Abstract
Behavioural studies in apraxic patients revealed dissociations between the processing of meaningful (MF) and meaningless (ML) gestures. Consequently, the existence of two differential neural mechanisms for the imitation of either gesture type has been postulated. While the indirect (semantic) route exclusively enables the imitation of MF gestures, the direct route can be used for the imitation of any gesture type, irrespective of meaning, and thus especially for ML gestures. Concerning neural correlates, it is debated which of the visuo-motor streams (i.e., the ventral steam, the ventro-dorsal stream, or the dorso-dorsal stream) supports the postulated indirect and direct imitation routes.
To probe the hypotheses that regions of the dorso-dorsal stream are involved differentially in the imitation of ML gestures and that regions of the ventro-dorsal stream are involved differentially in the imitation of MF gestures, we analysed behavioural (imitation of MF and ML finger gestures) and lesion data of 293 patients with a left hemisphere (LH) stroke.
Confirming previous work, the current sample of LH stroke patients imitated MF finger gestures better than ML finger gestures. The analysis using voxel-based lesion symptom mapping (VLSM) revealed that LH damage to dorso-dorsal stream areas was associated with an impaired imitation of ML finger gestures, whereas damage to ventro-dorsal regions was associated with a deficient imitation of MF finger gestures.
Accordingly, the analyses of the imitation of visually uniform and thus highly comparable MF and ML finger gestures support the dual-route model for gesture imitation at the behavioural and lesion level in a substantial patient sample. Furthermore, the data show that the direct route for ML finger gesture imitation depends on the dorso-dorsal visuo-motor stream while the indirect route for MF finger gesture imitation is related to regions of the ventro-dorsal visuo-motor stream.
Highlights
-
•
Identification of differential neural correlates for the imitation of meaningful and meaningless finger gestures.
-
•
Support for the dual-route model for gesture imitation in a substantial patient sample (n = 293).
-
•
Left hemispheric damage to dorso-dorsal stream areas is associated with an impaired imitation of meaningless finger gestures
-
•
Damage to ventro-dorsal regions is associated with a deficient imitation of meaningful finger gestures
1. Introduction
Imitation deficits are a common symptom in left hemisphere (LH) stroke patients with apraxia (Donkervoort et al., 2000). As a cognitive motor deficit, apraxia is characterized by a bilateral impairment of purposeful, skilled movements, such as imitation, but also pantomime and tool use, which cannot be explained by primary deficits of the sensorimotor system or disturbed communication due to aphasia (Dovern et al., 2012). Previous studies of apraxic patients suggested differences between the imitation of meaningful (MF) and meaningless (ML) gestures, i.e., clinically observable dissociations between impaired imitation of one but not the other gesture type (Bartolo et al., 2001; Goldenberg and Hagmann, 1997; Mengotti et al., 2013; Peigneux et al., 2000; Tessari et al., 2007). Consequently, two differential neural mechanisms for the imitation of ML and MF gestures have been postulated: the route underlying MF gesture imitation has been coined the indirect route since recognition of a MF gesture is thought to facilitate imitation by triggering recall of its meaning and configuration from memorized motor engrams. The second route has been termed the direct imitation route since it connects the visual analysis of a gesture directly to novel patterns without the involvement of semantic representations (Cubelli et al., 2000; Rothi et al., 1991; Rumiati and Tessari, 2002). Thus, the direct imitation route allows imitation of both gesture types irrespective of associated meaning. In addition, it is assumed that recognizing the meaning of a gesture triggers the use of the indirect imitation route, especially since imitating gestures via the indirect routes seems to be advantageous: stroke patients, as well as healthy subjects, usually imitate MF actions better than ML actions (Achilles et al., 2016; Dovern et al., 2011; Tessari and Rumiati, 2004). Within the framework of the dual-route model for gesture imitation, selective deficits in imitating ML gestures can be explained by a selective impairment of the direct route (Bartolo et al., 2001; Goldenberg and Hagmann, 1997; Mehler, 1987). On the other hand, a selective impairment in imitating MF gestures can be explained by an affection of the indirect semantic imitation route plus an additional difficulty to switch to the direct imitation route, which is putatively intact and would at least in principle allow imitation of any gesture including MF gestures (Bartolo et al., 2001; Tessari and Cubelli, 2014).
Concerning neural correlates, it has been postulated that the two imitation routes draw upon two differential, anatomically segregated visuo-motor streams, i.e., the dorsal stream and the ventral stream, described initially as processing pathways for vision-for-action (“where”) and vision-for-perception (“what”, Goodale and Milner, 1992; Mishkin and Ungerleider, 1982). Based on anatomical studies in monkeys (Rizzolatti and Matelli, 2003), an extension of the dual-stream model of action processing has been proposed with a subdivision of the dorsal stream into a dorso-dorsal and a ventro-dorsal stream (Binkofski and Buxbaum, 2013). In that context, the dorso-dorsal stream is presumed to constitute a “grasp system” supporting online-motor control (especially in the context of tool use), while the ventro-dorsal is thought to constitute the “use system” supporting long-term (tool) action representations (Binkofski and Buxbaum, 2013; Buxbaum and Kalénine, 2010; Dressing et al., 2018; Rizzolatti and Matelli, 2003). Considering analogies for gesture imitation it has been discussed that regions of the dorso-dorsal stream are primarily engaged during the imitation of ML gestures, while ventro-dorsal regions might especially be involved in the imitation of MF gestures due to associated semantic aspects (Binkofski and Buxbaum, 2013; Dressing et al., 2018; Hoeren et al., 2014).
Anatomically, both visuo-motor streams originate in the primary visual area (V1). The ventral stream then projects along the occipital and temporal cortices, including the fusiform gyrus (FFG), to the anterior temporal lobe (ATL, Binkofski and Buxbaum, 2013; Kleineberg et al., 2018; Mahon et al., 2007; Martin et al., 2016; Rizzolatti and Matelli, 2003), while the dorsal stream projects to the parietal cortex. Within the dorso-dorsal stream, the superior parietal lobule (SPL) and the posterior intraparietal sulcus (IPS) are considered especially important. On the other hand, the inferior parietal lobe (IPL) and parts of the anterior IPS are essential nodes within the ventro-dorsal stream (Binkofski et al., 1998; Binkofski and Buxbaum, 2013; Grefkes and Fink, 2005; Kalénine et al., 2010; Kleineberg et al., 2018; Sakreida et al., 2016).
Within the context of apraxia, previous studies have contributed not only to the development and refinement but also to the probing of the dual streams model, e.g., by contrasting lesions of stroke patients with different apraxic deficits such as impairments of imitation and pantomime (Dressing et al., 2018; Hoeren et al., 2014; Tessari et al., 2007; Weiss et al., 2014). For example, Hoeren and colleagues contrasted lesions of 98 LH stroke patients comparing lesion-symptom-associations for (supposedly) ML hand and finger gesture imitation and pantomime, i.e., MF actions, using voxel-based lesion symptom mapping (VLSM, Hoeren et al., 2014). Compatible with the dual route model, Hoeren et al. found that areas related to the dorso-dorsal stream (i.e., the posterior IPS and SPL) were more strongly associated with ML imitation deficits. On the other hand, pantomime deficits and particularly content errors were associated with regions of the ventro-dorsal and ventral streams such as the anterior IPL, posterior MTG, and fibres traversing the extreme capsule. These findings are consistent with results of a PET-study in healthy subjects showing differential activation of ventral and dorsal stream regions involved in MF and ML gesture processing, respectively (Rumiati et al., 2005).
