Abstract
The mammary gland in adult women consists of biologically distinct cell types that differ in their surface phenotypes. Isolation and molecular characterization of these subpopulations of mammary cells have provided extensive insights into their different transcriptional programs and regulation. This information is now serving as a baseline for interpreting the heterogeneous features of human breast cancers. Examination of breast cancer mutational profiles further indicates that most have undergone a complex evolutionary process even before being detected. The consequent intra‐tumoral as well as inter‐tumoral heterogeneity of these cancers thus poses major challenges to deriving information from early and hence likely pervasive changes in potential therapeutic interest. Recently described reproducible and efficient methods for generating human breast cancers de novo in immunodeficient mice transplanted with genetically altered primary cells now offer a promising alternative to investigate initial stages of human breast cancer development. In this review, we summarize current knowledge about key transcriptional regulatory processes operative in these partially characterized subpopulations of normal human mammary cells and effects of disrupting these processes in experimentally produced human breast cancers.
Keywords: breast cancer, chromatin, epigenomics, mammary, transcriptional regulation
Subject Categories: Cancer, Transcription
The normal adult human mammary gland
The adult human female mammary gland is a continuous branching tree of ducts that extend radially from the nipple and terminate in expanded alveolar structures frequently called lobules (Fig 1A). This structure is encased in a basement membrane and an outer layer of fibroblasts, all of which are embedded in a collagen‐rich stroma containing adipocytes, macrophages, lymphocytes, and blood and lymph vessels. The mammary gland, itself, consists of two layers of cells with different features and functions. The outer “basal” layer is made up of cells that are in direct contact with the basement membrane. These cells are also referred to as myoepithelial cells because they possess contractile, smooth muscle‐like properties. The inner “luminal” layer of the gland contains cells with quite different, polarized epithelial features and an ability to produce and secrete milk upon hormone induction.
Figure 1. Macro‐ and microscopic structure of the normal human breast.
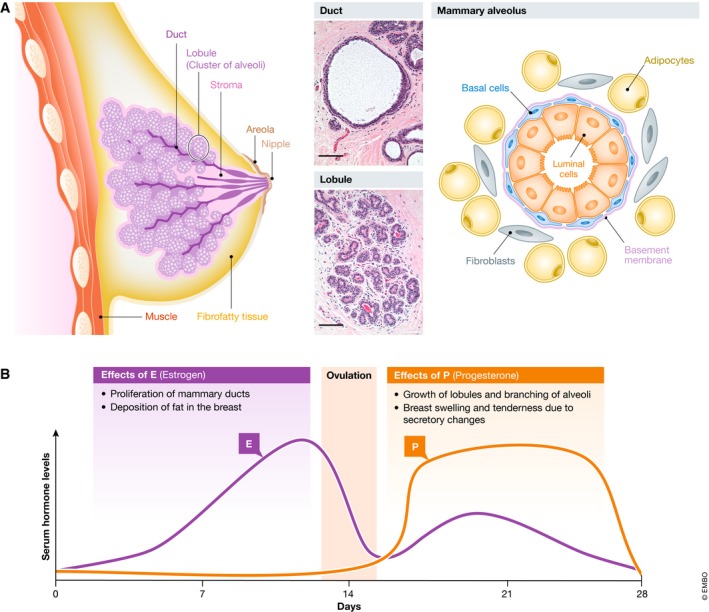
(A) Diagram showing the macroscopic structure of the human breast and histological sections of ducts and alveoli (scale bar = 100 μm). (B) Effects of serum hormone levels on the human mammary epithelium during the menstrual cycle.
The initial stages of development of the mammary gland that take place in humans before birth are not well documented, and hence, knowledge of these has had to rely on inferences drawn from studies of mice (Veltmaat et al, 2003; Spike et al, 2012; Makarem et al, 2013b). In that species, the mammary gland can be seen to originate in the embryo from cells in the ventral ectoderm that invade the underlying mesoderm to form a primitive branching structure. At this stage, the rudimentary gland is composed of cells with a mixture of properties that are associated with distinct cell types found in the adult mouse mammary gland. This primitive structure then expands rapidly after the onset of puberty. Thereafter, until menopause, the entire mammary gland in humans and mice alike undergoes continuous cyclical phases of expansion and involution under the control of changing levels of estrogen (E) and progesterone (P) (Fig 1B; Ramakrishnan et al, 2002). Current evidence indicates that the stimulatory effects of these hormones are exerted indirectly by activating paracrine signaling mechanisms that involve an upregulated production of amphiregulin by E, an induced secretion of RANKL by P, and an enhancing effect of hormonally controlled changes by WNT‐producing macrophages (Wilson et al, 2006; Asselin‐Labat et al, 2010; Brisken & O'Malley, 2010; Joshi et al, 2010; Roarty & Rosen, 2010; Visvader & Stingl, 2014; Arendt & Kuperwasser, 2015; Chakrabarti et al, 2018). Other growth factors implicated in regulating mammary gland development and homeostasis include members of the epidermal growth factor (EGF), insulin‐like growth factor (IGF), and fibroblast growth factor (FGF) families (Hynes & Watson, 2010).
The development of reproducible methods for isolating the different cell types that constitute the major components of the normal adult human mammary gland as separate suspensions of single viable cells was a key advance because it then enabled the further biological and molecular characterization of these different cell types. Most studies of normal human mammary cells have made use of discarded tissue obtained from women without known breast disease undergoing reduction mammoplasties. The pieces of tissue obtained are then subjected to a series of enzymatic dissociation and filtration steps, followed by removal of prevalent blood and endothelial cells using antibodies against CD45 and CD31. The three major cell types that constitute the mammary gland, plus remaining stromal fibroblasts, can then be separately isolated using flow cytometry according to their differential staining with antibodies to CD49f and EpCAM (Fig 2A). The three subpopulations of mammary cells obtained are typically referred to as basal cells (BCs), luminal progenitors (LPs), and luminal cells (LCs). Other antibody cocktails have also been used to obtain highly overlapping phenotypes with very similar biological and molecular properties (Raouf et al, 2008; Bachelard‐Cascales et al, 2010; Keller et al, 2012; Kannan et al, 2014; Nguyen et al, 2014; Fridriksdottir et al, 2015; Lawson et al, 2015; Britschgi et al, 2017), and additional markers have proven useful to subdivide these three subpopulations of human mammary cells even further (Eirew et al, 2012; Shehata et al, 2012; Knapp et al, 2017; Morel et al, 2017). However, the combination of antibodies to CD49f and EpCAM has generally been the most widely utilized.
Figure 2. Subpopulations of cells within the normal adult human mammary gland.
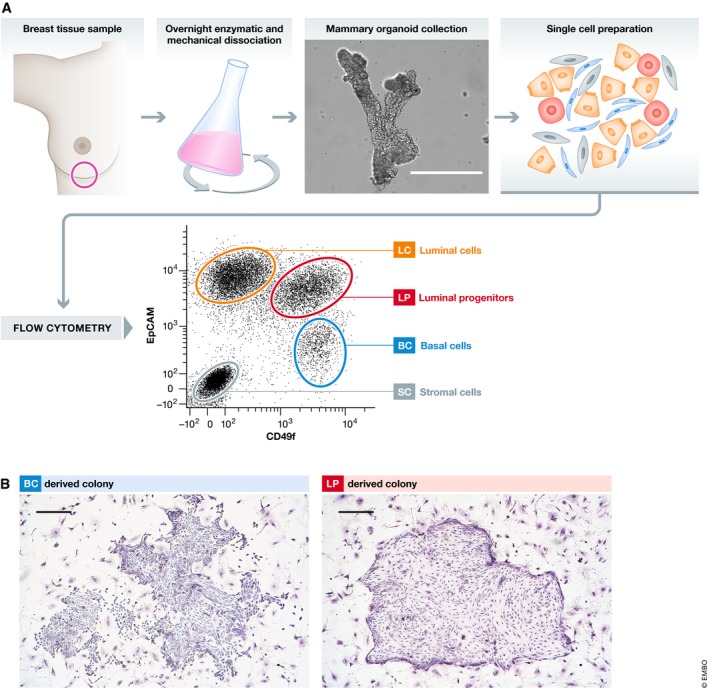
(A) Diagram showing the workflow for separating the four main cell populations present in the breast in addition to blood cells and endothelial cells (scale bar = 400 μm). (B) Examples of typical Giemsa‐stained colonies derived from BCs and LPs and assessed after 7–9 days in vitro (scale bar = 400 μm).
BCs are defined by their CD49f+EpCAMlow phenotype and are so‐named because they express numerous markers (e.g., KRT14, TP63, ACTA2/SMA, MME/CD10, and THY1/CD90) that distinguish cells of the basal layer from those of the luminal layer in histological preparations of normal human mammary tissue. In culture media containing insulin and EGF, as well as other supplements and a feeder layer of fibroblasts, ~ 10–20% of freshly isolated BCs plated at low density will produce readily visualized adherent colonies within 8–10 days (Fig 2B; Eirew et al, 2008; Kannan et al, 2013). Many of the individual colonies produced from BCs under these conditions will contain a mixture of cells expressing either basal or luminal markers (Stingl et al, 2001). A smaller fraction of the BCs (~ 0.1%) will produce bilayered epithelial structures that resemble the normal human mammary gland when injected directly into “humanized” fat pads (Kuperwasser et al, 2004; Proia & Kuperwasser, 2006; Lim et al, 2009) or when transplanted in collagen gels that are then inserted either under the kidney capsule (Eirew et al, 2008, 2010; Nguyen et al, 2015) or subcutaneously (Pellacani D and Eaves C, unpublished) in immunocompromised mice. In both of these sites, the regenerated human mammary gland‐like structures contain the same spectrum of EGF‐dependent in vitro mammary colony‐forming cells (CFCs) that are present in the normal human mammary gland, as well as rarer cells that can regenerate similar bilayered mammary gland structures and mammary CFCs upon transplantation into secondary hosts (Eirew et al, 2008; Lim et al, 2009; Nguyen et al, 2014). In addition, the regenerated human gland‐like structures will produce human milk proteins when appropriately hormonally stimulated (Eirew et al, 2008).
LPs and LCs are defined by their shared high expression of EpCAM, a well‐established marker of cells that constitute the luminal layer of mammary glands. Both LPs and LCs also express other markers histologically associated with the luminal layer (e.g., KRT8, KRT18, and MUC1). However, these EpCAM+ mammary cells can be readily subdivided according to their differential expression of CD49f (and CD117, c‐KIT). LC is the term assigned to the CD49f− cells within the EpCAM+ fraction, and they include most of the cells that express E and P receptors (ER/ESR1 and PR/PGR) and express low to undetectable levels of EGFR (Lim et al, 2009). Not surprisingly, LCs do not mount a significant direct signaling response to EGF (Knapp et al, 2017) and do not proliferate when exposed to EGF in vitro (Kannan et al, 2013, 2014). They are also incapable of reconstituting epithelial structures in vivo that contain clonogenic progeny (Eirew et al, 2008). However, it was recently reported that a small proportion (~ 0.4%) of EpCAM+CD271−CD166highCD117low human mammary cells, a phenotype expected to overlap with CD49f−EpCAMhi LCs, will form colonies in cultures containing inhibitors to the TGF‐β pathway (Fridriksdottir et al, 2015). Interestingly, cultures established from these cells could be expanded for 15 population doublings and their progeny continued to express ER and respond to E stimulation. In mice, similar evidence of the proliferative activity in vivo of non‐clonogenic LCs has also been obtained from BrdU incorporation studies (Giraddi et al, 2015). Together, these findings raise the possibility that at least some human mammary cells with a LC phenotype can proliferate when appropriately stimulated. Nevertheless, the relevance of these in vitro findings to events that underpin the cellular dynamics within the mammary gland of normal adult women remains obscure as, in situ, very few ER+ or PR+ mammary cells appear to be proliferating (Clarke et al, 1997; Stingl, 2011).
LPs are defined as the EpCAM+ cells that co‐express CD49f, suggesting that they might be an intermediate stage between BCs and LCs. However, these cells express other markers specific to the luminal layer of the epithelium assessed histologically, although only a minority express ER or PR (Lim et al, 2009). LPs are also distinct in their expression of high levels of CD117, a marker often used for their differential isolation (Fridriksdottir et al, 2015; Lawson et al, 2015). Approximately 50% of LPs also express KRT5/6 (Lim et al, 2009), a type of cytokeratin known to be expressed by cells in the basal layer of many types of epithelia (Purkis et al, 1990; Böcker et al, 1992). On average, 20–30% of LPs will generate colonies in vitro under the same conditions as BCs (Fig 2B). But, in this case, only cells with luminal features are produced (Stingl et al, 2005). A small proportion of LPs have also been reported to regenerate epithelial structures in vivo (Shehata et al, 2012), but the structures produced do not contain CFCs (Eirew et al, 2008).
Most LPs have very short telomeres and display a pronounced telomere‐associated DNA damage response, even in mammary cells obtained from women in their twenties (Kannan et al, 2013). Interestingly, some LPs expressing activated caspase‐3 will still show considerable subsequent proliferative activity in vitro (Knapp et al, 2017). LPs are also distinguished by elevated levels of reactive oxygen species (ROS) compared to LCs and BCs. In addition, they display an innately greater resistance to oxidative stress and a higher level of associated DNA damage (Kannan et al, 2014), two processes that have been proposed to accelerate telomere shortening (von Zglinicki, 2002; Richter & von Zglinicki, 2007), and predispose cells to transformation.
More recently, single‐cell mass cytometry (Knapp et al, 2017) and RNA sequencing methodologies (Nguyen et al, 2018) have provided further support for the segregation of normal human mammary epithelial cells into the same three main cell types. On the other hand, these studies have also highlighted their extensive molecular heterogeneity and the possible existence of new subsets within each (Shehata et al, 2012; Knapp et al, 2017; Nguyen et al, 2018). Nevertheless, pseudo‐temporal ordering of the available single‐cell transcriptional data produces a differentiation trajectory profile that separates into three main branches corresponding to the historically visualized distinction of cells produced in the normal adult human mammary gland (Nguyen et al, 2018).
