Abstract
The great ambition to treat cancer through harnessing a patient's own immune responses has started to become reality. Clinical trials have shown impressive results and some patients reaching the end of existing treatment options have achieved full remission. Yet the response rate even within the most promising trials remain at just 30–40% of patients. To date, the focus of immunotherapy research has been to identify tumour antigens, and to enhance activation of effector lymphocytes. Yet this is only the first step to effective immunotherapy for a broader range of patients. Activated cytotoxic T cells can only act on their tumour cell targets if they have free and easy access to all tumour regions. Solid tumours are complex, heterogeneous environments which vary greatly in their physical properties. We must now focus our efforts on understanding how factors such as the composition, density and geometry of tumour extracellular matrix acts to impede or promote immune cell infiltration and activation, and work to design novel pharmacological interventions which restore and enhance leucocyte trafficking within solid tumours.
This article is part of a discussion meeting issue ‘Forces in cancer: interdisciplinary approaches in tumour mechanobiology'.
Keywords: extracellular matrix, immunotherapy, fibroblast
1. Introduction
An early pioneer study in the 1890s by William Coley treating cancer patients with live bacteria, is one of the first examples demonstrating that boosting the patient's immune response against transformed cells was a possibility to cure cancer [1]. Many years later it is now widely demonstrated that the immune system can control tumour growth and that evading this specific immune response is a hallmark of cancer [2]. Over the last decades, scientists have developed a number of therapeutic avenues to exploit the possibility of harnessing immune responses to treat cancer.
Therapeutic cancer vaccines include inoculation of cancer cell lysates, isolated tumour-associated antigens and neoantigens, and autologous dendritic cells loaded with these same tumour antigens. Normally triggered by an adjuvant of choice, this approach generates an adaptive immune response against the tumour [3–6]. Inoculation of specifically designed chimeric antigen receptor T cells and NK cells could be considered a more targeted version of cancer vaccination [7,8]. Alternatively, spontaneous anti-tumour responses in cancer patients can be exploited by adoptive transfer of expanded populations of autologous tumour-infiltrating lymphocytes. Adjuvant treatment is still used today, and immunotherapy is the most successful therapy for non-muscle-invasive bladder cancer [9]. Last but not least, immune checkpoint blockade therapy neutralizes molecules that cancer cells and associated stromal cells use to dampen the immune response. The most popular and effective targets are CTLA-4 and PD-1 used alone, together, or in combination with other therapies [10].
The enormous potential of these therapies is backed up by a large number of studies using animal models. Nevertheless, efficacy in humans is not as good as expected. In order to work, all immunotherapy approaches have specific requirements to meet. One of these is the ability of effector immune cells to access the whole tumour. Here, we review the importance of the tumour stroma in shaping the tumour microenvironment and how this impacts the effectiveness of immunotherapy. We focus particularly in the extracellular matrix (ECM) composition and organization, how it creates both physical and signalling niches around tumours and its impact on immunological anti-tumoural responses.
2. The generation of the extracellular matrix in the tumour microenvironment
The ECM is composed of a network of macromolecules including fibrillar proteins, proteoglycans and glycoproteins that serve both biophysical and biochemical functions. It acts as a physical scaffold to maintain the structure and mechanical integrity of tissues, as well as an active signalling constituent through the sequestration and release of growth factors and cytokines [11]. The composition, anisotropy and biomechanics of the ECM is uniquely tailored to the specific function of the tissue.
The primary mediators of ECM deposition and maintenance are fibroblasts. In pathological contexts such as wound healing and fibrosis, fibroblasts are activated by soluble mediators like transforming growth factor beta (TGF-β) to increase ECM production and remodelling. In cancer, fibroblasts are chronically activated like a ‘wound that does not heal' resulting in severe desmoplasia, as well as dramatic changes in ECM composition and topography. The tumour microenvironment is typically enriched in fibrillar collagens, fibronectin, periostin, tenascin C, hyaluronan and versican, among others, and their upregulation is associated with poor prognosis [12–17]. At the structural level upregulation of the lysyl oxidase (LOX) family of enzymes elevates ECM cross-linking, and there is a progressive transition to ECM anisotropy or alignment which requires both cell-intrinsic factors such as polarity and actomyosin contractility, but also external factors such as the physical forces exerted by the growing tumour [18–20].
3. Extracellular matrix structures define tumour microenvironments
These pathological changes in ECM abundance, cross-linking and architecture modify the mechanics of tumour tissue, increasing tumour stiffness and ECM engagement. Integrin- and focal adhesion kinase (FAK)-dependant adhesions, in turn, stimulate proliferative signalling and inhibition of growth suppression and apoptosis in transformed cells [21–23]. The tumour-associated ECM also generates alignotactic, haptotactic and durotactic gradients that enhance invasion and metastasis. During the initial phase of metastasis tumour cells must depart the primary site and navigate toward blood and lymphatic vessels, and aligned collagen and fibronectin bundles generate permissive ‘highways' directing their migration and intravasation [20,24–27]. Stiffness and fibronectin gradients have also been shown to provide guidance cues to migrating normal and transformed breast epithelial cells [28–31]. Metastatic dissemination is also favoured by ECM rigidity by driving an epithelial to mesenchymal transition [32,33]. Tumour-associated desmoplasia could be explained as a foreign body response, a ‘walling off' of transformed cells through the generation of an obstructive barrier parallel to the invasive front. Conceivably this would act in a tumour suppressive manner, preventing tumour cell escape and inducing cell-cycle arrest through elevated compressive stress [34]. However, during tumour progression, the re-orientation of ECM bundles perpendicular to the tumour front is likely to counteract these initial effects [20]. Understanding the different functions of ECM in tumour progression and the balance between tumour-suppressive versus tumour-promoting functions will be necessary to designing therapeutic interventions.
