Abstract

The main pathway responsible for cellular regulation against oxidative stress is nuclear factor E2-related factor-2 (Nrf2) signaling. We previously synthesized and reported a novel vinyl sulfone (1) as an Nrf2 activator with therapeutic potential for Parkinson’s disease (PD). In this study, we changed the vinyl sulfone to vinyl sulfonamide or vinyl sulfonate to improve Nrf2 activating efficacy. We observed that the introduction of vinyl sulfonamide led to a reduction of the effects on Nrf2 activation, whereas vinyl sulfonate compounds exhibited superior activity compared to the vinyl sulfone compounds. Among the vinyl sulfonates, 3c exhibited 6.9- and 83.5-fold higher effects on Nrf2 activation than the corresponding vinyl sulfone (1) and vinyl sulfonamide (2c), respectively. Compound 3c was confirmed to induce expression of the Nrf2-dependent antioxidant enzymes at the protein level in cells. In addition, 3c mitigated PD-associated behavioral deficits by protecting DAergic neurons in the MPTP-induced mouse model of PD.
Keywords: Parkinson’s disease, antioxidant, anti-inflammatory, Nrf2/Keap1 pathway, MPTP mouse model, vinyl sulfone
Parkinson’s disease (PD) is the most prevalent neurodegenerative disorder featured clinically by motor deficits. PD patients have clinical features such as tremor, rigidity, slowness of movement, and postural disability.1,2 Most of the symptoms are caused by the death of dopaminergic (DAergic) neurons that produce chemical messengers called dopamine in the substantia nigra (SN) pars compacta.3,4 The etiology and pathogenesis of PD is not yet known, but several studies have suggested that oxidative stress is partly responsible for the loss of DAergic neurons and plays an important role in neurodegeneration of PD.5−7 Another factor contributing to the pathogenesis of PD has been thought to be neuro-inflammation. Microglial activation is linked to the loss of DAergic neurons, suggesting that neuronal inflammation contribute to the progressive degeneration.8,9 Several clinical studies have reported that the levels of inflammatory enzymes have been elevated in mouse models of PD and brain of PD patients.10−12
Nuclear factor E2-related factor-2 (Nrf2), encoded by gene NFE2L2, is a nuclear transcription factor that regulates cellular defense system against oxidative stress and has been shown to down-regulate inflammatory responses.13,14 Under normal conditions, Nrf2 adheres to Kelch-like ECH-associated protein 1 (Keap1) as a complex and undergoes ubiquitination and proteasomal degradation in cytoplasm. Under oxidative stress conditions, Nrf2 is liberated from Keap1 and translocated into the nucleus, which binds to the antioxidant response element (ARE) sequence in the DNA promoter region and begins transcription of the ARE-regulated antioxidant enzyme such as heme oxygenase-1 (HO-1), glutamate-cysteine ligase (GCL), and reduced nicotinamide adenine dinucleotide phosphate (NAD(P)H) quinone oxidoreductase 1 (NQO1).15−17
Although the precise mechanism associated with the Nrf2-mediated anti-inflammation pathway has not been elucidated in detail, recent studies reported that inflammatory mediators were overexpressed in the Nrf2 knockout mice and deficiency in transcription factor Nrf2 worsens inflammatory parameters in a mouse model of neurodegenerative diseases.18−20 The sole FDA-approved Nrf2 activator, dimethyl fumarate (DMF, brand name Tecfidera), shows effectiveness in treating multiple sclerosis (MS) by regulating inflammation.21,22 Taken together, targeting the Nrf2 pathway is an attractive therapeutic strategy for neurodegenerative diseases including PD through anti-inflammatory and antioxidant effects.
We previously synthesized and reported
vinyl sulfone scaffolds
as an Nrf2 activator.23−25 The lead compound (1) with the vinyl
sulfone scaffold induced expression of Nrf2-dependent antioxidant
enzyme and relieved the production of inflammatory mediators through
Nrf2 signaling.23,24 Compound 1 also
showed promising efficacy in the 1-methyl-4-phenyl-1,2,3,6,-tetrahydropyridine
(MPTP)-induced PD mouse model.
In this study, we replaced the sulfone moiety of compound 1 with a sulfonamide or sulfonate. Vinyl sulfonamides (2) and vinyl sulfonates (3) can be conveniently synthesized and also have an α,β-unsaturated sulfone group, which is a highly activated Michael acceptor and may be responsible for activating Nrf2. Here, we report the development, synthesis, and biological evaluation of a series of vinyl sulfonamides and vinyl sulfonates. We observed that vinyl sulfonate scaffold enhanced Nrf2 activating efficacy. The most potent compound (3c) was evaluated for the expression of antioxidant enzymes and attenuated the production of inflammatory cytokines in BV-2 microglial cells and SH-SY5Y human neuroblastoma cells. Finally, the compound was tested for in vivo therapeutic effects on parkinsonism in the MPTP-induced mouse model.
