Abstract
The serotonin system has been implicated in the pathophysiology of anorexia nervosa (AN). A recent report proposed that body image distortion (BID), a core symptom of AN, may relate to abnormalities of the serotonin system, especially the serotonin transporter (5HTT). Positron emission tomography (PET) studies of underweight patients with active AN reported alterations in serotonin receptors, but not 5HTT. Here, we aimed to disclose the clinicopathophysiology of AN by focusing on 5HTT and cognitive functions, including BID, in groups with active AN. Twenty-two underweight female patients with AN (12 restricting-type AN (ANR); 10 binge-eating/purging-type AN (ANBP)) and 20 age-matched healthy female subjects underwent PET with a 5HTT radioligand [11C]DASB. The binding potential (BPND) of [11C]DASB was estimated semiquantitatively, and clinical data from Raven's colored progressive matrices for general intelligence, the Stroop test for focused attention, the Iowa gambling task for decision making and a dot-probe task designed for BID were compared with the levels of BPND in different groups. [11C]DASB BPND was significantly decreased in the medial parietal cortex in patients with AN and in the dorsal raphe in patients with ANR compared with healthy subjects (p < .05 corrected). Patients with ANR showed a significantly negative correlation between [11C]DASB BPND in the dorsal raphe and performance on the dot-probe task (p < .05 corrected). While reduced 5HTT in the medial parietal cortex (the somatosensory association area) is pathophysiologically important in AN in general, additional 5HTT reduction in the dorsal raphe as seen in ANR is implicated for the clinicopathophysiological relevance.
Keywords: Anorexia nervosa, Serotonin transporter, Positron emission tomography, Body image distortion
Highlights
-
•
5HTT decreased in the parietal cortex in patients with AN.
-
•
5HTT decreased in the parietal cortex in patients with ANBP.
-
•
5HTT decreased in the parietal cortex and the dorsal raphe in patients with ANR.
-
•
Patients with AN were poor at responding to the test for body image distortion (BID).
-
•
5HTT in the dorsal raphe was associated with cognitive performance of BID.
1. Introduction
Anorexia nervosa (AN) is a psychiatric disorder characterized by a persistent restriction of energy intake, leading to significantly low body weight, an intense fear of gaining weight, and disturbances in the normal perception of body shape (American Psychiatric Association, 2000). The relative risk was >10 times as high in the relatives of patients with AN as in the relatives of healthy subjects (Strober et al., 2000). Two genome-wide association studies did not find a genome-wide significance, but the results strongly suggested that true findings exist, requiring larger sample sizes (Boraska et al., 2014; Wang et al., 2011). Risk factors for AN were birth-related perinatal complications, experiences of sexual abuse or physical neglect, higher levels of ineffectiveness and perfectionism, and negative self-evaluation (Jacobi et al., 2004). Women are >10 times more likely to suffer from AN than men (Javaras et al., 2015). Women with AN tend to exhibit more severe pathology and lower body weights than men with AN (Kinasz et al., 2016). The mortality rate for AN is higher than for many other psychiatric disorders (Hoang et al., 2014). Although various treatments (i.e., weight restoration and pharmacological and psychological treatments) have been proposed, reported long-term recovery rates have not exceeded 30% (Fichter et al., 2017). Therefore, the biological abnormalities underlying the persistence or progression of disease should be explored to address the lack of effective therapies for AN.
The restriction of energy intake normally reduces the levels of serotonin (5HT; 5-hydroxytryptamine) in the central nervous system, which induces hyperphagia and obesity (Burke and Heisler, 2015). Despite low levels of 5HT in the central nervous system in underweight patients with AN, energy intake does not increase, and body weight remains low (Kaye et al., 1984). Compared with healthy subjects, a 5HT metabolite 5-hydroxyindoleacetic acid, is significantly decreased in the cerebral spinal fluid in underweight patients with AN and it increases in the long-term recovered state (Kaye et al., 1984, 1991). In both underweight and recovered patients with AN but not in healthy subjects, acute depletion of tryptophan, a 5HT precursor amino acid, reduces the levels of 5HT in the central nervous system, which decreases anxiety (Kaye et al., 2003). The anxiolytic effect on tryptophan depletion might induce underweight patients with AN to restrict energy intake, leading to dietary-induced tryptophan reduction (Kaye et al., 2003). In contrast, in the periphery, a lower density of platelet 5HT2A receptor was reported in underweight patients with AN (Sigurdh et al., 2013). These lines of evidence suggest that the pathophysiology of AN resides in the 5HT system.
The 5HT system involves 14 or more receptor subtypes (e.g. 5HT1A, 5HT1B, 5HT2A, 5HT4, 5HT6, and 5HT7) and the 5HT transporter (5HTT). 5HT1A receptors play two roles as autoreceptors on the raphe nucleus producing 5HT and as postsynaptic receptors in several brain regions innervated by serotonergic projections (Beck et al., 1992; Hamon et al., 1990; Riad et al., 2000). 5HT1B receptors are autoreceptors on both serotonergic and nonserotonergic presynaptic terminals throughout the brain, inhibiting neurotransmitter release (Chenu et al., 2008; Ding et al., 2015; Hannon and Hoyer, 2008; Liu et al., 2015). 5HT2A receptors act as postsynaptic excitatory receptors located in broad brain regions (Savli et al., 2012). 5HT4 receptors are expressed in limbic regions and act to increase intracellular cAMP levels, which elevates neuronal activity (Hannon and Hoyer, 2008; Tanaka et al., 2012). 5HT6 receptors, located in the basal ganglia, cortex, hippocampus amygdala, and hypothalamus, are reported to be related to inducing an antidepressant-like effect (Carr et al., 2011; Hannon and Hoyer, 2008). 5HT7 receptors, present in the limbic and cortical regions, have been found to be involved with the hypothalamus-pituitary-adrenal axis (Hannon and Hoyer, 2008; Laplante et al., 2002). 5HTT mainly exists in the midbrain, brain stem, thalamus and basal ganglia, and it regulates the extracellular 5HT concentration through reuptake (Bel and Artigas, 1992; Savli et al., 2012).
Most previous positron emission tomography (PET) studies of underweight or recovered patients with AN have targeted the 5HT1A and 5HT2A receptors and 5HTT. 5HT1A receptors are increased in patients with AN irrespective of their condition (underweight or postrecovery, where a recovered state is defined as the normalization of body weight with at least a 1-year absence of AN symptoms) (Bailer et al., 2007b; Bailer et al., 2005; Galusca et al., 2008). The persistence of the increased 5HT1A receptors in patients with AN through underweight to postrecovery were considered to be consequences of malnutrition as part of the anxiolytic effect of tryptophan depletion. While 5HT2A receptors did not change in underweight patients with AN, they were decreased in recovered AN patients (Bailer et al., 2007b; Frank et al., 2002). Although 5HTT levels were reported to be unchanged in recovered patients with AN in an examination of five brain regions (the subgenual cingulate cortex, dorsal caudate, thalamus, antero-ventral striatum and dorsal raphe) (Bailer et al., 2007a), no PET studies of 5HTT have examined ailing underweight patients with active AN. In addition, biochemical studies of peripheral blood for gene polymorphisms of 5HTT and platelet 5HTT density have not shown consistent results in underweight patients with AN (Bruce et al., 2006; Sigurdh et al., 2013; Solmi et al., 2016). Because it is known that 5HTT is related to cognito-motivational functions in humans (Enge et al., 2014; Suzuki et al., 2008), the cognitive aspects specific to patients with AN need be explored in light of the molecular role of 5HTT in this disorder.
Disturbances in the normal perception of body shape, known as body image distortion (BID), are a core symptom of AN (Frank and Kaye, 2012; Gaudio et al., 2016). BID has been detected in body size estimations based on not only self-images in a mirror but also on self-memory in patients with AN (Overas et al., 2014). The body size estimation from self-memory can be modified by a virtual reality technique using a distorted image of one's own body in healthy subjects (Serino et al., 2016). Compared with healthy subjects, patients with AN recalled a lower number of emotional experiences and had more ambiguous episodic memories (Bomba et al., 2014). Many types of memory impairments (e.g., spatial, episodic and working memory impairments) have been observed in patients with AN (Bomba et al., 2014; Huber et al., 2015; Serino et al., 2015; Terhoeven et al., 2017; Weider et al., 2015). Spatial memory impairment has also been observed in 5HT1A or 5HT7 receptor knockout mice (Ballaz et al., 2007; Glikmann-Johnston et al., 2015; Sarkisyan and Hedlund, 2009). 5HTT knockout animals show episodic memory impairment (Olivier et al., 2009; Wu et al., 2016b). Blockade and ablation of the 5HT2A receptor affects retrieval of episodic memory in animals (Bekinschtein et al., 2013; Morici et al., 2015). 5HTT polymorphisms are associated with working memory impairment in healthy subjects (Anderson et al., 2012; Price et al., 2013). Because patients with AN are considered to be poor at correcting their body image based on self-memory due to these various memory impairments, some reviews have proposed that one possible reason for BID is body memory impairment, especially impairments in autobiographical allocentric memory of body-related events, reflecting a broader impairment in multisensory body integration (Dakanalis et al., 2016; Gadsby, 2017; Riva and Dakanalis, 2018). Moreover, Riva and Dakanalis suggested that BID is possibly involved in identifying internal bodily signals with their potential pleasant consequences (Riva and Dakanalis, 2018). Previous neuroimaging studies of the neural correlates of BID in patients with AN used visual tasks involving digitally distorted images of the subject's or another person's body. These fMRI studies assumed that BID reflects the presence of abnormal neural networks peculiar to AN (Gaudio et al., 2016). Interestingly, 5HTT knockdown animals show object and spatial memory impairment with diminished fear memory (Kalueff et al., 2010; Olivier et al., 2009; Wu et al., 2016b), and the short allele of the serotonin transporter gene has been associated with poor memory performance in healthy subjects (Price et al., 2013). BID is speculated to be related to impaired memory processes arising from 5HT system dysfunction, especially 5HTT hypofunction (Riva, 2016). To our knowledge, no previous PET studies of patients with active AN have evaluated BID or other cognitive assessments, even though many types of cognitive impairment have been reported in AN (e.g., decision making showed a large effect size) (Wu et al., 2014, Wu et al., 2016a). Thus, the combination of 5HTT alterations with cognitive impairments, including BID, in underweight patients with active AN is considered important for understanding its pathophysiology.
