Abstract
Breast cancer (BC) prognosis in BRCA1 and BRCA2 mutation carriers has been reported contradictorily, and the significance of variables influencing prognosis in sporadic BC is not established in BC patients with hereditary BRCA1/BRCA2 mutations. In this retrospective cohort study, we analyzed the effect of clinicopathological characteristics on BC prognosis (disease-free survival [DFS] and disease-specific survival [DSS]) in hereditary BRCA1/BRCA2 mutation carriers. We enrolled 234 BRCA1/BRCA2 mutation carriers and 899 non-carriers, of whom 191 carriers and 680 non-carriers, with complete data, were available for survival analyses. We found that patients with ER-positive tumors developed disease recurrence 2.3-times more likely when they carried a BRCA1/BRCA2 mutation (23/60; 38.3% ER-positive carriers vs. 74/445; 16.6% ER-positive non-carriers; p < 0.001). ER-positive mutation carriers also had a 3.4-times higher risk of death due to BC compared with ER-positive non-carriers (13/60; 21.7% vs. 28/445; 6.3%; p < 0.001). Moreover, prognosis in ER-negative BRCA1/BRCA2 mutation carriers was comparable with that in ER-positive non-carriers. Our study demonstrates that ER-positivity worsens BC prognosis in BRCA1/BRCA2 mutation carriers, while prognosis for carriers with ER-negative tumors (including early-onset) is significantly better and comparable with that in ER-positive, older BC non-carriers. These observations indicate that BRCA1/BRCA2 mutation carriers with ER-positive BC represent high-risk patients.
Keywords: breast cancer, BRCA1, BRCA2, germline mutations, estrogen receptor, survival
1. Introduction
Approximately 5–10% of all breast cancers (BC) have a hereditary background [1,2]. Mutations in BRCA1 or BRCA2 (BRCA1/BRCA2) account for most hereditary BC cases [3]. The proportion of BRCA1 vs. BRCA2 mutations is population-specific, with BRCA1 mutations being dominant among Czech patients [4]. Women carrying BRCA1/BRCA2 mutations have a 70–80% risk of BC development by age 80 [5,6]. Besides a high lifetime BC risk, mutation carriers are threatened by early BC onset and an increased risk of other cancers, including ovarian cancer (OC) [7].
Breast tumors are classified into distinct molecular subtypes with different prognoses and require specific therapeutic approaches [8,9]. Most BRCA1-associated BC cases have typical histopathological features including high-grade and triple-negative tumors [10,11,12,13]. Triple-negative breast cancer (TNBC) accounts for 15–20% of all BC cases and is associated with worse overall survival (OS) [14,15]. Pathological characteristics of BC in BRCA2 mutation carriers are less indicative (higher tumor grade, frequent ER-positivity, and HER-2 negativity), resembling sporadic tumors [11,12,13,16].
BC prognosis in BRCA1/BRCA2 mutation carriers has been contradictorily reported to be worse [13,17,18] or the same [19,20,21] as in patients with sporadic disease. Recent meta-analysis comparing survival in BC patients with BRCA1/BRCA2 mutations and non-carriers or unselected BC patients revealed that current evidence does not suggest worsened BC survival in mutation carriers [17]. The only prospective POSH (Prospective Outcomes in Sporadic versus Hereditary breast cancer) study of 2733 young-onset BC patients found no difference in OS in 338 BRCA1/BRCA2 mutation carriers [19]. Indeed, among 558 TNBC patients, BRCA1/BRCA2 mutation carriers had better OS than non-carriers at two years (95% vs. 91%; HR 0.59; 95% CI 0.35–0.99), but comparable at subsequent time points.
While germline BRCA1/BRCA2 mutations undoubtedly increase BC risk, it is unclear whether the overall prognosis and clinicopathological prognostic factors differ between mutation carriers and non-carriers. This question is of considerable clinical importance because the age at BC onset is over a decade lower among mutation carriers than in non-carriers [11,12,22].
To determine clinicopathological characteristics influencing BC prognosis (disease-free survival [DFS] and disease-specific survival [DSS]), we analyzed 1133 Czech BC patients, including 234 BRCA1/BRCA2 mutation carriers and 899 non-carriers.
2. Results
2.1. Patient and Tumor Characteristics
Among 1133 enrolled BC patients, 234 (19.5%) were carriers of pathogenic BRCA1 (N = 183) or BRCA2 (N = 51) mutations (Supplementary Table S1). The remaining 899 BC patients (74.8%) were non-carriers of mutations in the established cancer-susceptibility genes (incl. BRCA1, BRCA2, PALB2, CHEK2, ATM, TP53, RAD51C, RAD51D, BRIP1, MLH1, MLH3, NBN, NF1). Median follow-up of 1133 patients eligible for subsequent analyses was 9.8 years. A comparison of the clinicopathological characteristics of BRCA1/BRCA2 mutation carriers and non-carriers is summarized in Supplementary Table S2. BRCA1/BRCA2 mutation carriers were diagnosed with BC at an earlier age, with more advanced disease, different BC morphology, higher grade, and more frequent TNBC. These differences were driven mainly by BRCA1 mutation carriers, as BRCA2 mutation carriers differed from non-carriers only in terms of higher tumor stage and lower HER2-positivity. BRCA1/BRCA2 mutation carriers were more often treated by chemotherapy; however, surgical treatment and radiotherapy were comparable in both groups. BRCA1/BRCA2 mutation carriers also developed distant metastases and second BC more often than non-carriers, but the frequency of loco-regional recurrences was similar. Differences in age at diagnosis between mutation carriers and non-carriers are displayed in Supplementary Figure S1.
2.2. Prognosis and Long-Term Survival
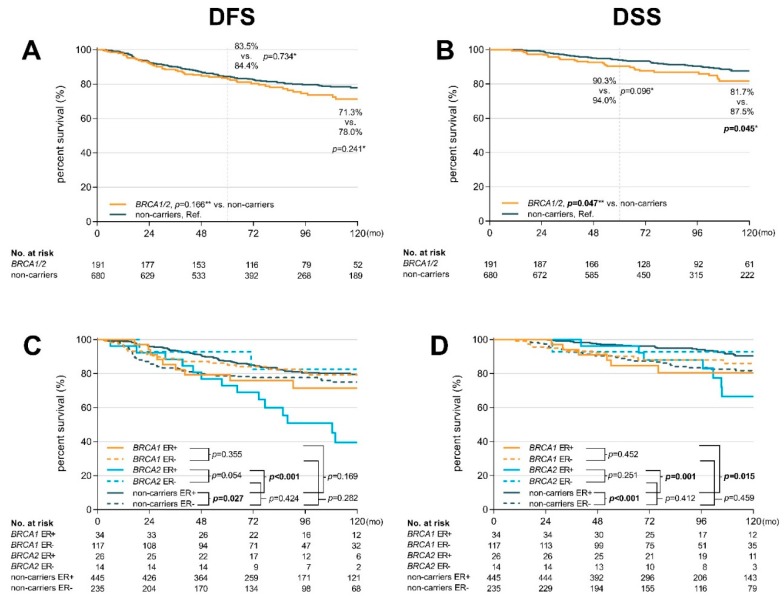
Complete clinicopathological data for survival analyses have been eligible for 191 mutation carriers (151 BRCA1 and 40 BRCA2 mutation carriers) and 680 non-carriers (Table 1) with median follow-up of 8.3 years. BRCA1/BRCA2 mutation carriers had marginally worsened DSS (HR 1.65 95% CI 1.01–2.70; p = 0.047; Figure 1B) with an absolute difference of 81.7% versus 87.5% (in non-carriers) after 10 years (p = 0.045), already apparent at 5 years. The non-significant difference in DFS (Figure 1A) reached absolute values of 71.3% versus 78.0% (p = 0.241) for BRCA1/BRCA2 mutation carriers and non-carriers, respectively. DFS was affected mainly by a higher relapse rate in carriers of mutations in BRCA2 (but not in BRCA1; Supplementary Figure S2).
Table 1.