Taken together, these findings are consistent with a hierarchically organized sequence of changes initiated in bipotent BCs that are able to generate progeny with either luminal or basal features. Cells with luminal features can then be phenotypically and biologically segregated into an intermediate, luminal‐restricted but EGF‐responsive state, and a state in which the capacity to proliferate in response to EGF has been lost. However, this model of a hierarchical differentiation process should not be viewed as necessarily reflecting a series of tightly co‐ordinated events and may also not reflect the operation of mechanisms that maintain these subpopulations under normal homeostatic conditions. Indeed, in the mouse, where analogous populations of BCs, LPs, and LCs have been identified, some luminal cells possess or can acquire the regenerative activity originally thought to be restricted to BCs (Shehata et al, 2012; Makarem et al, 2013a). In addition, in mice, in situ lineage‐tracing experiments suggest that both myoepithelial and luminal lineages can display self‐sustaining dynamics (Van Keymeulen et al, 2011), despite the continued presence and activity of transplantable cells with the bipotent regenerative properties of “stem cells” (Rios et al, 2014). Such findings are consistent with increasing evidence of an incomplete overlap of mechanisms that control mammary cell proliferative potential and those that determine whether their differentiated state will change (or not) with sequential divisions.
At the same time, it is important to recognize the caveats and assumptions inherent in available methods for associating functional and molecular properties of individual human mammary cells or the history of their acquisition and display. Deriving these associations is necessarily limited by an inability to undertake the requisite prospective lineage‐tracing experiments in humans. Accordingly, direct measurements of normal human mammary cell outputs in situ cannot be compared with the outputs that can be elicited from the same cells when they are exposed to highly stimulatory conditions in vitro or following their transplantation into mice. In addition, both flow cytometry and clonal assays have technical limitations of efficiency and specificity. They may also be compromised by the use of markers that are not co‐ordinately controlled by mechanisms that regulate their functional properties. However, these caveats may be partially reduced by the use of index‐sorting strategies to link molecular and functional properties more directly (Wilson et al, 2015), thereby circumventing the problem of assigning functions of rare cells present in bulk isolates.
Transcriptional differences between human mammary cell subsets
A variety of technologies have been used over the past 10 years to characterize the transcriptomes of BCs, LPs, and LCs isolated from normal adult female breast tissue (Bloushtain‐Qimron et al, 2008; Raouf et al, 2008; Lim et al, 2009, 2010; Maruyama et al, 2011; Shehata et al, 2012; Kannan et al, 2013; Gascard et al, 2015; Pellacani et al, 2016). These studies have revealed consistent differences in the activity of hundreds of genes in each of these phenotypically defined subsets. In turn, these studies have pointed to a number of differentially activated pathways that may regulate their different biological properties (Liu et al, 2005). For example, many components of the NOTCH pathway are expressed at different levels in BCs, LPs, and LCs, with some evidence of corresponding functional consequences (Dontu et al, 2004; Raouf et al, 2008). WNT pathway components also show differential patterns of expression, with biological evidence of their importance in maintaining a mammary stem cell state, at least as inferred from studies of the mouse mammary gland (Teulière et al, 2005; Roarty & Rosen, 2010; Zeng & Nusse, 2010; van Amerongen et al, 2012; Gu et al, 2013) with more limited, but consistent data for human cells (Arendt et al, 2014). Other pathways similarly implicated are the TGF‐β (Moses & Barcellos‐Hoff, 2011; Kahata et al, 2017) and the Hippo pathways (Chen et al, 2014; Pelissier et al, 2014; Skibinski et al, 2014; Shi et al, 2015; Britschgi et al, 2017). Importantly, all of these are variably deregulated in breast cancers (Howard & Ashworth, 2006).
Human mammary cell epigenomes reflect their transcriptional profiles
Several studies have now characterized the epigenomic features of human as well as mouse mammary cells (Maruyama et al, 2011; Choudhury et al, 2013; Dos Santos et al, 2015; Gascard et al, 2015; Huh et al, 2015; Pellacani et al, 2016; Shin et al, 2016; Lee et al, 2017). Early studies reported an association of differences in the H3K27me3 and DNA methylation of genes that are differently expressed in luminal and basal subsets (Maruyama et al, 2011). These genes include several that encode transcriptional regulators and/or other members of pathways of reported activity in the mammary gland. Subsequent analyses revealed DNA methylation to be a stable mark of exonic and intronic usage, with evidence of intron retention events specific to each subpopulation and linked to differences in protein expression (Gascard et al, 2015). The latter study also found many more hypo‐methylated enhancer elements in luminal cells (LPs + LCs) than in BCs and these were commonly associated with binding sites for FOXA1, GATA3, and ZNF217. These studies also indicated a higher overall transcriptional activity in the luminal cells. More extensive epigenomic characterization of highly purified human BCs, LPs, LCs and their associated stromal cells has now been derived from ChIP‐seq analyses of H3K4me1, H3K4me3, H3K27me3, H3K27ac, H3K36me3, and H3K9me3 marks on histones and accompanying whole‐genome bisulfite sequencing, with matching mRNA‐seq and miRNA‐seq data for the same cells (Pellacani et al, 2016). From these datasets, the chromatin landscape at putative enhancer sites of these different mammary cell types has been derived. Comparisons of these have also shown LPs to be intermediate between BCs and LCs, consistent with their different biological properties. Analysis of transcription factor binding sites (TFBS) and derived TF networks for each subpopulation has also enabled novel TFs to be identified as potential regulators of each subpopulation, in addition to others previously reported. Analysis of our more recently accrued epigenomic data has also provided new evidence of a bipartite TF network in LPs that includes elements of those operative in BCs and LCs (Fig 3A). In addition, this study showed that the epigenomic and transcriptional profiles of primary sources of normal human mammary cells are very different from those of established lines of immortalized but non‐tumorigenic mammary cells (Fig 3B; Pellacani et al, 2016). This latter finding highlights the caveats of relying on data from such immortalized cell lines to infer mechanisms controlling the biological properties of normal human mammary cells, and, conversely, the importance of analyzing primary isolates for this purpose.
Figure 3. Transcriptional regulation of normal human mammary cell subpopulations.
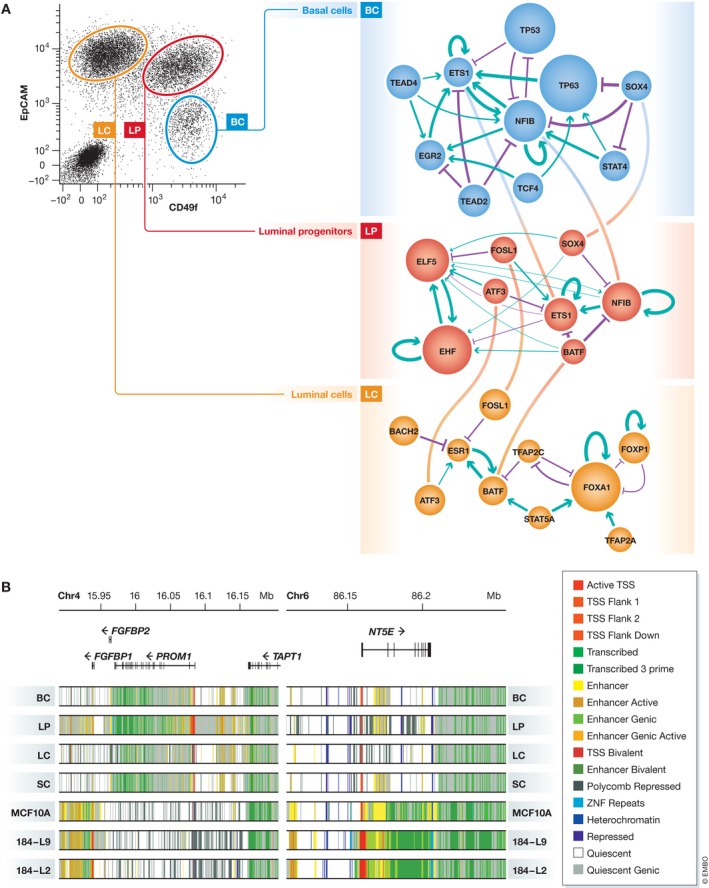
(A) TF regulatory networks constructed from the chromatin profiles at enhancers of BCs, LPs, and LCs. (B) Genome browser plots showing the differences in chromatin states defined for normal human mammary cell subpopulations and non‐tumorigenic mammary cell lines around the PROM1 and the NT5E genes.
Epigenomic and transcriptional changes related to aging and reproductive history
Aging and pregnancy are associated, respectively, with an increase and decrease in breast cancer risk. Several groups have therefore started dissecting the molecular changes evident in mammary cells obtained from donors of different ages or different reproductive histories. These include a report of an expansion with aging of defective multipotent progenitors that show altered interactions with extracellular matrix elements and in KRT14+ and CD49f+ luminal cells (Garbe et al, 2012; Pelissier et al, 2014). Accompanying transcriptome changes suggested an aging‐associated epigenomic deregulation, potentially mediated by changes in the microenvironment of the mammary gland (Miyano et al, 2017). Comparison of the transcriptomes of purified mammary cell subsets isolated from breast tissue of parous and nulliparous women has shown differences between the CD44+ cells from these two sources, with CDKN1B (p27) as one of the most differentially expressed genes (Choudhury et al, 2013). More extensive studies in mice have shown pregnancy to be associated with long‐lasting alterations in DNA methylation profiles at sequences enriched for STAT5 binding sites (Dos Santos et al, 2015).
Transcription factors regulating normal mammary cells
Epigenomic and transcriptional profiling of primary human mammary cells has also led to the identification of many candidate TFs that show subpopulation specificity. For example, several TFs are significantly elevated in only one of the three major subpopulations of normal human mammary cells (Fig 4A–C). In silico predictions further identify a differential enrichment of associated TFBSs at epigenetically defined promoter and enhancer regions in these cell types (Lim et al, 2010; Kannan et al, 2013; Gascard et al, 2015; Pellacani et al, 2016). Several studies in mice or human cell lines have also implicated a multitude of TFs to be involved in mammary cell development and differentiation. However, similar analyses of primary human cells are still very limited, although the strong correlations found between in silico predictions and results obtained from mice justify a brief overview of these.
Figure 4. Transcriptional regulators active in the normal human mammary gland and in human breast cancer.
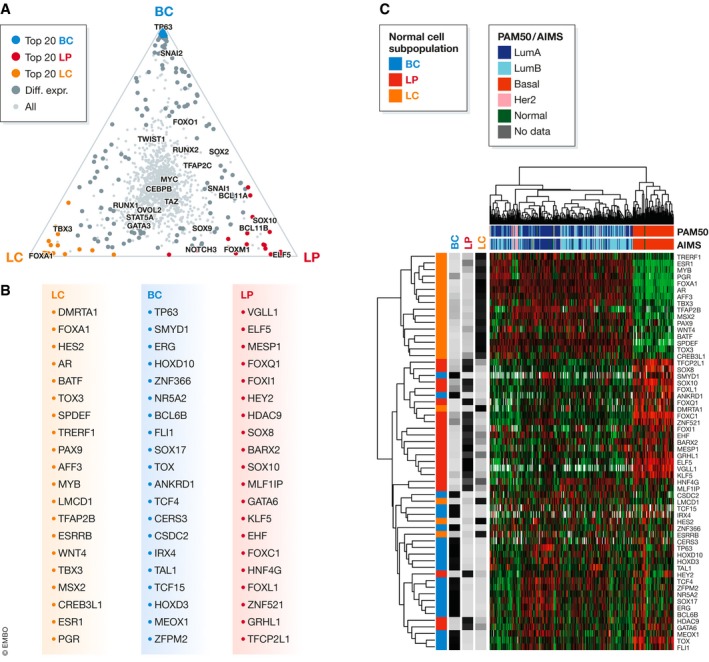
(A) Ternary plot of relative expression of all transcriptional regulators in normal human mammary cell subpopulations from a re‐analysis of the RNA‐seq data presented in Pellacani et al (2016). Transcriptional regulators discussed in the text are highlighted. (B) List of the top 20 transcriptional regulators most specific to each cell type highlighted in (A). (C) Clustering of the tumors profiled by RNA‐seq in Nik‐Zainal et al (2016) using the genes shown in (B).
One of the TFs implicated in modulating mouse mammary stem cell activity by acting directly on BCs is ∆Np63, a known regulator of normal stem cell maintenance in multiple epithelial tissues (Senoo et al, 2007). ∆Np63 appears to act by modulating several key pathways. These include enhancing WNT signaling by upregulating Fzd7 expression (Chakrabarti et al, 2014), activating Hedgehog signaling (Li et al, 2008; Memmi et al, 2015), and partially counteracting the effects of Notch signaling (Yalcin‐Ozuysal et al, 2010). TP63 expression in basal cells is also necessary during pregnancy and lactation: Genetic deletion of Trp63 in keratin 14‐expressing cells of the adult mouse leads to defects in luminal cell proliferation and differentiation, and failure to produce milk, due to lack of expression of the EGF family ligand NRG1 in basal cells which is required for ERBB4/STAT5A activation in luminal cells (Forster et al, 2014). Several SOX family TFs have likewise been implicated. For example, modulation of SOX9 expression was found to directly influence the ability of mouse mammary cells to produce organoid structures in vitro (Guo et al, 2012) and its conditional knockout impaired postnatal development of the gland (Malhotra et al, 2014). SOX10 is expressed specifically in mammary cells exhibiting the highest levels of stem/progenitor activity (Dravis et al, 2015) and SOX2 has also been implicated, albeit less directly, as its expression was induced by LGR4 downstream of WNT signaling (Wang et al, 2013).