AN is classified into two subtypes: binge-eating-/purging-type AN (ANBP), characterized by binge-eating or purging behavior, and restricting-type AN (ANR), characterized by restricting food consumption without binging or purging (American Psychiatric Association, 2000). Although the clinical features of these subtypes are different, most previous studies have combined both AN subtypes into a single group. Some studies have investigated the differences between subtypes. Patients with ANBP showed significant improvement in body mass index (BMI) and emotional dysregulation after treatments, but they had a significantly higher relapse rate compared with patients with ANR (Brambilla et al., 2007; Carter et al., 2012; Fioravanti et al., 2014). Although 5HTT availability in the antero-ventral striatum and dorsal raphe has been reported to be lower in patients with ANBP than in patients with ANR under postrecovery conditions (Bailer et al., 2007a), differences in 5HTT between subtypes before recovery (i.e., under active AN conditions) have not been investigated. While several meta-analyses have shown many types of cognitive impairments in patients with AN (Wu et al., 2014, Wu et al., 2016a), few studies have investigated any differences in cognitive impairments between the subtypes (Van Autreve and Vervaet, 2015). Detecting differences in 5HTT and cognitive ability between AN subtypes might be helpful for understanding the pathophysiology of different clinical features.
The purpose of this study was to investigate whether 5HTT was altered in underweight patients with AN and each subtype compared with healthy subjects and whether it correlated with the score of the cognitive assessments in each group, using PET measurement to evaluate 5HTT changes and the associations between 5HTT changes and cognitive impairment, specifically focusing on BID, in order to elucidate the clinicopathophysiology of the active AN condition.
2. Materials and methods
2.1. Participants
Because women exhibit a higher incidence of AN, more severe pathology, and lower body weights than men (Javaras et al., 2015; Kinasz et al., 2016), we recruited female patients with AN. The inclusion criteria for patients with AN were as follows: (1) diagnosed with AN according to the DSM-IV-TR, and (2) aged between 18 and 35 years. The exclusion criteria for patients with AN were as follows: (1) the presence of white matter changes on T2 and FLAIR magnetic resonance images; (2) the regular use of any medication with nootropic activity, such as antipsychotics, antidepressants, anxiolytics, and hypnotics, in the 3 months prior to PET measurement; (3) major medical illness; and (4) history of neurological or psychiatric disorders other than AN. Structured clinical interviews for the DSM-IV were used to assess the presence of psychiatric disorders (First et al., 1997; First et al., 2002). Age- and sex-matched healthy subjects who were free from any history of neurological or psychiatric disorders and did not drink or smoke regularly were also included in this study. Because previous studies have reported lower levels of 17β-estradiol in patients with AN and because 17β-estradiol has been found to affect cognitive function in healthy women (Graham et al., 2017; Ohwada et al., 2007), we measured the levels of 17β-estradiol in all participants and conducted PET measurements for the healthy female subjects within the first 10 days of the self-reported follicular phase, when 17β-estradiol is at the lowest levels in healthy women, to facilitate between-group comparisons without confounding from this hormonal factor. This study was conducted according to the Declaration of Helsinki. The study was approved by the Ethics Committee of the Hamamatsu University School of Medicine. We obtained written informed consent from all participants after informing them of the objectives, procedures and possible risks of the experimental procedure verbally and in writing. All of the participants were recruited from Hamamatsu University Hospital between April 3, 2013, and May 24, 2017.
2.2. Psychological and cognitive assessments
On the day of PET measurement, all of the participants underwent the following psychological and cognitive tests: psychological profiles were assessed using the Eating Disorder Inventory-2 to determine disease severity (DM, 1991), the Beck Depression Inventory-II to determine the level of depression (Beck et al., 1996), the State-Trait Anxiety Inventory to determine the level of anxiety (Spielberger et al., 1983), and the Yale-Brown Obsessive-Compulsive Scale to determine the level of obsession-compulsion (Goodman et al., 1989). The participants' cognitive profiles were assessed using Raven's colored progressive matrices for general intelligence to assess impaired general cognitive processing (JC, 1936), the Stroop test of focused attention to assess an attentional ability unrelated to BID using Stroop interference because of the positive correlation between the performance on the Stroop test and 5HTT availability of PET measurement (Madsen et al., 2011; Treisman and Fearnley, 1969), and the Iowa gambling task of net total score to assess decision making, which showed the largest effect size in patients with AN and an association with 5HTT gene polymorphisms (Bechara et al., 1994; Miu et al., 2012; Wu et al., 2016a).
2.3. Dot-probe task designed for BID
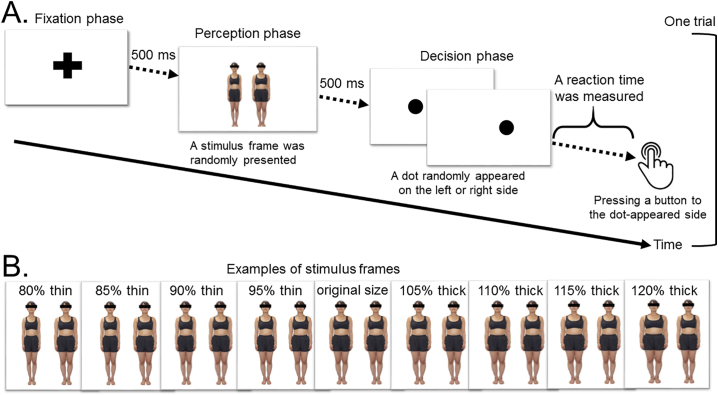
Because previous studies of BID revealed that patients with AN spent more time looking at body images and exhibited significant brain activation in response to pairs of distorted images of another person's body compared with healthy subjects (Horndasch et al., 2012; Miyake et al., 2010), we developed a computer-based cognitive test to measure reaction time in response to pairs of distorted images of another person's body in order to assess an attentional bias. This task is called the dot-probe task and was originally developed by MacLeod et al. (1988). Previous studies of the dot-probe task revealed an attentional bias in patients with AN using some kinds of parameters (i.e., reaction time, attentional bias score, and saccade latency) for various styles of body images (i.e., thin or obese images and images of the participant's or another person's body) (Blechert et al., 2010; Kim et al., 2014; Pona et al., 2017). To decide the parameter in this study, a preliminary analysis of the dot-probe task was conducted in 32 patients with AN (20 patients with ANR and 12 patients with ANBP) and 38 healthy subjects; it indicated that the statistical power of the reaction time for all distorted images was >0.8, suggesting that this measure was sufficient to detect differences (Supplementary Table 1). As shown in Fig. 1A, each trial of the dot-probe task consists of three phases: (1) a fixation phase, in which a fixation cross is presented for 500 milliseconds (ms); (2) a perception phase, in which a stimulus frame (a pair of computer-generated distorted images of another person's body) is randomly presented for 500 ms; and (3) a decision phase, in which participants are required to press a button as quickly as possible to indicate the side on which a dot randomly appeared. The dot appeared randomly on either the left or right side in a counterbalanced manner. We measured the reaction times between dot presentation and button-press and the numbers of correct and incorrect responses. The reaction times for incorrect answers were not included in the analysis. As shown in Fig. 1B, the stimulus frames contained a pair of images of a woman, one with her original body size and one with one of eight computer-generated distorted body sizes, ranging from 80% thin to 120% thick at 5% intervals (80% thin, 85% thin, 90% thin, 95% thin, 105% thick, 110% thick, 115% thick, and 120% thick). The distorted body images were presented on the left or right side at random in a counterbalanced manner. We prepared 256 pairs of distorted body images (eight different women × eight distorted body sizes × two right- or left-side distorted image positions × two right- or left-sided dot positions) and 16 pairs of original body-sized images (eight different women × two right- or left-side dot positions). Thus, each participant performed a total of 272 trials in the dot-probe task. We obtained the task data from the participants prior to their PET measurements on the same day.
Fig. 1.
Design of the dot-probe task.
(A) One trial of the dot-probe task consists of three phases (i.e., the fixation, perception, and decision phases). (B) The stimulus frames consist of a pair of computer-generated distorted images of another person's body.
2.4. Magnetic resonance imaging (MRI) acquisition
MRI was performed to determine the regions of concern for establishing volumes of interest (VOIs) and to investigate possible brain structural differences using a 1.5T MRI scanner (Signa HDxt, GE, Orlando, USA) with the following acquisition parameters: 3-dimensional mode sampling, TR/TE (25/minimum), 30° flip angle, 1.5-mm slice thickness with no gap and 256 × 128 matrices. The MRI parameters and a mobile PET gantry allowed us to reconstruct the PET images parallel to the intercommissural (ACPC) line without reslicing. Thus, we were able to locate the VOIs in the target regions of the original PET images (Ouchi et al., 1998).
2.5. PET data acquisition
All of the participants underwent PET scans with carbon 11-labeled 3-amino-4-(2-dimethylaminomethylphenylsulfanyl)-benzonitrile ([11C]DASB) after MRI scanning. We used a high-resolution brain PET scanner (SHR12000, Hamamatsu Photonics K.K., Hamamatsu, Japan) capable of yielding 47 tomographic images (Watanabe et al., 2002). After backprojection and filtering (Hanning filter, cut-off frequency of 0.2 cycles per pixel), the image resolution was 2.9 × 2.9 × 3.4 mm FWHM. The voxel of each reconstructed image measured 1.3 × 1.3 × 3.4 mm. After the intravenous injection of [11C]DASB (5 MBq/kg), dynamic PET scans of [11C]DASB with 38 frames (time frames: 4 × 30, 20 × 60, and 14 × 300 s) were performed over 92 min.
2.6. PET image data processing
The binding potential (BPND) of [11C]DASB was estimated based on an MRTM2, in which we used a normalized time activity curve based on the cerebellar cortex of each participant (Ichise et al., 2003). All BPND parametric PET images were generated using PMOD 3.2 software (PMOD Technologies, Ltd., Switzerland).
2.7. Statistical analysis
All statistical analyses were performed using SPSS, version 21 (IBM, Armonk, NY). Because the current AN group included patients with ANR and ANBP, we used t-tests to compare each parameter of the participants' characteristics in four combinations (i.e., patients with AN vs. healthy subjects, patients with ANR vs. healthy subjects, patients with ANBP vs. healthy subjects, and patients with ANR vs. patients with ANBP). The statistical threshold was assumed to be p < .05 corrected. Our other image analysis methods are described below.
2.8. SPM analysis
We examined the whole brain using a voxelwise analysis in SPM8 (Wellcome Department of Cognitive Neurology, London, UK). All [11C]DASB BPND parametric and MRI images were first normalized to the Montreal Neurological Institute (MNI) space and smoothed with an isotropic Gaussian kernel of 8 mm. The above-mentioned group comparisons were performed using t statistics on a voxel-by-voxel basis, with a statistical threshold of p < .001 uncorrected at the voxel level and p < .05 corrected for multiple comparisons using familywise error at the cluster level. In the t statistics for [11C]DASB BPND parametric images, BMI was used as a covariate. We used voxel-based morphometry (VBM) to evaluate possible brain volume alterations using the same statistical threshold.
