Abstract
A substantial burden of disease and mortality globally is attributable to both sleep disruption and low intakes of fruit and vegetable (FV) and there is increasing mechanistic and epidemiological evidence to support a reciprocal relationship between the two. This review provides an overview of experimental and observational studies assessing the relations between sleep and FV consumption from 52 human adult studies. Experimental studies are currently limited and show inconsistent results. Observational studies support a non-linear association with adults sleeping the recommended 7–9 hours/day having the highest intakes of FV. The potential mechanisms linking sleep and FV consumption are highlighted. Disrupted sleep influences FV consumption through homeostatic and non-homeostatic mechanisms. Conversely, FV consumption may influence sleep through polyphenol content via several potential pathways. Few human experimental studies have examined the effects of FV items and their polyphenols on sleep and there is a need for more studies to address this. An appreciation of the relationship between sleep and FV consumption may help optimize sleep and FV consumption and may reduce the burden of chronic diseases. This review provides implications for public health and directions for future work.
Keywords: sleep, fruits and vegetables, polyphenols, dietary intake, nutritional epidemiology
1. Introduction
Sleep is a universal need and humans spend about one-third of their lives asleep but its function remains to be fully elucidated. Sleep health encompasses sleep architecture [1], sleep duration, quality (efficiency which is the time in bed spent asleep, sleep onset latency (SOL) which is the amount of time it takes to fall asleep) [2], timing (sleep onset is the time sleeping starts and sleep offset is waking time), variability, daytime sleepiness, and napping [3]. However, most studies have focused on sleep duration since it is easier to report accurately by participants [4]. Sleep is regulated by a two-process model that interplay akin to an hourglass timer [5]. The two-processes include process S—which is the homeostatic drive to sleep which accumulates across the day, peaks before bedtime and dissipates throughout the night—and process C which is regulated by the circadian system [6].
Sleep disruption is defined as changes in sleep continuity, timing, or duration. It is intertwined with circadian rhythm disruption and their causes could be environmental, such as shift work and jetlag, and behavioral, such as the disruption of the fasting/feeding cycle and the rest/activity cycle [7]. The National Sleep Foundation (a US non-profit organization) recommends different sleep durations for individuals according to age. Adults aged between 18–64 years are recommended to sleep 7–9 h/day [8].
1.1. Economic Cost of Sleep Disruption and Low Intakes of FV
Hafner and colleagues reported the economic cost of insufficient sleep from 62,000 people in the UK, US, Canada, Germany, and Japan. Insufficient sleep costs $411 billion annually for the US, $138 billion for Japan, £40 billion for UK, $60 billion for Germany, and $21 billion for Canada [9]. Sleep disruption has detrimental consequences and identifying the factors that influence it is a public health priority.
Few studies have assessed the economic cost of “unhealthy diets” that include low consumption of FV, probably due to the conceptual challenges of its definition [10]. Popkin et al. defined an “unhealthy diet” as high in saturated and trans-fat, heavy alcohol drinking, and low consumption of whole grains and FV. Using this definition, the estimated annual cost of “unhealthy diets” for China was calculated as €3.5 billion per capita [11]. The economic burden attributable to low FV consumption in Australia was estimated to be $AUS 269 million [12]. For Canada, the economic burden of inadequate consumption of FV was $CAN 3.3 billion per year, of which 30% is direct for health-care costs and 69% is indirect costs due to productivity losses [13]. The estimates of the economic cost to the NHS in the UK in 2007 was £5.8 billion for “poor diet”, the consumption of <600 g/day of FV was one aspect of “poor diet” [14].
1.2. Sleep Disruption and Low Intakes of FV Are Associated with Morbidity and Mortality
There is growing evidence that sleep disruption has deleterious associations for health. The Centers for Disease Control and Prevention has declared “insufficient sleep” as a public health problem because it is associated with type 2 diabetes, heart disease, obesity, and depression [15]. Short sleep duration was associated with 38% increased risk of obesity in adults from 153 prospective studies in a meta-analysis [16]. Recent evidence from other meta-analyses found that long sleep duration was associated with an increased risk of obesity [4,17]. Sleep disruption was shown to increase the risk of other diseases including; cancer [18,19,20], type 2 diabetes mellitus [4,16,21], stroke [22], cardiovascular disease, and coronary heart disease [23,24]. A consistent U-shaped association was shown between sleep duration and mortality, short and long sleep durations were associated with an increased risk of mortality [4,16,25,26,27,28]. Collectively, sleep disruption is associated with an increased risk of diseases and mortality. These associations are partly mediated through changes in dietary intake including the low consumption of FV [29], thus exploring the associations between sleep and dietary intake is fundamental.
The reciprocal relationship between sleep and diet in humans has been studied since the 1980s [30,31,32]. Sleep disruption affects dietary intake [29,33,34] and dietary intake affects sleep [2,35,36]. With the reciprocal relationship in mind, The World Health Organization (WHO) recommends consuming 400 g or more of FV per day to improve overall health and reduce the risk of chronic diseases [37]. The recommended amount of FV consumption is different between countries [38,39,40,41,42]. Despite these recommendations, FV consumption remains below the recommended levels and below the WHO recommendations in many countries [37,43,44].
Increased consumption of FV has been shown to protect against type 2 diabetes [45], coronary heart disease [46], stroke [47], and some cancers [48]. Increasing FV consumption to 600 g/day could reduce the total worldwide burden of disease by 1.8%, reduce the burden of ischemic heart disease by 31%, ischemic stroke by 19%, stomach cancer by 19%, esophageal cancer by 20%, lung cancer by 12%, and colorectal cancer by 2% [49]. Recent evidence from a dose–response meta-analysis of prospective studies reported that the consumption of 800 g/day (10 portions per day) of FV are associated with lower risks of cardiovascular diseases, cancer, and all-cause mortality [50].
A substantial burden of premature deaths globally is attributable to low consumption of FV. In 2005, total worldwide mortality attributable to inadequate consumption of FV is estimated to be up to 2.635 million deaths per year [49]. In 2013, an estimated 5.6 million premature deaths worldwide may be attributable to FV intakes below 500 g/day and 7.8 million premature deaths to FV intakes below 800 g/day [50]. In 2017, an estimated 3.9 million deaths worldwide were attributable to inadequate FV consumption according to WHO [51]. These studies highlight the importance of FV consumption thus, identifying lifestyle factors which may influence FV intakes is a public health priority.
It is clear that both sleep disruption and low consumption of FV are economically burdensome and are attributable factors to morbidity and mortality. Consequently, bridging the scientific gap between them is essential and may have key public health implications. The aim of this review is to summarize the results from experimental and observational adult human studies assessing the relationship between sleep and FV consumption. Results from animal and in vitro studies are also included to support the potential mechanisms involved. This review will also highlight implications for public health and directions for future work. We used Medline, EMBASE, CINAHL, Cochrane, and PubMed databases (see Supplementary Material Table S1 for search terms used) to find published studies exploring the relationships between sleep and FV consumption. Hand searches of reference lists of retrieved articles were also undertaken. A total of 52 human studies were found and discussed below.
2. Sleep and Fruit and Vegetable Consumption
Several child and adolescent studies have assessed the association between sleep measures and dietary intake including FV consumption [52,53,54,55,56,57,58,59,60,61,62,63,64,65]. The association was shown to be positive in a recent meta-analysis [66]. Short sleep duration was associated with lower consumption of FV and an increased consumption of FV in children was associated with sleeping adequately. The associations between sleep measures and FV consumption are more consistent in children, however they are not well characterized in adults [29]. Sleep requirements differ between children, adolescents, and adults [8] and there is a need for more studies to assess this relationship in adults.
Experimental and observational adult studies assessing the association between sleep measures and FV consumption are summarized in Table 1 and are explained in detail in Table 2. Fifty-two studies were identified with only 10 experimental (interventional) studies including either sleep restriction or extension [67,68,69,70] or the effects of FV items on sleep measures [71,72,73,74,75,76] (Table 1).
Table 1.
Summary of human studies [references] assessing the association between sleep and FV consumption in adults.
| Study Type | |||||
|---|---|---|---|---|---|
| Observational | Experimental (Intervention) | ||||
| Cross-Sectional | Prospective | Sleep Restriction | Sleep Extension | Fruit Intervention | |
| Exposure/outcome not clearly stated | [89,97,98,99,100] | ||||
| Exposure | |||||
| Sleep | [82,84,90,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121] | [78,79,82] | [67,68,69] | [67,70] | |
| Diet including FV | [80,81,122,123,124,125,126,127,128,129] | [83] | [71,72,73,74,75,76] | ||
| Outcome | |||||
| Sleep | [80,81,122,123,124,125,126,127,128,129] | [83] | [71,72,73,74,75,76] | ||
| Diet including FV | [82,84,90,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121] | [78,79,82] | [67,68,69] | [67,70] | |
| Populations | |||||
| UK population | [82,84,90,109,117] | [82,83] | [75] | ||
| US population | [80,97,99,101,102,103,104,105,107,108,111,114,123,125,127] | [67,69] | [67,70] | [73] | |
| Other populations | [81,89,98,100,106,110,112,113,115,116,118,119,120,121,122,124,126,128,129] | [78,79] | [68] | [71,72,74,76] | |
| Sleep assessment | |||||
| Subjective | [80,81,82,84,89,90,97,98,99,100,101,102,103,105,106,107,108,109,111,112,113,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129] | [78,79,82,83] | [73] | ||
| Objective | [104,114] | [67,68,69] | [67,70] | [71,72,74,75,76] | |
| Sleep measurements | |||||
| Sleep duration | [80,81,82,84,89,90,97,99,100,101,102,105,106,107,108,109,110,111,112,113,114,115,116,117,118,120,121,123,125,126,127,128,129] | [78,82,83] | [67,68,69] | [67,70] | [71,72,73,74,75,76] |
| Sleep quality | [81,98,100,103,108,113,114,119,120,121,122,123,124,126] | [79] | [71,72,73,75,76] | ||
| Sleep timing | [104] | ||||
| Associations between sleep and FV | |||||
| Significant association | [80,81,82,84,89,97,99,100,101,102,103,104,105,106,109,111,114,115,116,117,118,120,122,123,124,126,128,129] | [78,79,82,83] | [67] | [71,72,73,74,75,76] | |
| No association | [90,98,107,108,110,112,113,119,121,125,127] | [68,69] | [67,70] | ||
| No control group | [70] | [71,72,74] | |||
| Primary objective of study was to assess associations between sleep and FV | [80,81,82,84] | [82,83] | [71,72,73,74,75,76] | ||
| Assessed non-linear associations between sleep and FV | [82,84] | [82] | |||
Legend: FV (fruits and vegetables), UK (United Kingdom), US (United States).
Table 2.
Adult human studies assessing the relationship between sleep measures and fruit and vegetable consumption.
| Author, Year (Ref) | Country | Population | Sample n | Exposure | Outcome | Adjusted Variables | Findings Reported on Sleep and FV | Comments |
|---|---|---|---|---|---|---|---|---|
| Cross-sectional studies | ||||||||
| Patel et al., 2006 [97] | United States | Nurse’s Health study | 68,183 | Subjective report of sleep duration. Sleep duration categorized to ≤5 h, 6 h, 7 h, 8 h, and ≥9 h | FFQ | No adjustment | FV consumption differed between sleep duration categories in baseline characteristics | Exposure and outcome not clearly stated. The significant difference in FV consumption between sleep duration groups could be due to the numerous categories of sleep duration |
| Adams and Colner 2008 [101] | United States | College students aged 18–25 years | 40,209 | Subjective report of sleep duration | FV consumption (servings/d) | Not clear | Sleep duration was a significant predictor for FV intakes, increased FV intake was positively associated with sleep duration | Sleep duration was combined in a physical health model based on health issues identified by the Centers for Disease Control and Prevention |
| Stamatakis and Brownson 2008 [102] | United States | Participants aged 20–92 from rural communities in Missouri, Tennessee, and Arkansas | 1203 | Subjective report of sleep duration. Sleep duration categorized to <7 h, 7–9 h, and ≥ 9 h | Self-report of FV consumption (servings/d) over the past month | Age, sex, ethnicity, education, marital status, and household income | Short sleep duration was associated with low FV consumption | |
| Buxton et al., 2009 [103] | United States | Motor freight workers | 542 | Sleep adequacy assessed by “How often during the past 4 weeks did you get enough sleep to feel rested upon waking up?” | 6 items of FV (servings/d) | Clustering of workers in trucking terminals through inclusion of terminal as a random effect | Adequate sleep was associated with more servings of FV | Several confounders were not adjusted for in the model |
| Baron et al., 2011 [104] | United states | Adults recruited from the community | 52 adults aged 18–71 years | Sleep timing assessed using logs and wrist actigraphy for 7 d | Food log in which participants recorded all food and drinks consumed for a 7 d period | Age and sleep duration | Sleep timing was independently associated with FV consumption. Later sleep timing was associated with fewer servings of FV | Exclusion criteria did not include shift workers, no participants reported shift work but this could cause report bias. Morning type diurnal preference participants were excluded providing no comparison with evening type participants |
| Kim et al., 2011 [105] | United States | Women aged 35–74 years | 27,983 | Subjective report of sleep duration | Eating pattern was self-reported and conventional eating and snack dominance scores were calculated, HEI calculated from FFQ | Age, race, income, education, employment, marital status, children, BMI, menopause status, smoking, alcohol, physical activity, health status, and stress | FV consumption (servings/d) were different among the four quartiles of conventional eating score. Short and long sleepers showed preponderance of snacks over meals related to lower intakes of FV | May have over adjusted and did not adjust for total energy intake |
| Haghighatdoost et al., 2012 [106] | Iran | Female university students aged 18–28 years | 410 | Subjective report of sleep duration. Sleep duration were categorized based on the tertiles of sleep duration: <6 h, 6–8 h, and >8 h | 168 items of FFQ. Diet diversity and HEI were calculated | No adjusted variables because the study was comparing dietary intake between tertiles of sleep duration | Consumption of fruits was significantly lower in the lowest tertile (<6 h) compared to the highest tertile (>8 h). Diversity scores of FV were significantly lower among participants in the lowest tertile | |
| Hoefelmann et al., 2012 [122] | Brazil | Workers part of a national survey | 47,477 | Self-report of FV (servings/week) | Subjective report of sleep quality | Socio-demographic indicators negative perception of health, wellbeing, stress, and self-reported morbidities | Inadequate FV consumption was associated with poor sleep quality | |
| Mosca and Aggarwal, 2012 [107] | United States | Men older than 40 years and women older than 50 years | 371 | Subjective report of sleep duration and snoring (yes, no).Sleep duration categorized to (<6 h/d) and (≥6 h/d) | <5 or ≥5 servings/d of FV | Age, sex, ethnicity, and marital status | No difference was shown between sleep duration categories and FV consumption. Snoring was associated with consuming less than 5 servings/day of FV | Assessment method of FV was not mentioned, may be self-report using a standardized questionnaire |
| Tu et al., 2012 [89] | China | Chinese women aged 40–70 years from the Shanghai Women’s Health Study | 68,832 | Subjective report of sleep duration. Sleep duration categorized; ≤4 h, 5 h,6 h, 8 h, 9 h, and ≥10 h | FFQ | Age, education level, occupational status, history of night-shift work, annual income, menopausal status, marital status, and number of live births | Fruit intake was inversely associated with short sleep duration. FV consumption was not associated with long sleep | Exposure and outcome not clearly stated |
| Beydoun et al., 2014 [123] | United States | Adults aged 20–85 from the NHANES | 2459 | Two 24-h dietary recalls. FV consumption (cup equivalent/d) | Subjective report of sleep | No adjustment | Very short, short and long sleepers consumed less FV compared to normal | |
| Katagiri et al., 2014 [124] | Japan | Middle-aged female workers aged 34–65 years | 3129 | 151-item self-administered diet history questionnaire | PSQI | Physical activity, CES-D score, employment, smoking, and BMI | High intake of vegetables were associated with good sleep quality | Analyses was not adjusted for several potential confounders e.g., age, total energy intake, SES, and ethnicity |
| Mota et al., 2014 [98] | Brazil | Resident physicians | 72 | Sleepiness assessed using the ESS. Sleep quality assessed using PSQI | Food diary for 3 non-consecutive days. FV consumption calculated using AHEI | Age and BMI | FV consumption were not correlated with ESS and PSQI | Exposure/outcome not clearly stated. Pearson correlation was used, does not provide predictions [130]. Analyses were not adjusted for several potential confounders |
| Chang et al., 2015 [108] | United States | Overweight and obese pregnant women | 213 | Sleep was assessed by PSQI | 7 items of FV assessed by the Rapid Food Screener | Not stated may be due to the use of Pearson correlation and path analyses (to investigate the mediating roles) | Sleep duration and sleep quality were not associated with FV intake in three trimesters. SOL was related to FV in the first and third trimester | |
| Grandner et al., 2015 [125] | United States | Nationally representative adults | 323,047 | Daily servings of FV from the BRFSS | Self-report of perceived insufficient sleep | Not clear | Consuming <1 or 1–3 servings of FV was not associated with insufficient sleep | Adjusted variables were not clearly reported |
| Kurotani et al., 2015 [126] | Japan | Workers aged 18–70 years | 2025 | 52-item diet history questionnaire. Healthy DPs included vegetables, mushrooms, potatoes, seaweeds, soy products, and eggs | Subjective report of seep duration, difficulty initiating and maintaining sleep, and sleep quality | Age, sex, site, shift work, employment, marital status, BMI, smoking, alcohol, physical activity, diabetic treatment, energy intake, skipping meals, habitual snacking at night | An inverse association was found between the healthy DPs and difficulties falling asleep at least once a week and persisted after excluding participants with severe depressive symptoms | May have over adjusted |
| Mossavar-Rahmani et al., 2015 [99] | United states | Hispanic/Latino participants aged 18–74 years | 11,888 | Subjective report on sleeping and waking times. Sleep duration categorized: short ≥3 h and <6 h, intermediate >6 h and ≤9 h, long >9 h and ≤ 14 h | Two 24-h dietary recalls. AHEI-2010 scores for diet quality | Age, sex, Hispanic/Latino background, income, employment status, education, depressive symptomology, and years lived in the US | Short sleepers had a lower quality diet compared to intermediate sleepers with significantly lower intakes of vegetables. Long sleepers had lower intakes of FV compared to intermediate sleepers | Exposure and outcome not clearly stated |
| Patterson et al., 2016 [109] | United Kingdom | Adults aged 40–69 from the UK Biobank | 439,933 | Subjective report of sleep duration categorized; very short ≤4 h, short 5–6 h, adequate 7–8 h, and long ≥9 h | Self-report of FV consumption for the previous year | Age, sex, ethnicity, attended college, and employment | Longer sleep duration was negatively associated with daily fruit intake, but positively associated with vegetable intake | FV consumption for the previous year may cause over/under reporting |
| Quick et al., 2016 [127] | United States | College students aged 18–24 years | 1252 | FV consumption over the past month (cups/day) | PSQI. Sleep duration categorized; <7 h/night, 7–8 h/night and ≥8 h/night | Sex, ethnicity, work time pressures, negative affect, and sleep disturbances | No difference was found in FV consumption between sleep duration groups | |
| Silva et al., 2016 [110] | Brazil | Students aged 18–39 | 204 | Perceived sleep debt calculated (preferred weekday sleep duration-self reported weekday sleep duration) | FFQ | Age, BMI, and sex | FV consumption were not associated with perceived sleep debt | |
| Xiao et al., 2016 [111] | United States | Women within 5 years of childbirth aged 20–44 years | 896 | Subjective report of sleep duration. Sleep duration was categorized to ≤6 h, 7–8 h, and long ≥9 h | Diet was assessed by two 24-h dietary recalls. Diet quality was measured by HEI-2010 | Age, ethnicity, education, marital status, poverty income ratio, weight status, years after recent childbirth, smoking, physical activity, depressive symptoms, history of breastfeeding, and diagnoses of chronic diseases | Short sleep duration was not associated with FV consumption. Long sleep duration was associated with lower consumption of total fruit and whole fruit | May have over adjusted |
| Doo and Kim 2017 [112] | Korea | Pre and post-menopausal women | 17,841 | Subjective report of sleep duration. Sleep duration categorized to short (≤6.9 h/d) and adequate (≥7 h/d) | One 24-h recall | Age, education, household income, diseases, smoking, alcohol, and physical activity | No differences were observed in FV consumption by sleep duration | |
| * Duke et al., 2017 [80] | United States | Pregnant | 2942 | FV consumption, 4 questions from the BRFSS | Subjective report of sleep duration | Age, ethnicity, education, exercise, marital status, income, employment | Orange and green vegetables were inversely associated with sleep duration. Total FV were not associated with sleep duration. Odds of meeting or exceeding sleep recommendation increased with each unit increase in total FV (OR = 1.05 95% CI 1.003, 1.092) | Recall of FV intakes was for the past month which is based on memory and may cause over or underreporting |
| Kleiser et al., 2017 [113] | Bavaria, Germany | Bavarian adults aged ≥18 | 814 | PSQI | Three 24-h dietary recalls (2 weekdays, 1 weekend day) | Age, sex BMI, education, smoking physical activity, TV/PC use, and season | Sleep duration was not associated with FV consumption | |
| Mossavar-Rahmani et al., 2017[114] | United States | Hispanic/Latino participants aged 18–74 years from 4 US cities | 2140 | Sleep measured by actigraphy for 7 consecutive days. Sleep duration categorized; short (<6 h), intermediate (= 6 and <8 h) and long (≥ 8 h). Sleep fragmentation index calculated | Two 24-h dietary recalls. AHEI-2010 scores for diet quality | Age, sex, site, ethnic background, employment depression, and log daily energy intake | Whole fruit intake differed between sleep duration groups with lowest intakes in short sleepers. Sleep efficiency was positively associated with whole fruit intake and sleep fragmentation index was negatively associated with whole fruit intake | |
