Abstract
Selective evolutionary pressure shapes the processes and genes that enable cancer survival and expansion in a tumour-suppressive environment. A distinguishing lethal feature of malignant cancer is its dissemination and seeding of metastatic foci. A key requirement for this process is the acquisition of a migratory/invasive ability. However, how the migratory phenotype is selected for during the natural evolution of cancer and what advantage, if any, it might provide to the growing malignant cells remain open issues. In this opinion piece, we discuss three possible answers to these issues. We will examine lines of evidence from mathematical modelling of cancer evolution that indicate that migration is an intrinsic selectable property of malignant cells that directly impacts on growth dynamics and cancer geometry. Second, we will argue that migratory phenotypes can emerge as an adaptive response to unfavourable growth conditions and endow cells not only with the ability to move/invade, but also with specific metastatic traits, including drug resistance, self-renewal and survival. Finally, we will discuss the possibility that migratory phenotypes are coincidental events that emerge by happenstance in the natural evolution of cancer.
This article is part of a discussion meeting issue ‘Forces in cancer: interdisciplinary approaches in tumour mechanobiology’.
Keywords: cell migration, endocytosis, collective motility, cancer evolution
1. Introduction
The distinguishing feature of solid malignant tumours is their dissemination from their primary site to seed metastatic foci. While traditionally this process was considered to be a late event in the natural history of cancer development, numerous studies have indicated that early cancer dissemination is frequent, and can often occur when the primary lesion is small and formed by a loose ensemble of highly proliferating and hyper-motile cells [1–4]. These cells can infiltrate blood or lymphatic vessels to disperse to distal sites. There, they might remain dormant for years, before re-awakening to undergo a fast-proliferating, metastasis-generating phase [5], which represents the main unmet clinical challenge and the yet-to-be-defeated cause of cancer recurrence and, ultimately, of cancer death.
During this journey, the ability of cancerous malignancies to detach from the primary early lesion and migrate throughout interstitial tissues represents an acquired trait to initiate the dissemination cascade that eventually leads to the seeding of metastatic foci. However, frequently, aggressive tumours have already disseminated their lethal, albeit dormant, load at the time of diagnosis [4,6]. Additionally, 5–10% of all cases of metastatic cancers are cancers of unknown primary origin, where the metastatic lesion is detected before the primary tumour [7,8]. Thus, attempts to target the migration phenotype of cancer malignancy are often seen as trying to ‘shut the stable door after the horse has bolted’. These observations further raise the issue as to whether the ability to move is selected for during the natural evolution of cancer, and what advantage, if any, this might provide to the growing malignant cells that must constantly compete with each other as well as with the surrounding host cells in a naturally unwelcoming, tumour-suppressive environment. Or, stated differently, why would the migratory ability of cancer cells be advantageous in the first place?
In this short opinion piece, without aiming to be comprehensive and exhaustive, we examine three distinct possible answers to this conundrum, the solution of which is likely to have broader implications not only for our understanding of cancer evolution and metastasis formation, but also in the design of specific anti-tumour therapeutic strategies. We will do so by examining a few selected examples. First, we consider the evidence, which mostly stems from mathematical modelling of cancer evolution, and points to the concept that cell migration is an intrinsic selectable property of malignant cells. This property is so intimately intertwined with more obvious evolution-driving cancer traits, such as cell proliferation and survival, that it directly impacts not only on the potential of malignant cells to disseminate, but also on their growth dynamics, ultimately providing a selective advantage [9–14]. Second, we argue that migratory phenotypes might emerge as an adaptive response to unfavourable growth conditions, including mechanically challenging microenvironments, which endow cells not only with the ability to move/invade, but also with specific metastatic traits, including drug resistance, self-renewal and survival. Finally, we will discuss the possibility that migratory phenotypes are coincidental events that emerge by happenstance in the natural evolution of cancer. This might occur owing to either accumulation of passenger mutations that happen to foster motility [15] or as non-genetic coincidental responses of cancers to selective growth conditions.
(a). The inextricable link between cell growth and migration
Cells that divide often do not move, which suggests that migration and proliferation are dichotomous, nearly opposing behaviours at least at the single-cell levels. Malignant cells must choose between these alternative behaviours at any given time during the natural evolution of cancerous masses [16,17], begging the questions as to what are the cues that drives this decision one way or the other, and how cells balance proliferation and migration to maintain tumour mass.
Molecularly, at least in single-cell analyses in vitro, it has been shown recently that a critical determinant in the decision-making process of whether a cell divides or migrates in response to a potent mitogen, such as epidermal growth factor (EGF), is membrane trafficking of the cognate EGF receptor (EGFR). Following EGF stimulation, the EGFR is activated and promotes a variety of cellular responses, including cell proliferation, survival, apoptosis, differentiation and migration [18]. To select among these diverse outcomes, the cell requires additional contextual information, which includes the intrinsic state of the cell (e.g. the cell cycle phase) and its extrinsic cues (e.g. additional extracellular signals, interactions with other cells or the surrounding matrix) that inform the cell about its microenvironment and promote its adaptation. Processing of this information occurs in part through a variety of biochemical feedback loops [19], although it is also strongly influenced by membrane trafficking [20]. Indeed, the activated EGFR is rapidly internalized and routed through the endocytic pathways to either lysosomal degradation or to return to the plasma membrane via recycling. This self-sustained vesicular recycling of EGFR generates positive feedback that prolongs plasma-membrane AKT (protein kinase B) signalling, which, in turn, promotes cell migration [21]. Impairing cell migration through activation of the EPH receptor, which is activated when two opposing cells encounter each other [22], inhibits EGFR recycling. This traps the EGFR in the endosomes, which reduces AKT activation, and specifically inhibits cell migration, while leaving proliferation unaffected. Thus, vesicular trafficking might be key in the regulation of the proliferation/migration decision-making process, particularly when individual cells come into contact with each other, thus partly accounting for the well-established phenomenon of contact inhibition of locomotion. It must be pointed out, however, that in dense tissues or tumour masses, EGFR signalling and internalization are severely perturbed [23–25], and what is detected at the single-cell level might not apply in multicellular ensembles.
Consistently, numerous malignant cells migrate and disseminate every day from a multicellular neoplasm, which indicates that the evolution of cell migration is not a limiting step [26]. Additionally, migratory-mediated fitness reduction applies only if tumours are considered as homogeneous masses. However, intratumoural cellular and environmental heterogeneity is the norm rather than the exception [27]. Using ecological principles from migratory animal populations, modelling studies have shown that by increasing the ability of a cancer clone to sample different environments with variable resources in the primary tumour mass, cell motility can provide a selective advantage, as it allows the cancer cell clone to experience more favourable environments [9]. Not surprisingly, for instance, oxygen deprivation frequently occurs in poorly vascularized inner tumour masses, and it is a powerful instigator of migratory programmes and fosters metastatic dissemination [28].
