Abstract
Despite the wide application of adherence as a concept, the definition, evaluation and improvement of the adherence to treatment by patients with chronic obstructive pulmonary disease (COPD) still present some challenges. First, it is necessary to clearly define the concepts of treatment adherence, compliance and persistence. Second, it is critical to consider the various methods of evaluating and quantifying adherence when interpreting adherence studies. In addition, the advantages and disadvantages of the different ways of measuring treatment adherence should be taken into account. Another subject of some debate is the number of variables associated with COPD treatment adherence. Adherence is a complex concept that goes beyond the dosage or the use of inhalation devices, and a number of variables are involved in determining adherence, from the clinical aspects of the disease to the patient’s confidence in the doctor’s expertise and the level of social support experienced by the patient. Notably, despite these challenges, the importance of adherence has been well established by clinical trials and routine clinical practice. The available evidence consistently shows the substantial impact that a lack of adherence has on the control of the disease and its long-term prognosis. For these reasons, the correct evaluation of therapeutic adherence should be a key objective in clinical interviews of patients. In recent years, various initiatives for improving adherence have been explored. All these initiatives have been based on patient education. Therefore, health care professionals should be aware of the issues pertaining to adherence and take the opportunity to educate patients each time they contact the health care system.
Keywords: COPD, adherence, inhaled therapy, medication reminders
Introduction
The approach to the treatment of chronic diseases is characterized by pharmacological interventions that are often prolonged. These interventions have demonstrated to be effective through well-designed clinical trials that have evaluated the therapeutic efficacy of active treatment under close surveillance. Therefore, the therapeutic recommendations are based on studies with good adherence to treatment. However, in real life, this therapeutic adherence is far from perfect, especially in the long term.1 Therefore, long-term adherence to therapies emerges as a relevant challenge in daily clinical practice.2 Long-term adherence is defined by the World Health Organization as “the extent to which a person’s behavior—taking medication, following a diet, and/or executing lifestyle changes—corresponds with agreed recommendations from a health care provider”.3 The definition of adherence is related to the extent to which a patient executes the prescribed therapeutic regime and the persistence of that accurate execution over time. However, although the concept seems clear, the operationalization of these definitions and the methods for evaluating this adherence varies among studies and clinical settings.4,5 Notably, the discussion of different but related concepts, including adherence, compliance, concordance, persistence and the associated terms, eg, poor, suboptimal or low, may increase confusion.6,7
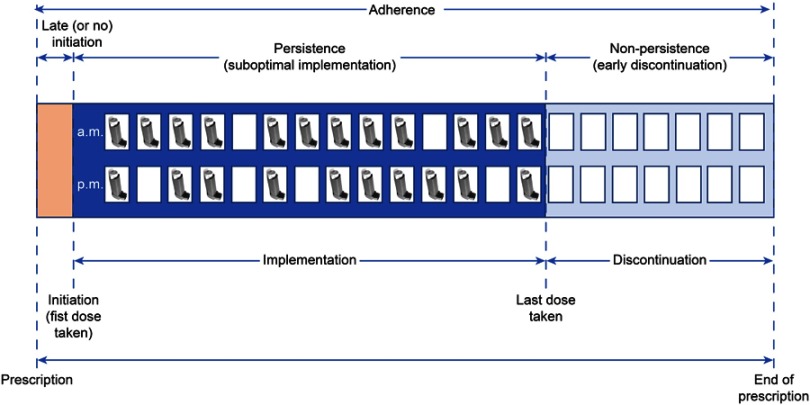
The assessment of prescriptions has been described to follow the outline shown in Figure 1.8,9 From the initiation of the prescription to its end, there are several moments during a course of treatment that delimit adherence. These are the prescribed beginning of the course of treatment, the initiation of the treatment by the patient, the implementation of the treatment by the patient, the moment when the patient decides to stop taking the treatment and the prescribed end of the course of treatment. In an ideal case, the prescribed start and end of the treatment course should coincide when the patient begins and finishes the course. Unfortunately, this is not always the case. Depending on the patient’s behavior during these periods, different situations can be identified, such as the late onset (or even non-onset), suboptimal implementation of treatment, or early discontinuation of treatment. Although most studies evaluate to suboptimal implementation, it is important to also be aware of delayed onset or non-persistence as forms of non-adherence to treatment.