It is, however, noteworthy that the tests used to assess finger imitation (the finger imitation test by Goldenberg and Hagmann (1997) adopted in the study by Hoeren and colleagues) includes MF as well as ML finger configurations (Achilles et al., 2016).
Furthermore, Tessari and colleagues showed by lesion subtraction analysis an involvement of regions of the ventral stream in stroke patients with selective deficits in MF gesture imitation and involvement of regions of the dorsal stream in those patients with impaired ML gesture imitation (Tessari et al., 2007). Note that this analysis was based on six cases only.
Considering the implications and limitations of previous studies in this field, we here set out to probe in a large patient sample of LH stroke patients (n = 293) the hypothesis that ventro-dorsal lesions are preferably associated with MF gesture imitation deficits and that dorso-dorsal lesions are instead associated with deficits in imitating ML gestures. To this end, we used the finger configurations of the Goldenberg finger imitation test (1996) that are comparable in many respects but can be divided into ML and MF finger gestures (Achilles et al., 2016). First, we aimed at confirming the prediction of the dual-route model of imitation, i.e., that meaning influences stroke patients' performance in the finger gesture imitation task. Second, we performed voxel-based lesion symptom mapping (VLSM) to identify the (differential) lesion sites that were associated with deficits in imitating MF versus ML finger gestures and related them to regions of the ventro-dorsal and dorso-dorsal streams. Third, to underline the validity of our findings for MF and ML finger configurations, we aimed at replicating lesion-symptom associations for impaired hand gesture imitation that have been consistently observed across multiple studies (see supplementary material).
2. Material and methods
2.1. Patient sample
We retrospectively analysed finger imitation scores and lesion maps of 293 patients (93 women; age [mean ± standard deviation, SD] = 57 ± 14 years; time since stroke at the assessment = 9 ± 19 months [range: 0 to 127 months]) who had suffered a single (first ever) unilateral left-hemispheric stroke. All patients were right-handed before the stroke (Oldfield, 1971), did not suffer from any other neurological or psychiatric diseases (e.g., depression), and were between 18 and 95 years old when assessed. Patients were not included if the severity of aphasia prevented informed consent to participate or the understanding of the imitation tasks.
Patients were classified as having a finger imitation deficit if they scored below the cut-off for the finger imitation test, as proposed initially by Goldenberg (see Section 2.2). Accordingly, 61 (21%) of the 293 LH stroke patients exhibited a finger imitation deficit.
Patients had given written informed consent for participating in the original studies on motor cognition from which these data were drawn. Each of the original studies had been approved by the local ethics committee and had been performed following the Declaration of Helsinki. Additionally, these retrospective analyses were approved by the institutional review board. Behavioural and lesion data of subsets of the current 293 patients were reported before in two other retrospective analyses comparing hand and finger imitation tests (n = 190, Achilles et al., 2017) and investigating the dissociation between MF and ML finger gesture imitation in LH and right hemisphere (RH) stroke at the behavioural level only (n = 132, Achilles et al., 2016).
2.2. Testing procedures
As described in Achilles et al. (2017) all patients were assessed with the test of imitating finger gestures by Goldenberg (1996): the examiner sits opposite to the patient and demonstrates the ten finger gestures in a mirror-like fashion. The examiner uses the hand opposite to the patient's non-paretic ipsilesional hand, which the patient is supposed to use for the imitation. After the first demonstration of each gesture, the examiner forms a fist (neutral gesture), and the patient is asked to imitate the previously shown gesture. Two points are allocated for an exact imitation, based solely on the final gesture (self-corrections or hesitations do not influence the score). If the imitation is incorrect, the examiner repeats the demonstration of the gesture and then returns to the neutral gesture (fist) again. The patient is then asked to imitate the gesture once more. One point is allocated for a correct imitation in this second trial, and no points are awarded if the patient fails at the second attempt again. A patient is considered to suffer from a finger imitation deficit if the total imitation score for the ten finger gestures is 16 or less of the 20 possible points. (Goldenberg, 1996).
2.3. Analysis of the effect of meaning on the stroke patients' finger gesture imitation performance
Using the meaning scores previously established in Achilles et al. (2016), which reflect how many healthy subjects attribute meaning to one of the ten Goldenberg finger gestures (in %), we analysed how its meaningfulness influenced the imitation of a given finger gesture. For this reason, the mean imitation scores of the two finger gestures (F03 and F09) that are clearly ML (i.e., these two finger gestures meant something to only 7% or 2% of healthy participants) and of the three finger gestures (F05, F06 and F08) that are clearly MF (i.e., these three finger gestures have been attributed with a meaning by 98% of healthy subjects), were calculated and subjected to an ANOVA with the within-subject factor MEANING (two levels: MF, ML), and the between-subject factor finger IMITATION DEFICIT (two levels: present, not present). Note that we subjected the mean imitation scores to all analyses in order to account for the different number of MF (n = 2) and ML (n = 3) finger gestures (i.e., the mean imitation score would be maximally 2, if all patients imitated the gestures correctly, and minimally 0, if all patients failed to imitate them entirely). All statistical analyses were performed using IBM SPSS statistics version 24. Data were analysed with a mixed-design analysis of variance (ANOVA) and are reported at a significance level of p < .05 for all analyses. Where appropriate, degrees of freedom were Greenhouse–Geisser corrected.
2.4. Imaging procedures and lesion mapping
Lesion mapping was performed using either clinical MRI (n = 198) or CT (n = 95) scans.
Lesions were delineated manually on axial slices of a T1-weighted template MRI scan from the Montreal Neurological Institute (MNI) using the MRIcron software package with a 1 × 1-mm in-plane resolution. Lesions were mapped onto the slices in steps of 5 mm in MNI space using the identical or the closest matching axial slices of each individual's CT or MRI. Detailed scanning sequences varied across the sample, which was aggregated from several smaller studies.
2.5. Voxel-based lesion-symptom mapping (VLSM)
VLSM was carried out using the NiiStat toolbox for MATLAB (https://www.nitrc.org/projects/niistat/, version 1.1, 2018-06-20). We assessed lesion-symptom associations for the overall score in the finger imitation task, and the mean scores of the three MF and the two ML finger gestures, as well as for the difference between the MF and ML finger configurations (and vice versa), the latter to test for task-specific lesion-symptom-interactions. For comparative reasons, we also report lesion data of the Goldenberg hand imitation task in the supplementary material. In VLSM, t-tests on the behavioural scores are performed at each voxel, with groups defined by the presence or absence of damage in each voxel (Bates et al., 2003). Thereby voxels in which damage is associated with a task deficit can be identified. Only voxels where at least 10% of the 293 (n = 29) patients had a lesion were included in the analysis. Voxels were related to brain regions (visual inspection of voxel-region-overlap) based on the “JHU-atlas” (Faria et al., 2012) as used in (Fridriksson et al., 2018; Yourganov et al., 2015).
