Abstract

Despite the perceived stability of the C–F bond, chemical instability and drug-metabolizing enzymes can lead to its cleavage. The resulting release of fluoride and formation of certain metabolites may cause safety issues and warrant the medicinal chemists’ attention.
Keywords: Fluorine, defluorination, reactive metabolite, CYP TDI
Fluorine is an indispensable tool in the medicinal chemists’ toolbox. Due to its small size and strong electron-withdrawing property, fluorine is widely used in medicinal chemistry to improve a molecule’s potency and permeability, modulate its pKa and lipophilicity, and control its conformation. Of the 38 small molecule drugs that were approved by the FDA in 2018, 18 contain fluorine. As the C–F bond-dissociation energy (BDE) is very high (typically 109 kcal/mol or above), fluorine is often used by medicinal chemists to block a metabolic soft spot, which can reduce a molecule’s metabolic clearance and/or prevent the formation of reactive metabolites. However, such high BDE measures the homolytic cleavage of the C–F bond, and dissociation of fluorine from carbon is typically heterolytic under physiological conditions. In the presence of a nucleophile or drug-metabolizing enzymes, the release of fluoride can be facile, which is often observed in the development of 18F-labeled positron emission tomography (PET) tracers, as the fate of the radioactive fluoride can be more easily tracked. For a drug suffering from significant C–F bond cleavage, fluoride’s strong affinity for bones may lead to safety issues such as skeletal fluorosis. For example, there is strong clinical evidence that long-term use of the antifungal voriconazole can increase plasma fluoride levels and lead to painful periostitis/exostoses in patients.1 In addition to fluoride, other toxic metabolites may also form following C–F bond cleavage, which can cause drug safety concerns. In this Viewpoint, some examples involving the decomposition and metabolism of fluorinated compounds are highlighted to draw the medicinal chemists’ attention to such issues. In many cases, a combined understanding of physical organic chemistry and drug metabolism can help the medicinal chemist predict chemical instability of the parent molecule and/or structures of potential metabolites, prioritize assays to test such liabilities, and design compounds to address these issues.
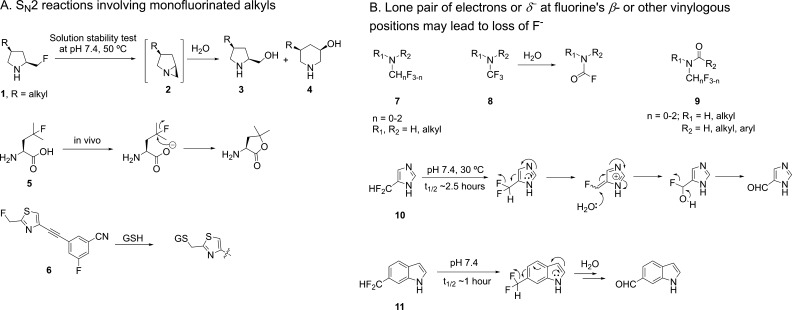
A drug substance’s stability is of paramount importance as it has a direct impact on the drug’s purity, efficacy, and toxicity. A monofluoroalkyl group in the presence of an intramolecular nucleophile may come with intrinsic instability as the Calkyl–F bond can be cleaved in SN2 reactions. Such potential problems can often be predicted with organic chemistry arrow-pushing. In a Novartis project, the stability of several compounds containing 2-(fluoromethyl)pyrrolidine, as represented by 1 (Figure 1A), was assessed in stress tests. While the solid material’s stability at elevated temperature was acceptable, in solution 60–90% decomposition occurred at pH 7.4 and 50 °C after 7 days. This issue led to the deprioritization of these compounds, as the final candidate would require a solution formulation for intravenous dosing. In contrast, structurally similar compounds without the fluoromethyl group showed less than 2% decomposition under the same conditions. LC-MS suggested the fluorine was displaced by a hydroxyl group to form the SN2-like product 3 and the ring-expanded product 4, implicating aziridine 2 as the intermediate. Another example illustrating the instability of the monofluoroalkyl group with an intramolecular nucleophile is 4-fluoroleucine (5), the carboxylate of which can displace the tertiary fluorine.2 Extending the distance between the carboxylate and the fluorine by one carbon greatly reduced defluorination, as the six-membered ring lactone was much slower to form. These two examples demonstrate that for monofluorinated alkyl carbons, a neighboring nucleophile may be a concern, and the chemical stability needs to be assessed.
Figure 1.
Chemical instability of several fluorine-containing structures.
