Abstract
Cell division, the purpose of which is to enable cell replication, and in particular to distribute complete, accurate copies of genetic material to daughter cells, is essential for the propagation of life. At a morphological level, division not only necessitates duplication of cellular structures, but it also relies on polar segregation of this material followed by physical scission of the parent cell. For these fundamental changes in cell shape and positioning to be achieved, mechanisms are required to link the cell cycle to the modulation of cytoarchitecture. Outside of mitosis, the three main cytoskeletal networks not only endow cells with a physical cytoplasmic skeleton, but they also provide a mechanism for spatio-temporal sensing via integrin-associated adhesion complexes and site-directed delivery of cargoes. During mitosis, some interphase functions are retained, but the architecture of the cytoskeleton changes dramatically, and there is a need to generate a mitotic spindle for chromosome segregation. An economical solution is to re-use existing cytoskeletal molecules: transcellular actin stress fibres remodel to create a rigid cortex and a cytokinetic furrow, while unipolar radial microtubules become the primary components of the bipolar spindle. This remodelling implies the existence of specific mechanisms that link the cell-cycle machinery to the control of adhesion and the cytoskeleton. In this article, we review the intimate three-way connection between microenvironmental sensing, adhesion signalling and cell proliferation, particularly in the contexts of normal growth control and aberrant tumour progression. As the morphological changes that occur during mitosis are ancient, the mechanisms linking the cell cycle to the cytoskeleton/adhesion signalling network are likely to be primordial in nature and we discuss recent advances that have elucidated elements of this link. A particular focus is the connection between CDK1 and cell adhesion.
This article is part of a discussion meeting issue ‘Forces in cancer: interdisciplinary approaches in tumour mechanobiology’.
Keywords: adhesion, cytoskeleton, integrin, cell cycle, cyclin-dependent kinase 1, checkpoint
1. Introduction
(a). Stromal rigidity and tumour progression
A defining characteristic of malignancy is the loss of adhesion dependence of proliferation, which implies that the mechanisms normally controlling the coordination between adhesion and cell division are subverted. In this context, many tumours develop in a highly rigid tissue context, which has the potential to interfere with the normally carefully controlled morphological changes that take place during mitosis. In particular, the stromal microenvironment of many carcinomas is characterized by a dense desmoplastic response and evidence is accumulating for a correlation between stromal density and poor clinical outcome [1–3]. This connection is best established in the breast, where intermediate and high mammographic density have been linked to a significantly elevated risk of local recurrence [4]. A high stromal index in pancreatic and colorectal adenocarcinoma also inversely correlates with survival [5–8]. Stratification of human breast, pancreatic and colorectal carcinomas according to the gene expression and protein profile of their constituent stromal cells is predictive of treatment outcome [9–14]. Mammographic density correlates with extracellular matrix (ECM) rigidity [15], and changes in breast tumour- and stroma-derived ECM components contribute causally to metastatic spread [16].
Stromal rigidity is controlled by a combination of paracrine stimulation of ECM deposition and covalent cross-linking [17–21]. A number of the most abundant ECM molecules and ECM cross-linking enzymes are elevated in tumour stroma and correlate with poor patient prognosis [22–24]. Conversely, inhibition or reversal of these changes reduces tumour growth and increases survival in animal models, e.g. enzymatic destruction of hyaluronan [25], genetic deletion of the collagen-binding integrin α11β1 [26], pharmacological inhibition of lysyl oxidase (LOX) [26–31], knockdown of tissue transglutaminase [22,32] and induction of stromal quiescence using the vitamin D receptor ligand calcipotriol [33]. Treatment with gemcitabine in combination with hyaluronidase treatment, vitamin D receptor agonism or LOX inhibition results in increased overall survival in mouse models [30,34]. Recent studies imply a direct role for the ECM in resistance to small molecule therapeutics through effects on stromal fibroblasts [35]. Thus, while it is established that anti-adhesive agents are efficacious in conjunction with other treatment regimens [36–42], stromal normalization offers an additional route to therapy.
(b). Transduction of tissue rigidity into intracellular signals by adhesion receptors
At a cellular level, early studies demonstrated that cell shape is directly coupled to cell division and fate for fibroblasts, epithelial and endothelial cells [43–49], and gene expression patterns and cell phenotype can be altered by choice of adhesive substrate [29,50,51]. The mechanical properties of the ECM also alter differentiation and morphogenesis in mammary and endothelial cell models [52–54]. Mesenchymal stem cells are tuned to differentiate in response to the mechanical properties of their environment, with flattened cells undergoing osteogenesis and round cells favouring adipogenesis [55], and direct presentation of synthetic matrices of increasing rigidity stimulates first neuronal, then myogenic and finally osteogenic differentiation [56–60]. Application of external force or shear stress also modulates both mesenchymal stem cell [55,61] and human embryonic stem cell fate [62].
Stromal rigidity is primarily sensed by integrin-associated cell–ECM adhesion complexes in vitro and in vivo that are distributed focally rather than diffusely [63,64]. These adhesion nexi transmit short-range tensile and elastic force across the plasma membrane, and interpret long-range alterations in tissue flow [65]. The adhesion nexus functions as a mechanosensitive molecular clutch in two- and three-dimensional ECMs [66,67]. Data from both literature curation [68–70] and mass spectrometric analysis of the adhesion nexus [71–76] demonstrate that a small number of proteins (tens) establish its framework and a larger cohort of more transient proteins (hundreds) tune its function to intra- and extracellular stimuli [77]. Analysis of the protein–protein interaction network of the adhesion nexus identifies four interconnected axes that relay force to the cytoskeleton [73,75–79]. Candidate sensors of mechanical force include LIM domain-containing proteins that bind strain sites in actin [80–83], integrins themselves as they form force-stabilized catch bonds that undergo cyclic mechanical reinforcement [84,85], and cytoskeletal adaptors, such as vinculin, talin and p130Cas, which undergo force-dependent activation [86–93]. Therefore, the composition and physical characteristics of the ECM can have profound effects on cellular signalling and behaviour via changes in adhesion complex signalling.
(c). Regulation of cell-cycle progression by adhesion signalling
For most cells in multicellular organisms, the ECM anchorage dependence of normal cell growth and the propensity of tumour cells to evade this requirement have been established for many decades (figure 1) [94,95]. During the commitment phase of the cell cycle, sustained adhesion signalling is required to initiate DNA synthesis [96,97] and suppress apoptosis [95,98]. Integrin-dependent signalling is required for cell-cycle progression during the G1 phase, in particular, the induction of cyclin D1 and the downregulation of cyclin-dependent kinase (CDK) inhibitors [46,99–101]. There is accumulating evidence that extracellular force can feed into cell-cycle checkpoints: a focal adhesion kinase (FAK)/Rac signalling module relays force-dependent signals to the G1/S checkpoint [100], increased ECM rigidity affects cell-cycle progression by activating the Hippo pathway [102,103], FAK is required to reorientate the mitotic spindle in response to mechanical compression [104] and mechanical stretching drives the ATR kinase to the nuclear envelope where it prevents replication errors [105].
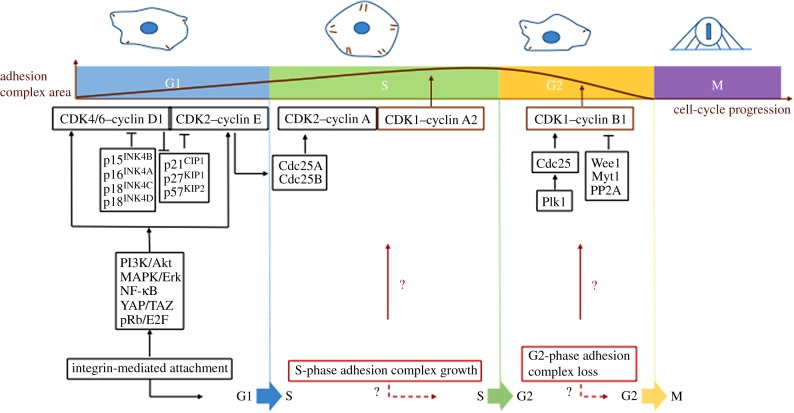
Figure 1.
Cross-talk between adhesion complexes and the cell-cycle machinery. Progress through S phase is associated with a CDK1–cyclin A2-dependent increase in adhesion complex area. Increased expression of cyclin B1 and inhibition of CDK1–cyclin B1 by Wee1/Myt1 results in a reduction in adhesion complexes in G2 prior to complete loss following mitotic cell rounding. Integrin-mediated attachment is required for the G1–S transition via the induction of cyclin D1 and cyclin E expression through the signals shown, but it remains unclear how adhesion signalling influences the S–G2 and G2–M transitions, and how adhesion complex turnover feeds into the cell-cycle regulation machinery.
During the replication and division phases of the cell cycle, major changes in cell shape, adhesiveness and cytoskeletal architecture are obligatory for chromosome segregation and cytokinesis [106–109]. These changes are highly conserved, implying the existence of a primordial regulatory mechanism. Across all metazoa, the remodelling events can be so extensive that cells become round and virtually lose their adhesion. Despite the risks to tissue integrity, the optimally symmetrical geometry of a sphere appears to enable the high degree of precision required for chromosome capture and division plane orientation [110–112]. Persistent adhesion and frustrated rounding prolong division and increase aneuploidy [113–116]. Aneuploidy is a common feature of human cancer [117,118], suggesting that its origin may be not only genetic, but also due to an aberrant physical microenvironment and the ability of cells to interpret this environment via integrin-dependent adhesion signalling.
2. Links between the cell-cycle machinery and adhesion
(a). Cell adhesion changes in a cell-cycle-dependent manner
Aside from the identification of anchorage-dependent growth in normal cells and the influence of adhesion on mitosis, very little is known about how adhesion complexes are regulated during cell-cycle progression or how adhesion signalling influences the transition between cell-cycle phases (figure 1). We have recently demonstrated that, as cells progress through S phase, adhesion complex area increases alongside the formation of robust actin stress fibres. Subsequently, adhesion complex area decreases and stress fibres disassemble when cells enter G2 [119]. These changes in adhesion complexes and the cytoskeleton correlate with changes in traction forces observed in cells progressing through the cell cycle [120] and with the observation that in epithelial monolayers, cellular tension is decreased in G2 several hours prior to mitosis [121].
