Abstract
The amyloid precursor protein (APP) is the parent polypeptide from which amyloid-beta (Aβ) peptides, key etiological agents of Alzheimer’s disease (AD), are generated by sequential proteolytic processing involving β- and γ-secretases. APP mutations underlie familial, early-onset AD, and the involvement of APP in AD pathology has been extensively studied. However, APP has important physiological roles in the mammalian brain, particularly its modulation of synaptic functions and neuronal survival. Recent works have now shown that APP could directly modulate γ-aminobutyric acid (GABA) neurotransmission in two broad ways. Firstly, APP is shown to interact with and modulate the levels and activity of the neuron-specific Potassium-Chloride (K+-Cl−) cotransporter KCC2/SLC12A5. The latter is key to the maintenance of neuronal chloride (Cl−) levels and the GABA reversal potential (EGABA), and is therefore important for postsynaptic GABAergic inhibition through the ionotropic GABAA receptors. Secondly, APP binds to the sushi domain of metabotropic GABAB receptor 1a (GABABR1a). In this regard, APP complexes and is co-transported with GABAB receptor dimers bearing GABABR1a to the axonal presynaptic plasma membrane. On the other hand, secreted (s)APP generated by secretase cleavages could act as a GABABR1a-binding ligand that modulates presynaptic vesicle release. The discovery of these novel roles and activities of APP in GABAergic neurotransmission underlies the physiological importance of APP in postnatal brain function.
Keywords: amyloid precursor protein (APP), amyloid-beta (Aβ), gamma-aminobutyric acid (GABA), GABA receptor, potassium chloride cotransporter 2 (KCC2)
1. Introduction
Alzheimer’s disease (AD) [1] is the most prevalent cause for aging-associated dementia [2]. The amyloid cascade hypothesis [3] posits that the accumulation and deposition of the amyloid-beta (Aβ) peptides in the brain parenchyma is a crucial step in disease development [4]. Aβ peptides are generated from the amyloid precursor protein (APP) through sequential cleavages by the β-secretase Beta-site APP Cleaving Enzyme 1 (BACE1) and the Presenilin-containing γ-secretase complex [5]. However, a first APP cleavage by the α-secretase ADAM10 [6] would effectively preclude Aβ formation. Much of the AD research over the years has focused on attempts to better understand the BACE1-γ-secretase-mediated amyloidogenic pathway, as well as searching for means to inhibit APP proteolysis or to decrease amyloid load. Although it is now clear that proteolytic processing of APP is complex [6,7,8,9] and no clinical trial of anti-Aβ drugs have shown any clear benefits to date [10], Aβ remains a prime AD therapeutic target [11,12] and continues to garner research efforts and interests.
APP is itself known to have a range of activities in the brain that are indicative of its physiological importance [13,14,15]. Mammals have three paralogous genes which encode APP and two APP-like proteins (APLP1 and APLP2) [16]. Although APP knockout in mice produce viable and fertile offspring, APP-deficient adult mice exhibit decreased locomotor activity compared to wild-type, as well as signs of neuroinflammation [17]. Various combinations of genetic deficiencies of the three members of the APP family resulted in early postnatal death and neurodevelopmental defects [18,19], attesting to both overlapping as well as non-redundant functions of the APP paralogues. Although fairly ubiquitous in its expression, a good number of physiological roles for APP and its non-amyloid cleavage products are known to affect neurons and neurotransmission. These include neurite/axon outgrowth [20,21,22], axonal guidance [23], neural cell adhesion [24,25], neuronal survival [26,27,28], and neural progenitor cell-fate determination [29,30]. Most importantly, APP is involved in the modulation of synaptic neurotransmission and plasticity. Both pre- and post-synaptic protein compositions are altered in neurons bearing APP mutant transgenes [31], or those in APP knockout [32] mice. The changes include reductions in the key postsynaptic neurotransmission components Postsynaptic density protein 95 (PSD-95) and the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) subunit GluR1. APP is a synaptic adhesion molecule [25,33,34] and has both presynaptic [35,36] and postsynaptic [37] localization and functions [38]. Aβ is well known for causing pathological dysregulation of postsynaptic trafficking of both the AMPA [39] and N-Methyl-d-aspartate (NMDA) [40]-type glutamate receptors. Notably, APP also has physiological roles in the function and trafficking of these glutamate receptors [41,42,43] and may thus be important for synaptic plasticity and learning/memory [44,45,46,47,48]. The actions of APP at the synapse are also known to be mediated by the secreted (s)APPs, mainly sAPPα generated by α-secretase cleavage [49,50,51,52,53].
γ-aminobutyric acid (GABA), a major inhibitory neurotransmitter in the brain, shapes brain tissue activity and provides a balancing stability to neural systems and networks [54] by preventing uncontrolled hyper-excitation (such as those occurring during epileptic episodes [55]). GABAergic neurotransmission is mediated by the ionotropic GABAA receptors (GABAAR) [56], as well as the metabotropic GABAB receptors (GABABR) [57]. GABAAR functions as ligand-gated chloride (Cl−) channels and whether GABA binding would be depolarizing or hyperpolarizing is largely determined by intracellular Cl− concentrations and the GABA reversal potential (EGABA). Resting Cl− concentration in central nervous system (CNS) neurons is determined by the activity of two major cation-chloride cotransporters, namely the Cl− influx-mediating Na+-K+-2Cl− cotransporter 1 (NKCC1) and the efflux-mediating K+-Cl− cotransporter 2 (KCC2) [58]. In the adult brain, GABA is mainly hyperpolarizing and inhibitory, but it is primarily depolarizing and excitatory in developing neurons, as demonstrated using rat embryonic and neonatal cortical slices [59]. This is largely because embryonic or immature neurons have high levels of NKCC1 but low levels of KCC2. However, KCC2 expression is developmentally upregulated in mature neurons, resulting in an increase in intracellular Cl−, with GABA thus becoming hyperpolarizing and inhibitory [60]. Changes in KCC2 expression and activity may thus underlie neuropathological conditions [61,62,63] associated with weakened GABA signaling due to a positive shift in EGABA.
Other than modulating the activity of excitatory glutamate receptors, recent works have now shown that APP could also directly modulate GABA neurotransmission via its interaction with KCC2 and its alteration of intracellular Cl− [64,65]. Furthermore, APP or its soluble cleavage product could interact with GABABR to modulate presynaptic GABABR-mediated inhibition or presynaptic vesicle release [66,67]. In the paragraphs that follow, an update of these findings is provided and the new perspectives brought about by these findings are discussed.
2. Amyloid Precursor Protein (APP) and Gamma-Aminobutyric Acid (GABA)ergic Neurotransmission
There are some earlier indications that APP modulates GABAergic transmission. In the loss-of-function context of the APP knockout mouse, an impairment in synaptic plasticity, as demonstrated by deficiencies in Long-term potentiation (LTP) formation [68,69] and behavioral/learning deficits [70], is associated with a reduction in GABA-elicited inhibitory post-synaptic currents [69]. Also, theta-gamma oscillation phase-amplitude coupling involving inhibitory transmission was strongly diminished in recordings from the parietal cortex and hippocampus of APP knockout mice [71]. APP is highly expressed in the GABAergic neurons in the neurogenic dentate gyrus, and selective deletion of APP in GABAergic, but not glutamatergic neurons disrupted adult hippocampal neurogenesis [72]. In this regard, it is notable that the excitatory activity of GABA on newborn neurons at the dentate gyrus is critical for synapse formation and dendritic development [73] and APP would thus play a role in GABA transmission for newborn neurons in embryonic neonatal as well as adult neurogenic settings. APP also appears to interact with and regulate the levels of Ca(v)1.2, the channel pore subunit of L-type calcium channels downstream of depolarizing GABA neurotransmission in neurons of the striatum and hippocampus. Changes in GABAergic short-term plasticity in these neurons with the loss of APP may therefore be related to this interaction [74]. Taken together, perturbations in GABAergic inhibitory transmission in CNS neurons resulting from the loss of APP attested to the latter’s function in modulating the former.