Besides displacement by an intramolecular nucleophile, an activated fluorine may also be susceptible to biological nucleophiles such as glutathione (GSH). For example, compound 6’s half-life in rat brain homogenate is only 20 min due to the displacement of fluorine by GSH.3 Considering this reactivity, such compounds may alkylate biological targets and lead to toxicity, especially if the fluorine is positioned close to a cysteine’s thiol when the drug molecule is bound to an off-target protein. Therefore, compounds with an electronically activated fluorine, such as those at benzylic, allylic, and a carbonyl’s α positions, may warrant activity-based proteomics to assess their reactivity. Certainly, besides the electronic factor, steric hindrance around the fluorine also has an impact on such moieties’ reactivity toward nucleophiles, and the safety risk can be further reduced if the dose is low. Chemical instability resulting from direct nucleophilic displacement of fluorine as mentioned above is probably limited to monofluorinated alkyl groups, as gem-difluoroalkyl and trifluoromethyl groups have much reduced reactivity in SN2 reactions due to each additional fluorine’s destabilizing effect on the partially positive carbon in the transition state.
In addition to nucleophilic displacement of fluorine, defluorination can also result from a lone pair of electrons or δ– at the fluorine’s β-position. For example, β-fluoro carbonyl compounds with an acidic α-proton are often unstable and eliminate HF. Fluoromethylamines (7, Figure 1B), including trifluoromethylamine 8, are also prone to decomposition due to the lone pair on the nitrogen.4 Such amines’ chemical stability can be improved by masking the amine as an amide (9) since the electron density on the nitrogen is greatly reduced. However, the improvement might not be sufficient to meet the standard of a marketed drug. Therefore, early stability tests are recommended to derisk such compounds.
Sometimes the δ– causing fluoride loss and compound decomposition is more distal to the fluorine. For instance, 5-difluoromethylimidazole (10)5 and 6-difluoromethylindole (11)6 suffer from defluorination in aqueous buffer solutions at physiological pH. The decomposition is fast and raises the concern for any compounds containing such substructures. Interestingly the solution stability of 6-substituted 1H-indole increases in the order of CH2F < CHF2 ≪ CF3.6 Loss of fluoride is unlikely limited to the substitution patterns shown in Figure 1B, as proper arrow-pushing shows that a fluoromethyl or difluoromethyl group at other positions of 1H-imidazole and 1H-indole may also be subject to defluorination. Furthermore, there may be other CH2F- and CF2H-substituted heteroaryls that suffer from such stability issues. Overall, the examples above demonstrate that neighboring group participation and lone pair/δ– at the fluorine’s β position (or other vinylogous positions) can facilitate the heterolytic cleavage of the Calkyl–F bond.
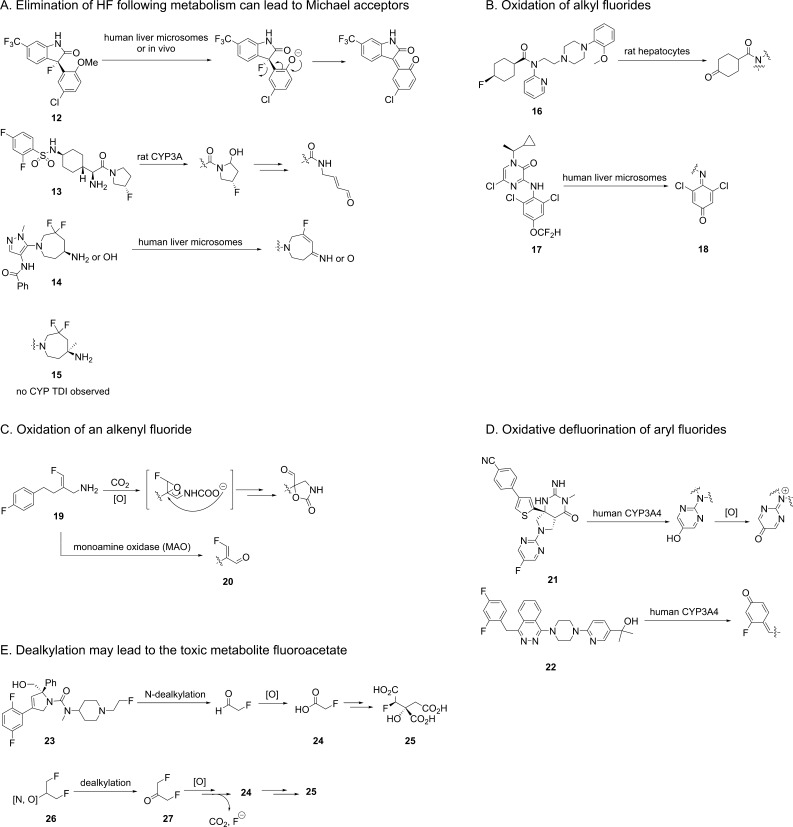
Besides chemical instability, metabolism can also liberate fluoride from fluorine-containing compounds through various mechanisms. Thus, when designing fluorinated compounds, one should also consider the sites of metabolism and the potential instability of fluorinated metabolites. For certain alkyl fluorides, metabolism can generate a nearby acidic group, which upon deprotonation may eliminate fluoride and form a Michael acceptor. Historically, the rational design of mechanism-based inactivators of pyridoxal phosphate (PLP)-dependent enzymes, such