Abstract
Class III peroxidases (PODs), commonly known as secretable class III plant peroxidases, are plant-specific enzymes that play critical roles in not only plant growth and development but also the responses to biotic and abiotic stress. In this study, we identified 198 nonredundant POD genes, designated GhPODs, with 180 PODs being predicted to secrete into apoplast. These POD genes were divided into 10 sub-groups based on their phylogenetic relationships. We performed systematic bioinformatic analysis of the POD genes, including analysis of gene structures, phylogenetic relationships, and gene expression profiles. The GhPODs are unevenly distributed on both upland cotton sub-genome A and D chromosomes. Additionally, these genes have undergone 15 segmental and 12 tandem duplication events, indicating that both segmental and tandem duplication contributed to the expansion of the POD gene family in upland cotton. Ka/Ks analysis suggested that most duplicated GhPODs experienced negative selection, with limited functional divergence during the duplication events. High-throughput RNA-seq data indicated that most highly expressed genes might play significant roles in root, stem, leaf, and fiber development. Under K or P deficiency conditions, PODs showed different expression patterns in cotton root and leaf. This study provides useful information for further functional analysis of the POD gene family in upland cotton.
Keywords: cotton, class III peroxidases, POD, gene duplication, nutrient deficiency
1. Introduction
Peroxidases (EC 1.11.1.x) are encoded by multigenic families and are involved in several important physiological and developmental processes. Among them, class III peroxidases (EC 1.11.1.7), belonging to the haem peroxidase subfamily, exist only in plants and have an extremely widespread presence in the plant kingdom [1]. They are members of a large multigenic family with more than 200 members in switchgrass [2], 93 in Populus [3], 138 in rice [4], and 73 in Arabidopsis [5]. The nomenclature of Class III plant peroxidase is not unified and various abbreviations were reported, such as POX [6,7], GPX [8], Prx [5], ClassIIIPRX [2], and POD [9,10]. Hereafter, class III peroxidases were abbreviated as PODs.
PODs are involved in a broad range of physiological processes such as auxin metabolism, lignin and suberin formation, cross-linking of cell wall components, phytoalexin synthesis, defense against biotic or abiotic stress, cell elongation, and the metabolism of reactive nitrogen species and reactive oxygen species (ROS), throughout the plant life cycle from the early stage of germination to the final step of senescence [11,12,13]. It is probably due to their high number of enzymatic isoforms and to the versatility of their enzyme-catalyzed reactions.
PODs are mainly considered as secreted/apoplastic/cell wall proteins, but vacuolar isoforms also exist [14]. Apoplastic POD can be further classified into three major categories based on their chemical and physical association with cell walls and available extraction methods: water soluble and loosely ionically bound, tightly ionically bound, and covalently bound [15]. The specific function of each member of the family is still elusive. Furthermore, they usually show dual enzymatic activities. For example, some consume ROS and others produce ROS; some loosen the cell wall and others stiffen the cell wall [14]. Therefore, they play a pivotal role in cellular growth and response to biotic and abiotic stresses. The comprehensive researches are necessary to explore the role of POD in plant growth and defense.
Widely cultivated in more than 100 countries, cotton is considered one of the most important fiber-producing and economic crops around the world. Suboptimal phosphorus (P) and potassium (K) availability, widely present in agriculture, negatively influences cotton growth and development and reduces cotton fiber yield and quality [16,17]. PODs participate in cotton growth and development, cotton defense against biotic and abiotic stresses, and fiber development. For example, GhPOX1 play an important role during fiber cell elongation possibly mediated by ROS production [6]. The cotton flower-specifically expressed pod, predominantly in pollen, suggested that peroxidase is involved in the male reproductive processes of angiosperms [18]. Two PODs from cotton play a role in the oxidative burst response of cotton to bacterial blight [19]. Up to now, however, no genome-wide characterization of the pod family and their responses to PK deficiency has been performed in cotton. The recently published genome sequence of Gossypium hirsutum L. acc. TM-1, a tetraploid cotton species [20], provides us with a great opportunity to identify and characterize pods in the cotton genome and to explore the expression profiles of pods under PK deficiency conditions.
In the present study, we performed for the first time the comprehensive analysis and responses to PK deficiency of the pod family in G. hirsutum. A total of 198 non-redundant POD encoding genes were identified in the genome of G. hirsutum. and were subsequently subjected to a systematic genomic analysis, including studies on phylogenetic relationships, on chromosome location, on gene duplication status, on substitution rates, on gene structures, on expression profiling and secretion traits, and on responses to PK deficiency in cotton leaf and root. The differentiation of functions of GhPODs were predicted on the basis of the expression profiles of pod members and the phylogenetic analysis among the POD proteins in G. hirsutum. Additionally, we analyzed whether the expansion of the pod family in G. hirsutum was caused by segmental duplication and/or tandem duplication. In summary, our genome-wide analysis of the POD gene family will contribute to future studies on the functional differentiation of POD proteins in different physiological processes of G. hirsutum; the differential responses to PK deficiency will benefit the elucidation of the relationship of physiological processes such as root elongation and branching, leaf senescence, and ROS modulation and with specific POD isoenzymes under PK deficiency.
2. Materials and Methods
2.1. Sequence Retrieval for POD Proteins in Cotton
The local BLAST database was established with protein sequences of upland cotton (G. hirsutum L. acc. TM-1) whole genome (download from http://mascotton.njau.edu.cn). The protein sequences of POD family members in the genome of Arabidopsis were retrieved from the TAIR database (http://www.arabidopsis. org/). The candidate sequences of POD in cotton were acquired by BLASTP with each of the 73 different amino acid sequences of Arabidopsis POD gene family as query sequences (screening threshold value/E-Value: 1e−10). To verify the reliability of the initial results, the acquired candidate sequences were further submitted against PFAM (http://pfam.xfam.org/) to verify the domains for identifying the POD gene family members in cotton. The theoretical molecular weights (MWs) and isoelectric points (pIs) of the proteins were collected through an online program (http://www.ebi.ac.uk/Tools/seqstats/emboss_pepstats/).
2.2. Phylogenetic Analysis
Multiple sequence alignments were conducted on the amino acid sequences of POD proteins in G. hirsutum genomes using Cluster W of MEGA 5.0 software with the default settings [21]. Subsequently, the software was employed to construct an unrooted phylogenetic tree based on alignments using the Neighbor-Joining (NJ) method with the following parameters: model (p-distance), bootstrap (1000 replicates), and gap/missing data (pairwise deletion).
2.3. Gene Structure Analysis
The genomic and CDS sequences of cotton PODs, extracted from G. hirsutum genome databases, were compared by using the Gene Structure Display Server program (http://gsds.cbi.pku.edu.cn/) to infer the exon/intron organization of POD genes.
2.4. Analysis of Chromosomal Location and Gene Duplication
Information about the physical locations of all POD genes on chromosomes was obtained through BLASTn searches against the G. hirsutum genome database. All GhPOD genes were then mapped on the chromosomes using the software MapInspect (http://mapinspect.software.informer.com). The detection of POD gene duplication events was also carried out and paralogous POD gene pairs were identified based on the alignment results. The criteria were as follows: the shorter sequence covers over 80% of the longer sequence after alignment and the minimum identity of aligned regions is equal to or above 80%. In addition, to explore the selection pressures among POD duplicated genes, we calculated the nonsynonymous mutation rate (Ka), synonymous mutation rate (Ks), and Ka/Ks values for the duplicated gene pairs with Mega 5.0.
2.5. Cotton Culture and Expression Analysis of POD Genes under PK Deficiency
Cotton (G. hirsutum L. TM-1) was planted in a growth chamber (day/night of 14/10 h with temperature 30/25 °C and photo intensity 450 µmol/m2·s) under liquid culture. The solution composition was as follows (mmol/L): 2.5 Ca(NO3)2, 1 MgSO4, 0.5 NH4H2PO4, 2.5 KCl, 2 NaCl, 2 × 10−4 CuSO4, 1 × 10−3 ZnSO4, 0.1 EDTA-FeNa, 2 × 10−2 H3BO3, 5 × 10−6 (NH4)6Mo7O24 and 1 × 10−3 MnSO4. The seedlings with four expanded leaves were treated separately with the original solution (control), low K solution (0.05 KCl with NaCl to balance the Cl ion and others the same as in the original solution), and low P solution (0.005 NH4H2PO4 with NH4Cl to balance the NH4+ and others the same as in the original solution). On the 7th day of treatment, the third leaf from the uppermost was counted and the young roots of all treatments were sampled and stored in −80 °C for RNA extraction and gene expression analysis.
Expression profiles of POD genes response to PK deficiency were analyzed by using the Illumina Hiseq2000 (Illumina, San Diego, CA, USA) to perform high-throughput RNA-seq of the root and leaf of control, P deficiency, and K-deficiency. In total, 26.95 Gb of raw RNA-seq data were generated (BGI-Tech., Shenzhen, China). RNA-seq reads were mapped to the cotton genotype TM-1 genome using Tophat (v2.0.8; Top Hat, Toronto, Canada). To measure the gene expression level in sampled tissues, we calculated the expression of each gene using FPKM (Fragments per Kilobase of exon model per Million mapped reads) with Cufflinks (v2.1.1; http://cole-trapnell-lab.github.io/cufflinks/). We analyzed the POD gene expression changes in root and leaf under control, P-deficiency, and K-deficiency by using software MultiExperiment Viewer (MeV; http://mev.tm4.org).
2.6. Localization of POD Proteins
Secretion of POD proteins to the apoplast or to the vacuole were predicted by combinations of using SignalP (www.cbs.dtu.dk/services/SignalP/) with a signal peptide, SecretomeP (www.cbs.dtu.dk/services/SecretomeP) without a signal peptide and TargetP (www.cbs.dtu.dk/services/TargetP). The secreted POD proteins were further investigated in the xylem saps, separately, from field cotton [8] and chamber cotton.