Some findings in the context of APP over-expression are also in support of its role in GABAergic neurotransmission. Controllable transgenic over-expression of APP in transgenic mice from birth (but not over-expression in adults) resulted in epileptiform electroencephalogram abnormalities which are not related to Aβ levels or plaque load, and are unaffected by a γ-secretase inhibitor [75]. In a mouse model of Down syndrome (DS), with mice harboring an extra chromosome 16 on which APP is located, GABAAR signaling was in fact found to be excitatory rather than inhibitory in hippocampal slices from the DS mice [76]. This appears to be associated with an increase in hippocampal NKCC1 expression and an inhibition of NKCC1 activity was able to reverse the phenotype. Taken as a whole, APP over-expression appears to have the effect of altering GABAergic neurotransmission by shifting the neuronal GABA reversal potential. In the section below, new findings on how APP influences this shift are discussed.
3. APP’s Modulation of GABAergic Neurotransmission through Potassium Chloride Cotransporter 2 (KCC2)
In investigating changes in NKCC1 and KCC2 levels and GABA responses in rat cortical neurons in culture, Doshina et al. [65] noted an increase in KCC2 and a decrease in NKCC1 levels with increasing days in vitro (DIV). These changes corresponded with a reduction in the neurons’ GABA depolarizing potential beginning 7 DIV, and which became greatly reduced by 13–17 days in vitro (DIV). Adenoviral vector-mediated over-expression of human APP in these neurons decreased both the transcript and protein levels of KCC2. However, unlike previous findings in the chromosome 16 trisomy mice [76], NKCC1 levels were unaffected by APP over-expression. Downregulation of KCC2 by APP elicited a more depolarizing GABA response, as indicated by an increase in intracellular Ca2+ due to signaling downstream from GABAAR [77] in late DIV neurons. However, unlike previous observations made with APP knockout mice [74], there were no significant changes in the levels of Ca(v)1.2. The notion that APP-induced changes in GABA response are due mainly to changes in intracellular Cl− resulting from KCC2 downregulation is supported by its reversal by the NKCC1 inhibitor bumetanide. Importantly, the authors showed some in vivo relevance of their findings in culture neurons by showing that Adenovirus-associated virus (AAV) construct-based transduction of APP in brains of mouse pups also reduced KCC2 levels without affecting NKCC1.
How does over-expressed APP downregulate KCC2? This APP activity is independent of the APP intracellular domain (AICD) (which is known for its transcriptional activities [78]), APP’s extracellular domain, or γ-secretase cleavage. However, APP over-expression is correlated with a decrease in the expression of upstream stimulating factor 1 (USF1), a known transcriptional regulator of the KCC2-encoding SLC12A5 gene [79]. Although it is unclear at the moment how APP affects the expression of USF1, the findings indicate that it is an important factor in maintaining KCC2 levels, intracellular Cl−, and EGABA in adult brain neurons.
In another report, Chen et al. [64] noted a depolarizing shift of EGABA in hippocampal slices of APP knockout mouse. By patching a glutamatergic neuron in a hippocampal culture and recording for post-synaptic unitary inhibitory postsynaptic current (uIPSC) of neighboring GABAergic interneurons, the mean uIPSC amplitude is found to be significantly reduced in APP knockout neurons compared to wild-type. Interestingly, analysis of hippocampal tissue lysates revealed a significant and specific reduction in the levels of the α1-subunit of GABAAR (which mediates fast inhibition). As with Doshina et al. [65], Chen et al. also noted a reduction in total and plasma membrane KCC2 levels (but not NKCC1) in an APP-deficient hippocampus. Both KCC2 levels and function could in fact be restored pharmacologically by Cl− extrusion enhancers such as CLP257 and CLP290 [80]. Importantly, restoration of normal KCC2 expression and function in APP-deficient mice with the CLPs reversed the changes in EGABA and GABAAR α1 levels as well as GABAAR mediated inhibition. The changes observed in APP-deficient neurons could thus be largely attributed to the reduction of KCC2 levels and activity, although it is yet unclear why GABAAR α1 levels were specifically reduced in the absence of APP.
On the other hand, Chen et al. [64] elucidated a different mechanism for APP deficiency-induced reduction in KCC2. The authors showed with co-transfection experiments that full-length APP, but not its proteolytic fragments, stabilized KCC2 levels. Functional expression of KCC2 at the neuronal cell surface is necessary for its Cl− efflux activity, and the trafficking of KCC2 to the cell surface and its subsequent endocytic internalization is regulated by different cellular mechanisms, with defects in these known to underlie a range of neuropathological conditions [58]. One such regulatory mechanism is the tyrosine phosphorylation of KCC2 mediated by tyrosine kinases, such as Src [81,82,83], which promotes KCC2 internalization from the plasma membrane and its subsequent lysosomal degradation. Interestingly, Chen et al. found that APP and KCC2 interacts physically by co-immunoprecipitation and proximity ligation assays. Moreover, levels of KCC2 tyrosine phosphorylation are increased in the absence of APP, correlating with its lower levels, and this is effectively reduced by a Src family tyrosine kinase inhibitor. It appears that APP’s interaction with KCC2 may limit its tyrosine phosphorylation, thus maintaining the former’s expression and activity at the plasma membrane. Increased tyrosine phosphorylation, however, is not the only reason why KCC2 is reduced in APP-deficient cells, as the levels of non-phosphorylatable mutants of KCC2 (Y903A and Y1087A) are still low in cells not co-expressing APP. Notably, the levels of ubiquitinated KCC2 in an APP-deficient hippocampus are significantly increased compared to wild-type, and the proteasome inhibitor MG132 increased levels of the mutant KCC2 only in the absence but not in the presence of the co-expressed APP. APP–KCC2 interactions thus appear to also limit KCC2 ubiquitination.
The findings of the two reports discussed above indicated that APP could be a physiological regulator of KCC2 expression and function, which would be consequently critical for neuronal intracellular Cl− concentrations and inhibitory neurotransmission. It appears that APP could regulate EGABA by modulating KCC2 levels in different ways, both influencing the latter’s transcript level through a major transcription factor as well as enhancing KCC2′s plasma membrane stability through limiting its susceptibility to post-translational modifications in the form of tyrosine phosphorylation and ubiquitination.
4. APP’s Modulation of Presynaptic GABAB Receptor (GABABR) Activity
Presynaptic glutamate and GABA receptors modulate neurotransmitter release [84] and the action of presynaptic GABABR in this regard has been well-documented [85,86,87]. The two subtypes of GABABR, namely GABABR1 and GABABR2, typically form functional heterodimers. There are two isoforms of GABABR1, GABABR1a and GABABR1b, which differ by the presence of two N-terminal sushi domain repeats that are unique to GABABR1a [88]. These sushi repeats confer differential plasma membrane domain targeting of GABABRs. While GABABR1b-containing GABABRs are targeted dendritically and mediate postsynaptic inhibition, GABABR1a-containing GABABR are axonal and inhibit glutamate release from the presynaptic plasma membrane [88]. The sushi repeats appear to aid axonal targeting in this regard [89,90]. Proteomics analyses have shown that GABABR1a/GABABR2 receptors co-purify with the kinesin-1 motor adapters, like the c-Jun N-terminal kinase-interacting protein (JIP) and Calsyntenin [91], attesting to the notion that these are trafficked to axons via kinesin-1-mediated axonal transport. However, as the sushi domains of GABABR1a are extracellular/luminal, they need to be linked to the cytoplasmic kinesin-1 by yet-to-be-identified transmembrane domain-containing proteins. A high-resolution proteomics screen has in fact identified some potential sushi domain interacting membrane proteins, including APP [91].