Figure 1.
Concepts pertaining to the evaluation of adherence. Adapted with permission from Vrijens B, De Geest S, Hughes DA, et al. A new taxonomy for describing and defining adherence to medications. Br J Clin Pharmacol. 2012;73(5):691–705. © 2012 The Authors. British Journal of Clinical Pharmacology © 2012 The British Pharmacological Society.8
Although adherence and compliance are terms often used in a similar way, they have slightly different connotations. It has been argued that while adherence assumes that the patient agrees with the recommendations, compliance is related to a more passive attitude on the part of the patient.10 Additionally, the concept of treatment persistence is defined as “the duration of time from the beginning to the interruption of therapy” and refers to the act of undergoing treatment throughout the time for which it has been prescribed (Figure 1).4
Assessing treatment adherence in patients with COPD
The measurement of adherence is a real challenge in the clinical evaluation of patients and in clinical research. First, there is currently no consistency in the method by which adherence is assessed in studies on COPD.11 The use of different methods of assessing adherence would provide different results. Second, the majority of drugs used for COPD are inhaled, and inhaled drugs carry different implications for the evaluation of adherence than do oral medications.12 During the study of medication adherence, each case has to be qualitatively defined as being adherent or non-adherent, which depends substantially on the method of measurement used and the period during which adherence is assessed (Figure 1). In these analyses, a common metric for adherence is the percent of the total doses of medication taken. This percentage is calculated as the number of doses taken within a given time divided by the number of doses prescribed by a physician during that same time.10 Although there is no universal agreement on the threshold that defines good adherence, traditionally, a cutoff value of 80% has been used to dichotomize adherent and non-adherent patients.13
There are two different approaches in studies evaluating adherence: retrospective evaluation through the use of administrative databases and prospective evaluation at the patient level. Each of these approaches has its own tools. The evaluation of administrative databases has its own challenges, including the correct identification of COPD and the inhaled therapies in a codified database. Additionally, the design of the database is set before the study is performed, so the study is a post hoc analysis of previously recorded data. Therefore, different indicators have been created to evaluate treatment adherence. The proportion of days covered is a percentage calculated as the number of days of maintenance medication divided by the duration of therapy. The medication possession ratio is the ratio of the number of days for which a patient actually has the medication divided by the total number of days a patient is observed, typically measured over six to 12 months. Another objective adherence measurement is the medication refill adherence or medication refill rate.5 This score is calculated by dividing the total days’ supply by the number of days the patient was prescribed the medication during the implementation phase (Figure 1). These metrics can identify patients at risk for treatment failure. However, they are retrospective by nature, and the records may be incomplete or difficult to access.14 Additionally, with regard to inhaled medication, systems do not provide data on the number of inhalations per day, and the withdrawal of medication from the pharmacy by a patient does not necessarily mean that the patient is actually inhaling the medication. Finally, the lack of the prescribed daily dose in most of these databases becomes another disadvantage.
For the prospective evaluation of adherence at the patient level, several methods have been described that can be classified as either subjective or objective (Table 1). Subjective measurements involve asking patients, caregivers or doctors about the use of the medications. Objective methods involve clinical monitoring (eg, counting the doses, using questionnaires, or examining the dispensing records of the pharmacy), electronic monitoring (eg, using electronic medication monitoring systems), and biochemical monitoring (eg, measurement of the medication or a metabolite in urine, serum or different tissues).10 Of note, although the evaluation of the therapeutic response can provide an idea regarding adherence to the treatment (for example, measuring heart rate to evaluate the use of ß-blockers), the variability in the therapeutic response to treatment in patients with COPD is wide and influenced by many aspects of the disease.15,16 For these reasons, therapeutic outcomes are not generally used as an adequate measure of adherence to treatment in patients with COPD.
Table 1.
Methods of evaluating medication adherence in COPD at the patient level
| Direct methods | Indirect methods | ||
|---|---|---|---|
| Subjective |
|
||
| Objective | Clinical monitoring |
|
|
| Electronic monitoring |
|
|
|
| Biochemical monitoring |
|
|
|
Other authors have classified these prospective methods as being either direct or indirect (Table 1), with direct methods providing evidence that the patient has taken the medication and serving as the most accurate measurement of adherence.14 Despite the different options for assessing adherence, there is currently no single method that is considered the reference standard. Therefore, using combination of methods is sometimes necessary to maximize the accuracy of the assessment.
