Abstract
BRCA1 gene mutation increases risk of hereditary breast/ovarian carcinogenesis. Calcium propionate is the food preservative. Superoxide dismutase enzyme protects oxidative stress in human. This report studied about the effects of 3300 del A-1061 Ter BRCA1 mutation at exon 11 and calcium propionate toxicity on parameters of oxidative stress induction and cause of breast cancer. The effects of 3300 del A-1061 Ter BRCA1 frameshift mutation on oxidative stress protection were studied by MTT dye reduction assay and superoxide dismutase activity assay. Calcium propionate and 3300 del A-1061 Ter BRCA1 frameshift mutation effects on superoxide dismutase activity were studied by superoxide dismutase activity assay on breast cancer cell line. The results showed that this mutation caused oxidative stress through superoxide dismutase activity inhibition (P < 0.05) and calcium propionate effected on superoxide dismutase activity in breast cancer cells (P < 0.05). 3300 del A-1061 Ter BRCA1 mutation and the chemical toxicity effect on oxidative stress and may lead to breast carcinogenesis.
Keywords: Oxidative stress, 3300 del A-1061 Ter BRCA1 mutation, calcium propionate
Introduction
Genetics plays a significant role by causing a hereditary breast cancer syndrome. This includes those who carry the BRCA1 and BRCA2 gene mutations. These mutations account for up to 90% of the total genetic influence with a risk of breast cancer of 60-80% in those affected [1]. In the presence of BRCA1, cells could sense and repair DNA lesions, which ensures genomic integrity and prevents tumorigenesis. However, the roles for BRCA1 include the regulation of cell cycle progression, DNA damage signaling and repair, maintenance of genomic integrity, and the regulation of various transcriptional pathways are established but the specific functions of the BRCA1 gene that contribute to tumor suppression are unclear [2]. The 3300 del A-1061 Ter frameshift BRCA1 mutation at exon 11 of BRCA1 was observed in 2002 and this mutation type caused breast/ovarian carcinogenesis [3]. The defect caused by this mutation is still unknown at present.
The chemical substances can induce cancer in human. Calcium propionate is an organic salt formed by the reaction of calcium hydroxide with propionic acid (also known as propanoic acid). Its chemical formula is Ca(OOCCH2CH3)2. The compound occurs in either crystalline or powder form. It is soluble in water and only very slightly soluble in alcohol. Metabolism of propionate begins with its conversion to propionyl coenzyme A and can directly enter neither beta oxidation nor the citric acid cycles. Calcium propionate is used as a food preservative and listed as E 282. It is used in breads and other baked goods because of its ability to inhibit the growth of molds and other microorganisms. It is not toxic to these organisms. It prevents them from reproducing and posing a health risk to humans. Propionic acid occurs naturally in some foods and acts as a preservative in those foods. Some types of cheese, for example, contain as much as 1% natural propionic acid. In agriculture, it is used, among other things, to prevent milk fever in cows and as a feed supplement. Calcium propionate is effective against both Bacillus mesentericus rope and mold. The tobacco industry has also used calcium propionate as a preservative in some of its products [4]. Calcium propionate addition to poultry litter along with water activity amelioration was an effective tool for controlling aflatoxin production in poultry feed [5]. Calcium propionate can cause allergies, intolerances, eczema, migrane headaches and skin rashes.
Superoxide dismutase (SOD, EC 1.15.1.1) is one of the most important antioxidative enzymes. Three forms of superoxide dismutase are present in humans, in all other mammals, and most chordates. SOD1 is located in the cytoplasm, SOD2 in the mitochondria, and SOD3 is extracellular. The first is a dimer (consists of two units), whereas the others are tetramers (four subunits). SOD1 and SOD3 contain copper and zinc, whereas SOD2, the mitochondrial enzyme, has manganese in its reactive center. The genes are located on chromosomes 21, 6, and 4, respectively. It alternately catalyzes the dismutation (or partitioning) of the superoxide (O2 -) radical into either ordinary molecular oxygen (O2) or hydrogen peroxide (H2O2). Superoxide is one of the main reactive oxygen species (ROS) in the cell. It is produced as a by-product of oxygen metabolism and, if not regulated, causes many types of cell damage [6]. Hydrogen peroxide is converted into water and oxygen by catalase. The SOD is an important antioxidant enzyme in nearly all living cells exposed to oxygen. The physiological importance of SODs is illustrated by the severe pathologies due to loss of SOD in genetically engineered mice to lack these enzymes. Mutations in SOD1 can cause familial Amyotrophic Lateral Sclerosis (ALS). Mice lacking SOD1 develop a wide range of pathologies, including hepatocellular carcinoma, an acceleration of age-related muscle mass loss, an earlier incidence of cataracts and a reduced lifespan. The inactivation of SOD2 in mice causes perinatal lethality. The extracellular superoxide dismutase (SOD3, ecSOD) contributes to the development of hypertension. Diminished SOD3 activity has been linked to lung diseases such as Acute Respiratory Distress Syndrome (ARDS) or Chronic Obstructive Pulmonary Disease (COPD) [7].
