Abstract
The aging process includes impairment in mitochondrial function, a reduction in anti-oxidant activity, and an increase in oxidative stress, marked by an increase in reactive oxygen species (ROS) production. Oxidative damage to macromolecules including DNA and electron transport proteins likely increases ROS production resulting in further damage. This oxidative theory of cell aging is supported by the fact that diseases associated with the aging process are marked by increased oxidative stress. Coenzyme Q10 (CoQ10) levels fall with aging in the human but this is not seen in all species or all tissues. It is unknown whether lower CoQ10 levels have a part to play in aging and disease or whether it is an inconsequential cellular response to aging. Despite the current lay public interest in supplementing with CoQ10, there is currently not enough evidence to recommend CoQ10 supplementation as an anti-aging anti-oxidant therapy.
Keywords: coenzyme Q10, aging, age-related diseases, mitochondrial dysfunction
1. Introduction
CoQ10 was first described in 1955, named ubiquitous quinone, a small lipophilic molecule located widely in cell membranes [1], and in 1957 its function as an electron carrier in the mitochondrial electron transport chain was reported [2]. The role in human disease was unknown for 20 years until in 1986 a benefit of CoQ10 treatment was reported in Kearns–Sayre syndrome [3]. Initially, therapeutic use of CoQ10 was focused on the oxidative phosphorylation (OXPHOS) defects in which there is documented CoQ10 deficiency [4] and in the group of CoQ10 synthesis disorders [5]. These conditions provided evidence for efficacy and safety of treatment with CoQ10 [6]. Subsequent larger-scale trials in Parkinson disease [7] and other neurodegenerative diseases have shown safety but no convincing benefit.
In the last decade, CoQ10 functions in membranes throughout the cell where antioxidant and signaling roles predominate have been of increasing interest [8]. There is growing evidence that oxidative stress is a major component of cellular senescence [9]. This multifactorial process involves DNA injury [10], protein and lipid damage, and activation of signaling pathways associated with aging [11]. Recently, the CoQ10 antioxidant effect has been shown to reduce markers for cardiovascular disease (CVD) and inflammation, the main components of atherosclerotic vascular disease [12].
It is suggested that CoQ10 supplementation can improve the symptoms of mitochondrial diseases and of aging because of an improvement in bioenergetics [12,13].
Our objective is to review, from a translational perspective, data regarding the association of CoQ10 and aging. Are the aging process and mitochondrial progressive failure related and can CoQ10 supplementation decelerate aging?
2. CoQ10
2.1. What Is It?
Coenzyme Q10, CoQ10 or ubiquinone (2,3 dimethoxy-5-methyl-6-decaprenyl-1,4-benzoquinone) is a small lipophilic structure, composed of a benzoquinone ring and an isoprenoid side-chain and it is found universally in cell membranes. In humans, synthesis occurs utilizing a collection of enzymes (complex Q) located in the mitochondrial matrix membrane [14]. The benzoquinone ring is derived from 4-hydroxybenzoic acid, while 10 isoprenes are derived from mevalonic acid (from the cholesterol synthesis pathway). The quinone ring is the functional group in the molecule, responsible for carrying electrons to complex III. CoQ10 (ubiquinone) is reversely reduced to ubiquinol. The polyisoprenoid tail is very lipophilic and localizes to hydrophobic membranes. The length of the isoprenyl chain is variable between species with 10 isoprenes forming human CoQ10 whilst rodents predominantly have CoQ9 [15].
2.2. Function
CoQ10 is widely distributed in all cell membranes and forms a critical component of the electron transport chain (ETC) transporting electrons between complexes I/II and III [13]. In rat liver the largest ubiquinone (CoQ9) concentration is found in the Golgi vesicles (2.62 μg/mg) followed by mitochondrial matrix membrane and lysosomes (with levels of 1.86 in each structure) [16].
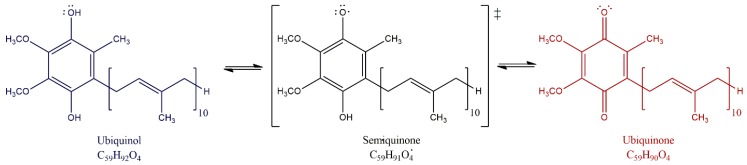
The major function of ubiquinone is in the mitochondrial ETC. CoQ10 accepts electrons from different donors, including complex I (reduced nicotinamide adenine dinucleotide [NADH]-coenzyme Q oxidoreductase), complex II (succinate dehydrogenase), the oxidation of fatty acids and branched-chain amino acids via flavin-linked dehydrogenases and electron transfer factor Q oxidoreductase (ETF-QO) to complex III (ubiquinone-cytochrome c oxidoreductase) [17,18]. CoQ10 cycles between its three chemical forms: completely oxidized (ubiquinone), a semi-oxidized intermediate free radical (semiquinone) and a completely reduced form (ubiquinol) as shown in Figure 1 [19,20]. By moving within the mitochondrial membrane, the proton-motive Q cycle allows proton pumping at complex III helping to generate the proton motive force for adenosine triphosphate (ATP) production.
Figure 1.
Redox forms of CoQ10. ubiquinone (oxidized form), ubiquinol (reduced form), and semiquinone (semi-oxidized). The Q cycle within the matrix membrane allows proton transfer from the mitochondrial matrix to the intermembrane space helping to generate the electrochemical gradient for ATP production.
The interaction of the CoQ pool with the ETC has in recent years been shown to be more complex with the recognition that mitochondrial supercomplexes composed mostly of complexes I/III, I/III/IV, and III/IV interact physically forming respirasomes. It seems likely that both single complexes within the matrix membrane and supercomplexes coexist in a dynamic state. The factors controlling assembly and disassembly of supercomplexes are not known although cardiolipin appears to play a role. There is evidence that in the I/III supercomplex intercomplex binding of CoQ shuttles electrons from complex I to III. This bound CoQ may be in equilibrium with the free CoQ pool. [21].
CoQ10 supplementation has been shown to have epigenetic effects in genes involved with signaling, intermediary metabolism, transport, transcription control, disease mutation, phosphorylation, and embryonal development indicating a role in modulation of gene expression [22,23].
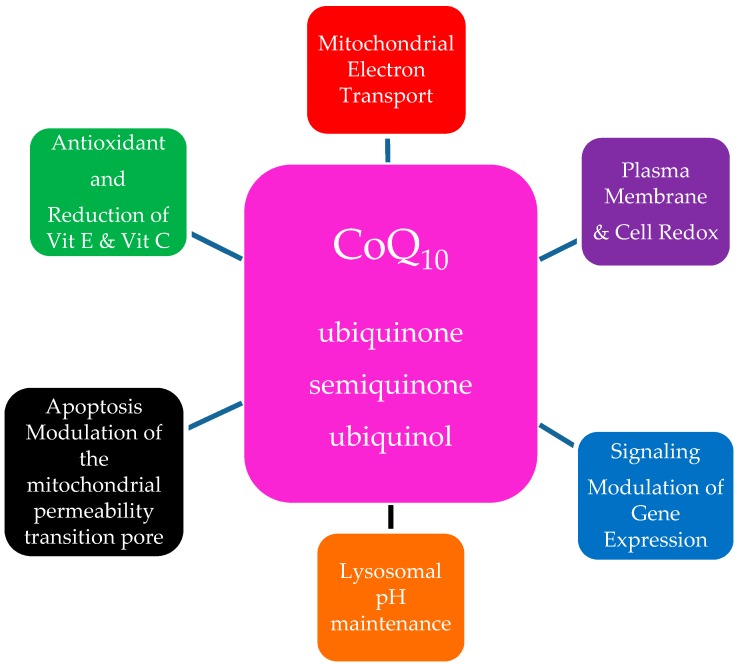
In addition to its major function in the ETC, CoQ10 has an important anti-oxidant role stabilizing the plasma membrane and other intracellular membranes protecting membrane phospholipids from peroxidation [13]. Ubiquinone and semiquinone are also involved with recycling of other anti-oxidant molecules, reducing α-tocopherol and ascorbate contributing to redox balance in the cell. Diminished CoQ10 levels in aging likely contribute to membrane peroxidation injury. There is evidence that part of its anti-oxidant effect occurs by enhancing the enzymatic activity of the antioxidant proteins superoxide dismutase and glutathione peroxidase [24]. Recent publications associate ubiquinol with protection of plasma low density lipoproteins (LDL) from oxidation, an important anti-atherogenic effect [25]. The pro-oxidant role of CoQ10 is a signaling function involved in gene expression but the mechanism of this function is not fully understood [26,27]. Other functions include modulation of the permeability transition pore, thus playing a role in apoptosis [28]. CoQ10′s main functions are summarized in Figure 2.
Figure 2.
The Multiple Roles of Ubiquinone in the Cell.
Chronic inflammation is a frequent aging-related problem. CoQ10, by reduction of free radicals, reduces the activation of NF-κB (nuclear factor kappa-light-chain-enhancer of activated B cells) cells and consequently reduces the release of pro-inflammatory cytokines mainly tumor necrosis factor alpha (TNF-α) and interleukin 6 (IL-6) [29]. Aging-related reduced CoQ10 levels may contribute to inflammation and there is accumulating evidence of secondary anti-inflammatory effects of CoQ10 supplementation. A recent meta-analysis provided evidence that CoQ10 supplementation significantly reduced the inflammatory markers CRP (C-reactive protein), IL-6 and TNF-α [30]. Another recent publication reported that patients with metabolic diseases (obesity, type 2 diabetes, metabolic syndrome, cardiovascular disease, and nonalcoholic fatty liver disease) had a significant decrease in TNF-α plasma levels with CoQ10 supplementation but not CRP or IL-6 [31]. CoQ10 was found to have an anti-inflammatory function via epigenetic effects on expression of genes related to NFkappaB1 (NFk-B1) [32]. CoQ10 has a hepatoprotective and neuroprotective effect in a rat model of non-alcoholic steatohepatitis [33] apparently through an adenosine 5′ monophosphate-activated protein kinase (AMPK) activation mechanism and in humans a randomized trial showed that supplementation of CoQ10 improved biomarkers for inflammation in nonalcoholic fatty liver disease (NAFLD) [34]. CoQ10 supplementation in patients with antiphospholipid syndrome has been found to attenuate levels of pro-inflammatory and thrombotic markers, with evidence of endothelial and mitochondrial function improvement [35]. In Down syndrome patients, chronic neuro-inflammatory changes have been proposed as a possible accelerator of Alzheimer disease [36]. These include high levels of interleukin 6 and tumor necrosis factor α along with decreased levels of CoQ10. A positive correlation between CoQ10 and intelligence quotient levels was also reported [37]. CoQ10 treatment in Down syndrome cells is associated with improved DNA repair mechanisms and DNA protection [38].
Cardiovascular disease is a common aging-related problem. There is a considerable body of evidence supporting a role for CoQ10 in cardiovascular function including a correlation of low endomyocardial levels with severity of heart failure and an improvement in cardiac contractility with CoQ10 treatment [39]. Improvement in lipid profiles (a major contributor to cardiovascular disease) has been reported with CoQ10 treatment [40].
CoQ10 has other important functions, participating in metabolic pathways as an electron receptor: (1) CoQ10 is a co-factor for dihydro-orotate dehydrogenase, an enzyme involved in the de novo pyrimidine biosynthesis [41]. (2) During the process of sulfide oxidation CoQ10 accepts electrons from the enzyme sulfide-quinone reductase to convert sulfide into thiosulfate [42]. (3) The oxidation from choline to glycine is catalyzed by choline dehydrogenase in the inner mitochondrial membrane, and CoQ10 is proposed to be the electron acceptor for this reaction [43]. (4) Proline dehydrogenase donates electrons from FAD and NAD+ to CoQ10 during process of synthesis of proline and arginine [21,44].