2.9. VOI analysis
To explore any differences in 5HTT, we manually set VOIs in the dorsal raphe, thalamus, dorsal caudate, antero-ventral striatum and subgenual cingulate, which were reported to have high 5HTT availability in healthy individuals and were investigated in the previous PET study in recovered patients with AN (Bailer et al., 2007a; Spies et al., 2015). Examples of VOIs are shown in Supplementary Fig. 1. We obtained [11C]DASB BPND values for each VOI and conducted comparisons between the patients with AN and the healthy subjects using t-tests with the Bonferroni correction for multiple comparisons and among the two subtypes and healthy subjects using one-way ANOVA. We estimated the statistical power for each VOI in a post hoc manner with G*Power 3.1 software (http://www.gpower.hhu.de/) (Faul et al., 2007). To investigate any associations between 5HTT availability and cognitive impairments, we examined the correlations of the [11C]DASB BPND values in each VOI with the score of the cognitive assessments in each group using a regression analysis with the Bonferroni correction for multiple comparisons with a statistical threshold of p < .0025 (= 0.05/20) uncorrected because the correlations consisted of 5 VOIs and 4 cognitive assessments.
3. Results
3.1. Participant characteristics
The participants' characteristics are shown in Table 1. Twenty-two female patients with active AN (12 with ANR and 10 with ANBP) and 20 age-matched healthy female subjects participated in our study. The current BMI and education history of the patients with AN, ANR, and ANBP were significantly lower than those of the healthy subjects, and the patients with ANR had significantly lower current BMIs than the patients with ANBP. The scores on all of the psychological profiles for the patients with AN, ANR, and ANBP were significantly higher than those of the healthy subjects. Regarding the cognitive profiles, the scores for Raven's colored progressive matrices and the Stroop interference test were not significantly different among the groups. The net total scores on the Iowa gambling task were significantly lower in the patients with AN, ANR, and ANBP compared with the healthy subjects. Although the number of incorrect answers on the dot-probe task was not significantly different among the groups (Supplementary Table 2), the reaction times on the dot-probe task were significantly longer in the patients with AN, ANR, and ANBP compared with the healthy subjects. Sixteen patients with AN (eight patients with ANR and eight with ANBP) were nootropic naïve, and three patients with AN smoked and/or drank alcohol regularly (two patients with ANBP smoked and drank, one patient with ANBP smoked, and one patient with ANBP drank) (Supplementary Table 3).
Table 1.
Characteristics of participants.
| All participants |
AN subtypes |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Healthy subjects |
Patients with AN |
p value (vs. healthy subjects) |
Patients with ANR |
p value (vs. healthy subjects) |
Patients with ANBP |
p value (vs. healthy subjects) |
p value (vs. patients with ANR) |
|||||
| (n = 20) |
(n = 22) |
(n = 12) |
(n = 10) |
|||||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |||||
| Demographic profiles | ||||||||||||
| Age, years | 22.4 | 3.2 | 24.8 | 6.0 | 0.10 | 24.3 | 6.0 | 0.30 | 25.4 | 6.2 | 0.17 | 0.69 |
| Current BMI, kg/m2 | 20.5 | 2.2 | 14.4 | 1.4 | < 0.001 | 13.8 | 1.3 | < 0.001 | 15.2 | 1.3 | < 0.001 | < 0.05 |
| Disease duration, years | na | na | 5.2 | 5.7 | na | 4.2 | 5.0 | na | 6.4 | 6.5 | na | 0.37 |
| Length of amenorrhea, years | na | na | 3.2 | 3.9 | na | 3.3 | 4.6 | na | 3.2 | 3.1 | na | 0.92 |
| Education history, years | 14.8 | 1.7 | 13.0 | 1.9 | < 0.005 | 12.9 | 1.6 | < 0.005 | 13.2 | 2.3 | < 0.05 | 0.74 |
| 17β estradiol, ng/l | 83.0 | 29.5 | 90.8 | 20.2 | 0.35 | 92.8 | 14.5 | 0.31 | 87.9 | 26.9 | 0.70 | 0.61 |
| Psychological profiles | ||||||||||||
| Eating disorder inventory-2 | 27.7 | 20.6 | 115.5 | 70.3 | < 0.001 | 99.6 | 52.5 | < 0.001 | 134.7 | 86.2 | < 0.005 | 0.25 |
| Beck depression inventory-II | 4.9 | 4.2 | 26.1 | 13.7 | < 0.001 | 21.4 | 10.5 | < 0.001 | 31.7 | 15.4 | < 0.001 | 0.08 |
| State anxiety score of STAI | 34.6 | 5.1 | 54.3 | 11.1 | < 0.001 | 54.8 | 10.9 | < 0.001 | 53.8 | 12.0 | < 0.001 | 0.83 |
| Trait anxiety score of STAI | 40.9 | 8.7 | 58.7 | 14.1 | < 0.001 | 55.7 | 14.3 | < 0.001 | 62.4 | 13.6 | < 0.001 | 0.28 |
| Y-BOCS | 17.5 | 2.8 | 13.3 | 11.6 | < 0.001 | 9.8 | 10.8 | < 0.05 | 17.4 | 11.7 | < 0.005 | 0.13 |
| Cognitive profiles | ||||||||||||
| RCPM | 34.5 | 1.7 | 34.6 | 1.5 | 0.78 | 34.5 | 1.6 | 0.83 | 34.6 | 1.4 | 0.81 | 0.98 |
| Stroop interference | 5.4 | 6.9 | 6.1 | 8.9 | 0.77 | 7.5 | 6.0 | 0.40 | 4.6 | 11.7 | 0.80 | 0.46 |
| Net total score of Iowa gambling task | 17.9 | 19.4 | −3.6 | 22.3 | < 0.005 | −1.8 | 24.0 | < 0.05 | −5.8 | 21.1 | < 0.05 | 0.69 |
| Reaction time in dot-probe task, milliseconds | 408.7 | 55.2 | 470.6 | 59.9 | < 0.005 | 452.8 | 58.5 | < 0.05 | 491.9 | 57.1 | < 0.001 | 0.13 |
Abbreviations: AN, anorexia nervosa; ANR, restricting-type AN; ANBP, binge-eating/purging-type AN; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); STAI, state-trait anxiety inventory; Y-BOCS, Yale-Brown Obsessive Compulsive Scale; RCPM, Raven's colored progressive matrices; na, not applicable.
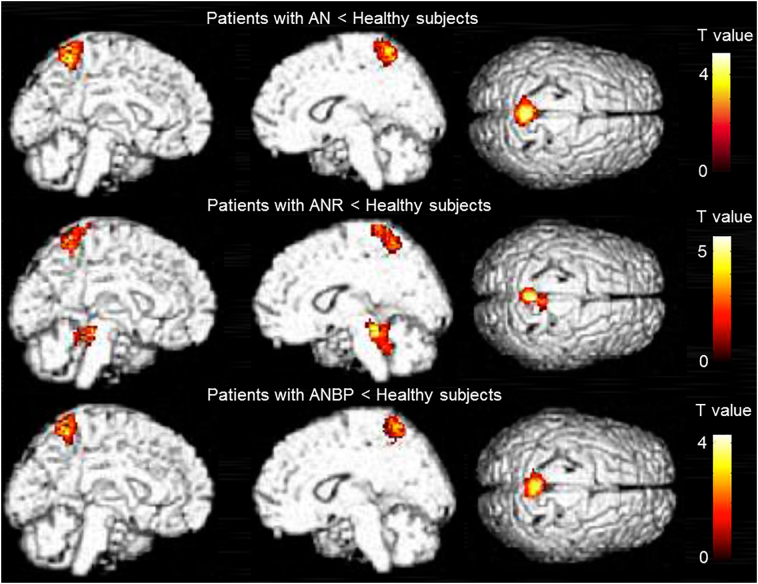
3.2. SPM analysis
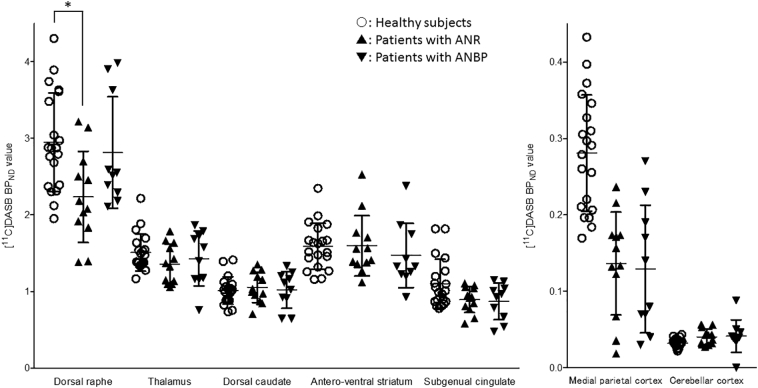
The results of the SPM analysis are presented in Table 2 and Fig. 2. Compared with the healthy subjects, the patients with AN, ANR, and ANBP showed significantly lower BPND values of [11C]DASB in the medial parietal cortex (i.e., the precuneus or paracentral lobule), and the patients with ANR showed significantly lower BPND values in the dorsal midbrain (i.e., the upper and lower dorsal midbrain). There were no significant differences in the BPND values of [11C]DASB between the patients with ANR and ANBP (Table 2). The results of the SPM and VBM analyses did not overlap for each between-group comparison (Supplementary Figs. 2, 3, and 4). We show the BPND values of the medial parietal cortex and cerebellar cortex using scatter plots in Fig. 3 and Supplementary Fig. 5.
Table 2.
Results of SPM analysis.
| Cluster level (p < .05 corrected) |
Peak level (p < .001 uncorrected) |
||||
|---|---|---|---|---|---|
| kE | p value | T value | MNI coordinates (x, y, z) | Anatomical name | |
| Patients with AN vs. healthy subjects | 690 | 0.004 | 4.95 | 1, −54, 64 | Right precuneus (BA 7) |
| Patients with ANR vs. healthy subjects | 484 | 0.011 | 5.70 | 6, −38, −16 | Right upper dorsal midbrain |
| 4.84 | 10, −45, −30 | Right lower dorsal midbrain | |||
| 455 | 0.014 | 4.62 | 2, −52, 62 | Right precuneus (BA 7) | |
| 4.22 | 5, −38, 78 | Right paracentral lobule (BA 1) | |||
| 4.08 | 2, −44, 72 | Right paracentral lobule (BA 1) | |||
| Patients with ANBP vs. healthy subjects | 503 | 0.019 | 4.31 | −2, −54, 62 | Left precuneus (BA 7) |
| 4.27 | 4, −52, 58 | Right precuneus (BA 7) | |||
| Patients with ANBP vs. patients with ANP | None | ||||
Abbreviations: AN, Anorexia nervosa; ANR, restricting-type AN; ANBP, binge-eating/purging-type AN; BA, Brodmann area; MNI coordinates, Montreal Neurological Institute coordinates.
Fig. 2.
Regions of significantly decreased [11C]DASB binding potential (BPND) values in the patients with AN, ANR, and ANBP compared with healthy subjects.
