Abstract
In addition to several well-established breast cancer (BC) susceptibility genes, the contribution of other candidate genes to BC risk remains mostly undefined. BARD1 is a potentially predisposing BC gene, however, the rarity of its mutations and an insufficient family/study size have hampered corroboration and estimation of the associated cancer risks. To clarify the role of BARD1 mutations in BC predisposition, a comprehensive case-control association study of a recurring nonsense mutation c.1690C>T (p.Q564X) was performed, comprising ~14,000 unselected BC patients and ~5900 controls from Polish and Belarusian populations. For comparisons, two BARD1 variants of unknown significance were also genotyped. We detected the highest number of BARD1 variants in BC cases in any individual BARD1-specific study, including 38 p.Q564X mutations. The p.Q564X was associated with a moderately increased risk of BC (OR = 2.30, p = 0.04). The estimated risk was even higher for triple-negative BC and bilateral BC. As expected, the two tested variants of unknown significance did not show significant associations with BC risk. Our study provides substantial evidence for the association of a deleterious BARD1 mutation with BC as a low/moderate risk allele. The p.Q564X was shown to be a Central European recurrent mutation with potential relevance for future genetic testing.
Keywords: breast cancer, BARD1, genotyping, p.Q564X, p.R658C, p.R659R, breast cancer risk
1. Introduction
Breast cancer (BC) is the most common cancer diagnosed in women worldwide, with over 1.5 million new cases diagnosed each year [1]. Most BCs occur sporadically, potentially as a result of interactions between multiple environmental, lifestyle, hormonal and genetic factors. However, approximately 5–10% of BCs present with a familial aggregation due to hereditary mutations in highly penetrant genes. Familial BC frequently co-occurs and shares some genetic background with epithelial ovarian cancer (OC). Other indicators of hereditary BC are younger age of onset, bilateral disease, and triple-negative BC (TNBC). Mutations in high-risk genes, including BRCA1 [2] and BRCA2 [3], as well as in a moderate-risk PALB2 gene [4], are responsible for approximately half of familial BC cases. Mutations in CDH1 [5], PTEN [6], and TP53 [7], which are associated with hereditary diffuse cancer, Cowden disease, and Li-Fraumeni syndrome, respectively, also contribute to high-risk predisposition of BC but are very rare. Low-penetrance genes, common polymorphisms [8,9], copy number variants [10,11,12], and epigenetic alterations [13,14] on familial BC risk are also suggested as constitutive risk factors but do not explain the missing fraction of familial heritability.
Genes encoding proteins that interact with BRCA1 and BRCA2 in different DNA damage response and tumor suppressor processes are among the candidate BC and/or OC susceptibility genes. One such gene that has been intensively studied is BRCA1-associated RING domain 1 (BARD1) [15], because the BARD1 protein shares both structural and functional similarities with BRCA1 and because the interaction between BARD1 and BRCA1 plays an important role in maintaining the stability and manifestation of the tumor suppressor function of BRCA1 [16].
In humans, the BARD1 gene spans a region of ~80 kb on the long arm of chromosome 2 (2q34-35) and comprises 11 exons. The gene encodes a protein of 777 amino acids that contains one N-terminal RING-finger domain, two C-terminal tandem BRCT domains (homologous to corresponding domains in BRCA1), and three Ankyrin (ANK) repeat domains. BARD1 may act as a tumor suppressor in the BRCA1-dependent pathways, which is associated with specific BRCA1/BARD1 heterodimer formation via N-terminal RING-finger domains. The BRCA1/BARD1 heterodimer demonstrates ubiquitin ligase activity, which functions in DNA damage response pathways, cell cycle regulation, and chromatin structural and hormone signaling modulation (for review see [17,18]). Because BARD1 may interact with other molecules implicated in genome integrity, a BRCA1-independent tumor suppressor functions for BARD1 have also been suggested. Examples include the interaction between BARD1 and p53, which stabilizes p53, facilitates p53 phosphorylation, and induces p53 apoptotic activity in response to DNA damage [19,20].
The hitherto reported BARD1 mutation screening studies of familial and unselected BC and/or OC cases has led to the identification of numerous BARD1 sequence variants [21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54]. Additionally, BARD1 common variants have been associated with an aggressive subset of human neuroblastomas [55,56], lung cancer [57] and colon cancer [58]. BARD1 mutations identified in BC cases include deleterious and potentially deleterious mutations that lead to premature termination of translation, disruption of protein structure/function, or alternative splicing. Some of these mutations have been shown to cosegregate in families with cancer [22,24,33]. Despite these observations, none of the abovementioned studies (including our own [36,37]) have provided strong, statistically supported data for the role of BARD1 mutations in cancer predisposition. This lack of data is due mostly to the following limitations: (i) most of the studies were not performed in a case-control manner (i.e., the control samples were either not screened at all or not analyzed in the same way as the case samples), (ii) a relatively small group of patients were analyzed (usually 100–300), and consequently, a small number of mutations were identified in individual studies, preventing reliable risk estimates, and (iii) in cases of mutations identified in families, the families were too small to perform formal linkage (mutation-cancer co-segregation) analysis. More recently, several studies reporting results for targeted sequencing with cancer-predisposition gene panels, including BARD1, have been published [35,39,40,41,42,43,44,45,46,47,48,49,50,51,52,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73]. The large-scale and cumulative character of some of these studies, including our own meta-analysis [74], allowed us to estimate the BARD1-attributed BC risk. However, in most of these studies, the risk was estimated by comparisons of mutation frequency in BC cases to that in controls from publicly available databases, not well matched in terms of the population studied (geographical region) and/or the methodology used. Such analysis may be affected by population stratification, e.g., due to an unequal distribution of founder mutations, which frequently occur in BC predisposition genes. Due to insufficient evidence of a BARD1 association with BC risk, BARD1 was not included in the recently proposed consensus multigene panel by the UK Cancer Genetics Group for BC testing [75]. Also, although BARD1 was included in the most recent version of “The National Comprehensive Cancer Network (NCCN) Guidelines for Genetic/Familial High-Risk Assessment: Breast and Ovarian” as a gene that may be linked with increased breast cancer risk, still due to insufficient evidence, no management recommendations for women with a pathogenic BARD1 variant was provided at this time.
To overcome the abovementioned limitations, we took advantage of a deleterious nonsense mutation, namely, c.1690C>T (p.Q564X), identified in the Polish population in several independent studies [36,37,76], to provide a reliable estimation of BC risk associated with BARD1 mutations. We performed an association analysis of the mutation in two large cohorts recruited from the POLISH and closely related BELARUSIAN populations, by genotyping ~14,000 BC patients and ~5900 control samples. Additionally, in the POLISH group, we also analyzed two rare BARD1 variants of unknown significance (also recurring in the Polish population), a missense variant c.1972C>T (p.R658C) and a synonymous variant c.1977A>G (p.R659R). The analysis showed that the definitively deleterious BARD1 mutation is associated with low/moderate BC risk (OR ~2) and that the risk is further increased in the group of patients with a risk for heritable BC (OR ~3), including TNBC, bilateral BC, early diagnosis of BC, and familial BC/OC.
2. Results
2.1. Selection of BARD1 Variants
For our genetic association study, we selected three BARD1 sequence variants: (i) a deleterious nonsense mutation p.Q564X, located in exon VIII, and two variants of unknown significance, (ii) missense variant p.R658C, located in exon X, and (iii) synonymous variant p.R659R, located in exon X. Based on preliminary results, we estimated the frequency of occurrence of each variant in the Polish population to be ~0.3%, ~0.5%, and ~0.4%, respectively. The ClinVar status of the p.Q564X mutation is defined as pathogenic, while both the p.R658C and p.R659R variants are listed as benign/likely benign/uncertain significance [77]. At present, according to different databases, the frequency of these variants in the European population ranges from 0.01–0.02%, 1.56–1.78%, and 0.59–1.02%, respectively (Table 1). As shown in Table 1, the variants of unknown significance also occur in non-European populations, while the frequency of the nonsense mutation is limited to European populations.
Table 1.
The frequencies of selected BARD1 variants in European and other populations.
| Population | Database (Population) |
p.Q564X M+/N (%) |
p.R658C M+/N (%) |
p.R659R M+/N (%) |
|---|---|---|---|---|
| European | EVS (European American) |
0/4300 (−) |
67/4300 (1.558) |
28/4300 (0.651) |
| FLOSSIES (European) |
1/7325 (0.014) |
130/7325 (1.775) |
75/7325 (1.024) |
|
| ExAC (European non-Finnish) |
6/33,368 (0.018) |
535/33,352 (1.604) |
196/33,357 (0.588) |
|
| gnomAD (European non-Finnish) |
7/64,526 (0.011) |
1078/64,546 (1.670) |
397/64,564 (0.615) |
|
| Other | gnomAD (All populations) |
7/141,343 (0.005) |
2302/141,376 (1.628) |
575/141,400 (0.407) |
| gnomAD (European Finnish) |
0/12,543 (−) |
316/12,557 (2.516) |
33/12,559 (0.263) |
|
| gnomAD (Ashkenazi Jewish) |
0/5184 (−) |
0/5183 (−) |
81/5183 (1.563) |
|
| gnomAD (East Asian) |
0/9977 (−) |
224/9977 (2.245) |
0/9977 (−) |
|
| gnomAD (South Asian) |
0/15,307 (−) |
124/15,306 (0.810) |
1/15,306 (0.007) |
|
| gnomAD (African) |
0/12,481 (−) |
59/12,481 (0.473) |
9/12,484 (0.072) |
|
| gnomAD (Latino) |
0/17,719 (−) |
444/17,718 (2.506) |
30/17,719 (0.169) |
|
| gnomAD (Other) |
0/3606 (−) |
57/3608 (1.580) |
24/3608 (0.665) |
EVS, Exome Variant Server; ExAC, Exome Aggregation Consortium; gnomAD, The Genome Aggregation Database; M+, number of subjects with a particular variant; N, number of all analyzed subjects.