as γ-aminobutyric acid (GABA) aminotransferase and ornithine decarboxylase, has taken advantage of such in situ generated Michael acceptors from alkyl fluorides. Three such examples are illustrated (Figure 2A) in which O-demethylation (12),7N-dealkylation (13),8 and amine/alcohol oxidation (14)9 generated Michael acceptors. In all three cases, formation of GSH adducts and/or cytochrome P450 (CYP) time-dependent inhibition (TDI) were observed. With a good mechanistic understanding, such issues can be successfully addressed. For example, the addition of a methyl group in 15 effectively prevented the amine’s oxidation and formation of the Michael acceptor.
Figure 2.
Metabolism of fluorinated compounds that produces fluoride and other toxic metabolites.
In addition to the elimination of HF, CYP-mediated hydroxylation at fluorinated aliphatic carbons can also release fluoride and produce toxic metabolites. Although fluorine’s strong σ inductive effect destabilizes the α-radical, its π conjugation can compensate, and mono- and difluoromethyl radicals are in fact more stable than the nonfluorinated methyl radical.10 Therefore, CYP-mediated hydroxylation can preferably happen at fluorinated alkyl carbon atoms. For example, significant defluorination of fluorocyclohexane 16 (Figure 2B) was observed both in rat hepatocytes and in vivo, which was likely mediated by CYPs.11 For the CRF-R1 inhibitor 17, a minor metabolic pathway in human liver microsomes is hydroxylation at the difluoromethyl carbon.12 This process generated the reactive quinone imine 18, although the fate of the apparently cleaved CF2H group was not reported.
Fluorinated alkenes are sometimes applied in medicinal chemistry, especially as bioisosteres of amides. Such moieties are not immune to CYP-mediated metabolism. An example is 19, which in dogs and humans underwent epoxidation of the fluorinated double bond, followed by epoxide opening and loss of fluoride (Figure 2C).13 Similar to 14, another potential liability of fluorinated alkenes with an allylic amine is oxidation of the amine to the imine/aldehyde, which in this case formed the activated Michael acceptor 20.
Besides alkyl and alkenyl fluorides, aryl fluorides may also undergo oxidative defluorination by various enzymes to generate phenol-like metabolites and release fluoride. Therefore, sometimes simply blocking a metabolic soft spot on an aromatic ring with fluorine may not be effective, and the hydroxyaryl can still form, which may lead further to reactive metabolites such as quinone, quinone imine, and quinone methide. Two such examples (21, 22) demonstrate this oxidative defluorination process, resulting in CYP TDI/GSH adducts (Figure 2D).14,15
Besides the aforementioned toxicities resulting from released fluoride and reactive metabolites, molecules bearing N- or O-2-fluoroethyl or 1,3-difluoro-2-propyl substituents also pose great concerns. Specifically, such fluorine-containing toxicophores can release fluoroacetate (24), which forms (−)-erythro-2-fluorocitrate (25), a highly potent inhibitor of aconitase (EC 4.2.1.3) that interrupts the tricarboxylic acid cycle (Figure 2E). Fluoroacetate has been used as a rodent poison and the human oral LD50 is 2–10 mg/kg, close to NaCN. In the case of 23, the metabolism to fluoroacetate killed rodents receiving an intravenous bolus dose of 12 mg/kg.16 For compounds containing the substructure 26, dealkylation may lead to 27, which is further metabolized to 24 and 25.17 Therefore, for molecules that could generate such metabolites, early metabolite identification is essential.