3. Results
3.1. Behavioural results
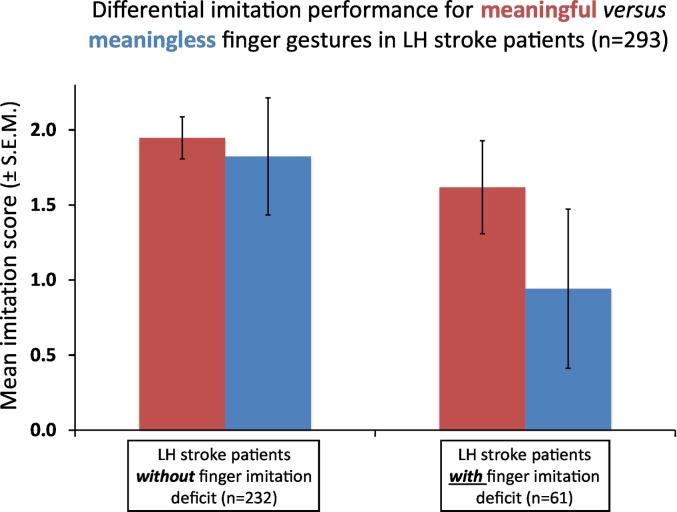
Our behavioural results (see Fig. 1) reproduced the findings of our previous work on this topic (Achilles et al., 2016): in addition to the expected main effect of IMITATION DEFICIT (F (1, 291) = 412.33, p < .001; note that this factor – by definition – separated stroke patients with and without finger imitation deficits), the ANOVA revealed a significant main effect of MEANING (F (1, 291) = 165.99, p < .001) with worse imitation of the two ML finger gestures compared to imitation of the three MF finger gestures (mean imitation score ± SD: ML finger gestures: 1.64 ± 0.51; MF finger gestures: 1.88 ± 0.25). The interaction MEANING by IMITATION DEFICIT (F (1, 291) = 79.14, p < .001) was also significant. Post-hoc t-tests revealed that the difference between patients with and without imitation deficit was significant for both MF (mean imitation score ± SD.: patients without imitation deficit: 1.94 ± 0.14; patients with imitation deficit: 1.82 ± 0.31; t (291) = 10.57, p < .001) and ML gesture imitation (mean imitation score ± SD.: patients without imitation deficit: 1.62 ± 0.39; patients with imitation deficit: 0.94 ± 0.53; t (291) = 16.87, p < .001). However, the difference in ML gesture imitation performance between patients with and without a finger imitation deficit (mean difference ± S.E.M. difference: 0.68 ± 0.09) was larger than the respective difference in MF gesture imitation performance (mean difference ± S.E.M. difference: 0.12 ± 0.02, t (291) = 8.90, p < .001). Thus, the difference between patients with and without an imitation deficit was more pronounced when imitating ML gestures compared to MF gestures constituting the significant interaction MEANING by IMITATION DEFICIT. Note that regression analyses did not reveal any significant relationship between the nuisance variables time post stroke, age, and lesion size with (the overall) finger and hand imitation scores (all p > .1).
Fig. 1.
Behavioural results. Assessment of the interaction of MEANING by IMITATION DEFICIT (F (1, 291) = 79.14, p < .001): the difference in imitation performance between patients with (n = 61) and without (n = 232) imitation deficit was significant for both MF (mean imitation score ± SD.: patients without imitation deficit: 1.94 ± 0.14; patients with imitation deficit: 1.82 ± 0.31; t (291) = 10.57, p < .001) and ML gesture imitation (mean imitation score ± SD.: patients without imitation deficit: 1.62 ± 0.39; patients with imitation deficit: 0.94 ± 0.53; t (291) = 16.87, p < .001). However, the difference between patients with and without imitation deficits was significantly larger for ML (ΔML, mean difference ± S.E.M. difference: 0.68 ± 0.09) compared to MF (ΔMF, mean difference ± S.E.M. difference: 0.12 ± 0.02, t (291) = 8.90, p < .001) gestures.
To ensure that the replication of our previous results was not simply driven by the effect of meaning on imitation in the 132 patients, who had been included in our previously published study (Achilles et al., 2016), we also repeated the above described analyses with the behavioural data of the 161 new patients who had not been included in the previous analyses. This analysis yielded the same pattern of results.
3.2. Dissociations between the imitation of ML and MF finger gestures
In total, seven of the 293 LH stroke patients exhibited a score of 0 points for the imitation of the two ML finger gestures, i.e., these patients completely failed to imitate the two-finger gestures that were rated as ML. Two of those seven patients showed a preserved imitation of the three finger gestures, which were classified as MF. The inverse dissociation (preserved imitation of ML finger gestures, impaired imitation of MF finger gestures) was not observed in our sample of 293 stroke patients. Note that in the whole sample, no patient entirely failed in imitating the MF finger gestures.
3.3. VLSM results
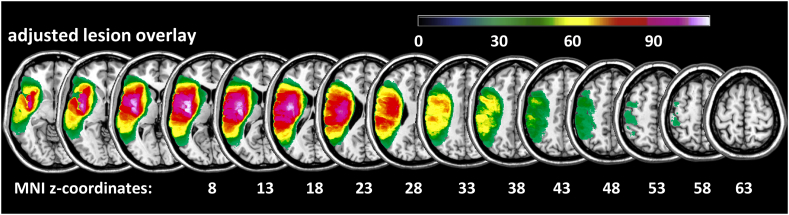
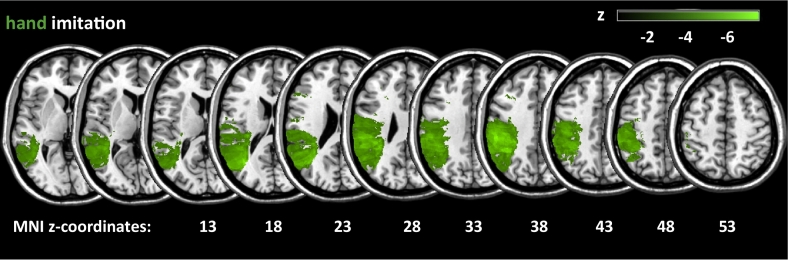
The lesion overlay in Fig. 2 displays voxels where at least 10% (n = 29) of all 293 LH stroke patients had a lesion (i.e., Fig. 2 displays those voxels which were subjected to the VLSM for finger imitation). Note the lesion coverage of the vascular territory of the left middle cerebral artery (MCA).
Fig. 2.
Lesion overlay. Lesion overlay plot for all 293 LH stroke patients where at least 10% (n = 29) of the patients had a lesion, i.e. displayed are only those voxels which were subjected to the VLSM.
