Abstract
Hepatocellular carcinoma (HCC) is the sixth most common type of cancer, with an increasing mortality rate. Aberrant expression of fibroblast growth factor 19–fibroblast growth factor receptor 4 (FGF19–FGFR4) is reported to be an oncogenic-driver pathway for HCC patients. Thus, the FGF19–FGFR4 signaling pathway is a promising target for the treatment of HCC. Several pan-FGFR (1–4) and FGFR4-specific inhibitors are in different phases of clinical trials. In this review, we summarize the information, recent developments, binding modes, selectivity, and clinical trial phases of different available FGFR4/pan-FGF inhibitors. We also discuss future perspectives and highlight the points that should be addressed to improve the efficacy of these inhibitors.
Keywords: prognosis, FGF19, FGFR4, HCC, inhibitors
1. Introduction
Hepatocellular carcinoma (HCC) is the sixth most common type of cancer, with the fourth highest mortality rate [1]. Despite advancements in therapeutic strategies, the response rate and overall survival rate are still low [2]. The most common cause of HCC is liver cirrhosis from any etiology including hepatitis B and hepatitis C infection, excessive alcohol consumption, diabetes mellitus, and non-alcoholic fatty liver disease [3]. Moreover, various molecular pathways are involved in the initiation and progression of HCC [4]. With respect to these pathways, there is evidence demonstrating the role of fibroblast growth factor pathway genes in HCC prognosis [5].
The fibroblast growth factors (FGFs) family comprises a large family of growth factors that are found in different multicellular organisms [6]. The FGFs signal through four transmembrane tyrosine kinase fibroblast growth factor receptors (FGFRs) namely FGFR1, FGFR2, FGFR3, and FGFR4 [7]. FGFs–FGFRs are involved in regulation of many biological processes such as embryonic development, cell proliferation, differentiation, and tissue repair [8]. FGF–FGFR dysregulation is also widely reported in different types of diseases, disorders, and cancers [9]. Notably, aberrant expression of FGF19/FGFR4 contributes to HCC progression [10].
Since sorafenib marked a new era in molecularly targeted therapy in advanced HCC [11], various drugs such as lenvatinib, regorafenib, cabozantinib, nivolumab, and ramucirumab have subsequently demonstrated overall survival benefits for patients [12,13,14,15,16]. However, the treatment outcome of metastatic HCC is still unsatisfactory, with a median overall survival below 15 months [12]. Thus, more effective treatment options for advanced HCC are needed. This can be achieved by a better understanding of the underlying genetic mechanisms involved in HCC. This review aims to provide comprehensive landscape of current information available on the FGF19–FGFR4 pathway. It also discusses recent advancements on FGF19–FGFR4 inhibitors in HCC. The data is obtained by systematic analysis of the literature and by using different text-mining approaches.
2. Overview of FGFR4 and FGF19
2.1. Structure and Function of FGFR4
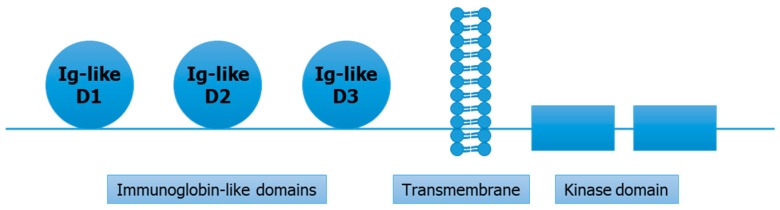
Fibroblast growth factor receptor 4 (FGFR4) is a protein coding gene and is a member of tyrosine kinase receptors family. The human FGFR4 gene is located on chromosome 5 and measures 11.41 bp in length [17]. The FGFR4 protein coded by two full transcripts of FGFR4 gene consists of ~800 amino acids, with molecular weight of around 95–110 kDa [18]. The structure of FGFR4 proteins contains three immunoglobin-like domains (D1–D3), a transmembrane domain, and the kinase domain [19]. (Figure 1) Among these immunoglobin-like domains, first two have role in receptor auto-inhibition, while the third domain is involved in specific binding of ligands [20]. The kinase domain (intracellular) is important in activation of downstream pathways [21]. Further, the kinase domain comprises the N-terminal (smaller) and C-terminal (larger) canonical domains [22]. FGF receptors differ from each other in tissue specificity and ligand-binding affinity. However, good identity scores are found between the kinase domains of FGFR4 and other FGF receptors [22]. The expression of FGFR4 is highly tissue-specific due to its unique ligand binding affinity [23]. At a functional level, FGFR4 is predominantly involved in regeneration of muscles, regulation of lipid metabolism, bile acid biosynthesis, cell proliferation, differentiation, glucose uptake, and myogenesis [24]. Of note, it is reported that FGFR4 is mostly expressed in liver tissue [25].
Figure 1.
Structural overview of fibroblast growth factor receptor 4 (FGFR4) protein.
2.2. FGFR4 in Cancer
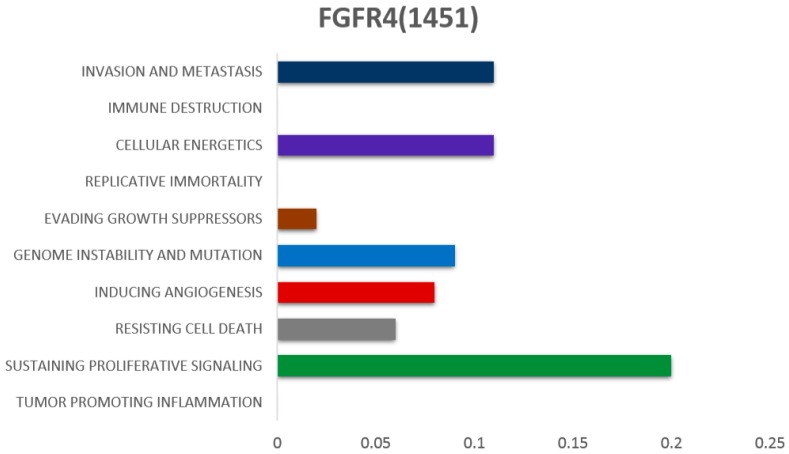
FGFR4 exerts a combination of biological effects that contribute to different hallmarks of cancer (Figure 2) [26]. Functional analysis demonstrated induction of both increased local growth and enhanced metastasis by mutated FGFR4 [27]. Xu et al. described germline mutations in FGFR4 i.e., glycine to arginine transition at position 388 in the transmembrane domain of FGFR4 receptor, which results in the formation of FGFR4 arg388 allele, leading to higher cancer risk [28]. Due to broad ligand binding spectrum of FGFR4, it is reportedly involved in multiple tumor types including HCC, breast cancer, colorectal cancer, rhabdomyosarcoma, and lung cancer [29,30,31,32,33].
Figure 2.