4. Extracellular matrix and immune infiltration
Just as the composition of the ECM determines architecture and compartmentalization of healthy tissues, the newly generated ECM around tumours also impacts tumour composition, including the spread of blood and lymphatic vessels and infiltration of immune cells. Many studies have also shown the relevance of the ECM in the regulation of the immune response in different pathological processes. ECM is a range of complex structures that can both provide a route through tissues, and a physical barrier to cell migration. This depends greatly on the patterns of ECM fibres, since T cells actively migrate along matrix fibres meaning that directionality of ECM fibres dictates leucocyte migration [35]. Additionally, ECM components can bind specific immune receptors, affecting leucocyte proliferation, polarization/differentiation and trafficking. For example, glucosaminoglycans and proteoglycans can act as functional ligands directly regulating recruitment and activation of innate and adaptive immune cells [36,37]. The duality of ECM to be either protective or tumour promoting means that we need a nuanced and carefully studied approach to ECM as a target for enhancing immunotherapy, but the potential benefits of getting this right are immense.
5. Extracellular matrix control of angiogenesis and lymphangiogenesis
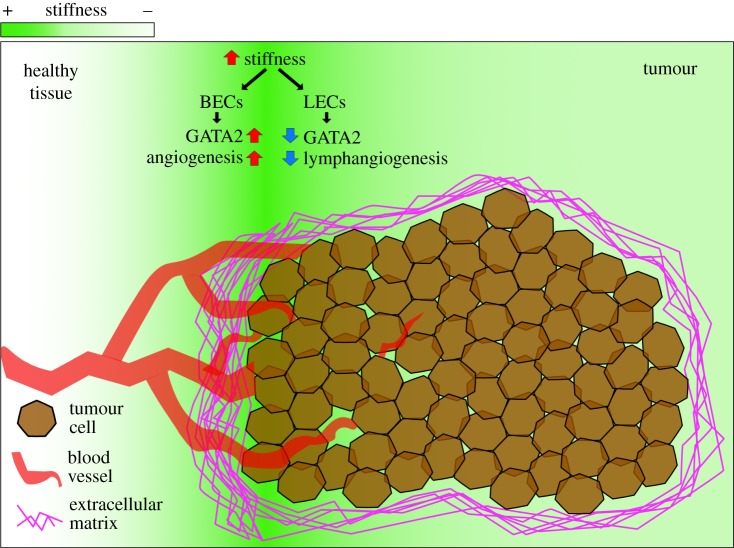
For immune infiltration, there must be an adequate blood supply surrounding the tumour for leucocytes to be recruited from. These vessels are the major routes of traffic for immune cells infiltrating the tumour site. The abundance and intrinsic properties of the tumour vasculature conditions leucocyte infiltration [38–40]. ECM components regulate angiogenesis by both binding angiogenic factors such as VEGFs [41], and by affecting the elasticity of tissues. Stiffer ECM promotes angiogenesis via increased expression of VEGFR2 in endothelial cells (figure 1), positively regulated by p190RhoGAP/GATA2 [42]. Furthermore, mechanosensing counteracts the antiproliferative role of IL-1β on endothelial cells, suggesting that stiff tissues dictates angiogenesis also under inflammatory stress [43]. Interestingly, lymphatic vessel development seems to respond in a reverse manner. Soft tissues (0.2–0.3 kPa) induce GATA2 expression in lymphatic endothelial cell (LEC) precursors and enhances their response to VEGF-C, promoting LEC migration and vessel sprouting [44]. Atomic force microscopy on human breast cancer has shown that stiffness gradients are formed in solid tumours, being generally stiffer than surrounding tissue (0.4 kPa healthy versus 1.2 kPa tumour), with the invasive front being the stiffest [45,46]. These mechanical gradients influence where and when new vessels form during tumour progression (figure 1), and therefore the access routes for immune infiltrate. Fankhauser et al. recently demonstrated that VEGF-C treatment potentiates immunotherapy by attracting naive T cells, which are locally activated upon immunotherapy-induced tumour cell killing [47]. Interestingly, targeting the tumour vasculature can also improve immune therapy [39,48]. Overall, careful characterization of tumour vasculature remodelling will determine the value of combined therapies.
Figure 1.
Differential tissue stiffness in cancer impacts angiogenesis. Tissue stiffness varies dramatically from healthy (0.4 kPa) to tumoral (1.2 kPa) tissue, with an increase in stromal stiffness heterogeneity in the invasive region. ECM rigidity induces blood vessel sprouting via upregulation of GATA2 and increased VEGFR2/VEGF-A signalling in blood endothelial cells (BECs). On the contrary, ECM rigidity might suppress lymphangiogenesis in a similar manner, since lymphatic endothelial cells (LECs) present lower levels of GATA2 and decreased VEGFR3/VEGF-C response in stiffer substrates. This may lead to angiogenic hot spots across the tumour tissue.
