Abstract
For type 1 diabetes patients with inadequate glycemic control, one treatment option is to increase the insulin dose (scenario 1), which should not give rise to a “metabolic imbalance.” A second option is additional treatment with a sodium–glucose cotransporter 2 inhibitor, which might lead to a “metabolic imbalance” (scenario 2). A reduction in insulin dose in addition to administration of a sodium–glucose cotransporter 2 inhibitor might further increase the “metabolic imbalance” (scenario 3).
Sodium–glucose cotransporter 2 (SGLT2) inhibitors, the newest class of oral antidiabetes drugs for type 2 diabetes, ameliorate hyperglycemia primarily by preventing the reabsorption of urinary glucose in the kidneys1. Within less than 5 years, abundant evidence for favorable characteristics of SGLT2 inhibitors has accumulated. Of note, large‐scale clinical trials have shown that these drugs prevent cardiovascular events, at least in cohorts at high risk for such events, and that they slow the decline in renal function1. Whereas metabolic and humoral changes induced secondarily by SGLT2 inhibition likely contribute to the beneficial effects of these drugs1, the full picture regarding such changes remains unclear.
Diabetic ketoacidosis (DKA) is a rare but serious complication of diabetes. Insufficient insulin action in adipose tissue results in exaggerated lipolysis and the consequent production of free fatty acids that serve as a source of ketone bodies in the liver. In the liver, insulin inhibits beta‐oxidation by stimulating the activity of acetyl‐CoA carboxylase and inhibiting that of carnitine palmitoyltransferase‐I, which in turn suppresses the production of ketone bodies. Glucagon counteracts these effects of insulin on the hepatic acetyl‐CoA carboxylase–carnitine palmitoyltransferase‐I pathway, and thereby promotes the production of ketone bodies. Both insufficient insulin action and excessive glucagon action can thus contribute to the development of DKA.
Treatment with SGLT2 inhibitors is often associated with an increase in the blood concentration of ketone bodies1. Given that these drugs lower blood glucose levels through a mechanism independent of insulin, their amelioration of hyperglycemia leads to the suppression of insulin secretion from pancreatic β‐cells. Furthermore, SGLT2 inhibitors stimulate the secretion of glucagon, an effect that might be secondary, at least in part, to the attenuation of insulin secretion. A meta‐analysis showed, however, that SGLT2 inhibitors do not significantly increase the incidence of DKA2, indicating that the elevated production of ketone bodies is adequately compensated for in most individuals. Importantly, when DKA does occur in patients taking SGLT2 inhibitors, it often presents with an uncommon feature. Whereas DKA without hyperglycemia, or euglycemic DKA, has long been recognized as a rare condition overall, it occurs not so infrequently in individuals taking SGLT2 inhibitors, with 30–50% of DKA cases taking this form in patients on these drugs3, 4. Given the lack of symptoms related to hyperglycemia, it is often difficult to notice the development of euglycemic DKA. Healthcare providers who prescribe SGLT2 inhibitors should thus recognize the possibility for development of this distinct form of DKA. Several factors are thought to contribute to SGLT2 inhibitor‐related DKA, including the profound impairment of insulin secretion, a lean body composition, long‐term starvation, carbohydrate restriction and termination of or a reduction in insulin or insulin secretagogue administration3.
The SGLT2 inhibitor, ipragliflozin, was recently approved for the treatment of type 1 diabetes in Japan. Although the results of clinical trials for ipragliflozin in type 1 diabetes are not yet publicly available, those for other SGLT2 inhibitors have shown that the drugs reduce glycated hemoglobin levels, body mass and the required insulin dose in patients with type 1 diabetes. However, such patients are at a higher risk for DKA than are type 2 diabetes patients. Whereas the incidence of SGLT2 inhibitor‐related DKA was <0.1% in randomized controlled trials with type 2 diabetes patients4, the incidence increased to ~4–6% in those with type 1 diabetes5, 6. Furthermore, a meta‐analysis showed that the administration of SGLT2 inhibitors significantly increases the incidence of DKA in patients with type 1 diabetes7. SGLT2 inhibitor‐related DKA in type 1 diabetes patients also often develops as euglycemic DKA, with eight of 21 cases (38%) and five of 12 cases (42%) of DKA meeting the general criterion of euglycemic DKA (blood glucose concentration of <250 mg/dL) in randomized controlled trials of dapagliflozin and canagliflozin, respectively5, 6.