3. Results
3.1. Identification of POD Genes
We used the 73 Arabidopsis POD genes to acquire 264 cotton POD genes by BLASTP and further verified their domains with PFAM. A total of 198 non-redundant POD genes with conserved POD domains were identified in cotton. This number is greater than that in Arabidopsis (73) [5], Populus (93) [3], Chinese Pear (94) [22], maize (119) [23], and rice (138) ([4]; but it was similar with that in switchgrass (200) [2]. For convenience, we assigned names to these POD genes (GhPOD01-198) according to their chromosomal positions. The length of the 198 newly identified POD proteins varies from 160 to 1098 amino acid (aa) with an average of 332 aa. There is only one POD containing more than 672 aa. The isoelectric point (PI) varied from 4.12 to 10.50 with a mean of 7.73 and >7.0 of 67.2% POD proteins. Other information of chromosomal location, molecular weight (MW) gene size, coding sequence (CDs) size of each GhPOD gene/protein is shown in Table 1.
Table 1.
The 198 POD genes identified in cotton and their sequence characteristics and location.
| Protein Name/ID | Chr Location | Gene/CDS Size (bp) | PL (aa)/MW (Kda)/PI | SignalP/SeretomeP/TargetP |
|---|---|---|---|---|
| GhPOD01/Gh_A01G1388 | chrA01:87059677-87066826 | 7150/867 | 288/31.86/5.64 | N/S/- |
| GhPOD02/Gh_A01G1487 | chrA01:90340312-90341927 | 1616/972 | 323/34.9/5.69 | S/N/S |
| GhPOD03/Gh_A01G2012 | chrA01:175229-177095 | 1867/975 | 324/35.23/9.57 | S/N/- |
| GhPOD04/Gh_A02G0542 | chrA02:8131024-8132267 | 1244/1002 | 333/36.13/4.12 | S/N/S |
| GhPOD05/Gh_A02G0651 | chrA02:10404884-10407370 | 2487/1029 | 342/37.15/5.16 | S/N/S |
| GhPOD06/Gh_A02G0927 | chrA02:35288344-35290345 | 2002/1020 | 339/37.96/8.3 | S/N/S |
| GhPOD07/Gh_A02G1203 | chrA02:69325920-69327130 | 1211/984 | 327/35.33/8.2 | N/N/S |
| GhPOD08/Gh_A02G1466 | chrA02:80770130-80772880 | 2751/993 | 330/36.02/9.44 | S/N/S |
| GhPOD09/Gh_A02G1648 | chrA02:82884707-82887518 | 2812/909 | 302/33.98/9.11 | N/S/- |
| GhPOD10/Gh_A02G1663 | chrA02:82988074-82989301 | 1228/954 | 317/34.25/9.27 | S/N/S |
| GhPOD11/Gh_A03G0199 | chrA03:3093785-3095982 | 2198/969 | 322/34.57/9.93 | S/N/S |
| GhPOD12/Gh_A03G0200 | chrA03:3102427-3103937 | 1511/744 | 247/26.58/5.73 | N/S/C |
| GhPOD13/Gh_A03G0944 | chrA03:60522384-60523564 | 1181/1008 | 335/36.76/5.19 | S/N/S |
| GhPOD14/Gh_A03G0960 | chrA03:61923097-61977652 | 54556/1971 | 656/71.66/8.68 | S/N/S |
| GhPOD15/Gh_A03G1517 | chrA03:95777005-95778170 | 1166/984 | 327/35.68/9.58 | S/N/S |
| GhPOD16/Gh_A03G1519 | chrA03:95814422-95816062 | 1641/723 | 240/25.77/6.99 | S/N/S |
| GhPOD17/Gh_A03G1812 | chrA03:99201877-99205979 | 4103/867 | 288/31.94/7.2 | N/N/- |
| GhPOD18/Gh_A03G2152 | chrA03:16442-17606 | 1165/984 | 327/35.9/8.94 | S/N/S |
| GhPOD19/Gh_A03G2153 | chrA03:28781-29958 | 1178/987 | 328/35.15/7.68 | S/N/S |
| GhPOD20/Gh_A04G0639 | chrA04:44156365-44158523 | 2159/990 | 329/37.28/7.27 | S/N/S |
| GhPOD21/Gh_A04G0963 | chrA04:59248781-59249939 | 1159/996 | 331/36/8.26 | S/N/S |
| GhPOD22/Gh_A04G1453 | chrA04:7944-10075 | 2132/1020 | 339/37.62/7.73 | N/S/S |
| GhPOD23/Gh_A05G0093 | chrA05:1157788-1159020 | 1233/963 | 320/34.93/7.34 | S/N/S |
| GhPOD24/Gh_A05G0507 | chrA05:5454946-5456283 | 1338/999 | 332/36.14/5.05 | N/N/S |
| GhPOD25/Gh_A05G0661 | chrA05:6938388-6950337 | 11950/1014 | 337/36.17/6.73 | S/N/S |
| GhPOD26/Gh_A05G0863 | chrA05:8607073-8608956 | 1884/753 | 250/27.59/6.35 | N/S/- |
| GhPOD27/Gh_A05G1328 | chrA05:13602610-13604358 | 1749/1053 | 350/38.23/9.51 | S/N/S |
| GhPOD28/Gh_A05G1452 | chrA05:15020810-15022360 | 1551/999 | 332/37.66/8.54 | S/N/S |
| GhPOD29/Gh_A05G1479 | chrA05:15196748-15197863 | 1116/1011 | 336/36.82/8.25 | N/S/- |
| GhPOD30/Gh_A05G1577 | chrA05:16171654-16172991 | 1338/951 | 316/34.19/9.5 | S/N/S |
| GhPOD31/Gh_A05G1635 | chrA05:16892245-16893617 | 1373/1050 | 349/37.73/7.7 | S/N/S |
| GhPOD32/Gh_A05G2401 | chrA05:29807925-29809249 | 1325/972 | 323/34.96/9.47 | S/N/S |
| GhPOD33/Gh_A05G2945 | chrA05:72277688-72279245 | 1558/972 | 323/35.2/4.67 | S/N/S |
| GhPOD34/Gh_A05G3141 | chrA05:81059455-81060420 | 966/966 | 321/35.48/8.86 | S/N/S |
| GhPOD35/Gh_A05G3239 | chrA05:84740299-84741875 | 1577/972 | 323/35.83/5.06 | N/S/S |
| GhPOD36/Gh_A05G3489 | chrA05:90428029-90429659 | 1631/1014 | 337/36.06/9.57 | S/N/S |
| GhPOD37/Gh_A05G3726 | chrA05:122462-125928 | 3467/1182 | 393/42.73/9.35 | N/S/C |
| GhPOD38/Gh_A06G0019 | chrA06:84546-85807 | 1262/954 | 317/34.23/9.28 | S/N/S |
| GhPOD39/Gh_A06G0383 | chrA06:6376778-6380906 | 4129/1167 | 388/42.25/7.68 | N/S/C |
| GhPOD40/Gh_A06G0929 | chrA06:38044180-38045572 | 1393/1038 | 345/37.15/4.4 | S/N/S |
| GhPOD41/Gh_A06G1006 | chrA06:49554553-49556908 | 2356/987 | 328/35.73/8.27 | S/N/S |
| GhPOD42/Gh_A06G2046 | chrA06:45925-48981 | 3057/960 | 319/34.42/8.88 | N/S/C |
| GhPOD43/Gh_A07G0012 | chrA07:136360-137613 | 1254/996 | 331/36.45/9.58 | S/N/S |
| GhPOD44/Gh_A07G0275 | chrA07:3399593-3400898 | 1306/996 | 331/35.82/4.82 | S/N/- |
| GhPOD45/Gh_A07G1997 | chrA07:76170501-76172204 | 1704/1026 | 341/37.65/8.37 | S/N/S |
| GhPOD46/Gh_A07G2090 | chrA07:77469444-77471552 | 2109/1101 | 366/40.24/6.74 | N/S/- |
| GhPOD47/Gh_A07G2109 | chrA07:77657753-77658858 | 1106/966 | 321/34.93/4.26 | S/N/S |
| GhPOD48/Gh_A07G2110 | chrA07:77659654-77660804 | 1151/975 | 324/35.43/7.68 | S/N/S |
| GhPOD49/Gh_A08G0345 | chrA08:4189416-4190503 | 1088/1002 | 333/35.9/7.9 | S/N/S |
| GhPOD50/Gh_A08G0347 | chrA08:4289654-4290385 | 732/732 | 243/25.94/7.89 | N/S/- |
| GhPOD51/Gh_A08G0711 | chrA08:17779406-17781156 | 1751/999 | 332/35.79/5.71 | N/S/S |
| GhPOD52/Gh_A08G0712 | chrA08:18361761-18367521 | 5761/1056 | 351/38.2/4.88 | S/N/S |
| GhPOD53/Gh_A08G0714 | chrA08:18413356-18418531 | 5176/1032 | 343/36.85/4.51 | S/N/S |
| GhPOD54/Gh_A08G0747 | chrA08:24039157-24042529 | 3373/990 | 329/36.31/10.43 | S/N/S |
| GhPOD55/Gh_A08G1744 | chrA08:97409160-97411219 | 2060/753 | 250/27.54/5.6 | N/N/- |
| GhPOD56/Gh_A08G1745 | chrA08:97440700-97442620 | 1921/726 | 241/26.72/7.74 | N/N/- |
| GhPOD57/Gh_A08G1746 | chrA08:97442829-97446411 | 3583/867 | 288/32.07/6.91 | N/N/- |
| GhPOD58/Gh_A08G1806 | chrA08:98615798-98617217 | 1420/960 | 319/34.42/5.68 | S/N/S |