6. Immune filtration determined by extracellular matrix structure
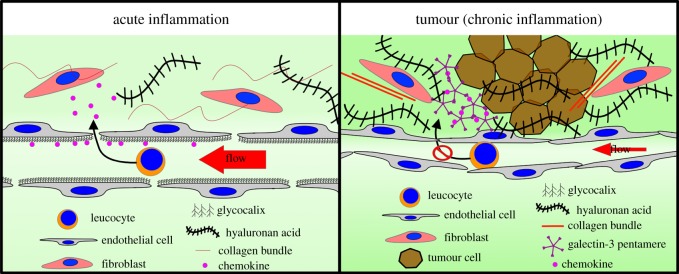
The presence of capillaries in the tumour microenvironment does not necessarily ensure intratumoural blood flow, since high interstitial pressure and solid-stress causes anomalous hydrodynamic blood flow [49], and ECM structures can accumulate to form physical barriers [35]. For example, hyalunoranic acid (HA), which plays essential roles in tumour growth [50] and is associated with poor prognosis, also increases the tumour interstitial fluid pressure (tIFP) impairing vascular function and hindering access of drugs and immune cells (figure 2) [51]. Targeting hyalunoran increases efficacy of immunotherapy by increasing infiltration of cytotoxic T cells [52]. Both cancer cells and cancer-associated fibroblasts (CAFs) are considered sources of HA and studies have shown that contact between both cell types promotes high HA production [53,54]. A relatively large number of secreted factors induce HA synthesis, such as platelet-derived growth factor (PDGF), fibroblast growth factor-2 (FGF-2), epidermal growth factor (EGF), TGF-β, cytokines and some chemokines [55]. Extracellular ATP and UTP also upregulate hyaluronic acid synthase 2 (HAS2) in human epidermal keratinocytes [56,57]. On the other hand, other secreted factors lower HA production in fibroblasts, such as IL-10 and IFN alpha [58]. Many of these signalling molecules are produced by leucocytes [59]; however, the complex interplay between leucocytes and fibroblasts, and the inflammatory microenvironment is still not fully understood.
Figure 2.
Immune infiltration. Similar osmotic pressures between fenestrated small capillaries and adjacent tissue enables normal exchange of small molecules in organs. During acute inflammation, this allows diffusion and gradient formation of chemoattractants that are partially trapped by the luminal glycocalyx of the endothelium, assisting leucocyte recruitment into the inflammation site. Cancer development represents a chronic inflammatory response in which vasculature is affected in a number of ways. Tumour growth and excess of ECM components such as collagens and hyaluronan acid increases interstitial fluid pressure that hinders molecule exchange. Blood vessels become tortuous, impeding normal flow and extravasation of leucocytes. Furthermore, cancer cells can induce loss of the luminal glycocalyx in endothelial cells, impeding the formation of chemoattractant gradients, which are retained within the tissue bound to tumour-derived galectin-3 lattices.
Physical constraints are not the only mechanism by which abnormal ECM impedes leucocyte recruitment. ECM-affiliated proteins [60] can sequester growth factors and chemoattractants leading to defects in leucocyte extravasation (figure 2). For example, secretion of galectin-3 by tumour cells binds the glycans of glycoproteins and forms lattices by oligomerization. These lattices sequester other glycosylated molecules such as IFN gamma, inhibiting formation of a functional gradient, and blocking T cell recruitment [61]. Galectin-3 targeting augments the efficacy of T cell therapy, also demonstrating the impact of this mechanism. These sink-like structures may apply to other glycosylated proteins such as chemokines, affecting tumour infiltration of other leucocytes. Chemokine availability is also influenced by the glycocalyx, which retains glycosylated proteins on the surface of cells, essential for the establishment of chemokine gradients [62]. Oligomerization of chemokines can drive glycocalyx cross-linking, establishing a mechanism that can alter the physical properties of cells and ECM [62,63]. In an in vitro system, lung tumour cell-derived TNF alpha, disrupted the endothelial glycocalyx via activation of endothelial heparanase [64], affecting its capacity to present chemokines [65]. It is, therefore, important to assess the glycocalyx status of tumour vasculature in order to maximize recruitment of immune cells for immunotherapy.
7. Matrix-immune response feedback
With 275 protein-coding genes (195 glycoproteins, 36 proteoglycans and 44 collagens), elements of the core matrisome [66], there exists an immense array of ligand domains for specific receptors expressed by infiltrating immune cells. A wealth of studies has shown how these ECM-ligands regulate the adaptive immune response, with pathogen recognition receptors and adhesion molecules as key regulators [67]. Apart from acting as ligands, the ECM scaffolding and mechanoproperties can directly modulate the anti-tumour immune response. High substrate stiffness induces expression of the immune suppressor molecule PD-L1 in a number of tumour cells, which is blocked when actin polymerization is inhibited [68]. Inhibitory PD-1 ligands are also expressed by tumour stromal cells, including CAFs [69]. Although the mechanism is not characterized, these findings shed light on the regulation of PD-L1 expression by the ECM, relevant for immune evasion and selective depletion of tumour-specific CD8+ cytotoxic cells.
Tumour-draining lymph nodes (TDLNs) represent an important immunological barrier against cancer, being privileged sites for generating tumour-specific immune responses [70]. Leucocyte–fibroblastic stroma interactions in LNs also provide a model system to study the signalling between these cells within tumours and how these influence ECM remodelling. TDLNs often present an immunosuppressive profile characterized by overrepresentation of regulatory CD4+ T cells [71,72]. This inhibitory profile of TDLNs can be reverted by TDLN-targeted adjuvant treatment, which induces Th1 responses and results in higher frequencies of intratumoural CD8+ cells, slowing down tumour growth in the murine B16–F10 melanoma model [73].
Evidence shows that abnormal ECM composition in TDLNs may affect anti-tumour immune response. In breast cancer, metastatic TDLNs present accumulation of subcapsular collagen I and III [74] and fibrosis in metastatic LNs is also strongly correlated with poor prognosis in colorectal cancer [75]. More specifically, increased levels of collagen and hyaluronic acid in non-metastatic TDLNs correlated with high bulk tissue elasticity and viscoelasticity, and with elevated intranodal pressures [76]. In pre-metastatic TDLNs, the lymphoid stromal population of fibroblastic reticular cells (FRCs) is increased in number and gradually reprogrammed towards a CAF-like phenotype in response to tumour factors. Importantly, TDLN FRCs present differential regulation of ECM genes and lower expression of IL-7 and CCL21, key factors in T cell homeostasis [77–79]. In these studies, loss of IL-7 correlated with low numbers of LN T cells, which may lead to poor anti-tumour responses. It is, therefore, important to study which cellular interactions might be inducing fibrosis in TDLNs and whether the fibrotic status of TDLNs may affect the response to immunotherapy. These mechanisms may be similar to those controlling ECM production within and surrounding the primary tumours.
