Abstract
Bacterial infections have caused serious threats to public health due to the antimicrobial resistance in bacteria. Recently, gold nanoclusters (AuNCs) have been extensively investigated for biomedical applications because of their superior structural and optical properties. Great efforts have demonstrated that AuNCs conjugated with various surface ligands are promising antimicrobial agents owing to their high biocompatibility, polyvalent effect, easy modification and photothermal stability. In this review, we have highlighted the recent achievements for the utilizations of AuNCs as the antimicrobial agents. We have classified the antimicrobial AuNCs by their surface ligands including small molecules (<900 Daltons) and macromolecules (>900 Daltons). Moreover, the antimicrobial activities and mechanisms of AuNCs have been introduced into two main categories of small molecules and macromolecules, respectively. In accordance with the advancements of antimicrobial AuNCs, we further provided conclusions of current challenges and recommendations of future perspectives of antimicrobial AuNCs for fundamental researches and clinical applications.
Keywords: gold nanoclusters, antimicrobial agent, small molecule, macromolecule, antimicrobial mechanism
1. Introduction
Treatment of bacterial infection is facing challenge against antimicrobial resistance [1,2,3,4]. The antimicrobial resistance in bacteria remains growing for many reasons included overuse and misuse of antibiotics and the spread of bacteria by various routes [5,6,7]. Therefore, the issue of antimicrobial resistance constitutes a serious risk to public health. According to previous study, the threat of antimicrobial resistance will represent the first cause of death with around ten million per year in 2050 [8,9]. Among different solutions to overcome the antimicrobial resistance, the developments of new antimicrobial agents are critically needed [10,11,12]. Nanomaterials with large surface area and facile functionalization have exhibited superior physical and chemical properties for applications in catalysis, electronic and medicine [13,14,15,16,17,18,19,20]. Recently, organic and inorganic nanomaterials offer an alternative approach to treat infectious diseases caused by bacteria [21,22,23,24,25,26,27]. The antibacterial mechanisms of nanomaterials have been demonstrated, such as the binding between nanomaterials and bacteria for bacterial membrane disruption, photothermal heat generation to kill bacteria by light irradiation onto nanomaterials, photocatalytic production of reactive oxygen species (ROS) via nanomaterials and release of metals ions from nanomaterials to disrupt cellular components of bacteria [28,29,30,31,32,33].
Recent advancements have been focused on the utilizations of metal nanoclusters including gold, silver and copper as the antibacterial agents for bacterial infections [34,35,36,37,38]. Among the metal nanoclusters, gold nanoclusters (AuNCs) have exhibited unique optical and structural properties for the biomedical applications in imaging, detection, and therapy [39,40,41,42,43,44,45]. For the application in therapy, AuNCs conjugated with various surface ligands have been extensively applied as the antimicrobial agents owing to their high biocompatibility, polyvalent effect, easy modification and photothermal stability [46,47,48,49,50,51,52,53,54]. The ligands of amino acids, peptides, antibiotics, antibodies, enzymes, DNA and so forth have been demonstrated for the syntheses of AuNCs [55,56,57,58,59,60,61,62,63,64,65,66]. In this review, we focus on recent achievements dealing with antimicrobial activity of AuNCs capped by various ligands. The ligands embracing different chemical structures were grouped into small molecules (<900 Daltons) and macromolecules (>900 Daltons) [67,68]. In molecular biology and pharmacology, small molecules are commonly defined for the organic compound with the molecular weight lower than 900 Daltons. Small molecules can be used to regulate a biological process [69]. Due to the small size, small molecules are able to penetrate across cell membranes to reach targets in the bacterial cell. In contrast to small molecules, macromolecules (>900 Daltons) are complex and usually exhibit therapeutic effect [70]. Therefore, in this review, AuNCs are classified by their surface ligands included small molecules and macromolecules for the explanation of the antibacterial mechanism of AuNCs (Table 1). The details of ligand size effects and antibacterial mechanisms of ligand-protected AuNCs are also discussed in this review. Finally, challenges and perspectives about antimicrobial AuNCs are provided.
Table 1.
Ligands and antibacterial mechanisms of AuNCs in this review.
| Types | Ligands (Molecular Weight) | Antibacterial Mechanisms | References |
|---|---|---|---|
| Small molecules | 6-Mercaptohexanoic acid (148 Da) | Increase of ROS generation by MHA-AuNCs to kill bacteria | [71] |
| Glutathione (307 Da) | Optimal radius of DPAu/AMD for the increase of cell uptake | [72] | |
| Allium cepa L. (Mixture) | Increase of the interaction between AuNCs and bacterial membrane | [73] | |
| Mannose (180 Da) | Bacterial aggregations | [74] | |
| Quaternary ammonium (282 Da) | Increase of ROS generation by QA-AuNCs with positive charge | [75] | |
| 4-Amino-2-mercaptopyrimidine (127 Da) | Increase of ROS generation by AuDHMP with positive charge | [76] | |
| 6-Mercaptohexanoic acid (148 Da) | Increase of ROS generation by Au25NCs with negative charge | [77] | |
| Macromolecules | Lysozyme (143000 Da) | Hydrolysis of bacterial cell wall by lysozyme-AuNCs | [78] |
| Lysozyme (143000 Da) | Multivalent interactions between AuNC-L-Amp and bacterial | [79] | |
| Vancomycin (1449 Da) | Delivery of vancomycin into bacteria by AuNC@Van | [80] | |
| Vancomycin (1449 Da) | Delivery of vancomycin into bacteria by Au-SGaa-Van | [81] | |
| G4NH2 & G4OH (14266 & 14277 Da) | Inhibition of LPS aggregation | [82] | |
| Bacitracin (1422 Da) | Damage of cell wall and increase of ROS production by AuNCs@Bacitracin | [83] |
2. Small Molecule-Conjugated AuNCs
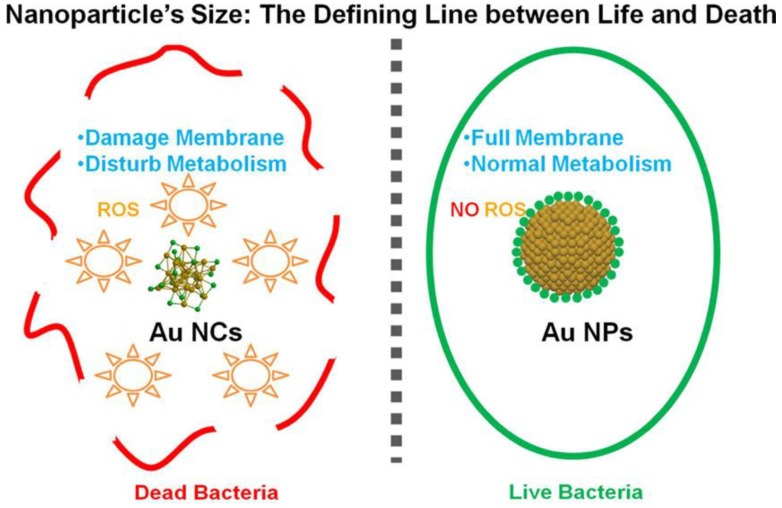
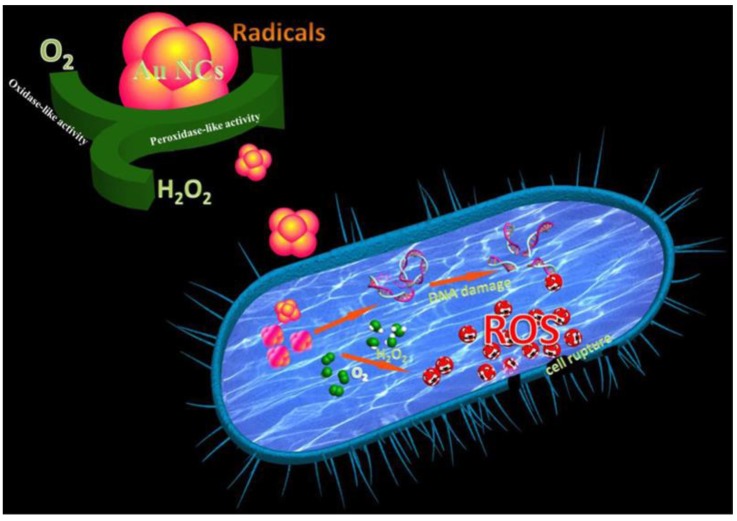
In recent years, small molecules containing thiol, amine and hydroxyl groups have been used as the ligands to synthesize AuNCs because of low cost, easy accessibility and facile modification. These AuNCs have revealed promising potential as the antimicrobial agents. For example, AuNCs protected by 6-mercaptohexanoic acid (MHA-AuNCs) have been prepared and used as an antimicrobial agent [71]. Zheng et al. have compared the antimicrobial activities of MHA-conjugated gold nanoparticles (MHA-AuNPs), MHA-AuNCs and Au(I)-MHA complexes for Gram-positive Staphylococcus aureus (S. aureus). After incubation with S. aureus, MHA-AuNCs have shown superior bacterial killing efficiency ∼95% of the S. aureus. In comparison with MHA-AuNCs, the bacterial killing efficiencies of MHA-AuNPs and Au(I)-MHA complexes are ∼3% and ∼5% for S. aureus, respectively. For the Gram-negative type Escherichia coli (E. coli), the bacterial killing efficiencies of MHA-AuNCs, MHA-AuNPs and Au(I)-MHA complexes are individually ∼96%, ∼2% and ∼3%. Herein, MHA-AuNPs have shown no significant antimicrobial activity. However, gold nanoparticles can be used as an antibiotic carrier. The gold nanoparticles with large surface area allow them to conjugate a large number of antibiotics for efficiently against various strains of bacteria [84,85,86,87]. In comparison with gold nanoparticles, the antimicrobial activity of MHA-AuNCs is attributed to their ultra-small size for the improvement of interaction with bacteria. After the internalization of MHA-AuNCs in bacteria, the interaction between MHA-AuNCs and bacteria could cause a metabolic imbalance to result in the increase of intracellular ROS production to eventually kill bacteria (Figure 1) [88].