| GhPOD59/Gh_A08G1950 | chrA08:100557586-100558616 | 1031/951 | 316/34.48/9.77 | S/N/S |
| GhPOD60/Gh_A08G2028 | chrA08:101461027-101462441 | 1415/969 | 322/34.96/7.75 | S/N/S |
| GhPOD61/Gh_A09G0591 | chrA09:48054359-48058181 | 3823/777 | 258/28.28/8.82 | N/S/- |
| GhPOD62/Gh_A09G1202 | chrA09:63774095-63775448 | 1354/1020 | 339/36.01/5.24 | S/N/S |
| GhPOD63/Gh_A09G1415 | chrA09:67412511-67414343 | 1833/990 | 329/37.12/6.58 | S/N/S |
| GhPOD64/Gh_A09G2334 | chrA09:93316-94482 | 1167/951 | 316/34.58/10.02 | S/N/S |
| GhPOD65/Gh_A09G2396 | chrA09:35011-35983 | 973/906 | 301/33.44/8.46 | S/N/S |
| GhPOD66/Gh_A10G0565 | chrA10:7031511-7032708 | 1198/1041 | 346/38.29/6.03 | S/N/S |
| GhPOD67/Gh_A10G0810 | chrA10:16610264-16611518 | 1255/993 | 330/35.91/9.88 | S/N/M |
| GhPOD68/Gh_A10G1317 | chrA10:69770818-69772754 | 1937/966 | 321/33.76/8.33 | S/N/S |
| GhPOD69/Gh_A10G1318 | chrA10:69892772-69893920 | 1149/954 | 317/34.64/9.07 | S/N/S |
| GhPOD70/Gh_A10G1537 | chrA10:84415649-84417960 | 2312/972 | 323/35.59/8.64 | S/N/S |
| GhPOD71/Gh_A10G1626 | chrA10:87538433-87544412 | 5980/909 | 302/33.3/10.36 | N/S/- |
| GhPOD72/Gh_A10G1627 | chrA10:87544899-87546095 | 1197/987 | 328/35.3/9.64 | S/N/S |
| GhPOD73/Gh_A10G2288 | chrA10:1167442-1169647 | 2206/984 | 327/35.78/10.03 | S/N/S |
| GhPOD74/Gh_A10G2290 | chrA10:1189614-1190983 | 1370/993 | 330/36.03/8.94 | S/N/S |
| GhPOD75/Gh_A11G0400 | chrA11:3718938-3720282 | 1345/996 | 331/36.51/9.37 | S/N/S |
| GhPOD76/Gh_A11G0523 | chrA11:4917637-4918870 | 1234/927 | 308/34.89/9.73 | N/S/S |
| GhPOD77/Gh_A11G1669 | chrA11:25291980-25293232 | 1253/1005 | 334/36.93/8.46 | S/N/S |
| GhPOD78/Gh_A11G1859 | chrA11:45617267-45622682 | 5416/930 | 309/34.02/4.72 | N/N/- |
| GhPOD79/Gh_A11G2043 | chrA11:63260806-63261288 | 483/483 | 160/17.1/4.89 | N/S/- |
| GhPOD80/Gh_A11G3132 | chrA11:6967-8208 | 1242/966 | 321/34.97/8 | S/N/S |
| GhPOD81/Gh_A12G0055 | chrA12:735887-756659 | 20773/972 | 323/35.31/8.26 | S/N/S |
| GhPOD82/Gh_A12G0056 | chrA12:760206-761426 | 1221/972 | 323/35.56/8.99 | S/N/S |
| GhPOD83/Gh_A12G0695 | chrA12:26988350-26989090 | 741/561 | 186/20.48/6.66 | N/S/- |
| GhPOD84/Gh_A12G0795 | chrA12:45772636-45776292 | 3657/1068 | 355/39.81/5.12 | S/N/S |
| GhPOD85/Gh_A12G1441 | chrA12:73250331-73252001 | 1671/1023 | 340/37.17/9.36 | N/N/M |
| GhPOD86/Gh_A12G1915 | chrA12:81887117-81888429 | 1313/1014 | 337/37.91/6 | S/N/S |
| GhPOD87/Gh_A12G2221 | chrA12:84939580-84946625 | 7046/3291 | 1096/122.66/7.51 | N/N/- |
| GhPOD88/Gh_A12G2370 | chrA12:86207288-86208988 | 1701/972 | 323/35.63/8.88 | S/N/S |
| GhPOD89/Gh_A12G2508 | chrA12:87451770-87452774 | 1005/1005 | 334/37.17/7.73 | S/N/S |
| GhPOD90/Gh_A12G2622 | chrA12:49766-51061 | 1296/1011 | 336/37.08/5.05 | S/N/S |
| GhPOD91/Gh_A13G0039 | chrA13:393916-396005 | 2090/978 | 325/35.42/9.01 | S/N/S |
| GhPOD92/Gh_A13G0772 | chrA13:33291935-33293456 | 1522/975 | 324/34.9/6.65 | S/N/S |
| GhPOD93/Gh_A13G0773 | chrA13:33310901-33312163 | 1263/987 | 328/35.42/7.36 | S/N/S |
| GhPOD94/Gh_A13G2003 | chrA13:79484281-79487307 | 3027/762 | 253/28/5.19 | N/N/- |
| GhPOD95/Gh_D01G0112 | chrD01:855518-857368 | 1851/975 | 324/35.25/9.56 | S/N/- |
| GhPOD96/Gh_D01G1310 | chrD01:36863811-36864806 | 996/996 | 331/36.9/8.19 | S/N/S |
| GhPOD97/Gh_D01G1317 | chrD01:37193921-37194913 | 993/993 | 330/36.56/7.96 | S/N/S |
| GhPOD98/Gh_D01G1359 | chrD01:40012715-40014005 | 1291/969 | 322/34.7/7.93 | S/N/S |
| GhPOD99/Gh_D01G1632 | chrD01:51431707-51437024 | 5318/867 | 288/31.78/5.81 | N/S/- |
| GhPOD100/Gh_D01G1726 | chrD01:53949584-53951128 | 1545/972 | 323/34.9/5.4 | S/N/S |
| GhPOD101/Gh_D02G0606 | chrD02:8255229-8256474 | 1246/1014 | 337/36.66/4.13 | S/N/S |
| GhPOD102/Gh_D02G0695 | chrD02:9863175-9865761 | 2587/1116 | 371/40.62/7.7 | N/S/M |
| GhPOD103/Gh_D02G1110 | chrD02:31109209-31111160 | 1952/1020 | 339/37.91/8.45 | S/N/S |
| GhPOD104/Gh_D02G1327 | chrD02:43855329-43856508 | 1180/1008 | 335/36.76/5.38 | S/N/S |
| GhPOD105/Gh_D02G1345 | chrD02:44875923-44877373 | 1451/990 | 329/36.19/9.18 | S/N/S |
| GhPOD106/Gh_D02G1346 | chrD02:44991381-44992620 | 1240/984 | 327/35.44/7.72 | S/N/S |
| GhPOD107/Gh_D02G1997 | chrD02:64059836-64065386 | 5551/987 | 328/35.24/7.94 | S/N/S |
| GhPOD108/Gh_D02G1998 | chrD02:64081876-64083040 | 1165/984 | 327/35.7/9.37 | S/N/S |
| GhPOD109/Gh_D02G2245 | chrD02:66344682-66348942 | 4261/867 | 288/31.96/7.2 | N/N/- |
| GhPOD110/Gh_D03G0059 | chrD03:390493-391719 | 1227/954 | 317/34.27/9.27 | S/N/S |
| GhPOD111/Gh_D03G0074 | chrD03:531888-534705 | 2818/912 | 303/33.96/8.22 | N/S/- |
| GhPOD112/Gh_D03G0246 | chrD03:2618435-2620271 | 1837/993 | 330/35.99/9.44 | S/N/S |
| GhPOD113/Gh_D03G0641 | chrD03:19207034-19208004 | 971/699 | 232/25.15/8.87 | N/N/S |
| GhPOD114/Gh_D03G1381 | chrD03:42417094-42420536 | 3443/1158 | 385/42.12/7.65 | N/S/- |
| GhPOD115/Gh_D03G1382 | chrD03:42426148-42428382 | 2235/969 | 322/34.55/9.72 | S/N/S |
| GhPOD116/Gh_D04G0130 | chrD04:1796590-1798244 | 1655/1014 | 337/36.04/9.22 | S/N/S |
| GhPOD117/Gh_D04G0735 | chrD04:15156949-15158096 | 1148/972 | 323/35.41/8.64 | S/N/S |
| GhPOD118/Gh_D04G1101 | chrD04:36441991-36444139 | 2149/987 | 328/37.19/6.73 | S/N/S |
| GhPOD119/Gh_D04G1116 | chrD04:36784560-36786018 | 1459/723 | 240/26.55/6.51 | N/N/- |
| GhPOD120/Gh_D04G1506 | chrD04:46932159-46969978 | 37820/2016 | 671/72.76/7.71 | S/N/S |
| GhPOD121/Gh_D04G1593 | chrD04:48023771-48025856 | 2086/1020 | 339/37.4/7.06 | N/N/S |
| GhPOD122/Gh_D05G0154 | chrD05:1545607-1546840 | 1234/978 | 325/35.03/7.99 | S/N/S |
| GhPOD123/Gh_D05G0626 | chrD05:5026931-5028271 | 1341/1002 | 333/36.15/5.05 | N/N/S |
| GhPOD124/Gh_D05G0807 | chrD05:6771535-6772831 | 1297/1014 | 337/36.1/6.73 | S/N/S |
| GhPOD125/Gh_D05G1498 | chrD05:13463061-13464814 | 1754/1059 | 352/38.37/9.62 | N/S/C |
| GhPOD126/Gh_D05G1626 | chrD05:14640100-14641647 | 1548/999 | 332/37.65/8.54 | S/N/S |
| GhPOD127/Gh_D05G1755 | chrD05:15837493-15838825 | 1333/951 | 316/34.22/9.47 | S/N/S |
| GhPOD128/Gh_D05G1817 | chrD05:16541131-16542504 | 1374/1050 | 349/37.76/7.7 | S/N/S |
| GhPOD129/Gh_D05G2244 | chrD05:21543390-21547747 | 4358/1218 | 405/43.82/9.15 | N/S/C |
| GhPOD130/Gh_D05G2666 | chrD05:27862573-27863898 | 1326/972 | 323/34.85/8.92 | S/N/S |