Clinicopathological characteristics of BRCA1/BRCA2 mutation carriers (together and separately) and non-carriers of mutations in cancer-susceptibility genes for whom complete clinicopathological data were available for subsequent univariate and multivariable analyses.
|
BRCA1/2 Carriers (N = 191) |
BRCA1 Carriers (N = 151) |
BRCA2 Carriers (N = 40) |
Non-Carriers (N = 680) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | %* | p | N | %* | P | N | %* | p | N | %* | p | |
| Median age at diagnosis | ||||||||||||
| year (25–75% percentile) | 37.1 (32.3–43.8) | <0.001 | 36.9 (31.9–42.6) | <0.001 | 38.7 (32.9–50.8) | 0.645 | 40.2 (33.5–49.6) | Ref. | ||||
| Age diagnosis categories (known) | ||||||||||||
| <35 years | 78 | (40.8) | <0.001 | 64 | (42.4)) | <0.001 | 14 | (35.0) | 0.601 | 213 | (31.3) | Ref. |
| 35–44 years | 72 | (37.7) | 58 | (38.4) | 14 | (35.0) | 209 | (30.7) | ||||
| ≥45 years | 41 | (21.5) | 29 | (19.2) | 12 | (30.0) | 258 | (38.0) | ||||
| Menopausal status | ||||||||||||
| Pre | 168 | (88.0) | 0.003 | 136 | (90.1) | <0.001 | 32 | (80.0) | 0.792 | 532 | (78.2) | Ref. |
| post | 23 | (12.0) | 15 | (9.9) | 8 | (20.0) | 148 | (21.8) | ||||
| Primary tumor (T) | ||||||||||||
| T1 (<2 cm) | 71 | (37.2) | 0.001 | 52 | (34.4) | <0.001 | 19 | (47.5) | 0.093 | 355 | (52.2) | Ref. |
| T2 (2–5 cm) | 84 | (44.0) | 73 | (48.3) | 11 | (27.5) | 244 | (35.9) | ||||
| T3 (>5 cm) | 24 | (12.6) | 17 | (11.3) | 7 | (17.5) | 62 | (9.1) | ||||
| T4 | 12 | (6.3) | 9 | (6.0) | 3 | (7.5) | 19 | (2.8) | ||||
| Regional lymphatic node (N) | ||||||||||||
| N0 | 100 | (52.4) | 0.296 | 85 | (56.3) | 0.206 | 15 | (37.5) | 0.077 | 387 | (56.9) | Ref. |
| N1 | 78 | (40.8) | 55 | (36.4) | 23 | (57.5) | 256 | (37.6) | ||||
| N2 | 8 | (4.2) | 6 | (4.0) | 2 | (5.0) | 30 | (4.4) | ||||
| N3 | 5 | (2.6) | 5 | (3.3) | 0 | (0.0) | 7 | (1.0) | ||||
| Tumor stage | ||||||||||||
| I (T1N0–1mi) | 52 | (27.2) | <0.001 | 41 | (27.2) | 0.002 | 11 | (27.5) | 0.022 | 282 | (41.5) | Ref. |
| II (T2–3N0, T1–2N1) | 97 | (50.8) | 79 | (52.3) | 18 | (45.0) | 310 | (45.6) | ||||
| III (T3N1, TXN2–3, T4NX) | 42 | (22.0) | 31 | (20.5) | 11 | (27.5) | 88 | (12.9) | ||||
| Breast tumor morphology | ||||||||||||
| ductal | 168 | (88.0) | 0.023 | 131 | (86.8) | 0.005 | 37 | (92.5) | 0.325 | 574 | (84.4) | Ref. |
| lobular | 6 | (3.1) | 3 | (2.0) | 3 | (7.5) | 52 | (7.6) | ||||
| medullar | 15 | (7.9) | 15 | (9.9) | 0 | (0.0) | 33 | (4.9) | ||||
| other | 2 | (1.0) | 2 | (1.3) | 0 | (0.0) | 21 | (3.1) | ||||
| Grade | ||||||||||||
| low (1) | 7 | (3.7) | <0.001 | 4 | (2.6) | <0.001 | 3 | (7.5) | 0.554 | 91 | (13.4) | Ref. |
| intermediate (2) | 59 | (30.9) | 40 | (26.5) | 19 | (47.5) | 311 | (45.7) | ||||
| high (3) | 125 | (65.4) | 107 | (70.9) | 18 | (45.0) | 278 | (40.9) | ||||
| ER status | ||||||||||||
| positive | 60 | (31.4) | <0.001 | 34 | (22.5) | <0.001 | 26 | (65.0) | >0.99 | 445 | (65.4) | Ref. |
| PR status | ||||||||||||
| positive | 61 | (31.9) | <0.001 | 34 | (22.5) | <0.001 | 27 | (67.5) | 0.616 | 428 | (62.9) | Ref. |
| HER-2 status | ||||||||||||
| positive | 13 | (6.8) | <0.001 | 9 | (6.0) | <0.001 | 4 | (10.0) | 0.052 | 164 | (24.1) | Ref. |
| TNBC | ||||||||||||
| yes | 114 | (59.7) | <0.001 | 105 | (69.5) | <0.001 | 9 | (22.5) | 0.690 | 138 | (20.3) | Ref. |
| Surgery—primary tumor | ||||||||||||
| mastectomy | 91 | (47.6) | 0.774 | 70 | (46.4) | 0.980 | 21 | (52.5) | 0.458 | 316 | (46.5) | Ref. |
| breast-conserving surgery | 100 | (52.4) | 81 | (53.6) | 19 | (47.5) | 364 | (53.5) | ||||
| Surgery—lymphatic nodes | ||||||||||||
| axillary dissection | 149 | (78.0) | 0.051 | 117 | (77.5) | 0.102 | 32 | (80.0) | 0.215 | 482 | (70.9) | Ref. |
| sentinel node biopsy | 42 | (22.0) | 34 | (22.5) | 8 | (20.0) | 198 | (29.1) | ||||
| Radiotherapy | ||||||||||||
| yes | 132 | (69.1) | 0.759 | 108 | (71.5) | 0.391 | 24 | (60.0) | 0.297 | 462 | (67.9) | Ref. |
| Chemotherapy type | ||||||||||||
| Antra + Tax | 122 | (63.9) | <0.001 | 97 | (64.2) | <0.001 | 25 | (62.5) | 0.096 | 308 | (45.3) | Ref. |
| Antra | 50 | (26.2) | 39 | (25.8) | 11 | (27.5) | 195 | (28.7) | ||||
| Other | 7 | (3.7) | 6 | (4.0) | 1 | (2.5) | 32 | (4.7) | ||||
| No chemotherapy | 12 | (6.3) | 9 | (6.0) | 3 | (7.5) | 145 | (21.3) | ||||
| Endocrine therapy ** | ||||||||||||
| TAM monotherapy | 21 | (35.0) | 0.951 | 14 | (41.2) | 0.947 | 7 | (26.9) | 0.745 | 165 | (37.1) | Ref. |
| AI monotherapy | 8 | (13.4) | 4 | (11.8) | 4 | (15.4) | 65 | (14.6) | ||||
| LHRH analogues + TAM | 29 | (48.3) | 15 | (44.1) | 14 | (53.8) | 204 | (45.8) | ||||
| LHRH analogues + AI | 2 | (3.3) | 1 | (2.9) | 1 | (3.9) | 11 | (2.5) | ||||
| Event during follow-up *** | ||||||||||||
| loco-regional recurrence | 5 | (2.6) | 0.036 | 5 | (3.3) | 0.122 | 0 | (0.0) | - | 45 | (6.6) | Ref. |
| distant metastasis | 41 | (21.5) | 0.001 | 25 | (16.6) | 0.150 | 16 | (40.0) | <0.001 | 83 | (12.2) | Ref. |
| second breast cancer | 24 | (12.6) | 0.009 | 34 | (22.5) | <0.001 | 9 | (22.5) | <0.001 | 46 | (6.8) | Ref. |
| second tumors | 7 | (3.7) | 0.322 | 10 | (6.6) | 0.570 | 2 | (5.0) | 0.905 | 37 | (5.4) | Ref. |
| Median of follow-up | ||||||||||||
| median (25–75% percentile) | 8.6 (6.0–12.1) | 0.235 | 8.2 (5.7–11.8) | 0.733 | 9.4 (6.9–13.4) | 0.031 | 8.2 (5.6–11.8) | Ref. | ||||
| Breast cancer related death | ||||||||||||
| yes | 28 | (14.7) | 0.507 | 16 | (10.6) | 0.655 | 12 | (30.0) | <0.001 | 64 | (9.4) | Ref. |
* % = percentage of known; ** N = number of patients with ER-positive BC; *** patient could be counted in more than one event. pre—premenopausal; post—postmenopausal; TNBC—triple-negative BC; Antra—anthracyclines; Tax—taxanes; TAM—tamoxifen; AI—aromatase inhibitor; LHRH—luteinizing hormone-releasing hormone; Ref—reference. Bold: statistically significant differences.
Figure 1.