The association of FGFR4 with different hallmarks of cancer, as reported in the literature. (Scales of bars from left to right represent the lowest to highest number of associations reported)
2.3. Structure and Function of FGF19
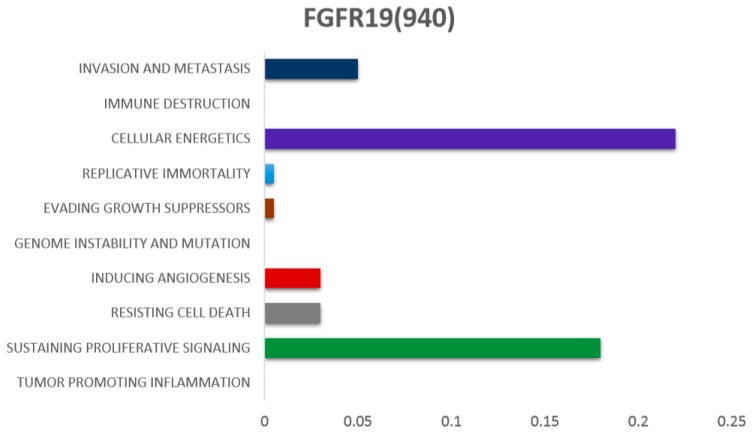
Out of three endogenous fibroblast growth factors (FGF19, FGF21, and FGF23), FGF19 binds to FGFR4 with highest affinity [34]. The human FGF19 gene is located on chromosome 11q13. In mice, the FGF15 gene is an orthologue of the human FGF19 gene [6]. The farnesoid X receptor (FXR) is activated by the secretion of bile acid from the gall bladder to the small intestine, which ultimately stimulates FGF19 secretion from the ileum [35,36]. The primary roles of FGF19 are found in bile acid synthesis, gallbladder filling, glycogen synthesis, gluconeogenesis, and protein synthesis [37]. FGF19 contributes to several hallmarks of cancer (Figure 3). Interestingly, FGF19 and FGF21 (endogenous fibroblast growth factors) are also most commonly involved in regulation of different functions occurring in liver [38]. Nicholes et al. demonstrated in transgenic mice that overexpression of FGF19 is involved in liver dysplasia [39]. In our recent study, amplification of FGF19 was found to be significantly associated with cirrhosis and also increased the risk of HCC [40]. Similarly, in our other study we used the fluorescence in situ hybridization technique and found the similar oncogenic patterns of FGF19 in HCC [41]. Copy number amplification of FGF19 is also highly reported in The Cancer Genome Atlas (TCGA) data [42]. Notably, the role of FGF19 at expression level is also frequently reported in HCC prognosis [43,44].
Figure 3.
The association of FGF19 with different hallmarks of cancer, as reported in the literature. (Scales of bars from left to right represent the lowest to highest number of associations reported)
2.4. Mechanism of FGFR4 Activation
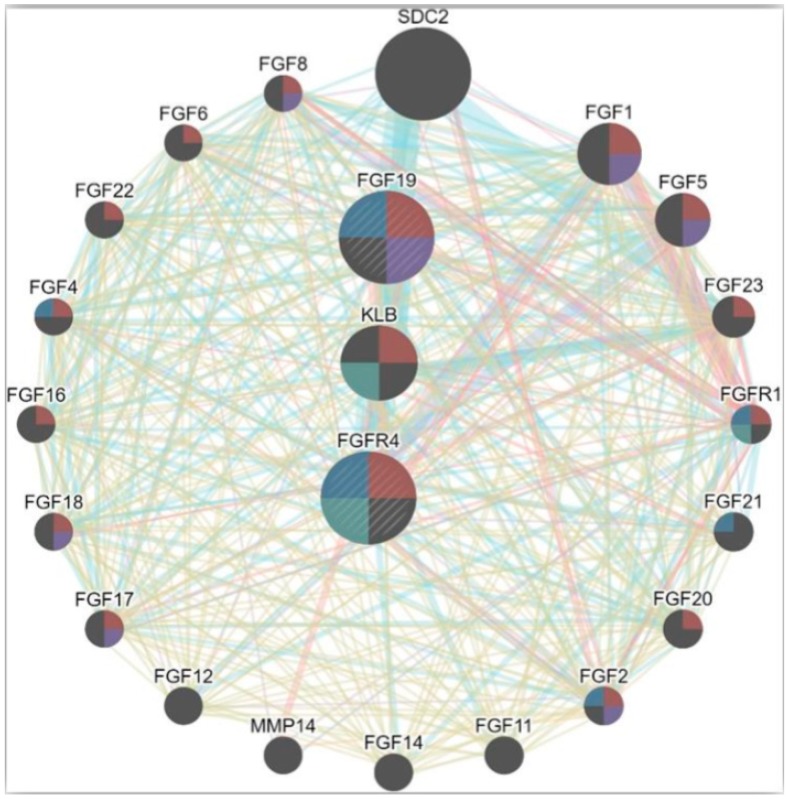
Specific ligand receptor binding spectrum in FGFs lead to autophosphorylation and formation of multiple complex [45]. FGFR4 is regulated using its co-receptor klotho-beta (KLB) (a transmembrane protein) [46]. The involvement of KLB co-receptor is reported in hepatocytes and adipose and pancreatic tissues [47]. FGFR4 and KLB are found to be overexpressed in mature hepatocytes [48]. In addition, KLB is required for FGF19–FGFR4 complex activation [49] (Figure 4).
Figure 4.
Interaction network of FGFR4 with different genes with high potency and functional similarity. The interaction network is based on various parameters including co-expression, genetic interactivity, shared protein domains, co-localization and physical interactions.
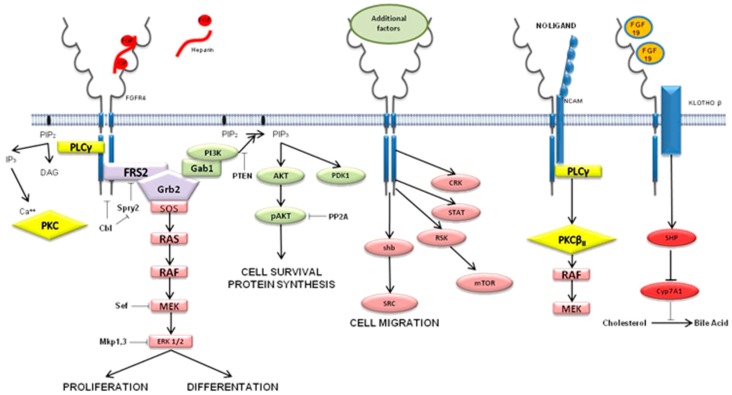
FGFR4 related pathways have predominant involvement in proliferation, differentiation, survival, and migration of cells. (Figure 4) Multiple signaling cascades such as GSK3β/β-catenin, PI3K/AKT, PLCγ/DAG/PKC, and RAS/RAF/MAPK are modulated by FGFR4 activation [10,50,51] (Figure 5).
Figure 5.
Involvement of FGFR4-related signaling pathways. Involvement in cell proliferation is depicted on the far left; next to it the cell survival signaling pathway is shown, and on the right side the cell migration pathway is explained (adapted from Atlas of Genetics and Cytogenetics in Oncology and Haematology).
FGFR4 selectively binds FGF19 ligand [49,52]. FGF19 is also reported as a functional partner of FGFR4, with the highest score in analysis through the STRING (https://string-db.org/) database.
2.5. FGF19–FGFR4 Pathway in HCC
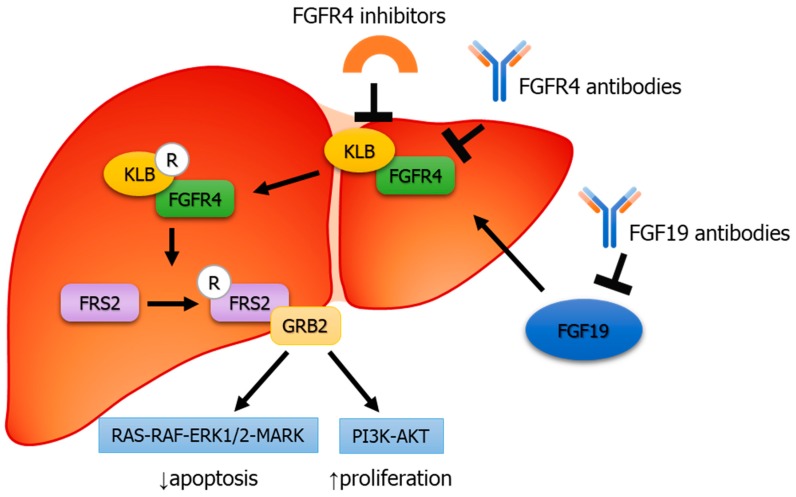
FGF19/FGFR4 activation leads to the formation of FGF receptor substrate 2 (FRS2) and growth factor receptor-bound protein 2 (GRB2) complex, ultimately activating Ras–Raf–ERK1/2MAPK and PI3K–Akt pathways. (Figure 6) These pathways are predominantly involved in tumor proliferation and anti-apoptosis. (Figure 6).