Results and Discussion
We further optimized previously developed compound 1 by modifying the α,β-unsaturated sulfone position according to two categories: vinyl sulfonamides (2) or vinyl sulfonates (3). The synthetic pathway of vinyl sulfonamides (2) and vinyl sulfonates (3) are described in Scheme 1. Various styrenes (4a–4d) were prepared by the addition of sulfuryl chloride (SO2Cl2) in dimethylformamide (DMF) for 3 h at 90 °C to produce (E)-2-phenylethene-1-sulfonyl chlorides (5a–5d). Condensation of 5a–5d with various anilines or phenols in the presence of triethylamine (TEA) in dichloromethane (CH2Cl2) for 5 h at room temperature provided the sulfonamides 2a–2e and the sulfonates 3a–3k, respectively.
Scheme 1. Synthesis of Compounds 2 and 3.
Reagents and conditions: (a) SO2Cl2, DMF, 0 °C to r.t., 3 h; (b) Et3N, CH2Cl2, r.t., 5 h.
The synthesized compounds were evaluated for Nrf2 activation in the optimized cell-based system. As we previously reported, this assay system assesses the ability of synthesized compounds to free Nrf2 from Keap1 and induce Nrf2 translocation to the nucleus using a genetically engineered stable cell-line.25,26 The efficacy of Nrf2 activation is described in Table 1 as EC50 values. In this assay, the previously developed compound 1 was confirmed to increase Nrf2 activation (1, EC50 = 0.530 μM).
Table 1. EC50 Values against Nrf2 Activation of Synthesized Compounds.
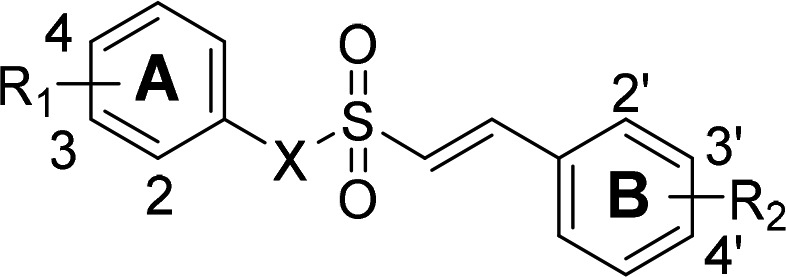
| compd. | X | R1 | R2 | Nrf2 EC50 (μM)a |
|---|---|---|---|---|
| 2a | N | -H | -H | >10 |
| 2b | N | -H | 2′-Cl | >10 |
| 2c | N | 2-OMe | 2′-Cl | 6.350 ± 0.039 |
| 2d | N | 3-OMe | 2′-Cl | >10 |
| 2e | N | 4-OMe | 2′-Cl | >10 |
| 3a | O | -H | -H | 1.040 ± 0.015 |
| 3b | O | 4-OMe | -H | 1.480 ± 0.047 |
| 3c | O | 2-OMe | 2′-Cl | 0.076 ± 0.009 |
| 3d | O | 3-OMe | 2′-Cl | 0.165 ± 0.026 |
| 3e | O | 4-OMe | 2′-Cl | 0.237 ± 0.015 |
| 3f | O | 2-OMe | 3′-Cl | 0.698 ± 0.016 |
| 3g | O | 3-OMe | 3′-Cl | 0.943 ± 0.050 |
| 3h | O | 4-OMe | 3′-Cl | 0.948 ± 0.028 |
| 3i | O | 2-OMe | 4′-Cl | 1.334 ± 0.024 |
| 3j | O | 3-OMe | 4′-Cl | 1.778 ± 0.030 |
| 3k | O | 4-OMe | 4′-Cl | 1.155 ± 0.036 |
| 1b | 2-OMe | 2′-Cl | 0.530 ± 0.025 | |
| SFNc | 0.580 ± 0.024 |
The Nrf2 functional assay was accomplished by a PathHunter U2OS Nrf2 nuclear translocation cell line (93-0821C3, DiscoveRx). U2OS cells were plated at 13,000 cells/well in triplicate with various compound concentrations for 6 h. Nrf2 translocation, which is activation-dependent, was determined using a cell-based functional assay of the mean ± standard error half maximal effective concentration (EC50) values.