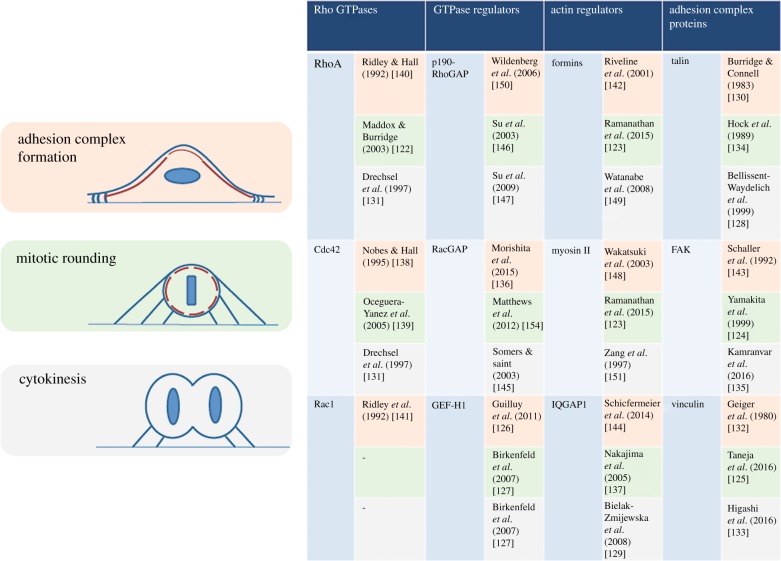
A number of proteins that regulate adhesion complexes and the actin cytoskeleton make key contributions to cell division (figure 2); for example, the activation of RhoA and the promotion of myosin-dependent contractility are required for both mitotic cell rounding and cytokinesis to occur [122,152–154]. Furthermore, formin activity is required to maintain cortical actin in mitotic cells [123]. Therefore, it is logical for the actomyosin machinery that regulates adhesion complexes to be redistributed and recycled for use during mitosis, where generation of a round morphology is critical for spindle positioning and chromosome capture [110,155,156]. Preventing the reduction in adhesion complexes observed in G2 leads to fewer cells entering mitosis and aberrant cell division [113,119,157], highlighting that these changes in adhesion and the cytoskeleton are key events that occur in G2 in preparation for entry into mitosis.
Figure 2.
Examples of adhesion-associated proteins that are re-used during mitotic cell rounding and cytokinesis. A number of proteins that regulate the actin cytoskeleton and adhesion complexes also play key roles in regulating mitotic cell rounding and cytokinesis. Four groups of proteins are highlighted and representative examples are presented in the table above together with key publications [122–151]. The background colour of the references matches the role of the protein in adhesion complex formation, mitotic cell rounding or cytokinesis regulation. The reuse of these regulators highlights the fundamental role of cross-talk between the cell-cycle machinery and adhesion complex signalling.
(b). Cyclin-dependent kinase 1 regulates cell adhesion
These observations demonstrate a reciprocal link between the cell-cycle machinery and adhesion complexes/cytoskeleton. This link from the cell-cycle machinery to adhesion complexes is primarily mediated by CDK1 [74,119]. CDK1 is a promiscuous serine/threonine kinase that has been shown to phosphorylate a wide range of substrates during mitosis [158,159] and ultimately drive the major changes in cell morphology associated with mitosis. A number of known adhesion complex proteins and regulators of the cytoskeleton are phosphorylated by CDK1 [124,160–163] and a recent phosphoproteomic study suggested that a high proportion of protein phosphorylation sites identified within adhesion complexes may be attributed to CDK1 (185 sites out of 1109 detected phosphorylation sites; 16.7%) [74]. These observations are consistent with an additional non-mitotic role for CDK1 in regulating adhesion and the cytoskeleton. Consistent with this dual function of CDK1 is the identification of the formin FMNL2 as a novel CDK1 substrate that is phosphorylated during both interphase and mitosis [119]. The regulation of CDK1 activity and association with other proteins such as cyclins therefore represent an elegant solution to the question of how changes in adhesion complexes are coordinated with cell-cycle progression. The ability of CDK1 to maintain adhesion complexes requires cyclin A2 [119,164], whereas the induction of cyclin B1 in G2 leads to increased levels of Wee1-dependent inactive cyclin–CDK1 complexes [119,165,166]. CDK1 activity is therefore reduced in G2 and coordinated with induction of cyclin B1 expression. Upon activation of cyclin B1–CDK1, the first event that occurs in mitotic entry is mitotic cell rounding [167]; therefore, it is tempting to suggest CDK1 has cyclin-dependent effects on adhesion complexes. When associated with cyclin B1, CDK1 drives adhesion complex disassembly during mitotic entry, but during interphase when associated with cyclin A2–CDK1 promotes adhesion complex formation. This hypothesis goes hand in hand with the idea that it is the cyclin that CDKs associate with that confers substrate specificity [168–171] and also suggests that the role for CDK1 in regulating all stages of cell-cycle progression in yeast [172–174] has in part been conserved in mammalian cells; in particular, the role for CDK1 in regulating the yeast cytoskeleton [175–180] along with entry into mitosis.
(c). Cell adhesion during mitosis
Upon activation of cyclin B1–CDK1 and translocation of this active complex into the nucleus, cells disassemble adhesion complexes, round up and enter mitosis. It is established that adhesion geometry prior to mitosis can inform the positioning of the mitotic spindle [111] and spatial memory between cell generations [181]. Furthermore, integrin-mediated adhesion is important in cytokinesis and the respreading and repulsive migration of daughter cells [125,182–185]. These observations are consistent with the presence of a mitotic anchor that stabilizes mitotic cells and also provides a footprint upon which dividing cells can form new adhesion complexes that facilitate cytokinesis and daughter cell respreading.
Much of the previous work on mitotic adhesion has focused on cells dividing on fibronectin and the role of β1 integrins that localize to the detached cell cortex [186] and to the cleavage furrow during cytokinesis [182–185,187]. While β1 integrin influences cytokinesis, it is not observed interacting with the ECM during earlier stages of mitosis other than in cell ‘tails’ that have not fully retracted into the cell body [188]. However, in cells attached to vitronectin or in cell culture dishes, the integrin αVβ5 is preferentially used by cells to mediate cell–ECM attachment [189]. αVβ5 is found in two distinct structures: classical focal adhesions that are positive for consensus adhesome components [77] and associated with actin fibres and novel structures termed reticular adhesions [189]. These reticular adhesions form and mediate cell adhesion in the absence of actin fibres and talin, and therefore represent a unique mechanism by which adhesion may be maintained during mitosis. Indeed, αVβ5-positive structures remain associated with ECM during mitotic cell rounding where they localize at the tips of retraction fibres and beneath the cell body. These complexes subsequently provide a footprint over which daughter cells respread following cytokinesis, and perturbation of reticular adhesions leads to aberrant cell division due to defects in mitotic axis orientation, cytokinesis and respreading [189]. Therefore, αVβ5-positive reticular adhesion complexes are essential for the normal progression of mitosis in cultured cells.
3. Future directions
(a). Regulation of cell-cycle-dependent adhesion transitions
Having established a novel fundamental link between cell-cycle progression and cell–ECM adhesion, a number of questions are outstanding. For example, how is adhesion complex growth in S phase promoted and what is its functional significance? This adhesion complex growth requires cyclin A2, so it is logical that it is coordinated with the induction of cyclin A2 expression during S phase [190–193]. Association of cyclin A2 with CDK1 requires phosphorylation of CDK1 by the CDK-activating kinase (CAK) [194], a complex of CDK7 with cyclin H [195], so it is possible that CAK plays a role in regulating adhesion complexes. Furthermore, identification of S-phase-specific CDK1 phosphorylation substrates would provide potential mechanisms by which the growth of adhesion complexes and induction of actin stress fibres is achieved. One potential candidate for this is the Rho GEF GEF-H1, which plays a role in facilitating force transduction through adhesion complexes [126] and is also phosphorylated by CDK1 during cytokinesis [127], although given the promiscuity of CDK1, there are likely to be a host of target proteins that are able to influence this process.
The induction of adhesion complexes and actin stress fibres may subsequently influence progression through S phase and into G2 by promoting downstream signalling events that regulate this process. For example, the transcription factors YAP/TAZ and SRF/MAL are activated by cellular tension and an increase in the F/G actin ratio, respectively [103,196]. These pathways may therefore be activated in S phase to facilitate expression of downstream target genes involved in cell-cycle progression. Alternatively, these changes in adhesion complexes and actin may influence gene transcription by exerting force on the nucleus and altering nuclear mechanics. The inhibition of FAK kinase activity leads to a reduction in cell proliferation [197] and FAK phosphorylation and activation increase as cells enter S phase (MC Jones 2019, unpublished data); however, how FAK subsequently influences cell-cycle progression has yet to be determined. It is therefore possible that signalling events activated downstream of adhesion complex formation are able to directly influence factors that mediate the transition from S into G2.
How cyclin B1–CDK1 drives the disassembly of residual adhesion complexes upon mitotic entry remains poorly understood, although a number of adhesion components have been shown to be phosphorylated during mitosis [124,161–163] and, in the case of α-parvin, FAK, paxillin and p130Cas, this phosphorylation is reversed following cytokinesis to allow daughter cell spreading [124,161,163]. This suggests that active cyclin B1–CDK1 undertakes a programme of adhesion complex protein phosphorylation that drives rapid disassembly. Alternatively, the high activation of cortical RhoA downstream of CDK1-dependent Ect2 phosphorylation [154] that drives mitotic cell rounding may result in the rapid loss of adhesion complexes. In this regard, it would be interesting to determine whether adhesion complex disassembly is still observed in compressed mitotic cells that are unable to round up. Entry into mitosis does not lead to the disassembly of reticular adhesions, so these adhesion complexes would appear to be regulated in a different way to focal adhesions; however, whether they are altered in a cell-cycle-dependent manner has yet to be determined. The balance of focal to reticular adhesions may therefore be an important consideration when determining the influence of individual adhesion proteins and signalling events to cell-cycle progression and the accuracy of cell division.
