar]
- 186.Hara S, Yoda E, Sasaki Y, Nakatani Y, Kuwata H. Calcium-independent phospholipase A2gamma (iPLA2gamma) and its roles in cellular functions and diseases. Biochim Biophys Acta Mol Cell Biol Lipids. 2018. [DOI] [PubMed] [Google Scholar]
- 187.Moon SH, Liu X, Cedars AM, et al. Heart failure-induced activation of phospholipase iPLA2gamma generates hydroxyeicosatetraenoic acids opening the mitochondrial permeability transition pore. J Biol Chem. 2018;293:115–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Moon SH, Jenkins CM, Liu X, Guan S, Mancuso DJ, Gross RW. Activation of mitochondrial calcium-independent phospholipase A2gamma (iPLA2gamma) by divalent cations mediating arachido-nate release and production of downstream eicosanoids. J Biol Chem. 2012;287:14880–14895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Iyer SS, He Q, Janczy JR, et al. Mitochondrial cardiolipin is required for Nlrp3 inflammasome activation. Immunity. 2013;39:311–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Brezinski ME, Serhan CN. Selective incorporation of (15S)-hydroxyeicosatetraenoic acid in phosphatidylinositol of human neutrophils: agonist-induced deacylation and transformation of stored hydroxyeicosanoids. Proc Natl Acad Sci USA. 1990;87: 6248–6252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Pawlowski NA, Scott WA, Andreach M, Cohn ZA. Uptake and metabolism of monohydroxy-eicosatetraenoic acids by macrophages. J Exp Med. 1982;155:1653–1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Joulain C, Meskini N, Anker G, Lagarde M, Prigent AF. Esterification of 12(S)-hydroxy-5,8,10,14-eicosatetraenoic acid into the phospholipids of human peripheral blood mononuclear cells: inhibition of the proliferative response. J Cell Physiol. 1995;164: 154–163. [DOI] [PubMed] [Google Scholar]
- 193.Fogh K, Hansen ES, Herlin T, et al. 15-Hydroxy-eicosatetraenoic acid (15-HETE) inhibits carrageenan-induced experimental arthritis and reduces synovial fluid leukotriene B4 (LTB4). Prostaglandins. 1989;37:213–228. [DOI] [PubMed] [Google Scholar]
- 194.Capdevila JH, Falck JR, Harris RC. Cytochrome P450 and arachidonic acid bioactivation. Molecular and functional properties of the arachi-donate monooxygenase. J Lipid Res. 2000;41:163–181. [PubMed] [Google Scholar]
- 195.Spector AA, Fang X, Snyder GD, Weintraub NL. Epoxyeicosatrienoic acids (EETs): metabolism and biochemical function. Prog Lipid Res. 2004;43:55–90. [DOI] [PubMed] [Google Scholar]
- 196.Klett EL, Chen S, Edin ML, et al. Diminished acyl-CoA synthetase isoform 4 activity in INS 832/13 cells reduces cellular epoxyeicosatrienoic acid levels and results in impaired glucose-stimulated insulin secretion. J Biol Chem. 2013;288:21618–21629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Shearer GC, Newman JW. Lipoprotein lipase releases esterified oxylipins from very low-density lipoproteins. Prostaglandins Leukot Essent Fatty Acids. 2008;79:215–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Mettler FA, Jr, Voelz GL. Major radiation exposure–what to expect and how to respond. N Engl J Med. 2002;346:1554–1561. [DOI] [PubMed] [Google Scholar]
- 199.Garg S, Boerma M, Wang J, et al. Influence of sublethal total-body irradiation on immune cell populations in the intestinal mucosa. Radiat Res. 2010;173:469–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Dennis EA, Cao J, Hsu YH, Magrioti V, Kokotos G. Phospholipase A2 enzymes: physical structure, biological function, disease implication, chemical inhibition, and therapeutic intervention. Chem Rev. 2011;111:6130–6185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Tyurina YY, Kisin ER, Murray A, et al. Global phospholipidomics analysis reveals selective pulmonary peroxidation profiles upon inhalation of single-walled carbon nanotubes. ACS Nano. 2011;5: 7342–7353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Shvedova AA, Kisin E, Murray AR, et al. Inhalation vs. aspiration of single-walled carbon nanotubes in C57BL/6 mice: inflammation, fibrosis, oxidative stress, and mutagenesis. Am J Physiol Lung Cell Mol Physiol. 2008;295:L552–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Kerr JF, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972;26:239–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Krysko DV, Vandenabeele P. Clearance of dead cells: mechanisms, immune responses and implication in the development of diseases. Apoptosis. 2010;15:995–997. [DOI] [PubMed] [Google Scholar]