| Pérez-Rodrigo et al., 2017 [128] | Spain | Adults aged 18–64 | 1617 | 24-h diet recall, a 3-day food record aided by a tablet device. Four DPs identified; traditional (high in FV), Mediterranean (high in FV), snack and dairy | Subjective report of sleep duration. Three lifestyle patterns identified; “Mixed diet-physically active-low sedentary lifestyle pattern”, a “Not poor diet-low physical activity-low sedentary lifestyle pattern”, and a “Poor diet-low physical activity-sedentary lifestyle pattern” | Age | Sleep duration differed between the 3 lifestyle patterns in men and women. In both men and women, mean sleep duration was the highest in the “Not poor diet-low physical activity-low sedentary lifestyle pattern” | Two DPs were identified with high intakes of FV. Analyses was not adjusted for several potential confounders |
| Potter et al., 2017 [90] | United Kingdom | Adults aged 19–65 years from the NDNS | 1615 | Subjective report of sleep duration | 4-day food diary | Age, sex, smoking, ethnicity, and SES | Sleep duration was not associated with FV consumption | Did not adjust for total energy intake. Non-linear associations not explored between sleep and diet |
| Timmermans et al., 2017 [115] | Europe | Adults | 5900 | Subjective report of sleep duration | FFQ | Age, sex, education and self-rated health | Longer sleep duration was associated with lower fruit consumption | |
| Van Lee et al., 2017 [100] | Singapore | Pregnant women | 497 | PSQI | One 24-h recall at 26–28 weeks of gestation. HEI-SGP to measure diet quality. DPs included FV and white rice pattern | Alcohol, physical activity, household income, education, ethnicity, energy intake, age, and gravidity | Good sleep quality was associated with better diet quality and greater adherence to the FV and white rice pattern compared to poor sleep quality | Exposure and outcome not clearly stated |
| Wang et al., 2017 [129] | China | Older adults aged 60–79 years | 4115 | Inadequate fruit intake was defined as adults who ate fruit less than three times per week | Subjective report of sleep duration. Sleep duration was categorized to <7 h/d, 7–8 h/d and >8 h/d | All independent variables of socio-demographic and lifestyle variables were included in the same model thus adjusting for each other | Inadequate intake of fruits was positively associated with short and long sleep durations | The definition of inadequate fruit was not based on a reference |
| Gebski et al., 2018 [116] | Polish adults | Adults aged 21–65 years | 1007 adults | Subjective report of sleep duration | Frequency of consumption of selected food groups including FV. Five DPs were derived including FV pattern and FV juices | Age, education and place of residence | In weekdays, short sleep duration was associated with lower odds of FV DP in men. In weekends, short sleep duration was associated with higher odds of FV DP in women | Analyses was not adjusted for several potential confounders |
| * Lee et al., 2018 [81] | China | Older adults aged ≥65 years | 5911 | Subjective report of the frequency of FV consumption | Subjective report of sleep duration and quality. Sleep duration categorized; short (<7 h), recommended (7–8 h) and long (>8 h) | Age, sex, marital status, education, alcohol, smoking, exercise, household income, community, and province | Frequent FV consumption were associated with better sleep quality. Less frequent FV consumption was associated with short sleep and long sleep compared to the reference | Did not test for non-linear associations. Dietary recall may cause over or under reporting |
| * Noorwali et al., 2018 [84] | United Kingdom | Adults aged 19–65 years from the National Diet and Nutrition Survey | 1612 | Subjective report of sleep duration categorized to short (<7 h/d), reference (7–8 h/d) long (>8 h/d) | 4-day food diaries. Foods containing FV were disaggregated into their components to help assess total FV. | Age, sex, SES, smoking, ethnicity, and total energy intake | Sleep duration was non-linearly associated with FV consumption with short and long sleepers having lower intakes compared to the reference group | Assessed non-linear associations and used FV biomarkers |
| Patterson et al., 2018 [117] | United Kingdom | Adults aged 40–69 enrolled in the UK Biobank | 438,933 | Subjective report of sleep duration. Sleep duration was categorized to ≤6 h/d, 7–8 h/d and ≥9 h/d | FFQ. Variables combined and a binary variable created to (<5 servings/d, ≥5 servings/d) | Age, sex, ethnicity, employment, shift work, education, urban vs. rural residence | Long sleepers with had a 62% higher odds of eating <5 servings/d of FV compared with adequate sleepers | Sleep duration and chronotype were used together as independent variables suggesting interactive effects |
| Peltzer et al., 2018 [118] | South Africa | Participants aged ≥ 40 years | 4725 | Subjective report of sleep duration. Sleep duration categorized to <7 h/d, 7–8 h/d and ≥9 h/d | Self-report of FV consumption. Inadequate FV consumption: having <5 servings/day | Not stated | Consumption of <5 servings/day of FV were associated with higher odds of short sleep duration | Authors state adjusted multinomial logistic regression but did not state the confounders |
| Tan et al., 2018 [119] | Germany and Netherlands | Participants aged 20–85 years | 790 | Subjective report of restful sleep and sleep quality | Self-report of FV consumption. “During the last weeks, did you eat five portions of FV per day?” The answers were based on a five-point Likert scale | Age, sex, BMI, country of origin, employment status, marital status, and education | Restful sleep was not associated with FV consumption however, in combination, restful sleep, physical activity, and FV intake were associated with increased sleep quality | |
| Vézina-Im et al., 2018 [120] | Canada | Women of child bearing age 18–44 years | 9749 | Subjective report of sleep duration and quality. Sleep duration was categorized to <7 h/night and ≥ 7 h/night | 6-item questionnaire to assess FV consumption | No adjustment | FV intake was associated with higher odds of having adequate sleep duration and quality sleep | |
| Vézina-Im et al., 2018 [121] | Canada | Women of child bearing age 18–44 years | 9749 | Subjective report of sleep duration and quality. Sleep duration was categorized to <7 h/night and ≥ 7 h/night | 6-item questionnaire to assess FV consumption | Age, ethnicity, education, household income, marital status, employment, parity, region, season, mood disorder, FV intake, physical activity, smoking, and alcohol | FV consumption was included as an adjustment between sleep duration and quality with BMI. FV consumption was not associated in the relationship between sleep duration and quality with BMI ≥25 | This study assessed the association between sleep duration and quality with BMI adjusting for several covariates including FV intakes |
| Prospective studies | ||||||||
| Imaki et al., 2002 [78] | Japan (6 year follow-up) | Male employees aged 20–59 years | 2000 | Multiple choice questionnaire: hours of sleep, (1) ≤6 h, (2) 6.1–8.9 h, (3) ≥9 h | 7 items of dietary habits including vegetable intakes in the diet (1) ample (2) none | No adjustment | The percentage of participants who slept 6 h or less consumed less vegetables compared to 6.1–8.9 h during the 6-year period of study | This study did not use any analyses for prediction such as regression analyses and only compared the intakes using percentages |
| Huang et al., 2013 [79] | Taiwan (10 year follow-up) | Elderly aged ≥65 years | 1865 | Subjective report of sleep quality categorized; poor, fair or good | 24-h dietary recall and FFQ. Dietary diversity score derived from 6 items including FV | Age, education, BMI, physical activity, and use of sleeping pills | Female poor sleepers consumed fewer vegetables compared to fair or good sleepers. Dietary diversity score and sleep quality interacted and modulated mortality with sex differences | |
| * Noorwali et al., 2018 [82] | United Kingdom | Middle aged women from the UK Women’s Cohort Study | Cross-sectional = 12,159 Prospective = 463 | Subjective report of sleep duration categorized to short (≤6 h/d) recommended (7–9 h/d) long (≥9 h/d) | 4-day food diaries | Age, SES, smoking, ethnicity, and total energy intake | Sleep duration was non-linearly associated with FV consumption in cross-sectional and prospective analyses with those sleeping the recommended 7–9 h having the highest intakes | First prospective study. Assessed non-linear associations and used FV biomarkers |
| * Noorwali et al., 2018 [83] | United Kingdom | Middle aged women from the UK Women’s Cohort Study | 13,958 | FV items from FFQ and their polyphenol content matched from Phenol Explorer database | Subjective report of sleep duration | Age, SES, smoking, ethnicity and total energy intake | FV consumption and their polyphenol content were inversely associated with sleep duration | First prospective study to examine the association between polyphenols from FV and sleep duration |
| Sleep restriction and extension studies | ||||||||
| Spiegel et al., 2004 [67] | United States | Healthy young men | 12 | Men were assigned to either 4 h of sleep for 2 consecutive nights or 10 h of sleep for 2 consecutive nights | Participants were provided with standard hospital meals and completed a visual analogue scale for hunger and appetite for various food categories including FV | No adjustment | Appetite rating for FV increased following sleep restriction by 17% (p = 0.07) for fruit and fruit juices and 21% for vegetables (p = 0.02) compared to sleep extension | Short intervention period and small sample size |
| Sleep restriction and extension studies | ||||||||
| Heath et al., 2012 [68] | Australia | Healthy males | 24 | Participants lived 12 consecutive days in a sleep laboratory. 14 participants were sleep restricted to 4 h (severe), 10 participants were restricted to 6 h of sleep (moderate) | Participants were served 3 meals and 5–6 snacks daily. Snacks included 3 categories; sweet, savoury and healthy (1 piece of fresh fruit and 1 packet of 40 g of dried fruit and nuts) | No adjustment | No effects of sleep restriction were found on healthy snack consumption | Short intervention period and small sample size |
| Spaeth et al., 2014 [69] | United States | Healthy adults aged 21–50 years | 44 | In laboratory sleep restriction to 4 h (04:00–08:00 a.m.) for 5 consecutive nights. Participants wore actigraph | Participants selected their meals and snacks by choosing from various menu options, selecting additional food and drink available in the laboratory suite | Age | Calories consumed from FV and salad did not differ between baselines and sleep restriction | |
| Sleep extension studies | ||||||||
| Tasali et al., 2014 [70] | United States | Overweight young adults reporting sleep <6.5 h/d | 10 | Habitual sleep was followed for 1 week and intervention was extending sleep to 8.5 h for 2 weeks by behavioral counselling on sleep hygiene | Desire for various foods including FV was assessed using visual analog scales | No adjusted variables | Extended sleep did not change the desire for FV | No control group. Short intervention period and small sample size |
| Fruit intervention studies | ||||||||
| * Garrido et al., 2009 [71] | Spain | Young, middle-aged, and elderly | 18 | Powdered freeze-dried nutraceutical product diluted in 125 mL water equivalent to 141 g Jerte Valley cherries, consumed twice a day for 3 consecutive days | Sleep was assessed by actigraphy. Participants wore it 3 days before the trial, during 3 days of trial, and 1 day afterwards. | No adjusted variables | After intervention, sleep duration increased compared to baseline. Immobility increased and nocturnal activity decreased in young and elderly compared to baseline | No control group. Short intervention period and small sample size |
| * Garrido et al., 2010 [72] | Spain | Middle-aged and elderly Caucasian | 12 | 200 g of 7 different cultivars of cherries twice a day for three days | Wrist actigraphy wore 3 days before the trial and during 3 days of the trial | No adjusted variables | Sleep duration and immobility increased after intervention, the number of awakenings, sleep latency, and nocturnal activity decreased | No control group. Short intervention period and small sample size |
| * Pigeon et al., 2010 [73] | United States | Healthy older adults aged ≥65 years with insomnia | 15 | Tart cherry juice blend or placebo consumed for 2 weeks twice a day in the morning between 8:00–10:00 a.m. and in the evening 1–2 h before bedtime | Sleep was assessed by an ISI and sleep diaries | No adjusted variables | Within groups, tart cherry juice improved ISI, SOL, sleep duration, sleep efficiency and wake after sleep onset. Between groups, tart cherry juice reduced the ISI score and wake after sleep onset with no difference in SOL, sleep duration, and sleep efficiency | Short intervention period and small sample size |
| * Lin et al., 2011 [74] | Taiwan | Participants self-reporting sleep disturbance aged 20–55 years | 24 | Two kiwifruits consumed 1 h before bedtime for 4 weeks | CPSQI, sleep diary, and actigraph | No adjusted variables | After intervention, Actigraph and sleep diary showed that sleep duration and efficiency increased compared to baseline. Sleep diary showed a decrease in CPSQI score, waking time after sleep onset, and SOL | No control group. Participants included only 2 males and 22 females. Kiwifruit consumption on sleep may differ by sex |
| * Howatson et al., 2012 [75] | United Kingdom | Healthy adults | 20 | Participants consumed a tart cherry juice concentrate or placebo for 7 d | Sleep quality recorded by actigraphy and online subjective sleep diaries were collected | No adjusted variables | Sleep diary showed that cherry juice intake decreased napping time. Actigraphy showed that cherry juice increased time in bed, sleep duration, and sleep efficiency | Short intervention period and small sample size |
| * Garrido et al., 2013 [76] | Spain | Young middle-aged and elderly | 30 | Jerte Valley cherry based product (JVCP) consumed twice a day as lunch and dinner desserts for 5 d or a placebo | Sleep was assessed by actigraphy. Participants wore it 5 d before the trial, during 5 d of trial and 5 d afterwards. | No adjusted variables | JVCP increased sleep duration and immobility in young, middle-aged and elderly compared to baseline and placebo. JVCP increased sleep efficiency in elderly compared to baseline. SOL decreased in middle-aged and elderly | Short intervention period and small sample size |
Legend: AHEI (Adapted Healthy Eating Index); AHEI-2010 (Alternative Healthy Eating Index); ANOVA (analyses of variance); BMI (body mass index); BRFSS (Behavioral Risk Factor surveillance System); CES-D (Centre for Epidemiological Studies Depression scale); CPSQI (Chinese version of the Pittsburgh Sleep Quality Index); CVD (cardio vascular disease); d (day); DPs (dietary patterns); ESS (Epworth Sleepiness Scale); FFQ (food frequency questionnaire); FV (fruit and vegetable);g (gram); h (hour); HEI (Healthy Eating Index); HEI-SGP (Healthy Eating Index for Pregnant women in Singapore); ISI (Insomnia Severity Index); JVCP (Jerte Valley cherry based product); n (number); NDNS (National Diet and Nutrition Survey); NHANES (National Health and Nutrition Examination Surveys); OR (odds ratio); PSQI (Pittsburgh Sleep Quality Inventory); Ref (reference); SES (socio-economic status); SOL (sleep onset latency). * BOLD row, Key paper with main objective assessing the association between sleep measures and fruit and vegetable consumption.
2.1. Sleep Affects FV Consumption: Experimental Studies
Sleep restriction and extension (increasing sleep duration) studies and their effects on FV consumption are summarized in Table 2. Sleep restriction in young healthy men increased appetite for FV by 17% for fruit and fruit juices and 21% for vegetables compared to sleep extension [67]. In contrast, sleep restriction had no effect on healthy snack intake composed of 1 piece of fresh fruit and 1 packet of 40 g of dried fruit and nuts in healthy Australian men [68]. Similarly, calories consumed from FV and salad did not differ between sleep restriction and baseline. However, there was an interaction between race and sleep for FV intakes and salad with African Americans consuming fewer calories from FV and salad during baseline but it did not differ from whites during sleep restriction [69]. Tasali and colleagues studied the effects of sleep extension using a home based approach in 10 overweight adults on the desire for various foods including FV, however, the study did not have a control group. Sleep extension did not change the desire for FV [70]. There is a need for more experimental studies to clarify the effects of sleep disruption on FV consumption.
2.2. Fruit Affects Sleep: Experimental Studies
Few studies assessed the effects of tart cherry juice and products [71,72,73,75] and kiwifruit [74] on sleep measures. However some studies had no control group to compare the effects of cherry [71,72] and kiwifruit [74] on sleep measures, whereas other studies included a control group [73,75,76]. The previous studies included a small sample size and a short period of intervention and did not meet the scoring of methodological quality to be included in a systematic review of dietary interventions targeting sleep behavior [77]. There is a need for more interventional studies to identify the effects of FV on sleep measures.
2.3. Observational Studies
Table 1 shows that all studies included were cross-sectional apart from two prospective studies [78,79] that had different objectives, including assessment of the association between sleep duration and lifestyle factors [78] and sleep quality and survival in elderly [79]. Most of the studies were conducted in US populations and only two observational studies had their primary objective to assess the association between sleep duration and FV consumption in pregnant women [80] and Chinese older adults (≥65 years) [81]. We conducted the other two prospective studies between sleep duration and FV consumption in UK adults [82,83]. Testing for non-linear associations has been recommended between sleep measures and dietary intakes [29], however—apart from our studies [82,84]—no study assessed non-linear associations (Table 1). We showed that sleep duration (exposure) was non-linearly associated with FV consumption (outcome) with short and long sleepers consuming less FV compared to those sleeping 7–8 h/day in a representative sample of UK adults [84]. This study strengthens the notion that people sleeping the recommended hours have a healthier lifestyle compared to short and long sleepers [85,86,87,88,89]. Potter et al. used the same dataset and found no association between sleep duration and FV consumption [90], this may be because non-linear associations were not explored between sleep duration and FV consumption. Our study [84] reinforces the need for non-linear exploration between sleep and diet in future studies.
Causal relationships cannot be inferred from cross-sectional studies and prospective studies help to clarify associations. Among UK adults, no study has assessed the associations between sleep duration and FV consumption, as well as the non-linear associations. Therefore, we addressed this question by exploring the non-linear prospective associations between sleep duration and FV consumption using a large cohort (~13,000 women) namely the UK Women’s Cohort study (UKWCS) [82]. Interestingly, cross-sectional and prospective analyses were consistent with the National Diet and Nutrition Survey (NDNS) analyses [84]. Although sleep duration was categorized differently than the NDNS analyses due to different sample sizes, we used a continuous variable of sleep duration to assess the non-linear associations in both studies and modelled this association using restricted cubic splines. Additionally, both studies assessed FV consumption using a four-day food diary and self-report of sleep duration providing more consistency. Interestingly, our prospective analyses [82] confirmed the cross-sectional associations [84] with those sleeping the recommended hours (~7–9 h/day) having the highest intakes of FV. These findings add a novel association to the literature and provide new insights to consider in experimental studies addressing the relationship between sleep and diet.
2.4. Studies Supporting the Inverse U-shaped Association between Sleep Duration and FV Consumption
The inverse U-shaped association we found between sleep duration and FV consumption [82,84] may be supported by the U-shaped association found in other studies between sleep disruption and unfavourable behaviors and characteristics. In a representative sample of US adults, sleep complaints were associated with sleep duration in a U-shaped relationship. Short sleepers and long sleepers reported sleep problems and those sleeping 7–8 h reported fewer sleep problems [87]. Other characteristics including smoking, alcohol drinking, and physical inactivity were associated with short and long sleep durations [85,86]. This was also shown in Swedish women with short and long sleepers being physically inactive, smokers, physiologically distressed, and having increased waist circumference compared to normal sleepers [88]. Both short and long sleep duration were negatively associated with education level, family income, leisure-time and physical activity in Chinese women [89] and a large Chinese adult population [91]. In Japanese adults, the U-shaped association between sleep duration and health were explained by the U-shaped association between sleep duration and disrupted sleep with psychosocial stress from work and family life. Short sleep duration was associated with long work hours and high work–family conflict, whereas long sleep was associated with daily alcohol drinking. Participants sleeping ~8 h had the lowest prevalence of poor sleep and unfavorable behaviors and characteristics [92]. Interestingly, the U-shaped association was found between sleep duration and serum lipid profiles in Chinese women [93], between sleep duration and diabetic retinopathy [94], and sleep duration and the risk of falls [95].
Overall, the inverse U-shaped associations observed in the previous studies may explain our findings of the inverse U-shaped association between sleep duration and FV consumption. A nutritious diet including high intakes of FV are considered one of the main keys to a healthy lifestyle [96]. Therefore, the previous studies showed that sleeping the recommended hours is associated with a healthier lifestyle, supporting our findings of higher intakes of FV in participants sleeping ~7–9 h/day. The association between sleep disruption and FV consumption may be part of the complex puzzle of the U-shaped association between sleep measures, morbidity, and mortality. Future research exploring whether FV consumption acts as a mediator between sleep disruption and morbidity is necessary to clarify the underlying mechanisms.
3. Chronotype and Fruit and Vegetable Consumption
Chronotype has been defined as “An individual’s phase angle of entrainment (for example, the timing of core body temperature nadir relative to dawn)” [7] which is the preference in timing of activity and sleep referred to as morning or evening type [131]. Chronotype has been assessed by various methods such as the Horne and Östberg’s Morningness–Eveningness Questionnaire (MEQ) [132]. However, the main limitation of MEQ is the unavailability of sleep timing estimates. This has been developed to the Munich Chronotype Questionnaire (MCTQ) [131] that uses mid-sleep time on non-work days as an estimate of chronotype after correcting for sleep debt on work days.