(b). The ‘adhesion checkpoint’
Ultimately, we hypothesize that cell-cycle-dependent changes in adhesion complexes and the cytoskeleton are essential for cell-cycle progression and division. The disruption of the actin cytoskeleton leads to arrest of cells in S phase [198,199] and increased cell adhesion in G2 leads to fewer cells entering mitosis and perturbed cell division [119,157]. This demonstrates that adhesion signalling is able to feed into cell-cycle checkpoints and in instances of aberrant adhesion signalling, cells are able to alter cell-cycle dynamics. This is consistent with a recent study identifying cellular tension in epithelial layers as being the key determinant of cell-cycle phase length [121]. The primary G1/S-phase checkpoint is characterized by hyperphosphorylation of Rb by cyclin D-CDK4/6 and cyclin E-CDK2 and activation of E2F-dependent transcription, with members of the INK4 and CIP/KIP CDK inhibitor families being able to exert checkpoint control. Non-adherent cells are unable to activate cyclin D and cyclin E complexes due to increased levels of p21 Cip1 and p27 Kip1 [200] and decreased levels of C-Myc [201] and consequently are unable to progress through S phase. Similarly, knockdown of talin-1 leads to reduced proliferation as a consequence of increased p21 expression [202] and disruption of the actin cytoskeleton results in retinoblastoma protein (Rb) hypophosphorylation. Furthermore, S-phase progression can also be enhanced by increasing actin stress fibres as a consequence of disrupting microtubules with nocodazole [203]. Specifically, perturbing the growth of adhesion complexes seen in S phase may therefore lead to G1/S-phase checkpoint activation and alter cell-cycle progression into G2.
The maintenance of adhesion complexes in G2 perturbs activation of cyclin B1–CDK1 [157] and results in fewer cells entering mitosis, suggesting that adhesion signalling is also able to impact upon the G2/M checkpoint. Given that increased cell adhesion and perturbation of αVβ5-positive mitotic adhesion complexes also leads to defects in division orientation and cytokinesis [119,189], this suggests that changes in adhesion signalling may also impact upon the spindle assembly checkpoint (SAC) during mitosis. Understanding the cell-cycle checkpoint signalling events that are influenced by adhesion complex signalling during G2 and mitosis is therefore a key avenue of future investigation. Likewise, how other cell-cycle regulators are able to influence adhesion complexes and the cytoskeleton remains to be determined. Greatwall kinase/MASTL has been identified as a negative regulator of β1-integrin function [204] and p27 Kip1 influences adhesion complexes and cell migration via modulation of RhoA activity [205]. Therefore, it is likely that the coordination of cell-cycle control with that of adhesion complexes and the cytoskeleton is complex and multi-layered, with a number of proteins being able to influence these processes.
Taken together, these observations suggest that normal cells are able to sense their surrounding ECM environment through integrin-associated adhesion complexes and determine whether to proceed through S phase or enter mitosis. In this regard, two distinct ‘adhesion checkpoints' that contribute to cell-cycle progression are present. In proliferative disorders such as cancer, these checkpoints are likely to be dysregulated. Changes in ECM composition and stiffness that alter adhesion signalling could impact upon the ability of cells to progress through S phase and accurately divide, leading to increased aneuploidy and contributing to tumour progression. Reciprocally, changes in cell-cycle signalling will also alter how cells perceive their ECM environment and could therefore impact upon a number of processes linked to tumour progression such as survival, invasion and colonization of metastatic niches. Developing a deeper understanding of this reciprocal relationship between cell-cycle and adhesion signalling will therefore contribute to our understanding of how tumour progression occurs and could also lead to novel therapeutic strategies targeting tumour matrix signalling alongside the use of anti-proliferative drugs.
Data accessibility
This article has no additional data.
Authors' contributions
M.J.H. developed the concept behind the review and all authors contributed equally to its writing.
Competing interests
We declare we have no competing interests.
Funding
This work was supported by Cancer Research UK (grant no. C13329/A21671) to M.J.H.
References
- 1.Pickup MW, Mouw JK, Weaver VM. 2014. The extracellular matrix modulates the hallmarks of cancer. EMBO Rep. 15, 1243–1253. ( 10.15252/embr.201439246) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sherratt MJ, McConnell JC, Streuli CH. 2016. Raised mammographic density: causative mechanisms and biological consequences. Breast Cancer Res. 18, 45 ( 10.1186/s13058-016-0701-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tlsty TD, Coussens LM. 2006. Tumor stroma and regulation of cancer development. Annu. Rev. Pathol. 1, 119–150. ( 10.1146/annurev.pathol.1.110304.100224) [DOI] [PubMed] [Google Scholar]
- 4.Cil T, Fishell E, Hanna W, Sun P, Rawlinson E, Narod SA, McCready DR. 2009. Mammographic density and the risk of breast cancer recurrence after breast-conserving surgery. Cancer 115, 5780–5787. ( 10.1002/cncr.24638) [DOI] [PubMed] [Google Scholar]
- 5.Erkan M, et al. 2008. The activated stroma index is a novel and independent prognostic marker in pancreatic ductal adenocarcinoma. Clin. Gastroenterol. Hepatol. 6, 1155–1161. ( 10.1016/j.cgh.2008.05.006) [DOI] [PubMed] [Google Scholar]
- 6.Guinney J, et al. 2015. The consensus molecular subtypes of colorectal cancer. Nat. Med. 21, 1350–1356. ( 10.1038/nm.3967) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Waghray M, Yalamanchili M, Magliano MP, Simeone DM. 2013. Deciphering the role of stroma in pancreatic cancer. Curr. Opin. Gastroenterol. 29, 537–543. ( 10.1097/MOG.0b013e328363affe) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Whatcott CJ, et al. 2015. Desmoplasia in primary tumors and metastatic lesions of pancreatic cancer. Clin. Cancer Res. 21, 3561–3568. ( 10.1158/1078-0432.CCR-14-1051) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bergamaschi A, et al. 2008. Extracellular matrix signature identifies breast cancer subgroups with different clinical outcome. J. Pathol. 214, 357–367. ( 10.1002/path.2278) [DOI] [PubMed] [Google Scholar]
- 10.Finak G, et al. 2008. Stromal gene expression predicts clinical outcome in breast cancer. Nat. Med. 14, 518–527. ( 10.1038/nm1764) [DOI] [PubMed] [Google Scholar]
- 11.Haider S, et al. 2014. A multi-gene signature predicts outcome in patients with pancreatic ductal adenocarcinoma. Genome Med. 6, 105 ( 10.1186/s13073-014-0105-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Isella C, et al. 2015. Stromal contribution to the colorectal cancer transcriptome. Nat. Genet. 47, 312–319. ( 10.1038/ng.3224) [DOI] [PubMed] [Google Scholar]
- 13.Jamieson NB, Carter CR, McKay CJ, Oien KA. 2011. Tissue biomarkers for prognosis in pancreatic ductal adenocarcinoma: a systematic review and meta-analysis. Clin. Cancer Res. 17, 3316–3331. ( 10.1158/1078-0432.CCR-10-3284) [DOI] [PubMed] [Google Scholar]
- 14.Ohuchida K, et al. 2007. Over-expression of S100A2 in pancreatic cancer correlates with progression and poor prognosis. J. Pathol. 213, 275–282. ( 10.1002/path.2250) [DOI] [PubMed] [Google Scholar]
- 15.McConnell JC, et al. 2016. Increased peri-ductal collagen micro-organization may contribute to raised mammographic density. Breast Cancer Res. 18, 5 ( 10.1186/s13058-015-0664-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Naba A, Clauser KR, Lamar JM, Carr SA, Hynes RO. 2014. Extracellular matrix signatures of human mammary carcinoma identify novel metastasis promoters. Elife 3, e01308 ( 10.7554/eLife.01308) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Calon A, Tauriello DV, Batlle E. 2014. TGF-beta in CAF-mediated tumor growth and metastasis. Semin. Cancer Biol. 25, 15–22. ( 10.1016/j.semcancer.2013.12.008) [DOI] [PubMed] [Google Scholar]
- 18.Ikushima H, Miyazono K. 2010. TGFbeta signalling: a complex web in cancer progression. Nat. Rev. Cancer 10, 415–424. ( 10.1038/nrc2853) [DOI] [PubMed] [Google Scholar]
- 19.Javelaud D, Pierrat MJ, Mauviel A. 2012. Crosstalk between TGF-beta and hedgehog signaling in cancer. FEBS Lett. 586, 2016–2025. ( 10.1016/j.febslet.2012.05.011) [DOI] [PubMed] [Google Scholar]
- 20.Tian H, Callahan CA, Dupree KJ, Darbonne WC, Ahn CP, Scales SJ, De Sauvage FJ. 2009. Hedgehog signaling is restricted to the stromal compartment during pancreatic carcinogenesis. Proc. Natl Acad. Sci. USA 106, 4254–4259. ( 10.1073/pnas.0813203106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yauch RL, et al. 2008. A paracrine requirement for hedgehog signalling in cancer. Nature 455, 406–410. ( 10.1038/nature07275) [DOI] [PubMed] [Google Scholar]