Figure 1.
Antimicrobial activity of MHA-AuNCs due to the increase of interaction between MHA-AuNCs and bacteria. Reproduced with permission from Reference [71]. Copyright © 2017, American Chemical Society.
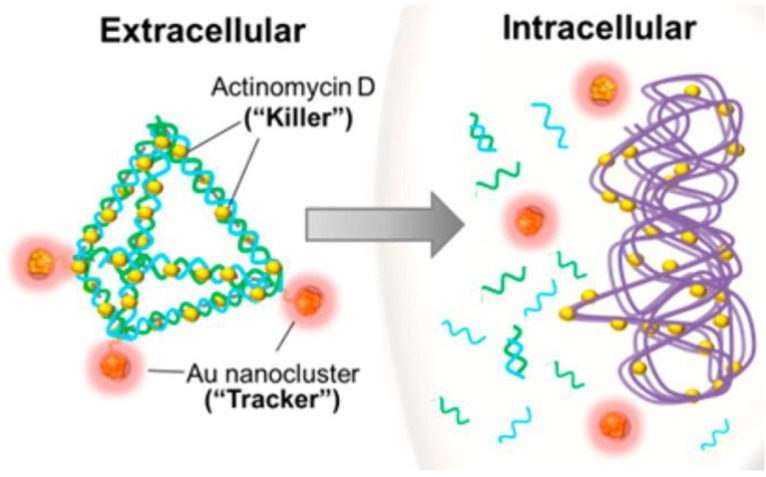
DNA nanopyramid (DP) is one of DNA nanostructures used in nanomedicine as delivery carrier [89]. Setyawati et al. have used DP as the scaffold to incorporate glutathione-protected AuNCs and Actinomycin D (AMD) to form a nanotheranostic agent (DPAu/AMD) as shown in Figure 2 [72]. The nanotheranostic agent of DPAu/AMD has been applied against E. coli and S. aureus. The result indicates that DPAu/AMD show a significant killing efficiency compared to that of the free AMD treatment for both of E. coli and S. aureus. The DPAu/AMD improve antibacterial effect by reduction of 65% of S. aureus population compared to that of 42% for the free AMD. For E. coli, the bacterial reductions of DPAu/AMD and free AMD are 48% and 14%, respectively. In comparison with free AMD, the high antibacterial effect of DPAu/AMD can be attributed to that the optimal radius of DPAu/AMD (38.3 nm) can increase the cell uptake for bacteria [90,91].
Figure 2.
Representative scheme of DPAu/AMD as a nanotheranostic agent. Reproduced with permission from Reference [72]. Copyright © 2014, American Chemical Society.
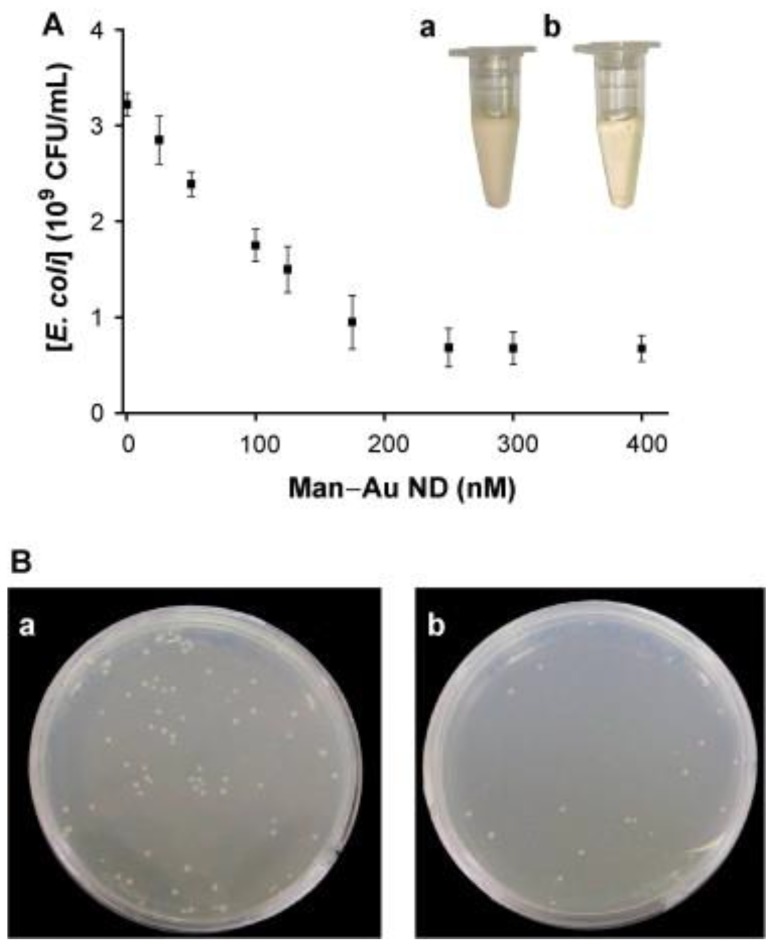
Sinha et al. have developed a one-pot fabrication with properties including simple, novel, green, economic, environment friendly and convenient for preparation of AuNCs with Allium cepa L. (AcL) conjugation [73]. The peel extraction of AcL has biomolecules such as flavonoids, carbohydrates, saponins, amino acid cysteine, sulphoxides, γ-glutamyl peptides and vitamins. The biomolecules with thiol groups in the peel extraction of AcL have been used to reduce the precursor of Au (III) to form Au (I) and Au (0) for the formation of AuNCs [92]. In this work, the antibacterial activities of AuNCs, AcL and Tetracycline antibiotic have been investigated against Gram-negative E. coli. Results show that AuNCs have the highest bacterial killing efficiency, followed by Tetracycline antibiotic and then extraction of AcL the least. The highest antibacterial activity of AuNCs can be attributed to that the large surface area and easy penetration ability of AuNCs can increase the interaction between AuNCs and bacterial membrane to result in the death of bacteria. To combat bacteria, water-soluble biofunctional AuNCs conjugated with mannose (Man-AuNCs) have been developed for the sensitive and selective detection and bacterial inhibition of E. coli. (Figure 3) [74]. The mannose ligands conjugated on the surfaces of Man-AuNCs have induced strong multivalent interactions between Man-AuNCs and FimH proteins located on the bacterial pili of E. coli. The bacterial aggregations caused by Man-AuNCs lead to the inhibition of the growth of E. coli. The antibacterial activity of Man-AuNCs has been shown in Figure 3. The growth curve of E. coli in sterile LB media has shown a very low growth rate of E. coli after incubated with Man-AuNCs (>250 nM) as shown in Figure 3A. In Figure 3B, the number of colonies on the LB agar plates of untreated and Man-AuNCs-treated E. coli have been calculated to be 78 and 18 colony-forming unit (CFU), respectively. In this work, the Man-AuNCs have great potential for use as an antibacterial agent due to high ligand density of mannose on the surface of Man-AuNCs for multivalent interactions with E. coli.
Figure 3.
(A) Antibacterial effect of Man-AuNCs with their concentrations from 0 to 400 nM. (B) Colony formation of E. coli on LB agar plates in the (a) absence and (b) presence of Man-AuNCs (250 nM). Insets of Figure 3A indicate photographs of E. coli (1.0×108 CFU/mL) grown for 10 h in the LB medium in the (a) absence and (b) presence of Man-AuNCs (250 nM). Reproduced with permission from Reference [74]. Copyright © 2011, Elsevier.
