Abstract
Calcium (Ca2+) signaling and the modulation of intracellular calcium ([Ca2+]i) levels play critical roles in several key processes that regulate cellular survival, growth, differentiation, metabolism, and death in normal cells. On the other hand, aberrant Ca2+-signaling and loss of [Ca2+]i homeostasis contributes to tumor initiation proliferation, angiogenesis, and other key processes that support tumor progression in several different cancers. Currently, chemically and functionally distinct drugs are used as chemotherapeutic agents in the treatment and management of cancer among which certain anti-cancer drugs reportedly suppress pro-survival signals and activate pro-apoptotic signaling through modulation of Ca2+-signaling-dependent mechanisms. Most importantly, the modulation of [Ca2+]i levels via the endoplasmic reticulum-mitochondrial axis and corresponding action of channels and pumps within the plasma membrane play an important role in the survival and death of cancer cells. The endoplasmic reticulum-mitochondrial axis is of prime importance when considering Ca2+-signaling-dependent anti-cancer drug targets. This review discusses how calcium signaling is targeted by anti-cancer drugs and highlights the role of calcium signaling in epigenetic modification and the Warburg effect in tumorigenesis.
Keywords: Intracellular calcium, anti-cancer drugs, apoptosis, proliferation
1. Intracellular Calcium Homeostasis and Calcium Signaling
Intracellular calcium ([Ca2+]i) is an important second messenger involved in cellular functions of muscles, neurons, immune cells, oocytes and others, modulating enzyme secretion, gene activation, proliferation, apoptosis, cell cycle progression, fertilization, and release of neurotransmitters [1,2,3]. Aberrant [Ca2+]i-signaling has been implicated in diseases such as Alzheimer’s, cancer, congestive heart failure, and diabetes [4,5,6]. In cancer, [Ca2+]i-signaling is involved in various processes of tumorigenesis such as proliferation, migration, angiogenesis, and evasion of apoptosis [7,8].
The concentration of Ca2+ in different compartments of a cell differs greatly ([Ca2+]i: 100 nM; extracellular calcium [Ca2+]o: 1–1.5mM; in the endoplasmic reticulum (ER): 0.5–1 mM; in the mitochondria: 100–200 nM (but can accumulate 10–20 fold upon activation) [9]. [Ca2+]i is tightly maintained at a low concentration by numerous regulating mechanisms, that include both active and passive mechanisms, e.g., channels or pumps on the plasma membrane, ER, mitochondria, and cytosol (Figure 1) [10,11,12,13]. In addition to channels and pumps, [Ca2+]i is modulated by a heterogeneous group of calcium binding proteins (CBP), including S100 proteins, calmodulin, and calcineurin. Binding of Ca2+ with CBP regulates signal transduction and gene expression [14]. Undoubtedly, the nucleus acts as a major compartment in which Ca2+ is sequestered. Nuclear Ca2+ has specific biological functions involved in gene expression and regulation [15]. The involvement of the nucleus in [Ca2+]i-regulation and its role in cell proliferation is extensively reviewed by Resende and colleagues [16]. Importantly, some studies proposed nuclear calcium signaling as an independent entity producing localized calcium-signaling and triggering the transcription of genes related to cell proliferation [17]. On the contrary, it is reported that nuclear [Ca2+] is dependent on changes in [Ca2+]i [18,19].
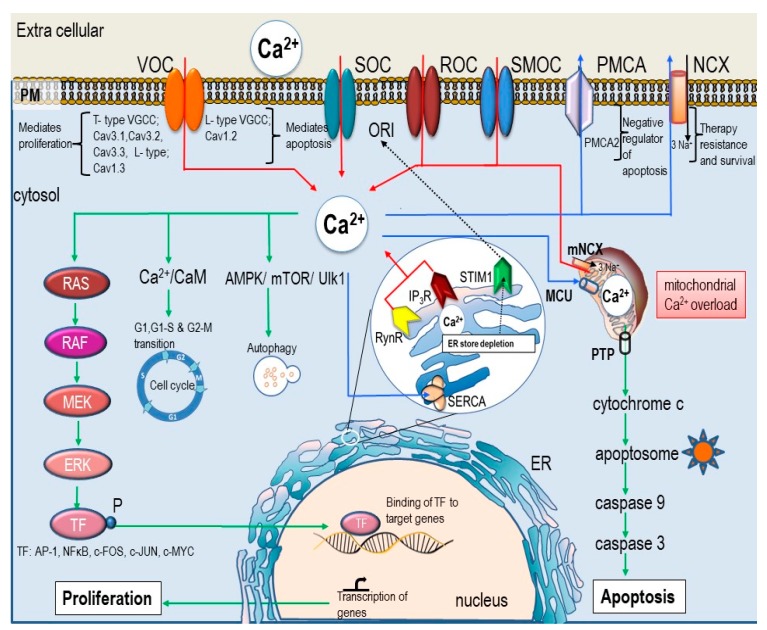
Figure 1.
Ca2+-signaling in proliferation and apoptosis. [Ca2+]i homeostasis of the cell is strictly regulated by various channels and pumps on plasma membrane, ER, and mitochondria. Intracellular calcium flux is indicated by arrows where red arrows indicate influx of Ca2+ ions, blue arrows indicate Ca2+ efflux and sequestration of Ca2+ into the stores, and green arrows indicate cell signaling pathways which are activated by [Ca2+]i. Primarily Ca2+ influx from the extra-cellular space is controlled by VOC (voltage operated calcium channel), SOC (store-operated Orai channels), SMOC (second messenger-operated channel), or ROC (receptor-operated channel), and Ca2+ efflux is mediated via (NCX (Na+/Ca2+ exchanger) and PMCA (plasma membrane Ca2+-ATPase). Ca2+ release from the ER stores is facilitated via RyR (ryanodine receptors) and IP3R (IP3 receptor). Uptake of calcium from the cytosol is an energy driven process mediated by SERCA (sarcoplasmic/ER Ca2+-ATPase). Additionally, mitochondria and associated proteins (including mitochondrial calcium uniporter (MCU), voltage dependent anion channel (VDAC), and mitochondrial Na+/Ca2+ exchanger (mNCX) are also related to [Ca2+]i-regulation. Mitochondrial-Ca2+ controls ATP synthesis, apoptosis, ROS generation, and biosynthesis and can determine the fate of the cell. The concentration of Ca2+ in the cytosol is maintained at a low level (100 nM) in comparison to the extra cellular Ca2+ (1mM). Any change in the [Ca2+]i results in signal transduction initiating various cellular process such as apoptosis, proliferation, and cell division. The type of signal depends on the duration, amplitude, localization, frequency, and oscillation. Sustained high level of Ca2+ in the mitochondria causes the release of cytochrome c and subsequently triggers death signals via caspase activation. More Ca2+ influx from the ion channels on the plasma membrane can trigger either proliferation (via T-type) or apoptosis (via L-type). Increased Ca2+ entry through the SOC channel promotes proliferation [32].
[Ca2+]i-signaling is initiated by the entry of Ca2+ from an extracellular pool or by releasing Ca2+ from ER stores or mitochondria. This increases [Ca2+]i from 100 nM (at rest) to approximately 1000 nM generating an “ON” signal for multiple processes. As a prolonged increase in [Ca2+]i may be harmful, the [Ca2+]i signals are spatially and temporally regulated [7]. Calcium binding proteins (Ca2+/calmodulin-dependent protein kinase II (CAMKII) and protein kinase C) decode the Ca2+ signals to various cellular processes [20,21]. With the completion of the cellular responses, an “OFF” mechanism restores the low concentration of [Ca2+]i. [Ca2+]i-signaling is involved in both proliferation and apoptosis. Ca2+-oscillations stimulate cell proliferation via Ca2+ sensitive transcription factor (NFAT) and conversely, an increase in [Ca2+]i for a longer duration activates apoptosis [22].
Abnormalities in [Ca2+]i-signaling are associated with various cancers and is also implicated in therapy resistance [23,24,25]. An extensive review by Cui et al. broadly outlines calcium regulating proteins altered in specific cancer types and enlist those compounds targeting calcium-signaling [7]. In this review we analyze the anti-cancer action of selected agents targeting the calcium dependent pathways regulating proliferation and apoptosis. Here, we emphasize the role of calcium-signaling in proliferation and apoptosis and in addition, highlight calcium dependent modification of tumor energy metabolism and epigenetic modification of genes by anti-cancer agents.
2. [Ca2+]i -Signaling in Cell Proliferation and Apoptosis
[Ca2+]i is a versatile second messenger in both proliferation and cell death. [Ca2+]i-signaling involves the participation of various proteins combined differently depending upon the type of cellular process initiated (Figure 1). [Ca2+]i-signaling is spatially and temporally distinct for proliferation or apoptosis [26]. Transition of a normal cell to malignant cell involves altered function, translation, and expression of various proteins involved in the calcium regulation and signaling. Therefore, aberrant regulation of [Ca2+]i levels may lead to uncontrolled proliferation and inhibition of apoptosis and thus contribute to carcinogenesis [27].
2.1. [Ca2+]i -Signaling and Cell Proliferation
[Ca2+]i-signaling mediated by the channels on the plasma membrane and by exchange of Ca2+ between the spatially and temporally separated ER and mitochondria determines the type of down-stream signaling which will be activated. The following section focuses on the association between proliferation and extracellular calcium and the influence of Ca2+-channels on proliferation. We will also discuss store-operated calcium entry, the sarco/endoplasmic reticulum calcium ATPase (SERCA), and the ER and mitochondrial axis in proliferation.
2.2. [Ca2+]o in Cell Proliferation
Extracellular calcium ([Ca2+]o) modulates various cellular processes via calcium channels and extracellular calcium-sensing G-protein coupled receptors, which include calcium-sensing receptor (CaSR) and GPRC6a [21]. Past studies describe [Ca2+]o as a key regulator of proliferation in chicken fibroblast [28]. A significant difference in the proliferation rate of normal vs. transformed chicken fibroblast is associated with changes of [Ca2+]o. Similar observations were made in mouse 3T3 cells, with cell proliferation being dependent on [Ca2+]o, while a calcium driven mechanism initiated DNA synthesis and cell cycle progression that ultimately resulted in cell division [29,30]. Moreover, the influence of [Ca2+]o and its role in proliferation is reviewed in detail by Borowiec [30], emphasizing that [Ca2+]o potentially exerts biological actions via sensor proteins on the plasma membrane. CaSR senses [Ca2+]o and thus triggers the influx of Ca2+ through specific channels and regulates Ca2+ absorption and homeostasis in various organs. A reduction of Ca2+ influx by blocking calcium channels at the plasma membrane (PM) or reduction of [Ca2+]o attenuates cell proliferation [31].
2.3. Role of Ca2+ Channels and Pumps in Proliferation
Ion channels are characterized as protein microchannels regulating intracellular concentrations of ions, contributing to the signaling pathways and influencing the overall behavior of cells [33]. Calcium selective channels are abundantly expressed on the plasma membrane which regulates Ca2+-influx. Voltage-gated calcium channels (VGCCs) (also known as voltage operated channels (VOC) (see Figure 1 and Figure 2) sense the depolarization of membrane potentials and open their calcium-selective channel pore, allowing Ca2+ entry into the cell, thus contributing to physiological processes [33,34]. Based on their electrophysiological properties, VGCCs are classified into 5 groups: L-type (Cav1), T-type (Cav3), N-type (Cav2), P-type (Cav2), and R-type (Cav2). In non-excitable cells, VGCCs [33] are associated with the regulation of cell proliferation and apoptosis [35]. Aberrant function of VGCCs is related to the progression of different malignancies. Phan et al., in their review, discuss altered VGCC family genes and its link to 19 different cancer types including brain, kidney, breast, and lung cancers [34]. It highlights the downregulation of specific VGCC family-genes in different cancers and emphasizes their role as tumor suppressor genes. Among the different VGCCs, L-type channels show a strong association with proliferation and migration of cancer cells. Moreover, estrogen can modulate the expression of ion channels. A higher expression of L-type channels (Cav1.3) correlates with an increase in 17β-estradiol level in endometrial cancer (EC). The mechanism behind Ca2+-induced cell proliferation involves the interaction of estrogen binding to G-protein coupled estrogen receptor (GPER) causing the L-type channel to open and allow Ca2+-entry, followed by downstream activation of the ERK1/2-CREB pathway promoting cell proliferation. L-type channels are also involved in migration of EC cells [35,36]. Additionally, a high level of estrogen is associated with increased breast cancer (BC) risk. The mechanism of estrogen-BC risk is linked to a pathway similar to EC proliferation. Importantly, estrogen induced a dose-dependent increase in the expression of Cav1.3 in MCF-7 cells. The silencing of G protein-coupled estrogen receptor 30 (GPER1/GPR30) mediated via siRNA transfection abolished Ca2+-influx as well as proliferation in BC, which may be an indication of a crucial role of Ca2+ entry through L-type channels in cell proliferation and breast cancer progression [29]. Furthermore, overexpression of the Calcium voltage-gated channel subunit alpha1 D gene (CACNA1D) is associated with prostate cancer progression. Importantly, [Ca2+]i measurements showed that an androgen stimulated Ca2+ influx was sensitive to nifedipine, indicating that L-type calcium channels were responsible for the androgen-stimulated Ca2+-influx in LNCaP cells. Interestingly, blocking of L-type channels resulted in decreased cell growth [37].