- 22.Eckert RL, Fisher ML, Grun D, Adhikary G, Xu W, Kerr C. 2015. Transglutaminase is a tumor cell and cancer stem cell survival factor. Mol. Carcinog. 54, 947–958. ( 10.1002/mc.22375) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Erler JT, Bennewith KL, Cox TR, Lang G, Bird D, Koong A, Le Q-T, Giaccia AJ. 2009. Hypoxia-induced lysyl oxidase is a critical mediator of bone marrow cell recruitment to form the premetastatic niche. Cancer Cell 15, 35–44. ( 10.1016/j.ccr.2008.11.012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Venning FA, Wullkopf L, Erler JT. 2015. Targeting ECM disrupts cancer progression. Front. Oncol. 5, 224 ( 10.3389/fonc.2015.00224) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Provenzano PP, Cuevas C, Chang AE, Goel VK, Von Hoff DD, Hingorani SR. 2012. Enzymatic targeting of the stroma ablates physical barriers to treatment of pancreatic ductal adenocarcinoma. Cancer Cell 21, 418–429. ( 10.1016/j.ccr.2012.01.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Navab R, et al. 2016. Integrin alpha11beta1 regulates cancer stromal stiffness and promotes tumorigenicity and metastasis in non-small cell lung cancer. Oncogene 35, 1899–1908. ( 10.1038/onc.2015.254) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baker AM, Cox TR, Bird D, Lang G, Murray GI, Sun X-F, Southall SM, Wilson JR, Erler JT. 2011. The role of lysyl oxidase in SRC-dependent proliferation and metastasis of colorectal cancer. J. Natl Cancer Inst. 103, 407–424. ( 10.1093/jnci/djq569) [DOI] [PubMed] [Google Scholar]
- 28.Cox TR, Bird D, Baker A-M, Barker HE, Ho MW-Y, Lang G, Erler JT. 2013. LOX-mediated collagen crosslinking is responsible for fibrosis-enhanced metastasis. Cancer Res. 73, 1721–1732. ( 10.1158/0008-5472.CAN-12-2233) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Levental KR, et al. 2009. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell 139, 891–906. ( 10.1016/j.cell.2009.10.027) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miller BW, et al. 2015. Targeting the LOX/hypoxia axis reverses many of the features that make pancreatic cancer deadly: inhibition of LOX abrogates metastasis and enhances drug efficacy. EMBO Mol. Med. 7, 1063–1076. ( 10.15252/emmm.201404827) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Payne SL, Fogelgren B, Hess AR, Seftor EA, Wiley EL, Fong SFT, Csiszar K, Hendrix MJC, Kirschmann DA. 2005. Lysyl oxidase regulates breast cancer cell migration and adhesion through a hydrogen peroxide-mediated mechanism. Cancer Res. 65, 11 429–11 436. ( 10.1158/0008-5472.CAN-05-1274) [DOI] [PubMed] [Google Scholar]
- 32.Lee J, Condello S, Yakubov B, Emerson R, Caperell-Grant A, Hitomi K, Xie J, Matei D. 2015. Tissue transglutaminase mediated tumor-stroma interaction promotes pancreatic cancer progression. Clin. Cancer Res. 21, 4482–4493. ( 10.1158/1078-0432.CCR-15-0226) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sherman MH, et al. 2014. Vitamin D receptor-mediated stromal reprogramming suppresses pancreatitis and enhances pancreatic cancer therapy. Cell 159, 80–93. ( 10.1016/j.cell.2014.08.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Michl P, Gress TM. 2012. Improving drug delivery to pancreatic cancer: breaching the stromal fortress by targeting hyaluronic acid. Gut 61, 1377–1379. ( 10.1136/gutjnl-2012-302604) [DOI] [PubMed] [Google Scholar]
- 35.Hirata E, Girotti MR, Viros A, Hooper S, Spencer-Dene B, Matsuda M, Larkin J, Marais R, Sahai E. 2015. Intravital imaging reveals how BRAF inhibition generates drug-tolerant microenvironments with high integrin beta1/FAK signaling. Cancer Cell 27, 574–588. ( 10.1016/j.ccell.2015.03.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Abdollahi A, et al. 2005. Inhibition of alpha(v)beta(3) integrin survival signaling enhances antiangiogenic and antitumor effects of radiotherapy. Clin. Cancer Res. 11, 6270–6279. ( 10.1158/1078-0432.CCR-04-1223) [DOI] [PubMed] [Google Scholar]
- 37.Burke PA, DeNardo SJ, Miers LA, Lamborn KR, Matzku S, DeNardo GL. 2002. Cilengitide targeting of alpha(v)beta(3) integrin receptor synergizes with radioimmunotherapy to increase efficacy and apoptosis in breast cancer xenografts. Cancer Res. 62, 4263–4272. ( 10.1089/cbr.2000.15.71) [DOI] [PubMed] [Google Scholar]
- 38.McLean GW, Carragher NO, Avizienyte E, Evans J, Brunton VG, Frame MC. 2005. The role of focal-adhesion kinase in cancer—a new therapeutic opportunity. Nat. Rev. Cancer 5, 505–515. ( 10.1038/nrc1647) [DOI] [PubMed] [Google Scholar]
- 39.Roberts WG, et al. 2008. Antitumor activity and pharmacology of a selective focal adhesion kinase inhibitor, PF-562,271. Cancer Res. 68, 1935–1944. ( 10.1158/0008-5472.CAN-07-5155) [DOI] [PubMed] [Google Scholar]
- 40.Shapiro IM, et al. 2014. Merlin deficiency predicts FAK inhibitor sensitivity: a synthetic lethal relationship. Sci. Transl. Med. 6, 237ra68 ( 10.1126/scitranslmed.3008639) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tavora B, et al. 2014. Endothelial-cell FAK targeting sensitizes tumours to DNA-damaging therapy. Nature 514, 112–116. ( 10.1038/nature13541) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wong PP, et al. 2015. Dual-action combination therapy enhances angiogenesis while reducing tumor growth and spread. Cancer Cell 27, 123–137. ( 10.1016/j.ccell.2014.10.015) [DOI] [PubMed] [Google Scholar]
- 43.Chen CS, Mrksich M, Huang S, Whitesides GM, Ingber DE. 1997. Geometric control of cell life and death. Science 276, 1425–1428. ( 10.1126/science.276.5317.1425) [DOI] [PubMed] [Google Scholar]
- 44.Folkman J, Moscona A. 1978. Role of cell shape in growth control. Nature 273, 345–349. ( 10.1038/273345a0) [DOI] [PubMed] [Google Scholar]
- 45.Goffin JM, Pittet P, Csucs G, Lussi JW, Meister J-J, Hinz B. 2006. Focal adhesion size controls tension-dependent recruitment of alpha-smooth muscle actin to stress fibers. J. Cell Biol. 172, 259–268. ( 10.1083/jcb.200506179) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Klein EA, et al. 2009. Cell-cycle control by physiological matrix elasticity and in vivo tissue stiffening. Curr. Biol. 19, 1511–1518. ( 10.1016/j.cub.2009.07.069) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kostic A, Sheetz MP. 2006. Fibronectin rigidity response through Fyn and p130Cas recruitment to the leading edge. Mol. Biol. Cell 17, 2684–2695. ( 10.1091/mbc.e05-12-1161) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Polte TR, Eichler GS, Wang N, Ingber DE. 2004. Extracellular matrix controls myosin light chain phosphorylation and cell contractility through modulation of cell shape and cytoskeletal prestress. Am. J. Physiol. Cell Physiol. 286, C518–C528. ( 10.1152/ajpcell.00280.2003) [DOI] [PubMed] [Google Scholar]
- 49.Watt FM, Jordan PW, O'Neill CH. 1988. Cell shape controls terminal differentiation of human epidermal keratinocytes. Proc. Natl Acad. Sci. USA 85, 5576–5580. ( 10.1073/pnas.85.15.5576) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Huhtala P, Humphries MJ, McCarthy JB, Tremble PM, Werb Z, Damsky CH. 1995. Cooperative signaling by alpha 5 beta 1 and alpha 4 beta 1 integrins regulates metalloproteinase gene expression in fibroblasts adhering to fibronectin. J. Cell Biol. 129, 867–879. ( 10.1083/jcb.129.3.867) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Streuli CH, Bailey N, Bissell MJ. 1991. Control of mammary epithelial differentiation: basement membrane induces tissue-specific gene expression in the absence of cell-cell interaction and morphological polarity. J. Cell Biol. 115, 1383–1395. ( 10.1083/jcb.115.5.1383) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Discher DE, Mooney DJ, Zandstra PW. 2009. Growth factors, matrices, and forces combine and control stem cells. Science 324, 1673–1677. ( 10.1126/science.1171643) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gehler S, et al. 2009. Filamin A-beta1 integrin complex tunes epithelial cell response to matrix tension. Mol. Biol. Cell 20, 3224–3238. ( 10.1091/mbc.e08-12-1186) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mammoto A, Connor KM, Mammoto T, Yung CW, Huh D, Aderman CM, Mostoslavsky G, Smith LEH, Ingber DE. 2009. A mechanosensitive transcriptional mechanism that controls angiogenesis. Nature 457, 1103–1108. ( 10.1038/nature07765) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McBeath R, Pirone DM, Nelson CM, Bhadriraju K, Chen CS. 2004. Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev. Cell 6, 483–495. ( 10.1016/S1534-5807(04)00075-9) [DOI] [PubMed] [Google Scholar]