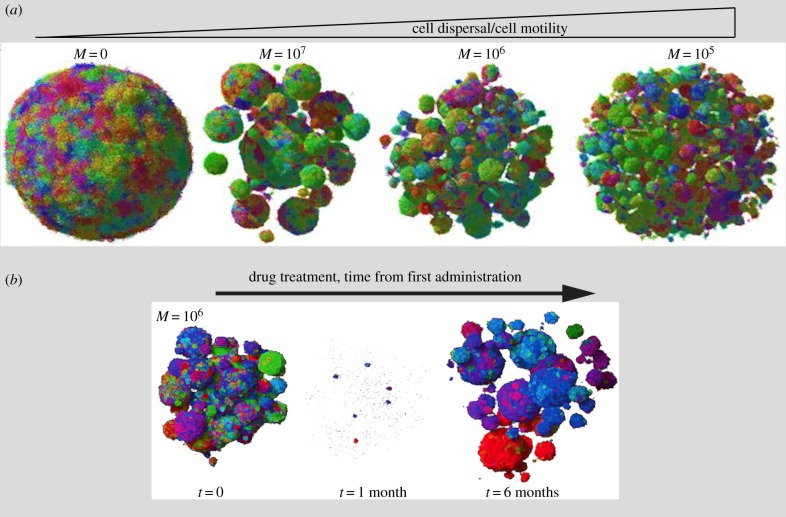
A more recent modelling of solid tumour growth combined the ecological–evolutionary principles of animal and bacterial populations with genetic tumour heterogeneity and changes in the three-dimensional (3D) architecture of tumours [13]. Using this model, the authors explored a set of realistic parameters to identify the factors that better described the evolution of tumours in a 3D setting, with particular focus on tumour geometry and cellular composition. If tumour cells are relatively immobile, then as they proliferate, they will give rise to dense and overcrowded homogeneous masses. Under this condition, the lack of available space causes cells to slow their replication rates and limits proliferation to the periphery, which leads to the slow expansion of a nearly perfect sphere, unlike what is observed experimentally. Adding cell migration or cell dispersal to this picture, however, was sufficient to cause drastic acceleration of the growth of the tumour cells that then underwent exponential expansion, with the formation of heterogeneously shaped tumour masses, much like what is observed for 3D invasive spheroids of various solid malignancies (figure 1a). Cell motility or turnover was also shown to accelerate the emergence of clones with mutated genes that conferred resistance to simulated drug treatments. Frequently, these genetic alterations are passenger mutations that can become drivers under selective pressure, such as that imposed by drug treatments. These, now-fitter clones rapidly expand through cell turnover and dispersal, to occupy the space left by drug-sensitive cells (figure 1b). Collectively, these modelling approaches highlight the pivotal importance of local increases in cell motility, not just in driving dissemination, but also in the control of tumour growth dynamics and shape, and the emergence of genetically fitter clones. Experimental validation of these predictions remains to be provided, but the availability of mosaically expressing multicoloured tumour cells that can be monitored in real time in animals through intravital microscopy might provide experimental support of this model.
Figure 1.
Cell motility impact on 3D tumour growth dynamics. (a) Shape evolution of a tumour mass composed of n = 1 × 107 cells as a function of the individual cell movement probability, where M = 0 indicates low or no probability of movement. The colours reflect the degree of genetic similarity. The three-dimensional tumour with M = 0 becomes spherical and small (tumour size not to scale, as tumours with M = 1 × 105 are much larger than those with M = 0). As the individual cell movement probability increases, tumours lose their spherical shape and become an ensemble of small clonal balls. (b) Emergence of drug resistant clones in tumours composed of moving cells. Simulation of the growth dynamics of tumours with a higher probability of movement M = 106 performed before and after administration of a typical targeted therapy at time t = 0, at t = 1 month and at t = 6 months. Drug treatments cause the loss of most of the tumour cells, and the few (probably pre-existing) resistant clones remain after one month of drug exposure. Intrinsic cell motion facilitates regrowth of the lesions to their original size six months after the treatment. Both cartoons are adapted from [13], copyright (2018), with permission from Elsevier.
The proliferation versus migration dichotomy does not appear to apply during a specific phase of the metastatic cascade when primary cancer masses extend multicellular strands or clusters that invade the surrounding stroma through a combination of collective motility and expansive proliferation, termed invasive growth [29]. This process has long been shown to occur in response to stimulation with various soluble scattering cues, such as hepatocyte growth factor (HGF), also known as scatter factor [30]. HGF is known to act as a potent morphogenic cue during embryogenesis, and it becomes a trigger for individual cancer dissemination by promoting a programme of invasive growth [29]. A similar, but multicellular, programme has also been directly documented, at least in animal models, through real-time microscopy analysis of the growth evolution of experimentally xeno-transplanted tumours [31]. In these cases, multicellular structures actively invade and collectively migrate, while undergoing cell division resolving the dichotomy of the two processes through a coordinated division of labour [31].
This disparate set of observations can be conceptually interpreted within a unifying framework which posits that cell motility and proliferation are dichotomous, non-necessarily opposing phenotypes, particularly within a growing tumour mass. Indeed, in multicellular malignancies, cell motility appears as an intrinsic favourable, if not positively selected trait that promotes the rapid expansion of cancerous mass as well as the emergence of fitter clones. As such, cell motility might be a valuable target to hit to improve cancer therapies.
(b). Migratory phenotypes are an adaptive response to unfavourable growth conditions: from epithelial-to-mesenchymal transition to jamming-to-unjamming transition
The available evidence, however, suggests that cell motility driving metastatic spreading should not be regarded as a genetic trait that is specifically selected during tumorigenesis. In particular, no major genetic differential mutations have been identified between primary tumours and metastases [32–34]. Topographic single-cell sequences of synchronous breast ductal carcinoma in situ (DCIS) and invasive ductal carcinoma have further revealed that: (i) genome evolution occurs within the ducts, before the tumour cells escape the basement membrane; and (ii) one or more clones can escape through the basement membrane into the adjacent tissues to establish an invasive tumour mass, which is consistent with a model of multiclonal invasion [35]. Stated differently, once malignant clones that have evolved individually in a primary tumour breach the basement membrane, they tend to co-migrate to established local outgrowths. Moreover, cancer cell dissemination can occur early during tumour progression, which indicates that the potential to form tumours at distant sites is intimately connected to mechanisms of tumour growth [1–4] (see also §1a). Emerging evidence suggests that the capacity to disseminate might be part of the adaptive responses of cancer cells to unfavourable micro-environmental conditions, which include hypoxia and scarcity of nutrients, to signals from the tumour-associated stroma, and to oxidative and genotoxic stress induced by anti-cancer treatments [36]. The influence of the structural/mechanical properties is also pivotal in this response, as these provide key survival, proliferative and invasive signals [37]. For example, a carcinoma must adapt to intrinsic tumour-suppressive conditions. These arise from the mechanical constraints imposed by excessive growth of tumours that are encased by the relatively rigid architectural organization of the tissue of origin. This is also frequently reinforced by a desmoplastic reaction, which is characterized by accumulation of fibrillar collagen around a tumour. A case in point is provided, once again, by breast cancer lesions. A significantly increasing fraction of human breast cancers currently diagnosed since the adoption of mammographic population-based screening is indeed DCIS [38]. DCIS grows within the confined space of the mammary duct, where it can show highly cohesive growth associated with extreme cell packing and a density that exerts mechanical stress, and thus suppresses cell motility and tumour progression. However, nearly 30% of these lesions can escape this tumour-suppressive environment to become invasive ductal carcinoma [39], through the acquisition of migratory phenotypes. These, we would argue, are adaptive mechanical responses that coincidentally endow metastatic traits and chemo-resistance to tumours. One of these responses implies a drastic change in the identity of a carcinoma, from epithelial-like to mesenchymal-like state. This is commonly referred to as epithelial-to-mesenchymal transition (EMT), which is a mechanism that transiently equips individual cancer cells not only with migratory/invasive ability, but also with increased resistance to drug treatments. Furthermore, they can also gain stemness potential at the expense of rapid proliferation. This process can be reversed at later stages, from the mesenchymal state back to a more epithelial identity, through mesenchymal-to-epithelial transition (MET), to thus facilitate regrowth at a distal site [40]. Indeed, it is now clear that, rather than being a binary switch from epithelial to mesenchymal behaviour, EMT and MET are graded processes with a range of different outcomes. This balance can thus give rise to cells that have various combinations of epithelial and mesenchymal features, which are frequently referred to as undergoing a plastic transition state [41]. Additionally, mounting evidence indicates that cells that undergo EMT or MET experience a constantly changing set of mechanical cues, which together control how these cells sense and respond to other signals from their microenvironment. We refer the reader to a recent comprehensive review for details of the types of mechanical inputs and mechanotransduction pathways that can either initiate or reinforce the EMT/MET-plastic rewiring of cell identity during both organ development and cancer dissemination [42].