8. Therapeutic opportunities
Given the contribution of ECM to tumour progression, many have reasonably hypothesized that targeting the fibroblastic stroma might offer some therapeutic benefit [80,81]. Targeting the fibroblast-activation protein with the neutralizing antibody sibrotuzumab has unfortunately failed to show efficacy in a phase II trial for the treatment of metastatic colorectal cancer [82]. More promisingly, the anti-fibrotic agent pirfenidone inhibits tumour-promoting actions of CAFs and increases vascular functionality and perfusion, improving doxorubicin chemotherapy treatment in two different cancer models [83,84]. In a landmark study by Olive et al., targeting tumour stroma cross-talk using Sonic HedgeHog (SHH) inhibitors improved drug delivery and response in murine PDAC [85]. Despite this promise, phase II clinical trials of an SHH inhibitor have so far been ineffective. More recent studies have enabled a more nuanced picture. Pharmacological and genetic ablation of fibroblast SHH signalling transiently stabilized tumours but ultimately accelerated disease progression [86]. Similarly, genetic depletion of activated fibroblasts gave rise to tumours that were less differentiated, more invasive and overall more aggressive [87]. These studies and others have highlighted the context-dependent role of the stroma and associated ECM, seemingly acting in both tumour-promoting and tumour-suppressive roles.
As an alternative to targeting the cancer-associated fibroblasts themselves, a number of therapies have focused on the ECM directly, targeting either specific ECM components or structural modifications like cross-linking. Most attention has focused on the latter using a monoclonal antibody against LOXL2 (GS-6624/simtuzumab) or small molecule inhibitor of transglutaminase 2. While pre-clinical investigations were promising, phase II clinical trials with simtuzumab in both cancer and fibrosis have so far displayed no clear benefit for patients. A study using a PEGylated enzyme against hyaluronan in pancreatic adenocarcinoma may provide some hope [88]. A recent phase II study demonstrated a significant increase in objective response and a three-month extension in median overall survival in patients with high hyaluronan [89].
Another approach has been to target ECM associated mechano-signalling in cancer cells directly using ligand mimetics or blocking antibodies against integrins. Cilengitide, a small peptide targeting αvβ3 showed promise in phase II trials in patients with glioblastoma, but unfortunately demonstrated limited efficacy in phase III [90]. An antibody against αvβ6 has also been trialled in idiopathic lung fibrosis to prevent integrin-mediated release of TGF-β1; however, the results of the phase II study (NCT01371305) are yet to be published. Signalling nodes downstream of integrins also offer additional points of therapeutic intervention. The tyrosine kinase FAK is activated upon ECM engagement by integrins, and works primarily through Src and downstream Rho/ROCK, ERK, PI3K and YAP to promote further ECM deposition, cell contractility, growth and survival. Small molecule inhibitors of FAK have been developed to disrupt its kinase function through either direct inhibition of the ATP-binding site or allosteric interference. Two of these (PF-04554878/VS-6063 and GSK2256098) are currently in early-stage clinical trials. Like other kinase inhibitors, these drugs are challenged by the structural ubiquity of the catalytic domain which confers undesirable cross-reactivity. Another recent approach has been to target specific scaffolding interactions of the kinase target which should give rise to greater selectivity.
9. Concluding remarks
The key role that the ECM plays in tumour progression is undisputed. Nevertheless, targeting the ECM is yet to prove of therapeutic benefit. While combined therapies might be the future, we need to increase our understanding of ECM composition and structure that impacts the efficacy of immunotherapy. Furthermore, arrival of immune cells in the tumour microenvironment will cause changes to stromal cell behaviour, in turn, feeding back to the immune response. A better understanding of these complex reciprocal interactions will be essential in order to design new effective therapeutic approaches.
Data accessibility
This article has no additional data.
Authors' contributions
V.G.M. and D.P. wrote the manuscript. S.E.A. provided input to planning and editing the manuscript.
Competing interests
We declare we have no competing interests.
Funding
This work is supported by Cancer Research UK grant no. CRUK-A19763 to S.E.A.