Care is thus obviously warranted with the administration of SGLT2 inhibitors to patients with type 1 diabetes. Are there any precautions that can be taken to avoid the development of DKA in such patients treated with these drugs? The characteristics of the 12 patients who developed serious DKA events during the randomized controlled trial of canagliflozin for type 1 diabetes were reported6. The baseline glycated hemoglobin level, duration of diabetes, history of DKA and reduction in body mass did not differ between these individuals and those who did not develop DKA. Information regarding the reduction in insulin dose at the time of the events was not available, but the reduction in insulin dose at the end of the 18‐week trial was greater in patients who took a high dose of the drug (300 mg) and developed DKA than in those who did not develop DKA. Possible contributing factors to the development of DKA included acute illness (pneumonia, influenza, sepsis, gastroenteritis, nonspecified viral infection and tooth extraction with a root canal), insulin pump malfunction, intake of a low‐carbohydrate diet, increased alcohol consumption and non‐compliance with insulin therapy6. Furthermore, cases of SGLT2 inhibitor‐related DKA in patients with type 1 diabetes in clinical practice, in which the drugs were used off‐label, have been reported (Table 1)8, 9, 10, 11, 12. The insulin dose was reduced in seven of eight cases for which such dose information was available. Of the 13 cases, 11 (85%) were euglycemic DKA, and common contributing factors for DKA were reported in some of these cases.
Table 1.
Cases of diabetic ketoacidosis in type 1 diabetes patients treated with sodium–glucose cotransporter 2 inhibitors
| Case | Age, years (sex) | BMI (kg/m2) | HbA1c (%) | BG (mg/dL) | Insulin dose reduction | BW loss | Possible contributing factors | Ref |
|---|---|---|---|---|---|---|---|---|
| 1 | 40 (F) | 26.5 | 11.4 | 220 | Yes (~50%) | NA | Febrile illness, appetite loss, temporary further reduction in insulin dose | 8 |
| 2 | 27 (F) | 24.3 | 7.8 | 150 | Yes (10–15%) | NA | Temporary cessation of basal insulin due to decrease in BG level | 8 |
| 3 | 28 (F) | 25.9 | 8.0 | 224 | Yes | NA | Alcohol consumption | 8 |
| 4 | 31 (F) | 33.2 | 7.0 | 125 | Yes | NA | Exaggerated physical activity (walk for 12 h) | 8 |
| 5 | 55 (F) | 22.0 | 7.2 | 190 | NA | NA | NA | 8 |
| 6 | 26 (F) | 22.0 | 6.6 | 150 | Yes (~25%) | NA | NA | 8 |
| 7 | 39 (F) | 26.1 | 7.0 | 233 | NA | NA | Upper respiratory infection | 8 |
| 8 | 29 (M) | NA | 9.1 | 177 | NA | NA | NA | 9 |
| 9 | 27 (F) | NA | 9.4 | 234 | Yes (~30%) | Yes (13.6 kg) | NA | 10 |
| 10 | 51 (M) | NA | 9.9 | 657 | No (increase of ~15%) | Yes (7.3 kg) | NA | 10 |
| 11 | 27 (F) | NA | 8.4 | 132 | Yes | Yes (4 lb) | Starvation for 1 day? | 11 |
| 12 | NA | NA | NA | <250† | NA | NA | Insulin pump site failure | 12 |
| 13 | NA | NA | NA | 250–300† | NA | NA | Omited basal insulin | 12 |
†Determined with a continuous glucose‐monitoring device. BG, blood glucose; BMI, body mass index; BW, bodyweight; F, female; M, male; NA, information not available; Ref, reference.