One important caveat is that while EMT has become recognized as the overarching mechanism that enables dissemination of single tumour cells [43,44], invasion by an epithelial-derived carcinoma frequently involves collective migration of cohesive cohorts of cells into adjacent tissues, rather than dispersal of individual carcinoma cells [45,46]. Indeed, this is further supported by the following: (i) breast carcinomas frequently disseminate as epithelial collectives, by maintaining their tight cell–cell interactions [40,47]; (ii) circulating cancer cells spread as epithelial cell clusters, and by doing so, they show increased metastatic seeding potential [48]; (iii) histopathological studies have indicated that human DCIS can invade as collective strands or clusters with E-cadherin-based cellular junctions [49,50]; and (iv) late-stage HER2-expressing murine mammary cancers can undergo kinetic arrest and have reduced metastatic potential as a consequence of increased cell density and packing [1,2]. These findings further imply that mechanisms that can overcome the kinetically silent state of a late-stage aggressive tumour might promote collective migratory mechanisms of cancer dissemination, without the need to invoke changes to cell identity or rewiring of transcriptional programmes (e.g. EMT-like programmes).
An emerging physical framework that is known as ‘cell jamming’ aims to capture and simplify into unifying principles the mechanical and biochemical mechanisms that govern collective motion, or more specifically, the transition of a multicellular ensemble from a rigid, dense or ‘jammed’ state, into a fluid, dynamic, motile or ‘unjammed’ state [51–54]. For example, a variety of multicellular collectives have been shown to acquire structural and dynamic physical properties that are surprisingly similar to those of amorphous viscoelastic materials [45,51–54]. During collective motility, cells can flow like a fluid, but as the cell density rises owing to proliferation, the motion of each cell is constrained by the crowding from its neighbours, which forces them to move as groups [55–57]. At a critical density, the motility ceases and the collectives jam or rigidify, to thus undergo liquid (unjammed)-to-solid (jammed) transition [51–54]. It has been proposed that this transition ensures the correct development of elasticity and of barrier properties of epithelial tissues, and also to act as a strong suppressive mechanism for aberrant growth of oncogenic clones [51]. The reverse, known as jamming-to-unjamming transition (JUT), might instead represent a complementary gateway to cell migration with respect to EMT, which enables tissues to escape the caging imposed by the crowded cellular landscape of the mature epithelia [51]. Indeed, if cells retain an epithelial phenotype throughout all stages of the metastatic process, then the idea of EMT as the sole or principal gateway to cellular migration does not hold. However, the molecular mechanisms and cellular processes that cells use to control the jamming transition are poorly defined. It is also even less clear whether JUT occurs in a 3D environment and during tumour progression, and whether JUT can elicit the durable genetic or epigenetic changes that are required to confer tumours with the relevant metastatic traits.
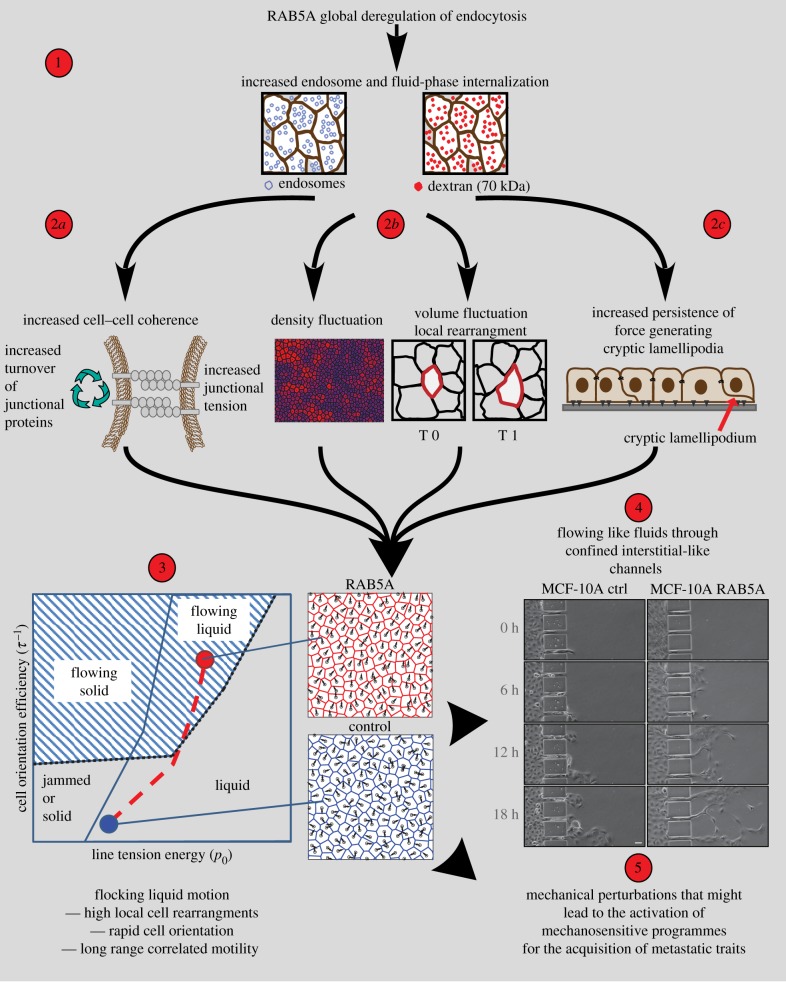
In an attempt to address the first of these issues, we and others have recently characterized endocytic-dependent, mechanically driven mechanisms that promote the transition from a jammed to a fluid (i.e. unjammed) state in epithelial monolayers [58–63]. Specifically, we have shown that a master regulator of endocytosis, RAB5A, is highly expressed in aggressive human breast cancer [64], with levels that are sufficient to promote JUT through changes to the mechanical properties of the tissue [61]. Perturbations in RAB5A levels in normal and tumorigenic mammary epithelia were sufficient to re-awaken the motility of the otherwise solid-like and kinetically arrested (i.e. jammed) monolayers [61]. RAB5A expression promoted millimetre-scale, coherent and ballistic movement of multicellular streams that flowed like flocking fluids. RAB5A also resulted in the extension of oriented and persistent protrusions. The combination of these effects allows cell monolayers to acquire a fluid-like character, and delays any transition to a jammed/solid state (figure 2). Molecularly, impairing endocytosis or micropinocytosis, or increasing fluid efflux, abrogated the RAB5A-induced collective motility, which supports the concept that perturbation of multiple trafficking routes that impact upon different signalling and biomechanical pathways is necessary for JUT [61,67]. A simple computational model that was based on mechanical junctional tension and included an active cell reorientation mechanism for the velocity of self-propelled cells identified regimes of monolayer dynamics that can explain endocytic re-awakening of cell movement in terms of the combination of large-scale directed migration and local unjamming [58,60,61]. These features lead to a ‘flocking’ (or flowing) fluid mode of collective cell migration (figure 2(3)). The model further provides a quantitative framework that supports the concept that small variations in fundamental cellular properties (e.g. cell self-propulsion, junctional tension, packing density) are sufficient to tip the dynamic state of collective structures from solid to liquid, or to flowing liquid. Biologically, this might provide both innate cell plasticity and adaptability, as the emerging properties of a collective structure. Consistently, as a consequence of these emerging material properties, RAB5A-expressing monolayers are not only efficient in directed movement during wound closure and epiboly gastrulation movement during zebrafish development [61], but they also show a high degree of plasticity that allows them to migrate under physical constraints that are typical of the interstitial tissue architecture [61]. Tumour cells, on the other hand, might exploit this ‘mechanical flexibility’ to activate key steps in the metastatic process without the need to change their genetic make-up or cell identity. They would thus require significantly less-drastic events than EMT (or the reverse, MET) to disseminate. Consistently, recently, we combined biophysical and biochemical analysis to study endocytic-mediated unjamming in a variety of malignant epithelial 3D collectives in breast cancer, such as spheroid models of breast DCIS, and ex vivo slices of orthotopically implanted DCIS. We found that RAB5A-mediated endocytic perturbations are sufficient to spark a flocking-liquid mode of 3D motility that causes tumour masses embedded in thick collagen type I matrix to undergo a highly persistent and coordinated rotation. This is accompanied by an augmented mechanical stress exerted by the cells on the extracellular matrix, which leads to its remodelling, and the concurrent ‘fluidification’ of cells in the close proximity of the remodelled matrix. The combination of these two effects results in the collective invasion of otherwise jammed carcinoma [67].