The images show regions of significantly decreased [11C]DASB binding potential (BPND) values with a significance threshold set at p < .001 uncorrected at the voxel level and p < .05 corrected for multiple comparisons using familywise error at the cluster level.
Fig. 3.
[11C]DASB binding potential (BPND) in the dorsal raphe, thalamus, dorsal caudate, antero-ventral striatum, subgenual cingulate, medial parietal cortex and cerebellar cortex among the subtypes of anorexia nervosa and the healthy subjects.
The open circles represent healthy subjects, the closed triangles represent patients with restricting-type anorexia nervosa (ANR), and the closed inverted triangles represent patients with binge-eating-/purging-type anorexia nervosa (ANBP). Error bars represent the standard errors. An asterisk (*) indicates significance at p < .05 using one-way ANOVA.
3.3. VOI analysis
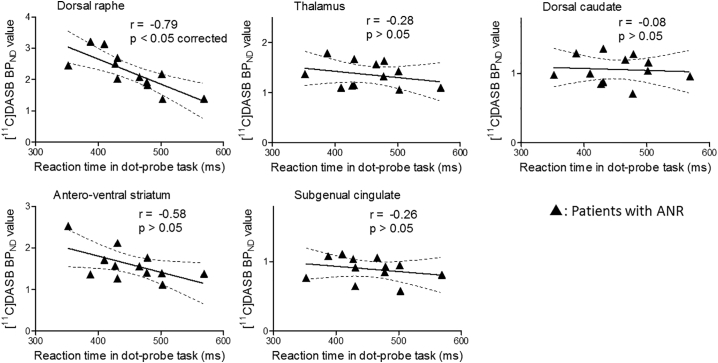
Compared with the healthy subjects, the patients with AN did not show significant differences in [11C]DASB BPND values in the dorsal raphe, thalamus, dorsal caudate, antero-ventral striatum, or subgenual cingulate (Supplementary Fig. 5). The patients with ANR showed significantly lower BPND values of [11C]DASB in the dorsal raphe compared with the healthy subjects (Fig. 3). There were no significant differences in the [11C]DASB BPND values between patients with ANBP and healthy subjects (Fig. 3). We show the mean BPND values of [11C]DASB and the statistical power for each VOI in Supplementary Fig. 6. The statistical power in the dorsal raphe comparing the patients with ANR with the healthy subjects was 0.9. We conducted the regression analysis between the [11C]DASB BPND values in each VOI and the scores of the cognitive assessments with the Bonferroni correction. Only in the patients with ANR, not in the patients with ANBP, was a significantly negative correlation observed between the [11C]DASB BPND values and the reaction time on the dot-probe task in the dorsal raphe (r = −0.79, p < .05 corrected) (Fig. 4 and Supplementary Fig. 7). We did not find any other significant correlations between the BPND values of each VOI and the scores of the other cognitive assessments.
Fig. 4.
Correlations of the patients with restricting-type anorexia nervosa between [11C]DASB binding potential (BPND) values and the reaction times on the dot-probe task in the dorsal raphe, thalamus, dorsal caudate, antero-ventral striatum and subgenual cingulate.
The closed triangles represent patients with restricting-type anorexia nervosa (ANR) in the scattergrams. The significance threshold was set at p < .05 with the Bonferroni correction for multiple comparisons.
4. Discussion
To the best of our knowledge, this is the first study to use PET to examine 5HTT alterations and their association with cognitive processing in patients with active AN, ANR and ANBP. The results of the SPM analysis showed significant reductions in [11C]DASB BPND values in the medial parietal cortex of patients with AN, ANR, and ANBP and in the dorsal midbrain (raphe) of patients with ANR. The results of the VOI analysis revealed that the [11C]DASB BPND values in the dorsal raphe of patients with ANR were significantly decreased and negatively correlated with reaction times on the dot-probe task, which indicated the presence of a biological-cognitive connection of relevance in ANR.
The current results revealed decreased 5HTT availability in the medial parietal cortex of patients with AN compared with healthy subjects (Table 2 and Fig. 2). In rodents, chronic food restriction was reported to induce 5HTT reduction in the somatosensory cortex, which is equivalent to the lateral and medial parietal cortex in human subjects (Medina-Aguirre et al., 2008). Previous fMRI studies of underweight patients with AN reported associations between altered functional connectivity in the somatosensory cortex and impaired visuospatial ability, which is thought to be involved with BID (Favaro et al., 2012; Gaudio et al., 2016). Because a fMRI study of healthy subjects also suggested that the medial parietal cortex might be involved in encoding spatial allocentric memory (Baumann and Mattingley, 2010), 5HTT reduction in this region might play a possible role in recalling the smaller number of ambiguous emotional memories in patients with AN (Bomba et al., 2014). Additionally, because some meta-analyses of neuroimaging studies showed that the precuneus, a part of the medial parietal cortex, was related to some tasks on self-face recognition or self- and external agency (Platek et al., 2008; Sperduti et al., 2011), 5HTT alteration in this region might be associated with self-representation. Consistently, our statistics showed a significant reduction in 5HTT in this region (the somatosensory association area) in both subtypes (Table 2 and Fig. 2). Collectively, a series of these findings support that 5HTT reduction in the medial parietal cortex is a key pathophysiological factor in AN in general.
Compared with healthy subjects, patients with ANR showed decreased 5HTT availability in the dorsal midbrain in the SPM and VOI analyses (Table 2, Fig. 2 and Fig. 3). The statistical power in the dorsal raphe comparing the patients with ANR with the healthy subjects was >0.8, suggesting that our sample size was sufficient to detect alterations in the region in this study. The similarity of the 5HTT reduction in the SPM and VOI analyses indicated that the dorsal midbrain examined in the SPM analysis mainly represented the dorsal raphe region. In animal experiments, chronic food restriction caused 5HTT reductions in the rat dorsal raphe (Jahng et al., 2007), and these reductions were normalized by the administration of the 5HT precursor tryptophan (Pratelli et al., 2017). Previous single photon emission computed tomography studies showed that 5HTT availability in the thalamus is not altered in food-restricted weight loss and is not correlated with BMI in obese human subjects without any history of psychiatric disorders (Nam et al., 2018; Versteeg et al., 2017b), while 5HTT availability in the midbrain is correlated negatively with BMI in obese healthy human subjects and positively in nonobese ones (Nam et al., 2018), suggesting that 5HTT reduction in a limited 5HTT-rich region (i.e., the midbrain) might be the key etiology of AN. This finding is significantly different from the 5HTT reduction over the extensive 5HTT-rich regions, such as the midbrain, thalamus and amygdala, as seen in depression (Spies et al., 2015). The positive correlation between 5HTT availability in the midbrain and BMI in nonobese healthy human subjects suggests that the underweight state itself may be associated with changes in 5HTT availability as seen in our study.
The hormone insulin is also important with regard to body mass. In the cultured rat pineal gland, insulin increased tryptophan activity leading to the modulation of 5HT synthesis (Garcia et al., 2008). Measuring concentrations in the rat central nervous system by microdialysis showed that 5HT increased insulin levels and vice versa without altered peripheral levels of insulin and glucose (Orosco et al., 2000). 5HT1B receptor knockout or 5HTT knockout mice showed higher insulin secretion than wild-type mice, leading to insulin resistance (Chen et al., 2012; Nonogaki et al., 1998). Obese human subjects with high 5HT2A receptor gene methylation have high insulin levels (Perez-Cornago et al., 2014). 5HTT availability in the diencephalon is reduced in insulin-resistant obese human subjects (Versteeg et al., 2017a) In these lines of evidence, these associations might be changed due to malnutrition and provide a possible explanation for 5HTT alterations in AN. In addition, a previous PET study showed normal levels of 5HTT availability in the dorsal raphe in each recovered subtype compared with healthy subjects (Bailer et al., 2007a), indicating that the 5HTT availability in the dorsal raphe decreased in underweight patients with ANR and was normalized in the recovered patients. Because the 5HTT availability in the dorsal raphe was not altered in recovered patients with ANR, decreased 5HTT availability in this region in underweight patients may be a pathophysiological sign of premorbid features or the postonset progression of the disease. Additionally, because in the dorsal cochlear nucleus (a well-established brainstem region of sensory integration) that receives a dense serotonergic input from the dorsal raphe, 5HT specifically decreased input from auditory nerve fibers among the other multisensory inputs (Tang and Trussell, 2017), the 5HTT reduction in the dorsal raphe might impair the multisensory integration, leading to a broader deficit in patients with ANR. Thus, a difference in susceptibility of the dorsal raphe between ANR and ANBP might suggest the severity of the disease in view of multisensory integration. At the moment, there is no clinical report on the effectiveness of improving decreased 5HTT for medicating AN. Because it was reported that 5HTT reduction in the dorsal raphe could be normalized in an animal experiment (Pratelli et al., 2017), further study on the normalization or elevation of the reduced 5HTT in this region might help patients with ANR.
Regarding the cognitive measures used in the current study, the net total scores on the Iowa gambling task and the reaction time on the dot-probe task differed significantly between the patient groups and the healthy subjects (Table 1). The correlation analyses (levels of tracer binding vs. cognitive impairments) used in the present study indicated that lower 5HTT availability in the dorsal raphe in patients with ANR was associated with longer reaction times on the dot-probe task (Fig. 4). A recent SPECT study revealed that lower 5HTT binding in the diencephalon was associated with longer reaction times in response to visual stimuli related to food in healthy lean male subjects (Koopman et al., 2016). These correlational findings support the notion of a clinicopathological linkage between BID and 5HTT hypofunction in the dorsal raphe. Based on previous reports and the current findings (i.e., a significant 5HTT reduction in the dorsal raphe, which was associated with BID in the present study, and the absence of this reduction in a postrecovery condition in a previous study (Bailer et al., 2007a), 5HTT stimulation in this region may be a therapeutic intervention target. This possibility should be investigated in future studies.
Our results and previous findings indicate that 5HT system alterations in patients with AN take the forms of increased 5HT1A receptors, decreased 5HT2A receptors and regional 5HTT reduction. Antidepressants, which block 5HTT function, are sometimes prescribed for patients with AN in clinical practice, but their efficacy has been controversial (Garner et al., 2016; Marvanova and Gramith, 2018). In patients with depression, a gene polymorphism study showed a poor antidepressant response in cases of low 5HTT function (Manoharan et al., 2016). Thus, 5HTT hypofunction might partly account for the limited response to antidepressants in patients with AN. Moreover, a previous trial of antidepressants with tryptophan, a 5HT precursor amino acid, to augment 5HT levels in the central nervous system failed to show effectiveness in patients with AN (Barbarich et al., 2004). Current psychopharmacological use of antidepressants targeting 5HTT function might be limited. No beneficial outcome in the trial to boost 5HT levels in the blood by tryptophan load may be ascribed to the alterations of 5HT1A and 5HT2A receptors. Currently, instead of blocking 5HTT function and correcting 5HT levels, a partial agonist of the 5HT1A receptor (e.g., Aripiprazole) has been shown to be effective in patients with AN (Frank, 2016; Frank et al., 2017; Marzola et al., 2015). Experimentally, the long-term administration of a selective 5HT2A receptor antagonist was shown to upregulate decreased 5HT2A receptors in animal studies (Rinaldi-Carmona et al., 1993; Yadav et al., 2011). Taken together, these findings indicate that although no method for stimulating 5HTT has been used in practice, collecting evidence regarding 5HTT from clinical and preclinical studies is necessary for advancing the pharmacological treatment of AN.