| GhPOD131/Gh_D05G3256 | chrD05:51004089-51005627 | 1539/951 | 316/33.69/8.5 | S/N/S |
| GhPOD132/Gh_D05G3875 | chrD05:76982-78848 | 1867/753 | 250/27.57/6.04 | N/S/- |
| GhPOD133/Gh_D06G0413 | chrD06:5889650-5894261 | 4612/1353 | 450/49.69/7.3 | N/S/C |
| GhPOD134/Gh_D06G1049 | chrD06:22550424-22553640 | 3217/1008 | 335/36.1/9.95 | N/S/C |
| GhPOD135/Gh_D06G1170 | chrD06:28537753-28539152 | 1400/1038 | 345/37.22/4.46 | S/N/S |
| GhPOD136/Gh_D06G1200 | chrD06:30337737-30340827 | 3091/1050 | 349/37.92/8.39 | S/N/S |
| GhPOD137/Gh_D07G0019 | chrD07:200078-201330 | 1253/996 | 331/36.42/9.11 | S/N/S |
| GhPOD138/Gh_D07G0331 | chrD07:3515650-3516955 | 1306/996 | 331/35.86/4.66 | S/N/S |
| GhPOD139/Gh_D07G1629 | chrD07:32319582-32320538 | 957/792 | 263/28.65/9.4 | N/S/- |
| GhPOD140/Gh_D07G1630 | chrD07:32393320-32394431 | 1112/939 | 312/34.24/9.89 | S/N/S |
| GhPOD141/Gh_D07G2217 | chrD07:53125200-53126909 | 1710/1026 | 341/37.7/8.37 | S/N/S |
| GhPOD142/Gh_D07G2320 | chrD07:54647068-54648167 | 1100/966 | 321/35.08/4.35 | S/N/S |
| GhPOD143/Gh_D07G2321 | chrD07:54648867-54650018 | 1152/975 | 324/35.39/7.24 | S/N/S |
| GhPOD144/Gh_D08G0441 | chrD08:4640562-4641585 | 1024/858 | 285/30.9/8.72 | N/S/C |
| GhPOD145/Gh_D08G0442 | chrD08:4670537-4671627 | 1091/1005 | 334/35.7/8.55 | S/N/S |
| GhPOD146/Gh_D08G0443 | chrD08:4687890-4688979 | 1090/1005 | 334/35.66/8.55 | S/N/S |
| GhPOD147/Gh_D08G0829 | chrD08:13789103-13790820 | 1718/999 | 332/35.8/6.5 | S/N/S |
| GhPOD148/Gh_D08G0832 | chrD08:14037040-14038366 | 1327/1065 | 354/38.22/4.6 | S/N/S |
| GhPOD149/Gh_D08G2093 | chrD08:59783046-59784995 | 1950/741 | 246/27.13/6.04 | N/S/- |
| GhPOD150/Gh_D08G2094 | chrD08:59804611-59806683 | 2073/738 | 245/27.03/5.9 | N/N/- |
| GhPOD151/Gh_D08G2095 | chrD08:59806893-59810058 | 3166/879 | 292/32.56/6.95 | N/N/- |
| GhPOD152/Gh_D08G2167 | chrD08:61090294-61091717 | 1424/960 | 319/34.38/5.68 | S/N/S |
| GhPOD153/Gh_D08G2330 | chrD08:63032857-63033881 | 1025/945 | 314/34.22/9.44 | S/N/S |
| GhPOD154/Gh_D08G2420 | chrD08:64145436-64146846 | 1411/969 | 322/34.92/7.75 | S/N/S |
| GhPOD155/Gh_D09G0590 | chrD09:28473937-28477562 | 3626/990 | 329/36.6/9.74 | S/N/S |
| GhPOD156/Gh_D09G1208 | chrD09:39009612-39010932 | 1321/1020 | 339/35.98/4.95 | S/N/S |
| GhPOD157/Gh_D09G1420 | chrD09:41767263-41769101 | 1839/987 | 328/36.92/6.28 | S/N/S |
| GhPOD158/Gh_D09G1611 | chrD09:43695508-43696494 | 987/987 | 328/36.13/6.76 | S/N/S |
| GhPOD159/Gh_D09G2046 | chrD09:47806422-47807579 | 1158/951 | 316/34.5/9.58 | S/N/S |
| GhPOD160/Gh_D09G2047 | chrD09:47809072-47810196 | 1125/885 | 294/31.91/9.35 | S/N/S |
| GhPOD161/Gh_D09G2048 | chrD09:47810407-47821212 | 10806/2019 | 672/73.64/9.25 | S/N/S |
| GhPOD162/Gh_D09G2418 | chrD09:1692-2815 | 1124/1026 | 341/37.69/9.36 | S/N/S |
| GhPOD163/Gh_D10G0605 | chrD10:6317691-6318891 | 1201/1041 | 346/38.32/6.12 | S/N/S |
| GhPOD164/Gh_D10G0951 | chrD10:12771913-12773186 | 1274/993 | 330/36.01/9.99 | S/N/M |
| GhPOD165/Gh_D10G1157 | chrD10:19241093-19242236 | 1144/954 | 317/34.55/9.06 | S/N/S |
| GhPOD166/Gh_D10G1158 | chrD10:19299752-19300772 | 1021/705 | 234/24.46/8.21 | N/S/- |
| GhPOD167/Gh_D10G1643 | chrD10:45423530-45424891 | 1362/993 | 330/36.1/8.98 | S/N/S |
| GhPOD168/Gh_D10G1644 | chrD10:45445937-45448111 | 2175/984 | 327/35.72/10.04 | S/N/S |
| GhPOD169/Gh_D10G1784 | chrD10:50389073-50391342 | 2270/972 | 323/35.64/8.8 | S/N/S |
| GhPOD170/Gh_D10G1880 | chrD10:52703553-52704762 | 1210/1041 | 346/37.99/8.78 | S/N/S |
| GhPOD171/Gh_D10G1881 | chrD10:52813459-52814911 | 1453/972 | 323/35.59/10.39 | S/N/S |
| GhPOD172/Gh_D10G1882 | chrD10:52815399-52816603 | 1205/987 | 328/35.25/9.64 | S/N/S |
| GhPOD173/Gh_D11G0463 | chrD11:3963558-3964899 | 1342/996 | 331/36.57/9.22 | S/N/S |
| GhPOD174/Gh_D11G0607 | chrD11:5302020-5303236 | 1217/978 | 325/36.13/8.95 | N/S/S |
| GhPOD175/Gh_D11G1824 | chrD11:20830683-20831933 | 1251/969 | 322/35.55/8.46 | S/N/S |
| GhPOD176/Gh_D11G2151 | chrD11:32727567-32730117 | 2551/972 | 323/35.51/4.66 | S/N/S |
| GhPOD177/Gh_D11G2183 | chrD11:34071064-34072304 | 1241/966 | 321/34.9/7.74 | S/N/S |
| GhPOD178/Gh_D11G2348 | chrD11:45744028-45745072 | 1045/957 | 318/35.11/10.5 | S/N/M |
| GhPOD179/Gh_D12G0069 | chrD12:815867-853649 | 37783/1764 | 587/64.17/7.56 | N/S/- |
| GhPOD180/Gh_D12G0070 | chrD12:860573-861841 | 1269/972 | 323/35.07/6.5 | S/N/S |
| GhPOD181/Gh_D12G0071 | chrD12:866590-867867 | 1278/1029 | 342/37.73/8.99 | N/S/S |
| GhPOD182/Gh_D12G0072 | chrD12:876401-877438 | 1038/789 | 262/28.85/8.06 | N/S/- |
| GhPOD183/Gh_D12G0699 | chrD12:15282699-15283971 | 1273/993 | 330/35.78/8.49 | S/N/S |
| GhPOD184/Gh_D12G0788 | chrD12:22644947-22646101 | 1155/1068 | 355/39.65/4.9 | S/N/S |
| GhPOD185/Gh_D12G0853 | chrD12:27599646-27601009 | 1364/1050 | 349/38.71/6.76 | S/N/S |
| GhPOD186/Gh_D12G1559 | chrD12:46570546-46572226 | 1681/1023 | 340/37.12/9.36 | N/S/M |
| GhPOD187/Gh_D12G1577 | chrD12:46856738-46864694 | 7957/1803 | 600/68.11/6.77 | N/S/- |
| GhPOD188/Gh_D12G2095 | chrD12:53969820-53971114 | 1295/1002 | 333/37.49/5.48 | S/N/S |
| GhPOD189/Gh_D12G2504 | chrD12:57927732-57929447 | 1716/972 | 323/35.48/8.72 | S/N/S |
| GhPOD190/Gh_D12G2635 | chrD12:59085174-59086178 | 1005/1005 | 334/37.2/7.99 | S/N/S |
| GhPOD191/Gh_D13G0054 | chrD13:451962-454035 | 2074/978 | 325/35.63/9.66 | S/N/S |
| GhPOD192/Gh_D13G0906 | chrD13:18641774-18643302 | 1529/978 | 325/34.95/6.77 | S/N/S |
| GhPOD193/Gh_D13G0907 | chrD13:18659873-18661135 | 1263/987 | 328/35.47/7.41 | S/N/S |
| GhPOD194/Gh_D13G0909 | chrD13:18677172-18678434 | 1263/987 | 328/35.5/7.41 | S/N/S |
| GhPOD195/Gh_D13G2402 | chrD13:60076810-60079762 | 2953/750 | 249/27.31/5.17 | N/N/- |
| GhPOD196/Gh_Sca005203G02 | chrSca:12082-12672 | 591/591 | 196/22.12/8.79 | N/S/- |
| GhPOD197/Gh_Sca005268G01 | chrSca:3248-4662 | 1415/960 | 319/34.49/7.93 | S/N/S |
| GhPOD198/Gh_Sca007173G01 | chrSca:8026-8850 | 825/825 | 275/30.21/6.74 | S/N/S |
Chr: chromosome; CDS: coding sequence; PL: peptide length; MW: molecular weight; N: non-secreted into the apoplast; S: secreted into apoplast with signal peptide in SignalP, without signal peptide but with non-classical secretion mode in SeretomeP and with signal peptide in TargetP; C: chloroplast; M: mitochondria; -: unsure location).