Figure 2.
The ‘nuts and bolts’ of endocytic-mediated jamming transition, and its biological consequences. (1) Global perturbations of endosomal function through elevation of the master regulator of early endosomes, RAB5A, alter endosomal numbers and macropinocytic internalization [61]. (2a–c) These alterations, in turn, can cause: increased turnover of junctional proteins (e.g. E-cadherin) and junctional tension (2a); greater volume and density fluctuations, as hallmarks of liquid-to-solid-like transition (2b); and increased formation of RAC1-dependent, polarized protrusions that can extend beneath neighbouring cells (also called cryptic lamellipodia), which promotes cell self-propulsion (2c). (3) The combination of these cellular and mechanical alterations influences the kinematics of epithelial monolayers, which can be understood through mathematical modelling. The simulation is based on a self-propelled Voronoi model with two main components: the target shape of each individual cell (p0, ratio between perimeter and square-root of the area), which is the result of competition between intracellular adhesion and cortical tension [61]; and the inverse of the reorientation time that each individual cell takes to align to the local direction of motion, τ−1. These components generate a phase diagram (bottom left) that explains endocytic re-awakening of movement in jammed epithelia, in terms of a combination of large-scale directed migration in the presence of local cell re-arrangement, which lead to a ‘flocking’ (or flowing) liquid mode of migration. (4) This transition in the mode of movement enables RAB5A-expressing epithelial monolayers to flow through micro-fabricated narrow slits that mimic the confined channels encountered during interstitial migration. (5) This might promote mechanosensitive programmes, such as the YAP1/TAZ axis [65], for the acquisition of metastatic traits. Adapted from [66], copyright (2018), with permission from Elsevier.
It should be noted here that a number of the alterations that characterize EMT are expected to impact on the mechanical and kinematic properties of cell collectives, such as changes in cell–cell and cell–matrix interactions, or in cell self-propulsion and motility. This is particularly relevant in the context of heterogeneous tumour ensembles, where cells that retain epithelial features coexist with those that have undergone partial or full mesenchymal transition, with as-yet unexplored consequences for the overall tissue dynamics. Thus, ultimately, EMT, JUT and collective cell motion might actually have much less sharply defined boundaries, and might even be slightly different aspects of the same process of tissue adaptation to specific mechanical inputs.
In keeping with this concept, and in analogy to EMT, to be instrumental in tumour dissemination, mechanically driven, collective invasive strategies must provide cells with metastatic traits that will include tumour-initiating growth capacity, a self-renewal programme, drug resistance and re-awakening from dormancy [68]. Whether this is the case remains to be addressed; nevertheless, we note that changes in tissue mechanics and in the ability of cells within a tissue to transmit forces both to the substrate and long range through cell–cell adhesion are pivotal in the control of the jamming transition and for the remodelling of extracellular matrix surrounding tumour to enhance their invasiveness [65]. These mechanical alterations might be sufficient to activate mechanosensitive transcriptional programmes, such as those elicited by the mechanotransducers YAP1 and TAZ [65]. The activation of YAP1 and TAZ is emerging as essential for tumour growth and metastatic colonization [69–74], and this has also been shown to initiate a subsequent EMT-like programme [75–78].
(c). Migration phenotypes emerge by happenstance in the natural evolution of a cancer: the case of macropinocytosis
Our understanding of the molecular mechanisms that drive cell motility has undergone an exponential increase more recently. A number of critical pathways and genes can be categorized based on their roles in the control of the critical steps of the cell migration cycle: (i) extension of the leading edge; (ii) adhesion to matrix contacts; (iii) contraction of the cytoplasm; and (iv) release from the contact sites to enable rear-end retraction and forward sliding of the cell body [79].
These basic fundamental steps have been deduced from the study of cells ‘crawling’ on flat 2D surfaces [79], and they will also apply, with some variations, to malignant cells in complex 3D environments. These cells must negotiate the intricacy of fibrillar collagen-rich networks, through activation of a pericellular proteolytic programme [80], and they must move on single fibres [81], squeeze through narrow pre-existing or newly formed slits and channels or move in a highly coordinated collective fashion [45].
As a consequence of the recognized complexity of cell motility, increasing numbers of genes have been implicated in migration, and particularly in three dimensions. This has revealed the extraordinary flexibility of cancer cells in rewiring their gene programmes in order to adapt to diverse and dynamically changing micro-environmental conditions. Remarkably, however, despite being essential for migration, the vast majority of these genes have rarely been ascribed the role of drivers of cancer development, albeit a number of them are mutated in diverse malignancies.
The case of the key founding RHO GTPase family members, CDC42, RAC1 and RHOA, is particularly relevant in this context [82]. These GTPases are pivotal in orchestrating the signalling pathways that are essential for cell migration, from acquisition of polarity (e.g. CDC42) to the extension of cell protrusions (e.g. RAC1) and generation of actomyosin contractility (e.g. RHOA) [82]. In cancers, the activity of these GTPases is frequently dysregulated, and genetic studies have supported the importance of their actions in the various phases of tumour initiation and progression [83,84]. However, at variance with respect to the genes coding for RAS GTPases, mutated variants of RHO GTPases are rarely found to act as drivers in cancers, with the notable exception of RAC1-P29S, which is a spontaneously activating cancer-associated GTPase defined in approximately 5% of sun-exposure melanomas [85]. Thus, various cancer cells might modulate the expression or activity of their essential migratory genes as part of an adaptive response to challenging micro-environmental conditions, rather than for the intrinsic pro-motility functions of these genes.
The process of macropinocytosis can be considered as a remarkable case in point [86]. Macropinocytosis is an endocytic route through which large macromolecules and solutes are internalized following remodelling of the actin cytoskeleton, which generates the force to extend the membrane protrusions and ruffles that are necessary for this process [86]. Macropinocytosis activity is increased following activation of potent oncogenes, such as KRAS and v-SRC, and it depends on the small RHO GTPase RAC1, which is also a master regulator of branched actin polymerization in crawling-cell migration [87–90]. Consistent with this, macropinocytosis has been implicated in cancer cell motility, extracellular matrix degradation and metastasis formation [86,91].
However, whether macropinocytosis has positive or negative effects on cell migration remains under debate, and its diverse biological outcomes might depend on the cell type. For example, macropinocytosis is very active in professional phagocytic cells, including macrophages and dendritic cells [92,93], and it mediates chemotaxis in highly motile cells, such as neutrophils [94]. Dendritic cells, in particular, appear to regulate the use of macropinocytosis according to their status. Following maturation and activation, dendritic cells switch from their macropinocytic, antigen-sampling, but kinetically inactive state to a hyper-motile, chemotactic phase. This is essential to guide their movement towards lymph vessels and nodes, so as to mount an efficient adaptive immune response [95,96]. Thus, at least in some specialized contexts, macropinocytosis is a trade-off for efficient, directed cell migration [97].
In non-professional, migratory cells, however, macropinocytosis (or at least some form of this process) has invariably been associated with enhanced crawling-cell movement. We have recently shown that circular dorsal ruffles represent a specialized set of ARP2/3-dependent protrusions [98,99]. These protrusions are sites of macropinocytic internalization, and they have dynamic features (i.e. rapid and recurrent wave-like behaviour) that are typical of an oscillatory excitable system that can be biased by chemical cues. They therefore act as steering devices to drive efficient chemotactic migration. Additionally, during stimulated fibroblast migration, integrins have been shown to undergo rapid trafficking via circular dorsal ruffles and macropinocytosis [100].