Our study has some limitations. First, some of the patients with AN in the current study engaged in habitual smoking and drinking. However, no significant effect of smoking or drinking on [11C]DASB BPND was reported in a previous study (Erritzoe et al., 2010). Second, the current results revealed a significant difference in BMI between the ANR and ANBP groups. We minimized the effects of differences in body weight by including BMI as a covariate in the SPM analysis. Additionally, the present VBM analysis revealed no effect of brain atrophy on [11C]DASB BPND levels in the target region in AN patients. Third, this study had a cross-sectional design. To clarify any differences in 5HTT vulnerability between the two subtypes, it may be useful to conduct a longitudinal study of both AN subtypes rather than a cross-sectional study. Fourth, although we used the reaction time for all distorted body image in our dot-probe task to assess an attentional bias based on our preliminary analysis (Supplementary Table 1), previous studies of the dot-probe task evaluating an attentional bias in patients with AN used some kinds of parameters (i.e., reaction time, attentional bias score, and saccade latency) for various styles of body image (i.e., thin or obese images and images of the participant's or another person's body) (Blechert et al., 2010; Kim et al., 2014; Pona et al., 2017). To investigate the meaning of the relationship between the reaction time for all images and the 5HTT availability in this study, we will need to evaluate each parameter for each body image in the future. Finally, although the distorted body images used in our study were similar to those used in previous fMRI studies (Miyake et al., 2010), a correlational study may be required to further elucidate the associations between the dot-probe task and the neural networks involved in BID in underweight patients with active AN.
5. Conclusion
We found significantly decreased 5HTT availability in the medial parietal cortex (the somatosensory association area) in underweight patients with both active AN subtypes, indicating that 5HTT vulnerability in this region might be pathophysiologically important in AN in general. In addition to this alteration, an involvement of 5HTT reduction in the dorsal raphe highlights the significance of the clinicopathophysiological aspect as seen in patients with active ANR, suggesting possible involvement with impaired multisensory integration. Hence, a therapeutic intervention on this raphe 5HTT hypofunction may help ameliorate BID-related symptoms in active ANR. The 5HT system contributes to the pathophysiology of AN.
Declaration of Competing Interests
None.
Acknowledgments
This work was supported by JSPS KAKENHI Grant Numbers JP26860921, JP16K10210, JP16H06402 (Willdynamics) and 17H042470001, 19K08014.
We thank Mitsuru Kikuchi, MD, PhD (Department of Psychiatry and Neurobiology, Graduate School of Medical Science, Kanazawa University, Japan), and Kei Omata, PhD (National Center of Neurology and Psychiatry, Japan) for their assistance in the dot-probe task. We also express our gratitude toward all the study participants and the staff of the Hamamatsu Photonics Medical Foundation, the Department of Psychiatry and Neurology, Hamamatsu University School of Medicine and the Shizuoka Prefectural Treatment and Support Center for Eating Disorders for their assistance with data collection.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2019.101928.
Appendix A. Supplementary date
Supplementary material
References
- American Psychiatric Association . 4th ed. American Psychiatric Publishing; Washington, DC: 2000. Diagnostic and Statistical Manual of Mental Disorders. text revision. [Google Scholar]
- Anderson D.E., Bell T.A., Awh E. Polymorphisms in the 5-HTTLPR gene mediate storage capacity of visual working memory. J. Cogn. Neurosci. 2012;24:1069–1076. doi: 10.1162/jocn_a_00207. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Bailer U.F., Frank G.K., Henry S.E., Price J.C., Meltzer C.C., Weissfeld L., Mathis C.A., Drevets W.C., Wagner A., Hoge J., Ziolko S.K., McConaha C.W., Kaye W.H. Altered brain serotonin 5-HT1A receptor binding after recovery from anorexia nervosa measured by positron emission tomography and [carbonyl11C]WAY-100635. Arch. Gen. Psychiatry. 2005;62:1032–1041. doi: 10.1001/archpsyc.62.9.1032. [DOI] [PubMed] [Google Scholar]
- Bailer U.F., Frank G.K., Henry S.E., Price J.C., Meltzer C.C., Becker C., Ziolko S.K., Mathis C.A., Wagner A., Barbarich-Marsteller N.C., Putnam K., Kaye W.H. Serotonin transporter binding after recovery from eating disorders. Psychopharmacology. 2007;195:315–324. doi: 10.1007/s00213-007-0896-7. [DOI] [PubMed] [Google Scholar]
- Bailer U.F., Frank G.K., Henry S.E., Price J.C., Meltzer C.C., Mathis C.A., Wagner A., Thornton L., Hoge J., Ziolko S.K., Becker C.R., McConaha C.W., Kaye W.H. Exaggerated 5-HT1A but normal 5-HT2A receptor activity in individuals ill with anorexia nervosa. Biol. Psychiatry. 2007;61:1090–1099. doi: 10.1016/j.biopsych.2006.07.018. [DOI] [PubMed] [Google Scholar]
- Ballaz S.J., Akil H., Watson S.J. The 5-HT7 receptor: role in novel object discrimination and relation to novelty-seeking behavior. Neuroscience. 2007;149:192–202. doi: 10.1016/j.neuroscience.2007.07.043. [DOI] [PubMed] [Google Scholar]
- Barbarich N.C., McConaha C.W., Halmi K.A., Gendall K., Sunday S.R., Gaskill J., La Via M., Frank G.K., Brooks S., Plotnicov K.H., Kaye W.H. Use of nutritional supplements to increase the efficacy of fluoxetine in the treatment of anorexia nervosa. Int. J. Eat. Disord. 2004;35:10–15. doi: 10.1002/eat.10235. [DOI] [PubMed] [Google Scholar]
- Baumann O., Mattingley J.B. Medial parietal cortex encodes perceived heading direction in humans. J. Neurosci. 2010;30:12897–12901. doi: 10.1523/JNEUROSCI.3077-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A., Damasio A.R., Damasio H., Anderson S.W. Insensitivity to future consequences following damage to human prefrontal cortex. Cognition. 1994;50:7–15. doi: 10.1016/0010-0277(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Beck S.G., Choi K.C., List T.J. Comparison of 5-hydroxytryptamine1A-mediated hyperpolarization in CA1 and CA3 hippocampal pyramidal cells. J. Pharmacol. Exp. Ther. 1992;263:350–359. [PubMed] [Google Scholar]
- Beck A.T., Steer R.A., Ball R., Ranieri W. Comparison of beck depression inventories -IA and -II in psychiatric outpatients. J. Pers. Assess. 1996;67:588–597. doi: 10.1207/s15327752jpa6703_13. [DOI] [PubMed] [Google Scholar]
- Bekinschtein P., Renner M.C., Gonzalez M.C., Weisstaub N. Role of medial prefrontal cortex serotonin 2A receptors in the control of retrieval of recognition memory in rats. J. Neurosci. 2013;33:15716–15725. doi: 10.1523/JNEUROSCI.2087-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bel N., Artigas F. Fluvoxamine preferentially increases extracellular 5-hydroxytryptamine in the raphe nuclei: an in vivo microdialysis study. Eur. J. Pharmacol. 1992;229:101–103. doi: 10.1016/0014-2999(92)90292-c. [DOI] [PubMed] [Google Scholar]
- Blechert J., Ansorge U., Tuschen-Caffier B. A body-related dot-probe task reveals distinct attentional patterns for bulimia nervosa and anorexia nervosa. J. Abnorm. Psychol. 2010;119:575–585. doi: 10.1037/a0019531. [DOI] [PubMed] [Google Scholar]
- Bomba M., Marfone M., Brivio E., Oggiano S., Broggi F., Neri F., Nacinovich R. Autobiographical memory in adolescent girls with anorexia nervosa. Eur. Eat. Disord. Rev. 2014;22:479–486. doi: 10.1002/erv.2321. [DOI] [PubMed] [Google Scholar]
- Boraska V., Franklin C.S., Floyd J.A., Thornton L.M., Huckins L.M., Southam L., Rayner N.W., Tachmazidou I., Klump K.L., Treasure J., Lewis C.M., Schmidt U., Tozzi F., Kiezebrink K., Hebebrand J., Gorwood P., Adan R.A., Kas M.J., Favaro A., Santonastaso P., Fernandez-Aranda F., Gratacos M., Rybakowski F., Dmitrzak-Weglarz M., Kaprio J., Keski-Rahkonen A., Raevuori A., Van Furth E.F., Slof-Op ’t Landt M.C., Hudson J.I., Reichborn-Kjennerud T., Knudsen G.P., Monteleone P., Kaplan A.S., Karwautz A., Hakonarson H., Berrettini W.H., Guo Y., Li D., Schork N.J., Komaki G., Ando T., Inoko H., Esko T., Fischer K., Mannik K., Metspalu A., Baker J.H., Cone R.D., Dackor J., DeSocio J.E., Hilliard C.E., O’Toole J.K., Pantel J., Szatkiewicz J.P., Taico C., Zerwas S., Trace S.E., Davis O.S., Helder S., Buhren K., Burghardt R., de Zwaan M., Egberts K., Ehrlich S., Herpertz-Dahlmann B., Herzog W., Imgart H., Scherag A., Scherag S., Zipfel S., Boni C., Ramoz N., Versini A., Brandys M.K., Danner U.N., de Kovel C., Hendriks J., Koeleman B.P., Ophoff R.A., Strengman E., van Elburg A.A., Bruson A., Clementi M., Degortes D., Forzan M., Tenconi E., Docampo E., Escaramis G., Jimenez-Murcia S., Lissowska J., Rajewski A., Szeszenia-Dabrowska N., Slopien A., Hauser J., Karhunen L., Meulenbelt I., Slagboom P.E., Tortorella A., Maj M., Dedoussis G., Dikeos D., Gonidakis F., Tziouvas K., Tsitsika A., Papezova H., Slachtova L., Martaskova D., Kennedy J.L., Levitan R.D., Yilmaz Z., Huemer J., Koubek D., Merl E., Wagner G., Lichtenstein P., Breen G., Cohen-Woods S., Farmer A., McGuffin P., Cichon S., Giegling I., Herms S., Rujescu D., Schreiber S., Wichmann H.E., Dina C., Sladek R., Gambaro G., Soranzo N., Julia A., Marsal S., Rabionet R., Gaborieau V., Dick D.M., Palotie A., Ripatti S., Widen E., Andreassen O.A., Espeseth T., Lundervold A., Reinvang I., Steen V.M., Le Hellard S., Mattingsdal M., Ntalla I., Bencko V., Foretova L., Janout V., Navratilova M., Gallinger S., Pinto D., Scherer S.W., Aschauer H., Carlberg L., Schosser A., Alfredsson L., Ding B., Klareskog L., Padyukov L., Courtet P., Guillaume S., Jaussent I., Finan C., Kalsi G., Roberts M., Logan D.W., Peltonen L., Ritchie G.R., Barrett J.C., Estivill X., Hinney A., Sullivan P.F., Collier D.A., Zeggini E., Bulik C.M. A genome-wide association study of anorexia nervosa. Mol. Psychiatry. 2014;19:1085–1094. doi: 10.1038/mp.2013.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brambilla F., Garcia C.S., Fassino S., Daga G.A., Favaro A., Santonastaso P., Ramaciotti C., Bondi E., Mellado C., Borriello R., Monteleone P. Olanzapine therapy in anorexia nervosa: psychobiological effects. Int. Clin. Psychopharmacol. 2007;22:197–204. doi: 10.1097/YIC.0b013e328080ca31. [DOI] [PubMed] [Google Scholar]
