Abstract
Background: Melatonin (Mel) has lower levels and can be used as monotherapy in schizophrenia. Mel alleviated liver steatosis induced by atypical antipsychotics. Goals: To investigate Mel effect as monotherapy and addon treatment on ketamine-induced behavioral changes in rat schizophrenia model and olanzapine (Ola)-induced metabolic derangement. Methods: 24 male rats divided into four groups; C: control; O: Ola; OM: Ola plus Mel and M: Mel. All groups treated orally daily for 25 days. We measured activities of daily life (ADL) and rat performance in radial arm water maze (RAWM) before and after ketamine (Ket) injection, serum level of liver enzymes, lipoproteins, sugar, inflammatory markers and liver histopathology. Results: Ket significantly reduced burrowing and hoarding behavior, increased working memory errors (WME) and time to reach target (TRT). Ola antagonized the deleterious effects of Ket on ADL, WME and TRT. Mel monotherapy significantly reduced burrowing and doesn’t affect hoarding, WME or TRT in RAWM. Significant rise in ALT, AST, IL-1 beta, IL-6, IL-10, TNF-alpha, LDL, TGs and hepatic steatosis score (HSS) in O compared to C group. Co administration of Mel significantly decreased ALT, AST, IL-1 beta, IL-6 and TNF alpha. Insignificant difference in IL-10, TGs or LDL and significant improvement in HSS in OM compared to O group. Insignificant change in HDL or blood sugar in both O and OM groups compared to C group detected. Conclusion: Although ineffective as monotherapy, Mel co administration provides promising natural way to improve Ola-induced hepatic derangement in psychotic disorders.
Keywords: Schizophrenia, olanzapine (Ola), melatonin (Mel), radial arm water maze (RAWM), activities of daily life (ADL), liver steatosis
Introduction
Schizophrenia is chronic cognitive disease whose etiology is multifactorial and not well understood. The role of melatonin (Mel) in treatment of schizophrenia is based on lower night concentration of this natural hormone in schizophrenia, its use as biological marker and the use of pineal extracts in treatment of dementia [1]. However, previous research reported controversy about concentration of Mel in schizophrenia or the effect of antipsychotic drugs on Mel level [2]. Moreover, Mel used to treat sleep disorders in schizophrenia and prevent metabolic complication of olanzapine (Ola) in patients with acute disease onset [3]. Recent study reported that Mel alleviated liver steatosis and cirrhosis through its anti-inflammatory and antioxidant properties [4].
However, previous studies highlighted that the protective effect of Mel is more significantly higher in bipolar disorder than schizophrenia patients and recommended further studies in this concern [5,6]. Most of the previous studies used Mel in a dose rang of (10-20 mg/Kg IP) [7,8]. One old study showed that Mel augmented phenobarbitone-induced sleep at a dose of 20 mg/Kg IP in both mice and rats [9]. The central goal of the current study is to investigate Mel effect as monotherapy and addon treatment on ketamine-induced behavioral changes of rat schizophrenia model using tests of daily life activities (ADL) and radial arm water maze (RAWM). Moreover, the present work tested the effect of Mel on Ola-induced metabolic derangement and hepatic steatosis. We measured serum level of liver enzymes; alanine transaminase (ALT) and aspartate transaminase (AST), inflammatory cytokines (IL-1 beta, IL-6, TNF-alpha and IL-10), parameters of dyslipidemia (LDL, HDL, TGs), serum sugar and histopathological steatosis score.
Methods
Drugs and chemicals
Melatonin (Mel Product No. 7903 (n-Acetyl-5-Methoxy tryptamine) purchased from Puritan’s Pride Egypt © 2019, (http://www.puritanspride.com.eg/) 24 mg mashed and dissolved in 24 ml distilled water freshly prepared and given at concentration of 10 mg/Kg/day orally daily by gavage for 25 days [10]. Olanzapine (Ola 10 mg tablet purchased from S.A.E., Badr City, Egypt 3018010101). The tab of Ola mashed and dissolved in 10 ml distilled water and given at dose of 1 mg/kg/day orally by gavage for 25 days [11]. Ketamine hydrochloride ampoule (Ket 10 ml at 50 mg/ml injection; Batch No. 10194, USP Rotex Medica, Trittau) was given as single IP injection of 20 mg/Kg 30 minutes before behavioral testing [12].
Animal grouping and experimental approach
Twenty-four young adult male Albino rats (125-225 gm) purchased from and kept at Assiut University Animal Core Facility, Assiut, Egypt. Rats were transported to Physiology lab one hour before behavioral testing. They were kept under temperature of 25°C, 12 hr light/dark cycle, normal rat chow and water Ad Libitum except for hoarding experiment. All applicable national and international guidelines were followed strictly. The protocol was approved by the Local Ethical Committee of Assiut University, Assiut, Egypt. Surgery was done under ketamine anesthesia, and all efforts were made to minimize suffering.
Rats divided into four groups six animals each; C group: control received distilled water; O group: treated with Ola (1 mg/Kg); OM group: received Ola (1 mg/Kg) plus Mel (10 mg/Kg) and M group: received Mel (10 mg/Kg) only. Treatment administered by gavage for 25 days in the early morning time between 8-10 am. Behavioral testing started 1 week after onset of treatment. We measured rat performance in ADL and RAWM. Rats subjected to behavioral tests twice before and after IP injection of single dose of Ketamine (Ket) 20 mg/Kg IP half an hour before testing. The group names after ketamine injection reported as K: Ket; KO: Ket plus Ola; KM: Ket plus Mel; and KOM: Ket plus Ola plus Mel (Figure 1).
Figure 1.
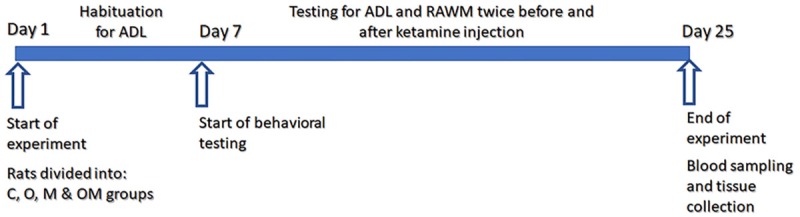
Schematic diagram of experimental work flow. C: control group; O: olanzapine group; M: melatonin group; OM: olanzapine plus melatonin group; ADL: activities of daily life testing (hoarding and burrowing); RAWM: radial arm water maze; N=6 rats in each group.