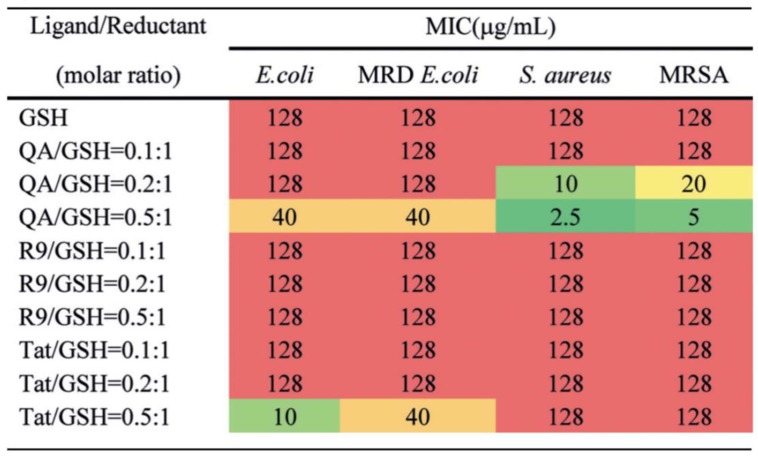
Recently, Xie et al. have synthesized and functionalized AuNCs using positive ligands including quaternary ammonium (QA-AuNCs), nona-arginine peptide (R9-AuNCs) and the transactivator of transcription peptide (Tat-AuNCs) by one-pot synthesis with glutathione as the reductant [75]. The antibacterial activities of AuNCs have been investigated by measuring their minimal inhibitory concentrations (MICs) in Gram-positive S. aureus, methicillin-resistant Staphylococcus aureus (MRSA), Gram-negative E. coli and multidrug-resistant E. coli. With the changes of ligand/reductant (L/R) ratio, the QA-AuNCs with an L/R ratio of 0.5:1 has exhibited superior antibacterial effect for the four targeting bacteria (Figure 4). The antibacterial mechanism of QA-AuNCs can be ascribed to the fact that the positive charge on the surface of QA-AuNCs can promote electrostatic adsorption onto bacterial cell membrane with negative charge. Additionally, then QA-AuNCs have induced disruption of membrane integrity, increase of membrane permeability and dissipation of the membrane potential of S. aureus. Eventually, QA-AuNCs can improve the generation of ROS and cause the death of bacteria [93]. Overall, QA-AuNCs have shown promising potential as the antibacterial agent using physicochemical mechanism for the skin infection model and the bacteremia model caused by MRSA [9,94].
Figure 4.
Antibacterial activities of AuNCs conjugated with different ligands by measuring their MICs. The lower MIC of AuNCs show higher antibacterial activity. Reproduced with permission from Reference [75]. Copyright © 2018, WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Moreover, four ligands which are analogues of mercaptopyrimidine including 4-amino-2-mercaptopyrimidine (AMP), 4,6-diamino-2-mercaptopyrimidine (DAMP), 4-amino-6-hydroxyl-2-mercaptopyrimidine (AHMP), and 4,6-dihydroxyl-2-mercaptopyrimidine (DHMP) have been used to synthesize mercaptopyrimidine conjugated AuNCs to combat multidrug-resistant bacteria [76]. For these AuNCs, DHMP-conjugated AuNCs (AuDHMP) have exhibited negative charge and the others AuNCs of AuAMP, AuDAMP and AuAHMP have shown positive charges. The zeta potentials for AuDHMP, AuAMP, AuDAMP and AuAHMP are −38.6 ± 1.8, +33.6 ± 1.4, +37.6 ± 1.1 and +12.7 ± 0.7 mV, respectively. All AuNCs have revealed antimicrobial activities against E. coli ATCC 35218 (Gram-negative bacteria) and S. aureus ATCC 29213 (Gram-positive bacteria). The AuDAMP have the best performance of antimicrobial activity compared to AuAMP, AuAHMP and AuDHMP because the high positive surface charge of AuDAMP can facilitate their electrostatic adsorption onto the surface of bacteria to increase internalization of AuDAMP into bacteria. Furthermore, AuDAMP also can fight mutli-drug resistant bacteria such as E. coli, Acinetobacter baumannii (A. baumannii), Pseudomonas aeruginosa, Klebsiella pneumonia (K. pneumonia), methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant Enterococcus faecium (E. faecium). To kill bacteria, the mechanisms of antimicrobial AuDAMP have been demonstrated by the combination of cell membrane destruction, DNA damage and ROS generation caused by AuDAMP to bacteria (Figure 5) [95,96,97].
Figure 5.
The mechanisms of cell membrane destruction, DNA damage and ROS generation for AuDAMP to kill bacteria. Reproduced with permission from Reference [76]. Copyright © 2018, American Chemical Society.
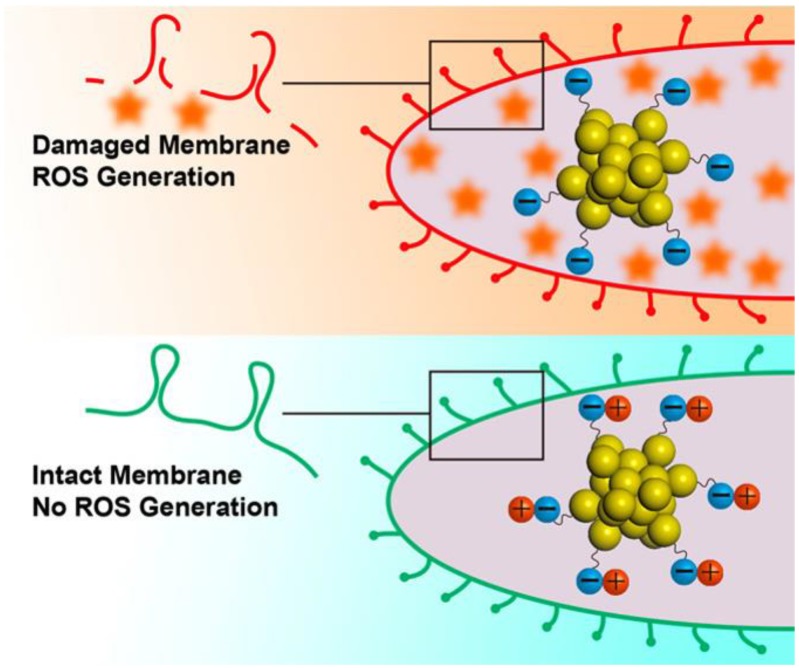
Nanomaterial-based antimicrobial agents with positive surface charges are generally considered to lead higher antimicrobial activities as shown in the example of AuDAMP. However, Zheng et al. have prepared five types of Au25NCs with negative surface charges including Au25NCs protected by MHA (Type I), Au25NCs protected by p-mercaptobenzoic acid (MBA) (Type II), Au25NCs protected by cysteine (Cys) (Type III), Au25NCs protected by MHA and cysteamine (Cystm) (Type IV) and Au25NCs protected by MHA and 2-mercaptoethanol (AuMetH) (Type V) [77]. By the designs of surface ligands, Au25NCs with more negative surface charges on the surface could induce more ROS generation to react with metabolic enzyme of bacteria and then to kill the bacteria (Figure 6) [98,99]. The results in this work indicate that surface charge of AuNCs plays a pivotal role in antimicrobial properties.
Figure 6.
Surface ligand chemistry of AuNCs could determine their antimicrobial ability. Reproduced with permission from Reference [77]. Copyright © 2018, American Chemical Society.
3. Macromolecule-Conjugated AuNCs
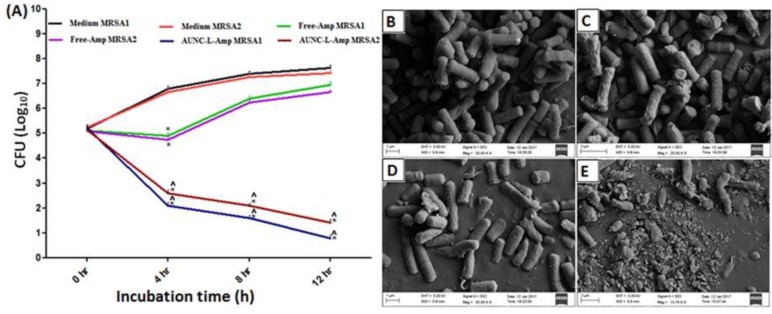
Macromolecules are also commonly used as the surface ligands to prepare AuNCs for antibacterial applications. With the conjugations of macromolecules, AuNCs have shown various antibacterial effects. Recently, Chen et al. have synthesized lysozyme capped AuNCs (lysozyme-AuNCs) as an antimicrobial agent [78]. The enzyme of lysozyme can hydrolyze the cell walls of pathogenic bacteria [100,101,102]. The lysozyme-AuNCs have exhibited bacteriostatic effects against pan-drug-resistant Acinetobacter baumannii (A. baumannii) and vancomycin-resistant Enterococcus faecalis (E. faecalis) because of the multivalent interactions of the Lysozyme-AuNCs with the target bacteria. Furthermore, lysozyme conjugated AuNCs have been functionalized with ampicillin (AuNC-L-Amp) to combat MRSA and other non-resistant bacteria [79]. In this work, AuNC-L-Amp have been proved to overcome the increased β-lactamase at the site of MRSA and then the multivalent binding of AuNC-L-Amp onto the bacterial surface can be applied to enhance the permeation of AuNC-L-Amp into bacteria. The AuNC-L-Amp have shown a significant enhancement (50–89% fold increase) of antimicrobial activity compared to that of free-Amp for nonresistant bacterial pathogens. The AuNC-L-Amp have also revealed antimicrobial activity for MRSA, but free-Amp and AuNC-L have exhibited no significant antimicrobial activity for MRSA (Figure 7). The mechanism for the use of AuNC-L-Amp as the antimicrobial agent can be ascribed to the reasons including the increase of Amp concentration in bacteria, multivalent presentation of antibiotics, hydrolysis of cell wall by lysozyme, dysfunction of the bacterial efflux pump and ions released from AuNCs to inhibit bacterial growth [103,104,105,106].