2.3. Sources of CoQ10
2.3.1. Internal Biosynthesis
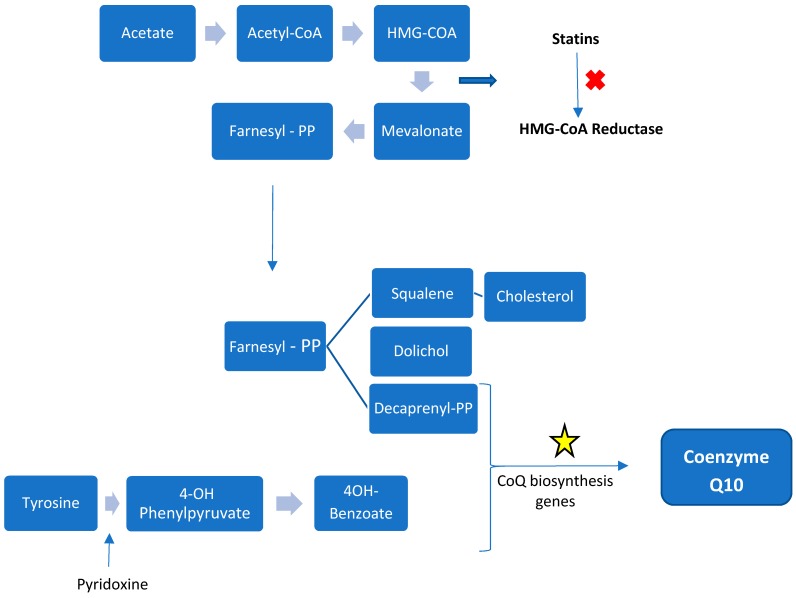
CoQ10 is the only lipid-soluble antioxidant synthetized by the human body [13]. The majority of CoQ10 comes from the internal synthesis, from tyrosine or phenylalanine (benzoquinone ring) and mevalonic acid (isoprenoid side-chain). Synthesis occurs in all tissues studied. In humans synthesis occurs utilizing a collection of enzymes (complex Q) located in the mitochondrial matrix membrane and in the endoplasmic reticulum The mevalonic acid pathway is responsible for cholesterol synthesis with 3-hydroxy-3-methyl-glutaryl-coenzyme A (HMG-CoA) reductase (the site of statin inhibition) as the regulatory step. CoQ10 derived from dietary intake becomes more important with aging as endogenous production decreases [13].
At least 14 genes are involved in CoQ10′s biosynthesis in yeast with 18 genes so far reported in humans. Many are homologues of genes identified in c. cerevisiae. These synthetic proteins are nuclear encoded and require mitochondrial targeting sequences for entry into the matrix or the inner mitochondrial membrane [14]. There is assembly of many of the components into a ‘supercomplex’ termed Complex Q in the mammal. Mitochondrial synthesis is thought to occur in all cells containing mitochondria but also in other organelles including the endoplasmic reticulum and peroxisomes [45]. Figure 3 summarizes the mammalian CoQ10 biosynthesis pathway.
Figure 3.
Schematic representation of CoQ biosynthesis. The isoprenoid side-chain derives from the cholesterol and dolichol synthetic pathway from mevalonate, the benzoquinone ring is derived from tyrosine metabolism. The star symbolizes the genes involved in the final synthesis of CoQ10.
2.3.2. External Sources
CoQ10 is widely found in many animal protein sources (pork, lamb, beef, chicken, fish), vegetables (spinach, pea, broccoli, cauliflower), fruits (orange, strawberry, apple) and cereals (rye, wheat) [13]. Heart, chicken leg, herring, and trout contain particularly high amounts of CoQ10. Daily intake between 3 and 5 mg is considered adequate and whilst external supplementation increases plasma levels, supplementation was not thought to increase tissue levels of CoQ10 in tissues with normal synthetic capacity [46], although evidence of treatment efficacy in a variety of human diseases does suggest that tissue uptake can occur [13,47].
2.3.3. Absorption and Transport
CoQ10 absorption is slow and occurs in the small intestine; in its reduced form, ubiquinol is 3 to 4 times better absorbed than the oxidized form, ubiquinone [48]. The absorption of CoQ10 can be increased if administered with food intake, mainly with lipids because of its lipophilic structure [13,49]. After absorption by the enterocytes, CoQ10 passes through lymphatic vessels and reaches the plasma, where it circulates bound to lipoproteins (LDL). Because of this, plasma measurements of CoQ10 should be corrected for lipoproteins levels. Between 80 and 95% of plasma circulating CoQ10 is in the reduced ubiquinol form. [48,50].
2.4. Tissue Levels and Distribution of CoQ10
Although the major CoQ10 plasma form is ubiquinol, laboratory measurements, in general, report the CoQ10 total level [50]. Lymphocyte and platelet levels may give some insight into levels in less accessible tissues such as heart, muscle, and brain [51]. The CoQ10 level varies in different tissues. Tissues with a higher metabolic rate and mitochondrial content (heart, kidney, liver, and muscle) have high levels of CoQ10, for example the level is 8 μg/g in lung and 114 μg/g in heart [13]. Tissue levels largely reflect the results of synthesis and degradation of CoQ10. Following intestinal absorption, liver and lipoprotein concentrations increase, but without a change in the level in heart and kidney noted in early studies [52]. However, there is some evidence that chronic administration can increase tissue levels [53]. In a study where rats were chronically fed a large dose of 150 mg/kg/day of CoQ10 for 13 weeks, small but significant increases in both CoQ9 and CoQ10 were found in all tissues measured [54]. No such data exist for young or aged human tissues; however, the accumulated evidence of benefit of CoQ10 therapy in human disease states does suggest that tissue levels can be increased by oral administration. The number, size, and structure of the mitochondria in each cell determines the tissue level of CoQ10 with highest levels found in tissues with high energy demands and a high mitochondrial content [13,48].
The gold standard for the diagnosis of CoQ10 deficiency is based on the measurement of muscle level by high-performance liquid chromatography (HLPC) [55,56]. Plasma levels of CoQ10 range between 0.40 and 1.91μmol/L (0.34–1.65μg/mL) in controls [57] but do not match tissue levels, reflecting consumption much more than endogenous synthesis. The diagnosis of CoQ10 deficiency requires tissue measurement [58]. There is evidence suggesting that the CoQ10 level in mononuclear cells can be correlated to muscle measurements [45,56]. Plasma CoQ10 levels are increased in some physiological conditions (cold adaptation and exercise) and in some diseases (paraneoplastic nodules, Alzheimer’s disease, and prion disease). Levels are decreased in aging. [45].
2.5. Causes of Reduction
Conditions associated with CoQ10 deficiency can be divided into three main groups: (1) CoQ10 nutritional deficiency, including intake of CoQ10 itself and nutrients and vitamins necessary for its synthesis (vitamin B6 is a cofactor in the pathway of CoQ10 biosynthesis); (2) CoQ10 synthesis genes (COQ family genes: COQ1 and the Complex Q genes including the mammalian homolog of the yeast Coq11 gene; the Complex I subunit NDUFA9), and acquired disorders impairing CoQ10 synthesis (statin use) [59,60,61]; and (3) medical conditions associated with decreased levels of CoQ10 [45,46]. In this last category, a variety of conditions have been reported with low CoQ10 including neurodegenerative disorders Friedreich’s ataxia, Nieman–Pick type C disease, and Parkinson Disease. In other disorders such as Alzheimer disease, diabetes, cancer, fibromyalgia, and cardiovascular diseases, elevations in plasma CoQ10 levels may be a stress response. [45,46]. Defects in genes which may be associated with reduced CoQ10 levels can be included in this group (APTX, ETFDH, BRAF).
Special attention should be given to the important observation that CoQ10 deficiency is potentially reversible if the supplementation starts before the appearance of the symptoms, when brain and kidney have not sustained permanent damage [46,62].
2.6. Supplementation
2.6.1. Dosing
A recent trend over the last few years has been to supplement adult patients with mitochondrial diseases with high doses of oral CoQ10 or ubiquinol, up to 1200 mg/day or higher. For the pediatric population, doses between 5 and 10 mg/kg/day of ubiquinol are recommended [63]. For a dose of 10 mg/kg/day, plasma levels range between 5 and 10 µg/mL 3–4 weeks after the beginning of the supplementation [53,63,64]. As explained above, there are important differences in bioavailability of the different formulations of CoQ10 used; ubiquinol (the reduced form) is 3 to 4 times better absorbed than ubiquinone (oxidized form) [48]. Primary CoQ10 diseases tend to respond to supplementation but may require very high doses. Also, some patients with secondary CoQ10 dysfunction were reported to improve some symptoms with CoQ10 supplementation. Examples are cardiomyopathy in organic acidurias (25 mg/kg/day with improvement in the cardiomyopathy) [65], glutaric acidemia type II (500 mg per day of CoQ10 along with riboflavin with improvement in strength, lactate, and creatine kinase levels) [59], ataxia oculomotor apraxia type 1 (200–600 mg with reported improvement in strength, ataxia, and cessation of seizures in one patient) [60], GLUT-1 deficiency (30 mg/kg/day with improvement in the ataxia and nystagmus) [66].
Exogenous administration of CoQ10 reportedly does not raise tissue levels above normal in healthy young individuals, except for two tissues (liver and spleen) [13]; however, this traditional view may be wrong given the improvement in multi-organ symptoms in a variety of disorders with mitochondrial dysfunction when treated with CoQ [67].
2.6.2. Safety and Adverse Events
Although the majority of studies have not shown convincing enough scientific evidence to support treatment with CoQ10 in specific diseases, they do provide evidence that oral supplementation is safe and well tolerated. One of the largest trials was a phase III randomized, placebo-controlled, double-blind clinical trial at 67 North American sites by the Parkinson Study Group using doses of CoQ10 up to 2400 mg/day demonstrated the safety of this dose [7]. Evidence suggests that supplementation does not inhibit endogenous production [13]. Previous studies had reported only mild side effects such as gastrointestinal symptoms, mainly nausea, with CoQ10 supplementation [13,68].
3. Aging
3.1. Physiology of Mitochondrial Involvement in the Process of Aging
Human aging is a normal multifactorial process resulting from the interaction of genetic and environmental factors. It is characterized by multi-organ system functional decline in association with the risk of age-related diseases (dementia, neurodegenerative disorders, osteoporosis, arthritis, diabetes, cardiovascular disease, age-related hearing loss, and cancer) [13,68,69,70,71].
A common hypothesis to explain some of the pathophysiology of age and degenerative diseases is an oxidative imbalance between the production of reactive oxygen species (hydrogen peroxide: H2O2, the oxygen-derived free radicals superoxide: O2•−, and hydroxyl radical: HO•), and antioxidant mechanisms such as superoxide dismutase, catalase, glutathione peroxidase, ascorbic acid, tocopherol, glutathione, and CoQ10, leading to a state of oxidative stress [72,73,74,75,76,77,78,79,80]. A number of animal models support this theory with shortened survival in mice lacking superoxide dismutase 1 (SOD1) and a lethal phenotype in mice lacking superoxide dismutase 2 (SOD2) [81,82].
As mitochondria are the main source of reactive oxygen species (ROS) production though OXPHOS supercomplex activity in the cristae of the inner mitochondrial membrane (mainly at complex I and III), this organelle is the major target of ROS damage. Mitochondrial DNA (mtDNA) is particularly vulnerable with a high mutation rate and limited mtDNA repair mechanisms [69,74]. The continuous production and accumulation of mitochondrial ROS is the basis for “the free radical theory of aging” [70,71]. Although the accumulation of ROS has a major effect on DNA (strand breaks, oxidation of bases, damage in sites coding for ETC proteins), other structures of the cell are also damaged: lipids, membranes, proteins (leading to dysfunction of the ETC, inadequate ATP production, and further ROS production) [72,73,74,75]. There is evidence that impaired mitochondrial machinery produces more oxidative stress and more ROS production, resulting in a vicious cycle [76,83]. Electrons leaking from impaired OXPHOS react with oxygen molecules to form the free radical superoxide [80]. There is also an effect on mitochondrial dynamics with impairment of fission, contributing to mitochondrial enlargement, which reduces recycling through mitophagy leading to a reduction in ATP generation [73]. Impairment of the mitochondrial–lysosomal axis occurs with aging, with accumulation of lipofuscin inside lysosomes with senescence. Lipofuscin accumulation is postulated to limit the ability of lysosomes to participate in mitophagy [73].
Considerable evidence supports the relationship between ROS accumulation and mitochondrial dysfunction leading to aging (the mitochondrial free radical theory of aging): ROS production increases in aged humans and animals [70,84], imbalance in the levels of pro and anti-oxidant substances occurs [85] with high levels of oxidized and damaged macromolecules (proteins, lipids, and DNA) [86]. There seems to be a convincing relationship between high ROS levels and longevity in humans and animals [87]; however, the exact role of ROS remains unclear as some animal models report a failure to increase longevity with ROS reduction and others link high levels of ROS and longevity. It remains unclear what the contribution of ROS generation is versus epigenetic factors modulating genes related to the protection from effects of aging [77,88,89]. Evidence against the oxidative theory of aging comes from some animal models where longevity is unaffected by increased ROS production. Some of these studies are detailed below.
