Abstract
The larches, the Larix genus of plants are known as a natural source of taxifolin (dihydroquercetin), and extracts of its taxifolin rich xylem are used in dietary supplements to maintain health. In the present study, to assess biological activities of a methanol extract of the Japanese larch, Larix kaempferi (LK-ME), the effects of LK-ME on cell viability, inflammatory cytokine expression, and glycation were investigated. The effects of taxifolin which is known to be a main compound of LK-ME, and its related flavonoids, quercetin and luteolin were also examined. The results show that taxifolin exhibits lower growth inhibition activity and lesser induction activity of inflammatory cytokines in a human monocyte derived cell line, THP-1 cells, while in vitro anti-glycation activities of taxifolin were inhibiting at comparable levels to those of quercetin and luteolin. The growth inhibition and the cytokine induction activities, and the anti-glycation effects of LK-ME are assumed to have properties similar to taxifolin. The results of high performance liquid chromatography (HPLC) analysis indicated that taxifolin was detected as the main peak of LK-ME at the absorbance of 280 nm, and the concentration of taxifolin was measured as 3.12 mg/ml. The actual concentration of taxifolin in LK-ME is lower than the concentration estimated from the IC50 values calculated by the results of glycation assays, suggesting that other compounds contained in LK-ME are involved in the anti-glycation activity.
Keywords: Food science, Functional foods, Protein glycation, Immune response, Glycation, Larix kaempferi, Taxifolin, Flavonoids, Dihydroquercetin
1. Introduction
Japanese larch, Larix kaempferi is a deciduous needle leaved tree and afforested in Hokkaido and Nagano, Japan on a large scale. Among other Larix genus plants, Larix sibirica and Larix gmelinii are known to contain abundant taxifolin (dihydroquercetin), a flavonoid, in the xylem, and taxifolin rich extracts of these larch xylems are used in a dietary supplement [1, 2, 3]. Taxifolin is known to be an anti-oxidative agent [4], and beneficial effects of taxifolin have been reported. Previous reports showed possible beneficial effects of taxifolin using animal models, including improvement of microcirculation [5], hepatoprotective effects [6], anti-viral activity [7], and prevention of diabetic nephropathy [8] as well as cardiomyopathy [9]. Further, in vitro studies demonstrated that taxifolin exhibits anti-bacterial [10], anti-fungal [11], and anti-parasitic [12] effects, and taxifolin inhibits acetylcholinesterase and carbonic anhydrase isoenzymes [13]. Taxifolin also inhibits oligomer formation of amyloid β proteins in mice, and is thought to be effective to prevent Alzheimer related diseases [14]. Similar to L. sibirica and L. gmelinii, the xylem of L. kaempferi is also known to contain much taxifolin [15]. At present L. kaempferi extracts have not been used in dietary supplements, and the effects of L. kaempferi extracts on the health of humans or animals are not well known.
Advanced glycation end products (AGEs) are produced by non-enzymatic reactions (Maillard reaction) of sugars and proteins. Excess energy intake, especially over-consumption of hydrocarbons increases blood sugar levels followed by induced glycation reactions. Elevated blood AGE levels are found in patients with diabetes mellitus [16], and it is thought to be involved in the disease onset of diabetic complications, such as in diabetic neuropathy [17], diabetic retinopathy and diabetic nephropathy [18]. In addition, accumulation of AGEs is known to progress with aging [16, 19]. It has been reported that accumulation of AGEs in the frontal lobe is found in Alzheimer dementia patients [20]. Overall, inhibition of production and accumulation of AGEs are thought to be important to prevent age related diseases.
In the present study, to evaluate potential of L. kaempferi extract to use for a supplement to maintain health of humans and animals, the effects of L. kaempferi methanol extract (LK-ME) on cell viability, induction of inflammatory cytokine mRNAs, and inhibition activity of glycation were investigated. These effects were also examined on taxifolin, a main compound of LK-ME, and compared with other taxifolin related flavonoids, quercetin and luteolin.