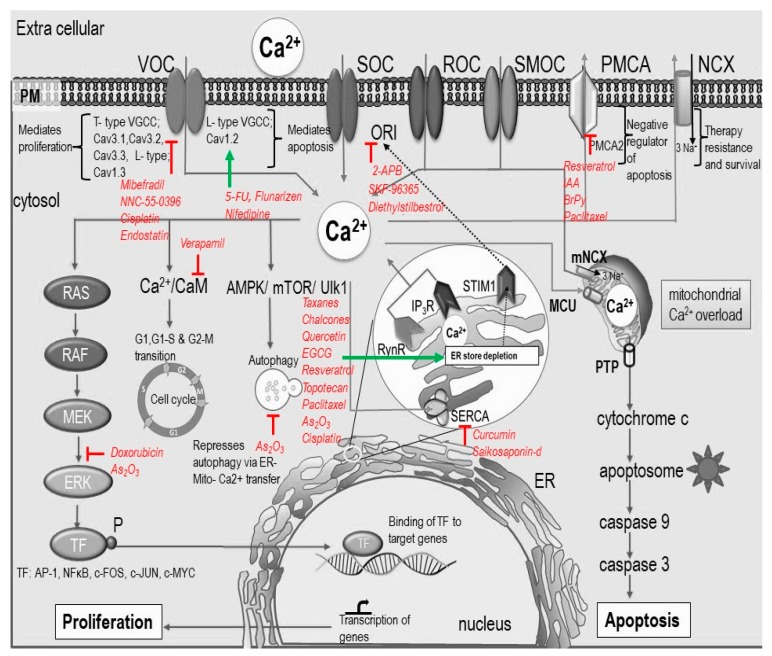
Figure 2.
Intra-cellular calcium response to anti-cancer agents. This schematic representation shows the effect of anti-cancer agents on calcium signaling and its downstream effect on apoptosis and proliferation. Anti-cancer drugs can target different calcium channels and pumps on the plasma membrane, ER, and mitochondria. ER-mitochondrial Ca2+ transfer plays an important role in apoptosis and many anti-cancer drugs target ER to induce apoptosis. Anti-cancer drugs can also be anti-proliferative (e.g., Doxorubicin) by targeting Ca2+-signaling that regulate various proliferation pathways and cell cycle progression. Green arrows represent activation and red lines represent a block.
Similarly, T-type channels are important in various physiological processes like secretion of transmitters and hormones [38]. At the cellular level, these channels regulate cell cycle progression, proliferation, and survival [39,40]. Studies show varying expression of T-type channels in cell cycle phases. A maximum expression (90% increase) of T-type channels is evident in the S-phase and at the beginning of M-phase of the cell cycle. Moreover, increased expression of T-type channels is observed in cancer of breast, bladder, lung, and liver [40]. Knockdown experiments of these channels in MCF-7 breast cancer cells resulted in two different outcomes: The silencing of Cav3.1 enhanced proliferation, and the silencing of Cav3.2 decreased proliferation [41]. As a result, Cav3.1 gene appears to have a tumor suppressor role while Cav3.2 possesses pro survival activity [41]. VGCCs are thus highly expressed in various cancers and thus contribute to the regulation of carcinogenesis. Despite the fact that molecular mechanisms of their activity is still not clear, VGCCs may represent a diagnostic marker and therapeutic target in the cancer treatment [34]. Moreover, PMCA is a major efflux pathway in [Ca2+]i-regulation, and impaired PMCA function results in Ca2+ overload and cell death. Glycolytic inhibition and subsequent ATP depletion impairs PMCA function and induces cells to undergo apoptosis [42].
2.4. Store Operated Calcium Entry in Cell Proliferation
Store operated calcium channels (SOC) on the plasma membrane generate calcium signals in a variety of cell types [43] and are considered to be the main calcium entry in non-excitable cells. Moreover, SOC entry controls a wide range of physiological functions such as apoptosis, proliferation, and migration [44]. Significantly, the activation of SOC entry is mediated via internal Ca2+-efflux [8]. Ca2+ stored in the ER and the mitochondria actively contributes to [Ca2+]i -signals as the Ca2+ influx is triggered by a calcium release from the ER which is mediated through stimulation of surface receptors [45]. Two important proteins, STIM1 on the ER (calcium sensor) and Orai1 (pore forming protein) of the calcium release-activated channels (CRAC) on the plasma membrane are involved in SOC entry. Ca2+-influx mediated by SOC entry activates “NFAT” transcription factor via STIM1 and Orai1, driving the proliferation pathway [45]. Proliferation is also positively regulated by SOCE (store operated calcium entry) through PI3K/Akt pathway [46]. A dysregulated STIM1/Orai1/SOC axis is implicated in many pathological conditions such as SCID (severe combined immune deficiency syndrome); cardiovascular and pulmonary diseases; and cancer of the breast, colon, and, esophagus [44]. In breast cancer, STIM1 and STIM2 contribute to invasion, migration, and SOC-dependent TGFβ-mediated EMT (epithelial-mesenchymal transition), and their overexpression significantly correlates with the poor survival [47]. Breast cancer is associated with an increased level of Orai1 and knockdown of Orai1 using siRNA in MCF-7 and MDA-MB-231 cells showed reduced SOC activity and decreased number of viable cells [48]. Moreover, in the ER positive BC cell line MCF-7 and T47D knockdown of Orai3 resulted in cell cycle arrest at G1 phase and induction of apoptosis [49]. Furthermore, in colon cancer cells the SOC-dependent migration was mediated by a complex of protein involving SK3/TRPC1/Orai1 [50]. Additionally, inhibition of SOC activity by non-steroidal anti-inflammatory drugs (NSAIDs) attenuated proliferation in the HRT-18 colon cancer cell line [51]. As the SOC entry is considered to be primary Ca2+ entry mechanism in most cancer types (thus contributing to cancer cell migration, invasiveness, and metastasis), there is a high interest in development of selective SOC entry blockers to prevent cancer metastasis [52,53].
2.5. SERCA in Cell Proliferation
SERCA is a calcium pump located in endoplasmic reticulum which maintains a low calcium level and thus enables signaling of various physiological processes [54]. Inhibition of SERCA by thapsigargin empties the ER-Ca2+ store and inhibits proliferation. Importantly, different isoforms of SERCA show altered expression in different types of cancers. For instance, SERCA2 is linked to the malignant progression of colorectal SW480 cells while its overexpression is associated with increased proliferation and migration of cancer cells mediated via activation of MAPK and AKT signaling pathways [55]. A close link between SERCA inhibition by thapsigargin and the ER calcium pool content depends on the epidermal growth factor (EGF) concentration, as was observed in LNCaP prostate cancer cells. The results confirmed the increase of ER-Ca2+ in the presence of EGF (200 ± 19 μM) when compared to control without EGF (90 ± 5 μM). In addition, higher expression of SERCA2b in the presence of growth factors such as EGF, DHT (dihydrotestosterone) serum, and the ER-Ca2+-pool concentration is important in regulating proliferation of LNCaP human prostate cancer cells [56]. A significant difference in [Ca2+]i homeostasis is observed in normal human bronchial epithelial (NHBE) cells vs. different lung cancers. Lung cancer cells showed lower ER-Ca2+-content which correlated with reduced expression of SERCA2b and increased expression of IP3R and calreticulin (a calcium buffering protein in the ER) [57].
2.6. ER and Mitochondrial Axis in Proliferation
High resolution microscopy images show a dynamic network of ER and mitochondria juxtaposed at various domains. These are identified as microdomains with high [Ca2+]i related to IP3 mediated Ca2+-release and are important determinants of [Ca2+]i-signaling, thus controlling survival, autophagy, apoptosis, metastasis, and invasiveness [58,59]. Mitochondrial calcium homeostasis is essential for cell survival and metabolic regulation [60,61]. Therefore, mitochondria play a dual role in death and survival, and dysregulation of mitochondrial Ca2+-homeostasis is associated with various diseases including cancer [62]. Importantly, mitochondrial calcium uniporter (MCU) is the channel enabling accumulation of Ca2+ in the matrix [62]. Aberrant expression or functioning of MCU complex contributes to cancer [62]. Significantly, integrity of the mitochondrial membrane, a sustained mitochondrial membrane potential, mitochondrial Ca2+ homeostasis regulated by Na+/Ca2+ exchanger, and MCU may ensure cell survival. Conversely, loss of mitochondrial potential, Ca2+ overload and opening of the permeability transition pores (PTP) activates an apoptotic cascade [63].
2.7. [Ca2+]i and Apoptosis
Programmed cell death, also known as “apoptosis”, is crucial for such normal cell physiological functions as turnover and cell death. There are two main signaling pathways (intrinsic and extrinsic) that culminate in apoptosis [64]. Mitochondria have a dual role in cell death and survival which is well documented. The intrinsic pathway is marked by mitochondrial Ca2+ overload, which results in the release of cytochrome c. Cytochrome c then combines with APAF-1 and, along with ATP and procaspase 9, forms the apoptosome, followed by activation of procaspase 9 to active caspase 9, which triggers the activation of effector caspases downstream, ultimately resulting in the death of the cell. In addition to caspases, calpains are another class of cysteine proteases which require calcium for their activation. Importantly, calpains mediate apoptosis as a response of ER stress [61]. In fact, mitochondria, as the main provider of cellular energy, also function as a regulator of [Ca2+]i. There is a calcium crosstalk in the domain between ER and mitochondria known as mitochondria-associated membrane (MAM). Importantly, tumor cells modify their MAM which leads to alterations of tumor homeostasis and consequently to promotion of migration, invasiveness, metastasis, resistance to apoptosis, and induction of EMT [63]. Furthermore, [Ca2+]i homeostasis also may be influenced by Bcl-2 proteins. Overall, members of the Bcl-2 family play an important role in cell death and survival via modulation of Ca2+-transport at the ER, plasma membrane, or mitochondria [65].
Furthermore, VDAC1 (voltage-dependent anion channel 1), which is located on the outer mitochondrial membrane (OMM), allows efflux and influx of ions and metabolites. It has a significant role in cell survival and death. It facilitates mitochondrial-mediated cell death in association with apoptotic proteins, causes the release of cytochrome c, and induces apoptosis. Furthermore, [Ca2+]i rise elevates VDAC1 expression and positively modulate apoptosis [66,67]. SOCE is also involved in apoptosis as SOC channels are activated or opened upon depletion of Ca2+ from the ER store. This causes an influx of Ca2+ from the extracellular pool, resulting in increased [Ca2+]i which is related to activation of apoptosis. The release of Ca2+ from ER store as well as influx of Ca2+ through the SOC channels influence apoptosis. As reported by Wertz and Dixit, the release of Ca2+ from the intracellular store alone activated apoptosis via caspase 3/7 in LNCaP prostate cancer cells [68]. Similarly, Skryma et al. demonstrated no requirement for the Ca2+ entry from the store in thapsigargin-treated LNCaP cells [22]. Importantly, the events that contributed to apoptosis following ER calcium depletion led to ER stress and initiation of death signals [69]. In this finding an exclusive relationship between ER-Ca2+ store/bcl-2/apoptosis was identified in LNCaP cells treated with thapsigargin [69]. Moreover, inhibition of SERCA by thapsigargin in prostate and breast cancer cells led to drainage of the ER-Ca2+ stores, which was followed by reduction in cell proliferation and consequent apoptosis [69].
3. Targeting Calcium Signaling for Anti-Cancer Therapy
Anti-cancer drugs have multiple modes of action and, based on their mechanism of action, can interfere at the DNA level, act as antimetabolites, inhibit enzyme synthesis, and inhibit microtubule function. In the past decade, much attention has been drawn to calcium signaling. Altered calcium signaling is crucial for the development and progression of cancer through the induction of proliferation, invasion, and metastasis [70]. Figure 2 and Table 1 summarize anti-cancer drugs and their [Ca2+]i mediated induction of cell death.
