Abstract
The research was targeted to investigate the effect of nano-TiO2 (anatase) on germination, vigour index, stress enzymes and mitotic cell cycle profile in lentils (Lens culinaris Medik.). Seed germination results indicated that TiO2 NP (Nanoparticle) at lowest concentration promotes seed germination, vigour index and biomass; however, at higher concentrations, they showed significant reduction in growth parameters and photosynthetic pigments in concentration-dependent manner. NP treatments triggered an excessive formation of reactive oxygen species (ROS) which was evident from increased production of stress enzymes, lipid peroxidation, augmented DNA damage and aberrant mitotic cell division. The results exhibit a dose-dependent modification of NP- mediated oxidative stress and genotoxicity in lentil.
Keywords: Agriculture, Environmental science, Nanomaterials, Materials science, Plant biology, Genotoxicity, TiO2 nanoparticles, Antioxidant enzymes, Lens culinaris
1. Introduction
Nanoscience is the study that deals in manipulation of materials at nanoscale, where properties differ significantly from the bulk material. Particle size and distribution are the most important characteristics of nanoparticle (NP) and are the major factors for the determination of it's in vivo distribution, biological fate, toxicity and the targeting ability of NP systems [1]. Effects of engineered nanoparticles on plants are of great concern because of their crucial interaction with the environment [2] and can have adverse effects on land and aquatic biota. Titanium oxide (TiO2) is among the top ten most produced engineered nanomaterials (ENMs) by mass [3] and was included in the list of ENMs of priority for immediate testing by the Organization for Economic Cooperation and Development (OECD) [4]. Titanium oxide NPs (TiO2) possesses many unique properties compared to it's bulk counterpart having high surface area. They have an ability to pass the cell membrane of living organisms because of their small size and are extensively used in cosmetics, printing ink, self-cleaning ceramics, sensing materials, glass, medicines, antiseptic, and a wide variety of industrial materials. The unproportionate use of these nanoparticles result into their accumulation in the soil, producers and ultimately entering the food chain since plants serve as a potential pathway for the transportation of Nanoparticles (NPs) [5] from primary producers (plants) to high trophic-level consumers [6]. Several studies have reported that injected or inhaled TiO2 NPs can migrate to several organs through circulation and impose adverse effects on organisms [7]. Therefore potential effects of these NPs on plants, especially on edible crop plants, should be evaluated before their widespread application.
Zheng et al. [8], Gao et al. [9], have shown that nano-sized TiO2 can have a positive effect on the growth of spinach when administered to the activity of several enzymes and to promote the adsorption of nitrate, acceleration of the transformation of inorganic into organic nitrogen. But at higher concentration of TiO2, seed germination, plant growth and physiological activities related to it may be adversely affected.
Plants possess several tissue antioxidants for protection against the potentially cytotoxic forms of activated oxygen. The harmful effects of free radicals produced as a result of oxidation are neutralized by the enzymatic antioxidant defences such as superoxide dismutase (SOD), glutathione peroxidase (GPx) and catalase (CAT). It is believed that nanoparticles increase the oxidative stress which mediates damage to cell structures, including lipids, membranes, proteins, and DNA. Ruffini Castiglione et al. [10] considered oxidative stress and oxidative damage as indicators of possible cytotoxicity caused by these nanoparticles.
The reports from previous studies have updated our knowledge regarding the toxicological impact of nanomaterial but still, the fate of release of these nanoparticles and their consequences on the plant system is poorly understood. There is also a paucity of literature regarding cytogenetic toxicity and dose-response relationship of nanoparticles in plant systems. Lentil is an economically important pulse crop with high protein content, widely used protein source for human, particularly for vegetarians, they are the only source of protein. Thus lentils are an important part of Indian crop. There are a lot of studies on heavy metal pollution on these crops but the problem of engineered NPs pollution is a new class of chemical pollution. Plant genotoxicity assays can be used in risk assessment for detection of environmental mutagens. So this research is an effort to examine the possible effects of anatase nanoparticles on germination, growth, genotoxicity and its role in inducing oxidative stress in lentil.
2. Materials & methods
2.1. TiO2 nanoparticles
The above-mentioned TiO2 NPs were obtained purchased from Sigma-Aldrich, USA, under the name of Titanium (IV) oxide, Anatase. According to manufacturer's data, the physical characteristics of the particles include specific surface area 45–55 m2/g, particle size <25 nm, mp 1825 °C (lit.) and density 3.9 g/mL at 25 °C (lit.). Characterisation of NP was done by UV–Vis optical spectra using Lambda 35 instrument (Perkin-Elmer).
2.2. Plant materials and treatments
The study was carried at Department of Botany, Aligarh Muslim University, India. Seeds of lentil were procured from National Seed Corporation (NSC), New Delhi, India. Fresh and healthy seeds were surface sterilized with 5% NaOCl for 10 min to ensure surface sterility and were rinsed thoroughly with distilled water several times. Seeds were then treated with five different concentrations (25, 50, 75, 100, 200 μg/mL) of TiO2 nanoparticle suspension for 24 h and 50 seeds per petri plate (size 200 × 20mm) in triplicates were allowed to germinate on wet cotton, at a controlled temperature of 25 ± 1 °C in the dark for 24 hours. The seeds were checked for germination and sprouts were used for growth and vigour studies. Another set of 75 seeds in replicates of three (25 seeds in each replicate) for each concentration were also sown in earthen pots for biochemical studies and allowed to grow under greenhouse condition. Control plants without treatment were also grown for comparative studies.
