Abstract
The utilization of microalgae as a source of carotenoid productions has gained increasing popularity due to its advantages, such as a relatively fast turnaround time. In this study, a newly discovered Coelastrum sp. TISTR 9501RE was characterized and investigated for its taxonomical identity and carotenoid profile. To the best of our knowledge, this report was the first to fully investigate the carotenoid profiles in a microalga of the genus Coelastrum. Upon use of limited nutrients as a stress condition, the strain was able to produce astaxanthin, canthaxanthin, and lutein, as the major carotenoid components. Additionally, the carotenoid esters were found to be all astaxanthin derivatives, and β-carotene was not significantly present under this stress condition. Importantly, we also demonstrated that this practical stress condition could be combined with simple growing factors, such as ambient sunlight and temperature, to achieve even more focused carotenoid profiles, i.e., increased overall amounts of the aforementioned carotenoids with fewer minor components and chlorophylls. In addition, this green microalga was capable of tolerating a wide range of salinity. Therefore, this study paved the way for more investigations and developments on this fascinating strain, which will be reported in due course.
Keywords: Coelastrum, microalgae, astaxanthin, canthaxanthin, lutein, carotenoids
1. Introduction
Carotenoids are an important class of natural tetraterpenes found in several plants, algae, fungi, and bacteria [1,2,3,4,5]. To date, there are several hundred characterized carotenoids found in various sources, some of which were found to play important roles in their respective organisms. Examples include being an integral part of the photosynthesis unit [6,7], or playing photoprotective roles [8,9,10]. Interestingly, the discovery of these aforementioned roles simultaneously inspired the investigation of these compounds for use in other applications. Consequently, several carotenoids, some of which are depicted in Figure 1, have found uses in a number of areas [11,12,13,14]. For instance, astaxanthin was found to be the strongest antioxidant carotenoid in nature, with power that was several-fold stronger than that of β-carotene [4,15,16]. Thus, it is currently in high demand for use in the nutraceutical, pharmaceutical, and cosmetic industries [17,18,19,20,21]. Another example is canthaxanthin, which was found to serve as a food colorant in animal feed for several food sources, such as poultry and aquatic animals [22,23,24]. Lastly, lutein has been demonstrated to exhibit crucial roles in human eye health [25,26,27]. Clearly, these attractive properties of carotenoids have led to an increasing demand for them, and thus the development of economic, high-yielding productions of carotenoids are continuously being studied [4,5,28,29,30,31,32].
Figure 1.
Chemical structures of important carotenoids with various applications.
Despite the fact that the chemical syntheses of some carotenoids are available, these methods have limited utilization in real applications owing to health concerns arising from the use of pure compounds instead of isomeric mixtures that typically exist in natural sources [4,33]. Hence, naturally obtained carotenoids are usually preferred, leading to the research and development of efficient carotenoid extraction methods from natural sources [4,5,31]. Nevertheless, except for some cases like lutein extraction from Marigold [34,35], microalgal sources have become more important than plants as a source of carotenoids [2,4,13,29]. This is due to the fact that microalgae possess a desirable balance between being autotrophs capable of producing a range of carotenoids, and having biotechnological-related advantages, such as fast growth and easier genetic manipulation. Prominent examples include astaxanthin from Haematococcus pluvialis and Xanthophyllomyces dendrorhous, and β-carotene from Dunaliella salina, both of which have been utilized in actual commercial production [36,37,38].
Notably, some enhancement cues are typically required for substantial production of the carotenoids of interest, which is usually in the form of stress conditions. Heat, light, high salt concentrations, and depleted nitrogen supply are common stress conditions used to induce carotenoid production in microalgae [30,39,40,41,42,43]. Interestingly, Coelastrum is a genus of green algae that was also found to be capable of producing carotenoids, although there have been few reports that investigated the carotenoid profiles of this genus. Coelastrum sp. HA-1, an isolated microalga from Bohai Bay, China, was cultivated under nitrogen-limiting conditions and it produced astaxanthin at a level of 6.36 mg/g dried cell weight [44]. The cultivation of Coelastrum cf. pseudomicroporum in urban wastewater under salt stress conditions produced carotenoids at a level of 33.4 ± 19.86 pg cell−1 [45]. Lastly, Soares et al. reported that Coelastrum sphaericum provided a set of carotenoids with the prominent components being astaxanthin and lutein [46].
In this study, a strain of Coelastrum sp. was isolated and investigated for its ability to enhance carotenoid production under stress conditions. The induction condition was a drastic reduction of the nutrient contents, which could also be viewed as a practical advantage in a real production process (cost reduction). Notably, in contrast to the aforementioned studies [44,45], this study serves as the first example of this genus, where the carotenoid profile is characterized by both HPLC-PDA (photodiode array detection) and LC-MS. Molecular identification of this microalgal strain, its growth profiles, and the carotenoid identifications and quantifications are discussed herein.
2. Results and Discussion
2.1. Morphology and Genetic Identification of the Microalga Coelastrum sp. TISTR 9501RE
Isolated from a coastal ecosystem in the northern part of Thailand, this strain was discovered from the screening of strains that are capable of enhancing carotenoid accumulations upon the stress conditions of interest. In this regard, we employed a nutrient-depleted condition to induce the carotenoid biosynthesis. Notably, the condition used was an overall reduction of the required nutrient (BG11), such that it was only one-fourth of the normal formula. This provided the added benefit of drastically reducing the overall production costs for larger scale production. Apparently, this led to an obvious concern regarding whether this reduced nutrient had overly affected the growth of the microalga. Hence, we sought to observe the growth rates under both conditions. The results (Figure S1) showed that the growth, albeit being unsurprisingly suppressed, could still reach OD730 at around 1.0. Compared to other studies [44], this level of cell mass was at an acceptable level. Prior to the induction condition, the algal colonies on the BG11 agar exhibited a dull-shiny texture, olive green color, and circular shape. Microscopic morphology observations showed that the algal cells were spherical vegetative cells varying between 8 and 15 µm in diameter (Figure 2A). The colonies were also spherical (Figure 2B). After 14 days of growth on one-fourth BG11 agar, there was an obvious accumulation of a bright orange color, which we hypothesized to be carotenoid-based pigments (Figure 2C). Furthermore, we also demonstrated that this microalga was capable of tolerating a wide range of salinities (up to the relevant concentration in the ocean at 500 mM NaCl) (Figure S2), thereby paving the way for more diverse applications. Given its spherical shape, this algal strain was preliminarily hypothesized to belong to the genus Coelastrum [47].