Behavioral tests
Radial arm water maze (RAWM)
Rats were tested over fifteen trials repeated for three days. At the third day Ket (20 mg/Kg) injected IP half an hour before testing. In each trial, we calculated the time to reach the pedestal (TRT), working and reference memory error (WME and RME respectively). The average of three consecutive trials are presented as five blocks on each day and named (C, O, M, OM) on the first and second days and (K, KO, and KOM) on the third day [13].
Activities of daily life
Rats tested in groups during habituation that was done on the first week of testing. On the second week rats tested separately for burrowing and hoarding [14].
Burrowing
We used tube filled with 800 gm of sand with elevated front end and closed back end put in the home cage three hours before dark. The test results were calculated the next morning by weighing the tube at the beginning and the end of the test.
Hoarding
Rats had an access to 100 gm of regular rat chow during the dark phase through long cylindrical wire tubes connected to their home cages and closed with doors during the daytime. Rats were fasted during the day time. The amount of food collected the next morning calculated by subtraction of the weight of the wired tubes at the beginning and the end of the test.
Biochemical parameters
At the end of the experiment, rats fasted for twelve hours and anaesthetized with ketamine (50 mg/Kg). Blood sampling and serum isolation done and stored at -20°C for further analysis. Serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were detected using ELISA kits (ALT FL IFCC and AST FL IFCC; purchased from Chema Diagnostica, www.chema.com). Serum Interleukin-1beta, Interleukin-6, Interleukin-10 and TNF-alpha were determined using ELIZA kits; Rat IL-1 beta (Cat. No. K0331212); Rat IL-6 (Cat. No. K0331229); Rat IL-10 (Cat No. K0332134); Rat TNF-alpha (Cat No. K0331196) purchased from (Koma Biotech INC., www.komabiotech.com). Absorbance was read using Automated ELIZA plate reader (Robonite-Readwell-India). Serum lipid profile detected using chemical methods low density lipoproteins (LDL-direct FL Cat No. DL F080 CH); high density lipoproteins (HDL-Precipitating Reagent Cat. No. CD 040 CH); triglycerides (Triglycerides FL Cat No. TR F100 CH) and glucose level (Glucose FL Cat No. GL F400 CH) all purchased from (Chema Diagnostica, www.chema.com). All biochemical analysis done following Manufacturer’s instruction and read using Biochemistry analyzer (Robonite Prietest-touch-India).
Histopathology and scoring
After animal sacrifice liver samples were taken from six animals per group and fixed in 10% of neutral buffered formalin at room temperature for 24 h. At least three different sections were taken per liver and routinely processed and embedded in paraffin blocks for sectioning. Sections (4 µm thick) were de-waxed, stained with hematoxylin and eosin (H&E) according to standard protocol. At least five microscopic fields were evaluated at magnification (X400) to score the specimens. All sections were evaluated for degree of steatosis, pattern of steatosis (Micro or macrovacuolar), degree of portal inflammation, lobular inflammation, vascular congestion and hepatocyte necrosis. Each specimen was scored using a scale (0: none < 5%; 1: mild 5-33%; 2: moderate 34-66%; 3: > severe 66%) for each criterion [15].
Statistical analysis
GraphPad Prism 5 (GraphPad Software Inc., La Jolla, CA, USA) was used for data analysis. Data were presented as mean +/-SEM. Data were compared among groups using One Way ANOVA or Two-Way ANOVA with Bonferroni Multiple Comparison as posthoc test as appropriate. A (P) value of less than 0.05 was considered to represent a statistically significant difference.
Results
Effect of melatonin vs olanzapine on activities of daily life (ADL) in ketamine -induced rat model of schizophrenia
The current study showed that Mel monotherapy significantly reduced burrowing and doesn’t affect hoarding. Mel addon to Ola was not superior to Ola alone in improving Ket-induced deterioration in hoarding behavior.
Effect of melatonin vs olanzapine on burrowing behavior in rat model of schizophrenia
We found significant reduction in the amount of sand burrowed in K group compared to C, O or KO groups (P<0.05). Significant reduction in the amount of sand burrowed in M group (P<0.001) compared to C, O and KO groups. Significant reduction in the amount of sand burrowed in KM group compared to C, O or KO groups (P<0.05). Insignificant difference in amount of sand burrowed between C, O, KO or KOM groups (P>0.05). Insignificant difference in amount of sand burrowed between M, K, KM or KOM groups (P>0.05) (Figure 2A).
Figure 2.
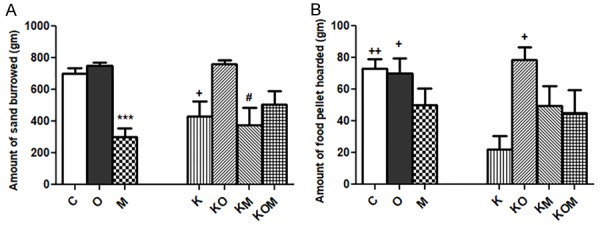
Effect of melatonin on activities of daily life (ADL). A: burrowing; B: hoarding; data represent mean ± standard error (M ± SEM); C: control group; O: olanzapine group; M: melatonin group; K: Ket; KO: Ket+Ola; KM: Ket+Mel; KOM: Ket+Ola+Mel; One-Way ANOVA with Bonferroni Multiple Comparison posthoc test; ***P<0.001; ++P<0.01; +P<0.05; #P<0.05; (*) P value of M vs C or O or KO; (+) P value of K vs C, O or KO; (#) P value of KM vs C, O or KO; P value of <.05 is considered significant; N=6 in each group.
Effect of melatonin vs olanzapine on hoarding behavior in rat model of schizophrenia
Significant reduction in the amount of food hoarded in K group compared to C (P<0.01), O (P<0.05) and KO (P<0.05) groups. Insignificant difference in amount of food hoarded between C, O, KO, KOM, M or KM groups (P>0.05) (Figure 2B).
Effect of co administration of melatonin and olanzapine on rat performance in radial arm water maze (RAWM)
The present work showed that Mel addon to Ola was not superior to Ola alone in improving the Ket-induced deterioration in rat performance in RAWM.
Effect of co administration of melatonin and olanzapine on time to reach target (TRT) in rat model of schizophrenia
Significant decrease in time to reach target (TRT) in KO (P<0.01) and KOM (P<0.001) groups compared to K group on the third day. Insignificant difference in TRT between KO and KOM groups block 1-5 (P>0.05) on the third day. Significant decrease in TRT in O (P<0.001) and OM (P<0.001) groups compared to C group in block 1 on the second day. Significant decrease in TRT in M group (P<0.05) and O group (P<0.001) compared to C group in block 2 on the second day. Significant decrease in TRT in O group (P<0.001) compared to C group in block 1 on the first day (Figure 3A).