Oxidative stress is caused by an imbalance in the redox status of the human body. Oxidative stress is known to be important in the development of aging and the certain diseases related to advanced age. Reactive oxygen species (ROS) affects different signaling pathways, including growth factors and pathways of mitosis, and controls many cellular processes, including cell proliferation, and uncontrolled growth of cells leading to carcinogenesis. Reactive oxygen species (ROS) can have multiple effects in the initiation stage of carcinogenesis mediating carcinogen activation, causing DNA damage, and interfering with the DNA damage response [8]. The imbalance between generation of reactive oxygen species and its detoxification by biological system lead to impairment of damage repair by cells. Increased oxidative stress caused by reactive oxygen species can reduce the antioxidant defense of the body against angiogenesis and metastasis in cancer cells that are the main process in cancer development [9].
Mammary tissues are exposed to a significant level of oxidative stress, during hormone induced growth and metabolism. Hormonally driven proliferation in cancer with cellular metabolism can trigger the formation of reactive oxygen species, which in turn can produce oxidative DNA damage. The accumulation of oxidative DNA damage can be a critical initiating event in carcinogenesis [10].
This report describes investigation of the effects of 3300 del A-1061 Ter BRCA1 frameshift mutation and calcium propionate toxicity on parameters of oxidative stress induction. This BRCA1 frameshift mutation and calcium propionate toxicity may involve in oxidative stress induction and breast carcinogenesis in human.
Materials and methods
Effect of 3300 del A-1061 Ter BRCA1 frameshift mutation on oxidative stress protection
BRCA1 function defect caused by oxidative stress protection failure was studied by survival assay and superoxide dismutase activity assay.
BRCA1 expression vectors, cell line and culture
Wild-type BRCA1 expression vector (wt.BRCA1) consisting of the full-length BRCA1 cDNA within the pcDNA3 mammalian expression vector (GeneArt® gene synthesis service, Invitrogen, Germany) and 3300 del A-1061 Ter BRCA1 expression vector (GeneArt® gene synthesis service, Invitrogen, Germany) were used in this study.
The human BRCA1-mutated breast cancer cell line HCC1937 was utilized in an in vitro culture model of breast cancer. Human breast cancer cell line (HCC1937) was grown in DMEM supplemented with 5% fetal calf serum, L-glutamine (5 mmol/L), nonessential amino acids (5 mmol/L), penicillin (100 units/mL) and streptomycin (100 µg/mL).
Survival assay by MTT dye reduction
Wild-type BRCA1 expression vector (wt.BRCA1) and 3300 del A-1061 Ter BRCA1 expression vector were co-transfected in each cell culture flask. Both flasks were further treated with MTT dye reduction assay for studying BRCA1-dependent DNA repair defect caused by oxidative stress protection failure. Sub-confluent proliferating cells in 96-well dishes were treated with different doses of H2O2 (Sigma Chemical Co.,St. Louis, MO, USA.) conc. 100, 200, 300 and 400 nM for 24 h incubation time and then assayed for MTT dye reduction, a measure of mitochondrial viability. Cell viability was normalized to 0 dose control cells. Cell viability values were calculated as means ± SE. Statistical comparisons were made and P < 0.05 was regarded as statistically significant.
Superoxide dismutase (SOD) activity assay
Wild-type BRCA1 expression vector (wt.BRCA1) and 3300 del A-1061 Ter BRCA1 expression vector were co-transfected in each cell culture flask. Sub-confluent proliferating cells in 96-well dishes were detected about the effect of BRCA1 mutation on superoxide dismutase activity change by SOD activity assay kit (BioVision).