2. Results
2.1. Quantification of the taxifolin concentration in LK-ME
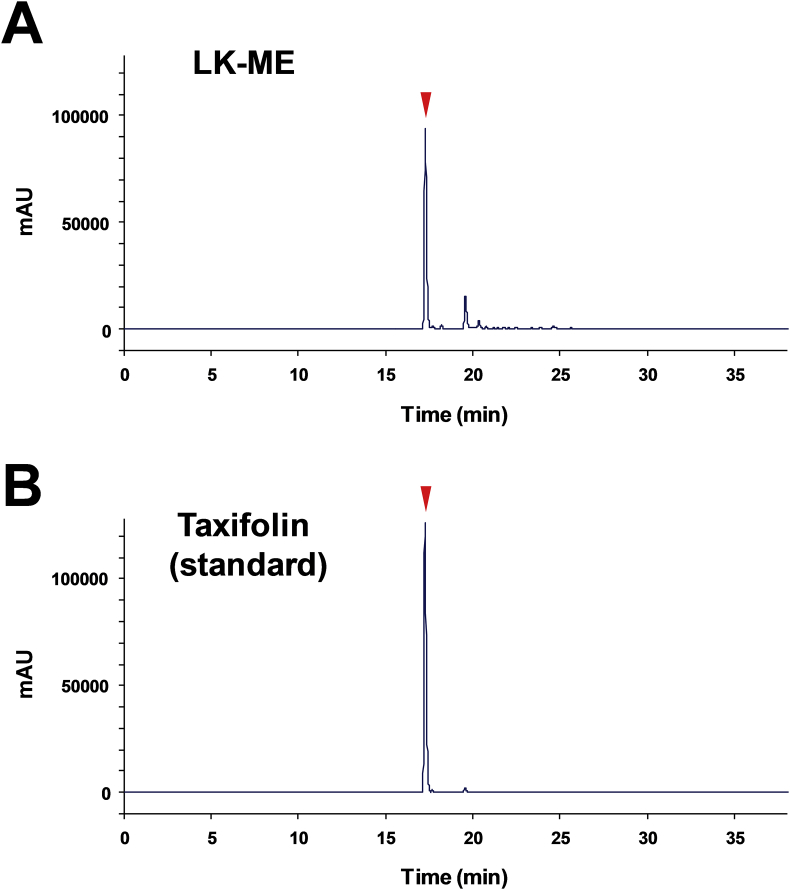
A methanol extract of L. kaempferi saw dust was used as LK-ME in this study. In this extraction condition, taxifolin is thought to be effectively extracted from the saw dust of L. kaempferi, and to substantiate this, and assess the quality of the extract, the concentration of taxifolin in LK-ME was quantified using high performance liquid chromatography (HPLC). As shown in Fig. 1A, taxifolin in LK-ME was detected as the main peak. The purity of the standard taxifolin was was calculated as 95.4% (Fig. 1B). The concentration of taxifolin in LK-ME was calculated as 3.12 mg/ml by a comparison with the peak area and the purity of taxifolin standard solution (20 μg/ml).
Fig. 1.
HPLC analysis of the taxifolin concentration in LK-ME. LK-ME (A) and purified taxifolin (B, 20 μg/ml) were loaded onto an octa decyl silyl column (YMC-Pack Pro C18, YMC, Kyoto, Japan), and eluted by a linear gradient of phosphate buffer and acetonitrile. The eluted compounds including taxifolin were detected spectrophotometrically at column wavelength of 280 nm. Taxifolin peak is indicated with arrow head.
2.2. The effects of LK-ME on THP-1 cells
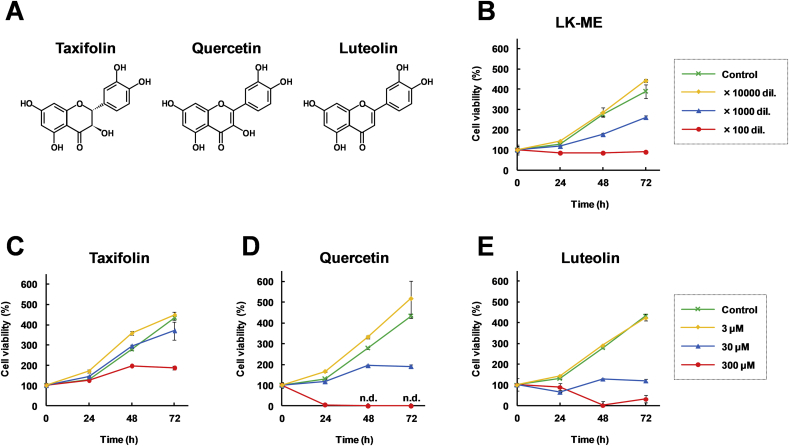
To investigate the effects of LK-ME on immune cells, the effects of LK-ME on the cell viability of a monocyte derived cell-line, THP-1 cells [21], were examined. Effects of taxifolin, a main component in the xylem of L. kaempferi, and the taxifolin related compounds, quercetin and luteolin on the cell viability were also examined. The chemical structures of taxifolin, quercetin, and luteolin are shown in Fig. 2A. The results show that LK-ME inhibits growth of THP-1 cells in a dose dependent manner (Fig. 1B). The inhibition by taxifolin on the growth of THP-1 cells was weaker than those of quercetin and luteolin (Fig. 1 C-E). The growth inhibition activity of a 100-fold dilution of LK-ME was slightly higher than that of 300 μM Taxifolin.