Figure 6.
Binding mechanism of FGF19 to FGFR4 leads to FRS2 along with recruitment of growth factor receptor-bound protein 2 (GRB2), ultimately leading to activation of the Ras–Raf–ERK1/2 MAPK and PI3K–Akt pathways.
As discussed, frequent studies reported the anomalous expression of FGF19–FGFR4 complex enhances the progression of HCC [31,44]. In a study conducted on mice model, Cui et al. suggested FGF19 as a potential therapeutic target for the treatment of HCC [53]. FGFR4 dysregulation and its correlation with TGF-β1 also suggested FGFR4 as potential therapeutic target of HCC patients with invasiveness and metastasis [43,54].
3. Targeting FGF19–FGFR4 in HCC
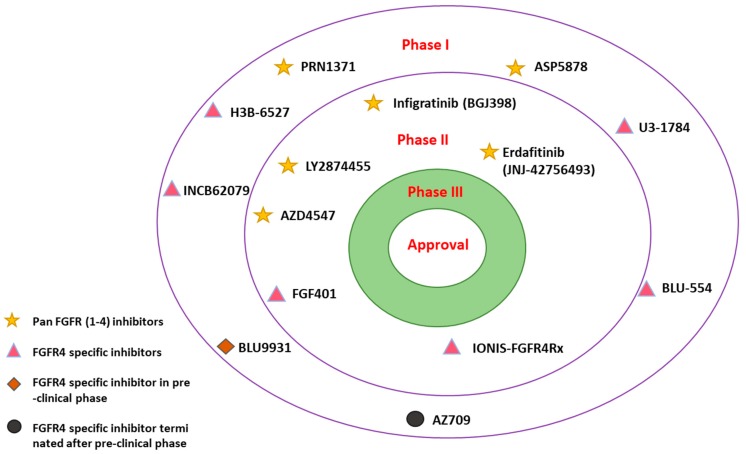
FGF19/FGFR4 inhibition is thought to lead to anti-tumor activities [55]. Thus, several FGFR (1–4) inhibitors are under trial for different types of malignancies including HCC [56] (Figure 7).
Figure 7.
Selected overview of pan-FGFRs and FGFR4-specific inhibitors in different stages of clinical trials for hepatocellular carcinoma (HCC).
3.1. Pan-FGFR (1–4) Inhibitors
Multiple pan-FGFR (1–4) inhibitors are under-development in different phases of clinical trials (Figure 7). LY2874455 (NCT01212107), AZD4547 (NCT02038673), infigratinib (NCT02160041), and erdafitinib (NCT02365597) drugs are designed to target pan-FGFRs and are in phase II of development and clinical trials (Table 1).
Table 1.
Pan-FGFR inhibitors in different phases of clinical trials.
| Drug | Company | Indication | Drug Target | Study Phase | Route of Administration | Clinical Trial ID |
|---|---|---|---|---|---|---|
| LY2874455 | Eli Lilly | Advanced and metastatic cancers | Pan-FGFR (1–4) inhibitor | Phase II | Oral | NCT01212107 |
| AZD4547 | Astra Zeneca | Stage IV squamous cell lung cancer | Pan-FGFR (1–4) inhibitor | Phase II | Oral | NCT02965378 |
| ER+ breast cancer | NCT01791985 | |||||
| Muscle-invasive bladder cancer (MIBC) | Phase I | NCT02546661 | ||||
| Infigratinib (BGJ398) | Novartis Pharmaceuticals | Tumors with FGFR genetic alterations | Pan-FGFR (1–4) inhibitor | Phase II | Oral | NCT02160041 |
| Advanced or metastatic cholangiocarcinoma |
Phase II | NCT02150967 | ||||
| Recurrent resectable or unresectable glioblastoma | Phase II | NCT01975701 | ||||
| Solid tumor | Phase I | NCT01697605 | ||||
| Advanced solid malignancies | Phase I | NCT01004224 | ||||
| Erdafitinib (JNJ-42756493) | Janssen Pharmaceuticals | Urothelial cancer Advanced hepatocellular carcinoma | Pan-FGFR (1–4) inhibitor | Phase II | Oral | NCT02365597 |
| Advanced non-small lung cancer Esophageal cancer | NCT02699606 | |||||
| Lymphoma | NCT02952573 | |||||
| PRN1371 | Prinicipia Biopharma Inc. | Solid tumor | Pan-FGFR (1–4) inhibitor | Phase I | Oral | NCT02608125 |
| ASP5878 | Astellas | Solid tumor | Pan-FGFR (1–4) inhibitor | Phase I | Oral | NCT02038673 |
ER+ breast cancer: estrogen-receptor-positive breast cancer.
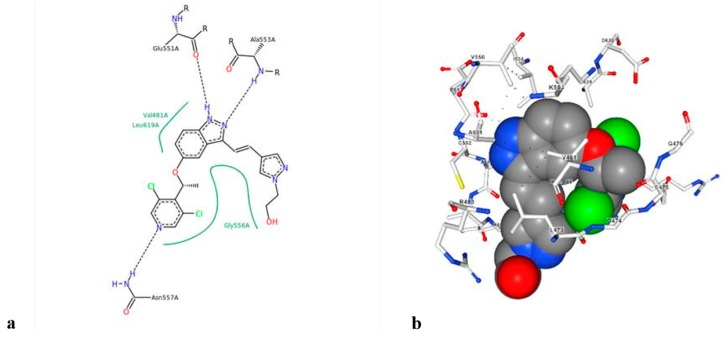
LY2874455 is a small molecule inhibitor developed by Eli Lilly [53] (Figure 8). It has shown promising effects against advanced and metastatic cancers such as myelomas, lung, bladder, and gastric cancer [57]. Its highly effective inhibitory action suggests that it can be effective potential drug for HCC in the near future.
Figure 8.
(a) Structure of LY2874455, and (b) binding mode of LY2874455 with the FGFR4 kinase domain (PDB code 5JKG).
AZD4547 was developed to specifically target pan-FGFR (1–4) in solid tumors. However, AZD4547 showed good efficacy against FGFR (1–3) but weaker activity against FGFR4 [58], suggesting low efficacy when specifically targeting FGFR4.
Infigratinib (BGJ398), which targets FGFR (1–3) with high affinity and FGFR4 with less affinity, was developed by Novartis Pharmaceuticals. It is currently in phase II for tumors with alteration of FGFR and for glioblastomas, solid tumors, hematologic malignancies, and advanced cholangiocarcinoma. Infigratinib showed an effective response against FGFR signaling pathways in HCC [59]. However, FDA-approved clinical trials are yet to be conducted for infigratinib in HCC [59].