- Bruce K.R., Steiger H., Ng Ying Kin N.M., Israel M. Reduced platelet [3H]paroxetine binding in anorexia nervosa: relationship to eating symptoms and personality pathology. Psychiatry Res. 2006;142:225–232. doi: 10.1016/j.psychres.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Burke L.K., Heisler L.K. 5-hydroxytryptamine medications for the treatment of obesity. J. Neuroendocrinol. 2015;27:389–398. doi: 10.1111/jne.12287. [DOI] [PubMed] [Google Scholar]
- Carr G.V., Schechter L.E., Lucki I. Antidepressant and anxiolytic effects of selective 5-HT6 receptor agonists in rats. Psychopharmacology. 2011;213:499–507. doi: 10.1007/s00213-010-1798-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter J.C., Mercer-Lynn K.B., Norwood S.J., Bewell-Weiss C.V., Crosby R.D., Woodside D.B., Olmsted M.P. A prospective study of predictors of relapse in anorexia nervosa: implications for relapse prevention. Psychiatry Res. 2012;200:518–523. doi: 10.1016/j.psychres.2012.04.037. [DOI] [PubMed] [Google Scholar]
- Chen X., Margolis K.J., Gershon M.D., Schwartz G.J., Sze J.Y. Reduced serotonin reuptake transporter (SERT) function causes insulin resistance and hepatic steatosis independent of food intake. PLoS One. 2012;7 doi: 10.1371/journal.pone.0032511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chenu F., David D.J., Leroux-Nicollet I., Le Maitre E., Gardier A.M., Bourin M. Serotonin1B heteroreceptor activation induces an antidepressant-like effect in mice with an alteration of the serotonergic system. J. Psychiatry Neurosci. 2008;33:541–550. [PMC free article] [PubMed] [Google Scholar]
- Dakanalis A., Gaudio S., Serino S., Clerici M., Carrà G., Riva G. Body-image distortion in anorexia nervosa. Nat. Rev. Dis. Primers. 2016;2 [Google Scholar]
- Ding S., Li L., Zhou F.M. Robust presynaptic serotonin 5-HT(1B) receptor inhibition of the striatonigral output and its sensitization by chronic fluoxetine treatment. J. Neurophysiol. 2015;113:3397–3409. doi: 10.1152/jn.00831.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DM G. 1991. EDI-2. Eating Disorder Inventory-2. (Professional manual) [Google Scholar]
- Enge S., Fleischhauer M., Lesch K.P., Reif A., Strobel A. Variation in key genes of serotonin and norepinephrine function predicts gamma-band activity during goal-directed attention. Cereb. Cortex. 2014;24:1195–1205. doi: 10.1093/cercor/bhs398. [DOI] [PubMed] [Google Scholar]
- Erritzoe D., Frokjaer V.G., Haahr M.T., Kalbitzer J., Svarer C., Holst K.K., Hansen D.L., Jernigan T.L., Lehel S., Knudsen G.M. Cerebral serotonin transporter binding is inversely related to body mass index. Neuroimage. 2010;52:284–289. doi: 10.1016/j.neuroimage.2010.03.086. [DOI] [PubMed] [Google Scholar]
- Faul F., Erdfelder E., Lang A.G., Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods. 2007;39:175–191. doi: 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- Favaro A., Santonastaso P., Manara R., Bosello R., Bommarito G., Tenconi E., Di Salle F. Disruption of visuospatial and somatosensory functional connectivity in anorexia nervosa. Biol. Psychiatry. 2012;72:864–870. doi: 10.1016/j.biopsych.2012.04.025. [DOI] [PubMed] [Google Scholar]
- Fichter M.M., Quadflieg N., Crosby R.D., Koch S. Long-term outcome of anorexia nervosa: results from a large clinical longitudinal study. Int. J. Eat. Disord. 2017 Sep;50(9):1018–1030. doi: 10.1002/eat.22736. Epub 2017 Jun 23. [DOI] [PubMed] [Google Scholar]
- Fioravanti G., Castellini G., Lo Sauro C., Ianni S., Montanelli L., Rotella F., Faravelli C., Ricca V. Course and moderators of emotional eating in anorectic and bulimic patients: a follow-up study. Eat. Behav. 2014;15:192–196. doi: 10.1016/j.eatbeh.2014.01.006. [DOI] [PubMed] [Google Scholar]
- First M., Gibbon M., Spitzer R.L., Williams J.B.W., Benjamin L.S. American Psychiatric Press, Inc; 1997. Structured Clinical Interview for DSM-IV Axis II Personality Disorders, (SCID-II) [Google Scholar]
- First M.B., Spitzer R.L., Miriam G., Williams J.B.W. Patient edition. New York State Psychiatric Institute; 2002. Structured Clinical Interview for DSM-IV-TR axis I Disorders, Research Version. (SCID-I/P). Biometrics Research. [Google Scholar]
- Frank G.K. Aripiprazole, a partial dopamine agonist to improve adolescent anorexia nervosa-A case series. Int. J. Eat. Disord. 2016;49:529–533. doi: 10.1002/eat.22485. [DOI] [PubMed] [Google Scholar]
- Frank G.K., Kaye W.H. Current status of functional imaging in eating disorders. Int. J. Eat. Disord. 2012;45:723–736. doi: 10.1002/eat.22016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank G.K., Kaye W.H., Meltzer C.C., Price J.C., Greer P., McConaha C., Skovira K. Reduced 5-HT2A receptor binding after recovery from anorexia nervosa. Biol. Psychiatry. 2002;52:896–906. doi: 10.1016/s0006-3223(02)01378-1. [DOI] [PubMed] [Google Scholar]
- Frank G.K., Shott M.E., Hagman J.O., Schiel M.A., DeGuzman M.C., Rossi B. The partial dopamine D2 receptor agonist aripiprazole is associated with weight gain in adolescent anorexia nervosa. Int. J. Eat. Disord. 2017;50:447–450. doi: 10.1002/eat.22704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadsby S. Distorted body representations in anorexia nervosa. Conscious. Cogn. 2017;51:17–33. doi: 10.1016/j.concog.2017.02.015. [DOI] [PubMed] [Google Scholar]
- Galusca B., Costes N., Zito N.G., Peyron R., Bossu C., Lang F., Le Bars D., Estour B. Organic background of restrictive-type anorexia nervosa suggested by increased serotonin 1A receptor binding in right frontotemporal cortex of both lean and recovered patients: [18F]MPPF PET scan study. Biol. Psychiatry. 2008;64:1009–1013. doi: 10.1016/j.biopsych.2008.06.006. [DOI] [PubMed] [Google Scholar]
- Garcia R.A., Afeche S.C., Scialfa J.H., do Amaral F.G., dos Santos S.H., Lima F.B., Young M.E., Cipolla-Neto J. Insulin modulates norepinephrine-mediated melatonin synthesis in cultured rat pineal gland. Life Sci. 2008;82:108–114. doi: 10.1016/j.lfs.2007.10.016. [DOI] [PubMed] [Google Scholar]
- Garner D.M., Anderson M.L., Keiper C.D., Whynott R., Parker L. Psychotropic medications in adult and adolescent eating disorders: clinical practice versus evidence-based recommendations. Eat. Weight Disord. 2016;21:395–402. doi: 10.1007/s40519-016-0253-0. [DOI] [PubMed] [Google Scholar]
- Gaudio S., Wiemerslage L., Brooks S.J., Schioth H.B. A systematic review of resting-state functional-MRI studies in anorexia nervosa: evidence for functional connectivity impairment in cognitive control and visuospatial and body-signal integration. Neurosci. Biobehav. Rev. 2016;71:578–589. doi: 10.1016/j.neubiorev.2016.09.032. [DOI] [PubMed] [Google Scholar]
- Glikmann-Johnston Y., Saling M.M., Reutens D.C., Stout J.C. Hippocampal 5-HT1A receptor and spatial learning and memory. Front. Pharmacol. 2015;6:289. doi: 10.3389/fphar.2015.00289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman W.K., Price L.H., Rasmussen S.A., Mazure C., Fleischmann R.L., Hill C.L., Heninger G.R., Charney D.S. The Yale-Brown obsessive compulsive scale. I. Development, use, and reliability. Arch. Gen. Psychiatry. 1989;46:1006–1011. doi: 10.1001/archpsyc.1989.01810110048007. [DOI] [PubMed] [Google Scholar]
- Graham B.M., Ash C., Den M.L. High endogenous estradiol is associated with enhanced cognitive emotion regulation of physiological conditioned fear responses in women. Psychoneuroendocrinology. 2017;80:7–14. doi: 10.1016/j.psyneuen.2017.02.023. [DOI] [PubMed] [Google Scholar]
- Hamon M., Lanfumey L., el Mestikawy S., Boni C., Miquel M.C., Bolanos F., Schechter L., Gozlan H. The main features of central 5-HT1 receptors. Neuropsychopharmacology. 1990;3:349–360. [PubMed] [Google Scholar]
- Hannon J., Hoyer D. Molecular biology of 5-HT receptors. Behav. Brain Res. 2008;195:198–213. doi: 10.1016/j.bbr.2008.03.020. [DOI] [PubMed] [Google Scholar]
- Hoang U., Goldacre M., James A. Mortality following hospital discharge with a diagnosis of eating disorder: national record linkage study, England, 2001–2009. Int. J. Eat. Disord. 2014;47:507–515. doi: 10.1002/eat.22249. [DOI] [PubMed] [Google Scholar]