Fig. 2.
Effect of LK-ME treatment on the THP-1 cell viability. A. Chemical structure of taxifolin, quercetin, and luteolin. B-E. THP-1 cells were grown in media containing the indicated concentrations (rightmost panels) of LK-ME (B), taxifolin (C), quercetin (D), and luteolin (E). At the time points indicated in the figure, the cell viability was measured using a Cell Counting Kit-8. The data represent the percentages of cell viability compared with untreated control cells at the initial time point. Error bars indicate standard deviations (n = 3). n.d.: not detected.
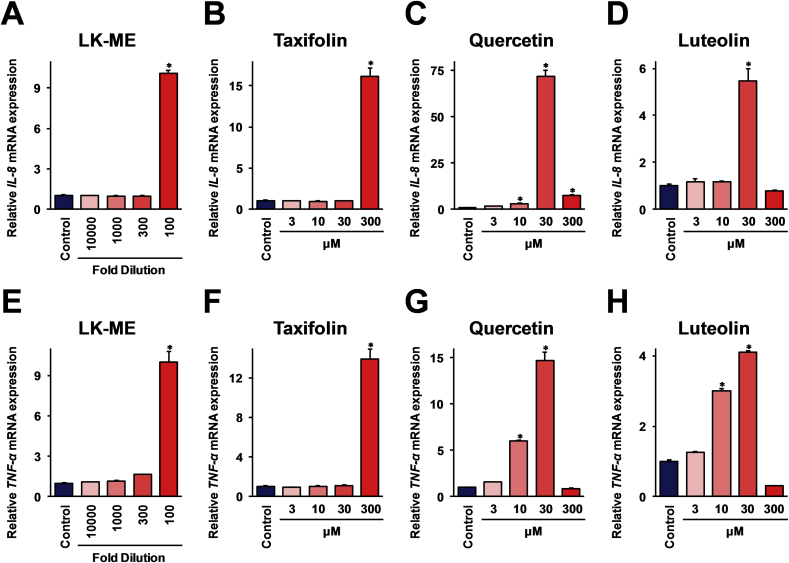
Next, the immune stimulation activity of LK-ME against THP-1 cells was investigated using real-time RT-PCR analysis. Here, THP-1 cells were stimulated with LK-ME, taxifolin, quercetin, or luteolin, and the mRNA expressions of inflammatory cytokines, interleukin-8 (IL-8), and tumor necrosis factor-α (TNF-α), were monitored. As shown in Fig. 3, the results indicate that the expressions of IL-8 and TNF-α mRNAs are significantly increased after the stimulation with LK-ME, as well as with taxifolin, quercetin, and luteolin. The induction activities of IL-8 and TNF-α mRNAs were different for these compounds, and these mRNAs were more effectively induced after stimulation with luteolin and quercetin than that of taxifolin.
Fig. 3.
The expressions of IL-8 and TNF-α mRNAs after stimulation with LK-ME. THP-1 cells were stimulated with LK-ME (A, E), taxifolin (B, F), quercetin (C, G), and luteolin (D, H) at the concentrations indicated in the figure. After 6 h of stimulation, the cells were harvested, and total RNA isolated from the cells subjected to real-time RT-PCR analysis using specific primer sets for IL-8 (A–E) and TNF-α (E–H) mRNA. The data are indicated as relative expression values compared with the expression in unstimulated control cells after normalization with the GAPDH mRNA expression. Error bars indicate standard deviations calculated from three independent experiments, and the asterisks (*) indicate that the difference is statistically significant (p < 0.01) and larger than two-fold, compared with that of the control.