Figure 7.
(A) Antimicrobial activities of AuNC-L-Amp and free-Amp at different incubation times for MRSA1 and 2. SEM images of MRSA incubation with (B) PBS, (C) AuNC-L, (D) Free-Amp and (E) AuNC-L-Amp. SEM images indicate that AuNC-L and free-Amp did not cause the changes of bacterial morphology. On the other hand, AuNC-L-Amp induced the cellular structure of MRSA. Reproduced with permission from Reference [79]. Copyright © 2018, Springer Nature.
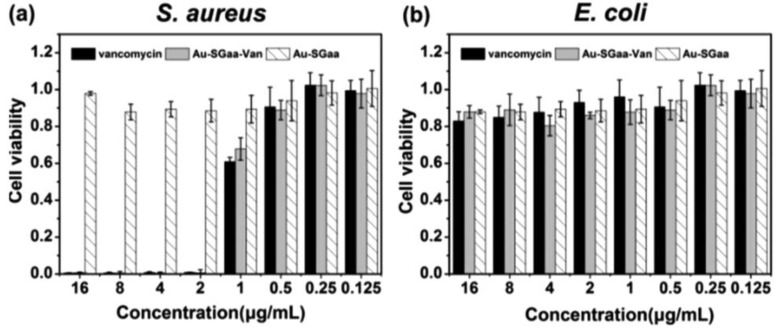
Antibiotic of vancomycin for all Gram-positive bacteria has been used as a template and reducing agent to synthesize vancomycin-bound AuNCs (AuNC@Van) [80]. Antibacterial ability of AuNC@Van has been evaluated on Gram-negative E. coli and Gram-positive S. aureus. The results indicate that AuNC@Van has good antimicrobial activity for both E. coli and S. aureus. To further study the antibacterial mechanism of AuNC@Van, Liang et al. have investigated the morphological changes of E. coli and S. aureus incubated with AuNC@Van by SEM. After incubation with AuNC@Van for 48 h, the bacterial cell wall has shown wrinkle and destruction. Afterward, because of the damage of bacterial cell wall, vancomycin on the surface of AuNC@Van can easily penetrate into bacteria to enhance its antimicrobial activity. Moreover, Li et al. have chosen a pentapeptide γ-ECGDADA (GSHaa) to prepare AuNCs (Au-SGaa, SGaa denotes dehydrogenated GSHaa) and then Au-SGaa have been conjugated with vancomycin (Au-SGaa-Van) [81]. In this study, the Gram-positive bacteria S. aureus and Gram-negative bacteria E. coli have been selected to assess antibacterial activity of Au-SGaa-Van. As shown in Figure 8, Au-SGaa-Van and vancomycin have shown significant antibacterial effect against S. aureus. However, there is no antibacterial activity of Au-SGaa-Van for E. coli. The results indicate that antibacterial activity of the Au-SGaa-Van is caused by the vancomycin on their surface.
Figure 8.
(a) Antibacterial activity of Au-SGaa-Van, vancomycin and Au-SGaa against Gram-positive S. aureus. (b) Antibacterial activity of Au-SGaa-Van, vancomycin and Au-SGaa against Gram-negative E. coli. Reproduced with permission from Reference [81]. Copyright © 2018, Royal Society of Chemistry.
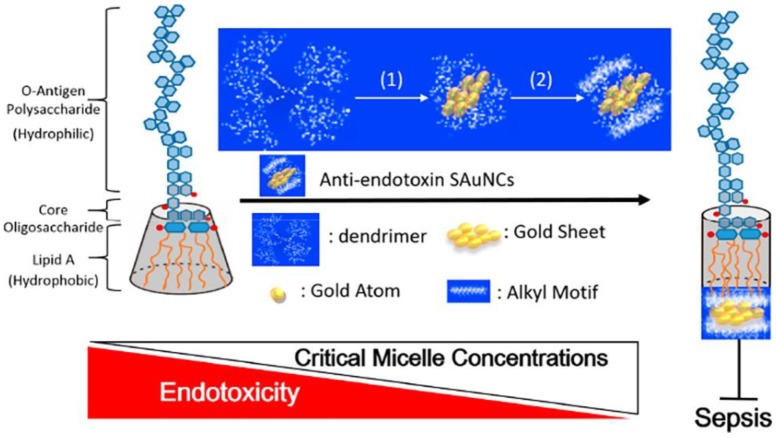
Furthermore, Liao et al. have constructed AuNCs to inhibit endotoxin activity by blocking on active site of lipopolysaccharide (LPS) [82]. LPS is one of constituents of Gram-negative bacteria responsible of sepsis to humans [107]. They have decorated subnanometer gold clusters (SAuNCs) using methyl and ethyl groups to synthesize SAuNC-M and SAuNC-E, respectively. Additionally, hydrophilic SAuNCs (SAuNC-A) and hydrophobic SAuNCs (SAuNC-H) have been synthesized. The SAuNC-M and SAuNC-E have caused the inhibition of LPS aggregation but SAuNC-A and SAuNC-H have been validated to produce LPS aggregation [108]. The endotoxin activity can be effectively blocked by SAuNCs including SAuNC-M and SAuNC-E as means to fight sepsis. Results of their work have shown that the antiendotoxin SAuNC-M and SAuNC-E could be the efficacious antimicrobial agents to prevent sepsis due to infection from Gram-negative bacteria (Figure 9).
Figure 9.
Illustration of interaction between SAuNCs, lipid A of LPS and sepsis progression. Reproduced with permission from Reference [82]. Copyright © 2018, American Chemical Society.
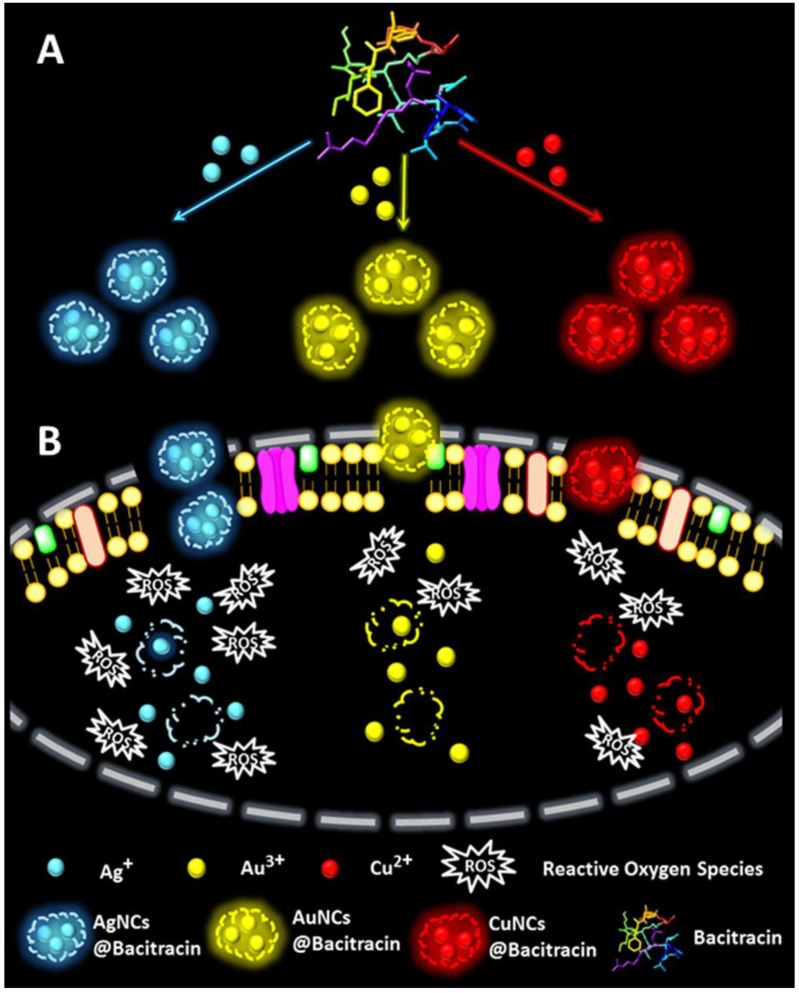
The bacitracin-directed silver, gold and copper nanoclusters (AgNCs@Bacitracin, AuNCs@Bacitracin and CuNCs@Bacitracin) have been obtained by Wang and coworkers [83]. The antibacterial activities of these nanoclusters have been investigated by the use of S. aureus. The antibacterial mechanism of these nanoclusters have been demonstrated with the coordination between bacitracin and the metallic atoms. In this work, the AgNCs@Bacitracin, AuNCs@ Bacitracin and CuNCs@Bacitracin have respectively revealed 72.3%, 26.6% and 30.5% of the damage of bacterial cell wall. Furthermore, AgNCs@Bacitracin, AuNCs@Bacitracin and CuNCs@Bacitracin have caused the increases of the intracellular ROS production leading to the bacterial death (Figure 10). Taking the advantages together, the nanoclusters of AgNCs@Bacitracin, AuNCs@Bacitracin and CuNCs@Bacitracin have shown superior antibacterial activities because of the damage of bacterial cell wall and the increase of intracellular ROS production. Additionally, bacitracin on the surface of nanoclusters has also cooperated with metallic atoms to improve antibacterial activity of nanoclusters. Among these three nanoclusters, AgNCs@Bacitracin have shown the best antibacterial activity compared to that of AuNCs@Bacitracin and CuNCs@Bacitracin. Although sliver-based nanoclusters have exhibited higher antibacterial activity compared to that of gold-based nanoclusters, gold-based nanoclusters are still the most promising metallic antibacterial agent due to their remarkable advantages such as high biocompatibility, polyvalent effect, easy modification and photothermal stability.