2.2. The Frequency of BARD1 Variants and BC Risk Estimates
All selected variants were screened in 12,476 BC cases and 4707 controls from the Polish population (POLISH group). Additionally, the p.Q564X mutation was screened in 1459 BC cases and 1189 controls from the Belarusian population (BELARUSIAN group). The size of the study predicted almost 100% and ~80% power to detect the effect of the mutation at the level of an OR of 3 and 2, respectively. The p.Q564X mutation was identified in 34 cases (0.27%) and seven controls (0.15%) in the POLISH group (OR = 1.83, 95%CI: 0.81–4.14, p = 0.14) and in four cases (0.27%) and 0 controls (0%) in the BELARUSIAN group.
A cumulative OR equals 2.30 (95%CI: 1.03–5.15, p = 0.04), which classifies BARD1 as a low/moderate BC susceptibility gene (Table 2). The stratification of BC patients into clinically defined BC subtypes showed an even higher risk for TNBC (16.1% of BC patients; OR = 3.62, 95%CI: 1.21–10.78, p = 0.02) and bilateral BC (4.4% of BC patients; OR = 5.10, 95%CI: 1.31–19.78, p = 0.02). The cumulative OR for the group of patients with increased risk of hereditary BC, including TNBC, bilateral BC, early diagnosis, and/or familial BC/OC, was 2.94 (95%CI: 1.21–7.14, p = 0.02) (Table 3). The indicators of hereditary BC, namely, younger age at diagnosis (≤40 years) and positive family history for BC and/or OC, were also associated with increased BC risk, though individually, these associations were not statistically significant due to the low number of cases. As shown in Table 2; Table 3, adjustment for the studied cohorts did not substantially change the statistical estimates. The frequencies of the missense variant p.R658C and the synonymous p.R659R variant did not differ significantly between the analyzed the BC case and control groups (80/12476, 0.64% vs. 26/4707, 0.55%, and 49/12476, 0.39% vs. 14/4707, 0.30%, respectively), suggesting a lack of their association with BC (OR = 1.16 and OR = 1.32, respectively).
Table 2.
The frequencies and effect sizes of the three tested BARD1 variants.
| Study Population | Group | p.Q564X | p.R658C | p.R659R | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M + /N (%) | OR | 95%CI | p-Value | M+/N (%) | OR | 95%CI | p-Value | M+/N (%) | OR | 95%CI | p-Value | ||
| Controls | P | 7/4707 (0.15) | - | - | - | 26/4707 (0.55) | - | - | - | 14/4707 (0.30) | - | - | - |
| B | 0/1189 (0.00) | - | - | - | - | - | - | - | - | - | - | - | |
| P + B | 7/5896 (0.12) | - | - | - | - | - | - | - | - | - | - | - | |
| All BC patients | P | 34/12,476 (0.27) | 1.83 | 0.81–4.14 | 0.14 | 80/12,476 (0.64) | 1.16 | 0.75–1.81 | 0.51 | 49/12,476 (0.39) | 1.32 | 0.73–2.40 | 0.36 |
| B | 4/1459 (0.27) | n.a. | n.a. | n.a. | - | - | - | - | - | - | - | - | |
| P + B | 38/13,935 (0.27) | 2.30 | 1.03–5.15 | 0.04 | - | - | - | - | - | - | - | - | |
| 2.24 * | 0.99–5.03 * | 0.05 * | - | - | - | - | - | - | |||||
| 2.12 ** | 0.97–4.62 ** | 0.06 ** | - | - | - | - | - | - | |||||
Table 3.
Prevalence of BARD1 variants and the associated BC risk in the groups of patients stratified by BC subtypes.
| Feature | Group | p.Q564X | p.R658C | p.R659R | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M+/N (%) | OR | 95%CI | p-Value | M+/N (%) | OR | 95%CI | p-Value | M+/N (%) | OR | 95%CI | p-Value | ||
| TNBC | P | 6/1120 (0.54) | 3.62 | 1.21–10.78 | 0.02 | 6/1120 (0.54) | 0.97 | 0.40–2.36 | 0.95 | 2/1120 (0.18) | 0.60 | 0.14–2.64 | 0.50 |
| Bilateral BC | P | 2/447 (0.45) | 3.02 | 0.63–14.57 | 0.17 | 4/447 (0.89) | 1.63 | 0.56–4.68 | 0.37 | 0/447 (0.00) | 0.36 | 0.02–6.07 | 0.48 |
| P + B | 3/498 (0.60) | 5.10 | 1.31–19.78 | 0.02 | - | - | - | - | - | - | - | - | |
| 4.85 * | 1.24–18.93 * | 0.02 * | - | - | - | - | - | - | |||||
| 5.22 ** | 1.50–18.19 ** | 0.01 ** | - | - | - | - | - | - | |||||
| BC diagnosed ≤40 y.o. | P | 4/1286 (0.31) | 2.09 | 0.61–7.17 | 0.24 | 7/1286 (0.54) | 0.99 | 0.43–2.28 | 0.97 | 5/1286 (0.39) | 1.30 | 0.47–3.64 | 0.61 |
| P + B | 5/1640 (0.30) | 2.57 | 0.82–8.12 | 0.11 | - | - | - | - | - | - | - | - | |
| 2.60 * | 0.82–8.21 * | 0.10 * | - | - | - | - | - | - | |||||
| 2.66 ** | 0.88–8.03 ** | 0.08 ** | - | - | - | - | - | - | |||||
| ≥1 BC/OC relatives | P | 5/2191 (0.23) | 1.54 | 0.49–4.84 | 0.46 | 14/2191 (0.64) | 1.16 | 0.60–2.22 | 0.66 | 3/2191 (0.14) | 0.46 | 0.13–1.60 | 0.22 |
| P + B | 6/2620 (0.23) | 1.93 | 0.65–5.75 | 0.24 | - | - | - | - | - | - | - | - | |
| 1.88 * | 0.63–5.60 * | 0.26 * | - | - | - | - | - | - | |||||
| 1.92 ** | 0.67–5.46 ** | 0.22 ** | - | - | - | - | - | - | |||||
| “Hereditary” BC risk patients *** | P | 13/4130 (0.31) | 2.12 | 0.85–5.32 | 0.11 | 30/4130 (0.73) | 1.32 | 0.78–2.23 | 0.31 | 9/4130 (0.22) | 0.73 | 0.32–1.69 | 0.47 |
| P + B | 16/4600 (0.35) | 2.94 | 1.21–7.14 | 0.02 | - | - | - | - | - | - | - | - | |
| 2.93 * | 1.20–7.19 * | 0.02 * | - | - | - | - | - | - | |||||
| 2.77 ** | 1.18–6.49 ** | 0.02 ** | - | - | - | - | - | - | |||||
Table 2 & Table 3: P: POLISH group; B: BELARUSIAN group; P + B: POLISH and BELARUSIAN GROUP; M+: number of patients with a particular variants; N: number of all genotyped patients; y.o.: years old; *: adjusted for the origin of the study; **: a Mantel-Haenszel method under the fixed effects model (please note that this analysis may be biased due to no mutations in the BELARUSIAN control group); ***: TNBC# and/or bilateral BC and/or BC diagnosed ≤40 y.o. and/or ≥1 BC/OC relative; #: TNBC status was not determined in the BELARUSIAN group.
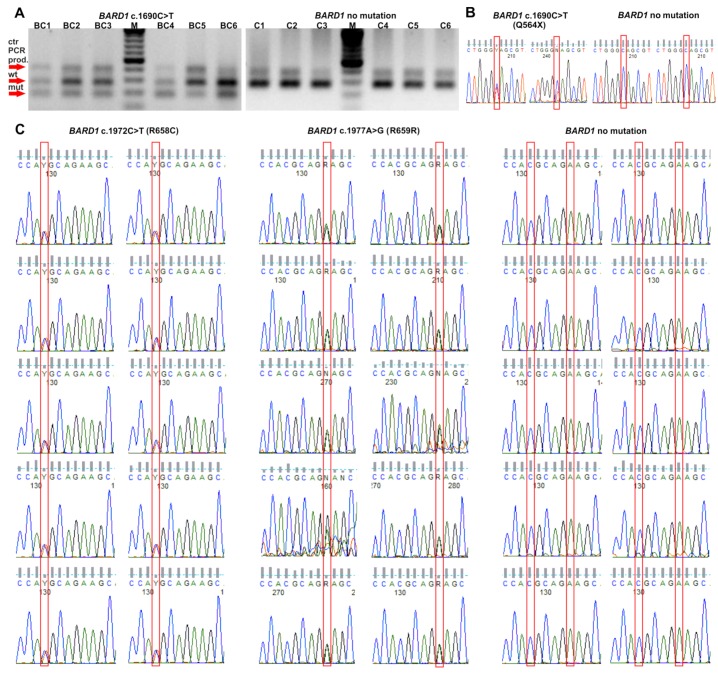
None of the analyzed subjects was a carrier of more than one of the tested variants. Validation of the genotyping results performed with alternative techniques in the limited number of positive and negative subjects confirmed all detected variants and proved 100% sensitivity and specificity of the genotyping results (see Section 4 and Figure 1).