Growing evidence supports the idea that increased levels of ROS are associated with the specific biochemical pathway that improves longevity, at least in some species [90]. C. elegans, a nematode mutant model clk-1 (COQ7 equivalent gene in humans), has higher longevity associated with higher ROS production. C. elegans with a point mutation in the gene isp-1, responsible for an iron-sulfur mitochondrial complex, also demonstrate an increase in life span [91]. These observations point out the multiple roles of ROS particularly as signaling molecules triggering protective pathways. Some mouse models of defective CoQ synthesis are difficult to square with the oxidative theory of aging. Mice with only one copy of the gene Mclk1 (equivalent of mammals COQ7 and C. elegans clk-1 model) have higher production of ROS in the mitochondria (although a normal level of total ROS in the body), a higher level of protection of the immune system (from some infections and also tumorigenesis) and increased longevity [92]. Homozygous knockout of the Mclk1 gene is embryonic lethal but utilizing a Tamoxifen dependent transgene mouse KO activated at 2 months of age a multisystemic disorder (heart, kidneys, and skeletal muscles) with a decline in ubiquinol levels is produced. At 8 months this “ubiquinol deficit” animal presented normal levels of some factors associated with oxidative stress (catalase, F2-isoprostanes, DNA oxidative damage, SOD1, and SOD2). Diet supplementation at 9 months with an analogue of the ubiquinol precursor 4-hydroxybenzoic acid rescued the clinical phenotype [93]. Another mouse model heterozygous for the Sod2 gene (with decreased Mn-superoxide dismutase activity) does show oxidative injury (increased tumor incidence and DNA damage) but does not decrease longevity [94]. The concept that mitochondrial dysfunction may be a consequence of aging factors rather than a cause is also supported by observations of sarcopenia in rat and human muscles with other factors such as denervation playing a role [95].
Further studies are needed to understand the balance of ROS as an agent of oxidative injury and its signaling epigenetic role modulating genes related to the protection of effects of aging. It may be that after the saturation of the mechanisms of protection, the ROS-stress protective cascade can no longer prevent oxidative damage [88].
It is not surprising that given the contradictory evidence for a central role for ROS in aging, evidence supporting the utility of anti-oxidant therapies in aging (such as CoQ10) remains unclear.
3.2. CoQ10 and Aging, CoQ10 Deficiency in Advanced Age, Evidence for Beneficial Supplementation
Published results from various research groups about CoQ levels and lifespan are often at variance, model dependent and do not support a similar pattern in all species [96,97,98].
3.2.1. C. elegans
Studies in the nematode C. elegans have produced unexpected results compared to mammals. As discussed above, a nematode mutant model clk-1 (mammals COQ7 equivalent gene), with low production of CoQ8, has an extension in longevity compared with the wild strain but requires dietary Q supplementation [99]. This diet does trigger a Dauer long-lived larval anerobic state. Other studies with CoQ gene knockouts confirm that deficiency in CoQ (less than 50%) also leads to an increase in lifespan, and a possible explanation is that less ROS production occurs in the case of moderate CoQ deficiency [100,101], but with more severe depletion of CoQ, longevity would be affected [100,102]. This finding was also confirmed in human cells, with two different studies from the same group reporting that fibroblasts with mutations on PDSS2 (homologue of yeast coq1) with less than 12 and 20% of CoQ10 of control cells had decreased synthesis of ATP without increase in the levels of ROS. However, when the defect was of 30%, with partial defect in the synthesis of ATP the levels of ROS were higher. The explanation proposed centered on the severity of the deficiency of coenzyme Q as an oxphos modulator [103,104].
3.2.2. Rodent Models
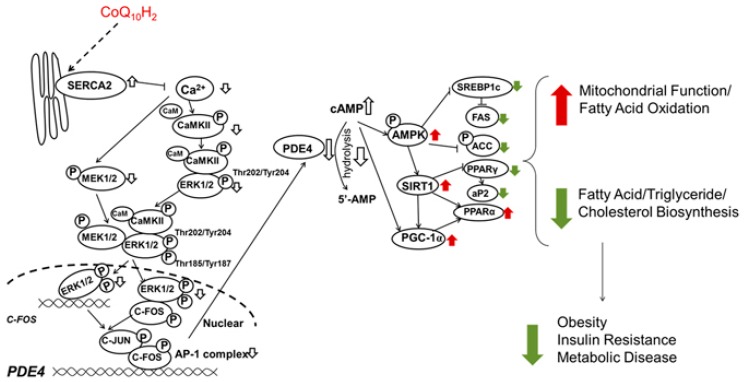
A diet supplemented with Ubiquinol-10 in the senescence-accelerated mouse prone 1 (SAMP1) reduced markers of oxidative stress (ratio of reduced and oxidized glutathione—GSH/GSSG), decelerated the normal decline in expression of genes (Sirt1, Sirt3, and Pgc-1a, and Ppara), and their respective proteins related to mitochondrial function during aging [105]. This treatment also increased auditory brainstem response hearing loss. The proposed mechanism of CoQ10 benefit as an anti-oxidant agent in this aging mouse is through cyclic adenosine monophosphate (cAMP). There is an enhancement in sirtuin genes, and PGC-1α (peroxisome proliferator-activated receptor gamma coactivator 1-alpha) with increased complex I and IV activity and reduce oxidative stress. Despite all these beneficial effects, there was no significant change in the overall lifespans compared to the control animals [105]. These findings of increased cAMP, SIRT1 (sirtuin 1) expression, and PGC-1α in reducing parameters of oxidative stress and increasing mitochondrial function were also confirmed in other experiments with ubiquinol supplementation in senescence-accelerated mice, with added benefits in obesity, insulin resistance, and metabolic syndrome, (hypothesized mechanism in Figure 4 [106]). PPARα (peroxisome proliferator-activated receptors) signaling and lipid metabolism gene expression changes in liver of C57BL6J mice was reported after one-week supplementation with ubiquinol (250 mg/kg BW/day) [23].
Figure 4.
Proposed mechanism by which ubiquinol improves metabolic function and inhibits insulin resistance in KKAy mice (a mouse model of obesity and diabetes). Ubiquinol inhibited phosphorylation of CaMKII (Ca2+/calmodulin-dependent protein kinase II) in the liver resulting in inhibition of C-FOS transcriptional activity and inhibition of PDE4 gene expression. Increased cAMP increases AMPK (AMP-activated protein kinase) activity resulting in SIRT1 and PGC-1α increased mitochondrial function and inhibition of lipid synthesis. (Adapted from Xu H. et al. 2017 [106] with permission).
Interestingly mice with a single copy of the Coq7 synthesis gene demonstrated increased longevity [107]. However the mechanism is unclear and several studies report that dietary supplementation with CoQ10 or ubiquinol in various rat models improves mechanisms involved with mitochondrial biogenesis, including parameters of oxidative stress [105,108,109,110,111].
3.2.3. Mammals and Tissue CoQ levels
In mammals there is a tendency to ubiquinone to be reduced with age, but this finding depends on the tissue investigated and also the species [97]. Early studies suggested that tissue levels of ubiquinol were endogenously produced with little change with dietary supplementation except in liver and spleen; however, subsequent studies in rodents confirm that oral supplementation with CoQ10 does increase tissue levels of both CoQ10 and CoQ9 in skeletal muscle, heart, and kidney [26], and when high doses (200 mg/kg) are used for 2 months in rats, brain levels are increased and are neuroprotective [112].
In humans, there is also a lack of consistent data. One study did not find a relationship between aging and CoQ10 plasma levels in elderly women [113]. Others report that plasma and tissue levels change over time, with a peak in pancreas and adrenal by 1 year of age and in the brain, heart, and lung by 20 years. After this peak, levels decrease over time [114]. A decrease in brain CoQ10 was confirmed in other studies [115,116]. Only 50% of the myocardial CoQ10 endogenous production remains by the age of 80 [114]. Serum total CoQ10 and ascorbic acid levels were decreased in centenarians compared with 76-year-old controls. An elevation of the CoQ10 binding protein prosaposin was also noted presumably in an attempt to compensate for low CoQ10 levels [117]. The authors conclude that CoQ10 supplementation could be beneficial for centenarians. However, it is not known if low tissue and plasma CoQ10 levels contribute to or are a side effect of aging.
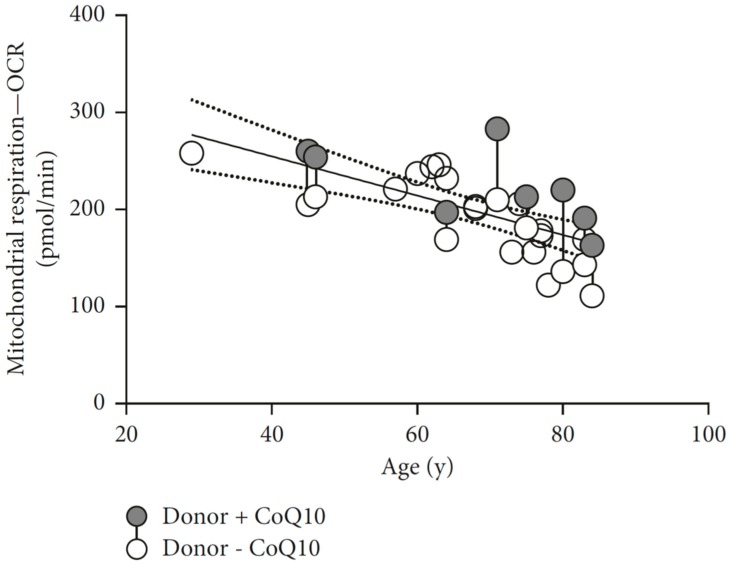
Reduction of CoQ10 with age is postulated to result from reduction in biosynthesis coupled with an increase in degradation attributed to age-related modification in lipid membranes, which alters quinone behavior [118]. This reduction of CoQ10 levels has a tissue/organ specificity with reported high levels of CoQ10 in the brain mitochondria from old rats [26], and reduced level in the muscle [98]. A recent paper showed age-related reduction in mitochondrial respiration parameters and ATP production in epithelial cells, rescued with CoQ10 administration. Both cited that parameters decrease as age increases, as seen in Figure 5, with the estimate of 10% reduction in mitochondrial respiration every ten years [70].
Figure 5.
Age-related decline in the oxygen consumption rate in epithelial tissue measured 16 h after collection in a Seahorse XF analyzer. The gray circles show treated samples, the white circles untreated samples, and the connected circles represent samples from the same donor. Significant improvement with 100 μM CoQ10 incubation. (Adapted from Schniertshauer et al. 2018 [70] with permission).
High CoQ10 concentration was reported to be associated with higher physical activity, lipid peroxidation, and lower oxidized LDL levels in elderly people. The same publication describes higher levels of plasma CoQ10 in elderly (more than 50 years old) people compared with young (less than 30 years) [119]. The same group confirmed lower lipid peroxidation and lower oxidized LDL in young adults and also showed that CoQ10 is lower in obese elderly patients [120].
In another study, prolonged CoQ10 supplementation for 4 years in community-dwelling elderly was associated not only with an improvement in health-related quality of life but more importantly with a lower “more days out of hospital“ rate. In this study, subjects were co-supplemented with selenium [121].
Although the association between CoQ10 and muscle power is not well established, one study showed a positive relation between CoQ10/cholesterol levels and hand grip, and lower ubiquinol levels in patients with less muscle strength. This study was undertaken to evaluate a possible relationship between low levels of CoQ10 and ubiquinol in sarcopenia [122].
Despite the multiple reports on the effects of CoQ10 supplementation on aging-related oxidative markers, reduction in biomarkers related to inflammation and in DNA repair mechanisms reports there is a great need for more controlled studies in an older population to determine effectiveness of CoQ10 as an anti-aging therapy [97] and also to determine the tissue CoQ10 levels in the human species during senescence.
3.3. CoQ10 and Specific Conditions Associated with Age
The case for beneficial effects of CoQ10 or ubiquinol supplementation is stronger for a number of aging-related diseases many of which have documented mitochondrial dysfunction or CoQ10 deficiency. Despite this, the majority of studies proving therapeutic effects of CoQ10 supplementation were carried out in animal models [25].
Early studies were carried out with CoQ10 although often information on the formulation used is lacking (it is known that ubiquinol can be 3 to 4 times better absorbed than ubiquinone). Also, the doses used in most of the studies were lower than the doses studied for primary CoQ10 deficiency (up to 1200 mg/day) and in secondary CoQ10 deficiency studies. The formulation and absorption will affect studies of treatment efficacy. A colloidal-Q10 formulation has been shown to have a better enteral absorption and bioavailability in human tissues, and also the vehicle of the active ingredient can impact the results of trials [123].
