Abstract
Background: A number of teams have investigated the association between the mode of anesthesia and the long-term outcomes after cancer surgeries, with inconsistent conclusions. We conducted this systematic review and meta-analysis to summarize the currently available findings of clinical studies on the long-term outcomes after cancer surgery under inhalational anesthesia with volatile anesthetics (VA) and total intravenous anesthesia (TIVA) with propofol. Methods: We systematically searched PubMed, Central, EMBASE, CINAHL, Google Scholar, Web of Science citation index, US clinical trials register, UK clinical trials register, Australia and New Zealand Clinical trials register for clinical studies comparing postoperative outcomes of VA and TIVA. The included outcomes were all-cause mortality, recurrence and recurrence free survival. Meta-analysis was done using the generic inverse variance method. Results: The overall pooled hazard ratio for all-cause mortality was in favor of TIVA [Harzard ratio (HR) 0.73, 95% confidence interval (CI) 0.60 to 0.89], so was the recurrence free survival (HR 1.22, 95% CI 1.07 to 1.41). The subgroup analysis of mortality in different cancer types did not show any remarkable difference between the intravenous or volatile anesthesia. There was also no significant difference in recurrence. Conclusion: Our meta-analysis suggests that TIVA is associated with lower all-cause mortality after cancer surgeries. As cancers of different origins can respond very differently to pharmacological intervention, more clinical trials are needed in each cancer types in order to substantiate the role of anesthesia in cancer surgery prognosis.
Keywords: Cancer, volatile anesthetics, propofol, survival, recurrence, patient outcome
Background
It is estimated that 17 million new cases of cancer were diagnosed worldwide in 2018, and 9.6 million died from cancer [1]. In the higher income countries, cancers are some of the leading causes of death [2]. Newer evidence have emerged which suggests that handling of tumor and the stress response to surgery may promote hematogenous cancer dissemination and alter the immune response to the disseminated cancer cells [3]. Perioperative factors such as anesthesia, analgesia, blood transfusion and temperature control could all interact with and impact on the surgical outcome [4-6]. Among them, one of the main modifiable factors in anesthesia is the choice of volatile anesthetics or intravenous anesthetic for the maintenance of general anesthesia. While total intravenous anesthesia (TIVA) with propofol has a slightly higher risk of intraoperative awareness and intraoperative hypotension, it also allows for faster emergence, reduced risk of postoperative nausea and vomiting [7]. In addition, more recent studies suggest that propofol may have some anti-tumor properties [8]. In contrast, volatile anesthetics (VA) such as isoflurane and sevoflurane have been reported to promote the proliferation and migration of various cancer cell lines in vitro [9-11], and increase the tumor load in vivo [12]. It therefore stands to reason that propofol TIVA may reduce cancer cell dissemination during surgery, reduce cancer recurrence and increase patient survival.
In the past few years, several studies have compared long-term outcomes of patients operated with inhalational anesthesia and TIVA and reported varying degrees of success with TIVA. In this systematic review and meta-analysis, we compile the current evidence on the long-term cancer recurrence and survival of patients after surgery with TIVA or VA.
Methods
Search strategy
This study conformed to the Preferred Reporting Items for Systematic reviews and Meta-analysis (PRISMA) statement [13]. We used search terms ‘TIVA’, ‘total intravenous anesthesia’, ‘propofol’, ‘volatile anesthesia’, ‘cancer’, ‘malignancy’, ‘neoplasm’, ‘tumor’, ‘survival’, ‘recurrence’, ‘mortality’ ‘progression’, ‘death’, ‘metastasis’ and their Boolean combinations in PubMed, Central, EMBASE, CINAHL, Google Scholar, Web of Science citation index, US clinical trials register, UK clinical trials register, Australia and New Zealand clinical trials register. We did not impose any language at the time of the literature search. All searches were conducted independently by two authors and discrepancies were discussed after the search process.
The inclusion criteria were: 1. Clinical studies which compared the long term (more than one year from the time of the surgery) all-cause mortality and recurrence after surgery with volatile anesthesia or propofol TIVA. We included both prospective and retrospective studies in the systematic review and meta-analysis. 2. Comparison must be reported as a risk estimate [Hazard ratio (HR) or Relative risk (RR)] with measure of precision. Alternatively, it must be possible to derive the risk estimate from the reported data. Studies which did not include data in a suitable format were excluded from the meta-analysis. Exclusion criteria was studies with regional anesthesia in one of the study arms, as regional anesthesia it self maybe associated with better postoperative outcomes [5].
In addition, we also collated all the ongoing clinical trials from the searched trials registers to aid future reviews.
Data extraction
Data extraction was conducted using standardized pro-forma and checked by a second author (ZJ and RL). Extracted data included bibliographical information (author, year, PubMed ID), study design (prospective or retrospective study, number of patients in the VA and TIVA cohort, follow up length) and the outcomes (mortality, recurrence, metastasis, and whether the multivariate regression was used to eliminate potential confounding factors).
We used the Quality of Prognostic Studies (QUIPs) tool for assessing the quality of the included studies. QUIPs is a 6-item questionnaire designed for assessing prognostic studies; it could be applied to both prospective and retrospective studies. Each item represents a risk category, and can be determined to be low, medium or high risk [14]. All assessments were done by two authors independently but at the same time, any disagreement was discussed with and resolved by a third author (JL).