Table 1.
Interference of anti-cancer agents with different mechanisms of calcium homeostasis. This table illustrates which calcium mediating processes are involved, the actual concentrations (doses) needed, the experimental model, and whether apoptosis or proliferations are involved.
| Drug Class | Drugs | Axis/Mechanism of Induction of Cell Death | Concentration Range | Apoptosis | Proliferation | Cell Line | References |
|---|---|---|---|---|---|---|---|
| Platinum agents (cytotoxic alkylating agent) | Cisplatin | [Ca2+]i ↑↑ by influx of extra cellular calcium. [Ca2+]i ↑↑ / ER stress / mitochondrial Ca2+ over load / caspase 3 activation. |
1 µM 5 µg/ml |
↑↑ | ↓↓ | MCF-7 SH-SY5Y HeLa-S3 |
[31,71,72,73] |
| Anti-metabolites | 5-Fluorouracil | Ca2+-CaM-p53 activation, Ca2+ influx partially through L-type Ca2+ channel. Ca2+ entry through TRPV1 / mitochondrial ROS production / caspase 8. |
768 μM, 25 μM |
↑↑ | ↓↓ | HCT116 MCF-7 |
[74,75] |
| Inorganic arsenic compounds | As2O3 | IP3R, RyR / [Ca2+]i ↑↑ / DNA damage / caspase 3. ↑↑ER–mitochondrial Ca2+ transfer. ↓↓ ERK1 and ERK2 |
1 µM | ↑↑ | ↓↓ | SH-SY5Y Pml-/- mice NB4 cells U937 |
[31,76,77,78] |
| Anthracyclines | Doxorubicin | [Ca2+]i modulation - ERK1/2 inactivation, activation of pro apoptotic BIM pathway and mitochondrial Ca2+ overload. | 500 nM–1 µM | ↑↑ | ↓↓ | MDA-MB-231 | [79] |
| Taxanes |
Paclitaxel
Docetaxel |
In activation of PMCA2/calcineurin A and activation of calcineurin A /NFAT pathway/ ↑↑ pro-apoptotic protein Fas ligand. External calcium influx, inhibition of bcl2/ IP3R-ER-[Ca2+]i. |
1 nM 10-6 M |
↑↑ | MDA-MB-231 MCF-7 MDA-MB-468 |
[80,81] | |
| Natural compounds |
Chalcones
Quercetin EGCG Piceatannol Etoposide; (semi-synthetic) Resveratrol Curcumin Saikosaponin-d |
Ca 2+ / ER stress / caspase 12. G-protein / IP3R-ER-[Ca2+]i, / modulation of p53 / transcription of pro-apoptotic genes. SERCA↓↓ activity / [Ca2+]i ↑↑ / increased mitochondrial Ca2+ uptake. SERCA ↓↓ / [Ca2+]i ↑↑ / ER stress / Autophagy mediated cell death. |
30–40 µM 50–100 µM 10 µM |
↑↑ | L1210 MDA-MB-231 MYCN2 HeLa SW480 (colon) MCF-7 |
[55,82,83,84,85,86] | |
| Camptothecin analog | Topotecan | Increased [Ca2+]i, altered expression of calcium regulating proteins. | 0.01 µM | ↑↑ | ↓↓ | SH-SY5Y | [87] |
| Hormonal receptor modulator | Tamoxifen | [Ca2+]i ↑↑ by influx of extra cellular calcium and release of Ca2+ from multiple stores. VGCC |
5–10 µM | ↑↑ | MCF-7 MG63 ZR-75-1 SCC BFTC |
[88,89,90,91,92] | |
| DNA methylation and HDAC modulators |
TSA
Azacitidine Digitoxin Pyrithion zinc Disulfiram |
↑↑SERCA3 / apoptosis. SOC / [Ca2+]i ↑↑ / CamK / via MeCP2 / reactivation of tumor suppressor genes. |
50 nM–5 µM | ↑↑ | KATO-III (gastric carcinoma) YB5 (colon) |
[93,94] |
3.1. [Ca2+]o Influences Drug Efficiency
[Ca2+]o influences the mechanism of action of paclitaxel in inducing apoptosis in breast cancer cells. Apoptosis induced by low dose paclitaxel is independent of [Ca2+]o whereas for high dose paclitaxel, normal [Ca2+]o is critical for inducing apoptosis in MDA-MB-468 TNBC cells, which is mediated via capacitative Ca2+-entry [81]. Similarly, low [Ca2+]o reduced anti-cancer action of tamoxifen (TM) on HepG2, human hepatoblastoma cells. To understand the route of Ca2+ entry, Ca2+ channel blockers nifedipine and verapamil were used, but neither of them reversed the effect of TM, while flufenamic effectively reversed the effect of TM. A non-selective cation channel (NSCC) blocker caused significant inhibition of TM-induced apoptosis, indicating NSCC as the main route of Ca2+-influx [95]. Modifying the [Ca2+]o improved the cytotoxic action of CDDP and topotecan [87].
3.2. Anti-Cancer Drugs-Induced [Ca2+]i Modulation Triggers Apoptosis
3.2.1. Platinum Drugs
Cisplatin (CDDP; cis-diamminedichloridoplatinum) is used in treatment of solid tumors and modulates [Ca2+]i. It blocks voltage gated channels at higher concentrations [96] but allows calcium entry through other channels [31] and releases calcium from the stores [97]. In neuroblastoma (NB) cells, CDDP alters the expression of key calcium regulating proteins such as RyR and IP3R [98]. HeLa cells, when treated with CDDP for 16 h, showed an increase in [Ca2+ ]i followed by an increased expression of VDAC1 and oligomerization of VDAC1-triggered cytochrome c release and concomitant apoptosis [67]. An increased expression of VDAC1 sensitizes cancer cells to anti-cancer drugs via Ca2+-dependent mechanism. A study in HeLa S3 cells revealed a rise in [Ca2+]i following CDDP treatment, which correlated with the activation of calpain, an important molecule in the induction of apoptosis [31]. Increase in CDDP concentration caused an increase in [Ca2+]i in MCF-7 breast cancer cells [72]. S100A9, a calcium-binding protein, is associated with cancer progression and has been reported to influence squamous cervical cancer cells’ sensitivity to CDDP treatment. Here, down-regulation of S100A9 protein resulted in increased cell death potentiated by altering pro survival AKT/ERK-FOXO1-Nanog signaling pathway [99]. Another study emphasized the role of NCX and NCXK (K+ dependent Na+/Ca2+-exchangers) in cisplatin chemotherapy of ovary carcinoma cells. In this study using A2780 ovarian cancer cell line, the expression of NCX 3 NCKX4, NCKX5, and NCKX6 isoform was higher in the CDDP resistant cell line. NCX inhibitor KB-R7943 (10 μM) significantly improved CDDP sensitivity there by confirming the role of NCX in therapy resistance [100]. Most of the anti-cancer drugs have side effects. Oxaliplatin, a platinum-based drug, undergoes activation with the release of oxalate and the generation of oxalate metabolite is often associated with oxaliplatin associated peripheral neuropathy. Schulze et al. examined the mechanism of oxaliplatin mediated calcium signaling and its role in oxaliplatin-induced peripheral neuropathy. A prolonged exposure to the drug induced changes in the ER calcium load and IP3R mediated calcium signaling, though there was no activation of cellular temperature sensors—TRP channels [101]. Strategies to selectively target cancer cells by sparing the normal cells is a wise approach. The new platinum drug [Pt(O,O′-acac)(γ-acac)(DMS)] (PtAcD) is selectively toxic on the breast cancer cell line and less toxic on normal breast cells due to differentially activated JNK and p38 [102]. Research on next generation platinum drugs (e.g., (Pt(IV) carboxylate complexes) are underway with more efficacy and less side effects.
3.2.2. Anti-Metabolites
5-Fluorouracil (5-FU), an anti-metabolite which interferes with DNA synthesis and repair, is used for treatment of breast, skin, pancreatic cancer, etc. Its mechanism of apoptosis induction in HCT116 involved the Ca2+-influx from extracellular space, which was detected as early as 1.5 h after exposure to 5-fluorouracil. Consequently, activated p53 was detected after 5 h of 5-FU treatment followed by activation of caspase and induction of apoptosis. Elevation of [Ca2+]i is an early event in the apoptosis of HCT116 mediated via Ca2+-CaM-p53 axis [74]. Another study reported the involvement of TRPV 1 channel in 5-FU induced cell death in MCF-7 cells, which involved rise in [Ca2+]i followed by mitochondrial ROS production and caspase activation [75]. TRPV1 is a non-specific cation channel sensitive to temperature, pH, and capsaicin.
3.2.3. Inorganic Arsenic Compounds
Arsenic trioxide (As2O3) is an anti-cancer drug used for the treatment of hematologic cancers such as chronic myelogenous leukemia (CML) [103]. Various studies have reported As2O3 modulating different Ca2+-dependent/independent signal transduction pathways, resulting in the induction of apoptosis, inhibition of angiogenesis, and proliferation [104]. Receptors on the ER (IP3R and ryanodine R) are involved in the induction of apoptosis by elevating [Ca2+]i. Treatment with 1 µM As2O3 in SH-SY5Y NB cells elevated [Ca2+]i by ER-Ca2+ store depletion [24]. Calcium imaging showed three types of signals: A slow and steady increase in [Ca2+]i, transient calcium elevation, and spikes. Mechanistic studies have revealed increased H2O2 level, Bax expression, growth arrest at the G1 phase of the cell cycle, and inhibition of vascular endothelial growth factor (VEGF) upon As2O3 treatment [105,106]. Missiroli et al. [77] elucidated a Ca2+-dependent mechanism that coordinately regulated apoptosis and autophagy in cancer. They investigated pml, a tumor suppressor gene related to the pathogenesis of acute promyelocytic leukemia. PML protein and its association with ER-mitochondrial contact sites were important in repressing autophagy, and treatment with As2O3 was shown to reduce the autophagy efficiency by modulating PML protein in NB4 leukemia cells [77]. Similar to CDDP treatment in HeLa cells, increased expression of VDAC1 is observed with As2O3 treatment in SKOV-3 cells (human ovarian carcinoma) and A549 cells (human lung adenocarcinoma alveolar basal epithelium).
3.2.4. Anthracyclines
Studies on doxorubicin, a chemotherapeutic drug belonging to the category anthracyclines, reported contrasting observations on the effect of Ca2+ on doxorubicin cytotoxicity. A counteracting mechanism of Ca2+ concentration on doxorubicin activity was reported by Nguyen et al. [107]. Here, [Ca2+]o concentration dependently suppressed the cytotoxic activity of doxorubicin in MCF-7 breast cancer cell line. High [Ca2+]o (100 and 140 mM CaCl2) almost completely rescued the cells treated with 0.2 µg/mL of doxorubicin (LD50). Mechanism revealed that Ca2+ activated V-ATPase (Vacuolar-type H+-ATPase), reduced the bioavailability of drug by acidifying the intracellular organelles and sequestering doxorubicin into subcellular compartments [107]. The finding emphasizes the observation that high [Ca2+]o confers insensitivity to doxorubicin treatment. In another study, silencing of PMCA2 calcium channel increases the sensitivity of MDA-MB-231 to doxorubicin treatment. Doxorubicin and simvastatin treatment in breast cancer cells caused a persistent release of Ca2+, resulting in activation of proapoptotic BIM pathway and subsequent overload of mitochondrial Ca2+ triggering apoptosis. In addition, both drugs significantly reduced the activation of pro survival pathway ERK1/2 signaling, which is calcium-dependent in doxorubicin treatment [79].
3.2.5. Taxanes
Paclitaxel is an anti-cancer drug used for the treatment of solid tumors. In neuroblastoma cells (SH-SY5Y), paclitaxel at a low concentration (sub-micromolar concentration) induced Ca2+-oscillations independent of [Ca2+]o or mitochondrial calcium but dependent on IP3R mediated calcium influx from ER. The molecular basis of paclitaxel induced cytosolic Ca2+-oscillation is due to the enhanced binding of protein neuronal Ca2+-sensor 1 (NCS-1) to IP3R [108]. Conversely, in MD-MBA-468 breast cancer cells under normal [Ca2+]o, Ca2+ entered from the extracellular pool following low dose paclitaxel (10−7 M) treatment and was independent of ER store, but high dose paclitaxel (10−6 M) induced Ca2+-influx extracellularly and a gradual depletion of ER store occurred, culminating in apoptosis. The mechanism of paclitaxel-mediated apoptosis is dependent on [Ca2+]o and dosage of the drug [81].