Figure 2.
Light microscopic images of Coelastrum sp. TISTR 9501RE; (A) Green vegetative cells; (B) A spherical colony; (C) Carotenoids accumulating cells.
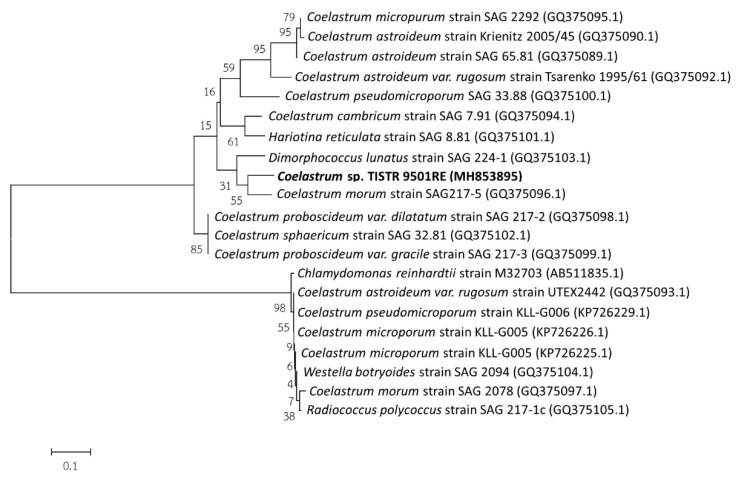
To better confirm its identity, standard genetic identification was accomplished using genomic sequencing of the internal transcribed spacer (ITS) 1 region of the 5.8S ribosomal RNA gene. To verify its taxonomical position, the ITS1 of the 5.8S rRNA gene was compared to sequences in public databases (e.g., GenBank). Similar sequences were used to construct independent molecular phylogenetic trees based on the ITS1-5.8s-ITS2 sequence. The reliability of the phylogenetic tree was evaluated using neighbor joining analysis. As shown in Figure 3, the ITS1-5.8s-ITS2 sequence comparison revealed that strain TISTR 9501RE was grouped together with other Coelastrum species in the same clade, thereby confirming that this strain was a species in the genus Coelastrum. Based on both the morphological and molecular evidence, this microalga was named Coelastrum sp. TISTR 9501RE, which is a member of the green algae (Chlorophyta).
Figure 3.
Maximum likelihood (ML) tree from the sequence-structure analysis of the ITS sequence data. The tree was generated by the neighbor joining method using the Molecular Evolutionary Genetic Analysis (MEGA6) software. The bootstrap value is expressed as a percentage of 100 replicates. The nucleotide sequence accession numbers are indicated in parentheses.
2.2. Carotenoid Profiles of Coelastrum sp. TISTR 9501RE
To characterize the molecular components of the putative carotenoid mixture observed from the morphological change of the cell, an extraction procedure by bead-beating [4,31] was employed for both the control (normal nutrient strength) condition and the nutrient-depleted condition. Thereafter, the obtained pigment mixtures were subjected to total chlorophyll determination via UV-vis spectroscopy. This revealed that the nutrient-depleted condition resulted in a significant decrease in chlorophyll production in the Coelastrum sp. TISTR 9501RE. That is, the total chlorophyll content substantially decreased from 5.64 ± 0.12 mg of total chlorophylls per one gram of dried weight of the cells (mg/g DW) to 3.89 ± 0.03 mg/g DW when the nutrient amount was restricted. This could also be illustrated by calculation of the chlorophyll a and b contents, which clearly showed that the amounts of both compounds were significantly reduced (chlorophyll a: 5.08 ± 0.10 to 3.37 ± 0.02 mg/g DW; chlorophyll b: 1.09 ± 0.02 to 0.92 ± 0.01 mg/g DW). Nevertheless, these numbers merely served as a rough guideline, and the identifications of the specific types of compounds were deemed to be more important.
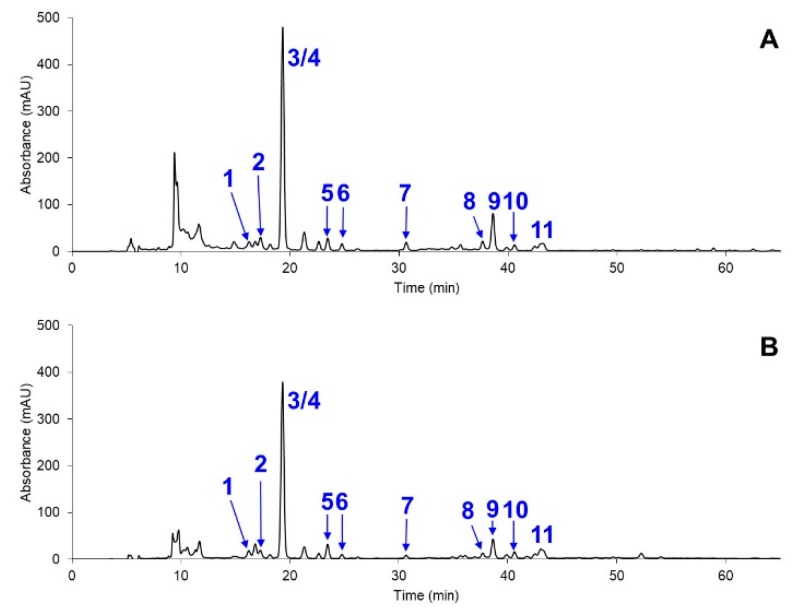
To gain more insight on the composition of the carotenoid mixtures, high performance liquid chromatography with photodiode array detection (HPLC-PDA) and liquid chromatography–mass spectrometry (LC-MS) experiments were conducted. These data, along with some comparisons from previous literature [48], resulted in the identification of the carotenoids of interest; most of which are shown in Figure 4, and Table 1; Table 2. Overall, three carotenoids of interest, namely astaxanthin (16.2 min), lutein (19.3 min, co-eluted with chlorophyll b), and canthaxanthin (23.5 min), were produced in appreciable amounts in this strain. Other prominent peaks included chlorophylls and carotenoid esters (putatively derived from astaxanthin), along with some unidentified steroid and glyceride species (as suggested by MS data not shown) scattered throughout the chromatograms. Compared to the control conditions, the nutrient-depleted microalgae showed discernible changes in the carotenoid biosynthesis. For instance, the syntheses of the unidentified steroid-related species around 10 min were suppressed, and the amounts of all the chlorophyll-related species, e.g., peak 6 and 9, were significantly decreased. On the other hand, the syntheses of all the aforementioned carotenoids were increased, as quantified by the LC-MS (see below). This suggested that the astaxanthin biosynthesis pathway could be legitimately up-regulated, as canthaxanthin was also produced in a significant amount. This was in-line with the pathway of H. pluvialis [49], where echinenone and canthaxanthin were direct precursors to astaxanthin. Furthermore, it seemed that fatty acid esters, which were commonly found in several organisms, were all derivatives of astaxanthin. Although many of them were present in minute amounts (there were in fact some additional astaxanthin esters in small but MS-detectable amounts that are not shown in Table 1 and Table 2), the combined amounts of all the esters could be considered as a significant addition to the free form, thereby confirming the enhanced production of astaxanthin.