Generic questionnaires
Self-report questionnaires provide an opportunity to obtain information regarding medication adherence directly from the patient, caregiver or doctor. However, some limitations include the need for a patient-questioner connection based on shared confidence and the lack of usefulness when the patient is unaware of their nonadherent behavior. Therefore, self-reported questionnaires generally tend to overestimate adherence.17 At present, there are numerous questionnaires for the evaluation of adherence to treatment. Similar to the questionnaires for the evaluation of health-related quality of life, adherence-related questionnaires can also be divided into the categories of generic or specific. A non-exhaustive discussion of some of the latest questionnaires that have been used to measure adherence in patients with COPD follows.
Morisky medication adherence scale (MMAS) or Morisky–Green scale
The MMAS is a 4-item, self-reported, generic questionnaire with 4 items that explore the following four reasons for a lack of adherence: forgetting the medication, carelessness about the medication and stopping the medication if the patient feels worse or better. The total score ranges from 0 to 4, with higher scores indicating better adherence. Good adherence is defined as a score of 4. Although the MMAS was first developed to evaluate medication adherence in patients with hypertension,18 it is one of the most widely used instruments to measure adherence in patients with chronic diseases, including COPD. The four questions of the instrument have been adapted for use with inhaled medication.19 The authors later developed an 8-item version20 that has been used in respiratory medicine.21
Measure of treatment adherence (MTA)
Developed in Portuguese, the MTA consists of seven generic items that assess common patterns of both intentional and unintentional non-adherent behavior. Answers are evaluated on a 6-point Likert scale. The total score ranges from 6 to 42, and higher scores indicate better self-reported adherence. Non-adherence is defined by a score ≤5.22
Beliefs about medicines questionnaire (BMQ)
The BMQ is a generic 11-item questionnaire that includes two five-item scales. The 5-item necessity scale assesses patients’ beliefs about the necessity of the prescribed medication and the 6-item concern scale evaluates patients’ concerns about the potential adverse consequences of taking the medication; both are answered on a 5-point Likert scale. Total scores range from 5 to 25 on the necessity scale and 6 to 30 on the concern scale.23 The BMQ has been tested in patients with COPD in Portugal.22
Adherence starts with knowledge 20 (ASK-20)
The ASK-20 is a self-administered, generic questionnaire that was designed to evaluate barriers to medication adherence. The questionnaire is composed of 20 items in which patients are asked to evaluate their potential barriers to medication adherence using a 5-point scale. Higher scores indicate stronger barriers to adherence for all items except 7–12, which must be scored in reverse to have the same direction as the others.24 A shorter version with 12 items (ASK-12) has been validated.25 The ASK-20 has been used in patients with COPD and asthma26
Adherence to refills and medications scale (ARMS)
The ARMS is a 12-item adherence questionnaire, and the total score ranges from 12 to 48 points. Lower scores indicate better adherence.27 The ARMS has been used to evaluate adherence in patients with sleep disorders and COPD.28
Medication adherence report scale (MARS)
The MARS consists of 5 items, each of which evaluates patterns of intentional and nonintentional nonadherent behavior with regard to the principal maintenance therapy. Each item is scored on a 5-point Likert scale. Total scores range from 5 to 25, with stronger adherence associated with higher scores. The MARS has been used in two COPD studies.29,30
Specific questionnaires
Test of the adherence to inhalers (TAI)
The TAI is a specific questionnaire designed to assess the adherence to inhaled medication. It consists of two complementary questionnaires with two domains, namely, the patient and the health professional domains.31 The 10-item TAI patient questionnaire was designed to assess the nonadherence level. Each item is scored from 1 to 5, with higher values indicating the best possible score. Therefore, the total score ranges from 10 to 50. The questionnaire specifically evaluates erratic and deliberate nonadherent behavioral patterns. The additional 2-item TAI health professional questionnaire assesses the non-adherence patterns observed by the health care professional. Each item is scored as either 1 (non-adherent) or 2 (adherent), and the total score ranges from 2 to 4. These items are designed to identify two possible causes of non-adherent behavior that are identified if one of the two items is scored as a 1.32 The TAI has been used to explore differences in adherence between patients with COPD and asthma33 The level of agreement between the 10-item TAI and the generic 8-item MMAS has been found to be only moderate (Kappa index: 0.42; agreement: 64.7%), however.21
Clinical monitoring
Directly observed therapy