Many of these studies in mice have associated expression of SOX TFs with the acquisition of features characteristic of mesenchymal cells in a process resembling an embryonic epithelial–mesenchymal transition (EMT). In fact, the possession of mesenchymal features has been frequently associated with mammary stem cell activity, both during development and subsequently throughout adulthood (Mani et al, 2008; Guen et al, 2017), although this is still controversial (Sikandar et al, 2017). Nevertheless, many other TFs associated with EMT have been directly linked to changes in the clonogenic or repopulating activity of mouse mammary cells. Of these, SNAI2 (SLUG) has been reported to cooperate with SOX9 (Guo et al, 2012) in regulating the transition of mouse mammary stem cells to short‐term progenitors (Phillips et al, 2014). SNAI1 (SNAIL) is another member of this group, and it was found to regulate the spindle orientation machinery in mammary stem cells responding to SLIT2/ROBO1 signaling (Ballard et al, 2015). OVOL2, a transcriptional repressor, was likewise reported to restrict activation of EMT (Watanabe et al, 2014). More recently, another transcription factor, ZEB1, was shown to be expressed at high levels in a fraction of mammary BCs (Nguyen et al, 2018) and associated with cells expressing protein C receptor (ProCR; Wang et al, 2015). ZEB1 was also recently reported to have a protective role against oncogene‐induced DNA damage in normal human mammary epithelial cells (Morel et al, 2017). Other TFs involved in mammary stem cell function include FOXO1 (Sreekumar et al, 2017), RUNX2 (Ferrari et al, 2015), MYC (Hynes & Stoelzle, 2009; Moumen et al, 2012), CEBPB (C/EBPβ; LaMarca et al, 2010), BCL11A (Khaled et al, 2015), and BCL11B (Miller et al, 2018).
TFs implicated in regulating luminal cell production and maintenance have also been identified. Of these, GATA3 was found to have an essential role in controlling the morphogenesis of the mammary gland in the mouse embryo, during puberty, and in adult life (Kouros‐Mehr et al, 2006; Asselin‐Labat et al, 2007). In addition, GATA3 promoted differentiation of cells within the luminal lineage in mice, potentially through a positive regulatory loop with ESR1 (Eeckhoute et al, 2007). FOXA1 was found to be involved in hormone‐induced mammary ductal invasion (Bernardo et al, 2010), but did not affect lobulo‐alveolar maturation and milk production. ELF5 was shown to be necessary for alveologenesis during pregnancy (Choi et al, 2009), and its deletion led to an accumulation of cells with mixed basal/luminal molecular phenotypes (Chakrabarti et al, 2012b). ELF5 was found to suppress EMT by down‐regulating transcription of SNAI2 (Chakrabarti et al, 2012a). ELF5 also acted directly in LPs (Yamaji et al, 2009) to influence expression of STAT5A (Choi et al, 2009), another TF involved in alveologenesis (Liu et al, 1997). Contrary to the effects of RUNX2, RUNX1 was shown to induce the appearance of ER+ luminal cells at least partially through the modulation of ELF5 and FOXA1 expression (van Bragt et al, 2014), potentially downstream of the p38α kinase (Del Barco Barrantes et al, 2018).
Notably, the Hippo pathway regulator TAZ, together with many other TFs, has recently emerged as a negative regulator of luminal differentiation in primary human cells (Skibinski et al, 2014). Other TFs and chromatin modifiers necessary for correct human luminal cell differentiation include TFAP2C (Cyr et al, 2015), TBX3 (Arendt et al, 2014), NOTCH3 (Raouf et al, 2008), FOXM1 (Carr et al, 2012), and KDM6A (Yoo et al, 2016). However, many “potential” TFs identified more recently from genome‐wide epigenomic analyses of both human and mouse mammary gland cells remain poorly characterized.
Cellular and molecular heterogeneity of human breast cancers
Breast cancers arise from single cells as aberrant clones of progeny that undergo a continuous process of evolution, demarcated by distributed genetic and epigenetic alterations in successive generations of daughter cells (Balani et al, 2017). Those that maintain and/or confer a selective growth advantage promote successive waves of subclonal expansion depending on local conditions and/or exposure to therapeutic agents. Such a complex history of subclonal evolution leading to the production of billions of genetically heterogeneous cells in human breast cancers has been dramatically revealed from genomic DNA sequence data (Nik‐Zainal et al, 2012; Eirew et al, 2014). And this profound inter‐tumor as well as intra‐tumor heterogeneity is further exacerbated by the metastatic process in which subclones differentially populated different sites.
Breast cancers are currently classified clinically on the basis of their extent and confinement, or not, within the basement membrane that surrounds the normal mammary gland, the proliferative activity and presence of nuclear abnormalities in the malignant cells, and their expression of ER, PR, and HER2. Global gene expression profiling has led to the identification of five major subtypes (Perou et al, 2000) that can now be distinguished based on the measurement of transcript levels of just 50 genes (PAM50; Parker et al, 2009; Nielsen et al, 2010; Chia et al, 2012). Notably, many of these detect the same perturbed features that have long been recognized histologically (Table 1). The five major subtypes thus identified are referred to as follows: basal‐like, luminal A, and luminal B, normal‐like, and claudin‐low tumors. More recently, additional subdivisions have come from analyses of both genomic sequencing data (Cancer Genome Atlas Network, 2012; Curtis et al, 2012) and altered epigenomic marks (Holm et al, 2010, 2016; Kamalakaran et al, 2011).
Table 1.
List of the genes used for the PAM50 classification
| Symbol | Histology | BC vs. LP | BC vs. LC | LC vs. LP |
|---|---|---|---|---|
| ACTR3B | ||||
| ANLN | ||||
| BAG1 | ||||
| BCL2 | DN | UP | ||
| BIRC5 | ||||
| BLVRA | UP | |||
| CCNB1 | ||||
| CCNE1 | ||||
| CDC20 | ||||
| CDC6 | ||||
| CDH3 | ||||
| CENPF | ||||
| CEP55 | ||||
| CXXC5 | UP | |||
| EGFR | UP | DN | ||
| ERBB2 | ✓ | |||
| ESR1 | ✓ | DN | DN | |
| EXO1 | ||||
| FGFR4 | ||||
| FOXA1 | DN | UP | ||
| FOXC1 | DN | |||
| GPR160 | DN | UP | ||
| GRB7 | ||||
| KIF2C | ||||
| KRT14 | UP | UP | ||
| KRT17 | UP | UP | ||
| KRT5 | UP | UP | DN | |
| MAPT | ||||
| MDM2 | ||||
| MELK | ||||
| MIA | DN | DN | ||
| MKI67 | ✓ | |||
| MLPH | DN | DN | UP | |
| MMP11 | ||||
| MYBL2 | ||||
| MYC | ||||
| NAT1 | DN | UP | ||
| NDC80 | ||||
| NUF2 | ||||
| ORC6 | ||||
| PGR | ✓ | UP | ||
| PHGDH | DN | |||
| PTTG1 | UP | |||
| RRM2 | ||||
| SFRP1 | UP | DN | ||
| SLC39A6 | UP | |||
| TMEM45B | DN | UP | ||
| TYMS | ||||
| UBE2C | ||||
| UBE2T |
Gene products used routinely in histological studies (✓) and transcripts increased (UP) or decreased (DN) in mammary epithelial cells are marked. Differential gene expression data are based on the RNA‐seq data presented in Pellacani et al (2016).
Interestingly, the expression profiles of the five main cancer subtypes are correlated with expression profiles of BCs, LPs, and LCs (Table 1). Even the PAM50 signature relies on an assessment of many gene transcripts (e.g., FOXA1, PGR, ESR1, KRT14, KRT5, EGFR, FOXC1, and MIA) that are normally present at different levels in BCs, LPs, and LCs. Generally, the transcriptional profiles of basal‐like breast cancers are closest to those of LPs, those of luminal A and B cancers to LCs, and claudin‐low cancers to BCs. These findings reinforce the concept that malignancies represent perturbations of the normal tissue from which they arise and frequently retain many components of the transcriptional regulatory networks that control cell production, differentiation, and death in the normal human mammary gland.
Altered transcriptional regulation in human breast cancers
Breast cancer “drivers” is a term that has been used to refer to mutations that are found repeatedly, suggesting they contribute to the malignant properties of the cells. In contrast, “passenger mutations” is a term often assigned to mutations that are rare and do not appear to be relevant to the genesis or progression of the malignant population. It is notable that a majority of the most frequently encountered mutations affect genes linked directly or indirectly to transcriptional regulation (Nik‐Zainal et al, 2016; Zacksenhaus et al, 2017).
One of the most frequently altered transcriptional regulators is GATA3 (mutated in > 10% of cases; Cancer Genome Atlas Network, 2012), most often in ER+ breast cancers (Fig 5; Nik‐Zainal et al, 2016). Both clinical and experimental lines of evidence link mutations in GATA3 directly to breast cancer development and progression. Expression of GATA3 has been associated with a favorable prognosis, although this is still debated (Chou et al, 2010; Takaku et al, 2018), and similarly, in mice and cell lines, a heightened expression reduces tumorigenesis, suppresses metastasis, and promotes expression of a luminal molecular signature. In contrast, a loss of GATA3 has been found to accelerate tumor progression (Asselin‐Labat et al, 2011; Chou et al, 2013).
Figure 5. Frequency of genomic alteration of GATA3, MYC, FOXA1, and KMT2C in human breast cancer subtypes.
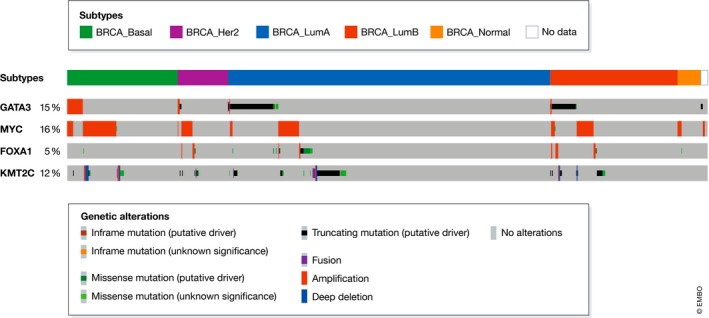
Heatmap showing the frequency of genomic alterations detected in GATA3, MYC, FOXA1, and KMT2C in human breast cancer subtypes. Data are drawn from the 993 breast cancer cases in the TCGA PanCancer Atlas study analyzed and plotted via cBioportal (http://www.cbioportal.org).
In > 15% of breast cancers, MYC is amplified. This is generally associated with an unfavorable clinical prognosis (Deming et al, 2000) and an ER− breast cancer phenotype (Fig 5; Nik‐Zainal et al, 2016). MYC is one of the most intensively studied oncogenes (Fallah et al, 2017). Of particular note is recent evidence that overexpression of MYC in immortalized human mammary cells triggers a reprogramming of the epigenome that confers tumor‐initiating proprieties and a down‐regulation of luminal‐specific TFs and genes (Poli et al, 2018). MYC activity has also been shown recently to be influenced by its interaction with EPIC1, a long non‐coding RNA, that is upregulated in many cancers (Wang et al, 2018). Interestingly, MYC amplification was also reported to be a frequent event in the genesis of transformants from primary human mammary cells (Elenbaas et al, 2001) and in radiation‐induced mammary cell lines (Wade et al, 2015).
FOXA1 is a TF that is mutated or amplified less frequently in human breast cancers (~ 2% mutated and ~ 1% amplified; Cancer Genome Atlas Network, 2012; Robinson et al, 2013) and usually found to be altered in ER+ tumors (Fig 5; Nik‐Zainal et al, 2016). FOXA1 is a main regulator of steroid receptor function in cancer (Augello et al, 2011), and it regulates ER signaling in breast cancer (Carroll et al, 2005; Lupien et al, 2008). FOXA1 mediates ESR1 binding and transcriptional activity (Hurtado et al, 2011), and its expression is associated with superior breast cancer outcomes (Shou et al, 2016). Molecularly, FOXA1 can recruit KMT2C (MLL3) to deposit H3K4me1 on FOXA1‐bound enhancers (Jozwik et al, 2016).
KMT2C is another frequently mutated transcriptional regulator in breast cancer, with a mutational spectrum consistent with a loss‐of‐function role (Wang et al, 2011; Ellis et al, 2012; Cancer Genome Atlas Research Network, 2015). Functionally, it is the catalytic component of a complex called COMPASS (complex of proteins associated with Set1) or ASCOM (ASC‐2‐ and MLL3‐containing complex) and responsible for the monomethylation of H3K4 (Herz et al, 2014). In mouse models, Mll3 deletion in the mammary gland results in hyperplasia and expansion of cells with basal features in transplant experiments, and an acceleration of PI3K‐driven tumorigenesis (Zhang et al, 2016), supporting its role as a tumor suppressor.
Many other histone methyltransferases are deregulated in breast cancer by genetic alteration (Michalak & Visvader, 2016) and thereby contribute to an increased emergence of epigenomic alterations in breast cancer. Consequent changes in the epigenomes of analyzed human breast cancers have revealed more than 100 frequently hyper‐ or hypo‐methylated gene promoters and pronounced global DNA hypo‐methylation (Davalos et al, 2017; Pasculli et al, 2018), and the functional implication of these changes is now starting to be investigated using CRISPR/Cas9 systems (Saunderson et al, 2017). These findings are particularly interesting clinically, as they may offer new biomarkers of risk, prognosis, and treatment response (Pouliot et al, 2015; Terry et al, 2016) that can be robustly measured at relatively low cost (Cheuk et al, 2017). However, downstream transcriptional alterations are not consistently predicted and many exceptions to the general inverse correlation between promoter methylation and gene expression exist. In addition, expression of many frequently hypermethylated genes in breast cancer cells is already repressed in normal mammary cells, usually by polycomb group proteins depositing H3K27me3 (Sproul et al, 2011).
Comparisons of the DNA methylation profiles of individual breast cancers have shown they are highly heterogeneous. However, when subjected to unsupervised clustering, these profiles subdivide into groups that correspond largely to established transcriptionally defined breast cancer subtypes with corresponding similarities to normal human mammary subpopulations (Holm et al, 2010, 2016; Kamalakaran et al, 2011). From these, specific DNA methylation signatures with prognostic potential have been derived for luminal B and basal‐like subtypes (Stefansson et al, 2015).