Figure 10.
(A) Illustration of preparations of AgNCs@Bacitracin, AuNCs@Bacitracin, and CuNCs@Bacitracin. (B) Bacteria incubated with AgNCs@Bacitracin, AuNCs@Bacitracin, or CuNCs@Bacitracin. Reproduced with permission from Reference [83]. Copyright © 2019, American Chemical Society.
4. Challenges and Opportunities
In this mini review, we have summarized recent achievements of AuNCs conjugated with small molecules and macromolecules for the applications as the antimicrobial agents (Table 1). These studies have demonstrated that AuNCs can be the potential antimicrobial agents because of their high biocompatibility, polyvalent effect, easy modification and photothermal stability. Although different AuNCs have been proven as the antimicrobial agents, however, their antimicrobial activities still need to be improved. The first challenge to improve the antimicrobial activity of AuNCs is to prepare AuNCs conjugated with antimicrobial surface ligands. The antimicrobial activity can be enhanced by the use of synergistic effect between AuNCs and antimicrobial surface ligands. The second challenge for antimicrobial AuNCs is to increase their cell uptake. With the controls of surface ligands, AuNCs can bear positive charge and negative charge and even to have target-specific property for bacteria to increase the cell uptake of AuNCs. The third challenge for antimicrobial AuNCs is to investigate the details of antimicrobial mechanisms in bacteria. Until now, there are various mechanisms to explain the antimicrobial performance of AuNCs. Therefore, experimental and theoretical investigations of the metabolisms of AuNCs in bacteria are still required for better understanding their antimicrobial activity. Overall, to realize the antimicrobial agents of AuNCs, a lot of work still need to be completed for the improvement of antimicrobial activity of AuNCs to meet the requirement in clinic application. With extensive investigations, we believe that AuNCs can be applied as the significant antimicrobial agents in clinic in the near future.
Acknowledgements
This work was supported by DP2-108-21121-01-N-09-03, MOST107-2113-M-038-004 and Taipei Medical University.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- 1.Holmes A.H., Moore L.S.P., Sundsfjord A., Steinbakk M., Regmi S., Karkey A., Guerin P.J., Piddock L.J.V. Understanding the mechanisms and drivers of antimicrobial resistance. Lancet. 2016;387:176–187. doi: 10.1016/S0140-6736(15)00473-0. [DOI] [PubMed] [Google Scholar]
- 2.Balouiri M., Sadiki M., Ibnsouda S.K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2016;6:71–79. doi: 10.1016/j.jpha.2015.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Laxminarayan R., Matsoso P., Pant S., Brower C., Rottingen J.A., Klugman K., Davies S. Access to effective antimicrobials: A worldwide challenge. Lancet. 2016;387:168–175. doi: 10.1016/S0140-6736(15)00474-2. [DOI] [PubMed] [Google Scholar]
- 4.Duran N., Duran M., de Jesus M.B., Seabra A.B., Favaro W.J., Nakazato G. Silver nanoparticles: A new view on mechanistic aspects on antimicrobial activity. Nanomed.-Nanotechnol. Biol. Med. 2016;12:789–799. doi: 10.1016/j.nano.2015.11.016. [DOI] [PubMed] [Google Scholar]
- 5.Fletcher S. Understanding the contribution of environmental factors in the spread of antimicrobial resistance. Environ. Health Prev. Med. 2015;20:243–252. doi: 10.1007/s12199-015-0468-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Andersson D.I., Hughes D., Kubicek-Sutherland J.Z. Mechanisms and consequences of bacterial resistance to antimicrobial peptides. Drug Resist. Updat. 2016;26:43–57. doi: 10.1016/j.drup.2016.04.002. [DOI] [PubMed] [Google Scholar]
- 7.Zhu Y.G., Zhao Y., Li B., Huang C.L., Zhang S.Y., Yu S., Chen Y.S., Zhang T., Gillings M.R., Su J.Q. Continental-scale pollution of estuaries with antibiotic resistance genes. Nat. Microbiol. 2017;2:16270. doi: 10.1038/nmicrobiol.2016.270. [DOI] [PubMed] [Google Scholar]
- 8.Kraker M.E.A., Stewardson A.J., Harbarth S. Will 10 million people die a year due to antimicrobial resistance by 2050? PLOS Med. 2016;13:e1002184. doi: 10.1371/journal.pmed.1002184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O’Neill J. Review on Antimicrobial Resistance; London, UK: 2014. Review on antimicrobial resistance antimicrobial resistance: Tackling a crisis for the health and wealth of nations. [Google Scholar]
- 10.Rasool K., Helal M., Ali A., Ren C.E., Gogotsi Y., Mahmoud K.A. Antibacterial activity of ti3c2tx mxene. ACS Nano. 2016;10:3674–3684. doi: 10.1021/acsnano.6b00181. [DOI] [PubMed] [Google Scholar]
- 11.Ahmed S., Ahmad M., Swami B.L., Ikram S. A review on plants extract mediated synthesis of silver nanoparticles for antimicrobial applications: A green expertise. J. Adv. Res. 2016;7:17–28. doi: 10.1016/j.jare.2015.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Czaplewski L., Bax R., Clokie M., Dawson M., Fairhead H., Fischetti V.A., Foster S., Gilmore B.F., Hancock R.E.W., Harper D., et al. Alternatives to antibiotics-a pipeline portfolio review. Lancet Infect. Dis. 2016;16:239–251. doi: 10.1016/S1473-3099(15)00466-1. [DOI] [PubMed] [Google Scholar]
- 13.Shen K., Chen X.D., Chen J.Y., Li Y.W. Development of mof-derived carbon-based nanomaterials for efficient catalysis. ACS Catal. 2016;6:5887–5903. doi: 10.1021/acscatal.6b01222. [DOI] [Google Scholar]
- 14.Tan C.L., Cao X.H., Wu X.J., He Q.Y., Yang J., Zhang X., Chen J.Z., Zhao W., Han S.K., Nam G.H., et al. Recent advances in ultrathin two-dimensional nanomaterials. Chem. Rev. 2017;117:6225–6331. doi: 10.1021/acs.chemrev.6b00558. [DOI] [PubMed] [Google Scholar]
- 15.Niu L.Y., Coleman J.N., Zhang H., Shin H., Chhowalla M., Zheng Z.J. Production of two-dimensional nanomaterials via liquid-based direct exfoliation. Small. 2016;12:272–293. doi: 10.1002/smll.201502207. [DOI] [PubMed] [Google Scholar]
- 16.Kuo T.-R., Liao H.-J., Chen Y.-T., Wei C.-Y., Chang C.-C., Chen Y.-C., Chang Y.-H., Lin J.-C., Lee Y.-C., Wen C.-Y. Extended visible to near-infrared harvesting of earth-abundant fes2-tio2 heterostructures for highly active photocatalytic hydrogen evolution. Green Chem. 2018;20:1640–1647. doi: 10.1039/C7GC03173D. [DOI] [Google Scholar]
- 17.Choi S., Lee H., Ghaffari R., Hyeon T., Kim D.H. Recent advances in flexible and stretchable bio-electronic devices integrated with nanomaterials. Adv. Mater. 2016;28:4203–4218. doi: 10.1002/adma.201504150. [DOI] [PubMed] [Google Scholar]
- 18.Zhu J., Hersam M.C. Assembly and electronic applications of colloidal nanomaterials. Adv. Mater. 2017;29:1603895. doi: 10.1002/adma.201603895. [DOI] [PubMed] [Google Scholar]
- 19.Shin T.H., Cheon J. Synergism of nanomaterials with physical stimuli for biology and medicine. Acc. Chem. Res. 2017;50:567–572. doi: 10.1021/acs.accounts.6b00559. [DOI] [PubMed] [Google Scholar]
- 20.Wen A.M., Steinmetz N.F. Design of virus-based nanomaterials for medicine, biotechnology, and energy. Chem. Soc. Rev. 2016;45:4074–4126. doi: 10.1039/C5CS00287G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lemire J.A., Harrison J.J., Turner R.J. Antimicrobial activity of metals: Mechanisms, molecular targets and applications. Nat. Rev. Microbiol. 2013;11:371. doi: 10.1038/nrmicro3028. [DOI] [PubMed] [Google Scholar]