Compound 1 developed as an Nrf2 activator in a previous study.24
SFN, sulforaphane: a well-known potent Nrf2 activator.
First, we replaced the sulfone moiety with sulfonamide and observed that Nrf2 activation was minimal. In our previous study, we found that the addition of the methoxy (OMe) and chloride (Cl) functional groups improved Nrf2 activation. We systematically attached a methoxy group to the 2-, 3-, and 4-positions of the A ring and a chloride group to the 2′-, 3′-, and 4′-positions of the B ring. We found that addition of a methoxy group to the 2-position of the A ring and a chloride to the 2′-position of the B ring enhanced the Nrf2 activation relative to compound 2a (2c EC50 = 6.35 μM vs 2a EC50 > 10 μM). Next, we substituted the sulfone moiety with sulfonate. Impressively, compound 3a showed Nrf2 activation in comparison to that of compound 2a (3a EC50 = 1.04 μM). We again systematically introduced the methoxy and chloride functional groups and observed Nrf2 activation. Similar to the results of a previous study, the vinyl sulfonates exhibited the most potent Nrf2 activation when 2-OMe (2-OMe > 3-OMe > 4-OMe) was added to R1 of the A ring and 2′-Cl (2′-Cl > 3′-Cl > 4′-Cl) was inserted into R2 of the B ring. The compounds 3c–3e with 2′-Cl on B ring exhibited potent Nrf2 activation compared to compound 1. In these 11 series of compounds, 3c with 2-OMe on A ring showed about 7-fold increase in Nrf2 activation than compound 1 (3c, EC50 = 0.076 μM, vs 1, EC50 = 0.530 μM). Thus, we selected 3c for further biological studies because it exhibited the most potent effect on Nrf2 activation (EC50 = 0.076 μM; Figure 1a) and favorable metabolic stabilities (Table S1 in the Supporting Information).
Figure 1.
Nrf2 translocation to the nucleus is activated by 3c. (a) Nrf2 translocation assay of engineered U2OS cells was treated with various concentrations of 3c. (b) Viability assay of BV-2 cells treated with 3c for 6 h. (c) After treatment with 3c (0.3–10 μM), Nrf2 levels were analyzed by Western blotting using whole BV-2 microglial cells (d) and nuclear extracts. All experiments were performed at least in duplicate cultures, and the data were averaged and expressed as fold induction of untreated control ± SEM. ****P < 0.001 vs untreated control (paired t test).
The cytotoxic potential of 3c was assessed, and nearly 100% cell survival was observed up to a concentration of 10 μM (98.0 ± 1.2% at 1 μM, 97.6 ± 2.4% at 3 μM, 95.9 ± 1.6% at 10 μM; Figure 1b). We then examined the effects on Nrf2 activation and expression of antioxidant enzymes in vitro. The results indicated that 3c increased Nrf2 activation in a concentration-dependent manner. Figure 1c shows that treatment with 3c significantly increased nuclear Nrf2 protein levels in a concentration-dependent manner (4.0 ± 0.01-fold at 0.3 μM, 17.6 ± 0.04-fold at 10 μM). We also confirmed that total Nrf2 protein levels increased in a concentration-dependent manner (3.3 ± 0.02-fold at 0.3 μM, 6.7 ± 0.03-fold at 10 μM; Figure 1d).
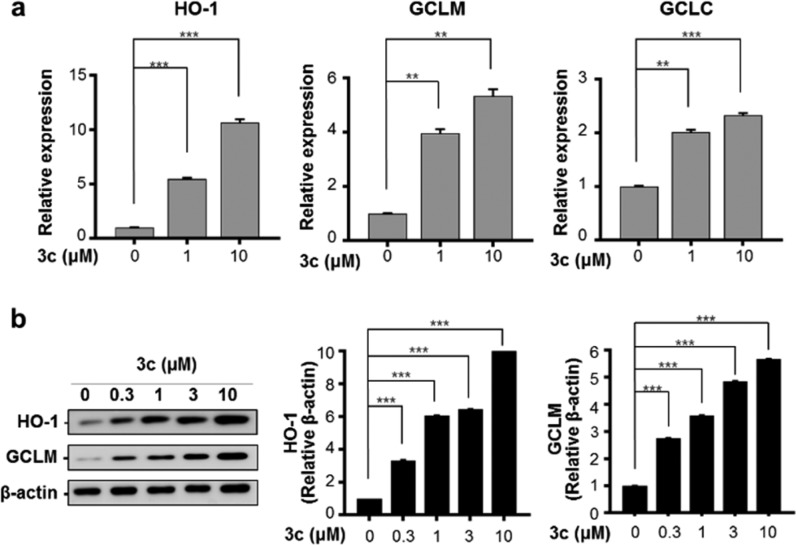
We investigated whether the expression of ARE-targeted antioxidant enzymes was induced by Nrf2 activation. The enzyme HO-1 degrades heme to carbon monoxide, iron ions, and biliverdin. HO-1 activity ultimately has important physiological functions associated with cell protection.27,28 GCL, an antioxidant enzyme in the biosynthetic pathway for major cells, consists of modulatory (GCLM) and catalytic (GCLC) subunits.29 Real-time quantitative polymerase chain reaction (RT-qPCR) analysis revealed that the mRNA levels of HO-1, GCLM, and GCLC were upregulated by treatment with 3c (10.7-, 5.3-, and 2.3-fold, respectively; Figure 2a). Western blot analysis indicated that HO-1 and GCLM protein levels were significantly increased by 10.3-fold and 5.6-fold following treatment with 3c, respectively (Figure 2b).