Dinamarca et al. [66] have now further investigated APP, as well as two other proteins that bind with high affinity to the sushi domains of GABABR1a and form distinct complexes with GABABR. These molecules are of interest as they could potentially function in linking GABABR1a to the axonal-targeting motor protein-adaptor complex. Among these sushi domain interactors, only the loss of APP impaired GABABR-mediated presynaptic inhibition. In this regard, the GABABR agonist baclofen was less able to reduce the amplitude of the evoked excitatory postsynaptic current (EPSC), as well as the frequency of miniature EPSC, in APP-deficient compared to wild-type hippocampal slices. APP was previously known to associate with both JIP [92] and calsyntenin [93], and confirmation of the interactions in this regard attested to APP’s potential to function as a transmembrane linker that facilitates axonal transport of GABABR. Interestingly, complex formation with GABABRs stabilizes APP at the cell surface and appears to reduce amyloidogenic processing of APP to Aβ. Thus, other than APP serving a GABABR axonal transport role to the presynaptic plasma membrane, the APP-GABABR complex formation may potentially also influence APP proteolysis and Aβ formation.
If full-length APP could interact with GABABR1a, sAPPs which encompass APP’s ectodomain, might be able to do likewise. A recent proteomics screen by Rice et al. [67] has indeed uncovered that sAPPα’s extension domain (ExD) [94] binds directly to the sushi 1 domain of GABABR1a. This sAPPα–GABABR1a interaction reduced the release probability of synaptic vesicles and suppressed synaptic transmission. This inhibition of synaptic vesicle release underlies sAPPα’s apparent enhancement of short-term plasticity at Schaffer collateral synapses of hippocampal slices in a GABABR1a-dependent manner. In fact, a 17–amino acid peptide within sAPPα’s ExD was able to replace sAPPα’s activity in this regard, and when infused into the hippocampal region suppressed in vivo spontaneous neuronal activity of CA1 pyramidal cells in mice. These findings indicate that GABABR1a act as a high-affinity synaptic receptor for sAPPα, mediating a physiological role for sAPPs in modulating synaptic transmission.
5. New Perspectives
The findings described above (summarized in Figure 1) provided some fresh perspectives on APP’s role in GABAergic neurotransmission. That APP could modulate KCC2 levels (and thus intracellular Cl− levels) either at the transcriptional or post-translational level would mean that the former has an important role to play in inhibitory neurotransmission, not just through the ionotropic GABAAR but also through the glycine receptor [95], another ligand-gated chloride channel. APP could thus moderate neuropathological conditions by maintaining intracellular Cl− levels and attenuate depolarizing shifts in EGABA, which would heighten excitation-based neuropathology. However, the actual in vivo relevance of APP’s function in this regard remains to be determined. Future work shall reveal whether there are changes in APP levels under neuropathological conditions that might lead to changes in KCC2 levels and intracellular Cl−, or APP polymorphisms that might predispose an individual to conditions like epileptic seizures or neuropathic pain. From a cellular and molecular perspective, much remains to be learned with regard to the physical and functional interactions between APP and KCC2. As alluded to above, how APP deficiency affects KCC2 transcription is not yet fully deciphered. Furthermore, the nature and dynamics of the APP–KCC2 interaction that effectively reduces KCC2 access by tyrosine kinases and ubiquitin ligases remains unclear, and whether this interaction has, in turn, any bearings on APP’s proteolytic processing is also not known.
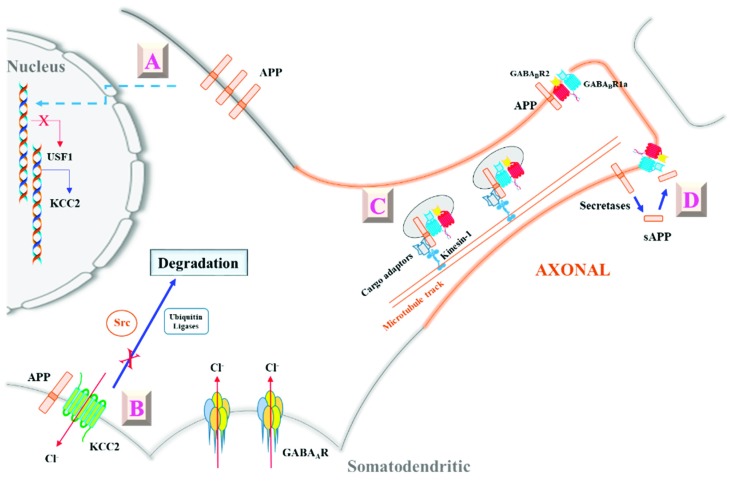
Figure 1.
A schematic diagram illustrating amyloid precursor protein’s (APP’s) modulation of γ-aminobutyric acid (GABA)ergic neurotransmission via its interaction with potassium chloride cotransporter 2 (KCC2) and GABAB receptor 1a (GABABR1a). A: APP modulates KCC2 expression by suppressing the levels of a KCC2 transcription factor UCF-1 in an unknown manner [65]. B: APP can also interact directly with KCC2, and maintains its levels and stability by inhibiting tyrosine phosphorylation and ubiquitination-based degradation [64]. APP’s modulation of KCC2 levels alters intracellular Cl− and shifts EGABA, thereby affecting inhibitory signaling through GABAARs. C: APP’s interaction with GABABR1a’s sushi domain allows it to effectively aid axon targeting of GABABR1a-GABABR2 dimers [66]. D: Furthermore, secreted (s)APP generated by secretase cleavage could bind as a ligand to GABABR1a to modulate GABABR’s presynaptic roles [67]. See text for more details.
APP’s high-affinity interaction with the sushi domain of GABABR1a gives rise to two new functional perspectives pertaining to metabotropic GABAergic signaling, particularly at the axonal presynaptic compartment where GABABR1a-GABABR2 dimers are selectively targeted to. The basis of this selective axonal targeting could now be, at least partly, attributed to GABABR1a’s interaction with APP as the latter is primarily an axonal protein by way of its engagement of the axonal trafficking kinesin-1 and the motor adaptors JIP and Calsyntenin. This presynaptic targeting of GABABR is important for the latter’s modulation of excitatory neurotransmitter release in hippocampal neurons [96]. Intriguingly, APP proteolytic products containing the APP ectodomain harboring the sushi domain interacting motif (the ExD) could also bind GABABR1a at the presynaptic compartment. sAPP, acting as an agonistic GABABR ligand, thus provides an added dimension to the regulation of GABABR activity at the presynaptic compartment. Again, the extent to which this sAPP-based modulation occurs in vivo is not yet clear. Presumably though, sAPP’s effect on postsynaptic GABABR signaling would be minimal, as these would largely consist of GABABR1b-GABABR2 which lack the sushi domain [88]. Like APP’s modulation of KCC2 levels and stability, the gross effect of APP’s action on GABABR appears therefore to be one that limits excitatory neurotransmission.
There are two neurological implications associated with the findings discussed above. The first pertains to the role of APP and its proteolytic products in synaptic plasticity. In this regard, it is notable that (1) APP proteolytic processing is enhanced by synaptic activity [97,98] and (2) GABABR is an important mediator of homeostatic synaptic plasticity [96]. As pointed out by Rice et al., the authors’ observation “...raised the possibility that the sAPP-GABABR1a interaction acts as an activity-dependent negative-feedback mechanism to suppress synaptic release and maintain proper homeostatic control of neural circuits” [67]. That APP and its cleavage product have key roles in homeostatic plasticity is very much in line with the abundance of APP at the synapse [99], as well as the synaptic deficits associated with loss of APP in mice [68,69], the latter of which could be at least partially rescued by sAPPα [44,100]. GABABR has also been implicated in several neurological disorders [101,102] and is a recognized therapeutic target in this regard [103]. The APP-interacting sushi domain could thus be a drug target that is unique to presynaptic GABABR1a.