Given its role in solute and nutrient uptake, macropinocytosis has been directly implicated in the regulation of the mTOR pathway and cell metabolism [101,102]. Indeed, in addition to cancer cell migration and dissemination, macropinocytosis appears to be used by malignant cells to also satisfy their demanding metabolic needs [102]. Metabolic alterations of cancer cells have, indeed, emerged as paramount for development and progression of neoplastic transformation [103]. A case in point is represented by pancreatic ductal adenocarcinoma. The reduced cellularity and increased presence of a stromal component of the pancreatic ductal adenocarcinoma microenvironment lead to high interstitial pressure, which induces physical and oxidative stress and tumour hypo-perfusion, with a consequent limited supply of both oxygen and nutrients [104,105]. To support cancer viability under these conditions, oncogenic insults, and specifically KRAS-G12 mutants (mutKRAS) (the prevalent oncogenes in these tumours), activate several pathways to use alternative sources of nutrients, among which there is macropinocytosis. Accumulating evidence indicates that macropinocytosis is, indeed, one of the main mechanisms by which mutKRAS pancreatic ductal adenocarcinoma cells scavenge protein and lipid sources. These are degraded in lysosomes, to refill the amino acid pools, and to fuel mitochondrial metabolism and lipid biosynthesis [88,106,107]. Ultimately, this enables cell survival in nutrient-deprived tumour microenvironments, and promotes resistance to inhibitors of the amino acid sensor mTOR [101]. Consistent with this, the inhibition of macropinocytosis has been shown to impair cell growth of mutKRAS pancreatic ductal carcinoma cells in vitro and as tumour growth in vivo. Additionally, macropinocytosis is activated following amino acid starvation and mTOR inhibition [101]. This argues that this endocytic process is selected for by various cancers, including prostate tumours [108], to adapt to and survive in microenvironments characterized by limited nutrient availability. Of note, a number of molecules that are required to activate macropinocytosis in cancer, including KRAS, RAC1 and their upstream regulators (e.g. guanine nucleotide exchange factor DOCK1; [109]), and the downstream effectors that regulate actin dynamics at the leading edge, are also essential to drive cell movement [86]. These observations suggest that migratory genes and motility phenotypes might emerge coincidentally, as by happenstance, in the natural evolution of cancer. These are subsequently exploited by disseminating malignant cells for their pro-migratory and pro-invasive properties.
2. Concluding remarks
We began by questioning whether cell migration is a trait that, if not actively selected for, is at least beneficial during the natural history of cancer development, much like proliferation or survival. The answer is clearly complex. There is little doubt that for the process of metastatization, malignant cells must acquire the ability to migrate through a set of extremely diverse micro-environmental conditions. This diversity drives the emergence of multiple and flexible modes of locomotion fostered by a vast number of motility molecules. Much less obvious is whether these motility molecular traits are advantageous, and as such under some sort of selective pressure in the context of the growing primary tumour mass. Indeed, if one considered cancer as an ensemble of individual entities in constant competition with their neighbours, it is not immediately obvious which kind of benefit the ability of moving might provide. However, tumours are ecologically complex multicellular structures with rich intracellular dynamics and composed of highly heterogeneous clones. Within this context, cell motion has been proposed to be a critical determinant of the growth dynamics of tumour masses, causing a drastic acceleration of proliferation by favouring the emergence of heterogeneous fitter clones, which can rapidly expand through cell turnover and dispersal to occupy the space left by lesser fit cells.
Migration in epithelial-derived carcinoma might also directly emerge as a favourable adaptive process in response to challenging micro-environmental biomechanical conditions. This, at the individual cell level, might be sufficient to trigger a transition in cell identity from an epithelial, sessile to a hyper-motile mesenchymal state or EMT thought to be essential to drive tumours' cell dissemination [43,44]. Alternatively, this adaptive response might promote a switch in the material property of packed carcinoma, from a solid, kinetically-arrested (i.e. jammed) phase to a liquid (i.e. unjammed)-like phase that would favour the migration and dispersal of epithelial, multicellular strands and clusters. Both these motility transitions might be associated with the acquisition of traits (i.e. self-renewal or drug resistance) that would increase the fitness of dispersed cells, thus favouring the seeding and growth of distal metastatic foci.
Finally, we reviewed evidence that migration may emerge indirectly, almost by happenstance, as a consequence once again of tumour cell adaptation to, for example, limited availability of nutritional components. These conditions force metabolically demanding tumours to activate pathways to use alternative sources of nutrients, among which there is macropinocytosis. Macropinocytosis requires the upregulation of force-generating actin cytoskeletal remodellers, which, in addition to driving membrane deformation for the engulfment of proteinaceous and lipid material, might also be exploited to propel migratory and explorative behaviours, ultimately promoting cancer cell dispersal.
Thus, migration, regardless of the way it becomes activated, should be viewed as a mechanism to promote tumour cell fitness, and as such should be a valuable therapeutic target to hit in the battle to curb the development of aggressive metastatic malignancies.
Data accessibility
This article has no additional data.
Authors' contributions
All authors edited and wrote the manuscript.
Competing interests
We declare we have no competing interests.
Funding
The authors are in debt to support from: the Associazione Italiana per la Ricerca sul Cancro (AIRC) (IG#18621); and the Italian Ministry of Health (RF-2013-02358446) and Italian Ministry of Education and University (PRIN-2017HWTP2K_003) to G.S. S.B. is supported by a Fellowship from AIRC; C.M. and A.R. are partially supported by Fellowships from the University of Milan; S.M. is supported by a Fellowship from the Milan Polytechnic.
References
- 1.Hosseini H, et al. 2016. Early dissemination seeds metastasis in breast cancer. Nature 540, 552 ( 10.1038/nature20785) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harper KL, et al. 2016. Mechanism of early dissemination and metastasis in Her2+ mammary cancer. Nature 540, 588 ( 10.1038/nature20609) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rhim AD, et al. 2012. EMT and dissemination precede pancreatic tumor formation. Cell 148, 349–361. ( 10.1016/j.cell.2011.11.025) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sanger N, Effenberger KE, Riethdorf S, Van Haasteren V, Gauwerky J, Wiegratz I, Strebhardt K, Kaufmann M, Pantel K. 2011. Disseminated tumor cells in the bone marrow of patients with ductal carcinoma in situ. Int. J. Cancer. 129, 2522–2526. ( 10.1002/ijc.25895) [DOI] [PubMed] [Google Scholar]