Effect of calcium propionate on superoxide dismutase activity through SKBR3 breast cancer cell line
Cell line, culture and superoxide dismutase activity assay
Breast cancer cell line (SKBR3) was grown in DMEM supplemented with 5% fetal calf serum, L-glutamine (5 mmol/L), nonessential amino acids (5 mmol/L), penicillin (100 units/mL) and streptomycin (100 µg/mL).
Sub-confluent proliferating cells in 96-well dishes were treated with calcium propionate conc. 0.5, 1.0, 1.5 and 2.0 mg/mL for 24 h incubation time. After 24 h incubation time, cells were investigated about the effect of calcium propionate on superoxide dismutase activity change by SOD activity assay kit (BioVision).
Effect of 3300 del A-1061 Ter BRCA1 frameshift mutation and calcium propionate on superoxide dismutase activity through SKBR3 breast cancer cell line
Cell line, culture and superoxide dismutase activity assay
Breast cancer cell line (SKBR3) was grown in DMEM supplemented with 5% fetal calf serum, L-glutamine (5 mmol/L), nonessential amino acids (5 mmol/L), penicillin (100 units/mL) and streptomycin (100 µg/mL).
The 3300 del A-1061 Ter BRCA1 expression vector was transfected in the cell culture flask and sub-confluent proliferating cells in 96-well dishes were treated with calcium propionate conc. 0.5, 1.0, 1.5 and 2.0 mg/mL for 24 h incubation time. After 24 h incubation time, cells were investigated about the effect of 3300 del A-1061 Ter BRCA1 frameshift mutation and calcium propionate on superoxide dismutase activity by SOD activity assay kit (BioVision).
Statistical analysis
The percentage of wild-type cell viability was compared with the percentage of mutant cell viability at different H2O2 concentrations and test by ANOVA. The percentages of inhibition activity of SOD by this mutation type and calcium propionate were test by ANOVA and P < 0.05 was regarded as statistically significant.
Results
The effect of 3300 del A-1061 Ter BRCA1 frameshift mutation on oxidative stress protection
For survival assay, the wt.BRCA1 cells were significantly more resistant to H2O2 but the mutant cells could not resist. The percentage of mutant cell viability in different H2O2 concentrations was less than the percentage of wild-type cell viability (P < 0.05) (Figure 1). The results showed that 3300 del A-1061 Ter BRCA1 frameshift mutation effected on oxidative stress protection.
Figure 1.
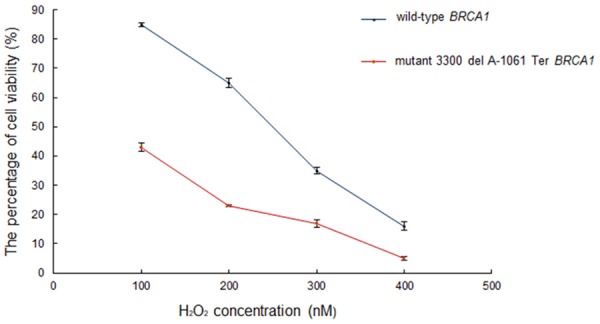
The percentage of wild-type cell viability in comparison with the percentage of mutant cell viability at different H2O2 concentrations.
The percentage of inhibition activity of superoxide dismutase caused by this mutation was reduced (P < 0.05) (Figure 2). The wt.BRCA1 mediated protection against H2O2 for DNA repair. This BRCA1 mutation affected DNA damage repair.
Figure 2.
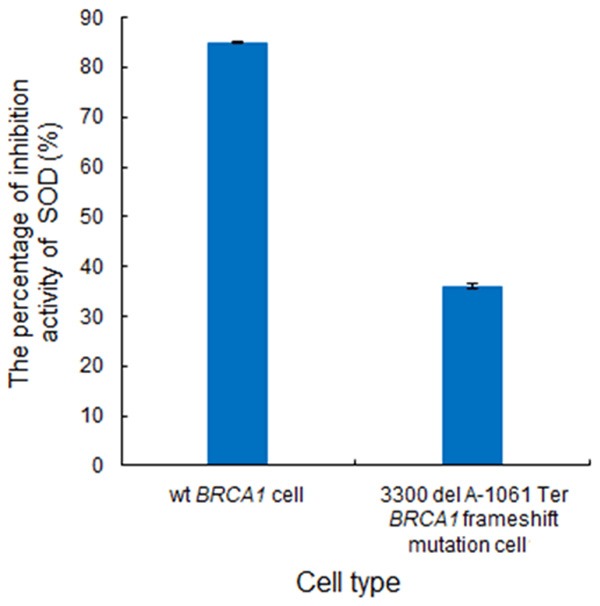
The percentage of inhibition activity of SOD induced by 3300 del A-1061 Ter BRCA1 frameshift mutation in HCC1937 cell line.