2.3. Growth studies
The parameters such as germination, seedling height, root length and vigour index were studied on 14 days old lentil seedlings after 24 h acute exposure of anatase NP. Vigour index (VI) was calculated by the procedure described by Abdul Baki and Anderson [11].
2.4. Photosynthetic pigment measurement
Estimation of chlorophyll was done by Acetone method [12]. Chlorophyll was extracted in 80% acetone and the absorbance was read at 663 and 645nm. The amount of chlorophyll was calculated using the formula.
Chlorophyll a (mg/g) = 12.7 (A663) − 2.69 (A645) × V/1000 × W
Chlorophyll b (mg/g) = 22.9 (A 645) − 4.68 (A 663) × V/1000 × W
Total chlorophyll (mg/g) = 20.2(A 645) + 8.02(A 663) × V/1000 × W
Carotenoid (mg/g) = 7.6 (A 480) – 1.49 (A 510) × V/1000 × W
Where
A = absorbance at specific wavelengths
V = final volume of chlorophyll extract
W = fresh weight of tissue extracted
2.5. Hydrogen peroxide scavenging effects
Hydrogen peroxide scavenging was determined [13] spectrophotometrically at 230 nm. The methanolic solution of leaf extracts at a concentration of 100 μg/mL was added to hydrogen peroxide solution (0.6 mL; 40 mM), prepared in phosphate buffer (pH 7.4). Final volume of the solution was made up to 3 mL and incubated for 10 min. Phosphate buffer solution without H2O2, served as a blank. Absorbance of all the compounds was measured at 230 nm. The extent of H2O2 scavenging of the plant extracts was calculated as
Hydrogen peroxide scavenging (%) = (A0 – A1) × 100/A0
A0 – Absorbance of control
A1 – Absorbance in the presence of plant extract
2.6. Lipid peroxidation
Lipid peroxidation was estimated following the method of Madhav Rao and Sresty [14]. Frozen 0.2 g of leaf samples were homogenized in 2 mL of 0.1% trichloroacetic acid (TCA) and centrifuged at 10,000 rpm for 10 min to pellet debris. Aliquots of supernatant were mixed with 4 mL of 20% TCA containing 0.5% thiobarbituric acid and 100 μL of butylated hydroxyl toluene in 4 % ethanol and incubated at 90 °C for 1 hr. After the tubes were cooled in an ice bath, they were centrifuged at 10,000 rpm for 5min. The supernatant was then measured at 532 nm, unspecific turbidity of the sample was measured at 600 nm and subtracted with the absorbance at 532 nm. The solution of 0.25% TBA in 10% TCA served as a blank. TBARS (thiobarbituric acid-reactive substance) content was expressed as nmol/g fresh weight (fw) using an extinction coefficient of 155 mM−1cm−1.
2.7. Superoxide dismutase (SOD) and catalase (CAT) activity
SOD activity was measured according to the protocol of Yu and Rengel [15] with slight modification. One unit of SOD activity was defined as the amount of enzyme that causes 50% inhibition of the photochemical reduction of NBT. Total CAT activity was analyzed by measuring the consumption of H2O2 at 240 nm according to the method of Gallego et al [16]. Enzymatic activities of SOD and CAT were expressed as U mg− 1 protein.
2.8. Chromosomal aberration studies
For cytological analysis, treated as well as control root tips were hydrolysed in 1 N HCl at 60 °C for five minutes. The root tips of lentil were then fixed in aceto alcohol (3 alcohol: 1 acetic acid) and were stored in 70% alcohol. Root tips were then squashed and stained in a 2% aceto carmine stain. The slides were scored for chromosomal aberrations. Abnormalities were counted at each phase of mitosis. Some of the common anomalies scored were stickiness, stray chromosomes, fragments, bridges, lagging chromosomes, C-mitosis etc.
2.9. Data analysis
The effect of TiO2 NPs was determined by one-way analysis of variance (ANOVA) using software SPSS 17.0 for windows 7. The difference of mean values between the treatment and control were analysed using One-way analysis of variance (ANOVA). The means of the treatments were compared by the least significant difference (LSD) test (P < 0.05). The spread of values is shown in the figures as standard errors of the means.
3. Results
3.1. Nanoparticles characterization
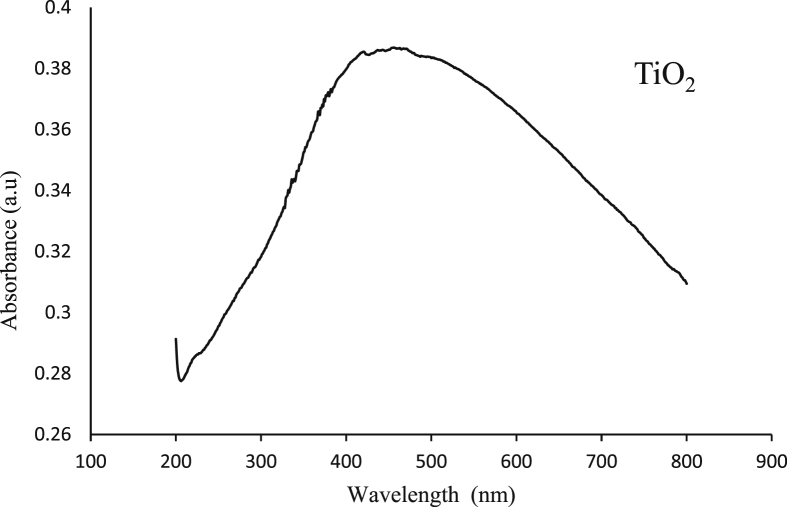
The nanomaterial was dispersed thoroughly in Millipore water using ultra-sonicator. Fig. 1 shows the absorbance of the sample in aa nano range between 200 to 800 nm at room temperature. A peak at 500 nm wavelength was observed with an absorbance of 0.39 (<1), which shows that it exhibits good absorbance at UV region [17]. Since with increase in particle size energy decreases, the nanoparticles, which have a size less than the bulk compound, shows shift in the UV-Visible spectra of nanoparticles which may be considered as the confirmatory test for the solution exhibiting properties of nanoparticle.