We also subjected the hand imitation scores to a VLSM in order to assure the validity of our findings (see Supplementary material, Supplementary Fig. 1).
When we subjected the overall finger imitation scores to a VLSM, no voxels survived corrected thresholds. Thus, all of the following analyses are reported at the uncorrected threshold of p < .05. Note that previous studies on finger gesture imitation used uncorrected thresholds too (Dovern et al., 2011; Goldenberg and Randerath, 2015).
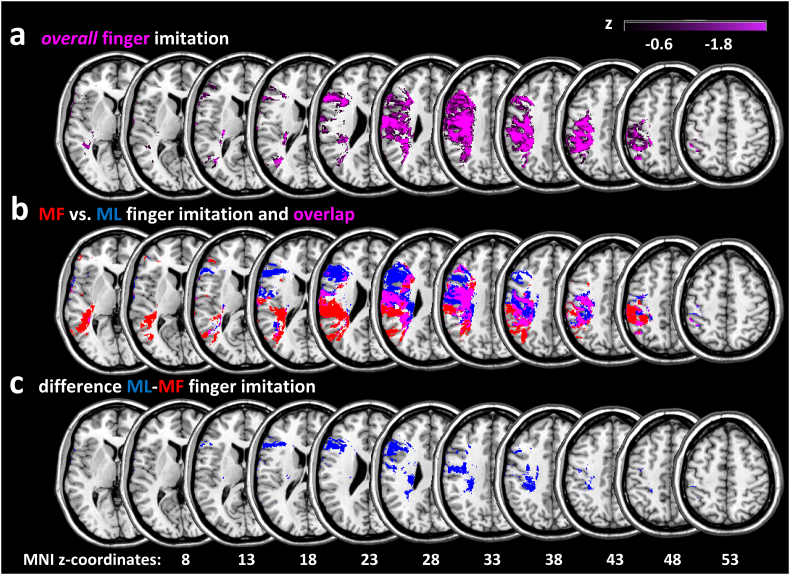
Fig. 3a displays voxels where damage was associated with poor performance in the overall finger imitation task. Note that more negative T-values indicate stronger lesion-symptom-association. Voxels, where damage was associated with overall finger imitation deficits, were mainly located in the inferior frontal gyrus (IFG, max. T-value (pars triangularis): −3.03), the middle frontal gyrus (MFG, max. T-value: −3.20), pre- and postcentral gyrus (max. T-values: −4.25 and −4.49 respectively), the superior temporal gyrus (STG, max. T-value −2.64), the posterior middle temporal gyrus (pMTG, max. T-value −2.71), the middle occipital gyrus (MOG, max. T-value: −3.43), the superior longitudinal fasciculus (SLF, max. T-value: −4.17), as well as the supramarginal (SMG, max. T-value: −3.45) and angular (AG, max. T-value: −3.63) gyrus of the inferior parietal lobe (IPL), and the superior parietal gyrus (SPG, max. T-value: −2.64).
Fig. 3.
VLSM analysis results. a) Results of the VLSM analysis for the overall finger imitation scores. VLSM-parameter: minimum lesion overlay 10% (n = 29), level of significance p < .05, uncorrected. Please note: this analysis did not reveal any significant voxels at p < .05 after controlling for multiple comparisons using FDR-correction. Thus, in all analyses on finger imitation, results are reported at the uncorrected significance level of p < .05. b) Results of the separate VLSM for the mean imitation scores of a) the three meaningful (MF, red) and the two meaningless (ML, blue) gestures. Magenta voxels represent the overlap between both VLSM (VLSM-parameter: see Fig. 2a). c) Results of the VLSM for the difference between the mean imitation scores of ML minus MF finger gesture imitation (VLSM-parameter: see Fig. 2a). Note that there were no voxels associated with the difference MF minus ML finger imitation at the significance level of p < .05, uncorrected.
The results of the VLSM, in which we subjected the mean imitation scores of the three MF gestures (red areas) and the two ML gestures (blue areas), are displayed in Fig. 3b. T-values of the regions resulting from the VLSM analyses of MF and ML finger imitation deficits are listed in Table 1. Besides, the region-specific T-values are ranked according to the lesion-symptom-association strength in a given VSLM analysis (here: MF, ML; see Table 1). The VLSM for the difference scores of “ML minus MF” finger gesture imitation is shown in Fig. 3c. Note that there were no voxels associated with the reverse difference (i.e., “MF minus ML”).
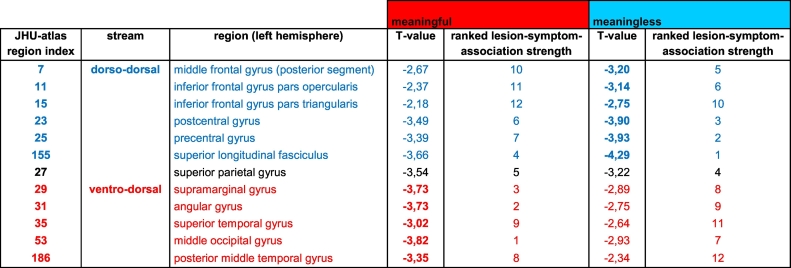
Table 1.
Summary of relevant lesion-symptom-associations for deficits in imitating ML and MF finger gestures.
The upper part of the table lists the regions commonly associated with the dorso-dorsal processing stream (depicted in blue). The regions of the ventro-dorsal processing stream are depicted in red and listed in the lower part of the table. The first column indicates the index number of a given region in the JHU-atlas. The last four columns list the T-values as well as the corresponding rank for the regions that were revealed by the VLSM analyses of meaningful (MF) and meaningless (ML) finger imitation, respectively. Note that for both analyses, more negative T-values indicate stronger lesion-symptom-associations. Accordingly, the regions and thus the lesion-symptom-associations were ranked from the lowest (negative) T-value (i.e., strongest association, rank 1) to the highest (negative) T-value (i.e., weakest association, rank 12) - separately for both analyses.
The table shows that for the VLSM analysis of deficits in ML finger gesture imitation, regions of the dorso-dorsal processing stream achieve higher T-values and higher ranks than the regions of the ventro-dorsal processing stream. For the VLSM analysis of imitation deficits for MF finger gestures, the inverse pattern was observed. Notably, this detailed analysis also revealed that the superior parietal gyrus (SPG, depicted in black) was implicated in both MF and ML finger gesture imitation.
Voxels associated with a poor performance of both MF and ML finger gesture imitation mainly overlapped in the pre- and postcentral gyrus, IFG, SLF, IPL, and the SPG (see Fig. 3b, pink areas).