3.2.6. Glucocorticoids
Glucocorticoid (GC), a class of drugs that have multiple physiological effects such as immune suppression, cytotoxicity, and anti-inflammatory effects, is commonly used for hematological malignancies like leukemia, lymphoma, and myeloma [109]. GC modulates [Ca2+]i homeostasis in B lymphocytes, while [Ca2+]i has a complex role in the induction of apoptosis by glucocorticoids. Release of Ca2+ from the stores initiates apoptosis in GC-treated lymphocytes. Here, Ca2+-dependent proteases such as calpain were activated, and blocking of calpain activation prevented GC-induced cell death [109]. Similar to doxorubicin, [Ca2+]i attenuated dexamethasone sensitivity in acute lymphoblastic leukemia (ALL) cells. Dexamethasone increased [Ca2+]i mainly by SOC-operated calcium entry [110].
3.2.7. Natural Compounds
Analysis of studies based on some natural compounds showed a Ca2+-dependent anti-cancer effect (see Table 1 and Figure 2). For example, Resveratrol induced Ca2+-dependent apoptosis through a biphasic [Ca2+]i-rise involving mitochondria, activation of calpain, and decreased SERCA activity [85,111]. Saikosaponin-d, a natural SERCA inhibitor, induced Ca2+ dependent autophagy mediated cell death via CaMKKb-AMPK-mTOR pathway [86]. In another study, epibrassinolide, a polyhydroxylated sterol derivative, induced Ca2+ sequestration and thus caused an alteration in the ER pathway, consequent ER stress, and progress to apoptosis [112]. Using a model of endometriosis, Park et al. described that luteolin exerts antiproliferative and apoptotic effects in pre-neoplastic human endometrial cells VK2/E6E7 and End1/E6E7. The anti-cancer effects of luteolin were linked with an increase in cytosolic calcium levels, ROS production, and lipid peroxidation in cells and altered regulation of PI3K/AKT and MAPK cell signaling, as well as the expression of CCNE1 [113]. Using the same model and cells, the same group showed that delphinidin exerts proapoptotic and antiproliferative effects mediated by MAPK and PI3K/AKT signaling proteins. These effects were accompanied by a decrease in the phosphorylation of ERK1/2, AKT, p70S6K, S6 and an increase in the phosphorylation of p38 MAPK and p90RSK [114]. Delphinidin induced apoptosis in human endometrial cells by decreasing the mitochondrial membrane potential and increasing the [Ca2+]i. Safrole-mediated apoptotic cell death in HSC-3 cells was associated with an increase of cytosolic Ca2+ levels, a decrease in the mitochondrial membrane potential, and activation of Fas-dependent pathways [115]. Curcumin induced apoptosis in HepG2 cell, which was associated with the disruption of mitochondrial membrane potential and disturbance of intracellular free Ca2+ concentration [116]. Natural products are potential drug candidates which can be considered for combination therapy as this strategy can increase sensitivity to chemotherapy by targeting multiple pathways and, therefore, reduce the side effects.
3.2.8. Hormonal Receptor Modulator
Tamoxifen (TM) is an estrogen receptor modulator commonly used for breast cancer prevention and treatment. Data from several sources have identified [Ca2+]i modulation by tamoxifen, consistent with cytotoxicity in tumor cells [89,91,92]. In ZR-75-1 human breast cancer cells, treatment with tamoxifen at a concentration above 2 μM induced an early [Ca2+]i rise due to release from stores as well as entry from extracellular space [91]. A detailed study by Zhang et al. on calcium dynamics in tamoxifen-treated breast cancer cells and glioma cells revealed increase in [Ca2+]i level and spatial expansion of calcium waves by 30–150%. Mechanistic studies revealed tamoxifen-induced calcium-depended cytotoxicity facilitated via enhanced purinergic signaling [88]. Furthermore, in human osteosarcoma cells a sustained increase in [Ca2+]i due to release of calcium from multiple stores (phospholipase C-independent manner) was recorded following 1 µM tamoxifen treatment [90]. The antiproliferative effect of tamoxifen in human non-melanoma skin cancer cells A431, DJM-1, and HSC-1 is also related to an increase of [Ca2+]i [92].
3.2.9. Epigenetic Modulators
Recent studies discovered a prominent role of epigenetic deregulation linked to cancers. The most important epigenetic changes, DNA methylation and histone post-translational modification (methylation and acetylation), result in gene silencing of tumor suppressor genes (TSG) or up/down regulation of other genes. A recent article highlights the role of [Ca2+]i-signaling in re-activating tumor suppressor genes via CamK. Reactivation of silenced TSG by epigenetic drug azatidine revealed a novel mechanism, which traced back to Ca2+-dependent activation of CamK and subsequent release of MeCP2 methyl-binding protein from promotion of the silenced genes [94]. Epigenetic silencing of gene ATP2A3, which codes for SERCA3, is down-regulated in gastric and colon tumors. Treatment of KATO-III cells with butyrate, trichostatin A, and 5-azacytidine increased the expression of SERCA3 and was correlated with increased apoptosis and decreased viability [93]. Mitochondrial Ca2+-overload is suggested as a key trigger for programmed cell death. MCU, together with its regulatory subunits, mitochondrial calcium uptake 1 (MICU1) and mitochondrial calcium uniporter regulator 1 (MCUR1), which are responsible for mitochondrial-Ca2+ entry, provide novel molecular tools to evaluate this process. Regarding epigenetic modulations of these mechanisms, recent data have also demonstrated that miR-25 reduces mitochondrial Ca2+ uptake via MCU, resulting in suppression of apoptosis. It is shown that down-regulation of MCU in human colon cancer cells correlates with miR-25 aberrant expression, pointing the importance of mitochondrial Ca2+ regulation in apoptosis.
3.3. Calcium Dependent Modulation of Aerobic Glycolysis by Anti-Cancer Agents
Warburg effect (aerobic glycolysis) is a mechanism observed in tumor energy metabolism promoting tumor growth and survival. Here, cells observe excess uptake of glucose and break down of glucose to pyruvate and lactate even in the presence of oxygen and a functional mitochondria [117,118]. Hence cancer cells exhibit altered Ca2+-dependent ATPase function. Chakraborty et al. (2017) documented that Mitochondrial Calcium Uptake 1 (MICU1/CBARA1), the gatekeeper of mitochondrial Ca2+-uptake, forces aerobic glycolysis and chemoresistance in ovarian cancer cells [119]. Another study showed that an increased expression of transient receptor potential canonical channel (TRPC5) enhances [Ca2+]i level and results in chemoresistance and suppressed apoptosis in human colorectal cancer (CRC) cells [120]. In the follow-up of this study, Wang et al. demonstrated the crucial role of glycolysis in TRPC5 induced chemoresistance in CRC cells through maintaining [Ca2+]i homeostasis [121]. In vitro studies using dichloroacetate (DCA) demonstrated a shift in the metabolism from glycolysis to glucose oxidation in HeLa cells [122]. The changes in the metabolism modality led to increased intracellular H2O2 and pH levels, a drop in mitochondrial membrane potential, and the increase of caspase 3 and 9. Regarding mechanism of action, the increased Kv1.5 expression and decreased [Ca2+]i appointed a positive feedback loop that caused the decrease in tonic inhibition of caspases. CDDP in combination with DCA exhibited synergy [122]. In vitro studies conducted in human PDAC cell lines (pancreatic cancer) treated with glycolytic inhibitors BrPy 500 μm (3-bromopyruvate) and sodium iodoacetate 2 mm (IAA) resulted in glycolytic inhibition and ATP depletion. As a result, ATP depletion lead to impaired PMCA function and [Ca2+]i overload inducing cell death [42] (Figure 2). Furthermore, dexamethasone and prednisolone belonging to the class of glucocorticoids in ALL proved to shift cells’ energy metabolism by suppressing glycolysis and increasing the anti-cancer cancer effect of etoposide [123].
3.4. Calcium Modulators in Combination with Anti-Cancer Agents
Tumor heterogeneity can be attributed to the cancer stem cell, tumor microenvironment, gene mutation, and epi-genetic changes. Consequently, cancer cells exposed to chemotherapeutic drugs are often not completely eliminated, and a few cells survive, generating resistant cancer cells. Originally, cancer was treated using single drugs but with the relapse and development of resistance to chemotherapy more trials with drug combinations yielded improved results. Hence, for an effective treatment, the tumor is exposed to a combination of anti-cancer agents targeting different pathways [124]. An important mechanism by which anti-cancer drugs induce cytotoxicity is by interfering with [Ca2+]i either by binding or bringing conformational changes to calcium modulating proteins, including channels and pumps located at the plasma membrane, ER, or cytosol [23,25]. A considerable amount of literature has been published on the effect of calcium modulators on cell proliferation and apoptosis [125]. Over the years, research has emphasized the use of calcium modulators for the enhancement of anti-cancer drug efficiency [7]. A report in 1989 claimed a synergistic action of nifedipine with CDDP in B16a-Pt (cisplatin resistant murine tumor cell line) by a mechanism independent of VGCCs [126]. A novel approach using Riluzole, an activator of Ca2+-activated K+ channel (KCa3.1) and an inhibitor of Kv11.1 increased the CDDP drug uptake and reversed CDDP resistance in the colorectal cancer cell line [127]. An extensive in vitro study in neuroblastoma chemotherapy using CDDP and topotecan showed enhanced cytotoxic effects with combinations of pharmacological modulators of [Ca2+]i regulating proteins [87]. Synergistic action with a panel of calcium channel modulators (cyclosporine A, thapsigargin, dantrolene, 2-APB) in CDDP/topotecan treatment showed promising results in neuroblastoma cell lines SH-SY5Y, NLF and IMR-32 [87]. For example, thapsigargin showed the highest cytotoxic effect by facilitating store-operated calcium entry and triggered apoptosis via ER-mitochondrial axis. Similarly, among the other modulators tested were cyclosporine A and 2-Aminoethoxydiphenyl borate (2-APB), which also induced ER stress and enhanced the cytotoxic effect of both CDDP and topotecan in neuroblastoma cell. 2-APB activates SOC mediated Ca2+-entry at a lower concentration, inhibits IP3R, and modulates TRP channels. Live cell calcium imaging studies performed in MCF-7cells pre-treated (30 min) with calcium channel modulators (caffeine, nimodipine, and ionomycin) showed decrease in CDDP-induced [Ca2+]i rise [72]. 1,2-Bis(2-aminophenoxy)ethane-N,N,N’,N’-tetraacetic acid tetrakis (acetoxymethyl ester) BAPTA-AM is a membrane-permeable calcium chelator widely used for [Ca2+ ]i-signaling studies. A study in HeLa cells (human cervical cancer) indicated that combined treatment with (BAPTA-AM) or 2-APB attenuated CDDP-mediated [Ca2+]i rise and apoptosis [31]. The above observations show the role of specific calcium channels in CDDP mediated [Ca2+]i rise. As in the case of dexamethasone, BAPTA-AM synergistically enhanced the cytotoxicity of dexamethasone in ALL cell lines [110]. On the contrary, another group reported that doxorubicin efficiency was compromised in the presence of BAPTA-AM in MDA-MB-231 breast cancer cells by preventing the inhibition of ERK1/2 phosphorylation, and was consistent with the attenuation of [Ca2+ ]i rise and decrease in cell death [79]. Huang et al. identified a [Ca2+]i-dependent apoptotic mechanism in PC3 human prostate cancer cells exposed to thapsigargin (1–10 µM). Here, the rise in [Ca2+]i was attributed to both Ca2+-influx from extracellular environment and depletion of ER stores [128]. Combination treatment with TRAIL (70 and 35 ng/mL) and thapsigargin (0.3 and 0.6 μM) induced apoptosis and inhibited migration, invasion, and adhesion of ESCC cell lines (esophageal squamous cell carcinoma), demonstrating that this combination induces both apoptosis and inhibits metastasis [129]. In MCF-7 and MDA-MB-468 cells, apoptosis was triggered mainly due to a secondary and a delayed (12–36 h) calcium response to thapsigargin (1 µM) [130]. Calcium modulating drugs can be used for enhancement of anti-cancer drug effect, whether it can mitigate side effects of anti-cancer treatment should be explored. Nifedipine, a dihydropyridine-type Ca2+-channel blocker improved the blood circulation and reduced hypoxia and hence achieved a better drug delivery towards tumor in an isolated limb perfusion experiment performed in rat bearing tumors [131]. Similarly, verapamil, a calcium channel blocker, improved the sensitivity of tumor to radiation by modifying the tumor vasculature [132].