Chronotype determinants include genetic (non-modifiable) and environmental factors (modifiable) [133]. Non-modifiable determinants include rare cases of chronotype disorders such as advanced sleep-phase syndrome [134,135]. Other non-modifiable determinants include race [136], sex [137], and age [138]. Environmental factors that influence chronotype include light exposure, social interactions, urban/rural areas, and variations in the LD cycle across different latitudes and time zones [133].
Later chronotype (evening type) has been associated with less healthy behaviors such as smoking [109], physical inactivity with sedentary behavior [117], and consuming more alcohol and caffeine (from coffee and cola) compared to early chronotypes [139]. Later chronotype was associated with higher risks of some diseases such as CVD [140] type 2 diabetes [141], metabolic disorders [142], bipolar disorder [143], and obesity [117]. In a recent study conducted using the UK Biobank, a large prospective population based cohort study including 433,268 adults, later chronotype was associated with higher odds of psychological disorders, diabetes, neurological disorders, gastrointestinal disorders, and respiratory disorders. Additionally, later chronotype was associated with an increased risk of all-cause mortality compared to earlier chronotype [144]. These findings are of concern to public health and thus studies assessing the associations between chronotype and other lifestyle behaviors such as FV consumption are necessary.
Inadequate intakes of FV were associated with later chronotype in a cross-sectional study in UK adolescents [59] and US adolescents [145]. In other cross-sectional studies, later chronotype assessed by MEQ and MCTQ was associated with lower intakes of vegetables in Japanese women [146,147]. Similarly, later chronotype was associated with lower intakes of green, yellow, white vegetables, and fruits in Japanese nurses [148]. A representative sample of Finnish adults showed that later chronotypes assessed by a shortened version of MEQ consumed less fruit [149].
Patterson et al. found that early chronotypes consumed more servings of FV compared to later chronotypes in UK adults from the UK Biobank project [109]. Chronotype was self-reported by asking participants “Do you consider yourself to be (1) definitely a morning person, (2) more a morning than an evening person, (3) more an evening than a morning person, (4) definitely an evening person”. This was consistent with another recent study conducted by Patterson et al. using the UK Biobank data with a difference of including sleep duration and chronotype as independent variables suggesting an interactive effects between sleep homeostatic and circadian influence. Later chronotype and longer sleep was associated with higher odds of consuming <5 servings/days of FV compared with adequate sleep and earlier chronotype. However, earlier chronotypes and adequate sleep was associated with lower odds for all cardiovascular risk behaviors including tobacco use, physical inactivity, highly sedentary behavior, and overweight/obesity except FV consumption <5 servings/day [117].
In contrast, no association was found between chronotype and vegetables and salad in German adolescents [150]. Earlier chronotype assessed by MEQ was associated with lower intakes of vegetables and no association with fruit intake [151]. No association was found between chronotype and FV consumption among Brazilian undergraduate students [110].
The previous studies show that later chronotypes tend to consume unhealthy diets with low intakes of FV. However, the results are contradictory and a main limitation of the previous studies is the lack of usage of objective methods to measure chronotype such as actimetry and validated dietary assessment methods. There is a necessity to assess the associations between chronotype and FV consumption using validated objective methods.
4. Mechanisms for the Relationship between Sleep and Fruit and Vegetable Consumption
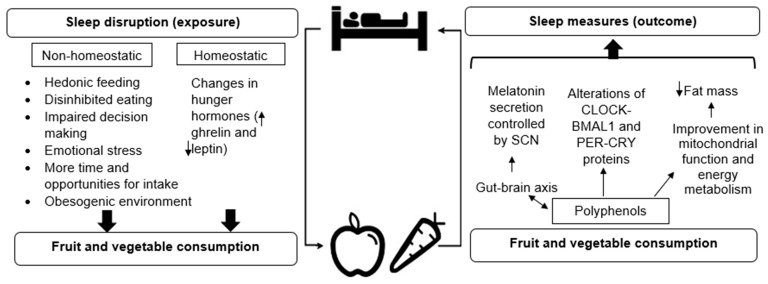
The potential mechanisms underlying the reciprocal relationship between sleep and FV consumption are shown in Figure 1. Several mechanisms have been proposed of the reciprocal relationship between sleep disruption and dietary intake that may subsequently lead to obesity and metabolic diseases [35,152,153,154,155,156,157].
Figure 1.
Potential reciprocal mechanisms between sleep duration and fruit and vegetable consumption. Sleep disruption may influence dietary intake through non-homeostatic and homeostatic mechanisms. On the other hand, FV consumption may influence sleep through their polyphenol content through several potential pathways. With further research, other potential mechanisms may be identified. Legend: SCN (suprachiasmatic nuclei); CLOCK (circadian locomotor output cycles kaput); BMAL1 (brain and muscle aryl hydrocarbon receptor nuclear translocator-like 1); PER (period); CRY (cryptochrome).
4.1. Homeostatic Mechanisms
Sleep disruption may influence dietary intake through non-homeostatic and homeostatic mechanisms (Figure 1). Homeostatic mechanisms include energy homeostasis mediated by satiety hormonal changes ghrelin and leptin. Leptin sends satiety signals to the appetite control centers in the brain and ghrelin sends signals from the stomach to the brain stimulating an increase in appetite [158].
A number of studies have observed associations between sleep disruption on leptin and ghrelin levels. In a laboratory study on 10 healthy men, Mullington et al. observed a reduction in diurnal amplitude of leptin during the days of sleep deprivation [159]. Interestingly, amplitudes of leptin returned to normal in the period of sleep recovery. Similarly, leptin levels decreased when sleep was restricted to 4 h in 11 adults [160]. Furthermore, sleep restriction reduced leptin by 18% and increased ghrelin by 28% in 12 healthy men [67]. Other laboratory studies indicated an increase of ghrelin after sleep restriction [161,162,163,164]. However, the effects of sleep restriction on ghrelin and leptin are contradictory [164,165,166,167,168,169] with a suggestion of sex differences [170]. The variability in ghrelin and leptin responses to sleep restriction may be due to the small sample sizes, differences in timing of blood chemistry and analyses and variability in sleep restriction hours.
With respect to sleep and FV consumption mechanisms, laboratory studies showed that disrupted sleep changes appetite-related hormones ghrelin and leptin, which may increase the preference for energy-dense foods [33] probably leading to lower consumption of FV.
4.2. Non-Homeostatic Mechanisms
Non-homeostatic mechanisms have been supported with observational and experimental studies [171]. In a meta-analysis, sleep deprivation was one of the most prominent lifestyle determinants of increased food intake [172]. People eat more after sleep loss to compensate for the additional energetic cost of wakefulness [173]. Consistently, sleep deprivation increased food purchasing in men with preference to energy-dense, rewarding foods [174]. This preference for energy-dense foods may potentially lead to lower intakes of FV. Recent evidence suggests that similar to sleep restriction, long sleep duration may impair energy homeostasis through unhealthy dietary choices, leading to potentially lower intakes of FV [175].
Non-homeostatic mechanisms linking sleep disruption with FV consumption include hedonic feeding (Figure 1), which is the consumption of food to obtain pleasure in the absence of energy deficit [176]. To study the effects of sleep disruption on non-homeostatic reward-driven behavior, brain imaging studies were conducted supporting the non-homeostatic hypothesis [177]. After one night of sleep deprivation, brain activity changed in response to food stimuli and was associated with an increase in appetite [178]. Furthermore, sleep restriction to 4 h for 6 days increased the neuronal response to food stimuli and activated brain regions associated with reward [179].
Daytime sleepiness reduced the activation of ventromedial prefrontal cortex, a brain region involved in the ability to inhibit and control emotions and behavior, when participants were shown “high calorie food” compared to “low calorie food” images (included fresh salad and FV). Additionally, this reduction in prefrontal activation predicted over-eating in women [180]. Sleep restriction increased the neuronal response to “unhealthy” food images compared with “healthy” food images (that included FV) [181]. Consistently, following sleep restriction, appetite sensations and food reward increased compared to controls [182]. The previous brain imaging and experimental studies of sleep restriction provide some non-homeostatic mechanisms for sleep disruption, enhancing hedonic stimulus processing in the brain and altering brain connectivity leading to food reward, food craving, and affecting food decisions. The enhanced reward mechanism may promote energy-dense food consumption, leading to lower intakes of FV.
It has been shown that high disinhibited eating (tendency toward overeating in response to different stimuli; for example the presence of palatable food or emotional stress [183]) mediated the relationship between disrupted sleep and weight gain [184,185,186]. The mediating effect of disinhibition between disrupted sleep (short/long sleep durations, poor sleep quality) and weight status may be due to over-eating and less healthful food choices [187]. In a cross-sectional study of 187 women and their children, disinhibition scores (higher scores indicate higher disinhibition) _were negatively associated with FV consumption in both mothers and their children [188]. Consistently, in a prospective study of 2 year follow-up of men, disinhibition scores were negatively associated with fruit intake [189]. These studies provide evidence that sleep disruption may lower the intakes of FV through the mediating effects of disinhibited eating.
Furthermore, emotional eating and stress were shown to influence the association between sleep duration and dietary intake [190]. Disrupted sleep increases emotional reactivity [191] leading to an increase in dietary intake specifically energy-dense foods to improve the mood and stress of individuals with their pleasing effects through the opioidergic, dopaminergic, and serotonergic systems [192] resulting in potentially lower intakes of FV. Sleep disruption deficits impulse control [193] that plays a major role in inhibiting appetitive thoughts and behaviors [156], when impulse control is altered this results in impaired decision making leading to excess dietary intake, energy-dense foods for reward and potentially lower intakes of FV. Sleep disruption accompanied with an obesogenic environment—“ the sum of influences that the surroundings, opportunities, or conditions of life have on promoting obesity in individuals or populations” [194]—may enhance behaviors including irregular eating with fewer main meals, more frequent energy-dense snacking, and altered time of intake leading to potentially lower intakes of FV [29].
4.3. Mechanisms for Effects of Polyphenols on Sleep
4.3.1. Animal Studies
With the reciprocal relationship between sleep and dietary intake in mind, FV consumption may influence sleep measures through their polyphenol content through several potential pathways. Polyphenols are phytochemicals that are abundant in our diets and have a probable preventive role from CVD [195], ischemic heart disease [196], stroke [197], and cancer [198]. Polyphenol profiles are complex in foods and mostly contain multiple classes of polyphenols in a single plant. The main sources of polyphenols are FV, tea, coffee, red wine, cereals, grains, and soy beans however, bioavailability differ extremely between the various polyphenols. Polyphenols are classified and sub-classified based on the number of phenol rings that they contain and of the structural elements that bind these rings to one another. The main classes of polyphenols are flavonoids, phenolic acids, stilbenes, lignans, and other polyphenols [199].
The direct and indirect effects of flavonoids in the brain including cerebrovascular blood flow and synaptic plasticity that improve learning and memory have been previously reviewed [200] and the role of sleep on memory has been highlighted [201]; however, there is a lack of studies linking sleep with polyphenols. Some animal studies (Table 3) have investigated the effects of different types of polyphenols on clock genes, circadian rhythms, and sleep/wake cycle with few studies conducted in humans (see Section 4.3.2).
Table 3.
Summary of animal and in vitro studies [references] assessing the effects of polyphenols on sleep and their potential mechanisms.
| Potential Mechanism Assessed | Sleep Assessed | |||||
|---|---|---|---|---|---|---|
| Serotonin 1A Receptor | GABA Receptors | Circadian Rhythms | Clock Gene Expression | Sleep/Wake Cycles | Sleep Duration | |
| In vivo | [202] | [203,204,205,206,207] | [208,209,210] | [211,212,213,214,215] | [202,204,205,206,207,216,217,218,219] | [202,203,205,206,207,216,217,218,220] |
| In vitro | [221] | [222] | ||||
| Polyphenol | ||||||
| Flavonoids | [202] | [205] | [221] | [202,205] | [202,205,220] | |
| Resveratrol | [209,210] | [212,213] | [219] | |||
| Phenolic acids | [220] | |||||
| GSPEs | [211,214,215] | |||||
| Phlorotannins * | [203,204] | [204] | [203] | |||
| Triphlorethol A* | [216] | [216] | ||||
| Red cabbage extracts | [217] | [217] | ||||
| Kiwifruit extracts | [206] | [206] | [206] | |||
| Romaine lettuce | [218] | [218] | ||||
| Tea polyphenols | [207] | [222] | [207] | [207] | ||
| Cherry | [208] | |||||
Legend: GSPEs (Grape seed proanthocyanidins extracts), GABA (γ-aminobutyric acid), * Sea weed polyphenols.
The first potential mechanism of how polyphenols from FV consumption may affect sleep measures is through the gut–brain axis (Figure 1) via serotonin and γ-aminobutyric acid (GABA) receptors, consequently affecting nocturnal secretion of melatonin. Spinosin, a C-glycoside flavonoid of semen Ziziphi spinosae, a herb that has been used to treat insomnia and other diseases, reduced SOL and increased non-rapid eye movement sleep and sleep duration and increased rapid-eye movement sleep time via serotonin 1A receptor (5-hydroxytryptamine, 5-HT1A) in Male Sprague–Dawley rats [202].
Other studies found that different polyphenols modulated sleep via GABA receptors. Polyphenols such as phlorotannins [203,204] and triphlorethol A (seaweed polyphenols) [216], red cabbage extracts [217], and kiwifruit extracts [206] decreased SOL and increased sleep duration via GABA receptors in mice. Other polyphenols such as bioflavonoids extracts from Rhus parviflora referred as Tintidikah, a medicinal plant used in south Asia, were the most potent components in decreasing SOL and increasing sleep duration via GABA receptors [205]. Seeds of Ziziphus mauritiana, a hypnotic widely used in Asian countries, contained flavonoids and phenolic acids that increased sleep duration in mice administered with sodium pentobarbital [220]. Furthermore, the seed and leaf extracts derived from romaine lettuce potentiated the pentobarbital-induced sleeping behavior in mice [218]. In contrast, GABA in black tea did not decrease SOL induced by sodium barbital—a hypnotic—in mice, but SOL was decreased and sleep duration was increased with sodium pentobarbital, a hypnotic [207]. Collectively, the previous animal studies found that different polyphenols via serotonin and GABA receptors decreased SOL and increased sleep duration however, further human studies are required to confirm this.
Since the circadian system and their clock genes are intertwined with the sleep/wake cycle [7], the second potential mechanism of how polyphenols derived from FV consumption may influence sleep is through their effects on circadian rhythms, clock gene expression, and peripheral clocks (Figure 1). An animal study investigated the effects of resveratrol, a dietary polyphenol present in a variety of foods including FV, on circadian period and body temperature [209]. Compared to controls, resveratrol supplementation for 2 weeks in constant dark condition in primate grey mouse lemur shortened free-running period, reduced mean body temperature and locomotor activity indicating that resveratrol supplementation influences the circadian clock of those animals. Limitations of the study including the short intervention period and small number of mice (n = 13) requires further exploration. However, Pifferi et al. extended the resveratrol supplementation for 4 weeks in another study [210] and observed a reduction of locomotor activity onset in dark conditions, suggesting a better synchronization.
The effects of resveratrol supplementation on clock genes was investigated in several animal studies. The expression of clock genes Period (PER) 1, PER 2, and brain and muscle aryl hydrocarbon receptor nuclear translocator-like 1 (BMAL1) were increased in cultured Rat-1 cells with resveratrol for 8 h [212]. Resveratrol reversed the change induced by high-fat feeding in the expression of reverse erythroblastosis (REV-Erbα), a nuclear receptor, in adipose tissue indicating that resveratrol polyphenol targets the clock genes and thus influences sleep [213].
Pifferi et al. observed an increased proportion of active-wake time during the resting phase (light) of the sleep/wake cycle after 3 weeks of resveratrol supplementation in mice. Negligible changes in active-wake time during the active phase (dark) of the sleep wake cycle suggested that resveratrol activity depends largely on the time of administration [219]. This was consistent with another study that noted that resveratrol administration on male rats behaved as an antioxidant during the night and as a pro-oxidant during day-time [223].
Furthermore, grape seed proanthocyanidin extract (GSPE) treatment maintained nocturnal melatonin levels and modulated the circadian rhythms when it was administered at the start of the day, rather than at night [211]. GSPE administration for 21 days in healthy rats and in rats with diet-induced obesity, clock genes were overexpressed positively with a dose-dependent manner. In addition, BMAL1 protein increased and PER 2 was overexpressed whereas Rev-Erbα was repressed in the liver, gut, and white adipose tissue in healthy rats. This was also observed in the liver and gut of diet-induced obesity rats [214]. GSPE administration modulated clock genes in rat liver by increasing BMAL1 only when administered when the light were turned off suggesting also time-dependency effects [215]. The effectiveness of polyphenols during periods of the day could be due to the discrepant functionality of the suprachiasmatic nuclei (SCN). It has been shown that SCN cells are extensively coupled during the day, when the cells exhibit synchronous neural activity, but minimally coupled during the night, when the cells are electrically silent [224].
Tea polyphenols were capable of manipulating circadian clock genes by enhancing BMAL1 and ameliorated neural redox imbalance and mitochondrial dysfunction [222]. The intake of cherry nutraceutical product decreased diurnal activity and increased nocturnal activity in young and old rats (representative of nocturnal animals). In contrast, the opposite effects were observed for ringdoves (representative of diurnal animals), indicating that effects are modulated depending on the nature of the animals’ circadian rhythms [208]. The previous animal studies showed that polyphenol administration modulated the circadian system through circadian rhythms, clocks, and the sleep/wake cycle with dose and time dependency and possible sex differences providing insight that polyphenols may influence sleep measures.
Since the metabolic state of a cell is coupled to the molecular clock, diet may modify rhythmic cellular activities [6]. In light of this, the third potential mechanism of how polyphenols from FV may affect sleep is by activation of pathways that promote silent mating type information regulation 2 homolog 1 (SIRT1) protein expression [225]. SIRT1 modulates the ventromedial hypothalamic clock, a brain region that contains neuronal food-synchronized clocks that contribute to regulation of the circadian rhythm in feeding behavior [226]. SIRT1 has a central role for reactive oxygen species mainly produced as a consequence of mitochondrial functions [227]. It has been identified that several polyphenols, such as resveratrol, act as dietary activators of SIRT1 [225]. In turn, SIRT1 binds CLOCK-BMAL1 and promote the degradation of PER 2 [228] thus influencing sleep. Alternatively, it has been suggested that resveratrol through its action on SIRT1 improves mitochondrial function and energy metabolism by decreasing fat mass, leading to changes in sleep [219].
The previous animal studies showed that polyphenols modulated sleep through several potential mechanisms however, there is a need for human studies to confirm these mechanisms.
4.3.2. Human Experimental and Observational Studies
The effects of FV consumption on sleep may be due to their high content of melatonin and serotonin [229]. Tart cherry juice has been shown to increase urinary melatonin concentrations in humans [73]; however, this is yet to be confirmed. Alternatively, the effects of polyphenols on sleep measures may be through their antioxidant content reducing oxidative stress and improving sleep quality [2]. St-Onge suggested that plant based diets improve mitochondrial function, energy metabolism, body composition, lower body fat and abdominal adiposity, consequently this may potentially improve sleep quality [230]. However, this was not specifically for FV consumption but diets high in plants.
The effects of different polyphenols on sleep architecture and sleep measures were conducted in few human studies (Table 4). Human experimental studies provide conflicting results with some showing an improvement in sleep measures after polyphenol administration and others not showing any effects. These mixed results may be due to the diverse intervention periods, different types of polyphenols, and doses. The longest intervention period was 90 days [231] and longer intervention studies are required. Furthermore, polyphenol effects from supplements differ from their effects from foods relatively due to their bioavailability and concentration [232]. Another probable reason for the distinctive results is the small number of participants, different study designs, and participants. More effects were shown in participants reporting sleep disturbances, pre-hypertensives, and memory impairment than healthy adults. Experimental trials on participants with sleep problems differ from healthy free-living individuals; therefore, it is necessary to consider the potential for non-representative samples taking part in experimental studies.
Table 4.