- 22.Zazo H., Colino C.I., Lanao J.M. Current applications of nanoparticles in infectious diseases. J. Control. Release. 2016;224:86–102. doi: 10.1016/j.jconrel.2016.01.008. [DOI] [PubMed] [Google Scholar]
- 23.Li Q.L., Mahendra S., Lyon D.Y., Brunet L., Liga M.V., Li D., Alvarez P.J.J. Antimicrobial nanomaterials for water disinfection and microbial control: Potential applications and implications. Water Res. 2008;42:4591–4602. doi: 10.1016/j.watres.2008.08.015. [DOI] [PubMed] [Google Scholar]
- 24.Miao H., Teng Z., Wang C., Chong H., Wang G. Recent progress in two-dimensional antimicrobial nanomaterials. Chem. Euro. J. 2019;25:929–944. doi: 10.1002/chem.201801983. [DOI] [PubMed] [Google Scholar]
- 25.Ji H.W., Sun H.J., Qu X.G. Antibacterial applications of graphene-based nanomaterials: Recent achievements and challenges. Adv. Drug Deliv. Rev. 2016;105:176–189. doi: 10.1016/j.addr.2016.04.009. [DOI] [PubMed] [Google Scholar]
- 26.Kumar R., Umar A., Kumar G., Nalwa H.S. Antimicrobial properties of zno nanomaterials: A review. Ceram. Int. 2017;43:3940–3961. doi: 10.1016/j.ceramint.2016.12.062. [DOI] [Google Scholar]
- 27.Zou X.F., Zhang L., Wang Z.J., Luo Y. Mechanisms of the antimicrobial activities of graphene materials. J. Am. Chem. Soc. 2016;138:2064–2077. doi: 10.1021/jacs.5b11411. [DOI] [PubMed] [Google Scholar]
- 28.Zhang Y., Ali S.F., Dervishi E., Xu Y., Li Z., Casciano D., Biris A.S. Cytotoxicity effects of graphene and single-wall carbon nanotubes in neural phaeochromocytoma-derived pc12 cells. ACS Nano. 2010;4:3181–3186. doi: 10.1021/nn1007176. [DOI] [PubMed] [Google Scholar]
- 29.Qi Z., Bharate P., Lai C.-H., Ziem B., Böttcher C., Schulz A., Beckert F., Hatting B., Mülhaupt R., Seeberger P.H. Multivalency at interfaces: Supramolecular carbohydrate-functionalized graphene derivatives for bacterial capture, release, and disinfection. Nano Lett. 2015;15:6051–6057. doi: 10.1021/acs.nanolett.5b02256. [DOI] [PubMed] [Google Scholar]
- 30.Jia Y., Zhan S., Ma S., Zhou Q. Fabrication of tio2–bi2wo6 binanosheet for enhanced solar photocatalytic disinfection of e. Coli: Insights on the mechanism. ACS Appl. Mater. Interfaces. 2016;8:6841–6851. doi: 10.1021/acsami.6b00004. [DOI] [PubMed] [Google Scholar]
- 31.Wang X., Cao L., Lu F., Meziani M.J., Li H., Qi G., Zhou B., Harruff B.A., Kermarrec F., Sun Y.-P. Photoinduced electron transfers with carbon dots. Chem. Commun. 2009:3774–3776. doi: 10.1039/b906252a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kittler S., Greulich C., Diendorf J., Koller M., Epple M. Toxicity of silver nanoparticles increases during storage because of slow dissolution under release of silver ions. Chem. Mater. 2010;22:4548–4554. doi: 10.1021/cm100023p. [DOI] [Google Scholar]
- 33.Zhu W., Li Z., Zhou Y., Yan X. Deposition of silver nanoparticles onto two dimensional biocl nanodiscs for enhanced visible light photocatalytic and biocidal activities. RSC Adv. 2016;6:64911–64920. doi: 10.1039/C6RA09964E. [DOI] [Google Scholar]
- 34.Wang H.-Y., Hua X.-W., Wu F.-G., Li B., Liu P., Gu N., Wang Z., Chen Z. Synthesis of ultrastable copper sulfide nanoclusters via trapping the reaction intermediate: Potential anticancer and antibacterial applications. ACS Appl. Mater. Interfaces. 2015;7:7082–7092. doi: 10.1021/acsami.5b01214. [DOI] [PubMed] [Google Scholar]
- 35.Javani S., Lorca R., Latorre A., Flors C., Cortajarena A.L., Somoza Á. Antibacterial activity of DNA-stabilized silver nanoclusters tuned by oligonucleotide sequence. ACS Appl. Mater. Interfaces. 2016;8:10147–10154. doi: 10.1021/acsami.6b00670. [DOI] [PubMed] [Google Scholar]
- 36.Zheng K.Y., Setyawati M.I., Lim T.P., Leong D.T., Xie J.P. Antimicrobial cluster bombs: Silver nanoclusters packed with daptomycin. ACS Nano. 2016;10:7934–7942. doi: 10.1021/acsnano.6b03862. [DOI] [PubMed] [Google Scholar]
- 37.Liu Y.F., Wang L., Bu C.P., Wang G.Q., Zhang Y.H., Fang S.M., Shi W.Z. Synthesis of luminescent ag nanoclusters with antibacterial activity. J. Nanomater. 2015;2015:792095. doi: 10.1155/2015/792095. [DOI] [Google Scholar]
- 38.Vimbela G.V., Ngo S.M., Fraze C., Yang L., Stout D.A. Antibacterial properties and toxicity from metallic nanomaterials. Int. J. Nanomed. 2017;12:3941–3965. doi: 10.2147/IJN.S134526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Qian H., Zhu M., Wu Z., Jin R. Quantum sized gold nanoclusters with atomic precision. Acc. Chem. Res. 2012;45:1470–1479. doi: 10.1021/ar200331z. [DOI] [PubMed] [Google Scholar]
- 40.Jin R., Zeng C., Zhou M., Chen Y. Atomically precise colloidal metal nanoclusters and nanoparticles: Fundamentals and opportunities. Chem. Rev. 2016;116:10346–103413. doi: 10.1021/acs.chemrev.5b00703. [DOI] [PubMed] [Google Scholar]
- 41.Zhou X., Xu W., Liu G., Panda D., Chen P. Size-dependent catalytic activity and dynamics of gold nanoparticles at the single-molecule level. J. Am. Chem. Soc. 2010;132:138–146. doi: 10.1021/ja904307n. [DOI] [PubMed] [Google Scholar]
- 42.Saha K., Agasti S.S., Kim C., Li X., Rotello V.M. Gold nanoparticles in chemical and biological sensing. Chem. Rev. 2012;112:2739–2779. doi: 10.1021/cr2001178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang Z., Ross R.D., Roeder R.K. Preparation of functionalized gold nanoparticles as a targeted x-ray contrast agent for damaged bone tissue. Nanoscale. 2010;2:582–586. doi: 10.1039/b9nr00317g. [DOI] [PubMed] [Google Scholar]
- 44.Cheng T.M., Chu H.L., Lee Y.C., Wang D.Y., Chang C.C., Chung K.L., Yen H.C., Hsiao C.W., Pan X.Y., Kuo T.R., et al. Quantitative analysis of glucose metabolic cleavage in glucose transporters overexpressed cancer cells by target-specific fluorescent gold nanoclusters. Anal. Chem. 2018;90:3974–3980. doi: 10.1021/acs.analchem.7b04961. [DOI] [PubMed] [Google Scholar]
- 45.Kaur N., Aditya R.N., Singh A., Kuo T.R. Biomedical applications for gold nanoclusters: Recent developments and future perspectives. Nanoscale Res. Lett. 2018;13:302. doi: 10.1186/s11671-018-2725-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li G., Jin R. Atomically precise gold nanoclusters as new model catalysts. Acc. Chem. Res. 2013;46:1749–1758. doi: 10.1021/ar300213z. [DOI] [PubMed] [Google Scholar]
- 47.Lin C.-A.J., Yang T.-Y., Lee C.-H., Huang S.H., Sperling R.A., Zanella M., Li J.K., Shen J.-L., Wang H.-H., Yeh H.-I. Synthesis, characterization, and bioconjugation of fluorescent gold nanoclusters toward biological labeling applications. ACS Nano. 2009;3:395–401. doi: 10.1021/nn800632j. [DOI] [PubMed] [Google Scholar]
- 48.Wen F., Dong Y., Feng L., Wang S., Zhang S., Zhang X. Horseradish peroxidase functionalized fluorescent gold nanoclusters for hydrogen peroxide sensing. Anal. Chem. 2011;83:1193–1196. doi: 10.1021/ac1031447. [DOI] [PubMed] [Google Scholar]
- 49.Chen W., Chen S. Oxygen electroreduction catalyzed by gold nanoclusters: Strong core size effects. Angew. Chem. Int. Ed. 2009;48:4386–4389. doi: 10.1002/anie.200901185. [DOI] [PubMed] [Google Scholar]
- 50.Miyamura H., Matsubara R., Miyazaki Y., Kobayashi S. Aerobic oxidation of alcohols at room temperature and atmospheric conditions catalyzed by reusable gold nanoclusters stabilized by the benzene rings of polystyrene derivatives. Angew. Chem. Int. Ed. 2007;46:4151–4154. doi: 10.1002/anie.200700080. [DOI] [PubMed] [Google Scholar]