Interestingly, DNA sequence alterations that do not occur within regions that encode protein sequences directly (non‐coding mutations) represent ~ 98% of mutations in cancer and most still remain poorly characterized. Of these, mutations occurring in cis‐regulatory elements (i.e., enhancers and promoters) are of particular interest, as they can directly alter expression of associated gene products, by directly or indirectly altering DNA binding of TFs (Deplancke et al, 2016; Shi et al, 2016). Such mutations are frequent in breast cancer (Bailey et al, 2016; Zhou et al, 2016; Rheinbay et al, 2017; Gyorffy et al, 2018), but their significance is generally unclear (Nik‐Zainal et al, 2016). However, mutations in ESR1 enhancer sequences found in ~ 7% of breast cancers have now been shown to be responsible for altering ESR1 expression by modulating TF binding activity (Bailey et al, 2016). In addition, a single‐nucleotide variant in one of these enhancer sequences has been associated with increased breast cancer risk. Mutations in the promoter of FOXA1 that cause its overexpression through increased E2F binding constitute a second documented example of a biologically relevant mutation in a cis‐element in some breast cancer genomes (Rheinbay et al, 2017). Variants linked to increased breast cancer risk have been found in distal regulatory elements of genes whose expression is modulated by FOXA1 (Cowper‐Sal Lari et al, 2012).
Breast cancers also contain many cell types that are not part of the malignant population but, nevertheless, interact with them and co‐evolve with them, adding further to the complexity and heterogeneity of breast tumors (Hanahan & Weinberg, 2011). These additional cell types include components of the blood and lymph vasculature, tissue macrophages and lymphocytes, and various stromal fibroblasts and their derivatives. Both the infiltrating leukocytes and resident cancer‐associated fibroblasts (CAFs) are now well established as playing significant roles in modulating breast cancer cell growth and plasticity through direct interactions as well as through their secretion of growth factors, cytokines, and extracellular matrix components (Allinen et al, 2004; Aboussekhra, 2011; Place et al, 2011; Esquivel‐Velázquez et al, 2015; Qiao et al, 2016).
One of the best characterized mechanisms of CAF modulation of human breast cancer cells is mediated by their secretion of TGF‐β. Recently, this has been updated to include the suppression of adjacent normal mammary cells (Chatterjee et al, 2018) and the promotion of EMT in a xenografted breast cancer cell line through the transactivation of a HOX transcript antisense RNA (Ren et al, 2018). A third recently described role of CAFs is their induction of a FOXA1‐mediated creation of a hormone‐sensitive, luminal gene regulatory program in basal‐like breast cancers in response to PDGF secretion by the tumor cells (Roswall et al, 2018). Loss of TP53 in stromal fibroblasts has also been shown to promote breast tumor development in vivo through the production of SDF‐1 (Addadi et al, 2010). Additional reported mechanisms include the altered expression in CAFs of non‐coding RNAs and microRNAs (Verghese et al, 2013; Shah et al, 2015; Ren et al, 2018). Other components of the tumor microenvironment, including tumor‐associated macrophages, have been implicated in tumor promotion through the expression of TFEB (Fang et al, 2017).
Transcriptional deregulation during the initiation of breast cancers
Early events important to the genesis of human breast cancer are still limited and largely extrapolated from transgenic mouse models. Information derived from studies of human cancers has been largely limited to retrospective analyses of prevalent changes in established tumors (Futreal et al, 2004; Nik‐Zainal et al, 2012), or a few analyses of preneoplastic mammary cells were obtained from carriers of BRCA1 mutations (Lim et al, 2009; Proia et al, 2011; Choudhury et al, 2013) or from samples of ductal carcinoma in situ (DCIS; Yeong et al, 2017). Events that accompany the acquisition of malignant properties by immortalized, but non‐tumorigenic, human mammary cell lines have also been described (Debnath et al, 2003; Leung & Brugge, 2012). More recently, experimental models initiated directly with primary human mammary cells have been reported.
Transgenically controlled overexpression of potential culprit genes in mice, including overexpression of MYC and HER2, was important in providing the first experimental evidence that oncogene overexpression alone could induce the formation of malignant tumors (Stewart et al, 1984; Muller et al, 1988; Bouchard et al, 1989). Since then, derivative approaches are now able to model metastasis due to expression of co‐operating oncogenes (Sinn et al, 1987; Guy et al, 1992; Podsypanina et al, 2008; Adams et al, 2011) and assess mechanisms of pathway perturbation including TGF‐β and WNT (Pierce et al, 1995; Li et al, 2000). The introduction of conditional and inducible systems to drive the expression of transgenes has enabled these models to be further refined (Sandgren et al, 1995; Moody et al, 2002; Podsypanina et al, 2008; Menezes et al, 2014; Rutkowski et al, 2014), including a model in mice of invasive lobular breast cancer created using CRISPR/Cas9‐mediated disruption of PTEN (Annunziato et al, 2016).
However, a major criticism of these mouse models of breast cancer is the very ease with which the tumors can be generated. They also frequently lack the genetic complexity of human breast cancers, and their similarities to their human counterparts are often restricted to specific sites within the tumors produced (Cardiff et al, 2000; Hollern et al, 2018). In addition, their pathology may be highly dependent on the promoters used to drive expression of the oncogenic transgene and few display highly invasive properties (Cardiff et al, 2000). Gene expression differences in mice are also notable (Pfefferle et al, 2013), and some types of human breast cancer have not yet been possible to model in mice. For example, although ER+ tumors account for the majority of all human breast cancers, stably ER+ mouse mammary tumors have been difficult to obtain and the genetic changes that lead to ER expression in mouse tumors are frequently not characteristic of patients’ ER+ tumors (Mohibi et al, 2011).
Immortalized cell lines, and the MCF10A line in particular, have also been used for modeling the human mammary cell transformation process also because of their ease of use and manipulation and their availability in virtually unlimited numbers. MCF10A cells were generated by immortalizing human mammary cells obtained from a donor with benign fibrocystic disease (Soule et al, 1990). Forced expression of multiple cancer genes in these cells has been found to induce some features of transformation (recently reviewed in Balani et al, 2017). Notably, aggressively tumorigenic lines have been derived from MCF10A cells forced to overexpress HRAS and passaged in vivo, and their extensive characterization has revealed the presence of a number of predicted driver mutations (Maguire et al, 2016). However, their controlled modification has not recapitulated the phenotypic, genomic, and functional heterogeneity found in most spontaneously arising human breast cancers (Kaur & Dufour, 2012).
Analysis of DCIS has been another strategy used to investigate early events leading to invasive breast cancer. Initial transgenic mouse models of DCIS were obtained by driving expression of the SV40 large tumor antigen with the mouse WAP promoter that becomes highly active in terminal lobular luminal cells in pregnant mice (Schulze‐Garg et al, 2000). More recently, in vivo models of human DCIS have also been developed by the intraductal injection of mice with experimentally transformed human cell lines (Behbod et al, 2009) or primary DCIS samples from patients (Valdez et al, 2011). These models generally recapitulate the histology and heterogeneity of the human disease, including occasional examples of disease progression indicated by cellular invasion into the surrounding stroma.
Experimental models of de novo mammary tumorigenesis starting from isolated primary cells from normal tissues are particularly attractive because they avoid species differences and concerns of extrapolating from human immortalized cell lines. However, there are very few reports of genetic perturbations that consistently yield fully malignant human mammary cells in transplanted female immunodeficient mouse hosts (either NOD/SCID or NRG—NOD‐Rag1 −/−‐IL2Rγc −/− mice). Interestingly, most of those that have been reported have used different combinations of oncogenes, cell types, and sites of injection, with or without added fibroblasts. Immunohistological analyses of tumors produced from human EpCAM+ luminal cells transduced with either TP53R175H + CCND1 + myristoylated PIK3CA + KRASG12V or SV40 T antigen + KRASG12V transplanted into “humanized” fat pads of NOD/SCID mice (obtained by added injection of human fibroblasts) suggested the tumors most closely resemble ductal carcinomas with predominant luminal features, including expression of ERα, CK8/18, and CK19. In contrast, the same manipulation of CD10+ (basal) cells caused them to acquire squamous and metaplastic features with reduced ERα and CK19 expression and robust expression of the basal marker, CK14 (Keller et al, 2012). On the other hand, we have found that transduction of either normal human BCs or LPs (but not LCs or SCs from the same mammoplasty samples) with just a KRAS G12D‐encoding vector produces serially transplantable invasive ductal carcinomas rapidly and at high efficiency in mice using injection sites under the kidney capsule or subcutaneously (Nguyen et al, 2015). These KRAS G12D‐derived tumors are also highly heterogeneous with variable proportions of cells positive for ERα, Ki67, EGFR, CK14, and CK8/18, independently of their BC or LP cell of origin (Nguyen et al, 2015).
Use of a DNA barcoding strategy, to track the clonal dynamics of the primary and secondary KRAS G12D‐derived tumors, showed them to be consistently and highly polyclonal, regardless of the initial cell type transduced (Nguyen et al, 2015). The median size of the few clones found in both primary and secondary tumors derived from the same initial inoculum was larger than most of the clones appearing only after a first passage. Interestingly, normal human mammary cells transduced with the same tracking vector also showed a delayed appearance of new and larger clones in the “normal” structures obtained in secondary as compared to primary recipients of the same original cells (Nguyen et al, 2014). The invasive nature of the primary clones but their general lack of perpetuation in secondary implants contrasts with the conventional concept of the oncogenic process, in which the control of invasive properties by human mammary cells is usually modeled as property that is acquired after deregulated growth has created a large “premalignant” population from which a more advanced derivative then arises. Taken together, these findings thus challenge previous assumptions of a requirement for a multi‐step selective process during which the genetic and/or epigenetic changes needed to obtain a continuously growing invasive tumor are successively accrued.
Transcriptional profiling of the polyclonal KRAS G12D‐induced primary tumors we have described has shown they are characterized by a global deregulation of gene expression that is largely but not completely independent of the cell type used to initiate them (Nguyen et al, 2015). A similar result was found for tumors derived by transducing primary cells from the same normal donors with SV40 T antigen + KRASG12V (Keller et al, 2012) or cells from donors with a different BRCA1 mutation status using vectors encoding TP53R175H + CCND1 + myristoylated PIK3CA + KRASG12V (Proia et al, 2011). Thus, the initiating cell type may not necessarily make a major contribution to the transcriptional profile of the cells constituting the bulk of any breast cancer. Such a concept is of interest as it challenges the idea that globally acquired molecular profiles of breast cancers will provide informative indications of the cell of origin or the cells from which relapses are most likely to emerge.
Conclusions
Heterogeneity is a pronounced feature of human breast cancer genomes and epigenomes. These variable features likely explain the corresponding heterogeneity evident in the transcriptomes of these malignant populations. The multitude of these alterations, plus the still partial elucidation of the molecular networks governing the properties of normal human mammary cells, still obscures identification of critical initial transforming events. Nevertheless, early changes that lead to human breast cancer development remain important potential targets for more effective strategies. Expansion of de novo models now appears possible with established robust transduction protocols and new screening approaches on the horizon. The coupling of these strategies with clonal analyses, highly multiplexed gene manipulations, and exposure to small molecules thus holds new promise for the future more rapid identification of targetable mechanisms critical to breast cancer development.
Conflict of interest
The authors declare that they have no conflict of interest.
Acknowledgements
This review was prepared with support from the BC Cancer Foundation Strategic Priorities fund, grant #702851 from the Canadian Cancer Society Research Institute and grant #22416 jointly funded by The Cancer Research Society and the Canadian Institutes of Health Research (CIHR). S Tan held a CIHR Banting and Best Research Studentship.