The second neurological implication concerns APP’s role in AD pathology, beyond that of being a source of Aβ. APP has been shown to act as a cellular receptor for Aβ [104,105,106] and is known to mediate the pathological effects of Aβ and tau [107,108] in AD models. On the other hand, sAPPα’s neuronal pro-survivor activity is well known [26,28] and in this regard appears to be antagonistic to the neurotoxic nature of Aβ. APP’s role in AD pathology could thus be rather context-dependent. Interestingly, GABABR antagonists can improve memory and enhance cognition [109], and have some demonstrated benefits in animal models and patients with mild cognitive impairment (MCI) [110]. Given that hyper-excitability, interneuron dysfunctions, and network abnormalities are features often associated with, and could precede full clinical onset of, AD [111], APP and sAPPα are therefore potentially useful in countering MCI and certain aspects of AD pathology, as demonstrated recently in a mouse AD model [52]. Furthermore, as α-secretase cleavage and sAPPα generation effectively exclude BACE1 processing, therapeutic strategies that enhance α-secretase processing have been proposed to be beneficial to AD in terms of a lowering of amyloid load and enhancing neuroprotection [112,113]. These possibilities, however, remain to be more fully explored. Conversely, APP’s interaction with KCC2 and GABABR1a at the plasma membrane might influence proteolytic processing of the former in a manner that is AD-relevant. While a reduction of proteolysis of APP to Aβ is shown to result from its interaction with GABABR1a [66], the situation is less clear for KCC2. These are all points that might be worth pursuing from a therapeutics perspective.
The growing appreciation of APP’s activity in modulating both the excitatory and inhibitory neurotransmission suggests that it has fundamental, non-pathological roles in the development, maintenance, and functioning of the mammalian CNS. This fresh perspective would guide future investigations and may even help to innovate disease intervention strategies.
Acknowledgments
B.L.T. is supported by the NUS Graduate School for Integrative Sciences and Engineering (NGS) and the Office of Deputy President, Research & Technology (ODPRT) of the National University of Singapore.
Funding
This research received no external funding.
Conflicts of Interest
The author declares no conflict of interest.
References
- 1.Scheltens P., Blennow K., Breteler M.M.B., de Strooper B., Frisoni G.B., Salloway S., Van der Flier W.M. Alzheimer’s disease. Lancet. 2016;388:505–517. doi: 10.1016/S0140-6736(15)01124-1. [DOI] [PubMed] [Google Scholar]
- 2.Alzheimer’s Association Alzheimer’s Association 2016 Alzheimer’s disease facts and figures. Alzheimers Dement. 2016;12:459–509. doi: 10.1016/j.jalz.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 3.Hardy J.A., Higgins G.A. Alzheimer’s disease: The amyloid cascade hypothesis. Science. 1992;256:184–185. doi: 10.1126/science.1566067. [DOI] [PubMed] [Google Scholar]
- 4.Selkoe D.J., Hardy J. The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol. Med. 2016;8:595–608. doi: 10.15252/emmm.201606210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Strooper B., Iwatsubo T., Wolfe M.S. Presenilins and γ-secretase: Structure, function, and role in Alzheimer Disease. Cold Spring Harb. Perspect. Med. 2012;2:a006304. doi: 10.1101/cshperspect.a006304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kuhn P.H., Wang H., Dislich B., Colombo A., Zeitschel U., Ellwart J.W., Kremmer E., Rossner S., Lichtenthaler S.F. ADAM10 is the physiologically relevant, constitutive alpha-secretase of the amyloid precursor protein in primary neurons. EMBO J. 2010;29:3020–3032. doi: 10.1038/emboj.2010.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Andrew R.J., Kellett K.A.B., Thinakaran G., Hooper N.M. A Greek Tragedy: The Growing Complexity of Alzheimer Amyloid Precursor Protein Proteolysis. J. Biol. Chem. 2016;291:19235–19244. doi: 10.1074/jbc.R116.746032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Willem M., Tahirovic S., Busche M.A., Ovsepian S.V., Chafai M., Kootar S., Hornburg D., Evans L.D.B., Moore S., Daria A., et al. η-Secretase processing of APP inhibits neuronal activity in the hippocampus. Nature. 2015;526:443–447. doi: 10.1038/nature14864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang Z., Song M., Liu X., Su Kang S., Duong D.M., Seyfried N.T., Cao X., Cheng L., Sun Y.E., Ping Yu S., et al. Delta-secretase cleaves amyloid precursor protein and regulates the pathogenesis in Alzheimer’s disease. Nat. Commun. 2015;6:8762. doi: 10.1038/ncomms9762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mehta D., Jackson R., Paul G., Shi J., Sabbagh M. Why do trials for Alzheimer’s disease drugs keep failing? A discontinued drug perspective for 2010–2015. Expert Opin. Investig. Drugs. 2017;26:735–739. doi: 10.1080/13543784.2017.1323868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Panza F., Lozupone M., Dibello V., Greco A., Daniele A., Seripa D., Logroscino G., Imbimbo B.P. Are antibodies directed against amyloid-β (Aβ) oligomers the last call for the Aβ hypothesis of Alzheimer’s disease? Immunotherapy. 2019;11:3–6. doi: 10.2217/imt-2018-0119. [DOI] [PubMed] [Google Scholar]
- 12.Abbott A., Dolgin E. Failed Alzheimer’s trial does not kill leading theory of disease. Nature. 2016;540:15–16. doi: 10.1038/nature.2016.21045. [DOI] [PubMed] [Google Scholar]
- 13.Van der Kant R., Goldstein L.S.B. Cellular functions of the amyloid precursor protein from development to dementia. Dev. Cell. 2015;32:502–515. doi: 10.1016/j.devcel.2015.01.022. [DOI] [PubMed] [Google Scholar]
- 14.Lopez Sanchez M.I.G., van Wijngaarden P., Trounce I.A. Amyloid precursor protein-mediated mitochondrial regulation and Alzheimer’s disease. Br. J. Pharmacol. 2018 doi: 10.1111/bph.14554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Müller U.C., Zheng H. Physiological functions of APP family proteins. Cold Spring Harb. Perspect. Med. 2012;2:a006288. doi: 10.1101/cshperspect.a006288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shariati S.A.M., De Strooper B. Redundancy and divergence in the amyloid precursor protein family. FEBS Lett. 2013;587:2036–2045. doi: 10.1016/j.febslet.2013.05.026. [DOI] [PubMed] [Google Scholar]
- 17.Zheng H., Jiang M., Trumbauer M.E., Sirinathsinghji D.J., Hopkins R., Smith D.W., Heavens R.P., Dawson G.R., Boyce S., Conner M.W., et al. beta-Amyloid precursor protein-deficient mice show reactive gliosis and decreased locomotor activity. Cell. 1995;81:525–531. doi: 10.1016/0092-8674(95)90073-X. [DOI] [PubMed] [Google Scholar]