3.3.1. Neurodegenerative Disorders
Neurodegenerative diseases involve neuro-inflammation and oxidative stress, with ROS accumulation and mitochondrial dysfunction [83,124,125].
The data supporting mitochondrial cofactor supplementation in aging and the aging-related diseases, including Alzheimer disease, Parkinson disease, amyotrophic lateral sclerosis, and multiple sclerosis, are addressed in focused review articles in this supplement. A systematic review published in 2014 reviewed 16 articles and concluded that there is no available data from randomized controlled trials (RCTs) to support the use of mitochondrial supplements for Parkinson disease, atypical Parkinsonism, Huntington disease, and Friedreich ataxia [126].
Previous studies have shown that patients with Lewy’s body disease and amyotrophic lateral sclerosis have lower CoQ10 plasma levels and Alzheimer disease patients have reduced levels of CoQ10 in the cerebral spinal fluid [127,128].
In Huntington disease, increased levels of cortical lactate were observed, which were normalized after 2 months of Coenzyme Q10 oral supplementation at a dose of 360 mg/day. In another publication, after discontinuation of the supplement, the lactate levels rose again [67,124]. This study does provide evidence for CNS penetration of administered CoQ10 in humans. These and other data lead to a multicenter RCT with 609 patients, which could not identify a benefit in Functional Capacity Score and time to death after 60 months of 2400 mg per day of CoQ10 supplementation compared to placebo [129]. Thus, currently, there is insufficient evidence to support CoQ10 supplementation as a treatment to delay neurodegeneration in Huntington disease even in the early stages of the disease.
A similar situation occurred in Parkinson disease. An early study found that patients had decreased levels of α-tocopherol and CoQ10 in plasma and cerebrospinal fluid with increased levels of lipoprotein oxidation compared to controls in a 2004 study of 161 subjects. [130]. Mitochondrial complexes I and II/III were reduced in platelet mitochondria from early Parkinson disease patients [131]. A more recent study confirmed the association of Parkinson’s disease and CoQ10 deficiency using Functional Intracellular Assay, when compared to matched controls for age and gender [132]. A phase 2 study in 80 subjects at early or mid-stage Parkinson disease showed a beneficial effect from supplementation with CoQ10 in progressive doses of 300, 600, 1200 mg/day for 16 months. There was an improvement in the ADL UPDRS (Activity Of Daily Living Unified Parkinson Disease Rating Scale) and Schwab and England scales compared to placebo (+11.99), with highest dose (+6.69) having the best effect [133]. This lead to a phase III randomized, placebo-controlled, double-blind clinical trial at 67 North American sites by the Parkinson Study Group which used doses up to 2400 mg/day of CoQ10 vitamin E in the dose of 1200 IU/d (to enhance the absorption of the lipophilic coenzyme) however this large study did not find convincing evidence to support CoQ10 treatment for this disease [7]. A systematic review and meta-analysis from 2016 could not find sufficient evidence in five selected RCTs to support the use of ubiquinone (300 mg/day to 2400 mg/day) to decelerate the progression of Parkinson’s disease or improve symptoms [134].
In aged, cognitively impaired mice, CoQ10 supplementation does improve special learning and attenuates oxidative damage [135]. In Alzheimer Disease, the Alzheimer’s Disease Cooperative Study could not identify benefits in the levels of oxidative stress and neurodegeneration biomarkers in CSF (cerebrospinal fluid) with a dose of 400 mg of CoQ10 3 times/day for 16 weeks for patients [136]. To date, chronic large-scale trials of CoQ10 have not been carried out in Alzheimer disease.
3.3.2. Cardiovascular Disease
A meta-analysis reviewed randomized controlled trials in healthy adults; two trials reported reduction in systolic blood pressure, and no evidence for reduction in diastolic blood pressure was reported. Another trial included in this meta-analysis failed to show an effect of CoQ10 supplementation on the lipid profile (LDL: low-density lipoprotein cholesterol, HDL: high-density lipoprotein cholesterol, and triglycerides) [137]. Another meta-analysis from 2016 concluded that there is not enough evidence to support CoQ10 use to treat hypertension [138]. For dyslipidemia, no relationship was found between CoQ levels in hyperlipidemic and normolipidemic, older women and no association with body mass index was found [139]. Statins are well known to reduce CoQ10 levels because of inhibition of 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase. This enzyme is the first step in the mevalonic acid synthesis pathway, which is necessary for the synthesis of cholesterol but also the isoprenoid side-chain of CoQ10 [140].
Patients with mitochondrial myopathy do not tolerate statin treatment, often developing exercise intolerance and myalgia when treated with statins. These symptoms may improve with CoQ10 oral supplementation [141,142,143]. Statins were also found related to a reduction in complex III activity. However, the exact mechanism for the mitochondrial myotoxicity of statins is not well understood and other factors than CoQ10 can be involved, such as genetic polymorphisms [144].
There is evidence that cardiac function in the elderly may be improved by CoQ10 treatment. This effect may be related to the documented fall in cardiac CoQ10 levels in the aged heart [114]. A meta-analysis reviewed eight clinical trials and concluded that patients submitted to cardiopulmonary bypass have a decreased requirement for ionotropic drugs and a reduced chance of ventricular arrhythmia if receiving CoQ10 supplementation [145].
The best case for CoQ10 supplementation is in cardiovascular disease. It was recently reported that 12 years after a 4-year double-blind treatment protocol in the elderly co-supplemented with selenium (200 µg) and CoQ10 (200 mg/day) (ubiquinone in a softgel vehicle of vegetable oils) the treated subjects still had a reduced cardiovascular mortality (38.7% in placebo-treated group versus 28.1% in CoQ-treated group) [146]. The Q-SYMBIO trial enrolled 420 patients and showed that CoQ10 100 mg 3 times daily for 2 years plus standard therapy reduced the risk of cardiovascular events, in this study measured as cardiovascular mortality (16% in placebo and 9% in the treated group) and hospitalization for heart failure compared to placebo, and also a positive change in the New York Heart Association (NYHA) functional classification (26% in placebo and 15% in the treated group) [147]. A recent review of the use of CoQ10 in heart failure is recommended [39].
3.3.3. Endothelial Dysfunction
Endothelial function may be a risk factor for coronary artery disease and atherosclerosis. CoQ10 together with a Mediterranean diet was shown to improve markers of endothelial function in elderly patients [148,149].
3.3.4. Renal Disease
Although ubiquinol can improve the endothelial dysfunction associated with the diabetic kidney disease (systolic blood pressure and urinary albumin) [150], and a trial with CoQ10 supplementation (1200 mg/day in dialysis patients) identified a reduction in a plasma indicator of oxidative stress (F2-isoprostane) [151], a meta-analysis failed to prove CoQ10 efficacy in avoiding the progression of diabetic kidney disease [152].
3.3.5. Inflammation
Many aging-related diseases share a common physiologic pathway of chronic inflammation leading to oxidative stress, such as cardiovascular diseases, diabetes, cancer, and chronic kidney disease.
A recent meta-analysis reports a reduction in the plasma inflammatory biomarkers, C-reactive protein, IL-6, and TNF-α, after CoQ10 supplementation (60 to 500 mg/day, formulations described as CoQ10 or ubiquinol), for 1 week to 4 months in different inflammatory disorders (cardio and cerebral vascular disease, multiple sclerosis, obesity, renal failure, rheumatoid arthritis, diabetes, and fatty liver disease). The same review reports that CoQ10 decreases other biomarkers for inflammation and inflammatory cytokines [30]. Although CoQ10 treatment has been shown to improve markers of inflammation, a benefit of chronic treatment for the diseases associated with inflammation has not been demonstrated.
CoQ10 (100 mg/day) supplementation for 2 months decreased the levels of TFN-α in rheumatoid arthritis patients compared to placebo, [153]. A more recent systematic review and meta-analysis reported CoQ10 supplementation between 60 to 300 mg/day (no extra information about formulations) was associated with a slightly drop in C-reactive protein levels and a significant decrease in in IL-6 levels [154].
Down syndrome patients have an abnormal pro-inflammatory profile (increased IL-6 and TNF-α) [36,37] and reduced CoQ10 levels, and supplementation reduced markers of oxidative stress and mitochondrial dysfunction [38,155].
3.3.6. Osteoporosis
Animal and human studies have demonstrated that benefits of CoQ10 supplementation have a beneficial profile for osteoporosis [156,157,158].
3.3.7. Cancer
There is evidence of the relationship between some cancers and reduced CoQ10 levels in blood, particularly breast cancer [159], myeloma [160], melanoma [161], and follicular and papillary thyroid carcinomas [162]. There is also evidence that CoQ10 supplementation modulates phospholipid hydroperoxide glutathione peroxidase gene expression, free radical production, and can decelerate the growth of tumor cells in a prostate cancer line (PC3 line) [163].
4. Conclusions
There is still much to be learned about the pathophysiology of the process of aging. There are well documented reductions of tissue CoQ10 in senescence. It is not known if low CoQ10 is an effect of aging, perhaps matching the fall in mitochondrial electron transport function or a contributing cause to the aging process. There is accumulating evidence that some diseases of aging may benefit from supplemental ubiquinol or CoQ10 treatment. Studies to date have supported the safety and the potential of CoQ10 in reducing oxidative stress biomarkers. There remains a lack of adequate large-scale clinical trials preferably utilizing ubiquinol as the better absorbed form of CoQ10. Despite the lack of evidence, large numbers of people in the population are taking CoQ10 and other vitamins and cofactors in the hope that these agents will slow senescence and expand longevity.
Acknowledgments
Thank you to the many patients and families who have taught the authors so much including their experiences with CoQ10 treatment.