Kaplan-Meier plots showing disease-free survival (DFS) (A) and disease-specific survival (DSS) (B) in BRCA1/2 mutation carriers and non-carriers; DFS (C) DSS (D) of BRCA1 mutation carriers, BRCA2 mutation carriers, and non-carriers classified according to the ER status. * p-values calculated by χ2 test (number of events at the end of follow-up interval); ** p-values calculated by log-rank test (considering whole follow-up period).
Next, we compared the effect of clinicopathological characteristics on survival between BRCA1/BRCA2 mutation carriers and non-carriers by the Mantel–Haenszel test (Table 2). Older BRCA1/BRCA2 mutation carriers had increased risk of disease recurrence (DFS), including patients ≥35 years (HR 1.81 95% CI 1.10–2.99), patients ≥45 years (HR 3.98 95% CI 1.62–9.81), and postmenopausal patients (HR 3.72 95% CI 1.16–11.90), when compared with age-matched non-carriers. Mutation carriers with ER-positive tumors also had significantly worse DFS (HR 3.14 95% CI 1.69–5.81; p = 0.003) than non-carriers with ER-positive tumors, with similar significant differences also detected for DSS (HR 5.70 95% CI 2.27–14.4; p < 0.001). The opposite prognostic effect in BRCA1/BRCA2 mutation carriers and non-carriers was not limited to ER status only as we have observed the same trend with age at diagnosis and menopausal status. Subsequent analyses within each group confirmed the observed differences in DFS with similar trends for DSS (Supplementary Table S3). These univariate analyses also confirmed the expected negative impact of increased tumor stage on survival in both analyzed groups.
Table 2.
Analysis of 10-year DFS and DSS using the Mantel–Haenszel test comparing variables between BRCA1/2 mutation carriers and non-carriers.
| Disease Free Survival (DFS) Analysis | Disease Specific Survival (DSS) Analysis | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BRCA1/2 Carriers | Non-Carriers | BRCA1/2 Carriers | Non-Carriers | ||||||||||||||||||
| Pts No. |
Ev No. |
Ev % |
HR | 95% CI | p | Pts No. |
Ev No. |
Ev % |
Pts No. |
Ev No. |
Ev % |
HR | 95% CI | p | Pts No. |
Ev No. |
Ev % |
||||
| All pts | 191 | 46 | 24.1 | 1.29 | 0.90–1.84 | 0.166 | 680 | 128 | 18.8 | Ref. | 191 | 28 | 14.7 | 1.65 | 1.01–2.70 | 0.047 | 680 | 64 | 9.4 | Ref. | |
| Age at diagnosis | <35 | 78 | 18 | 23.1 | 0.82 | 0.50–1.35 | 0.431 | 213 | 60 | 28.2 | Ref. | 78 | 12 | 15.4 | 1.09 | 0.55–2.13 | 0.813 | 213 | 32 | 15.0 | Ref. |
| ≥35 | 113 | 28 | 24.8 | 1.81 | 1.10–2.99 | 0.021 | 467 | 68 | 14.6 | Ref. | 113 | 16 | 14.2 | 2.29 | 1.13–4.65 | 0.021 | 467 | 32 | 6.9 | Ref. | |
| <45 | 150 | 34 | 22.7 | 0.93 | 0.63–1.36 | 0.690 | 422 | 100 | 23.7 | Ref. | 150 | 21 | 14.0 | 1.21 | 0.71–2.05 | 0.489 | 422 | 49 | 11.6 | Ref. | |
| ≥45 | 41 | 12 | 29.3 | 3.98 | 1.62–9.81 | 0.003 | 258 | 28 | 10.9 | Ref. | 41 | 7 | 17.1 | 4.48 | 1.34–15.0 | 0.015 | 258 | 15 | 5.8 | Ref. | |
| Menopausal status | pre | 168 | 39 | 23.2 | 1.10 | 0.76–1.60 | 0.621 | 532 | 119 | 22.4 | Ref. | 168 | 23 | 13.7 | 1.36 | 0.81–2.29 | 0.247 | 532 | 55 | 10.3 | Ref. |
| post | 23 | 7 | 30.4 | 3.72 | 1.16–11.9 | 0.027 | 148 | 18 | 12.2 | Ref. | 23 | 5 | 21.7 | 5.95 | 1.32–26.8 | 0.020 | 148 | 9 | 6.1 | Ref. | |
| Tumor size | T1 | 71 | 11 | 15.5 | 1.23 | 0.61–2.47 | 0.569 | 355 | 45 | 12.7 | Ref. | 71 | 4 | 5.6 | 1.13 | 0.37–3.74 | 0.835 | 355 | 18 | 5.1 | Ref. |
| T2 | 84 | 20 | 23.8 | 1.07 | 0.63–1.81 | 0.802 | 244 | 52 | 21.3 | Ref. | 84 | 12 | 14.3 | 1.17 | 0.58–2.36 | 0.653 | 244 | 29 | 11.9 | Ref. | |
| T3 | 24 | 9 | 37.5 | 0.90 | 0.42–1.92 | 0.787 | 62 | 23 | 37.1 | Ref. | 24 | 7 | 29.2 | 1.30 | 0.50–3.41 | 0.594 | 62 | 13 | 21.0 | Ref. | |
| T4 | 12 | 6 | 50.0 | 1.42 | 0.47–4.29 | 0.530 | 19 | 8 | 42.1 | Ref. | 12 | 5 | 41.7 | 2.22 | 0.57–8.62 | 0.249 | 19 | 4 | 21.1 | Ref. | |
| Nodal status | N0 | 100 | 17 | 17.0 | 1.26 | 0.70–2.26 | 0.435 | 387 | 51 | 13.2 | Ref. | 100 | 11 | 11.0 | 2.55 | 1.06–6.12 | 0.037 | 387 | 19 | 4.9 | Ref. |
| N1 | 78 | 25 | 32.1 | 1.37 | 0.83–2.25 | 0.217 | 256 | 64 | 25.0 | Ref. | 78 | 15 | 19.2 | 1.42 | 0.74–2.72 | 0.285 | 256 | 38 | 14.8 | Ref. | |
| N2 | 8 | 2 | 25.0 | 0.72 | 0.18–2.90 | 0.647 | 30 | 9 | 30.0 | Ref. | 8 | 1 | 12.5 | 0.86 | 0.11–7.02 | 0.890 | 30 | 4 | 13.3 | Ref. | |
| N3 | 5 | 2 | 40.0 | 0.65 | 0.13–3.30 | 0.605 | 7 | 4 | 57.1 | Ref. | 5 | 1 | 20.0 | 0.40 | 0.05–3.07 | 0.382 | 7 | 3 | 42.9 | Ref. | |
| Tumor stage | I | 52 | 7 | 13.5 | 1.42 | 0.57–3.54 | 0.457 | 282 | 28 | 9.9 | Ref. | 52 | 2 | 3.8 | 1.35 | 0.25–7.31 | 0.730 | 282 | 8 | 2.8 | Ref. |
| II | 97 | 23 | 23.7 | 1.09 | 0.67–1.77 | 0.733 | 310 | 65 | 21.0 | Ref. | 97 | 15 | 15.5 | 1.26 | 0.67–2.37 | 0.472 | 310 | 37 | 11.9 | Ref. | |
| III | 42 | 16 | 38.1 | 0.89 | 0.50–1.60 | 0.699 | 88 | 35 | 39.8 | Ref. | 42 | 11 | 26.2 | 1.22 | 0.57–2.63 | 0.608 | 88 | 19 | 21.6 | Ref. | |
| Tumor grade | 1 | 7 | 2 | 28.6 | 9.83 | 0.77–125.4 | 0.079 | 91 | 6 | 6.6 | Ref. | 7 | 1 | 14.3 | - | - | - | 91 | 1 | 1.1 | Ref. |
| 2 | 59 | 16 | 27.1 | 1.44 | 0.78–2.66 | 0.240 | 311 | 59 | 19.0 | Ref. | 59 | 9 | 15.3 | 2.71 | 1.03–7.13 | 0.044 | 311 | 21 | 6.8 | Ref. | |
| 3 | 125 | 28 | 22.4 | 0.96 | 0.61–1.49 | 0.841 | 278 | 63 | 22.7 | Ref. | 125 | 18 | 14.4 | 0.94 | 0.54–1.62 | 0.824 | 278 | 42 | 15.1 | Ref. | |
| ER status | pos | 60 | 23 | 38.3 | 3.14 | 1.69–5.81 | <0.001 | 445 | 74 | 16.6 | Ref. | 60 | 13 | 21.7 | 5.70 | 2.27–14.4 | <0.001 | 445 | 28 | 6.3 | Ref. |
| neg | 131 | 23 | 17.6 | 0.75 | 0.47–1.19 | 0.218 | 235 | 54 | 23.0 | Ref. | 131 | 15 | 11.5 | 0.76 | 0.43–1.35 | 0.354 | 235 | 36 | 15.3 | Ref. | |
| PR status | pos | 62 | 22 | 35.5 | 2.52 | 1.38–4.60 | 0.003 | 428 | 76 | 17.8 | Ref. | 62 | 10 | 16.1 | 2.85 | 1.44–7.14 | 0.026 | 428 | 30 | 7.0 | Ref. |
| neg | 129 | 24 | 18.6 | 0.87 | 0.54–1.40 | 0.566 | 252 | 52 | 20.6 | Ref. | 129 | 18 | 14.0 | 1.02 | 0.57–1.80 | 0.954 | 252 | 34 | 13.5 | Ref. | |
| HER-2 status | pos | 13 | 4 | 30.8 | 1.68 | 0.49–5.78 | 0.413 | 164 | 34 | 20.7 | Ref. | 13 | 2 | 15.4 | 1.44 | 0.27–7.60 | 0.668 | 164 | 19 | 11.6 | Ref. |
| neg | 129 | 24 | 18.6 | 0.87 | 0.54–1.40 | 0.566 | 252 | 52 | 20.6 | Ref. | 129 | 18 | 14.0 | 1.02 | 0.57–1.80 | 0.954 | 252 | 34 | 13.5 | Ref. | |
| TNBC | yes | 114 | 20 | 17.5 | 0.77 | 0.44–1.35 | 0.367 | 138 | 30 | 21.7 | Ref. | 114 | 14 | 12.3 | 0.78 | 0.40–1.53 | 0.475 | 138 | 21 | 15.2 | Ref. |
| no | 77 | 26 | 33.8 | 2.27 | 1.32–3.88 | 0.003 | 542 | 98 | 18.1 | Ref. | 77 | 14 | 18.2 | 2.92 | 1.34–6.38 | 0.007 | 542 | 43 | 7.9 | Ref. | |
Pts No.—number of patients; Ev No.—number of events; Ev %—percentage of events; HR—hazard ratio; 95% CI—95% confidential interval; pre—premenopausal; post—postmenopausal; pos—positive; neg—negative; Ref.—reference. Bold: statistically significant differences.