Figure 1.
Validation of BARD1 variants. (A) Validation of p.Q564X BARD1 mutation in randomly selected carriers and noncarriers with the tetra-primer ARMS-PCR assay [BC: breast cancer patients; C: controls; M: marker, GeneRuler 100 bp Plus DNA Ladder (Thermo Fisher Scientific, Waltham, MA, USA)] and (B) with Sanger sequencing. (C) Validation of p.R658C and p.R659R BARD1 variants in randomly selected carriers and noncarriers with Sanger sequencing. See the Materials and Methods section for details.
2.3. Predictive Value of BARD1 Variants
In the next step, we compared the frequencies of selected variants in the different BC subgroups. Carriers and noncarriers of the p.Q564X mutation have a similar average age at diagnosis (54.8 and 54.1 years, respectively). As shown in Table S1, there was only a small nonsignificant increase in the mutation frequency in patients with vs. without a family history of BC and/or OC (0.23% vs. 0.30%, respectively). In the assessment of the molecular BC subtype, a higher incidence of the mutation was found in the patients with a progesterone receptor-negative (PR−) BC (0.55% vs. 0.24%, p = 0.03), estrogen receptor-negative (ER−) BC (0.43% vs. 0.29%) and TNBC (0.54% vs. 0.33%) than in the corresponding patients with a receptor-positive BC. However, due to the low number of cases in the subgroups, only the difference between the PR− and PR+ BC patients was significant. There was also no difference in p.Q564X mutation prevalence between the human epidermal growth factor receptor 2-positive (HER2+) and HER2-negative (HER−) BC patients.
There were no evident changes in the p.R658C and p.R659R prevalences after stratifying BC patients in terms of different clinical features (Table S2). The exceptions were a higher frequency of the p.R659R variant in patients with PR+ than in patients with PR- BC (0.44% vs. 0.17%, 0.07) and a higher frequency of p.R659R in patients with tubulolobular BC than in patients with other histological types of BC (1.72% vs. 0.30%, p = 0.05), however, these results were only borderline significant and may be accidental due to the small number of patients in each group. We also noticed a higher incidence of p.R658C variants among deceased patients than in those who were still alive (1.02% vs. 0.57%, p = 0.03).
Besides the presented data on population frequency and case-control study, we analyzed functional significance of the selected variants with the use of a wide panel of bioinformatics tools evaluating a potential effect of the particular variants on evolutionary conservation of an altered amino acid, RNA structure, or structure/function of a protein (Table S3).
3. Discussion
Since the discovery of BARD1 about 20 years ago [15], many studies have been carried out to try to understand its role in cancer. Because BARD1 functions in the same molecular pathways as BRCA1, BARD1 is considered a good candidate as a BC susceptibility gene.
The identification of recurrent BARD1 variants in the Polish population [36,37,76] motivated us to thoroughly examine the association between BARD1 and BC, as a founder-mutation-based study is likely to be successful in the search for and confirmation of infrequently mutated BC susceptibility genes. Similar approaches have been applied previously to investigate the contributions of recurrent variants to conferring cancer risk. The examples include the genotyping of c.5932G>T (p.E1978X) in ATM [78], c.1667_1667+3delAGTA in RECQL [79], c.509_510delGA [80,81], and c.172_175delTTGT in PALB2 [81], for which the studies revealed associations with an elevated BC risk, and c.576+1G>A in RAD51D [82], which was associated with OC risk. To our knowledge, this is the first study of BC risk attributed to individual BARD1 variants. It is important as the type and location of a particular mutation may alter the cancer risk estimate, as was previously shown for BRCA1/2 mutations [83,84,85,86,87].
Considering the interaction between BARD1 and BRCA1 and the similarities in their structures, it is surprising that BARD1 mutations are relatively rare in BC patients compared to BRCA1 mutations. In spite of this, some of the studies for identified BARD1 mutations suggested a weak association with BC, although individual studies have been largely unsuccessful to provide convincing and statistically supported proof of this association. This may due to an insufficient sample/study size and a lack of a geographically matched population controls, complicating the interpretation of the results. For rare mutations of lower penetrance, which have a “low/moderate” effect on phenotype, it is especially difficult to demonstrate their causative nature and thereby confirm their contributions to BC, therefore, a very large case-control analysis should be performed. As whole exome or even whole gene analysis can be expensive for a sufficient number of cases and controls, the analysis of founder mutations may be the more cost-efficient solution.
In this large case-control study, we provided strong evidence that BARD1 is a low/moderate cancer susceptibility gene. The large collection of BC cases and controls allowed us to uncover an approximately two-fold increase in BC risk (OR = 2.3, p = 0.04) associated with the presence of the p.Q564X mutation. The analysis also showed that p.Q564X is recurrent, potentially a founder mutation, at least in Polish and Belarusian populations. It has to be noted, however, that further haplotype analyses are needed to prove the founder effect of the Q564X mutation. The p.Q564X frequency determined here was 25× and 14× higher in the Polish BC patients and controls, respectively, than in the gnomAD Non-Finnish European population [88] (0.27% and 0.15% vs. 0.011%, respectively) (see Table 1 and Table 2). The mutation seems to be less frequent (was not detectable) in other control populations. Additionally, the recent conference report of the German Consortium for Hereditary Breast and Ovarian Cancer revealed the p.Q564X mutation in 6 out of 14 BARD1 mutation carriers in Germany [89]. This suggests that p.Q564X may have been spread in the wider Central European population.
The cumulative loss-of-function mutation frequency of BARD1 was reported to range between 0.12% and 0.49% in other studies analyzing at least 1000 BC patients with multigene panels (screening the whole coding sequence of BARD1) [42,43,45,51,59,61,74]. These results emphasize the frequent occurrence of a single p.Q564X mutation (0.27%) in the tested populations. Although other estimates of BC risk associated with BARD1 mutations were either statistically underpowered or used imperfectly matched controls, they are largely in line with the risk estimates of this study. Thompson et al. suggested that BARD1 mutations were associated with moderate BC risk (OR = 3.0, p = 0.62) by comparing 2000 BRCA1/2-negative BC patients with geographically matched controls, though not reaching statistical significance [45]. Similar results were obtained in cumulative large-scale analyses performed by Couch et al. (OR = 2.2, p = 0.002) [59] and Slavin et al. (OR = 3.2, p = 0.012) [43], as well as in our own recently published meta-analysis (OR = 2.3, p < 0.0001) [74].
Some evidence for a higher effect size of p.Q564X in BC (OR = 2.9, p = 0.02) was obtained for BC patients characterized by features associated with hereditary BC, including TNBC, bilateral BC, early diagnosis (under 40 years old) and a positive BC/OC family history. More specifically, mutation carriers have a higher risk (OR = 3.6, p = 0.02) of TNBC, a tumor phenotype associated with a hereditary disease cause, a more aggressive disease course, an increased recurrence risk and a poor five-year survival rate than patients with other BC subtypes. Our results are in line with the previous study by Buys et al. [61], which demonstrated that BARD1 mutation prevalence was higher among women with TNBC (3.3%) than among women with other BC subtypes (1.7%). Also, Shimelis et al. [90] reported that pathogenic variants in BARD1 were enriched by more than threefold in TNBC patients (0.67%) compared to non-TNBC patients (0.18%), suggesting that BARD1 is a predominantly TNBC predisposition gene. An even higher effect sizes for the association of BARD1 mutations with TNBC were reported in Shimelis et al. [90] (OR = 5.92) and Castera et al. [91] (OR = 11.27), although the estimates by the latter study were based on a low number of mutation carriers.
Our study also indicated a likely contribution of BARD1 mutations to bilateral BC (OR = 4.85, p = 0.02), which is another indicator of hereditary BC, in addition to TNBC. This tendency was also noticed in a study by Castera et al. [91] (OR = 6.92), however, the result did not reach statistical significance. The results of our study add to previous evidence that the contributions of particular genes to the development of specific subtypes of BC may differ. The differences may not be noticeable in an overall analysis, therefore, stratification BC tumor type (e.g., bilateral BC, TNBC) should be performed to reliably estimate BC risk.
Although the prevalence of the synonymous and missense variants analyzed in our study was slightly increased in the BC group, there was no significant association, which supports their benign/likely benign/uncertain significance status in the ClinVar database. We tested these variants in addition to p.Q564X as they had been suggested to have some functional or pathological effects. For example, p.R659R was shown to impair several exonic splicing enhancer motifs in exon 10, resulting in a transcript lacking exons 2–9 (r.[=,159_1903del], p.Cys53_Trp635delinsfsX12) [36]. However, aberrant splicing occurs only in a fraction of transcripts. Also, the p.R658C variant, which affects conserved amino acids, was detected before in BC patients [33,37,76], and a partial co-segregation of the variant with BC/OC in two small families was shown [33]. Additionally, the p.R658C variant, has been shown to correlate with a risk of lung cancer (OR = 1.55) [57]. From our results, we cannot definitively assess the role of p.R659R and p.R658C in BC predisposition, however more than three-fold increases in risk would be safely excluded from the upper confidence limits in our study.
Because the BARD1 contribution to BC risk has been determined by us to be at the borderline of low and moderate levels, it may suggest that BARD1 mutations contribute to a polygenic model [92,93], like for mutations of lower penetrance in other genes, including CHEK2 or ATM.