Fig. 1.
Absorption spectrum of TiO2 nanoparticles.
3.2. Effects of TiO2 NPs on growth and seedling biomass
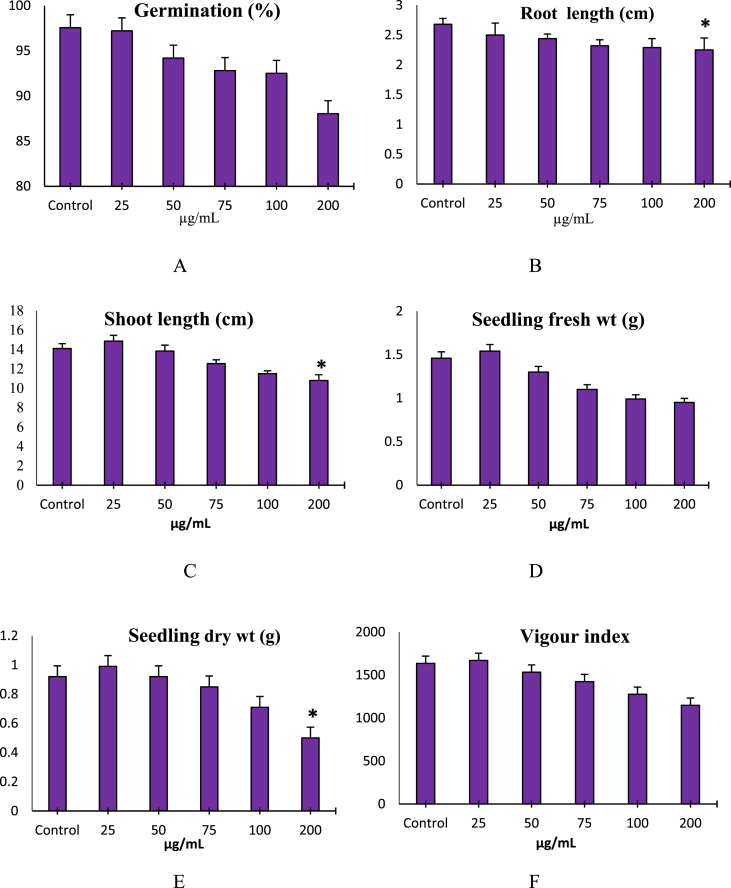
Fig. 2 shows the effect of nano-TiO2 on germination in 6 days old seedling. Average seed germination in control was 97.55% and decreased with the increasing concentrations of NP (Fig. 3A). Besides reduction in germination percentage, delayed germination was also observed at higher concentrations possibly due to change in metabolic activity of the cells, though the germination was delayed insignificantly for 2–3 days but not retarded. Anatase NPs had a toxic effect on lentil seedlings at higher concentrations as shown in the root length values. Significant decrease in the root length was observed at 200 μg/mL (Fig. 3B). Shoot length increased insignificantly in 25 μg/mL compared to control, where it was 14.09cm (Fig. 3C). However, at the highest concentration, it decreased significantly to 10.80 cm (Fig. 3C). Similarly seedling dry weight was also found to increase slightly at the lowest concentration and then decrease in dose-dependent manner; however, the decrease was significant at the highest concentration (200 μg/mL) of nano-TiO2 (Fig. 3E). Mean values for seedling vigour in response to seedling length was also dose-dependent and decreased with the increasing concentrations except at 25 μg/mL, where it increased insignificantly over control due to increase in the mean values of seedling shoot length (Fig. 3F).
Fig. 2.
Effect of TiO2 nanoparticles on seed germination.
Fig. 3.
Effect of nano-TiO2 on Seed germination (A); Root length (B); Shoot length (C); Seedling Fresh weight (D); Seedling Dry weight (E) and Vigour Index (F). Vertical bars represent Mean ± SE; * represents statistical significance at p < 0.05.