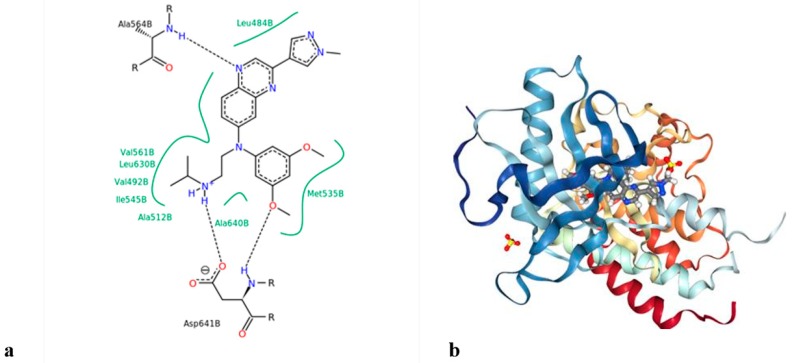
Janssen Pharmaceuticals reported erdafitinib (JNJ-42756493), a pan-FGFR (1–4) inhibitor (Figure 9), which is currently under phase II of clinical trials for advanced HCC. It significantly inhibited FGFR-overexpressing tumor cells in HCC [60].
Figure 9.
(a) Structure of JNJ-42756493 (b) Interaction of JNJ-42756493 with FGFR1 (PDB code 5EW8).
PRN1371 (NCT02608125) and ASP5878 (NCT02038673) are drugs designed to target pan-FGFRs and are in phase I of development and clinical trials. PRN1371 was developed by Principia Biopharma Inc. for solid tumors. It is an irreversible inhibitor that specifically targets FGFRs. The inhibitory action of this drug has been reported in many tumor types like HCC, gastric, and lung cancer [61]. Astellas developed ASP5878 to target pan-FGFRs (1–4) in solid tumors. Importantly, ASP5878 also inhibited HCC cell lines exhibiting overexpression of FGF19 in the pre-clinical phase. In addition, this small inhibitor molecule improved the efficacy of sorafenib [62].
3.2. FGFR4-Specific Inhibitors
As discussed, the overexpression of FGFR4 is most frequently reported receptor compared to FGFR (1–3) in HCC initiation and progression. However, selectivity of pan-FGFR inhibitors is comparatively lower for FGFR4. Thus, Prieto-Dominguez et al. outlined different targeted therapeutics available for the FGF19–FGFR4 complex [29]. A number of drugs are under different phases of clinical trials which specifically target FGF19/FGFR4. Two potential drug candidates in the phase II stage of clinical trials, namely IONIS-FGFR4Rx (NCT02476019) and FGF-401 (NCT02325739), are reported (Table 2).
Table 2.
FGFR4-specific inhibitors under different phases of clinical trials.
| Drug | Company | Indication | Drug Target | Study Phase | Route of Administration | Clinical Trial ID |
|---|---|---|---|---|---|---|
| IONIS-FGFR4Rx | Ionis Pharmaceuticals | Obesity and insulin sensitivity | FGFR4-specific | Phase II | Subcutaneous | NCT02476019 |
| FGF401 | Novartis AG | Hepatocellular carcinoma Solid malignancies | FGFR4-specific | Phase II (recruiting status) | Oral | NCT02325739 |
| H3B-6527 | H3 Biomedicine Inc. | Hepatocellular carcinoma | FGFR4-specific | Phase I | Oral | NCT02834780 |
| U3-1784 | Daiichi Sankyo Inc. | Advanced solid tumor Hepatocellular carcinoma | FGFR4-specific | Phase I (Terminated) | Intravenous | NCT02690350 |
| BLU-554 | Blueprint Medicines Corp. | Hepatocellular carcinoma (orphan drug designation for HCC by the U.S. FDA) | FGFR4-specific | Phase I | Oral | NCT02508467 |
| AZ709 | AstraZeneca | Hepatocellular carcinoma | FGFR4-specific | Inactive (Pre-clinical) | Unspecified |
U.S. FDA: U.S. Food and Drug Administration.
IONIS-FGFR4Rx, previously known as ISIS-FGFR4Rx, exhibited antisense inhibitor activity against FGFR4 [59]. IONIS-FGFR4Rx has undergone a phase II clinical trial for obesity, specifically targeting FGFR4 in liver and fat tissues. It is not only effective in reducing obesity but also improves insulin sensitivity [63]. Thus, we suggest that conducting trials with IONIS-FGFR4Rx in HCC patients may give significant results.
FGF401 was developed by Novartis and specifically targets FGFR4 in HCC patients. According to the most recent update, FGF401 is in phase II of clinical trials for HCC, expected to be completed by the year 2020. FGF401, with an IC50, exhibited at least 1000-fold potency for inhibiting FGFR4 kinase activity compared to other FGFRs (1–3) [64].
H3B-6527 (NCT02834780), U3-1784 (NCT02690350), and BLU-554 (NCT02508467) are reported to be in phase I clinical trials to specifically target FGFR4.
H3B-6527 is a small inhibitor molecule developed by H3 Biomedicine Inc for targeting FGFR4-overexpression in advanced HCC and cholangiocarcinoma (IHCC) patients. In preclinical trials, H3B-6527 proved to be effective in terms of repressing tumor growth in a xenograft model of HCC which exhibited activated aberrant FGF19–FGFR4 signaling [65].
The human monoclonal drug U3-1784 is under-development by Daiichi Sankyo Inc for HCC and other solid tumors. This antibody specifically binds to FGFR4 and is most effective (approximately 90%) in FGF19-expressing models, suggesting it as a potential drug for HCC with an activated FGF19–FGFR4 pathway. However, according to a recent update, the clinical trials for this drug have been terminated [66].
BLU-554, a FGFR4-specific inhibitor, is under recruiting phase by Blueprint Medicines Corp. for HCC and cholangiocarcinoma patients. In addition, it was also granted an orphan drug designation in 2015 by the U.S. FDA for HCC [67].
Lastly, AZ709 showed good selective inhibition of FGFR4 in HCC, as recently reported by AstraZeneca, and is in the preclinical stage of development. However, no progress has been reported on this drug to date (reported at the 2013 NCRI Cancer Conference, Liverpool, UK).
3.3. Irreversible FGFR4 Inhibitors
Two irreversible FGFR4 inhibitors have also been recently reported, including INCB62079 (ClinicalTrials.gov Identifier: NCT03144661) and BLU9931 [68] (Figure 7, Table 3). INCB62079, developed by the Incyte Corporation, showed effective dose-dependent and compound-selective activity against cancer cells exhibiting active FGF19–FGFR4. Additionally, it showed good efficacy in Hep3b hepatocellular cancer xenograft model in pre-clinical trial phase. INCB62079 is currently in phase I clinical trials (ClinicalTrials.gov Identifier: NCT03144661) for HCC.
Table 3.
FGFR4-specific irreversible inhibitors under different phases of clinical trials.
| Drug | Company | Indication | Drug Target | Study Phase | Route of Administration | Clinical Trial ID |
|---|---|---|---|---|---|---|
| INCB62079 | Incyte Corporation | Liver cancer |
FGFR4-specific (irreversible) |
Phase I | Unspecified | NCT03144661 |
| BLU9931 | Blueprint Medicines Corp. | Hepatocellular carcinoma |
FGFR4-specific (irreversible) |
Pre-clinical | Oral |
Blueprint Medicines Corp reported the remarkable drug BLU9931, a small irreversible inhibitor of FGFR4. It is currently in the pre-clinical stage of development for HCC and has not been approved by the U.S. FDA. In the preclinical trial phase, BLU9931 exhibited potent antitumor activity in mice with an HCC tumor xenograft with amplified FGF19 and high expression of FGF19 at the mRNA level. Recently, it has been reported that FGF19 shows resistance to sorafenib, but BLU9931 is involved in improving sorafenib efficacy by inactivating FGFR4 signaling [68].
