Abstract
A 64‐year‐old woman developed type 1 diabetes 23 years after the diagnosis of idiopathic CD4 lymphocytopenia. To investigate the etiological interaction between idiopathic CD4 lymphocytopenia and type 1 diabetes, we carried out a longitudinal analysis related to islet‐specific autoimmunity. Anti‐glutamic acid decarboxylase antibody had been already weakly positive for at least 16 years and started rising at 6 months before the onset of type 1 diabetes. The seroconversion of anti‐insulinoma‐associated antigen‐2 antibody and insulin autoantibody occurred at the time of onset. The ratio of CD8/CD4 had been gradually increasing for 8 years before type 1 diabetes onset. Notably, islet‐specific glucose‐6‐phosphatase catalytic subunit‐related protein‐reactive CD8+ T cells were detected at type 1 diabetes onset, and the frequency was higher than that in 15 non‐diabetic controls (6.75% vs 0.49 ± 0.78%, mean ± SD). The present type 1 diabetes patient, presented with idiopathic CD4 lymphocytopenia and showed an elevated number of CD8+ T cells, including the islet antigen‐specific CD8+ T cells that might contribute to autoimmune destruction of pancreatic β‐cells.
Keywords: Idiopathic CD4 lymphocytopenia, T cells, Type 1 diabetes
Introduction
Idiopathic CD4 lymphocytopenia (ICL) is characterized by decreased CD4+ T cells without any specific cause of lymphocytopenia, such as HIV infection, malignant tumors or CD4+ T‐cell reduction‐related medications1, 2. Previous reports have reported several autoimmune diseases accompanying ICL1, 2. We herein report the first case of type 1 diabetes presenting with ICL, using longitudinal analyses related to islet‐specific autoimmunity.
Case Report
In 1994, a 41‐year‐old woman was diagnosed with ICL and administered interleukin‐2 intermittently at the dosage of 350,000–700,000 units/day between 2002 and 2004, for recovery of the CD4+ T‐cell numbers. In May 2017 (aged 64 years), she presented with a 2‐week history of thirst, polyuria and general malaise. Laboratory tests revealed the levels of postprandial plasma glucose and glycated hemoglobin to be 517 mg/dL and 12.2%, respectively, in the presence of metabolic acidosis, hence indicating diabetic ketoacidosis. As her serum C‐peptide level had decreased and anti‐glutamic acid decarboxylase antibody was positive, she was diagnosed with type 1 diabetes. She had HLA‐A*24:02/*26:01, DRB1*09:01/*15:02 and DQB1*03:03/*06:01 (Table S1).
Levels of islet‐related autoantibodies were estimated by enzyme‐linked immunosorbent assay using serum samples frozen since 2001. The glutamic acid decarboxylase antibody titer was between 15 and 50 U/mL until 9 months before the onset of type 1 diabetes, after which it started rising, and reached 1,400 U/mL at onset. Both insulinoma‐associated antigen‐2 antibody and insulin autoantibody were negative until 3 months before the onset, and seroconversion occurred at onset. Zinc transporter‐8 antibody was negative, both before and at onset (Figure 1a).
Figure 1.