The effect of calcium propionate on superoxide dismutase activity through SKBR3 breast cancer cell line
The results showed that calcium propionate could effect on superoxide dismutase activity change through SKBR3 breast cancer cell line. The percentages of inhibition activity of superoxide dismutase caused by calcium propionate conc. 0.5 to 2.0 mg/mL were reduced (P < 0.05) (Table 1). This chemical toxicity effected antioxidant enzyme activity.
Table 1.
The percentage of inhibition activity of SOD induced by calcium propionate conc. 0.5, 1.0, 1.5 and 2.0 mg/mL in SKBR3 cell line
| Calcium propionate concentration (mg/mL) | The percentage of inhibition activity of SOD ± SE (%) |
|---|---|
| 0 | 57.2 ±0.5 |
| 0.5 | 51.9 ± 0.2 |
| 1.0 | 46 ± 0.4 |
| 1.5 | 44 ± 0.3 |
| 2.0 | 43 ± 0.1 |
Effect of 3300 del A-1061 Ter BRCA1 frameshift mutation and calcium propionate on superoxide dismutase activity through SKBR3 breast cancer cell line
The results showed that calcium propionate and 3300 del A-1061 Ter BRCA1 frameshift mutation effected on superoxide dismutase activity change through SKBR3 breast cancer cell line. The percentages of inhibition activity of superoxide dismutase caused by 3300 del A-1061 Ter BRCA1 frameshift mutation and calcium propionate (conc. 0.5, 1.0, 1.5 and 2.0 mg/mL) were reduced (P < 0.05) (Table 2).
Table 2.
The percentage of inhibition activity of SOD induced by 3300 del A-1061 Ter BRCA1 frameshift mutation and calcium propionate conc. 0.5, 1.0, 1.5 and 2.0 mg/mL in SKBR3 cell line
| The 3300 del A-1061 Ter BRCA1 frameshift mutant and the various concentrations of calcium propionate (mg/mL) | The percentage of inhibition activity of SOD ± SE (%) |
|---|---|
| 0 | 41 ± 0.4 |
| 0.5 | 38 ± 0.5 |
| 1.0 | 35 ± 0.2 |
| 1.5 | 32 ± 0.4 |
| 2.0 | 30 ± 0.6 |
Discussion
BRCA1 is phosphorylated at multiple residues by various kinases including ATM, ATR, the checkpoint kinase 2 (CHK2), the DNA-dependent protein kinase, the cyclin-dependent kinases (CDKs), the aurora kinase A and AKT. The BRCA1 and its associated kinases sense oxidative stress and activate tumor suppression activities. It has an antioxidant role in response to oxidative stress in which it regulates the expression of multiple genes, including antioxidants such as glutathione S-transferase, peroxidases. It could be one of the key protein in DNA damage response. In the presence of BRCA1, cells could sense and repair DNA lesions, which ensures genomic integrity and prevents tumorigenesis, whereas cancer associated BRCA1 mutations disrupt normal DNA damage response. The BRCA1 can suppress the nuclease activity of MRE11 and BRCA1 is required for ATM-dependent phosphorylation of NBS1 following DNA damage [11,12]. BRCA1 relocates to DNA damage sites and forms nuclear foci following DNA double-strand breaks (DSBs) [13]. The BRCA1-deficient cells were hypersensitive to DNA damage agents and impaired DNA damage repair, further suggesting that BRCA1 plays an important role in DNA repair [13-16]. Accumulated DNA damage causes genomic instability and leads to tumorigenesis. The BRCA1 may prevent cancer development by enhancing antioxidant defenses, thereby protecting cells against damage caused by exogenous and/or endogenous reactive oxygen species. The ROS can directly induce oxidized nucleotides that are subsequently converted to DSBs during replication and then repaired via homologous recombination (HR). Oxidative stress in hormonally-driven breast epithelium can promote genetic instability in cells with a loss of function of the BRCA1 pathway of homologous recombination and hence promote carcinogenesis.