The number of identified BARD1 mutation carriers is, to our knowledge, the highest reported to date in an individual BARD1 mutation-specific study. Even though, it has to be noted that the risk estimates are only borderline significant, and more research data from large sequencing studies will be useful to validate these findings. However, as discussed above, the results obtained from our case-control study in founder populations should be very helpful to corroborate the role of BARD1 as a breast cancer susceptibility gene.
4. Materials and Methods
4.1. Study Population
The genetic association study for the selected BARD1 variants was performed in two Slavic populations, the POLISH group and the BELARUSIAN group. The POLISH group comprised 12,476 BC cases (unselected for familial history of the disease) and 4707 cancer-free adult female controls from the International Hereditary Cancer Center (IHCC) cohort (Szczecin, Poland). Patients were diagnosed with invasive BC between 1996 and 2012 at one of 18 different hospitals in Poland. Patients diagnosed with ductal or lobular carcinoma in situ were excluded, with the exception of patients diagnosed with ductal carcinoma in situ with microinvasion. The average age of BC diagnosis was 54 years. Information regarding family history was collected at the time of enrollment for 92.7% of IHCC patients, and 18.9% of them had at least one first- or second-degree relative with BC and/or OC. Additionally, data on the tumor histological and molecular type, disease bilaterality, and lymph node status were collected, when possible.
The BELARUSIAN group comprised 1459 BC patients unselected for familial history of the disease and 1189 cancer-free female controls from the Hannover-Minsk Breast Cancer Study (HMBCS). BC patients from the HMBCS were diagnosed during the years of 1998–2008 at the Belarussian Institute for Oncology and Medical Radiology Aleksandrov N.N. in Minsk or at one of the regional oncology centers in Gomel, Mogilev, Grodno, Brest, or Vitebsk, Belarus [87]. The average age of BC diagnosis was 48 years, and 29.4% of BC patients had a family history of BC and/or OC (first- or second-degree relatives). All subjects were Caucasians of European ancestry and ethnic Poles or Belarusians in the POLISH or BELARUSIAN group, respectively. Written informed consent was obtained from all subjects, and the study was approved by the appropriate local ethics committees, namely, the Institutional Review Board of Pomeranian Medical University, Szczecin, Poland; the Ethics Commission of the State Organization ‘Institute for Hereditary Diseases’, Ministry of Health, Republic of Belarus; and the Institutional Review Board at Hannover Medical School, Hannover, Germany. The ethical approval for: (i) the POLISH group has the identifier no. BN-001/33/04 (23 February 2004; Pomeranian Medical University, Szczecin, Poland) and for (ii) the BELARUSIAN group has the identifier no. 6079 (last renewal 9 February 2016; Hannover Medical School, Hannover, Germany). More detailed characteristics of the BC patients and tumors in both the POLISH and BELARUSIAN groups are provided in Table 4.
Table 4.
Clinical characteristics of the BC patients.
| Feature | POLISH Group n = 12,476 | BELARUSIAN Group n = 1459 | ||
|---|---|---|---|---|
| n of Cases in Which Feature Status was Determined | n and (%) of Positive Cases | n of Cases in Which Feature Status was Determined | n and (%) of Positive Cases | |
| Age at diagnosis (years) | ||||
| ≤40 | 12,459 | 1286 (10.3) | 1459 | 354 (24.3) |
| 41–50 | 12,459 | 4394 (35.3) | 1459 | 520 (35.6) |
| 51–60 | 12,459 | 3248 (26.1) | 1459 | 329 (22.5) |
| 61–70 | 12,459 | 2226 (17.9) | 1459 | 176 (12.1) |
| ≥71 | 12,459 | 1305 (10.5) | 1459 | 80 (5.5) |
| Number of relatives with BC | ||||
| 0 | 11,564 | 9593 (83.0) | 1459 | 1214 (83.2) |
| 1 | 11,564 | 1508 (13.0) | 1459 | 236 (16.2) |
| 2 | 11,564 | 344 (3.0) | 1459 | 9 (0.6) |
| ≥3 | 11,564 | 119 (1.0) | 1459 | 0 (0.0) |
| Numbers of relatives with OC | ||||
| 0 | 11,564 | 11,263 (97.4) | 1459 | 1457 (99.9) |
| ≥1 | 11,564 | 301 (2.6) | 1459 | 2 (0.1) |
| Histological type of BC | ||||
| Ductal, grade 3 | 8670 | 1998 (23.0) | 525 | 42 (8.0) |
| Ductal, grade 1–2 | 8670 | 4057 (46.8) | 525 | 179 (34.1) |
| Ductal, grade unknown | 8670 | 664 (7.7) | 525 | 165 (31.4) |
| Medullary | 8670 | 287 (3.3) | 525 | 10 (1.9) |
| Lobular | 8670 | 1243 (14.3) | 525 | 108 (20.6) |
| Tubulolobular | 8670 | 116 (1.3) | 525 | 21 (4.0) |
| DCIS with microinvasion | 8670 | 305 (3.5) | 525 | 0 (0.0) |
| Molecular type of BC | ||||
| Oestrogen receptor-positive | 8572 | 5970 (69.6) | 875 | 676 (77.3) |
| Progesterone receptor-positive | 8270 | 5885 (71.2) | n.d. | n.d. |
| HER2-positive | 7215 | 1270 (17.6) | n.d. | n.d. |
| TNBC | 6937 | 1120 (16.1) | n.d. | n.d. |
| Size (cm) | ||||
| <1 | 7909 | 914 (11.6) | n.d. | n.d. |
| 1–1.9 | 7909 | 3204 (40.5) | n.d. | n.d. |
| 2–4.9 | 7909 | 3462 (43.8) | n.d. | n.d. |
| ≥5 | 7909 | 329 (4.2) | n.d. | n.d. |
| Other features | ||||
| Bilateral BC | 9787 | 447 (4.6) | 1459 | 51 (3.5) |
| Lymph node-positive | 8162 | 3551 (43.5) | n.d. | n.d. |
| Vital status (deceased) | 12,349 | 2066 (16.7) | n.d. | n.d. |
HER2, human epidermal growth factor receptor 2; n.d., not determined.
4.2. Variant Screening
Genotyping of the BARD1 variants in the POLISH group was performed with the TaqMan assay, according to general recommendations, utilizing the LightCycler® Real-Time PCR 480 System (Roche Life Science, Penzberg, Germany). Genotyping of the p.Q564X mutation in the BELARUSIAN group was accomplished using allele-specific SNP-type assays and Dynamic Arrays on a Fluidigm Biomark platform (Fluidigm, South San Francisco, CA, USA). The TaqMan primer and probe sets are shown in Table S4.
4.3. Validation of Genotyping Results
A small group of samples, which were either positive or negative for the presence of a particular variant, from both the BC and control series, were reanalyzed with alternative methods. The p.Q564X mutation identified in POLISH samples was validated with the tetra-primer amplification refractory mutation system PCR (tetra-primer ARMS-PCR) assay, designed and performed according to previously published guidelines [94]. The analysis was performed in 18 randomly selected carriers (13 from the BC group and 5 from the control group) and 20 noncarriers from the control group. The results were independently validated by Sanger sequencing analysis on an ABI Prism 3130 genetic analyzer (Applied Biosystems, Carlsbad, CA, USA) according to the manufacturer’s general recommendations. The BELARUSIAN p.Q564X mutation carriers were validated by Sanger sequencing. The p.R658C and p.R659R variants were reanalyzed in 10 carriers (for each mutation) and 10 noncarriers by Sanger sequencing. The primer sets used for confirmation of selected BARD1 variants are shown in Table S5.
4.4. Statistical Analysis
The association between BARD1 variants and BC risk was assessed using odds ratios (ORs) and 95% confidence intervals (CIs) derived from univariate logistic regression models. When combining the POLISH and BELARUSIAN groups, appropriate OR adjustments were applied. The significance of the differences between the variant carrier and noncarrier groups was determined using Fisher’s exact test and the X2 test, as appropriate for category size. All statistical tests were two-sided, and a p = 0.05 was considered to be the significance threshold. Analyses were performed using MedCalc Statistical Software version 18.11 (MedCalc Software bvba, Ostend, Belgium; http://www.medcalc.org).
5. Conclusions
This is the first study of individual BARD1 variants in the context of BC risk. It shows that deleterious BARD1 mutations contribute to low/moderate BC risk, thus confirming the role of BARD1 in BC predisposition. An even higher effect size of the BARD1 mutations was observed for TNBC and bilateral BC, i.e., subgroups attributed to hereditary BC. Our results also indicate that the BC-predisposing role of variants of unknown significance should be interpreted with caution and that effect of these mutations need to be proven individually before consideration in BC risk evaluations.
Acknowledgments
The authors would like to acknowledge the Genome Aggregation Database (gnomAD) and thank the groups that provided exome and genome variant data to this resource. A full list of contributing groups can be found at http://gnomad.broadinstitute.org/about. The authors would like to thank contributors to the FLOSSIES project: The King Lab at the University of Washington; Color Genomics; and the Women’s Health Initiative. The authors would like to thank the contributors to the NHLBI GO Exome Sequencing Project and its ongoing studies who produced and provided exome variant calls for comparison: The Lung GO Sequencing Project (HL-102923), the WHI Sequencing Project (HL-102924), the Broad GO Sequencing Project (HL-102925), the Seattle GO Sequencing Project (HL-102926) and the Heart GO Sequencing Project (HL-103010).