Figure 3.
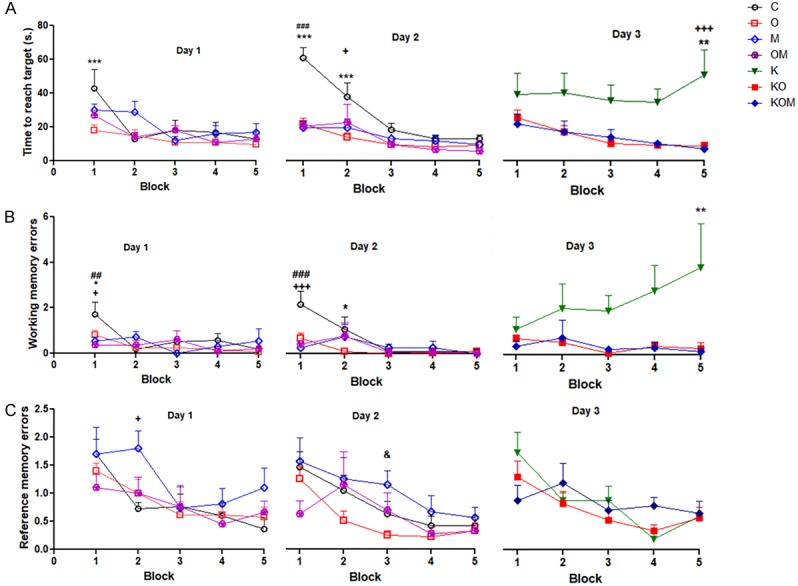
Effect of melatonin as addon treatment to olanzapine on rat performance in radial arm water maze (RAWM). A: Time to reach target (TRT); B: Working memory errors (WME); C: Reference memory errors (RME); data represent mean ± standard error (M ± SEM); C: control group; O: Ola group; M: Mel group; K: Ket; KO: Ket+Ola; KOM: Ket+Ola+Mel; Two-way ANOVA with Bonferroni Multiple Comparison posthoc test; ***P<0.001; **P<0.01; *P<0.05; +++P<0.001; ++P<0.01; +P<0.05; ###P<0.001; ##P<0.01; #P<0.05; &P<0.05; (*) P of C vs O or K vs KO; (+) P of C vs M or K vs KM; (#) P of C vs OM or KO vs KOM; (&) P of O vs M; P value of <.05 is considered significant; N = 6 in each group.
Effect of co administration of melatonin and olanzapine on working memory errors (WME) in rat model of schizophrenia
Significant decrease in working memory errors (WME) in KO group (P<0.01) and KOM group (P<0.05) compared to C group block 5 on the third day. Insignificant difference in WME between KO and KOM groups block 1-5 (P>0.05) on the third day. Significant decrease in WME in M and OM groups compared to C group (P<0.001) block 1 on the second day. Significant decrease in WME in O group compared to C group block 2 on the second day. Significant decrease in working memory errors in O, M and OM groups compared to C group (P<0.05) block 1 on the first day (Figure 3B).
Effect of co administration of melatonin and olanzapine on reference memory errors (RME) in rat model of schizophrenia
Insignificant difference in RME between K, KO or KOM blocks 1-5 on the third day. Significant difference between O group and M group (P<0.05) block 3 on the second day. Significant difference between C group and M group (P<0.05) block 2 on the first day (Figure 3C).
Effect of co administration of melatonin and olanzapine on serum level of liver enzymes, lipid profile and inflammatory cytokines
We found significant rise in ALT, AST, IL-1 beta, IL-6, IL-10, TNF-alpha, LDL and TGs in O group compared to controls. Co administration of Mel significantly decreased ALT, AST, IL-1 beta, IL-6 and TNF alpha compared to Ola alone. Insignificant difference in IL-10, TGs and LDL in OM group compared to O group was demonstrated.
Effect of co administration of melatonin and olanzapine on serum level of liver enzymes
Significant rise of alanine transaminase (ALT) (P<0.001) and aspartate transaminase (AST) (P<0.01) in Ola group if compared with C group. Co administration of melatonin caused significant decrease in ALT (P<0.001) and AST (P<0.01) in OM group compared to O group. Insignificant difference in serum ALT and AST levels between OM group and C group (P>0.05) was found (Figure 4).
Figure 4.
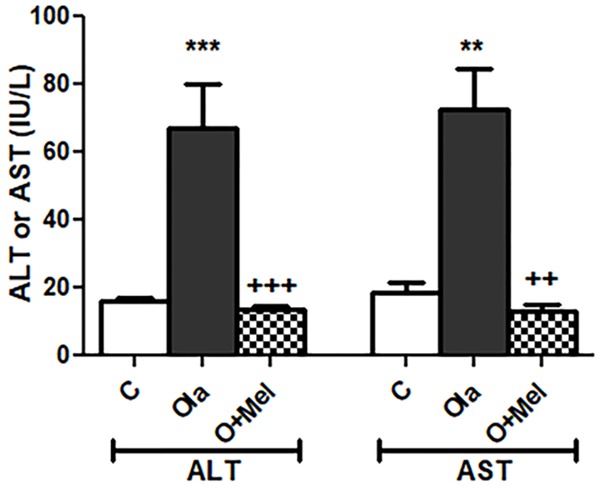
Effect of co administration of melatonin (Mel) and olanzapine (Ola) on serum level of liver enzymes; data represent mean ± standard error (M ± SEM); C: control group; O: olanzapine group: OM: olanzapine and melatonin group; alanine transaminase (ALT); aspartate transaminase (AST); international unit per liter (IU/L); One Way ANOVA with Bonferroni Multiple Comparison posthoc test; ***P<0.001; **P<0.01; +++P<0.001; ++P<0.01; (*) P value of O vs C group; (+) P value of OM vs O group; P value of <.05 is considered significant; N=6 in each group.
Effect of co administration of melatonin and olanzapine on serum lipoproteins and sugar
Significant rise of low-density lipoproteins (LDL) (P<0.01) and triglycerides (P<0.001) in O group if compared with C group. Significant rise of LDL (P<0.001) and triglycerides (P<0.001) in OM group if compared with C group. Insignificant difference in serum high density lipoproteins (HDL) or sugar level between three studied groups was obtained (P>0.05) (Figure 5).
Figure 5.