- 5.Aguirre-Ghiso JA, Sosa MS. 2018. Emerging topics on disseminated cancer cell dormancy and the paradigm of metastasis. Annu. Rev. Cancer Biol. 2, 377–393. ( 10.1146/annurev-cancerbio-030617-050446) [DOI] [Google Scholar]
- 6.Friberg S, Nystrom A. 2015. Cancer metastases: early dissemination and late recurrences. Cancer Growth Metastasis 8, 43–49. ( 10.4137/CGM.S31244) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Varadhachary GR, Raber MN. 2014. Cancer of unknown primary site. N. Engl. J. Med. 371, 757–765. ( 10.1056/NEJMra1303917) [DOI] [PubMed] [Google Scholar]
- 8.Pavlidis N, Pentheroudakis G. 2012. Cancer of unknown primary site. Lancet 379, 1428–1435. ( 10.1016/S0140-6736(11)61178-1) [DOI] [PubMed] [Google Scholar]
- 9.Chen J, Sprouffske K, Huang Q, Maley CC. 2011. Solving the puzzle of metastasis: the evolution of cell migration in neoplasms. PLoS ONE 6, e17933 ( 10.1371/journal.pone.0017933) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rodriguez-Brenes IA, Komarova NL, Wodarz D. 2013. Tumor growth dynamics: insights into evolutionary processes. Trends Ecol. Evol. 28, 597–604. ( 10.1016/j.tree.2013.05.020) [DOI] [PubMed] [Google Scholar]
- 11.Rodriguez-Brenes IA, Komarova NL, Wodarz D. 2011. Evolutionary dynamics of feedback escape and the development of stem-cell-driven cancers. Proc. Natl Acad. Sci. USA 108, 18 983–18 988. ( 10.1073/pnas.1107621108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Manem VS, Kohandel M, Komarova NL, Sivaloganathan S. 2014. Spatial invasion dynamics on random and unstructured meshes: implications for heterogeneous tumor populations. J. Theor. Biol. 349, 66–73. ( 10.1016/j.jtbi.2014.01.009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Waclaw B, Bozic I, Pittman ME, Hruban RH, Vogelstein B, Nowak MA. 2015. A spatial model predicts that dispersal and cell turnover limit intratumour heterogeneity. Nature 525, 261–264. ( 10.1038/nature14971) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rozhok AI, DeGregori J. 2015. Toward an evolutionary model of cancer: considering the mechanisms that govern the fate of somatic mutations. Proc. Natl Acad. Sci. USA 112, 8914–8921. ( 10.1073/pnas.1501713112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Talavera D, Taylor MS, Thornton JM. 2010. The (non)malignancy of cancerous amino acidic substitutions. Proteins 78, 518–529. ( 10.1002/prot.22574) [DOI] [PubMed] [Google Scholar]
- 16.Giese A, Bjerkvig R, Berens ME, Westphal M. 2003. Cost of migration: invasion of malignant gliomas and implications for treatment. J. Clin. Oncol. 21, 1624–1636. ( 10.1200/JCO.2003.05.063) [DOI] [PubMed] [Google Scholar]
- 17.Tzamali E, Grekas G, Marias K, Sakkalis V. 2014. Exploring the competition between proliferative and invasive cancer phenotypes in a continuous spatial model. PLoS ONE 9, e103191 ( 10.1371/journal.pone.0103191) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Avraham R, Yarden Y. 2011. Feedback regulation of EGFR signalling: decision making by early and delayed loops. Nat. Rev. Mol. Cell Biol. 12, 104–117. ( 10.1038/nrm3048) [DOI] [PubMed] [Google Scholar]
- 19.Segatto O, Anastasi S, Alema S. 2011. Regulation of epidermal growth factor receptor signalling by inducible feedback inhibitors. J. Cell Sci. 124(Pt 11), 1785–1793. ( 10.1242/jcs.083303) [DOI] [PubMed] [Google Scholar]
- 20.Bakker J, Spits M, Neefjes J, Berlin I. 2017. The EGFR odyssey—from activation to destruction in space and time. J. Cell Sci. 130, 4087–4096. ( 10.1242/jcs.209197) [DOI] [PubMed] [Google Scholar]
- 21.Stallaert W, Bruggemann Y, Sabet O, Baak L, Gattiglio M, Bastiaens PIH. 2018. Contact inhibitory Eph signaling suppresses EGF-promoted cell migration by decoupling EGFR activity from vesicular recycling. Sci. Signal. 11, eaat0114 ( 10.1126/scisignal.aat0114) [DOI] [PubMed] [Google Scholar]
- 22.Lisabeth EM, Falivelli G, Pasquale EB. 2013. Eph receptor signaling and ephrins. Cold Spring Harb. Perspect. Biol. 5, a009159 ( 10.1101/cshperspect.a009159) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Casaletto JB, McClatchey AI. 2012. Spatial regulation of receptor tyrosine kinases in development and cancer. Nat. Rev. Cancer. 12, 387–400. ( 10.1038/nrc3277) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Curto M, Cole BK, Lallemand D, Liu CH, McClatchey AI. 2007. Contact-dependent inhibition of EGFR signaling by Nf2/Merlin. J. Cell Biol. 177, 893–903. ( 10.1083/jcb.200703010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chiasson-MacKenzie C, et al. 2015. NF2/Merlin mediates contact-dependent inhibition of EGFR mobility and internalization via cortical actomyosin. J. Cell Biol. 211, 391–405. ( 10.1083/jcb.201503081) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Butler LM, et al. 2000. Suberoylanilide hydroxamic acid, an inhibitor of histone deacetylase, suppresses the growth of prostate cancer cells in vitro and in vivo. Cancer Res. 60, 5165–5170. [PubMed] [Google Scholar]
- 27.Marusyk A, Polyak K. 2010. Tumor heterogeneity: causes and consequences. Biochim. Biophys. Acta. 1805, 105–117. ( 10.1016/j.bbcan.2009.11.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schito L, Semenza GL. 2016. Hypoxia-inducible factors: master regulators of cancer progression. Trends Cancer. 2, 758–770. ( 10.1016/j.trecan.2016.10.016) [DOI] [PubMed] [Google Scholar]
- 29.Boccaccio C, Comoglio PM. 2006. Invasive growth: a MET-driven genetic programme for cancer and stem cells. Nat. Rev. Cancer. 6, 637–645. ( 10.1038/nrc1912) [DOI] [PubMed] [Google Scholar]
- 30.Nakamura T, Teramoto H, Ichihara A. 1986. Purification and characterization of a growth factor from rat platelets for mature parenchymal hepatocytes in primary cultures. Proc. Natl Acad. Sci. USA 83, 6489–6493. ( 10.1073/pnas.83.17.6489) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Haeger A, Wolf K, Zegers MM, Friedl P. 2015. Collective cell migration: guidance principles and hierarchies. Trends Cell Biol. 25, 556–566. ( 10.1016/j.tcb.2015.06.003) [DOI] [PubMed] [Google Scholar]
- 32.Klein CA. 2009. Parallel progression of primary tumours and metastases. Nat. Rev. Cancer. 9, 302–312. ( 10.1038/nrc2627) [DOI] [PubMed] [Google Scholar]