Mainly dorso-dorsal regions were explicitly associated with poorer performance in the ML finger gesture imitation: the pre- (max. T-value: −3.93) and postcentral gyrus (max. T-value: −3.90), and the SLF (max. T-value: −4.29, see blue areas in Fig. 3a). On the other hand, more ventro-dorsal regions were implicated explicitly in the imitation of MF finger gesture, particularly the SMG (max. T-value: −3.73) and the AG (max. T-value: −3.73), the MOG (max. T-value: −3.82), and the posterior MTG (max. T-value: −3.35, see red areas in Fig. 3a). The VLSM for the difference score “ML minus MF” finger gesture imitation indicated that especially damage to voxels in the IFG (max. T-values: −2.54) and the pre- (max. T-values: −2.87) and postcentral gyrus (max. T-values: −3.27) as well as the SLF (max. T-values: −3.27) was associated with worse ML finger gesture imitation relative to MF gesture imitation. Note that small difference scores for ML minus MF were either due to small scores in ML finger gesture imitation or large scores in MF finger gesture imitation. On the other hand, there were no voxels at this threshold where damage predicted worse MF finger gesture imitation relative to ML finger gesture imitation.
Consistently, Table 1 clearly shows that for the VLSM analysis of imitation deficits for meaningless (ML) finger gestures regions of the dorso-dorsal processing stream achieve more negative T-values and higher ranks than the regions of the ventro-dorsal processing stream. For the VLSM analysis of imitation deficits for MF finger gestures, the inverse pattern was observed. Notably, this detailed analysis also revealed that the superior parietal gyrus (SPG) was implicated in both MF and ML finger gesture imitation (see Table 1).
4. Discussion
There are two main findings of this study: first of all, behavioural analyses of the data of this large sample of 293 patients (including data of 161 patients not reported before) confirmed the notion that the meaning of a gesture affects finger imitation performance in (LH) stroke patients, especially in those patients with a finger imitation deficit (Achilles et al., 2016). The second main finding concerns the involvement of regions of the ventro-dorsal and dorso-dorsal streams in the imitation of MF and ML finger gestures. While deficits in the overall finger imitation task, which includes MF and ML finger gestures covered a large proportion of both streams (Binkofski and Buxbaum, 2013), there were specific lesion patterns associated with deficient imitation of ML and MF finger gestures. The former was predominantly associated with lesions affecting the dorso-dorsal stream, while the latter was related to lesions affecting the ventro-dorsal stream. Taken together, these data support the dual-route model of gesture imitation at the behavioural as well as the lesion level. Since the current behavioural results replicate previously published findings (Achilles et al., 2016, Achilles et al., 2017; albeit in a now even larger cohort of LH stroke patients), we will focus the following discussion on the lesion mapping results.
VLSM in the current sample of 293 patients with LH stroke revealed that lesioned voxels mainly in the following regions were associated with overall finger imitation deficits: IFG, posterior STG, the STG itself, SLF, the posterior MTG, MOG, MFG, pre- and postcentral gyrus as well as the SMG, AG, and SPG. The implication of this large set of regions in the task of imitating finger configurations is in good accordance with findings of other studies investigating imitation deficits in stroke patients (Buxbaum et al., 2014; Dovern et al., 2011; Hoeren et al., 2014; Mengotti et al., 2013; Weiss et al., 2014). Furthermore, it is evident that voxels associated with deficits in the overall finger imitation task, which includes MF and ML finger gestures, covered a large proportion of both the ventro-dorsal and dorso-dorsal stream (Binkofski and Buxbaum, 2013). For the overall finger imitation task, the strongest lesion-symptom-associations (indicated by the respective T-values) were found in regions of the dorso-dorsal stream and for the IPL (max. T-values for SMG: −3.45 and AG: −3.63). The three highest lesion-symptom-associations as indicated by the max. T-value (the more negative the T-value, the stronger the lesion-symptom-association) were found in the pre- (max. T-value: −4.25) and postcentral gyrus (max. T-value: −4.49) as well as the SLF (max. T-value: −4.17). These three regions are considered to belong to the dorso-dorsal processing stream. Interestingly, this finding is directly related to a previous behavioural study by Tessari and Rumiati. In that study, the importance of the direct imitation route when (as in the current study) MF and ML gestures were presented in a mixed manner was stressed (Tessari and Rumiati, 2004), since the direct imitation route, which is associated with the dorso-dorsal stream, allows imitation of both MF and ML gestures. As shown by Tessari and Rumiati (2004), in imitation tasks where MF and ML gestures are presented in a mixed manner, rather than in blocks of either gesture type, it seems to be advantageous concerning cognitive resources to rely on the imitation route, which allows imitation of both gesture types, rather than switching between the indirect and direct imitation route.
Further analyses revealed that voxels specifically associated with ML finger gesture imitation deficits were mainly located in the pre- and postcentral gyrus, the SLF and the IFG pars triangularis and opercularis. All these regions are considered to be part of the dorso-dorsal stream. On the other hand, more ventro-dorsal regions were implicated in MF finger gesture imitation, particularly the SMG and the AG of the IPL, the MOG, and the posterior superior and middle temporal gyri. Therefore, the distribution of lesions associated with MF or ML finger imitation deficits converges with the predictions derived from the visuo-motor streams model: while the dorso-dorsal stream is considered to support online-motor control specifically relevant during the imitation of ML gestures, regions of the ventro-dorsal stream were implicated in the imitation of MF gestures with associated semantic aspects (Binkofski and Buxbaum, 2013; Dressing et al., 2018; Hoeren et al., 2014). Note that the SPL, which is implicated in visuo-motor online motor control (Pisella et al., 2000), a process that is especially relevant for finger coordination and imitation, was involved in both MF and ML finger imitation (as well overall finger imitation). A prerequisite for the correct imitation of a given finger gesture is the precise visuo-spatial analysis of the perceptually similar finger gestures (Goldenberg, 1999). On the other hand, the same precision is demanded when the participant is configuring the individual fingers to match the template gesture in the imitation process. Therefore, finger gesture imitation puts high demands on movement precision and thus (on-line) corrections of movements. Exactly these motor processes are supported by the SPG (Desmurget et al., 1999).
Moreover, support for the dual pathway model of imitation can be derived from the results of the VLSM for the difference scores ML minus MF finger gesture imitation (and vice versa). The VLSM with the difference score ML minus MF finger imitation indicated that damage to voxels in the IFG and the pre- and postcentral cortex was associated with worse ML gesture imitation. Thus, it can be reasoned that these brain regions of the dorso-dorsal stream correspond to the direct imitation route, which is used explicitly for ML finger gesture imitation, since MF gestures can also be imitated via the indirect imitation route, which is - as shown here - associated with ventro-dorsal stream regions. On the other hand, there were no voxels, where damage predicted worse MF than ML finger gesture imitation – even at the uncorrected threshold of p < .05. This finding is consistent with the observation that single case analyses (in the examined large patient sample, n = 293) revealed not a single patient with deficient MF, but spared ML gesture imitation (see also Achilles et al., 2016). The dual-route model of gesture imitation can also explain this behavioural pattern: worse imitation of MF gestures requires a selective deficit of the indirect imitation route and additional problems to switch to the intact direct imitation route, which would still allow the imitation of any gesture irrespective of its meaning (Bartolo et al., 2001; Rumiati et al., 2005). Our data indicate that at the group and single case level the occurrence of both deficits in a given patient, i.e., a deficient indirect imitation route and problems in switching to the direct route, is very rare. This notion is also reflected in the small number of reported cases (n = 4) in the literature with deficient MF, but preserved ML gesture imitation (Bartolo et al., 2001; Mehler, 1987; Ochipa et al., 1994).