In this study, The percentages of inhibition activity of superoxide dismutase in mutant cells were reduced compairing with wt.cells. BRCA1-deficient cells (which contained 3300 del A-1061 Ter BRCA1 mutation) were sensitive to H2O2 treatment by accumulating oxidative damage and double strand break (DSBs) in S-phase. These deficient cells were susceptible to DSB induced by oxidative stress and modeled by hydrogen peroxide. The accumulation of oxidative DNA damage can be a critical initiating event in carcinogenesis. 3300 del A-1061 Ter BRCA1 frameshift mutation effects on the failure of oxidative stress protection and lead to breast carcinogenesis. This is the first report about the effect of 3300 del A-1061 Ter BRCA1 frameshift mutation on breast carcinogenesis.
Calcium propionate is the chemical food preservative. The irritability, restlessness, inattention and sleep disturbance in some children may be caused by the food preservative in healthy foods consumed daily [17]. The chemical food preservative caused hematological, biochemical alterations and liver injury in male Wistar rats [18]. Oxidative stress is associated with increased production of oxidizing species or a significant decrease in the effectiveness of antioxidant defenses. The reactive species produced in oxidative stress can cause direct damage to the DNA and are therefore mutagenic, and it may also suppress apoptosis and promote proliferation, invasiveness and metastasis [6]. Alkylation of DNA bases has been postulated for inducing DNA damage [19]. Calcium propionate caused oxidative stress in cells and reduced SOD activity for cell protection in this study. The mechanism of calcium propionate induced DNA damage is presently unknown. The risk of various environmental factors such as the chemical substance (calcium propionate) can potentially induce carcinogenesis by oxidative stress induction.
When we studied about the effect of 3300 del A-1061 Ter BRCA1 frameshift mutation combined with the various calcium propionate concentrations together, we found that they could reduce SOD activity in cells. This BRCA1 frameshift mutation effect combined with the chemical toxicity may lead to carcinogenesis in human.
Oxidative stress causes carcinogenesis through genomic instability by way of DNA-damage and uncontrolled cell proliferation. The regulation of oxidative stress response by BRCA1 may constitute a wheel of the chariot of the BRCA1 tumor suppression. The environmental factors such as the chemical substance might cause carcinogenesis by reduce superoxide dismutase activity and affect oxidative stress. This study suggests that gene mutation and the chemical toxicity effect on oxidative stress. Failure for oxidative stress protection may cause breast carcinogenesis in human.
Acknowledgements
This work was supported by Thammasat University research grant 2016 and 2017.
Disclosure of conflict of interest
None.
References
- 1.King MC, Marks JH, Mandell JB. Breast and ovarian cancer risk due to inherited mutations in BRCA1 and BRCA2. Science. 2003;302:643–646. doi: 10.1126/science.1088759. [DOI] [PubMed] [Google Scholar]
- 2.Yun MH, Hiom K. Understanding the functions of BRCA1 in the DNA-damage response. Biochem Soc Trans. 2009;37:597–604. doi: 10.1042/BST0370597. [DOI] [PubMed] [Google Scholar]
- 3.Patmasiriwat P, Bhothisuwan K, Sinilnikova OM, Chopin S, Methakijvaroon S, Badzioch M, Padungsutt P, Vattanaviboon P, Vattanasapt V, Szabo C, Saunders GF, Goldgar D, Lenoir GM. Analysis of breast cancer susceptibility genes BRCA1 and BRCA2 in Thai familial and isolated early-onset breast and ovarian cancer. Hum Mutat. 2002;20:230. doi: 10.1002/humu.9049. [DOI] [PubMed] [Google Scholar]