Statistical analysis
Meta-analysis was conducted for any outcomes reported in more than one study. Otherwise it is reported descriptively. Review Manager (RevMan) Version 5.3 (Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014) was used for the pooled analysis. For each outcome, the pooled hazard ratio (HR) of TIVA against VA was computed from the hazard ratios of the individual studies using generic inverse variance method with 95% confidence interval (CI) [15]. In studies which did not conduct formal survival studies and report the hazard ratio, relative risk value was used as the estimate for hazard ratio using methods described by Tiemey et al [16]. Heterogeneity was assessed using Cochrane’s I2 statistic, expressed as a percentage term; higher percentage suggests higher degree of heterogeneity [17].
Due to the inherent heterogeneity in the cancer types and stages, we used the random effect model for all outcomes. In addition, subgroup analyses were conducted for organ involved and for prospective against retrospective studies. Due to the small number of studies for each organ system and the inherent heterogeneity in cancer stages, we used random effect models in all subgroup analyses. Publication bias was assessed using Egger’s regression (statistical significance indicates high probability of publication bias) using statistical package provided by Suurmond et al. Egger’s regression is expressed as a p-value, and P < 0.05 is considered significant likelihood of bias [18,19].
Results
Description of included studies
The last literature search was done on May 20th, 2019. Our literature search process identified a total of 1257 studies, of which 32 passed the title and abstract screening. There were 17 duplicates which were removed. Of the remaining studies, three were removed on further reading, two used epidural anesthesia, which is independently associated with better postoperative outcomes; one was a study protocol (see Figure 1).
Figure 1.
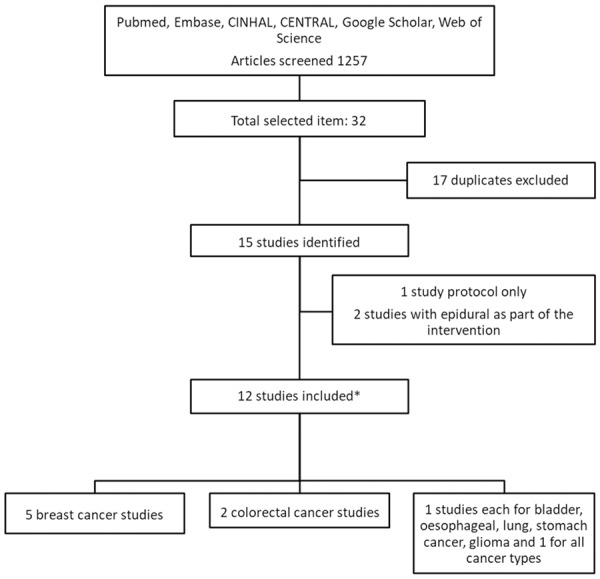
Flow chart of the literature search. *: one study included subgroups of breast and colorectal cancer.
Twelve studies were included in the meta-analysis, and their characteristics was summarized in Table 1 [20-31]. There were two prospective studies, with sample size between 28 and 80 participants, and ten retrospective studies, with sample size between 294 and 7030 case records. In terms of cancer type, there were five studies on breast cancer surgery and two studies on colorectal cancer surgery. In addition, there was one study each for esophageal, bladder, gastric, glioma, and lung cancer surgery outcomes. The study by Enlund et al included cases of both breast and colorectal surgery and reported the outcomes separately [21]. The study by Wigmore included 7030 case records of patients who had any elective cancer surgery [27]. The median follow-up of the studies ranges from 1 year to over 5 years.
Table 1.
Characteristics of included trials
| Study ID | Methods | Participants | Interventions | Outcomes | Notes |
|---|---|---|---|---|---|
| Dong 2019 [20] | Single centre retrospective analysis of hospital records | 294 High grade glioma patients operated 2012-2016 | Propofol vs sevoflurane | Survival, progression of disease | |
| Enlund 2014 [21] | Single centre retrospective analysis of hospital records | 2,838 Breast and colorectal cancer patients operated between 1998-2010 | Propofol vs sevoflurane | Survival | |
| Jun 2017 [22] | Single centre retrospective analysis of hospital records | 922 Esophageal cancer patients operated between 2005-2015 | Propofol vs volatiles | Survival, recurrence free survival | Propensity score matched subgroup analysis |
| Kim 2017 [23] | Single centre retrospective analysis of hospital records | 2,729 Breast cancer patients operated between 2005-2010 | Propofol vs volatiles | Survival, recurrence | Propensity matched |
| Lee 2016 [24] | Single centre retrospective analysis of hospital records | 325 Breast cancer patients operated between 2007-2008 | Propofol vs sevoflurane | Survival, recurrence | |
| Oh 2018 [25] | Single centre retrospective analysis of hospital records | 943 Lung cancer patients operated between 2003-2012 | Propofol vs sevoflurane | Survival, recurrence | Propensity matched |
| Sofra 2013 [26] | RCT, unclear blinding | 28 Bladder cancer patients operated between 2010-2011 | Propofol vs sevoflurane | Survival | |
| Wigmore 2016 [27] | Single centre retrospective analysis of hospital records | 7030 Patients for all cancer surgery between 2010-2013 | Propofol vs volatiles | Survival | Propensity matched |
| Wu 2018 [28] | Single centre retrospective analysis of hospital records | 1363 Colon cancer patients operated between 2005-2014 | Propofol vs Desflurane | Survival, recurrence | Propensity matched |
| Yan 2018 [29] | RCT, blinding not clear | 80 Breast cancer patients operated in 2016 | Propofol vs sevoflurane | Survival, recurrence, recurrence free survival | |
| Yoo 2018 [31] | Single centre retrospective analysis of hospital records | 5331 Breast cancer patients operated between 2005-2013 | Propofol vs volatiles | Survival and recurrence free survival | Propensity matched |
| Zheng 2018 [30] | Single centre retrospective analysis of hospital records | 2,856 Gastric cancer patients operated between 2007-2012 | Propofol vs sevoflurane | Survival | Propensity matched |
The risk of bias assessment for each study is displayed in Figure 2. The main sources of potential bias we encountered during the assessment were with the study participant attrition, either due to authors not reporting the number of cases lost to follow up [22-25,32] or due to considerably uneven incidence of censoring in the cohorts [21]. Another source of bias was study confounding, mainly due to authors not reporting the tumor stage and comorbidities [21,23,27].