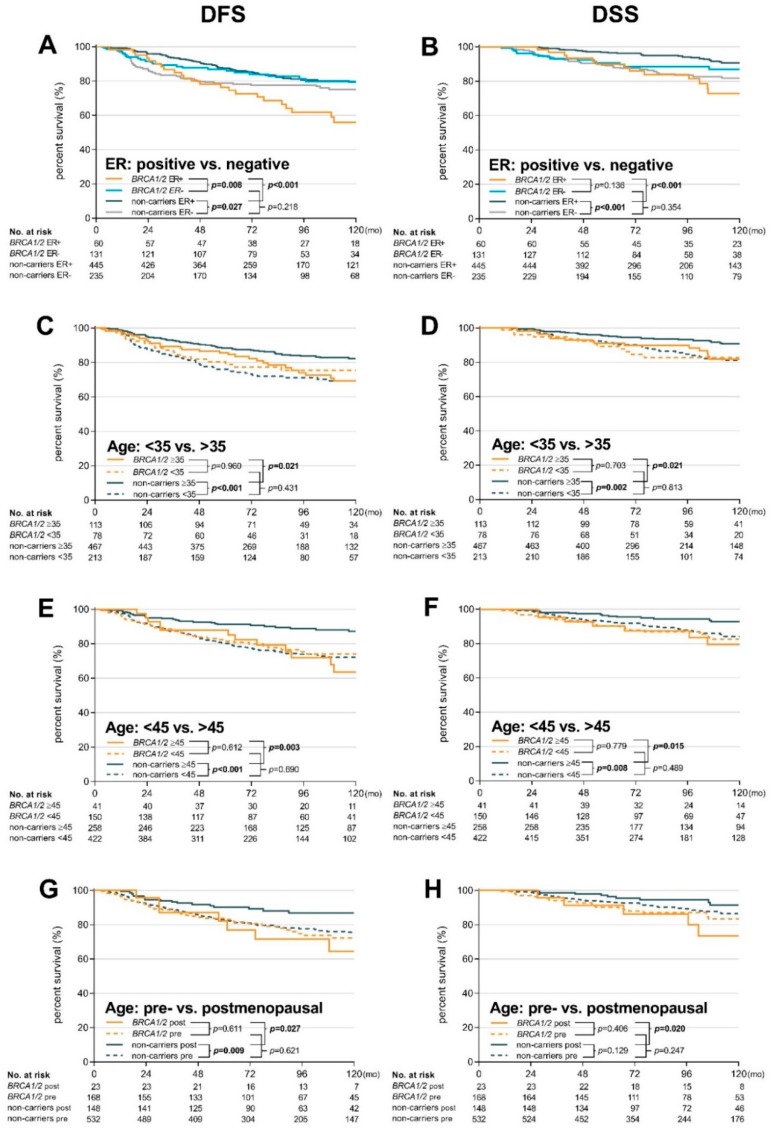
To more closely evaluate the opposite effects of age, menopausal status and ER status on survival of BRCA1/BRCA2 mutation carriers and non-carriers, we plotted Kaplan–Meier curves to visualize the dynamics of survival data from Table 2 and Supplementary Table S3. The survival curves showed that ER-positivity worsened DFS and DSS in BRCA1/BRCA2 mutation carriers, while the age at diagnosis (positively correlating with the risk) and menopausal status only slightly modified the risk (Figure 2). In contrast, younger age at diagnosis or pre-menopausal status worsened DFS and DSS in non-carriers and ER status only modified the course of survival curves and earlier recurrence in ER-negative patients. Importantly, the negative effect of ER-positivity on survival was comparable between BRCA1 and BRCA2 mutation carriers (Figure 1C,D).
Figure 2.
Kaplan–Meier plots of DFS and DSS for: (A,B) ER status (positive vs. negative); (C,D) age (<35 vs. ≥35); (E,F) age (<45 vs. ≥45); and (G,H) menopausal status (pre- vs. post-menopausal).
Based on these observations, we further analyzed the combined impact of ER-positivity with age at BC onset (or menopausal status). In non-carriers, 10-year DFS and DSS were significantly worsened in younger patients’ subgroups (<35; <45; and premenopausal, respectively) with ER-negative patients relapsing substantially earlier, as expected (Supplementary Figure S3).
In contrast, 10-year DFS was not significantly influenced by age or menopausal status in BRCA1/BRCA2 mutation carriers, but patients with ER-positive tumors had worsened DFS (with a similar non-significant trend for DSS), compared with patients with ER-negative BC (Supplementary Figure S4). Patients with BRCA1/BRCA2 mutations diagnosed with ER-positive tumors at ≥35 years showed a significantly increased risk of recurrence (HR 2.53 95% CI 1.15–5.57), compared with ER-negative mutation carriers of the same age. Similarly, increased risk of recurrence was shown for ER-positive patients ≥45 years (HR 4.03 95% CI 1.36–12.00), compared with ER-negative patients of the same age.
A direct comparison of the combined effects of ER status with age at disease onset (or menopausal status) on survival in BRCA1/BRCA2 mutation carriers and non-carriers is shown in Supplementary Figure S5. BRCA1/BRCA2 mutation carriers with ER-positive tumors diagnosed at ≥35 years had worse DFS (HR 4.56 95% CI 2.00–10.37) and DSS (HR 8.24 95% CI 2.37–28.72) than ER-positive non-carriers of the same age. Similarly, BRCA1/BRCA2 mutation carriers with ER-positive tumors diagnosed at ≥45 years or with post-menopausal BC also faced increased risk of recurrence.
The recurrence risk in young (<35 years) ER-negative patients was higher in non-carriers (HR 1.93 95% CI 1.03–3.61; p = 0.039) than in BRCA1/BRCA2 mutation carriers, with the same trend observed for DSS. The non-significant trend was also observed in patients diagnosed at <45 years, but not in premenopausal patients.