Apart from the drugs reported in different clinical trials, different studies are underway to find new potent inhibitors against FGF19/FGFR. For instance, Cheuk et al. developed a chimeric antibody 3A11ScFvFc (mice antibody Fv + Human IgG1Fc) to specifically target FGFR4 in HCC [69]. Chen et al. found ABSK-011 to be involved in suppressing high FGFR4 expression, which ultimately results in HCC tumor suppression. ABSK-011, acting as irreversible inhibitor, selectively modifies cys552, which is the residue present within the active site of FGFR4. Of note, safety studies have also been conducted for this inhibitor [70]. Lee et al. examined the effect of the HM81422 inhibitor on the FGFR4–FGFR19 pathway. They successfully demonstrated that HM81422 can potentially target FGFR4 activated pathways. However, further elucidation is still required to understand the role of this inhibitor in HCC [71]. Furthermore, different pharmacological approaches suggested significant involvement of the drug sorafenib in inhibiting tyrosine kinase pathways. Initially, Gao et al. reported sorafenib as potential tyrosine kinase inhibitor which improves overall survival rate in HCC patients [68]. Later, Matsuki et al. revealed that sorafenib has no particular effect on the oncogenic FGF signaling pathway. However, the involvement of the drug lenvatinib was also recently reported [68]. Lenvatinib reportedly inhibits FGF pathways in HCC cell lines. Of note, studies suggested that it can be used as a pan-FGFR (1–4) inhibitor [68]. However, the specificity of lenvatinib against the FGF19–FGFR4 signaling pathway still remains unclear [72].
4. Discussion and Conclusions
Compelling evidence supports the involvement of the FGF19–FGFR4 signaling pathway in HCC [43]. Therefore, this pathway is considered to be a promising therapeutic target for the treatment of HCC. Interestingly, a number of different inhibitors and drugs have been reported to target FGF and FGFR signaling pathways. Despite promising advancements, it is still challenging to completely address all the underlying perspectives of this pathway. These perspectives, if clearly addressed, can improve the efficacy and potency of drugs available for HCC. The detailed analysis of available data revealed that FGFR4 is structurally distinct from other FGF receptors (1–3) and also exhibits variable inhibition potency towards different available FGFR drugs [73]. Perhaps, this distinct characteristic of FGFR4 should be exploited in depth to develop FGFR4-specific inhibitors to improve drug efficacy for HCC. Importantly, the evidence derived from primates suggests that anti-FGF19 antibody treatment is mostly accompanied with dose-related liver toxicity [74]. Therefore, the likelihood of adverse effects of FGF/FGFR drugs should be properly envisaged to assure best possible and safe outcomes along with reduced dose-dependent side effects.
In addition, the correlation of FGF19 gene amplification and HCC is reported to be highly significant, and it is consequently thought to act as potential biomarker for HCC [75]. Therefore, copy number gain of FGF19 and FGFR4 should be taken into consideration when designing potential inhibitors of these genes and their pathways.
Conceptually, it is shown that the patients having elevated bile acid concentrations and diabetes have a higher risk of developing HCC [44,53]. Therefore, these complications should be taken into account along with the inhibition of FGF19–FGFR4 pathways to avoid potential adverse impacts and minimize safety risks in HCC patients.
Overall, the degree of FGF–FGFR inhibition in HCC is not satisfactory. This perhaps gives an indication towards elucidating other factors that are simultaneously involved in the FGF–FGFR signaling pathway. For instance, KLB (the co-receptor of FGFR4) is reportedly considered as a novel drug candidate as it is mostly found involved in inducing FGFR4 overexpression and is also found in an elevated state in HCC [46,76]. Thus, in the future klotho-specific inhibitors can be considered to potentially maximize antitumor and therapeutic benefits in HCC by terminating FGF19-binding to FGFR4. Lastly, developing drugs that act on key SNPs of FGFR4 i.e., Gly388 to Arg388, may also be clinically relevant.
In conclusion, most of the FGFR4-specific inhibitors are in pre-clinical phases. Progression of these potential inhibitors to advance clinical trial phases coupled with comprehensive research and improvements can revolutionize the available therapeutic options for HCC.
Author Contributions
S.-M.A. and F.H. contributed to the conception and design of the study. A.R. performed the literature review. A.R. and F.H. wrote the manuscript. A.R., I.P., and S.-M.A. contributed in revising the manuscript.
Funding
This research was supported by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute, funded by the Ministry of Health and Welfare (HI16C1985) and by a grant from Gachon University Gil Medical Center (FRD2016-20). The research was also funded by Higher Education Commission of Pakistan through National Research Program For Universities (NRPU # 7036).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Huang S., He X. The role of microRNAs in liver cancer progression. Br. J. Cancer. 2011;104:235–240. doi: 10.1038/sj.bjc.6606010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kulik L., El-Serag H.B. Epidemiology and Management of Hepatocellular Carcinoma. Gastroenterology. 2019;156:477–491. doi: 10.1053/j.gastro.2018.08.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moeini A., Cornellà H., Villanueva A. Emerging Signaling Pathways in Hepatocellular Carcinoma. LIC. 2012;1:83–93. doi: 10.1159/000342405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zheng N., Wei W., Wang Z. Emerging roles of FGF signaling in hepatocellular carcinoma. Transl. Cancer Res. 2016;5:1–6. [PMC free article] [PubMed] [Google Scholar]
- 6.Ornitz D.M., Itoh N. Fibroblast growth factors. Genome Biol. 2001;2:reviews3005.1–reviews3005.12. doi: 10.1186/gb-2001-2-3-reviews3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Itoh N., Ornitz D.M. Fibroblast growth factors: From molecular evolution to roles in development, metabolism and disease. J. Biochem. 2011;149:121–130. doi: 10.1093/jb/mvq121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wilkie A., Morriss-Kay G.M., Yvonne Jones E., Heath J.K. Functions of fibroblast growth factors and their receptors. Curr. Biol. 1995;5:500–507. doi: 10.1016/S0960-9822(95)00102-3. [DOI] [PubMed] [Google Scholar]
- 9.Turner N., Grose R. Fibroblast growth factor signalling: From development to cancer. Nat. Rev. Cancer. 2010;10:116–129. doi: 10.1038/nrc2780. [DOI] [PubMed] [Google Scholar]
- 10.Zhao H., Lv F., Liang G., Huang X., Wu G., Zhang W., Yu L., Shi L., Teng Y. FGF19 promotes epithelial-mesenchymal transition in hepatocellular carcinoma cells by modulating the GSK3β/β- catenin signaling cascade via FGFR4 activation. Oncotarget. 2015;7:13575–13586. doi: 10.18632/oncotarget.6185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Llovet J.M., Hilgard P., de Oliveira A.C., Forner A., Zeuzem S., Galle P.R., Häussinger D., Moscovici M. Sorafenib in Advanced Hepatocellular Carcinoma. N. Engl. J. Med. 2008;13 doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 12.Kudo M., Finn R.S., Qin S., Han K.-H., Ikeda K., Piscaglia F., Baron A., Park J.-W., Han G., Jassem J., et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: A randomised phase 3 non-inferiority trial. Lancet. 2018;391:1163–1173. doi: 10.1016/S0140-6736(18)30207-1. [DOI] [PubMed] [Google Scholar]
- 13.Bruix J., Qin S., Merle P., Granito A., Huang Y.-H., Bodoky G., Pracht M., Yokosuka O., Rosmorduc O., Breder V., et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389:56–66. doi: 10.1016/S0140-6736(16)32453-9. [DOI] [PubMed] [Google Scholar]
- 14.Abou-Alfa G.K., Meyer T., Cheng A.-L., El-Khoueiry A.B., Rimassa L., Ryoo B.-Y., Cicin I., Merle P., Chen Y., Park J.-W., et al. Cabozantinib in Patients with Advanced and Progressing Hepatocellular Carcinoma. N. Engl. J. Med. 2018;379:54–63. doi: 10.1056/NEJMoa1717002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhu A.X., Kang Y.-K., Yen C.-J., Finn R.S., Galle P.R., Llovet J.M., Assenat E., Brandi G., Pracht M., Lim H.Y., et al. Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased α-fetoprotein concentrations (REACH-2): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019;20:282–296. doi: 10.1016/S1470-2045(18)30937-9. [DOI] [PubMed] [Google Scholar]
- 16.El-Khoueiry A.B., Sangro B., Yau T., Crocenzi T.S., Kudo M., Hsu C., Kim T.-Y., Choo S.-P., Trojan J., Welling T.H., et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): An open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017;389:2492–2502. doi: 10.1016/S0140-6736(17)31046-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Transcript: FGFR4-201 (ENST00000292408.8)—Protein summary—Homo sapiens—Ensembl genome browser 95. [(accessed on 19 January 2019)]; Available online: https://asia.ensembl.org/Homo_sapiens/Transcript/ProteinSummary?g=ENSG00000160867;r=5:177086886-177098144;t=ENST00000292408.