Figure 2.
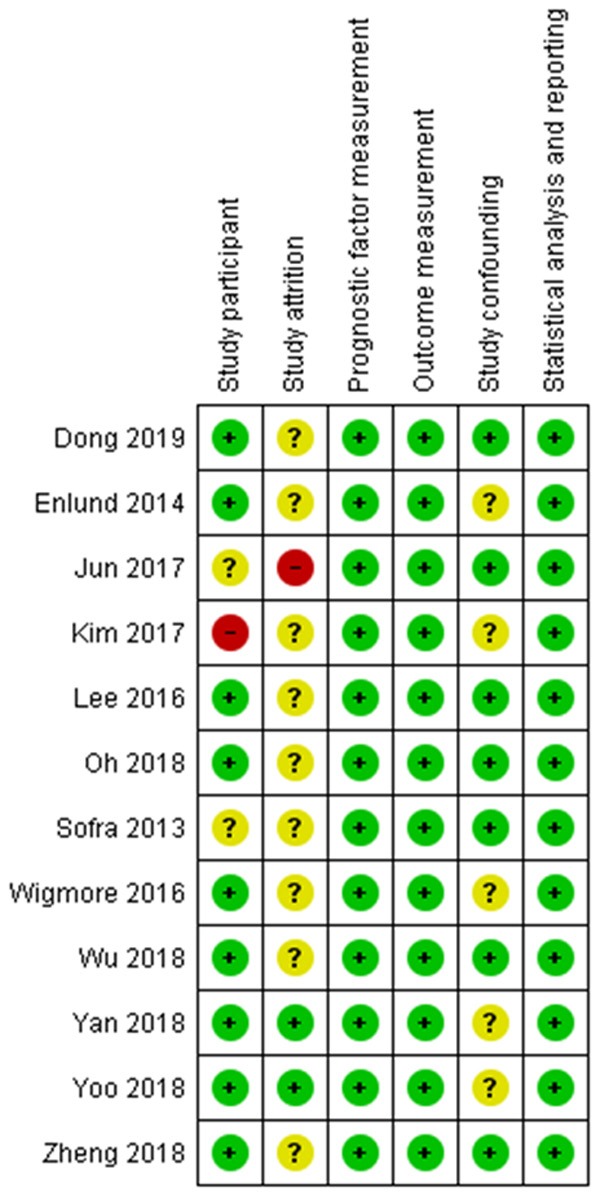
Risk of bias assessment summary.
We also identified seven ongoing clinical trials, their characteristics as well as estimated completion times are listed in Table 2.
Table 2.
List of ongoing clinical trials comparing TIVA to volatile aneasthesia
| Trial identifier | Cancer type | Estimated enrollment | Study arms | Outcomes | Trial status and estimated completion date |
|---|---|---|---|---|---|
| NCT03193710 | Colorectal cancer | 260 | Propofol vs Sevoflurane | Cancer free survival, Recurrence, metastasis up to 5 years | Recruiting, October 2023 |
| NCT03034096 | All cancer surgery | 2000 | Propofol vs volatiles | Mortality, recurrence free survival at least 2 years | Recruiting, December 2020 |
| NCT02839668 | Breast cancer | 120 | Propofol vs sevoflurane; +/- Lidocaine | Survival up to 1 year | Ongoing, December 2018 |
| NCT02786329 | Colorectal cancer | 450 | Propofol vs sevoflurane | Recurrence up to 5 years | Recruiting, December 2021 |
| NCT02756312 | Malignant Glioma | 500 | Propofol vs volatiles | Progression free survival up to 1 year | Ongoing, December 2018 |
| NCT01975064 | Breast, colorectal cancer | 8000 | Propofol vs sevoflurane | Survival after 5 years | Recruiting, December 2022 |
| NCT02660411 | All cancer surgery | 1200 | Propofol vs sevoflurane | Survival and recurrence free survival up to 3 years | Ongoing, December 2020 |
All-cause mortality
All twelve studies reported risk estimates for all-cause mortality. Both of the prospective studies reported the raw mortality rate at the end of the study. Nine of the ten retrospective studies conducted formal survival analysis and reported mortality HR based on multivariate regression, but the study by Lee et al [24] only reported the raw mortality rate at the end of the follow-up period.