- 205.Wyllie AH. Glucocorticoid-induced thymocyte apoptosis is associated with endogenous endonuclease activation. Nature. 1980;284:555–556. [DOI] [PubMed] [Google Scholar]
- 206.Enari M, Sakahira H, Yokoyama H, Okawa K, Iwamatsu A, Nagata S. A caspase-activated DNase that degrades DNA during apoptosis, and its inhibitor ICAD. Nature. 1998;391:43–50. [DOI] [PubMed] [Google Scholar]
- 207.Liu X, Kim CN, Yang J, Jemmerson R, Wang X. Induction of apoptotic program in cell-free extracts: requirement for dATP and cytochrome c. Cell. 1996;86:147–157. [DOI] [PubMed] [Google Scholar]
- 208.Jiang X, Wang X. Cytochrome C-mediated apoptosis. Annu Rev Biochem. 2004;73:87–106. [DOI] [PubMed] [Google Scholar]
- 209.Maguire JJ, Tyurina YY, Mohammadyani D, et al. Known unknowns of cardiolipin signaling: the best is yet to come. Biochim Biophys Acta Mol Cell Biol Lipids. 2017;1862:8–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Krysko O, Vandenabeele P, Krysko DV, Bachert C. Impairment of phagocytosis of apoptotic cells and its role in chronic airway diseases. Apoptosis. 2010;15:1137–1146. [DOI] [PubMed] [Google Scholar]
- 211.Henson PM. Cell removal: efferocytosis. Annu Rev Cell Dev Biol. 2017;33:127–144. [DOI] [PubMed] [Google Scholar]
- 212.Mishchenko T, Mitroshina E, Balalaeva I, Krysko O, Vedunova M, Krysko DV. An emerging role for nanomaterials in increasing immunogenicity of cancer cell death. Biochim Biophys Acta Rev Cancer. 2018;1871:99–108. [DOI] [PubMed] [Google Scholar]
- 213.Krysko O, Love Aaes T, Bachert C, Vandenabeele P, Krysko DV. Many faces of DAMPs in cancer therapy. Cell Death Dis. 2013;4:e631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Krysko DV, Agostinis P, Krysko O, et al. Emerging role of damage-associated molecular patterns derived from mitochondria in inflammation. Trends Immunol. 2011;32:157–164. [DOI] [PubMed] [Google Scholar]
- 215.Chakraborty K, Raundhal M, Chen BB, et al. The mito-DAMP cardiolipin blocks IL-10 production causing persistent inflammation during bacterial pneumonia. Nat Commun. 2017;8:13944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Buland JR, Wasserloos KJ, Tyurin VA, et al. Biosynthesis of oxidized lipid mediators via lipoprotein-associated phospholipase A2 hydrolysis of extracellular cardiolipin induces endothelial toxicity. Am J Physiol Lung Cell Mol Physiol. 2016;311:L303–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Vanden Berghe T, Linkermann A, Jouan-Lanhouet S, Walczak H, Vandenabeele P. Regulated necrosis: the expanding network of non-apoptotic cell death pathways. Nat Rev Mol Cell Biol. 2014;15: 135–147. [DOI] [PubMed] [Google Scholar]
- 218.Galluzzi L, Vitale I, Aaronson SA, et al. Molecular mechanisms of cell death: recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018;25:486–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Moreno-Gonzalez G, Vandenabeele P, Krysko DV. Necroptosis: a novel cell death modality and its potential relevance for critical care medicine. Am J Respir Crit Care Med. 2016;194:415–428. [DOI] [PubMed] [Google Scholar]
- 220.Dondelinger Y, Declercq W, Montessuit S, et al. MLKL compromises plasma membrane integrity by binding to phosphatidylinositol phosphates. Cell Rep. 2014;7:971–981. [DOI] [PubMed] [Google Scholar]
- 221.Quarato G, Guy CS, Grace CR, et al. Sequential engagement of distinct MLKL phosphatidylinositol-binding sites executes necroptosis. Mol Cell. 2016;61:589–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Shin YK, Kim J, Yang Y. Switch for the necroptotic permeation pore. Structure. 2014;22:1374–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Cai Z, Jitkaew S, Zhao J, et al. Plasma membrane translocation of trimerized MLKL protein is required for TNF-induced necroptosis. Nat Cell Biol. 2014;16:55–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Cozza G, Rossetto M, Bosello-Travain V, et al. Glutathione peroxidase 4-catalyzed reduction of lipid hydroperoxides in membranes: the polar head of membrane phospholipids binds the enzyme and addresses the fatty acid hydroperoxide group toward the redox center. Free Radic Biol Med. 2017;112:1–11. [DOI] [PubMed] [Google Scholar]