Collectively, the combination of calcium channel modulators with anti-cancer drugs can have a beneficial effect with some combination of drugs.
4. Conclusions and Future Directions
Cancer research reveals that [Ca2+]i-signaling has substantial effects on proliferation and apoptosis. Prolonged increase in [Ca2+]i triggers apoptotic cascade and is the principle behind the action of many anti-cancer drugs. In the context of cancer, many drugs such as CDDP, topotecan, and As2O3 induce apoptosis depending on extra- and intracellular calcium. The modification of either [Ca2+]o or [Ca2+]i by calcium chelating agents can significantly augment the action of anti-cancer drugs such as doxorubicin and dexamethasone. This observation is relevant in a clinical context as caution should be exercised when using calcium-altering drugs and diets. Calcium signaling plays a dual role in cell survival and death. ER–mitochondrial Ca2+ fluxes are crucial in steps (cancer hallmarks) leading to cancer growth, and it is the most targeted site for many of the anti-cancer drugs (Figure 2). ER-mitochondrial Ca2+-flux, SOC entry, channels, and pumps on the PM coordinately control the fate of the cell. Hence, components of the calcium tool kit are potential targets of anti-cancer drugs. Nuclear calcium controls gene regulation, but its dependence on cytosolic calcium is a subject of debate. Hence, a drug which modulates [Ca2+]i may or may not affect nuclear calcium, but there is no adequate information on this.
Dysregulated expression of many calcium regulation proteins is associated with migration, proliferation, apoptosis, angiogenesis, and invasion in cancer. However, it is beyond the scope of this review to discuss all the calcium signaling components dysregulated in various cancers. A growing body of evidence emphasizes targeting altered calcium homeostasis in cancer as a potential tool in cancer chemotherapy by modulating either the expression of calcium regulating proteins or their function. CDDP and topotecan are drugs identified to modulate the expression of IP3R and ryanodine in cancer chemotherapy, however, there are multiple calcium channel inhibitors/activators, which block/activate the function of various channels and thus modulate the apoptosis and proliferation of the cells. In this regard, many combinations of classical drugs with calcium channel regulators have improved efficacy within cancer chemotherapy. Moreover, a wide variety of drugs derived from natural compounds have proapoptotic and antiproliferative modes of action, such as topotecan, anthracyclins, and taxanes, and all modulate calcium signaling. Curcumin, safrole, luteolin, delphinidin, and other phytochemicals can potentially serve as molecules for developing novel anti-cancer drugs targeting proliferation and apoptosis in cancer cell via regulation of cytosolic Ca2+-levels. Reversing the glycolytic phenotype may trigger apoptosis in tumor cells, and thus represents an attractive therapeutic tool for anti-cancer clinical strategies. Selective targeting of the key functional enzymes of glucose metabolism through the elevation of [Ca2+]i level, which could lead to [Ca2+]i overload and promote apoptosis, is a new challenge for oncological research. Targeting epigenetic pathways is a newer approach in cancer chemotherapy, and using the calcium signaling pathway to reactivate silenced genes was recently introduced. In this regard, certain calcium-modulating agents have been proven to epigenetically increase the protein phosphorylation included in apoptosis/cell-cycle signaling, which is repressed in several cancers such as in the liver, pancreatic, breast, or prostate.
An increasing number of studies have identified and listed various proteins in the calcium signaling tool kit which are altered in various cancers [1]. Studies emphasize the important role of calcium cell signaling and various calcium regulating proteins in cancer development. However, further investigations are needed to explore precise molecular mechanisms of their anti-cancer action. Developing drugs against targets involving the calcium signaling tool kit is challenging as these proteins are ubiquitously present in all types of cells. It is important to critically classify those targets that are relevant to each type of cancer.
Acknowledgments
We thank Steven Stay (Weill Cornell Medicine-Qatar, Doha, Qatar) for language corrections and linguistic and grammatical suggestions.
Abbreviations
| [Ca2+]i | intracellular calcium |
| [Ca2+]o | extra cellular calcium |
| ALL | acute lymphoblastic leukemia |
| APAF-1 | apoptotic protease activating factor 1 |
| As2O3 | arsenic trioxide |
| ATP | adenosine triphosphate |
| BrPy | 3-bromopyruvate |
| Calreticulin | calcium buffering protein in the ER |
| CamK | Ca2+/calmodulin-dependent protein kinase |
| CaSR | calcium sensing receptor |
| CDDP | cis-diamminedichloridoplatinum(II) |
| CML | chronic myelogenous leukemia |
| CRAC | channel Calcium release-activated channels |
| CREB | cAMP response element-binding protein |
| DJM-1 | a human skin squamous carcinoma cell line |
| EC | endometrial cancer |
| EGF | epidermal growth factor |
| EGCG | Epigallocatechin gallate |
| EMT | epithelial–mesenchymal transition |
| ER | endoplasmic reticulum |
| ERK1/2 | extracellular signal–regulated kinases |
| GC | glucocorticoid |
| GPER | G protein-coupled estrogen receptor |
| HSC-1 | a human skin squamous carcinoma cell line |
| IAA | sodium iodoacetate |
| IP3R | inositol trisphosphate receptor |
| IAA | sodium iodoacetate |
| MAPK | mitogen-activated protein kinase |
| MCU | mitochondrial calcium uniporter |
| mTOR | mechanistic target of rapamycin |
| NB | neuroblastoma |
| NCS-1 | neuronal Ca2+ sensor 1 |
| NHBE | normal human bronchial epithelial |
| NSAID | non-steroidal anti-inflammatory drugs |
| Orai1 | calcium release-activated calcium channel protein 1 |
| PMCA | plasma membrane calcium ATPase |
| PML | promyelocytic leukemia protein |
| PTP | permeability transition pore |
| RyR | ryanodine receptors |
| SCID | severe combined immune deficiency syndrome |
| SERCA | sarco/endoplasmic reticulum Ca2+-ATPase |
| SK3 | small-conductance calcium-activated potassium channel |
| SOC | store operated channel |
| STIM1 | stromal interaction molecule 1 |
| TGFβ | transforming growth factor beta |
| TM | tamoxifen |
| TF | transcription factor |
| TSA | trichostatin A |
| TRPC1 | transient receptor potential channel 1 |
| TRPV1 | transient receptor potential vanilloid 1 |
| V-ATPase | vacuolar-type H+ -ATPase |
| VDAC1 | voltage-dependent anion channel |
| VGCC | voltage gated calcium channel |
| VGEF | vascular endothelial growth factor |
| VOC | voltage operated calcium channel |
| 2-APB | 2-Aminoethoxydiphenyl borate |
| 5-FU | 5-Fluorouracil |
Author Contributions
The authors contributed as follows: Conceptualization, E.V. and D.B.; Literature Review and Resources, E.V.; S.M.S.; P.K. (Peter Kubatka) and A.L.; Writing—Original Draft Preparation, E.V.; Writing—Review and Editing, E.V.; S.M.S.; Z.S.; P.K. (Peter Kruzliak); A.L.; J.B; P.P.; P.K. (Peter Kubatka) and D.B.; Figure Preparation and Editing, E.V.; D.B.; Visualization, E.V.; S.M.S. and D.B.; Supervision, D.B. All authors reviewed the results and approved the final version of the manuscript.
Funding
This work was supported by the Bridge Funding Grant (Nov 2017–Dec 2018) from the Qatar National Research Fund (QNRF) and Weill Cornell Medicine-Qatar, Qatar Foundation, Doha, Qatar. The statements made herein are solely the responsibility of the authors.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Bootman M.D., Rietdorf K., Hardy H., Dautova Y., Corps E., Pierro C., Stapleton E., Kang E., Proudfoot D. eLS. John Wiley & Sons, Ltd.; Hoboken, NJ, USA: 2012. Calcium Signalling and Regulation of Cell Function. [DOI] [Google Scholar]
- 2.Munaron L., Antoniotti S., Lovisolo D. Intracellular calcium signals and control of cell proliferation: How many mechanisms? J. Cell. Mol. Med. 2004;8:161–168. doi: 10.1111/j.1582-4934.2004.tb00271.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miyazaki S. Calcium signalling during mammalian fertilization. Ciba Found. Symp. 1995;188:235–247; discussion 247–251. doi: 10.1002/9780470514696.ch13. [DOI] [PubMed] [Google Scholar]
- 4.Bezprozvanny I. Calcium signaling and neurodegenerative diseases. Trends Mol. Med. 2009;15:89–100. doi: 10.1016/j.molmed.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gilon P., Chae H.-Y., Rutter G.A., Ravier M.A. Calcium signaling in pancreatic β-cells in health and in Type 2 diabetes. Cell Calcium. 2014;56:340–361. doi: 10.1016/j.ceca.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 6.Luo M., Anderson Mark E. Mechanisms of Altered Ca2+ Handling in Heart Failure. Circ. Res. 2013;113:690–708. doi: 10.1161/CIRCRESAHA.113.301651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cui C., Merritt R., Fu L., Pan Z. Targeting calcium signaling in cancer therapy. Acta Pharm. Sin. B. 2017;7:3–17. doi: 10.1016/j.apsb.2016.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang W., Ren Y., Wang L., Zhao W., Dong X., Pan J., Gao H., Tian Y. Orai1 and Stim1 Mediate the Majority of Store-Operated Calcium Entry in Multiple Myeloma and Have Strong Implications for Adverse Prognosis. Cell. Physiol. Biochem. 2018;48:2273–2285. doi: 10.1159/000492645. [DOI] [PubMed] [Google Scholar]
- 9.Berridge M.J., Lipp P., Bootman M.D. The versatility and universality of calcium signalling. Nat. Rev. Mol. Cell Biol. 2000;1:11–21. doi: 10.1038/35036035. [DOI] [PubMed] [Google Scholar]
- 10.Bagur R., Hajnóczky G. Intracellular Ca(2+) Sensing: Its Role in Calcium Homeostasis and Signaling. Mol. Cell. 2017;66:780–788. doi: 10.1016/j.molcel.2017.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reddish F.N., Miller C.L., Gorkhali R., Yang J.J. Calcium Dynamics Mediated by the Endoplasmic/Sarcoplasmic Reticulum and Related Diseases. Int. J. Mol. Sci. 2017;18:1024. doi: 10.3390/ijms18051024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Williams J.A., Hou Y., Ni H.-M., Ding W.-X. Role of intracellular calcium in proteasome inhibitor-induced endoplasmic reticulum stress, autophagy, and cell death. Pharm. Res. 2013;30:2279–2289. doi: 10.1007/s11095-013-1139-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rizzuto R., Marchi S., Bonora M., Aguiari P., Bononi A., De Stefani D., Giorgi C., Leo S., Rimessi A., Siviero R., et al. Ca(2+) transfer from the ER to mitochondria: When, how and why. Biochim. Biophys. Acta. 2009;1787:1342–1351. doi: 10.1016/j.bbabio.2009.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yáñez M., Gil-Longo J., Campos-Toimil M. Calcium Binding Proteins. In: Islam M.S., editor. Calcium Signaling. Springer; Dordrecht, The Netherlands: 2012. pp. 461–482. [DOI] [PubMed] [Google Scholar]