Figure 2.
Compound 3c induces gene expression of Nrf2-dependent antioxidant enzymes in BV-2 cells. (a) BV-2 cells were exposed to various concentrations of 3c for 3 h followed by RT-qPCR analysis of HO-1, GCLM, and GCLC expression. (b) BV-2 cells were treated with 3c for 12 h, and protein levels of HO-1, GCLM, and GCLC were measured via Western blot analysis. The expression levels of antioxidant enzymes were normalized to β-actin. All experiments were performed at least in duplicate cultures, and the data were averaged and expressed as fold induction of untreated control ± SEM. **P < 0.01, *** P < 0.005 (paired t test).
We also confirmed the antioxidant effects of 3c in SH-SY5Y cells. SH-SY5Y cells have been extensively used to investigate DAergic cell death induced by oxidative damage in PD studies.30
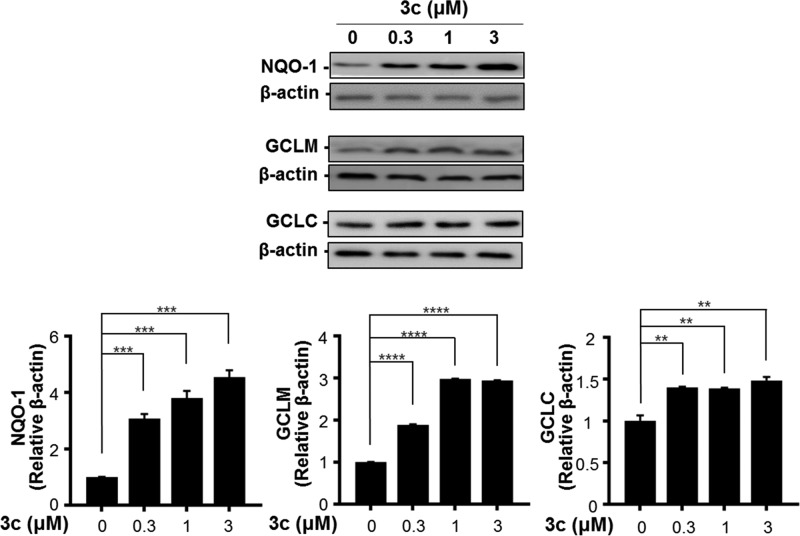
NQO1 is a crucial factor in the metabolism and toxicity of quinone and acts as a detoxification agent in mitochondrial phase 2 metabolism. Western blot analysis revealed that the protein level of NQO1 increased 4.7-fold by treating with 3c (Figure 3). As observed in BV-2 cells, GCL protein levels were also concentration-dependently increased by treatment with 3c in SH-SY5Y cells. Both GCLM and GCLC protein levels were significantly upregulated by treatment with 3c, reaching 2.9- and 1.5-fold induction, respectively. These results indicate that 3c quantitatively up-regulates the Nrf2/ARE pathway in BV-2 cells and SH-SY5Y.
Figure 3.
Compound 3c affects gene expression of Nrf2-dependent antioxidant enzymes in SH-SY5Y cells. SH-SY5Y cells were treated with 3c for 6 or 24 h and harvested for Western blot analysis. NQO1, GCLM, and GCLC protein levels were presented as relative amounts using β-actin as a loading control. All experiments were performed at least in duplicate cultures, and the data were averaged and expressed as fold induction of untreated control ± SEM. **P < 0.01, ***P < 0.005, ****P < 0.001 (paired t test).