- Horndasch S., Kratz O., Holczinger A., Heinrich H., Honig F., Noth E., Moll G.H. “Looks do matter”--visual attentional biases in adolescent girls with eating disorders viewing body images. Psychiatry Res. 2012;198:321–323. doi: 10.1016/j.psychres.2011.12.029. [DOI] [PubMed] [Google Scholar]
- Huber J., Salatsch C., Ingenerf K., Schmid C., Maatouk I., Weisbrod M., Herzog W., Friederich H.-C., Nikendei C. Characteristics of disorder-related autobiographical memory in acute anorexia nervosa patients. Eur. Eat. Disord. Rev. 2015;23:379–389. doi: 10.1002/erv.2379. [DOI] [PubMed] [Google Scholar]
- Ichise M., Liow J.S., Lu J.Q., Takano A., Model K., Toyama H., Suhara T., Suzuki K., Innis R.B., Carson R.E. Linearized reference tissue parametric imaging methods: application to [11C]DASB positron emission tomography studies of the serotonin transporter in human brain. J. Cereb. Blood Flow Metab. 2003;23:1096–1112. doi: 10.1097/01.WCB.0000085441.37552.CA. [DOI] [PubMed] [Google Scholar]
- Jacobi C., Hayward C., de Zwaan M., Kraemer H.C., Agras W.S. Coming to terms with risk factors for eating disorders: application of risk terminology and suggestions for a general taxonomy. Psychol. Bull. 2004;130:19–65. doi: 10.1037/0033-2909.130.1.19. [DOI] [PubMed] [Google Scholar]
- Jahng J.W., Kim J.G., Kim H.J., Kim B.T., Kang D.W., Lee J.H. Chronic food restriction in young rats results in depression- and anxiety-like behaviors with decreased expression of serotonin reuptake transporter. Brain Res. 2007;1150:100–107. doi: 10.1016/j.brainres.2007.02.080. [DOI] [PubMed] [Google Scholar]
- Javaras K.N., Runfola C.D., Thornton L.M., Agerbo E., Birgegard A., Norring C., Yao S., Rastam M., Larsson H., Lichtenstein P., Bulik C.M. Sex- and age-specific incidence of healthcare-register-recorded eating disorders in the complete swedish 1979-2001 birth cohort. Int. J. Eat. Disord. 2015;48:1070–1081. doi: 10.1002/eat.22467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JC R. 1936. Mental Tests Used in Genetic Studies: The Performance of Related Individuals on Tests Mainly Educative and Mainly Reproductive. (MSc Thesis) [Google Scholar]
- Kalueff A.V., Olivier J.D., Nonkes L.J., Homberg J.R. Conserved role for the serotonin transporter gene in rat and mouse neurobehavioral endophenotypes. Neurosci. Biobehav. Rev. 2010;34:373–386. doi: 10.1016/j.neubiorev.2009.08.003. [DOI] [PubMed] [Google Scholar]
- Kaye W.H., Ebert M.H., Raleigh M., Lake R. Abnormalities in CNS monoamine metabolism in anorexia nervosa. Arch. Gen. Psychiatry. 1984;41:350–355. doi: 10.1001/archpsyc.1984.01790150040007. [DOI] [PubMed] [Google Scholar]
- Kaye W.H., Gwirtsman H.E., George D.T., Ebert M.H. Altered serotonin activity in anorexia nervosa after long-term weight restoration. Does elevated cerebrospinal fluid 5-hydroxyindoleacetic acid level correlate with rigid and obsessive behavior? Arch. Gen. Psychiatry. 1991;48:556–562. doi: 10.1001/archpsyc.1991.01810300068010. [DOI] [PubMed] [Google Scholar]
- Kaye W.H., Barbarich N.C., Putnam K., Gendall K.A., Fernstrom J., Fernstrom M., McConaha C.W., Kishore A. Anxiolytic effects of acute tryptophan depletion in anorexia nervosa. Int. J. Eat. Disord. 2003;33:257–270. doi: 10.1002/eat.10135. [DOI] [PubMed] [Google Scholar]
- Kim Y.R., Kim C.H., Cardi V., Eom J.S., Seong Y., Treasure J. Intranasal oxytocin attenuates attentional bias for eating and fat shape stimuli in patients with anorexia nervosa. Psychoneuroendocrinology. 2014;44:133–142. doi: 10.1016/j.psyneuen.2014.02.019. [DOI] [PubMed] [Google Scholar]
- Kinasz K., Accurso E.C., Kass A.E., Le Grange D. Does sex matter in the clinical presentation of eating disorders in youth? J. Adolesc. Health. 2016;58:410–416. doi: 10.1016/j.jadohealth.2015.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koopman K.E., Roefs A., Elbers D.C., Fliers E., Booij J., Serlie M.J., la Fleur S.E. Brain dopamine and serotonin transporter binding are associated with visual attention bias for food in lean men. Psychol. Med. 2016;46:1707–1717. doi: 10.1017/S0033291716000222. [DOI] [PubMed] [Google Scholar]
- Laplante P., Diorio J., Meaney M.J. Serotonin regulates hippocampal glucocorticoid receptor expression via a 5-HT7 receptor. Brain Res. Dev. Brain Res. 2002;139:199–203. doi: 10.1016/s0165-3806(02)00550-3. [DOI] [PubMed] [Google Scholar]
- Liu Y., Kelly M.A., Sexton T.J., Neumaier J.F. 5-HT1B autoreceptors differentially modulate the expression of conditioned fear in a circuit-specific manner. Neuroscience. 2015;298:436–447. doi: 10.1016/j.neuroscience.2015.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLeod C., Mathews A. Anxiety and the allocation of attention to threat. Q. J. Exp. Psychol. A. 1988;40:653–670. doi: 10.1080/14640748808402292. [DOI] [PubMed] [Google Scholar]
- Madsen K., Erritzoe D., Mortensen E.L., Gade A., Madsen J., Baare W., Knudsen G.M., Hasselbalch S.G. Cognitive function is related to fronto-striatal serotonin transporter levels--a brain PET study in young healthy subjects. Psychopharmacology. 2011;213:573–581. doi: 10.1007/s00213-010-1926-4. [DOI] [PubMed] [Google Scholar]
- Manoharan A., Shewade D.G., Rajkumar R.P., Adithan S. Serotonin transporter gene (SLC6A4) polymorphisms are associated with response to fluoxetine in south Indian major depressive disorder patients. Eur. J. Clin. Pharmacol. 2016;72:1215–1220. doi: 10.1007/s00228-016-2099-9. [DOI] [PubMed] [Google Scholar]
- Marvanova M., Gramith K. Role of antidepressants in the treatment of adults with anorexia nervosa. Ment. Health Clin. 2018;8:127–137. doi: 10.9740/mhc.2018.05.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzola E., Desedime N., Giovannone C., Amianto F., Fassino S., Abbate-Daga G. Atypical antipsychotics as augmentation therapy in anorexia nervosa. PLoS One. 2015;10 doi: 10.1371/journal.pone.0125569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina-Aguirre I., Gutierrez-Ospina G., Hernandez-Rodriguez J., Boyzo A., Manjarrez-Gutierrez G. Development of 5-HT(1B), SERT and thalamo-cortical afferents in early nutrionally restricted rats: an emerging explanation for delayed barrel formation. Int. J. Dev. Neurosci. 2008;26:225–231. doi: 10.1016/j.ijdevneu.2007.12.002. [DOI] [PubMed] [Google Scholar]
- Miu A.C., Crisan L.G., Chis A., Ungureanu L., Druga B., Vulturar R. Somatic markers mediate the effect of serotonin transporter gene polymorphisms on Iowa Gambling Task. Genes Brain Behav. 2012;11:398–403. doi: 10.1111/j.1601-183X.2012.00774.x. [DOI] [PubMed] [Google Scholar]
- Miyake Y., Okamoto Y., Onoda K., Kurosaki M., Shirao N., Okamoto Y., Yamawaki S. Brain activation during the perception of distorted body images in eating disorders. Psychiatry Res. 2010;181:183–192. doi: 10.1016/j.pscychresns.2009.09.001. [DOI] [PubMed] [Google Scholar]
- Morici J.F., Ciccia L., Malleret G., Gingrich J.A., Bekinschtein P., Weisstaub N.V. Serotonin 2a receptor and serotonin 1a receptor interact within the medial prefrontal cortex during recognition memory in mice. Front. Pharmacol. 2015;6:298. doi: 10.3389/fphar.2015.00298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam S.B., Kim K., Kim B.S., Im H.J., Lee S.H., Kim S.J., Kim I.J., Pak K. The effect of obesity on the availabilities of dopamine and serotonin transporters. Sci. Rep. 2018;8:4924. doi: 10.1038/s41598-018-22814-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonogaki K., Strack A.M., Dallman M.F., Tecott L.H. Leptin-independent hyperphagia and type 2 diabetes in mice with a mutated serotonin 5-HT2C receptor gene. Nat. Med. 1998;4:1152–1156. doi: 10.1038/2647. [DOI] [PubMed] [Google Scholar]
- Ohwada R., Hotta M., Sato K., Shibasaki T., Takano K. The relationship between serum levels of estradiol and osteoprotegerin in patients with anorexia nervosa. Endocr. J. 2007;54:953–959. doi: 10.1507/endocrj.k07-034. [DOI] [PubMed] [Google Scholar]
- Olivier J.D., Jans L.A., Blokland A., Broers N.J., Homberg J.R., Ellenbroek B.A., Cools A.R. Serotonin transporter deficiency in rats contributes to impaired object memory. Genes Brain Behav. 2009;8:829–834. doi: 10.1111/j.1601-183X.2009.00530.x. [DOI] [PubMed] [Google Scholar]
- Orosco M., Rouch C., Gerozissis K. Activation of hypothalamic insulin by serotonin is the primary event of the insulin-serotonin interaction involved in the control of feeding. Brain Res. 2000;872:64–70. doi: 10.1016/s0006-8993(00)02449-5. [DOI] [PubMed] [Google Scholar]
- Ouchi Y., Nobezawa S., Okada H., Yoshikawa E., Futatsubashi M., Kaneko M. Altered glucose metabolism in the hippocampal head in memory impairment. Neurology. 1998;51:136–142. doi: 10.1212/wnl.51.1.136. [DOI] [PubMed] [Google Scholar]
- Overas M., Kapstad H., Brunborg C., Landro N.I., Lask B. Memory versus perception of body size in patients with anorexia nervosa and healthy controls. Eur. Eat. Disord. Rev. 2014;22:109–115. doi: 10.1002/erv.2276. [DOI] [PubMed] [Google Scholar]
- Perez-Cornago A., Mansego M.L., Zulet M.A., Martinez J.A. DNA hypermethylation of the serotonin receptor type-2A gene is associated with a worse response to a weight loss intervention in subjects with metabolic syndrome. Nutrients. 2014;6:2387–2403. doi: 10.3390/nu6062387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platek S.M., Wathne K., Tierney N.G., Thomson J.W. Neural correlates of self-face recognition: an effect-location meta-analysis. Brain Res. 2008;1232:173–184. doi: 10.1016/j.brainres.2008.07.010. [DOI] [PubMed] [Google Scholar]