- 18.Partanen J., Mäkelä T.P., Eerola E., Korhonen J., Hirvonen H., Claesson-Welsh L., Alitalo K. FGFR-4, a novel acidic fibroblast growth factor receptor with a distinct expression pattern. EMBO J. 1991;10:1347–1354. doi: 10.1002/j.1460-2075.1991.tb07654.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mohammadi M., Olsen S.K., Ibrahimi O.A. Structural basis for fibroblast growth factor receptor activation. Cytok. Growth Factor Rev. 2005;16:107–137. doi: 10.1016/j.cytogfr.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 20.Wang F., Kan M., Yan G., Xu J., McKeehan W.L. Alternately Spliced NH2-terminal Immunoglobulin-like Loop I in the Ectodomain of the Fibroblast Growth Factor (FGF) Receptor 1 Lowers Affinity for both Heparin and FGF-1. J. Biol. Chem. 1995;270:10231–10235. doi: 10.1074/jbc.270.17.10231. [DOI] [PubMed] [Google Scholar]
- 21.Ornitz D.M., Itoh N. The Fibroblast Growth Factor signaling pathway. Wiley Interdiscip. Rev. Dev. Biol. 2015;4:215–266. doi: 10.1002/wdev.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tucker J.A., Klein T., Breed J., Breeze A.L., Overman R., Phillips C., Norman R.A. Structural Insights into FGFR Kinase Isoform Selectivity: Diverse Binding Modes of AZD4547 and Ponatinib in Complex with FGFR1 and FGFR4. Structure. 2014;22:1764–1774. doi: 10.1016/j.str.2014.09.019. [DOI] [PubMed] [Google Scholar]
- 23.Horlick R.A., Stack S.L., Cooke G.M. Cloning, expression and tissue distribution of the gene encoding rat fibroblast growth factor receptor subtype 4. Gene. 1992;120:291–295. doi: 10.1016/0378-1119(92)90108-2. [DOI] [PubMed] [Google Scholar]
- 24.Reference G.H. FGFR4 Gene. [(accessed on 19 January 2019)]; Available online: https://ghr.nlm.nih.gov/gene/FGFR4.
- 25.Hughes S.E. Differential Expression of the Fibroblast Growth Factor Receptor (FGFR) Multigene Family in Normal Human Adult Tissues. J. Histochem. Cytochem. 1997;45:1005–1019. doi: 10.1177/002215549704500710. [DOI] [PubMed] [Google Scholar]
- 26.Gross S., Rahal R., Stransky N., Lengauer C., Hoeflich K.P. Targeting cancer with kinase inhibitors. J. Clin. Investig. 2015;125:1780–1789. doi: 10.1172/JCI76094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Greenman C., Stephens P., Smith R., Dalgliesh G.L., Hunter C., Bignell G., Davies H., Teague J., Butler A., Stevens C., et al. Patterns of somatic mutation in human cancer genomes. Nature. 2007;446:153–158. doi: 10.1038/nature05610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu W., Li Y., Wang X., Chen B., Wang Y., Liu S., Xu J., Zhao W., Wu J. FGFR4 transmembrane domain polymorphism and cancer risk: A meta-analysis including 8555 subjects. Eur. J. Cancer. 2010;46:3332–3338. doi: 10.1016/j.ejca.2010.06.017. [DOI] [PubMed] [Google Scholar]
- 29.Ye Y.-W., Zhang X., Zhou Y., Wu J., Zhao C., Yuan L., Wang G., Du C., Wang C., Shi Y. The correlations between the expression of FGFR4 protein and clinicopathological parameters as well as prognosis of gastric cancer patients. J. Surg. Oncol. 2012;106:872–879. doi: 10.1002/jso.23153. [DOI] [PubMed] [Google Scholar]
- 30.Spinola M., Leoni V.P., Tanuma J., Pettinicchio A., Frattini M., Signoroni S., Agresti R., Giovanazzi R., Pilotti S., Bertario L., et al. FGFR4 Gly388Arg polymorphism and prognosis of breast and colorectal cancer. Oncol. Rep. 2005;14:415–419. doi: 10.3892/or.14.2.415. [DOI] [PubMed] [Google Scholar]
- 31.Matakidou A., el Galta R., Rudd M.F., Webb E.L., Bridle H., Eisen T., Houlston R.S. Further observations on the relationship between the FGFR4 Gly388Arg polymorphism and lung cancer prognosis. Br. J. Cancer. 2007;96:1904–1907. doi: 10.1038/sj.bjc.6603816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vi J.G.T., Cheuk A.T., Tsang P.S., Chung J.-Y., Song Y.K., Desai K., Yu Y., Chen Q.-R., Shah K., Youngblood V., et al. Identification of FGFR4-activating mutations in human rhabdomyosarcomas that promote metastasis in xenotransplanted models. J. Clin. Investig. 2009;119:3395–3407. doi: 10.1172/JCI39703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sheu M.-J., Hsieh M.-J., Chiang W.-L., Yang S.-F., Lee H.-L., Lee L.-M., Yeh C.-B. Fibroblast Growth Factor Receptor 4 Polymorphism Is Associated with Liver Cirrhosis in Hepatocarcinoma. PLoS ONE. 2015;10:e0122961. doi: 10.1371/journal.pone.0122961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee K.J., Jang Y.O., Cha S.-K., Kim M.Y., Park K.-S., Eom Y.W., Baik S.K. Expression of Fibroblast Growth Factor 21 and β-Klotho Regulates Hepatic Fibrosis through the Nuclear Factor-κB and c-Jun N-Terminal Kinase Pathways. Gut Liver. 2018;12:449–456. doi: 10.5009/gnl17443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu W.-Y., Xie D.-M., Zhu G.-Q., Huang G.-Q., Lin Y.-Q., Wang L.-R., Shi K.-Q., Hu B., Braddock M., Chen Y.-P., et al. Targeting fibroblast growth factor 19 in liver disease: A potential biomarker and therapeutic target. Expert Opin. Ther. Targets. 2015;19:675–685. doi: 10.1517/14728222.2014.997711. [DOI] [PubMed] [Google Scholar]
- 36.Kurosu H., Choi M., Ogawa Y., Dickson A.S., Goetz R., Eliseenkova A.V., Mohammadi M., Rosenblatt K.P., Kliewer S.A., Kuro-o M. Tissue-specific Expression of βKlotho and Fibroblast Growth Factor (FGF) Receptor Isoforms Determines Metabolic Activity of FGF19 and FGF21. J. Biol. Chem. 2007;282:26687–26695. doi: 10.1074/jbc.M704165200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kir S., Kliewer S.A., Mangelsdorf D.J. Roles of FGF19 in Liver Metabolism. Cold Spring Harb. Symp. Quant. Biol. 2011;76:139–144. doi: 10.1101/sqb.2011.76.010710. [DOI] [PubMed] [Google Scholar]
- 38.Fukumoto S. Actions and Mode of Actions of FGF19 Subfamily Members. Endocr. J. 2008;55:23–31. doi: 10.1507/endocrj.KR07E-002. [DOI] [PubMed] [Google Scholar]