- 33.Ding L, et al. 2010. Genome remodelling in a basal-like breast cancer metastasis and xenograft. Nature 464, 999–1005. ( 10.1038/nature08989) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jones S, et al. 2008. Comparative lesion sequencing provides insights into tumor evolution. Proc. Natl Acad. Sci. USA 105, 4283–4288. ( 10.1073/pnas.0712345105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Casasent AK, et al. 2018. Multiclonal invasion in breast tumors identified by topographic single cell sequencing. Cell 172, 205–217. ( 10.1016/j.cell.2017.12.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lambert AW, Pattabiraman DR, Weinberg RA. 2017. Emerging biological principles of metastasis. Cell 168, 670–691. ( 10.1016/j.cell.2016.11.037) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kai F, Laklai H, Weaver VM. 2016. Force matters: biomechanical regulation of cell invasion and migration in disease. Trends Cell Biol. 26, 486–497. ( 10.1016/j.tcb.2016.03.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gorringe KL, Fox SB. 2017. Ductal carcinoma in situ biology, biomarkers, and diagnosis. Front. Oncol. 7, 248 ( 10.3389/fonc.2017.00248) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cowell CF, Weigelt B, Sakr RA, Ng CK, Hicks J, King TA, Reis-Filho JS. 2013. Progression from ductal carcinoma in situ to invasive breast cancer: revisited. Mol. Oncol. 7, 859–869. ( 10.1016/j.molonc.2013.07.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cheung KJ, Gabrielson E, Werb Z, Ewald AJ. 2013. Collective invasion in breast cancer requires a conserved basal epithelial program. Cell 155, 1639–1651. ( 10.1016/j.cell.2013.11.029) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grigore AD, Jolly MK, Jia D, Farach-Carson MC, Levine H. 2016. Tumor budding: the name is EMT. Partial EMT. J. Clin. Med. 5, 51 ( 10.3390/jcm5050051) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Przybyla L, Muncie JM, Weaver VM. 2016. Mechanical control of epithelial-to-mesenchymal transitions in development and cancer. Annu. Rev. Cell Dev. Biol. 32, 527–554. ( 10.1146/annurev-cellbio-111315-125150) [DOI] [PubMed] [Google Scholar]
- 43.Ye X, Weinberg RA. 2015. Epithelial–mesenchymal plasticity: a central regulator of cancer progression. Trends Cell Biol. 25, 675–686. ( 10.1016/j.tcb.2015.07.012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nieto MA, Huang RY, Jackson RA, Thiery JP. 2016. Emt: 2016. Cell 166, 21–45. ( 10.1016/j.cell.2016.06.028) [DOI] [PubMed] [Google Scholar]
- 45.Friedl P, Locker J, Sahai E, Segall JE. 2012. Classifying collective cancer cell invasion. Nat. Cell Biol. 14, 777–783. ( 10.1038/ncb2548) [DOI] [PubMed] [Google Scholar]
- 46.Cheung KJ, Ewald AJ. 2016. A collective route to metastasis: seeding by tumor cell clusters. Science 352, 167–169. ( 10.1126/science.aaf6546) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cheung KJ, et al. 2016. Polyclonal breast cancer metastases arise from collective dissemination of keratin 14-expressing tumor cell clusters. Proc. Natl Acad. Sci. USA 113, E854–E863. ( 10.1073/pnas.1508541113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Aceto N, et al. 2014. Circulating tumor cell clusters are oligoclonal precursors of breast cancer metastasis. Cell 158, 1110–1122. ( 10.1016/j.cell.2014.07.013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Khalil AA, Ilina O, Gritsenko PG, Bult P, Span PN, Friedl P. 2017. Collective invasion in ductal and lobular breast cancer associates with distant metastasis. Clin. Exp. Metastasis. 34, 421–429. ( 10.1007/s10585-017-9858-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Clark AG, Vignjevic DM. 2015. Modes of cancer cell invasion and the role of the microenvironment. Curr. Opin. Cell Biol. 36, 13–22. ( 10.1016/j.ceb.2015.06.004) [DOI] [PubMed] [Google Scholar]
- 51.Park JA, Atia L, Mitchel JA, Fredberg JJ, Butler JP. 2016. Collective migration and cell jamming in asthma, cancer and development. J. Cell Sci. 129, 3375–3383. ( 10.1242/jcs.187922) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tambe DT, Fredberg JJ. 2015. And I hope you like jamming too. New J. Phys. 17, 091001 ( 10.1088/1367-2630/17/9/091001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Park JA, et al. 2015. Unjamming and cell shape in the asthmatic airway epithelium. Nat. Mater. 14, 1040–1048. ( 10.1038/nmat4357) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sadati M, Nourhani A, Fredberg JJ, Taheri Qazvini N. 2014. Glass-like dynamics in the cell and in cellular collectives. Wiley Interdiscip. Rev. Syst. Biol. Med. 6, 137–149. ( 10.1002/wsbm.1258) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Szabo B, Szollosi GJ, Gonci B, Juranyi Z, Selmeczi D, Vicsek T. 2006. Phase transition in the collective migration of tissue cells: experiment and model. Phys. Rev. E Stat. Nonlin. Soft Matter Phys. 74(6 Pt 1), 061908 ( 10.1103/PhysRevE.74.061908) [DOI] [PubMed] [Google Scholar]
- 56.Angelini TE, Hannezo E, Trepat X, Fredberg JJ, Weitz DA. 2010. Cell migration driven by cooperative substrate deformation patterns. Phys. Rev. Lett. 104, 168104 ( 10.1103/PhysRevLett.104.168104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Angelini TE, Hannezo E, Trepat X, Marquez M, Fredberg JJ, Weitz DA. 2011. Glass-like dynamics of collective cell migration. Proc. Natl Acad. Sci. USA 108, 4714–4719. ( 10.1073/pnas.1010059108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Giavazzi F, Paoluzzi M, Macchi M, Bi D, Scita G, Manning ML, Cerbino R, Marchetti MC. 2018. Flocking transitions in confluent tissues. Soft Matter 14, 3471–3477. ( 10.1039/C8SM00126J) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chepizhko O, Lionetti MC, Malinverno C, Giampietro C, Scita G, Zapperi S, La Porta CAM. 2018. From jamming to collective cell migration through a boundary induced transition. Soft Matter 14, 3774–3782. ( 10.1039/C8SM00128F) [DOI] [PubMed] [Google Scholar]
- 60.Giavazzi F, Malinverno C, Corallino S, Ginelli F, Scita G, Cerbino R. 2017. Giant fluctuations and structural effects in a flocking epithelium. J. Phys. D: Appl. Phys. 50, 384003 ( 10.1088/1361-6463/aa7f8e) [DOI] [Google Scholar]
- 61.Malinverno C, et al. 2017. Endocytic reawakening of motility in jammed epithelia. Nat. Mater. 16, 587–596. ( 10.1038/nmat4848) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chepizhko O, et al. 2016. Bursts of activity in collective cell migration. Proc. Natl Acad. Sci. USA 113, 11 408–11 413. ( 10.1073/pnas.1600503113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kuriyama S, Theveneau E, Benedetto A, Parsons M, Tanaka M, Charras G, Kabla A, Mayor R. 2014. In vivo collective cell migration requires an LPAR2-dependent increase in tissue fluidity. J. Cell Biol. 206, 113–127. ( 10.1083/jcb.201402093) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Frittoli E, et al. 2014. A RAB5/RAB4 recycling circuitry induces a proteolytic invasive program and promotes tumor dissemination. J. Cell Biol. 206, 307–328. ( 10.1083/jcb.201403127) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Totaro A, Panciera T, Piccolo S. 2018. YAP/TAZ upstream signals and downstream responses. Nat. Cell Biol. 20, 888–899. ( 10.1038/s41556-018-0142-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sigismund S, Scita G. 2018. The ‘endocytic matrix reloaded’ and its impact on the plasticity of migratory strategies. Curr. Opin. Cell Biol. 54, 9–17. ( 10.1016/j.ceb.2018.02.006) [DOI] [PubMed] [Google Scholar]
- 67.Palamidessi A, et al. 2018. Unjamming overcomes kinetic and proliferation arrest in terminally differentiated cells and promotes collective motility of carcinoma. bioRxiv. 388553.