Adult human interventional studies exploring the effects of polyphenols on sleep.
| Author, Year (REF) | Study Type | Population | Sample n | Polyphenol Intervention | Intervention Period | Findings Reported on Polyphenol Effect on Sleep |
|---|---|---|---|---|---|---|
| Kuratsune et al., 2010 [233] | Double-blind, placebo-controlled, cross-over | Healthy men with mild sleep complaint | 21 | Crocetin, active carotenoid | Two intervention periods of 2 weeks each separated by a 2-week washout | Actigraphic data showed a reduction in the number of wakening episodes compared to placebo. Subjective data showed improvement in sleep quality |
| Wightman et al., 2015 [234] | Randomized, double-blind, placebo-controlled, parallel | Adults aged 18–30 years | 60 | Resveratrol | 28 days | No effect on PSQI score or its seven factors |
| Park et al., 2017 [235] | Double-blind, placebo-controlled, cross-over | Healthy adults | 9 | Chlorogenic acids, most abundant polyphenol in coffee | 5 days | Shortened SOL compared with the control with no effect on sleep architecture |
| Herrlinger et al., 2018 [231] | Double-blind, placebo-controlled, parallel | Older adults with age associated memory impairment | 90 | Spearmint extract containing 24% total polyphenols | 90 days | Improved the ability to fall asleep, alertness, and behavior following wakefulness compared to controls |
| Um et al., 2018 [236] | Randomized, double-blind, placebo-controlled, parallel | Adults with subjective sleep disturbances | 24 | Phlorotannin | One week | Sleep duration increased compared to placebo, however no effects were shown on the total PSQI score |
| Romain et al., 2017 [237] | Randomized, double-blind, placebo-controlled, parallel | Overweight and obese adults | 33 | Holisfiit®, a polyphenol-rich extract-based food supplement developed from FV | 16 weeks | Awakening during the night improved by 38%, total sleep duration by 50%, and sleep quality by 43% compared to baseline and subjective sleep complaints improved significantly compared to controls |
| Uddin et al., 2018 [238] | Randomized, double-blind, placebo-controlled, cross-over | Pre-hypertensive adults | 12 | Fruitflow® supplements, tomato extract | 24-h period | Both systolic and diastolic blood pressure were lower after FruitFlow® consumption compared to placebo in the wake period whereas during the sleep period, the effect was only shown for systolic blood pressure only |
| Grassi et al., 2016 [239] | Randomized, double-blind, cross-over | Healthy adults | 32 | Flavanol-rich chocolate | Consumption of (high or poor flavanol chocolate bars) after one night of total sleep deprivation | High-flavanol chocolate bar reduced high systolic and diastolic blood pressure caused by sleep deprivation compared to low-flavanol chocolate bar consumption |
| Bigelman et al., 2011 [240] | Randomized, double-blind, placebo-controlled, cross-over | Healthy adults conducting military physical training | 58 | Quercetin | 6 weeks | No effects on sleep quality |
Legend: PSQI (Pittsburgh Sleep Quality Inventory), SOL (sleep onset latency), REF (reference), n (number).
Few observational studies have assessed the associations between isoflavones, a polyphenol mainly found in soybeans and legumes, with sleep measures [241,242]. Cui et al. assessed the cross-sectional association between isoflavone intake and self-reported sleep duration and quality in 1076 Japanese adults [241]. High intakes of isoflavones were associated with adequate sleep duration (7–8 h) and better sleep quality. In contrast, a longitudinal study showed that the highest quartile of soy isoflavone intake was associated with lower odds of long sleep duration (≥9 h/night) and lower odds of falling asleep during daytime in women only. There was a persistent inverse association between isoflavone intake and sleep duration suggesting these effects are due to the estrogenic contents of isoflavones [242]. These inverse associations were consistent with our study exploring the prospective associations between polyphenols derived from FV and sleep duration in UK women [83]. To our knowledge, our study is the first prospective study to explore associations between FV items and their polyphenol content with sleep duration.
Whilst FV consumption may have an immediate effect on sleep, it may also have a longer term impact. Greenwood et al. assessed the stability of dietary patterns in women from the UKWCS using cluster analysis at baseline and after 5 years. Results showed that there was moderate stability in dietary patterns in the UKWCS [243] and in other studies [244,245,246,247]. Thus, exploring the longitudinal associations between FV consumption and sleep duration using the UKWCS was appropriate.
5. Public Health Implications
With the reciprocal relationship between sleep and FV in mind, this review has two main implications that may contribute to public health. Healthy lifestyle patterns have focused mainly on dietary intake and physical activity however, recently, awareness of sleep as a healthy behavior has been raised [248,249]. A first implication, dietary guidelines could include information on sleep and chronotype. A natural starting point is improving sleep hygiene by recommending behavioral and environmental practices to promote better sleep. These practices include optimising temperature, bedding, mattresses, and sound. Sleep hygiene education have shown effective enhancement of sleep quality and decreased daytime sleepiness in adults [250,251] and children [252,253]. Dietary guidelines and nutrition professionals could promote better sleep by eliminating or reducing caffeinated foods and beverages before bedtime, smoking cessation, massage therapy, dim or reduce bright lights during dark hours, engage in physical activity throughout the day and have consistent sleeping and waking times [254].
If future studies continue to support previous findings that later chronotype initiates lower consumption of FV and less healthy behaviors, governments should revise their guidelines accordingly. Dietary recommendations tailored to late chronotypes would ultimately be another worthwhile development. Such recommendations may include pre-planning and preparation of meals to prioritize and increase the consumption of FV. Furthermore, if future studies support that the timing of FV consumption may impact sleep measures, recommendations on the optimal time of FV consumption alongside the 5-a-day guidelines [255] will be informative. However, as few human studies have addressed this question, a greater body of evidence would be required before such recommendations could be proposed.
Promoting FV consumption is a key objective of food and nutrition policy interventions conducted by governments and non-governments. The success of campaigns and interventions conducted around the world in terms of increasing the daily consumption of FV remain modest [256]. The second implication is the incorporation of sleep screening in GP practices, hospitals, weight-loss programs, and campaigns targeting higher consumption of FV. Sleep screening questions on timing, duration, difficulty falling asleep, waking up at night, refreshed feeling upon waking and sleepiness during the day should be included [254]. If desired answers are not received, further assessment can be conducted by using the Pittsburgh Sleep Quality Inventory (PSQI) [257]. Participants with an indication of poor sleep or sleep disorders should be referred to sleep clinicians.
6. Directions for Future Work
Exploring the links between sleep and FV consumption are scarce and future studies are required to take into account several factors (Table 5).
Table 5.
Recommendations for future studies investigating the relationship between sleep and FV consumption.
| Recommendations |
|---|
| 1. Consider individual differences of sleep by exploring optimal sleep duration in a laboratory. |
| 2. Explore the effects of sleep disruption on FV consumption based on genetic disparities. |
| 3. Explore the effects of different FV items (individually and combined) on sleep measures. |
| 4. Compare the effects of FV consumption at specific time points on sleep measures. |
| 5. Explore the effects of other FV components (e.g., micronutrients, moisture, fiber, polyphenols, antioxidants and offsetting energy intake) on sleep measures and architecture. |
| 6. Identify a biomarker of polyphenol consumption to be used in studies exploring the relations between sleep and FV. |
| 7. Consider food matrix by comparing the effects of polyphenol administration between participants fasting, consuming a complex meal and consuming FV items. |
| 8. Controlling for other sources of polyphenols such as coffee, tea, red wine, soy, and chocolate. |
| 9. Group FV items based on similarity of total antioxidant and explore their effects on sleep and compare their effects with antioxidants from supplements. |
| 10. Identify objective markers of antioxidant intake and compare their levels across different sleep durations, quality, and chronotypes. |
| 11. In RCT exploring the effects of FV on sleep, selection of FV items that undertaken similar food processing and handling methods to account for antioxidant preservation is necessary. |
| 12. Investigate the effects of sleep timing, taking chronotype into account, on FV consumption. |
| 13. Non-linear relationships between FV and sleep need to be considered in observational and sleep restriction/extension studies. |
| 14. Explore the relations between sleep and FV consumption in other populations such as; different ethnicities, elderly, clinical populations, shift workers, and less-developed countries. |
| 15. Study whether FV consumption is substituted with convenient desserts and sweets in response to sleep disruption. |
| 16. Identify how to optimize exercise protocols to increase FV consumption and improve sleep. |
| 17. Compare the effects of sleep hygiene education and digital cognitive behavioral therapy on FV consumption in sleep extension studies. |
| 18. Identify the role of stress and self-control in the relationship between sleep and FV consumption. |
Legend: FV (fruit and vegetable), RCT (randomized controlled trials).
It would be productive to reach agreement on the best ways to assess sleep duration and chronotype. Although the recommendations for sleep duration have been provided [8], individual differences need to be considered by exploring optimal sleep duration in a laboratory. Next, the difference between optimal sleep duration and habitual sleep duration can be obtained, this difference represents potential sleep loss which is not clearly recognized by individuals. Previous findings confirmed that evaluating optimal sleep duration may be a useful clinical marker of sleep loss and individual differences [258]. However, laboratory experiments of sleep deprivation have shown that individuals have differential neurobehavioral vulnerability to sleep loss suggesting a polygenetic phenotype [259,260]. Omics (transcriptomics, epigenomics, and metabolomics) approaches were suggested as biomarkers for identifying differential vulnerability to sleep loss [261]. Identification of such markers will provide a viable means to determine those individuals who may need more habitual sleep or who may need to prevent or mitigate sleep deprivation through lifestyle choices and effective interventions and countermeasures (e.g., caffeine, naps, etc.). Variation in sleep measures and chronotype are related to circadian clock genes and non-circadian genes [262]. Sleep and diet research may need to explore the effects of sleep disruption on FV consumption based on genetic disparities.
One of our studies focused on FV items and their polyphenol content with sleep duration [83]. It would be informative to explore the effects on other sleep measures (sleep timing and quality). Furthermore, future intervention studies comparing the effects of different FV items (individually and combined) on sleep measures will be instructive and may be beneficial in identifying the underlying mechanisms. Previous studies found that activities done during the day have an effect on sleep [263,264,265,266,267]. Related to this, future studies may compare the effects of FV consumption at specified time points on sleep measures. Nevertheless, little is currently known about associations between many FV items and sleep measures.
Adherence to a Mediterranean diet (the highest tertile) was associated with better sleep quality compared to those in the lowest tertile [268]. It could be that different FV items may have different effects on sleep measures due to their moisture, water content [269], fiber, polyphenols, and antioxidants [270]. Lower consumption of dietary fiber was associated with less slow-wave sleep (deep sleep) [271,272] however, few studies explored the effects of different polyphenols on sleep architecture (see Section 4.3.2) and more studies will be instructive. People who eat several servings of FV per day, their total polyphenol intake reaches ~1 g/d [199]. The assessment of polyphenol intake is difficult to evaluate by using similar methods to dietary assessment due to their bioavailability and bio-efficacy variances [232]. Therefore, biomarkers for polyphenol exposure would be very useful. Limited studies found that food matrix affects the bioavailability of polyphenols and thus influence their absorption [273,274]. Future studies may compare the effects of different polyphenols on sleep measures between fasting participants and participants consuming a complex meal or FV items. Consideration of the dietary fiber content of FV items in these studies are necessary because dietary fiber stimulates intestinal fermentation that may influence the production of microbial metabolites that may have consequences on the absorption of polyphenols [199]. Another factor to consider in studies exploring the effects of polyphenols from FV on sleep, is controlling for other sources of high polyphenol content from foods such as coffee, tea, red wine, soy, and chocolate.
The antioxidant properties of polyphenols have been widely studied [275]. However, it is uncertain whether increased antioxidant nutrient intake or supplementation would modify sleep. Nonetheless, studies reported reduced antioxidant capacity in serum of patients with obstructive sleep apnea (OSA) [276], reduced dietary intake of antioxidants in veterans with OSA [277]. Related to OSA, participants with the metabolic syndrome had reduced serum concentrations of antioxidants [278]. Furthermore, antioxidant nutrient intake from high consumption of FV or supplement intake were proposed as potential moderators of cognitive decline and CVD from OSA [279]. In a recent cross-sectional study conducted among 3941 Korean men, short sleepers (<6 h) with low consumption of dietary antioxidant had a higher risk of obesity than those with a high consumption of dietary antioxidants [280]. These results suggest that the increased risk of obesity associated with short sleep duration may be modified by the consumption of dietary antioxidants. In light of this, as a starting point, epidemiological studies could subgroup FV based on similarity of total antioxidant capacity to explore their relationship with sleep. Ten subgroups of FV were proposed based on food component and classification variables (botanic family, plant part, color, and total antioxidant capacity) [281]. Furthermore, long-term prospective randomized controlled clinical trials can study the effects of antioxidants from FV combined based on the 10 groups, or antioxidants from supplements in individuals with short, recommended, and long sleep duration. In addition, other sleep measures (sleep architecture, quality, and timing) could be explored in relation to antioxidant intake. Objective markers of antioxidant intake and oxidative stress should be initiated and compared across persons with short, recommended, and long sleep durations, and also compared across persons with poor/good sleep quality and those with different chronotypes. In those randomized controlled trials, it is necessary to select FV that undertaken similar food processing and handling methods to overcome their effects on preservation of antioxidants [282].
Sleep restriction studies (Table 2) provide conflicting results on their effects on FV consumption and there are a lack of studies assessing the timing of sleep in relation to FV consumption, more studies investigating the timing of sleep and taking into account chronotype are required to clarify this relationship. Studies exploring the effects of sleep extension on FV consumption (Table 1) are few, more studies are needed to help clarify the underlying mechanisms between sleep disruption and FV consumption. Non-linear relationships between FV and sleep need to be considered in sleep restriction/extension studies. A recent study showed the feasibility of extending sleep by sleep hygiene intervention and their effects on dietary intake and energy balance [283]. The results showed that sleep extension led to reduced intakes of free sugars compared to controls. It would be interesting to conduct a similar study and explore the effects of sleep extension in habitual short sleepers on FV consumption. One factor that may influence the effects of sleep extension interventions on diet is chronotype. Therefore, comparing the effects of sleep restriction/extension on FV consumption between different chronotypes is essential.
Additional studies of effects of sleep restriction/extension on FV consumption in different populations would also be useful. Differences in sleep between different races and ethnicities have been reported. Black individuals tend to have shorter sleep durations and poorer sleep quality than white individuals [284,285,286,287]. It has been proposed that the underlying mechanisms of ethnic/racial disparities in sleep include several potential biological, psycho-behavioral, sociocultural, and environmental factors [286,287]. These potential mediators are important to explore in future research conducted in different race/ethnic individuals assessing associations between sleep measures and FV consumption. Other populations to consider could include elderly people, clinical populations, shift workers, and less-developed countries. Little is known about sleep and FV consumption in these people, to my knowledge.
A study explored the effects of short and long sleep durations on taste preference in healthy adults. Habitual long-sleepers preferred sweeter stimuli following sleep restriction while sleep extension did not change taste preference in habitual short-sleepers [288]. However, in adolescents, sweet foods were more appealing after sleep restriction [289]. This may be an explanation for the low intakes of FV in short and long sleepers in our studies [82,84]. Because FV require some preparation, convenience and time are important factors today as the pace of life has increased, therefore, people tend to buy products that require minimum preparation [256]. In light of this, the question of whether short and long sleepers substitute FV with off the shelf and convenient desserts and sweets is not well understood and will be a valuable point of inquiry to address in future studies that assess the association between sleep measures and FV consumption.
Reciprocity between sleep and exercise exists; sleep disruption could impair an individual’s capacity for exercise and increase the risk of exercise-induced injuries, conversely, acute and regular exercise effect sleep architecture and measures depending on numerous factors; sex, age, fitness level, BMI, intensity and duration of exercise, time of day and environment (indoor or outdoor) [290]. The results are conflicting and effects of exercise on sleep were mostly shown in people with sleep disorders and trivial improvement in sleep in individuals with good sleep [254,290]. There is not enough evidence that exercise may effect FV consumption and there is a paucity of human studies on this subject, it is important to study how to optimize exercise protocols to increase FV consumption and improve sleep.
Studying the complex relationship between sleep and diet needs to take into consideration numerous components. Current usage of digital devices is unprecedented and keeping pace with the digital revolution we are experiencing is fundamental. Future sleep extension studies need to compare between the effects of digital cognitive behavioral therapy [291] and sleep hygiene education on sleep, quality of life, and psychological well-being. Another factor to consider in future studies is stress. Mental stress and depression have increased dramatically over the last 50 years. Stress has been found to be associated with disrupted sleep and increase desire for palatable food, thus causing obesity. On the other hand, improving sleep patterns and nutritional status may reduce the severity of stress and mental disorders [292]. This highlights the importance of the need to further examine the complex relationship between sleep, diet, and stress. Related to this, sleep and self-control are intertwined, sleep disrupted individuals are at an increased risk of impaired decision making, including dietary selections [293]. Self-control has two effects on healthy behavior (such as physical activity, eating healthily (high intakes of FV for example), reducing alcohol intake and not smoking); an indirect effect mediated by intentions and a moderated effect on the intention–health behavior relationship. Hagger et al. proposed several pathways of how sleep may affect health behavior in the context of the health self-control model [294]. People with better sleep quality and sufficient duration will be more likely to be able to form intentions to engage in healthy behaviors. The underlying mechanism for this effect is because better sleep (quality and quantity) provide individuals with sufficient cognitive resources for more effective planning. In light of this, does self-control have a role in the association between sleep duration and FV consumption? Future studies addressing this will be extremely valuable in clarifying the underlying mechanisms.
7. Conclusions
Based on health psychology research from five decades, a nutritious diet and sleep moderation and optimism are two of the main keys to a long, happy, healthy, and productive life [96]. The substantial attribution of both sleep disruption and low intakes of FV on the global burden of diseases are well documented and understanding the reciprocal relationship between them is necessary. In this review, we have provided epidemiological evidence (cross-sectional and prospective) in adults that sleep duration is non-linearly associated with FV consumption with short and long sleepers consuming less FV and sleeping the recommended ~7–9 h/day is associated with higher intakes of FV in a sub-group of UK adults. Experimental studies are limited and there is a need for robust intervention studies of sleep (restriction/extension) on FV consumption and also intervention studies of the effects of FV consumption on sleep.
This review provided potential reciprocal mechanisms linking sleep disruption with FV consumption. Disrupted sleep may influence FV consumption through homeostatic and non-homeostatic mechanisms. On the other hand, FV consumption and their polyphenol content may alter sleep measures through the gut–brain axis, their influence on circadian rhythms; clock gene expression and peripheral clocks, and by the improvement of mitochondrial function and energy metabolism by decreasing fat mass leading to changes in sleep, unidentified mechanisms may exist. With further research, interactions between sleep measures and FV consumption may be clarified and potentially reduce the burden of chronic diseases and premature deaths.
Supplementary Materials
The following are available online at https://www.mdpi.com/2072-6643/11/6/1382/s1, Table S1: Search terms used for literature review.
Author Contributions
Conceptualization, E.N.; Writing—review and editing, E.N., L.H., and J.C.; Visualization, E.N.; Supervision, L.H. and J.C.
Funding
E.N. is in receipt of a scholarship from Umm Al-Qura University, Makkah, Saudi Arabia.
Conflicts of Interest
J.C. is a director of the University of Leeds spin out company Dietary Assessment. The founding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the review.
References
- 1.Colten H.R., Altevogt B.M. Sleep Disorders and Sleep Deprivation: An Unmet Public Health Problem. Academies Press; Washington, DC, USA: 2006. [PubMed] [Google Scholar]
- 2.St-Onge M.P., Mikic A., Pietrolungo C.E. Effects of Diet on Sleep Quality. Adv. Nutr. 2016;7:938–949. doi: 10.3945/an.116.012336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ogilvie R.P., Patel S.R. The epidemiology of sleep and obesity. Sleep Health. 2017;3:383–388. doi: 10.1016/j.sleh.2017.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jike M., Itani O., Watanabe N., Buysse D.J., Kaneita Y. Long sleep duration and health outcomes: A systematic review, meta-analysis and meta-regression. Sleep Med. Rev. 2018;39:25–36. doi: 10.1016/j.smrv.2017.06.011. [DOI] [PubMed] [Google Scholar]
- 5.Borbély A. A two process model of sleep regulation. Hum. Neurobiol. 1982;1:195–204. [PubMed] [Google Scholar]
- 6.Potter G.D., Cade J.E., Grant P.J., Hardie L.J. Nutrition and the circadian system. Br. J. Nutr. 2016;116:434–442. doi: 10.1017/S0007114516002117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Potter G.D., Skene D.J., Arendt J., Cade J.E., Grant P.J., Hardie L.J. Circadian Rhythm and Sleep Disruption: Causes, Metabolic Consequences, and Countermeasures. Endocr. Rev. 2016;37:584–608. doi: 10.1210/er.2016-1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hirshkowitz M., Whiton K., Albert S.M., Alessi C., Bruni O., DonCarlos L., Hazen N., Herman J., Katz E.S., Kheirandish-Gozal L., et al. National Sleep Foundation’s sleep time duration recommendations: Methodology and results summary. Sleep Health. 2015;1:40–43. doi: 10.1016/j.sleh.2014.12.010. [DOI] [PubMed] [Google Scholar]
- 9.Hafner M., Stepanek M., Taylor J., Troxel W.M., van Stolk C. Why Sleep Matters-The Economic Costs of Insufficient Sleep: A Cross-Country Comparative Analysis. Rand Health Q. 2017;6:11. [PMC free article] [PubMed] [Google Scholar]
- 10.Candari C.J., Cylus J., Nolte E. Assessing the Economic Costs of Unhealthy Diets and Low Physical Activity: An Evidence Review and Proposed Framework. WHO Regional Office for Europe; Copenhagen, Denmark: 2017. [(accessed on 2 January 2019)]. Available online: http://www.euro.who.int/__data/assets/pdf_file/0004/342166/Unhealthy-Diets-ePDF-v1.pdf?ua=1. [PubMed] [Google Scholar]
- 11.Popkin B.M., Kim S., Rusev E.R., Du S., Zizza C. Measuring the full economic costs of diet, physical activity and obesity-related chronic diseases. Obes. Rev. 2006;7:271–293. doi: 10.1111/j.1467-789X.2006.00230.x. [DOI] [PubMed] [Google Scholar]
- 12.Cadilhac D.A., Magnus A., Sheppard L., Cumming T.B., Pearce D.C., Carter R. The societal benefits of reducing six behavioural risk factors: An economic modelling study from Australia. BMC Public Health. 2011;11:483. doi: 10.1186/1471-2458-11-483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ekwaru J.P., Ohinmaa A., Loehr S., Setayeshgar S., Thanh N.X., Veugelers P.J. The economic burden of inadequate consumption of vegetables and fruit in Canada. Public Health Nutr. 2017;20:515–523. doi: 10.1017/S1368980016002846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scarborough P., Bhatnagar P., Wickramasinghe K.K., Allender S., Foster C., Rayner M. The economic burden of ill health due to diet, physical inactivity, smoking, alcohol and obesity in the UK: An update to 2006-07 NHS costs. J. Public Health. 2011;33:527–535. doi: 10.1093/pubmed/fdr033. [DOI] [PubMed] [Google Scholar]
- 15.Centers for Disease Control and Prevention Sleep and Sleep Disorders. [(accessed on 25 January 2019)];2018 Available online: https://www.cdc.gov/sleep/index.html.