2.3. Anti-glycation activity of LK-ME
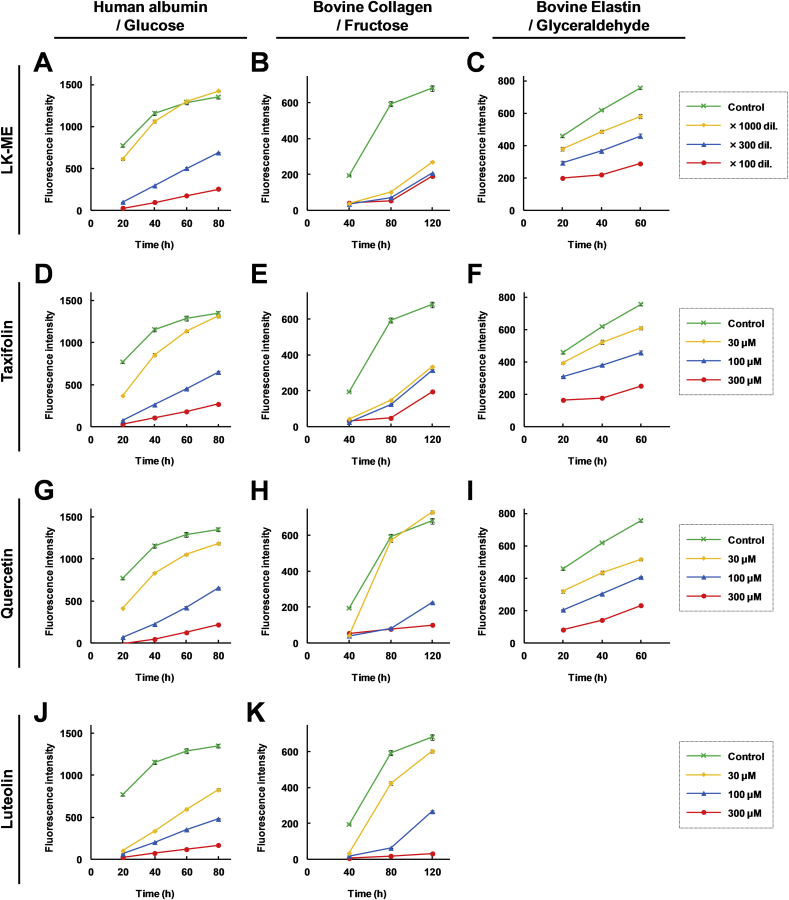
To investigate the anti-glycation activity of LK-ME, glucose, fructose, and glyceraldehyde were reacted with albumin, collagen, and elastin, respectively. Production of glycated proteins were determined by measurement of the fluorescence intensity. The anti-glycation activities of taxifolin, quercetin, and luteolin were also examined. As shown in Fig. 4A-C, LK-ME inhibited glycation in a dose dependent manner. Quercetin is known to be an anti-glycation agent [22]. Similar to quercetin (Fig. 4G-I), taxifolin (Fig. 4D-F) and luteolin (Fig. 4J and K) also exhibited anti-glycation activities in a dose dependent manner. The half maximal inhibitory concentration (IC50) calculated from these data are shown in Table 1. In the albumin-glucose reaction, luteolin exhibits the strongest anti-glycation activity. Taxifolin was more efficiently inhibiting glycation in the collagen-fructose reaction than quercetin and luteolin, and in the elastin-glyceraldehyde reaction more effectively than quercetin.
Fig. 4.
Effect of LK-ME on glycation. Human albumin and glucose (left column), bovine collagen and Fructose (middle column), and bovine elastin and glyceraldehyde (right column) were incubated with the indicated concentrations of LK-ME (A–C), taxifolin (D–F), quercetin (G–I), and luteolin (J, K). At the indicated time points, the concentrations of advanced glycation end products (AGEs) was measured by monitoring the fluorescence intensity (excitation: 365 nm, emission: 410–460 nm) using a multimode microplate reader. Error bars indicate standard deviations (n = 3).
Table 1.
The half maximal inhibitory concentration (IC50) of LK-ME against production of AGEs.
| LK-ME | Taxifolin | Quercetin | Luteolin | |
|---|---|---|---|---|
| Albumin-Glucose | ×474.97 dil. | 46.34 μM | 47.28 μM | 19.55 μM |
| 2.11 μl/ml | 14.10 μg/ml | 14.29 μg/ml | 5.60 μg/ml | |
| Collagen-Fructose | ×3214.56 dil. | 15.92μM | 48.76 μM | 41.42 μM |
| 0.31 μl/ml | 4.83 μg/ml | 14.74 μg/ml | 11.86 μg/ml | |
| Elastin-Glyceraldehyde | ×102.14 dil. | 293.32 μM | 597.30 μM | n.d. |
| 9.80 μl/ml | 89.24 μg/ml | 180.53 μg/ml | n.d. |
These IC50 values were calculated from the results of the glycation assay (Fig. 3). n.d.: no data.
3. Discussion
In present study, the effects of LK-ME on cell growth, induction of inflammatory cytokines, and glycation were investigated. Overall, the biological activities of LK-ME investigated in this study were similar to those of its main compound, taxifolin. The inhibition activity to cell growth and the induction activities of IL-8 and TNF-α mRNA of taxifolin were weaker than those of quercetin and luteolin, while the anti-glycation activities of taxifolin were comparable to those of quercetin and luteolin. LK-ME is assumed to exhibit the following attractive properties: low cytotoxicity, low inflammatory activity, and strong anti-glycation activity, as those determined for taxifolin.