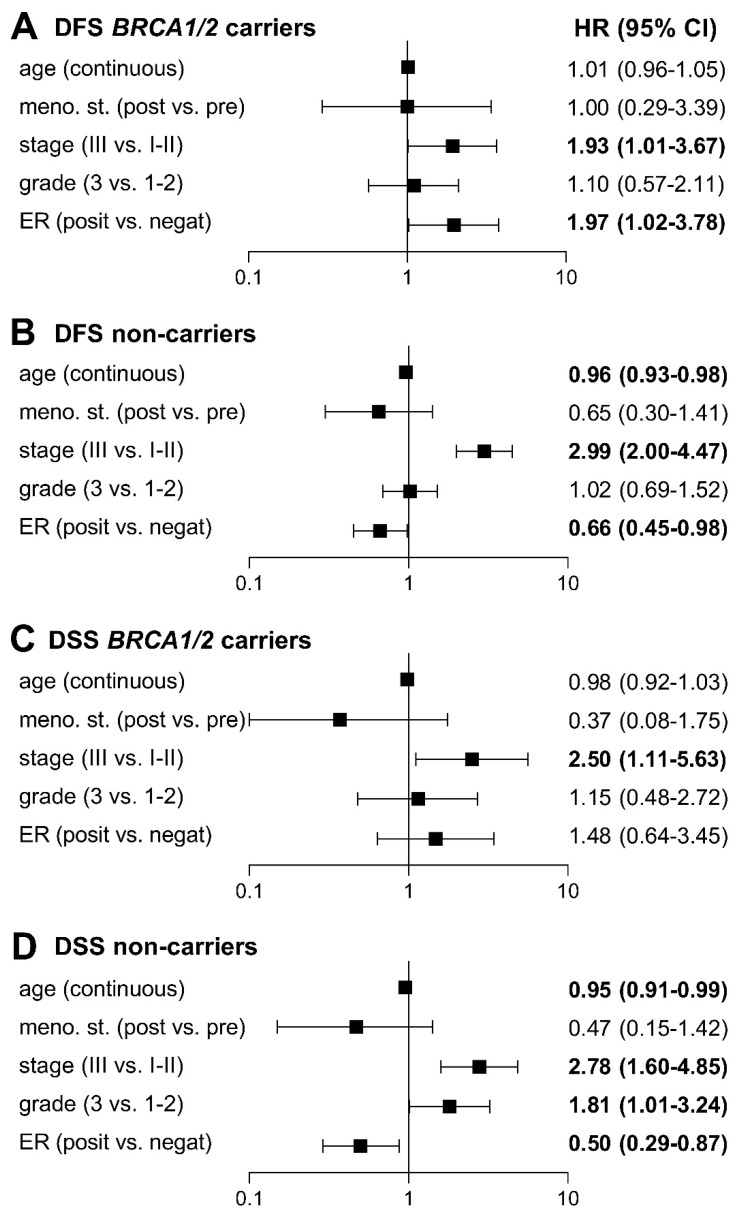
To exclude potential bias resulting from differences in baseline clinicopathological characteristics, we analyzed BRCA1/BRCA2 mutation carriers and non-carriers by multivariable Cox proportional-hazard models considering significantly different covariates from univariate Cox analyses (Supplementary Table S4). The Cox univariate analysis confirmed differences found by the Mantel–Haenszel test (Supplementary Table S5), identifying age at diagnosis, menopausal status, stage, grade, and ER status as statistically significant covariates. We excluded tumor size, nodal and PR status from the multivariable analysis as these covariates directly correlated with tumor stage and ER status. All six models in the multivariable analysis, differing in age (continuous; <35 vs. ≥35 years; <45 vs. ≥45 years) and tumor stage (II–III vs. I; III vs. I–II), confirmed tumor stage as the strongest risk factor (Supplementary Table S5) in both groups, while ER-positivity emerged as a statistically significant negative prognostic factor (with an effect comparable with advanced stage) for recurrence in BRCA1/BRCA2 mutation carriers. In contrast, ER-positivity reduced the risk in non-carriers (Figure 3). Age at diagnosis was inversely associated with the risk of recurrence in non-carriers only. While ER-positivity non-significantly increased the risk of death in BRCA1/BRCA2 mutation carriers, it was a strong protective factor in non-carriers. Other variables that negatively affected DSS in non-carriers were only younger age at diagnosis and tumor grade 3.
Figure 3.
Forest plots of multivariable hazard ratios from model D (Supplementary Table S5) for DFS (A,B) and DSS (C,D) in BRCA1/2 carriers and non-carriers adjusted for age (continuous), menopausal status (post vs. premenopausal), stage (III vs. I–II), grade (3 vs. 1–2), and ER status (positive vs. negative). Bold: statistically significant differences.
3. Discussion
Our initial analysis revealed slightly worsened DFS and DSS in BRCA1/BRCA2 mutation carriers compared with non-carriers; however, the difference was less than 10% at 10 years and marginally significant for DSS only (HR = 1.65 95% CI 1.01–2.70). A similar observation was recently reported in a meta-analysis by Baretta and colleagues, showing decreased BC-specific survival (HR = 1.42 95% CI 1.05–1.92) but non-significantly changed DFS for BRCA1/BRCA2 mutation carriers [18]. A meta-analysis by van den Broek revealed a non-significant trend towards a survival disadvantage for BC outcomes in BRCA1/BRCA2 mutation carriers [17]. A recent prospective analysis by Copson and colleagues found no significant differences in OS for BRCA1/BRCA2 mutation carriers [19]. All these data indicate that BRCA1/BRCA2 mutation carriers have only a slight prognostic disadvantage over non-carriers. However, this conclusion is clinically contra-intuitive because we and others have noted that BRCA1 mutation carriers (predominating among our mutation-positive patients) are mostly young, TNBC patients who otherwise represent a subpopulation of BC patients with poor prognoses [23,24,25]. This indicates differences between the effects of hormonal receptor status or age at disease onset on BRCA1/BRCA2 mutation carriers and non-carriers.
Indeed, we observed an inverse correlation between ER status and age in these two groups in initial univariate analyses (Table 2). As expected, the risk of disease recurrence was higher in ER-negative, younger (or premenopausal) non-carriers. Surprisingly, the same was true for ER-positive BRCA1/BRCA2 mutation carriers who developed disease recurrence 2.3-times more likely (23/60; 38.3%) than ER-positive non-carriers (74/445; 16.6%; p < 0.001). ER-positive BRCA1/BRCA2 mutation carriers also had 3.4-times higher risk of death due to BC compared with ER-positive non-carriers (13/60; 21.7% vs. 28/445; 6.3%; p < 0.001). We must emphasize that the prognosis for ER-negative BRCA1/BRCA2 mutation carriers was comparable with that for ER-positive non-carriers. Inferior survival associated with ER-positive tumors was also reported in a prospective POSH study in BRCA1 mutation carriers (HR = 1.96 95% CI 1.41–2.71) and BRCA2 mutation carriers (HR = 2.24 95% CI 1.56–3.22) at 10 years [19]. Interestingly, BRCA2 ER-positive patients in our study contributed to a worsened prognosis more than BRCA1 ER-positive patients (Supplementary Figure S2). Jonasson and colleagues showed decreased DSS (HR = 1.61 95% CI 1.11–2.35) in BC patients carrying the Icelandic founder 999del5 BRCA2 mutation, which was even more pronounced in ER-positive patients (HR = 1.92 95% CI 1.20–3.05) [16]. Schmidt and colleagues reported inferior OS in Dutch ER-positive BC patients with BRCA2 mutations (HR = 2.04 95% CI 1.22–3.39) but not BRCA1 mutations [13]. Recent data by Metcalfe and colleagues also found worsened survival in ER-positive BC BRCA2 mutation carriers (the 20-year survival rate was 62.2% and 83.7% (p = 0.03) for ER-positive and ER-negative patients, respectively) [26]. All these data indicate that the prognostic role of ER-positivity differs between BRCA1/BRCA2 mutation carriers and non-carriers.
Interestingly, Lips and colleagues have shown that ER-positive tumors in both BRCA1 and BRCA2 mutation carriers share similar specific genomic profiles of DNA somatic copy number alterations, different from those in ER-positive sporadic tumors and in ER-negative tumors in BRCA1 mutation carriers [27]. A high number of loss-of-heterozygosity events at the BRCA1 genomic locus in ER-positive tumors from BRCA1 mutation carriers found in this study (83%) and in a study by Tung and colleagues (81%) indicates that BRCA1 impairment directly contributes to the formation of ER-positive tumors [28]. The mechanism explaining how ER signaling can contribute to worsened BC progression in BRCA1/BRCA2 mutation carriers is unknown; however, preclinical data demonstrated estrogen-dependent progression of mammary tumorigenesis in BRCA1-defficient cells [29,30]. Shah and colleagues have analyzed OncotypeDX in BRCA1/BRCA2 mutation carriers with ER-positive tumors and found a high proportion of patients with a high recurrence score who may benefit from adjuvant chemotherapy [31].