- 16.Itani O., Jike M., Watanabe N., Kaneita Y. Short sleep duration and health outcomes: A systematic review, meta-analysis, and meta-regression. Sleep Med. 2017;32:246–256. doi: 10.1016/j.sleep.2016.08.006. [DOI] [PubMed] [Google Scholar]
- 17.Liu W., Zhang R., Tan A., Ye B., Zhang X., Wang Y., Zou Y., Ma L., Chen G., Li R., et al. Long sleep duration predicts a higher risk of obesity in adults: A meta-analysis of prospective cohort studies. J. Public Health. 2018;135:1–11. doi: 10.1093/pubmed/fdy135. [DOI] [PubMed] [Google Scholar]
- 18.Zhao H., Yin J.Y., Yang W.S., Qin Q., Li T.T., Shi Y., Deng Q., Wei S., Liu L., Wang X., et al. Sleep duration and cancer risk: A systematic review and meta-analysis of prospective studies. Asian Pac. J. Cancer Prev. 2013;14:7509–7515. doi: 10.7314/APJCP.2013.14.12.7509. [DOI] [PubMed] [Google Scholar]
- 19.Erren T.C., Morfeld P., Foster R.G., Reiter R.J., Gross J.V., Westermann I.K. Sleep and cancer: Synthesis of experimental data and meta-analyses of cancer incidence among some 1,500,000 study individuals in 13 countries. Chronobiol. Int. 2016;33:325–350. doi: 10.3109/07420528.2016.1149486. [DOI] [PubMed] [Google Scholar]
- 20.Ma Q.Q., Yao Q., Lin L., Chen G.C., Yu J.B. Sleep duration and total cancer mortality: A meta-analysis of prospective studies. Sleep Med. 2016;27:39–44. doi: 10.1016/j.sleep.2016.06.036. [DOI] [PubMed] [Google Scholar]
- 21.Cappuccio F.P., D’Elia L., Strazzullo P., Miller M.A. Quantity and quality of sleep and incidence of type 2 diabetes: A systematic review and meta-analysis. Diabetes Care. 2010;33:414–420. doi: 10.2337/dc09-1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li W., Wang D., Cao S., Yin X., Gong Y., Gan Y., Zhou Y., Lu Z. Sleep duration and risk of stroke events and stroke mortality: A systematic review and meta-analysis of prospective cohort studies. Int. J. Cardiol. 2016;223:870–876. doi: 10.1016/j.ijcard.2016.08.302. [DOI] [PubMed] [Google Scholar]
- 23.Cappuccio F.P., Cooper D., D’Elia L., Strazzullo P., Miller M.A. Sleep duration predicts cardiovascular outcomes: A systematic review and meta-analysis of prospective studies. Eur. Heart J. 2011;32:1484–1492. doi: 10.1093/eurheartj/ehr007. [DOI] [PubMed] [Google Scholar]
- 24.Kwok C.S., Kontopantelis E., Kuligowski G., Gray M., Muhyaldeen A., Gale C.P., Peat G.M., Cleator J., Chew-Graham C., Loke Y.K., et al. Self-Reported Sleep Duration and Quality and Cardiovascular Disease and Mortality: A Dose-Response Meta-Analysis. J. Am. Heart Assoc. 2018;7:e008552. doi: 10.1161/JAHA.118.008552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cappuccio F.P., D’Elia L., Strazzullo P., Miller M.A. Sleep duration and all-cause mortality: A systematic review and meta-analysis of prospective studies. Sleep. 2010;33:585–592. doi: 10.1093/sleep/33.5.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.da Silva A.A., de Mello R.G., Schaan C.W., Fuchs F.D., Redline S., Fuchs S.C. Sleep duration and mortality in the elderly: A systematic review with meta-analysis. BMJ Open. 2016;6:e008119. doi: 10.1136/bmjopen-2015-008119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gallicchio L., Kalesan B. Sleep duration and mortality: A systematic review and meta-analysis. J. Sleep Res. 2009;18:148–158. doi: 10.1111/j.1365-2869.2008.00732.x. [DOI] [PubMed] [Google Scholar]
- 28.Liu T.Z., Xu C., Rota M., Cai H., Zhang C., Shi M.J., Yuan R.X., Weng H., Meng X.Y., Kwong J.S., et al. Sleep duration and risk of all-cause mortality: A flexible, non-linear, meta-regression of 40 prospective cohort studies. Sleep Med. Rev. 2017;32:28–36. doi: 10.1016/j.smrv.2016.02.005. [DOI] [PubMed] [Google Scholar]
- 29.Dashti H.S., Scheer F.A., Jacques P.F., Lamon-Fava S., Ordovas J.M. Short sleep duration and dietary intake: Epidemiologic evidence, mechanisms, and health implications. Adv. Nutr. 2015;6:648–659. doi: 10.3945/an.115.008623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Crisp A. Sleep, Activity, Nutrition and Mood. Br. J. Psychiatry. 1980;137:1–7. doi: 10.1192/bjp.137.1.1. [DOI] [PubMed] [Google Scholar]
- 31.Hicks R., Rozette E. Habitual sleep duration and eating disorders in college students. Percept. Mot. Skills. 1986;62:209–210. doi: 10.2466/pms.1986.62.1.209. [DOI] [PubMed] [Google Scholar]
- 32.Hicks R., McTighe S., Juarez M. Sleep duration and eating behaviors of college students. Percept. Mot. Skills. 1986;62:25–26. doi: 10.2466/pms.1986.62.1.25. [DOI] [PubMed] [Google Scholar]
- 33.Al Khatib H.K., Harding S.V., Darzi J., Pot G.K. The effects of partial sleep deprivation on energy balance: A systematic review and meta-analysis. Eur. J. Clin. Nutr. 2017;71:614–624. doi: 10.1038/ejcn.2016.201. [DOI] [PubMed] [Google Scholar]
- 34.Capers P.L., Fobian A.D., Kaiser K.A., Borah R., Allison D.B. A systematic review and meta-analysis of randomized controlled trials of the impact of sleep duration on adiposity and components of energy balance. Obes. Rev. 2015;16:771–782. doi: 10.1111/obr.12296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peuhkuri K., Sihvola N., Korpela R. Diet promotes sleep duration and quality. Nutr. Res. 2012;32:309–319. doi: 10.1016/j.nutres.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 36.Ji X., Grandner M.A., Liu J. The relationship between micronutrient status and sleep patterns: A systematic review. Public Health Nutr. 2017;20:687–701. doi: 10.1017/S1368980016002603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.WHO Diet Nutrition and the Prevention of Chronic Diseases. [(accessed on 5 September 2016)]; Available online: http://apps.who.int/iris/bitstream/handle/10665/42665/WHO_TRS_916.pdf?sequence=1.
- 38.United States Department of Agriculture Choose My Plate. [(accessed on 5 February 2019)]; Available online: https://www.choosemyplate.gov.
- 39.Australian Institute of Health and Welfare Australia’s Food & Nutrition. [(accessed on 8 February 2019)]; Available online: https://www.aihw.gov.au/getmedia/0c26b1458–1fa-4a94-af38-d52515885a07/12504.pdf.aspx?inline=true.
- 40.Health Canada Eating Well with Canada’s Food Guide. [(accessed on 8 February 2019)]; Available online: https://www.canada.ca/content/dam/hc-sc/migration/hc-sc/fn-an/alt_formats/hpfb-dgpsa/pdf/food-guide-aliment/print_eatwell_bienmang-eng.pdf.
- 41.Food and Agriculture Organization of the United Nations The Official Dietary Guidelines (Danish: De Officielle Kostråd) [(accessed on 7 February 2019)]; Available online: http://www.fao.org/nutrition/education/food-dietary-guidelines/regions/countries/denmark/en/
- 42.Public Health England in Association with the Welsh Government, Food Standards Scotland and the Food Standards Agency in Northern Ireland. [(accessed on 9 February 2019)]; Eatwell Guide. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/528193/Eatwell_guide_colour.pdf.
- 43.WHO Regional Office for Europe Comparative Analysis of Nutrition Policies in WHO European Member States. [(accessed on 6 January 2019)]; Available online: https://www.who.int/nutrition/publications/policies/comparative_analysis_european/en/
- 44.Hall J.N., Moore S., Harper S.B., Lynch J.W. Global Variability in Fruit and Vegetable Consumption. Am. J. Prev. Med. 2009;36:402–409. doi: 10.1016/j.amepre.2009.01.029. [DOI] [PubMed] [Google Scholar]
- 45.Muraki I., Imamura F., Manson J.E., Hu F.B., Willett W.C., van Dam R.M., Sun Q. Fruit consumption and risk of type 2 diabetes: Results from three prospective longitudinal cohort studies. BMJ. 2013;347:f5001. doi: 10.1136/bmj.f5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.He F., Nowson C., Lucas M., MacGregor G. Increased consumption of fruit and vegetables is related to a reduced risk of coronary heart disease: Meta-analysis of cohort studies. J. Hum. Hypertens. 2007;21:717–728. doi: 10.1038/sj.jhh.1002212. [DOI] [PubMed] [Google Scholar]
- 47.Hu D., Huang J., Wang Y., Zhang D., Qu Y. Fruits and vegetables consumption and risk of stroke: A meta-analysis of prospective cohort studies. Stroke. 2014;45:1613–1619. doi: 10.1161/STROKEAHA.114.004836. [DOI] [PubMed] [Google Scholar]
- 48.World Cancer Research Fund/American Institute for Cancer Research Food, Nutrition, Physical Activity and the Prevention of Cancer: A Global Perspective. [(accessed on 6 January 2016)]; Available online: http://discovery.ucl.ac.uk/4841/1/4841.pdf.
- 49.Lock K., Pomerleau J., Causer L., Altmann D.R., McKee M. The global burden of disease attributable to low consumption of fruit and vegetables: Implications for the global strategy on diet. Bull. World Health Organ. 2005;83:100–108. [PMC free article] [PubMed] [Google Scholar]
- 50.Aune D., Giovannucci E., Boffetta P., Fadnes L.T., Keum N., Norat T., Greenwood D.C., Riboli E., Vatten L.J., Tonstad S. Fruit and vegetable intake and the risk of cardiovascular disease, total cancer and all-cause mortality-a systematic review and dose-response meta-analysis of prospective studies. Int. J. Epidemiol. 2017;46:1029–1056. doi: 10.1093/ije/dyw319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.WHO Increasing Fruit and Vegetable Consumption to Reduce the Risk of Noncommunicable Diseases. [(accessed on 8 February 2019)]; Available online: https://www.who.int/elena/titles/fruit_vegetables_ncds/en/
- 52.Garaulet M., Ortega F.B., Ruiz J.R., Rey-Lopez J.P., Beghin L., Manios Y., Cuenca-Garcia M., Plada M., Diethelm K., Kafatos A., et al. Short sleep duration is associated with increased obesity markers in European adolescents: Effect of physical activity and dietary habits. The HELENA study. Int J. Obes. 2011;35:1308–1317. doi: 10.1038/ijo.2011.149. [DOI] [PubMed] [Google Scholar]
- 53.Golley R., Maher C., Matricciani L., Olds T. Sleep duration or bedtime? Exploring the association between sleep timing behaviour, diet and BMI in children and adolescents. Int J. Obes. 2013;37:546–551. doi: 10.1038/ijo.2012.212. [DOI] [PubMed] [Google Scholar]
- 54.Hoppe C., Rothausen B.W., Biltoft-Jensen A., Matthiessen J., Groth M.V., Chaput J.P., Tetens I. Relationship between sleep duration and dietary intake in 4-to 14-year-old Danish children. J. Nutr. Sci. 2013;2:1–7. doi: 10.1017/jns.2013.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Beebe D.W., Simon S., Summer S., Hemmer S., Strotman D., Dolan L.M. Dietary intake following experimentally restricted sleep in adolescents. Sleep. 2013;36:827–834. doi: 10.5665/sleep.2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Franckle R.L., Falbe J., Gortmaker S., Ganter C., Taveras E.M., Land T., Davison K.K. Insufficient sleep among elementary and middle school students is linked with elevated soda consumption and other unhealthy dietary behaviors. Prev. Med. 2015;74:36–41. doi: 10.1016/j.ypmed.2015.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bornhorst C., Wijnhoven T.M., Kunesova M., Yngve A., Rito A.I., Lissner L., Duleva V., Petrauskiene A., Breda J. WHO European Childhood Obesity Surveillance Initiative: Associations between sleep duration, screen time and food consumption frequencies. BMC Public Health. 2015;15:442. doi: 10.1186/s12889-015-1793-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cao M., Zhu Y., He B., Yang W., Chen Y., Ma J., Jing J. Association between sleep duration and obesity is age- and gender-dependent in Chinese urban children aged 6–18 years: A cross-sectional study. BMC Public Health. 2015;15 doi: 10.1186/s12889-015-2359-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Arora T., Taheri S. Associations among late chronotype, body mass index and dietary behaviors in young adolescents. Int. J. Obes. 2015;39:39–44. doi: 10.1038/ijo.2014.157. [DOI] [PubMed] [Google Scholar]
- 60.Ferranti R., Marventano S., Castellano S., Giogianni G., Nolfo F., Rametta S., Matalone M., Mistretta A. Sleep quality and duration is related with diet and obesity in young adolescent living in Sicily, Southern Italy. Sleep Sci. 2016;9:117–122. doi: 10.1016/j.slsci.2016.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Khan M.K.A., Faught E.L., Chu Y.L., Ekwaru J.P., Storey K.E., Veugelers P.J. Is it nutrients, food items, diet quality or eating behaviours that are responsible for the association of children’s diet with sleep? J. Sleep Res. 2017;26:468–476. doi: 10.1111/jsr.12466. [DOI] [PubMed] [Google Scholar]
- 62.Asarnow L.D., Greer S.M., Walker M.P., Harvey A.G. The Impact of Sleep Improvement on Food Choices in Adolescents with Late Bedtimes. J. Adolesc. Health. 2017;60:570–576. doi: 10.1016/j.jadohealth.2016.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gong Q.H., Li H., Zhang X.H., Zhang T., Cui J., Xu G.Z. Associations between sleep duration and physical activity and dietary behaviors in Chinese adolescents: Results from the Youth Behavioral Risk Factor Surveys of 2015. Sleep Med. 2017;37:168–173. doi: 10.1016/j.sleep.2017.06.024. [DOI] [PubMed] [Google Scholar]
- 64.Harrex H.A.L., Skeaff S.A., Black K.E., Davison B.K., Haszard J.J., Meredith-Jones K., Quigg R., Saeedi P., Stoner L., Wong J.E., et al. Sleep timing is associated with diet and physical activity levels in 9-11-year-old children from Dunedin, New Zealand: The PEDALS study. J. Sleep Res. 2018;27:e12634. doi: 10.1111/jsr.12634. [DOI] [PubMed] [Google Scholar]
- 65.Kaar J.L., Schmiege S.J., Vadiveloo M., Simon S.L., Tovar A. Sleep duration mediates the relationship between health behavior patterns and obesity. Sleep Health. 2018;4:442–447. doi: 10.1016/j.sleh.2018.07.004. [DOI] [PubMed] [Google Scholar]
- 66.Cordova F.V., Barja S., Brockmann P.E. Consequences of short sleep duration on the dietary intake in children: A systematic review and metanalysis. Sleep Med. Rev. 2018;42:68–84. doi: 10.1016/j.smrv.2018.05.006. [DOI] [PubMed] [Google Scholar]
- 67.Spiegel K., Tasali E., Penev P., Van Cauter E. Brief communication: Sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann. Intern. Med. 2004;141:846–850. doi: 10.7326/0003-4819-141-11-200412070-00008. [DOI] [PubMed] [Google Scholar]
- 68.Heath G., Roach G.D., Dorrian J., Ferguson S.A., Darwent D., Sargent C. The effect of sleep restriction on snacking behaviour during a week of simulated shiftwork. Accid. Anal. Prev. 2012;45:62–67. doi: 10.1016/j.aap.2011.09.028. [DOI] [PubMed] [Google Scholar]
- 69.Spaeth A.M., Dinges D.F., Goel N. Sex and race differences in caloric intake during sleep restriction in healthy adults. Am. J. Clin. Nutr. 2014;100:559–566. doi: 10.3945/ajcn.114.086579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tasali E., Chapotot F., Wroblewski K., Schoeller D. The effects of extended bedtimes on sleep duration and food desire in overweight young adults: A home-based intervention. Appetite. 2014;80:220–224. doi: 10.1016/j.appet.2014.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Garrido M., Espino J., González-Gómez D., Lozano M., Cubero J., Toribio-Delgado A.F., Maynar-Mariño J.I., Terrón M.P., oz J.L., Pariente J.A., et al. A nutraceutical product based on Jerte Valley cherries improves sleep and augments the antioxidant status in humans. E Spen. Eur. E J. Clin. Nutr. Metab. 2009;4:e321–e323. doi: 10.1016/j.eclnm.2009.09.003. [DOI] [Google Scholar]
- 72.Garrido M., Paredes S.D., Cubero J., Lozano M., Toribio-Delgado A.F., Munoz J.L., Reiter R.J., Barriga C., Rodriguez A.B. Jerte Valley cherry-enriched diets improve nocturnal rest and increase 6-sulfatoxymelatonin and total antioxidant capacity in the urine of middle-aged and elderly humans. J. Gerontol. A Biol. Sci. Med. Sci. 2010;65:909–914. doi: 10.1093/gerona/glq099. [DOI] [PubMed] [Google Scholar]
- 73.Pigeon W., Carr M., Gorman C., Perlis M. Effects of a Tart Cherry Juice Beverage on the Sleep of Older Adults with Insomnia: A Pilot Study. J. Med. Food. 2010;13:579–583. doi: 10.1089/jmf.2009.0096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lin H., Tsai P., Fang S., Liu J. Effect of kiwifruit consumption on sleep quality in adults with sleep problems. Asia Pac. J. Clin. Nutr. 2011;20:169–174. [PubMed] [Google Scholar]
- 75.Howatson G., Bell P.G., Tallent J., Middleton B., McHugh M.P., Ellis J. Effect of tart cherry juice (Prunus cerasus) on melatonin levels and enhanced sleep quality. Eur. J. Nutr. 2012;51:909–916. doi: 10.1007/s00394-011-0263-7. [DOI] [PubMed] [Google Scholar]
- 76.Garrido M., González-Gómez D., Lozano M., Barriga C., Paredes S., Rodríguez A. A jerte valley cherry product provides beneficial effects on sleep quality. Influence on aging. J. Nutr. Health Aging. 2013;17:553–560. doi: 10.1007/s12603-013-0029-4. [DOI] [PubMed] [Google Scholar]
- 77.Knowlden A.P., Hackman C.L., Sharma M. Systematic Review of Dietary Interventions Targeting Sleep Behavior. J. Altern Complement. Med. 2016;22:349–362. doi: 10.1089/acm.2015.0238. [DOI] [PubMed] [Google Scholar]
- 78.Imaki M., Hatanaka Y., Ogawa Y., Yoshida Y., Tanada S. An epidemiological study on relationship between the hours of sleep and life style factors in japanese factory workers. J. Physiol. Anthropol. Appl. Human Sci. 2002;21:115–120. doi: 10.2114/jpa.21.115. [DOI] [PubMed] [Google Scholar]
- 79.Huang Y.C., Wahlqvist M.L., Lee M.S. Sleep quality in the survival of elderly taiwanese: Roles for dietary diversity and pyridoxine in men and women. J. Am. Coll Nutr. 2013;32:417–427. doi: 10.1080/07315724.2013.848158. [DOI] [PubMed] [Google Scholar]
- 80.Duke C.H., Williamson J.A., Snook K.R., Finch K.C., Sullivan K.L. Association Between Fruit and Vegetable Consumption and Sleep Quantity in Pregnant Women. Matern. Child. Health J. 2017;21:966–973. doi: 10.1007/s10995-016-2247-y. [DOI] [PubMed] [Google Scholar]
- 81.Lee Y.H., Chang Y.C., Lee Y.T., Shelley M., Liu C.T. Dietary patterns with fresh fruits and vegetables consumption and quality of sleep among older adults in mainland China. Sleep Biol. Rhythms. 2018;16:293–305. doi: 10.1007/s41105-018-0163-9. [DOI] [Google Scholar]
- 82.Noorwali E.A., Hardie L.J., Cade J.E. Recommended sleep duration is associated with higher consumption of fruits and vegetables; cross-sectional and prospective analyses from the UK Women’s Cohort Study. J. Sleep Sci. Pract. 2018;2:13. doi: 10.1186/s41606-018-0032-0. [DOI] [Google Scholar]