The EMBO Journal (2019) 38: e100330
References
- Aboussekhra A (2011) Role of cancer‐associated fibroblasts in breast cancer development and prognosis. Int J Dev Biol 55: 841–849 [DOI] [PubMed] [Google Scholar]
- Adams JR, Xu K, Liu JC, Agamez NMR, Loch AJ, Wong RG, Wang W, Wright KL, Lane TF, Zacksenhaus E, Egan SE (2011) Cooperation between Pik3ca and p53 mutations in mouse mammary tumor formation. Cancer Res 71: 2706–2717 [DOI] [PubMed] [Google Scholar]
- Addadi Y, Moskovits N, Granot D, Lozano G, Carmi Y, Apte RN, Neeman M, Oren M (2010) p53 status in stromal fibroblasts modulates tumor growth in an SDF1‐dependent manner. Cancer Res 70: 9650–9658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allinen M, Beroukhim R, Cai L, Brennan C, Lahti‐Domenici J, Huang H, Porter D, Hu M, Chin L, Richardson A, Schnitt S, Sellers WR, Polyak K (2004) Molecular characterization of the tumor microenvironment in breast cancer. Cancer Cell 6: 17–32 [DOI] [PubMed] [Google Scholar]
- van Amerongen R, Bowman AN, Nusse R (2012) Developmental stage and time dictate the fate of Wnt/β‐catenin‐responsive stem cells in the mammary gland. Cell Stem Cell 11: 387–400 [DOI] [PubMed] [Google Scholar]
- Annunziato S, Kas SM, Nethe M, Yücel H, Del Bravo J, Pritchard C, Bin Ali R, van Gerwen B, Siteur B, Drenth AP, Schut E, van de Ven M, Boelens MC, Klarenbeek S, Huijbers IJ, van Miltenburg MH, Jonkers J (2016) Modeling invasive lobular breast carcinoma by CRISPR/Cas9‐mediated somatic genome editing of the mammary gland. Genes Dev 30: 1470–1480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arendt LM, St Laurent J, Wronski A, Caballero S, Lyle SR, Naber SP, Kuperwasser C (2014) Human breast progenitor cell numbers are regulated by WNT and TBX3. PLoS One 9: e111442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arendt LM, Kuperwasser C (2015) Form and function: how estrogen and progesterone regulate the mammary epithelial hierarchy. J Mammary Gland Biol Neoplasia 20: 9–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asselin‐Labat M‐L, Sutherland KD, Barker H, Thomas R, Shackleton M, Forrest NC, Hartley L, Robb L, Grosveld FG, van der Wees J, Lindeman GJ, Visvader JE (2007) Gata‐3 is an essential regulator of mammary‐gland morphogenesis and luminal‐cell differentiation. Nat Cell Biol 9: 201–209 [DOI] [PubMed] [Google Scholar]
- Asselin‐Labat M‐L, Vaillant F, Sheridan JM, Pal B, Wu D, Simpson ER, Yasuda H, Smyth GK, Martin TJ, Lindeman GJ, Visvader JE (2010) Control of mammary stem cell function by steroid hormone signalling. Nature 465: 798–802 [DOI] [PubMed] [Google Scholar]
- Asselin‐Labat M‐L, Sutherland KD, Vaillant F, Gyorki DE, Wu D, Holroyd S, Breslin K, Ward T, Shi W, Bath ML, Deb S, Fox SB, Smyth GK, Lindeman GJ, Visvader JE (2011) Gata‐3 negatively regulates the tumor‐initiating capacity of mammary luminal progenitor cells and targets the putative tumor suppressor caspase‐14. Mol Cell Biol 31: 4609–4622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augello MA, Hickey TE, Knudsen KE (2011) FOXA1: master of steroid receptor function in cancer. EMBO J 30: 3885–3894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachelard‐Cascales E, Chapellier M, Delay E, Pochon G, Voeltzel T, Puisieux A, Caron de Fromentel C, Maguer‐Satta V (2010) The CD10 enzyme is a key player to identify and regulate human mammary stem cells. Stem Cells 28: 1081–1088 [DOI] [PubMed] [Google Scholar]
- Bailey SD, Desai K, Kron KJ, Mazrooei P, Sinnott‐Armstrong NA, Treloar AE, Dowar M, Thu KL, Cescon DW, Silvester J, Yang SYC, Wu X, Pezo RC, Haibe‐Kains B, Mak TW, Bedard PL, Pugh TJ, Sallari RC, Lupien M (2016) Noncoding somatic and inherited single‐nucleotide variants converge to promote ESR1 expression in breast cancer. Nat Genet 48: 1260–1266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balani S, Nguyen LV, Eaves CJ (2017) Modeling the process of human tumorigenesis. Nat Commun 8: 15422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballard MS, Zhu A, Iwai N, Stensrud M, Mapps A, Postiglione MP, Knoblich JA, Hinck L (2015) Mammary stem cell self‐renewal is regulated by Slit2/Robo1 signaling through SNAI1 and mINSC. Cell Rep 13: 290–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behbod F, Kittrell FS, LaMarca H, Edwards D, Kerbawy S, Heestand JC, Young E, Mukhopadhyay P, Yeh H‐W, Allred DC, Hu M, Polyak K, Rosen JM, Medina D (2009) An intraductal human‐in‐mouse transplantation model mimics the subtypes of ductal carcinoma in situ . Breast Cancer Res 11: R66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardo GM, Lozada KL, Miedler JD, Harburg G, Hewitt SC, Mosley JD, Godwin AK, Korach KS, Visvader JE, Kaestner KH, Abdul‐Karim FW, Montano MM, Keri RA (2010) FOXA1 is an essential determinant of ERalpha expression and mammary ductal morphogenesis. Development 137: 2045–2054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloushtain‐Qimron N, Yao J, Snyder EL, Shipitsin M, Campbell LL, Mani SA, Hu M, Chen H, Ustyansky V, Antosiewicz JE, Argani P, Halushka MK, Thomson JA, Pharoah P, Porgador A, Sukumar S, Parsons R, Richardson AL, Stampfer MR, Gelman RS et al (2008) Cell type‐specific DNA methylation patterns in the human breast. Proc Natl Acad Sci USA 105: 14076–14081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böcker W, Bier B, Freytag G, Brömmelkamp B, Jarasch ED, Edel G, Dockhorn‐Dworniczak B, Schmid KW (1992) An immunohistochemical study of the breast using antibodies to basal and luminal keratins, alpha‐smooth muscle actin, vimentin, collagen IV and laminin. Part I: normal breast and benign proliferative lesions. Virchows Arch A Pathol Anat Histopathol 421: 315–322 [DOI] [PubMed] [Google Scholar]
- Bouchard L, Lamarre L, Tremblay PJ, Jolicoeur P (1989) Stochastic appearance of mammary tumors in transgenic mice carrying the MMTV/c‐neu oncogene. Cell 57: 931–936 [DOI] [PubMed] [Google Scholar]
- van Bragt MPA, Hu X, Xie Y, Li Z (2014) RUNX1, a transcription factor mutated in breast cancer, controls the fate of ER‐positive mammary luminal cells. Elife 4: e03881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brisken C, O'Malley B (2010) Hormone action in the mammary gland. Cold Spring Harb Perspect Biol 2: a003178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britschgi A, Duss S, Kim S, Couto JP, Brinkhaus H, Koren S, De Silva D, Mertz KD, Kaup D, Varga Z, Voshol H, Vissieres A, Leroy C, Roloff T, Stadler MB, Scheel CH, Miraglia LJ, Orth AP, Bonamy GMC, Reddy VA et al (2017) The Hippo kinases LATS1 and 2 control human breast cell fate via crosstalk with ERα. Nature 541: 541–545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cancer Genome Atlas Network (2012) Comprehensive molecular portraits of human breast tumours. Nature 490: 61–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cancer Genome Atlas Research Network (2015) The molecular taxonomy of primary prostate cancer. Cell 163: 1011–1025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardiff RD, Anver MR, Gusterson BA, Hennighausen L, Jensen RA, Merino MJ, Rehm S, Russo J, Tavassoli FA, Wakefield LM, Ward JM, Green JE (2000) The mammary pathology of genetically engineered mice: the consensus report and recommendations from the Annapolis meeting. Oncogene 19: 968–988 [DOI] [PubMed] [Google Scholar]
- Carr JR, Kiefer MM, Park HJ, Li J, Wang Z, Fontanarosa J, DeWaal D, Kopanja D, Benevolenskaya EV, Guzman G, Raychaudhuri P (2012) FoxM1 regulates mammary luminal cell fate. Cell Rep 1: 715–729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll JS, Liu XS, Brodsky AS, Li W, Meyer CA, Szary AJ, Eeckhoute J, Shao W, Hestermann EV, Geistlinger TR, Fox EA, Silver PA, Brown M (2005) Chromosome‐wide mapping of estrogen receptor binding reveals long‐range regulation requiring the forkhead protein FoxA1. Cell 122: 33–43 [DOI] [PubMed] [Google Scholar]
- Chakrabarti R, Hwang J, Andres Blanco M, Wei Y, Lukačišin M, Romano R‐A, Smalley K, Liu S, Yang Q, Ibrahim T, Mercatali L, Amadori D, Haffty BG, Sinha S, Kang Y (2012a) Elf5 inhibits the epithelial‐mesenchymal transition in mammary gland development and breast cancer metastasis by transcriptionally repressing Snail2. Nat Cell Biol 14: 1212–1222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti R, Wei Y, Romano R‐A, DeCoste C, Kang Y, Sinha S (2012b) Elf5 regulates mammary gland stem/progenitor cell fate by influencing notch signaling. Stem Cells 30: 1496–1508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti R, Wei Y, Hwang J, Hang X, Andres Blanco M, Choudhury A, Tiede B, Romano R‐A, DeCoste C, Mercatali L, Ibrahim T, Amadori D, Kannan N, Eaves CJ, Sinha S, Kang Y (2014) ΔNp63 promotes stem cell activity in mammary gland development and basal‐like breast cancer by enhancing Fzd7 expression and Wnt signalling. Nat Cell Biol 16: 1004–1015, 1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti R, Celià‐Terrassa T, Kumar S, Hang X, Wei Y, Choudhury A, Hwang J, Peng J, Nixon B, Grady JJ, DeCoste C, Gao J, van Es JH, Li MO, Aifantis I, Clevers HC, Kang Y (2018) Notch ligand Dll1 mediates cross‐talk between mammary stem cells and the macrophageal niche. Science 360: eaan4153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee S, Basak P, Buchel E, Safneck J, Murphy LC, Mowat M, Kung SK, Eirew P, Eaves CJ, Raouf A (2018) Breast cancers activate stromal fibroblast‐induced suppression of progenitors in adjacent normal tissue. Stem Cell Reports 10: 196–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Zhang N, Gray RS, Li H, Ewald AJ, Zahnow CA, Pan D (2014) A temporal requirement for Hippo signaling in mammary gland differentiation, growth, and tumorigenesis. Genes Dev 28: 432–437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheuk IWY, Shin VY, Kwong A (2017) Detection of methylated circulating DNA as noninvasive biomarkers for breast cancer diagnosis. J Breast Cancer 20: 12–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chia SK, Bramwell VH, Tu D, Shepherd LE, Jiang S, Vickery T, Mardis E, Leung S, Ung K, Pritchard KI, Parker JS, Bernard PS, Perou CM, Ellis MJ, Nielsen TO (2012) A 50‐gene intrinsic subtype classifier for prognosis and prediction of benefit from adjuvant tamoxifen. Clin Cancer Res 18: 4465–4472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi YS, Chakrabarti R, Escamilla‐Hernandez R, Sinha S (2009) Elf5 conditional knockout mice reveal its role as a master regulator in mammary alveolar development: failure of Stat5 activation and functional differentiation in the absence of Elf5. Dev Biol 329: 227–241 [DOI] [PubMed] [Google Scholar]
- Chou J, Provot S, Werb Z (2010) GATA3 in development and cancer differentiation: cells GATA have it!. J Cell Physiol 222: 42–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou J, Lin JH, Brenot A, Kim J‐W, Provot S, Werb Z (2013) GATA3 suppresses metastasis and modulates the tumour microenvironment by regulating microRNA‐29b expression. Nat Cell Biol 15: 201–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhury S, Almendro V, Merino VF, Wu Z, Maruyama R, Su Y, Martins FC, Fackler MJ, Bessarabova M, Kowalczyk A, Conway T, Beresford‐Smith B, Macintyre G, Cheng Y‐K, Lopez‐Bujanda Z, Kaspi A, Hu R, Robens J, Nikolskaya T, Haakensen VD et al (2013) Molecular profiling of human mammary gland links breast cancer risk to a p27(+) cell population with progenitor characteristics. Cell Stem Cell 13: 117–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke RB, Howell A, Potten CS, Anderson E (1997) Dissociation between steroid receptor expression and cell proliferation in the human breast. Cancer Res 57: 4987–4991 [PubMed] [Google Scholar]
- Cowper‐Sal Lari R, Zhang X, Wright JB, Bailey SD, Cole MD, Eeckhoute J, Moore JH, Lupien M (2012) Breast cancer risk‐associated SNPs modulate the affinity of chromatin for FOXA1 and alter gene expression. Nat Genet 44: 1191–1198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis C, Shah SP, Chin S‐F, Turashvili G, Rueda OM, Dunning MJ, Speed D, Lynch AG, Samarajiwa S, Yuan Y, Gräf S, Ha G, Haffari G, Bashashati A, Russell R, McKinney S, METABRIC Group , Langerød A, Green A, Provenzano E et al (2012) The genomic and transcriptomic architecture of 2,000 breast tumours reveals novel subgroups. Nature 486: 346–352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyr AR, Kulak MV, Park JM, Bogachek MV, Spanheimer PM, Woodfield GW, White‐Baer LS, O'Malley YQ, Sugg SL, Olivier AK, Zhang W, Domann FE, Weigel RJ (2015) TFAP2C governs the luminal epithelial phenotype in mammary development and carcinogenesis. Oncogene 34: 436–444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davalos V, Martinez‐Cardus A, Esteller M (2017) The epigenomic revolution in breast cancer: from single‐gene to genome‐wide next‐generation approaches. Am J Pathol 187: 2163–2174 [DOI] [PubMed] [Google Scholar]
- Debnath J, Muthuswamy SK, Brugge JS (2003) Morphogenesis and oncogenesis of MCF‐10A mammary epithelial acini grown in three‐dimensional basement membrane cultures. Methods 30: 256–268 [DOI] [PubMed] [Google Scholar]
- Del Barco Barrantes I, Stephan‐Otto Attolini C, Slobodnyuk K, Igea A, Gregorio S, Gawrzak S, Gomis RR, Nebreda AR (2018) Regulation of mammary luminal cell fate and tumorigenesis by p38α. Stem Cell Reports 10: 257–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deming SL, Nass SJ, Dickson RB, Trock BJ (2000) C‐myc amplification in breast cancer: a meta‐analysis of its occurrence and prognostic relevance. Br J Cancer 83: 1688–1695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deplancke B, Alpern D, Gardeux V (2016) The genetics of transcription factor DNA binding variation. Cell 166: 538–554 [DOI] [PubMed] [Google Scholar]