3.2. Phylogenetic Analysis
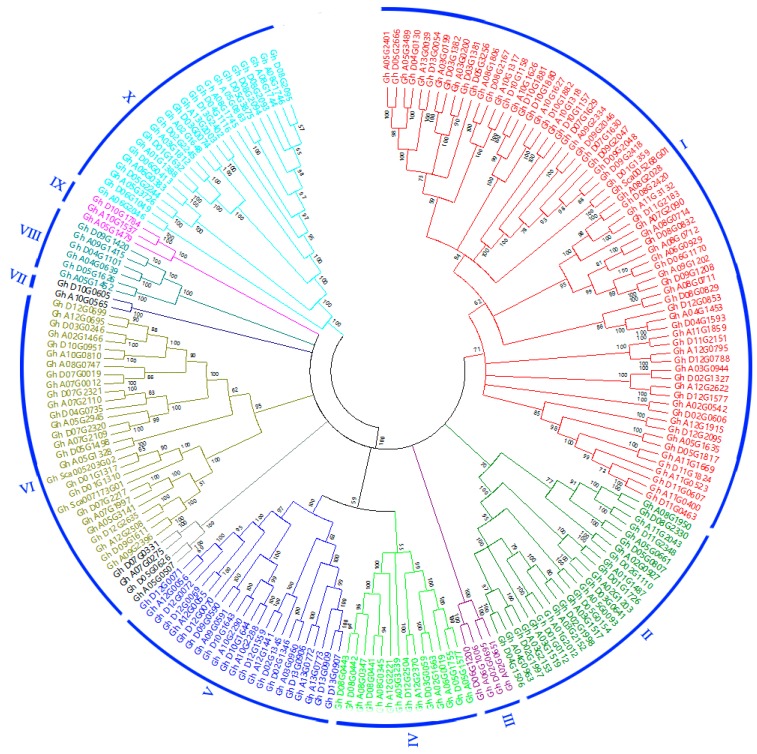
The phylogenetic tree was constructed with the NJ method based on multiple sequence alignment of entire amino acid sequence of 198 upland cotton POD protein sequences in order to acquire a better understanding of evolutionary history and the phylogenetic relationship of POD in upland cotton. Based on the phylogenetic tree (Figure 1), we identified 10 major clusters with high bootstrap probabilities (BPs) ranging from 59 to 100%, among them six clusters had 100% BPs, one had 95% BPs, and two had 71% or nearly 71% BPs (Figure 1). The POD genes were not evenly distributed in some groups in upland cotton, Cluster I had the most members (68), which could be divided into seven subgroups, but Cluster VII only had two members (Figure 1 and Figure 2).
Figure 1.
Phylogenetic relationship of the 198 identified upland cotton POD genes. Unrooted tree constructed using MEGA5.02 by the Neighbor-Joining (NJ) method. Bootstrap values (above 50%) from 1000 replicates are indicated at each node. The tree shows 10 major phylogenetic subfamilies (subfamilies I to X).
Figure 2.
Exon–intron structures of the 198 identified upland cotton POD genes. Exons and introns are indicated by yellow cylinder bars and black lines, respectively.
3.3. Gene Structures
POD genes with classical conserved intron/exon gene structure were observed. The coding sequence of 100 of the 198 peroxidase genes are disrupted by three introns at conserved positions (Figure 2). However, variations in this basic gene structure were observed for another 98 of the family, implicating loss of one or more introns (64) or gain of one or more introns (34). Forty genes lost one of the three putative ancestral introns, while fifteen genes lost two introns. Additionally, nine genes (among them, eight genes were closely related, belonged to VI subgroup) were devoid of any introns. In comparison with the classical three introns, the number of POD genes gaining one to nine more introns (except eight), were 5, 3, 2, 4, 13, 2, 4, and 1, respectively. These differences may be derived from a single intron loss or gain events during the long evolutionary period. In addition, 23 of 24 genes with more than seven introns constitute group X, which contains the largest numbers of introns. Sub-clusters with conserved intron/exon gene structure were also observed.
3.4. Chromosomal Location and Gene Duplication
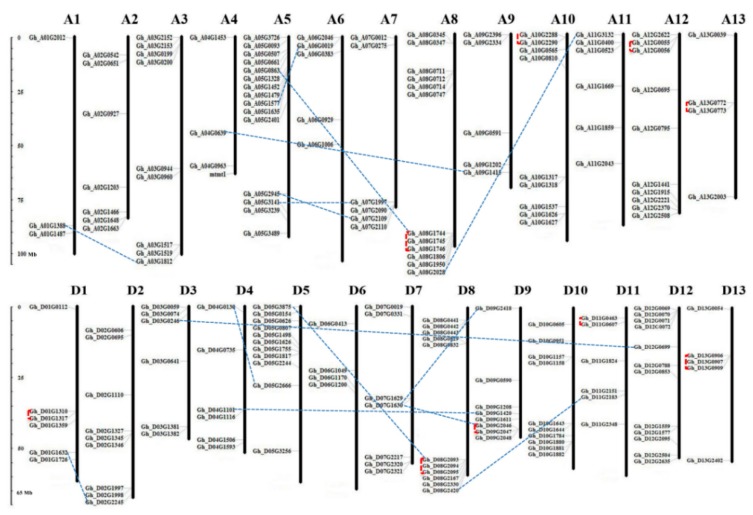
To investigate the genome organization and distribution of GhPODs on different subgenomes and chromosomes (CH) in upland cotton, a chromosome map was constructed. Among 198 GhPOD genes, 94 and 101 were located in subgenomes A and D, respectively. The other three POD genes were located on scaffolds. GhPOD genes unequally distributes in both subgenome A and D. For subgenome A, CHA5 has the most POD members (15), and ChA1 and CHA4 have the least but same members (3); for subgenome D, CHD12 has the most POD members (12), and CHD6 has the least members (4). In addition, the majority of chromosomes, especially for subgenome A, exhibit a relatively high density of GhPOD genes, which tends to assemble at the bottoms or the tops, such as CHA3, 5, 7, 8, 12 and CHD3, 5, 8, 9.
Gene duplications, including segmental and tandem duplication, are one of the primary driving forces in genome evolution [24]. In this study, 27 duplicated gene pairs were identified (Figure 3, Table 2); among them, 12 and 15 GhPOD gene pairs were in subgenome A and subgenome D, respectively. A total of 15 gene pairs (29 genes) were localized to segmentally duplicated regions, while 9 gene clusters (12 gene pairs or duplication events; 21 genes) are arranged in tandem repeats [Segmental duplication (Gh_D07G1630/Gh_D09G2418) (I); (Gh_D07G1630/Gh_D09G2046) (I); Gh_A08G2028/Gh_A11G3132) (I); Gh_D08G2420/Gh_D11G2183 (I); Gh_D04G0130/Gh_D05G2666 (I); Gh_A05G1577/Gh_A06G0019 (IV); Gh_D03G0246/Gh_D12G0699 (VI); Gh_D04G1101/Gh_D09G1420 (VI); Gh_A04G0639/Gh_A09G1415 (VI); Gh_A05G2945/Gh_A07G2109 (VIII); Gh_A05G3141/Gh_A07G1997 (VIII); Gh_D01G1632/Gh_D02G2245 (X); Gh_D05G3875/Gh_D08G2093 (X); Gh_A01G1388/Gh_A03G1812 (X); Gh_A05G0863/Gh_A08G1744 (X); Tandem duplication (Gh_D09G2046/Gh_D09G2047 (I); Gh_D11G0463/Gh_D11G0607 (I); Gh_D13G0906/Gh_D13G0907/Gh_D13G0909 (IV); Gh_A10G2288/Gh_A10G2290 (IV); Gh_A12G0055/Gh_A12G0056 (IV); Gh_A13G0772/Gh_A13G0773 (IV); Gh_D01G1310/Gh_D01G1317 (VI); Gh_A08G1744/Gh_A08G1745/Gh_A08G1746 (X); Gh_D08G2093/Gh_D08G2094/Gh_D082095 (X)].
Figure 3.
Chromosomal location and gene duplication events of 198 POD genes on 26 upland cotton chromosomes. Chromosome types and numbers are indicated at the top of each bar. The scale on the left is in mega-bases. The gene ID on the left side of each chromosome correspond to the approximate locations of each POD gene. The segmentally duplicated genes are connected by dashed blue lines, and the tandemly duplicated gene clusters are marked by red square bracket with dashed line.