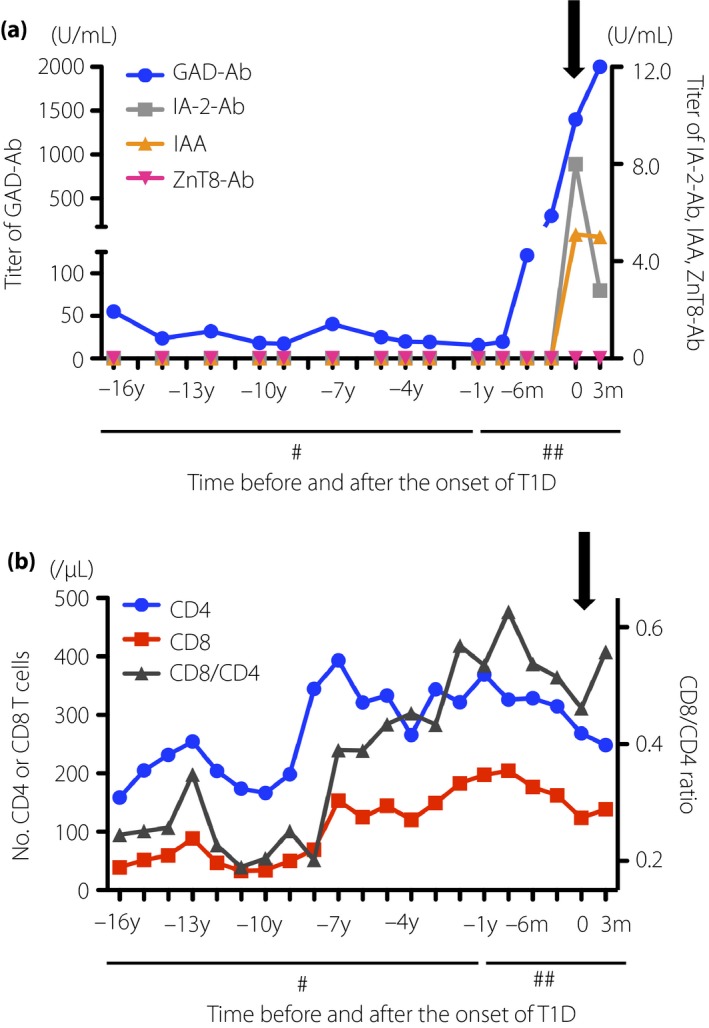
(a) Change in titer of islet‐related autoantibodies, glutamic acid decarboxylase antibody (GAD‐Ab), insulinoma‐associated antigen‐2 antibody (IA‐2Ab), insulin autoantibody (IAA) and zinc transporter‐8 antibody (ZnT8‐Ab), and (b) the number of T lymphocytes. #Each tick interval is 1 year; ##each tick interval is 3 months. Black arrows indicate the time of onset of type 1 diabetes.
The number of CD4+ T cells was approximately 150–250/μL from 16 to 9 years before the onset of type 1 diabetes, except for the period of interleukin‐2 therapy that induced transient elevation of lymphocyte number. Subsequently, it increased and remained approximately 250–450/μL until the onset of type 1 diabetes. The number of CD8+ T cells and ratio of CD8/CD4 were <50/μL and <0.30, respectively, between 16 and 8 years before the onset of type 1 diabetes. Subsequently, those numbers gradually increased to approximately 120–200/μL and 0.40–0.60, respectively, until the onset of type 1 diabetes (Figure 1b). As the number of CD8+ cells was elevated, we investigated whether islet antigen‐specific CD8+ T cells could be detected in the peripheral blood of the patient. It was analyzed at the onset of type 1 diabetes, alongside 15 non‐diabetic controls, as previously described3. The method and the peptide list for the experiments are shown in Appendix S1 and Table S2, respectively. Approximately 6.75% of islet‐specific glucose‐6‐phosphatase catalytic subunit‐related protein (IGRP)‐C3‐reactive CD8+ T cells produced interferon‐gamma in the patient. In contrast, a non‐diabetic individual showed fewer interferon‐gamma‐producing CD8+ T cells in response to all the peptide clusters, including IGRP‐C3 (Figure 2a). Furthermore, the percentage of IGRP‐C3‐specific CD8+ T cells in the patient was higher than that in the 15 non‐diabetic controls (6.75% vs 0.49 ± 0.78%, mean ± SD; Figure 2b).
Figure 2.