The age at disease onset negatively correlates with the risk of cancer-related death in unselected BC patients [24,25]. While the negative prognostic effect of younger age was clearly apparent in all age categories for non-carriers also in our study, it did not affect disease recurrence or survival in BRCA1/BRCA2 mutation carriers (Figure 1). The results of univariate analyses revealed that age at BC onset does not play an important prognostic role in BRCA1/BRCA2 mutation carriers and indicated that age should not be considered as a factor influencing patients’ treatment approaches. This observation will require further evaluation in larger cohorts because carriers of BRCA1/BRCA2 mutation develop BC significantly earlier than non-carriers and many published studies focused primarily on early-onset BC patients or had enriched this subgroup as a result of criteria for genetic testing [10,13,19].
The multivariable analysis confirmed an inverse prognostic effect of ER-positivity and the age at disease onset on BRCA1/BRCA2 mutation carriers against non-carriers. The HR in ER-positive patients for disease recurrence was 1.97 (95% CI 1.02–3.78) in BRCA1/BRCA2 carriers and 0.66 (95% CI 0.45–0.98) in non-carriers with a similar trend for survival (Figure 3). The multivariable analysis also showed a significant age-dependent decrease in risk of recurrence and cancer-related death in non-carriers but not in BRCA1/BRCA2 carriers.
Hormone receptor-positive BC (regardless of the BRCA1/BRCA2 mutation status) is currently considered a cancer with a favorable prognosis, allowing the omission of adjuvant chemotherapy, shorter course of adjuvant hormonal treatment (no longer than five years), and other “de-escalation” approaches. In contrast, our data suggest that ER-positive BRCA1/BRCA2 mutation carriers exert an extremely dismal prognosis. We suppose that this patient group should be considered a high-risk group for BC recurrence and BC-related death.
Our study has several strengths. All genotyping was done in a single center using systematic counseling and testing criteria. We analyzed a homogenous set of BC patients excluding patients with breast and ovarian/pancreatic cancer duplicity and patients carrying mutations in non-BRCA1/BRCA2 BC-susceptibility genes. Furthermore, data were highly consistent as only 18.4% of BRCA1/BRCA2 carriers and 24.4% of non-carriers (mainly those enrolled before 2005) were excluded due to incomplete data (a proportion comparable with a prospective POSH trial) [19]. We used DSS instead of OS (to exclude death events from non-BC causes) for more accurate survival analyses. Study limitations included a limited sample size; however, only four out of 66 studies evaluated in a meta-analysis by van den Broek and colleagues surpassed the number of 191 mutation carriers analyzed in our study [17]. A subsequent meta-analysis of BRCA1/BRCA2 mutation carriers’ prognoses by Baretta and colleagues identified 60 studies and revealed the median number of BRCA1/BRCA2 mutation carriers as 39.5 (range 5–326). The size limitation affected especially BRCA2 mutation carriers (representing a minority population compared with BRCA1 mutation carriers in the Czech Republic [4]) and BRCA1 mutation carriers with ER-positive BC (representing 22.5% of all BRCA1 mutation carriers in our study). Further limitations resulted from the study design and a retrospective character of data. We performed a two-round, independent review of all clinicopathological data (irrespectively to the BRCA1/BRCA2 mutation status) obtained from the medical records. However, we cannot exclude a potential selection bias as 23.2% of all enrolled patients were excluded due to incomplete data from the univariate and multivariable analyses. On the other hand, no patient was excluded due to the loss of follow-up from the survival analyses. Retrospective study design is also sensitive to changes made in diagnostics and treatment procedures during the 19-year study period (1997–2015). Histopathological assessments for ER, PR, and HER-2 positivity have not been identical for all patients throughout the study period. Potential bias represent changes in BC treatment guidelines (especially for chemotherapy administration) during the study period, including the introduction of taxanes and trastuzumab, routinely available in the last 15 years. We are also aware that a further extension of the median follow-up (currently 9.8 years) will be necessary to better evaluate the effect of ER-positivity on DSS. Future studies screening BRCA1/BRCA2 mutations in an unselected BC population prospectively should be conducted to further examine differences in clinicopathological characteristics in BRCA1/BRCA2-positive and BRCA1/BRCA2-negative patients in the general BC population.
4. Materials and Methods
4.1. Patient Characteristics
We enrolled 1133 unrelated, female BC patients (including 234 BRCA1/BRCA2 mutation carriers and 899 non-carriers) who were tested for the presence of mutations in BRCA1/BRCA2 and other cancer-susceptibility genes at the Laboratory of Oncogenetics, First Faculty of Medicine, Charles University, in 1997–2015. All patients met national criteria for genetic/familial high-risk assessment in BC/OC (Supplementary Table S6) to be eligible for genetic counseling and testing. Patients with duplicity of breast and ovarian/pancreatic cancer were not enrolled because of the extensive impact on cancer prognosis. We also excluded a heterogeneous group of patients carrying mutations in non-BRCA1/BRCA2 BC/OC-susceptibility genes. Clinicopathological data (Supplementary Table S2) were retrieved from clinical documentation and independently reviewed by a two-round evaluation (last assessed November, 2018). All patients were Caucasians of Czech origin. The study was approved by the Ethics Committee of the General University Hospital, Prague (ethic code:1858/14 S-IV).
4.2. Molecular Analysis
An analysis of mutations in BC-susceptibility genes was initially performed using a protein truncation test or direct sequencing, the presence of large genomic BRCA1/BRCA2 rearrangements was analyzed by multiplex ligation-dependent probe amplification (MRC Holland, Amsterdam, the Netherlands), as described previously [32,33,34]. As of 2015, all samples were analyzed using the custom-designed CZECANCA panel (NimbleGen/Roche, Pleasanton, CA, USA) targeting 219 cancer-susceptible genes on MiSeq (Illumina, San Diego, CA, USA) [35]. The bioinformatics analysis included the identification of pathogenic mutations (single nucleotide variants described as pathogenic in ClinVar, non-sense, frame-shift, splicing-site alterations, and copy number variants) using a pipeline described recently [35,36].
4.3. Statistical Methods
Categorical variables (including age, menopausal status, tumor stage, tumor size, nodal status, morphology, tumor grade, ER, PR, HER-2, BC subtypes, surgery, radiotherapy, chemotherapy, endocrine therapy, event during follow-up, median follow-up, and death due to BC) were compared between BRCA1/BRCA2 mutation carriers and non-carriers using χ2 or Fisher exact tests, where appropriate. Continuous variables (age at diagnosis and follow-up period) were tested by the Mann–Whitney test.
The Kaplan–Meier product-limit method was used for survival analyses and differences were tested using the log-rank and Mantel–Haenszel tests. BC patients with carcinoma in situ (N = 46) or primarily metastatic BC (N = 25) were excluded from survival analyses. DFS was defined as the interval between BC diagnosis and the first loco-regional or distant recurrence or the last follow-up. The development of a second tumor was not considered a DFS event. DSS was defined as the interval between BC diagnosis and death from BC or the last follow-up.
Univariate analyses of categorical variables (age, menopausal status, stage, grade, ER, PR, HER-2 status, and TNBC) and multivariate analyses (age, menopausal status, stage, grade, and ER status) were performed using Cox proportional hazard regression.
All analyses were performed using the GraphPad Prism v8.0.1 (GraphPad Software, San Diego, CA, USA) and Statistica v12 (StatSoft, Palo Alto, CA, USA) programs. Two-sided p values < 0.05 and 95% confidence intervals (CI) excluding 1 were considered statistically significant.
5. Conclusions
The present study indicates that BRCA1/BRCA2 mutation carriers with ER-positive tumors have a poor prognosis with increased BC recurrence and BC-related death rate; therefore, their specific treatment (surgical and pharmacological prevention) should be considered. The BC prognosis for these patients is worse than that for young ER-negative BC non-carriers. In contrast, the prognosis for BRCA1/BRCA2 mutation carriers with ER-negative tumors, even with early BC onset, is comparable with ER-positive, older BC non-carriers, who are generally considered lower-risk patients.