The results of the cell viability analysis showed that the THP-1 cell viability was more strongly inhibited after treatment with quercetin and luteolin than taxifolin (Fig. 2B-E). Correlated with the growth inhibition activities, IL-8 and TNF-α mRNAs were more strongly induced after stimulation with quercetin and luteolin when compared with that of taxifolin (Fig. 3). The TNF-α and IL-8 are known to be inflammatory cytokines induced by various stressors [23, 24, 25]. Based on this, the stress induced after stimulated with the flavonoids is thought to be involved in the induction of TNF-α and IL-8, and the stress induction after treatment with taxifolin is assumed to be less pronounced than that of the other flavonoids examined in this study, quercetin and luteolin.
The results of the HPLC analysis showed that taxifolin was detected as the major compound which absorbs at 280 nm in LK-ME used in this study (Fig. 1). There are several minor peaks in the chromatogram chart of LK-ME. A previous report showed that a metanol extract of L. kaempferi contains dihydrokaempferol, naringenin, 4-Hydroxybenzaldehyde, p-Coumaryl aldehyde as minor compounds [15]. Therefore, the minor peaks found in the HPLC chart are thought to be including these compounds.
The results of the cell viability analysis indicate that the growth inhibition activity of a 1,000-fold dilution of LK-ME is equivalent to 30–300 μM of taxifolin (Fig. 2B and C). This gives the taxifolin concentration in LK-ME, estimated by the growth inhibition activity, as 9.1–91.3 mg/ml. The IL-8 and TNF-α mRNA induction activities of a 100-fold dilution of LK-ME extract are comparable to 300 μM of taxifolin (Fig. 3). The taxifolin concentration in LK-ME is estimated as < 9.1 mg/ml by the mRNA induction activities. Further, based on the IC50 values indicated in Table 1, anti-glycation activities of LK-ME against albumin-glucose, collagen-fructose, and elastin-glyceraldehyde were equivalent to 6.70, 15.51, and 9.12 mg/ml taxifolin, respectively. The actual concentration of taxifolin in LK-ME was calculated as 3.12 mg/ml by the HPLC analysis, and these concentrations of taxifolin in LK-ME estimated by the experimental results are higher than the concentrated quantities determined by the HPLC analysis. The difference between the estimated concentrations between the experimental results and the actual concentrations suggest that other compounds contained in LK-ME are involved in the biological activities investigated in this study.
Japanese larch, L. kaempferi is thought to be a good source of taxifolin, and as shown in this study, taxifolin is simply extracted from the saw dust of the xylem of L. kaempferi. Although, further investigations including toxicity tests are required before it becomes possible to use L. kaempferi extract as a dietary supplement, the results shown in this study suggest L. kaempferi extract to be of promise for a supplement to maintain health of humans and also animals.
4. Materials and methods
4.1. Preparetion of L. kaempferi extract (LK-ME)
LK-ME used in this study was prepared by methanol extraction from saw dust of L. kaempferi. Saw dust of L. kaempferi was obtained from the Forestry cooperative of Shimokawa town, Hokkaido, Japan. The saw dust (7.43 g) was extracted with 40 ml of methanol overnight at room temperature. The debris was removed by centrifugation, and the supernatant was filtrated with a 0.45 μm filter. Then, 1 ml of the extract was dried, resolved into 150 μl of dimethyl sulfoxide (DMSO, for assays using cultured cells) or ethanol (for glycation assay), and used in this study.
Purified taxifolin (AdooQ Bioscience, Irvine, CA, USA), quercetin (Sigma-Aldrich, St. Louis, MO, USA), and luteolin (Tokyo Chemical Industry, Tokyo, Japan) were purchased from commercially available products.
4.2. Quantitation of taxifolin concentrations in LK-ME
The concentration of taxifolin in LK-ME was quantified using high performance liquid chromatography (HPLC). The HPLC system (Shimadzu Corporation, Kyoto, Japan) consist of a Model LC-20AD high pressure pump, a Model CTO-20AC column oven, a Model SIL-20AC total-volume injection-type auto-sampler, and a Model SPD-20A variable wavelength UV–Vis detector. Samples were separated using YMC-Pack Pro C18 (internal diameter: 3.0 mm, length: 150 mm, YMC, Kyoto, Japan) at 40 °C and the mobile phase consisted of 10 mM phosphoric acid (A) and acetonitrile (B) at 0.5 ml/min flow rates. Purified taxifolin (20 μg/ml) was used as the standard compound, and the concentration of taxifolin in LK-ME was calculated by the peak area of absorbance units at 280 nm compared with the standard. The HPLC analysis was performed by the Biodynamic Plant Institute, Sapporo, Hokkaido, Japan.
4.3. Cell culture and monitoring of cell viability
A human monocyte-derived cell line, THP-1 cells (ATCC TIB-202) [21] were grown and maintained in RPMI 1640 medium supplemented with 10% fetal bovine serum, 100 U/ml penicillin, 100 mg/ml streptomycin (Life Technologies, Carlsbad, CA, USA), The cells were grown at 37 °C in 5% CO2 in a humidified incubator. The cell viability was monitored using a Cell Counting Kit-8 (Dojindo, Kumamoto, Japan) in accordance with the manufacturer instructions.