Given the rapid glucose‐lowering effect of SGLT2 inhibitors, a reduction in insulin dose is an important consideration to avoid hypoglycemia when the drugs are administered to insulin‐treated patients. In most randomized controlled trials of SGLT2 inhibitors for type 1 diabetes, the insulin dose was reduced, likely accounting for the fact that the incidence of hypoglycemia was not increased6. Insulin not only lowers blood glucose, however, but also inhibits catabolism. Rather, it is more correct to say that insulin lowers blood glucose as a result of its inhibition of catabolism and stimulation of anabolism. Given that SGLT2 inhibitors do not have an anticatabolic effect, the administration of these drugs can result in an imbalance between “glucose‐lowering” and “anticatabolic” effects (Figure 1). A smaller amount of insulin is usually required to prevent exaggerated catabolism than to maintain glycemia, and SGLT2 inhibitors can thus be administered safely in most cases. It should be borne in mind, however, that SGLT2 inhibitors generate a “metabolic imbalance,” even though it might be latent. The serum level of ketone bodies was found to be greater in type 1 diabetes patients who reduced their insulin dose by >20% after the initiation of SGLT2 inhibitors than in those who reduced it by ≤20%, suggesting that caution is warranted in patients who reduce their insulin dose by the larger amount13. The package insert for ipragliflozin states to “pay attention to excessive reduction of insulin dose” when the drug is administered to patients with type 1 diabetes. However, it is difficult to determine whether a reduction in insulin dose is “excessive” or not; even if the reduction is not excessive with regard to the “glucose‐lowering effect,” it might be excessive in terms of the “anticatabolic effect.”
Figure 1.
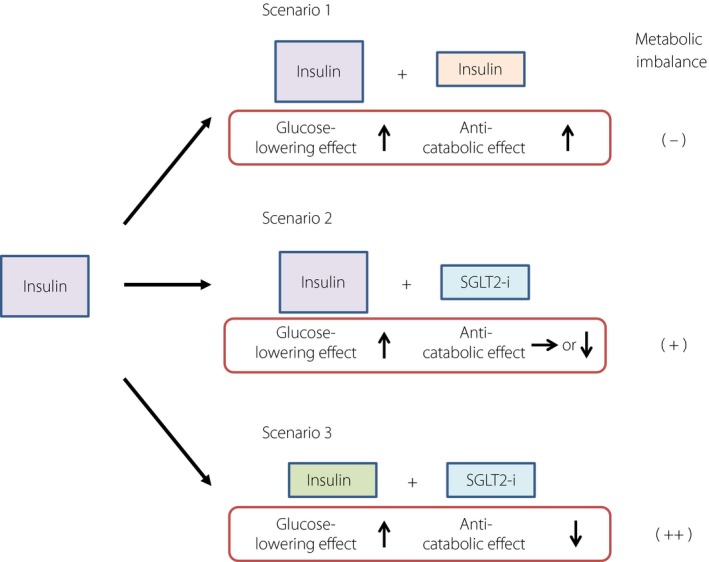
Metabolic imbalance triggered by sodium–glucose cotransporter 2 (SGLT2) inhibitors. For type 1 diabetes patients with inadequate glycemic control, one treatment option is to increase the insulin dose (scenario 1), which should not give rise to a “metabolic imbalance.” A second option is additional treatment with an SGLT2 inhibitor (SGLT2‐i), which might lead to a “metabolic imbalance” (scenario 2). A reduction in insulin dose in addition to administration of an SGLT2 inhibitor might further increase the “metabolic imbalance” (scenario 3).