3.3. Effect of nanoparticles on pigment system
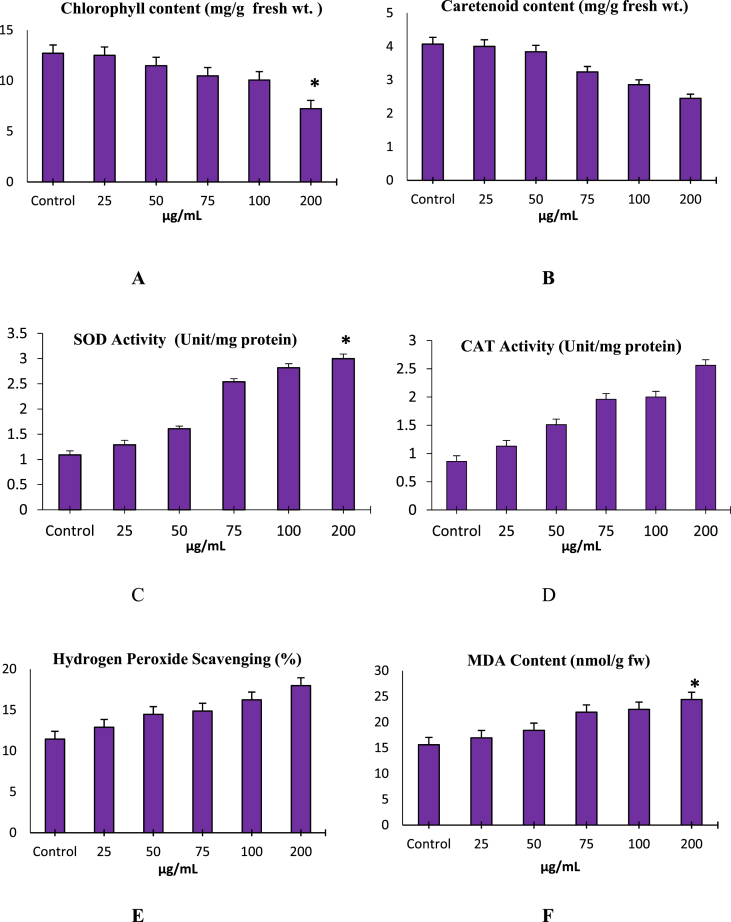
TiO2 NP at optimum temperature inhibits chlorophyll and carotenoid contents. The decrease in total chlorophyll content was significant at the highest concentration (Fig. 4A) and this result could cause an overall inhibition in the process of photosynthesis.
Fig. 4.
Effect of TiO2 nanoparticles on Chlorophyll content (A) Carotenoid content (B). SOD activity (C); CAT activity (D); Hydrogen peroxide scavenging (E) and Lipid peroxidation (MDA concentration) (F); Vertical bars represent Mean ± SE; * represents significance at p<0.05.
3.4. Effect of nano-TiO2 on stress enzymes (SOD, catalase), H2O2 scavenging and lipid peroxidation
The enzyme, SOD has been known to produce as the first line of defence against generated reactive oxygen species (ROS) during oxidative stress. A gradual increase in SOD and CAT activities were observed in treated populations when compared with the control (Fig 4 C, D). The Significant (P < 0.05) increase in SOD at highest concentration indicates increased generation of ROS and conversion of superoxide anion free radicals into hydrogen peroxide which is displayed in our result by increased H2O2 scavenging. Hydroxyl radical scavenging effect of anatase nanoparticles shows activity in dose-dependent manner and increased insignificantly with the increasing concentrations of nanoparticles (Fig. 4E). Change in malondialdehyde (MDA) concentration has been used as a parameter to assess oxidative stress damages to lipid membranes [18]. An increase in the level of lipid peroxidation measured as TBARS in leaves was dose-dependent. The higher level of MDA means higher level of cellular damage and occurrence of oxidative stress which was recorded at 200 μg/mL (Fig. 4 F). Accumulation of high level of MDA indicates nano-TiO2 induced stress.