4.4. Monitoring the production of advanced glycation end products (AGEs).
Human serum albumin (Fraction V; Nacalai tesque, Kyoto, Japan), elastin derived from bovine neck ligament (MP Biomedicals, Irvine, CA, USA), and collagen derived from bovine skin (Type I, acid soluble; Nippi, Tokyo, Japan) were purchased as commercially available products. To solubilize elastin, 100 mg of bovine elastin was heated in 1ml of 0.1N NaOH at 99 °C, and the supernatant recovered. After repeating this step twice, the remaining pellets were autoclaved in 1 ml of 0.1N NaOH, and the supernatant was collected. This step was also repeated twice, and then the collected supernatant containing solubilized elastin was neutralized using 1N HCl, and sterilized using a 0.22 μm filter. The protein concentration was measured using a Pierce BCA Protein Assay Kit (Thermo Fisher Scientific Waltham, MA, USA) according to the manufacturer instructions, and used in this study.
The production of AGEs was measured by monitoring the increment in fluorescence intensity. Human albumin (8 mg/ml) and glucose (0.2 M), bovine collagen (0.3 mg/ml) and fructose (0.2 M), or bovine elastin (0.5 mg/ml) and glyceraldehyde (0.05 M) were reacted in 0.05 M phosphate buffer (pH 7.4) with a series of concentrations of LK-ME, taxifolin, quercetin, or luteolin at 60 °C. After the incubation, the fluorescence intensity (excitation: 365 nm emission: 410–460 nm) was measured using a multimode microplate reader (GloMax Multi Detection System; Promega, Madison, WI, USA).
The half maximal inhibitory concentration (IC50) values were calculated using the IC50 calculator, a web based program developed by AAT Bioquest, Sunnyvale, CA, USA (https://www.aatbio.com/tools/ic50-calculator).
4.5. Real-time reverse transcriptase polymerase chain reaction (RT-PCR) analysis
The total RNA was extracted from the cultured cells using TRIzol reagent (Invitrogen, Carlsbad, CA, USA). The isolated total RNA was treated with DNaseI (Takara, Otsu, Shiga, Japan) and then subjected to oligo-dT- and random-primed reverse transcription using ReverTra Ace (Toyobo, Osaka, Japan). Real-time PCR was performed using Thunderbird SYBR qPCR Mix (Toyobo), and the PCR reactions and analysis of the mRNA expressions were performed using the CFX96 Real-Time PCR Detection System (Bio-Rad, Hercules, CA, USA). The procedures were performed according to the manufacturer protocols. The following listed specific primer sets for IL-8, TNF-α, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNAs were used in this study. TNF-α sense primer 5′- CCCAGGGACCTCTCTCTAATC -3’; TNF-α antisense primer 5′- ATGGCTACAGGCTTGTCACT -3’; IL-8 sense primer 5′- GTGCAGTTTTGCCAAGGAGT -3’; IL-8 antisense primer 5′- CTCTGCACCCAGTTTTCCTT -3’; GAPDH sense primer 5′- TTCTTTTGCGTCGCCAGCCG -3’; and GAPDH antisense primer 5′- GGTGACCAGGCGCCCAATACG -3’.
4.6. Statistical analysis
To determine statistically significant differences between data pairs, a two-tailed unpaired Student's t-test was performed in this study.
Declarations
Author contribution statement
Daisuke Muramatsu: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Hirofumi Uchiyama: Conceived and designed the experiments; Performed the experiments.
Hiroshi Kida: Contributed reagents, materials, analysis tools or data; Wrote the paper.
Atsushi Iwai: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This study was funded by Aureo Co., Ltd., Kimitsu, Japan and Aureo-Science Co., Ltd., Sapporo, Japan. The funders had no role in the study design, data collection, or analysis, decision to publish, or preparation of the manuscript.
Competing interest statement
The authors declare the following conflicts of interest: Daisuke Muramatsu, Hirofumi Uchiyama and Atsushi Iwai are employees of Aureo-Science Co., Ltd., and Atsushi Iwai is an employee of Aureo Co., Ltd.
Additional information
No additional information is available for this paper.