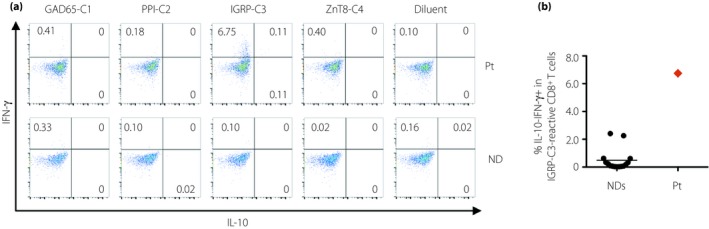
(a)The frequencies of cytokine‐producing CD8+ T cells in response to islet antigen peptide clusters, glutamic acid decarboxylase (GAD)65‐C1, preproinsulin (PPI)‐C2, islet‐specific glucose‐6‐phosphatase catalytic subunit‐related protein (IGRP)‐C3 and zinc transporter‐8 antibody (ZnT8‐C4), with peptide diluent as a negative control in the patient with type 1 diabetes, presenting with idiopathic CD4 lymphocytopenia, and a non‐diabetic individual. Intracytoplasmic cytokine detection assay was carried out after 7‐day stimulation with islet antigen peptide clusters in the presence of interleukin‐2 (IL‐2), gated to LIVE/DEAD − CD3+ CD8+ T‐cell populations. (b)Comparison of the frequencies of islet‐specific glucose‐6‐phosphatase catalytic subunit‐related protein (IGRP)‐C3‐specific interferon‐gamma (IFN‐γ)‐producing CD8+ T cells between the patient and non‐diabetic individuals (n = 15). CD4, CD4+ T cells; CD8, CD8+ T cells; IL‐10, interleukin‐10; IFN‐γ, interferon‐gamma; Pt, patient with type 1 diabetes, presenting with idiopathic CD4 lymphocytopenia; ND, non‐diabetic individual.
The study protocol was approved by the ethics committee of the National Center for Global Health and Medicine (registration no. NCGM‐A‐000200‐00). Written informed consent was obtained from all participants.
Discussion
Although 14–35% of patients with ICL have accompanying autoimmune diseases, such as Sjögren's syndrome, psoriasis and chronic thyroiditis1, 2, no case of type 1 diabetes with ICL had yet been reported. Type 1 diabetes is caused by autoimmune destruction of pancreatic β‐cells; autoreactive T cells play a major role in the process. Before or at the onset of type 1 diabetes, islet‐related autoantibodies are usually detected in blood as biomarkers for diagnosis. Here, we investigated the mechanistic relationship between the onset of type 1 diabetes and the course of ICL.
In the analysis of islet autoantibodies, multiple autoantibodies, including insulin autoantibody and insulinoma‐associated antigen‐2 antibody, appeared at the onset of type 1 diabetes, after low titers of glutamic acid decarboxylase antibody. Ziegler et al.4 reported the seroconversion of multiple islet autoantibodies to be associated with a higher frequency and faster progression of type 1 diabetes, compared with that of single or no autoantibodies. In the present patient, we speculated that lower magnitude of autoimmunity existed decades before its onset, followed by the antigen spread and rapid β‐cell destruction.
Takarabe et al.5 reported autoimmune diabetes as an immune reconstruction inflammatory syndrome, caused by the increase of CD4+ T cells, after highly aggressive anti‐retroviral treatment for lymphocytopenia related to HIV infection. The autoimmune process activated by CD4+ T‐cell expansion might pose a risk for the development of autoimmune diseases. Additionally, homeostatic expansion of CD4+ T cells after lymphocytopenia might facilitate autoimmunity6. However, in the pathogenesis of human type 1 diabetes, CD8+ cytotoxic T cells play an essential role in the destruction of β‐cells7, and the ones specific for islet antigens, such as insulin and IGRP, infiltrate into insulitic lesions8. The present patient showed an elevated number of CD8+ T cells before the onset of type 1 diabetes, along with a high frequency of IGRP‐specific CD8+ T cells. In 8.3‐NOD, IGRP‐specific CD8+ T cells were identified as a prevalent population of pathogenic CD8+ T cells in autoimmune diabetes9. In addition, 65% of pediatric patients with type 1 diabetes showed CD8+ T‐cell responses specific for at least one epitope of IGRP, unlike that in the healthy controls10. According to these reports, IGRP‐reactive CD8+ T cells might play a central role for autoimmune destruction of pancreatic β‐cells.