Figure 4.
Chromatograms (representative data from three experiments at 450 nm) of the carotenoid extract from Coelastrum sp. TISTR 9501RE, with (A) the control condition, and (B) the nutrient depleted condition. Identities of the peaks can be found in Table 1 and Table 2.
Table 1.
Carotenoid profiles based on the LC-MS data of the extract of the control conditions from Coelastrum sp. TISTR 9501RE (a representative set of data from three experiments).
| Entry | Identity * | Retention Time ** | Proposed Formula | Precursor Mass | Found at Mass | Mass Error (ppm) |
|---|---|---|---|---|---|---|
| 1 | All-trans-astaxanthin | 16.24 | C40H52O4 | 597.3938 | 597.3936 | −0.48 |
| 2 | Violaxanthin isomer | 17.28 | C40H56O4 | 601.4251 | 601.4234 | −2.91 |
| 3 | All-trans-lutein | 19.32 | C40H56O2 | 569.4353 | 569.4317 | −6.34 |
| 4 | Chlorophyll b | 19.32 | C55H70N4O6Mg | 907.5219 | 907.5214 | −0.45 |
| 5 | All-trans-Canthaxanthin | 23.45 | C40H52O2 | 565.4040 | 565.4030 | −1.75 |
| 6 | Chlorophyll a | 25.65 | C55H72N4O5Mg | 893.5426 | 893.5393 | −3.66 |
| 7 | AME C18:4 isomer | 30.66 | C58H78O5 | 855.5922 | 855.5877 | −5.27 |
| 8 | AME C18:1 isomer | 37.67 | C58H84O5 | 861.6392 | 861.6346 | −5.29 |
| 9 | Chlorophyll b epimer | 38.62 | C55H70N4O6Mg | 907.5219 | 907.5294 | 8.32 |
| 10 | AME C18:2 isomer | 41.76 | C58H82O5 | 859.6235 | 859.6211 | −2.85 |
| 11 | Chlorophyll a epimer | 42.96 | C55H72N4O5Mg | 893.5426 | 893.5474 | 5.43 |
* AME = Astaxanthin monoester. C18:n indicates an unsaturated fatty acyl part, with n being the number of double bonds in the molecule. ** Data from the HPLC-PDA run.
Table 2.
Carotenoid profiles based on the LC-MS data of the extract of the nutrient-depleted conditions from Coelastrum sp. TISTR 9501RE (a representative set of data from three experiments).
| Entry | Identity * | Retention Time ** | Proposed Formula | Precursor Mass | Found at Mass | Mass Error (ppm) |
|---|---|---|---|---|---|---|
| 1 | All-trans-astaxanthin | 16.22 | C40H52O4 | 597.3938 | 597.3935 | −0.53 |
| 2 | Violaxanthin isomer | 17.28 | C40H56O4 | 601.4251 | 601.4251 | −0.04 |
| 3 | All-trans-lutein | 19.33 | C40H56O2 | 569.4353 | 569.4327 | −4.54 |
| 4 | Chlorophyll b | 19.33 | C55H70N4O6Mg | 907.5219 | 907.5218 | −0.03 |
| 5 | All-trans-Canthaxanthin | 23.46 | C40H52O2 | 565.4040 | 565.4035 | −0.87 |
| 6 | Chlorophyll a | 25.65 | C55H72N4O5Mg | 893.5426 | 893.5425 | −0.12 |
| 7 | AME C18:4 isomer | 30.67 | C58H78O5 | 855.5922 | 855.5918 | −0.51 |
| 8 | AME C18:1 isomer | 37.69 | C58H84O5 | 861.6392 | 861.6392 | 0.02 |
| 9 | Chlorophyll b epimer | 38.64 | C55H70N4O6Mg | 907.5220 | 907.5329 | 12.2 |
| 10 | AME C18:2 isomer | 41.77 | C58H82O5 | 859.6235 | 859.6231 | −0.49 |
| 11 | Chlorophyll a epimer | 43.00 | C55H72N4O5Mg | 893.5426 | 893.5507 | 9.04 |
* AME = Astaxanthin monoester. C18:n indicates an unsaturated fatty acyl part, with n being the number of double bonds in the molecule. ** Data from the HPLC-PDA run.
Thereafter, some quantitative studies were conducted to allow for further comparisons with previous studies. In this regard, we prepared calibration plots (Figure S3) and quantified three compounds, namely astaxanthin, canthaxanthin, and lutein, using LC-MS experiments. This provided the benefit of not having to develop a new method, since certain compounds were co-eluted with other components, thereby causing some quantification errors if HPLC-PDA were to be used. As a result, we determined that the strain Coelastrum sp. TISTR 9501RE produced all the compounds in higher amounts when stressed with limited nutrients (Table 3). However, as alluded above, the total astaxanthin was higher owing to the conjugation with various fatty acids.
Table 3.