- Pona A.A., Jones A.C., Masterson T.L., Ben-Porath D.D. Biases in attention and memory for body shape images in eating disorders. Eat. Weight Disord. 2017 doi: 10.1007/s40519-017-0472-z. [DOI] [PubMed] [Google Scholar]
- Pratelli M., Migliarini S., Pelosi B., Napolitano F., Usiello A., Pasqualetti M. Perturbation of serotonin homeostasis during adulthood affects serotonergic neuronal circuitry. eNeuro. 2017;4 doi: 10.1523/ENEURO.0376-16.2017. e0376-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price J.S., Strong J., Eliassen J., McQueeny T., Miller M., Padula C.B., Shear P., Lisdahl K. Serotonin transporter gene moderates associations between mood, memory and hippocampal volume. Behav. Brain Res. 2013;242:158–165. doi: 10.1016/j.bbr.2012.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riad M., Garcia S., Watkins K.C., Jodoin N., Doucet E., Langlois X., el Mestikawy S., Hamon M., Descarries L. Somatodendritic localization of 5-HT1A and preterminal axonal localization of 5-HT1B serotonin receptors in adult rat brain. J. Comp. Neurol. 2000;417:181–194. [PubMed] [Google Scholar]
- Rinaldi-Carmona M., Bouaboula M., Congy C., Oury-Donat F., Simiand J., Shire D., Casellas P., Soubrie P., Breliere J.C., Le Fur G. Up-regulation of 5-HT2 receptors in the rat brain by repeated administration of SR 46349B, a selective 5-HT2 receptor antagonist. Eur. J. Pharmacol. 1993;246:73–80. doi: 10.1016/0922-4106(93)90012-x. [DOI] [PubMed] [Google Scholar]
- Riva G. Neurobiology of anorexia nervosa: serotonin dysfunctions link self-starvation with body image disturbances through an impaired body memory. Front. Hum. Neurosci. 2016;10:600. doi: 10.3389/fnhum.2016.00600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riva G., Dakanalis A. Altered processing and integration of multisensory bodily representations and signals in eating disorders: a possible path toward the understanding of their underlying causes. Front. Hum. Neurosci. 2018;12:49. doi: 10.3389/fnhum.2018.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkisyan G., Hedlund P.B. The 5-HT7 receptor is involved in allocentric spatial memory information processing. Behav. Brain Res. 2009;202:26–31. doi: 10.1016/j.bbr.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savli M., Bauer A., Mitterhauser M., Ding Y.S., Hahn A., Kroll T., Neumeister A., Haeusler D., Ungersboeck J., Henry S., Isfahani S.A., Rattay F., Wadsak W., Kasper S., Lanzenberger R. Normative database of the serotonergic system in healthy subjects using multi-tracer PET. Neuroimage. 2012;63:447–459. doi: 10.1016/j.neuroimage.2012.07.001. [DOI] [PubMed] [Google Scholar]
- Serino S., Dakanalis A., Gaudio S., Carra G., Cipresso P., Clerici M., Riva G. Out of body, out of space: impaired reference frame processing in eating disorders. Psychiatry Res. 2015;230:732–734. doi: 10.1016/j.psychres.2015.10.025. [DOI] [PubMed] [Google Scholar]
- Serino S., Pedroli E., Keizer A., Triberti S., Dakanalis A., Pallavicini F., Chirico A., Riva G. Virtual reality body swapping: a tool for modifying the allocentric memory of the body. Cyberpsychol. Behav. Soc. Netw. 2016;19:127–133. doi: 10.1089/cyber.2015.0229. [DOI] [PubMed] [Google Scholar]
- Sigurdh J., Allard P., Spigset O., Hagglof B. Platelet serotonin transporter and 5-HT2A receptor binding in adolescents with eating disorders. Int. J. Neurosci. 2013;123:333–338. doi: 10.3109/00207454.2012.761215. [DOI] [PubMed] [Google Scholar]
- Solmi M., Gallicchio D., Collantoni E., Correll C.U., Clementi M., Pinato C., Forzan M., Cassina M., Fontana F., Giannunzio V., Piva I., Siani R., Salvo P., Santonastaso P., Tenconi E., Veronese N., Favaro A. Serotonin transporter gene polymorphism in eating disorders: data from a new biobank and META-analysis of previous studies. World J. Biol. Psychiatry. 2016;17:244–257. doi: 10.3109/15622975.2015.1126675. [DOI] [PubMed] [Google Scholar]
- Sperduti M., Delaveau P., Fossati P., Nadel J. Different brain structures related to self- and external-agency attribution: a brief review and meta-analysis. Brain Struct. Funct. 2011;216:151–157. doi: 10.1007/s00429-010-0298-1. [DOI] [PubMed] [Google Scholar]
- Spielberger C.D., Gorsuch R.L., Lushene R., Vagg P.R., Jacobs G.A. Consulting Psychologists Press; 1983. Manual for the State-Trait Anxiety Inventory. [Google Scholar]
- Spies M., Knudsen G.M., Lanzenberger R., Kasper S. The serotonin transporter in psychiatric disorders: insights from PET imaging. Lancet Psychiatry. 2015;2:743–755. doi: 10.1016/S2215-0366(15)00232-1. [DOI] [PubMed] [Google Scholar]
- Strober M., Freeman R., Lampert C., Diamond J., Kaye W. Controlled family study of anorexia nervosa and bulimia nervosa: evidence of shared liability and transmission of partial syndromes. Am. J. Psychiatry. 2000;157:393–401. doi: 10.1176/appi.ajp.157.3.393. [DOI] [PubMed] [Google Scholar]
- Suzuki A., Matsumoto Y., Oshino S., Kamata M., Goto K., Otani K. Combination of the serotonin transporter and norepinephrine transporter gene promoter polymorphisms might influence harm avoidance and novelty seeking in healthy females. Neurosci. Lett. 2008;439:52–55. doi: 10.1016/j.neulet.2008.04.088. [DOI] [PubMed] [Google Scholar]
- Tanaka K.F., Samuels B.A., Hen R. Serotonin receptor expression along the dorsal-ventral axis of mouse hippocampus. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2012;367:2395–2401. doi: 10.1098/rstb.2012.0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Z.Q., Trussell L.O. Serotonergic modulation of sensory representation in a central multisensory circuit is pathway specific. Cell Rep. 2017;20:1844–1854. doi: 10.1016/j.celrep.2017.07.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terhoeven V., Kallen U., Ingenerf K., Aschenbrenner S., Weisbrod M., Herzog W., Brockmeyer T., Friederich H.-C., Nikendei C. Meaningful memory in acute anorexia nervosa patients—comparing recall, learning, and recognition of semantically related and semantically unrelated word stimuli. Eur. Eat. Disord. Rev. 2017;25:89–97. doi: 10.1002/erv.2496. [DOI] [PubMed] [Google Scholar]
- Treisman A., Fearnley S. The Stroop test: selective attention to colours and words. Nature. 1969;222:437–439. doi: 10.1038/222437a0. [DOI] [PubMed] [Google Scholar]
- Van Autreve S., Vervaet M. Are there differences in central coherence and set shifting across the subtypes of anorexia nervosa?: a systematic review. J. Nerv. Ment. Dis. 2015;203:774–780. doi: 10.1097/NMD.0000000000000366. [DOI] [PubMed] [Google Scholar]
- Versteeg R.I., Koopman K.E., Booij J., Ackermans M.T., Unmehopa U.A., Fliers E., la Fleur S.E., Serlie M.J. Serotonin transporter binding in the diencephalon is reduced in insulin-resistant obese humans. Neuroendocrinology. 2017;105:141–149. doi: 10.1159/000450549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Versteeg R.I., Schrantee A., Adriaanse S.M., Unmehopa U.A., Booij J., Reneman L., Fliers E., la Fleur S.E., Serlie M.J. Timing of caloric intake during weight loss differentially affects striatal dopamine transporter and thalamic serotonin transporter binding. FASEB J. 2017 Oct;31(10):4545–4554. doi: 10.1096/fj.201601234R. Epub 2017 Jul 5. [DOI] [PubMed] [Google Scholar]
- Wang K., Zhang H., Bloss C.S., Duvvuri V., Kaye W., Schork N.J., Berrettini W., Hakonarson H. A genome-wide association study on common SNPs and rare CNVs in anorexia nervosa. Mol. Psychiatry. 2011;16:949–959. doi: 10.1038/mp.2010.107. [DOI] [PubMed] [Google Scholar]
- Watanabe M., Shimizu K., Omura T., Takahashi M., Kosugi T., Yoshikawa E., Sato N., Okada H., T. Y. A new high-resolution PET scanner dedicated to brain research. IEEE Trans. Nucl. Sci. 2002;49:634–639. [Google Scholar]
- Weider S., Indredavik M.S., Lydersen S., Hestad K. Neuropsychological function in patients with anorexia nervosa or bulimia nervosa. Int. J. Eat. Disord. 2015;48:397–405. doi: 10.1002/eat.22283. [DOI] [PubMed] [Google Scholar]
- Wu M., Brockmeyer T., Hartmann M., Skunde M., Herzog W., Friederich H.C. Set-shifting ability across the spectrum of eating disorders and in overweight and obesity: a systematic review and meta-analysis. Psychol. Med. 2014;44:3365–3385. doi: 10.1017/S0033291714000294. [DOI] [PubMed] [Google Scholar]
- Wu M., Brockmeyer T., Hartmann M., Skunde M., Herzog W., Friederich H.C. Reward-related decision making in eating and weight disorders: a systematic review and meta-analysis of the evidence from neuropsychological studies. Neurosci. Biobehav. Rev. 2016;61:177–196. doi: 10.1016/j.neubiorev.2015.11.017. [DOI] [PubMed] [Google Scholar]
- Wu Z.M., Zheng C.H., Zhu Z.H., Wu F.T., Ni G.L., Liang Y. SiRNA-mediated serotonin transporter knockdown in the dorsal raphe nucleus rescues single prolonged stress-induced hippocampal autophagy in rats. J. Neurol. Sci. 2016;360:133–140. doi: 10.1016/j.jns.2015.11.056. [DOI] [PubMed] [Google Scholar]
- Yadav P.N., Kroeze W.K., Farrell M.S., Roth B.L. Antagonist functional selectivity: 5-HT2A serotonin receptor antagonists differentially regulate 5-HT2A receptor protein level in vivo. J. Pharmacol. Exp. Ther. 2011;339:99–105. doi: 10.1124/jpet.111.183780. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material