References
- 1.McCarthy EF. 2006. The toxins of William B. Coley and the treatment of bone and soft-tissue sarcomas. Iowa Orthop. J. 26, 154–158. [PMC free article] [PubMed] [Google Scholar]
- 2.Hanahan D, Weinberg RA. 2011. Hallmarks of cancer: the next generation. Cell 144, 646–674. ( 10.1016/j.cell.2011.02.013) [DOI] [PubMed] [Google Scholar]
- 3.Melief CJM, van Hall T, Arens R, Ossendorp F, van der Burg SH. 2015. Therapeutic cancer vaccines. J. Clin. Invest. 125, 3401–3412. ( 10.1172/JCI80009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schlom J. 2012. Therapeutic cancer vaccines: current status and moving forward. J. Natl Cancer Inst. 104, 599–613. ( 10.1093/jnci/djs033) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thomas S, Prendergast GC. 2016. Cancer vaccines: a brief overview. Methods Mol. Biol. 1403, 755–761. ( 10.1007/978-1-4939-3387-7_43) [DOI] [PubMed] [Google Scholar]
- 6.Banday AH, Jeelani S, Hruby VJ. 2015. Cancer vaccine adjuvants–recent clinical progress and future perspectives. Immunopharmacol. Immunotoxicol. 37, 1–11. ( 10.3109/08923973.2014.971963) [DOI] [PubMed] [Google Scholar]
- 7.Ye B, Stary CM, Li X, Gao Q, Kang C, Xiong X. 2018. Engineering chimeric antigen receptor-T cells for cancer treatment. Mol. Cancer 17, 32 ( 10.1186/s12943-018-0814-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mehta RS, Rezvani K. 2018. Chimeric antigen receptor expressing natural killer cells for the immunotherapy of cancer. Front. Immunol. 9, 283 ( 10.3389/fimmu.2018.00283) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weijers Y, Arentsen HC, Arends TJH, Witjes JA. 2015. Management of low-risk and intermediate-risk non-muscle-invasive bladder carcinoma. Hematol. Oncol. Clin. North Am. 29, 219–25– vii. ( 10.1016/j.hoc.2014.11.001) [DOI] [PubMed] [Google Scholar]
- 10.Yan Y, Kumar AB, Finnes H, Markovic SN, Park S, Dronca RS, Dong H. 2018. Combining immune checkpoint inhibitors with conventional cancer therapy. Front. Immunol. 9, 1739 ( 10.3389/fimmu.2018.01739) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hynes RO. 2009. The extracellular matrix: not just pretty fibrils. Science 326, 1216–1219. ( 10.1126/science.1176009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pearce OMT, et al. 2018. Deconstruction of a metastatic tumor microenvironment reveals a common matrix response in human cancers. Cancer Discov. 8, 304–319. ( 10.1158/2159-8290.CD-17-0284) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.González-González L, Alonso J. 2018. Periostin: a matricellular protein with multiple functions in cancer development and progression. Front. Oncol. 8, 57 ( 10.3389/fonc.2018.00225) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lowy CM, Oskarsson T. 2015. Tenascin C in metastasis: a view from the invasive front. Cell Adhes. Migration 9, 112–124. ( 10.1080/19336918.2015.1008331) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chanmee T, Ontong P, Itano N. 2016. Hyaluronan: a modulator of the tumor microenvironment. Cancer Lett. 375, 20–30. ( 10.1016/j.canlet.2016.02.031) [DOI] [PubMed] [Google Scholar]
- 16.Ricciardelli C, Sakko AJ, Ween MP, Russell DL, Horsfall DJ. 2009. The biological role and regulation of versican levels in cancer. Cancer Metastasis Rev. 28, 233–245. ( 10.1007/s10555-009-9182-y) [DOI] [PubMed] [Google Scholar]
- 17.Mayorca-Guiliani AE, Madsen CD, Cox TR, Horton ER, Venning FA, Erler JT. 2017. ISDoT: in situ decellularization of tissues for high-resolution imaging and proteomic analysis of native extracellular matrix. Nat. Med. 23, 890–898. ( 10.1038/nm.4352) [DOI] [PubMed] [Google Scholar]
- 18.Drifka CR, Loeffler AG, Mathewson K, Keikhosravi A, Eickhoff JC, Liu Y, Weber SM, Kao WJ, Eliceiri KW. 2016. Highly aligned stromal collagen is a negative prognostic factor following pancreatic ductal adenocarcinoma resection. Oncotarget 7, 76 197–76 213. ( 10.18632/oncotarget.12772) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Conklin MW, Eickhoff JC, Riching KM, Pehlke CA, Eliceiri KW, Provenzano PP, Friedl A, Keely PJ. 2011. Aligned collagen is a prognostic signature for survival in human breast carcinoma. Am. J. Pathol. 178, 1221–1232. ( 10.1016/j.ajpath.2010.11.076) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Provenzano PP, Eliceiri KW, Campbell JM, Inman DR, White JG, Keely PJ. 2006. Collagen reorganization at the tumor-stromal interface facilitates local invasion. BMC Med. 4, 223 ( 10.1186/1741-7015-4-38) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klein EA, et al. 2009. Cell-cycle control by physiological matrix elasticity and in vivo tissue stiffening. Current Biol. 19, 1511–1518. ( 10.1016/j.cub.2009.07.069) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mouw JK, et al. 2014. Tissue mechanics modulate microRNA-dependent PTEN expression to regulate malignant progression. Nat. Med. 20, 360–367. ( 10.1038/nm.3497) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pickup MW, Mouw JK, Weaver VM. 2014. The extracellular matrix modulates the hallmarks of cancer. EMBO Rep. 15, 1243–1253. ( 10.15252/embr.201439246) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Riching KM, et al. 2014. 