Acknowledgments
We thank our patients for contributing to this study. We thank our clinical colleagues Karel Kubat, Frantisek Lehanka, Jitka Jakesova, Jiri Forster, Radovan Turyna, Michael Frank, Michaela Zugarova, Ivo Michalicka, Zuzana Valkovicova, Sarka Lukesova, Petr Klepetko, Tomas Svoboda, Lenka Lisnerova, Eugen Kubala, Petra Rihova, Tomas Vlasek, Karel Odrazka, Ivona Mrazova, Josef Kvech, Helena Milcova, Klara Janouskova, Denisa Vitaskova, Magdalena Dzivjakova, Libor Hanus, Pavel Krystof, Barbora Otavova for their help with clinical data collection, Ondrej Havranek and Tomas Buchler for their valuable comments on the manuscript, Stanislav Kormunda for statistical analyses support and Jan Flemr for language editing.
Supplementary Materials
The following are available online at https://www.mdpi.com/2072-6694/11/6/738/s1, Figure S1: Differences in the age at diagnosis in mutation carriers and non-carriers in particular breast cancer subtypes, Figure S2: Kaplan–Meier plots showing DFS and DSS in BRCA1 mutation carriers, BRCA2 mutation carriers, and non-carriers, Figure S3: Kaplan–Meier plots of DFS and DSS in BRCA1/2 mutations non-carriers considering a combined impact of ER-status and age at onset or menopausal status on survival, Figure S4: Kaplan–Meier plots of DFS and DSS in BRCA1/2 mutation carriers considering a combined impact of ER-status and age at onset or menopausal status on survival, Figure S5. Kaplan–Meier plots of DFS and DSS in BRCA1/2 mutation carriers and non-carriers considering a combined effect of ER-positivity or ER-negativity and age at disease onset or menopausal status on survival, Table S1: List of pathogenic mutations identified in the BRCA1 and BRCA2 genes in analyzed breast cancer patients, Table S2: Clinicopathological characteristics of all BRCA1/BRCA2 mutation carriers (together and separately) and non-carriers of mutations in cancer-susceptibility genes, Table S3: Analysis of 10-year DFS and DSS using the Mantel–Haenszel test comparing variables within subgroups of BRCA1/2 mutation carriers and non-carriers, respectively, Table S4: Analysis of 10-year DFS and DSS using a univariate Cox regression model comparing variables within subgroups of BRCA1/2 mutation carriers and non-carriers, Table S5: Analysis of 10-year DFS and DSS using multivariable Cox proportional-hazard models adjusted for age, menopausal status, stage, tumor grade and ER status, Table S6: The indication criteria for genetic testing of hereditary BC/OC predisposition in the Czech Republic.
Author Contributions
Conceptualization, M.V. and Z.K.; methodology, M.V. and Z.K.; software, P.Z.; validation, P.K., J.S., M.J. and P.Z.; formal analysis, M.V. and P.Z.; investigation, M.V., P.K., J.S., M.J., J.K., M.Z., Z.B., P.T., L.P., M.M., L.K., J.K., J.N., B.K., M.C., M.B., M.S., D.S.-M., V.C., R.K., L.M., S.A., L.S., K.L. and M.B.; data curation, M.V. and P.Z.; writing—original draft preparation, M.V. and Z.K.; writing—review and editing, J.N., P.K., J.S., M.J., M.S., L.P., M.Z., P.T., Z.B., L.K., M.M., J.K. and P.Z.; visualization, M.V. and Z.K.; supervision, Z.K. and L.P.; project administration, Z.K.; Funding Acquisition, Z.K. and L.P.
Funding
The study was supported by grants by the Ministry of Health of the Czech Republic NV15-28830A, and the grants by Charles University PROGRES Q28/LF1 and SVV2018/260367.
Conflicts of Interest
The authors declare no potential conflicts of interest.
References
- 1.Kwon J.S., Gutierrez-Barrera A.M., Young D., Sun C.C., Daniels M.S., Lu K.H., Arun B. Expanding the criteria for BRCA mutation testing in breast cancer survivors. J. Clin. Oncol. 2010;28:4214–4220. doi: 10.1200/JCO.2010.28.0719. [DOI] [PubMed] [Google Scholar]
- 2.Begg C.B., Haile R.W., Borg A., Malone K.E., Concannon P., Thomas D.C., Langholz B., Bernstein L., Olsen J.H., Lynch C.F., et al. Variation of breast cancer risk among BRCA1/2 carriers. JAMA. 2008;299:194–201. doi: 10.1001/jama.2007.55-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Szabo C.I., King M.C. Population genetics of BRCA1 and BRCA2. Am. J. Hum. Genet. 1997;60:1013–1020. [PMC free article] [PubMed] [Google Scholar]
- 4.Pohlreich P., Zikan M., Stribrna J., Kleibl Z., Janatova M., Kotlas J., Zidovska J., Novotny J., Petruzelka L., Szabo C., et al. High proportion of recurrent germline mutations in the BRCA1 gene in breast and ovarian cancer patients from the Prague area. Breast Cancer Res. 2005;7:R728–R736. doi: 10.1186/bcr1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.King M.C., Marks J.H., Mandell J.B. New York Breast Cancer Study G. Breast and ovarian cancer risks due to inherited mutations in BRCA1 and BRCA2. Science. 2003;302:643–646. doi: 10.1126/science.1088759. [DOI] [PubMed] [Google Scholar]
- 6.Kuchenbaecker K.B., Hopper J.L., Barnes D.R., Phillips K.A., Mooij T.M., Roos-Blom M.J., Jervis S., van Leeuwen F.E., Milne R.L., Andrieu N., et al. Risks of Breast, Ovarian, and Contralateral Breast Cancer for BRCA1 and BRCA2 Mutation Carriers. JAMA. 2017;317:2402–2416. doi: 10.1001/jama.2017.7112. [DOI] [PubMed] [Google Scholar]
- 7.Kleibl Z., Kristensen V.N. Women at high risk of breast cancer: Molecular characteristics, clinical presentation and management. Breast. 2016;28:136–144. doi: 10.1016/j.breast.2016.05.006. [DOI] [PubMed] [Google Scholar]
- 8.Parker J.S., Mullins M., Cheang M.C., Leung S., Voduc D., Vickery T., Davies S., Fauron C., He X., Hu Z., et al. Supervised risk predictor of breast cancer based on intrinsic subtypes. J. Clin. Oncol. 2009;27:1160–1167. doi: 10.1200/JCO.2008.18.1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kennecke H., Yerushalmi R., Woods R., Cheang M.C., Voduc D., Speers C.H., Nielsen T.O., Gelmon K. Metastatic behavior of breast cancer subtypes. J. Clin. Oncol. 2010;28:3271–3277. doi: 10.1200/JCO.2009.25.9820. [DOI] [PubMed] [Google Scholar]
- 10.Huzarski T., Byrski T., Gronwald J., Górski B., Domagala P., Cybulski C., Oszurek O., Szwiec M., Gugala K., Stawicka M., et al. Ten-year survival in patients with BRCA1-negative and BRCA1-positive breast cancer. J. Clin. Oncol. 2013;31:3191–3196. doi: 10.1200/JCO.2012.45.3571. [DOI] [PubMed] [Google Scholar]
- 11.Spurdle A.B., Couch F.J., Parsons M.T., McGuffog L., Barrowdale D., Bolla M.K., Wang Q., Healey S., Schmutzler R., Wappenschmidt B., et al. Refined histopathological predictors of BRCA1 and BRCA2 mutation status: A large-scale analysis of breast cancer characteristics from the BCAC, CIMBA, and ENIGMA consortia. Breast Cancer Res. 2014;16:3419. doi: 10.1186/s13058-014-0474-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sun J., Meng H., Yao L., Lv M., Bai J., Zhang J., Wang L., Ouyang T., Li J., Wang T., et al. Germline Mutations in Cancer Susceptibility Genes in a Large Series of Unselected Breast Cancer Patients. Clin. Cancer Res. 2017;23:6113–6119. doi: 10.1158/1078-0432.CCR-16-3227. [DOI] [PubMed] [Google Scholar]
- 13.Schmidt M.K., van den Broek A.J., Tollenaar R.A., Smit V.T., Westenend P.J., Brinkhuis M., Oosterhuis W.J., Wesseling J., Janssen-Heijnen M.L., Jobsen J.J., et al. Breast Cancer Survival of BRCA1/BRCA2 Mutation Carriers in a Hospital-Based Cohort of Young Women. J. Natl. Cancer Inst. 2017;109:djw329. doi: 10.1093/jnci/djw329. [DOI] [PubMed] [Google Scholar]