- 56.Boontheekul T, Hill EE, Kong H-J, Mooney DJ. 2007. Regulating myoblast phenotype through controlled gel stiffness and degradation. Tissue Eng. 13, 1431–1442. ( 10.1089/ten.2006.0356) [DOI] [PubMed] [Google Scholar]
- 57.Engler AJ, Sen S, Sweeney HL, Discher DE. 2006. Matrix elasticity directs stem cell lineage specification. Cell 126, 677–689. ( 10.1016/j.cell.2006.06.044) [DOI] [PubMed] [Google Scholar]
- 58.Khatiwala CB, Kim PD, Peyton SR, Putnam AJ. 2009. ECM compliance regulates osteogenesis by influencing MAPK signaling downstream of RhoA and ROCK. J. Bone Miner. Res. 24, 886–898. ( 10.1359/jbmr.081240) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rowlands AS, George PA, Cooper-White JJ. 2008. Directing osteogenic and myogenic differentiation of MSCs: interplay of stiffness and adhesive ligand presentation. Am. J. Physiol. Cell Physiol. 295, C1037–C1044. ( 10.1152/ajpcell.67.2008) [DOI] [PubMed] [Google Scholar]
- 60.Saha K, Keung AJ, Irwin EF, Li Y, Little L, Schaffer DV, Healy KE. 2008. Substrate modulus directs neural stem cell behavior. Biophys. J. 95, 4426–4438. ( 10.1529/biophysj.108.132217) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Arnsdorf EJ, Tummala P, Kwon RY, Jacobs CR. 2009. Mechanically induced osteogenic differentiation—the role of RhoA, ROCKII and cytoskeletal dynamics. J. Cell Sci. 122, 546–553. ( 10.1242/jcs.036293) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Peerani R, Rao BM, Bauwens C, Yin T, Wood GA, Nagy A, Kumacheva E, Zandstra PW. 2007. Niche-mediated control of human embryonic stem cell self-renewal and differentiation. EMBO J. 26, 4744–4755. ( 10.1038/sj.emboj.7601896) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cukierman E, Pankov R, Stevens DR, Yamada KM. 2001. Taking cell-matrix adhesions to the third dimension. Science 294, 1708–1712. ( 10.1126/science.1064829) [DOI] [PubMed] [Google Scholar]
- 64.Geiger B, Bershadsky A, Pankov R, Yamada KM. 2001. Transmembrane crosstalk between the extracellular matrix—cytoskeleton crosstalk. Nat. Rev. Mol. Cell Biol. 2, 793–805. ( 10.1038/35099066) [DOI] [PubMed] [Google Scholar]
- 65.Ray RP, Matamoro-Vidal A, Ribeiro PS, Tapon N, Houle D, Salazar-Ciudad I, Thompson BJ. 2015. Patterned anchorage to the apical extracellular matrix defines tissue shape in the developing appendages of Drosophila. Dev. Cell 34, 310–322. ( 10.1016/j.devcel.2015.06.019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Case LB, Waterman CM. 2015. Integration of actin dynamics and cell adhesion by a three-dimensional, mechanosensitive molecular clutch. Nat. Cell Biol. 17, 955–963. ( 10.1038/ncb3191) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Doyle AD, Yamada KM. 2016. Mechanosensing via cell-matrix adhesions in 3D microenvironments. Exp. Cell Res. 343, 60–66. ( 10.1016/j.yexcr.2015.10.033) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Winograd-Katz SE, Fässler R, Geiger B, Legate KR. 2014. The integrin adhesome: from genes and proteins to human disease. Nat. Rev. Mol. Cell Biol. 15, 273–288. ( 10.1038/nrm3769) [DOI] [PubMed] [Google Scholar]
- 69.Zaidel-Bar R, Geiger B. 2010. The switchable integrin adhesome. J. Cell Sci. 123, 1385–1388. ( 10.1242/jcs.066183) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zaidel-Bar R, Itzkovitz S, Ma'ayan A, Iyengar R, Geiger B. 2007. Functional atlas of the integrin adhesome. Nat. Cell Biol. 9, 858–867. ( 10.1038/ncb0807-858) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Byron A, et al. 2015. A proteomic approach reveals integrin activation state-dependent control of microtubule cortical targeting. Nat. Commun. 6, 6135 ( 10.1038/ncomms7135) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Humphries JD, Byron A, Bass MD, Craig SE, Pinney JW, Knight D, Humphries MJ. 2009. Proteomic analysis of integrin-associated complexes identifies RCC2 as a dual regulator of Rac1 and Arf6. Sci. Signal 2, ra51 ( 10.1126/scisignal.2000396) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kuo JC, Han X, Hsiao C-T, Yates Iii JR, Waterman CM. 2011. Analysis of the myosin-II-responsive focal adhesion proteome reveals a role for beta-Pix in negative regulation of focal adhesion maturation. Nat. Cell Biol. 13, 383–393. ( 10.1038/ncb2216) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Robertson J, Jacquemet G, Byron A, Jones MC, Warwood S, Selley JN, Knight D, Humphries JD, Humphries MJ. 2015. Defining the phospho-adhesome through the phosphoproteomic analysis of integrin signalling. Nat. Commun. 6, 6265 ( 10.1038/ncomms7265) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schiller HB, Friedel CC, Boulegue C, Fässler R. 2011. Quantitative proteomics of the integrin adhesome show a myosin II-dependent recruitment of LIM domain proteins. EMBO Rep. 12, 259–266. ( 10.1038/embor.2011.5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Schiller HB, et al. 2013. beta1(1)- and alpha(v)-class integrins cooperate to regulate myosin II during rigidity sensing of fibronectin-based microenvironments. Nat. Cell Biol. 15, 625–636. ( 10.1038/ncb2747) [DOI] [PubMed] [Google Scholar]
- 77.Horton ER, et al. 2015. Definition of a consensus integrin adhesome and its dynamics during adhesion complex assembly and disassembly. Nat. Cell Biol. 17, 1577–1587. ( 10.1038/ncb3257) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ng DH, Humphries JD, Byron A, Millon-Frémillon A, Humphries MJ. 2014. Microtubule-dependent modulation of adhesion complex composition. PLoS ONE 9, e115213 ( 10.1371/journal.pone.0115213) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yue J, Xie M, Gou X, Lee P, Schneider MD, Wu X. 2014. Microtubules regulate focal adhesion dynamics through MAP4K4. Dev. Cell 31, 572–585. ( 10.1016/j.devcel.2014.10.025) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Schiller HB, Fassler R. 2013. Mechanosensitivity and compositional dynamics of cell-matrix adhesions. EMBO Rep. 14, 509–519. ( 10.1038/embor.2013.49) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Smith MA, Blankman E, Gardel ML, Luettjohann L, Waterman CM, Beckerle MC. 2010. A zyxin-mediated mechanism for actin stress fiber maintenance and repair. Dev. Cell 19, 365–376. ( 10.1016/j.devcel.2010.08.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Smith MA, Hoffman LM, Beckerle MC. 2014. LIM proteins in actin cytoskeleton mechanoresponse. Trends Cell Biol. 24, 575–583. ( 10.1016/j.tcb.2014.04.009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Uemura A, Nguyen T-N, Steele AN, Yamada S. 2011. The LIM domain of zyxin is sufficient for force-induced accumulation of zyxin during cell migration. Biophys. J. 101, 1069–1075. ( 10.1016/j.bpj.2011.08.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kong F, García AJ, Mould AP, Humphries MJ, Zhu C. 2009. Demonstration of catch bonds between an integrin and its ligand. J. Cell Biol. 185, 1275–1284. ( 10.1083/jcb.200810002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kong F, Li Z, Parks WM, Dumbauld DW, García AJ, Mould AP, Humphries MJ, Zhu C. 2013. Cyclic mechanical reinforcement of integrin-ligand interactions. Mol. Cell 49, 1060–1068. ( 10.1016/j.molcel.2013.01.015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Austen K, et al. 2015. Extracellular rigidity sensing by talin isoform-specific mechanical linkages. Nat. Cell Biol. 17, 1597–1606. ( 10.1038/ncb3268) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Carisey A, et al. 2013. Vinculin regulates the recruitment and release of core focal adhesion proteins in a force-dependent manner. Curr. Biol. 23, 271–281. ( 10.1016/j.cub.2013.01.009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.del Rio A, Perez-Jimenez R, Liu R, Roca-Cusachs P, Fernandez JM, Sheetz MP. 2009. Stretching single talin rod molecules activates vinculin binding. Science 323, 638–641. ( 10.1126/science.1162912) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Friedland JC, Lee MH, Boettiger D. 2009. Mechanically activated integrin switch controls alpha(5)beta(1) function. Science 323, 642–644. ( 10.1126/science.1168441) [DOI] [PubMed] [Google Scholar]
- 90.Grashoff C, et al. 2010. Measuring mechanical tension across vinculin reveals regulation of focal adhesion dynamics. Nature 466, 263–266. ( 10.1038/nature09198) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hytonen VP, Wehrle-Haller B. 2016. Mechanosensing in cell-matrix adhesions—converting tension into chemical signals. Exp. Cell Res. 343, 35–41. ( 10.1016/j.yexcr.2015.10.027) [DOI] [PubMed] [Google Scholar]