Funding
I.P.d.B. is recipient of a postdoctoral fellowship from the North American Mitochondrial Disease Consortium (NAMDC) supported by National Institute of Health (NIH) U54 grant NS078059 and RHH is also supported by this grant.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Festenstein G.N., Heaton F.W., Lowe J.S., Morton R.A. A constituent of the unsaponifiable portion of animal tissue lipids (λmax. Mμ.) Biochem. J. 1955;59:558–566. doi: 10.1042/bj0590558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Crane F.L. Electron transport and cytochromes of sub-cellular particles from cauliflower buds. Plant Physiol. 1957;32:619–625. doi: 10.1104/pp.32.6.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ogasahara S., Nishikawa Y., Yorifuji S., Soga F., Nakamura Y., Takahashi M., Hashimoto S., Kono N., Tarui S. Treatment of Kearns-Sayre syndrome with coenzyme Q10. Neurology. 1986;36:45–53. doi: 10.1212/WNL.36.1.45. [DOI] [PubMed] [Google Scholar]
- 4.Miles M.V., Miles L., Tang P.H., Horn P.S., Steele P.E., DeGrauw A.J., Wong B.L., Bove K.E. Systematic evaluation of muscle coenzyme Q10 content in children with mitochondrial respiratory chain enzyme deficiencies. Mitochondrion. 2008;8:170–180. doi: 10.1016/j.mito.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 5.Hirano M., Garone C., Quinzii C.M. CoQ(10) deficiencies and MNGIE: Two treatable mitochondrial disorders. Biochim. Biophys. Acta. 2012;1820:625–631. doi: 10.1016/j.bbagen.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marin S.E., Haas R.H. Coenzyme Q10 and the Treatment of Mitochondrial Disease. In: Hargreaves I.P., Hargreaves A.K., editors. Coenzyme Q10: From Fact to Fiction. Nova Science Publishers; Hauppauge, NY, USA: 2015. pp. 85–108. [Google Scholar]
- 7.Parkinson Study Group QE3 Investigators. Beal M.F., Oakes D., Shoulson I., Henchcliffe C., Galpern W.R., Haas R., Juncos J.L., Nutt J.G., Voss T.S., et al. A randomized clinical trial of high-dosage coenzyme Q10 in early Parkinson disease: No evidence of benefit. JAMA Neurol. 2014;71:543–552. doi: 10.1001/jamaneurol.2014.131. [DOI] [PubMed] [Google Scholar]
- 8.Navas P., Villalba J.M., de Cabo R. The importance of plasma membrane coenzyme Q in aging and stress responses. Mitochondrion. 2007;7:S34–S40. doi: 10.1016/j.mito.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 9.Huo J., Xu Z., Hosoe K., Kubo H., Miyahara H., Dai J., Mori M., Sawashita J., Higuchi K. Coenzyme Q10 prevents senescence and dysfunction caused by oxidative stress in vascular endothelial cells. Oxid. Med. Cell. Longev. 2018;2018:1–15. doi: 10.1155/2018/3181759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang W., Hui R., Yang S. Telomeres, cardiovascular aging, and potential intervention for cellular senescence. Sci. China Life Sci. 2014;57:858–862. doi: 10.1007/s11427-014-4700-8. [DOI] [PubMed] [Google Scholar]
- 11.Davalli P., Mitic T., Caporali A., Lauriola A., D’Arca D. Ros, cell senescence, and novel molecular mechanisms in aging and age-related diseases. Oxid. Med. Cell. Longev. 2016;2016 doi: 10.1155/2016/3565127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hernández-Camacho J.D., Bernier M., López-Lluch G., Navas P. Coenzyme Q10 supplementation in aging and disease. Front. Physiol. 2018 doi: 10.3389/fphys.2018.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gutierrez-Mariscal F.M., Yubero-Serrano E.M., Villalba J.M., Lopez-Miranda J. Coenzyme Q10: From bench to clinic in aging diseases, a translational review. Crit. Rev. Food Sci. Nutr. 2018 doi: 10.1080/10408398.2018.1442316. [DOI] [PubMed] [Google Scholar]
- 14.Awad A.M., Bradley M.C., Fernandez-Del-Rio L., Nag A., Tsui H.S., Clarke C.F. Coenzyme Q10 deficiencies: Pathways in yeast and humans. Essays Biochem. 2018;62:361–376. doi: 10.1042/EBC20170106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gale P.H., Erickson R.E., Page A.C., Jr., Folkers K. Coenzyme Q. Li. New data on the distribution of coenzyme Q in nature. Arch. Biochem. Biophys. 1964;104:169–172. doi: 10.1016/S0003-9861(64)80051-5. [DOI] [PubMed] [Google Scholar]
- 16.Kalen A., Norling B., Appelkvist E.L., Dallner G. Ubiquinone biosynthesis by the microsomal fraction from rat liver. Biochim. Biophys. Acta. 1987;926:70–78. doi: 10.1016/0304-4165(87)90183-8. [DOI] [PubMed] [Google Scholar]
- 17.Lenaz G., Fato R., Di Bernardo S., Jarreta D., Costa A., Genova M.L., Parenti Castelli G. Localization and mobility of coenzyme Q in lipid bilayers and membranes. BioFactors. 1999;9:87–93. doi: 10.1002/biof.5520090202. [DOI] [PubMed] [Google Scholar]
- 18.Olson R.E., Rudney H. Biosynthesis of ubiquinone. Vitam Horm. 1983;40:1–43. doi: 10.1016/s0083-6729(08)60431-8. [DOI] [PubMed] [Google Scholar]
- 19.Alcazar-Fabra M., Navas P., Brea-Calvo G. Coenzyme Q biosynthesis and its role in the respiratory chain structure. Biochim. Biophys. Acta. 2016;1857:1073–1078. doi: 10.1016/j.bbabio.2016.03.010. [DOI] [PubMed] [Google Scholar]
- 20.Lenaz G., Fato R., Castelluccio C., Genova M.L., Bovina C., Estornell E., Valls V., Pallotti F., Parenti Castelli G. The function of coenzyme Q in mitochondria. Clin. Investig. 1993;71:S66–S70. doi: 10.1007/BF00226843. [DOI] [PubMed] [Google Scholar]
- 21.Enriquez J.A., Lenaz G. Coenzyme Q and the respiratory chain: Coenzyme Q pool and mitochondrial supercomplexes. Mol. Syndromol. 2014;5:119–140. doi: 10.1159/000363364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Groneberg D.A., Kindermann B., Althammer M., Klapper M., Vormann J., Littarru G.P., Doring F. Coenzyme Q10 affects expression of genes involved in cell signalling, metabolism and transport in human caco-2 cells. Int. J. Biochem. Cell Biol. 2005;37:1208–1218. doi: 10.1016/j.biocel.2004.11.017. [DOI] [PubMed] [Google Scholar]
- 23.Schmelzer C., Kitano M., Hosoe K., Döring F. Ubiquinol affects the expression of genes involved in pparα signalling and lipid metabolism without changes in methylation of CpG promoter islands in the liver of mice. J. Clin. Biochem. Nutr. 2012;50:119–126. doi: 10.3164/jcbn.11-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blatt T., Littarru G.P. Biochemical rationale and experimental data on the antiaging properties of CoQ(10) at skin level. BioFactors. 2011;37:381–385. doi: 10.1002/biof.169. [DOI] [PubMed] [Google Scholar]
- 25.Lopez-Lluch G., Rodriguez-Aguilera J.C., Santos-Ocana C., Navas P. Is coenzyme Q a key factor in aging? Mech. Ageing Dev. 2010;131:225–235. doi: 10.1016/j.mad.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 26.Sohal R.S., Kamzalov S., Sumien N., Ferguson M., Rebrin I., Heinrich K.R., Forster M.J. Effect of coenzyme Q10 intake on endogenous coenzyme Q content, mitochondrial electron transport chain, antioxidative defenses, and life span of mice. Free Radic. Biol. Med. 2006;40:480–487. doi: 10.1016/j.freeradbiomed.2005.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Crane F.L. Discovery of ubiquinone (coenzyme Q) and an overview of function. Mitochondrion. 2007;7:S2–S7. doi: 10.1016/j.mito.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 28.Belliere J., Devun F., Cottet-Rousselle C., Batandier C., Leverve X., Fontaine E. Prerequisites for ubiquinone analogs to prevent mitochondrial permeability transition-induced cell death. J. Bioenerg. Biomembr. 2012;44:207–212. doi: 10.1007/s10863-012-9406-7. [DOI] [PubMed] [Google Scholar]
- 29.Olivieri F., Lazzarini R., Babini L., Prattichizzo F., Rippo M.R., Tiano L., Di Nuzzo S., Graciotti L., Festa R., Bruge F., et al. Anti-inflammatory effect of ubiquinol-10 on young and senescent endothelial cells via mir-146a modulation. Free Radic. Biol. Med. 2013;63:410–420. doi: 10.1016/j.freeradbiomed.2013.05.033. [DOI] [PubMed] [Google Scholar]
- 30.Fan L., Feng Y., Chen G.C., Qin L.Q., Fu C.L., Chen L.H. Effects of coenzyme Q10 supplementation on inflammatory markers: A systematic review and meta-analysis of randomized controlled trials. Pharmacol. Res. 2017;119:128–136. doi: 10.1016/j.phrs.2017.01.032. [DOI] [PubMed] [Google Scholar]
- 31.Zhai J., Bo Y., Lu Y., Liu C., Zhang L. Effects of coenzyme Q10 on markers of inflammation: A systematic review and meta-analysis. PLoS ONE. 2017;12:e0170172. doi: 10.1371/journal.pone.0170172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schmelzer C., Lindner I., Rimbach G., Niklowitz P., Menke T., Doring F. Functions of coenzyme Q10 in inflammation and gene expression. BioFactors. 2008;32:179–183. doi: 10.1002/biof.5520320121. [DOI] [PubMed] [Google Scholar]
- 33.Saleh D.O., Ahmed R.F., Amin M.M. Modulatory role of co-enzyme Q10 on methionine and choline deficient diet-induced non-alcoholic steatohepatitis (nash) in albino rats. Appl. Physiol. Nutr. Metabol. 2017;42:243–249. doi: 10.1139/apnm-2016-0320. [DOI] [PubMed] [Google Scholar]
- 34.Farhangi M.A., Alipour B., Jafarvand E., Khoshbaten M. Oral coenzyme Q10 supplementation in patients with nonalcoholic fatty liver disease: Effects on serum vaspin, chemerin, pentraxin 3, insulin resistance and oxidative stress. Arch. Med. Res. 2014;45:589–595. doi: 10.1016/j.arcmed.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 35.Perez-Sanchez C., Aguirre M.A., Ruiz-Limon P., Abalos-Aguilera M.C., Jimenez-Gomez Y., Arias-de la Rosa I., Rodriguez-Ariza A., Fernandez-Del Rio L., Gonzalez-Reyes J.A., Segui P., et al. Ubiquinol effects on antiphospholipid syndrome prothrombotic profile: A randomized, placebo-controlled trial. Arterioscler. Thromb. Vasc. Biol. 2017;37:1923–1932. doi: 10.1161/ATVBAHA.117.309225. [DOI] [PubMed] [Google Scholar]
- 36.Wilcock D.M., Griffin W.S. Down’s syndrome, neuroinflammation, and Alzheimer neuropathogenesis. J. Neuroinflamm. 2013;10:84. doi: 10.1186/1742-2094-10-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zaki M.E., El-Bassyouni H.T., Tosson A.M., Youness E., Hussein J. Coenzyme Q10 and pro-inflammatory markers in children with Down syndrome: Clinical and biochemical aspects. J. Pediatr. 2017;93:100–104. doi: 10.1016/j.jped.2016.04.012. [DOI] [PubMed] [Google Scholar]
- 38.Tiano L., Carnevali P., Padella L., Santoro L., Principi F., Bruge F., Carle F., Gesuita R., Gabrielli O., Littarru G.P. Effect of coenzyme Q10 in mitigating oxidative DNA damage in Down syndrome patients, a double blind randomized controlled trial. Neurobiol. Aging. 2011;32:2103–2105. doi: 10.1016/j.neurobiolaging.2009.11.016. [DOI] [PubMed] [Google Scholar]
- 39.Sharma A., Fonarow G.C., Butler J., Ezekowitz J.A., Felker G.M. Coenzyme Q10 and heart failure: A state-of-the-art review. Circ. Heart Fail. 2016;9:e002639. doi: 10.1161/CIRCHEARTFAILURE.115.002639. [DOI] [PubMed] [Google Scholar]