Table 2.
The Ka, Ks, and Ka/Ks values for the 27 gene pairs.
| Paralogous Pairs | Ks | Ka | Ka/Ks | Duplicate Type |
|---|---|---|---|---|
| Gh_A01G1388-Gh_A03G1812 | 0.446 | 0.065 | 0.15 | Segmental |
| Gh_D03G0246-Gh_D12G0699 | 1.037 | 0.099 | 0.10 | Segmental |
| Gh_D04G0130-Gh_D05G2666 | 0.573 | 0.086 | 0.15 | Segmental |
| Gh_D04G1101-Gh_D09G1420 | 0.452 | 0.095 | 0.21 | Segmental |
| Gh_D05G3875-Gh_D08G2093 | 0.373 | 0.03 | 0.08 | Segmental |
| Gh_D07G1630-Gh_D09G2046 | 0.298 | 0.063 | 0.21 | Segmental |
| Gh_D07G1630-Gh_D09G2418 | 0.205 | 0.046 | 0.22 | Segmental |
| Gh_D08G2420-Gh_D11G2183 | 0.479 | 0.066 | 0.14 | Segmental |
| Gh_A04G0639-Gh_A09G1415 | 0.486 | 0.095 | 0.20 | Segmental |
| Gh_A05G0863-Gh_A08G1744 | 0.148 | 0.074 | 0.50 | Segmental |
| Gh_A05G1577-Gh_A06G0019 | 0.508 | 0.07 | 0.14 | Segmental |
| Gh_A05G2945-Gh_A07G2109 | 0.79 | 0.096 | 0.12 | Segmental |
| Gh_A05G3141-Gh_A07G1997 | 0.721 | 0.079 | 0.11 | Segmental |
| Gh_A08G2028-Gh_A11G3132 | 0.483 | 0.073 | 0.15 | Segmental |
| Gh_D01G1632-Gh_D02G2245 | 0.452 | 0.063 | 0.14 | Segmental |
| Gh_D08G2093-Gh_D08G2094 | 0.087 | 0.011 | 0.13 | Tandem |
| Gh_D08G2094-Gh_D08G2095 | 0.087 | 0.017 | 0.20 | Tandem |
| Gh_D09G2046-Gh_D09G2047 | 0.355 | 0.046 | 0.13 | Tandem |
| Gh_D11G0463-Gh_D11G0607 | 0.129 | 0.039 | 0.30 | Tandem |
| Gh_D13G0906-Gh_D13G0907 | 0.205 | 0.07 | 0.34 | Tandem |
| Gh_D13G0907-Gh_D13G0909 | 0.009 | 0.001 | 0.11 | Tandem |
| Gh_A08G1744-Gh_A08G1745 | 0.039 | 0.039 | 1.00 | Tandem |
| Gh_A08G1745-Gh_A08G1746 | 0.033 | 0.033 | 1.00 | Tandem |
| Gh_A10G2288-Gh_A10G2290 | 0.306 | 0.093 | 0.30 | Tandem |
| Gh_A12G0055-Gh_A12G0056 | 0.289 | 0.061 | 0.21 | Tandem |
| Gh_A13G0772-Gh_A13G0773 | 0.205 | 0.076 | 0.37 | Tandem |
| Gh_D01G1310-Gh_D01G1317 | 0.018 | 0.008 | 0.44 | Tandem |
To explore the selection pressures among duplicated POD genes, we calculated the Ka, Ks, and Ka/Ks values for 27 identified gene pairs (Table 2). In general, Ka/Ks > 1 indicates positive selection, Ka/Ks = 1 indicates neutral selection, and Ka/Ks < 1 indicates negative selection. The Ka/Ks ratios of most GhPOD gene pairs were <1 except for two pairs (GhPOD55 and GhPOD56; GhPOD56 and GhPOD57) with Ka/Ks = 1, suggesting that these gene pairs were evolved under negative selection in upland cotton.
3.5. Responses of POD Genes to PK Deficiency in Cotton Roots and Leaves
Among 198 GhPOD genes, the expression of 30 genes was not detected in all samples including roots and leaves under control conditions, and under K and P deficient conditions. In roots, K and P deficiency, respectively, induced 10 and 11 POD gene expression from zero to slight level (FPKM value < 1). In leaves, K and P deficiency, respectively, induced 28 and 11 POD gene expression from zero to slight level (FPKM value < 1 for most of them). For roots, compared with controls, 12 and 42 POD gene expression, respectively, was above 2-fold and below 0.5-fold under K deficiency; the expression of 18 POD genes, respectively, was above 2-fold and below 0.5-fold under P deficiency. In leaves, compared with controls, the expression of 25 and 16 POD genes, respectively, was above 2-fold and below 0.5-fold being subjected to K deficiency; the expression of 12 and 33 POD genes, respectively, was above 2-fold and below 0.5-fold being subjected to P deficiency. The same POD gene expressed itself with obviously different patterns in leaves or roots under K or P deficiency. For example, under K deficiency, the expression of Gh_A08G1806 was 0.2 times as much as the controls; however, under P deficiency, it was 6.1 times as much as controls in roots. K deficiency induced the expression of Gh_A08G1806 from zero to 1.3 FPKM, but P deficiency did not change its transcription level.
3.6. Secret Traits of Cotton POD
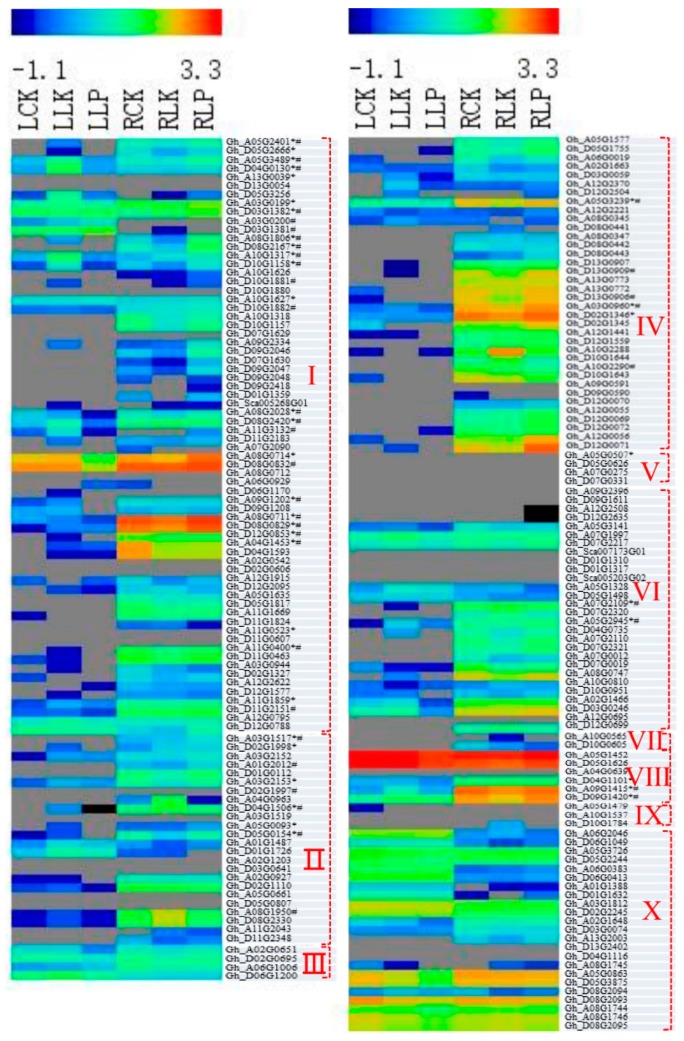
Among 198 GhPOD genes, 142 POD enzymes were predicted with signal peptide by using SignalP, 147 with signal peptide by using TargetP, another 38 POD enzymes were predicted being secreted into apoplast with SecretomeP among no signal peptides by SignalP prediction (Table 1). In xylem sap, 61 POD enzyme isoforms were identified. Among them, 31 isoforms were found not only in field conditions but also in greenhouse conditions (Figure 4).
Figure 4.
Responses of gene expression in cotton root and leaf to PK deficiency. Values are log10 FPKM. * the POD protein was detected in the field cotton xylem sap; # the POD protein was detected in the greenhouse cotton.
4. Discussion
4.1. Identification of Cotton POD Genes and Their Expansion
Members of POD gene family are involved in the regulation of a variety of biological processes. POD proteins are classified into apoplast type and vacuole type [25]. Apoplast type PODs participate in plant cell wall lignification, defense to abiotic and biotic stresses, plant growth and development, etc. The majority of PODs (90%) was predicted to be secreted to apoplast by using SignalP plus SecretomeP, and 61 PODs in total were detected in cotton xylem sap from adult cotton plants in the field and cotton seedlings in the greenhouse, indicating their different roles in cotton growth and development. The predictive tools for localization show very different results, indicating the fact that plant localization signals are very variable.
Previously, Delannoy et al. (2003) characterized nine POD genes, found them showing differential expressions in response to the pathogen and suggested that they may have various functions in cotton defense to bacterial blight disease [9]. Furthermore, Delannoy et al. (2006) analyzed 12 POD genes from cotton and found two of them played a role in the oxidative burst response of cotton to bacterial blight [19]. Mei et al. (2009) also investigated 10 POD genes in cotton fiber development [6].