Even though the current results are in good accordance with the literature supporting the dual pathway model of imitation, some limitations have to be considered. First of all, concerning the task under investigation, i.e., finger imitation, it has to be noted that the number of finger gestures was small, i.e., ten different gestures (with the emphasis on the five MF and ML gestures). However, this is at least in part compensated for by the large patient sample (n = 293). Moreover, the distribution of voxels associated with poor performance in the finger imitation task per se (including all ten finger gestures) was similar to the combined results of the two VLSMs separately investigating the two ML and the three MF finger gestures. This finding implies that not too much information was lost by subjecting only half of the ten finger gestures to the VLSM for MF and ML finger gesture imitation. Furthermore, the findings of the current study probed the results of Hoeren and colleagues who analysed the imitation of finger and hand gestures although hand gestures are ML and finger gestures are - at least in part - MF. Moreover, the current results extend the findings of Tessari et al. (2007), who reported a lesion overlay analysis in only six patients with selective deficits in imitating ML versus MF gestures, by investigating a large patient sample (n = 293) and adopting a statistically more profound approach, i.e., VLSM.
Concerning the VLSM-results it is essential to note that they need to be considered with some caution. No voxels survived the finger imitation analyses after correction for multiple comparisons, although our sample with data of 293 LH stroke patients was huge. Correction for multiple comparisons is recommended for several reasons (de Haan and Karnath, 2018; Mirman et al., 2018; Shahid et al., 2017; Sperber and Karnath, 2018). However, concerning the finger imitation task under investigation, several lesion studies also used uncorrected thresholds (Dovern et al., 2011; Goldenberg and Randerath, 2015) or lower (corrected) thresholds than those for hand imitation (e.g., finger imitation: FDR p < .05, hand imitation: FDR p < .01 (Hoeren et al., 2014)). Thus, a lack of power seems unlikely, also with regards to the robust significant and literature-consistent results of the VLSM for hand imitation deficits (see supplementary material and e.g. (Goldenberg and Randerath, 2015)). The lack of significant voxels at the corrected level for finger imitation deficits in VLSM might instead be related to the notion that a wide-spread network of brain regions is involved in the finger imitation task. This consideration is lent credence by a recent study showing that in large patient samples false negative results can arise when a given deficit can be caused by different lesion sites (Gajardo-Vidal et al., 2018). In line with this notion, studies on finger imitation have commonly implicated a wide-spread network of brain regions, even including the right hemisphere, which was not assessed in this study (Goldenberg, 2009; Goldenberg et al., 2009; Goldenberg and Randerath, 2015; Goldenberg and Strauss, 2002; Hartmann et al., 2005; Hermsdörfer et al., 2001).
Besides these aspects, also the method used to delineate the stroke lesions needs consideration. All lesions were marked manually, which is still the standard of lesion demarcation (Wilke et al., 2011). However, this approach is observer-dependent. Further, the data were derived from several studies, i.e., different investigators were involved, although all lesions were cross-checked by a second investigator experienced in lesion delineation to ensure reliability. Finally, delineated lesion size may vary as a function of scan resolution, which differs within and across the two scan modalities (CT versus MRI) (de Haan et al., 2015; de Haan and Karnath, 2018).
5. Conclusion
Confirming, but also clearly extending previous behavioural findings, our current results based upon a large cohort of patients with LH stroke (n = 293) strongly support current cognitive (dual-route) models of imitation (Cubelli et al., 2000; Rothi et al., 1991; Rumiati and Tessari, 2002). Furthermore, our lesion mapping data relate the concept of visuo-motor streams to the dual-route model of imitation. The results suggest that damage of the dorso-dorsal stream leads to deficits in ML finger gesture imitation (presumably caused by a dysfunction of the direct imitation route), while deficits in MF finger gesture imitation (putatively related to a dysfunction of the indirect imitation route) are associated with damage of the ventro-dorsal stream.
Glossary
- AG
angular gyrus
- aIPS
anterior intraparietal sulcus
- ATL
anterior temporal lobe/pole
- FDR
false discovery rate
- FFG
fusiform gyrus
- IFG
inferior frontal gyrus
- IPL
inferior parietal lobe
- LH
left-hemispheric/left hemisphere
- MF
meaningful
- MFG
middle frontal gyrus
- ML
meaningless
- MOG
middle occipital gyrus
- pMTG
posterior middle temporal gyrus
- pSTG
posterior superior temporal gyrus
- SLF
superior longitudinal fasciculus
- SMG
supramarginal gyrus
- SPG
superior parietal gyrus
- SPL
superior parietal lobule
- STG
superior temporal gyrus
- V1
primary visual area
- VLSM
voxel-based lesion-symptom-mapping
The following are the supplementary data related to this article.
Supplementary Fig. 1.
Results of the VLSM analysis for hand imitation scores. Note that only 291 of the 293 patients, of whom finger imitation data are reported in the main article, were included in this analysis, since hand imitation scores of two patients were not available. VLSM-parameter: minimum lesion overlay 10% (n = 29), level of significance p < 0.01, FDR-corrected for multiple comparisons.
Supplementary material
Acknowledgements
Elisabeth Achilles was supported by the Medical Faculty of the University of Cologne with a Gerok-position for clinical research. Gereon R. Fink gratefully acknowledges support by the Marga and Walter Boll Foundation.