- 18.Herms J., Anliker B., Heber S., Ring S., Fuhrmann M., Kretzschmar H., Sisodia S., Müller U. Cortical dysplasia resembling human type 2 lissencephaly in mice lacking all three APP family members. EMBO J. 2004;23:4106–4115. doi: 10.1038/sj.emboj.7600390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heber S., Herms J., Gajic V., Hainfellner J., Aguzzi A., Rülicke T., von Kretzschmar H., von Koch C., Sisodia S., Tremml P., et al. Mice with combined gene knock-outs reveal essential and partially redundant functions of amyloid precursor protein family members. J. Neurosci. 2000;20:7951–7963. doi: 10.1523/JNEUROSCI.20-21-07951.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoareau C., Borrell V., Soriano E., Krebs M.O., Prochiantz A., Allinquant B. Amyloid precursor protein cytoplasmic domain antagonizes reelin neurite outgrowth inhibition of hippocampal neurons. Neurobiol. Aging. 2008;29:542–553. doi: 10.1016/j.neurobiolaging.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 21.Rama N., Goldschneider D., Corset V., Lambert J., Pays L., Mehlen P. Amyloid precursor protein regulates netrin-1-mediated commissural axon outgrowth. J. Biol. Chem. 2012;287:30014–30023. doi: 10.1074/jbc.M111.324780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen K., Lu H., Gao T., Xue X., Wang C., Miao F. Synergic interaction between amyloid precursor protein and neural cell adhesion molecule promotes neurite outgrowth. Oncotarget. 2016;7:14199–14206. doi: 10.18632/oncotarget.7348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang B., Li H., Mutlu S.A., Bowser D.A., Moore M.J., Wang M.C., Zheng H. The Amyloid Precursor Protein is a conserved receptor for Slit to mediate axon guidance. eNeuro. 2017;4 doi: 10.1523/ENEURO.0185-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Soba P., Eggert S., Wagner K., Zentgraf H., Siehl K., Kreger S., Löwer A., Langer A., Merdes G., Paro R., et al. Homo- and heterodimerization of APP family members promotes intercellular adhesion. EMBO J. 2005;24:3624–3634. doi: 10.1038/sj.emboj.7600824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sosa L.J., Cáceres A., Dupraz S., Oksdath M., Quiroga S., Lorenzo A. The physiological role of the amyloid precursor protein as an adhesion molecule in the developing nervous system. J. Neurochem. 2017;143:11–29. doi: 10.1111/jnc.14122. [DOI] [PubMed] [Google Scholar]
- 26.Corrigan F., Vink R., Blumbergs P.C., Masters C.L., Cappai R., van den Heuvel C. sAPPα rescues deficits in amyloid precursor protein knockout mice following focal traumatic brain injury. J. Neurochem. 2012;122:208–220. doi: 10.1111/j.1471-4159.2012.07761.x. [DOI] [PubMed] [Google Scholar]
- 27.Nikolaev A., McLaughlin T., O’Leary D.D.M., Tessier-Lavigne M. APP binds DR6 to trigger axon pruning and neuron death via distinct caspases. Nature. 2009;457:981–989. doi: 10.1038/nature07767. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 28.Milosch N., Tanriöver G., Kundu A., Rami A., François J.C., Baumkötter F., Weyer S.W., Samanta A., Jäschke A., Brod F., et al. Holo-APP and G-protein-mediated signaling are required for sAPPα-induced activation of the Akt survival pathway. Cell Death Dis. 2014;5:e1391. doi: 10.1038/cddis.2014.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Coronel R., Bernabeu-Zornoza A., Palmer C., Muñiz-Moreno M., Zambrano A., Cano E., Liste I. Role of Amyloid Precursor Protein (APP) and Its derivatives in the biology and cell fate specification of neural stem cells. Mol. Neurobiol. 2018;55:7107–7117. doi: 10.1007/s12035-018-0914-2. [DOI] [PubMed] [Google Scholar]
- 30.Coronel R., Lachgar M., Bernabeu-Zornoza A., Palmer C., Domínguez-Alvaro M., Revilla A., Ocaña I., Fernández A., Martínez-Serrano A., Cano E., et al. Neuronal and glial differentiation of human neural stem cells is regulated by Amyloid Precursor Protein (APP) levels. Mol. Neurobiol. 2019;56:1248–1261. doi: 10.1007/s12035-018-1167-9. [DOI] [PubMed] [Google Scholar]
- 31.Almeida C.G., Tampellini D., Takahashi R.H., Greengard P., Lin M.T., Snyder E.M., Gouras G.K. Beta-amyloid accumulation in APP mutant neurons reduces PSD-95 and GluR1 in synapses. Neurobiol. Dis. 2005;20:187–198. doi: 10.1016/j.nbd.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 32.Martinsson I., Capetillo-Zarate E., Faideau M., Willén K., Esteras N., Frykman S., Tjernberg L.O., Gouras G.K. APP depletion alters selective pre- and post-synaptic proteins. Mol. Cell Neurosci. 2019;95:86–95. doi: 10.1016/j.mcn.2019.02.003. [DOI] [PubMed] [Google Scholar]
- 33.Schilling S., Mehr A., Ludewig S., Stephan J., Zimmermann M., August A., Strecker P., Korte M., Koo E.H., Müller U.C., et al. APLP1 Is a synaptic cell adhesion molecule, supporting maintenance of dendritic spines and basal synaptic transmission. J. Neurosci. 2017;37:5345–5365. doi: 10.1523/JNEUROSCI.1875-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stahl R., Schilling S., Soba P., Rupp C., Hartmann T., Wagner K., Merdes G., Eggert S., Kins S. Shedding of APP limits its synaptogenic activity and cell adhesion properties. Front. Cell Neurosci. 2014;8:410. doi: 10.3389/fncel.2014.00410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Laßek M., Weingarten J., Einsfelder U., Brendel P., Müller U., Volknandt W. Amyloid precursor proteins are constituents of the presynaptic active zone. J. Neurochem. 2013;127:48–56. doi: 10.1111/jnc.12358. [DOI] [PubMed] [Google Scholar]
- 36.Fanutza T., Del Prete D., Ford M.J., Castillo P.E., D’Adamio L. APP and APLP2 interact with the synaptic release machinery and facilitate transmitter release at hippocampal synapses. eLife. 2015;4:e09743. doi: 10.7554/eLife.09743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang Z., Wang B., Yang L., Guo Q., Aithmitti N., Songyang Z., Zheng H. Presynaptic and postsynaptic interaction of the amyloid precursor protein promotes peripheral and central synaptogenesis. J. Neurosci. 2009;29:10788–10801. doi: 10.1523/JNEUROSCI.2132-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tyan S.H., Shih A.Y.J., Walsh J.J., Maruyama H., Sarsoza F., Ku L., Eggert S., Hof P.R., Koo E.H., Dickstein D.L. Amyloid precursor protein (APP) regulates synaptic structure and function. Mol. Cell Neurosci. 2012;51:43–52. doi: 10.1016/j.mcn.2012.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gu Z., Liu W., Yan Z. {beta}-Amyloid impairs AMPA receptor trafficking and function by reducing Ca2+/calmodulin-dependent protein kinase II synaptic distribution. J. Biol. Chem. 2009;284:10639–10649. doi: 10.1074/jbc.M806508200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Snyder E.M., Nong Y., Almeida C.G., Paul S., Moran T., Choi E.Y., Nairn A.C., Salter M.W., Lombroso P.J., Gouras G.K., et al. Regulation of NMDA receptor trafficking by amyloid-beta. Nat. Neurosci. 2005;8:1051–1058. doi: 10.1038/nn1503. [DOI] [PubMed] [Google Scholar]
- 41.Cousins S.L., Hoey S.E.A., Anne Stephenson F., Perkinton M.S. Amyloid precursor protein 695 associates with assembled NR2A- and NR2B-containing NMDA receptors to result in the enhancement of their cell surface delivery. J. Neurochem. 2009;111:1501–1513. doi: 10.1111/j.1471-4159.2009.06424.x. [DOI] [PubMed] [Google Scholar]