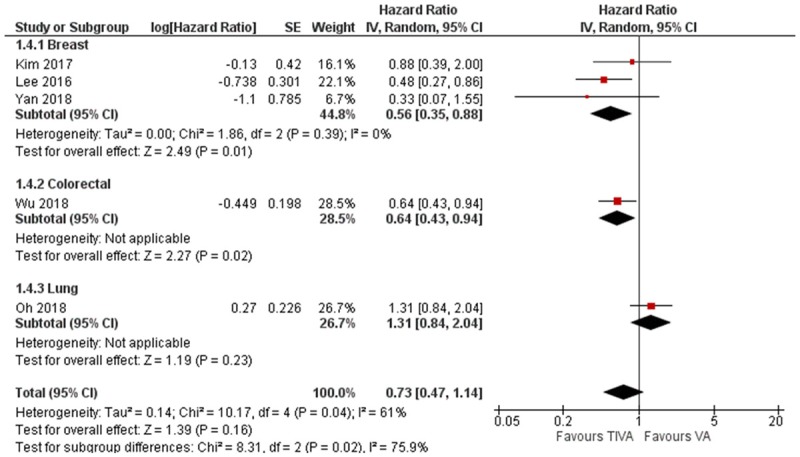
The pooled HR for mortality demonstrated significant difference in favor of the TIVA cohort, there was however considerable heterogeneity (HR = 0.73, 95% CI 0.60 to 0.89, I2 = 79%, Egger’s regression P = 0.78) (Figure 3). Due to the significant heterogeneity amongst the studies, we conducted a subgroup analysis for the different organ involvement. This did not demonstrate any significant difference between TIVA and VA (Breast cancer: HR = 1.14, 95% CI 0.92 to 1.40, Colorectal cancer: HR = 0.57, 95% CI 0.23 to 1.41) (Figure 4).
Figure 3.
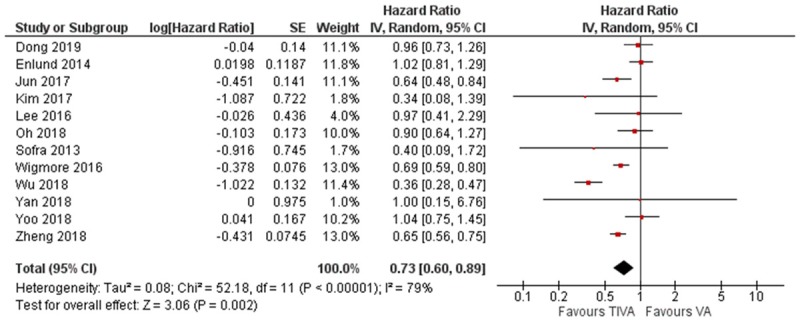
Forest plot of all-cause mortality in TIVA and VA cohorts.
Figure 4.
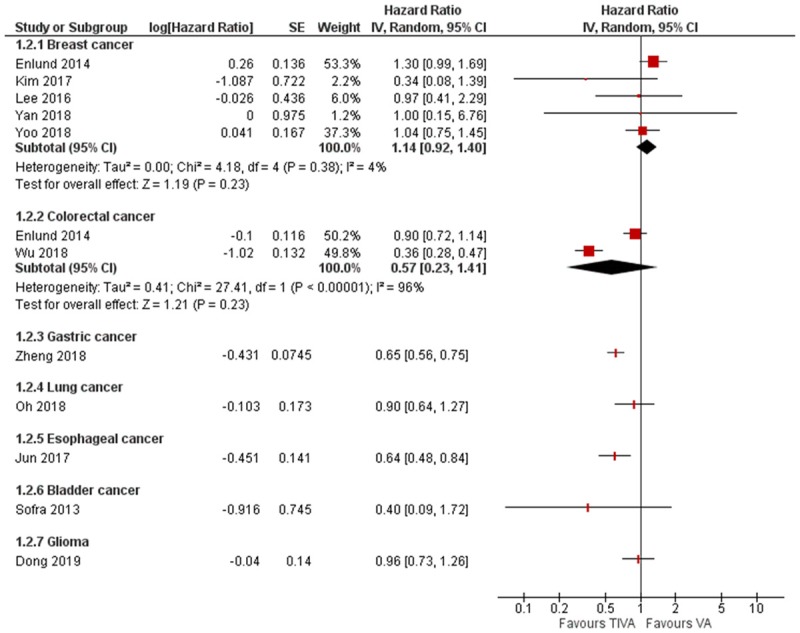
Subgroup analysis of mortality according to cancer types.
We then conducted a subgroup analysis comparing the retrospective studies which conducted formal survival analysis, and studies which only reported raw mortality data. The subgroup analysis found significant HR in favor of TIVA in the survival analysis studies, but not in the raw mortality data studies (survival analysis data: HR = 0.73, 95% CI 0.59 to 0.90, I2 = 84%; raw mortality data: HR = 0.80, 95% CI 0.40 to 1.60, I2 = 0%). There were more studies in the survival analysis subgroup, and the studies in the survival analysis subgroup had comparatively smaller confidence interval, which may have contributed to the difference (Figure 5).
Figure 5.
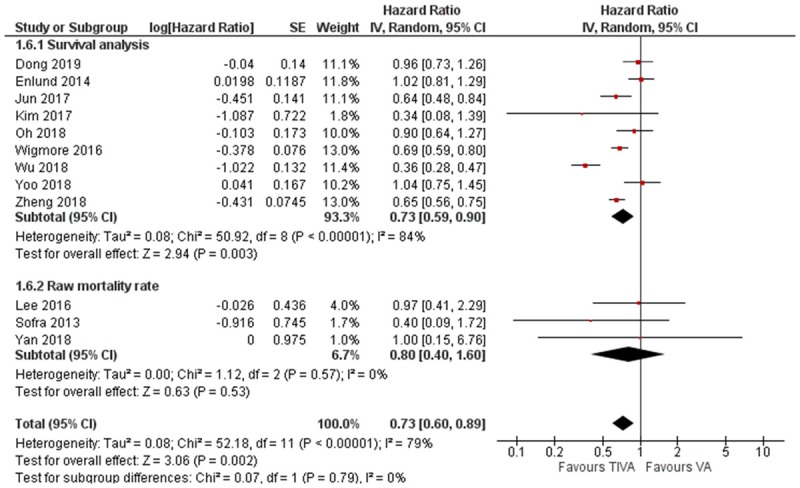
Subgroup analysis of mortality according to study design and analysis.