- 51.Tsunoyama H., Sakurai H., Negishi Y., Tsukuda T. Size-specific catalytic activity of polymer-stabilized gold nanoclusters for aerobic alcohol oxidation in water. J. Am. Chem. Soc. 2005;127:9374–9375. doi: 10.1021/ja052161e. [DOI] [PubMed] [Google Scholar]
- 52.Xie J., Zheng Y., Ying J.Y. Protein-directed synthesis of highly fluorescent gold nanoclusters. J. Am. Chem. Soc. 2009;131:888–889. doi: 10.1021/ja806804u. [DOI] [PubMed] [Google Scholar]
- 53.Herzing A.A., Kiely C.J., Carley A.F., Landon P., Hutchings G.J. Identification of active gold nanoclusters on iron oxide supports for co oxidation. Science. 2008;321:1331–1335. doi: 10.1126/science.1159639. [DOI] [PubMed] [Google Scholar]
- 54.Garzón I., Michaelian K., Beltrán M., Posada-Amarillas A., Ordejón P., Artacho E., Sánchez-Portal D., Soler J. Lowest energy structures of gold nanoclusters. Phys. Rev. Lett. 1998;81:1600. doi: 10.1103/PhysRevLett.81.1600. [DOI] [PubMed] [Google Scholar]
- 55.Li C.-H., Kuo T.-R., Su H.-J., Lai W.-Y., Yang P.-C., Chen J.-S., Wang D.-Y., Wu Y.-C., Chen C.-C. Fluorescence-guided probes of aptamer-targeted gold nanoparticles with computed tomography imaging accesses for in vivo tumor resection. Sci. Rep. 2015;5:15675. doi: 10.1038/srep15675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yahia-Ammar A., Sierra D., Merola F., Hildebrandt N., Le Guevel X. Self-assembled gold nanoclusters for bright fluorescence imaging and enhanced drug delivery. ACS Nano. 2016;10:2591–2599. doi: 10.1021/acsnano.5b07596. [DOI] [PubMed] [Google Scholar]
- 57.Bootharaju M.S., Joshi C.P., Parida M.R., Mohammed O.F., Bakr O.M. Templated atom-precise galvanic synthesis and structure elucidation of a [ag24au(sr)18]− nanocluster. Angew. Chem. Int. Ed. 2016;55:922–926. doi: 10.1002/anie.201509381. [DOI] [PubMed] [Google Scholar]
- 58.Higaki T., Liu C., Zeng C.J., Jin R.X., Chen Y.X., Rosi N.L., Jin R.C. Controlling the atomic structure of au-30 nanoclusters by a ligand-based strategy. Angew. Chem. Int. Ed. 2016;55:6694–6697. doi: 10.1002/anie.201601947. [DOI] [PubMed] [Google Scholar]
- 59.Kang X., Wang S.X., Song Y.B., Jin S., Sun G.D., Yu H.Z., Zhu M.Z. Bimetallic au2cu6 nanoclusters: Strong luminescence induced by the aggregation of copper(i) complexes with gold(0) species. Angew. Chem. Int. Ed. 2016;55:3611–3614. doi: 10.1002/anie.201600241. [DOI] [PubMed] [Google Scholar]
- 60.Wang Y.Z., De S., Yan N. Rational control of nano-scale metal-catalysts for biomass conversion. Chem. Commun. 2016;52:6210–6224. doi: 10.1039/C6CC00336B. [DOI] [PubMed] [Google Scholar]
- 61.Chen Y.X., Liu C., Tang Q., Zeng C.J., Higaki T., Das A., Jiang D.E., Rosi N.L., Jin R.C. Isomerism in au28(sr)20 nanocluster and stable structures. J. Am. Chem. Soc. 2016;138:1482–1485. doi: 10.1021/jacs.5b12094. [DOI] [PubMed] [Google Scholar]
- 62.Wang Y., Wan X.K., Ren L.T., Su H.F., Li G., Malola S., Lin S.C., Tang Z.C., Hakkinen H., Teo B.K., et al. Atomically precise alkynyl-protected metal nanoclusters as a model catalyst: Observation of promoting effect of surface ligands on catalysis by metal nanoparticles. J. Am. Chem. Soc. 2016;138:3278–3281. doi: 10.1021/jacs.5b12730. [DOI] [PubMed] [Google Scholar]
- 63.Zeng C.J., Chen Y.X., Iida K., Nobusada K., Kirschbaum K., Lambright K.J., Jin R.C. Gold quantum boxes: On the periodicities and the quantum confinement in the au-28, au-36, au-44, and au-52 magic series. J. Am. Chem. Soc. 2016;138:3950–3953. doi: 10.1021/jacs.5b12747. [DOI] [PubMed] [Google Scholar]
- 64.Yao Q.F., Yuan X., Fung V., Yu Y., Leong D.T., Jiang D.E., Xie J.P. Understanding seed-mediated growth of gold nanoclusters at molecular level. Nat. Commun. 2017;8:927. doi: 10.1038/s41467-017-00970-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Govindaraju S., Ankireddy S.R., Viswanath B., Kim J., Yun K. Fluorescent gold nanoclusters for selective detection of dopamine in cerebrospinal fluid. Sci. Rep. 2017;7 doi: 10.1038/srep40298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Weng B., Lu K.-Q., Tang Z., Chen H.M., Xu Y.-J. Stabilizing ultrasmall au clusters for enhanced photoredox catalysis. Nat. Commun. 2018;9:1543. doi: 10.1038/s41467-018-04020-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yamgar R.S., Nivid Y., Nalawade S., Mandewale M., Atram R., Sawant S.S. Novel zinc (ii) complexes of heterocyclic ligands as antimicrobial agents: Synthesis, characterisation, and antimicrobial studies. Bioinorg. Chem. Appl. 2014;2014:276598. doi: 10.1155/2014/276598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fernando S., Fernando T., Stefanik M., Eyer L., Ruzek D. An approach for zika virus inhibition using homology structure of the envelope protein. Mol. Biotechnol. 2016;58:801–806. doi: 10.1007/s12033-016-9979-1. [DOI] [PubMed] [Google Scholar]
- 69.Nwibo D.D., Levi C.A., Nwibo M.I. Small molecule drugs; down but not out: A future for medical research and therapeutics. J. Appl. Dent. 2015;14:70–77. [Google Scholar]
- 70.Hay M., Thomas D.W., Craighead J.L., Economides C., Rosenthal J. Clinical development success rates for investigational drugs. Nat. Biotechnol. 2014;32:40. doi: 10.1038/nbt.2786. [DOI] [PubMed] [Google Scholar]
- 71.Zheng K., Setyawati M.I., Leong D.T., Xie J. Antimicrobial gold nanoclusters. ACS Nano. 2017;11:6904–6910. doi: 10.1021/acsnano.7b02035. [DOI] [PubMed] [Google Scholar]
- 72.Setyawati M.I., Kutty R.V., Tay C.Y., Yuan X., Xie J., Leong D.T. Novel theranostic DNA nanoscaffolds for the simultaneous detection and killing of escherichia coli and staphylococcus aureus. ACS Appl. Mater. Interfaces. 2014;6:21822–21831. doi: 10.1021/am502591c. [DOI] [PubMed] [Google Scholar]
- 73.Sinha T., Ahmaruzzaman M. A new and facile strategy for the one-pot fabrication of luminescent gold nanoclusters and their prospective application. RSC Adv. 2016;6:44–56. doi: 10.1039/C5RA21495E. [DOI] [Google Scholar]
- 74.Tseng Y.-T., Chang H.-T., Chen C.-T., Chen C.-H., Huang C.-C. Preparation of highly luminescent mannose–gold nanodots for detection and inhibition of growth of escherichia coli. Biosens. Bioelectron. 2011;27:95–100. doi: 10.1016/j.bios.2011.06.021. [DOI] [PubMed] [Google Scholar]
- 75.Xie Y., Liu Y., Yang J., Liu Y., Hu F., Zhu K., Jiang X. Gold nanoclusters for targeting methicillin-resistant staphylococcus aureus in vivo. Angew. Chem. Int. Ed. Engl. 2018;57:3958–3962. doi: 10.1002/anie.201712878. [DOI] [PubMed] [Google Scholar]
- 76.Zheng Y., Liu W., Qin Z., Chen Y., Jiang H., Wang X. Mercaptopyrimidine-conjugated gold nanoclusters as nanoantibiotics for combating multidrug-resistant superbugs. Bioconjug. Chem. 2018;29:3094–3103. doi: 10.1021/acs.bioconjchem.8b00452. [DOI] [PubMed] [Google Scholar]
- 77.Zheng K., Setyawati M.I., Leong D.T., Xie J. Surface ligand chemistry of gold nanoclusters determines their antimicrobial ability. Chem. Mater. 2018;30:2800–2808. doi: 10.1021/acs.chemmater.8b00667. [DOI] [Google Scholar]
- 78.Chen W.Y., Lin J.Y., Chen W.J., Luo L., Wei-Guang Diau E., Chen Y.C. Functional gold nanoclusters as antimicrobial agents for antibiotic-resistant bacteria. Nanomedicine. 2010;5:755–764. doi: 10.2217/nnm.10.43. [DOI] [PubMed] [Google Scholar]