- 225.Parisi LR, Li N, Atilla-Gokcumen GE. Very long chain fatty acids are functionally involved in necroptosis. Cell Chem Biol. 2017;24: 1445–1454 e8. [DOI] [PubMed] [Google Scholar]
- 226.Krysko O, Aaes TL, Kagan VE, et al. Necroptotic cell death in anti-cancer therapy. Immunol Rev. 2017;280:207–219. [DOI] [PubMed] [Google Scholar]
- 227.Yatim N, Jusforgues-Saklani H, Orozco S, et al. RIPK1 and NF-kappaB signaling in dying cells determines cross-priming of CD8(+) T cells. Science. 2015;350:328–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Aaes TL, Kaczmarek A, Delvaeye T, et al. Vaccination with necroptotic cancer cells induces efficient anti-tumor immunity. Cell Rep. 2016;15:274–287. [DOI] [PubMed] [Google Scholar]
- 229.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. [DOI] [PubMed] [Google Scholar]
- 230.Dixon SJ, Lemberg KM, Lamprecht MR, et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149: 1060–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Stockwell BR, Friedmann Angeli JP, Bayir H, et al. Ferroptosis: a regulated cell death nexus linking metabolism, redox biology, and disease. Cell. 2017;171:273–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.D’Herde K, Krysko DV. Ferroptosis: oxidized PEs trigger death. NatChem Biol. 2017;13:4–5. [DOI] [PubMed] [Google Scholar]
- 233.Dar HH, Tyurina YY, Mikulska-Ruminska K, et al. Pseudomonas aeruginosa utilizes host polyunsaturated phosphatidylethanolamines to trigger theft-ferroptosis in bronchial epithelium. J Clin Invest. 2018;128:4639–4653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Dixon SJ, Winter GE, Musavi LS, et al. Human haploid cell genetics reveals roles for lipid metabolism genes in nonapoptotic cell death. ACS Chem Biol. 2015;10:1604–1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Yuan H, Li X, Zhang X, Kang R, Tang D. Identification of ACSL4 as a biomarker and contributor of ferroptosis. Biochem Biophys Res Commun. 2016;478:1338–1343. [DOI] [PubMed] [Google Scholar]
- 236.Seiler A, Schneider M, Forster H, et al. Glutathione peroxidase 4 senses and translates oxidative stress into 12/15-lipoxygenase dependent- and AIF-mediated cell death. Cell Metab. 2008;8: 237–248. [DOI] [PubMed] [Google Scholar]
- 237.Probst L, Dachert J, Schenk B, Fulda S. Lipoxygenase inhibitors protect acute lymphoblastic leukemia cells from ferroptotic cell death. Biochem Pharmacol. 2017;140:41–52. [DOI] [PubMed] [Google Scholar]
- 238.Shintoku R, Takigawa Y, Yamada K, et al. Lipoxygenase-mediated generation of lipid peroxides enhances ferroptosis induced by erastin and RSL3. Cancer Sci. 2017;108:2187–2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Friedmann Angeli JP, Schneider M, Proneth B, et al. Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice. Nat Cell Biol. 2014;16:1180–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Skouta R, Dixon SJ, Wang J. Ferrostatins inhibit oxidative lipid damage and cell death in diverse disease models. J Am Chem Soc. 2014;136:4551–4556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Sarhan M, Land WG, Tonnus W, Hugo CP, Linkermann A. Origin and consequences of necroinflammation. Physiol Rev. 2018;98: 727–780. [DOI] [PubMed] [Google Scholar]
- 242.Martin-Sanchez D, Ruiz-Andres O, Poveda J, et al. Ferroptosis, but not necroptosis, is important in nephrotoxic folic acid-induced AKI. J Am Soc Nephrol. 2017;28:218–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Tyurina YY, Tyurin VA, Kaynar AM, et al. Oxidative lipidomics of hyperoxic acute lung injury: mass spectrometric characterization of cardiolipin and phosphatidylserine peroxidation. Am J Physiol Lung Cell Mol Physiol. 2010;299:L73–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Tyurina YY, Tyurin VA, Kapralova VI, et al. Oxidative lipidomics of gamma-radiation-induced lung injury: mass spectrometric characterization of cardiolipin and phosphatidylserine peroxidation. Radiat Res. 2011;175:610–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Tyurina YY, Tyurin VA, Epperly MW, Greenberger JS, Kagan VE. Oxidative lipidomics of gamma-irradiation-induced intestinal injury. Free Radic Biol Med. 2008;44:299–314. [DOI] [PubMed] [Google Scholar]