- 92.Klapholz B, Herbert SL, Wellmann J, Johnson R, Parsons M, Brown NH. 2015. Alternative mechanisms for talin to mediate integrin function. Curr. Biol. 25, 847–857. ( 10.1016/j.cub.2015.01.043) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sawada Y, Tamada M, Dubin-Thaler BJ, Cherniavskaya O, Sakai R, Tanaka S, Sheetz MP. 2006. Force sensing by mechanical extension of the Src family kinase substrate p130Cas. Cell 127, 1015–1026. ( 10.1016/j.cell.2006.09.044) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Freedman VH, Shin SI. 1974. Cellular tumorigenicity in nude mice: correlation with cell growth in semi-solid medium. Cell 3, 355–359. ( 10.1016/0092-8674(74)90050-6) [DOI] [PubMed] [Google Scholar]
- 95.Meredith JE Jr, Fazeli B, Schwartz MA. 1993. The extracellular matrix as a cell survival factor. Mol. Biol. Cell 4, 953–961. ( 10.1091/mbc.4.9.953) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Assoian RK. 1997. Anchorage-dependent cell cycle progression. J. Cell Biol. 136, 1–4. ( 10.1083/jcb.136.1.1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Schwartz MA, Assoian RK. 2001. Integrins and cell proliferation: regulation of cyclin-dependent kinases via cytoplasmic signaling pathways. J. Cell Sci. 114, 2553–2560. [DOI] [PubMed] [Google Scholar]
- 98.Frisch SM, Ruoslahti E. 1997. Integrins and anoikis. Curr. Opin. Cell Biol. 9, 701–706. ( 10.1016/S0955-0674(97)80124-X) [DOI] [PubMed] [Google Scholar]
- 99.Assoian RK, Schwartz MA. 2001. Coordinate signaling by integrins and receptor tyrosine kinases in the regulation of G1 phase cell-cycle progression. Curr. Opin. Genet. Dev. 11, 48–53. ( 10.1016/S0959-437X(00)00155-6) [DOI] [PubMed] [Google Scholar]
- 100.Bae YH, Mui KL, Hsu BY, Liu S-L, Cretu A, Razinia Z, Xu T, Pure E, Assoian RK. 2014. A FAK-Cas-Rac-lamellipodin signaling module transduces extracellular matrix stiffness into mechanosensitive cell cycling. Sci. Signal 7, ra57 ( 10.1126/scisignal.2004838) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Moreno-Layseca P, Streuli CH. 2014. Signalling pathways linking integrins with cell cycle progression. Matrix Biol. 34, 144–153. ( 10.1016/j.matbio.2013.10.011) [DOI] [PubMed] [Google Scholar]
- 102.Aragona M, Panciera T, Manfrin A, Giulitti S, Michielin F, Elvassore N, Dupont S, Piccolo S. 2013. A mechanical checkpoint controls multicellular growth through YAP/TAZ regulation by actin-processing factors. Cell 154, 1047–1059. ( 10.1016/j.cell.2013.07.042) [DOI] [PubMed] [Google Scholar]
- 103.Dupont S, et al. 2011. Role of YAP/TAZ in mechanotransduction. Nature 474, 179–183. ( 10.1038/nature10137) [DOI] [PubMed] [Google Scholar]
- 104.Petridou NI, Skourides PA. 2014. FAK transduces extracellular forces that orient the mitotic spindle and control tissue morphogenesis. Nat. Commun. 5, 5240 ( 10.1038/ncomms6240) [DOI] [PubMed] [Google Scholar]
- 105.Kumar A, et al. 2014. ATR mediates a checkpoint at the nuclear envelope in response to mechanical stress. Cell 158, 633–646. ( 10.1016/j.cell.2014.05.046) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Cadart C, Zlotek-Zlotkiewicz E, Le Berre M, Piel M, Matthews HK. 2014. Exploring the function of cell shape and size during mitosis. Dev. Cell 29, 159–169. ( 10.1016/j.devcel.2014.04.009) [DOI] [PubMed] [Google Scholar]
- 107.Fededa JP, Gerlich DW. 2012. Molecular control of animal cell cytokinesis. Nat. Cell Biol. 14, 440–447. ( 10.1038/ncb2482) [DOI] [PubMed] [Google Scholar]
- 108.Lancaster OM, Baum B. 2014. Shaping up to divide: coordinating actin and microtubule cytoskeletal remodelling during mitosis. Semin. Cell Dev. Biol. 34, 109–115. ( 10.1016/j.semcdb.2014.02.015) [DOI] [PubMed] [Google Scholar]
- 109.Lesman A, Notbohm J, Tirrell DA, Ravichandran G. 2014. Contractile forces regulate cell division in three-dimensional environments. J. Cell Biol. 205, 155–162. ( 10.1083/jcb.201309029) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lancaster OM, Le Berre M, Dimitracopoulos A, Bonazzi D, Zlotek-Zlotkiewicz E, Picone R, Duke T, Piel M, Baum B. 2013. Mitotic rounding alters cell geometry to ensure efficient bipolar spindle formation. Dev. Cell 25, 270–283. ( 10.1016/j.devcel.2013.03.014) [DOI] [PubMed] [Google Scholar]
- 111.Thery M, Racine V, Pépin A, Piel M, Chen Y, Sibarita J-B, Bornens M. 2005. The extracellular matrix guides the orientation of the cell division axis. Nat. Cell Biol. 7, 947–953. ( 10.1038/ncb1307) [DOI] [PubMed] [Google Scholar]
- 112.Toyoshima F, Nishida E. 2007. Spindle orientation in animal cell mitosis: roles of integrin in the control of spindle axis. J. Cell. Physiol. 213, 407–411. ( 10.1002/jcp.21227) [DOI] [PubMed] [Google Scholar]
- 113.Dao VT, Dupuy AG, Gavet O, Caron E, de Gunzburg J. 2009. Dynamic changes in Rap1 activity are required for cell retraction and spreading during mitosis. J. Cell Sci. 122, 2996–3004. ( 10.1242/jcs.041301) [DOI] [PubMed] [Google Scholar]
- 114.Jefford CE, Irminger-Finger I. 2006. Mechanisms of chromosome instability in cancers. Crit. Rev. Oncol. Hematol. 59, 1–14. ( 10.1016/j.critrevonc.2006.02.005) [DOI] [PubMed] [Google Scholar]
- 115.Xi W, Schmidt CK, Sanchez S, Gracias DH, Carazo-Salas RE, Jackson SP, Schmidt OG. 2014. Rolled-up functionalized nanomembranes as three-dimensional cavities for single cell studies. Nano Lett. 14, 4197–4204. ( 10.1021/nl4042565) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Yennek S, Burute M, Théry M, Tajbakhsh S. 2014. Cell adhesion geometry regulates non-random DNA segregation and asymmetric cell fates in mouse skeletal muscle stem cells. Cell Rep. 7, 961–970. ( 10.1016/j.celrep.2014.04.016) [DOI] [PubMed] [Google Scholar]
- 117.Beroukhim R, et al. 2010. The landscape of somatic copy-number alteration across human cancers. Nature 463, 899–905. ( 10.1038/nature08822) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Santaguida S, Amon A. 2015. Short- and long-term effects of chromosome mis-segregation and aneuploidy. Nat. Rev. Mol. Cell Biol. 16, 473–485. ( 10.1038/nrm4025) [DOI] [PubMed] [Google Scholar]
- 119.Jones MC, Askari JA, Humphries JD, Humphries MJ. 2018. Cell adhesion is regulated by CDK1 during the cell cycle. J. Cell Biol. 217, 3203–3218. ( 10.1083/jcb.201802088) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Vianay B, et al. 2018. Variation in traction forces during cell cycle progression. Biol. Cell 110, 91–96. ( 10.1111/boc.201800006) [DOI] [PubMed] [Google Scholar]
- 121.Uroz M, Wistorf S, Serra-Picamal X, Conte V, Sales-Pardo M, Roca-Cusachs P, Guimerã R, Trepat X. 2018. Regulation of cell cycle progression by cell-cell and cell-matrix forces. Nat. Cell Biol. 20, 646–654. ( 10.1038/s41556-018-0107-2) [DOI] [PubMed] [Google Scholar]
- 122.Maddox AS, Burridge K. 2003. RhoA is required for cortical retraction and rigidity during mitotic cell rounding. J. Cell Biol. 160, 255–265. ( 10.1083/jcb.200207130) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ramanathan SP, Helenius J, Stewart MP, Cattin CJ, Hyman AA, Muller DJ. 2015. Cdk1-dependent mitotic enrichment of cortical myosin II promotes cell rounding against confinement. Nat. Cell Biol. 17, 148–159. ( 10.1038/ncb3098) [DOI] [PubMed] [Google Scholar]
- 124.Yamakita Y, Totsukawa G, Yamashiro S, Fry D, Zhang X, Hanks SK, Matsumura F. 1999. Dissociation of FAK/p130CAS/c-Src complex during mitosis: role of mitosis-specific serine phosphorylation of FAK. J. Cell Biol. 144, 315–324. ( 10.1083/jcb.144.2.315) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Taneja N, Fenix AM, Rathbun L, Millis BA, Tyska MJ, Hehnly H, Burnette DT. 2016. Focal adhesions control cleavage furrow shape and spindle tilt during mitosis. Sci. Rep. 6, 29846 ( 10.1038/srep29846) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Guilluy C, Swaminathan V, Garcia-Mata R, O'Brien ET, Superfine R, Burridge K. 2011. The Rho GEFs LARG and GEF-H1 regulate the mechanical response to force on integrins. Nat. Cell Biol. 13, 722–727. ( 10.1038/ncb2254) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Birkenfeld J, Nalbant P, Bohl BP, Pertz O, Hahn KM, Bokoch GM. 2007. GEF-H1 modulates localized RhoA activation during cytokinesis under the control of mitotic kinases. Dev. Cell 12, 699–712. ( 10.1016/j.devcel.2007.03.014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Bellissent-Waydelich A, Vanier M, Albigès-Rizo C, Simon-Assmann P. 1999. Talin concentrates to the midbody region during mammalian cell cytokinesis. J. Histochem. Cytochem. 47, 1357–1368. ( 10.1177/002215549904701102) [DOI] [PubMed] [Google Scholar]
- 129.Bielak-Zmijewska A, Kolano A, Szczepanska K, Maleszewski M, Borsuk E. 2008. Cdc42 protein acts upstream of IQGAP1 and regulates cytokinesis in mouse oocytes and embryos. Dev. Biol. 322, 21–32. ( 10.1016/j.ydbio.2008.06.039) [DOI] [PubMed] [Google Scholar]
- 130.Burridge K, Connell L. 1983. Talin: a cytoskeletal component concentrated in adhesion plaques and other sites of actin-membrane interaction. Cell Motil. 3, 405–417. ( 10.1002/cm.970030509) [DOI] [PubMed] [Google Scholar]