Supplementary Materials
The following are available online at https://www.mdpi.com/2072-6694/11/6/740/s1, Table S1: Characteristics of BC patients in terms of different clinical features and the status of the p.Q564X BARD1 mutation (carrier vs. noncarrier), Table S2: Characteristics of BC patients in terms of different clinical features and the status of p.R658C and p.R659R BARD1 variants (carriers vs. noncarriers), Table S3: The computational analyses of the BARD1 variants selected for the analysis, Table S4: Primers and probes used for BARD1 variants genotyping with the TaqMan assay, Table S5: Primers used for validation of BARD1 variants.
Author Contributions
M.S. (Malwina Suszynska)—contributed to the study design, designed and performed experiments, including mutations validation and Sanger sequencing, contributed to data management, performed statistical analysis, wrote and edited the manuscript, including tables and Supplementary Materials; W.K.—designed TaqMan assays, performed the mutations genotyping in POLISH samples; D.W.—participated in the mutation genotyping; A.J.—contributed to the collection of samples and clinical characterization; T.H.—contributed to the collection of samples and clinical characterization; J.G.—contributed to the collection of samples and clinical characterization; T.D. (Tadeusz Debniak)—contributed to the collection of samples and clinical characterization; M.S. (Marek Szwiec)—contributed to the collection of samples and clinical characterization; M.R.—contributed reference samples and clinical characterization; K.K.—contributed to conceiving the study and critical revision of the manuscript; S.N.—contributed to conceiving the study and critical revision of the manuscript; N.B.—contributed to the collection of samples, genotyping of BELARUSIAN samples and data management; T.D. (Thilo Dörk)—contributed to the study design, data analysis and critical revision of the manuscript; J.L.—contributed to the study design and critical revision of the manuscript; C.C.—contributed to the study design and critical revision of the manuscript; P.K.—contributed to conceiving and design of the study, obtained funding, coordinated and supervised the study; participated in the manuscript preparation. All authors read and approved the final manuscript.
Funding
This research was funded by the Polish National Science Centre (NCN; 2015/17/B/NZ2/01182, and 2016/22/A/NZ2/00184) and the German Research Foundation (Do 761/10).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Torre L.A., Bray F., Siegel R.L., Ferlay J., Lortet-Tieulent J., Jemal A. Global cancer statistics, 2012. CA Cancer J. Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Miki Y., Swensen J., Shattuck-Eidens D., Futreal P.A., Harshman K., Tavtigian S., Liu Q., Cochran C., Bennett L.M., Ding W., et al. A strong candidate for the breast and ovarian cancer susceptibility gene BRCA1. Science. 1994;266:66–71. doi: 10.1126/science.7545954. [DOI] [PubMed] [Google Scholar]
- 3.Wooster R., Bignell G., Lancaster J., Swift S., Seal S., Mangion J., Collins N., Gregory S., Gumbs C., Micklem G. Identification of the breast cancer susceptibility gene BRCA2. Nature. 1995;378:789–792. doi: 10.1038/378789a0. [DOI] [PubMed] [Google Scholar]
- 4.Rahman N., Seal S., Thompson D., Kelly P., Renwick A., Elliott A., Reid S., Spanova K., Barfoot R., Chagtai T., et al. PALB2, which encodes a BRCA2-interacting protein, is a breast cancer susceptibility gene. Nat. Genet. 2007;39:165–167. doi: 10.1038/ng1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pharoah P.D., Guilford P., Caldas C., International Gastric Cancer Linkage Consortium Incidence of gastric cancer and breast cancer in CDH1 (E-cadherin) mutation carriers from hereditary diffuse gastric cancer families. Gastroenterology. 2001;121:1348–1353. doi: 10.1053/gast.2001.29611. [DOI] [PubMed] [Google Scholar]
- 6.Tan M.H., Mester J.L., Ngeow J., Rybicki L.A., Orloff M.S., Eng C. Lifetime cancer risks in individuals with germline PTEN mutations. Clin. Cancer Res. 2012;18:400–407. doi: 10.1158/1078-0432.CCR-11-2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mai P.L., Best A.F., Peters J.A., DeCastro R.M., Khincha P.P., Loud J.T., Bremer R.C., Rosenberg P.S., Savage S.A. Risks of first and subsequent cancers among TP53 mutation carriers in the National Cancer Institute Li-Fraumeni syndrome cohort. Cancer. 2016;122:3673–3681. doi: 10.1002/cncr.30248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johnson N., Fletcher O., Palles C., Rudd M., Webb E., Sellick G., dos Santos Silva I., McCormack V., Gibson L., Fraser A., et al. Counting potentially functional variants in BRCA1, BRCA2 and ATM predicts breast cancer susceptibility. Hum. Mol. Genet. 2007;16:1051–1057. doi: 10.1093/hmg/ddm050. [DOI] [PubMed] [Google Scholar]
- 9.Michailidou K., Hall P., Gonzalez-Neira A., Ghoussaini M., Dennis J., Milne R.L., Schmidt M.K., Chang-Claude J., Bojesen S.E., Bolla M.K., et al. Large-scale genotyping identifies 41 new loci associated with breast cancer risk. Nat. Genet. 2013;45:353–361. doi: 10.1038/ng.2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kumaran M., Krishnan P., Cass C.E., Hubaux R., Lam W., Yasui Y., Damaraju S. Breast cancer associated germline structural variants harboring small noncoding RNAs impact post-transcriptional gene regulation. Sci. Rep. 2018;8:7529. doi: 10.1038/s41598-018-25801-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krepischi A.C., Achatz M.I., Santos E.M., Costa S.S., Lisboa B.C., Brentani H., Santos T.M., Goncalves A., Nobrega A.F., Pearson P.L., et al. Germline DNA copy number variation in familial and early-onset breast cancer. Breast Cancer Res. 2012;14:R24. doi: 10.1186/bcr3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wellcome Trust Case Control Consortium. Craddock N., Hurles M.E., Cardin N., Pearson R.D., Plagnol V., Robson S., Vukcevic D., Barnes C., Conrad D.F., et al. Genome-wide association study of CNVs in 16,000 cases of eight common diseases and 3000 shared controls. Nature. 2010;464:713–720. doi: 10.1038/nature08979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Joo J.E., Dowty J.G., Milne R.L., Wong E.M., Dugue P.A., English D., Hopper J.L., Goldgar D.E., Giles G.G., Southey M.C., et al. Heritable DNA methylation marks associated with susceptibility to breast cancer. Nat. Commun. 2018;9:867. doi: 10.1038/s41467-018-03058-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Severi G., Southey M.C., English D.R., Jung C.H., Lonie A., McLean C., Tsimiklis H., Hopper J.L., Giles G.G., Baglietto L. Epigenome-wide methylation in DNA from peripheral blood as a marker of risk for breast cancer. Breast Cancer Res. Treat. 2014;148:665–673. doi: 10.1007/s10549-014-3209-y. [DOI] [PubMed] [Google Scholar]
- 15.Wu L.C., Wang Z.W., Tsan J.T., Spillman M.A., Phung A., Xu X.L., Yang M.C., Hwang L.Y., Bowcock A.M., Baer R. Identification of a RING protein that can interact in vivo with the BRCA1 gene product. Nat. Genet. 1996;14:430–440. doi: 10.1038/ng1296-430. [DOI] [PubMed] [Google Scholar]
- 16.Hashizume R., Fukuda M., Maeda I., Nishikawa H., Oyake D., Yabuki Y., Ogata H., Ohta T. The RING heterodimer BRCA1-BARD1 is a ubiquitin ligase inactivated by a breast cancer-derived mutation. J. Biol. Chem. 2001;276:14537–14540. doi: 10.1074/jbc.C000881200. [DOI] [PubMed] [Google Scholar]
- 17.Irminger-Finger I., Ratajska M., Pilyugin M. New concepts on BARD1: Regulator of BRCA pathways and beyond. Int. J. Biochem. Cell. Biol. 2016;72:1–17. doi: 10.1016/j.biocel.2015.12.008. [DOI] [PubMed] [Google Scholar]
- 18.Cimmino F., Formicola D., Capasso M. Dualistic Role of BARD1 in Cancer. Genes. 2017;8:375. doi: 10.3390/genes8120375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feki A., Jefford C.E., Berardi P., Wu J.Y., Cartier L., Krause K.H., Irminger-Finger I. BARD1 induces apoptosis by catalysing phosphorylation of p53 by DNA-damage response kinase. Oncogene. 2005;24:3726–3736. doi: 10.1038/sj.onc.1208491. [DOI] [PubMed] [Google Scholar]