Systematic and comprehensive analyses of POD gene families have been published for Populus trichocarpa [3], Zea mays [23], Arabidopsis thaliana [5], and Oryza sativa [4]. The genome data of allotetraploid upland cotton [20] provides a useful tool for analysis of the upland cotton POD gene family. In our study, 198 POD genes were identified and characterized in upland cotton. The number in the upland cotton is higher than that in Arabidopsis (73) [5], Poplar (93) [3], Chinese Pear (94) [22], maize (119) [23], and rice (138) [4] and similar to that in switchgrass (more than 200) [2]. This is probably due to the fact that upland cotton and switchgrass are tetraploid with larger genomes containing two-type sub-genomes (respectively, 26 chromosomes, A and D; 18, A and B), and that Arabidopsis, maize and rice were diploid with smaller genomes (respectively, 5 chromosomes; 10; 12). However, this cannot explain the fact that the tetraploid Populus with 19 chromosomes in the genome has only 93 POD isoforms.
Gene duplications are one of the primary driving forces in the evolution of genomes and genetic systems [24]. Certain studies have shown that segmental duplication was largely responsible for the expansion of cotton gene families such as the TCP transcription factors in G. raimondii, YABBY and GhHsp20 in G. hirsutum [26,27,28]. By contrast, tandem duplication has contributed significantly to the expansion of this gene family in poplar [3]. However, for nsLTPs, both tandem and segmental duplication contributed to its expansion in G. arboreum and G. hirsutum, while tandem duplication was the dominant pattern in G. raimondii [29]. Interestingly, in this study, we determined that the number of GhPOD genes involved in segmental duplication and tandem duplication is similar, suggesting that both segmental and tandem duplications were equal contributors to the expansion of the POD gene family in upland cotton. It showed a similar gene duplication with POD genes in maize [23]. The Ka/Ks < 1 of the most GhPOD duplicated pairs showed that negative selection may be largely responsible for maintaining the functions of upland cotton POD enzymes.
Phylogenetic analysis of the GHPOD gene family revealed that the exon/intron structures of these genes are relatively conserved due to one half of the GhPOD genes with the 4 exons/3 introns structures. Similarly, 48 of the 73 (65.8%) peroxidase encoding genes in Arabidopsis consist of 4 exons/3 introns [5], and 38 of 138 (27.5%) in rice constitute this structure. It suggests a common ancestral gene with a classical pattern of 4 exons/3 introns [4]. Many studies have shown that introns were specifically inserted into plants and were retained in the genome during the course of evolution [30]. Another half of GhPOD genes gained or lost one or more introns from the POD coding region in a subfamily specific manner, and what is most serious is that some genes contain no introns. This extreme case for POD genes also exist in Arabidopsis [5], rice [4], maize [23], which might be explained either by the loss of all introns or by the occurrence of a reverse transcription event followed by the integration of the cDNA copy back in the genome, as described in mammals, yeast, and maize [31,32]. It is well known that the structural diversity of genes drives the evolution of multigene families. Also, the differences in these characteristics detected between different subfamilies suggest that upland cotton POD members are functionally diversified.
4.2. Expression Profiles of GhPOD Genes
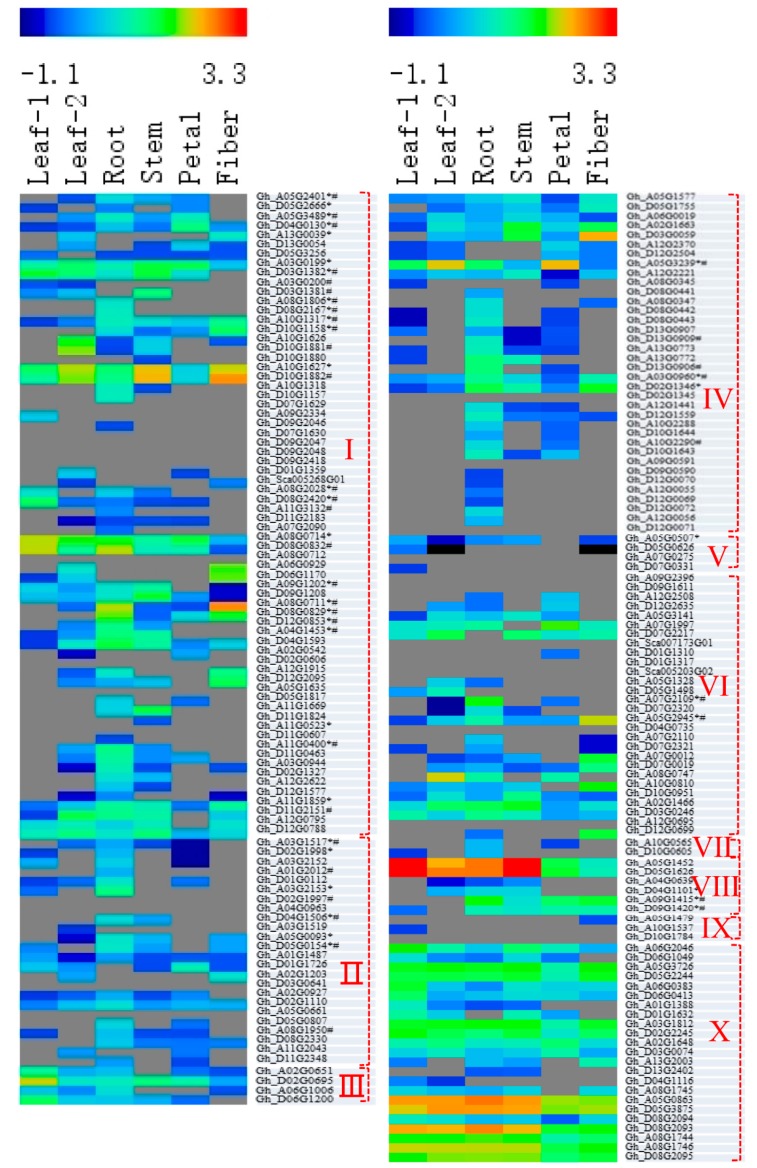
Gene expression patterns can provide important clues about gene function. We used publicly available [20] (https://www.ncbi.nlm.nih.gov/sra/?term=PRJNA248163) and our own genome-wide transcripts profiling data from upland cotton tissues as a resource to investigate the expression patterns of GhPODs. Based on the public data, 28 of the 198 identified GhPOD genes were not expressed in leaves, roots, stems, petals or fibers (Figure 4 and Figure 5), indicating their functional loss among all organs. In comparison between our results and the results of public data by using data from normal culture conditions, 56, 35, and 19 of the 198 GhPOD genes, respectively, exhibited no expression in leaves or/and roots (Figure 4 and Figure 5). It indicated that a part of GhPODs are expressed coincidently under different conditions or at different developmental stages. Few POD genes demonstrate tissue or organ specificity. In the Arabidopsis genome, 73 POD genes have been annotated, 65 of which were expressed in various tissues, and only three (AtPrx12, AtPrx62, AtPrx65) identified as specific to roots [33]. In the upland cotton genome, 17 of 198 POD genes were identified as specific to roots. However, only 4 and 1 were expressed in leaves and fibers, respectively (Figure 4 and Figure 5).
Figure 5.
Expression profiles of POD genes across different cotton tissues. Values are log10 FPKM. * the POD protein was detected in the field cotton xylem sap; # the POD protein was detected in the greenhouse cotton. Sources of the samples are as follows: leaf-1 (true leaves, Accession: SRX849561); leaf-2 (leaves at 2-week-old plants, Accession: SRX797901); root (roots at 2-week-old plants, Accession: SRX797899); stem (stems at 2-week-old plants, Accession: SRX797900); petals (petals of mature flowers, Accession: SRX797903); fibers (fibers of 25 days post-anthesis).
PODs are expressed in different patterns when facing different biotic and abiotic stresses [19,23,34,35]. This was also confirmed in roots and leaves when plants were subjected to K or P deficiency. For example, expression level of gene Gh_D10G1158 decreased obviously in roots under K deficiency and was about 30% of the controls; under P deficiency, its expression was increased obviously and was 202% as much as controls. Conversely, its expression level was increased by 20.9-fold in the leaf under K deficiency, but very few of them show expression changes under P deficiency. Additionally, different subfamily genes showed obviously different responses to K or P deficiency. In comparison with the controls, 16.7% and 45.2% of GhPOD genes had, respectively, more than 2 and less than 0.5 times the expression level in the root under K deficit in the I subfamily; 48.0% and 33.3% of GhPOD genes had, respectively, more than 2 and less than 0.5 times the expression level in the root under P deficit in the I subfamily. Interestingly, few GhPOD genes with higher expression level with FPKM > 30 showed changes of more than 2 or less than 0.5 times in leaf or root under K or P deficit, indicating that these genes play important roles in the maintenance of basic plant growth.
Author Contributions
P.D. and Z.Z. designed the experiments and wrote the manuscript, B.Z., G.W. and M.C. contribute substantially in data analysis and interpretation of the data. All authors reviewed and approved the final manuscript.