- 131.Drechsel DN, Hyman AA, Hall A, Glotzer M. 1997. A requirement for Rho and Cdc42 during cytokinesis in Xenopus embryos. Curr. Biol. 7, 12–23. ( 10.1016/S0960-9822(06)00023-6) [DOI] [PubMed] [Google Scholar]
- 132.Geiger B, Tokuyasu KT, Dutton AH, Singer SJ. 1980. Vinculin, an intracellular protein localized at specialized sites where microfilament bundles terminate at cell membranes. Proc. Natl Acad. Sci. USA 77, 4127–4131. ( 10.1073/pnas.77.7.4127) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Higashi T, Arnold TR, Stephenson RE, Dinshaw KM, Miller AL. 2016. Maintenance of the epithelial barrier and remodeling of cell–cell junctions during cytokinesis. Curr. Biol. 26, 1829–1842. ( 10.1016/j.cub.2016.05.036) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hock RS, Sanger JM, Sanger JW. 1989. Talin dynamics in living microinjected nonmuscle cells. Cell Motil. Cytoskeleton 14, 271–287. ( 10.1002/cm.970140213) [DOI] [PubMed] [Google Scholar]
- 135.Kamranvar SA, Gupta DK, Huang Y, Gupta RK, Johansson S. 2016. Integrin signaling via FAK-Src controls cytokinetic abscission by decelerating PLK1 degradation and subsequent recruitment of CEP55 at the midbody. Oncotarget 7, 30 820–30 830. ( 10.18632/oncotarget.9003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Morishita Y, Tsutsumi K, Ohta Y. 2015. Phosphorylation of serine 402 regulates RacGAP protein activity of FilGAP protein. J. Biol. Chem. 290, 26 328–26 338. ( 10.1074/jbc.M115.666875) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Nakajima E, Suzuki K, Takahashi K. 2005. Mitotic dissociation of IQGAP1 from Rac-bound β1-integrin is mediated by protein phosphatase 2A. Biochem. Biophys. Res. Commun. 326, 249–253. ( 10.1016/j.bbrc.2004.11.023) [DOI] [PubMed] [Google Scholar]
- 138.Nobes CD, Hall A. 1995. Rho, rac, and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell 81, 53–62. ( 10.1016/0092-8674(95)90370-4) [DOI] [PubMed] [Google Scholar]
- 139.Oceguera-Yanez F, Kimura K, Yasuda S, Higashida C, Kitamura T, Hiraoka Y, Haraguchi T, Narumiya S. 2005. Ect2 and MgcRacGAP regulate the activation and function of Cdc42 in mitosis. J. Cell Biol. 168, 221–232. ( 10.1083/jcb.200408085) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ridley AJ, Hall A. 1992. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell 70, 389–399. ( 10.1016/0092-8674(92)90163-7) [DOI] [PubMed] [Google Scholar]
- 141.Ridley AJ, Paterson HF, Johnston CL, Diekmann D, Hall A. 1992. The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell 70, 401–410. ( 10.1016/0092-8674(92)90164-8) [DOI] [PubMed] [Google Scholar]
- 142.Riveline D, Zamir E, Balaban NQ, Schwarz US, Ishizaki T, Narumiya S, Kam Z, Geiger B, Bershadsky AD. 2001. Focal contacts as mechanosensors: externally applied local mechanical force induces growth of focal contacts by an mDia1-dependent and ROCK-independent mechanism. J. Cell Biol. 153, 1175–1186. ( 10.1083/jcb.153.6.1175) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Schaller MD, Borgman CA, Cobb BS, Vines RR, Reynolds AB, Parsons JT. 1992. pp125FAK a structurally distinctive protein-tyrosine kinase associated with focal adhesions. Proc. Natl Acad. Sci. USA 89, 5192–5196. ( 10.1073/pnas.89.11.5192) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Schiefermeier N, et al. 2014. The late endosomal p14-MP1 (LAMTOR2/3) complex regulates focal adhesion dynamics during cell migration. J. Cell Biol. 205, 525–540. ( 10.1083/jcb.201310043) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Somers WG, Saint R. 2003. A RhoGEF and Rho family GTPase-activating protein complex links the contractile ring to cortical microtubules at the onset of cytokinesis. Dev. Cell 4, 29–39. ( 10.1016/S1534-5807(02)00402-1) [DOI] [PubMed] [Google Scholar]
- 146.Su L, Agati JM, Parsons SJ. 2003. p190RhoGAP is cell cycle regulated and affects cytokinesis. J. Cell Biol. 163, 571–582. ( 10.1083/jcb.200308007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Su L, Pertz O, Mikawa M, Hahn K, Parsons SJ. 2009. p190RhoGAP negatively regulates Rho activity at the cleavage furrow of mitotic cells. Exp. Cell Res. 315, 1347–1359. ( 10.1016/j.yexcr.2009.02.014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Wakatsuki T, Wysolmerski RB, Elson EL. 2003. Mechanics of cell spreading: role of myosin II. J. Cell Sci. 116, 1617–1625. ( 10.1242/jcs.00340) [DOI] [PubMed] [Google Scholar]
- 149.Watanabe S, Ando Y, Yasuda S, Hosoya H, Watanabe N, Ishizaki T, Chang F. 2008. mDia2 induces the actin scaffold for the contractile ring and stabilizes its position during cytokinesis in NIH 3T3 cells. Mol. Biol. Cell 19, 2328–2338. ( 10.1091/mbc.e07-10-1086) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Wildenberg GA, Dohn MR, Carnahan RH, Davis MA, Lobdell NA, Settleman J, Reynolds AB. 2006. p120-catenin and p190RhoGAP regulate cell-cell adhesion by coordinating antagonism between Rac and Rho. Cell 127, 1027–1039. ( 10.1016/j.cell.2006.09.046) [DOI] [PubMed] [Google Scholar]
- 151.Zang JH, Cavet G, Sabry JH, Wagner P, Moores SL, Spudich JA. 1997. On the role of myosin-II in cytokinesis: division of Dictyostelium cells under adhesive and nonadhesive conditions. Mol. Biol. Cell 8, 2617–2629. ( 10.1091/mbc.8.12.2617) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Breznau EB, Semack AC, Higashi T, Hardin JD. 2015. MgcRacGAP restricts active RhoA at the cytokinetic furrow and both RhoA and Rac1 at cell–cell junctions in epithelial cells. Mol. Biol. Cell 26, 2439–2455. ( 10.1091/mbc.E14-11-1553) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Glotzer M. 2005. The molecular requirements for cytokinesis. Science 307, 1735–1739. ( 10.1126/science.1096896) [DOI] [PubMed] [Google Scholar]
- 154.Matthews HK, Delabre U, Rohn JL, Guck J, Kunda P, Baum B. 2012. Changes in Ect2 localization couple actomyosin-dependent cell shape changes to mitotic progression. Dev. Cell 23, 371–383. ( 10.1016/j.devcel.2012.06.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Kunda P, Baum B. 2009. The actin cytoskeleton in spindle assembly and positioning. Trends Cell Biol. 19, 174–179. ( 10.1016/j.tcb.2009.01.006) [DOI] [PubMed] [Google Scholar]
- 156.Kunda P, Pelling AE, Liu T, Baum B. 2008. Moesin controls cortical rigidity, cell rounding, and spindle morphogenesis during mitosis. Curr. Biol. 18, 91–101. ( 10.1016/j.cub.2007.12.051) [DOI] [PubMed] [Google Scholar]
- 157.Marchesi S, et al. 2014. DEPDC1B coordinates de-adhesion events and cell-cycle progression at mitosis. Dev. Cell 31, 420–433. ( 10.1016/j.devcel.2014.09.009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Malumbres M. 2014. Cyclin-dependent kinases. Genome Biol. 15, 122 ( 10.1186/gb4184) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Blethrow JD, Glavy JS, Morgan DO, Shokat KM. 2008. Covalent capture of kinase-specific phosphopeptides reveals Cdk1-cyclin B substrates. Proc. Natl Acad. Sci. USA 105, 1442–1447. ( 10.1073/pnas.0708966105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Cukier IH, Li Y, Lee JM. 2007. Cyclin B1/Cdk1 binds and phosphorylates Filamin A and regulates its ability to cross-link actin. FEBS Lett. 581, 1661–1672. ( 10.1016/j.febslet.2007.03.041) [DOI] [PubMed] [Google Scholar]
- 161.Curtis M, Nikolopoulos SN, Turner CE. 2002. Actopaxin is phosphorylated during mitosis and is a substrate for cyclin B1/cdc2 kinase. Biochem. J. 363(Pt 2), 233–242. ( 10.1042/bj3630233) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Zhou J, et al. 2018. Zyxin promotes colon cancer tumorigenesis in a mitotic phosphorylation-dependent manner and through CDK8-mediated YAP activation. Proc. Natl Acad. Sci. USA 115, E6760–E6769. ( 10.1073/pnas.1800621115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Yamaguchi R, Mazaki Y, Hirota K, Hashimoto S, Sabe H. 1997. Mitosis specific serine phosphorylation and downregulation of one of the focal adhesion protein, paxillin. Oncogene 15, 1753–1761. ( 10.1038/sj.onc.1201345) [DOI] [PubMed] [Google Scholar]
- 164.Arsic N, et al. 2012. A novel function for Cyclin A2: control of cell invasion via RhoA signaling. J. Cell Biol. 196, 147–162. ( 10.1083/jcb.201102085) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Gould KL, Nurse P. 1989. Tyrosine phosphorylation of the fission yeast cdc2+ protein kinase regulates entry into mitosis. Nature 342, 39–45. ( 10.1038/342039a0) [DOI] [PubMed] [Google Scholar]
- 166.Parker LL, Piwnica-Worms H. 1992. Inactivation of the p34cdc2-cyclin B complex by the human WEE1 tyrosine kinase. Science 257, 1955–1957. ( 10.1126/science.1384126) [DOI] [PubMed] [Google Scholar]