We had a certain limitation in the present study. As we could not obtain peripheral blood mononuclear cells from the patient before type 1 diabetes onset, we could not compare the frequencies of islet‐specific CD8+ T cells and the numbers or functions of regulatory T cells. In addition, the majority of antigen peptides we used in the present study were selected based on the findings from Caucasian patients with HLA‐A*2, despite study participants with HLA‐A*24 being included in our study. So further studies to evaluate A*24‐restricted responses, including binding assay, are required by using the samples from A*24‐positive Japanese type 1 diabetes patients. Finally, there is still the possibility that type 1 diabetes and ICL were incidentally complicated. In future, we need to carry out in‐depth immunological studies of similar patients, including pathological analyses for further investigation of disease etiology.
Disclosure
The authors declare no conflict of interest.
Supporting information
Appendix S1 | The method for the analysis of islet antigen‐specific CD8+ T‐cell responses
Table S1 | Laboratory data at the time of admission
Table S2 | Islet antigen‐specific peptide clusters
Acknowledgments
This study was supported by a KAKENHI (No. 17K09854) grant from the Japan Society for the Promotion of Science and by the Japan Diabetes Foundation. The authors thank the patient and non‐diabetic volunteers who participated in this study.
J Diabetes Investig 2019; 10: 1108–1111
References
- 1. Ahmad DS, Esmadi M, Steinmann WC. Idiopathic CD4 lymphocytopenia: spectrum of opportunistic infections, malignancies, and autoimmune diseases. Avicenna J Med 2013; 3: 37–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Regent A, Autran B, Carcelain G, et al Idiopathic CD4 lymphocytopenia: clinical and immunologic characteristics and follow‐up of 40 patients. Medicine (Baltimore) 2014; 93: 61–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chujo D, Nguyen TS, Foucat E, et al Adult‐onset type 1 diabetes patients display decreased IGRP‐specific Tr1 cells in blood. Clin Immunol 2015; 161: 270–277. [DOI] [PubMed] [Google Scholar]
- 4. Ziegler AG, Rewers M, Simell O, et al Seroconversion to multiple islet autoantibodies and risk of progression to diabetes in children. JAMA 2013; 309: 2473–2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Takarabe D, Rokukawa Y, Takahashi Y, et al Autoimmune diabetes in HIV‐infected patients on highly active antiretroviral therapy. J Clin Endocrinol Metab 2010; 95: 4056–4060. [DOI] [PubMed] [Google Scholar]
- 6. King C, Ilic A, Koelsch K, et al Homeostatic expansion of T cells during immune insufficiency generates autoimmunity. Cell 2004; 117: 265–277. [DOI] [PubMed] [Google Scholar]
- 7. Mallone R, Martinuzzi E, Blancou P, et al CD8+ T‐cell responses identify beta‐cell autoimmunity in human type 1 diabetes. Diabetes 2007; 56: 613–621. [DOI] [PubMed] [Google Scholar]
- 8. Coppieters KT, Dotta F, Amirian N, et al Demonstration of islet‐autoreactive CD8 T cells in insulitic lesions from recent onset and long‐term type 1 diabetes patients. J Exp Med 2012; 209: 51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lieberman SM, Evans AM, Han B, et al Identification of the beta cell antigen targeted by a prevalent population of pathogenic CD8+ T cells in autoimmune diabetes. Proc Natl Acad Sci USA 2003; 100: 8384–8388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jarchum I, Nichol L, Trucco M, et al Identification of novel IGRP epitopes targeted in type 1 diabetes patients. Clin Immunol 2008; 127: 359–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 | The method for the analysis of islet antigen‐specific CD8+ T‐cell responses
Table S1 | Laboratory data at the time of admission
Table S2 | Islet antigen‐specific peptide clusters