- 42.Cousins S.L., Dai W., Stephenson F.A. APLP1 and APLP2, members of the APP family of proteins, behave similarly to APP in that they associate with NMDA receptors and enhance NMDA receptor surface expression. J. Neurochem. 2015;133:879–885. doi: 10.1111/jnc.13063. [DOI] [PubMed] [Google Scholar]
- 43.Mockett B.G., Guévremont D., Elder M.K., Parfitt K.D., Peppercorn K., Morrissey J., Singh A., Hintz T.J., Kochen L., Tom Dieck S., et al. Glutamate receptor trafficking and protein synthesis mediate the facilitation of ltp by secreted amyloid precursor protein-alpha. J. Neurosci. 2019;39:3188–3203. doi: 10.1523/JNEUROSCI.1826-18.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hick M., Herrmann U., Weyer S.W., Mallm J.P., Tschäpe J.A., Borgers M., Mercken M., Roth F.C., Draguhn A., Slomianka L., et al. Acute function of secreted amyloid precursor protein fragment APPsα in synaptic plasticity. Acta Neuropathol. 2015;129:21–37. doi: 10.1007/s00401-014-1368-x. [DOI] [PubMed] [Google Scholar]
- 45.Taylor C.J., Ireland D.R., Ballagh I., Bourne K., Marechal N.M., Turner P.R., Bilkey D.K., Tate W.P., Abraham W.C. Endogenous secreted amyloid precursor protein-alpha regulates hippocampal NMDA receptor function, long-term potentiation and spatial memory. Neurobiol. Dis. 2008;31:250–260. doi: 10.1016/j.nbd.2008.04.011. [DOI] [PubMed] [Google Scholar]
- 46.Marik S.A., Olsen O., Tessier-Lavigne M., Gilbert C.D. Physiological role for amyloid precursor protein in adult experience-dependent plasticity. Proc. Natl. Acad. Sci. USA. 2016;113:7912–7917. doi: 10.1073/pnas.1604299113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Korte M., Herrmann U., Zhang X., Draguhn A. The role of APP and APLP for synaptic transmission, plasticity, and network function: Lessons from genetic mouse models. Exp. Brain Res. 2012;217:435–440. doi: 10.1007/s00221-011-2894-6. [DOI] [PubMed] [Google Scholar]
- 48.Preat T., Goguel V. Role of Drosophila Amyloid Precursor Protein in memory formation. Front. Mol Neurosci. 2016;9:142. doi: 10.3389/fnmol.2016.00142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xiong M., Jones O.D., Peppercorn K., Ohline S.M., Tate W.P., Abraham W.C. Secreted amyloid precursor protein-alpha can restore novel object location memory and hippocampal LTP in aged rats. Neurobiol. Learn Mem. 2017;138:291–299. doi: 10.1016/j.nlm.2016.08.002. [DOI] [PubMed] [Google Scholar]
- 50.Furukawa K., Barger S.W., Blalock E.M., Mattson M.P. Activation of K+ channels and suppression of neuronal activity by secreted beta-amyloid-precursor protein. Nature. 1996;379:74–78. doi: 10.1038/379074a0. [DOI] [PubMed] [Google Scholar]
- 51.Ishida A., Furukawa K., Keller J.N., Mattson M.P. Secreted form of beta-amyloid precursor protein shifts the frequency dependency for induction of LTD, and enhances LTP in hippocampal slices. Neuroreport. 1997;8:2133–2137. doi: 10.1097/00001756-199707070-00009. [DOI] [PubMed] [Google Scholar]
- 52.Tan V.T.Y., Mockett B.G., Ohline S.M., Parfitt K.D., Wicky H.E., Peppercorn K., Schoderboeck L., Yahaya M.F.B., Tate W.P., Hughes S.M., et al. Lentivirus-mediated expression of human secreted amyloid precursor protein-alpha prevents development of memory and plasticity deficits in a mouse model of Alzheimer’s disease. Mol. Brain. 2018;11:7. doi: 10.1186/s13041-018-0348-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Müller U.C., Deller T., Korte M. Not just amyloid: Physiological functions of the amyloid precursor protein family. Nat. Rev. Neurosci. 2017;18:281–298. doi: 10.1038/nrn.2017.29. [DOI] [PubMed] [Google Scholar]
- 54.Isaacson J.S., Scanziani M. How inhibition shapes cortical activity. Neuron. 2011;72:231–243. doi: 10.1016/j.neuron.2011.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hernandez C.C., Macdonald R.L. A structural look at GABA A receptor mutations linked to epilepsy syndromes. Brain Res. 2019 doi: 10.1016/j.brainres.2019.03.004. [DOI] [PubMed] [Google Scholar]
- 56.Chua H.C., Chebib M. GABAA receptors and the diversity in their structure and pharmacology. Adv. Pharmacol. 2017;79:1–34. doi: 10.1016/bs.apha.2017.03.003. [DOI] [PubMed] [Google Scholar]
- 57.Terunuma M. Diversity of structure and function of GABAB receptors: A Complexity of GABAB-mediated signaling. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2018;94:390–411. doi: 10.2183/pjab.94.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tang B.L. K+-Cl− co-transporter 2 (KCC2)—A membrane trafficking perspective. Mol. Membr. Biol. 2016;33:100–110. doi: 10.1080/09687688.2017.1393566. [DOI] [PubMed] [Google Scholar]
- 59.Owens D.F., Boyce L.H., Davis M.B., Kriegstein A.R. Excitatory GABA responses in embryonic and neonatal cortical slices demonstrated by gramicidin perforated-patch recordings and calcium imaging. J. Neurosci. 1996;16:6414–6423. doi: 10.1523/JNEUROSCI.16-20-06414.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rivera C., Voipio J., Payne J.A., Ruusuvuori E., Lahtinen H., Lamsa K., Pirvola U., Saarma M., Kaila K. The K+/Cl− co-transporter KCC2 renders GABA hyperpolarizing during neuronal maturation. Nature. 1999;397:251–255. doi: 10.1038/16697. [DOI] [PubMed] [Google Scholar]
- 61.Boulenguez P., Liabeuf S., Bos R., Bras H., Jean-Xavier C., Brocard C., Stil A., Darbon P., Cattaert D., Delpire E., et al. Down-regulation of the potassium-chloride cotransporter KCC2 contributes to spasticity after spinal cord injury. Nat. Med. 2010;16:302–307. doi: 10.1038/nm.2107. [DOI] [PubMed] [Google Scholar]
- 62.Ben-Ari Y., Khalilov I., Kahle K.T., Cherubini E. The GABA excitatory/inhibitory shift in brain maturation and neurological disorders. Neuroscientist. 2012;18:467–486. doi: 10.1177/1073858412438697. [DOI] [PubMed] [Google Scholar]