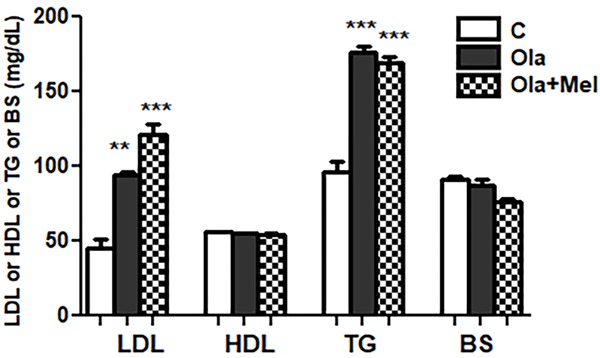
Effect of co administration of melatonin (Mel) and olanzapine (Ola) on serum lipoproteins and sugar levels; data represent mean ± standard error (M ± SEM); C: control group; O: Ola group: OM: Ola+ Mel group; low density lipoproteins (LDL); high density lipoproteins (HDL); triglycerides (TG); blood sugar (BS); milligram/deciliter (mg/dl); One Way ANOVA with Bonferroni Multiple Comparison posthoc test; ***P<0.001; **P<0.01; (*) P value of O or OM vs C group; P value of <.05 is considered significant; N=6 in each group.
Effect of co administration of melatonin and olanzapine on serum level of inflammatory cytokine
Significant rise of IL-1β, IL-6, IL-10 and TNF-α in O group (P<0.001) if compared with C group. Co administration of melatonin caused significant decrease in IL-1β, IL-6 and TNF-α in OM groups compared to O group (P<0.001). Insignificant difference in serum IL-10 level between O and OM group was obtained (P>0.05). Significant rise in serum IL-1β, IL-6, IL-10 levels in OM if compared with C group (P<0.001). Insignificant difference in TNF-α serum level in OM group when compared with C group (Figure 6).
Figure 6.
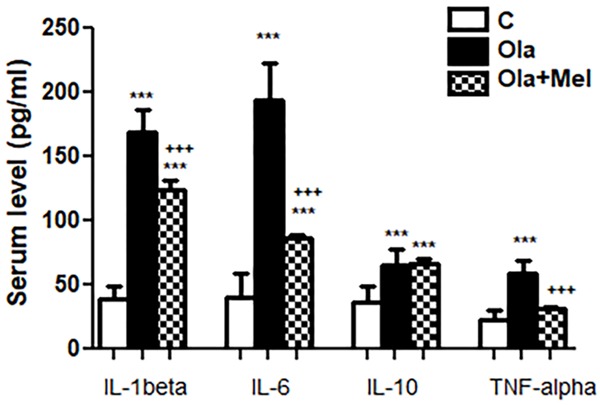
Effect of co administration of melatonin (Mel) and olanzapine (Ola) on serum level of inflammatory cytokines; data represent mean ± standard error (M ± SEM); C: control group; O: Ola group: OM: Ola+Mel group; Interleukin 1 beta (IL-1β); interleukin 6 (IL-6); interleukin 10 (IL-10); tumor necrosis factor alpha (TNF-α); Picogram/milliliter (pg/ml); One Way ANOVA with Bonferroni Multiple Comparison posthoc test; ***P<0.001; +++P<0.001; (*) P value of O or OM vs C group; (+) P value of OM vs O group; P value of <.05 is considered significant; N=6 in each group.
Effect of co administration of melatonin and olanzapine on liver histopathology and steatosis score
Significant rise hepatic damage score; steatosis, inflammatory infiltrate, congestion and necrosis in O group compared to controls. Co administration of Mel significantly decreased steatosis, congestion, cellular infiltration, necrosis and improved liver histopathology compared to Ola alone. Microscopic examination of liver sections obtained from control rats (C group) revealed normal liver architecture with the hepatocytes arranged in one thick plate that radiated away from central vein towards portal tracts. The cell plates were separated by liver blood sinusoids in which blood flow from portal space to central vein. The hepatocytes appeared polygonal with acidophilic cytoplasm and large round vesicular nuclei and prominent nucleoli (Figure 8A). Microscopic examination of liver sections obtained from Ola treated group (O group) revealed significant degree of liver affection in comparison to the C group (P<0.001) and M group (P<0.001) (Table 1; Figure 7). The pathologic changes include highest degree of steatosis mainly macrovacuolar pattern (Figure 8B), marked dilation and congestion of central vein and blood sinusoids (Figure 8C), lobular and portal inflammation together with spotty necrosis of hepatocytes (Figure 8D and 8E). Microscopic examination of liver sections obtained from Ola plus Mel treated group (OM group) showed significant protection when compared to Ola treated group in the form of significant reduction in lobular and portal inflammation with less cellular infiltrate (P<0.001), decreased congestion of vascular structures, absence of macrovacuolar steatosis of hepatocytes (P<0.001) and less necrosis (P<0.01) (Table 1; Figures 7, 8F-G). Microscopic examination of liver sections obtained from Mel treated group (M group) showed normal liver architecture without significant difference if compared to control group or OM group (P>0.05) (Table 1; Figures 7, 8H).
Figure 8.
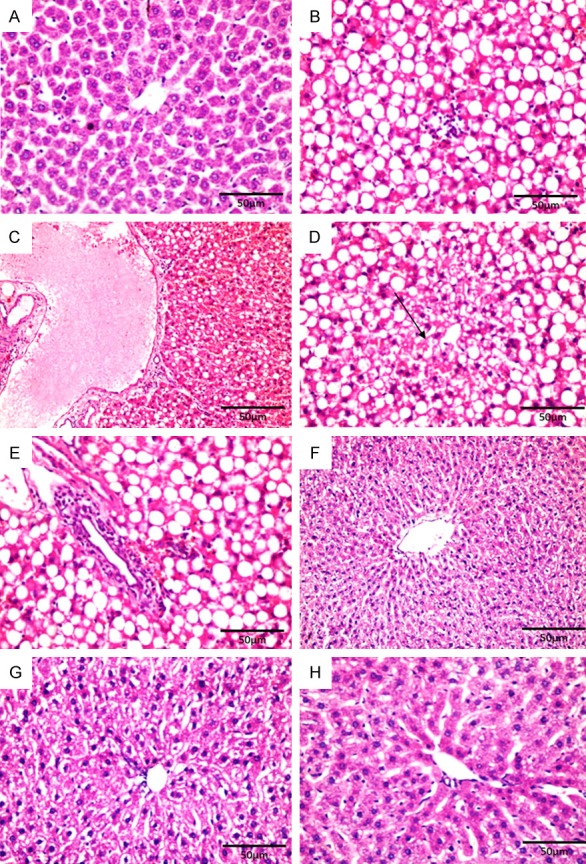
Photomicrographs of the liver stained with hematoxylin and eosin. C: control group showing normal hepatocytes around the central vein (A ×400). O: Ola group showing macrovacuolar steatosis and lobular hepatitis (B ×400), marked dilation and congestion of central vein and blood sinusoids (C ×200), spotty necrosis (straight arrow) (D ×400), portal tract expansion by inflammatory cells (E ×400). OM: Ola plus Mel group; a significant protection was observed (F ×200 & G ×400). M: Mel group showed normal liver architecture with no obvious abnormality (H ×400).