3.5. Chromosomal aberration studies
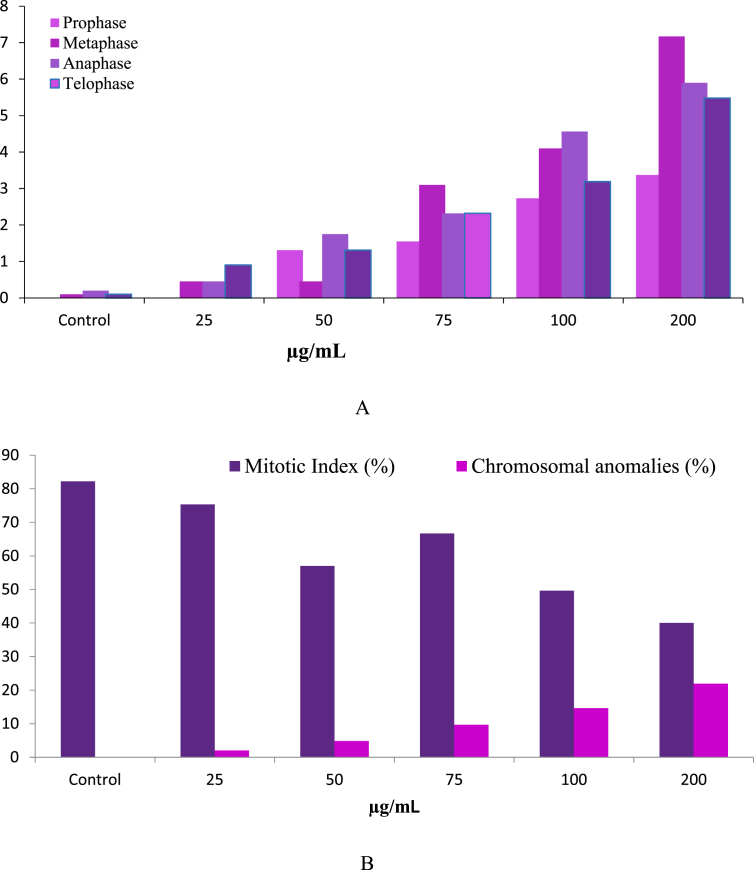
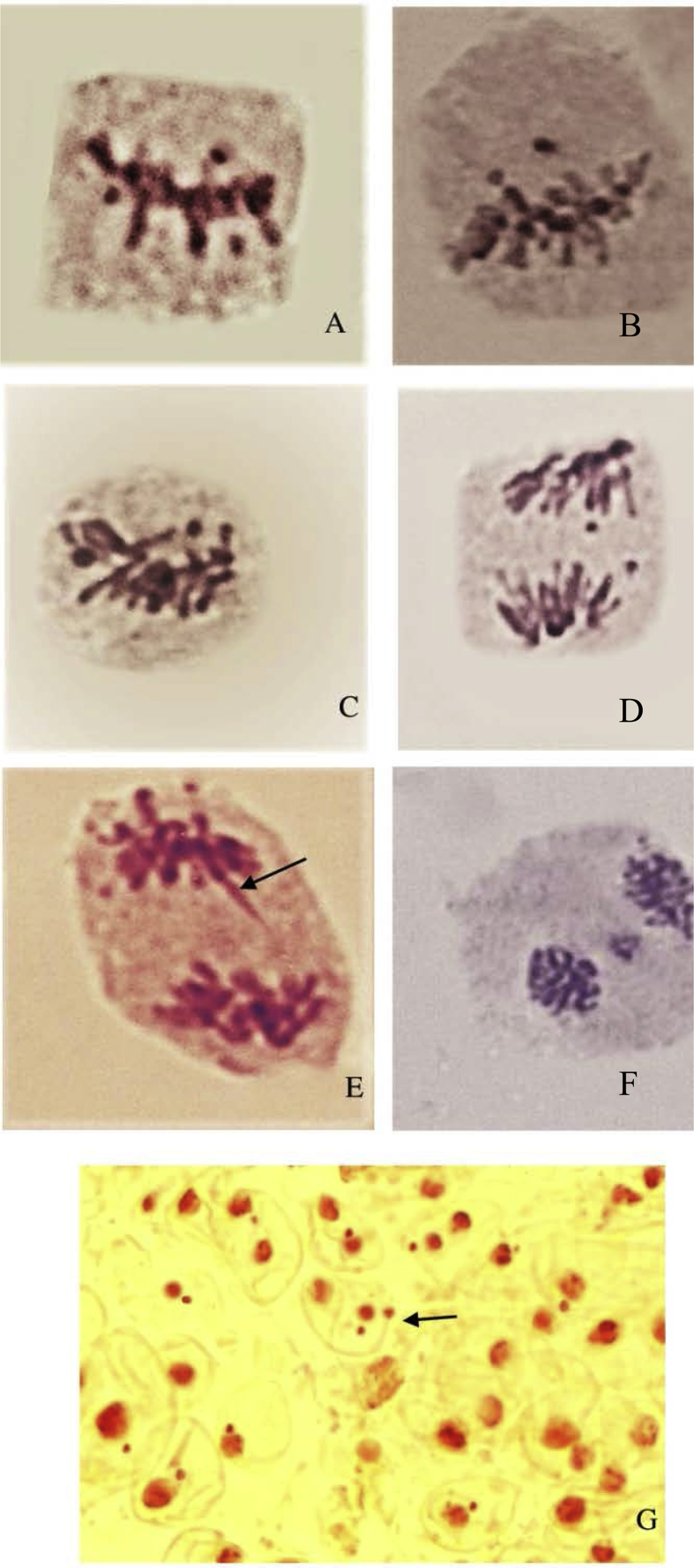
No chromatic aberrations were observed in the root tip cells of control plants. Frequency of chromosomal aberrations was dose-dependent and increase with the increasing concentration of nanoparticle suspension (Fig. 5A). Slides prepared as a part of experiment for scoring chromosomal anomalies were used for calculating the mitotic index. Chromatic aberration (CA) value was lowest at 25 μg/mL and increases along with the increasing concentration; highest value of CA (21.94%) was recorded at 200 μg/mL (Fig. 5B). The above result shows that the percentages of abnormal cells were directly proportional to the increasing concentrations of TiO2. Contrastingly Mitotic index (MI) was found to be highest in control (82.22%) and decreased with increasing concentration of nanoparticle solution (Fig. 5B). Treated plants showed abnormalities like stickiness and stray chromosomes (Fig. 6A, B), sticky chromosomes at metaphase (Fig. 6D), fragments (Fig. 6E), lagging chromosome with broken bridge at anaphase (Fig. 6E) laggard at telophase (Fig. 6 F), micronucleus (Fig. 6G) etc.
Fig. 5.
Frequency of chromosomal anomalies induced by Anatase TiO2 NP at different stages of mitotic cell division (A); Percent Mitotic index (MI) and Chromosomal Anomalies (CA) induced by TiO2 NP (B).
Fig. 6.
A, B: Stickiness and stray chromosomes at metaphase, C: Sticky chromosomes at metaphase, D: Fragments at anaphase, E: Lagging chromosome (marked) with broken bridge at anaphase, F: Laggard at telophase, G: Micronucleus.