Recurrence and recurrence-free survival
There were five studies which reported risk estimates for recurrence. Three studies were on breast cancer and one study each on lung and colon cancer. The pooled HR slightly favors TIVA, but it was not statistically significant (HR = 0.73, 95% CI 0.47 to 1.14, I2 = 61%, Figure 6). Subgroup analysis of the three breast cancer studies however was significantly in favor of TIVA (HR = 0.56, 95% CI 0.35 to 0.88, I2 = 0%, Egger’s regression P = 0.48, Figure 6). Three studies reported risk estimates for recurrence-free survival, two for breast cancer and one for esophagus cancer. The pooled HR significantly favors TIVA (HR = 1.22, 95% CI 1.07 to 1.40, I2 = 0%, Egger’s regression P = 0.81, Figure 7).
Figure 6.
Forest plot of cancer recurrence in TIVA and VA cohorts.
Figure 7.
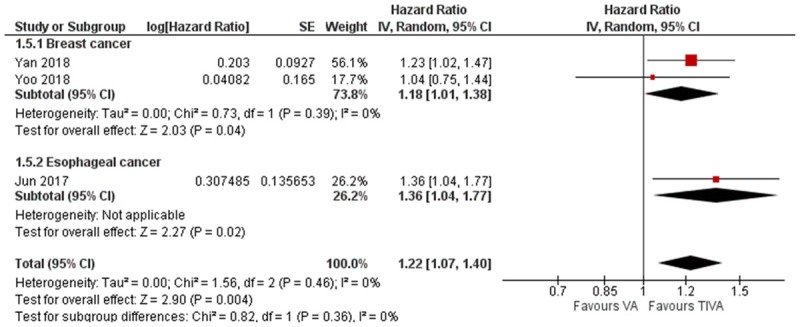
Forest plot of cancer recurrence free survival in TIVA and VA cohorts.
Discussion
Our meta-analysis suggests that in the clinical context, the choice of anesthetic agent for cancer surgery may affect long-term postoperative outcomes. TIVA appears to be associated with lower all-cause mortality and better recurrence-free survival than volatile anesthetics. This is consistent with the pre-clinical findings discussed above. It is important to consider that tumors may have various responses to the anesthetics. Indeed, the breast cancer subgroup analysis revealed lower recurrence rate but no difference in overall mortality with TIVA, different to the pooled study data. However, our attempt to conduct subgroup analysis was limited by the available number of viable subgroups (breast cancer and colorectal cancer) and number of studies (2 to 5 studies) in each sub-group. More studies are needed for each organ systems to have a sufficiently powered meta-analysis.
In order to assess the effect of study design on the heterogeneity of the results, we conducted subgroup analysis according to study design, the survival analysis studies (all retrospective studies) had a smaller confidence interval compared to the raw mortality rate studies (2 RCTs and 1 retrospective study), but the hazard ratio studies were similar. It is therefore unlikely that the study design is source of heterogeneity in the pooled analysis.
In contrast to overall mortality, the meta-analysis of cancer recurrence data did not demonstrate significant difference between TIVA and VA. This may be due to the following reasons. First, this could be due to the small number of studies and lack of statistical power. More studies are needed for each tumor types in order to accurately interpret the effect of mode of anesthesia. Second, there have been concerns that choice of anesthetic agent may have a direct impact on postoperative mortality, unrelated to its effect on cancer cells [33,34]. However, Uhlig et al conducted a meta-analysis of TIVA vs. VA in all surgery type and found no significant difference in mortality up to one year postoperatively [35]. Therefore, we suggest that the limited number of available studies reporting recurrence would still be the major reason for the differential observations between overall mortality and recurrence.
Some laboratory studies support that propofol is favorable to volatile anesthetics but remains controversial. Direct effects of anesthetics on various cancer cells have been explored for both volatile anesthetics and intravenous propofol, reviewed in [8,36,37]. Most studies focused on the changes of cancer cell phenotypes, including proliferation, migration, and invasion. Among reported molecular targets, Hypoxia-inducible factor 1-alpha (HIF-1α) is the most extensively studied one in laboratory. Isoflurane was shown to switch on HIF-1α signaling pathway in prostate cancer, lung cancer and renal cancer cells [10,38,39]. Sevoflurane was found to activate HIF-1α and downstream phosphorylated protein kinase B (p-Akt) in glioma stem cells [40]. In the opposite, propofol has been suggested to suppress the activation of HIF-1α induced by sevoflurane in prostate cancer cells [38]. To fully decipher the effect of anesthetics on cancer cells, carefully designed systematic analysis, as exampled in a study published by Huitink et al [41], are required to take into consideration of cancer heterogeneity and proper approaches to convert the identified targets into clinical application.
Another important aspect of anesthetics may affect the cancer patient outcome could be the impact on immunity. Volatile anesthetics have been shown to systemically impair immune function by inducing T-lymphocyte apoptosis, attenuating nature killer (NK) cell activity, decrease Th1/Th2 ratio, and increase levels of pro-tumorigenic cytokines and matrix metalloproteinase (MMPs) [42-44]. In contrast, propofol increased cytotoxic T-lymphocyte activity, preserved NK cell function, and decreased pro-tumorigenic cytokines [43-45]. Propofol also exhibited anti-inflammatory and anti-oxidation properties through inhibiting cyclooxygenase-2 (COX-2) and prostaglandin E2 (PGE2) function [46]. Preclinical studies as-to-now suggested that the volatile anesthetic-induced immunosuppression may be involved in cancer recurrence and metastasis, whereas propofol-based anesthesia has the opposite effect. The causal link between anesthetics, perioperative immunosuppression, and survival remains to be elucidated.