Do we need to be concerned about DKA if the dose of insulin is not reduced? The insulin dose was not decreased, but was instead increased, in a previously reported case of DKA in a patient with type 1 diabetes (Table 1, case 10)10. It is not clear whether DKA in this case was triggered by the SGLT2 inhibitor and the associated “metabolic imbalance” or by factors unrelated to the drug. One randomized controlled trial showed, however, that the addition of dapagliflozin to type 1 diabetes patients treated with insulin and liraglutide, a glucagon‐like peptide‐1 receptor agonist, resulted in an increase in the plasma concentrations of glucagon and ketone bodies in the absence of a significant reduction in insulin dose14. Although no DKA was reported in this trial, we should be aware of the possibility for an increase in ketone body levels in type 1 diabetes patients treated with SGLT2 inhibitor, even if the insulin dose is not reduced.
For type 1 diabetes patients with inadequate glycemic control, options for medication intensification have included an increase in insulin dose (Figure 1, scenario 1), which would not result in a “metabolic imbalance.” We now have the option of the addition of an SGLT2 inhibitor, which might give rise to a “metabolic imbalance” and increase the risk of hypoglycemia (Figure 1, scenario 2). If the dose of insulin is reduced to minimize this risk, the “metabolic imbalance” might be further increased (Figure 1, scenario 3). The combination of insulin with SGLT2 inhibitors is thus a trade‐off between a possible improvement in glycemic control with a reduction in insulin dose and body mass on the one hand, and the development of a “metabolic imbalance” on the other.
A foolproof approach to the prediction and prevention of SGLT2 inhibitor‐related DKA in patients with type 1 diabetes is not currently available, although a reduction in insulin dose is a key consideration. It is important that not only healthcare providers, but also patients themselves, fully recognize that SGLT2 inhibitors induce a “metabolic imbalance.” Patients should thus avoid precipitating factors for DKA as much as possible, and keep in mind that DKA might develop without hyperglycemia. Given that children, adolescents and young adults with type 1 diabetes develop DKA more frequently than do older patients15, healthcare workers should be especially cautious about prescribing SGLT2 inhibitors to younger individuals. SGLT2 inhibitors exert beneficial effects that other antidiabetes drugs do not possess, and the “metabolic imbalance” induced by this new class of drugs might be related to such beneficial actions1. It remains uncertain, however, whether similar outcomes should be expected in type 1 diabetes patients as in those with type 2 diabetes. The risks and benefits of these drugs for the treatment of type 1diabetes should thus be carefully weighed against each other.
Disclosure
WO has received research support from Abbot, Astellas, AstraZeneca, Boehringer Ingelheim, Daiichi Sankyo, Dainippon‐Sumitomo Pharma, Kyowa Kirin, Mitsubishi Tanabe Pharma, MSD, Novartis, Novo Nordisk Pharma, Ono Pharmaceutical, Sanofi, Taisho Toyama Pharmaceutical, Takeda Pharmaceutical and Teijin Pharma; and has received lecture fees from Abbot, Astellas, Boehringer Ingelheim, Dainippon‐Sumitomo Pharma, Mitsubishi Tanabe Pharma, MSD, Novartis and Takeda Pharmaceutical. YH has received lecture fees from Eli Lilly, Sanofi and Takeda Pharmaceutical.
Acknowledgment
We thank Yuko Okada, Yoshikazu Tamori and Takeshi Ohara for discussion and suggestions.