- 15.Bading H. Nuclear calcium signalling in the regulation of brain function. Nat. Rev. Neurosci. 2013;14:593–608. doi: 10.1038/nrn3531. [DOI] [PubMed] [Google Scholar]
- 16.Resende R.R., Andrade L.M., Oliveira A.G., Guimarães E.S., Guatimosim S., Leite M.F. Nucleoplasmic calcium signaling and cell proliferation: Calcium signaling in the nucleus. Cell Commun. Signal. 2013;11:14. doi: 10.1186/1478-811X-11-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leite M.F., Thrower E.C., Echevarria W., Koulen P., Hirata K., Bennett A.M., Ehrlich B.E., Nathanson M.H. Nuclear and cytosolic calcium are regulated independently. Proc. Natl. Acad. Sci. USA. 2003;100:2975–2980. doi: 10.1073/pnas.0536590100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Allbritton N.L., Oancea E., Kuhn M.A., Meyer T. Source of nuclear calcium signals. Proc. Natl. Acad. Sci. USA. 1994;91:12458–12462. doi: 10.1073/pnas.91.26.12458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Echevarria W., Leite M.F., Guerra M.T., Zipfel W.R., Nathanson M.H. Regulation of calcium signals in the nucleus by a nucleoplasmic reticulum. Nat. Cell Biol. 2003;5:440–446. doi: 10.1038/ncb980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ikura M., Osawa M., Ames J.B. The role of calcium-binding proteins in the control of transcription: Structure to function. BioEssays. 2002;24:625–636. doi: 10.1002/bies.10105. [DOI] [PubMed] [Google Scholar]
- 21.Hiraoki T., Vogel H.J. Structure and Function of Calcium-Binding Proteins. J. Cardiovasc. Pharmacol. 1987;10:S14–S31. doi: 10.1097/00005344-198710001-00004. [DOI] [PubMed] [Google Scholar]
- 22.Prevarskaya N., Skryma R., Bidaux G., Flourakis M., Shuba Y. Ion channels in death and differentiation of prostate cancer cells. Cell Death Differ. 2007;14:1295. doi: 10.1038/sj.cdd.4402162. [DOI] [PubMed] [Google Scholar]
- 23.Florea A.M., Busselberg D. Anti-cancer drugs interfere with intracellular calcium signaling. Neurotoxicology. 2009;30:803–810. doi: 10.1016/j.neuro.2009.04.014. [DOI] [PubMed] [Google Scholar]
- 24.Florea A.-M., Splettstoesser F., Büsselberg D. Arsenic trioxide (As2O3) induced calcium signals and cytotoxicity in two human cell lines: SY-5Y neuroblastoma and 293 embryonic kidney (HEK) Toxicol. Appl. Pharmacol. 2007;220:292–301. doi: 10.1016/j.taap.2007.01.022. [DOI] [PubMed] [Google Scholar]
- 25.Varghese E., Busselberg D. Auranofin, an anti-rheumatic gold compound, modulates apoptosis by elevating the intracellular calcium concentration ([Ca2+]i) in mcf-7 breast cancer cells. Cancers. 2014;6:2243–2258. doi: 10.3390/cancers6042243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Capiod T., Shuba Y., Skryma R., Prevarskaya N. Calcium Signalling and Disease. Volume 45. Springer; Dordrecht, The The Netherlands: 2007. Calcium signalling and cancer cell growth; pp. 405–427. [DOI] [PubMed] [Google Scholar]
- 27.Xu M., Seas A., Kiyani M., Ji K.S.Y., Bell H.N. A temporal examination of calcium signaling in cancer- from tumorigenesis, to immune evasion, and metastasis. Cell Biosci. 2018;8:25. doi: 10.1186/s13578-018-0223-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boynton A.L., Whitfield J.F., Isaacs R.J., Morton H.J. Control of 3T3 cell proliferation by calcium. In Vitro. 1974;10:12–17. doi: 10.1007/BF02615333. [DOI] [PubMed] [Google Scholar]
- 29.Pinto M.C.X., Kihara A.H., Goulart V.A.M., Tonelli F.M.P., Gomes K.N., Ulrich H., Resende R.R. Calcium signaling and cell proliferation. Cell. Signal. 2015;27:2139–2149. doi: 10.1016/j.cellsig.2015.08.006. [DOI] [PubMed] [Google Scholar]
- 30.Borowiec A.-S., Bidaux G., Pigat N., Goffin V., Bernichtein S., Capiod T. Calcium channels, external calcium concentration and cell proliferation. Eur. J. Pharmacol. 2014;739:19–25. doi: 10.1016/j.ejphar.2013.10.072. [DOI] [PubMed] [Google Scholar]
- 31.Splettstoesser F., Florea A.M., Busselberg D. IP(3) receptor antagonist, 2-APB, attenuates cisplatin induced Ca2+-influx in HeLa-S3 cells and prevents activation of calpain and induction of apoptosis. Br. J. Pharmacol. 2007;151:1176–1186. doi: 10.1038/sj.bjp.0707335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Capiod T. Extracellular Calcium Has Multiple Targets to Control Cell Proliferation. In: Rosado J.A., editor. Calcium Entry Pathways in Non-Excitable Cells. Springer International Publishing; Cham, Switzerland: 2016. pp. 133–156. [DOI] [PubMed] [Google Scholar]
- 33.Flucher B.E., Tuluc P. How and why are calcium currents curtailed in the skeletal muscle voltage-gated calcium channels? J. Physiol. 2017;595:1451–1463. doi: 10.1113/JP273423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Phan N.N., Wang C.-Y., Chen C.-F., Sun Z., Lai M.-D., Lin Y.-C. Voltage-gated calcium channels: Novel targets for cancer therapy. Oncol. Lett. 2017;14:2059–2074. doi: 10.3892/ol.2017.6457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hao J., Bao X., Jin B., Wang X., Mao Z., Li X., Wei L., Shen D., Wang J.-L. Ca2+ channel subunit α 1D promotes proliferation and migration of endometrial cancer cells mediated by 17β-estradiol via the G protein-coupled estrogen receptor. FASEB J. 2015;29:2883–2893. doi: 10.1096/fj.14-265603. [DOI] [PubMed] [Google Scholar]
- 36.Ji Y., Han Z., Shao L., Zhao Y. Ultrasound-targeted microbubble destruction of calcium channel subunit α 1D siRNA inhibits breast cancer via G protein-coupled receptor 30. Oncol. Rep. 2016;36:1886–1892. doi: 10.3892/or.2016.5031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen R., Zeng X., Zhang R., Huang J., Kuang X., Yang J., Liu J., Tawfik O., Brantley Thrasher J., Li B. Cav1.3 channel α1D protein is overexpressed and modulates androgen receptor transactivation in prostate cancers. Urol. Oncol. Semin. Orig. Investig. 2014;32:524–536. doi: 10.1016/j.urolonc.2013.05.011. [DOI] [PubMed] [Google Scholar]
- 38.Triggle D.J. The Physiological and Pharmacological Significance of Cardiovascular T-Type, Voltage-gated Calcium Channels. Am. J. Hypertens. 1998;11:80S–87S. doi: 10.1016/S0895-7061(98)00004-1. [DOI] [PubMed] [Google Scholar]
- 39.Antal L., Martin-Caraballo M. T-type Calcium Channels in Cancer. Cancers. 2019;11:134. doi: 10.3390/cancers11020134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dziegielewska B., Gray L.S., Dziegielewski J. T-type calcium channels blockers as new tools in cancer therapies. Pflügers Archiv. 2014;466:801–810. doi: 10.1007/s00424-014-1444-z. [DOI] [PubMed] [Google Scholar]
- 41.Ohkubo T., Yamazaki J. T-type voltage-activated calcium channel Cav3.1, but not Cav3.2, is involved in the inhibition of proliferation and apoptosis in MCF-7 human breast cancer cells. Int. J. Oncol. 2012;41:267–275. doi: 10.3892/ijo.2012.1422. [DOI] [PubMed] [Google Scholar]
- 42.James A.D., Chan A., Erice O., Siriwardena A.K., Bruce J.I.E. Glycolytic ATP fuels the plasma membrane calcium pump critical for pancreatic cancer cell survival. J. Biol. Chem. 2013;288:36007–36019. doi: 10.1074/jbc.M113.502948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Prakriya M., Lewis R.S. Store-Operated Calcium Channels. Physiol. Rev. 2015;95:1383–1436. doi: 10.1152/physrev.00020.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Giachini F.R., Lima V.V., Hannan J.L., Carneiro F.S., Webb R.C., Tostes R.C. STIM1/Orai1-mediated store-operated Ca2+ entry: The tip of the iceberg. Braz. J. Med. Biol. Res. 2011;44:1080–1087. doi: 10.1590/S0100-879X2011007500133. [DOI] [PubMed] [Google Scholar]
- 45.Lipskaia L., Hulot J.-S., Lompré A.-M. Role of sarco/endoplasmic reticulum calcium content and calcium ATPase activity in the control of cell growth and proliferation. Pflügers Archiv. 2009;457:673–685. doi: 10.1007/s00424-007-0428-7. [DOI] [PubMed] [Google Scholar]
- 46.Emeriau N., de Clippele M., Gailly P., Tajeddine N. Store operated calcium entry is altered by the inhibition of receptors tyrosine kinase. Oncotarget. 2018;9:16059–16073. doi: 10.18632/oncotarget.24685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang S., Miao Y., Zheng X., Gong Y., Zhang J., Zou F., Cai C. STIM1 and STIM2 differently regulate endogenous Ca2+ entry and promote TGF-β-induced EMT in breast cancer cells. Biochem. Biophys. Res. Commun. 2017;488:74–80. doi: 10.1016/j.bbrc.2017.05.009. [DOI] [PubMed] [Google Scholar]
- 48.McAndrew D., Grice D.M., Peters A.A., Davis F.M., Stewart T., Rice M., Smart C.E., Brown M.A., Kenny P.A., Roberts-Thomson S.J., et al. ORAI1-Mediated Calcium Influx in Lactation and in Breast Cancer. Mol. Cancer Ther. 2011;10:448–460. doi: 10.1158/1535-7163.MCT-10-0923. [DOI] [PubMed] [Google Scholar]
- 49.Faouzi M., Hague F., Potier M., Ahidouch A., Sevestre H., Ouadid-Ahidouch H. Down-regulation of Orai3 arrests cell-cycle progression and induces apoptosis in breast cancer cells but not in normal breast epithelial cells. J. Cell. Physiol. 2011;226:542–551. doi: 10.1002/jcp.22363. [DOI] [PubMed] [Google Scholar]
- 50.Weiss H., Amberger A., Widschwendter M., Margreiter R., Öfner D., Dietl P. Inhibition of store-operated calcium entry contributes to the anti-proliferative effect of non-steroidal anti-inflammatory drugs in human colon cancer cells. Int. J. Cancer. 2001;92:877–882. doi: 10.1002/ijc.1280. [DOI] [PubMed] [Google Scholar]
- 51.Mo P., Yang S. The store-operated calcium channels in cancer metastasis: From cell migration, invasion to metastatic colonization. Front. Biosci. 2018;23:1241–1256. doi: 10.2741/4641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yang Z., Pan L., Liu S., Li F., Lv W., Shu Y., Dong P. Inhibition of stromal-interacting molecule 1-mediated store-operated Ca(2+) entry as a novel strategy for the treatment of acquired imatinib-resistant gastrointestinal stromal tumors. Cancer Sci. 2018;109:2792–2800. doi: 10.1111/cas.13718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yang N., Tang Y., Wang F., Zhang H., Xu D., Shen Y., Sun S., Yang G. Blockade of store-operated Ca2+ entry inhibits hepatocarcinoma cell migration and invasion by regulating focal adhesion turnover. Cancer Lett. 2013;330:163–169. doi: 10.1016/j.canlet.2012.11.040. [DOI] [PubMed] [Google Scholar]
- 54.Primeau J.O., Armanious G.P., Fisher M.L.E., Young H.S. The SarcoEndoplasmic Reticulum Calcium ATPase. In: Harris J.R., Boekema E.J., editors. Membrane Protein Complexes: Structure and Function. Springer; Singapore: 2018. pp. 229–258. [DOI] [PubMed] [Google Scholar]
- 55.Fan L., Li A., Li W., Cai P., Yang B., Zhang M., Gu Y., Shu Y., Sun Y., Shen Y., et al. Novel role of Sarco/endoplasmic reticulum calcium ATPase 2 in development of colorectal cancer and its regulation by F36, a curcumin analog. Biomed. Pharmacother. 2014;68:1141–1148. doi: 10.1016/j.biopha.2014.10.014. [DOI] [PubMed] [Google Scholar]
- 56.Legrand G., Humez S., Slomianny C., Dewailly E., Vanden Abeele F., Mariot P., Wuytack F., Prevarskaya N. Ca2+ pools and cell growth. Evidence for sarcoendoplasmic Ca2+-ATPases 2B involvement in human prostate cancer cell growth control. J. Biol. Chem. 2001;276:47608–47614. doi: 10.1074/jbc.M107011200. [DOI] [PubMed] [Google Scholar]