Table 1.
Comparison of mean histopathological changes in the four groups
| Steatosis | Lobular inflammation | Portal inflammation | Venous Congestion | Necrosis | |
|---|---|---|---|---|---|
| C | 0.00±0.00 | 0.00±0.00 | 0.00±0.00 | 0.43±0.20 | 0.00±0.00 |
| O | 2.8±0.14a,b | 2.7±0.18a,b | 2.4±0.20a,b | 2.43±0.20a | 2.29±0.18a,c |
| OM | 0.43±0.20 | 0.43±0.20 | 0.43±0.20 | 1.29±0.18 | 0.43±0.20 |
| M | 0.14±1.43 | 0.00±0.00 | 0.29±0.18 | 0.43±0.20 | 0.00±0.00 |
C: control; O: Ola; OM: Ola plus Mel; M: Mel; TWO WAY ANOVA with Bonferroni multiple comparison posthoc;
P<0.001 of O group vs C or M groups;
P<0.001 of O group vs OM group;
P<0.01 of O group vs OM group;
N=6 in each group.
Figure 7.
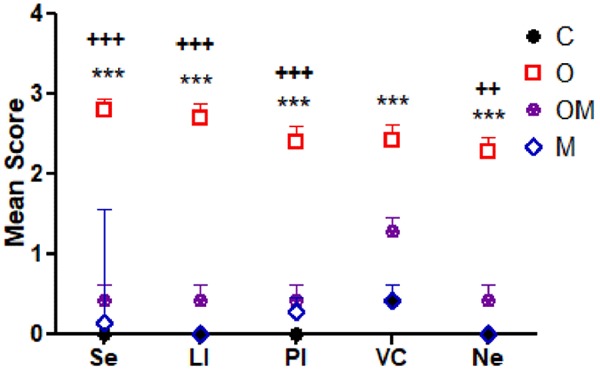
Comparison of mean histopathological changes in the four treated groups; data represent mean ± standard error (M ± SEM); C: control group; O: Ola group: OM: Ola+Mel group; M: Mel group; Se: steatosis; LI: lobular inflammation; PI: portal inflammation; VC: venous congestion; Ne: necrosis; Scoring scale: (0: none, 1: mild, 2: moderate, and 3: severe). Two Way ANOVA with Bonferroni Multiple Comparison posthoc test; ***P<0.001; +++P<0.001; ++P<0.01; (*) P value of O vs C or M group; (+) P value of C vs OM group; P value of <.05 is considered significant; N=6 in each group.
Discussion
Previous studies highlighted the beneficial effect of Mel as antipsychotic drug in schizophrenia specially in rearing and stereotypical behavior [16]. In the present study we tested the effect of Mel monotherapy and addon treatment to Ola on ADL and RAWM tests in rat model of schizophrenia. We found that Ket significantly reduced the amount of sand burrowed and the amount of food hoarded compared to controls. Mel monotherapy caused significant reduction in the amount of sand burrowed that matched the effect of Ket alone. Co-treatment of Ola and Ket significantly increased burrowing that matches controls. We found insignificant difference in amount of sand burrowed between C, O, KO or KOM groups. Although Mel monotherapy decreased hoarding, it didn’t significantly affect it compared to controls or Ket groups. While co-administration of Ola and Ket significantly increased the amount of food hoarded compared Ket alone, insignificant difference between K and KM groups found.
Up to our knowledge, no previous study tested the effect of Mel on burrowing or hoarding behavior in rodents. In line with our study, two previous studies highlighted that daily injection of Mel entrained the rhythm of free running in rats [17,18]. They highlighted that Mel effect on circadian rhythm was dose and time dependent with higher effect when given at the time of activity and with doses higher than 20 mg/Kg. Another study reported that the sedative effect of Mel was higher than 20 mg/Kg IP and motor incoordination on rotarod occurred at doses higher than 200 mg/Kg [9]. Moreover, Mel administration in rats at 2.5-10 mg/Kg significantly increased both slow wave and paradoxical sleep in dose dependent manner [19]. Previous study reported that Mel act as serotonin receptor antagonist and its co administration with AAD may augment the negative symptoms of schizophrenia [20]. In the current work, we found that Mel 10 mg/Kg orally administered in the morning time caused significant reduction in the burrowing and insignificant tendency to decrease of hoarding behavior at dark phase. Therefore, we may speculate that Mel-induced sedative effect or augmented negative symptoms hindered burrowing mainly but didn’t affect hoarding.
Moreover, we tested the effect of Mel as monotherapy and addon treatment to Ola and Ket on rat performance in RAWM. We found significant decrease in latency to pedestal and memory errors in all treated groups compared to ketamine group. Insignificant difference between C, M, O, OM groups on the first and second days was found. Both Ola alone and Ola plus Mel significantly improved Ket-induced prolongation in TRT and increased WME on the third day. Insignificant difference in RME between groups was found on days 1, 2 or 3. Several studies reported the beneficial role of Mel monotherapy and addon treatment on cognitive and memory tasks in rodents [21]. However, no previous study examined the effect of Mel as addon treatment to Ola in RAWM. We found that Mel monotherapy 10 mg/Kg didn’t improve performance in RAWM over control group or other groups. There was insignificant difference between the effect of Ola alone and Ola and Mel in antagonizing the Ket-induced impaired spatial fun-ction. Supporting us, it was reported that Mel monotherapy (10 mg/Kg IP 15 min before ethanol injection) didn’t improve the deleterious effects of ethanol on spatial memory in Morris water maze (MWM) despite it significantly improved performance compared to controls [22]. Another study showed that Mel treatment 30 mg/Kg IP for 10 days didn’t improve rat performance in passive avoidance task in rat model of Alzheimer’s disease (AD) [23]. Moreover, it was reported that both Mel and vitamin E co administration improved streptozotocin-induced deterioration of spatial memory of diabetic rats [24]. Taken together, we may speculate that Mel beneficial effect in the study Tuzcu and Baydas and in ours could be due to the effect of vitamin E or Ola respectively.