With the discovery of more innovative fluorination reagents and methods, the medicinal chemists are now equipped with powerful tools to install fluorine at many positions in a molecule to tackle various issues encountered in a drug discovery project. However, inappropriate placement of fluorine can cause stability and toxicity issues. This Viewpoint is certainly not meant to sound the alarm on all fluorinated compounds, and it is a fact that many fluorinated drugs have excellent stability and safety. The purpose of collecting these examples is to raise the awareness of common drug instability and metabolism issues leading to defluorination, as well as the resulting reactive/toxic metabolites. Clearly fluorine is not a “set and forget” solution, and follow-up studies are often warranted. As key members of a drug discovery project team, medicinal chemists need to recognize the potential liability of certain fluorine-containing substructures and initiate early profiling, before any problematic moieties become an essential part of a lead series. In addition, when CYP TDI or other toxicity is observed, recognizing such structural alerts may enable the medicinal chemist to quickly develop and test certain hypotheses, which could save a significant amount of time and cost.
Acknowledgments
The author thanks Professor Richard B. Silverman of Northwestern University for teaching the author how labile fluorine can be. The author is also grateful to Professor Ryan Altman at the University of Kansas and Dr. Stevan Djuric at AbbVie for offering their suggestions.
Views expressed in this Viewpoint are those of the author and not necessarily the views of the ACS.
The author declares no competing financial interest.
References
- Wermers R. A.; Cooper K.; Razonable R. R.; Deziel P. J.; Whitford G. M.; Kremers W. K.; Moyer T. P. Fluoride excess and periostitis in transplant patients receiving long-term voriconazole therapy. Clin. Infect. Dis. 2011, 52, 604–611. 10.1093/cid/ciq188. [DOI] [PubMed] [Google Scholar]
- Nodwell M. B.; Yang H.; Čolović M.; Yuan Z.; Merkens H.; Martin R. E.; Bénard F.; Schaffer P.; Britton R. 18F-fluorination of unactivated C–H bonds in branched aliphatic amino acids: direct synthesis of oncological positron emission tomography imaging agents. J. Am. Chem. Soc. 2017, 139, 3595–3598. 10.1021/jacs.6b11533. [DOI] [PubMed] [Google Scholar]
- Shetty H. U.; Zoghbi S. S.; Simeon F. G.; Liow J.-S.; Brown A. K.; Kannan P.; Innis R. B.; Pike V. W. Radiodefluorination of 3-fluoro-5-(2-(2-[18F](fluoromethyl)-thiazol-4-yl)ethynyl)benzonitrile ([18F]SP203), a radioligand for imaging brain metabotropic glutamate subtype-5 receptors with positron emission tomography, occurs by glutathionylation in rat brain. J. Pharmacol. Exp. Ther. 2008, 327, 727–735. 10.1124/jpet.108.143347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawelke G. Reaction of tetrakis(dimethylamino)ethylene with CF2Br2 in the presence of secondary amines, formation of N-trifluoromethyldialkylamines. J. Fluorine Chem. 1991, 52, 229–234. 10.1016/S0022-1139(00)80138-4. [DOI] [Google Scholar]
- Tuan E.; Kirk K. L. Fluorine reactivity in difluoromethylimidazoles. J. Fluorine Chem. 2006, 127, 980–982. 10.1016/j.jfluchem.2006.03.014. [DOI] [Google Scholar]
- Woolridge E. M.; Rokita S. E. Synthesis and reactivity of 6-(fluoromethyl)indole and 6-(difluoromethyl)indole. Tetrahedron Lett. 1989, 30, 6117–6120. 10.1016/S0040-4039(01)93319-2. [DOI] [Google Scholar]
- Zhang D.; Krishna R.; Wang L.; Zeng J.; Mitroka J.; Dai R.; Narasimhan N.; Reeves R. A.; Srinivas N. R.; Klunk L. J. Metabolism, pharmacokinetics, and protein covalent binding of radiolabeled Maxipost (BMS-204352) in humans. Drug Metab. Dispos. 2005, 33, 83–93. 10.1124/dmd.104.001412. [DOI] [PubMed] [Google Scholar]