- 57.Bergner A., Kellner J., Tufman A., Huber R.M. Endoplasmic reticulum Ca2+-homeostasis is altered in small and non-small cell lung cancer cell lines. J. Exp. Clin. Cancer Res. 2009;28:25. doi: 10.1186/1756-9966-28-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rizzuto R., Pinton P., Carrington W., Fay F.S., Fogarty K.E., Lifshitz L.M., Tuft R.A., Pozzan T. Close Contacts with the Endoplasmic Reticulum as Determinants of Mitochondrial Ca2+ Responses. Science. 1998;280:1763–1766. doi: 10.1126/science.280.5370.1763. [DOI] [PubMed] [Google Scholar]
- 59.Ivanova H., Kerkhofs M., La Rovere R.M., Bultynck G. Endoplasmic Reticulum-Mitochondrial Ca(2+) Fluxes Underlying Cancer Cell Survival. Front. Oncol. 2017;7:70. doi: 10.3389/fonc.2017.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tarasov A.I., Griffiths E.J., Rutter G.A. Regulation of ATP production by mitochondrial Ca(2+) Cell Calcium. 2012;52:28–35. doi: 10.1016/j.ceca.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Luongo T.S., Lambert J.P., Gross P., Nwokedi M., Lombardi A.A., Shanmughapriya S., Carpenter A.C., Kolmetzky D., Gao E., van Berlo J.H., et al. The mitochondrial Na(+)/Ca(2+) exchanger is essential for Ca(2+) homeostasis and viability. Nature. 2017;545:93–97. doi: 10.1038/nature22082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Marchi S., Vitto V.A.M., Danese A., Wieckowski M.R., Giorgi C., Pinton P. Mitochondrial calcium uniporter complex modulation in cancerogenesis. Cell Cycle. 2019;18:1068–1083. doi: 10.1080/15384101.2019.1612698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Romero-Garcia S., Prado-Garcia H. Mitochondrial calcium: Transport and modulation of cellular processes in homeostasis and cancer (Review) Int. J. Oncol. 2019;54:1155–1167. doi: 10.3892/ijo.2019.4696. [DOI] [PubMed] [Google Scholar]
- 64.Rathore R., McCallum J.E., Varghese E., Florea A.M., Busselberg D. Overcoming chemotherapy drug resistance by targeting inhibitors of apoptosis proteins (IAPs) Apoptosis. 2017;22:898–919. doi: 10.1007/s10495-017-1375-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vervliet T., Parys J.B., Bultynck G. Bcl-2 proteins and calcium signaling: Complexity beneath the surface. Oncogene. 2016;35:5079. doi: 10.1038/onc.2016.31. [DOI] [PubMed] [Google Scholar]
- 66.Shoshan-Barmatz V., Ben-Hail D., Admoni L., Krelin Y., Tripathi S.S. The mitochondrial voltage-dependent anion channel 1 in tumor cells. Biochim. Biophys. Acta Biomembr. 2015;1848:2547–2575. doi: 10.1016/j.bbamem.2014.10.040. [DOI] [PubMed] [Google Scholar]
- 67.Weisthal S., Keinan N., Ben-Hail D., Arif T., Shoshan-Barmatz V. Ca2+-mediated regulation of VDAC1 expression levels is associated with cell death induction. Biochim. Biophys. Acta Mol. Cell Res. 2014;1843:2270–2281. doi: 10.1016/j.bbamcr.2014.03.021. [DOI] [PubMed] [Google Scholar]
- 68.Wertz I.E., Dixit V.M. Characterization of Calcium Release-activated Apoptosis of LNCaP Prostate Cancer Cells. J. Biol. Chem. 2000;275:11470–11477. doi: 10.1074/jbc.275.15.11470. [DOI] [PubMed] [Google Scholar]
- 69.Sehgal P., Szalai P., Olesen C., Praetorius H.A., Nissen P., Christensen S.B., Engedal N., Møller J.V. Inhibition of the sarco/endoplasmic reticulum (ER) Ca(2+)-ATPase by thapsigargin analogs induces cell death via ER Ca(2+) depletion and the unfolded protein response. J. Biol. Chem. 2017;292:19656–19673. doi: 10.1074/jbc.M117.796920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Stewart T.A., Yapa K.T.D.S., Monteith G.R. Altered calcium signaling in cancer cells. Biochim. Biophys. Acta Biomembr. 2015;1848:2502–2511. doi: 10.1016/j.bbamem.2014.08.016. [DOI] [PubMed] [Google Scholar]
- 71.Günes D.A., Florea A.-M., Splettstoesser F., Büsselberg D. Co-application of arsenic trioxide (As2O3) and cisplatin (CDDP) on human SY-5Y neuroblastoma cells has differential effects on the intracellular calcium concentration ([Ca2+]i) and cytotoxicity. Neurotoxicology. 2009;30:194–202. doi: 10.1016/j.neuro.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 72.Al-Taweel N., Varghese E., Florea A.-M., Büsselberg D. Cisplatin (CDDP) triggers cell death of MCF-7 cells following disruption of intracellular calcium Ca2+ homeostasis. J. Toxicol. Sci. 2014;39:765–774. doi: 10.2131/jts.39.765. [DOI] [PubMed] [Google Scholar]
- 73.Shen L., Wen N., Xia M., Zhang Y.U., Liu W., Xu Y.E., Sun L. Calcium efflux from the endoplasmic reticulum regulates cisplatin-induced apoptosis in human cervical cancer HeLa cells. Oncol. Lett. 2016;11:2411–2419. doi: 10.3892/ol.2016.4278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Can G., Akpinar B., Baran Y., Zhivotovsky B., Olsson M. 5-Fluorouracil signaling through a calcium–calmodulin-dependent pathway is required for p53 activation and apoptosis in colon carcinoma cells. Oncogene. 2012;32:4529. doi: 10.1038/onc.2012.467. [DOI] [PubMed] [Google Scholar]
- 75.Deveci H.A., Nazıroğlu M., Nur G. 5-Fluorouracil-induced mitochondrial oxidative cytotoxicity and apoptosis are increased in MCF-7 human breast cancer cells by TRPV1 channel activation but not Hypericum perforatum treatment. Mol. Cell. Biochem. 2018;439:189–198. doi: 10.1007/s11010-017-3147-1. [DOI] [PubMed] [Google Scholar]
- 76.Kerkhofs M., Bittremieux M., Morciano G., Giorgi C., Pinton P., Parys J.B., Bultynck G. Emerging molecular mechanisms in chemotherapy: Ca2+ signaling at the mitochondria-associated endoplasmic reticulum membranes. Cell Death Dis. 2018;9:334. doi: 10.1038/s41419-017-0179-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Missiroli S., Bonora M., Patergnani S., Poletti F., Perrone M., Gafà R., Magri E., Raimondi A., Lanza G., Tacchetti C., et al. PML at Mitochondria-Associated Membranes Is Critical for the Repression of Autophagy and Cancer Development. Cell Rep. 2016;16:2415–2427. doi: 10.1016/j.celrep.2016.07.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Iwama K., Nakajo S., Aiuchi T., Nakaya K. Apoptosis induced by arsenic trioxide in leukemia U937 cells is dependent on activation of p38, inactivation of ERK and the Ca2+-dependent production of superoxide. Int. J. Cancer. 2001;92:518–526. doi: 10.1002/ijc.1220. [DOI] [PubMed] [Google Scholar]
- 79.Abdoul-Azize S., Buquet C., Li H., Picquenot J.-M., Vannier J.-P. Integration of Ca2+ signaling regulates the breast tumor cell response to simvastatin and doxorubicin. Oncogene. 2018;37:4979–4993. doi: 10.1038/s41388-018-0329-6. [DOI] [PubMed] [Google Scholar]
- 80.Blanc M.C., Holton M., Baggott R.R., Roux-Soro S.C., Armesilla A.L., Oceandy D., Cartwright E.J., Neyses L., Mohamed T.M.A., Brown S., et al. Disruption of the interaction between PMCA2 and calcineurin triggers apoptosis and enhances paclitaxel-induced cytotoxicity in breast cancer cells. Carcinogenesis. 2012;33:2362–2368. doi: 10.1093/carcin/bgs282. [DOI] [PubMed] [Google Scholar]
- 81.Pan Z., Avila A., Gollahon L. Paclitaxel induces apoptosis in breast cancer cells through different calcium--regulating mechanisms depending on external calcium conditions. Int. J. Mol. Sci. 2014;15:2672–2694. doi: 10.3390/ijms15022672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Winter E., Chiaradia L.D., Silva A.H., Nunes R.J., Yunes R.A., Creczynski-Pasa T.B. Involvement of extrinsic and intrinsic apoptotic pathways together with endoplasmic reticulum stress in cell death induced by naphthylchalcones in a leukemic cell line: Advantages of multi-target action. Toxicol. In Vitro. 2014;28:769–777. doi: 10.1016/j.tiv.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 83.van Ginkel P.R., Yan M.B., Bhattacharya S., Polans A.S., Kenealey J.D. Natural products induce a G protein-mediated calcium pathway activating p53 in cancer cells. Toxicol. Appl. Pharmacol. 2015;288:453–462. doi: 10.1016/j.taap.2015.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Varghese E., Samuel S.M., Abotaleb M., Cheema S., Mamtani R., Büsselberg D. The “Yin and Yang” of Natural Compounds in Anticancer Therapy of Triple-Negative Breast Cancers. Cancers. 2018;10:346. doi: 10.3390/cancers10100346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Madreiter-Sokolowski C.T., Gottschalk B., Parichatikanond W., Eroglu E., Klec C., Waldeck-Weiermair M., Malli R., Graier W.F. Resveratrol Specifically Kills Cancer Cells by a Devastating Increase in the Ca2+ Coupling Between the Greatly Tethered Endoplasmic Reticulum and Mitochondria. Cell. Physiol. Biochem. 2016;39:1404–1420. doi: 10.1159/000447844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wong V.K., Li T., Law B.Y., Ma E.D., Yip N.C., Michelangeli F., Law C.K., Zhang M.M., Lam K.Y., Chan P.L., et al. Saikosaponin-d, a novel SERCA inhibitor, induces autophagic cell death in apoptosis-defective cells. Cell Death Dis. 2013;4:e720. doi: 10.1038/cddis.2013.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Florea A.M., Varghese E., McCallum J.E., Mahgoub S., Helmy I., Varghese S., Gopinath N., Sass S., Theis F.J., Reifenberger G., et al. Calcium-regulatory proteins as modulators of chemotherapy in human neuroblastoma. Oncotarget. 2017;8:22876–22893. doi: 10.18632/oncotarget.15283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhang W., Couldwell W.T., Song H., Takano T., Lin J.H.C., Nedergaard M. Tamoxifen-induced Enhancement of Calcium Signaling in Glioma and MCF-7 Breast Cancer Cells. Cancer Res. 2000;60:5395. [PubMed] [Google Scholar]
- 89.Jan C.-R., Cheng J.-S., Chou K.-J., Wang S.-P., Lee K.C., Tang K.-Y., Tseng L.-L., Chiang H.-T. Dual Effect of Tamoxifen, an Anti-Breast-Cancer Drug, on Intracellular Ca2+ and Cytotoxicity in Intact Cells. Toxicol. Appl. Pharmacol. 2000;168:58–63. doi: 10.1006/taap.2000.9011. [DOI] [PubMed] [Google Scholar]
- 90.Lu Y.-C., Jiann B.-P., Chang H.-T., Huang J.-K., Chen W.-C., Su W., Jan C.-R. Effect of the Anti-Breast Cancer Drug Tamoxifen on Ca2+ Movement in Human Osteosarcoma Cells. Pharmacol. Toxicol. 2002;91:34–39. doi: 10.1034/j.1600-0773.2002.910106.x. [DOI] [PubMed] [Google Scholar]
- 91.Chang H.-T., Huang J.-K., Wang J.-L., Cheng J.-S., Lee K.-C., Lo Y.-K., Liu C.-P., Chou K.-J., Chen W.-C., Su W., et al. Tamoxifen-Induced Increases in Cytoplasmic Free Ca2+ Levels in Human Breast Cancer Cells. Breast Cancer Res. Treat. 2002;71:125–131. doi: 10.1023/A:1013807731642. [DOI] [PubMed] [Google Scholar]
- 92.Hasegawa G., Akatsuka K., Nakashima Y., Yokoe Y., Higo N., Shimonaka M. Tamoxifen inhibits the proliferation of non-melanoma skin cancer cells by increasing intracellular calcium concentration. Int. J. Oncol. 2018;53:2157–2166. doi: 10.3892/ijo.2018.4548. [DOI] [PubMed] [Google Scholar]
- 93.Meneses-Morales I., Izquierdo-Torres E., Flores-Peredo L., Rodríguez G., Hernández-Oliveras A., Zarain-Herzberg Á. Epigenetic regulation of the human ATP2A3 gene promoter in gastric and colon cancer cell lines. Mol. Carcinog. 2019;58:887–897. doi: 10.1002/mc.22978. [DOI] [PubMed] [Google Scholar]