- 40.Jorat M.V., Tabrizi R., Mirhosseini N., Lankarani K.B., Akbari M., Heydari S.T., Mottaghi R., Asemi Z. The effects of coenzyme Q10 supplementation on lipid profiles among patients with coronary artery disease: A systematic review and meta-analysis of randomized controlled trials. Lipids Health Dis. 2018;17:230. doi: 10.1186/s12944-018-0876-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lucas-Hourani M., Munier-Lehmann H., El Mazouni F., Malmquist N.A., Harpon J., Coutant E.P., Guillou S., Helynck O., Noel A., Scherf A., et al. Original 2-(3-alkoxy-1h-pyrazol-1-yl)azines inhibitors of human dihydroorotate dehydrogenase (dhodh) J. Med. Chem. 2015;58:5579–5598. doi: 10.1021/acs.jmedchem.5b00606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ziosi M., Di Meo I., Kleiner G., Gao X.H., Barca E., Sanchez-Quintero M.J., Tadesse S., Jiang H., Qiao C., Rodenburg R.J., et al. Coenzyme Q deficiency causes impairment of the sulfide oxidation pathway. EMBO Mol. Med. 2017;9:96–111. doi: 10.15252/emmm.201606356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Salvi F., Gadda G. Human choline dehydrogenase: Medical promises and biochemical challenges. Arch. Biochem. Biophys. 2013;537:243–252. doi: 10.1016/j.abb.2013.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Szabados L., Savoure A. Proline: A multifunctional amino acid. Trends Plant Sci. 2010;15:89–97. doi: 10.1016/j.tplants.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 45.Bentinger M., Tekle M., Dallner G. Coenzyme Q--biosynthesis and functions. Biochem. Biophys. Res. Commun. 2010;396:74–79. doi: 10.1016/j.bbrc.2010.02.147. [DOI] [PubMed] [Google Scholar]
- 46.Garrido-Maraver J., Cordero M.D., Oropesa-Avila M., Vega A.F., de la Mata M., Pavon A.D., Alcocer-Gomez E., Calero C.P., Paz M.V., Alanis M., et al. Clinical applications of coenzyme Q10. Front. Biosci. (Landmark Ed) 2014;19:619–633. doi: 10.2741/4231. [DOI] [PubMed] [Google Scholar]
- 47.Zozina V.I., Covantev S., Goroshko O.A., Krasnykh L.M., Kukes V.G. Coenzyme Q10 in cardiovascular and metabolic diseases: Current state of the problem. Curr. Cardiol. Rev. 2018;14:164–174. doi: 10.2174/1573403X14666180416115428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bhagavan H.N., Chopra R.K. Plasma coenzyme Q10 response to oral ingestion of coenzyme Q10 formulations. Mitochondrion. 2007;7:S78–S88. doi: 10.1016/j.mito.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 49.Ochiai A., Itagaki S., Kurokawa T., Kobayashi M., Hirano T., Iseki K. Improvement in intestinal coenzyme Q10 absorption by food intake. Yakugaku zasshi: J. Pharm. Soc. Jpn. 2007;127:1251–1254. doi: 10.1248/yakushi.127.1251. [DOI] [PubMed] [Google Scholar]
- 50.Mohr D., Bowry V.W., Stocker R. Dietary supplementation with coenzyme Q10 results in increased levels of ubiquinol-10 within circulating lipoproteins and increased resistance of human low-density lipoprotein to the initiation of lipid peroxidation. Biochim. Biophys. Acta. 1992;1126:247–254. doi: 10.1016/0005-2760(92)90237-P. [DOI] [PubMed] [Google Scholar]
- 51.Hargreaves I.P. Ubiquinone: Cholesterol’s reclusive cousin. Ann. Clin. Biochem. 2003;40:207–218. doi: 10.1258/000456303321610493. [DOI] [PubMed] [Google Scholar]
- 52.Ogasahara S., Engel A.G., Frens D., Mack D. Muscle coenzyme Q deficiency in familial mitochondrial encephalomyopathy. Proc. Natl. Acad. Sci. USA. 1989;86:2379–2382. doi: 10.1073/pnas.86.7.2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Miles M.V. The uptake and distribution of coenzyme Q10. Mitochondrion. 2007;7:S72–S77. doi: 10.1016/j.mito.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 54.Kwong L.K., Kamzalov S., Rebrin I., Bayne A.C., Jana C.K., Morris P., Forster M.J., Sohal R.S. Effects of coenzyme Q(10) administration on its tissue concentrations, mitochondrial oxidant generation, and oxidative stress in the rat. Free Radic. Biol. Med. 2002;33:627–638. doi: 10.1016/S0891-5849(02)00916-4. [DOI] [PubMed] [Google Scholar]
- 55.Niklowitz P., Menke T., Wiesel T., Mayatepek E., Zschocke J., Okun J.G., Andler W. Coenzyme Q10 in plasma and erythrocytes: Comparison of antioxidant levels in healthy probands after oral supplementation and in patients suffering from sickle cell anemia. Clin. Chim. Acta. 2002;326:155–161. doi: 10.1016/S0009-8981(02)00328-5. [DOI] [PubMed] [Google Scholar]
- 56.Duncan A.J., Heales S.J., Mills K., Eaton S., Land J.M., Hargreaves I.P. Determination of coenzyme Q10 status in blood mononuclear cells, skeletal muscle, and plasma by HPLC with di-propoxy-coenzyme Q10 as an internal standard. Clin. Chem. 2005;51:2380–2382. doi: 10.1373/clinchem.2005.054643. [DOI] [PubMed] [Google Scholar]
- 57.Bhagavan H.N., Chopra R.K. Coenzyme Q10: Absorption, tissue uptake, metabolism and pharmacokinetics. Free Radic. Res. 2006;40:445–453. doi: 10.1080/10715760600617843. [DOI] [PubMed] [Google Scholar]
- 58.Musumeci O., Naini A., Slonim A.E., Skavin N., Hadjigeorgiou G.L., Krawiecki N., Weissman B.M., Tsao C.Y., Mendell J.R., Shanske S., et al. Familial cerebellar ataxia with muscle coenzyme Q10 deficiency. Neurology. 2001;56:849–855. doi: 10.1212/WNL.56.7.849. [DOI] [PubMed] [Google Scholar]
- 59.Gempel K., Topaloglu H., Talim B., Schneiderat P., Schoser B.G., Hans V.H., Palmafy B., Kale G., Tokatli A., Quinzii C., et al. The myopathic form of coenzyme Q10 deficiency is caused by mutations in the electron-transferring-flavoprotein dehydrogenase (ETFDH) gene. Brain. 2007;130:2037–2044. doi: 10.1093/brain/awm054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Quinzii C.M., Kattah A.G., Naini A., Akman H.O., Mootha V.K., DiMauro S., Hirano M. Coenzyme Q deficiency and cerebellar ataxia associated with an aprataxin mutation. Neurology. 2005;64:539–541. doi: 10.1212/01.WNL.0000150588.75281.58. [DOI] [PubMed] [Google Scholar]
- 61.Ihara Y., Namba R., Kuroda S., Sato T., Shirabe T. Mitochondrial encephalomyopathy (MELAS): Pathological study and successful therapy with coenzyme Q10 and idebenone. J. Neurol. Sci. 1989;90:263–271. doi: 10.1016/0022-510X(89)90112-3. [DOI] [PubMed] [Google Scholar]
- 62.Quinzii C.M., Hirano M. Primary and secondary CoQ(10) deficiencies in humans. BioFactors. 2011;37:361–365. doi: 10.1002/biof.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hathcock J.N., Shao A. Risk assessment for coenzyme Q10 (ubiquinone) Regul. Toxicol. Pharmacol. 2006;45:282–288. doi: 10.1016/j.yrtph.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 64.Balreira A., Boczonadi V., Barca E., Pyle A., Bansagi B., Appleton M., Graham C., Hargreaves I.P., Rasic V.M., Lochmuller H., et al. Ano10 mutations cause ataxia and coenzyme Q(1)(0) deficiency. J. Neurol. 2014;261:2192–2198. doi: 10.1007/s00415-014-7476-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Baruteau J., Hargreaves I., Krywawych S., Chalasani A., Land J.M., Davison J.E., Kwok M.K., Christov G., Karimova A., Ashworth M., et al. Successful reversal of propionic acidaemia associated cardiomyopathy: Evidence for low myocardial coenzyme Q10 status and secondary mitochondrial dysfunction as an underlying pathophysiological mechanism. Mitochondrion. 2014;17:150–156. doi: 10.1016/j.mito.2014.07.001. [DOI] [PubMed] [Google Scholar]
- 66.Yubero D., O’Callaghan M., Montero R., Ormazabal A., Armstrong J., Espinos C., Rodriguez M.A., Jou C., Castejon E., Aracil M.A., et al. Association between coenzyme Q10 and glucose transporter (GLUT1) deficiency. BMC Pediatr. 2014;14:284. doi: 10.1186/s12887-014-0284-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Beal M.F. Coenzyme Q10 administration and its potential for treatment of neurodegenerative diseases. BioFactors. 1999;9:261–266. doi: 10.1002/biof.5520090222. [DOI] [PubMed] [Google Scholar]
- 68.Baggio E., Gandini R., Plancher A.C., Passeri M., Carmosino G. Italian multicenter study on the safety and efficacy of coenzyme Q10 as adjunctive therapy in heart failure. CoQ10 drug surveillance investigators. Mol. Asp. Med. 1994;15:s287–s294. doi: 10.1016/0098-2997(94)90040-X. [DOI] [PubMed] [Google Scholar]
- 69.Wallace D.C. A mitochondrial paradigm of metabolic and degenerative diseases, aging, and cancer: A dawn for evolutionary medicine. Annu. Rev. Genet. 2005;39:359–407. doi: 10.1146/annurev.genet.39.110304.095751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schniertshauer D., Gebhard D., Bergemann J. Age-dependent loss of mitochondrial function in epithelial tissue can be reversed by coenzyme Q10. J. Aging Res. 2018;2018:6354680. doi: 10.1155/2018/6354680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Harman D. The biologic clock: The mitochondria? J. Am. Geriatr. Soc. 1972;20:145–147. doi: 10.1111/j.1532-5415.1972.tb00787.x. [DOI] [PubMed] [Google Scholar]
- 72.Krutmann J., Schroeder P. Role of mitochondria in photoaging of human skin: The defective powerhouse model. J. Investig. Dermatol. Symp. Proc. 2009;14:44–49. doi: 10.1038/jidsymp.2009.1. [DOI] [PubMed] [Google Scholar]
- 73.Brunk U.T., Terman A. The mitochondrial-lysosomal axis theory of aging: Accumulation of damaged mitochondria as a result of imperfect autophagocytosis. Eur. J. Biochem. 2002;269:1996–2002. doi: 10.1046/j.1432-1033.2002.02869.x. [DOI] [PubMed] [Google Scholar]
- 74.Clayton D.A., Doda J.N., Friedberg E.C. The absence of a pyrimidine dimer repair mechanism in mammalian mitochondria. Proc. Natl. Acad. Sci. USA. 1974;71:2777–2781. doi: 10.1073/pnas.71.7.2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Demple B., Harrison L. Repair of oxidative damage to DNA: Enzymology and biology. Annu. Rev. Biochem. 1994;63:915–948. doi: 10.1146/annurev.bi.63.070194.004411. [DOI] [PubMed] [Google Scholar]
- 76.Barja G. Mitochondrial oxygen consumption and reactive oxygen species production are independently modulated: Implications for aging studies. Rejuv. Res. 2007;10:215–224. doi: 10.1089/rej.2006.0516. [DOI] [PubMed] [Google Scholar]
- 77.Miquel J. An update on the oxygen stress-mitochondrial mutation theory of aging: Genetic and evolutionary implications. Exp. Gerontol. 1998;33:113–126. doi: 10.1016/S0531-5565(97)00060-0. [DOI] [PubMed] [Google Scholar]
- 78.Sohal R.S., Mockett R.J., Orr W.C. Mechanisms of aging: An appraisal of the oxidative stress hypothesis. Free Radic. Biol. Med. 2002;33:575–586. doi: 10.1016/S0891-5849(02)00886-9. [DOI] [PubMed] [Google Scholar]
- 79.Maurya P.K., Noto C., Rizzo L.B., Rios A.C., Nunes S.O., Barbosa D.S., Sethi S., Zeni M., Mansur R.B., Maes M., et al. The role of oxidative and nitrosative stress in accelerated aging and major depressive disorder. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2016;65:134–144. doi: 10.1016/j.pnpbp.2015.08.016. [DOI] [PubMed] [Google Scholar]
- 80.Wallace D.C., Fan W., Procaccio V. Mitochondrial energetics and therapeutics. Annu. Rev. Pathol. 2010;5:297–348. doi: 10.1146/annurev.pathol.4.110807.092314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Elchuri S., Oberley T.D., Qi W., Eisenstein R.S., Jackson Roberts L., Van Remmen H., Epstein C.J., Huang T.T. Cuznsod deficiency leads to persistent and widespread oxidative damage and hepatocarcinogenesis later in life. Oncogene. 2005;24:367–380. doi: 10.1038/sj.onc.1208207. [DOI] [PubMed] [Google Scholar]
- 82.Li Y., Huang T.T., Carlson E.J., Melov S., Ursell P.C., Olson J.L., Noble L.J., Yoshimura M.P., Berger C., Chan P.H., et al. Dilated cardiomyopathy and neonatal lethality in mutant mice lacking manganese superoxide dismutase. Nat. Genet. 1995;11:376–381. doi: 10.1038/ng1295-376. [DOI] [PubMed] [Google Scholar]
- 83.Ma D., Stokes K., Mahngar K., Domazet-Damjanov D., Sikorska M., Pandey S. Inhibition of stress induced premature senescence in presenilin-1 mutated cells with water soluble coenzyme Q10. Mitochondrion. 2014;17:106–115. doi: 10.1016/j.mito.2014.07.004. [DOI] [PubMed] [Google Scholar]
- 84.Sohal R.S., Sohal B.H. Hydrogen peroxide release by mitochondria increases during aging. Mech. Ageing Dev. 1991;57:187–202. doi: 10.1016/0047-6374(91)90034-W. [DOI] [PubMed] [Google Scholar]
- 85.Asensi M., Sastre J., Pallardo F.V., Lloret A., Lehner M., Garcia-de-la Asuncion J., Vina J. Ratio of reduced to oxidized glutathione as indicator of oxidative stress status and DNA damage. Methods Enzymol. 1999;299:267–276. doi: 10.1016/s0076-6879(99)99026-2. [DOI] [PubMed] [Google Scholar]