- 83.Noorwali E.A., Hardie L.J., Cade J.E. Fruit and Vegetable Consumption and Their Polyphenol Content Are Inversely Associated with Sleep Duration: Prospective Associations from the UK Women’s Cohort Study. Nutrients. 2018;10:1803. doi: 10.3390/nu10111803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Noorwali E.A., Cade J.E., Burley V.J., Hardie L.J. The relationship between sleep duration and fruit/vegetable intakes in UK adults: A cross-sectional study from the National Diet and Nutrition Survey. BMJ Open. 2018;8:e020810. doi: 10.1136/bmjopen-2017-020810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bixler E. Sleep and society: An epidemiological perspective. Sleep Med. 2009;10:S3–S6. doi: 10.1016/j.sleep.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 86.Magee C.A., Iverson D.C., Caputi P. Factors associated with short and long sleep. Prev. Med. 2009;49:461–467. doi: 10.1016/j.ypmed.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 87.Grandner M., Kripke D. Self-reported sleep complaints with long and short sleep: A nationally representative sample. Psychosom. Med. 2004;66:239–241. doi: 10.1097/01.PSY.0000107881.53228.4D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Theorell-Haglow J., Berglund L., Janson C., Lindberg E. Sleep duration and central obesity in women-differences between short sleepers and long sleepers. Sleep Med. 2012;13:1079–1085. doi: 10.1016/j.sleep.2012.06.013. [DOI] [PubMed] [Google Scholar]
- 89.Tu X., Cai H., Gao Y.T., Wu X., Ji B.T., Yang G., Li H., Zheng W., Shu X.O. Sleep duration and its correlates in middle-aged and elderly Chinese women: The Shanghai Women’s Health Study. Sleep Med. 2012;13:1138–1145. doi: 10.1016/j.sleep.2012.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Potter G.D.M., Cade J.E., Hardie L.J. Longer sleep is associated with lower BMI and favorable metabolic profiles in UK adults: Findings from the National Diet and Nutrition Survey. PLoS ONE. 2017;12:e0182195. doi: 10.1371/journal.pone.0182195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wang S., Li B., Wu Y., Ungvari G.S., Ng C.H., Fu Y., Kou C., Yu Y., Sun H.Q., Xiang Y.T. Relationship of Sleep Duration with Sociodemographic Characteristics, Lifestyle, Mental Health, and Chronic Diseases in a Large Chinese Adult Population. J. Clin. Sleep Med. 2017;13:377–384. doi: 10.5664/jcsm.6484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sekine M., Tatsuse T., Cable N., Chandola T., Marmot M. U-shaped associations between time in bed and the physical and mental functioning of Japanese civil servants: The roles of work, family, behavioral and sleep quality characteristics. Sleep Med. 2014;15:1122–1131. doi: 10.1016/j.sleep.2014.04.012. [DOI] [PubMed] [Google Scholar]
- 93.Zhan Y., Chen R., Yu J. Sleep duration and abnormal serum lipids: The China Health and Nutrition Survey. Sleep Med. 2014;15:833–839. doi: 10.1016/j.sleep.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 94.Tan N.Y.Q., Chew M., Tham Y.C., Nguyen Q.D., Yasuda M., Cheng C.Y., Wong T.Y., Sabanayagam C. Associations between sleep duration, sleep quality and diabetic retinopathy. PLoS ONE. 2018;13:e0196399. doi: 10.1371/journal.pone.0196399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wu L., Sun D.L. Sleep duration and falls: A systemic review and meta-analysis of observational studies. J. Sleep Res. 2017;26:293–301. doi: 10.1111/jsr.12505. [DOI] [PubMed] [Google Scholar]
- 96.Johnson B.T., Acabchuk R.L. What are the keys to a longer, happier life? Answers from five decades of health psychology research. Soc. Sci. Med. 2018;196:218–226. doi: 10.1016/j.socscimed.2017.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Patel S.R., Malhotra A., White D.P., Gottlieb D.J., Hu F.B. Association between reduced sleep and weight gain in women. Am. J. Epidemiol. 2006;164:947–954. doi: 10.1093/aje/kwj280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mota M.C., Waterhouse J., De-Souza D.A., Rossato L.T., Silva C.M., Araujo M.B., Tufik S., de Mello M.T., Crispim C.A. Sleep pattern is associated with adipokine levels and nutritional markers in resident physicians. Chronobiol. Int. 2014;31:1130–1138. doi: 10.3109/07420528.2014.957300. [DOI] [PubMed] [Google Scholar]
- 99.Mossavar-Rahmani Y., Jung M., Patel S.R., Sotres-Alvarez D., Arens R., Ramos A., Redline S., Rock C.L., Van Horn L. Eating behavior by sleep duration in the Hispanic Community Health Study/Study of Latinos. Appetite. 2015;95:275–284. doi: 10.1016/j.appet.2015.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.van Lee L., Chia A.R., Loy S.L., Colega M., Tham E.K.H., Cai S., Yap F., Godfrey K.M., Teoh O.H., Goh D., et al. Sleep and Dietary Patterns in Pregnancy: Findings from the GUSTO Cohort. Int J. Environ. Res. Public Health. 2017;14:1409. doi: 10.3390/ijerph14111409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Adams T.B., Colner W. The association of multiple risk factors with fruit and vegetable intake among a nationwide sample of college students. J. Am. Coll. Health. 2008;56:455–461. doi: 10.3200/JACH.56.44.455-464. [DOI] [PubMed] [Google Scholar]
- 102.Stamatakis K.A., Brownson R.C. Sleep duration and obesity-related risk factors in the rural Midwest. Prev. Med. 2008;46:439–444. doi: 10.1016/j.ypmed.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Buxton O.M., Quintiliani L.M., Yang M.H., Ebbeling C.B., Stoddard A.M., Pereira L.K., Sorensen G. Association of sleep adequacy with more healthful food choices and positive workplace experiences among motor freight workers. Am. J. Public Health. 2009;99:S636–S643. doi: 10.2105/AJPH.2008.158501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Baron K.G., Reid K.J., Kern A.S., Zee P.C. Role of sleep timing in caloric intake and BMI. Obesity. 2011;19:1374–1381. doi: 10.1038/oby.2011.100. [DOI] [PubMed] [Google Scholar]
- 105.Kim S., DeRoo L.A., Sandler D.P. Eating patterns and nutritional characteristics associated with sleep duration. Public Health Nutr. 2011;14:889–895. doi: 10.1017/S136898001000296X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Haghighatdoost F., Karimi G., Esmaillzadeh A., Azadbakht L. Sleep deprivation is associated with lower diet quality indices and higher rate of general and central obesity among young female students in Iran. Nutrition. 2012;28:1146–1150. doi: 10.1016/j.nut.2012.04.015. [DOI] [PubMed] [Google Scholar]
- 107.Mosca M., Aggarwal B. Sleep duration, snoring habits, and cardiovascular disease risk factors in an ethnically diverse population. J. Cardiovasc. Nurs. 2012;27:263–269. doi: 10.1097/JCN.0b013e31821e7ad1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chang M.W., Brown R., Nitzke S., Smith B., Eghtedary K. Stress, sleep, depression and dietary intakes among low-income overweight and obese pregnant women. Matern. Child. Health J. 2015;19:1047–1059. doi: 10.1007/s10995-014-1604-y. [DOI] [PubMed] [Google Scholar]
- 109.Patterson F., Malone S.K., Lozano A., Grandner M.A., Hanlon A.L. Smoking, Screen-Based Sedentary Behavior, and Diet Associated with Habitual Sleep Duration and Chronotype: Data from the UK Biobank. Ann. Behav. Med. 2016;50:715–726. doi: 10.1007/s12160-016-9797-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Silva C.M., Mota M.C., Miranda M.T., Paim S.L., Waterhouse J., Crispim C.A. Chronotype, social jetlag and sleep debt are associated with dietary intake among Brazilian undergraduate students. Chronobiol. Int. 2016;33:740–748. doi: 10.3109/07420528.2016.1167712. [DOI] [PubMed] [Google Scholar]
- 111.Xiao R.S., Moore Simas T.A., Pagoto S.L., Person S.D., Rosal M.C., Waring M.E. Sleep Duration and Diet Quality Among Women within 5 Years of Childbirth in the United States: A Cross-Sectional Study. Matern. Child. Health J. 2016;20:1869–1877. doi: 10.1007/s10995-016-1991-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Doo M., Kim Y. The Risk of Being Obese According to Short Sleep Duration Is Modulated after Menopause in Korean Women. Nutrients. 2017;9:206. doi: 10.3390/nu9030206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kleiser C., Wawro N., Stelmach-Mardas M., Boeing H., Gedrich K., Himmerich H., Linseisen J. Are sleep duration, midpoint of sleep and sleep quality associated with dietary intake among Bavarian adults? Eur. J. Clin. Nutr. 2017;71:631–637. doi: 10.1038/ejcn.2016.264. [DOI] [PubMed] [Google Scholar]
- 114.Mossavar-Rahmani Y., Weng J., Wang R., Shaw P.A., Jung M., Sotres-Alvarez D., Castaneda S.F., Gallo L.C., Gellman M.D., Qi Q., et al. Actigraphic sleep measures and diet quality in the Hispanic Community Health Study/Study of Latinos Sueno ancillary study. J. Sleep Res. 2017;26:739–746. doi: 10.1111/jsr.12513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Timmermans M., Mackenbach J.D., Charreire H., Bardos H., Compernolle S., De Bourdeaudhuij I., Oppert J.M., Rutter H., McKee M., Lakerveld J. Exploring the mediating role of energy balance-related behaviours in the association between sleep duration and obesity in European adults. The SPOTLIGHT project. Prev. Med. 2017;100:25–32. doi: 10.1016/j.ypmed.2017.03.021. [DOI] [PubMed] [Google Scholar]
- 116.Gebski J., Jezewska-Zychowicz M., Guzek D., Swiatkowska M., Stangierska D., Plichta M. The Associations between Dietary Patterns and Short Sleep Duration in Polish Adults (LifeStyle Study) Int. J. Environ. Res. Public Health. 2018;15:2497. doi: 10.3390/ijerph15112497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Patterson F., Malone S.K., Grandner M.A., Lozano A., Perkett M., Hanlon A. Interactive effects of sleep duration and morning/evening preference on cardiovascular risk factors. Eur. J. Public Health. 2018;28:155–161. doi: 10.1093/eurpub/ckx029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Peltzer K., Pengpid S. Self-Reported Sleep Duration and Its Correlates with Sociodemographics, Health Behaviours, Poor Mental Health, and Chronic Conditions in Rural Persons 40 Years and Older in South Africa. Int. J. Environ. Res. Public Health. 2018;15:1357. doi: 10.3390/ijerph15071357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Tan S.L., Storm V., Reinwand D.A., Wienert J., de Vries H., Lippke S. Understanding the Positive Associations of Sleep, Physical Activity, Fruit and Vegetable Intake as Predictors of Quality of Life and Subjective Health Across Age Groups: A Theory Based, Cross-Sectional Web-Based Study. Front. Psychol. 2018;9:977. doi: 10.3389/fpsyg.2018.00977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Vezina-Im L.A., Lebel A., Gagnon P., Nicklas T.A., Baranowski T. Individual Correlates of Sleep Among Childbearing Age Women in Canada. Behav. Sleep Med. 2018:1–12. doi: 10.1080/15402002.2018.1435547. [DOI] [PubMed] [Google Scholar]
- 121.Vezina-Im L.A., Lebel A., Gagnon P., Nicklas T.A., Baranowski T. Association between sleep and overweight/obesity among women of childbearing age in Canada. Can. J. Public Health. 2018;109:516–526. doi: 10.17269/s41997-018-0071-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hoefelmann L.P., Lopes Ada S., Silva K.S., Silva S.G., Cabral L.G., Nahas M.V. Lifestyle, self-reported morbidities, and poor sleep quality among Brazilian workers. Sleep Med. 2012;13:1198–1201. doi: 10.1016/j.sleep.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 123.Beydoun M.A., Gamaldo A.A., Canas J.A., Beydoun H.A., Shah M.T., McNeely J.M., Zonderman A.B. Serum nutritional biomarkers and their associations with sleep among US adults in recent national surveys. PLoS ONE. 2014;9:e103490. doi: 10.1371/journal.pone.0103490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Katagiri R., Asakura K., Kobayashi S., Suga H., Sasaki S. Low Intake of Vegetables, High Intake of Confectionary, and Unhealthy Eating Habits are Associated with Poor Sleep Quality among Middle-aged Female Japanese Workers. J. Occup. Health. 2014;56:359–368. doi: 10.1539/joh.14-0051-OA. [DOI] [PubMed] [Google Scholar]
- 125.Grandner M.A., Jackson N.J., Izci-Balserak B., Gallagher R.A., Murray-Bachmann R., Williams N.J., Patel N.P., Jean-Louis G. Social and Behavioral Determinants of Perceived Insufficient Sleep. Front. Neurol. 2015;6:112. doi: 10.3389/fneur.2015.00112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kurotani K., Kochi T., Nanri A., Eguchi M., Kuwahara K., Tsuruoka H., Akter S., Ito R., Pham N.M., Kabe I., et al. Dietary patterns and sleep symptoms in Japanese workers: The Furukawa Nutrition and Health Study. Sleep Med. 2015;16:298–304. doi: 10.1016/j.sleep.2014.09.017. [DOI] [PubMed] [Google Scholar]
- 127.Quick V., Byrd-Bredbenner C., Shoff S., White A.A., Lohse B., Horacek T., Colby S., Brown O., Kidd T., Greene G. Relationships of Sleep Duration with Weight-Related Behaviors of U.S. College Students. Behav. Sleep Med. 2016;14:565–580. doi: 10.1080/15402002.2015.1065411. [DOI] [PubMed] [Google Scholar]
- 128.Perez-Rodrigo C., Gianzo-Citores M., Gil A., Gonzalez-Gross M., Ortega R.M., Serra-Majem L., Varela-Moreiras G., Aranceta-Bartrina J. Lifestyle Patterns and Weight Status in Spanish Adults: The ANIBES Study. Nutrients. 2017;9 doi: 10.3390/nu9060606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wang S., Wu Y., Ungvari G.S., Ng C.H., Forester B.P., Gatchel J.R., Chiu H.F.K., Kou C., Fu Y., Qi Y., et al. Sleep duration and its association with demographics, lifestyle factors, poor mental health and chronic diseases in older Chinese adults. Psychiatry Res. 2017;257:212–218. doi: 10.1016/j.psychres.2017.07.036. [DOI] [PubMed] [Google Scholar]
- 130.Altman D.G., Bland J.M. Measurement in Medicine-the Analysis of Method Comparison Studies. J. Roy. Stat. Soc. D-STA. 1983;32:307–317. doi: 10.2307/2987937. [DOI] [Google Scholar]
- 131.Roenneberg T., Wirz-Justice A., Merrow M. Life between clocks: Daily temporal patterns of human chronotypes. J. Biol Rhythms. 2003;18:80–90. doi: 10.1177/0748730402239679. [DOI] [PubMed] [Google Scholar]
- 132.Horne J.A., Ostberg O. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int. J. Chronobiol. 1976;4:97–110. [PubMed] [Google Scholar]
- 133.Almoosawi S., Vingeliene S., Gachon F., Voortman T., Palla L., Johnston J.D., Van Dam R.M., Darimont C., Karagounis L.G. Chronotype: Implications for Epidemiologic Studies on Chrono-Nutrition and Cardiometabolic Health. Adv. Nutr. 2019;10:30–42. doi: 10.1093/advances/nmy070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Von Schantz M. Phenotypic effects of genetic variability in human clock genes on circadian and sleep parameters. J. Genet. 2008;87:513–519. doi: 10.1007/s12041-008-0074-7. [DOI] [PubMed] [Google Scholar]
- 135.Smith M.R., Burgess H.J., Fogg L.F., Eastman C.I. Familial advanced sleep-phase syndrome: A short-period circadian rhythm variant in humans. Nat. Med. 1999;5:1062–1065. doi: 10.1038/12502. [DOI] [PubMed] [Google Scholar]
- 136.Smith M.R., Burgess H.J., Fogg L.F., Eastman C.I. Racial differences in the human endogenous circadian period. PLoS ONE. 2009;4:e6014. doi: 10.1371/journal.pone.0006014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lehnkering H., Siegmund R. Influence of chronotype, season, and sex of subject on sleep behavior of young adults. Chronobiol. Int. 2007;24:875–888. doi: 10.1080/07420520701648259. [DOI] [PubMed] [Google Scholar]
- 138.Roenneberg T., Allebrandt K.V., Merrow M., Vetter C. Social jetlag and obesity. Curr. Biol. 2012;22:939–943. doi: 10.1016/j.cub.2012.03.038. [DOI] [PubMed] [Google Scholar]
- 139.Adan A. Chronotype and personality factors in the daily consumption of alcohol and psychostimulants. Addiction. 1994;89:455–462. doi: 10.1111/j.1360-0443.1994.tb00926.x. [DOI] [PubMed] [Google Scholar]
- 140.Reutrakul S., Knutson K.L. Consequences of Circadian Disruption on Cardiometabolic Health. Sleep Med. Clin. 2015;10:455–468. doi: 10.1016/j.jsmc.2015.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Merikanto I., Lahti T., Puolijoki H., Vanhala M., Peltonen M., Laatikainen T., Vartiainen E., Salomaa V., Kronholm E., Partonen T. Associations of chronotype and sleep with cardiovascular diseases and type 2 diabetes. Chronobiol. Int. 2013;30:470–477. doi: 10.3109/07420528.2012.741171. [DOI] [PubMed] [Google Scholar]
- 142.Yu J.H., Yun C.H., Ahn J.H., Suh S., Cho H.J., Lee S.K., Yoo H.J., Seo J.A., Kim S.G., Choi K.M., et al. Evening Chronotype Is Associated with Metabolic Disorders and Body Composition in Middle-Aged Adults. J. Clin. Endocrinol. Metab. 2015;100:1494–1502. doi: 10.1210/jc.2014-3754. [DOI] [PubMed] [Google Scholar]
- 143.Melo M.C.A., Abreu R.L.C., Linhares Neto V.B., de Bruin P.F.C., de Bruin V.M.S. Chronotype and circadian rhythm in bipolar disorder: A systematic review. Sleep Med. Rev. 2017;34:46–58. doi: 10.1016/j.smrv.2016.06.007. [DOI] [PubMed] [Google Scholar]
- 144.Knutson K.L., von Schantz M. Associations between chronotype, morbidity and mortality in the UK Biobank cohort. Chronobiol. Int. 2018;35:1045–1053. doi: 10.1080/07420528.2018.1454458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Malone S.K., Zemel B., Compher C., Souders M., Chittams J., Thompson A.L., Lipman T.H. Characteristics Associated with Sleep Duration, Chronotype, and Social Jet Lag in Adolescents. J. Sch. Nurs. 2016;32:120–131. doi: 10.1177/1059840515603454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Sato-Mito N., Shibata S., Sasaki S., Sato K. Dietary intake is associated with human chronotype as assessed by both morningness-eveningness score and preferred midpoint of sleep in young Japanese women. Int. J. Food Sci. Nutr. 2011;62:525–532. doi: 10.3109/09637486.2011.560563. [DOI] [PubMed] [Google Scholar]
- 147.Sato-Mito N., Sasaki S., Murakami K., Okubo H., Takahashi Y., Shibata S., Yamada K., Sato K. The midpoint of sleep is associated with dietary intake and dietary behavior among young Japanese women. Sleep Med. 2011;12:289–294. doi: 10.1016/j.sleep.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 148.Yoshizaki T., Komatsu T., Tada Y., Hida A., Kawano Y., Togo F. Association of habitual dietary intake with morningness-eveningness and rotating shift work in Japanese female nurses. Chronobiol. Int. 2018;35:392–404. doi: 10.1080/07420528.2017.1410169. [DOI] [PubMed] [Google Scholar]
- 149.Kanerva N., Kronholm E., Partonen T., Ovaskainen M.L., Kaartinen N.E., Konttinen H., Broms U., Mannisto S. Tendency toward eveningness is associated with unhealthy dietary habits. Chronobiol. Int. 2012;29:920–927. doi: 10.3109/07420528.2012.699128. [DOI] [PubMed] [Google Scholar]
- 150.Fleig D., Randler C. Association between chronotype and diet in adolescents based on food logs. Eat. Behav. 2009;10:115–118. doi: 10.1016/j.eatbeh.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 151.Mota M.C., Waterhouse J., De-Souza D.A., Rossato L.T., Silva C.M., Araujo M.B., Tufik S., de Mello M.T., Crispim C.A. Association between chronotype, food intake and physical activity in medical residents. Chronobiol. Int. 2016;33:730–739. doi: 10.3109/07420528.2016.1167711. [DOI] [PubMed] [Google Scholar]