- 20.Irminger-Finger I., Leung W.C., Li J., Dubois-Dauphin M., Harb J., Feki A., Jefford C.E., Soriano J.V., Jaconi M., Montesano R., et al. Identification of BARD1 as mediator between proapoptotic stress and p53-dependent apoptosis. Mol. Cell. 2001;8:1255–1266. doi: 10.1016/S1097-2765(01)00406-3. [DOI] [PubMed] [Google Scholar]
- 21.Thai T.H., Du F., Tsan J.T., Jin Y., Phung A., Spillman M.A., Massa H.F., Muller C.Y., Ashfaq R., Mathis J.M., et al. Mutations in the BRCA1-associated RING domain (BARD1) gene in primary breast, ovarian and uterine cancers. Hum. Mol. Genet. 1998;7:195–202. doi: 10.1093/hmg/7.2.195. [DOI] [PubMed] [Google Scholar]
- 22.Ghimenti C., Sensi E., Presciuttini S., Brunetti I.M., Conte P., Bevilacqua G., Caligo M.A. Germline mutations of the BRCA1-associated ring domain (BARD1) gene in breast and breast/ovarian families negative for BRCA1 and BRCA2 alterations. Genes Chromosomes Cancer. 2002;33:235–242. doi: 10.1002/gcc.1223. [DOI] [PubMed] [Google Scholar]
- 23.Ishitobi M., Miyoshi Y., Hasegawa S., Egawa C., Tamaki Y., Monden M., Noguchi S. Mutational analysis of BARD1 in familial breast cancer patients in Japan. Cancer Lett. 2003;200:1–7. doi: 10.1016/S0304-3835(03)00387-2. [DOI] [PubMed] [Google Scholar]
- 24.Karppinen S.M., Heikkinen K., Rapakko K., Winqvist R. Mutation screening of the BARD1 gene: Evidence for involvement of the Cys557Ser allele in hereditary susceptibility to breast cancer. J. Med. Genet. 2004;41:e114. doi: 10.1136/jmg.2004.020669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sauer M.K., Andrulis I.L. Identification and characterization of missense alterations in the BRCA1 associated RING domain (BARD1) gene in breast and ovarian cancer. J. Med. Genet. 2005;42:633–638. doi: 10.1136/jmg.2004.030049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vahteristo P., Syrjakoski K., Heikkinen T., Eerola H., Aittomaki K., von Smitten K., Holli K., Blomqvist C., Kallioniemi O.P., Nevanlinna H. BARD1 variants Cys557Ser and Val507Met in breast cancer predisposition. Eur. J. Hum. Genet. 2006;14:167–172. doi: 10.1038/sj.ejhg.5201542. [DOI] [PubMed] [Google Scholar]
- 27.Karppinen S.M., Barkardottir R.B., Backenhorn K., Sydenham T., Syrjakoski K., Schleutker J., Ikonen T., Pylkas K., Rapakko K., Erkko H., et al. Nordic collaborative study of the BARD1 Cys557Ser allele in 3956 patients with cancer: Enrichment in familial BRCA1/BRCA2 mutation-negative breast cancer but not in other malignancies. J. Med. Genet. 2006;43:856–862. doi: 10.1136/jmg.2006.041731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huo X., Hu Z., Zhai X., Wang Y., Wang S., Wang X., Qin J., Chen W., Jin G., Liu J., et al. Common non-synonymous polymorphisms in the BRCA1 Associated RING Domain (BARD1) gene are associated with breast cancer susceptibility: A case-control analysis. Breast Cancer Res. Treat. 2007;102:329–337. doi: 10.1007/s10549-006-9332-7. [DOI] [PubMed] [Google Scholar]
- 29.Jakubowska A., Cybulski C., Szymanska A., Huzarski T., Byrski T., Gronwald J., Debniak T., Gorski B., Kowalska E., Narod S.A., et al. BARD1 and breast cancer in Poland. Breast Cancer Res. Treat. 2008;107:119–122. doi: 10.1007/s10549-007-9537-4. [DOI] [PubMed] [Google Scholar]
- 30.Gorringe K.L., Choong D.Y., Visvader J.E., Lindeman G.J., Campbell I.G. BARD1 variants are not associated with breast cancer risk in Australian familial breast cancer. Breast Cancer Res. Treat. 2008;111:505–509. doi: 10.1007/s10549-007-9799-x. [DOI] [PubMed] [Google Scholar]
- 31.Johnatty S.E., Beesley J., Chen X., Hopper J.L., Southey M.C., Giles G.G., Goldgar D.E., Chenevix-Trench G., Spurdle A.B., Australian Ovarian Cancer Study Group et al. The BARD1 Cys557Ser polymorphism and breast cancer risk: An Australian case-control and family analysis. Breast Cancer Res. Treat. 2009;115:145–150. doi: 10.1007/s10549-008-0045-y. [DOI] [PubMed] [Google Scholar]
- 32.Guenard F., Labrie Y., Ouellette G., Beauparlant C.J., Durocher F., BRCAs I. Genetic sequence variations of BRCA1-interacting genes AURKA, BAP1, BARD1 and DHX9 in French Canadian families with high risk of breast cancer. J. Hum. Genet. 2009;54:152–161. doi: 10.1038/jhg.2009.6. [DOI] [PubMed] [Google Scholar]
- 33.De Brakeleer S., De Greve J., Loris R., Janin N., Lissens W., Sermijn E., Teugels E. Cancer predisposing missense and protein truncating BARD1 mutations in non-BRCA1 or BRCA2 breast cancer families. Hum. Mutat. 2010;31:E1175–E1185. doi: 10.1002/humu.21200. [DOI] [PubMed] [Google Scholar]
- 34.Sabatier R., Adelaide J., Finetti P., Ferrari A., Huiart L., Sobol H., Chaffanet M., Birnbaum D., Bertucci F. BARD1 homozygous deletion, a possible alternative to BRCA1 mutation in basal breast cancer. Genes Chromosomes Cancer. 2010;49:1143–1151. doi: 10.1002/gcc.20822. [DOI] [PubMed] [Google Scholar]
- 35.Walsh T., Casadei S., Lee M.K., Pennil C.C., Nord A.S., Thornton A.M., Roeb W., Agnew K.J., Stray S.M., Wickramanayake A., et al. Mutations in 12 genes for inherited ovarian, fallopian tube, and peritoneal carcinoma identified by massively parallel sequencing. Proc. Natl. Acad. Sci. USA. 2011;108:18032–18037. doi: 10.1073/pnas.1115052108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ratajska M., Antoszewska E., Piskorz A., Brozek I., Borg A., Kusmierek H., Biernat W., Limon J. Cancer predisposing BARD1 mutations in breast-ovarian cancer families. Breast Cancer Res. Treat. 2012;131:89–97. doi: 10.1007/s10549-011-1403-8. [DOI] [PubMed] [Google Scholar]
- 37.Ratajska M., Matusiak M., Kuzniacka A., Wasag B., Brozek I., Biernat W., Koczkowska M., Debniak J., Sniadecki M., Kozlowski P., et al. Cancer predisposing BARD1 mutations affect exon skipping and are associated with overexpression of specific BARD1 isoforms. Oncol. Rep. 2015;34:2609–2617. doi: 10.3892/or.2015.4235. [DOI] [PubMed] [Google Scholar]
- 38.Rouleau E., Jesson B., Briaux A., Nogues C., Chabaud V., Demange L., Sokolowska J., Coulet F., Barouk-Simonet E., Bignon Y.J., et al. Rare germline large rearrangements in the BRCA1/2 genes and eight candidate genes in 472 patients with breast cancer predisposition. Breast Cancer Res. Treat. 2012;133:1179–1190. doi: 10.1007/s10549-012-2009-5. [DOI] [PubMed] [Google Scholar]
- 39.Castera L., Krieger S., Rousselin A., Legros A., Baumann J.J., Bruet O., Brault B., Fouillet R., Goardon N., Letac O., et al. Next-generation sequencing for the diagnosis of hereditary breast and ovarian cancer using genomic capture targeting multiple candidate genes. Eur. J. Hum. Genet. 2014;22:1305–1313. doi: 10.1038/ejhg.2014.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cybulski C., Lubinski J., Wokolorczyk D., Kuzniak W., Kashyap A., Sopik V., Huzarski T., Gronwald J., Byrski T., Szwiec M., et al. Mutations predisposing to breast cancer in 12 candidate genes in breast cancer patients from Poland. Clin. Genet. 2015;88:366–370. doi: 10.1111/cge.12524. [DOI] [PubMed] [Google Scholar]
- 41.Pennington K.P., Walsh T., Harrell M.I., Lee M.K., Pennil C.C., Rendi M.H., Thornton A., Norquist B.M., Casadei S., Nord A.S., et al. Germline and somatic mutations in homologous recombination genes predict platinum response and survival in ovarian, fallopian tube, and peritoneal carcinomas. Clin. Cancer Res. 2014;20:764–775. doi: 10.1158/1078-0432.CCR-13-2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Couch F.J., Hart S.N., Sharma P., Toland A.E., Wang X., Miron P., Olson J.E., Godwin A.K., Pankratz V.S., Olswold C., et al. Inherited mutations in 17 breast cancer susceptibility genes among a large triple-negative breast cancer cohort unselected for family history of breast cancer. J. Clin. Oncol. 2015;33:304–311. doi: 10.1200/JCO.2014.57.1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Slavin T.P., Maxwell K.N., Lilyquist J., Vijai J., Neuhausen S.L., Hart S.N., Ravichandran V., Thomas T., Maria A., Villano D., et al. The contribution of pathogenic variants in breast cancer susceptibility genes to familial breast cancer risk. NPJ Breast Cancer. 2017;3:22. doi: 10.1038/s41523-017-0024-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Eliade M., Skrzypski J., Baurand A., Jacquot C., Bertolone G., Loustalot C., Coutant C., Guy F., Fumoleau P., Duffourd Y., et al. The transfer of multigene panel testing for hereditary breast and ovarian cancer to healthcare: What are the implications for the management of patients and families? Oncotarget. 2017;8:1957–1971. doi: 10.18632/oncotarget.12699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thompson E.R., Rowley S.M., Li N., McInerny S., Devereux L., Wong-Brown M.W., Trainer A.H., Mitchell G., Scott R.J., James P.A., et al. Panel Testing for Familial Breast Cancer: Calibrating the Tension Between Research and Clinical Care. J. Clin. Oncol. 2016;34:1455–1459. doi: 10.1200/JCO.2015.63.7454. [DOI] [PubMed] [Google Scholar]