- Dontu G, Jackson KW, McNicholas E, Kawamura MJ, Abdallah WM, Wicha MS (2004) Role of Notch signaling in cell‐fate determination of human mammary stem/progenitor cells. Breast Cancer Res 6: R605–R615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dos Santos CO, Dolzhenko E, Hodges E, Smith AD, Hannon GJ (2015) An epigenetic memory of pregnancy in the mouse mammary gland. Cell Rep 11: 1102–1109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dravis C, Spike BT, Harrell JC, Johns C, Trejo CL, Southard‐Smith EM, Perou CM, Wahl GM (2015) Sox10 regulates stem/progenitor and mesenchymal cell states in mammary epithelial cells. Cell Rep 12: 2035–2048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eeckhoute J, Keeton EK, Lupien M, Krum SA, Carroll JS, Brown M (2007) Positive cross‐regulatory loop ties GATA‐3 to estrogen receptor alpha expression in breast cancer. Cancer Res 67: 6477–6483 [DOI] [PubMed] [Google Scholar]
- Eirew P, Stingl J, Raouf A, Turashvili G, Aparicio S, Emerman JT, Eaves CJ (2008) A method for quantifying normal human mammary epithelial stem cells with in vivo regenerative ability. Nat Med 14: 1384–1389 [DOI] [PubMed] [Google Scholar]
- Eirew P, Stingl J, Eaves CJ (2010) Quantitation of human mammary epithelial stem cells with in vivo regenerative properties using a subrenal capsule xenotransplantation assay. Nat Protoc 5: 1945–1956 [DOI] [PubMed] [Google Scholar]
- Eirew P, Kannan N, Knapp DJHF, Vaillant F, Emerman JT, Lindeman GJ, Visvader JE, Eaves CJ (2012) Aldehyde dehydrogenase activity is a biomarker of primitive normal human mammary luminal cells. Stem Cells 30: 344–348 [DOI] [PubMed] [Google Scholar]
- Eirew P, Steif A, Khattra J, Ha G, Yap D, Farahani H, Gelmon K, Chia S, Mar C, Wan A, Laks E, Biele J, Shumansky K, Rosner J, McPherson A, Nielsen C, Roth AJL, Lefebvre C, Bashashati A, de Souza C et al (2014) Dynamics of genomic clones in breast cancer patient xenografts at single‐cell resolution. Nature 518: 422–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elenbaas B, Spirio L, Koerner F, Fleming MD, Zimonjic DB, Donaher JL, Popescu NC, Hahn WC, Weinberg RA (2001) Human breast cancer cells generated by oncogenic transformation of primary mammary epithelial cells. Genes Dev 15: 50–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis MJ, Ding L, Shen D, Luo J, Suman VJ, Wallis JW, Van Tine BA, Hoog J, Goiffon RJ, Goldstein TC, Ng S, Lin L, Crowder R, Snider J, Ballman K, Weber J, Chen K, Koboldt DC, Kandoth C, Schierding WS et al (2012) Whole‐genome analysis informs breast cancer response to aromatase inhibition. Nature 486: 353–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esquivel‐Velázquez M, Ostoa‐Saloma P, Palacios‐Arreola MI, Nava‐Castro KE, Castro JI, Morales‐Montor J (2015) The role of cytokines in breast cancer development and progression. J Interferon Cytokine Res 35: 1–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallah Y, Brundage J, Allegakoen P, Shajahan‐Haq AN (2017) MYC‐driven pathways in breast cancer subtypes. Biomolecules 7: 53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang L, Hodge J, Saaoud F, Wang J, Iwanowycz S, Wang Y, Hui Y, Evans TD, Razani B, Fan D (2017) Transcriptional factor EB regulates macrophage polarization in the tumor microenvironment. Oncoimmunology 6: e1312042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari N, Riggio AI, Mason S, McDonald L, King A, Higgins T, Rosewell I, Neil JC, Smalley MJ, Sansom OJ, Morris JR, Cameron ER, Blyth K (2015) Runx2 contributes to the regenerative potential of the mammary epithelium. Sci Rep 5: 15658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster N, Saladi SV, van Bragt M, Sfondouris ME, Jones FE, Li Z, Ellisen LW (2014) Basal cell signaling by p63 controls luminal progenitor function and lactation via NRG1. Dev Cell 28: 147–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridriksdottir AJ, Kim J, Villadsen R, Klitgaard MC, Hopkinson BM, Petersen OW, Rønnov‐Jessen L (2015) Propagation of oestrogen receptor‐positive and oestrogen‐responsive normal human breast cells in culture. Nat Commun 6: 8786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futreal PA, Coin L, Marshall M, Down T, Hubbard T, Wooster R, Rahman N, Stratton MR (2004) A census of human cancer genes. Nat Rev Cancer 4: 177–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbe JC, Pepin F, Pelissier FA, Sputova K, Fridriksdottir AJ, Guo DE, Villadsen R, Park M, Petersen OW, Borowsky AD, Stampfer MR, LaBarge MA (2012) Accumulation of multipotent progenitors with a basal differentiation bias during aging of human mammary epithelia. Cancer Res 72: 3687–3701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gascard P, Bilenky M, Sigaroudinia M, Zhao J, Li L, Carles A, Delaney A, Tam A, Kamoh B, Cho S, Griffith M, Chu A, Robertson G, Cheung D, Li I, Heravi‐Moussavi A, Moksa M, Mingay M, Hussainkhel A, Davis B et al (2015) Epigenetic and transcriptional determinants of the human breast. Nat Commun 6: 6351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giraddi RR, Shehata M, Gallardo M, Blasco MA, Simons BD, Stingl J (2015) Stem and progenitor cell division kinetics during postnatal mouse mammary gland development. Nat Commun 6: 8487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu B, Watanabe K, Sun P, Fallahi M, Dai X (2013) Chromatin effector Pygo2 mediates Wnt‐notch crosstalk to suppress luminal/alveolar potential of mammary stem and basal cells. Cell Stem Cell 13: 48–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guen VJ, Chavarria TE, Kröger C, Ye X, Weinberg RA, Lees JA (2017) EMT programs promote basal mammary stem cell and tumor‐initiating cell stemness by inducing primary ciliogenesis and Hedgehog signaling. Proc Natl Acad Sci USA 114: E10532–E10539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W, Keckesova Z, Donaher JL, Shibue T, Tischler V, Reinhardt F, Itzkovitz S, Noske A, Zürrer‐Härdi U, Bell G, Tam W‐L, Mani SA, van Oudenaarden A, Weinberg RA (2012) Slug and Sox9 cooperatively determine the mammary stem cell state. Cell 148: 1015–1028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy CT, Cardiff RD, Muller WJ (1992) Induction of mammary tumors by expression of polyomavirus middle T oncogene: a transgenic mouse model for metastatic disease. Mol Cell Biol 12: 954–961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyorffy B, Pongor L, Bottai G, Li X, Budczies J, Szabó A, Hatzis C, Pusztai L, Santarpia L (2018) An integrative bioinformatics approach reveals coding and non‐coding gene variants associated with gene expression profiles and outcome in breast cancer molecular subtypes. Br J Cancer 118: 1107–1114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144: 646–674 [DOI] [PubMed] [Google Scholar]
- Herz H‐M, Hu D, Shilatifard A (2014) Enhancer malfunction in cancer. Mol Cell 53: 859–866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollern DP, Swiatnicki MR, Andrechek ER (2018) Histological subtypes of mouse mammary tumors reveal conserved relationships to human cancers. PLoS Genet 14: e1007135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm K, Hegardt C, Staaf J, Vallon‐Christersson J, Jönsson G, Olsson H, Borg Å, Ringnér M (2010) Molecular subtypes of breast cancer are associated with characteristic DNA methylation patterns. Breast Cancer Res 12: R36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm K, Staaf J, Lauss M, Aine M, Lindgren D, Bendahl P‐O, Vallon‐Christersson J, Barkardottir RB, Höglund M, Borg Å, Jönsson G, Ringnér M (2016) An integrated genomics analysis of epigenetic subtypes in human breast tumors links DNA methylation patterns to chromatin states in normal mammary cells. Breast Cancer Res 18: 27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard B, Ashworth A (2006) Signalling pathways implicated in early mammary gland morphogenesis and breast cancer. PLoS Genet 2: e112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh SJ, Clement K, Jee D, Merlini A, Choudhury S, Maruyama R, Yoo R, Chytil A, Boyle P, Ran FA, Moses HL, Barcellos‐Hoff MH, Jackson‐Grusby L, Meissner A, Polyak K (2015) Age‐ and pregnancy‐associated DNA methylation changes in mammary epithelial cells. Stem Cell Reports 4: 297–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurtado A, Holmes KA, Ross‐Innes CS, Schmidt D, Carroll JS (2011) FOXA1 is a key determinant of estrogen receptor function and endocrine response. Nat Genet 43: 27–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes NE, Stoelzle T (2009) Key signalling nodes in mammary gland development and cancer: Myc. Breast Cancer Res 11: 210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes NE, Watson CJ (2010) Mammary gland growth factors: roles in normal development and in cancer. Cold Spring Harb Perspect Biol 2: a003186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi PA, Jackson HW, Beristain AG, Di Grappa MA, Mote PA, Clarke CL, Stingl J, Waterhouse PD, Khokha R (2010) Progesterone induces adult mammary stem cell expansion. Nature 465: 803–807 [DOI] [PubMed] [Google Scholar]
- Jozwik KM, Chernukhin I, Serandour AA, Nagarajan S, Carroll JS (2016) FOXA1 directs H3K4 monomethylation at enhancers via recruitment of the methyltransferase MLL3. Cell Rep 17: 2715–2723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahata K, Maturi V, Moustakas A (2017) TGF‐β family signaling in ductal differentiation and branching morphogenesis. Cold Spring Harb Perspect Biol 10: a031997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamalakaran S, Varadan V, Giercksky Russnes HE, Levy D, Kendall J, Janevski A, Riggs M, Banerjee N, Synnestvedt M, Schlichting E, Kåresen R, Shama Prasada K, Rotti H, Rao R, Rao L, Eric Tang M‐H, Satyamoorthy K, Lucito R, Wigler M, Dimitrova N et al (2011) DNA methylation patterns in luminal breast cancers differ from non‐luminal subtypes and can identify relapse risk independent of other clinical variables. Mol Oncol 5: 77–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannan N, Huda N, Tu L, Droumeva R, Aubert G, Chavez E, Brinkman RR, Lansdorp P, Emerman J, Abe S, Eaves CJ, Gilley D (2013) The luminal progenitor compartment of the normal human mammary gland constitutes a unique site of telomere dysfunction. Stem Cell Reports 1: 28–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannan N, Nguyen LV, Makarem M, Dong Y, Shih K, Eirew P, Raouf A, Emerman JT, Eaves CJ (2014) Glutathione‐dependent and ‐independent oxidative stress‐control mechanisms distinguish normal human mammary epithelial cell subsets. Proc Natl Acad Sci USA 111: 7789–7794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur G, Dufour JM (2012) Cell lines: valuable tools or useless artifacts. Spermatogenesis 2: 1–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller PJ, Arendt LM, Skibinski A, Logvinenko T, Klebba I, Dong S, Smith AE, Prat A, Perou CM, Gilmore H, Schnitt S, Naber SP, Garlick JA, Kuperwasser C (2012) Defining the cellular precursors to human breast cancer. Proc Natl Acad Sci USA 109: 2772–2777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khaled WT, Choon Lee S, Stingl J, Chen X, Raza Ali H, Rueda OM, Hadi F, Wang J, Yu Y, Chin S‐F, Stratton M, Futreal A, Jenkins NA, Aparicio S, Copeland NG, Watson CJ, Caldas C, Liu P (2015) BCL11A is a triple‐negative breast cancer gene with critical functions in stem and progenitor cells. Nat Commun 6: 5987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp DJHF, Kannan N, Pellacani D, Eaves CJ (2017) Mass cytometric analysis reveals viable activated caspase‐3(+) luminal progenitors in the normal adult human mammary gland. Cell Rep 21: 1116–1126 [DOI] [PubMed] [Google Scholar]
- Kouros‐Mehr H, Slorach EM, Sternlicht MD, Werb Z (2006) GATA‐3 maintains the differentiation of the luminal cell fate in the mammary gland. Cell 127: 1041–1055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuperwasser C, Chavarria T, Wu M, Magrane G, Gray JW, Carey L, Richardson A, Weinberg RA (2004) Reconstruction of functionally normal and malignant human breast tissues in mice. Proc Natl Acad Sci USA 101: 4966–4971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaMarca HL, Visbal AP, Creighton CJ, Liu H, Zhang Y, Behbod F, Rosen JM (2010) CCAAT/enhancer binding protein beta regulates stem cell activity and specifies luminal cell fate in the mammary gland. Stem Cells 28: 535–544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson DA, Bhakta NR, Kessenbrock K, Prummel KD, Yu Y, Takai K, Zhou A, Eyob H, Balakrishnan S, Wang C‐Y, Yaswen P, Goga A, Werb Z (2015) Single‐cell analysis reveals a stem‐cell program in human metastatic breast cancer cells. Nature 526: 131–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HK, Willi M, Wang C, Yang CM, Smith HE, Liu C, Hennighausen L (2017) Functional assessment of CTCF sites at cytokine‐sensing mammary enhancers using CRISPR/Cas9 gene editing in mice. Nucleic Acids Res 45: 4606–4618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung CT, Brugge JS (2012) Outgrowth of single oncogene‐expressing cells from suppressive epithelial environments. Nature 482: 410–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Hively WP, Varmus HE (2000) Use of MMTV‐Wnt‐1 transgenic mice for studying the genetic basis of breast cancer. Oncogene 19: 1002–1009 [DOI] [PubMed] [Google Scholar]