- 14.Hicks D.G., Short S.M., Prescott N.L., Tarr S.M., Coleman K.A., Yoder B.J., Crowe J.P., Choueiri T.K., Dawsonm A.E., Budd G.T., et al. Breast cancers with brain metastases are more likely to be estrogen receptor negative, express the basal cytokeratin CK5/6, and overexpress HER2 or EGFR. Am. J. Surg. Pathol. 2006;30:1097–1104. doi: 10.1097/01.pas.0000213306.05811.b9. [DOI] [PubMed] [Google Scholar]
- 15.Lin N.U., Vanderplas A., Hughes M.E., Theriault R.L., Edge S.B., Wong Y.N., Blayney D.W., Niland J.C., Winer E.P., Weeks J.C. Clinicopathologic features, patterns of recurrence, and survival among women with triple-negative breast cancer in the National Comprehensive Cancer Network. Cancer. 2012;118:5463–5472. doi: 10.1002/cncr.27581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jonasson J.G., Stefansson O.A., Johannsson O.T., Sigurdsson H., Agnarsson B.A., Olafsdottir G.H., Alexiusdottir K.K., Stefansdottir H., Mitev R.M., Olafsdottir K., et al. Oestrogen receptor status, treatment and breast cancer prognosis in Icelandic BRCA2 mutation carriers. Br. J. Cancer. 2016;115:776–783. doi: 10.1038/bjc.2016.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van den Broek A.J., Schmidt M.K., van ‘t Veer L.J., Tollenaar R.A., van Leeuwen F.E. Worse breast cancer prognosis of BRCA1/BRCA2 mutation carriers: what’s the evidence? A systematic review with meta-analysis. PLoS One. 2015;10:e0120189. doi: 10.1371/journal.pone.0120189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baretta Z., Mocellin S., Goldin E., Olopade O.I., Huo D. Effect of BRCA germline mutations on breast cancer prognosis: A systematic review and meta-analysis. Medicine. 2016;95:e4975. doi: 10.1097/MD.0000000000004975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Copson E.R., Maishman T.C., Tapper W.J., Cutress R.I., Greville-Heygate S., Altman D.G., Eccles B., Gerty S., Durcan L.T., Jones L., et al. Germline BRCA mutation and outcome in young-onset breast cancer (POSH): A prospective cohort study. Lancet Oncol. 2018;19:169–180. doi: 10.1016/S1470-2045(17)30891-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rennert G., Bisland-Naggan S., Barnett-Griness O., Bar-Joseph N., Zhang S., Rennert H.S., Narod S.A. Clinical outcomes of breast cancer in carriers of BRCA1 and BRCA2 mutations. N. Engl. J. Med. 2007;357:115–123. doi: 10.1056/NEJMoa070608. [DOI] [PubMed] [Google Scholar]
- 21.Zhong Q., Peng H.L., Zhao X., Zhang L., Hwang W.T. Effects of BRCA1- and BRCA2-related mutations on ovarian and breast cancer survival: A meta-analysis. Clin. Cancer Res. 2015;21:211–220. doi: 10.1158/1078-0432.CCR-14-1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goodwin P.J., Phillips K.A., West D.W., Ennis M., Hopper J.L., John E.M., O’Malley F.P., Milne R.L., Andrulis I.L., Friedlander M.L., et al. Breast cancer prognosis in BRCA1 and BRCA2 mutation carriers: An International Prospective Breast Cancer Family Registry population-based cohort study. J. Clin. Oncol. 2012;30:19–26. doi: 10.1200/JCO.2010.33.0068. [DOI] [PubMed] [Google Scholar]
- 23.Carey L., Winer E., Viale G., Cameron D., Gianni L. Triple-negative breast cancer: Disease entity or title of convenience? Nat. Rev. Clin. Oncol. 2010;7:683–692. doi: 10.1038/nrclinonc.2010.154. [DOI] [PubMed] [Google Scholar]
- 24.Sopik V., Sun P., Narod S.A. The prognostic effect of estrogen receptor status differs for younger versus older breast cancer patients. Breast Cancer Res. Treat. 2017;165:391–402. doi: 10.1007/s10549-017-4333-2. [DOI] [PubMed] [Google Scholar]
- 25.Waks A.G., Winer E.P. Breast Cancer Treatment: A Review. JAMA. 2019;321:288–300. doi: 10.1001/jama.2018.19323. [DOI] [PubMed] [Google Scholar]
- 26.Metcalfe K., Lynch H.T., Foulkes W.D., Tung N., Olopade O.I., Eisen A., Lerner-Ellis J., Snyder C., Kim S.J., Sun P., et al. Oestrogen receptor status and survival in women with BRCA2-associated breast cancer. Br. J. Cancer. 2019;120:398–403. doi: 10.1038/s41416-019-0376-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lips E.H., Debipersad R.D., Scheerman C.E., Mulder L., Sonke G.S., van der Kolk L.E., Wesseling J., Hogervorst F.B., Nederlof P.M. BRCA1-Mutated Estrogen Receptor-Positive Breast Cancer Shows BRCAness, Suggesting Sensitivity to Drugs Targeting Homologous Recombination Deficiency. Clin. Cancer Res. 2017;23:1236–1241. doi: 10.1158/1078-0432.CCR-16-0198. [DOI] [PubMed] [Google Scholar]
- 28.Tung N., Wang Y., Collins L.C., Kaplan J., Li H., Gelman R., Comander A.H., Gallagher B., Fetten K., Krag K., et al. Estrogen receptor positive breast cancers in BRCA1 mutation carriers: Clinical risk factors and pathologic features. Breast Cancer Res. 2010;12:R12. doi: 10.1186/bcr2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang C., Bai F., Zhang L.H., Scott A., Li E., Pei X.H. Estrogen promotes estrogen receptor negative BRCA1-deficient tumor initiation and progression. Breast Cancer Res. 2018;20:74. doi: 10.1186/s13058-018-0996-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baek H.J., Kim S.E., Choi E.K., Kim J.K., Shin D.H., Park E.J., Kim T.H., Kim J.Y., Kim K.G., Deng C.X., et al. Inhibition of Estrogen Signaling Reduces the Incidence of BRCA1-associated Mammary Tumor Formation. Int. J. Biol. Sci. 2018;14:1755–1768. doi: 10.7150/ijbs.28142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shah P.D., Patil S., Dickler M.N., Offit K., Hudis C.A., Robson M.E. Twenty-one-gene recurrence score assay in BRCA-associated versus sporadic breast cancers: Differences based on germline mutation status. Cancer. 2016;122:1178–1184. doi: 10.1002/cncr.29903. [DOI] [PubMed] [Google Scholar]
- 32.Pohlreich P., Stribrna J., Kleibl Z., Zikan M., Kalbacova R., Petruzelka L., Konopasek B. Mutations of the BRCA1 gene in hereditary breast and ovarian cancer in the Czech Republic. Med. Princ. Pract. 2003;12:23–29. doi: 10.1159/000068163. [DOI] [PubMed] [Google Scholar]
- 33.Pohlreich P., Stribrna J., Ticha I., Soukupova J., Kleibl Z., Zikan M., Zimovjanova M., Kotlas J., Panczak A. Predisposing genes in hereditary breast and ovarian cancer in the Czech Republic. Eur. J. Cancer Suppl. 2010;8:16. doi: 10.1016/S1359-6349(10)70869-7. [DOI] [Google Scholar]
- 34.Ticha I., Kleibl Z., Stribrna J., Kotlas J., Zimovjanova M., Mateju M., Zikan M., Pohlreich P. Screening for genomic rearrangements in BRCA1 and BRCA2 genes in Czech high-risk breast/ovarian cancer patients: High proportion of population specific alterations in BRCA1 gene. Breast Cancer Res. Treat. 2010;124:337–347. doi: 10.1007/s10549-010-0745-y. [DOI] [PubMed] [Google Scholar]
- 35.Soukupova J., Zemankova P., Lhotova K., Janatova M., Borecka M., Stolarova L., Lhota F., Foretova L., Machackova E., Stranecky V., et al. Validation of CZECANCA (CZEch CAncer paNel for Clinical Application) for targeted NGS-based analysis of hereditary cancer syndromes. PLoS One. 2018;13:e0195761. doi: 10.1371/journal.pone.0195761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lhota F., Zemankova P., Kleiblova P., Soukupova J., Vocka M., Stranecky V., Janatova M., Hartmannova H., Hodanova K., Kmoch S., et al. Hereditary truncating mutations of DNA repair and other genes in BRCA1/BRCA2/PALB2-negatively tested breast cancer patients. Clin. Genet. 2016;90:324–333. doi: 10.1111/cge.12748. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.