Quantitative data from the LC-MS of three carotenoids (astaxanthin, lutein, and canthaxanthin) produced from Coelastrum sp. TISTR 9501RE under control and nutrient-depleted conditions. Data shown are averaged from three experiments.
| Compound | Amount (mg/g DW) | ||
|---|---|---|---|
| Control Condition | Nutrient-Depleted Condition | Nutrient-Depleted Condition (20,000-L Pond) | |
| All-trans-astaxanthin | 0.03 ± 0.001 | 0.11 ± 0.01 | 0.18 ± 0.004 |
| All-trans-lutein | 2.35 ± 0.05 | 4.18 ± 0.46 | 3.13 ± 0.07 |
| All-trans-Canthaxanthin | 0.27 ± 0.03 | 1.15 ± 0.10 | 1.37 ± 0.03 |
Interestingly, when compared to previous studies, the Coelastrum sp. TISTR 9501RE exhibited a unique carotenoid profile. For example, our strain yielded about one half of the amount of all-trans-astaxanthin as did H. pluvialis from Jin and coworkers (0.25 ± 0.04 mg/g DW vs. 0.11 ± 0.01 mg/g DW in our case) under that study’s nitrogen deficiency condition [50]. However, none of their conditions, including conditions with higher astaxanthin contents, yielded comparable amounts of canthaxanthin and lutein as obtained in our case. On the other hand, a dark condition from their study did provide a significant amount of lutein, but at the expense of the total absence of astaxanthin and canthaxanthin. Significantly higher amounts of astaxanthin could be obtained, although with other stress conditions requiring high energy, such as 6000-lx cool white fluorescent light. Interestingly, whilst H. pluvialis is well known as the most efficient astaxanthin producer, it has some notable drawbacks, including slow growth at room temperature, ease of contamination by other microalgae, and a high light requirement [51]. Hence, it is relatively uncommon to cultivate this strain in more relaxed, but large-scale conditions, such as the one demonstrated herein (see below), which has prompted researchers to find alternative species [52]. For more similar organisms, a report by Liu et al. [44] showed that Coelastrum sp. HA-1 could produce astaxanthin at 6.36 mg/g DW, although it was not possible to compare it with other carotenoid profiles, since there was no identification of other carotenoids at all. Similarly, the work by Minhas et al. reported the production of 1.17 mg/L of astaxanthin and 0.64 mg/g DW of lutein for Coelastrella sp. (P63), but there was no information on other carotenoid species [53]. Importantly, the currently reported Coelastrum sp. TISTR 9501RE produced a relatively limited amount of β-carotene, and only under the control condition (around 62.5 min in Figure 4A), which was in contrast to some of the aforementioned works. Another example is from Hu and coworkers [54], who reported the presence of five components, including astaxanthin, canthaxanthin, lutein, β-carotene, and adonirubin, without the identification of other carotenoids.
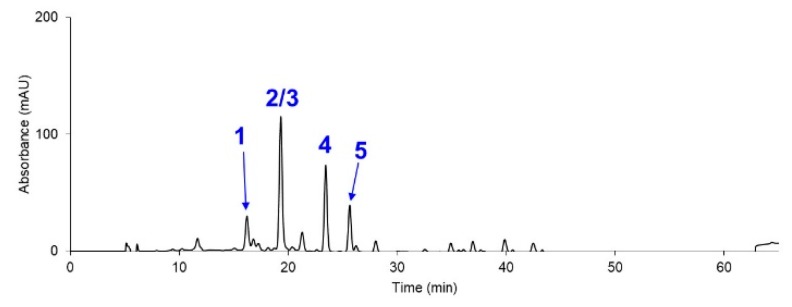
Last but not least, since the stress condition used in this study provided the practical advantage of consuming less nutrients, we explored the possibility of growing this microalga on a much larger scale. To demonstrate its great applicability, we employed ambient sunlight, ambient temperature (ranging from 30 to 35 °C), groundwater as the water source, paddle wheels for stirring without active air feeding, and the same reduced nutrient (1/4 diluted BG11) to cultivate the Coelastrum sp. TISTR 9501RE in a 20,000-L open raceway pond. Interestingly, the HPLC and LC-MS analyses (Figure 5 and Table 4) of the extract showed a drastically simpler carotenoid profile, where all the major peaks were only astaxanthin, lutein, and canthaxanthin (excluding two chlorophyll species). Given that these carotenoids are attractive candidates for nutraceutical applications [21] due to their potent antioxidant activities, this condition thereby served as a prime example for more extensive application in large-scale productions in the future.
Figure 5.
Chromatogram data (450 nm) of the carotenoid extract from the Coelastrum sp. TISTR 9501RE in a 20,000-L open raceway pond. Identities of the peaks can be found in Table 4.
Table 4.
Carotenoid profiles based on the LC-MS data of the extract of the nutrient-depleted condition from Coelastrum sp. TISTR 9501RE in a 20,000-L open raceway pond (a representative set of data from three experiments).
| Entry | Identity | Retention Time * | Proposed Formula | Precursor Mass | Found at Mass | Mass Error (ppm) |
|---|---|---|---|---|---|---|
| 1 | All-trans-astaxanthin | 16.21 | C40H52O4 | 597.3938 | 597.3935 | −0.60 |
| 2 | All-trans-lutein | 19.32 | C40H56O2 | 569.4353 | 569.4351 | −0.39 |
| 3 | Chlorophyll b | 19.32 | C55H70N4O6Mg | 907.5219 | 907.5227 | 0.88 |
| 4 | All-trans-Canthaxanthin | 23.44 | C40H52O2 | 565.4040 | 565.4044 | 0.65 |
| 5 | Chlorophyll a | 25.64 | C55H72N4O5Mg | 893.5426 | 893.5434 | 0.95 |
* Data from the HPLC-PDA run.
3. Materials and Methods
3.1. Microalgal Strain and Culture Conditions
The green microalga Coelastrum sp. was isolated from a coastal ecosystem in northern Thailand (obtained from the algae library of the Thailand Institute of Scientific and Technological Research (TISTR)). Cells were grown photoautotrophically (70 µmol m−2 s−1) in BG11 medium [55] or onto BG11 agar at 25 °C, unless otherwise stated. For carotenoids induction, the algal cells were inoculated at 3% (v/v) into 50 mL of BG11 and one-fourth strength BG11 medium (three replicates). All the flasks were shaken at 110 rpm under light 75–100 µmol m−2 s−1 for 14 days, with intermittent OD730 measurements for growth study (which was repeated for three independent experiments). The cells were harvested using centrifugation (12,000 × g, 10 min, 4 °C), and then dehydrated using a Flexi-Dry MP freeze dryer (Kinetics, Stone Ridge, NY, USA). The freeze-dried cells were used for carotenoids extraction (see below), and the subsequent HPLC analyses. The effect of salinity on growth of the microalgal strain was performed onto BG11 agar supplemented with different doses of NaCl (0, 0.15, 0.30, 0.40, and 0.5 M).