We investigated whether 3c relieves lipopolysaccharide (LPS)-induced inflammation by the down-regulation of nitric oxide (NO) synthase, iNOS, COX-2, IL-1β, and TNF-α in microglial cells. To induce an inflammatory response, BV-2 cells were stimulated with LPS.30 As shown in Figure 4, 3c significantly attenuated LPS-induced inflammatory enzymes and cytokines in BV-2 cells. Dramatic up-regulation of iNOS and COX-2 by LPS was suppressed by 3c at protein levels (Figure 4a). The upregulation of iNOS and IL-1β induced by LPS were also significantly reduced by 3c at mRNA levels (Figure 4b). NO, which plans an important role in the pathogenesis of inflammation, was used as an inflammatory marker.31 We found the increase in NO production induced by LPS was significantly diminished by treatment with 3c by ELISA (Figure 4c). In addition, LPS-induced elevation of IL-6 and TNF-α were attenuated by 3c in a concentration-dependent manner (Figure 4d).
Figure 4.
Expression levels of inflammation-related enzymes are decreased by 3c in the concentration-dependent manner. (a–b) BV-2 cells were treated with 3c for 6 h and subsequently exposed to 1 μg/mL LPS for 12 h. Protein and mRNA levels were quantitated and presented as relative levels. Data are presented as mean ± SD (n = 3). (c–d) BV-2 cells were treated with 3c and exposed to 1 μg/mL LPS for 24 h. All experiments were performed at least in duplicate cultures, and the data were averaged and expressed as relative values compared with the LPS-stimulated control ± SEM. **P < 0.01, ***P < 0.005, ****P < 0.001 vs LPS-stimulated control (paired t test).
We investigated whether 3c improves motor deficits using the MPTP-induced mouse model for PD. We evaluated the ability of mice to restore their motor ability by treatment with 3c (20 mg/kg, p.o.) for 3 consecutive days prior and subsequent to MPTP administration (four injections of MPTP, 2 h intervals; 20 mg/kg, i.p. injection, Figure 5a).
Figure 5.
Compound 3c attenuates motor dysfunction in MPTP-treated mice. (a) Experimental schedule and behavior test diagram. Mice were administered 3c (20 mg/kg) or vehicle and treated with MPTP (20 mg/kg) four times after 24 h. (b–c) Behavioral deficit recovery of the mice was evaluated by the vertical grid and coat-hanger tests. Results are shown as mean ± SEM (n = 10–12 per group). *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001, vs the MPTP-treated group (one-way ANOVA with Tukey’s multiple comparisons test).
PD-related motor dysfunction was tested using a coat-hanger test that included a vertical grid test32 established by our group. In behavioral tests, motor deficits in the MPTP-induced PD model were prevented by treatment with 3c. Specifically, the turning time, descent time, and total time in MPTP-treated mice were restored to control levels by 3c (Figure 5b).
Similarly, coat-hanger tests also exhibited that 3c-treated mice improved abnormal movement compared to MPTP-treated mice and showed similar scores to the vehicle control (Figure 5c). MPTP-treated mice remained in their bases or moved horizontally (score: 1.9 ± 0.1), whereas mice treated with 3c (score: 3.0 ± 0.2) showed a similar score to the vehicle control (score: 3.2 ± 0.2). Therefore, all data demonstrate that 3c is highly effective in preventing PD-related movement disorders in MPTP-treated mouse models of PD (detailed information for statistical analysis is shown in the Supporting Information, Table S2).
We found that 3c demonstrated antioxidant and anti-inflammatory effects in vitro, so we then tested the neuroprotective effects on DAergic neurons in vivo using the MPTP-induced PD mouse model. The number of tyrosine hydroxylase (TH)-negative neurons was reduced in MPTP-treated mice (up to 39 ± 8.3% of the treated vehicle), but not in 3c-co-treated mice (P > 0.001 vs vehicle treatment). While the TH-positive cell density in striatum and SN was reduced after MPTP injection compared to controls, the cell density was significantly restored in 3c-treated mice (Figure 6a,b). Compound 3c protected DAergic neurons in the striatum of the PD mouse model.
Figure 6.

Compound 3c protects DAnergic neurons in the MPTP-induced mouse model. (a) TH protein staining was performed using striatal and SN tissue sections of mice treated with vehicle or 3c (collected 7 days later). (b) Density of TH-positive neurons in the striatum and the number of TH-positive neurons in the SN were analyzed. Both results are shown as mean ± SEM. ****P < 0.0001, vs the MPTP-treated group (one-way ANOVA with Tukey’s multiple comparisons test).