References
- 1.Schauss A.G., Tselyico S.S., Kuznetsova V.A., Yegorova I. Toxicological and genotoxicity assessment of a dihydroquercetin-rich Dahurian larch tree (Larix gmelinii Rupr) extract (Lavitol) Int. J. Toxicol. 2015;34:162–181. doi: 10.1177/1091581815576975. [DOI] [PubMed] [Google Scholar]
- 2.Turck D., Bresson J., Burlingame B., Dean T., Fairweather-Tait S., Heinonen M., Hirsch-Ernst K.I., Mangelsdorf I., McArdle H.J., Naska A., Neuhäuser-Berthold M., Nowicka G., Pentieva K., Sanz Y., Siani A., Sjödin A., Stern M., Tomé D., Vinceti M., Willatts P., Engel K., Marchelli R., Pöting A., Poulsen M., Schlatter J., Gelbmann W., Van Loveren H. Scientific opinion on taxifolin-rich extract from dahurian larch (Larix gmelinii) EFSA J. 2017;15 [Google Scholar]
- 3.Turck D., Bresson J., Burlingame B., Dean T., Fairweather-Tait S., Heinonen M., Hirsch-Ernst K.I., Mangelsdorf I., McArdle H.J., Naska A., Neuhäuser-Berthold M., Nowicka G., Pentieva K., Sanz Y., Siani A., Sjödin A., Stern M., Tomé D., Vinceti M., Willatts P., Engel K., Marchelli R., Pöting A., Poulsen M., Schlatter J., Gelbmann W., Van Loveren H. Statement on the safety of taxifolin-rich extract from Dahurian Larch (Larix gmelinii) EFSA J. 2017;15 doi: 10.2903/j.efsa.2017.5059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Topal F., Nar M., Gocer H., Kalin P., Kocyigit U.M., Gülçin İ., Alwasel S.H. Antioxidant activity of taxifolin: an activity-structure relationship. J. Enzym. Inhib. Med. Chem. 2016;31:674–683. doi: 10.3109/14756366.2015.1057723. [DOI] [PubMed] [Google Scholar]
- 5.Plotnikov M.B., Aliev O.I., Sidekhmenova A.V., Shamanaev A.Y., Anishchenko A.M., Fomina T.I., Chernysheva G.A., Smol’yakova V.I., Arkhipov A.M. Dihydroquercetin improves microvascularization and microcirculation in the brain cortex of SHR rats during the development of arterial hypertension. Bull. Exp. Biol. Med. 2017;163:57–60. doi: 10.1007/s10517-017-3737-7. [DOI] [PubMed] [Google Scholar]
- 6.Chiu Y.J., Chou S.C., Chiu C.S., Kao C.P., Wu K.C., Chen C.J., Tsai J.C., Peng W.H. Hepatoprotective effect of the ethanol extract of Polygonum orientale on carbon tetrachloride-induced acute liver injury in mice. J. Food Drug Anal. 2017;26:369–379. doi: 10.1016/j.jfda.2017.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Galochkina A.V., Anikin V.B., Babkin V.A., Ostrouhova L.A., Zarubaev V.V. Virus-inhibiting activity of dihydroquercetin, a flavonoid from Larix sibirica, against coxsackievirus B4 in a model of viral pancreatitis. Arch. Virol. 2016;161:929–938. doi: 10.1007/s00705-016-2749-3. [DOI] [PubMed] [Google Scholar]
- 8.Ding T., Wang S., Zhang X., Zai W., Fan J., Chen W., Bian Q., Luan J., Shen Y., Zhang Y., Ju D., Mei X. Kidney protection effects of dihydroquercetin on diabetic nephropathy through suppressing ROS and NLRP3 inflammasome. Phytomedicine. 2018;41:45–53. doi: 10.1016/j.phymed.2018.01.026. [DOI] [PubMed] [Google Scholar]
- 9.Sun X., Chen R., Yang Z., Sun G., Wang M., Ma X., Yang L., Sun X. Taxifolin prevents diabetic cardiomyopathy in vivo and in vitro by inhibition of oxidative stress and cell apoptosis. Food Chem. Toxicol. 2014;63:221–232. doi: 10.1016/j.fct.2013.11.013. [DOI] [PubMed] [Google Scholar]
- 10.Kuspradini H., Mitsunaga T., Ohashi H. Antimicrobial activity against Streptococcus sobrinus and glucosyltransferase inhibitory activity of taxifolin and some flavanonol rhamnosides from kempas (Koompassia malaccensis) extracts. J. Wood Sci. 2009;55:308–313. [Google Scholar]
- 11.Mishra S., Singh S., Misra K. Restraining pathogenicity in Candida albicans by taxifolin as an inhibitor of ras1-pka pathway. Mycopathologia. 2017;182:953–965. doi: 10.1007/s11046-017-0170-4. [DOI] [PubMed] [Google Scholar]
- 12.Abugri D.A., Witola W.H., Russell A.E., Troy R.M. In vitro activity of the interaction between taxifolin (dihydroquercetin) and pyrimethamine against Toxoplasma gondii. Chem. Biol. Drug Des. 2018;91:194–201. doi: 10.1111/cbdd.13070. [DOI] [PubMed] [Google Scholar]