3D collagen alignment limits protrusions to enhance breast cancer cell persistence. Biophys. J. 107, 2546–2558. ( 10.1016/j.bpj.2014.10.035) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Provenzano PP, Inman DR, Eliceiri KW, Knittel JG, Yan L, Rueden CT, White JG, Keely PJ. 2008. Collagen density promotes mammary tumor initiation and progression. BMC Med. 6, 1159 ( 10.1186/1741-7015-6-11) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goetz JG, et al. 2011. Biomechanical remodeling of the microenvironment by stromal caveolin-1 favors tumor invasion and metastasis. Cell 146, 148–163. ( 10.1016/j.cell.2011.05.040) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Han W, et al. 2016. Oriented collagen fibers direct tumor cell intravasation. Proc. Natl Acad. Sci. USA 113, 11 208–11 213. ( 10.1073/pnas.1610347113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oudin MJ, et al. 2016. Tumor cell-driven extracellular matrix remodeling drives haptotaxis during metastatic progression. Cancer Discov. 6, 516–531. ( 10.1158/2159-8290.CD-15-1183) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sunyer R, et al. 2016. Collective cell durotaxis emerges from long-range intercellular force transmission. Science 353, 1157–1161. ( 10.1126/science.aaf7119) [DOI] [PubMed] [Google Scholar]
- 30.Parekh A, et al. 2011. Sensing and modulation of invadopodia across a wide range of rigidities. Biophys. J. 100, 573–582. ( 10.1016/j.bpj.2010.12.3733) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Erler JT, Bennewith KL, Nicolau M, Dornhöfer N, Kong C, Le Q-T, Chi J-TA, Jeffrey SS, Giaccia AJ. 2006. Lysyl oxidase is essential for hypoxia-induced metastasis. Nature 440, 1222–1226. ( 10.1038/nature04695) [DOI] [PubMed] [Google Scholar]
- 32.Leight JL, Wozniak MA, Chen S, Lynch ML, Chen CS. 2012. Matrix rigidity regulates a switch between TGF-β1-induced apoptosis and epithelial–mesenchymal transition. Mol. Biol. Cell 23, 781–791. ( 10.1091/mbc.e11-06-0537) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wei SC, et al. 2015. Matrix stiffness drives epithelial–mesenchymal transition and tumour metastasis through a TWIST1–G3BP2 mechanotransduction pathway. Nat. Cell Biol. 17, 678–688. ( 10.1038/ncb3157) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Delarue M, Montel F, Vignjevic D, Prost J, Joanny J-F, Cappello G. 2014. Compressive stress inhibits proliferation in tumor spheroids through a volume limitation. Biophys. J. 107, 1821–1828. ( 10.1016/j.bpj.2014.08.031) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Salmon H, Franciszkiewicz K, Damotte D, Dieu-Nosjean M-C, Validire P, Trautmann A, Mami-Chouaib F, Donnadieu E. 2012. Matrix architecture defines the preferential localization and migration of T cells into the stroma of human lung tumors. J. Clin. Invest. 122, 899–910. ( 10.1172/JCI45817) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mushtaq MU, Papadas A, Pagenkopf A, Flietner E, Morrow Z, Chaudhary SG, Asimakopoulos F. 2018. Tumor matrix remodeling and novel immunotherapies: the promise of matrix-derived immune biomarkers. J. ImmunoTher. Cancer 6, 205 ( 10.1186/s40425-018-0376-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wight TN, Frevert CW, Debley JS, Reeves SR, Parks WC, Ziegler SF. 2017. Interplay of extracellular matrix and leukocytes in lung inflammation. Cell. Immunol. 312, 1–14. ( 10.1016/j.cellimm.2016.12.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ganss R, Hanahan D. 1998. Tumor microenvironment can restrict the effectiveness of activated antitumor lymphocytes. Cancer Res. 58, 4673–4681. [PubMed] [Google Scholar]
- 39.Bocca P, et al. 2017. Bevacizumab-mediated tumor vasculature remodelling improves tumor infiltration and antitumor efficacy of GD2-CAR T cells in a human neuroblastoma preclinical model. OncoImmunology 7, e1378843 ( 10.1080/2162402X.2017.1378843) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schaaf MB, Garg AD, Agostinis P. 2018. Defining the role of the tumor vasculature in antitumor immunity and immunotherapy. Cell Death Dis. 9, 115 ( 10.1038/s41419-017-0061-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vempati P, Popel AS, Mac Gabhann F. 2014. Extracellular regulation of VEGF: isoforms, proteolysis, and vascular patterning. Cytokine Growth Factor Rev. 25, 1–19. ( 10.1016/j.cytogfr.2013.11.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mammoto A, Connor KM, Mammoto T, Yung CW, Huh D, Aderman CM, Mostoslavsky G, Smith LEH, Ingber DE. 2009. A mechanosensitive transcriptional mechanism that controls angiogenesis. Nature 457, 1103–1108. ( 10.1038/nature07765) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu J, Agarwal S. 2010. Mechanical signals activate vascular endothelial growth factor receptor-2 to upregulate endothelial cell proliferation during inflammation. J. Immunol. 185, 1215–1221. ( 10.4049/jimmunol.0903660) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Frye M, et al. 2018. Matrix stiffness controls lymphatic vessel formation through regulation of a GATA2-dependent transcriptional program. Nat. Commun. 9, 1511 ( 10.1038/s41467-018-03959-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lopez JI, Kang I, You W-K, McDonald DM, Weaver VM. 2011. In situ force mapping of mammary gland transformation. Integr. Biol. 3, 910–921. ( 10.1039/c1ib00043h) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Acerbi I, et al. 2015. Human breast cancer invasion and aggression correlates with ECM stiffening and immune cell infiltration. Integr. Biol. 7, 1120–1134. ( 10.1039/C5IB00040H) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fankhauser M, et al. 2017. Tumor lymphangiogenesis promotes T cell infiltration and potentiates immunotherapy in melanoma. Sci. Transl. Med. 9, eaal4712 ( 10.1126/scitranslmed.aal4712) [DOI] [PubMed] [Google Scholar]