4. Discussion
Metal NPs are hardly degraded as they possess strong bonds that allow them to stay in the environment for a longer time [19]. Therefore, their retention in the ecological system can be dangerous as they are highly reactive as compared to their bulk material and thus can be potentially toxic. Current research study was conducted to study the phytotoxicity of anatase nanoparticles on lentils since anatase nanoparticles are now considered as a “legacy nanoparticles” due to its widespread application in almost every field of application. Since with increase in size, energy decreases, the nanoparticles, which have a size less than the bulk compound, shows a blue shift in the UV-Visible absorption spectra due to the quantum size effect of TiO2 nanoparticles. This blue shift observed by the suspension may be considered as one of the confirmatory test for the existence of nanoparticle properties. Different endpoints have been proposed to evaluate NPs phytotoxicity, with germination rates being most widely used. Germination is not a specific response, but definitely an ultimate response dependent on genetic, biochemical, and physiological responses [20]. Literature review revealed a very contradictory result of nano-TiO2 showing both positive and negative response. For example, anatase exposure showed a positive effect on germination in Linum usitatissimum [21] and in Arabidopsis thaliana [22]. At higher concentrations, TiO2 NPs were shown to delay germination, reduce the mitotic index, and inhibit root elongation of Vicia narbonensis L. and Zea mays L [23]. In our study, the germination response at low concentration was somewhat near to control, however higher doses proved to be toxic affecting germination and vigour index (200 μg/mL). Our study was in support with the previous reports about the growth inhibiting effects of TiO2 on Vicia narbonensis [8]. It has been suggested that nanoparticles promotes the germination and growth of soybean by increasing the strength of roots and activity of nitrate reductase [24] however, at higher concentration it inhibits germination. The possible mechanism behind inhibition may be that nanoparticles being small-sized particles can be easily translocated inside the plant cells whereby it decreases nitrate reductase activity at higher concentration, consequently decreasing the ability of seeds to absorb water and fertilizers. These nanoparticles when present in higher concentrations damage the plant cell wall and plasma membrane thus penetrate and disrupt the physiological processes. At lowest concentration, the shoot length and seedling biomass increased slightly as compared to control showing that relatively low concentrations could promote the seedling growth, as supported by previous study in Lentil [25]. As the level of nanoparticles increased in the soil as their concentration increased in the environment of the plants as well. These results suggest that environmental pollution with nanoparticles is easily transferable to the plants and can enter into the food chain [26].
A significant diminution in photosynthetic pigment, chlorophyll was observed in 200 μg/mL (p < 0.05). The decrease in carotenoid content was non-significant at all concentrations. There are several proposed mechanisms for decrease in photosynthetic pigments, such as the nanoparticles can cause serious damage to the photosynthetic process and tend to decrease the number of thylakoids per grana, resulting in the reduced amount of PSII–LHCII complexes which play a crucial role in light-harvesting as well as ultrastructural organization of thylakoids [27]. The decline within the overall chlorophyll contents, as well as the growth inhibition, can be regarded as general responses associated with nanoparticle toxicity [28]. Mukherjee et al. [29] also observed low chlorophyll level in leaves treated with ZnO NPs, compared to the control plant. Similar reduction of total chlorophyll content in algal species treated with anatase (25 nm particle size) was reported by Sadiq et al. [30] Nanoparticles interfere with plant metabolism and different oxidative processes resulting in oxidative burst [31], which can induce many kinds of negative effects including membrane peroxidation, loss of ions, protein cleavage, and even DNA strand breakage [32]. Phytotoxicity of various nanoparticles is related to the imbalance between ROS production and scavenging in the cellular components of plants [33].
TiO2 catalyses the overproduction of ROS, hence the enzymes associated with it were found to increase many folds. The SOD catalyses the disproportionate superoxide anion free radicals into hydrogen peroxide and molecular oxygen. The significant increase in SOD activity at the highest concentration of NP demonstrates the increased free radical production and conversion of superoxide anion into hydrogen peroxide. However, hydrogen peroxide itself is not very reactive but gives rise to hydroxyl radical which, when accumulates at critical level causes damage to the cell. Therefore removal of H2O2 is very important for cell defence. An increase in H2O2 concentration in the treated plants indicates that in the presence of TiO2 NP, the efficiency of redox reactions increases. At higher concentration, the anatase nanoparticles also increase the activity of H2O2-metabolizing enzymes [34]. SOD activity was also reported to increase in plants exposed to various environmental stresses, including drought and metal toxicity [35]. MDA level significantly increased at higher concentrations in nano-stressed plants resulting in membrane damage. These findings are similar to those reported by Rico et al, [5] in rice seeds after treating with Cerium oxide nanoparticles (nCeO2).
Induction of chromosome alterations represents an effective method for analysis of genotoxicity. In the present study changes in chromosomal behaviour induced via nanoparticles have been extensively studied in order to assess the spectrum of chromosomal damage caused by them. Control plant showed no chromosomal aberrations while treated plants showed anomalies even at the lowest concentration. Occurrence of these abnormalities suggests their clastogenic, aneugenic and tubergenic effects [21] and are produced as a result of disturbances in spindle apparatus (bridges, anomalous metaphase, anaphase and telophase), chromosomal breakage (stray, fragments, micronuclei) etc [36, 37]. Hypothesis behind the occurrence of these root tip anomalies might be due to the fact that nanoparticles enter inside the plant through root tip cells, where it interferes with normal cell division and induces toxicity. Mitotic Index is a reliable parameter for estimating the frequency of cell division. In the present study decrease in mitotic index with increasing concentration was due to cdk genes which limit cdc2 expression. This is a common stress response leading to cell cycle arrest and prolonged S-phase progression [38]. Previous studies on plants as well as animal cells also demonstrated that nanoparticles accumulate in the cytoplasm and penetrate in sub-cellular structures, such as mitochondria and nucleus where it encourages production of ROS through disruption of mitochondrial respiratory chain leading to DNA breakages.
5. Conclusions
Cytotoxicity of nanoparticles was directly proportion to the concentration of treatments. Increased concentrations of TiO2-NPs is clearly indicating a negative impact on the plant physiology and genetics, suggesting that nanoparticles present in the environment are easily transferable to the plants and ultimately enter into the food chain. Precisely these TiO2-NPs inhibit plant growth by inducing oxidative stress and subsequently causing DNA damage, therefore, it poses a potential environmental risk. These results are significant in terms of disposal of nanoparticles and concentration at which they are harmful.