- 13.Gocer H., Topal F., Topal M., Küçük M., Teke D., Gülçin İ., Alwasel S.H., Supuran C.T. Acetylcholinesterase and carbonic anhydrase isoenzymes I and II inhibition profiles of taxifolin. J. Enzym. Inhib. Med. Chem. 2015;31:1–7. doi: 10.3109/14756366.2015.1036051. [DOI] [PubMed] [Google Scholar]
- 14.Saito S., Yamamoto Y., Maki T., Hattori Y., Ito H., Mizuno K., Harada-Shiba M., Kalaria R.N., Fukushima M., Takahashi R., Ihara M. Taxifolin inhibits amyloid-β oligomer formation and fully restores vascular integrity and memory in cerebral amyloid angiopathy. Acta Neuropathol. Commun. 2017;5:26. doi: 10.1186/s40478-017-0429-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kawanishi Y., Bito N., Nakada R., Imai T. Visualization of the distribution of flavonoids in Larix kaempferi wood by fluorescence microscopy. Mokuzai Gakkaishi. 2015;61:297–307. [Google Scholar]
- 16.Negre-Salvayre A., Salvayre R., Augé N., Pamplona R., Portero-Otín M. Hyperglycemia and glycation in diabetic complications. Antioxidants Redox Signal. 2009;11:3071–3109. doi: 10.1089/ars.2009.2484. [DOI] [PubMed] [Google Scholar]
- 17.Duran-Jimenez B., Dobler D., Moffatt S., Rabbani N., Streuli C.H., Thornalley P.J., Tomlinson D.R., Gardiner N.J. Advanced glycation end products in extracellular matrix proteins contribute to the failure of sensory nerve regeneration in diabetes. Diabetes. 2009;58:2893–2903. doi: 10.2337/db09-0320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Genuth S., Sun W., Cleary P., Sell D.R., Dahms W., Malone J., Sivitz W., Monnier V.M. Glycation and carboxymethyllysine levels in skin collagen predict the risk of future 10-year progression of diabetic retinopathy and nephropathy in the diabetes control and complications trial and epidemiology of diabetes interventions and complications participants with type 1 diabetes. Diabetes. 2005;54:3103–3111. doi: 10.2337/diabetes.54.11.3103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Atanasova M., Konova E., Baydanoff S., Atanasova M., Velkova A. Age-related changes in the glycation of human aortic elastin. Exp. Gerontol. 2004;39:249–254. doi: 10.1016/j.exger.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 20.Vitek M.P., Bhattacharya K., Glendening J.M., Stopa E., Vlassara H., Bucala R., Manogue K., Cerami A. Advanced glycation end products contribute to amyloidosis in Alzheimer disease. Proc. Natl. Acad. Sci. U.S.A. 1994;91:4766–4770. doi: 10.1073/pnas.91.11.4766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tsuchiya S., Yamabe M., Yamaguchi Y., Kobayashi Y., Konno T., Tada K. Establishment and characterization of a human acute monocytic leukemia cell line (THP-1) Int. J. Cancer. 1980;26:171–176. doi: 10.1002/ijc.2910260208. [DOI] [PubMed] [Google Scholar]
- 22.Li X., Zheng T., Sang S., Lv L. Quercetin inhibits advanced glycation end product formation by trapping methylglyoxal and glyoxal. J. Agric. Food Chem. 2014;62:12152–12158. doi: 10.1021/jf504132x. [DOI] [PubMed] [Google Scholar]
- 23.Verhasselt V., Goldman M., Willems F. Oxidative stress up-regulates IL-8 and TNF-α synthesis by human dendritic cells. Eur. J. Immunol. 1998;28:3886–3890. doi: 10.1002/(SICI)1521-4141(199811)28:11<3886::AID-IMMU3886>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 24.Li D.-Q., Luo L., Chen Z., Kim H.-S., Song X.J., Pflugfelder S.C. JNK and ERK MAP kinases mediate induction of IL-1β, TNF-α and IL-8 following hyperosmolar stress in human limbal epithelial cells. Exp. Eye Res. 2006;82:588–596. doi: 10.1016/j.exer.2005.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jain S.K., Rains J., Croad J., Larson B., Jones K. Curcumin supplementation lowers TNF-α, IL-6, IL-8, and MCP-1 secretion in high glucose-treated cultured monocytes and blood levels of TNF-α, IL-6, MCP-1, glucose, and glycosylated hemoglobin in diabetic rats. Antioxidants Redox Signal. 2009;11:241–249. doi: 10.1089/ars.2008.2140. [DOI] [PMC free article] [PubMed] [Google Scholar]