- 48.Hendry SA, Farnsworth RH, Solomon B, Achen MG, Stacker SA, Fox SB. 2016. The role of the tumor vasculature in the host immune response: implications for therapeutic strategies targeting the tumor microenvironment. Front. Immunol. 7, 621 ( 10.3389/fimmu.2016.00621) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Broders-Bondon F, Nguyen Ho-Bouldoires TH, Fernandez-Sanchez M-E, Farge E. 2018. Mechanotransduction in tumor progression: the dark side of the force. J. Cell Biol. 217, 1571–1587. ( 10.1083/jcb.201701039) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kultti A, Li X, Jiang P, Thompson CB, Frost GI, Shepard HM. 2012. Therapeutic targeting of hyaluronan in the tumor stroma. Cancers (Basel) 4, 873–903. ( 10.3390/cancers4030873) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jacobetz MA, et al. 2013. Hyaluronan impairs vascular function and drug delivery in a mouse model of pancreatic cancer. Gut 62, 112–120. ( 10.1136/gutjnl-2012-302529) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Guan X, Chen J, Hu Y, Lin L, Sun P, Tian H, Chen X. 2018. Highly enhanced cancer immunotherapy by combining nanovaccine with hyaluronidase. Biomaterials 171, 198–206. ( 10.1016/j.biomaterials.2018.04.039) [DOI] [PubMed] [Google Scholar]
- 53.Knudson W, Biswas C, Toole BP. 1984. Interactions between human tumor cells and fibroblasts stimulate hyaluronate synthesis. Proc. Natl Acad. Sci. USA 81, 6767–6771. ( 10.1073/pnas.81.21.6767) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McCarthy JB, El-Ashry D, Turley EA. 2018. Hyaluronan, cancer-associated fibroblasts and the tumor microenvironment in malignant progression. Front. Cell Dev. Biol. 6, 1110 ( 10.3389/fcell.2018.00048) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fouladi-Nashta AA, Raheem KA, Marei WF, Ghafari F, Hartshorne GM. 2017. Regulation and roles of the hyaluronan system in mammalian reproduction. Reproduction 153, R43–R58. ( 10.1530/REP-16-0240) [DOI] [PubMed] [Google Scholar]
- 56.Rauhala L, Jokela T, Kärnä R, Bart G, Takabe P, Oikari S, Tammi MI, Pasonen-Seppänen S, Tammi RH. 2018. Extracellular ATP activates hyaluronan synthase 2 (HAS2) in epidermal keratinocytes via P2Y2, Ca2+ signaling, and MAPK pathways. Biochem. J. 475, 1755–1772. ( 10.1042/BCJ20180054) [DOI] [PubMed] [Google Scholar]
- 57.Jokela T, Kärnä R, Rauhala L, Bart G, Pasonen-Seppänen S, Oikari S, Tammi MI, Tammi RH. 2017. Human keratinocytes respond to extracellular UTP by induction of hyaluronan synthase 2 expression and increased hyaluronan synthesis. J. Biol. Chem. 292, 4861–4872. ( 10.1074/jbc.M116.760322) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kim M-S, Song HJ, Lee SH, Lee CK. 2014. Comparative study of various growth factors and cytokines on type I collagen and hyaluronan production in human dermal fibroblasts. J. Cosmet. Dermatol. 13, 44–51. ( 10.1111/jocd.12073) [DOI] [PubMed] [Google Scholar]
- 59.Gaucherand L, Falk BA, Evanko SP, Workman G, Chan CK, Wight TN. 2017. Crosstalk between T lymphocytes and lung fibroblasts: generation of a hyaluronan-enriched extracellular matrix adhesive for monocytes. J. Cell. Biochem. 118, 2118–2130. ( 10.1002/jcb.25842) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Naba A, Clauser KR, Hoersch S, Liu H, Carr SA, Hynes RO. 2012. The matrisome: in silico definition and in vivo characterization by proteomics of normal and tumor extracellular matrices. Mol. Cell Proteomics 11, M111.014647 ( 10.1074/mcp.M111.014647) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gordon-Alonso M, Hirsch T, Wildmann C, van der Bruggen P. 2017. Galectin-3 captures interferon-gamma in the tumor matrix reducing chemokine gradient production and T-cell tumor infiltration. Nat. Commun. 8, 1 ( 10.1038/s41467-017-00925-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dyer DP, Migliorini E, Salanga CL, Thakar D, Handel TM, Richter RP. 2017. Differential structural remodelling of heparan sulfate by chemokines: the role of chemokine oligomerization. Open Biol. 7, 160286 ( 10.1098/rsob.160286) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Paszek MJ, et al. 2014. The cancer glycocalyx mechanically primes integrin-mediated growth and survival. Nature 511, 319–325. ( 10.1038/nature13535) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schmidt EP, et al. 2012. The pulmonary endothelial glycocalyx regulates neutrophil adhesion and lung injury during experimental sepsis. Nat. Med. 18, 1217–1223. ( 10.1038/nm.2843) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rai S, Nejadhamzeeigilani Z, Gutowski NJ, Whatmore JL. 2015. Loss of the endothelial glycocalyx is associated with increased E-selectin mediated adhesion of lung tumour cells to the brain microvascular endothelium. J. Exp. Clin. Cancer Res. 34, 105 ( 10.1186/s13046-015-0223-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Naba A, Clauser KR, Ding H, Whittaker CA, Carr SA, Hynes RO. 2016. The extracellular matrix: tools and insights for the ‘omics’ era. Matrix. Biol. 49, 10–24. ( 10.1016/j.matbio.2015.06.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.O'Dwyer DN, Gurczynski SJ, Moore BB. 2018. Pulmonary immunity and extracellular matrix interactions. Matrix Biol. 293, 966–981. ( 10.1016/j.matbio.2018.04.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Miyazawa A, Ito S, Asano S, Tanaka I, Sato M, Kondo M, Hasegawa Y. 2018. Regulation of PD-L1 expression by matrix stiffness in lung cancer cells. Biochem. Biophys. Res. Commun. 495, 2344–2349. ( 10.1016/j.bbrc.2017.12.115) [DOI] [PubMed] [Google Scholar]