- 63.Tang X., Kim J., Zhou L., Wengert E., Zhang L., Wu Z., Carromeu C., Muotri A.R., Marchetto M.C.N., Gage F.H., et al. KCC2 rescues functional deficits in human neurons derived from patients with Rett syndrome. Proc. Natl. Acad. Sci. USA. 2016;113:751–756. doi: 10.1073/pnas.1524013113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chen M., Wang J., Jiang J., Zheng X., Justice N.J., Wang K., Ran X., Li Y., Huo Q., Zhang J., et al. APP modulates KCC2 expression and function in hippocampal GABAergic inhibition. eLife. 2017;6 doi: 10.7554/eLife.20142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Doshina A., Gourgue F., Onizuka M., Opsomer R., Wang P., Ando K., Tasiaux B., Dewachter I., Kienlen-Campard P., Brion J.P., et al. Cortical cells reveal APP as a new player in the regulation of GABAergic neurotransmission. Sci. Rep. 2017;7:370. doi: 10.1038/s41598-017-00325-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dinamarca M.C., Raveh A., Schneider A., Fritzius T., Früh S., Rem P.D., Stawarski M., Lalanne T., Turecek R., Choo M., et al. Complex formation of APP with GABAB receptors links axonal trafficking to amyloidogenic processing. Nat Commun. 2019;10:1331. doi: 10.1038/s41467-019-09164-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rice H.C., de Malmazet D., Schreurs A., Frere S., Van Molle I., Volkov A.N., Creemers E., Vertkin I., Nys J., Ranaivoson F.M., et al. Secreted amyloid-β precursor protein functions as a GABABR1a ligand to modulate synaptic transmission. Science. 2019;363 doi: 10.1126/science.aao4827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dawson G.R., Seabrook G.R., Zheng H., Smith D.W., Graham S., O’Dowd G., Bowery B.J., Boyce S., Trumbauer M.E., Chen H.Y., et al. Age-related cognitive deficits, impaired long-term potentiation and reduction in synaptic marker density in mice lacking the beta-amyloid precursor protein. Neuroscience. 1999;90:1–13. doi: 10.1016/S0306-4522(98)00410-2. [DOI] [PubMed] [Google Scholar]
- 69.Seabrook G.R., Smith D.W., Bowery B.J., Easter A., Reynolds T., Fitzjohn S.M., Morton R.A., Zheng H., Dawson G.R., Sirinathsinghji D.J., et al. Mechanisms contributing to the deficits in hippocampal synaptic plasticity in mice lacking amyloid precursor protein. Neuropharmacology. 1999;38:349–359. doi: 10.1016/S0028-3908(98)00204-4. [DOI] [PubMed] [Google Scholar]
- 70.Senechal Y., Kelly P.H., Dev K.K. Amyloid precursor protein knockout mice show age-dependent deficits in passive avoidance learning. Behav. Brain Res. 2008;186:126–132. doi: 10.1016/j.bbr.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 71.Zhang X., Zhong W., Brankačk J., Weyer S.W., Müller U.C., Tort A.B.L., Draguhn A. Impaired theta-gamma coupling in APP-deficient mice. Sci. Rep. 2016;6:21948. doi: 10.1038/srep21948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang B., Wang Z., Sun L., Yang L., Li H., Cole A.L., Rodriguez-Rivera J., Lu H.C., Zheng H. The amyloid precursor protein controls adult hippocampal neurogenesis through GABAergic interneurons. J. Neurosci. 2014;34:13314–13325. doi: 10.1523/JNEUROSCI.2848-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ge S., Goh E.L.K., Sailor K.A., Kitabatake Y., Ming G.L., Song H. GABA regulates synaptic integration of newly generated neurons in the adult brain. Nature. 2006;439:589–593. doi: 10.1038/nature04404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yang L., Wang Z., Wang B., Justice N.J., Zheng H. Amyloid precursor protein regulates Cav1.2 L-type calcium channel levels and function to influence GABAergic short-term plasticity. J. Neurosci. 2009;29:15660–15668. doi: 10.1523/JNEUROSCI.4104-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Born H.A., Kim J.Y., Savjani R.R., Das P., Dabaghian Y.A., Guo Q., Yoo J.W., Schuler D.R., Cirrito J.R., Zheng H., et al. Genetic suppression of transgenic APP rescues hypersynchronous network activity in a mouse model of Alzheimer’s disease. J. Neurosci. 2014;34:3826–3840. doi: 10.1523/JNEUROSCI.5171-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Deidda G., Parrini M., Naskar S., Bozarth I.F., Contestabile A., Cancedda L. Reversing excitatory GABAAR signaling restores synaptic plasticity and memory in a mouse model of Down syndrome. Nat. Med. 2015;21:318–326. doi: 10.1038/nm.3827. [DOI] [PubMed] [Google Scholar]
- 77.Kim D.Y., Fenoglio K.A., Simeone T.A., Coons S.W., Wu J., Chang Y., Kerrigan J.F., Rho J.M. GABAA receptor-mediated activation of L-type calcium channels induces neuronal excitation in surgically resected human hypothalamic hamartomas. Epilepsia. 2008;49:861–871. doi: 10.1111/j.1528-1167.2007.01455.x. [DOI] [PubMed] [Google Scholar]
- 78.Müller T., Meyer H.E., Egensperger R., Marcus K. The amyloid precursor protein intracellular domain (AICD) as modulator of gene expression, apoptosis, and cytoskeletal dynamics-relevance for Alzheimer’s disease. Prog. Neurobiol. 2008;85:393–406. doi: 10.1016/j.pneurobio.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 79.Markkanen M., Uvarov P., Airaksinen M.S. Role of upstream stimulating factors in the transcriptional regulation of the neuron-specific K-Cl cotransporter KCC2. Brain Res. 2008;1236:8–15. doi: 10.1016/j.brainres.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 80.Gagnon M., Bergeron M.J., Lavertu G., Castonguay A., Tripathy S., Bonin R.P., Perez-Sanchez J., Boudreau D., Wang B., Dumas L., et al. Chloride extrusion enhancers as novel therapeutics for neurological diseases. Nat. Med. 2013;19:1524–1528. doi: 10.1038/nm.3356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wake H., Watanabe M., Moorhouse A.J., Kanematsu T., Horibe S., Matsukawa N., Asai K., Ojika K., Hirata M., Nabekura J. Early changes in KCC2 phosphorylation in response to neuronal stress result in functional downregulation. J. Neurosci. 2007;27:1642–1650. doi: 10.1523/JNEUROSCI.3104-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lee H.H.C., Jurd R., Moss S.J. Tyrosine phosphorylation regulates the membrane trafficking of the potassium chloride co-transporter KCC2. Mol. Cell Neurosci. 2010;45:173–179. doi: 10.1016/j.mcn.2010.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lee H.H.C., Deeb T.Z., Walker J.A., Davies P.A., Moss S.J. NMDA receptor activity downregulates KCC2 resulting in depolarizing GABAA receptor-mediated currents. Nat. Neurosci. 2011;14:736–743. doi: 10.1038/nn.2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Raiteri M. Presynaptic metabotropic glutamate and GABAB receptors. Handb. Exp. Pharmacol. 2008;184:373–407. doi: 10.1007/978-3-540-74805-2_12. [DOI] [PubMed] [Google Scholar]
- 85.Sakaba T., Neher E. Direct modulation of synaptic vesicle priming by GABA(B) receptor activation at a glutamatergic synapse. Nature. 2003;424:775–778. doi: 10.1038/nature01859. [DOI] [PubMed] [Google Scholar]
- 86.Chalifoux J.R., Carter A.G. GABAB receptors modulate NMDA receptor calcium signals in dendritic spines. Neuron. 2010;66:101–113. doi: 10.1016/j.neuron.2010.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chalifoux J.R., Carter A.G. GABAB receptor modulation of synaptic function. Curr. Opin. Neurobiol. 2011;21:339–344. doi: 10.1016/j.conb.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Vigot R., Barbieri S., Bräuner-Osborne H., Turecek R., Shigemoto R., Zhang Y.P., Luján R., Jacobson L.H., Biermann B., Fritschy J.M., et al. Differential compartmentalization and distinct functions of GABAB receptor variants. Neuron. 2006;50:589–601. doi: 10.1016/j.neuron.2006.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Biermann B., Ivankova-Susankova K., Bradaia A., Abdel Aziz S., Besseyrias V., Kapfhammer J.P., Missler M., Gassmann M., Bettler B. The Sushi domains of GABAB receptors function as axonal targeting signals. J. Neurosci. 2010;30:1385–1394. doi: 10.1523/JNEUROSCI.3172-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hannan S., Wilkins M.E., Smart T.G. Sushi domains confer distinct trafficking profiles on GABAB receptors. Proc. Natl. Acad. Sci. USA. 2012;109:12171–12176. doi: 10.1073/pnas.1201660109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Schwenk J., Pérez-Garci E., Schneider A., Kollewe A., Gauthier-Kemper A., Fritzius T., Raveh A., Dinamarca M.C., Hanuschkin A., Bildl W., et al. Modular composition and dynamics of native GABAB receptors identified by high-resolution proteomics. Nat. Neurosci. 2016;19:233–242. doi: 10.1038/nn.4198. [DOI] [PubMed] [Google Scholar]