We used the MPTP-induced PD model to determine whether 3c can suppress neuroinflammation in vivo. An ionized calcium-binding adapter molecule 1 (Iba-1) as a microglial marker was observed in the DAergic degeneration area of the SN. Immunofluorescence intensity of Iba-1 in MPTP-injected mice was 4-fold higher than that of saline-injected mice. Nonetheless, MPTP-injected mice treated with 3c significantly decreased Iba-1 immunoreactivity. In addition, TH-positive DAergic neurons were abundantly detected only in the absence of activated microglia in the merged image (Figure 7a,b).
Figure 7.

Compound 3c inhibited microglial activation in the MPTP-induced mouse model. Double immunofluorescence staining for TH (green) and Iba-1 (Red) in the brain 10 days after MPTP injection (i.p.). (a) TH and Iba-1 immunostaining was evident on the coronal section of SN. (b) Quantitative comparison of relative Iba-1 staining intensity between wild type (n = 9), 3c-treated (n = 9), and MPTP-treated (n = 9) mice at high magnification (×4). Data are shown as mean ± SEM. ***P < 0.001, **** P < 0.0001, vs the MPTP-treated group (one-way ANOVA with Tukey’s multiple comparisons test).
In conclusion, vinyl sulfonamide and vinyl sulfonate derivatives were developed and synthesized, and their effects on Nrf2 translocation and Nrf2 activation were biologically evaluated. Among them, the new vinyl sulfonate (3c) exhibited excellent Nrf2 activation (3c, EC50 = 76 nM). We demonstrated that selected 3c induces the expression of Nrf2-dependent antioxidant enzymes and prevents the production of inflammatory mediators. In addition, 3c displayed potent in vivo therapeutic efficacy against motor dysfunction in MPTP-induced PD mice. The recovery of TH expression and anti-inflammatory responses in the SN and striatum were observed in this mouse model. In summary, the novel Nrf2 activator 3c has potential as an effective therapeutic agent for the treatment of PD.
Experimental Procedures
Detailed synthetic procedures and analytical data for all compounds and experimental methods for biological studies are described in the Supporting Information.
Acknowledgments
This study was supported by a National Research Council of Science & Technology grant by the South Korean government (MSIP, No. CRC-15-04-KIST) and the National Research Foundation of Korea (NRF-2018M3A9C8016849).
Glossary
ABBREVIATIONS
- ARE
antioxidant response element
- DAergic
dopaminergic
- GCLC
glutamate-cysteine ligase catalytic subunit
- GCLM
glutamate-cysteine ligase regulatory subunit
- HO-1
heme oxygenase
- Iba-1
ionized calcium-binding adapter molecule 1
- Keap1
Kelch-like ECH-related protein 1
- LPS
lipopolysaccharide
- MPTP
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- MS
multiple sclerosis
- NO
nitric oxide
- NQO-1
NAD(P)H:quinone oxidoreductase 1
- Nrf2
nuclear factor E2-related factor-2
- PD
Parkinson’s disease
- SFN
sulforaphane
- SN
substantia nigra
- TH
tyrosine hydroxylase
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsmedchemlett.9b00163.
Experimental methods for synthesis and biological studies; instrumental data for the synthesized compounds; 1H- and 13C-NMR spectra of the final compounds (PDF)
Author Contributions
⊥ J.W.C. and S.J.S. contributed equally to this work.
The authors declare no competing financial interest.