- 39.Nicholes K., Guillet S., Tomlinson E., Hillan K., Wright B., Frantz G.D., Pham T.A., Dillard-Telm L., Tsai S.P., Stephan J.-P., et al. A Mouse Model of Hepatocellular Carcinoma: Ectopic Expression of Fibroblast Growth Factor 19 in Skeletal Muscle of Transgenic Mice. Am. J. Pathol. 2002;160:2295–2307. doi: 10.1016/S0002-9440(10)61177-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ahn S.-M., Jang S.J., Shim J.H., Kim D., Hong S.-M., Sung C.O., Baek D., Haq F., Ansari A.A., Lee S.Y., et al. Genomic portrait of resectable hepatocellular carcinomas: Implications of RB1 and FGF19 aberrations for patient stratification. Hepatology. 2014;60:1972–1982. doi: 10.1002/hep.27198. [DOI] [PubMed] [Google Scholar]
- 41.Kang H.J., Haq F., Sung C.O., Choi J., Hong S.-M., Eo S.-H., Jeong H.J., Shin J., Shim J.H., Lee H.C., et al. Characterization of Hepatocellular Carcinoma Patients with FGF19 Amplification Assessed by Fluorescence in situ Hybridization: A Large Cohort Study. LIC. 2019;8:12–23. doi: 10.1159/000488541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ally A., Balasundaram M., Carlsen R., Chuah E., Clarke A., Dhalla N., Holt R.A., Jones S.J.M., Lee D., Ma Y., et al. Comprehensive and Integrative Genomic Characterization of Hepatocellular Carcinoma. Cell. 2017;169:1327–1341. doi: 10.1016/j.cell.2017.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miura S., Mitsuhashi N., Shimizu H., Kimura F., Yoshidome H., Otsuka M., Kato A., Shida T., Okamura D., Miyazaki M. Fibroblast growth factor 19 expression correlates with tumor progression and poorer prognosis of hepatocellular carcinoma. BMC Cancer. 2012;12:56. doi: 10.1186/1471-2407-12-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wu X., Ge H., Lemon B., Vonderfecht S., Weiszmann J., Hecht R., Gupte J., Hager T., Wang Z., Lindberg R., et al. FGF19-induced Hepatocyte Proliferation Is Mediated through FGFR4 Activation. J. Biol. Chem. 2010;285:5165–5170. doi: 10.1074/jbc.M109.068783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Powers C.J., McLeskey S.W., Wellstein A. Fibroblast growth factors, their receptors and signaling. Endocr. Relat. Cancer. 2000;7:165–197. doi: 10.1677/erc.0.0070165. [DOI] [PubMed] [Google Scholar]
- 46.Poh W., Wong W., Ong H., Aung M.O., Lim S.G., Chua B.T., Ho H.K. Klotho-beta overexpression as a novel target for suppressing proliferation and fibroblast growth factor receptor-4 signaling in hepatocellular carcinoma. Mol. Cancer. 2012;11:14. doi: 10.1186/1476-4598-11-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ito S., Kinoshita S., Shiraishi N., Nakagawa S., Sekine S., Fujimori T., Nabeshima Y. Molecular cloning and expression analyses of mouse βklotho, which encodes a novel Klotho family protein. Mech. Dev. 2000;98:115–119. doi: 10.1016/S0925-4773(00)00439-1. [DOI] [PubMed] [Google Scholar]
- 48.Li Y., Zhang W., Doughtie A., Cui G., Li X., Pandit H., Yang Y., Li S., Martin R. Up-regulation of fibroblast growth factor 19 and its receptor associates with progression from fatty liver to hepatocellular carcinoma. Oncotarget. 2016;7:52329–52339. doi: 10.18632/oncotarget.10750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lin B.C., Wang M., Blackmore C., Desnoyers L.R. Liver-specific Activities of FGF19 Require Klotho beta. J. Biol. Chem. 2007;282:27277–27284. doi: 10.1074/jbc.M704244200. [DOI] [PubMed] [Google Scholar]
- 50.Pratsinis H., Armatas† A.A., Kletsas D. Response of Fetal and Adult Cells to Growth Factors. In: Bhattacharya N., Stubblefield P., editors. Human Fetal Tissue Transplantation. Springer; London, UK: 2013. pp. 65–77. [Google Scholar]
- 51.Tiong K.H., Tan B.S., Choo H.L., Chung F.F.-L., Hii L.-W., Tan S.H., Khor N.T.W., Wong S.F., See S.-J., Tan Y.-F., et al. Fibroblast growth factor receptor 4 (FGFR4) and fibroblast growth factor 19 (FGF19) autocrine enhance breast cancer cells survival. Oncotarget. 2016;7:57633–57650. doi: 10.18632/oncotarget.9328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xie M.-H., Holcomb I., Deuel B., Dowd P., Huang A., Vagts A., Foster J., Liang J., Brush J., Gu Q., et al. FGF-19, a Novel Fibroblast Growth Factor with Unique Specificity for FGFR4. Cytokine. 1999;11:729–735. doi: 10.1006/cyto.1999.0485. [DOI] [PubMed] [Google Scholar]
- 53.Cui G., Martin R.C., Jin H., Liu X., Pandit H., Zhao H., Cai L., Zhang P., Li W., Li Y. Up-regulation of FGF15/19 signaling promotes hepatocellular carcinoma in the background of fatty liver. J. Exp. Clin. Cancer Res. 2018;37:136. doi: 10.1186/s13046-018-0781-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ho H.K., Pok S., Streit S., Ruhe J.E., Hart S., Lim K.S., Loo H.L., Aung M.O., Lim S.G., Ullrich A. Fibroblast growth factor receptor 4 regulates proliferation, anti-apoptosis and alpha-fetoprotein secretion during hepatocellular carcinoma progression and represents a potential target for therapeutic intervention. J. Hepatol. 2009;50:118–127. doi: 10.1016/j.jhep.2008.08.015. [DOI] [PubMed] [Google Scholar]
- 55.Lin B.C., Desnoyers L.R. FGF19 and Cancer. In: Kuro-o M., editor. Endocrine FGFs and Klothos. Springer; New York, NY, USA: 2012. pp. 183–194. [Google Scholar]
- 56.Touat M., Ileana E., Postel-Vinay S., André F., Soria J.-C. Targeting FGFR Signaling in Cancer. Clin. Cancer Res. 2015;21:2684–2694. doi: 10.1158/1078-0432.CCR-14-2329. [DOI] [PubMed] [Google Scholar]
- 57.Michael M., Bang Y.-J., Park Y.S., Kang Y.-K., Kim T.M., Hamid O., Thornton D., Tate S.C., Raddad E., Tie J. A Phase 1 Study of LY2874455, an Oral Selective pan-FGFR Inhibitor, in Patients with Advanced Cancer. Target. Oncol. 2017;12:463–474. doi: 10.1007/s11523-017-0502-9. [DOI] [PubMed] [Google Scholar]