Funding
This research was supported by the National Natural Science Foundation of China (31571600 and 31271648).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Passardi F., Theiler G., Zamocky M., Cosio C., Rouhier N., Teixera F., Margis-Pinheiro M., Ioannidis V., Penel C., Falquet L., et al. PeroxiBase: The peroxidase database. Phytochemistry. 2007;68:1605–1611. doi: 10.1016/j.phytochem.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 2.Moural T.W., Lewis K.M., Barnaba C., Zhu F., Palmer N.A., Sarath G., Scully E.D., Jones J.P., Sattler S.E., Kang C. Characterization of class III peroxidases from switchgrass. Plant Physiol. 2017;173:417–433. doi: 10.1104/pp.16.01426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ren L.L., Liu Y.J., Liu H.J., Qian T.T., Qi L.W., Wang X.R., Zeng Q.Y. Subcellular relocalization and positive selection play key roles in the retention of duplicate genes of Populus class III peroxidase family. Plant Cell. 2014;26:2404–2419. doi: 10.1105/tpc.114.124750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Passardi F., Longet D., Penel C., Dunand C. The class III peroxidase multigenic family in rice and its evolution in land plants. Phytochemistry. 2004;65:1879–1893. doi: 10.1016/j.phytochem.2004.06.023. [DOI] [PubMed] [Google Scholar]
- 5.Tognolli M., Penel C., Greppin H., Simon P. Analysis and expression of the class III peroxidase large gene family in Arabidopsis thaliana. Gene. 2002;288:129–138. doi: 10.1016/S0378-1119(02)00465-1. [DOI] [PubMed] [Google Scholar]
- 6.Mei W., Qin Y., Song W., Li J., Zhu Y. Cotton GhPOX1 encoding plant class III peroxidase may be responsible for the high level of reactive oxygen species production that is related to cotton fiber elongation. J. Genet. Genom. 2009;36:141–150. doi: 10.1016/S1673-8527(08)60101-0. [DOI] [PubMed] [Google Scholar]
- 7.Martinez-Rubio R., Acebes J.L., Encina A., Karkonen A. Class III peroxidases in cellulose deficient cultured maize cells during cell wall remodeling. Physiol. Plant. 2018;164:45–55. doi: 10.1111/ppl.12710. [DOI] [PubMed] [Google Scholar]
- 8.Zhang Z., Xin W., Wang S., Zhang X., Dai H., Sun R., Frazier T., Zhang B., Wang Q. Xylem sap in cotton contains proteins that contribute to environmental stress response and cell wall development. Funct. Integr. Genom. 2015;15:17–26. doi: 10.1007/s10142-014-0395-y. [DOI] [PubMed] [Google Scholar]
- 9.Delannoy E., Jalloul l A., Assigbetse K., Marmey P., Geiger J.P., Lherminier J., Daniel J.F., Martinez C., Nicole M. Activity of class III peroxidases in the defense of cotton to bacterial blight. Mol. Plant Microbe Interact. 2003;16:1030–1038. doi: 10.1094/MPMI.2003.16.11.1030. [DOI] [PubMed] [Google Scholar]
- 10.Racz A., Hideg E., Czegeny G. Selective responses of class III plant peroxidase isoforms to environmentally relevant UV-B doses. J. Plant Physiol. 2018;221:101–106. doi: 10.1016/j.jplph.2017.12.010. [DOI] [PubMed] [Google Scholar]
- 11.Passardi F., Cosio C., Penel C., Dunand C. Peroxidases have more functions than a Swiss army knife. Plant Cell Rep. 2005;24:255–265. doi: 10.1007/s00299-005-0972-6. [DOI] [PubMed] [Google Scholar]
- 12.Almagro L., Gomez Ros L.V., Belchi-Navarro S., Bru R., Ros Barcelo A., Pedreno M.A. Class III peroxidases in plant defence reactions. J. Exp. Bot. 2009;60:377–390. doi: 10.1093/jxb/ern277. [DOI] [PubMed] [Google Scholar]
- 13.Marjamaa K., Hilden K., Kukkola E., Lehtonen M., Holkeri H., Haapaniemi P., Koutaniemi S., Teeri T.H., Fagerstedt K., Lundell T. Cloning, characterization and localization of three novel class III peroxidases in lignifying xylem of Norway spruce (Picea abies) Plant Mol. Biol. 2006;61:719–732. doi: 10.1007/s11103-006-0043-6. [DOI] [PubMed] [Google Scholar]
- 14.Francoz E., Ranocha P., Nguyen-Kim H., Jamet E., Burlat V., Dunand C. Roles of cell wall peroxidases in plant development. Phytochemistry. 2015;112:15–21. doi: 10.1016/j.phytochem.2014.07.020. [DOI] [PubMed] [Google Scholar]
- 15.Zhu J., Chen S., Alvarez S., Asirvatham V.S., Schachtman D.P., Wu Y., Sharp R.E. Cell wall proteome in the maize primary root elongation zone. I. Extraction and identification of water-soluble and lightly ionically bound proteins. Plant Physiol. 2006;140:311–325. doi: 10.1104/pp.105.070219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Girma K., Teal R.K., Freeman K.W., Boman R.K., Raun W.R. Cotton lint yield and quality as affected by applications of N, P, and K fertilizers. J. Cotton Sci. 2007;11:12–19. [Google Scholar]
- 17.Dong H., Kong X., Li W., Tang W., Zhang D. Effects of plant density and nitrogen and potassium fertilization on cotton yield and uptake of major nutrients in two fields with varying fertility. Field Crops Res. 2010;119:106–113. doi: 10.1016/j.fcr.2010.06.019. [DOI] [Google Scholar]
- 18.Chen D., Ding Y., Guo W., Zhang T. Molecular cloning and characterization of a flower-specific class III peroxidase gene in G. hirsutum. Mol. Biol. Rep. 2009;36:461–469. doi: 10.1007/s11033-007-9202-3. [DOI] [PubMed] [Google Scholar]
- 19.Delannoy E., Marmey P., Jalloul A., Etienne H., Nicole M. Molecular analysis of class III peroxidases from cotton. J. Cotton Sci. 2006;10:53–60. [Google Scholar]
- 20.Zhang T., Hu Y., Jiang W., Fang L., Guan X., Chen J., Zhang J., Saski C.A., Scheffler B.E., Stelly D.M., et al. Sequencing of allotetraploid cotton (Gossypium hirsutum L. acc. TM-1) provides a resource for fiber improvement. Nat. Biotechnol. 2015;33:531–537. doi: 10.1038/nbt.3207. [DOI] [PubMed] [Google Scholar]
- 21.Tamura K., Peterson D., Peterson N., Stecher G., Nei M., Kumar S. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 2011;28:2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cao Y., Han Y., Meng D., Li D., Jin Q., Lin Y., Cai Y. Structural, evolutionary, and functional analysis of the class III peroxidase gene family in Chinese Pear (Pyrus bretschneideri) Front. Plant Sci. 2016;7:1874. doi: 10.3389/fpls.2016.01874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang Y., Wang Q., Zhao Y., Han G., Zhu S. Systematic analysis of maize class III peroxidase gene family reveals a conserved subfamily involved in abiotic stress response. Gene. 2015;566:95–108. doi: 10.1016/j.gene.2015.04.041. [DOI] [PubMed] [Google Scholar]
- 24.Moore R.C., Purugganan M.D. The early stages of duplicate gene evolution. Proc. Natl. Acad. Sci. USA. 2003;100:15682–15687. doi: 10.1073/pnas.2535513100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matsui T., Tabayashi A., Iwano M., Shinmyo A., Kato K., Nakayama H. Activity of the C-terminal-dependent vacuolar sorting signal of horseradish peroxidase C1a is enhanced by its secondary structure. Plant Cell Physiol. 2011;52:413–420. doi: 10.1093/pcp/pcq205. [DOI] [PubMed] [Google Scholar]
- 26.Yang Z., Gong Q., Wang L., Jin Y., Xi J., Li Z., Qin W., Yang Z., Lu L., Chen Q., et al. Genome-wide study of YABBY genes in upland cotton and their expression patterns under different stresses. Front. Genet. 2018;9:33. doi: 10.3389/fgene.2018.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ma J., Wang Q., Sun R., Xie F., Jones D.C., Zhang B. Genome-wide identification and expression analysis of TCP transcription factors in Gossypium raimondii. Sci. Rep. 2014;4:6645. doi: 10.1038/srep06645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ma W., Zhao T., Li J., Liu B., Fang L., Hu Y., Zhang T. Identification and characterization of the GhHsp20 gene family in Gossypium hirsutum. Sci. Rep. 2016;6:32517. doi: 10.1038/srep32517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li F., Fan K., Ma F., Yue E., Bibi N., Wang M., Shen H., Hasan M.M., Wang X. Genomic identification and comparative expansion analysis of the non-specific lipid transfer protein gene family in Gossypium. Sci. Rep. 2016;6:38948. doi: 10.1038/srep38948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rogozin I.B., Wolf Y.I., Sorokin A.V., Mirkin B.G., Koonin E.V. Remarkable interkingdom conservation of intron positions and massive, lineage-specific intron loss and gain in eukaryotic evolution. Curr. Biol. 2003;13:1512–1517. doi: 10.1016/S0960-9822(03)00558-X. [DOI] [PubMed] [Google Scholar]
- 31.Robertson H.M. The large srh family of chemoreceptor genes in Caenorhabditis nematodes reveals processes of genome evolution involving large duplications and deletions and intron gains and losses. Genome Res. 2000;10:192–203. doi: 10.1101/gr.10.2.192. [DOI] [PubMed] [Google Scholar]
- 32.Trotman C.N. Introns-early: Slipping lately? Trends Genet. 1998;14:132–134. doi: 10.1016/S0168-9525(98)01400-0. [DOI] [PubMed] [Google Scholar]
- 33.Valerio L., De Meyer M., Penel C., Dunand C. Expression analysis of the Arabidopsis peroxidase multigenic family. Phytochemistry. 2004;65:1331–1342. doi: 10.1016/j.phytochem.2004.04.017. [DOI] [PubMed] [Google Scholar]
- 34.Zhang Z., Chao M., Wang S., Bu J., Tang J., Li F., Wang Q., Zhang B. Proteome quantification of cotton xylem sap suggests the mechanisms of potassium-deficiency-induced changes in plant resistance to environmental stresses. Sci. Rep. 2016;6:21060. doi: 10.1038/srep21060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shigeto J., Tsutsumi Y. Diverse functions and reactions of class III peroxidases. New Phytol. 2016;209:1395–1402. doi: 10.1111/nph.13738. [DOI] [PubMed] [Google Scholar]