- 167.Gavet O, Pines J. 2010. Progressive activation of CyclinB1-Cdk1 coordinates entry to mitosis. Dev. Cell 18, 533–543. ( 10.1016/j.devcel.2010.02.013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Peeper DS, Parker LL, Ewen ME, Toebes M, Hall FL, Xu M, Zantema A, Van Der Eb AJ, Piwnica-Worms H. 1993. A- and B-type cyclins differentially modulate substrate specificity of cyclin-cdk complexes. EMBO J. 12, 1947–1954. ( 10.1002/j.1460-2075.1993.tb05844.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Higashi H, Suzukitakahashi I, Taya Y, Segawa K, Nishimura S, Kitagawa M. 1995. Differences in substrate specificity between Cdk2-cyclin A and Cdk2-cyclin E in vitro. Biochem. Biophys. Res. Commun. 216, 520–525. ( 10.1006/bbrc.1995.2653) [DOI] [PubMed] [Google Scholar]
- 170.Lim S, Kaldis P. 2013. Cdks, cyclins and CKIs: roles beyond cell cycle regulation. Development 140, 3079–3093. ( 10.1242/dev.091744) [DOI] [PubMed] [Google Scholar]
- 171.Bloom J, Cross FR. 2007. Multiple levels of cyclin specificity in cell-cycle control. Nat. Rev. Mol. Cell Biol. 8, 149–160. ( 10.1038/nrm2105) [DOI] [PubMed] [Google Scholar]
- 172.Malumbres M, Barbacid M. 2005. Mammalian cyclin-dependent kinases. Trends Biochem. Sci. 30, 630–641. ( 10.1016/j.tibs.2005.09.005) [DOI] [PubMed] [Google Scholar]
- 173.Nurse P, Bissett Y. 1981. Gene required in G1 for commitment to cell cycle and in G2 for control of mitosis in fission yeast. Nature 292, 558–560. ( 10.1038/292558a0) [DOI] [PubMed] [Google Scholar]
- 174.Piggott JR, Rai R, Carter BL. 1982. A bifunctional gene product involved in two phases of the yeast cell cycle. Nature 298, 391–393. ( 10.1038/298391a0) [DOI] [PubMed] [Google Scholar]
- 175.Miao Y, Han X, Zheng L, Xie Y, Mu Y, Yates JR, Drubin DG. 2016. Fimbrin phosphorylation by metaphase Cdk1 regulates actin cable dynamics in budding yeast. Nat. Commun. 7, 11 265 ( 10.1038/ncomms11265) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Miao Y, Wong CCL, Mennella V, Michelot A, Agard DA, Holt LJ, Yates JR, Drubin DG. 2013. Cell-cycle regulation of formin-mediated actin cable assembly. Proc. Natl Acad. Sci. USA 110, E4446–E4455. ( 10.1073/pnas.1314000110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.McCusker D, Denison C, Anderson S, Egelhofer TA, Yates JR, Gygi SP, Kellogg DR. et al. 2007. Cdk1 coordinates cell-surface growth with the cell cycle. Nat. Cell Biol. 9, 506–515. ( 10.1038/ncb1568) [DOI] [PubMed] [Google Scholar]
- 178.Kono K, Nogami S, Abe M, Nishizawa M, Morishita S, Pellman D, Solomon M. 2008. G1/S cyclin-dependent kinase regulates small GTPase Rho1p through phosphorylation of RhoGEF Tus1p in Saccharomyces cerevisiae. Mol. Biol. Cell 19, 1763–1771. ( 10.1091/mbc.e07-09-0950) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Tang CS, Reed SI. 2002. Phosphorylation of the septin Cdc3 in G1 by the Cdc28 kinase is essential for efficient septin ring disassembly. Cell Cycle 1, 42–49. ( 10.4161/cc.1.1.99) [DOI] [PubMed] [Google Scholar]
- 180.Egelhofer TA, Villén J, McCusker D, Gygi SP, Kellogg DR. et al. 2008. The septins function in G1 pathways that influence the pattern of cell growth in budding yeast. PLoS ONE 3, e2022 ( 10.1371/journal.pone.0002022) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Thery M, Jiménez-Dalmaroni A, Racine V, Bornens M, Jülicher F. 2007. Experimental and theoretical study of mitotic spindle orientation. Nature 447, 493–496. ( 10.1038/nature05786) [DOI] [PubMed] [Google Scholar]
- 182.Mathew SS, Nieves B, Sequeira S, Sambandamoorthy S, Pumiglia K, Larsen M, Laflamme SE. 2014. Integrins promote cytokinesis through the RSK signaling axis. J. Cell Sci. 127, 534–545. ( 10.1242/jcs.133280) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Aszodi A, Hunziker EB, Brakebusch C, Fässler R. 2003. Beta1 integrins regulate chondrocyte rotation, G1 progression, and cytokinesis. Genes Dev. 17, 2465–2479. ( 10.1101/gad.277003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Hognas G, Tuomi S, Veltel S, Mattila E, Murumägi A, Edgren H, Kallioniemi O, Ivaska J. 2012. Cytokinesis failure due to derailed integrin traffic induces aneuploidy and oncogenic transformation in vitro and in vivo. Oncogene 31, 3597–3606. ( 10.1038/onc.2011.527) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Pellinen T, et al. 2008. Integrin trafficking regulated by Rab21 is necessary for cytokinesis. Dev. Cell 15, 371–385. ( 10.1016/j.devcel.2008.08.001) [DOI] [PubMed] [Google Scholar]
- 186.Petridou NI, Skourides PA. 2016. A ligand-independent integrin beta1 mechanosensory complex guides spindle orientation. Nat. Commun. 7, 10899 ( 10.1038/ncomms10899) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Reverte CG, Benware A, Jones CW, Laflamme SE. 2006. Perturbing integrin function inhibits microtubule growth from centrosomes, spindle assembly, and cytokinesis. J. Cell Biol. 174, 491–497. ( 10.1083/jcb.200603069) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Dix CL, et al. 2018. The role of mitotic cell-substrate adhesion re-modeling in animal cell division. Dev. Cell 45, 132–145.e3. ( 10.1016/j.devcel.2018.03.009) [DOI] [PubMed] [Google Scholar]
- 189.Lock JG, et al. 2017. Reticular adhesions: a new class of adhesion complex that mediates cell-matrix attachment during mitosis. bioRxiv ( 10.1101/234237) [DOI] [PubMed] [Google Scholar]
- 190.Barlat I, Fesquet D, Bréchot C, Henglein B, Dupuy d'Angeac A, Vié A, Blanchard JM. 1993. Loss of the G1-S control of cyclin A expression during tumoral progression of Chinese hamster lung fibroblasts. Cell Growth Differ. 4, 105–113. [PubMed] [Google Scholar]
- 191.Pines J, Hunter T. 1990. Human cyclin A is adenovirus E1A-associated protein p60 and behaves differently from cyclin B. Nature 346, 760–763. ( 10.1038/346760a0) [DOI] [PubMed] [Google Scholar]
- 192.Erlandsson F, Linnman C, Ekholm S, Bengtsson E, Zetterberg A. 2000. A detailed analysis of cyclin A accumulation at the G(1)/S border in normal and transformed cells. Exp. Cell Res. 259, 86–95. ( 10.1006/excr.2000.4889) [DOI] [PubMed] [Google Scholar]
- 193.Pagano M, Pepperkok R, Verde F, Ansorge W, Draetta G. 1992. Cyclin A is required at two points in the human cell cycle. EMBO J. 11, 961–971. ( 10.1002/j.1460-2075.1992.tb05135.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Desai D, Wessling HC, Fisher RP, Morgan DO. 1995. Effects of phosphorylation by CAK on cyclin binding by CDC2 and CDK2. Mol. Cell. Biol. 15, 345–350. ( 10.1128/MCB.15.1.345) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Fisher RP, Morgan DO. 1994. A novel cyclin associates with MO15/CDK7 to form the CDK-activating kinase. Cell 78, 713–724. ( 10.1016/0092-8674(94)90535-5) [DOI] [PubMed] [Google Scholar]
- 196.Miralles F, Posern G, Zaromytidou A-I, Treisman R. 2003. Actin dynamics control SRF activity by regulation of its coactivator MAL. Cell 113, 329–342. ( 10.1016/S0092-8674(03)00278-2) [DOI] [PubMed] [Google Scholar]
- 197.Horton ER, Humphries JD, James J, Jones MC, Askari JA, Humphries MJ. 2016. The integrin adhesome network at a glance. J. Cell Sci. 129, 4159–4163. ( 10.1242/jcs.192054) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Assoian RK, Zhu X. 1997. Cell anchorage and the cytoskeleton as partners in growth factor dependent cell cycle progression. Curr. Opin. Cell Biol. 9, 93–98. ( 10.1016/S0955-0674(97)80157-3) [DOI] [PubMed] [Google Scholar]
- 199.Huang S, Ingber DE. 2002. A discrete cell cycle checkpoint in late G(1) that is cytoskeleton-dependent and MAP kinase (Erk)-independent. Exp. Cell Res. 275, 255–264. ( 10.1006/excr.2002.5504) [DOI] [PubMed] [Google Scholar]
- 200.Zhu X, Ohtsubo M, Böhmer RM, Roberts JM, Assoian RK. 1996. Adhesion-dependent cell cycle progression linked to the expression of cyclin D1, activation of cyclin E-cdk2, and phosphorylation of the retinoblastoma protein. J. Cell Biol. 133, 391–403. ( 10.1083/jcb.133.2.391) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Benaud CM, Dickson RB. 2001. Adhesion-regulated G1 cell cycle arrest in epithelial cells requires the downregulation of c-Myc. Oncogene 20, 4554–4567. ( 10.1038/sj.onc.1204609) [DOI] [PubMed] [Google Scholar]
- 202.Wang P, Ballestrem C, Streuli CH. 2011. The C terminus of talin links integrins to cell cycle progression. J. Cell Biol. 195, 499–513. ( 10.1083/jcb.201104128) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Bershadsky A, Chausovsky A, Becker E, Lyubimova A, Geiger B. 1996. Involvement of microtubules in the control of adhesion-dependent signal transduction. Curr. Biol. 6, 1279–1289. ( 10.1016/S0960-9822(02)70714-8) [DOI] [PubMed] [Google Scholar]
- 204.Pellinen T, Rantala JK, Arjonen A, Mpindi J-P, Kallioniemi O, Ivaska J. 2012. A functional genetic screen reveals new regulators of beta1-integrin activity. J. Cell Sci. 125, 649–661. ( 10.1242/jcs.090704) [DOI] [PubMed] [Google Scholar]
- 205.Besson A, Gurian-West M, Schmidt A, Hall A, Roberts JM. 2004. p27Kip1 modulates cell migration through the regulation of RhoA activation. Genes Dev. 18, 862–876. ( 10.1101/gad.1185504) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.