In contrast to us, one study showed that melatonin at 100 mg/kg orally daily for 4 weeks ameliorated sleep deprivation-induced memory deficit in RAWM test [25]. Another study reported that Mel administration delayed reference memory deterioration in RAWM, degeneration of hippocampus CA1 area and decreased beta amyloid accumulation in prefrontal cortex of rat model of Alzheimer’s disease [26]. Moreover, melatonin 10 mg/Kg IP for 12 days significantly improved performance of diabetic rats in spatial navigation memory task through its antioxidant effect [27]. This controversy might be explained based on the higher doses of Mel used or different animal models.
Previous studies highlighted the protective effect of co administration of Mel and AAD on hepatic injury and metabolic syndrome that could be through improving sleep, anti-inflammatory, antioxidant or neuroprotective effect [28]. The present work showed significant reduction in serum liver enzymes (ALT and AST) levels and liver histopathology with Mel plus Ola co administration compared Ola alone. Along with the present study, several previous studies showed the protective effect of pre or post treatment with Mel on liver injury induced by toxins or pharmaceutical drugs including AAD in rodents [29]. A clinical study reported decreased liver enzymes particularly AST after six months of Mel treatment 5 mg twice daily in patients taking 20 mg daily dose of statins [30]. Previous literature reported improved lipid profile with Mel administration. We found significant rise in LDL, TGs and insignificant change in HDL and blood sugar in both Ola and Ola plus Mel groups compared to controls. Insignificant difference between Ola and Ola plus Mel groups was found concerning the previous parameters. Supporting us, recent clinical study reported that Mel as addon to AAD failed to correct lipid profile after 4 weeks of treatment, however, it significantly increased HDL, decreased blood pressure and fasting blood sugar after 8 weeks of treatment [31]. Another study reported that Mel administration at doses of 2.5-20 mg/Kg for 6 weeks had insignificant effect on elevated plasma lipids, however, it reduced blood sugar and TGs, food and water intake in diabetic rats after 8 weeks of Mel administration [32]. An old study showed that daily injection of Mel 4 mg/Kg IP for 10 days had insignificant effect on plasma lipoproteins and lipase activity in rats fed normal diet, however it decreased those parameters in high cholesterol diet fed rats [33]. In contrast, one study reported significant decrease in plasma LDL, TG, VLDL and increase in HDL with Mel treatment 10 mg/Kg for 30 days in high cholesterol fed rats [34]. It seems that Mel was effective at higher doses and longer duration of administration. Therefore, we suggest that the effect of Mel on plasma lipoproteins was dose, duration and model sensitive.
Previous studies reported the anti-inflammatory effect of Mel whether induced by pharmaceutical drugs, ischemia reperfusion (IR) or disease. In the present study we found that Ola caused significant rise in IL-1beta, IL-6, TNF alpha and IL-10 levels compared to controls. Mel co-administration significantly decreased the proinflammatory cytokines IL1beta, IL-6, TNF-alpha, lobular and portal inflammation compared to Ola alone and significantly raised IL-10 compared to controls. Along our study, a previous study showed that Mel reduced significantly plasma sugar and serum IL-6 in high sugar diet fed rats [35]. Another study reported that Mel administration at 10 mg/Kg IP half an hour and 70 min before hepatic IR decreased significantly IL1beta and increased IL1 receptor antagonist expression in rats [36]. Another study reported that Mel 10 mg/kg injection immediately before renal IR significantly decreased serum TNF-alpha and renal histopathology damage [37]. Moreover, Mel at 25 microg/ml in drinking water restored IL-4 and IL-10 levels and significantly reduced levels of IL-1β, IL-6, TNF-α, IFN-γ and CRP in high fat diet-induced rat model of metabolic syndrome [38]. Mel at 10 mg/Kg daily orally for 6 weeks significantly reduced IL-6, TNF-alpha, C reactive protein and oxidative stress in diabetic rats [39]. In addition, Mel injection at 25-50 mg/Kg half an hour before IR significantly decreased plasma TNF-alpha and increased IL-10 level in IR-induced pancreatitis [40].
The current study showed insignificant difference in TNF alpha between OM group and control group denoting complete restoration. However, we found significant rise in IL-1beta and IL-6 in OM group compared to C group indicating partial correction. Although IL-10 was raised significantly in O and OM groups compared to C group, we didn’t find significant difference between O group and OM group denoting that Mel co administration was not superior to Ola alone in elevating the anti-inflammatory cytokine IL-10. In contrast to us, most of the previous studies that used higher dose of Mel (10-50 mg/Kg) as monotherapy reported significant rise in the anti-inflammatory cytokine IL-10 [35,38,40]. This controversy might be explained by the higher dose of Mel or longer duration used in the previous studies. Collectively, the present work provided an evidence for lack of effect of Mel as monotherapy in ketamine-induced rat model of schizophrenia. It also showed the beneficial effect of Mel as addon treatment to olanzapine in reducing hepatic steatosis and inflammation histologically as well as serologically. It was reported that olanzapine administration to rats caused reduction in Mel secreted at night to almost half that was corrected by giving combined treatment with Mel [41]. The correction of Mel level in the previous study was associated with significant reduction in the weight gain and visceral fat. Therefore, we encourage further clinical as well as animal research using combined therapy of Mel and AAD to prove efficacy and safety of Mel as addon treatment in schizophrenia.
Conclusion
Previous work raised the question of administration of Mel as monotherapy and addon treatment in schizophrenia. The central goal of the current study was to investigate the effect of oral administration of small dose of Mel as monotherapy and addon treatment to AAD, Ola, in rat model of Ket-induced schizophrenia. We found that Mel monotherapy significantly reduced burrowing and doesn’t affect hoarding, WME or TRT in RAWM. Moreover, Mel addon to Ola was not superior to Ola alone in improving hoarding or RAWM tests. Significant rise in ALT, AST, IL-1 beta, IL-6, IL-10, TNF-alpha, LDL, TGs and hepatic steatosis and inflammatory infiltrate and necrosis in O group compared to controls. Co administration of Mel significantly decreased ALT, AST, IL-1 beta, IL-6 and TNF alpha and improved liver histopathology compared to Ola alone. Insignificant difference in IL-10, TGs and LDL in OM group compared to O group. Although Mel was ineffective as monotherapy, its addon treatment to Ola significantly reduced the Ola-induced elevation in liver enzymes, inflammatory cytokines and improved hepatic steatosis. We recommend further animal studies using higher doses of Mel as well as clinical studies to prove the efficacy and safety of Mel as addon treatment in schizophrenia.
Disclosure of conflict of interest
None.