- 46.Minion L.E., Dolinsky J.S., Chase D.M., Dunlop C.L., Chao E.C., Monk B.J. Hereditary predisposition to ovarian cancer, looking beyond BRCA1/BRCA2. Gynecol. Oncol. 2015;137:86–92. doi: 10.1016/j.ygyno.2015.01.537. [DOI] [PubMed] [Google Scholar]
- 47.Maxwell K.N., Wubbenhorst B., D’Andrea K., Garman B., Long J.M., Powers J., Rathbun K., Stopfer J.E., Zhu J., Bradbury A.R., et al. Prevalence of mutations in a panel of breast cancer susceptibility genes in BRCA1/2-negative patients with early-onset breast cancer. Genet. Med. 2015;17:630–638. doi: 10.1038/gim.2014.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pritzlaff M., Summerour P., McFarland R., Li S., Reineke P., Dolinsky J.S., Goldgar D.E., Shimelis H., Couch F.J., Chao E.C., et al. Male breast cancer in a multi-gene panel testing cohort: Insights and unexpected results. Breast Cancer Res. Treat. 2017;161:575–586. doi: 10.1007/s10549-016-4085-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Susswein L.R., Marshall M.L., Nusbaum R., Vogel Postula K.J., Weissman S.M., Yackowski L., Vaccari E.M., Bissonnette J., Booker J.K., Cremona M.L., et al. Pathogenic and likely pathogenic variant prevalence among the first 10,000 patients referred for next-generation cancer panel testing. Genet. Med. 2016;18:823–832. doi: 10.1038/gim.2015.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Norquist B.M., Harrell M.I., Brady M.F., Walsh T., Lee M.K., Gulsuner S., Bernards S.S., Casadei S., Yi Q., Burger R.A., et al. Inherited Mutations in Women With Ovarian Carcinoma. JAMA Oncol. 2016;2:482–490. doi: 10.1001/jamaoncol.2015.5495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tung N., Battelli C., Allen B., Kaldate R., Bhatnagar S., Bowles K., Timms K., Garber J.E., Herold C., Ellisen L., et al. Frequency of mutations in individuals with breast cancer referred for BRCA1 and BRCA2 testing using next-generation sequencing with a 25-gene panel. Cancer. 2015;121:25–33. doi: 10.1002/cncr.29010. [DOI] [PubMed] [Google Scholar]
- 52.Li J., Meeks H., Feng B.J., Healey S., Thorne H., Makunin I., Ellis J., kConFab I., Campbell I., Southey M., et al. Targeted massively parallel sequencing of a panel of putative breast cancer susceptibility genes in a large cohort of multiple-case breast and ovarian cancer families. J. Med. Genet. 2016;53:34–42. doi: 10.1136/jmedgenet-2015-103452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.De Brakeleer S., De Greve J., Desmedt C., Joris S., Sotiriou C., Piccart M., Pauwels I., Teugels E. Frequent incidence of BARD1-truncating mutations in germline DNA from triple-negative breast cancer patients. Clin. Genet. 2016;89:336–340. doi: 10.1111/cge.12620. [DOI] [PubMed] [Google Scholar]
- 54.Gass J., Tatro M., Blackburn P., Hines S., Atwal P.S. BARD1 nonsense variant c.1921C > T in a patient with recurrent breast cancer. Clin. Case Rep. 2017;5:104–107. doi: 10.1002/ccr3.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pugh T.J., Morozova O., Attiyeh E.F., Asgharzadeh S., Wei J.S., Auclair D., Carter S.L., Cibulskis K., Hanna M., Kiezun A., et al. The genetic landscape of high-risk neuroblastoma. Nat. Genet. 2013;45:279–284. doi: 10.1038/ng.2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Capasso M., Devoto M., Hou C., Asgharzadeh S., Glessner J.T., Attiyeh E.F., Mosse Y.P., Kim C., Diskin S.J., Cole K.A., et al. Common variations in BARD1 influence susceptibility to high-risk neuroblastoma. Nat. Genet. 2009;41:718–723. doi: 10.1038/ng.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rudd M.F., Webb E.L., Matakidou A., Sellick G.S., Williams R.D., Bridle H., Eisen T., Houlston R.S., Consortium G. Variants in the GH-IGF axis confer susceptibility to lung cancer. Genome Res. 2006;16:693–701. doi: 10.1101/gr.5120106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Esteban-Jurado C., Vila-Casadesus M., Garre P., Lozano J.J., Pristoupilova A., Beltran S., Munoz J., Ocana T., Balaguer F., Lopez-Ceron M., et al. Whole-exome sequencing identifies rare pathogenic variants in new predisposition genes for familial colorectal cancer. Genet. Med. 2015;17:131–142. doi: 10.1038/gim.2014.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Couch F.J., Shimelis H., Hu C., Hart S.N., Polley E.C., Na J., Hallberg E., Moore R., Thomas A., Lilyquist J., et al. Associations Between Cancer Predisposition Testing Panel Genes and Breast Cancer. JAMA Oncol. 2017;3:1190–1196. doi: 10.1001/jamaoncol.2017.0424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lincoln S.E., Kobayashi Y., Anderson M.J., Yang S., Desmond A.J., Mills M.A., Nilsen G.B., Jacobs K.B., Monzon F.A., Kurian A.W., et al. A Systematic Comparison of Traditional and Multigene Panel Testing for Hereditary Breast and Ovarian Cancer Genes in More Than 1000 Patients. J. Mol. Diagn. 2015;17:533–544. doi: 10.1016/j.jmoldx.2015.04.009. [DOI] [PubMed] [Google Scholar]
- 61.Buys S.S., Sandbach J.F., Gammon A., Patel G., Kidd J., Brown K.L., Sharma L., Saam J., Lancaster J., Daly M.B. A study of over 35,000 women with breast cancer tested with a 25-gene panel of hereditary cancer genes. Cancer. 2017;123:1721–1730. doi: 10.1002/cncr.30498. [DOI] [PubMed] [Google Scholar]
- 62.Tung N., Lin N.U., Kidd J., Allen B.A., Singh N., Wenstrup R.J., Hartman A.R., Winer E.P., Garber J.E. Frequency of Germline Mutations in 25 Cancer Susceptibility Genes in a Sequential Series of Patients With Breast Cancer. J. Clin. Oncol. 2016;34:1460–1468. doi: 10.1200/JCO.2015.65.0747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schroeder C., Faust U., Sturm M., Hackmann K., Grundmann K., Harmuth F., Bosse K., Kehrer M., Benkert T., Klink B., et al. HBOC multi-gene panel testing: Comparison of two sequencing centers. Breast Cancer Res. Treat. 2015;152:129–136. doi: 10.1007/s10549-015-3429-9. [DOI] [PubMed] [Google Scholar]
- 64.Kurian A.W., Hare E.E., Mills M.A., Kingham K.E., McPherson L., Whittemore A.S., McGuire V., Ladabaum U., Kobayashi Y., Lincoln S.E., et al. Clinical evaluation of a multiple-gene sequencing panel for hereditary cancer risk assessment. J. Clin. Oncol. 2014;32:2001–2009. doi: 10.1200/JCO.2013.53.6607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kwong A., Shin V.Y., Au C.H., Law F.B., Ho D.N., Ip B.K., Wong A.T., Lau S.S., To R.M., Choy G., et al. Detection of Germline Mutation in Hereditary Breast and/or Ovarian Cancers by Next-Generation Sequencing on a Four-Gene Panel. J. Mol. Diagn. 2016;18:580–594. doi: 10.1016/j.jmoldx.2016.03.005. [DOI] [PubMed] [Google Scholar]
- 66.Ramus S.J., Song H., Dicks E., Tyrer J.P., Rosenthal A.N., Intermaggio M.P., Fraser L., Gentry-Maharaj A., Hayward J., Philpott S., et al. Germline Mutations in the BRIP1, BARD1, PALB2, and NBN Genes in Women With Ovarian Cancer. J. Natl. Cancer Inst. 2015;107 doi: 10.1093/jnci/djv214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tedaldi G., Tebaldi M., Zampiga V., Danesi R., Arcangeli V., Ravegnani M., Cangini I., Pirini F., Petracci E., Rocca A., et al. Multiple-gene panel analysis in a case series of 255 women with hereditary breast and ovarian cancer. Oncotarget. 2017;8:47064–47075. doi: 10.18632/oncotarget.16791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Frey M.K., Sandler G., Sobolev R., Kim S.H., Chambers R., Bassett R.Y., Martineau J., Sapra K.J., Boyd L., Curtin J.P., et al. Multigene panels in Ashkenazi Jewish patients yield high rates of actionable mutations in multiple non-BRCA cancer-associated genes. Gynecol. Oncol. 2017;146:123–128. doi: 10.1016/j.ygyno.2017.04.009. [DOI] [PubMed] [Google Scholar]