- 152.Knutson K.L., Spiegel K., Penev P., Van Cauter E. The metabolic consequences of sleep deprivation. Sleep Med. Rev. 2007;11:163–178. doi: 10.1016/j.smrv.2007.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Patel S.R., Hu F.B. Short sleep duration and weight gain: A systematic review. Obesity. 2008;16:643–653. doi: 10.1038/oby.2007.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Chaput J.-P., Tremblay A. Insufficient Sleep as a Contributor to Weight Gain: An Update. Curr. Obes. Rep. 2012;1:245–256. doi: 10.1007/s13679-012-0026-7. [DOI] [Google Scholar]
- 155.Chaput J.P. Sleep patterns, diet quality and energy balance. Physiol. Behav. 2014;134:86–91. doi: 10.1016/j.physbeh.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 156.Lundahl A., Nelson T.D. Sleep and food intake: A multisystem review of mechanisms in children and adults. J. Health Psychol. 2015;20:794–805. doi: 10.1177/1359105315573427. [DOI] [PubMed] [Google Scholar]
- 157.Bayon V., Leger D., Gomez-Merino D., Vecchierini M.F., Chennaoui M. Sleep debt and obesity. Ann. Med. 2014;46:264–272. doi: 10.3109/07853890.2014.931103. [DOI] [PubMed] [Google Scholar]
- 158.Klok M.D., Jakobsdottir S., Drent M.L. The role of leptin and ghrelin in the regulation of food intake and body weight in humans: A review. Obes. Rev. 2007;8:213–214. doi: 10.1111/j.1467-789X.2006.00270.x. [DOI] [PubMed] [Google Scholar]
- 159.Mullington J., Chan J., Van Dongen H., Szuba M., Samaras J., Price N., Meier-Ewert H., Dinges D., Mantzoros C. Sleep Loss Reduces Diurnal Rhythm Amplitude of Leptin in Healthy Men. J. Neuroendocrinol. 2003;15:851–854. doi: 10.1046/j.1365-2826.2003.01069.x. [DOI] [PubMed] [Google Scholar]
- 160.Spiegel K., Leproult R., L’Hermite-Baleriaux M., Copinschi G., Penev P.D., Van Cauter E. Leptin levels are dependent on sleep duration: Relationships with sympathovagal balance, carbohydrate regulation, cortisol, and thyrotropin. J. Clin. Endocrinol. Metab. 2004;89:5762–5771. doi: 10.1210/jc.2004-1003. [DOI] [PubMed] [Google Scholar]
- 161.Schmid S.M., Hallschmid M., Jauch-Chara K., Born J., Schultes B. A single night of sleep deprivation increases ghrelin levels and feelings of hunger in normal-weight healthy men. J. Sleep Res. 2008;17:331–334. doi: 10.1111/j.1365-2869.2008.00662.x. [DOI] [PubMed] [Google Scholar]
- 162.Hogenkamp P.S., Nilsson E., Nilsson V.C., Chapman C.D., Vogel H., Lundberg L.S., Zarei S., Cedernaes J., Rangtell F.H., Broman J.E., et al. Acute sleep deprivation increases portion size and affects food choice in young men. Psychoneuroendocrinology. 2013;38:1668–1674. doi: 10.1016/j.psyneuen.2013.01.012. [DOI] [PubMed] [Google Scholar]
- 163.Hart C.N., Carskadon M.A., Considine R.V., Fava J.L., Lawton J., Raynor H.A., Jelalian E., Owens J., Wing R. Changes in Children’s Sleep Duration on Food Intake, Weight, and Leptin. Pediatrics. 2013;132:e1478. doi: 10.1542/peds.2013-1274. [DOI] [PubMed] [Google Scholar]
- 164.Broussard J.L., Kilkus J.M., Delebecque F., Abraham V., Day A., Whitmore H.R., Tasali E. Elevated ghrelin predicts food intake during experimental sleep restriction. Obesity. 2016;24:132–138. doi: 10.1002/oby.21321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Spiegel K., Leproult R., Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet. 1999;354:1435–1439. doi: 10.1016/S0140-6736(99)01376-8. [DOI] [PubMed] [Google Scholar]
- 166.Pejovic S., Vgontzas A.N., Basta M., Tsaoussoglou M., Zoumakis E., Vgontzas A., Bixler E.O., Chrousos G.P. Leptin and hunger levels in young healthy adults after one night of sleep loss. J. Sleep Res. 2010;19:552–558. doi: 10.1111/j.1365-2869.2010.00844.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Calvin A.D., Carter R.E., Adachi T., Macedo P.G., Albuquerque F.N., van der Walt C., Bukartyk J., Davison D.E., Levine J.A., Somers V.K. Effects of experimental sleep restriction on caloric intake and activity energy expenditure. Chest. 2013;144:79–86. doi: 10.1378/chest.12-2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Schussler P., Uhr M., Ising M., Weikel J.C., Schmid D.A., Held K., Mathias S., Steiger A. Nocturnal ghrelin, ACTH, GH and cortisol secretion after sleep deprivation in humans. Psychoneuroendocrinology. 2006;31:915–923. doi: 10.1016/j.psyneuen.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 169.Taheri S., Lin L., Austin D., Young T., Mignot E. Short sleep duration is associated with reduced leptin, elevated ghrelin, and increased body mass index. PLoS Med. 2004;1:e62. doi: 10.1371/journal.pmed.0010062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.St-Onge M.P. Impact of sleep duration on food intake regulation: Different mechanisms by sex? Obesity (Silver Spring) 2016;24:11. doi: 10.1002/oby.21374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Chaput J.P., St-Onge M.P. Increased food intake by insufficient sleep in humans: Are we jumping the gun on the hormonal explanation? Front. Endocrinol. 2014;5:116. doi: 10.3389/fendo.2014.00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Chapman C.D., Benedict C., Brooks S.J., Schioth H.B. Lifestyle determinants of the drive to eat: A meta-analysis. Am. J. Clin. Nutr. 2012;96:492–497. doi: 10.3945/ajcn.112.039750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Greer S.M., Goldstein A.N., Walker M.P. The impact of sleep deprivation on food desire in the human brain. Nat. Commun. 2013;4:2259. doi: 10.1038/ncomms3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Chapman C.D., Nilsson E.K., Nilsson V.C., Cedernaes J., Rangtell F.H., Vogel H., Dickson S.L., Broman J.E., Hogenkamp P.S., Schioth H.B., et al. Acute sleep deprivation increases food purchasing in men. Obesity. 2013;21:E555–E560. doi: 10.1002/oby.20579. [DOI] [PubMed] [Google Scholar]
- 175.Tan X., Chapman C.D., Cedernaes J., Benedict C. Association between long sleep duration and increased risk of obesity and type 2 diabetes: A review of possible mechanisms. Sleep Med. Rev. 2018;40:127–134. doi: 10.1016/j.smrv.2017.11.001. [DOI] [PubMed] [Google Scholar]
- 176.Lutter M., Nestler E.J. Homeostatic and hedonic signals interact in the regulation of food intake. J. Nutr. 2009;139:629–632. doi: 10.3945/jn.108.097618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Chaput J.P. Short sleep duration promoting overconsumption of food: A reward-driven eating behavior? Sleep. 2010;33:1135–1136. doi: 10.1093/sleep/33.9.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Benedict C., Brooks S.J., O’Daly O.G., Almen M.S., Morell A., Aberg K., Gingnell M., Schultes B., Hallschmid M., Broman J.E., et al. Acute sleep deprivation enhances the brain’s response to hedonic food stimuli: An fMRI study. J. Clin. Endocrinol. Metab. 2012;97:E443–E447. doi: 10.1210/jc.2011-2759. [DOI] [PubMed] [Google Scholar]
- 179.St-Onge M.P., McReynolds A., Trivedi Z.B., Roberts A.L., Sy M., Hirsch J. Sleep restriction leads to increased activation of brain regions sensitive to food stimuli. Am. J. Clin. Nutr. 2012;95:818–824. doi: 10.3945/ajcn.111.027383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Killgore W.D., Schwab Z.J., Weber M., Kipman M., Deldonno S.R., Weiner M.R., Rauch S.L. Daytime sleepiness affects prefrontal regulation of food intake. Neuroimage. 2013;71:216–223. doi: 10.1016/j.neuroimage.2013.01.018. [DOI] [PubMed] [Google Scholar]
- 181.St-Onge M.P., Wolfe S., Sy M., Shechter A., Hirsch J. Sleep restriction increases the neuronal response to unhealthy food in normal-weight individuals. Int. J. Obes. 2014;38:411–416. doi: 10.1038/ijo.2013.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.McNeil J., Forest G., Hintze L.J., Brunet J.F., Finlayson G., Blundell J.E., Doucet E. The effects of partial sleep restriction and altered sleep timing on appetite and food reward. Appetite. 2017;109:48–56. doi: 10.1016/j.appet.2016.11.020. [DOI] [PubMed] [Google Scholar]
- 183.Hays N.P., Roberts S.B. Aspects of eating behaviors "disinhibition" and "restraint" are related to weight gain and BMI in women. Obesity. 2008;16:52–58. doi: 10.1038/oby.2007.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Kelly N.R., Shomaker L.B., Radin R.M., Thompson K.A., Cassidy O.L., Brady S., Mehari R., Courville A.B., Chen K.Y., Galescu O.A., et al. Associations of sleep duration and quality with disinhibited eating behaviors in adolescent girls at-risk for type 2 diabetes. Eat. Behav. 2016;22:149–155. doi: 10.1016/j.eatbeh.2016.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Blumfield M.L., Bei B., Zimberg I.Z., Cain S.W. Dietary disinhibition mediates the relationship between poor sleep quality and body weight. Appetite. 2018;120:602–608. doi: 10.1016/j.appet.2017.10.022. [DOI] [PubMed] [Google Scholar]
- 186.Chaput J.P., Després J.P., Bouchard C., Tremblay A. The Association between Short Sleep Duration and Weight Gain is Dependent on Disinhibited eating Behavior in Adults. Sleep. 2011;34:1291–1297. doi: 10.5665/SLEEP.1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Bryant E.J., King N.A., Blundell J.E. Disinhibition: Its effects on appetite and weight regulation. Obes. Rev. 2008;9:409–419. doi: 10.1111/j.1467-789X.2007.00426.x. [DOI] [PubMed] [Google Scholar]
- 188.Contento I.R., Zybert P., Williams S.S. Relationship of cognitive restraint of eating and disinhibition to the quality of food choices of Latina women and their young children. Prev. Med. 2005;40:326–336. doi: 10.1016/j.ypmed.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 189.Borg P., Fogelholm M., Kukkonen-Harjula K. Food selection and eating behaviour during weight maintenance intervention and 2-y follow-up in obese men. Int. J. Obes. 2004;28:1548–1554. doi: 10.1038/sj.ijo.0802790. [DOI] [PubMed] [Google Scholar]
- 190.Dweck J.S., Jenkins S.M., Nolan L.J. The role of emotional eating and stress in the influence of short sleep on food consumption. Appetite. 2014;72:106–113. doi: 10.1016/j.appet.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 191.Beattie L., Kyle S.D., Espie C.A., Biello S.M. Social interactions, emotion and sleep: A systematic review and research agenda. Sleep Med. Rev. 2015;24:83–100. doi: 10.1016/j.smrv.2014.12.005. [DOI] [PubMed] [Google Scholar]
- 192.Gibson E.L. Emotional influences on food choice: Sensory, physiological and psychological pathways. Physiol. Behav. 2006;89:53–61. doi: 10.1016/j.physbeh.2006.01.024. [DOI] [PubMed] [Google Scholar]
- 193.Rossa K.R., Smith S.S., Allan A.C., Sullivan K.A. The effects of sleep restriction on executive inhibitory control and affect in young adults. J. Adolesc. Health. 2014;55:287–292. doi: 10.1016/j.jadohealth.2013.12.034. [DOI] [PubMed] [Google Scholar]
- 194.Swinburn B., Egger G. Preventive strategies against weight gain and obesity. Obes. Rev. 2002;3:289–301. doi: 10.1046/j.1467-789X.2002.00082.x. [DOI] [PubMed] [Google Scholar]
- 195.Khurana S., Venkataraman K., Hollingsworth A., Piche M., Tai T.C. Polyphenols: Benefits to the cardiovascular system in health and in aging. Nutrients. 2013;5:3779–3827. doi: 10.3390/nu5103779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Du G., Sun L., Zhao R., Du L., Song J., Zhang L., He G., Zhang Y., Zhang J. Polyphenols: Potential source of drugs for the treatment of ischaemic heart disease. Pharmacol. Ther. 2016;162:23–34. doi: 10.1016/j.pharmthera.2016.04.008. [DOI] [PubMed] [Google Scholar]
- 197.Tressera-Rimbau A., Arranz S., Eder M., Vallverdu-Queralt A. Dietary Polyphenols in the Prevention of Stroke. Oxid. Med. Cell Longev. 2017;2017:7467962. doi: 10.1155/2017/7467962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Zhou Y., Zheng J., Li Y., Xu D.P., Li S., Chen Y.M., Li H.B. Natural Polyphenols for Prevention and Treatment of Cancer. Nutrients. 2016;8:515. doi: 10.3390/nu8080515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Manach C., Scalbert A., Morand C., Rémésy C., Jiménez L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004;79:727–747. doi: 10.1093/ajcn/79.5.727. [DOI] [PubMed] [Google Scholar]
- 200.Rendeiro C., Rhodes J.S., Spencer J.P.E. The mechanisms of action of flavonoids in the brain: Direct versus indirect effects. Neurochem. Int. 2015;89:126–139. doi: 10.1016/j.neuint.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 201.Rasch B., Born J. About sleep’s role in memory. Physiol. Rev. 2013;93:681–766. doi: 10.1152/physrev.00032.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Wang L.E., Cui X.Y., Cui S.Y., Cao J.X., Zhang J., Zhang Y.H., Zhang Q.Y., Bai Y.J., Zhao Y.Y. Potentiating effect of spinosin, a C-glycoside flavonoid of Semen Ziziphi spinosae, on pentobarbital-induced sleep may be related to postsynaptic 5-HT(1A) receptors. Phytomedicine. 2010;17:404–409. doi: 10.1016/j.phymed.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 203.Cho S., Yang H., Jeon Y.J., Lee C.J., Jin Y.H., Baek N.I., Kim D., Kang S.M., Yoon M., Yong H., et al. Phlorotannins of the edible brown seaweed Ecklonia cava Kjellman induce sleep via positive allosteric modulation of gamma-aminobutyric acid type A-benzodiazepine receptor: A novel neurological activity of seaweed polyphenols. Food Chem. 2012;132:1133–1142. doi: 10.1016/j.foodchem.2011.08.040. [DOI] [PubMed] [Google Scholar]
- 204.Cho S., Yoon M., Pae A.N., Jin Y.H., Cho N.C., Takata Y., Urade Y., Kim S., Kim J.S., Yang H., et al. Marine polyphenol phlorotannins promote non-rapid eye movement sleep in mice via the benzodiazepine site of the GABAA receptor. Psychopharmacology. 2014;231:2825–2837. doi: 10.1007/s00213-014-3445-1. [DOI] [PubMed] [Google Scholar]
- 205.Shrestha S., Park J.H., Lee D.Y., Cho J.G., Cho S., Yang H.J., Yong H.I., Yoon M.S., Han D.S., Baek N.I. Rhus parviflora and its biflavonoid constituent, rhusflavone, induce sleep through the positive allosteric modulation of GABA(A)-benzodiazepine receptors. J. Ethnopharmacol. 2012;142:213–220. doi: 10.1016/j.jep.2012.04.047. [DOI] [PubMed] [Google Scholar]
- 206.Yang H., Lee Y.C., Han K.S., Singh H., Yoon M., Park J.H., Cho C.W., Cho S. Green and gold kiwifruit peel ethanol extracts potentiate pentobarbital-induced sleep in mice via a GABAergic mechanism. Food Chem. 2013;136:160–163. doi: 10.1016/j.foodchem.2012.07.111. [DOI] [PubMed] [Google Scholar]
- 207.Zhao W., Li Y., Ma W., Ge Y., Huang Y. A study on quality components and sleep-promoting effects of GABA black tea. Food Funct. 2015;6:3393–3398. doi: 10.1039/C5FO00265F. [DOI] [PubMed] [Google Scholar]
- 208.Delgado J., Terron M.P., Garrido M., Pariente J.A., Barriga C., Rodriguez A.B., Paredes S.D. Diets enriched with a Jerte Valley cherry-based nutraceutical product reinforce nocturnal behaviour in young and old animals of nocturnal (Rattus norvegicus) and diurnal (Streptopelia risoria) chronotypes. J. Anim. Physiol. Anim. Nutr. 2013;97:137–145. doi: 10.1111/j.1439-0396.2011.01251.x. [DOI] [PubMed] [Google Scholar]
- 209.Pifferi F., Dal-Pan A., Menaker M., Aujard F. Resveratrol dietary supplementation shortens the free-running circadian period and decreases body temperature in a prosimian primate. J. Biol. Rhythms. 2011;26:271–275. doi: 10.1177/0748730411401788. [DOI] [PubMed] [Google Scholar]
- 210.Pifferi F., Dal-Pan A., Languille S., Aujard F. Effects of resveratrol on daily rhythms of locomotor activity and body temperature in young and aged grey mouse lemurs. Oxid. Med. Cell Longev. 2013;2013:187301. doi: 10.1155/2013/187301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Ribas-Latre A., Del Bas J.M., Baselga-Escudero L., Casanova E., Arola-Arnal A., Salvado M.J., Arola L., Blade C. Dietary proanthocyanidins modulate melatonin levels in plasma and the expression pattern of clock genes in the hypothalamus of rats. Mol. Nutr. Food Res. 2015;59:865–878. doi: 10.1002/mnfr.201400571. [DOI] [PubMed] [Google Scholar]
- 212.Oike H., Kobori M. Resveratrol Regulates Circadian Clock Genes in Rat-1 Fibroblast Cells. Biosci. Biotechnol. Biochem. 2014;72:3038–3040. doi: 10.1271/bbb.80426. [DOI] [PubMed] [Google Scholar]
- 213.Miranda J., Portillo M.P., Madrid J.A., Arias N., Macarulla M.T., Garaulet M. Effects of resveratrol on changes induced by high-fat feeding on clock genes in rats. Br. J. Nutr. 2013;110:1421–1428. doi: 10.1017/S0007114513000755. [DOI] [PubMed] [Google Scholar]
- 214.Ribas-Latre A., Baselga-Escudero L., Casanova E., Arola-Arnal A., Salvado M.J., Arola L., Blade C. Chronic consumption of dietary proanthocyanidins modulates peripheral clocks in healthy and obese rats. J. Nutr. Biochem. 2015;26:112–119. doi: 10.1016/j.jnutbio.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 215.Ribas-Latre A., Baselga-Escudero L., Casanova E., Arola-Arnal A., Salvado M.J., Blade C., Arola L. Dietary proanthocyanidins modulate BMAL1 acetylation, Nampt expression and NAD levels in rat liver. Sci. Rep. 2015;5:10954. doi: 10.1038/srep10954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Yoon M., Cho S. Triphlorethol A, a Dietary Polyphenol from Seaweed, Decreases Sleep Latency and Increases Non-Rapid Eye Movement Sleep in Mice. Mar. Drugs. 2018;16:139. doi: 10.3390/md16050139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Hosseini A., Sobhanifar M.A., Forouzanfar F., Aghaee A., Rakhshandeh H. Hypnotic Effect of Red Cabbage (Brassica oleracea) on Pentobarbital-Induced Sleep in Mice. J. Pharm. Bioallied Sci. 2018;10:48–53. doi: 10.4103/jpbs.JPBS_215_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Kim H.D., Hong K.B., Noh D.O., Suh H.J. Sleep-inducing effect of lettuce (Lactuca sativa) varieties on pentobarbital-induced sleep. Food Sci. Biotechnol. 2017;26:807–814. doi: 10.1007/s10068-017-0107-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Pifferi F., Rahman A., Languille S., Auffret A., Babiloni C., Blin O., Lamberty Y., Richardson J.C., Aujard F. Effects of dietary resveratrol on the sleep-wake cycle in the non-human primate gray mouse lemur (Microcebus murinus) Chronobiol. Int. 2012;29:261–270. doi: 10.3109/07420528.2011.654019. [DOI] [PubMed] [Google Scholar]
- 220.San A.M., Thongpraditchote S., Sithisarn P., Gritsanapan W. Total Phenolics and Total Flavonoids Contents and Hypnotic Effect in Mice of Ziziphus mauritiana Lam. Seed Extract. Evid. Based Complement. Alternat. Med. 2013;2013:835854. doi: 10.1155/2013/835854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Shinozaki A., Misawa K., Ikeda Y., Haraguchi A., Kamagata M., Tahara Y., Shibata S. Potent Effects of Flavonoid Nobiletin on Amplitude, Period, and Phase of the Circadian Clock Rhythm in PER2::LUCIFERASE Mouse Embryonic Fibroblasts. PLoS ONE. 2017;12:e0170904. doi: 10.1371/journal.pone.0170904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Qi G., Mi Y., Fan R., Zhao B., Ren B., Liu X. Tea polyphenols ameliorates neural redox imbalance and mitochondrial dysfunction via mechanisms linking the key circadian regular Bmal1. Food Chem. Toxicol. 2017;110:189–199. doi: 10.1016/j.fct.2017.10.031. [DOI] [PubMed] [Google Scholar]