Supplementary Material
References
- Aarsland D.; Creese B.; Politis M.; Chaudhuri K. R.; Ffytche D.-H.; Weintraub D.; Ballard C. Cognitive decline in Parkinson disease. Nat. Rev. Neurol. 2017, 13 (4), 217–231. 10.1038/nrneurol.2017.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratwicke J.; Jahanshahi M.; Foltynie T. Parkinson’s disease dementia: a neural networks perspective. Brain 2015, 138 (6), 1454–76. 10.1093/brain/awv104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branch S. Y.; Chen C.; Sharma R.; Lechleiter J. D.; Li S.; Beckstead M. J. Dopaminergic neurons exhibit an age-dependent decline in electrophysiological parameters in the mitopark mouse model of Parkinson’s disease. J. Neurosci. 2016, 36 (14), 4026–37. 10.1523/JNEUROSCI.1395-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt Weisenhorn D. M.; Giesert F.; Wurst W. Diversity matters - heterogeneity of dopaminergic neurons in the ventral mesencephalon and its relation to Parkinson’s disease. J. Neurochem 2016, 139 (Suppl 1), 8–26. 10.1111/jnc.13670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asaithambi A.; Ay M.; Jin H.; Gosh A.; Anantharam V.; Kanthasamy A.; Kanthasamy A. G. Protein kinase D1 (PKD1) phosphorylation promotes dopaminergic neuronal survival during 6-OHDA-induced oxidative stress. PLoS One 2014, 9 (5), e96947 10.1371/journal.pone.0096947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias V.; Junn E.; Mouradian M. M. The role of oxidative stress in Parkinson’s disease. J. Parkinsons Dis 2013, 3 (4), 461–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou Y.; Peng S.; Li X.; Yao J.; Xu J.; Fang J. Honokiol alleviates oxidative stress-induced neurotoxicity via activation of Nrf2. ACS Chem. Neurosci. 2018, 9, 3108–16. 10.1021/acschemneuro.8b00290. [DOI] [PubMed] [Google Scholar]
- Sarkar S.; Raymick J.; Imam S. Neuroprotective and therapeutic strategies against Parkinson’s disease: recent perspectives. Int. J. Mol. Sci. 2016, 17 (6), 904. 10.3390/ijms17060904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amor S.; Puentes F.; Baker D.; van der Valk P. Inflammation in neurodegenerative diseases. Immunology 2010, 129 (2), 154–69. 10.1111/j.1365-2567.2009.03225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartels A. L.; Leenders K. L. Cyclooxygenase and neuroinflammation in Parkinson’s disease neurodegeneration. Curr. Neuropharmacol 2010, 8 (1), 62–8. 10.2174/157015910790909485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert T. W.; Soskolne C. L.; Bergum V.; Howell J.; Dossetor J. B. Ethical perspectives for public and environmental health: fostering autonomy and the right to know. Environ. Health Perspect. 2003, 111 (2), 133–7. 10.1289/ehp.4477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith G. E.; Rademacher D. J. MPTP mouse models of Parkinson’s disease: an update. J. Parkinsons Dis 2011, 1 (1), 19–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tebay L. E.; Robertson H.; Durant S. T.; Vitale S. R.; Penning T. M.; Dinkova-Kostova A. T.; Hayes J. D. Mechanisms of activation of the transcription factor Nrf2 by redox stressors, nutrient cues, and energy status and the pathways through which it attenuates degenerative disease. Free Radic Biol. Med. 2015, 88 (Pt B), 108–146. 10.1016/j.freeradbiomed.2015.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi E. H.; Suzuki T.; Funayama R.; Nagashima T.; Hayashi M.; Sekine H.; Tanaka N.; Moriguchi T.; Motohashi H.; Nakayama K.; Yamamoto M. Nrf2 suppresses macrophage inflammatory response by blocking proinflammatory cytokine transcription. Nat. Commun. 2016, 7, 11624. 10.1038/ncomms11624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird L.; Dinkova-Kostova A. T. The cytoprotective role of the Keap1-Nrf2 pathway. Arch. Toxicol. 2011, 85 (4), 241–72. 10.1007/s00204-011-0674-5. [DOI] [PubMed] [Google Scholar]