- 69.Crawford B., Adams S.B., Sittler T., van den Akker J., Chan S., Leitner O., Ryan L., Gil E., van ‘t Veer L. Multi-gene panel testing for hereditary cancer predisposition in unsolved high-risk breast and ovarian cancer patients. Breast Cancer Res. Treat. 2017;163:383–390. doi: 10.1007/s10549-017-4181-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Frey M.K., Kim S.H., Bassett R.Y., Martineau J., Dalton E., Chern J.Y., Blank S.V. Rescreening for genetic mutations using multi-gene panel testing in patients who previously underwent non-informative genetic screening. Gynecol. Oncol. 2015;139:211–215. doi: 10.1016/j.ygyno.2015.08.006. [DOI] [PubMed] [Google Scholar]
- 71.Lhota F., Zemankova P., Kleiblova P., Soukupova J., Vocka M., Stranecky V., Janatova M., Hartmannova H., Hodanova K., Kmoch S., et al. Hereditary truncating mutations of DNA repair and other genes in BRCA1/BRCA2/PALB2-negatively tested breast cancer patients. Clin. Genet. 2016;90:324–333. doi: 10.1111/cge.12748. [DOI] [PubMed] [Google Scholar]
- 72.Churpek J.E., Marquez R., Neistadt B., Claussen K., Lee M.K., Churpek M.M., Huo D., Weiner H., Bannerjee M., Godley L.A., et al. Inherited mutations in cancer susceptibility genes are common among survivors of breast cancer who develop therapy-related leukemia. Cancer. 2016;122:304–311. doi: 10.1002/cncr.29615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shirts B.H., Casadei S., Jacobson A.L., Lee M.K., Gulsuner S., Bennett R.L., Miller M., Hall S.A., Hampel H., Hisama F.M., et al. Improving performance of multigene panels for genomic analysis of cancer predisposition. Genet. Med. 2016;18:974–981. doi: 10.1038/gim.2015.212. [DOI] [PubMed] [Google Scholar]
- 74.Suszynska M., Klonowska K., Jasinska A.J., Kozlowski P. Large-scale meta-analysis of mutations identified in panels of breast/ovarian cancer-related genes - Providing evidence of cancer predisposition genes. Gynecol. Oncol. 2019 doi: 10.1016/j.ygyno.2019.01.027. [DOI] [PubMed] [Google Scholar]
- 75.Taylor A., Brady A.F., Frayling I.M., Hanson H., Tischkowitz M., Turnbull C., Side L., UK Cancer Genetics Group Consensus for genes to be included on cancer panel tests offered by UK genetics services: guidelines of the UK Cancer Genetics Group. J. Med. Genet. 2018;55:372–377. doi: 10.1136/jmedgenet-2017-105188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Klonowska K., Ratajska M., Czubak K., Kuzniacka A., Brozek I., Koczkowska M., Sniadecki M., Debniak J., Wydra D., Balut M., et al. Analysis of large mutations in BARD1 in patients with breast and/or ovarian cancer: The Polish population as an example. Sci. Rep. 2015;5:10424. doi: 10.1038/srep10424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Landrum M.J., Lee J.M., Benson M., Brown G.R., Chao C., Chitipiralla S., Gu B., Hart J., Hoffman D., Jang W., et al. ClinVar: Improving access to variant interpretations and supporting evidence. Nucleic Acids Res. 2018;46:D1062–D1067. doi: 10.1093/nar/gkx1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bogdanova N., Cybulski C., Bermisheva M., Datsyuk I., Yamini P., Hillemanns P., Antonenkova N.N., Khusnutdinova E., Lubinski J., Dork T. A nonsense mutation (E1978X) in the ATM gene is associated with breast cancer. Breast Cancer Res. Treat. 2009;118:207–211. doi: 10.1007/s10549-008-0189-9. [DOI] [PubMed] [Google Scholar]
- 79.Cybulski C., Carrot-Zhang J., Kluzniak W., Rivera B., Kashyap A., Wokolorczyk D., Giroux S., Nadaf J., Hamel N., Zhang S., et al. Germline RECQL mutations are associated with breast cancer susceptibility. Nat. Genet. 2015;47:643–646. doi: 10.1038/ng.3284. [DOI] [PubMed] [Google Scholar]
- 80.Noskowicz M., Bogdanova N., Bermisheva M., Takhirova Z., Antonenkova N., Khusnutdinova E., Bremer M., Christiansen H., Park-Simon T.W., Hillemanns P., et al. Prevalence of PALB2 mutation c.509_510delGA in unselected breast cancer patients from Central and Eastern Europe. Fam. Cancer. 2014;13:137–142. doi: 10.1007/s10689-013-9684-1. [DOI] [PubMed] [Google Scholar]
- 81.Cybulski C., Kluzniak W., Huzarski T., Wokolorczyk D., Kashyap A., Jakubowska A., Szwiec M., Byrski T., Debniak T., Gorski B., et al. Clinical outcomes in women with breast cancer and a PALB2 mutation: A prospective cohort analysis. Lancet Oncol. 2015;16:638–644. doi: 10.1016/S1470-2045(15)70142-7. [DOI] [PubMed] [Google Scholar]
- 82.Pelttari L.M., Kiiski J., Nurminen R., Kallioniemi A., Schleutker J., Gylfe A., Aaltonen L.A., Leminen A., Heikkila P., Blomqvist C., et al. A Finnish founder mutation in RAD51D: Analysis in breast, ovarian, prostate, and colorectal cancer. J. Med. Genet. 2012;49:429–432. doi: 10.1136/jmedgenet-2012-100852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gayther S.A., Warren W., Mazoyer S., Russell P.A., Harrington P.A., Chiano M., Seal S., Hamoudi R., van Rensburg E.J., Dunning A.M., et al. Germline mutations of the BRCA1 gene in breast and ovarian cancer families provide evidence for a genotype-phenotype correlation. Nat. Genet. 1995;11:428–433. doi: 10.1038/ng1295-428. [DOI] [PubMed] [Google Scholar]
- 84.Thompson D., Easton D., Breast Cancer Linkage C. Variation in cancer risks, by mutation position, in BRCA2 mutation carriers. Am. J. Hum. Genet. 2001;68:410–419. doi: 10.1086/318181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rebbeck T.R., Mitra N., Wan F., Sinilnikova O.M., Healey S., McGuffog L., Mazoyer S., Chenevix-Trench G., Easton D.F., Antoniou A.C., et al. Association of type and location of BRCA1 and BRCA2 mutations with risk of breast and ovarian cancer. JAMA. 2015;313:1347–1361. doi: 10.1001/jama.2014.5985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lubinski J., Phelan C.M., Ghadirian P., Lynch H.T., Garber J., Weber B., Tung N., Horsman D., Isaacs C., Monteiro A.N., et al. Cancer variation associated with the position of the mutation in the BRCA2 gene. Fam. Cancer. 2004;3:1–10. doi: 10.1023/B:FAME.0000026816.32400.45. [DOI] [PubMed] [Google Scholar]
- 87.Bogdanova N.V., Antonenkova N.N., Rogov Y.I., Karstens J.H., Hillemanns P., Dork T. High frequency and allele-specific differences of BRCA1 founder mutations in breast cancer and ovarian cancer patients from Belarus. Clin. Genet. 2010;78:364–372. doi: 10.1111/j.1399-0004.2010.01473.x. [DOI] [PubMed] [Google Scholar]
- 88.Lek M., Karczewski K.J., Minikel E.V., Samocha K.E., Banks E., Fennell T., O’Donnell-Luria A.H., Ware J.S., Hill A.J., Cummings B.B., et al. Analysis of protein-coding genetic variation in 60,706 humans. Nature. 2016;536:285–291. doi: 10.1038/nature19057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Weber-Lassalle N.B.J., Weber-Lassalle K., Klaschik K., Neidhardt G., Ernst C., Blümcke B., Klonowska K., Volk A., Kubisch C., Baber R., et al. Germline loss-of-function variants in the BARD1 gene are associated with familial breast cancer. Senologie Zeitschrift für Mammadiagnostik und Therapie. 2018;15:e51. doi: 10.1055/s-0038-1651818. [DOI] [Google Scholar]
- 90.Shimelis H., LaDuca H., Hu C., Hart S.N., Na J., Thomas A., Akinhanmi M., Moore R.M., Brauch H., Cox A., et al. Triple-Negative Breast Cancer Risk Genes Identified by Multigene Hereditary Cancer Panel Testing. J. Natl. Cancer Inst. 2018 doi: 10.1093/jnci/djy106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Castera L., Harter V., Muller E., Krieger S., Goardon N., Ricou A., Rousselin A., Paimparay G., Legros A., Bruet O., et al. Landscape of pathogenic variations in a panel of 34 genes and cancer risk estimation from 5131 HBOC families. Genet. Med. 2018 doi: 10.1038/s41436-018-0005-9. [DOI] [PubMed] [Google Scholar]
- 92.Antoniou A.C., Pharoah P.D., McMullan G., Day N.E., Ponder B.A., Easton D. Evidence for further breast cancer susceptibility genes in addition to BRCA1 and BRCA2 in a population-based study. Genet. Epidemiol. 2001;21:1–18. doi: 10.1002/gepi.1014. [DOI] [PubMed] [Google Scholar]
- 93.Pharoah P.D., Antoniou A., Bobrow M., Zimmern R.L., Easton D.F., Ponder B.A. Polygenic susceptibility to breast cancer and implications for prevention. Nat. Genet. 2002;31:33–36. doi: 10.1038/ng853. [DOI] [PubMed] [Google Scholar]
- 94.Medrano R.F., de Oliveira C.A. Guidelines for the tetra-primer ARMS-PCR technique development. Mol. Biotechnol. 2014;56:599–608. doi: 10.1007/s12033-014-9734-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.