- 69.Lakins MA, Ghorani E, Munir H, Martins CP, Shields JD. 2018. Cancer-associated fibroblasts induce antigen-specific deletion of CD8+ T cells to protect tumour cells. Nat. Commun. 9, 1960 ( 10.1038/s41467-018-03347-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yoshizawa H, Sakai K, Chang AE, Shu S. 1991. Activation by anti-CD3 of tumor-draining lymph node cells for specific adoptive immunotherapy. Cell. Immunol. 134, 473–479. ( 10.1016/0008-8749(91)90318-6) [DOI] [PubMed] [Google Scholar]
- 71.Alonso R, et al. 2018. Induction of anergic or regulatory tumor-specific CD4+ T cells in the tumor-draining lymph node. Nat. Commun. 9, 2113 ( 10.1038/s41467-018-04524-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Faghih Z, Erfani N, Haghshenas MR, Safaei A, Talei A-R, Ghaderi A. 2014. Immune profiles of CD4+ lymphocyte subsets in breast cancer tumor draining lymph nodes. Immunol. Lett. 158, 57–65. ( 10.1016/j.imlet.2013.11.021) [DOI] [PubMed] [Google Scholar]
- 73.Thomas SN, Vokali E, Lund AW, Hubbell JA, Swartz MA. 2014. Targeting the tumor-draining lymph node with adjuvanted nanoparticles reshapes the anti-tumor immune response. Biomaterials 35, 814–824. ( 10.1016/j.biomaterials.2013.10.003) [DOI] [PubMed] [Google Scholar]
- 74.Rizwan A, Bulte C, Kalaichelvan A, Cheng M, Krishnamachary B, Bhujwalla ZM, Jiang L, Glunde K. 2015. Metastatic breast cancer cells in lymph nodes increase nodal collagen density. Sci. Rep. 5, 10002 ( 10.1038/srep10002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ikuta D, Miyake T, Shimizu T, Sonoda H, Mukaisho K-I, Tokuda A, Ueki T, Sugihara H, Tani M. 2018. Fibrosis in metastatic lymph nodes is clinically correlated to poor prognosis in colorectal cancer. Oncotarget 9, 29 574–29 586. ( 10.18632/oncotarget.25636) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rohner NA, McClain J, Tuell SL, Warner A, Smith B, Yun Y, Mohan A, Sushnitha M, Thomas SN. 2015. Lymph node biophysical remodeling is associated with melanoma lymphatic drainage. FASEB J. 29, 4512–4522. ( 10.1096/fj.15-274761) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Riedel A, Shorthouse D, Haas L, Hall BA, Shields J. 2016. Tumor-induced stromal reprogramming drives lymph node transformation. Nat. Immunol. 17, 1118–1127. ( 10.1038/ni.3492) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gao J, Zhao L, Liu L, Yang Y, Guo B, Zhu B. 2017. Disrupted fibroblastic reticular cells and interleukin-7 expression in tumor draining lymph nodes. Oncol. Lett. 14, 2954–2960. ( 10.3892/ol.2017.6537) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Link A, Vogt TK, Favre S, Britschgi MR, Acha-Orbea H, Hinz B, Cyster JG, Luther SA. 2007. Fibroblastic reticular cells in lymph nodes regulate the homeostasis of naive T cells. Nat. Immunol. 8, 1255–1265. ( 10.1038/ni1513) [DOI] [PubMed] [Google Scholar]
- 80.Dykes SS, Hughes VS, Wiggins JM, Fasanya HO, Tanaka M, Siemann D. 2018. Stromal cells in breast cancer as a potential therapeutic target. Oncotarget 9, 23761 ( 10.18632/oncotarget.25245) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jiang H, Hegde S, DeNardo DG. 2017. Tumor-associated fibrosis as a regulator of tumor immunity and response to immunotherapy. Cancer Immunol. Immunother. 66, 1037–1048. ( 10.1007/s00262-017-2003-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hofheinz R-D, et al. 2003. Stromal antigen targeting by a humanised monoclonal antibody: an early phase II trial of sibrotuzumab in patients with metastatic colorectal cancer. Onkologie 26, 44–48. ( 10.1159/000069863) [DOI] [PubMed] [Google Scholar]
- 83.Takai K, Le A, Weaver VM, Werb Z. 2016. Targeting the cancer-associated fibroblasts as a treatment in triple-negative breast cancer. Oncotarget 7, 82 889–82 901. ( 10.18632/oncotarget.12658) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Polydorou C, Mpekris F, Papageorgis P, Voutouri C, Stylianopoulos T. 2017. Pirfenidone normalizes the tumor microenvironment to improve chemotherapy. Oncotarget 8, 24 506–24 517. ( 10.18632/oncotarget.15534) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Olive KP, et al. 2009. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science 324, 1457–1461. ( 10.1126/science.1171362) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rhim AD, et al. 2014. Stromal elements act to restrain, rather than support, pancreatic ductal adenocarcinoma. Cancer Cell 25, 735–747. ( 10.1016/j.ccr.2014.04.021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Özdemir BC, et al. 2014. Depletion of carcinoma-associated fibroblasts and fibrosis induces immunosuppression and accelerates pancreas cancer with reduced survival. Cancer Cell 25, 719–734. ( 10.1016/j.ccr.2014.04.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Provenzano PP, Cuevas C, Chang AE, Goel VK, von Hoff DD, Hingorani SR. 2012. Enzymatic targeting of the stroma ablates physical barriers to treatment of pancreatic ductal adenocarcinoma. Cancer Cell 21, 418–429. ( 10.1016/j.ccr.2012.01.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hingorani SR, et al. 2018. HALO 202: randomized phase II study of PEGPH20 plus nab-paclitaxel/gemcitabine versus nab-paclitaxel/gemcitabine in patients with untreated, metastatic pancreatic ductal adenocarcinoma. J. Clin. Oncol. 36, 359–366. ( 10.1200/JCO.2017.74.9564) [DOI] [PubMed] [Google Scholar]
- 90.Stupp R, et al. 2014. Cilengitide combined with standard treatment for patients with newly diagnosed glioblastoma with methylated MGMT promoter (CENTRIC EORTC 26 071–22 072 study): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 15, 1100–1108. ( 10.1016/S1470-2045(14)70379-1) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.