- Magesh S.; Chen Y.; Hu L. Small molecule modulators of Keap1-Nrf2-ARE pathway as potential preventive and therapeutic agents. Med. Res. Rev. 2012, 32 (4), 687–726. 10.1002/med.21257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Done A. J.; Traustadottir T. Nrf2 mediates redox adaptations to exercise. Redox Biol. 2016, 10, 191–199. 10.1016/j.redox.2016.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T.; Yamamoto M. Stress-sensing mechanisms and the physiological roles of the Keap1-Nrf2 system during cellular stress. J. Biol. Chem. 2017, 292 (41), 16817–16824. 10.1074/jbc.R117.800169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojo A. I.; Pajares M.; Rada P.; Nunez A.; Nevado-Holgado A. J.; Killik R.; Van Leuven F.; Ribe E.; Lovestone S.; Yamamoto M.; Cuadrado A. NRF2 deficiency replicates transcriptomic changes in Alzheimer’s patients and worsens APP and TAU pathology. Redox Biol. 2017, 13, 444–451. 10.1016/j.redox.2017.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojo A. I.; Pajares M.; Garcia-Yague A. J.; Buendia I.; Van Leuven F.; Yamamoto M.; Lopez M. G.; Cuadrado A. Deficiency in the transcription factor NRF2 worsens inflammatory parameters in a mouse model with combined tauopathy and amyloidopathy. Redox Biol. 2018, 18, 173–180. 10.1016/j.redox.2018.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burness C. B.; Deeks E. D. Dimethyl fumarate: a review of its use in patients with relapsing-remitting multiple sclerosis. CNS Drugs 2014, 28 (4), 373–87. 10.1007/s40263-014-0155-5. [DOI] [PubMed] [Google Scholar]
- Gold R.; Kappos L.; Arnold D. L.; Bar-Or A.; Giovannoni G.; Selmaj K.; Tornatore C.; Sweetser M. T.; Yang M.; Sheikh S. I.; Dawson K. T. Placebo-controlled phase 3 study of oral BG-12 for relapsing multiple sclerosis. N Engl J. Med. 2012, 367 (12), 1098–107. 10.1056/NEJMoa1114287. [DOI] [PubMed] [Google Scholar]
- Lee J. A.; Kim J. H.; Woo S. Y.; Son H. J.; Han S. H.; Jang B. K.; Choi J. W.; Kim D. J.; Park K. D.; Hwang O. A novel compound VSC2 has anti-inflammatory and antioxidant properties in microglia and in Parkinson’s disease animal model. Br. J. Pharmacol. 2015, 172 (4), 1087–1100. 10.1111/bph.12973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo S. Y.; Kim J. H.; Moon M. K.; Han S. H.; Yeon S. K.; Choi J. W.; Jang B. K.; Song H. J.; Kang Y. G.; Kim J. W.; Lee J.; Kim D. J.; Hwang O.; Park K. D. Discovery of vinyl sulfones as a novel class of neuroprotective agents toward Parkinson’s disease therapy. J. Med. Chem. 2014, 57 (4), 1473–87. 10.1021/jm401788m. [DOI] [PubMed] [Google Scholar]
- Choi J. W.; Kim S.; Park J. H.; Kim H. J.; Shin S. J.; Kim J. W.; Woo S. Y.; Lee C.; Han S. M.; Lee J.; Pae A. N.; Han G.; Park K. D. Optimization of vinyl sulfone derivatives as potent nuclear factor erythroid 2-related factor 2 (Nrf2) activators for Parkinson’s disease therapy. J. Med. Chem. 2019, 62, 811–830. 10.1021/acs.jmedchem.8b01527. [DOI] [PubMed] [Google Scholar]
- Park J. H.; Choi J. W.; Ju E. J.; Pae A. N.; Park K. D. Antioxidant and anti-Inflammatory activities of a natural compound, shizukahenriol, through Nrf2 activation. Molecules 2015, 20 (9), 15989–6003. 10.3390/molecules200915989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loboda A.; Damulewicz M.; Pyza E.; Jozkowicz A.; Dulak J. Role of Nrf2/HO-1 system in development, oxidative stress response and diseases: an evolutionarily conserved mechanism. Cell. Mol. Life Sci. 2016, 73 (17), 3221–47. 10.1007/s00018-016-2223-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H.; Wood J. T.; Whitten K. M.; Vadivel S. K.; Seng S.; Makriyannis A.; Avraham H. K. Inhibition of fatty acid amide hydrolase activates Nrf2 signalling and induces heme oxygenase 1 transcription in breast cancer cells. Br. J. Pharmacol. 2013, 170 (3), 489–505. 10.1111/bph.12111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi S.; Hisatsune A.; Kurauchi Y.; Seki T.; Katsuki H. Insulin-like growth factor 1 specifically up-regulates expression of modifier subunit of glutamate-cysteine ligase and enhances glutathione synthesis in SH-SY5Y cells. Eur. J. Pharmacol. 2016, 771, 99–106. 10.1016/j.ejphar.2015.12.013. [DOI] [PubMed] [Google Scholar]
- Kim S. S.; Lim J.; Bang Y.; Gal J.; Lee S. U.; Cho Y. C.; Yoon G.; Kang B. Y.; Cheon S. H.; Choi H. J. Licochalcone E activates Nrf2/antioxidant response element signaling pathway in both neuronal and microglial cells: therapeutic relevance to neurodegenerative disease. J. Nutr. Biochem. 2012, 23 (10), 1314–23. 10.1016/j.jnutbio.2011.07.012. [DOI] [PubMed] [Google Scholar]
- Sharma J. N.; Al-Omran A.; Parvathy S. S. Role of nitric oxide in inflammatory diseases. Inflammopharmacology 2007, 15 (6), 252–259. 10.1007/s10787-007-0013-x. [DOI] [PubMed] [Google Scholar]
- Kim S. T.; Son H. J.; Choi J. H.; Ji I. J.; Hwang O. Vertical grid test and modified horizontal grid test are sensitive methods for evaluating motor dysfunctions in the MPTP mouse model of Parkinson’s disease. Brain Res. 2010, 1306, 176–83. 10.1016/j.brainres.2009.09.103. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.