References
- 1. Kashiwagi A, Maegawa H. Metabolic and hemodynamic effects of sodium‐dependent glucose cotransporter 2 inhibitors on cardio‐renal protection in the treatment of patients with type 2 diabetes mellitus. J Diabetes Investig 2017; 8: 416–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Monami M, Nreu B, Zannoni S, et al Effects of SGLT‐2 inhibitors on diabetic ketoacidosis: a meta‐analysis of randomised controlled trials. Diabetes Res Clin Pract 2017; 130: 53–60. [DOI] [PubMed] [Google Scholar]
- 3. Ogawa W, Sakaguchi K. Euglycemic diabetic ketoacidosis induced by SGLT2 inhibitors: possible mechanism and contributing factors. J Diabetes Investig 2016; 7: 135–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bonora BM, Avogaro A, Fadini G. Sodium‐glucose co‐transporter‐2 inhibitors and diabetic ketoacidosis: an updated review of the literature. Diabetes Obes Metab 2018; 20: 25–33. [DOI] [PubMed] [Google Scholar]
- 5. Peters AL, Henry RR, Thakkar P, et al Diabetic ketoacidosis with canagliflozin, a sodium‐glucose cotransporter 2 inhibitor, in patients with type 1 diabetes. Diabetes Care 2016; 39: 532–538. [DOI] [PubMed] [Google Scholar]
- 6. Dandona P, Mathieu C, Phillip M, et al Efficacy and safety of dapagliflozin in patients with inadequately controlled type 1 diabetes: the DEPICT‐1 52‐Week Study. Diabetes Care 2018; 41: 2552–2559. [DOI] [PubMed] [Google Scholar]
- 7. Yamada T, Shojima N, Noma H, et al Sodium‐glucose co‐transporter‐2 inhibitors as add‐on therapy to insulin for type 1 diabetes mellitus: systematic review and meta‐analysis of randomized controlled trials. Diabetes Obes Metab 2018; 20: 1755–1761. [DOI] [PubMed] [Google Scholar]
- 8. Peters AL, Buschur EO, Buse JB, et al Euglycemic diabetic ketoacidosis: a potential complication of treatment with sodium‐glucose cotransporter 2 inhibition. Diabetes Care 2015; 38: 1687–1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tahir H, Wani A, Daruwalla V, et al Euglycemic diabetic ketoacidosis and severe acute kidney injury secondary to off label use of sodium glucose cotransporter‐2 inhibitor in a type‐1 diabetic patient. J Ayub Med Coll Abbottabad 2015; 27: 923–924. [PubMed] [Google Scholar]
- 10. Harati H, Sharma V, Motazedi A. Sodium‐glucose cotransporter 2 inhibitor‐associated diabetic ketoacidosis: report of two cases with hyperglycemic ketoacidosis in type 1 diabetes. J Diabetes 2016; 8: 165. [DOI] [PubMed] [Google Scholar]
- 11. Bader N, Mirza L. Euglycemic diabetic ketoacidosis in a 27 year‐old female patient with type‐1‐diabetes treated with sodium‐glucose cotransporter‐2 (SGLT2) inhibitor canagliflozin. Pak J Med Sci 2016; 32: 786–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Argento NB, Nakamura K. Glycemic effects of SGLT‐2 inhibitor canagliflozin in type 1 diabetes patients using the Dexcom G4 Platinum CGM. Endocr Pract 2016; 22: 315–322. [DOI] [PubMed] [Google Scholar]
- 13. Henry RR, Dandona PD, Pettus JP, et al Dapagliflozin in patients with type 1 diabetes: a post hoc analysis of the effect of insulin dose adjustments on 24‐hour continuously monitored mean glucose and fasting β‐hydroxybutyrate levels in a phase IIa pilot study. Diabetes Obes Metab 2017; 19: 814–821. [DOI] [PubMed] [Google Scholar]
- 14. Kuhadiya N, Ghanim H, Mehta A, et al Dapagliflozin as additional treatment on liraglutide and insulin in patients with type 1 diabetes. J Clin Endocrinol Metab 2016; 10: 3506–3515. [DOI] [PubMed] [Google Scholar]
- 15. Garg SK, Peters AL, Buse JB, et al Strategy for mitigating DKA risk in patients with type 1 diabetes on adjunctive treatment with SGLT inhibitors: a STICH protocol. Diabetes Technol Ther 2018; 20: 571–575. [DOI] [PubMed] [Google Scholar]