Regardless of the exact mechanism, the choice of TIVA or VA is a potentially modifiable factor in cancer management, the findings from our meta-analysis indicate that TIVA was associated with lower postoperative mortality. We need further prospective clinical trials to explicate the role of anesthetic agent on cancer prognosis. There are several ongoing clinical trials in this area which may shed some light on the topic (Table 2). However, the larger clinical trials involving common cancer types are not due to be completed for several years. We therefore believe summary of the current literature is necessary in the meantime in order to aid decision making.
Incidentally, Yap et al conducted a similar review concurrent to our meta-analysis and reported that overall survival and recurrence-free survival was significantly better with TIVA [47], which is consistent with the findings of our meta-analysis. However, our meta-analysis also included cancer recurrence as an additional outcome and found that the choice of anesthetic agent did not affect the risk of recurrence. In summary, more studies are needed in order to generate a more definitive conclusion.
Limitations
During the risk of bias assessments, we noticed that the most prominent sources of risk of bias were participant attrition and control of confounding factors. Most of the included studies either did not report their loss of participants during the follow-up periods or reported significantly more participant loss in one cohort than the other. In addition, five of twelve studies did not adequately take into account confounding factors such as patient co-morbidities or tumor grading. Those are issues for consideration for any groups looking to do further studies on the topic.
In addition to the risk assessment of the studies, this meta-analysis has a few important limitations. Firstly, this meta-analysis only covers limited types of cancer and there were only sufficient studies to conduct subgroup analysis on two cancer types. Given the diverse phenotype of cancerous cells, the results here should be interpreted with caution in clinical practice. Secondly, the included papers are mostly retrospective studies; this increases the risk of errors, for example bias in allocation to TIVA vs VA, or in selection of included cases, as well as potentially confounding factors. However, it is worth noting that seven of the ten retrospective studies included a propensity matched cohort, which does limit the risk of confounders to an extent. Lastly, while our meta-analysis has established a possible association, it does not infer causality or explains the underlying mechanism. We believe that more pre-clinical studies are needed in order to better understand the molecular mechanisms underlying the effect of anesthetic agents in cancer.
Conclusion
We conducted a meta-analysis of 12 studies, including more than 21,000 patients, which demonstrated that TIVA is associated with slightly lower mortality after cancer surgery, while its effect on recurrence and recurrence free survival remained inconclusive. More prospective clinical trials are needed to expand on the evidence base on anesthetic practice for cancer surgery.
Acknowledgements
We thank Yaohua He, MD, PhD (Senior Director, Head of Biostatistics, Taiho Oncology, Inc., Princeton, NJ, 08540) for statistic assistance. Our laboratory is funded by the Department of Anesthesiology at Stony Brook University.
Disclosure of conflict of interest
None.
References
- 1.Cancer research UK. Worldwide cancer statistics. 2018. Available from URL; https://www.cancerresearchuk.org/health-professional/cancer-statistics/worldwide-cancer.
- 2.WHO. The top 10 causes of death. 2018. Available from URL; https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death.
- 3.Tohme S, Simmons RL, Tsung A. Surgery for cancer: a trigger for metastases. Cancer Res. 2017;77:1548–1552. doi: 10.1158/0008-5472.CAN-16-1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Horowitz M, Neeman E, Sharon E, Ben-Eliyahu S. Exploiting the critical perioperative period to improve long-term cancer outcomes. Nat Rev Clin Oncol. 2015;12:213–226. doi: 10.1038/nrclinonc.2014.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sun Y, Li T, Gan TJ. The effects of perioperative regional anesthesia and analgesia on cancer recurrence and survival after oncology surgery: a systematic review and meta-analysis. Reg Anesth Pain Med. 2015;40:589–598. doi: 10.1097/AAP.0000000000000273. [DOI] [PubMed] [Google Scholar]
- 6.Kelbel I, Weiss M. Anaesthetics and immune function. Curr Opin Anaesthesiol. 2001;14:685–691. doi: 10.1097/00001503-200112000-00015. [DOI] [PubMed] [Google Scholar]
- 7.Al-Rifai Z, Mulvey D. Principles of total intravenous anaesthesia: practical aspects of using total intravenous anaesthesia. BJA Education. 2018;16:276–280. [Google Scholar]