Declarations
Author contribution statement
Zeba Khan: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Durre Shahwar, Rahul Chandel: Analyzed and interpreted the data.
M. Yunus. K Ansari: Contributed reagents, materials, analysis tools or data.
Funding statement
This work was supported by grants DST (Department of Science and Technology), Government of India for financial support through DST-PURSE programme (Phase II) at Aligarh Muslim University, India.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.Nel A., Xia T., Madler L., Li N. Toxic potential of materials at the nanolevel. Science. 2006;311:622–627. doi: 10.1126/science.1114397. [DOI] [PubMed] [Google Scholar]
- 2.Jacob D.L., Borchardt J.D., Navaratnam L., Otte M.L., Bezbaruah A.N. Uptake and translocation of Ti from nanoparticles in crops and wetland plants. Int. J. Phytoremediation. 2013;15:142–153. doi: 10.1080/15226514.2012.683209. [DOI] [PubMed] [Google Scholar]
- 3.Keller A., McFerran S., Lazareva A., Suh S. Global life cycle releases of engineered nanomaterials. J. Nano Res. 2013;15 1692. [Google Scholar]
- 4.OECD . OECD Environment, Health and Safety Publications Series on the Safety of Manufactured Nanomaterial; Paris: 2010. List of Manufactured Nanomaterials and List of End Points for Phase One of the Sponsorship Programme for the Testing of Manufactured Nanomaterials: Revision. [Google Scholar]
- 5.Rico C.M., Hong J., Morales M.I., Zhao L., Barrios A.C., Zhang J.Y., Peralta-Videa J.R., Gardea-Torresdey J.L. Effect of cerium oxide nanoparticles on rice: a study involving the antioxidant defence system and in vivo fluorescence imaging. Environ. Sci. Technol. 2013;47(11):5635–5642. doi: 10.1021/es401032m. [DOI] [PubMed] [Google Scholar]
- 6.Zhu H., Han J., Xiao J.Q., Jin Y. Uptake, translocation, and accumulation of manufactured iron oxide nanoparticles by pumpkin plants. J. Environ. Monit. 2008;10:713–717. doi: 10.1039/b805998e. [DOI] [PubMed] [Google Scholar]
- 7.Yu Q., Renge Z. Micronutrient deficiency influences plant growth and activities of superoxide dismutases in narrow-leafed lupins. Ann. Bot. 1999;83:175–182. [Google Scholar]
- 8.Zheng L., Hong F., Lv S., Liu C. Effect of Nano-TiO2 on strength of naturally aged seeds and growth of spinach. Biol. Trace Elem. Res. 2005;104:83–92. doi: 10.1385/BTER:104:1:083. [DOI] [PubMed] [Google Scholar]
- 9.Gao F., Liu C., Qu C., Zheng L., Yang F., Su M., Hong F. Was improvement of spinach growth by nano-TiO2 treatment related to the changes of Rubiscoactivase? Biometals. 2008;21:211–217. doi: 10.1007/s10534-007-9110-y. [DOI] [PubMed] [Google Scholar]
- 10.Ruffini C.M., Giorgetti L., Cremonini R., Bottega S., Spanò C. Impact of TiO2 nanoparticles on Vicia narbonensis L.: potential toxicity effects. Protoplasma. 2014 doi: 10.1007/s00709-014-0649-5. [DOI] [PubMed] [Google Scholar]
- 11.Abdul-Baki A.A., Anderson J.D. Vigor determination in Soybean seed by multiple criteria. Crop Sci. 1973;13:630–633. [Google Scholar]
- 12.Arnon D.I. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949;24:1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ruch R.J., Cheng S.J., Klaunig J.E. Prevention of cytotoxicity and inhibition of intracellular communication by antioxidant catechins isolated from Chinese green tea. Carcinogenesis. 1989;10:1003. doi: 10.1093/carcin/10.6.1003. [DOI] [PubMed] [Google Scholar]
- 14.Madhava Rao K.V., Sresty T.V.S. Antioxidative parameters in the seedlings of pigeon pea (Cajanus cajan L. Mill Spaugh) in response to Zn and Ni stresses. Plant Sci. 2000;157:113–128. doi: 10.1016/s0168-9452(00)00273-9. [DOI] [PubMed] [Google Scholar]
- 15.Yu Q., Rengel Z. Micronutrient deficiency influences plant growth and activities of superoxide dismutases in narrow-leafed lupins. Ann. Bot. 1999;83:175–182. 1999. [Google Scholar]
- 16.Gallego S.M., Benavides M.P., Tomaro M.L. Effect of heavy metal ion excess on sunflower leaves evidence for involvement of oxidative stress. Plant Sci. 1996;121:151–159. [Google Scholar]
- 17.Vijayalakshmi R., Rajendran V. Synthesis and characterization of nano-TiO2 via different methods. Arch. Appl. Sci. Res. 2012;4(2):1183–1190. [Google Scholar]
- 18.Arbona V., Hossain Z., López-Climent M.F., Pérez-Clemente R.M., Gómez-Cadenas A. Antioxidant enzymatic activity is linked to waterlogging stress tolerance in citrus. Physiol. Plant. 2008;132(4):452–466. doi: 10.1111/j.1399-3054.2007.01029.x. [DOI] [PubMed] [Google Scholar]
- 19.Svintradze D.V., Pidaparti R.M. A theoretical model for metal corrosion degradation. Int. J. Corros. 2010:279540. 2010. [Google Scholar]