- 94.Raynal N.J.M., Lee J.T., Wang Y., Beaudry A., Madireddi P., Garriga J., Malouf G.G., Dumont S., Dettman E.J., Gharibyan V., et al. Targeting Calcium Signaling Induces Epigenetic Reactivation of Tumor Suppressor Genes in Cancer. Cancer Res. 2016;76:1494–1505. doi: 10.1158/0008-5472.CAN-14-2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kim J.-A., Kang Y.S., Jung M.-W., Lee S.H., Lee Y.S. Involvement of Ca2+ influx in the mechanism of tamoxifen-induced apoptosis in HepG2 human hepatoblastoma cells. Cancer Lett. 1999;147:115–123. doi: 10.1016/S0304-3835(99)00284-0. [DOI] [PubMed] [Google Scholar]
- 96.Tomaszewski A., Büsselberg D. Cisplatin modulates voltage gated channel currents of dorsal root ganglion neurons of rats. Neurotoxicology. 2007;28:49–58. doi: 10.1016/j.neuro.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 97.Gualdani R., de Clippele M., Ratbi I., Gailly P., Tajeddine N. Store-Operated Calcium Entry Contributes to Cisplatin-Induced Cell Death in Non-Small Cell Lung Carcinoma. Cancers. 2019;11:430. doi: 10.3390/cancers11030430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Satheesh N.J., Büsselberg D. The role of intracellular calcium for the development and treatment of neuroblastoma. Cancers. 2015;7:823–848. doi: 10.3390/cancers7020811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhao C., Lu E., Hu X., Cheng H., Zhang J.-A., Zhu X. S100A9 regulates cisplatin chemosensitivity of squamous cervical cancer cells and related mechanism. Cancer Manag. Res. 2018;10:3753–3764. doi: 10.2147/CMAR.S168276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Pelzl L., Hosseinzadeh Z., Alzoubi K., Al-Maghout T., Schmidt S., Stournaras C., Lang F. Impact of Na+/Ca2+ Exchangers on Therapy Resistance of Ovary Carcinoma Cells. Cell. Physiol. Biochem. 2015;37:1857–1868. doi: 10.1159/000438547. [DOI] [PubMed] [Google Scholar]
- 101.Schulze C., McGowan M., Jordt S.-E., Ehrlich B.E. Prolonged oxaliplatin exposure alters intracellular calcium signaling: A new mechanism to explain oxaliplatin-associated peripheral neuropathy. Clin. Colorectal Cancer. 2011;10:126–133. doi: 10.1016/j.clcc.2011.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Muscella A., Vetrugno C., Fanizzi F.P., Manca C., De Pascali S.A., Marsigliante S. A new platinum(II) compound anticancer drug candidate with selective cytotoxicity for breast cancer cells. Cell Death Dis. 2013;4:e796. doi: 10.1038/cddis.2013.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Antman K.H. Introduction: The history of arsenic trioxide in cancer therapy. Oncologist. 2001;6(Suppl. 2):1–2. doi: 10.1634/theoncologist.6-suppl_2-1. [DOI] [PubMed] [Google Scholar]
- 104.Liu L.Z., Jiang Y., Carpenter R.L., Jing Y., Peiper S.C., Jiang B.H. Role and mechanism of arsenic in regulating angiogenesis. PLoS ONE. 2011;6:e20858. doi: 10.1371/journal.pone.0020858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Park W.H., Seol J.G., Kim E.S., Hyun J.M., Jung C.W., Lee C.C., Kim B.K., Lee Y.Y. Arsenic Trioxide-mediated Growth Inhibition in MC/CAR Myeloma Cells via Cell Cycle Arrest in Association with Induction of Cyclin-dependent Kinase Inhibitor, p21, and Apoptosis. Cancer Res. 2000;60:3065–3071. [PubMed] [Google Scholar]
- 106.Jing Y., Dai J., Chalmers-Redman R.M.E., Tatton W.G., Waxman S. Arsenic Trioxide Selectively Induces Acute Promyelocytic Leukemia Cell Apoptosis Via a Hydrogen Peroxide-Dependent Pathway. Blood. 1999;94:2102–2111. [PubMed] [Google Scholar]
- 107.Nguyen T.T.T., Lim Y.J., Fan M.H.M., Jackson R.A., Lim K.K., Ang W.H., Ban K.H.K., Chen E.S. Calcium modulation of doxorubicin cytotoxicity in yeast and human cells. Genes Cells. 2016;21:226–240. doi: 10.1111/gtc.12346. [DOI] [PubMed] [Google Scholar]
- 108.Boehmerle W., Splittgerber U., Lazarus M.B., McKenzie K.M., Johnston D.G., Austin D.J., Ehrlich B.E. Paclitaxel induces calcium oscillations via an inositol 1,4,5-trisphosphate receptor and neuronal calcium sensor 1-dependent mechanism. Proc. Natl. Acad. Sci. USA. 2006;103:18356–18361. doi: 10.1073/pnas.0607240103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Greenstein S., Ghias K., Krett N.L., Rosen S.T. Mechanisms of Glucocorticoid-mediated Apoptosis in Hematological Malignancies. Clin. Cancer Res. 2002;8:1681–1694. [PubMed] [Google Scholar]
- 110.Abdoul-Azize S., Dubus I., Vannier J.-P. Improvement of dexamethasone sensitivity by chelation of intracellular Ca2+ in pediatric acute lymphoblastic leukemia cells through the prosurvival kinase ERK1/2 deactivation. Oncotarget. 2017;8:27339–27352. doi: 10.18632/oncotarget.16039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sareen D., Darjatmoko S.R., Albert D.M., Polans A.S. Mitochondria, calcium, and calpain are key mediators of resveratrol-induced apoptosis in breast cancer. Mol. Pharmacol. 2007;72:1466–1475. doi: 10.1124/mol.107.039040. [DOI] [PubMed] [Google Scholar]
- 112.Obakan P., Barrero C., Coker-Gurkan A., Arisan E.D., Merali S., Palavan-Unsal N. SILAC-Based Mass Spectrometry Analysis Reveals That Epibrassinolide Induces Apoptosis via Activating Endoplasmic Reticulum Stress in Prostate Cancer Cells. PLoS ONE. 2015;10:e0135788. doi: 10.1371/journal.pone.0135788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Park S., Lim W., You S., Song G. Ameliorative effects of luteolin against endometriosis progression in vitro and in vivo. J. Nutr. Biochem. 2019;67:161–172. doi: 10.1016/j.jnutbio.2019.02.006. [DOI] [PubMed] [Google Scholar]
- 114.Park S., Lim W., Song G. Delphinidin induces antiproliferation and apoptosis of endometrial cells by regulating cytosolic calcium levels and mitochondrial membrane potential depolarization. J. Cell. Biochem. 2019;120:5072–5084. doi: 10.1002/jcb.27784. [DOI] [PubMed] [Google Scholar]
- 115.Yu F.S., Yang J.S., Yu C.S., Lu C.C., Chiang J.H., Lin C.W., Chung J.G. Safrole Induces Apoptosis in Human Oral Cancer HSC-3 Cells. J. Dent. Res. 2010;90:168–174. doi: 10.1177/0022034510384619. [DOI] [PubMed] [Google Scholar]
- 116.Wang M., Ruan Y., Chen Q., Li S., Wang Q., Cai J. Curcumin induced HepG2 cell apoptosis-associated mitochondrial membrane potential and intracellular free Ca2+ concentration. Eur. J. Pharmacol. 2011;650:41–47. doi: 10.1016/j.ejphar.2010.09.049. [DOI] [PubMed] [Google Scholar]
- 117.Liberti M.V., Locasale J.W. The Warburg Effect: How Does it Benefit Cancer Cells? Trends Biochem. Sci. 2016;41:211–218. doi: 10.1016/j.tibs.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Samuel S.M., Varghese E., Varghese S., Busselberg D. Challenges and perspectives in the treatment of diabetes associated breast cancer. Cancer Treat. Rev. 2018;70:98–111. doi: 10.1016/j.ctrv.2018.08.004. [DOI] [PubMed] [Google Scholar]
- 119.Chakraborty P.K., Mustafi S.B., Xiong X., Dwivedi S.K.D., Nesin V., Saha S., Zhang M., Dhanasekaran D., Jayaraman M., Mannel R., et al. MICU1 drives glycolysis and chemoresistance in ovarian cancer. Nat. Commun. 2017;8:14634. doi: 10.1038/ncomms14634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Chen Z., Tang C., Zhu Y., Xie M., He D., Pan Q., Zhang P., Hua D., Wang T., Jin L., et al. TrpC5 regulates differentiation through the Ca2+/Wnt5a signalling pathway in colorectal cancer. Clin. Sci. 2017;131:227–237. doi: 10.1042/CS20160759. [DOI] [PubMed] [Google Scholar]
- 121.Wang T., Ning K., Sun X., Zhang C., Jin L.-F., Hua D. Glycolysis is essential for chemoresistance induced by transient receptor potential channel C5 in colorectal cancer. BMC Cancer. 2018;18:207. doi: 10.1186/s12885-018-4123-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Xie J., Wang B.S., Yu D.H., Lu Q., Ma J., Qi H., Fang C., Chen H.Z. Dichloroacetate shifts the metabolism from glycolysis to glucose oxidation and exhibits synergistic growth inhibition with cisplatin in HeLa cells. Int. J. Oncol. 2011;38:409–417. doi: 10.3892/ijo.2010.851. [DOI] [PubMed] [Google Scholar]
- 123.Aoki S., Morita M., Hirao T., Yamaguchi M., Shiratori R., Kikuya M., Chibana H., Ito K. Shift in energy metabolism caused by glucocorticoids enhances the effect of cytotoxic anti-cancer drugs against acute lymphoblastic leukemia cells. Oncotarget. 2017;8:94271–94285. doi: 10.18632/oncotarget.21689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Freedman R.A., Tolaney S.M. Efficacy and safety in older patient subsets in studies of endocrine monotherapy versus combination therapy in patients with HR+/HER2−advanced breast cancer: A review. Breast Cancer Res. Treat. 2018;167:607–614. doi: 10.1007/s10549-017-4560-6. [DOI] [PubMed] [Google Scholar]
- 125.Mason R.P. Calcium channel blockers, apoptosis and cancer: Is there a biologic relationship? J. Am. Coll. Cardiol. 1999;34:1857–1866. doi: 10.1016/S0735-1097(99)00447-7. [DOI] [PubMed] [Google Scholar]
- 126.Onoda J.M., Nelson K.K., Taylor J.D., Honn K.V. Invivo Characterization of Combination Antitumor Chemotherapy with Calcium Channel Blockers and cis-Diamminedichloroplatinum(II) Cancer Res. 1989;49:2844–2850. [PubMed] [Google Scholar]
- 127.Pillozzi S., D’Amico M., Bartoli G., Gasparoli L., Petroni G., Crociani O., Marzo T., Guerriero A., Messori L., Severi M., et al. The combined activation of KCa3.1 and inhibition of Kv11.1/hERG1 currents contribute to overcome Cisplatin resistance in colorectal cancer cells. Br. J. Cancer. 2017;118:200. doi: 10.1038/bjc.2017.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Huang J.-K., Chou C.-T., Chang H.-T., Shu S.-S., Kuo C.-C., Tsai J.-Y., Liao W.-C., Wang J.-L., Lin K.-L., Lu Y.-C., et al. Effect of thapsigargin on Ca2+ fluxes and viability in human prostate cancer cells. J. Recept. Signal Transduct. 2011;31:247–255. doi: 10.3109/10799893.2011.563311. [DOI] [PubMed] [Google Scholar]
- 129.Ma Z., Fan C., Yang Y., Di S., Hu W., Li T., Zhu Y., Han J., Xin Z., Wu G., et al. Thapsigargin sensitizes human esophageal cancer to TRAIL-induced apoptosis via AMPK activation. Sci. Rep. 2016;6:35196. doi: 10.1038/srep35196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Jackisch C., Hahm H.A., Tombal B., McCloskey D., Butash K., Davidson N.E., Denmeade S.R. Delayed Micromolar Elevation in Intracellular Calcium Precedes Induction of Apoptosis in Thapsigargin-treated Breast Cancer Cells. Clin. Cancer Res. 2000;6:2844–2850. [PubMed] [Google Scholar]
- 131.Thews O., Hummel M., Kelleher D.K., Lecher B., Vaupel P. Nifedipine improves blood flow and oxygen supply, but not steady-state oxygenation of tumours in perfusion pressure-controlled isolated limb perfusion. Br. J. Cancer. 2002;87:1462–1469. doi: 10.1038/sj.bjc.6600611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wood P.J., Hirst D.G. Modification of tumour response by calcium antagonists in the SCVII/St tumour implanted at two different sites. Int. J. Radiat. Biol. 1989;56:355–367. doi: 10.1080/09553008914551511. [DOI] [PubMed] [Google Scholar]