- Xu S.; Zhu B.; Teffera Y.; Pan D. E.; Caldwell C. G.; Doss G.; Stearns R. A.; Evans D. C.; Beconi M. G. Metabolic activation of fluoropyrrolidine dipeptidyl peptidase-IV inhibitors by rat liver microsomes. Drug Metab. Dispos. 2005, 33, 121–130. 10.1124/dmd.104.001842. [DOI] [PubMed] [Google Scholar]
- Wang X.; Sun M.; New C.; Nam S.; Blackaby W. P.; Hodges A. J.; Nash D.; Matteucci M.; Lyssikatos J. P.; Fan P. W.; Tay S.; Chang J. H. Probing mechanisms of CYP3A time-dependent inhibition using a truncated model system. ACS Med. Chem. Lett. 2015, 6, 925–929. 10.1021/acsmedchemlett.5b00191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X.-K.; Li X.-Y.; Wang K.-Y. Reversal of the nature of substituent effect by changing the number of the α-substituent. Relative ease of formation of the three α-fluoromethyl radicals. J. Org. Chem. 1989, 54, 5648–5650. 10.1021/jo00285a003. [DOI] [Google Scholar]
- Magata Y.; Lang L.; Kiesewetter D. O.; Jagoda E. M.; Channing M. A.; Eckelman W. C. Biologically stable [18F]-labeled benzylfluoride derivatives. Nucl. Med. Biol. 2000, 27, 163–168. 10.1016/S0969-8051(99)00108-0. [DOI] [PubMed] [Google Scholar]
- Zhuo X.; Hartz R. A.; Bronson J. J.; Wong H.; Ahuja V. T.; Vrudhula V. M.; Leet J. E.; Huang S.; Macor J. E.; Shu Y.-Z. Comparative biotransformation of pyrazinone-containing corticotropin-releasing factor receptor-1 antagonists: minimizing the reactive metabolite formation. Drug Metab. Dispos. 2010, 38, 5–15. 10.1124/dmd.109.028910. [DOI] [PubMed] [Google Scholar]
- Dow J.; Piriou F.; Wolf E.; Dulery B. D.; Haegele K. D. Novel carbamate metabolites of mofegiline, a primary amine monoamine oxidase B inhibitor, in dogs and humans. Drug Metab. Dispos. 1994, 22, 738–749. [PubMed] [Google Scholar]
- Mandal M.; Mitra K.; Grotz D.; Lin X.; Palamanda J.; Kumari P.; Buevich A.; Caldwell J. P.; Chen X.; Cox K.; Favreau L.; Hyde L.; Kennedy M. E.; Kuvelkar R.; Liu X.; Mazzola R. D.; Parker E.; Rindgen D.; Sherer E.; Wang H.; Zhu Z.; Stamford A. W.; Cumming J. N. Overcoming time-dependent inhibition (TDI) of cytochrome P450 3A4 (CYP3A4) resulting from bioactivation of a fluoropyrimidine moiety. J. Med. Chem. 2018, 61, 10700–10708. 10.1021/acs.jmedchem.8b01326. [DOI] [PubMed] [Google Scholar]
- Gunduz M.; Argikar U. A.; Kamel A.; Colizza K.; Bushee J. L.; Cirello A.; Lombardo F.; Harriman S. Oxidative ipso substitution of 2,4-difluoro-benzylphthalazines: identification of a rare stable quinone methide and subsequent GSH conjugate. Drug Metab. Dispos. 2012, 40, 2074–2080. 10.1124/dmd.112.046268. [DOI] [PubMed] [Google Scholar]
- Cox C. D.; Coleman P. J.; Breslin M. J.; Whitman D. B.; Garbaccio R. M.; Fraley M. E.; Buser C. A.; Walsh E. S.; Hamilton K.; Schaber M. D.; Lobell R. B.; Tao W.; Davide J. P.; Diehl R. E.; Abrams M. T.; South V. J.; Huber H. E.; Torrent M.; Prueksaritanont T.; Li C.; Slaughter D. E.; Mahan E.; Fernandez-Metzler C.; Yan Y.; Kuo L. C.; Kohl N. E.; Hartman G. D. Kinesin spindle protein (KSP) inhibitors. 9. Discovery of (2S)-4-(2,5-difluorophenyl)-N-[(3R,4S)-3-fluoro-1-methylpiperidin-4-yl]-2-(hydroxymethyl)-N-methyl-2-phenyl-2,5-dihydro-1H-pyrrole-1-carboxamide (MK-0731) for the treatment of taxane-refractory cancer. J. Med. Chem. 2008, 51, 4239–4252. 10.1021/jm800386y. [DOI] [PubMed] [Google Scholar]
- Menon K. I.; Feldwick M. G.; Noakes P. S.; Mead R. J. The mode of toxic action of the pesticide gliftor: the metabolism of 1,3-difluoroacetone to (−)-erythro-fluorocitrate. J. Biochem. Mol. Toxicol. 2001, 15, 47–54. . [DOI] [PubMed] [Google Scholar]