For large-scale production, the algal cells (10% inoculum) were cultivated in a 20,000-L open raceway pond equipped with paddle wheels (to ensure thorough mixing) under natural sunlight in one-fourth strength BG11 medium for 14 days. Cells were harvested by precipitation and centrifugation, using a SSE80-06-077 centrifuge (at 6200 rpm as per the manufacturer’s instructions, GEA Westfalia Separator Group GmbH, Oelde, Germany), followed by freeze drying and storage at −20 °C before analysis.
3.2. Molecular Identification and Polyphasic Taxonomy Approaches
All molecular cloning methods were performed according to standard protocols described in Reference [56]. PCR amplification of the internal transcribed spacer (ITS) 1 of the 5.8S ribosomal RNA gene was performed with the following primers: ITS forward 1 5′-TCCGTAGGTGAACCTGCGG-3′ and ITS reverse4 5′-TCCTCCGCTTATTGATATGC-3′. DNA sequencing was performed using an ABI 310 Genetic Analyzer (Applied Biosystems, Foster City, CA, USA). The ITS1-5.8s-ITS2 sequence was deposited into the GenBank under accession number MH853895. The phylogenetic tree was constructed and analyzed by the neighbor-joining method using the Molecular Evolutionary Genetics Analysis (MEGA6) software (free of charge from http://www.megasoftware.net). The robustness of the tree was assessed using bootstrap analysis (100 replicates).
3.3. Carotenoids Extraction
Thereafter, 70-mg freeze-dried cell powder was dissolved in 1-mL acetone and mixed with 0.5-mm silica glass beads (BeadBug™, Sigma-Aldrich, St. Louis, MO, USA). The resulting suspension was subjected to alternating cycles of vortexing and sonication as described below.
The suspension was vortexed for 5 min, followed by 2 min centrifugation at 10,000 rpm. The supernatant was collected, and 1 mL of fresh acetone was added to the crude precipitate. This process was repeated one more time.
The suspension from step 1 was sonicated for 30 min, followed by 2 min centrifugation at 10,000 rpm. Then, the supernatant was collected, and 1 mL of fresh acetone was added to the crude precipitate.
Step 1 was repeated exactly as shown above.
Step 2 was repeated exactly as shown above.
Step 1 was repeated exactly as shown above.
Then, all the supernatant fractions were combined. Thereafter, the solvent from the resulting solution was removed using a rotary evaporator. After that, the dried pigment mixture was ready for further analyses.
3.4. Determination of the Overall Chlorophyll Content
The overall content of chlorophylls was determined by adapting the method from Ritchie [57]. Briefly, crude extract was added with acetone to create a solution at a concentration of 0.5 mg/mL. This solution was then subjected to absorption measurement at 630, 647, 664, and 691 nm using a Cary 100 Bio-UV visible spectrophotometer (Agilent, Santa Clara, CA, USA). The resulting absorbance data were used to calculate the content of chlorophylls and carotenoids based on the following formulae (as µg/mL) [57].
| Chlorophyll a = −0.3319A630 − 1.7485A647 + 11.9442A664 − 1.4306A691 |
| Chlorophyll b = −1.2825A630 + 19.8839A647 − 4.8860A664 − 2.3416A691 |
| Total Chlorophyll = 21.3877A630 + 10.3739A647 + 5.3805A664 + 5.5309A691 |
3.5. HPLC and LC-MS Analyses
A high performance liquid chromatograph (HPLC) with a photodiode array (PDA) detector was used to reveal the composition of the carotenoid mixtures. The following parameters were used in these experiments. Three separate experiments were performed for each sample, although one representative set was selected for illustration purposes in the results and discussion section.
Model: UltiMate 3000 HPLC ThermoFisher Scientific (Waltham, MA, USA); column: YMC30 reverse phase column (3 µm, ID 4.6 mm × 150 mm) (Kyoto, Japan); column temperature: 35 °C; injection volume: 10 µL; flow rate: 0.3 mL/min; mobile phase A: MeOH:MTBE (methyl t-butyl ether):H2O (81:15:4); mobile phase B: MeOH:MTBE:H2O (16:80.4:3.6). The time program can be found in Table S1 in the supplementary material.
Liquid chromatography–mass spectrometry (LC-MS) was performed to further characterize the carotenoids found in the mixture with the following parameters. Three separate experiments were performed for each sample, although one representative set was selected for illustration purposes in the results and discussion section.
Model: HPLC—ExionLC™ AD ultra-high performance liquid chromatograph (UHPLC); MS—SciEx X500R quadrupole time-of-flight MS (QTOF) (Framingham, MA, USA). MS parameters: mass range = 500–1300 m/z, positive mode; ion source gas 1 = 50 psi; ion source gas 2 = 50 psi; source temperature = 500 °C; spray voltage = 5500 V; declustering potential (DP) = 50 V; collision energy (CE) = 10 V.
The time programs for both the HPLC-PDA and LC-MS analyses were the same (Table S1). The concentrations of the mixtures used for the analysis were 10 and 2 mg/mL in acetone for the HPLC-PDA and LC-MS experiments, respectively. For the quantification experiments, standard solutions of the carotenoids of interest were prepared (500 ppm in acetonitrile for astaxanthin and canthaxanthin; 1000 ppm in acetone for lutein). Then each solution was serially diluted to different ranges that covered the relevant concentrations in the samples (0.5–8 ppm for astaxanthin, 1–20 ppm for canthaxanthin, and 10–150 ppm for lutein). All of these solutions were then analyzed and quantified by LC-MS in triplicates.
4. Conclusions
In conclusion, in this study, we reported the genetic identification and the study of carotenoid profiles of a new Coelastrum strain named Coelastrum sp. TISTR 9501RE. With the stress condition being the overall reduction in nutrients, the strain provided unique carotenoid compositions, with astaxanthin, canthaxanthin, and lutein being the major components. Interestingly, large-scale production could also be achieved under sustainable conditions such as ambient light, with even higher amounts of the desirable carotenoids. Further studies on a variety of stress conditions, especially the use of saltwater as a stress condition, and their effects on the biosynthesis pathways of carotenoids are an ongoing investigation in our group, so as to gain a better understanding and achieve more efficient carotenoid production.