Abbreviations
- Ola
Olanzapine
- Mel
Melatonin
- APDs
Atypical antipsychotic drugs
- Ket
Ketamine
- ALT
Alanine transaminase
- AST
Aspartate transaminase
- Tp
Total protein
- alb
Albumin
- RAWM
Radial arm water maze
- TRT
Time to reach target
- WME
Working memory error
- RME
Reference memory error
- LDL
Low density lipoproteins
- HDL
High density lipoproteins
- TGs
Triglycerids
- BS
Blood sugar
- HSS
Hepatic steatosis score
References
- 1.Sun X, Wang Y, Jiang N, Du Z, Sun H, Sun L. The potential role of melatonin on mental disorders: insights from physiology and pharmacology. Bipolar Disord. 2016;2:1. [Google Scholar]
- 2.Morera-Fumero AL, Abreu-Gonzalez P. Role of melatonin in schizophrenia. Int J Mol Sci. 2013;14:9037–9050. doi: 10.3390/ijms14059037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Modabbernia A, Heidari P, Soleimani R, Sobhani A, Roshan ZA, Taslimi S, Ashrafi M, Modabbernia MJ. Melatonin for prevention of metabolic side-effects of olanzapine in patients with first-episode schizophrenia: randomized double-blind placebo-controlled study. J Psychiatr Res. 2014;53:133–140. doi: 10.1016/j.jpsychires.2014.02.013. [DOI] [PubMed] [Google Scholar]
- 4.Bona S, Rodrigues G, Moreira AJ, Di Naso FC, Dias AS, Da Silveira TR, Marroni CA, Marroni NP. Antifibrogenic effect of melatonin in rats with experimental liver cirrhosis induced by carbon tetrachloride. JGH Open. 2018;2:117–123. doi: 10.1002/jgh3.12055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Igwe SC, Brigo F. Does melatonin and melatonin agonists improve the metabolic side effects of atypical antipsychotics? A systematic review and meta-analysis of randomized controlled trials. Clin Psychopharmacol Neurosci. 2018;16:235–245. doi: 10.9758/cpn.2018.16.3.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Romo-Nava F, Alvarez-Icaza González D, Fresán-Orellana A, Saracco Alvarez R, Becerra-Palars C, Moreno J, Ontiveros Uribe MP, Berlanga C, Heinze G, Buijs RM. Melatonin attenuates antipsychotic metabolic effects: an eight-week randomized, double-blind, parallel-group, placebo-controlled clinical trial. Bipolar Disord. 2014;16:410–21. doi: 10.1111/bdi.12196. [DOI] [PubMed] [Google Scholar]
- 7.Yılmaz H, Ertekin T, Atay E, Nisari M, Susar Güler H, Al Ö, Payas A, Yilmaz S. Antioxidant role of melatonin against nicotine’s teratogenic effects on embryonic bone development. Iran J Basic Med Sci. 2018;21:787–793. doi: 10.22038/IJBMS.2018.26705.6539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee B, Shim I, Lee H, Hahm DH. Melatonin ameliorates cognitive memory by regulation of cAMP-response element-binding protein expression and the anti-inflammatory response in a rat model of post-traumatic stress disorder. BMC Neurosci. 2018;19:38. doi: 10.1186/s12868-018-0439-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sugden D. Psychopharmacological effects of melatonin in mouse and rat. J Pharmacol Exp Ther. 1983;227:587–91. [PubMed] [Google Scholar]
- 10.Butun I, Ekmekci H, Ciftci O, Sonmez H, Caner M, Altug T, Kokoglu E. The effects of different doses of melatonin on lipid peroxidation in diet-induced hypercholesterolemic rats. Bratisl Lek Listy. 2013;114:129–32. doi: 10.4149/bll_2013_028. [DOI] [PubMed] [Google Scholar]
- 11.Mutlu O, Celikyurt IK, Ulak G, Tanyeri P, Akar FY, Erden F. Effects of olanzapine and clozapine on radial maze performance in naive and MK-801-treated mice. Arzneimittelforschung. 2012;62:4–8. doi: 10.1055/s-0031-1291360. [DOI] [PubMed] [Google Scholar]
- 12.Razoux F, Garcia R, Isabelle Léna I. Ketamine, at a dose that disrupts motor behavior and latent inhibition, enhances prefrontal cortex synaptic efficacy and glutamate release in the nucleus accumbens. Neuropsychopharmacology. 2007;32:719–27. doi: 10.1038/sj.npp.1301057. [DOI] [PubMed] [Google Scholar]
- 13.Alamed J, Wilcock DM, Diamond DM, Gordon MN, Morgan D. Two-day radial-arm water maze learning and memory task; robust resolution of amyloid-related memory deficits in transgenic mice. Nat Protoc. 2006;1:1671. doi: 10.1038/nprot.2006.275. [DOI] [PubMed] [Google Scholar]
- 14.Deacon RMJ. A novel approach to discovering treatment for Alzheimer’s disease. J Alzheimers Dis Parkinsonism. 2014;4:142. [Google Scholar]
- 15.Burt AD, Lackner C, Tiniakos DG. Diagnosis and assessment of NAFLD: definitions and histopathological classification. Semin Liver Dis. 2015;35:207–20. doi: 10.1055/s-0035-1562942. [DOI] [PubMed] [Google Scholar]
- 16.Erbas O, Elikucuk B, Solmaz V, Akseki HS, Semiz M. Antipsychotic-like effect of agomelatine in a rodent model. Düşünen Adam the Journal of Psychiatry and Neurological Sciences. 2015;28:140–146. [Google Scholar]
- 17.Redman J, Armstrong S, Ng KT. Free-running activity rhythms in the rat: entrainment by melatonin. Science. 1983;219:1089–91. doi: 10.1126/science.6823571. [DOI] [PubMed] [Google Scholar]
- 18.Armstrong SM, Redman J. Melatonin administration: effects on rodent circadian rhythms. Ciba Found Symp. 1985;117:188–207. doi: 10.1002/9780470720981.ch12. [DOI] [PubMed] [Google Scholar]
- 19.Holmes SW, Sugden D. Effects of melatonin on sleep and neurochemistry in the rat. Br J Pharmacol. 1982;76:95–101. doi: 10.1111/j.1476-5381.1982.tb09194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sandyk R, Kay SR. Down regulation of 5-HT2 receptors: possible role of melatonin and significance for negative schizophrenia. Int J Neurosci. 1991;56:209–214. doi: 10.3109/00207459108985419. [DOI] [PubMed] [Google Scholar]