- 4.Bintyihok A, Kositcharoenkul S. Effect of dietary calcium propionate on performance, hepatic enzyme activities and aflatoxin residues in broilers fed a diet containing low levels of aflatoxin B1. Toxicon. 2006;47:41–46. doi: 10.1016/j.toxicon.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 5.Alam S, Shah HU, Afzal M, Magan M. Influence of calcium propionate, water activity and storage time on mold incidence and aflatoxins production in broiler starter feed. Animal Feed Science and Technology. 2014;188:137–144. [Google Scholar]
- 6.Hu JJ, Dubin N, Kurland D, Ma BL, Roush GC. The effects of hydrogen peroxide on DNA repair activities. Mutat Res. 1995;336:193–201. doi: 10.1016/0921-8777(94)00054-a. [DOI] [PubMed] [Google Scholar]
- 7.Itzecka J. Superoxide dismutase-1 (SOD-1) gene mutation-dependent mechanisms of neural degeneration in amyotrophic lateral sclerosis. Neurol Neurochir Pol. 2001;35:461–469. [PubMed] [Google Scholar]
- 8.Lau AT, Wang Y, Chiu JF. Reactive oxygen species: current knowledge and applications in cancer research and therapeutic. J Cell Biochem. 2008;104:657–667. doi: 10.1002/jcb.21655. [DOI] [PubMed] [Google Scholar]
- 9.Nogueira V, Hay N. Molecular pathways: reactive oxygen species homeostasis in cancer cells and implications for cancer therapy. Clin Cancer Res. 2013;19:4309–4314. doi: 10.1158/1078-0432.CCR-12-1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jorgenson TC, Zhong W, Obertey TD. Redox imbalance and biochemical changes in cancer. Cancer Res. 2013;73:6118–6123. doi: 10.1158/0008-5472.CAN-13-1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yarden RI, Brody LC. BRCA1 interacts with components of the histone deacetylase complex. Proc Natl Acad Sci U S A. 1998;96:4983–4988. doi: 10.1073/pnas.96.9.4983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang Y, Cortez D, Yazdi P, Neff N, Elledge SJ, Qin J. BASC, a super complex of BRCA1-associated proteins involved in the recognition and repair of aberrant DNA structures. Genes Dev. 2000;14:927–939. [PMC free article] [PubMed] [Google Scholar]
- 13.Scully R, Chen J, Ochs RL, Keegan K, Hoekstra M, Feunteun J, Livingston DM. Dynamic changes of BRCA1 subnuclear location and phosphorylation state are initiated by DNA damage. Cell. 1997;90:425–435. doi: 10.1016/s0092-8674(00)80503-6. [DOI] [PubMed] [Google Scholar]
- 14.MacLachlan TK, Somasundaram K, Sgagias M, Shifman Y, Muschel RJ, Cowan KH, EL-Deiry WS. BRCA1 effects on the cell cycle and the DNA damage response are linked to altered gene expression. J Biol Chem. 2000;275:2777–2785. doi: 10.1074/jbc.275.4.2777. [DOI] [PubMed] [Google Scholar]
- 15.Rosen EM, Fan S, Pestell RG, Goldberg ID. The BRCA1 gene in breast cancer. J Cell Physiol. 2003;196:19–41. doi: 10.1002/jcp.10257. [DOI] [PubMed] [Google Scholar]
- 16.Xiong J, Fan S, Meng Q, Schramm L, Wang C, Bouzahza B, Zhou J, Zafonte B, Goldberg ID, Haddad BR, Pestell RG, Rosen EM. BRCA1 inhibition of telomerase activity in cultured cells. Mol Cell Biol. 2003;23:8668–8690. doi: 10.1128/MCB.23.23.8668-8690.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dengate S, Ruben A. Controlled trial of cumulative behavioural effects of a common bread preservative. J Paediatr Child Health. 2002;38:373–376. doi: 10.1046/j.1440-1754.2002.00009.x. [DOI] [PubMed] [Google Scholar]
- 18.Delgado González MF, Zamora González A, Gonsebatt ME, Mata Meza E, Vargas Garcia GG, Rincón Calleros EY, Morales Pérez R. Subacute intoxication with sodium nitrate induces hematological and biochemical alterations and liver injury in male wistar rats. Ecotoxicol Environ Saf. 2018;166:48–55. doi: 10.1016/j.ecoenv.2018.09.060. [DOI] [PubMed] [Google Scholar]
- 19.Schlatter J, Wurgler FE, Kranzlin R, Maier P, Holliger E, Graf U. The potential genotoxicity of sorbates: effect on cell cycle in vitro in V79 cells and somatic mutations in drosophila. Food Chem Toxicol. 1992;30:843–851. doi: 10.1016/0278-6915(92)90049-q. [DOI] [PubMed] [Google Scholar]