- 68.Wendt MK, Taylor MA, Schiemann BJ, Schiemann WP. 2011. Down-regulation of epithelial cadherin is required to initiate metastatic outgrowth of breast cancer. Mol. Biol. Cell. 22, 2423–2435. ( 10.1091/mbc.e11-04-0306) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yui S, et al. 2018. YAP/TAZ-dependent reprogramming of colonic epithelium links ECM remodeling to tissue regeneration. Cell Stem Cell 22, 35–49. ( 10.1016/j.stem.2017.11.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zanconato F, Cordenonsi M, Piccolo S. 2016. YAP/TAZ at the roots of cancer. Cancer Cell 29, 783–803. ( 10.1016/j.ccell.2016.05.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Warren JSA, Xiao Y, Lamar JM. 2018. YAP/TAZ activation as a target for treating metastatic cancer. Cancers (Basel) 10, 115 ( 10.3390/cancers10040115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Li H, Gumbiner BM. 2016. Deregulation of the Hippo pathway in mouse mammary stem cells promotes mammary tumorigenesis. Mamm. Genome. 27, 556–564. ( 10.1007/s00335-016-9662-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Panciera T, et al. 2016. Induction of expandable tissue-specific stem/progenitor cells through transient expression of YAP/TAZ. Cell Stem Cell 19, 725–737. ( 10.1016/j.stem.2016.08.009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Aragona M, Panciera T, Manfrin A, Giulitti S, Michielin F, Elvassore N, Dupont S, Piccolo S. 2013. A mechanical checkpoint controls multicellular growth through YAP/TAZ regulation by actin-processing factors. Cell 154, 1047–1059. ( 10.1016/j.cell.2013.07.042) [DOI] [PubMed] [Google Scholar]
- 75.Shao DD, et al. 2014. KRAS and YAP1 converge to regulate EMT and tumor survival. Cell 158, 171–184. ( 10.1016/j.cell.2014.06.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lei QY, Zhang H, Zhao B, Zha ZY, Bai F, Pei XH, Zhao S, Xiong Y, Guan K-L. 2008. TAZ promotes cell proliferation and epithelial–mesenchymal transition and is inhibited by the hippo pathway. Mol. Cell. Biol. 28, 2426–2436. ( 10.1128/MCB.01874-07) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lehmann W, et al. 2016. ZEB1 turns into a transcriptional activator by interacting with YAP1 in aggressive cancer types. Nat. Commun. 7, 10498 ( 10.1038/ncomms10498) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tang Y, Weiss SJ. 2017. Snail/Slug-YAP/TAZ complexes cooperatively regulate mesenchymal stem cell function and bone formation. Cell Cycle 16, 399–405. ( 10.1080/15384101.2017.1280643) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sheetz MP, Felsenfeld D, Galbraith CG, Choquet D. 1999. Cell migration as a five-step cycle. Biochem. Soc. Symp. 65, 233–243. [PubMed] [Google Scholar]
- 80.Friedl P, Wolf K. 2009. Proteolytic interstitial cell migration: a five-step process. Cancer Metastasis Rev. 28, 129–135. ( 10.1007/s10555-008-9174-3) [DOI] [PubMed] [Google Scholar]
- 81.Doyle AD, Wang FW, Matsumoto K, Yamada KM. 2009. One-dimensional topography underlies three-dimensional fibrillar cell migration. J. Cell Biol. 184, 481–490. ( 10.1083/jcb.200810041) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ridley AJ. 2001. Rho GTPases and cell migration. J. Cell Sci. 114(Pt 15), 2713–2722. [DOI] [PubMed] [Google Scholar]
- 83.Jansen S, Gosens R, Wieland T, Schmidt M. 2018. Paving the Rho in cancer metastasis: Rho GTPases and beyond. Pharmacol. Ther. 183, 1–21. ( 10.1016/j.pharmthera.2017.09.002) [DOI] [PubMed] [Google Scholar]
- 84.Porter AP, Papaioannou A, Malliri A. 2016. Deregulation of Rho GTPases in cancer. Small GTPases 7, 123–138. ( 10.1080/21541248.2016.1173767) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Davis MJ, Ha BH, Holman EC, Halaban R, Schlessinger J, Boggon TJ. 2013. RAC1P29S is a spontaneously activating cancer-associated GTPase. Proc. Natl Acad. Sci. USA 110, 912–917. ( 10.1073/pnas.1220895110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bloomfield G, Kay RR. 2016. Uses and abuses of macropinocytosis. J. Cell Sci. 129, 2697–2705. ( 10.1242/jcs.176149) [DOI] [PubMed] [Google Scholar]
- 87.Bar-Sagi D, Feramisco JR. 1986. Induction of membrane ruffling and fluid-phase pinocytosis in quiescent fibroblasts by ras proteins. Science 233, 1061–1068. ( 10.1126/science.3090687) [DOI] [PubMed] [Google Scholar]
- 88.Commisso C, et al. 2013. Macropinocytosis of protein is an amino acid supply route in Ras-transformed cells. Nature 497, 633–637. ( 10.1038/nature12138) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Veithen A, Cupers P, Baudhuin P, Courtoy PJ. 1996. v-Src induces constitutive macropinocytosis in rat fibroblasts. J. Cell Sci. 109 (Pt 8), 2005–2012. [DOI] [PubMed] [Google Scholar]
- 90.Kasahara K, Nakayama Y, Sato I, Ikeda K, Hoshino M, Endo T, Yamaguchi N. 2007. Role of Src-family kinases in formation and trafficking of macropinosomes. J. Cell. Physiol. 211, 220–232. ( 10.1002/jcp.20931) [DOI] [PubMed] [Google Scholar]
- 91.Ha KD, Bidlingmaier SM, Liu B. 2016. Macropinocytosis exploitation by cancers and cancer therapeutics. Front. Physiol. 7, 381 ( 10.1002/jcp.20931) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lim JP, Gleeson PA. 2011. Macropinocytosis: an endocytic pathway for internalising large gulps. Immunol. Cell Biol. 89, 836–843. ( 10.1038/icb.2011.20) [DOI] [PubMed] [Google Scholar]
- 93.Buckley CM, King JS. 2017. Drinking problems: mechanisms of macropinosome formation and maturation. FEBS J. 284, 3778–3790. ( 10.1111/febs.14115) [DOI] [PubMed] [Google Scholar]
- 94.Carpentier JL, Lew DP, Paccaud JP, Gil R, Iacopetta B, Kazatchkine M, Stendahl O, Pozzan T. 1991. Internalization pathway of C3b receptors in human neutrophils and its transmodulation by chemoattractant receptors stimulation. Cell Regul. 2, 41–55. ( 10.1091/mbc.2.1.41) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Burns S, Hardy SJ, Buddle J, Yong KL, Jones GE, Thrasher AJ. 2004. Maturation of DC is associated with changes in motile characteristics and adherence. Cell Motil. Cytoskeleton. 57, 118–132. ( 10.1002/cm.10163) [DOI] [PubMed] [Google Scholar]
- 96.Vargas P, et al. 2016. Innate control of actin nucleation determines two distinct migration behaviours in dendritic cells. Nat. Cell Biol. 18, 43–53. ( 10.1038/ncb3284) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Veltman DM, Lemieux MG, Knecht DA, Insall RH. 2014. PIP(3)-dependent macropinocytosis is incompatible with chemotaxis. J. Cell Biol. 204, 497–505. ( 10.1083/jcb.201309081) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Corallino S, et al. 2018. A RAB35-p85/PI3 K axis controls oscillatory apical protrusions required for efficient chemotactic migration. Nat. Commun. 9, 1475 ( 10.1038/s41467-018-03571-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zobel M, et al. 2018. A NUMB-EFA6B-ARF6 recycling route controls apically restricted cell protrusions and mesenchymal motility. J. Cell Biol. 217, 3161–3182. ( 10.1083/jcb.201802023) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gu Z, Noss EH, Hsu VW, Brenner MB. 2011. Integrins traffic rapidly via circular dorsal ruffles and macropinocytosis during stimulated cell migration. J. Cell Biol. 193, 61–70. ( 10.1083/jcb.201007003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yoshida S, Pacitto R, Inoki K, Swanson J. 2017. Macropinocytosis, mTORC1 and cellular growth control. Cell. Mol. Life Sci. 75, 1227–1239. ( 10.1007/s00018-017-2710-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Recouvreux MV, Commisso C. 2017. Macropinocytosis: a metabolic adaptation to nutrient stress in cancer. Front. Endocrinol. (Lausanne) 8, 261 ( 10.3389/fendo.2017.00261) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hanahan D, Weinberg RA. 2011. Hallmarks of cancer: the next generation. Cell 144, 646–674. ( 10.1016/j.cell.2011.02.013) [DOI] [PubMed] [Google Scholar]
- 104.Halbrook CJ, Lyssiotis CA. 2017. Employing metabolism to improve the diagnosis and treatment of pancreatic cancer. Cancer Cell 31, 5–19. ( 10.1016/j.ccell.2016.12.006) [DOI] [PubMed] [Google Scholar]
- 105.Hezel AF, Kimmelman AC, Stanger BZ, Bardeesy N, Depinho RA. 2006. Genetics and biology of pancreatic ductal adenocarcinoma. Genes Dev. 20, 1218–1249. ( 10.1101/gad.1415606) [DOI] [PubMed] [Google Scholar]
- 106.Palm W, Araki J, King B, DeMatteo RG, Thompson CB. 2017. Critical role for PI3-kinase in regulating the use of proteins as an amino acid source. Proc. Natl Acad. Sci. USA 114, E8628–E8636. ( 10.1073/pnas.1712726114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Davidson SM, et al. 2017. Direct evidence for cancer-cell-autonomous extracellular protein catabolism in pancreatic tumors. Nat. Med. 23, 235–241. ( 10.1038/nm.4256) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kim SM, et al. 2016. Targeting cancer metabolism by simultaneously disrupting parallel nutrient access pathways. J. Clin. Invest. 126, 4088–4102. ( 10.1172/JCI87148) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tajiri H, et al. 2017. Targeting Ras-driven cancer cell survival and invasion through selective inhibition of DOCK1. Cell Rep. 19, 969–980. ( 10.1016/j.celrep.2017.04.016) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.