- 58.Saka H., Kitagawa C., Kogure Y., Takahashi Y., Fujikawa K., Sagawa T., Iwasa S., Takahashi N., Fukao T., Tchinou C., et al. Safety, tolerability and pharmacokinetics of the fibroblast growth factor receptor inhibitor AZD4547 in Japanese patients with advanced solid tumours: A Phase I study. Investig. New Drugs. 2017;35:451–462. doi: 10.1007/s10637-016-0416-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Huynh H., Lee L.Y., Goh K.Y., Ong R., Hao H.-X., Huang A., Wang Y., Porta D.G., Chow P., Chung A. Infigratinib mediates vascular normalization, impairs metastasis and improves chemotherapy in hepatocellular carcinoma. Hepatology. 2018 doi: 10.1002/hep.30481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nishina T., Takahashi S., Iwasawa R., Noguchi H., Aoki M., Doi T. Safety, pharmacokinetic, and pharmacodynamics of erdafitinib, a pan-fibroblast growth factor receptor (FGFR) tyrosine kinase inhibitor, in patients with advanced or refractory solid tumors. Investig. New Drugs. 2018;36:424–434. doi: 10.1007/s10637-017-0514-4. [DOI] [PubMed] [Google Scholar]
- 61.Brameld K.A. Abstract SY30-01: Discovery of the highly selective covalent FGFR1-4 inhibitor PRN1371, currently in development for the treatment of solid tumors. Cancer Res. 2016;76:SY30-01. [Google Scholar]
- 62.Futami T., Okada H., Kihara R., Kawase T., Nakayama A., Suzuki T., Kameda M., Shindoh N., Terasaka T., Hirano M., et al. ASP5878, a Novel Inhibitor of FGFR1, 2, 3, and 4, Inhibits the Growth of FGF19-Expressing Hepatocellular Carcinoma. Mol. Cancer Ther. 2017;16:68–75. doi: 10.1158/1535-7163.MCT-16-0188. [DOI] [PubMed] [Google Scholar]
- 63.Martinussen C., Bojsen-Moller K.N., Svane M.S., Dejgaard T.F., Madsbad S. Emerging drugs for the treatment of obesity. Expert Opin. Emerg. Drugs. 2017;22:87–99. doi: 10.1080/14728214.2017.1269744. [DOI] [PubMed] [Google Scholar]
- 64.Weiss A., Porta D.G., Reimann F., Buhles A., Stamm C., Fairhurst R.A., Kinyamu-Akunda J., Sterker D., Murakami M., Wartmann M., et al. Abstract 2103: NVP-FGF401: Cellular and in vivo profile of a novel highly potent and selective FGFR4 inhibitor for the treatment of FGF19/FGFR4/KLB+ tumors. Cancer Res. 2017;77:2103. [Google Scholar]
- 65.Selvaraj A., Corcoran E., Coffey H., Prajapati S., Hao M.-H., Larsen N., Tsai J., Satoh T., Ichikawa K., Joshi J.J., et al. Abstract 3126: H3B6527, a selective and potent FGFR4 inhibitor for FGF19-driven hepatocellular carcinoma. Cancer Res. 2017;77:3126. doi: 10.1158/0008-5472.CAN-17-1865. [DOI] [PubMed] [Google Scholar]
- 66.Bartz R., Fukuchi K., Lange T., Gruner K., Ohtsuka T., Watanabe I., Hayashi S., Redondo-Müller M., Takahashi M., Agatsuma T., et al. Abstract 3852: U3-1784, a human anti-FGFR4 antibody for the treatment of cancer. Cancer Res. 2016;76:3852. [Google Scholar]
- 67.Kim R., Sharma S., Meyer T., Sarker D., Macarulla T., Sung M., Choo S.P., Shi H., Schmidt-Kittler O., Clifford C., et al. First-in-human study of BLU-554, a potent, highly-selective FGFR4 inhibitor designed for hepatocellular carcinoma (HCC) with FGFR4 pathway activation. Eur. J. Cancer. 2016;69:S41. doi: 10.1016/S0959-8049(16)32704-6. [DOI] [Google Scholar]
- 68.Gao L., Shay C., Lv F., Wang X., Teng Y. Implications of FGF19 on sorafenib-mediated nitric oxide production in hepatocellular carcinoma cells—A short report. Cell Oncol. 2018;41:85–91. doi: 10.1007/s13402-017-0354-4. [DOI] [PubMed] [Google Scholar]
- 69.Cheuk A., Shivaprasad N., Skarzynski M., Baskar S., Azorsa P., Khan J. Abstract 5618: Anti-FGFR4 antibody drug conjugate for immune therapy of rhabdomyosarcoma and hepatocellular carcinoma. Cancer Res. 2018;78:5618. [Google Scholar]
- 70.Chen Z. Abstract LB-272: Discovery and characterization of a novel FGFR4 Inhibitor for the treatment of hepatocellular carcinoma. Cancer Res. 2018;78:LB–272. [Google Scholar]
- 71.Lee J., Kang H., Koo K., Ha Y., Lim S.Y., Byun J.-Y., Yu H., Song T., Lee M., Jung S.H., et al. Abstract 4780: A novel, potent and selective FGFR4 inhibitor, HM81422 in hepatocellular carcinoma with FGFR4-driven pathway activation. Cancer Res. 2018;78:4780. [Google Scholar]
- 72.Matsuki M., Hoshi T., Yamamoto Y., Ikemori-Kawada M., Minoshima Y., Funahashi Y., Matsui J. Lenvatinib inhibits angiogenesis and tumor fibroblast growth factor signaling pathways in human hepatocellular carcinoma models. Cancer Med. 2018;7:2641–2653. doi: 10.1002/cam4.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ho H.K., Yeo A.H.L., Kang T.S., Chua B.T. Current strategies for inhibiting FGFR activities in clinical applications: Opportunities, challenges and toxicological considerations. Drug Discov. Today. 2014;19:51–62. doi: 10.1016/j.drudis.2013.07.021. [DOI] [PubMed] [Google Scholar]
- 74.Pai R., French D., Ma N., Hotzel K., Plise E., Salphati L., Setchell K.D.R., Ware J., Lauriault V., Schutt L., et al. Antibody-Mediated Inhibition of Fibroblast Growth Factor 19 Results in Increased Bile Acids Synthesis and Ileal Malabsorption of Bile Acids in Cynomolgus Monkeys. Toxicol. Sci. 2012;126:446–456. doi: 10.1093/toxsci/kfs011. [DOI] [PubMed] [Google Scholar]
- 75.Kaibori M., Sakai K., Ishizaki M., Matsushima H., De Velasco M.A., Matsui K., Iida H., Kitade H., Kwon A.-H., Nagano H., et al. Increased FGF19 copy number is frequently detected in hepatocellular carcinoma with a complete response after sorafenib treatment. Oncotarget. 2016;7:49091–49098. doi: 10.18632/oncotarget.10077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tang X., Wang Y., Fan Z., Ji G., Wang M., Lin J., Huang S., Meltzer S.J. Klotho: A tumor suppressor and modulator of the Wnt/β-catenin pathway in human hepatocellular carcinoma. Lab. Investig. 2016;96:197–205. doi: 10.1038/labinvest.2015.86. [DOI] [PMC free article] [PubMed] [Google Scholar]