- 79.Kalita S., Kandimalla R., Bhowal A.C., Kotoky J., Kundu S. Functionalization of beta-lactam antibiotic on lysozyme capped gold nanoclusters retrogress mrsa and its persisters following awakening. Sci. Rep. 2018;8:5778. doi: 10.1038/s41598-018-22736-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Liang J., Xiong H., Wang W., Wen W., Zhang X., Wang S. “Luminescent-off/on” sensing mechanism of antibiotic-capped gold nanoclusters to phosphate-containing metabolites and its antibacterial characteristics. Sensor. Actuat. B-Chem. 2018;255:2170–2178. doi: 10.1016/j.snb.2017.09.032. [DOI] [Google Scholar]
- 81.Li Q., Pan Y., Chen T., Du Y., Ge H., Zhang B., Xie J., Yu H., Zhu M. Design and mechanistic study of a novel gold nanocluster-based drug delivery system. Nanoscale. 2018;10:10166–10172. doi: 10.1039/C8NR02189A. [DOI] [PubMed] [Google Scholar]
- 82.Liao F.H., Wu T.H., Huang Y.T., Lin W.J., Su C.J., Jeng U.S., Kuo S.C., Lin S.Y. Subnanometer gold clusters adhere to lipid a for protection against endotoxin-induced sepsis. Nano Lett. 2018;18:2864–2869. doi: 10.1021/acs.nanolett.7b05464. [DOI] [PubMed] [Google Scholar]
- 83.Wang S., Wang Y., Peng Y., Yang X. Exploring the anti-bacteria performance of multi-color ag, au and cu nanoclusters. ACS Appl. Mater. Interfaces. 2019;11:8461–8469. doi: 10.1021/acsami.8b22143. [DOI] [PubMed] [Google Scholar]
- 84.Grace A.N., Pandian K. Antibacterial efficacy of aminoglycosidic antibiotics protected gold nanoparticles—a brief study. Colloids Surf. A. 2007;297:63–70. doi: 10.1016/j.colsurfa.2006.10.024. [DOI] [Google Scholar]
- 85.Rai A., Prabhune A., Perry C.C. Antibiotic mediated synthesis of gold nanoparticles with potent antimicrobial activity and their application in antimicrobial coatings. J. Mater. Chem. 2010;20:6789–6798. doi: 10.1039/c0jm00817f. [DOI] [Google Scholar]
- 86.Burygin G., Khlebtsov B., Shantrokha A., Dykman L., Bogatyrev V., Khlebtsov N. On the enhanced antibacterial activity of antibiotics mixed with gold nanoparticles. Nanoscale Res. Lett. 2009;4:794. doi: 10.1007/s11671-009-9316-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Li X., Robinson S.M., Gupta A., Saha K., Jiang Z., Moyano D.F., Sahar A., Riley M.A., Rotello V.M. Functional gold nanoparticles as potent antimicrobial agents against multi-drug-resistant bacteria. ACS Nano. 2014;8:10682–10686. doi: 10.1021/nn5042625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fang F.C. Antimicrobial reactive oxygen and nitrogen species: Concepts and controversies. Nat. Rev. Microbiol. 2004;2:820–832. doi: 10.1038/nrmicro1004. [DOI] [PubMed] [Google Scholar]
- 89.Li J., Fan C., Pei H., Shi J., Huang Q. Smart drug delivery nanocarriers with self-assembled DNA nanostructures. Adv. Mater. 2013;25:4386–4396. doi: 10.1002/adma.201300875. [DOI] [PubMed] [Google Scholar]
- 90.Zhang S., Li J., Lykotrafitis G., Bao G., Suresh S. Size-dependent endocytosis of nanoparticles. Adv. Mater. 2009;21:419–424. doi: 10.1002/adma.200801393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Dubnau D. DNA uptake in bacteria. Annu. Rev. Microbiol. 1999;53:217–244. doi: 10.1146/annurev.micro.53.1.217. [DOI] [PubMed] [Google Scholar]
- 92.Cui M., Zhao Y., Song Q. Synthesis, optical properties and applications of ultra-small luminescent gold nanoclusters. TrAC Trends Anal. Chem. 2014;57:73–82. doi: 10.1016/j.trac.2014.02.005. [DOI] [Google Scholar]
- 93.Lin Y., Ren J., Qu X. Catalytically active nanomaterials: A promising candidate for artificial enzymes. Acc. Chem. Res. 2014;47:1097–1105. doi: 10.1021/ar400250z. [DOI] [PubMed] [Google Scholar]
- 94.Liu Y., Ding S., Dietrich R., Märtlbauer E., Zhu K. A biosurfactant-inspired heptapeptide with improved specificity to kill mrsa. Angew. Chem. Int. Ed. 2017;56:1486–1490. doi: 10.1002/anie.201609277. [DOI] [PubMed] [Google Scholar]
- 95.Zhang X., Chen X., Yang J., Jia H.-R., Li Y.-H., Chen Z., Wu F.-G. Quaternized silicon nanoparticles with polarity-sensitive fluorescence for selectively imaging and killing gram-positive bacteria. Adv. Funct. Mater. 2016;26:5958–5970. doi: 10.1002/adfm.201602185. [DOI] [Google Scholar]
- 96.Zheng Y., Liu W., Chen Y., Jiang H., Yan H., Kosenko I., Chekulaeva L., Sivaev I., Bregadze V., Wang X. A highly potent antibacterial agent targeting methicillin-resistant staphylococcus aureus based on cobalt bis(1,2-dicarbollide) alkoxy derivative. Organometallics. 2017;36:3484–3490. doi: 10.1021/acs.organomet.7b00426. [DOI] [Google Scholar]
- 97.Shi M., Kwon H.S., Peng Z., Elder A., Yang H. Effects of surface chemistry on the generation of reactive oxygen species by copper nanoparticles. ACS Nano. 2012;6:2157–2164. doi: 10.1021/nn300445d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Brogden K.A. Antimicrobial peptides: Pore formers or metabolic inhibitors in bacteria? Nat. Rev. Microbiol. 2005;3:238. doi: 10.1038/nrmicro1098. [DOI] [PubMed] [Google Scholar]
- 99.Yeaman M.R., Yount N.Y. Mechanisms of antimicrobial peptide action and resistance. Pharmacol. Rev. 2003;55:27–55. doi: 10.1124/pr.55.1.2. [DOI] [PubMed] [Google Scholar]
- 100.Chan P.H., Wong S.Y., Lin S.H., Chen Y.C. Lysozyme-encapsulated gold nanocluster-based affinity mass spectrometry for pathogenic bacteria. Rapid Commun. Mass. Spectrom. 2013;27:2143–2148. doi: 10.1002/rcm.6674. [DOI] [PubMed] [Google Scholar]
- 101.Kiristi M., Singh V.V., Esteban-Fernandez de Avila B., Uygun M., Soto F., Aktas Uygun D., Wang J. Lysozyme-based antibacterial nanomotors. ACS Nano. 2015;9:9252–9259. doi: 10.1021/acsnano.5b04142. [DOI] [PubMed] [Google Scholar]
- 102.Alsaiari S.K., Hammami M.A., Croissant J.G., Omar H.W., Neelakanda P., Yapici T., Peinemann K.V., Khashab N.M. Colloidal gold nanoclusters spiked silica fillers in mixed matrix coatings: Simultaneous detection and inhibition of healthcare-associated infections. Adv. Healthc. Mater. 2017;6:1601135. doi: 10.1002/adhm.201601135. [DOI] [PubMed] [Google Scholar]
- 103.Kohanski M.A., Dwyer D.J., Collins J.J. How antibiotics kill bacteria: From targets to networks. Nat. Rev. Microbiol. 2010;8:423. doi: 10.1038/nrmicro2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Py B., Barras F. Building fe–s proteins: Bacterial strategies. Nat. Rev. Microbiol. 2010;8:436. doi: 10.1038/nrmicro2356. [DOI] [PubMed] [Google Scholar]
- 105.Turci F., Ghibaudi E., Colonna M., Boscolo B., Fenoglio I., Fubini B. An integrated approach to the study of the interaction between proteins and nanoparticles. Langmuir. 2010;26:8336–8346. doi: 10.1021/la904758j. [DOI] [PubMed] [Google Scholar]
- 106.Pu Y., Zhao Z., Li Y., Zou J., Ma Q., Zhao Y., Ke Y., Zhu Y., Chen H., Baker M.A. Enhanced efflux activity facilitates drug tolerance in dormant bacterial cells. Mol. Cell. 2016;62:284–294. doi: 10.1016/j.molcel.2016.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Park B.S., Lee J.-O. Recognition of lipopolysaccharide pattern by tlr4 complexes. Exp. Mol. Med. 2013;45:e66. doi: 10.1038/emm.2013.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Luo Y.-H., Wu Z.W., Tsai H.-T., Lin S.-Y., Lin P. Endotoxin nanovesicles: Hydrophilic gold nanodots control supramolecular lipopolysaccharide assembly for modulating immunological responses. Nano Lett. 2015;15:6446–6453. doi: 10.1021/acs.nanolett.5b01809. [DOI] [PubMed] [Google Scholar]