References
- Achilles E.I.S., Fink G.R., Fischer M.H., Dovern A., Held A., Timpert D.C., Schroeter C., Schuetz K., Kloetzsch C., Weiss P.H. Effect of meaning on apraxic finger imitation deficits. Neuropsychologia. 2016;82:74–83. doi: 10.1016/j.neuropsychologia.2015.12.022. [DOI] [PubMed] [Google Scholar]
- Achilles E.I.S., Weiss P.H., Fink G.R., Binder E., Price C.J., Hope T.M.H. Using multi-level Bayesian lesion-symptom mapping to probe the body-part-specificity of gesture imitation skills. NeuroImage. 2017;161:94–103. doi: 10.1016/j.neuroimage.2017.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartolo A., Cubelli R., Della Sala S., Drei S., Marchetti C. Double dissociation between meaningful and meaningless gesture reproduction in apraxia. Cortex. 2001;37:696–699. doi: 10.1016/s0010-9452(08)70617-8. [DOI] [PubMed] [Google Scholar]
- Bates E., Wilson S.M., Saygin A.P., Dick F., Sereno M.I., Knight R.T., Dronkers N.F. Voxel-based lesion-symptom mapping. Nat. Neurosci. 2003;6:448–450. doi: 10.1038/nn1050. [DOI] [PubMed] [Google Scholar]
- Binkofski F., Buxbaum L.J. Two action systems in the human brain. Brain Lang. 2013;127:222–229. doi: 10.1016/j.bandl.2012.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binkofski F., Dohle C., Posse S., Stephan K.M., Hefter H., Seitz R.J., Freund H.J. Human anterior intraparietal area subserves prehension: a combined lesion and functional MRI activation study. Neurology. 1998;50:1253–1259. doi: 10.1212/wnl.50.5.1253. [DOI] [PubMed] [Google Scholar]
- Buxbaum L.J., Kalénine S. Action knowledge, visuomotor activation, and embodiment in the two action systems. Ann. N. Y. Acad. Sci. 2010;1191:201–218. doi: 10.1111/j.1749-6632.2010.05447.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buxbaum L.J., Shapiro A.D., Coslett H.B. Critical brain regions for tool-related and imitative actions: a componential analysis. Brain. 2014 doi: 10.1093/brain/awu111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cubelli R., Marchetti C., Boscolo G., Della Sala S. Cognition in action: testing a model of limb apraxia. Brain Cogn. 2000;44:144–165. doi: 10.1006/brcg.2000.1226. [DOI] [PubMed] [Google Scholar]
- de Haan B., Karnath H.-O. A hitchhiker's guide to lesion-behaviour mapping. Neuropsychologia. 2018;115:5–16. doi: 10.1016/j.neuropsychologia.2017.10.021. [DOI] [PubMed] [Google Scholar]
- de Haan B., Clas P., Juenger H., Wilke M., Karnath H.-O. Fast semi-automated lesion demarcation in stroke. NeuroImage Clin. 2015;9:69–74. doi: 10.1016/j.nicl.2015.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmurget M., Epstein C.M., Turner R.S., Prablanc C., Alexander G.E., Grafton S.T. Role of the posterior parietal cortex in updating reaching movements to a visual target. Nat. Neurosci. 1999;2:563–567. doi: 10.1038/9219. [DOI] [PubMed] [Google Scholar]
- Donkervoort M., Dekker J., van den Ende E., Stehmann-Saris J.C., Deelman B.G. Prevalence of apraxia among patients with a first left hemisphere stroke in rehabilitation centres and nursing homes. Clin. Rehabil. 2000;14:130–136. doi: 10.1191/026921500668935800. [DOI] [PubMed] [Google Scholar]
- Dovern A., Fink G.R., Weiss P.H. How to diagnose and treat limb apraxia. Fortschr. Neurol. Psychiatr. 2011;79:345–357. doi: 10.1055/s-0029-1246097. [DOI] [PubMed] [Google Scholar]
- Dovern A., Fink G.R., Weiss P.H. Diagnosis and treatment of upper limb apraxia. J. Neurol. 2012 doi: 10.1007/s00415-011-6336-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dressing A., Nitschke K., Kümmerer D., Bormann T., Beume L., Schmidt C.S.M., Ludwig V.M., Mader I., Willmes K., Rijntjes M., Kaller C.P., Weiller C., Martin M. Distinct contributions of dorsal and ventral streams to imitation of tool-use and communicative gestures. Cereb. Cortex. 2018;28:474–492. doi: 10.1093/cercor/bhw383. [DOI] [PubMed] [Google Scholar]
- Faria A.V., Joel S.E., Zhang Y., Oishi K., van Zjil P.C.M., Miller M.I., Pekar J.J., Mori S. Atlas-based analysis of resting-state functional connectivity: evaluation for reproducibility and multi-modal anatomy–function correlation studies. NeuroImage. 2012;61:613–621. doi: 10.1016/j.neuroimage.2012.03.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridriksson J., den Ouden D.-B., Hillis A.E., Hickok G., Rorden C., Basilakos A., Yourganov G., Bonilha L. Anatomy of aphasia revisited. Brain J. Neurol. 2018 doi: 10.1093/brain/awx363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gajardo-Vidal A., Lorca-Puls D.L., Crinion J.T., White J., Seghier M.L., Leff A.P., Hope T.M.H., Ludersdorfer P., Green D.W., Bowman H., Price C.J. How distributed processing produces false negatives in voxel-based lesion-deficit analyses. Neuropsychologia. 2018;115:124–133. doi: 10.1016/j.neuropsychologia.2018.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldenberg G. Defective imitation of gestures in patients with damage in the left or right hemispheres. J. Neurol. Neurosurg. Psychiatry. 1996;61:176–180. doi: 10.1136/jnnp.61.2.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldenberg G. Matching and imitation of hand and finger postures in patients with damage in the left or right hemispheres. Neuropsychologia. 1999;37:559–566. doi: 10.1016/s0028-3932(98)00111-0. [DOI] [PubMed] [Google Scholar]
- Goldenberg G. Apraxia and the parietal lobes. Neuropsychologia. 2009;47:1449–1459. doi: 10.1016/j.neuropsychologia.2008.07.014. [DOI] [PubMed] [Google Scholar]
- Goldenberg G., Hagmann S. The meaning of meaningless gestures: a study of visuo-imitative apraxia. Neuropsychologia. 1997;35:333–341. doi: 10.1016/s0028-3932(96)00085-1. [DOI] [PubMed] [Google Scholar]
- Goldenberg G., Randerath J. Shared neural substrates of apraxia and aphasia. Neuropsychologia. 2015;75:40–49. doi: 10.1016/j.neuropsychologia.2015.05.017. [DOI] [PubMed] [Google Scholar]
- Goldenberg G., Strauss S. Hemisphere asymmetries for imitation of novel gestures. Neurology. 2002;59:893–897. doi: 10.1212/wnl.59.6.893. [DOI] [PubMed] [Google Scholar]
- Goldenberg G., Münsinger U., Karnath H.-O. Severity of neglect predicts accuracy of imitation in patients with right hemisphere lesions. Neuropsychologia. 2009;47:2948–2952. doi: 10.1016/j.neuropsychologia.2009.06.024. [DOI] [PubMed] [Google Scholar]
- Goodale M.A., Milner A.D. Separate visual pathways for perception and action. Trends Neurosci. 1992;15:20–25. doi: 10.1016/0166-2236(92)90344-8. [DOI] [PubMed] [Google Scholar]
- Grefkes C., Fink G.R. The functional organization of the intraparietal sulcus in humans and monkeys. J. Anat. 2005;207:3–17. doi: 10.1111/j.1469-7580.2005.00426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann K., Goldenberg G., Daumüller M., Hermsdörfer J. It takes the whole brain to make a cup of coffee: the neuropsychology of naturalistic actions involving technical devices. Neuropsychologia. 2005;43:625–637. doi: 10.1016/j.neuropsychologia.2004.07.015. [DOI] [PubMed] [Google Scholar]