- 8.Li R, Liu H, Dilger JP, Lin J. Effect of propofol on breast cancer cell, the immune system, and patient outcome. BMC Anesthesiol. 2018;18:77. doi: 10.1186/s12871-018-0543-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iwasaki M, Zhao H, Jaffer T, Unwith S, Benzonana L, Lian Q, Sakamoto A, Ma D. Volatile anaesthetics enhance the metastasis related cellular signalling including CXCR2 of ovarian cancer cells. Oncotarget. 2016;7:26042–26056. doi: 10.18632/oncotarget.8304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Benzonana LL, Perry NJ, Watts HR, Yang B, Perry IA, Coombes C, Takata M, Ma D. Isoflurane, a commonly used volatile anesthetic, enhances renal cancer growth and malignant potential via the hypoxia-inducible factor cellular signaling pathway in vitro. Anesthesiology. 2013;119:593–605. doi: 10.1097/ALN.0b013e31829e47fd. [DOI] [PubMed] [Google Scholar]
- 11.Luo X, Zhao H, Hennah L, Ning J, Liu J, Tu H, Ma D. Impact of isoflurane on malignant capability of ovarian cancer in vitro. Br J Anaesth. 2015;114:831–839. doi: 10.1093/bja/aeu408. [DOI] [PubMed] [Google Scholar]
- 12.Zhu M, Li M, Zhou Y, Dangelmajer S, Kahlert UD, Xie R, Xi Q, Shahveranov A, Ye D, Lei T. Isoflurane enhances the malignant potential of glioblastoma stem cells by promoting their viability, mobility in vitro and migratory capacity in vivo. Br J Anaesth. 2016;116:870–877. doi: 10.1093/bja/aew124. [DOI] [PubMed] [Google Scholar]
- 13.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hayden JA, van der Windt DA, Cartwright JL, Cote P, Bombardier C. Assessing bias in studies of prognostic factors. Ann Intern Med. 2013;158:280–286. doi: 10.7326/0003-4819-158-4-201302190-00009. [DOI] [PubMed] [Google Scholar]
- 15.Bland JM, Altman DG. Statistics notes. Logarithms. BMJ. 1996;312:700. doi: 10.1136/bmj.312.7032.700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16. doi: 10.1186/1745-6215-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Suurmond R, van Rhee H, Hak T. Introduction, comparison, and validation of meta-essentials: a free and simple tool for meta-analysis. Res Synth Methods. 2017;8:537–553. doi: 10.1002/jrsm.1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Orwin RG. A fail-safe N for effect size in meta-analysis. Journal of Educational Statistics. 1983;8:157–159. [Google Scholar]
- 20.Dong J, Zeng M, Ji N, Hao S, Zhou Y, Gao Z, Gu H, Zhang L, Ma D, Peng Y, Han R. Impact of anesthesia on long-term outcomes in patients with supratentorial high-grade glioma undergoing tumor resection: a retrospective cohort study. J Neurosurg Anesthesiol. 2019 doi: 10.1097/ANA.0000000000000588. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 21.Enlund M, Berglund A, Andreasson K, Cicek C, Enlund A, Bergkvist L. The choice of anaesthetic--sevoflurane or propofol--and outcome from cancer surgery: a retrospective analysis. Ups J Med Sci. 2014;119:251–261. doi: 10.3109/03009734.2014.922649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jun IJ, Jo JY, Kim JI, Chin JH, Kim WJ, Kim HR, Lee EH, Choi IC. Impact of anesthetic agents on overall and recurrence-free survival in patients undergoing esophageal cancer surgery: a retrospective observational study. Sci Rep. 2017;7:14020. doi: 10.1038/s41598-017-14147-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim MH, Kim DW, Kim JH, Lee KY, Park S, Yoo YC. Oncotarget. 2017. Does the type of anesthesia really affect the recurrence-free survival after breast cancer surgery? pp. 90477–90487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee JH, Kang SH, Kim Y, Kim HA, Kim BS. Effects of propofol-based total intravenous anesthesia on recurrence and overall survival in patients after modified radical mastectomy: a retrospective study. Korean J Anesthesiol. 2016;69:126–132. doi: 10.4097/kjae.2016.69.2.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oh TK, Kim K, Jheon S, Lee J, Do SH, Hwang JW, Song IA. Long-term oncologic outcomes for patients undergoing volatile versus intravenous anesthesia for non-small cell lung cancer surgery: a retrospective propensity matching analysis. Cancer Control. 2018;25:1073274818775360. doi: 10.1177/1073274818775360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sofra M, Fei PC, Fabrizi L, Marcelli ME, Claroni C, Gallucci M, Ensoli F, Forastiere E. Immunomodulatory effects of total intravenous and balanced inhalation anesthesia in patients with bladder cancer undergoing elective radical cystectomy: preliminary results. J Exp Clin Cancer Res. 2013;32:6. doi: 10.1186/1756-9966-32-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wigmore TJ, Mohammed K, Jhanji S. Long-term survival for patients undergoing volatile versus IV anesthesia for cancer surgery. Anesthesiology. 2016;124:69–79. doi: 10.1097/ALN.0000000000000936. [DOI] [PubMed] [Google Scholar]
- 28.Wu ZF, Lee MS, Wong CS, Lu CH, Huang YS, Lin KT, Lou YS, Lin C, Chang YC, Lai HC. Propofol-based total intravenous anesthesia is associated with better survival than desflurane anesthesia in colon cancer surgery. Anesthesiology. 2018;129:932–941. doi: 10.1097/ALN.0000000000002357. [DOI] [PubMed] [Google Scholar]