- 223.Gadacha W., Ben-Attia M., Bonnefont-Rousselot D., Aouani E., Ghanem-Boughanmi N., Touitou Y. Resveratrol opposite effects on rat tissue lipoperoxidation: Pro-oxidant during day-time and antioxidant at night. Redox Rep. 2009;14:154–158. doi: 10.1179/135100009X466131. [DOI] [PubMed] [Google Scholar]
- 224.Colwell C.S. Rhythmic coupling among cells in the suprachiasmatic nucleus. J. Neurobiol. 2000;43:379–388. doi: 10.1002/1097-4695(20000615)43:4<379::AID-NEU6>3.0.CO;2-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Ramis M.R., Esteban S., Miralles A., Tan D.X., Reiter R.J. Caloric restriction, resveratrol and melatonin: Role of SIRT1 and implications for aging and related-diseases. Mech. Ageing Dev. 2015;146:28–41. doi: 10.1016/j.mad.2015.03.008. [DOI] [PubMed] [Google Scholar]
- 226.Orozco-Solis R., Ramadori G., Coppari R., Sassone-Corsi P. SIRT1 relays nutritional inputs to the circadian clock through the sf1 neurons of the ventromedial hypothalamus. Endocrinology. 2015;156:2174–2184. doi: 10.1210/en.2014-1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Lagouge M., Argmann C., Gerhart-Hines Z., Meziane H., Lerin C., Daussin F., Messadeq N., Milne J., Lambert P., Elliott P., et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell. 2006;127:1109–1122. doi: 10.1016/j.cell.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 228.Asher G., Gatfield D., Stratmann M., Reinke H., Dibner C., Kreppel F., Mostoslavsky R., Alt F., Schibler U. SIRT1 regulates circadian clock gene expression through PER2 deacetylation. Cell. 2008;134:317–328. doi: 10.1016/j.cell.2008.06.050. [DOI] [PubMed] [Google Scholar]
- 229.Meng X., Li Y., Li S., Zhou Y., Gan R.Y., Xu D.P., Li H.B. Dietary Sources and Bioactivities of Melatonin. Nutrients. 2017;9 doi: 10.3390/nu9040367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.St-Onge M.P., Crawford A., Aggarwal B. Plant-based diets: Reducing cardiovascular risk by improving sleep quality? Curr. Sleep Med. Rep. 2018;4:74–78. doi: 10.1007/s40675-018-0103-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Herrlinger K.A., Nieman K.M., Sanoshy K.D., Fonseca B.A., Lasrado J.A., Schild A.L., Maki K.C., Wesnes K.A., Ceddia M.A. Spearmint Extract Improves Working Memory in Men and Women with Age-Associated Memory Impairment. J. Altern. Complement. Med. 2018;24:374–377. doi: 10.1089/acm.2016.0379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Manach C., Williamson G., Morand C., Scalbert A., Remesy C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am. J. Clin. Nutr. 2005;81:230S–242S. doi: 10.1093/ajcn/81.1.230S. [DOI] [PubMed] [Google Scholar]
- 233.Kuratsune H., Umigai N., Takeno R., Kajimoto Y., Nakano T. Effect of crocetin from Gardenia jasminoides Ellis on sleep: A pilot study. Phytomedicine. 2010;17:840–843. doi: 10.1016/j.phymed.2010.03.025. [DOI] [PubMed] [Google Scholar]
- 234.Wightman E.L., Haskell-Ramsay C.F., Reay J.L., Williamson G., Dew T., Zhang W., Kennedy D.O. The effects of chronic trans-resveratrol supplementation on aspects of cognitive function, mood, sleep, health and cerebral blood flow in healthy, young humans. Br. J. Nutr. 2015;114:1427–1437. doi: 10.1017/S0007114515003037. [DOI] [PubMed] [Google Scholar]
- 235.Park I., Ochiai R., Ogata H., Kayaba M., Hari S., Hibi M., Katsuragi Y., Satoh M., Tokuyama K. Effects of subacute ingestion of chlorogenic acids on sleep architecture and energy metabolism through activity of the autonomic nervous system: A randomised, placebo-controlled, double-blinded cross-over trial. Br. J. Nutr. 2017;117:979–984. doi: 10.1017/S0007114517000587. [DOI] [PubMed] [Google Scholar]
- 236.Um M.Y., Kim J.Y., Han J.K., Kim J., Yang H., Yoon M., Kim J., Kang S.W., Cho S. Phlorotannin supplement decreases wake after sleep onset in adults with self-reported sleep disturbance: A randomized, controlled, double-blind clinical and polysomnographic study. Phytother. Res. 2018;32:698–704. doi: 10.1002/ptr.6019. [DOI] [PubMed] [Google Scholar]
- 237.Romain C., Alcaraz P.E., Chung L.H., Cases J. Regular consumption of HolisFiit, a polyphenol-rich extract-based food supplement, improves mind and body well-being of overweight and slightly obese volunteers: A randomized, double-blind, parallel trial. Int. J. Food Sci. Nutr. 2017;68:840–848. doi: 10.1080/09637486.2017.1292221. [DOI] [PubMed] [Google Scholar]
- 238.Uddin M., Biswas D., Ghosh A., O’Kennedy N., Duttaroy A.K. Consumption of Fruitflow® lowers blood pressure in pre-hypertensive males: A randomised, placebo controlled, double blind, cross-over study. Int. J. Food Sci. Nutr. 2018;69:494–502. doi: 10.1080/09637486.2017.1376621. [DOI] [PubMed] [Google Scholar]
- 239.Grassi D., Socci V., Tempesta D., Ferri C., De Gennaro L., Desideri G., Ferrara M. Flavanol-rich chocolate acutely improves arterial function and working memory performance counteracting the effects of sleep deprivation in healthy individuals. J. Hypertens. 2016;34:1298–1308. doi: 10.1097/HJH.0000000000000926. [DOI] [PubMed] [Google Scholar]
- 240.Bigelman K.A., Chapman D.P., Freese E.C., Trilk J.L., Cureton K.J. Effects of 6 Weeks of Quercetin Supplementation on Energy, Fatigue, and Sleep in ROTC Cadets. Mil. Med. 2011;176:565–572. doi: 10.7205/MILMED-D-09-00230. [DOI] [PubMed] [Google Scholar]
- 241.Cui Y., Niu K., Huang C., Momma H., Guan L., Kobayashi Y., Guo H., Chujo M., Otomo A., Nagatomi R. Relationship between daily isoflavone intake and sleep in Japanese adults: A cross-sectional study. Nutr. J. 2015;14:127. doi: 10.1186/s12937-015-0117-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Cao Y., Taylor A.W., Zhen S., Adams R., Appleton S., Shi Z. Soy Isoflavone Intake and Sleep Parameters over 5 Years among Chinese Adults: Longitudinal Analysis from the Jiangsu Nutrition Study. J. Acad. Nutr. Diet. 2017;117:536–544. doi: 10.1016/j.jand.2016.10.016. [DOI] [PubMed] [Google Scholar]
- 243.Greenwood D., Gilthorpe M., Golding C., Cade J. Stability over time of dietary patterns in the UK Women’s Cohort Study. Proc. Nutr. Soc. 2003;62:89A. [Google Scholar]
- 244.Borland S.E., Robinson S.M., Crozier S.R., Inskip H.M., Group S.W.S.S. Stability of dietary patterns in young women over a 2-year period. Eur. J. Clin. Nutr. 2008;62:119–126. doi: 10.1038/sj.ejcn.1602684. [DOI] [PubMed] [Google Scholar]
- 245.Jankovic N., Steppel M.T., Kampman E., de Groot L.C., Boshuizen H.C., Soedamah-Muthu S.S., Kromhout D., Feskens E.J. Stability of dietary patterns assessed with reduced rank regression; the Zutphen Elderly Study. Nutr. J. 2014;13:30. doi: 10.1186/1475-2891-13-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Crozier S.R., Robinson S.M., Godfrey K.M., Cooper C., Inskip H.M. Women’s Dietary Patterns Change Little from Before to During Pregnancy. J. Nutr. 2009;139:1956–1963. doi: 10.3945/jn.109.109579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Newby P.K., Weismayer C., Akesson A., Tucker K.L., Wolk A. Long-term stability of food patterns identified by use of factor analysis among Swedish women. J. Nutr. 2006;136:626–633. doi: 10.1093/jn/136.3.626. [DOI] [PubMed] [Google Scholar]
- 248.Perry G.S., Patil S.P., Presley-Cantrell L.R. Raising Awareness of Sleep as a Healthy Behavior. Prev. Chronic Dis. 2013;10 doi: 10.5888/pcd10.130081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Heffron T.M. Sleep Well, Be Well: A National Health Priority. [(accessed on 13 April 2019)];2014 Available online: http://sleepeducation.org/news/2014/05/16/sleep-well-be-well-a-national-health-priority.
- 250.Kakinuma M., Takahashi M., Kato N., Aratake Y., Watanabe M., Ishikawa Y., Kojima R., Shibaoka M., Tanaka K. Effect of brief sleep hygiene education for workers of an information technology company. Ind. Health. 2010;48:758–765. doi: 10.2486/indhealth.MS1083. [DOI] [PubMed] [Google Scholar]
- 251.Chen P.H., Kuo H.Y., Chueh K.H. Sleep Hygiene Education: Efficacy on Sleep Quality in Working Women. J. Nurs. Res. 2010;18:283–289. doi: 10.1097/JNR.0b013e3181fbe3fd. [DOI] [PubMed] [Google Scholar]
- 252.Galland B.C., Mitchell E.A. Helping children sleep. Arch. Dis. Child. 2010;95:850–853. doi: 10.1136/adc.2009.162974. [DOI] [PubMed] [Google Scholar]
- 253.Tan E., Healey D., Gray A.R., Galland B.C. Sleep hygiene intervention for youth aged 10 to 18 years with problematic sleep: A before-after pilot study. BMC Pediatr. 2012;12:189. doi: 10.1186/1471-2431-12-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Golem D.L., Martin-Biggers J.T., Koenings M.M., Davis K.F., Byrd-Bredbenner C. An integrative review of sleep for nutrition professionals. Adv. Nutr. 2014;5:742–759. doi: 10.3945/an.114.006809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.The Eatwell Guide. [(accessed on 15 March 2019)]; Available online: https://www.nhs.uk/live-well/eat-well/the-eatwell-guide/
- 256.Rekhy R., McConchie R. Promoting consumption of fruit and vegetables for better health. Have campaigns delivered on the goals? Appetite. 2014;79:113–123. doi: 10.1016/j.appet.2014.04.012. [DOI] [PubMed] [Google Scholar]
- 257.Buysse D.J., Reynolds C.F., 3rd, Monk T.H., Berman S.R., Kupfer D.J. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 258.Kitamura S., Katayose Y., Nakazaki K., Motomura Y., Oba K., Katsunuma R., Terasawa Y., Enomoto M., Moriguchi Y., Hida A., et al. Estimating individual optimal sleep duration and potential sleep debt. Sci. Rep. 2016;6:35812. doi: 10.1038/srep35812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Goel N., Banks S., Mignot E., Dinges D.F. DQB1*0602 predicts interindividual differences in physiologic sleep, sleepiness, and fatigue. Neurology. 2010;75:1509–1519. doi: 10.1212/WNL.0b013e3181f9615d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Goel N., Dinges D.F. Behavioral and genetic markers of sleepiness. J. Clin Sleep Med. 2011;7:S19–S21. doi: 10.5664/jcsm.1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Goel N. “Omics” approaches for sleep and circadian rhythm research: Biomarkers for identifying differential vulnerability to sleep loss. Curr. Sleep Med. Rep. 2015;1:38–46. doi: 10.1007/s40675-014-0003-7. [DOI] [Google Scholar]
- 262.Goel N. Genetic Markers of Sleep and Sleepiness. Sleep Med. Clin. 2017;12:289–299. doi: 10.1016/j.jsmc.2017.03.005. [DOI] [PubMed] [Google Scholar]
- 263.Pot G.K. Sleep and dietary habits in the urban environment: The role of chrono-nutrition. Proc. Nutr. Soc. 2018;77:189–198. doi: 10.1017/S0029665117003974. [DOI] [PubMed] [Google Scholar]
- 264.Basner M., Fomberstein K.M., Razavi F.M., Banks S., William J.H., Rosa R.R., Dinges D.F. American time use survey: Sleep time and its relationship to waking activities. Sleep. 2007;30:1085–1095. doi: 10.1093/sleep/30.9.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Basner M., Spaeth A.M., Dinges D.F. Sociodemographic characteristics and waking activities and their role in the timing and duration of sleep. Sleep. 2014;37:1889–1906. doi: 10.5665/sleep.4238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Dautovich N.D., Shoji K.D., McCrae C.S. Variety is the Spice of Life: A Microlongitudinal Study Examining Age Differences in Intraindividual Variability in Daily Activities in Relation to Sleep Outcomes. J. Gerontol. B Psychol. Sci. Soc. Sci. 2015;70:581–590. doi: 10.1093/geronb/gbt120. [DOI] [PubMed] [Google Scholar]
- 267.Olds T.S., Maher C.A., Matricciani L. Sleep duration or bedtime? Exploring the relationship between sleep habits and weight status and activity patterns. Sleep. 2011;34:1299–1307. doi: 10.5665/SLEEP.1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Campanini M.Z., Guallar-Castillon P., Rodriguez-Artalejo F., Lopez-Garcia E. Mediterranean Diet and Changes in Sleep Duration and Indicators of Sleep Quality in Older Adults. Sleep. 2017;40 doi: 10.1093/sleep/zsw083. [DOI] [PubMed] [Google Scholar]
- 269.Grandner M.A., Jackson N., Gerstner J.R., Knutson K.L. Sleep symptoms associated with intake of specific dietary nutrients. J. Sleep Res. 2014;23:22–34. doi: 10.1111/jsr.12084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Slavin J.L., Lloyd B. Health benefits of fruits and vegetables. Adv. Nutr. 2012;3:506–516. doi: 10.3945/an.112.002154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.St-Onge M.P., Roberts A., Shechter A., Choudhury A.R. Fiber and Saturated Fat Are Associated with Sleep Arousals and Slow Wave Sleep. J. Clin. Sleep Med. 2016;12:19–24. doi: 10.5664/jcsm.5384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Spaeth A.M., Dinges D.F., Goel N. Objective Measurements of Energy Balance Are Associated with Sleep Architecture in Healthy Adults. Sleep. 2017;40 doi: 10.1093/sleep/zsw018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Mandalari G., Vardakou M., Faulks R., Bisignano C., Martorana M., Smeriglio A., Trombetta D. Food Matrix Effects of Polyphenol Bioaccessibility from Almond Skin during Simulated Human Digestion. Nutrients. 2016;8 doi: 10.3390/nu8090568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Bohn T. Dietary factors affecting polyphenol bioavailability. Nutr. Rev. 2014;72:429–452. doi: 10.1111/nure.12114. [DOI] [PubMed] [Google Scholar]
- 275.Scalbert A., Johnson I.T., Saltmarsh M. Polyphenols: Antioxidants and beyond. Am. J. Clin. Nutr. 2005;81:215s–217s. doi: 10.1093/ajcn/81.1.215S. [DOI] [PubMed] [Google Scholar]
- 276.Christou K., Moulas A.N., Pastaka C., Gourgoulianis K.I. Antioxidant capacity in obstructive sleep apnea patients. Sleep Med. 2003;4:225–228. doi: 10.1016/S1389-9457(02)00253-8. [DOI] [PubMed] [Google Scholar]
- 277.Baldwin C.M., Bell I.R., Kroesen K.W., Quan S.F. Differences in antioxidant intake in veterans with and without obstructive sleep apnea. Sleep. 2003;26:A212–A213. [Google Scholar]
- 278.Ford E.S., Mokdad A.H., Giles W.H., Brown D.W. The metabolic syndrome and antioxidant concentrations: Findings from the Third National Health and Nutrition Examination Survey. Diabetes. 2003;52:2346–2352. doi: 10.2337/diabetes.52.9.2346. [DOI] [PubMed] [Google Scholar]
- 279.Baldwin C.M., Bootzin R.R., Schwenke D.C., Quan S.F. Antioxidant nutrient intake and supplements as potential moderators of cognitive decline and cardiovascular disease in obstructive sleep apnea. Sleep Med. Rev. 2005;9:459–476. doi: 10.1016/j.smrv.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 280.Doo M., Kim Y. The Consumption of Dietary Antioxidant Vitamins Modifies the Risk of Obesity among Korean Men with Short Sleep Duration. Nutrients. 2017;9:780. doi: 10.3390/nu9070780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Pennington J.A.T., Fisher R.A. Food component profiles for fruit and vegetable subgroups. J. Food Compost. Anal. 2010;23:411–418. doi: 10.1016/j.jfca.2010.01.008. [DOI] [Google Scholar]
- 282.Al-Juhaimi F., Ghafoor K., Ozcan M.M., Jahurul M.H.A., Babiker E.E., Jinap S., Sahena F., Sharifudin M.S., Zaidul I.S.M. Effect of various food processing and handling methods on preservation of natural antioxidants in fruits and vegetables. J. Food Sci. Technol. 2018;55:3872–3880. doi: 10.1007/s13197-018-3370-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Al Khatib H.K., Hall W.L., Creedon A., Ooi E., Masri T., McGowan L., Harding S.V., Darzi J., Pot G.K. Sleep extension is a feasible lifestyle intervention in free-living adults who are habitually short sleepers: A potential strategy for decreasing intake of free sugars? A randomized controlled pilot study. Am. J. Clin. Nutr. 2018;107:43–53. doi: 10.1093/ajcn/nqx030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Ruiter M.E., Decoster J., Jacobs L., Lichstein K.L. Normal sleep in African-Americans and Caucasian-Americans: A meta-analysis. Sleep Med. 2011;12:209–214. doi: 10.1016/j.sleep.2010.12.010. [DOI] [PubMed] [Google Scholar]
- 285.Ruiter M.E., DeCoster J., Jacobs L., Lichstein K.L. Sleep Disorders in African Americans and Caucasian Americans: A Meta-Analysis. Behav. Sleep Med. 2010;8:246–259. doi: 10.1080/15402002.2010.509251. [DOI] [PubMed] [Google Scholar]
- 286.Petrov M.E., Lichstein K.L. Differences in sleep between black and white adults: An update and future directions. Sleep Med. 2016;18:74–81. doi: 10.1016/j.sleep.2015.01.011. [DOI] [PubMed] [Google Scholar]
- 287.Grandner M.A., Williams N.J., Knutson K.L., Roberts D., Jean-Louis G. Sleep disparity, race/ethnicity, and socioeconomic position. Sleep Med. 2016;18:71–78. doi: 10.1016/j.sleep.2015.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Smith S.L., Ludy M.J., Tucker R.M. Changes in taste preference and steps taken after sleep curtailment. Physiol. Behav. 2016;163:228–233. doi: 10.1016/j.physbeh.2016.05.002. [DOI] [PubMed] [Google Scholar]
- 289.Simon S.L., Field J., Miller L.E., DiFrancesco M., Beebe D.W. Sweet/dessert foods are more appealing to adolescents after sleep restriction. PLoS ONE. 2015;10:e0115434. doi: 10.1371/journal.pone.0115434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Chennaoui M., Arnal P.J., Sauvet F., Leger D. Sleep and exercise: A reciprocal issue? Sleep Med. Rev. 2015;20:59–72. doi: 10.1016/j.smrv.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 291.Espie C.A., Luik A.I., Cape J., Drake C.L., Siriwardena A.N., Ong J.C., Gordon C., Bostock S., Hames P., Nisbet M., et al. Digital Cognitive Behavioural Therapy for Insomnia versus sleep hygiene education: The impact of improved sleep on functional health, quality of life and psychological well-being. Study protocol for a randomised controlled trial. Trials. 2016;17:257. doi: 10.1186/s13063-016-1364-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Geiker N.R.W., Astrup A., Hjorth M.F., Sjodin A., Pijls L., Markus C.R. Does stress influence sleep patterns, food intake, weight gain, abdominal obesity and weight loss interventions and vice versa? Obes. Rev. 2018;19:81–97. doi: 10.1111/obr.12603. [DOI] [PubMed] [Google Scholar]
- 293.Pilcher J.J., Morris D.M., Donnelly J., Feigl H.B. Interactions between sleep habits and self-control. Front. Hum. Neurosci. 2015;9 doi: 10.3389/fnhum.2015.00284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Hagger M.S. Where does sleep fit in models of self-control and health behaviour? Stress Health. 2014;30:425–430. doi: 10.1002/smi.2624. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.