- 86.Halliwell B. The wanderings of a free radical. Free Radic. Biol. Med. 2009;46:531–542. doi: 10.1016/j.freeradbiomed.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 87.Lambert A.J., Boysen H.M., Buckingham J.A., Yang T., Podlutsky A., Austad S.N., Kunz T.H., Buffenstein R., Brand M.D. Low rates of hydrogen peroxide production by isolated heart mitochondria associate with long maximum lifespan in vertebrate homeotherms. Aging Cell. 2007;6:607–618. doi: 10.1111/j.1474-9726.2007.00312.x. [DOI] [PubMed] [Google Scholar]
- 88.Barja G. Updating the mitochondrial free radical theory of aging: An integrated view, key aspects, and confounding concepts. Antioxid. Redox Signal. 2013;19:1420–1445. doi: 10.1089/ars.2012.5148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hekimi S., Lapointe J., Wen Y. Taking a “good” look at free radicals in the aging process. Trends Cell Biol. 2011;21:569–576. doi: 10.1016/j.tcb.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wang Y., Hekimi S. Mitochondrial dysfunction and longevity in animals: Untangling the knot. Science. 2015;350:1204–1207. doi: 10.1126/science.aac4357. [DOI] [PubMed] [Google Scholar]
- 91.Feng J., Bussiere F., Hekimi S. Mitochondrial electron transport is a key determinant of life span in caenorhabditis elegans. Dev. Cell. 2001;1:633–644. doi: 10.1016/S1534-5807(01)00071-5. [DOI] [PubMed] [Google Scholar]
- 92.Hekimi S. Enhanced immunity in slowly aging mutant mice with high mitochondrial oxidative stress. Oncoimmunology. 2013;2:e23793. doi: 10.4161/onci.23793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wang Y., Oxer D., Hekimi S. Mitochondrial function and lifespan of mice with controlled ubiquinone biosynthesis. Nat. Commun. 2015;6:6393. doi: 10.1038/ncomms7393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Van Remmen H., Ikeno Y., Hamilton M., Pahlavani M., Wolf N., Thorpe S.R., Alderson N.L., Baynes J.W., Epstein C.J., Huang T.T., et al. Life-long reduction in mnsod activity results in increased DNA damage and higher incidence of cancer but does not accelerate aging. Physiol. Genom. 2003;16:29–37. doi: 10.1152/physiolgenomics.00122.2003. [DOI] [PubMed] [Google Scholar]
- 95.Hepple R.T. Mitochondrial involvement and impact in aging skeletal muscle. Front. Aging Neurosci. 2014;6:211. doi: 10.3389/fnagi.2014.00211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Buffenstein R., Edrey Y.H., Yang T., Mele J. The oxidative stress theory of aging: Embattled or invincible? Insights from non-traditional model organisms. Age. 2008;30:99–109. doi: 10.1007/s11357-008-9058-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Varela-Lopez A., Giampieri F., Battino M., Quiles J.L. Coenzyme Q and its role in the dietary therapy against aging. Molecules. 2016;21:373. doi: 10.3390/molecules21030373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sohal R.S., Forster M.J. Coenzyme Q, oxidative stress and aging. Mitochondrion. 2007;7:S103–S111. doi: 10.1016/j.mito.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jonassen T., Davis D.E., Larsen P.L., Clarke C.F. Reproductive fitness and quinone content of caenorhabditis elegans clk-1 mutants fed coenzyme Q isoforms of varying length. J. Biol. Chem. 2003;278:51735–51742. doi: 10.1074/jbc.M308760200. [DOI] [PubMed] [Google Scholar]
- 100.Asencio C., Navas P., Cabello J., Schnabel R., Cypser J.R., Johnson T.E., Rodriguez-Aguilera J.C. Coenzyme Q supports distinct developmental processes in caenorhabditis elegans. Mech. Ageing Dev. 2009;130:145–153. doi: 10.1016/j.mad.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 101.Gavilan A., Asencio C., Cabello J., Rodriguez-Aguilera J.C., Schnabel R., Navas P.C. Elegans knockouts in ubiquinone biosynthesis genes result in different phenotypes during larval development. BioFactors. 2005;25:21–29. doi: 10.1002/biof.5520250104. [DOI] [PubMed] [Google Scholar]
- 102.Hihi A.K., Gao Y., Hekimi S. Ubiquinone is necessary for caenorhabditis elegans development at mitochondrial and non-mitochondrial sites. J. Biol. Chem. 2002;277:2202–2206. doi: 10.1074/jbc.M109034200. [DOI] [PubMed] [Google Scholar]
- 103.Quinzii C.M., Lopez L.C., Von-Moltke J., Naini A., Krishna S., Schuelke M., Salviati L., Navas P., DiMauro S., Hirano M. Respiratory chain dysfunction and oxidative stress correlate with severity of primary CoQ10 deficiency. Faseb J. 2008;22:1874–1885. doi: 10.1096/fj.07-100149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Quinzii C.M., López L.C., Gilkerson R.W., Dorado B., Coku J., Naini A.B., Lagier-Tourenne C., Schuelke M., Salviati L., Carrozzo R., et al. Reactive oxygen species, oxidative stress, and cell death correlate with level of CoQ10 deficiency. Faseb J. 2010;24:3733–3743. doi: 10.1096/fj.09-152728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Tian G., Sawashita J., Kubo H., Nishio S.Y., Hashimoto S., Suzuki N., Yoshimura H., Tsuruoka M., Wang Y., Liu Y., et al. Ubiquinol-10 supplementation activates mitochondria functions to decelerate senescence in senescence-accelerated mice. Antioxid. Redox Signal. 2014;20:2606–2620. doi: 10.1089/ars.2013.5406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Xu Z., Huo J., Ding X., Yang M., Li L., Dai J., Hosoe K., Kubo H., Mori M., Higuchi K., et al. Coenzyme Q10 improves lipid metabolism and ameliorates obesity by regulating camkii-mediated pde4 inhibition. Sci. Rep. 2017;7:8253. doi: 10.1038/s41598-017-08899-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Liu X., Jiang N., Hughes B., Bigras E., Shoubridge E., Hekimi S. Evolutionary conservation of the clk-1-dependent mechanism of longevity: Loss of mclk1 increases cellular fitness and lifespan in mice. Genes Dev. 2005;19:2424–2434. doi: 10.1101/gad.1352905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ochoa J.J., Quiles J.L., Lopez-Frias M., Huertas J.R., Mataix J. Effect of lifelong coenzyme Q10 supplementation on age-related oxidative stress and mitochondrial function in liver and skeletal muscle of rats fed on a polyunsaturated fatty acid (PUFA)-rich diet. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2007;62:1211–1218. doi: 10.1093/gerona/62.11.1211. [DOI] [PubMed] [Google Scholar]
- 109.Quiles J.L., Ochoa J.J., Battino M., Gutierrez-Rios P., Nepomuceno E.A., Frias M.L., Huertas J.R., Mataix J. Life-long supplementation with a low dosage of coenzyme Q10 in the rat: Effects on antioxidant status and DNA damage. BioFactors. 2005;25:73–86. doi: 10.1002/biof.5520250109. [DOI] [PubMed] [Google Scholar]
- 110.Quiles J.L., Pamplona R., Ramirez-Tortosa M.C., Naudi A., Portero-Otin M., Araujo-Nepomuceno E., Lopez-Frias M., Battino M., Ochoa J.J. Coenzyme Q addition to an n-6 PUFA-rich diet resembles benefits on age-related mitochondrial DNA deletion and oxidative stress of a MUFA-rich diet in rat heart. Mech. Ageing Dev. 2010;131:38–47. doi: 10.1016/j.mad.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 111.Schmelzer C., Kubo H., Mori M., Sawashita J., Kitano M., Hosoe K., Boomgaarden I., Doring F., Higuchi K. Supplementation with the reduced form of Coenzyme Q10 decelerates phenotypic characteristics of senescence and induces a peroxisome proliferator-activated receptor-alpha gene expression signature in SAMP1 mice. Mol. Nutr. Food Res. 2010;54:805–815. doi: 10.1002/mnfr.200900155. [DOI] [PubMed] [Google Scholar]
- 112.Matthews R.T., Yang L., Browne S., Baik M., Beal M.F. Coenzyme Q10 administration increases brain mitochondrial concentrations and exerts neuroprotective effects. Proc. Natl. Acad. Sci. USA. 1998;95:8892–8897. doi: 10.1073/pnas.95.15.8892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wolters M., Hahn A. Plasma ubiquinone status and response to six-month supplementation combined with multivitamins in healthy elderly women--results of a randomized, double-blind, placebo-controlled study. Int. J. Vitam. Nutr. Res. 2003;73:207–214. doi: 10.1024/0300-9831.73.3.207. [DOI] [PubMed] [Google Scholar]
- 114.Kalen A., Appelkvist E.L., Dallner G. Age-related changes in the lipid compositions of rat and human tissues. Lipids. 1989;24:579–584. doi: 10.1007/BF02535072. [DOI] [PubMed] [Google Scholar]
- 115.Soderberg M., Edlund C., Kristensson K., Dallner G. Lipid compositions of different regions of the human brain during aging. J. Neurochem. 1990;54:415–423. doi: 10.1111/j.1471-4159.1990.tb01889.x. [DOI] [PubMed] [Google Scholar]
- 116.Edlund C., Soderberg M., Kristensson K., Dallner G. Ubiquinone, dolichol, and cholesterol metabolism in aging and Alzheimer’s disease. Biochem. Cell Biol. 1992;70:422–428. doi: 10.1139/o92-065. [DOI] [PubMed] [Google Scholar]
- 117.Nagase M., Yamamoto Y., Matsumoto N., Arai Y., Hirose N. Increased oxidative stress and coenzyme Q10 deficiency in centenarians. J. Clin. Biochem. Nutr. 2018;63:129–136. doi: 10.3164/jcbn.17.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bentinger M., Brismar K., Dallner G. The antioxidant role of coenzyme Q. Mitochondrion. 2007;7:S41–S50. doi: 10.1016/j.mito.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 119.Del Pozo-Cruz J., Rodriguez-Bies E., Ballesteros-Simarro M., Navas-Enamorado I., Tung B.T., Navas P., Lopez-Lluch G. Physical activity affects plasma coenzyme Q10 levels differently in young and old humans. Biogerontology. 2014;15:199–211. doi: 10.1007/s10522-013-9491-y. [DOI] [PubMed] [Google Scholar]
- 120.Del Pozo-Cruz J., Rodriguez-Bies E., Navas-Enamorado I., Del Pozo-Cruz B., Navas P., Lopez-Lluch G. Relationship between functional capacity and body mass index with plasma coenzyme Q10 and oxidative damage in community-dwelling elderly-people. Exp. Gerontol. 2014;52:46–54. doi: 10.1016/j.exger.2014.01.026. [DOI] [PubMed] [Google Scholar]
- 121.Johansson P., Dahlstrom O., Dahlstrom U., Alehagen U. Improved health-related quality of life, and more days out of hospital with supplementation with selenium and coenzyme Q10 combined. Results from a double blind, placebo-controlled prospective study. J. Nutr. Health Aging. 2015;19:870–877. doi: 10.1007/s12603-015-0509-9. [DOI] [PubMed] [Google Scholar]
- 122.Fischer A., Onur S., Niklowitz P., Menke T., Laudes M., Rimbach G., Doring F. Coenzyme Q10 status as a determinant of muscular strength in two independent cohorts. PLoS ONE. 2016;11:e0167124. doi: 10.1371/journal.pone.0167124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Liu Z.X., Artmann C. Relative bioavailability comparison of different coenzyme Q10 formulations with a novel delivery system. Altern. Ther. Health Med. 2009;15:42–46. [PubMed] [Google Scholar]
- 124.Koroshetz W.J., Jenkins B.G., Rosen B.R., Beal M.F. Energy metabolism defects in Huntington’s disease and effects of coenzyme Q10. Ann. Neurol. 1997;41:160–165. doi: 10.1002/ana.410410206. [DOI] [PubMed] [Google Scholar]
- 125.Balaban R.S., Nemoto S., Finkel T. Mitochondria, oxidants, and aging. Cell. 2005;120:483–495. doi: 10.1016/j.cell.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 126.Liu J., Wang L.N. Mitochondrial enhancement for neurodegenerative movement disorders: A systematic review of trials involving creatine, coenzyme Q10, idebenone and mitoquinone. CNS Drugs. 2014;28:63–68. doi: 10.1007/s40263-013-0124-4. [DOI] [PubMed] [Google Scholar]