- 92.Muresan Z., Muresan V. Coordinated transport of phosphorylated amyloid-beta precursor protein and c-Jun NH2-terminal kinase-interacting protein-1. J. Cell Biol. 2005;171:615–625. doi: 10.1083/jcb.200502043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Vagnoni A., Perkinton M.S., Gray E.H., Francis P.T., Noble W., Miller C.C.J. Calsyntenin-1 mediates axonal transport of the amyloid precursor protein and regulates Aβ production. Hum. Mol. Genet. 2012;21:2845–2854. doi: 10.1093/hmg/dds109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Coburger I., Dahms S.O., Roeser D., Gührs K.H., Hortschansky P., Than M.E. Analysis of the overall structure of the multi-domain amyloid precursor protein (APP) PLoS ONE. 2013;8:e81926. doi: 10.1371/journal.pone.0081926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lynch J.W. Molecular structure and function of the glycine receptor chloride channel. Physiol. Rev. 2004;84:1051–1095. doi: 10.1152/physrev.00042.2003. [DOI] [PubMed] [Google Scholar]
- 96.Vertkin I., Styr B., Slomowitz E., Ofir N., Shapira I., Berner D., Fedorova T., Laviv T., Barak-Broner N., Greitzer-Antes D., et al. GABAB receptor deficiency causes failure of neuronal homeostasis in hippocampal networks. Proc. Natl. Acad. Sci. USA. 2015;112:E3291–E3299. doi: 10.1073/pnas.1424810112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Nitsch R.M., Farber S.A., Growdon J.H., Wurtman R.J. Release of amyloid beta-protein precursor derivatives by electrical depolarization of rat hippocampal slices. Proc. Natl. Acad. Sci. USA. 1993;90:5191–5193. doi: 10.1073/pnas.90.11.5191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kamenetz F., Tomita T., Hsieh H., Seabrook G., Borchelt D., Iwatsubo T., Sisodia S., Malinow R. APP processing and synaptic function. Neuron. 2003;37:925–937. doi: 10.1016/S0896-6273(03)00124-7. [DOI] [PubMed] [Google Scholar]
- 99.Wilhelm B.G., Mandad S., Truckenbrodt S., Kröhnert K., Schäfer C., Rammner B., Koo S.J., Claßen G.A., Krauss M., Haucke V., et al. Composition of isolated synaptic boutons reveals the amounts of vesicle trafficking proteins. Science. 2014;344:1023–1028. doi: 10.1126/science.1252884. [DOI] [PubMed] [Google Scholar]
- 100.Ring S., Weyer S.W., Kilian S.B., Waldron E., Pietrzik C.U., Filippov M.A., Herms J., Buchholz C., Eckman C.B., Korte M., et al. The secreted beta-amyloid precursor protein ectodomain APPs alpha is sufficient to rescue the anatomical, behavioral, and electrophysiological abnormalities of APP-deficient mice. J. Neurosci. 2007;27:7817–7826. doi: 10.1523/JNEUROSCI.1026-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Heaney C.F., Kinney J.W. Role of GABA(B) receptors in learning and memory and neurological disorders. Neurosci. Biobehav. Rev. 2016;63:1–28. doi: 10.1016/j.neubiorev.2016.01.007. [DOI] [PubMed] [Google Scholar]
- 102.Malcangio M. GABA receptors and pain. Neuropharmacology. 2018;136:102–105. doi: 10.1016/j.neuropharm.2017.05.012. [DOI] [PubMed] [Google Scholar]
- 103.Benke D. GABAB receptor trafficking and interacting proteins: Targets for the development of highly specific therapeutic strategies to treat neurological disorders? Biochem. Pharmacol. 2013;86:1525–1530. doi: 10.1016/j.bcp.2013.09.016. [DOI] [PubMed] [Google Scholar]
- 104.Sola Vigo F., Kedikian G., Heredia L., Heredia F., Añel A.D., Rosa A.L., Lorenzo A. Amyloid-beta precursor protein mediates neuronal toxicity of amyloid beta through Go protein activation. Neurobiol Aging. 2009;30:1379–1392. doi: 10.1016/j.neurobiolaging.2007.11.017. [DOI] [PubMed] [Google Scholar]
- 105.Kedikian G., Heredia F., Salvador V.R., Raimunda D., Isoardi N., Heredia L., Lorenzo A. Secreted amyloid precursor protein and holo-APP bind amyloid beta through distinct domains eliciting different toxic responses on hippocampal neurons. J. Neurosci. Res. 2010;88:1795–1803. doi: 10.1002/jnr.22347. [DOI] [PubMed] [Google Scholar]
- 106.Wang Z., Jackson R.J., Hong W., Taylor W.M., Corbett G.T., Moreno A., Liu W., Li S., Frosch M.P., Slutsky I., et al. Human brain-derived Aβ oligomers bind to synapses and disrupt synaptic activity in a manner that requires APP. J. Neurosci. 2017;37:11947–11966. doi: 10.1523/JNEUROSCI.2009-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Takahashi M., Miyata H., Kametani F., Nonaka T., Akiyama H., Hisanaga S.I., Hasegawa M. Extracellular association of APP and tau fibrils induces intracellular aggregate formation of tau. Acta Neuropathol. 2015;129:895–907. doi: 10.1007/s00401-015-1415-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Puzzo D., Piacentini R., Fá M., Gulisano W., Li Puma D.D., Staniszewski A., Zhang H., Tropea M.R., Cocco S., Palmeri A., et al. LTP and memory impairment caused by extracellular Aβ and Tau oligomers is APP-dependent. eLife. 2017;6 doi: 10.7554/eLife.26991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Helm K.A., Haberman R.P., Dean S.L., Hoyt E.C., Melcher T., Lund P.K., Gallagher M. GABAB receptor antagonist SGS742 improves spatial memory and reduces protein binding to the cAMP response element (CRE) in the hippocampus. Neuropharmacology. 2005;48:956–964. doi: 10.1016/j.neuropharm.2005.01.019. [DOI] [PubMed] [Google Scholar]
- 110.Froestl W., Gallagher M., Jenkins H., Madrid A., Melcher T., Teichman S., Mondadori C.G., Pearlman R. SGS742: The first GABA(B) receptor antagonist in clinical trials. Biochem. Pharmacol. 2004;68:1479–1487. doi: 10.1016/j.bcp.2004.07.030. [DOI] [PubMed] [Google Scholar]
- 111.Palop J.J., Mucke L. Network abnormalities and interneuron dysfunction in Alzheimer disease. Nat. Rev. Neurosci. 2016;17:777–792. doi: 10.1038/nrn.2016.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Tang B.L. Alzheimer’s disease: Channeling APP to non-amyloidogenic processing. Biochem. Biophys. Res. Commun. 2005;331:375–378. doi: 10.1016/j.bbrc.2005.03.074. [DOI] [PubMed] [Google Scholar]
- 113.Qin W., Yang T., Ho L., Zhao Z., Wang J., Chen L., Zhao W., Thiyagarajan M., MacGrogan D., Rodgers J.T., et al. Neuronal SIRT1 activation as a novel mechanism underlying the prevention of Alzheimer disease amyloid neuropathology by calorie restriction. J. Biol. Chem. 2006;281:21745–21754. doi: 10.1074/jbc.M602909200. [DOI] [PubMed] [Google Scholar]