- 21.Zakaria R, Ahmad AH, Othman Z. The potential role of melatonin on memory function: lessons from rodent studies. Folia Biologica (Praha) 2016;62:181–187. doi: 10.14712/fb2016062050181. [DOI] [PubMed] [Google Scholar]
- 22.Gönenç S, Uysal N, Açikgöz O, Kayatekin BM, Sönmez A, Kiray M, Aksu I, Güleçer B, Topçu A, Semin I. Effects of melatonin on oxidative stress and spatial memory impairment induced by acute ethanol treatment in rats. Physiol Res. 2005;54:341–8. [PubMed] [Google Scholar]
- 23.Eslamizade MJ, Madjd Z, Rasoolijazi H, Saffarzadeh F, Pirhajati V, Aligholi H, Janahmadi M, Mehdizadeh M. Impaired memory and evidence of histopathology in CA1 pyramidal neurons through injection of Aβ1-42 peptides into the frontal cortices of rat. Basic Clin Neurosci. 2016;7:31–41. [PMC free article] [PubMed] [Google Scholar]
- 24.Tuzcu M, Baydas G. Effect of melatonin and vitamin E on diabetes-induced learning and memory impairment in rats. Eur J Pharmacol. 2006;537:106–10. doi: 10.1016/j.ejphar.2006.03.024. [DOI] [PubMed] [Google Scholar]
- 25.Alzoubi KH, Mayyas FA, Khabour OF, Bani Salama FM, Alhashimi FH, Mhaidat NM. Chronic melatonin treatment prevents memory impairment induced by chronic sleep deprivation. Mol. Neurobiol. 2016;53:3439–3447. doi: 10.1007/s12035-015-9286-z. [DOI] [PubMed] [Google Scholar]
- 26.Rudnitskaya EA, Muraleva NA, Maksimova KY, Kiseleva E, Kolosova NG, Stefanova NA. Melatonin attenuates memory impairment, amyloid-β accumulation, and neurodegeneration in a rat model of sporadic Alzheimer’s disease. J Alzheimers Dis. 2014;47:103–116. doi: 10.3233/JAD-150161. [DOI] [PubMed] [Google Scholar]
- 27.Babaei-Balderlou F, Zare S. Melatonin improves spatial navigation memory in male diabetic rats. Vet Res Forum. 2012;3:187–192. [PMC free article] [PubMed] [Google Scholar]
- 28.Porfirio MC, Gomes de Almeida JP, Stornelli M, Giovinazzo S, Purper-Ouakil D, Masi G. Can melatonin prevent or improve metabolic side effects during antipsychotic treatments? Neuropsychiatr Dis Treat. 2017;13:2167–2174. doi: 10.2147/NDT.S127564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang J, Meng X, Li Y, Zhou Y, Xu D, Li S, Li H. Effects of melatonin on liver injuries and diseases. Int J Mol Sci. 2017;18:673. doi: 10.3390/ijms18040673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chojnacki C, Błońska A, Chojnacki J. The effects of melatonin on elevated liver enzymes during statin treatment. Biomed Res Int. 2017;2017:3204504. doi: 10.1155/2017/3204504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Agahia M, Akasheha N, Ahmadvanda A, Akbarib H, Izadpanah F. Effect of melatonin in reducing second-generation antipsychotic metabolic effects: a double blind controlled clinical trial. Diabetes Metab Syndr. 2018;12:9–15. doi: 10.1016/j.dsx.2017.08.004. [DOI] [PubMed] [Google Scholar]
- 32.Bibak B, Khalili M, Rajaei Z, Soukhtanloo M, Hadjzadeh MA, Hayatdavoudi P. Effects of melatonin on biochemical factors and food and water consumption indiabetic rats. Adv Biomed Res. 2014;19:173. doi: 10.4103/2277-9175.139191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mori N, Aoyama H, Murase T, Murase T, Mori W. Antihypercholesterolemic effect of melatonin in rats. Acta Pathol Jpn. 1989;39:613–8. doi: 10.1111/j.1440-1827.1989.tb02407.x. [DOI] [PubMed] [Google Scholar]
- 34.Hussain SA. Effect of melatonin on cholesterol absorption in rats. J Pineal Res. 2007;42:267–71. doi: 10.1111/j.1600-079X.2006.00415.x. [DOI] [PubMed] [Google Scholar]
- 35.Zephy D, Lakshmi LJ, Muhamad F, Kasmi N, Bais PS. Effect of melatonin in high sugar diet fed male wistar rats on the levels of plasma glucose, magnesium, and interleukin-6. JMSCR. 2018;06:412–19. [Google Scholar]
- 36.Zhou H, Jiang C, Gu L, Liu Y, Sun L, Xu Q. Influence of melatonin on IL-1Ra gene and IL-1 expression in rats with liver ischemia reperfusion injury. Biomed Rep. 2016;4:667–672. doi: 10.3892/br.2016.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oguz E, Yilmaz Z, Ozbilge H, Baba F, Tabur S, Yerer MB, Hekimoglu A. Effects of melatonin on the serum levels of pro-inflammatory cytokines and tissue injury after renal ischemia reperfusion in rats. Ren Fail. 2015;37:318–322. doi: 10.3109/0886022X.2014.991263. [DOI] [PubMed] [Google Scholar]
- 38.Cano-Barquilla P, Pagano ES, Jimenez-Ortega V, Fernandez-Mateos P, Esquifino AI, Cardinali DP. Melatonin normalizes clinical and biochemical parameters of mild inflammation in diet-induced metabolic syndrome in rats. J Pineal Res. 2014;57:280–90. doi: 10.1111/jpi.12168. [DOI] [PubMed] [Google Scholar]
- 39.Agil A, Reiter RJ, Jiménez-Aranda A, Ibán-Arias R, Navarro-Alarcón M, Marchal JA, Adem A, Fernández-Vázquez G. Melatonin ameliorates low-grade inflammation and oxidative stress in young Zucker diabetic fatty rats. J Pineal Res. 2013;54:381–8. doi: 10.1111/jpi.12012. [DOI] [PubMed] [Google Scholar]
- 40.Jaworek J, Leja-Szpak A, Bonior J, Nawrot K, Tomaszewska R, Stachura J, Sendur R, Pawlik W, Brzozowski T, Konturek SJ. Protective effect of melatonin and its precursor L-tryptophan on acute pancreatitis induced by caerulein overstimulation or ischemia/reperfusion. J Pineal Res. 2003;34:40–52. doi: 10.1034/j.1600-079x.2003.02937.x. [DOI] [PubMed] [Google Scholar]
- 41.Raskind MA, Burke BL, Crites NJ, Tapp AM, Rasmussen DD. Olanzapine-induced weight gain and increased visceral adiposity is blocked by melatonin replacement therapy in rats. Neuropsychopharmacology. 2007;32:284–8. doi: 10.1038/sj.npp.1301093. [DOI] [PubMed] [Google Scholar]