- 127.Molina J.A., de Bustos F., Ortiz S., Del Ser T., Seijo M., Benito-Leon J., Oliva J.M., Perez S., Manzanares J. Serum levels of coenzyme Q in patients with Lewy body disease. J. Neural Transm. (Vienna, Austria : 1996) 2002;109:1195–1201. doi: 10.1007/s00702-001-0761-5. [DOI] [PubMed] [Google Scholar]
- 128.Isobe C., Abe T., Terayama Y. Levels of reduced and oxidized coenzyme Q-10 and 8-hydroxy-2’-deoxyguanosine in the cerebrospinal fluid of patients with living Parkinson’s disease demonstrate that mitochondrial oxidative damage and/or oxidative DNA damage contributes to the neurodegenerative process. Neurosci. Lett. 2010;469:159–163. doi: 10.1016/j.neulet.2009.11.065. [DOI] [PubMed] [Google Scholar]
- 129.McGarry A., McDermott M., Kieburtz K., de Blieck E.A., Beal F., Marder K., Ross C., Shoulson I., Gilbert P., Mallonee W.M., et al. A randomized, double-blind, placebo-controlled trial of coenzyme Q10 in Huntington disease. Neurology. 2017;88:152–159. doi: 10.1212/WNL.0000000000003478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Buhmann C., Arlt S., Kontush A., Moller-Bertram T., Sperber S., Oechsner M., Stuerenburg H.J., Beisiegel U. Plasma and CSF markers of oxidative stress are increased in Parkinson’s disease and influenced by antiparkinsonian medication. Neurobiol. Dis. 2004;15:160–170. doi: 10.1016/j.nbd.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 131.Haas R.H., Nasirian F., Nakano K., Ward D., Pay M., Hill R., Shults C.W. Low platelet mitochondrial complex I and complex II/III activity in early untreated Parkinson’s disease. Ann. Neurol. 1995;37:714–722. doi: 10.1002/ana.410370604. [DOI] [PubMed] [Google Scholar]
- 132.Mischley L.K., Allen J., Bradley R. Coenzyme Q10 deficiency in patients with Parkinson’s disease. J. Neurol. Sci. 2012;318:72–75. doi: 10.1016/j.jns.2012.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Shults C.W., Oakes D., Kieburtz K., Beal M.F., Haas R., Plumb S., Juncos J.L., Nutt J., Shoulson I., Carter J., et al. Effects of coenzyme Q10 in early Parkinson disease: Evidence of slowing of the functional decline. Arch. Neurol. 2002;59:1541–1550. doi: 10.1001/archneur.59.10.1541. [DOI] [PubMed] [Google Scholar]
- 134.Negida A., Menshawy A., El Ashal G., Elfouly Y., Hani Y., Hegazy Y., El Ghonimy S., Fouda S., Rashad Y. Coenzyme Q10 for patients with Parkinson’s disease: A systematic review and meta-analysis. CNS Neurol. Disord. Drug Targets. 2016;15:45–53. doi: 10.2174/1871527314666150821103306. [DOI] [PubMed] [Google Scholar]
- 135.Shetty R.A., Forster M.J., Sumien N. Coenzyme Q(10) supplementation reverses age-related impairments in spatial learning and lowers protein oxidation. Age. 2013;35:1821–1834. doi: 10.1007/s11357-012-9484-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Galasko D.R., Peskind E., Clark C.M., Quinn J.F., Ringman J.M., Jicha G.A., Cotman C., Cottrell B., Montine T.J., Thomas R.G., et al. Antioxidants for Alzheimer disease: A randomized clinical trial with cerebrospinal fluid biomarker measures. Arch. Neurol. 2012;69:836–841. doi: 10.1001/archneurol.2012.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Flowers N., Hartley L., Todkill D., Stranges S., Rees K. Co-enzyme Q10 supplementation for the primary prevention of cardiovascular disease. Cochrane Database Syst. Rev. 2014;12:Cd010405. doi: 10.1002/14651858.CD010405.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ho M.J., Li E.C., Wright J.M. Blood pressure lowering efficacy of coenzyme Q10 for primary hypertension. Cochrane Database Syst. Rev. 2016;3:Cd007435. doi: 10.1002/14651858.CD007435.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Hosoe K., Kitano M., Kishida H., Kubo H., Fujii K., Kitahara M. Study on safety and bioavailability of ubiquinol (Kaneka QH) after single and 4-week multiple oral administration to healthy volunteers. Regul. Toxicol. Pharmacol. 2007;47:19–28. doi: 10.1016/j.yrtph.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 140.Rundek T., Naini A., Sacco R., Coates K., DiMauro S. Atorvastatin decreases the coenzyme Q10 level in the blood of patients at risk for cardiovascular disease and stroke. Arch. Neurol. 2004;61:889–892. doi: 10.1001/archneur.61.6.889. [DOI] [PubMed] [Google Scholar]
- 141.Lamperti C., Naini A.B., Lucchini V., Prelle A., Bresolin N., Moggio M., Sciacco M., Kaufmann P., DiMauro S. Muscle coenzyme Q10 levels in statin-related myopathy. Arch. Neurol. 2005;62:1709–1712. doi: 10.1001/archneur.62.11.1709. [DOI] [PubMed] [Google Scholar]
- 142.Caso G., Kelly P., McNurlan M.A., Lawson W.E. Effect of coenzyme Q10 on myopathic symptoms in patients treated with statins. Am. J. Cardiol. 2007;99:1409–1412. doi: 10.1016/j.amjcard.2006.12.063. [DOI] [PubMed] [Google Scholar]
- 143.Fedacko J., Pella D., Fedackova P., Hanninen O., Tuomainen P., Jarcuska P., Lopuchovsky T., Jedlickova L., Merkovska L., Littarru G.P. Coenzyme Q(10) and selenium in statin-associated myopathy treatment. Can. J. Physiol. Pharmacol. 2013;91:165–170. doi: 10.1139/cjpp-2012-0118. [DOI] [PubMed] [Google Scholar]
- 144.Schirris T.J., Renkema G.H., Ritschel T., Voermans N.C., Bilos A., van Engelen B.G., Brandt U., Koopman W.J., Beyrath J.D., Rodenburg R.J., et al. Statin-induced myopathy is associated with mitochondrial complex III inhibition. Cell Metab. 2015;22:399–407. doi: 10.1016/j.cmet.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 145.de Frutos F., Gea A., Hernandez-Estefania R., Rabago G. Prophylactic treatment with coenzyme Q10 in patients undergoing cardiac surgery: Could an antioxidant reduce complications? A systematic review and meta-analysis. Interact. Cardiovasc. Thorac. Surg. 2015;20:254–259. doi: 10.1093/icvts/ivu334. [DOI] [PubMed] [Google Scholar]
- 146.Alehagen U., Aaseth J., Alexander J., Johansson P. Still reduced cardiovascular mortality 12 years after supplementation with selenium and coenzyme Q10 for four years: A validation of previous 10-year follow-up results of a prospective randomized double-blind placebo-controlled trial in elderly. PLoS ONE. 2018;13:e0193120. doi: 10.1371/journal.pone.0193120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Mortensen S.A., Rosenfeldt F., Kumar A., Dolliner P., Filipiak K.J., Pella D., Alehagen U., Steurer G., Littarru G.P. The effect of coenzyme Q10 on morbidity and mortality in chronic heart failure: Results from Q-SYMBIO: A randomized double-blind trial. JACC Heart Fail. 2014;2:641–649. doi: 10.1016/j.jchf.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 148.Yubero-Serrano E.M., Gonzalez-Guardia L., Rangel-Zuniga O., Delgado-Lista J., Gutierrez-Mariscal F.M., Perez-Martinez P., Delgado-Casado N., Cruz-Teno C., Tinahones F.J., Villalba J.M., et al. Mediterranean diet supplemented with coenzyme Q10 modifies the expression of proinflammatory and endoplasmic reticulum stress-related genes in elderly men and women. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2012;67:3–10. doi: 10.1093/gerona/glr167. [DOI] [PubMed] [Google Scholar]
- 149.Tsai H.Y., Lin C.P., Huang P.H., Li S.Y., Chen J.S., Lin F.Y., Chen J.W., Lin S.J. Coenzyme Q10 attenuates high glucose-induced endothelial progenitor cell dysfunction through AMP-activated protein kinase pathways. J. Diabetes Res. 2016;2016:6384759. doi: 10.1155/2016/6384759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Ishikawa A., Kawarazaki H., Ando K., Fujita M., Fujita T., Homma Y. Renal preservation effect of ubiquinol, the reduced form of coenzyme Q10. Clin. Exp. Nephrol. 2011;15:30–33. doi: 10.1007/s10157-010-0350-8. [DOI] [PubMed] [Google Scholar]
- 151.Rivara M.B., Yeung C.K., Robinson-Cohen C., Phillips B.R., Ruzinski J., Rock D., Linke L., Shen D.D., Ikizler T.A., Himmelfarb J. Effect of coenzyme Q10 on biomarkers of oxidative stress and cardiac function in hemodialysis patients: The CoQ10 biomarker trial. Am. J. Kidney Dis. 2017;69:389–399. doi: 10.1053/j.ajkd.2016.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Bolignano D., Cernaro V., Gembillo G., Baggetta R., Buemi M., D’Arrigo G. Antioxidant agents for delaying diabetic kidney disease progression: A systematic review and meta-analysis. PLoS ONE. 2017;12:e0178699. doi: 10.1371/journal.pone.0178699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Abdollahzad H., Aghdashi M.A., Asghari Jafarabadi M., Alipour B. Effects of coenzyme Q10 supplementation on inflammatory cytokines (tnf-alpha, il-6) and oxidative stress in rheumatoid arthritis patients: A randomized controlled trial. Arch. Med. Res. 2015;46:527–533. doi: 10.1016/j.arcmed.2015.08.006. [DOI] [PubMed] [Google Scholar]
- 154.Mazidi M., Kengne A.P., Banach M. Effects of coenzyme Q10 supplementation on plasma c-reactive protein concentrations: A systematic review and meta-analysis of randomized controlled trials. Pharmacol. Res. 2018;128:130–136. doi: 10.1016/j.phrs.2017.08.011. [DOI] [PubMed] [Google Scholar]
- 155.Tiano L., Busciglio J. Mitochondrial dysfunction and Down’s syndrome: Is there a role for coenzyme Q(10)? BioFactors. 2011;37:386–392. doi: 10.1002/biof.184. [DOI] [PubMed] [Google Scholar]
- 156.Zhang X.X., Qian K.J., Zhang Y., Wang Z.J., Yu Y.B., Liu X.J., Cao X.T., Liao Y.H., Zhang D.Y. Efficacy of coenzyme Q10 in mitigating spinal cord injury-induced osteoporosis. Mol. Med. Rep. 2015;12:3909–3915. doi: 10.3892/mmr.2015.3856. [DOI] [PubMed] [Google Scholar]
- 157.Moon H.J., Ko W.K., Jung M.S., Kim J.H., Lee W.J., Park K.S., Heo J.K., Bang J.B., Kwon I.K. Coenzyme Q10 regulates osteoclast and osteoblast differentiation. J. Food Sci. 2013;78:H785–H891. doi: 10.1111/1750-3841.12116. [DOI] [PubMed] [Google Scholar]
- 158.Zheng D., Cui C., Yu M., Li X., Wang L., Chen X., Lin Y. Coenzyme Q10 promotes osteoblast proliferation and differentiation and protects against ovariectomy-induced osteoporosis. Mol. Med. Rep. 2018;17:400–407. doi: 10.3892/mmr.2017.7907. [DOI] [PubMed] [Google Scholar]
- 159.Jolliet P., Simon N., Barre J., Pons J.Y., Boukef M., Paniel B.J., Tillement J.P. Plasma coenzyme Q10 concentrations in breast cancer: Prognosis and therapeutic consequences. Int. J. Clin. Pharmacol. Ther. 1998;36:506–509. [PubMed] [Google Scholar]
- 160.Folkers K., Osterborg A., Nylander M., Morita M., Mellstedt H. Activities of vitamin Q10 in animal models and a serious deficiency in patients with cancer. Biochem. Biophys. Res. Commun. 1997;234:296–299. doi: 10.1006/bbrc.1997.6522. [DOI] [PubMed] [Google Scholar]
- 161.Rusciani L., Proietti I., Paradisi A., Rusciani A., Guerriero G., Mammone A., De Gaetano A., Lippa S. Recombinant interferon alpha-2b and coenzyme Q10 as a postsurgical adjuvant therapy for melanoma: A 3-year trial with recombinant interferon-alpha and 5-year follow-up. Melanoma Res. 2007;17:177–183. doi: 10.1097/CMR.0b013e32818867a0. [DOI] [PubMed] [Google Scholar]
- 162.Mano T., Iwase K., Hayashi R., Hayakawa N., Uchimura K., Makino M., Nagata M., Sawai Y., Oda N., Hamada M., et al. Vitamin E and coenzyme Q concentrations in the thyroid tissues of patients with various thyroid disorders. Am. J. Med. Sci. 1998;315:230–232. doi: 10.1097/00000441-199804000-00002. [DOI] [PubMed] [Google Scholar]
- 163.Quiles J.L., Farquharson A.J., Ramirez-Tortosa M.C., Grant I., Milne L., Huertas J.R., Battino M., Mataix J., Wahle K.W. Coenzyme Q differentially modulates phospholipid hydroperoxide glutathione peroxidase gene expression and free radicals production in malignant and non-malignant prostate cells. BioFactors. 2003;18:265–270. doi: 10.1002/biof.5520180229. [DOI] [PubMed] [Google Scholar]