Supplementary Materials
The following are available online at https://www.mdpi.com/1660-3397/17/6/328/s1, Figure S1: Growth curves of Coelastrum sp. TISTR 9501RE under (A) the control condition, and (B) the nutrient-depleted condition. Data shown are averaged from three experiments, Figure S2: Effect of salinity on the growth and cell morphology of Coelastrum sp. TISTR 9501RE. (A) Effect of salinity on the growth of Coelastrum sp. TISTR 9501RE was performed onto BG11 agar supplemented with different doses of NaCl (0, 0.15, 0.30, 0.40, and 0.50 M). For each concentration, 20 µL of microalgal culture in BG11 (OD730 ~ 1) was streaked onto BG11 at an indicated NaCl concentration. Plate cultures were incubated photoautotrophically (70 µmol m−2 s−1) at 25 °C for 3 days. Salinity tolerance was scored by assessing the growth or lack of growth. (B) Light microscopic images of Coelastrum sp. TISTR 9501RE were grown in BG11 liquid medium supplemented with different concentrations of NaCl (0, 0.15, 0.30, 0.45 M). Microalgal culture in BG11 (OD730 ~ 1) was inoculated into new BG11 liquid medium at each indicated NaCl concentration. Incubation condition was performed in the same way as indicated in (A). The morphology of the cells was observed under a light microscope (Olympus BX51, Japan), Figure S3: Calibration plots for astaxanthin (top), canthaxanthin (middle), and lutein (bottom) quantifications. Data shown are averaged from three experiments, Table S1: The time program for the HPLC-PDA and LC-MS experiments.
Author Contributions
Conceptualization, R.W.-S. and T.P.; methodology, R.W.-S. and T.P.; validation, M.R., K.J., and P.K.; formal analysis, M.R. and K.J.; investigation, M.R. and K.J.; resources, P.K., S.S., R.W.-S., and T.P.; writing—original draft preparation, R.W.S. and T.P.; writing—review and editing, R.W.-S. and T.P.; visualization, S.S., M.R., R.W.-S., and T.P.; supervision, R.W.-S. and T.P.; project administration, R.W.-S. and T.P.; funding acquisition, T.P.
Funding
This research was funded by the Thailand Research Fund (MRG6280238). A PhD scholarship was provided to MR from the Development and Promotion of Science and Technology Talented Project (DPST), Thailand.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Maresca J.A., Graham J.E., Bryant D.A. The biochemical basis for structural diversity in the carotenoids of chlorophototrophic bacteria. Photosynth. Res. 2008;97:121–140. doi: 10.1007/s11120-008-9312-3. [DOI] [PubMed] [Google Scholar]
- 2.Guedes A.C., Amaro H.M., Malcata F.X. Microalgae as sources of carotenoids. Mar. Drugs. 2011;9:625–644. doi: 10.3390/md9040625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Avalos J., Carmen Limón M. Biological roles of fungal carotenoids. Curr. Genet. 2015;61:309–324. doi: 10.1007/s00294-014-0454-x. [DOI] [PubMed] [Google Scholar]
- 4.Gong M., Bassi A. Carotenoids from microalgae: A review of recent developments. Biotechnol. Adv. 2016;34:1396–1412. doi: 10.1016/j.biotechadv.2016.10.005. [DOI] [PubMed] [Google Scholar]
- 5.Adadi P., Barakova N.V., Krivoshapkina E.F. Selected methods of extracting carotenoids, characterization, and health concerns: A review. J. Agric. Food Chem. 2018;66:5925–5947. doi: 10.1021/acs.jafc.8b01407. [DOI] [PubMed] [Google Scholar]
- 6.Polívka T., Frank H.A. Molecular factors controlling photosynthetic light harvesting by carotenoids. Acc. Chem. Res. 2010;43:1125–1134. doi: 10.1021/ar100030m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Christaki E., Bonos E., Giannenas I., Florou-Paneri P. Functional properties of carotenoids originating from algae. J. Sci. Food Agric. 2013;93:5–11. doi: 10.1002/jsfa.5902. [DOI] [PubMed] [Google Scholar]
- 8.Wang B., Zarka A., Trebst A., Boussiba S. Astaxanthin accumulation in Haematococcus pluvialis (chlorophyceae) as an active photoprotective process under high irradiance. J. Phycol. 2003;39:1116–1124. doi: 10.1111/j.0022-3646.2003.03-043.x. [DOI] [Google Scholar]
- 9.Cazzonelli C.I. Carotenoids in nature: Insights from plants and beyond. Funct. Plant Biol. 2011;38:833–847. doi: 10.1071/FP11192. [DOI] [PubMed] [Google Scholar]
- 10.Erickson E., Wakao S., Niyogi K.K. Light stress and photoprotection in Chlamydomonas reinhardtii. Plant J. 2015;82:449–465. doi: 10.1111/tpj.12825. [DOI] [PubMed] [Google Scholar]
- 11.Breithaupt D.E. Modern application of xanthophylls in animal feeding—A review. Trends Food Sci. Technol. 2007;18:501–506. doi: 10.1016/j.tifs.2007.04.009. [DOI] [Google Scholar]
- 12.Vílchez C., Forján E., Cuaresma M., Bédmar F., Garbayo I., Vega J.M. Marine carotenoids: Biological functions and commercial applications. Mar. Drugs. 2011;9:319–333. doi: 10.3390/md9030319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Borowitzka M.A. High-value products from microalgae—Their development and commercialisation. J. Appl. Phycol. 2013;25:743–756. doi: 10.1007/s10811-013-9983-9. [DOI] [Google Scholar]
- 14.Sathasivam R., Ki J.-S. A review of the biological activities of microalgal carotenoids and their potential use in healthcare and cosmetic industries. Mar. Drugs. 2018;16:26. doi: 10.3390/md16010026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Naguib Y.M.A. Antioxidant activities of astaxanthin and related carotenoids. J. Agric. Food Chem. 2000;48:1150–1154. doi: 10.1021/jf991106k. [DOI] [PubMed] [Google Scholar]