- 246.Tyurina YY, Polimova AM, Maciel E, et al. LC/MS analysis of cardiolipins in substantia nigra and plasma of rotenone-treated rats: implication for mitochondrial dysfunction in Parkinson’s disease. Free Radic Res. 2015;49:681–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Tyurina YY, Winnica DE, Kapralova VI, Kapralov AA, Tyurin VA, Kagan VE. LC/MS characterization of rotenone induced cardiolipin oxidation in human lymphocytes: implications for mitochondrial dys-function associated with Parkinson’s disease. Mol Nutr Food Res. 2013;57:1410–1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Anthonymuthu TS, Kenny EM, Bayir H. Therapies targeting lipid peroxidation in traumatic brain injury. Brain Res. 2016;1640: 57–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Shohami E, Shapira Y, Yadid G, Reisfeld N, Yedgar S. Brain phospholipase A2 is activated after experimental closed head injury in the rat. J Neurochem. 1989;53:1541–1546. [DOI] [PubMed] [Google Scholar]
- 250.Anthonymuthu TS, Kenny EM, Amoscato AA, et al. Global assessment of oxidized free fatty acids in brain reveals an enzymatic predominance to oxidative signaling after trauma. Biochim Biophys Acta Mol Basis Dis. 2017;1863:2601–2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Davalos D, Grutzendler J, Yang G, et al. ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci. 2005;8:752–758. [DOI] [PubMed] [Google Scholar]
- 252.Ersahin M, Toklu HZ, Erzik C, et al. The anti-inflammatory and neuroprotective effects of ghrelin in subarachnoid hemorrhage-induced oxidative brain damage in rats. J Neurotrauma. 2010;27:1143–1155. [DOI] [PubMed] [Google Scholar]
- 253.Clark RS, Schiding JK, Kaczorowski SL, Marion DW, Kochanek PM. Neutrophil accumulation after traumatic brain injury in rats: comparison of weight drop and controlled cortical impact models. J Neuro-trauma. 1994;11:499–506. [DOI] [PubMed] [Google Scholar]
- 254.Kenny EM, Fidan E, Yang Q, et al. Ferroptosis contributes to neuronal death and functional outcome after traumatic brain injury. Crit Care Med. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Jiang J, Serinkan BF, Tyurina YY, et al. Peroxidation and externalization of phosphatidylserine associated with release of cytochrome c from mitochondria. Free Radic Biol Med. 2003;35:814–825. [DOI] [PubMed] [Google Scholar]
- 256.van der Meer-Janssen YP, van Galen J, Batenburg JJ, Helms JB. Lipids in host-pathogen interactions: pathogens exploit the complexity of the host cell lipidome. Prog Lipid Res. 2010;49:1–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Vromman F, Subtil A. Exploitation of host lipids by bacteria. Curr Opin Microbiol. 2014;17:38–45. [DOI] [PubMed] [Google Scholar]
- 258.Bomberger JM, Maceachran DP, Coutermarsh BA, Ye S, O’Toole GA, Stanton BA. Long-distance delivery of bacterial virulence factors by Pseudomonas aeruginosa outer membrane vesicles. PLoS Pathog. 2009;5:e1000382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Rutherford ST, Bassler BL. Bacterial quorum sensing: its role in virulence and possibilities for its control. Cold Spring Harb Perspect Med. 2012:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Song D, Meng J, Cheng J, et al. Pseudomonas aeruginosa quorum-sensing metabolite induces host immune cell death through cell surface lipid domain dissolution. Nat Microbiol. 2019;4:97–111. [DOI] [PubMed] [Google Scholar]
- 261.Rao GV, Tinkle S, Weissman DN, et al. Efficacy of a technique for exposing the mouse lung to particles aspirated from the pharynx. J Toxicol Environ Health A. 2003;66:1441–1452. [DOI] [PubMed] [Google Scholar]
- 262.Bronikowski MJ, Willis P, Colbert DT, Smith KA, Smalley RE. Gas-phase production of carbon single-walled nanotubes from carbon monoxide via the HiPco process: a parametric study. J Vac Sci Technol. 2001;19:1800. [Google Scholar]
- 263.Gorelik O, Nikolaev P, Arepalli S. Purification Procedures for Single-Wall Carbon Nanotubes, NASA Contractor Report NASA/CR-2000–208926. Washington, DC: NASA; 2001. [Google Scholar]
- 264.Fauland A, Trotzmuller M, Eberl A, et al. An improved SPE method for fractionation and identification of phospholipids. J Sep Sci. 2013;36:744–751. [DOI] [PubMed] [Google Scholar]
- 265.Kim J, Minkler PE, Salomon RG, Anderson VE, Hoppel CL. Cardiolipin: characterization of distinct oxidized molecular species. J Lipid Res. 2011;52:125–135. [DOI] [PMC free article] [PubMed] [Google Scholar]