- 29.Yan T, Zhang GH, Wang BN, Sun L, Zheng H. Effects of propofol/remifentanil-based total intravenous anesthesia versus sevoflurane-based inhalational anesthesia on the release of VEGF-C and TGF-beta and prognosis after breast cancer surgery: a prospective, randomized and controlled study. BMC Anesthesiol. 2018;18:131. doi: 10.1186/s12871-018-0588-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zheng X, Wang Y, Dong L, Zhao S, Wang L, Chen H, Xu Y, Wang G. Effects of propofol-based total intravenous anesthesia on gastric cancer: a retrospective study. Onco Targets Ther. 2018;11:1141–1148. doi: 10.2147/OTT.S156792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yoo S, Lee HB, Han W, Noh DY, Park SK, Kim WH, Kim JT. Total intravenous anesthesia versus inhalation anesthesia for breast cancer surgery: a retrospective cohort study. Anesthesiology. 2019;130:31–40. doi: 10.1097/ALN.0000000000002491. [DOI] [PubMed] [Google Scholar]
- 32.Zheng XY, Wang Y, Dong LL, Zhao S, Wang LP, Chen H, Xu Y, Wang GN. Effects of propofol-based total intravenous anesthesia on gastric cancer: a retrospective study. Onco Targets Ther. 2018;11:1141–1148. doi: 10.2147/OTT.S156792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang DF, Su X, Meng ZT, Li HL, Wang DX, Li XY, Maze M, Ma D. Impact of dexmedetomidine on long-term outcomes after noncardiac surgery in elderly: 3-year follow-up of a randomized controlled trial. Ann Surg. 2018 doi: 10.1097/SLA.0000000000002801. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 34.Huang WW, Zhu WZ, Mu DL, Ji XQ, Nie XL, Li XY, Wang DX, Ma D. Perioperative management may improve long-term survival in patients after lung cancer surgery: a retrospective cohort study. Anesth Analg. 2018;126:1666–1674. doi: 10.1213/ANE.0000000000002886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Uhlig C, Bluth T, Schwarz K, Deckert S, Heinrich L, De Hert S, Landoni G, Serpa Neto A, Schultz MJ, Pelosi P, Schmitt J, Gama de Abreu M. Effects of volatile anesthetics on mortality and postoperative pulmonary and other complications in patients undergoing surgery: a systematic review and meta-analysis. Anesthesiology. 2016;124:1230–1245. doi: 10.1097/ALN.0000000000001120. [DOI] [PubMed] [Google Scholar]
- 36.Evans DR, Fowler-Williams C, Ma D. Is volatile anesthesia during cancer surgery likely to increase the metastatic risk? Int Anesthesiol Clin. 2016;54:92–107. doi: 10.1097/AIA.0000000000000115. [DOI] [PubMed] [Google Scholar]
- 37.Ciechanowicz S, Zhao H, Chen Q, Cui J, Mi E, Mi E, Lian Q, Ma D. Differential effects of sevoflurane on the metastatic potential and chemosensitivity of non-small-cell lung adenocarcinoma and renal cell carcinoma in vitro. Br J Anaesth. 2018;120:368–375. doi: 10.1016/j.bja.2017.11.066. [DOI] [PubMed] [Google Scholar]
- 38.Huang H, Benzonana LL, Zhao H, Watts HR, Perry NJ, Bevan C, Brown R, Ma D. Prostate cancer cell malignancy via modulation of HIF-1alpha pathway with isoflurane and propofol alone and in combination. Br J Cancer. 2014;111:1338–1349. doi: 10.1038/bjc.2014.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang W, Shao X. Isoflurane promotes non-small cell lung cancer malignancy by activating the akt-mammalian target of rapamycin (mTOR) signaling pathway. Med Sci Monit. 2016;22:4644–4650. doi: 10.12659/MSM.898434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shi QY, Zhang SJ, Liu L, Chen QS, Yu LN, Zhang FJ, Yan M. Sevoflurane promotes the expansion of glioma stem cells through activation of hypoxia-inducible factors in vitro. Br J Anaesth. 2015;114:825–830. doi: 10.1093/bja/aeu402. [DOI] [PubMed] [Google Scholar]
- 41.Huitink J, Helmerikxs M, Nieuwland M, Loer S, Brugman W, Velds A, Sie D, Kerkhoven RM. Volatile anesthetics modulate gene expression in breast and brain tumor cells. Anesth Analg. 2010;111:1411–1415. doi: 10.1213/ANE.0b013e3181fa3533. [DOI] [PubMed] [Google Scholar]
- 42.Loop T, Dovi-Akue D, Frick M, Roesslein M, Egger L, Humar M, Hoetzel A, Schmidt R, Borner C, Pahl HL, Geiger KK, Pannen BH. Volatile anesthetics induce caspase-dependent, mitochondria-mediated apoptosis in human T lymphocytes in vitro. Anesthesiology. 2005;102:1147–1157. doi: 10.1097/00000542-200506000-00014. [DOI] [PubMed] [Google Scholar]
- 43.Desmond F, McCormack J, Mulligan N, Stokes M, Buggy DJ. Effect of anaesthetic technique on immune cell infiltration in breast cancer: a follow-up pilot analysis of a prospective, randomised, investigator-masked study. Anticancer Res. 2015;35:1311–1319. [PubMed] [Google Scholar]
- 44.Deegan CA, Murray D, Doran P, Moriarty DC, Sessler DI, Mascha E, Kavanagh BP, Buggy DJ. Anesthetic technique and the cytokine and matrix metalloproteinase response to primary breast cancer surgery. Reg Anesth Pain Med. 2010;35:490–495. doi: 10.1097/AAP.0b013e3181ef4d05. [DOI] [PubMed] [Google Scholar]
- 45.Kushida A, Inada T, Shingu K. Enhancement of antitumor immunity after propofol treatment in mice. Immunopharmacol Immunotoxicol. 2007;29:477–486. doi: 10.1080/08923970701675085. [DOI] [PubMed] [Google Scholar]
- 46.Kim JD, Ahn BM, Joo BS, Kwon JY, Chung HJ, Yu SB. Effect of propofol on prostaglandin E2 production and prostaglandin synthase-2 and cyclooxygenase-2 expressions in amniotic membrane cells. J Anesth. 2014;28:911–918. doi: 10.1007/s00540-014-1830-x. [DOI] [PubMed] [Google Scholar]
- 47.Yap A, Lopez-Olivo MA, Dubowitz J, Hiller J, Riedel B. Anesthetic technique and cancer outcomes: a meta-analysis of total intravenous versus volatile anesthesia. Can J Anaesth. 2019;66:546–561. doi: 10.1007/s12630-019-01330-x. [DOI] [PubMed] [Google Scholar]