- 16.Higuera-Ciapara I., Félix-Valenzuela L., Goycoolea F.M. Astaxanthin: A review of its chemistry and applications. Crit. Rev. Food Sci. Nutr. 2006;46:185–196. doi: 10.1080/10408690590957188. [DOI] [PubMed] [Google Scholar]
- 17.Yuan J.-P., Peng J., Yin K., Wang J.-H. Potential health-promoting effects of astaxanthin: A high-value carotenoid mostly from microalgae. Mol. Nutr. Food Res. 2011;55:150–165. doi: 10.1002/mnfr.201000414. [DOI] [PubMed] [Google Scholar]
- 18.Ambati R., Phang S.-M., Ravi S., Aswathanarayana R. Astaxanthin: Sources, extraction, stability, biological activities and its commercial applications—A review. Mar. Drugs. 2014;12:128. doi: 10.3390/md12010128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Visioli F., Artaria C. Astaxanthin in cardiovascular health and disease: Mechanisms of action, therapeutic merits, and knowledge gaps. Food Funct. 2017;8:39–63. doi: 10.1039/C6FO01721E. [DOI] [PubMed] [Google Scholar]
- 20.Davinelli S., Nielsen M., Scapagnini G. Astaxanthin in skin health, repair, and disease: A comprehensive review. Nutrients. 2018;10:522. doi: 10.3390/nu10040522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Viera I., Pérez-Gálvez A., Roca M. Bioaccessibility of marine carotenoids. Mar. Drugs. 2018;16:26. doi: 10.3390/md16100397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Torrissen O.J. Pigmentation of salmonids—Effect of carotenoids in eggs and start-feeding diet on survival and growth rate. Aquaculture. 1984;43:185–193. doi: 10.1016/0044-8486(84)90021-8. [DOI] [Google Scholar]
- 23.Surai A.P., Surai P.F., Steinberg W., Wakeman W.G., Speake B.K., Sparks N.H.C. Effect of canthaxanthin content of the maternal diet on the antioxidant system of the developing chick. Br. Poult. Sci. 2003;44:612–619. doi: 10.1080/00071660310001616200. [DOI] [PubMed] [Google Scholar]
- 24.Esatbeyoglu T., Rimbach G. Canthaxanthin: From molecule to function. Mol. Nutr. Food Res. 2017;61:1600469. doi: 10.1002/mnfr.201600469. [DOI] [PubMed] [Google Scholar]
- 25.Ma L., Lin X.-M. Effects of lutein and zeaxanthin on aspects of eye health. J. Sci. Food Agric. 2010;90:2–12. doi: 10.1002/jsfa.3785. [DOI] [PubMed] [Google Scholar]
- 26.Koushan K., Rusovici R., Li W., Ferguson L., Chalam K. The role of lutein in eye-related disease. Nutrients. 2013;5:1823–1829. doi: 10.3390/nu5051823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Manayi A., Abdollahi M., Raman T., Nabavi S.F., Habtemariam S., Daglia M., Nabavi S.M. Lutein and cataract: From bench to bedside. Crit. Rev. Biotechnol. 2016;36:829–839. doi: 10.3109/07388551.2015.1049510. [DOI] [PubMed] [Google Scholar]
- 28.Del Campo J.A., García-González M., Guerrero M.G. Outdoor cultivation of microalgae for carotenoid production: Current state and perspectives. Appl. Microbiol. Biotechnol. 2007;74:1163–1174. doi: 10.1007/s00253-007-0844-9. [DOI] [PubMed] [Google Scholar]
- 29.Varela J.C., Pereira H., Vila M., León R. Production of carotenoids by microalgae: Achievements and challenges. Photosynth. Res. 2015;125:423–436. doi: 10.1007/s11120-015-0149-2. [DOI] [PubMed] [Google Scholar]
- 30.Sun X.-M., Ren L.-J., Zhao Q.-Y., Ji X.-J., Huang H. Microalgae for the production of lipid and carotenoids: A review with focus on stress regulation and adaptation. Biotechnol. Biofuels. 2018;11:272. doi: 10.1186/s13068-018-1275-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saini R.K., Keum Y.-S. Carotenoid extraction methods: A review of recent developments. Food Chem. 2018;240:90–103. doi: 10.1016/j.foodchem.2017.07.099. [DOI] [PubMed] [Google Scholar]
- 32.Huang W., Lin Y., He M., Gong Y., Huang J. Induced high-yield production of zeaxanthin, lutein, and β-carotene by a mutant of Chlorella zofingiensis. J. Agric. Food Chem. 2018;66:891–897. doi: 10.1021/acs.jafc.7b05400. [DOI] [PubMed] [Google Scholar]
- 33.Patrick L. Beta carotene: The controversy continues. Altern. Med. Rev. 2000;5:530–545. [PubMed] [Google Scholar]
- 34.Lin J.-H., Lee D.-J., Chang J.-S. Lutein production from biomass: Marigold flowers versus microalgae. Bioresour. Technol. 2015;184:421–428. doi: 10.1016/j.biortech.2014.09.099. [DOI] [PubMed] [Google Scholar]
- 35.Sowbhagya H.B., Sampathu S.R., Krishnamurthy N. Natural colorant from marigold-chemistry and technology. Food Rev. Int. 2004;20:33–50. doi: 10.1081/FRI-120028829. [DOI] [Google Scholar]
- 36.Rodríguez-Sáiz M., de la Fuente J.L., Barredo J.L. Xanthophyllomyces dendrorhous for the industrial production of astaxanthin. Appl. Microbiol. Biotechnol. 2010;88:645–658. doi: 10.1007/s00253-010-2814-x. [DOI] [PubMed] [Google Scholar]
- 37.Li J., Zhu D., Niu J., Shen S., Wang G. An economic assessment of astaxanthin production by large scale cultivation of Haematococcus pluvialis. Biotechnol. Adv. 2011;29:568–574. doi: 10.1016/j.biotechadv.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 38.Hejazi M.A., Holwerda E., Wijffels R.H. Milking microalga Dunaliella salina for β-carotene production in two-phase bioreactors. Biotechnol. Bioeng. 2004;85:475–481. doi: 10.1002/bit.10914. [DOI] [PubMed] [Google Scholar]