- Li N, Singh S, Cherukuri P, Li H, Yuan Z, Ellisen LW, Wang B, Robbins D, DiRenzo J (2008) Reciprocal intraepithelial interactions between TP63 and hedgehog signaling regulate quiescence and activation of progenitor elaboration by mammary stem cells. Stem Cells 26: 1253–1264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim E, Vaillant F, Wu D, Forrest NC, Pal B, Hart AH, Asselin‐Labat M‐L, Gyorki DE, Ward T, Partanen A, Feleppa F, Huschtscha LI, Thorne HJ, kConFab , Fox SB, Yan M, French JD, Brown MA, Smyth GK, Visvader JE et al (2009) Aberrant luminal progenitors as the candidate target population for basal tumor development in BRCA1 mutation carriers. Nat Med 15: 907–913 [DOI] [PubMed] [Google Scholar]
- Lim E, Wu D, Pal B, Bouras T, Asselin‐Labat M‐L, Vaillant F, Yagita H, Lindeman GJ, Smyth GK, Visvader JE (2010) Transcriptome analyses of mouse and human mammary cell subpopulations reveal multiple conserved genes and pathways. Breast Cancer Res 12: R21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Robinson GW, Wagner K‐U, Garrett L, Wynshaw‐Boris A, Hennighausen L (1997) Stat5a is mandatory for adult mammary gland development and lactogenesis. Genes Dev 11: 179–186 [DOI] [PubMed] [Google Scholar]
- Liu S, Dontu G, Wicha MS (2005) Mammary stem cells, self‐renewal pathways, and carcinogenesis. Breast Cancer Res 7: 86–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupien M, Eeckhoute J, Meyer CA, Wang Q, Zhang Y, Li W, Carroll JS, Liu XS, Brown M (2008) FoxA1 translates epigenetic signatures into enhancer‐driven lineage‐specific transcription. Cell 132: 958–970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire SL, Peck B, Wai PT, Campbell J, Barker H, Gulati A, Daley F, Vyse S, Huang P, Lord CJ, Farnie G, Brennan K, Natrajan R (2016) Three‐dimensional modelling identifies novel genetic dependencies associated with breast cancer progression in the isogenic MCF10 model. J Pathol 240: 315–328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makarem M, Kannan N, Nguyen LV, Knapp DJHF, Balani S, Prater MD, Stingl J, Raouf A, Nemirovsky O, Eirew P, Eaves CJ (2013a) Developmental changes in the in vitro activated regenerative activity of primitive mammary epithelial cells. PLoS Biol 11: e1001630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makarem M, Spike BT, Dravis C, Kannan N, Wahl GM, Eaves CJ (2013b) Stem cells and the developing mammary gland. J Mammary Gland Biol Neoplasia 18: 209–219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhotra GK, Zhao X, Edwards E, Kopp JL, Naramura M, Sander M, Band H, Band V (2014) The role of Sox9 in mouse mammary gland development and maintenance of mammary stem and luminal progenitor cells. BMC Dev Biol 14: 47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mani SA, Guo W, Liao M‐J, Eaton EN, Ayyanan A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, Campbell LL, Polyak K, Brisken C, Yang J, Weinberg RA (2008) The epithelial‐mesenchymal transition generates cells with properties of stem cells. Cell 133: 704–715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama R, Choudhury S, Kowalczyk A, Bessarabova M, Beresford‐Smith B, Conway T, Kaspi A, Wu Z, Nikolskaya T, Merino VF, Lo P‐K, Liu XS, Nikolsky Y, Sukumar S, Haviv I, Polyak K (2011) Epigenetic regulation of cell type‐specific expression patterns in the human mammary epithelium. PLoS Genet 7: e1001369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Memmi EM, Sanarico AG, Giacobbe A, Peschiaroli A, Frezza V, Cicalese A, Pisati F, Tosoni D, Zhou H, Tonon G, Antonov A, Melino G, Pelicci PG, Bernassola F (2015) p63 Sustains self‐renewal of mammary cancer stem cells through regulation of Sonic Hedgehog signaling. Proc Natl Acad Sci USA 112: 3499–3504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menezes ME, Das SK, Emdad L, Windle JJ, Wang X‐Y, Sarkar D, Fisher PB (2014) Genetically engineered mice as experimental tools to dissect the critical events in breast cancer. Adv Cancer Res 121: 331–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalak EM, Visvader JE (2016) Dysregulation of histone methyltransferases in breast cancer – opportunities for new targeted therapies? Mol Oncol 10: 1497–1515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller DH, Jin DX, Sokol ES, Cabrera JR, Superville DA, Gorelov RA, Kuperwasser C, Gupta PB (2018) BCL11B drives human mammary stem cell self‐renewal in vitro by inhibiting basal differentiation. Stem Cell Reports 10: 1131–1145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyano M, Sayaman RW, Stoiber MH, Lin C‐H, Stampfer MR, Brown JB, LaBarge MA (2017) Age‐related gene expression in luminal epithelial cells is driven by a microenvironment made from myoepithelial cells. Aging (Albany NY) 9: 2026–2051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohibi S, Mirza S, Band H, Band V (2011) Mouse models of estrogen receptor‐positive breast cancer. J Carcinog 10: 35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moody SE, Sarkisian CJ, Hahn KT, Gunther EJ, Pickup S, Dugan KD, Innocent N, Cardiff RD, Schnall MD, Chodosh LA (2002) Conditional activation of Neu in the mammary epithelium of transgenic mice results in reversible pulmonary metastasis. Cancer Cell 2: 451–461 [DOI] [PubMed] [Google Scholar]
- Morel A‐P, Ginestier C, Pommier RM, Cabaud O, Ruiz E, Wicinski J, Devouassoux‐Shisheboran M, Combaret V, Finetti P, Chassot C, Pinatel C, Fauvet F, Saintigny P, Thomas E, Moyret‐Lalle C, Lachuer J, Despras E, Jauffret J‐L, Bertucci F, Guitton J et al (2017) A stemness‐related ZEB1‐MSRB3 axis governs cellular pliancy and breast cancer genome stability. Nat Med 23: 568–578 [DOI] [PubMed] [Google Scholar]
- Moses H, Barcellos‐Hoff MH (2011) TGF‐beta biology in mammary development and breast cancer. Cold Spring Harb Perspect Biol 3: a003277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moumen M, Chiche A, Deugnier M‐A, Petit V, Gandarillas A, Glukhova MA, Faraldo MM (2012) The proto‐oncogene Myc is essential for mammary stem cell function. Stem Cells 30: 1246–1254 [DOI] [PubMed] [Google Scholar]
- Muller WJ, Sinn E, Pattengale PK, Wallace R, Leder P (1988) Single‐step induction of mammary adenocarcinoma in transgenic mice bearing the activated c‐neu oncogene. Cell 54: 105–115 [DOI] [PubMed] [Google Scholar]
- Nguyen LV, Makarem M, Carles A, Moksa M, Kannan N, Pandoh P, Eirew P, Osako T, Kardel M, Cheung AMS, Kennedy W, Tse K, Zeng T, Zhao Y, Humphries RK, Aparicio S, Eaves CJ, Hirst M (2014) Clonal analysis via barcoding reveals diverse growth and differentiation of transplanted mouse and human mammary stem cells. Cell Stem Cell 14: 253–263 [DOI] [PubMed] [Google Scholar]
- Nguyen LV, Pellacani D, Lefort S, Kannan N, Osako T, Makarem M, Cox CL, Kennedy W, Beer PA, Carles A, Moksa M, Bilenky M, Balani S, Babovic S, Sun I, Rosin M, Aparicio S, Hirst M, Eaves CJ (2015) Barcoding reveals complex clonal dynamics of de novo transformed human mammary cells. Nature 528: 267–271 [DOI] [PubMed] [Google Scholar]
- Nguyen QH, Pervolarakis N, Blake K, Ma D, Davis RT, James N, Phung AT, Willey E, Kumar R, Jabart E, Driver I, Rock J, Goga A, Khan SA, Lawson DA, Werb Z, Kessenbrock K (2018) Profiling human breast epithelial cells using single cell RNA sequencing identifies cell diversity. Nat Commun 9: 2028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen TO, Parker JS, Leung S, Voduc D, Ebbert M, Vickery T, Davies SR, Snider J, Stijleman IJ, Reed J, Cheang MCU, Mardis ER, Perou CM, Bernard PS, Ellis MJ (2010) A comparison of PAM50 intrinsic subtyping with immunohistochemistry and clinical prognostic factors in tamoxifen‐treated estrogen receptor‐positive breast cancer. Clin Cancer Res 16: 5222–5232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nik‐Zainal S, Van Loo P, Wedge DC, Alexandrov LB, Greenman CD, Lau KW, Raine K, Jones D, Marshall J, Ramakrishna M, Shlien A, Cooke SL, Hinton J, Menzies A, Stebbings LA, Leroy C, Jia M, Rance R, Mudie LJ, Gamble SJ et al (2012) The life history of 21 breast cancers. Cell 149: 994–1007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nik‐Zainal S, Davies H, Staaf J, Ramakrishna M, Glodzik D, Zou X, Martincorena I, Alexandrov LB, Martin S, Wedge DC, Van Loo P, Ju YS, Smid M, Brinkman AB, Morganella S, Aure MR, Lingjærde OC, Langerød A, Ringnér M, Ahn S‐M et al (2016) Landscape of somatic mutations in 560 breast cancer whole‐genome sequences. Nature 534: 47–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker JS, Mullins M, Cheang MCU, Leung S, Voduc D, Vickery T, Davies S, Fauron C, He X, Hu Z, Quackenbush JF, Stijleman IJ, Palazzo J, Marron JS, Nobel AB, Mardis E, Nielsen TO, Ellis MJ, Perou CM, Bernard PS (2009) Supervised risk predictor of breast cancer based on intrinsic subtypes. J Clin Oncol 27: 1160–1167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasculli B, Barbano R, Parrella P (2018) Epigenetics of breast cancer: biology and clinical implication in the era of precision medicine. Semin Cancer Biol 51: 22–35 [DOI] [PubMed] [Google Scholar]
- Pelissier FA, Garbe JC, Ananthanarayanan B, Miyano M, Lin C, Jokela T, Kumar S, Stampfer MR, Lorens JB, LaBarge MA (2014) Age‐related dysfunction in mechanotransduction impairs differentiation of human mammary epithelial progenitors. Cell Rep 7: 1926–1939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellacani D, Bilenky M, Kannan N, Heravi‐Moussavi A, Knapp DJHF, Gakkhar S, Moksa M, Carles A, Moore R, Mungall AJ, Marra MA, Jones SJM, Aparicio S, Hirst M, Eaves CJ (2016) Analysis of normal human mammary epigenomes reveals cell‐specific active enhancer states and associated transcription factor networks. Cell Rep 17: 2060–2074 [DOI] [PubMed] [Google Scholar]
- Perou CM, Sørlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA, Fluge O, Pergamenschikov A, Williams C, Zhu SX, Lønning PE, Børresen‐Dale AL, Brown PO, Botstein D (2000) Molecular portraits of human breast tumours. Nature 406: 747–752 [DOI] [PubMed] [Google Scholar]
- Pfefferle AD, Herschkowitz JI, Usary J, Harrell JC, Spike BT, Adams JR, Torres‐Arzayus MI, Brown M, Egan SE, Wahl GM, Rosen JM, Perou CM (2013) Transcriptomic classification of genetically engineered mouse models of breast cancer identifies human subtype counterparts. Genome Biol 14: R125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips S, Prat A, Sedic M, Proia TA, Wronski A, Mazumdar S, Skibinski A, Shirley SH, Perou CM, Gill G, Gupta PB, Kuperwasser C (2014) Cell‐state transitions regulated by SLUG are critical for tissue regeneration and tumor initiation. Stem Cell Reports 2: 633–647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce DF, Gorska AE, Chytil A, Meise KS, Page DL, Coffey RJ, Moses HL (1995) Mammary tumor suppression by transforming growth factor beta 1 transgene expression. Proc Natl Acad Sci USA 92: 4254–4258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Place AE, Jin Huh S, Polyak K (2011) The microenvironment in breast cancer progression: biology and implications for treatment. Breast Cancer Res 13: 227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podsypanina K, Politi K, Beverly LJ, Varmus HE (2008) Oncogene cooperation in tumor maintenance and tumor recurrence in mouse mammary tumors induced by Myc and mutant Kras. Proc Natl Acad Sci USA 105: 5242–5247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poli V, Fagnocchi L, Fasciani A, Cherubini A, Mazzoleni S, Ferrillo S, Miluzio A, Gaudioso G, Vaira V, Turdo A, Giaggianesi M, Chinnici A, Lipari E, Bicciato S, Bosari S, Todaro M, Zippo A (2018) MYC‐driven epigenetic reprogramming favors the onset of tumorigenesis by inducing a stem cell‐like state. Nat Commun 9: 1024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouliot M‐C, Labrie Y, Diorio C, Durocher F (2015) The role of methylation in breast cancer susceptibility and treatment. Anticancer Res 35: 4569–4574 [PubMed] [Google Scholar]
- Proia DA, Kuperwasser C (2006) Reconstruction of human mammary tissues in a mouse model. Nat Protoc 1: 206–214 [DOI] [PubMed] [Google Scholar]
- Proia TA, Keller PJ, Gupta PB, Klebba I, Jones AD, Sedic M, Gilmore H, Tung N, Naber SP, Schnitt S, Lander ES, Kuperwasser C (2011) Genetic predisposition directs breast cancer phenotype by dictating progenitor cell fate. Cell Stem Cell 8: 149–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purkis PE, Steel JB, Mackenzie IC, Nathrath WB, Leigh IM, Lane EB (1990) Antibody markers of basal cells in complex epithelia. J Cell Sci 97(Pt 1): 39–50 [DOI] [PubMed] [Google Scholar]
- Qiao Y, He H, Jonsson P, Sinha I, Zhao C, Dahlman‐Wright K (2016) AP‐1 is a key regulator of proinflammatory cytokine TNFα‐mediated triple‐negative breast cancer progression. J Biol Chem 291: 5068–5079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramakrishnan R, Khan SA, Badve S (2002) Morphological changes in breast tissue with menstrual cycle. Mod Pathol 15: 1348–1356 [DOI] [PubMed] [Google Scholar]
- Raouf A, Zhao Y, To K, Stingl J, Delaney A, Barbara M, Iscove N, Jones S, McKinney S, Emerman J, Aparicio S, Marra MA, Eaves CJ (2008) Transcriptome analysis of the normal human mammary cell commitment and differentiation process. Cell Stem Cell 3: 109–118 [DOI] [PubMed] [Google Scholar]