- 20.Silva S., Oliveira H., Silva A.M.S., Santos C. The cytotoxic targets of anatase or rutile + anatase nanoparticles depend on the plant species. Biol. Plant. 2017;61:717–725. [Google Scholar]
- 21.Clement L., Hurel C., Marmier N. Toxicity of TiO2 − nanoparticles to cladocerans, algae, rotifers and plants effects of size and crystalline structure. Chemosphere. 2013;90:1083–1090. doi: 10.1016/j.chemosphere.2012.09.013. [DOI] [PubMed] [Google Scholar]
- 22.Tumburu L., Andersen C., Rygiewicz P., Reichman J. Phenotypic and genomic responses to titanium dioxide and cerium oxide nanoparticles in Arabidopsis germinants. Environ. Toxicol. Chem. 2015;34:70–83. doi: 10.1002/etc.2756. [DOI] [PubMed] [Google Scholar]
- 23.Ruffini Castiglione M., Giorgetti L., Geri C., Cremonini R. The effects of nano-TiO2 on seed germination, development and mitosis of root tip cells of Vicia narbonensis L. and Zea mays L. J. Nanoparticle Res. 2010;13:2443–2449. [Google Scholar]
- 24.Rezaei F., Moaveni P., Mozafari H. Effect of Different Concentrations and Time of Nano TiO2 Spraying on Quantitative and Qualitative yield of Soybean (Glycine max L.) at Shahr-e-Qods, Iran. Biol. Forum Int. J. 2015;7(1):957–964. [Google Scholar]
- 25.Khan Z., Ansari M.Y.K. Impact of Si engineered nanoparticles on seed germination vigour index and genotoxicity assessment via DNA damage of root tip cells in Lens culinaris. J. Plant Biochem. Physiol. 2018;6:3. [Google Scholar]
- 26.Miri A., Shakib E.S., Ebrahimi O., Sharifi-Rad J. Impacts of Nickel Nanoparticles on growth characteristics, photosynthetic pigment content and antioxidant activity of Coriandrum sativum L. Orient. J. Chem. 2017;33(3):1297–1303. [Google Scholar]
- 27.DA Costa M.V.J., Sharma P.K. Effect of copper oxide nanoparticles on growth, morphology, photosynthesis and antioxidant response in Oryza sativa. Photosynthetica. 2016;54(1):110–119. [Google Scholar]
- 28.Narendhran S., Rajiv P., Sivaraj Rajeshwari. Influence of zinc oxide nanoparticles on growth of Sesamum indicum L. in zinc-deficient soil. Int. J. Pharm. Pharm. Sci. 2016;8:365–371. [Google Scholar]
- 29.Mukherjee A., Peralta-Videa J.R., Bandyopadhyay S., Rico C.M., Zhao L., Gardea-Torresdey J.L. Physiological effect of nanoparticles ZnO in green peas (Pisum sativum L.) cultivated in soil. Metallomics. 2014;6:132–138. doi: 10.1039/c3mt00064h. [DOI] [PubMed] [Google Scholar]
- 30.Sadiq I.M., Swayamprava D., Chandrasekaran N., Mukherjee A. Ecotoxicity study of titania (TiO2) NPs on two microalgae species: scenedesmus sp. and Chlorella sp. Ecotoxicol. Environ. Saf. 2011;74:1180–1187. doi: 10.1016/j.ecoenv.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 31.Hossain Z., Mustafa G., Komatsu S. Plant responses to nanoparticle stress. Int. J. Mol. Sci. 2015;16(11):26644–26653. doi: 10.3390/ijms161125980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mittler R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002;7:405–410. doi: 10.1016/s1360-1385(02)02312-9. [DOI] [PubMed] [Google Scholar]
- 33.Oukarroum A., Barhoumi L., Pirastru L., Dewez D. Silver nanoparticle toxicity effect on growth and cellular viability of the aquatic plant Lemna gibba. Environ. Toxicol. Chem. 2013;32:902–907. doi: 10.1002/etc.2131. [DOI] [PubMed] [Google Scholar]
- 34.Sharma P., Bhatt D., Zaidi M.G.H. Silver Nanoparticle-Mediated Enhancement in Growth and Antioxidant Status of Brassica juncea. Appl. Biochem. Biotechnol. 2012;167:22–25. doi: 10.1007/s12010-012-9759-8. [DOI] [PubMed] [Google Scholar]
- 35.Mishra S., Jha A.B., Dubey R.S. Arsenite treatment induces oxidative stress, upregulates antioxidant system, and causes phytochelatin synthesis in rice seedlings. Protoplasma. 2011;248(3):565–577. doi: 10.1007/s00709-010-0210-0. [DOI] [PubMed] [Google Scholar]
- 36.Shahwar D., Ansari M.Y.K., Chaudhary S. Evaluation of genetic potentialof heavy metal in a proteinaceous crop (Lens culinaris Medik) in aspect to cyto-morphological parameters. J. Biol. Sci. 2018;18:208–215. [Google Scholar]
- 37.Khan Z., Ansari M.Y.K., Gupta H., Choudhary S. Dynamics of 2, 4-D in generation of cytomorphological variants in an important anticancerous and antihepatotoxic herb – Cichorium intybus L. Turk. J. Bot. 2009;33:383–387. [Google Scholar]
- 38.Kitsios G., Doonan J.H. Cyclin dependent protein kinases and stress responses in plants. Plant Signal. Behav. 2011;6(2):204–209. doi: 10.4161/psb.6.2.14835. [DOI] [PMC free article] [PubMed] [Google Scholar]