- 39.Kim S.-H., Liu K.-H., Lee S.-Y., Hong S.-J., Cho B.-K., Lee H., Lee C.-G., Choi H.-K. Effects of light intensity and nitrogen starvation on glycerolipid, glycerophospholipid, and carotenoid composition in Dunaliella tertiolecta culture. PLoS ONE. 2013;8:e72415. doi: 10.1371/journal.pone.0072415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Minyuk G., Chelebieva E., Chubchikova I., Dantsyuk N., Drobetskaya I., Sakhon E., Chekanov K., Solovchenko A. Stress-induced secondary carotenogenesis in Coelastrella rubescens (Scenedesmaceae, Chlorophyta), a producer of value-added keto-carotenoids. Algae. 2017;32:245–259. doi: 10.4490/algae.2017.32.8.6. [DOI] [Google Scholar]
- 41.Zhang P., Li Z., Lu L., Xiao Y., Liu J., Guo J., Fang F. Effects of stepwise nitrogen depletion on carotenoid content, fluorescence parameters and the cellular stoichiometry of Chlorella vulgaris. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017;181:30–38. doi: 10.1016/j.saa.2017.03.022. [DOI] [PubMed] [Google Scholar]
- 42.Huang J.J., Lin S., Xu W., Cheung P.C.K. Enhancement of the production of bioactive microalgal metabolites by ultraviolet radiation (UVA 365 nm) J. Agric. Food Chem. 2018;66:10215–10224. doi: 10.1021/acs.jafc.8b03789. [DOI] [PubMed] [Google Scholar]
- 43.Janchot K., Rauytanapanit M., Honda M., Hibino T., Sirisattha S., Praneenararat T., Kageyama H., Waditee-Sirisattha R. Effects of potassium chloride-induced stress on the carotenoids canthaxanthin, astaxanthin, and lipid accumulations in the green Chlorococcal microalga strain TISTR 9500. J. Eukaryot. Microbiol. 2019 doi: 10.1111/jeu.12726. [DOI] [PubMed] [Google Scholar]
- 44.Liu Z., Liu C., Hou Y., Chen S., Xiao D., Zhang J., Chen F. Isolation and characterization of a marine microalga for biofuel production with astaxanthin as a co-product. Energies. 2013;6:2759–2772. doi: 10.3390/en6062759. [DOI] [Google Scholar]
- 45.Úbeda B., Gálvez J.Á., Michel M., Bartual A. Microalgae cultivation in urban wastewater: Coelastrum cf. pseudomicroporum as a novel carotenoid source and a potential microalgae harvesting tool. Bioresour. Technol. 2017;228:210–217. doi: 10.1016/j.biortech.2016.12.095. [DOI] [PubMed] [Google Scholar]
- 46.Soares A.T., da Costa D.C., Vieira A.A.H., Antoniosi Filho N.R. Analysis of major carotenoids and fatty acid composition of freshwater microalgae. Heliyon. 2019;5:e01529. doi: 10.1016/j.heliyon.2019.e01529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Štenclová L., Fučíková K., Kaštovský J., Pažoutová M. Molecular and morphological delimitation and generic classification of the family Oocystaceae (Trebouxiophyceae, Chlorophyta) J. Phycol. 2017;53:1263–1282. doi: 10.1111/jpy.12581. [DOI] [PubMed] [Google Scholar]
- 48.Deli J., Gonda S., Nagy L.Z., Szabó I., Gulyás-Fekete G., Agócs A., Marton K., Vasas G. Carotenoid composition of three bloom-forming algae species. Food Res. Int. 2014;65:215–223. doi: 10.1016/j.foodres.2014.05.020. [DOI] [Google Scholar]
- 49.Han D., Li Y., Hu Q. Astaxanthin in microalgae: Pathways, functions and biotechnological implications. Algae. 2013;28:131–147. doi: 10.4490/algae.2013.28.2.131. [DOI] [Google Scholar]
- 50.Jin H., Lao Y.M., Zhou J., Zhang H.J., Cai Z.H. Simultaneous determination of 13 carotenoids by a simple C18 column-based ultra-high-pressure liquid chromatography method for carotenoid profiling in the astaxanthin-accumulating Haematococcus pluvialis. J. Chromatogr. A. 2017;1488:93–103. doi: 10.1016/j.chroma.2017.01.088. [DOI] [PubMed] [Google Scholar]
- 51.Masojídek J., Torzillo G. Mass cultivation of freshwater microalgae. In: Jørgensen S.E., Fath B.D., editors. Encyclopedia of Ecology. Academic Press; Oxford, UK: 2008. pp. 2226–2235. [Google Scholar]
- 52.Liu J., Sun Z., Gerken H., Liu Z., Jiang Y., Chen F. Chlorella zofingiensis as an alternative microalgal producer of astaxanthin: Biology and industrial potential. Mar. Drugs. 2014;12:3487–3515. doi: 10.3390/md12063487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Minhas A.K., Hodgson P., Barrow C.J., Sashidhar B., Adholeya A. The isolation and identification of new microalgal strains producing oil and carotenoid simultaneously with biofuel potential. Bioresour. Technol. 2016;211:556–565. doi: 10.1016/j.biortech.2016.03.121. [DOI] [PubMed] [Google Scholar]
- 54.Hu C.-W., Chuang L.-T., Yu P.-C., Chen C.-N.N. Pigment production by a new thermotolerant microalga Coelastrella sp. F50. Food Chem. 2013;138:2071–2078. doi: 10.1016/j.foodchem.2012.11.133. [DOI] [PubMed] [Google Scholar]
- 55.Stanier R.Y., Kunisawa R., Mandel M., Cohen-Bazire G. Purification and properties of unicellular blue-green algae (order Chroococcales) Bacteriol. Rev. 1971;35:171–205. doi: 10.1128/br.35.2.171-205.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sambrook J., Fritsch E.F., Maniatis T. Molecular Cloning: A Laboratory Manual. 2nd ed. Cold Spring Harbor Laboratory Press; New York, NY, USA: 1989. [Google Scholar]
- 57.Ritchie R.J. Universal chlorophyll equations for estimating chlorophylls a, b, c, and d and total chlorophylls in natural assemblages of photosynthetic organisms using acetone, methanol, or ethanol solvents. Photosynthetica. 2008;46:115–126. doi: 10.1007/s11099-008-0019-7. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.