- Ren Y, Jia H‐H, Xu Y‐Q, Zhou X, Zhao X‐H, Wang Y‐F, Song X, Zhu Z‐Y, Sun T, Dou Y, Tian W‐P, Zhao X‐L, Kang C‐S, Mei M (2018) Paracrine and epigenetic control of CAF‐induced metastasis: the role of HOTAIR stimulated by TGF‐ß1 secretion. Mol Cancer 17: 5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rheinbay E, Parasuraman P, Grimsby J, Tiao G, Engreitz JM, Kim J, Lawrence MS, Taylor‐Weiner A, Rodriguez‐Cuevas S, Rosenberg M, Hess J, Stewart C, Maruvka YE, Stojanov P, Cortés ML, Seepo S, Cibulskis C, Tracy A, Pugh TJ, Lee J et al (2017) Recurrent and functional regulatory mutations in breast cancer. Nature 547: 55–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter T, von Zglinicki T (2007) A continuous correlation between oxidative stress and telomere shortening in fibroblasts. Exp Gerontol 42: 1039–1042 [DOI] [PubMed] [Google Scholar]
- Rios AC, Fu NY, Lindeman GJ, Visvader JE (2014) In situ identification of bipotent stem cells in the mammary gland. Nature 506: 322–327 [DOI] [PubMed] [Google Scholar]
- Roarty K, Rosen JM (2010) Wnt and mammary stem cells: hormones cannot fly wingless. Curr Opin Pharmacol 10: 643–649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson JLL, Holmes KA, Carroll JS (2013) FOXA1 mutations in hormone‐dependent cancers. Front Oncol 3: 20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roswall P, Bocci M, Bartoschek M, Li H, Kristiansen G, Jansson S, Lehn S, Sjölund J, Reid S, Larsson C, Eriksson P, Anderberg C, Cortez E, Saal LH, Orsmark‐Pietras C, Cordero E, Haller BK, Häkkinen J, Burvenich IJG, Lim E et al (2018) Microenvironmental control of breast cancer subtype elicited through paracrine platelet‐derived growth factor‐CC signaling. Nat Med 24: 463–473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutkowski MR, Allegrezza MJ, Svoronos N, Tesone AJ, Stephen TL, Perales‐Puchalt A, Nguyen J, Zhang PJ, Fiering SN, Tchou J, Conejo‐Garcia JR (2014) Initiation of metastatic breast carcinoma by targeting of the ductal epithelium with adenovirus‐cre: a novel transgenic mouse model of breast cancer. J Vis Exp 85: e51171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandgren EP, Schroeder JA, Qui TH, Palmiter RD, Brinster RL, Lee DC (1995) Inhibition of mammary gland involution is associated with transforming growth factor alpha but not c‐myc‐induced tumorigenesis in transgenic mice. Cancer Res 55: 3915–3927 [PubMed] [Google Scholar]
- Saunderson EA, Stepper P, Gomm JJ, Hoa L, Morgan A, Allen MD, Jones JL, Gribben JG, Jurkowski TP, Ficz G (2017) Hit‐and‐run epigenetic editing prevents senescence entry in primary breast cells from healthy donors. Nat Commun 8: 1450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze‐Garg C, Löhler J, Gocht A, Deppert W (2000) A transgenic mouse model for the ductal carcinoma in situ (DCIS) of the mammary gland. Oncogene 19: 1028–1037 [DOI] [PubMed] [Google Scholar]
- Senoo M, Pinto F, Crum CP, McKeon F (2007) p63 Is essential for the proliferative potential of stem cells in stratified epithelia. Cell 129: 523–536 [DOI] [PubMed] [Google Scholar]
- Shah SH, Miller P, Garcia‐Contreras M, Ao Z, Machlin L, Issa E, El‐Ashry D (2015) Hierarchical paracrine interaction of breast cancer associated fibroblasts with cancer cells via hMAPK‐microRNAs to drive ER‐negative breast cancer phenotype. Cancer Biol Ther 16: 1671–1681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shehata M, Teschendorff A, Sharp G, Novcic N, Russell A, Avril S, Prater M, Eirew P, Caldas C, Watson CJ, Stingl J (2012) Phenotypic and functional characterization of the luminal cell hierarchy of the mammary gland. Breast Cancer Res 14: R134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi P, Feng J, Chen C (2015) Hippo pathway in mammary gland development and breast cancer. Acta Biochim Biophys Sin (Shanghai) 47: 53–59 [DOI] [PubMed] [Google Scholar]
- Shi W, Fornes O, Mathelier A, Wasserman WW (2016) Evaluating the impact of single nucleotide variants on transcription factor binding. Nucleic Acids Res 44: 10106–10116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin HY, Willi M, Yoo KH, Zeng X, Wang C, Metser G, Hennighausen L (2016) Hierarchy within the mammary STAT5‐driven Wap super‐enhancer. Nat Genet 48: 904–911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shou J, Lai Y, Xu J, Huang J (2016) Prognostic value of FOXA1 in breast cancer: a systematic review and meta‐analysis. Breast 27: 35–43 [DOI] [PubMed] [Google Scholar]
- Sikandar SS, Kuo AH, Kalisky T, Cai S, Zabala M, Hsieh RW, Lobo NA, Scheeren FA, Sim S, Qian D, Dirbas FM, Somlo G, Quake SR, Clarke MF (2017) Role of epithelial to mesenchymal transition associated genes in mammary gland regeneration and breast tumorigenesis. Nat Commun 8: 1669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinn E, Muller W, Pattengale P, Tepler I, Wallace R, Leder P (1987) Coexpression of MMTV/v‐Ha‐ras and MMTV/c‐myc genes in transgenic mice: synergistic action of oncogenes in vivo . Cell 49: 465–475 [DOI] [PubMed] [Google Scholar]
- Skibinski A, Breindel JL, Prat A, Galván P, Smith E, Rolfs A, Gupta PB, LaBaer J, Kuperwasser C (2014) The Hippo transducer TAZ interacts with the SWI/SNF complex to regulate breast epithelial lineage commitment. Cell Rep 6: 1–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soule HD, Maloney TM, Wolman SR, Peterson WD, Brenz R, McGrath CM, Russo J, Pauley RJ, Jones RF, Brooks SC (1990) Isolation and characterization of a spontaneously immortalized human breast epithelial cell line, MCF‐10. Cancer Res 50: 6075–6086 [PubMed] [Google Scholar]
- Spike BT, Engle DD, Lin JC, Cheung SK, La J, Wahl GM (2012) A mammary stem cell population identified and characterized in late embryogenesis reveals similarities to human breast cancer. Cell Stem Cell 10: 183–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sproul D, Nestor C, Culley J, Dickson JH, Dixon JM, Harrison DJ, Meehan RR, Sims AH, Ramsahoye BH (2011) Transcriptionally repressed genes become aberrantly methylated and distinguish tumors of different lineages in breast cancer. Proc Natl Acad Sci USA 108: 4364–4369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sreekumar A, Toneff MJ, Toh E, Roarty K, Creighton CJ, Belka GK, Lee D‐K, Xu J, Chodosh LA, Richards JS, Rosen JM (2017) WNT‐mediated regulation of FOXO1 constitutes a critical axis maintaining pubertal mammary stem cell homeostasis. Dev Cell 43: 436–448.e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefansson OA, Moran S, Gomez A, Sayols S, Arribas‐Jorba C, Sandoval J, Hilmarsdottir H, Olafsdottir E, Tryggvadottir L, Jonasson JG, Eyfjord J, Esteller M (2015) A DNA methylation‐based definition of biologically distinct breast cancer subtypes. Mol Oncol 9: 555–568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart TA, Pattengale PK, Leder P (1984) Spontaneous mammary adenocarcinomas in transgenic mice that carry and express MTV/myc fusion genes. Cell 38: 627–637 [DOI] [PubMed] [Google Scholar]
- Stingl J, Eaves CJ, Zandieh I, Emerman JT (2001) Characterization of bipotent mammary epithelial progenitor cells in normal adult human breast tissue. Breast Cancer Res Treat 67: 93–109 [DOI] [PubMed] [Google Scholar]
- Stingl J, Raouf A, Emerman JT, Eaves CJ (2005) Epithelial progenitors in the normal human mammary gland. J Mammary Gland Biol Neoplasia 10: 49–59 [DOI] [PubMed] [Google Scholar]
- Stingl J (2011) Estrogen and progesterone in normal mammary gland development and in cancer. Horm Cancer 2: 85–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takaku M, Grimm SA, Roberts JD, Chrysovergis K, Bennett BD, Myers P, Perera L, Tucker CJ, Perou CM, Wade PA (2018) GATA3 zinc finger 2 mutations reprogram the breast cancer transcriptional network. Nat Commun 9: 1059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry MB, McDonald JA, Wu HC, Eng S, Santella RM (2016) Epigenetic biomarkers of breast cancer risk: across the breast cancer prevention continuum. Adv Exp Med Biol 882: 33–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teulière J, Faraldo MM, Deugnier M‐A, Shtutman M, Ben‐Ze'ev A, Thiery JP, Glukhova MA (2005) Targeted activation of beta‐catenin signaling in basal mammary epithelial cells affects mammary development and leads to hyperplasia. Development 132: 267–277 [DOI] [PubMed] [Google Scholar]
- Valdez KE, Fan F, Smith W, Allred DC, Medina D, Behbod F (2011) Human primary ductal carcinoma in situ (DCIS) subtype‐specific pathology is preserved in a mouse intraductal (MIND) xenograft model. J Pathol 225: 565–573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Keymeulen A, Rocha AS, Ousset M, Beck B, Bouvencourt G, Rock J, Sharma N, Dekoninck S, Blanpain C (2011) Distinct stem cells contribute to mammary gland development and maintenance. Nature 479: 189–193 [DOI] [PubMed] [Google Scholar]
- Veltmaat JM, Mailleux AA, Thiery JP, Bellusci S (2003) Mouse embryonic mammogenesis as a model for the molecular regulation of pattern formation. Differentiation 71: 1–17 [DOI] [PubMed] [Google Scholar]
- Verghese ET, Drury R, Green CA, Holliday DL, Lu X, Nash C, Speirs V, Thorne JL, Thygesen HH, Zougman A, Hull MA, Hanby AM, Hughes TA (2013) MiR‐26b is down‐regulated in carcinoma‐associated fibroblasts from ER‐positive breast cancers leading to enhanced cell migration and invasion. J Pathol 231: 388–399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visvader JE, Stingl J (2014) Mammary stem cells and the differentiation hierarchy: current status and perspectives. Genes Dev 28: 1143–1158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade MA, Sunter NJ, Fordham SE, Long A, Masic D, Russell LJ, Harrison CJ, Rand V, Elstob C, Bown N, Rowe D, Lowe C, Cuthbert G, Bennett S, Crosier S, Bacon CM, Onel K, Scott K, Scott D, Travis LB et al (2015) c‐MYC is a radiosensitive locus in human breast cells. Oncogene 34: 4985–4994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X‐X, Fu L, Li X, Wu X, Zhu Z, Fu L, Dong J‐T (2011) Somatic mutations of the mixed‐lineage leukemia 3 (MLL3) gene in primary breast cancers. Pathol Oncol Res 17: 429–433 [DOI] [PubMed] [Google Scholar]
- Wang Y, Dong J, Li D, Lai L, Siwko S, Li Y, Liu M (2013) Lgr4 regulates mammary gland development and stem cell activity through the pluripotency transcription factor Sox2. Stem Cells 31: 1921–1931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Cai C, Dong X, Yu QC, Zhang X‐O, Yang L, Zeng YA (2015) Identification of multipotent mammary stem cells by protein C receptor expression. Nature 517: 81–84 [DOI] [PubMed] [Google Scholar]
- Wang Z, Yang B, Zhang M, Guo W, Wu Z, Wang Y, Jia L, Li S, Cancer Genome Atlas Research Network , Xie W, Yang D (2018) lncRNA epigenetic landscape analysis identifies EPIC1 as an oncogenic lncRNA that interacts with MYC and promotes cell‐cycle progression in cancer. Cancer Cell 33: 706–720.e9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe K, Villarreal‐Ponce A, Sun P, Salmans ML, Fallahi M, Andersen B, Dai X (2014) Mammary morphogenesis and regeneration require the inhibition of EMT at terminal end buds by Ovol2 transcriptional repressor. Dev Cell 29: 59–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson CL, Sims AH, Howell A, Miller CJ, Clarke RB (2006) Effects of oestrogen on gene expression in epithelium and stroma of normal human breast tissue. Endocr Relat Cancer 13: 617–628 [DOI] [PubMed] [Google Scholar]
- Wilson NK, Kent DG, Buettner F, Shehata M, Macaulay IC, Calero‐Nieto FJ, Sánchez Castillo M, Oedekoven CA, Diamanti E, Schulte R, Ponting CP, Voet T, Caldas C, Stingl J, Green AR, Theis FJ, Göttgens B (2015) Combined single‐cell functional and gene expression analysis resolves heterogeneity within stem cell populations. Cell Stem Cell 16: 712–724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yalcin‐Ozuysal O, Fiche M, Guitierrez M, Wagner K‐U, Raffoul W, Brisken C (2010) Antagonistic roles of Notch and p63 in controlling mammary epithelial cell fates. Cell Death Differ 17: 1600–1612 [DOI] [PubMed] [Google Scholar]
- Yamaji D, Na R, Feuermann Y, Pechhold S, Chen W, Robinson GW, Hennighausen L (2009) Development of mammary luminal progenitor cells is controlled by the transcription factor STAT5A. Genes Dev 23: 2382–2387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeong J, Thike AA, Tan PH, Iqbal J (2017) Identifying progression predictors of breast ductal carcinoma in situ . J Clin Pathol 70: 102–108 [DOI] [PubMed] [Google Scholar]
- Yoo KH, Oh S, Kang K, Wang C, Robinson GW, Ge K, Hennighausen L (2016) Histone demethylase KDM6A controls the mammary luminal lineage through enzyme‐independent mechanisms. Mol Cell Biol 36: 2108–2120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacksenhaus E, Liu JC, Jiang Z, Yao Y, Xia L, Shrestha M, Ben‐David Y (2017) Transcription factors in breast cancer‐lessons from recent genomic analyses and therapeutic implications. Adv Protein Chem Struct Biol 107: 223–273 [DOI] [PubMed] [Google Scholar]
- Zeng YA, Nusse R (2010) Wnt proteins are self‐renewal factors for mammary stem cells and promote their long‐term expansion in culture. Cell Stem Cell 6: 568–577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Zglinicki T (2002) Oxidative stress shortens telomeres. Trends Biochem Sci 27: 339–344 [DOI] [PubMed] [Google Scholar]
- Zhang Z, Christin JR, Wang C, Ge K, Oktay MH, Guo W (2016) Mammary‐stem‐cell‐based somatic mouse models reveal breast cancer drivers causing cell fate dysregulation. Cell Rep 16: 3146–3156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou S, Treloar AE, Lupien M (2016) Emergence of the noncoding cancer genome: a target of genetic and epigenetic alterations. Cancer Discov 6: 1215–1229 [DOI] [PMC free article] [PubMed] [Google Scholar]