- Hermsdörfer J., Goldenberg G., Wachsmuth C., Conrad B., Ceballos-Baumann A.O., Bartenstein P., Schwaiger M., Boecker H. Cortical correlates of gesture processing: clues to the cerebral mechanisms underlying apraxia during the imitation of meaningless gestures. NeuroImage. 2001;14:149–161. doi: 10.1006/nimg.2001.0796. [DOI] [PubMed] [Google Scholar]
- Hoeren M., Kummerer D., Bormann T., Beume L., Ludwig V.M., Vry M.-S., Mader I., Rijntjes M., Kaller C.P., Weiller C. Neural bases of imitation and pantomime in acute stroke patients: distinct streams for praxis. Brain. 2014 doi: 10.1093/brain/awu203. [DOI] [PubMed] [Google Scholar]
- Kalénine S., Buxbaum L.J., Coslett H.B. Critical brain regions for action recognition: lesion symptom mapping in left hemisphere stroke. Brain J. Neurol. 2010;133:3269–3280. doi: 10.1093/brain/awq210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleineberg N.N., Dovern A., Binder E., Grefkes C., Eickhoff S.B., Fink G.R., Weiss P.H. Action and semantic tool knowledge - effective connectivity in the underlying neural networks. Hum. Brain Mapp. 2018 doi: 10.1002/hbm.24188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahon B.Z., Milleville S.C., Negri G.A.L., Rumiati R.I., Caramazza A., Martin A. Action-related properties shape object representations in the ventral stream. Neuron. 2007;55:507–520. doi: 10.1016/j.neuron.2007.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin M., Dressing A., Bormann T., Schmidt C.S.M., Kümmerer D., Beume L., Saur D., Mader I., Rijntjes M., Kaller C.P., Weiller C. Componential network for the recognition of tool-associated actions: evidence from voxel-based lesion-symptom mapping in acute stroke patients. Cereb. Cortex N. Y. N. 2016:1991. doi: 10.1093/cercor/bhw226. [DOI] [PubMed] [Google Scholar]
- Mehler M.F. Visuo-imitative apraxia. Neurology. 1987;37:129. [Google Scholar]
- Mengotti P., Corradi-Dell'acqua C., Negri G.A.L., Ukmar M., Pesavento V., Rumiati R.I. Selective imitation impairments differentially interact with language processing. Brain J. Neurol. 2013;136:2602–2618. doi: 10.1093/brain/awt194. [DOI] [PubMed] [Google Scholar]
- Mirman D., Landrigan J.-F., Kokolis S., Verillo S., Ferrara C., Pustina D. Corrections for multiple comparisons in voxel-based lesion-symptom mapping. Neuropsychologia. 2018;115:112–123. doi: 10.1016/j.neuropsychologia.2017.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishkin M., Ungerleider L.G. Contribution of striate inputs to the visuospatial functions of parieto-preoccipital cortex in monkeys. Behav. Brain Res. 1982;6:57–77. doi: 10.1016/0166-4328(82)90081-x. [DOI] [PubMed] [Google Scholar]
- Ochipa C., Rothi L.J., Heilman K.M. Conduction apraxia. J. Neurol. Neurosurg. Psychiatry. 1994;57:1241–1244. doi: 10.1136/jnnp.57.10.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield R.C. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Peigneux P., Salmon E., van der Linden M., Garraux G., Aerts J., Delfiore G., Degueldre C., Luxen A., Orban G., Franck G. The role of lateral occipitotemporal junction and area MT/V5 in the visual analysis of upper-limb postures. NeuroImage. 2000;11:644–655. doi: 10.1006/nimg.2000.0578. [DOI] [PubMed] [Google Scholar]
- Pisella L., Gréa H., Tilikete C., Vighetto A., Desmurget M., Rode G., Boisson D., Rossetti Y. An “automatic pilot” for the hand in human posterior parietal cortex: toward reinterpreting optic ataxia. Nat. Neurosci. 2000;3:729–736. doi: 10.1038/76694. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G., Matelli M. Two different streams form the dorsal visual system: anatomy and functions. Exp. Brain Res. 2003;153:146–157. doi: 10.1007/s00221-003-1588-0. [DOI] [PubMed] [Google Scholar]
- Rothi L.J.G., Ochipa C., Heilman K.M. A cognitive neuropsychological model of limb praxis. Cogn. Neuropsychol. 1991;8:443–458. [Google Scholar]
- Rumiati R.I., Tessari A. Imitation of novel and well-known actions. Exp. Brain Res. 2002;142:425–433. doi: 10.1007/s00221-001-0956-x. [DOI] [PubMed] [Google Scholar]
- Rumiati R.I., Weiss P.H., Tessari A., Assmus A., Zilles K., Herzog H., Fink G.R. Common and differential neural mechanisms supporting imitation of meaningful and meaningless actions. J. Cogn. Neurosci. 2005;17:1420–1431. doi: 10.1162/0898929054985374. [DOI] [PubMed] [Google Scholar]
- Sakreida K., Effnert I., Thill S., Menz M.M., Jirak D., Eickhoff C.R., Ziemke T., Eickhoff S.B., Borghi A.M., Binkofski F. Affordance processing in segregated parieto-frontal dorsal stream sub-pathways. Neurosci. Biobehav. Rev. 2016;69:89–112. doi: 10.1016/j.neubiorev.2016.07.032. [DOI] [PubMed] [Google Scholar]
- Shahid H., Sebastian R., Schnur T.T., Hanayik T., Wright A., Tippett D.C., Fridriksson J., Rorden C., Hillis A.E. Important considerations in lesion-symptom mapping: illustrations from studies of word comprehension. Hum. Brain Mapp. 2017;38:2990–3000. doi: 10.1002/hbm.23567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperber C., Karnath H.-O. On the validity of lesion-behaviour mapping methods. Neuropsychologia. 2018;115:17–24. doi: 10.1016/j.neuropsychologia.2017.07.035. [DOI] [PubMed] [Google Scholar]
- Tessari A., Cubelli R. Route selection in action imitation: a matter of strategic choice? Cortex. 2014;57:277–278. doi: 10.1016/j.cortex.2014.01.014. [DOI] [PubMed] [Google Scholar]
- Tessari A., Rumiati R.I. The strategic control of multiple routes in imitation of actions. J. Exp. Psychol. 2004;30:1107–1116. doi: 10.1037/0096-1523.30.6.1107. [DOI] [PubMed] [Google Scholar]
- Tessari A., Canessa N., Ukmar M., Rumiati R.I. Neuropsychological evidence for a strategic control of multiple routes in imitation. Brain. 2007;130:1111–1126. doi: 10.1093/brain/awm003. [DOI] [PubMed] [Google Scholar]
- Weiss P.H., Ubben S.D., Kaesberg S., Kalbe E., Kessler J., Liebig T., Fink G.R. Where language meets meaningful action: a combined behavior and lesion analysis of aphasia and apraxia. Brain Struct. Funct. 2016:563–576. doi: 10.1007/s00429-014-0925-3. [DOI] [PubMed] [Google Scholar]
- Wilke M., de Haan B., Juenger H., Karnath H.-O. Manual, semi-automated, and automated delineation of chronic brain lesions: a comparison of methods. NeuroImage. 2011;56:2038–2046. doi: 10.1016/j.neuroimage.2011.04.014. [DOI] [PubMed] [Google Scholar]
- Yourganov G., Smith K.G., Fridriksson J., Rorden C. Predicting aphasia type from BRAIN damage measured with structural MRI. Cortex J. Devoted Study Nerv. Syst. Behav. 2015;73:203–215. doi: 10.1016/j.cortex.2015.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material