The most reliable method of ensuring adherence to medication is directly observing the therapy, meaning that the patient receives each dose in the presence of a third person.14 This form of medication administration has the added advantage that adverse effects can be documented immediately after drug administration. In addition, inhaled medication allows the evaluation of the inhalation technique and can serve as an educational intervention. However, one main limitation is that many resources are needed to observe each patient directly. For this reason, it is usually reserved for the context of clinical trials or in diseases with a high impact on public health.34
Dose counting
Another possibility for clinical monitoring is dose counting at prespecified time intervals. Although the system is considered accurate and objective and provides long-term adherence information, a number of problems have been identified, namely, it can be time consuming for those cases with a considerable number of medications, and patients can intentionally withhold medications or forget to bring some or all the medications to the visit.14 In the case of inhalers, the availability of a dose counter facilitates this system of adherence monitoring. However, some potential problems include the possibility of wasting inhalations without taking them, the dose counter being too small for the patient to see, and poor inhalation technique giving false results.
Electronic monitoring
The availability of technological advancements together with the expansion of the Internet and information and communication technologies in recent decades has had an impact on the evaluation of therapeutic adherence. Several initiatives have been tested. With regard to oral medication, two initiatives have been described, namely, the electronic microchip (consisting of incorporating a specially designed nontoxic microchip within each dose of a drug that allows monitoring) and medication event monitoring systems (consisting of a microchip integrated in the medication packaging that records the opening date and time).14 These have not been used in patients with COPD so far.
Electronic devices
There are different electronic devices that provide information on the correctness of the patient inhaler technique and treatment adherence. One example is an electronic audio recording device called an INhaler Compliance Assessment (INCA) device (Figure 2). The INCA is an electronic device that is attached to the Diskus inhaler. By recording sound during the use of the inhaler, it can objectively evaluate different aspects of inhaler use every time the patient uses the inhaler, including the moment of use, the time elapsed between doses, and the accuracy of inhaler use.35 A recent study has evaluated this device, helping clinicians learn why a prescribed inhaler may not be effective and devising strategies to improve adherence in patients with COPD.36 Additionally, different electronic devices have been tested for their ability to evaluate inhalation technique.37
Figure 2.
The INCA system. which is an open-access article distributed under the terms of the creative commons attribution license, permitting its unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Note: Reproduced from D’Arcy S, MacHale E, Seheult J, Holmes MS, Hughes C, Sulaiman I, et al. A method to assess adherence in inhaler use through analysis of acoustic recordings of inhaler events. PLoS One. 2014;9(6): e98701. Creative Commons license and disclaimer available from: http://creativecommons.org/licenses/by/4.0/legalcode.35
Biochemical measurements
Measurement of the medication or its metabolites in urine or serum
The determination of drug-derived metabolites or the concentration of the drug itself in the serum or urine constitutes an objective and relevant way of exploring exposure to a drug. Evaluation of the therapeutic adherence through the determination of the concentration of the drug or its metabolites in plasma is a direct and objective method of evaluation. During the development of drugs, the systemic bioavailability of the drugs or their metabolites is evaluated.38–40 However, this is a technique that has a relatively high cost and that is not exempt from the so-called white-coat adherence, which occurs when patients change their behavior before scheduled tests. Therefore, this type of evaluation of medication adherence has not been implemented in clinical practice in COPD and has limited usefulness in research.
Measurement of medication or its metabolite in tissues
A recent study evaluated the measurement of drug residues in hair samples to assess inhaled medication adherence in patients with asthma or COPD. The authors found that inhaled drugs were detected in hair for 72% of subjects, including inhaled formoterol and vilanterol. This test showed a dose response with different impacts depending on hair color.41
Adherence in clinical trials
Achieving an optimal therapeutic adherence is extremely important in the execution of a clinical trial because the degree of adherence is directly related to the magnitude of the effect of the treatment under study. It is known that stronger adherence increases the size of the effect, while insufficient medication adherence can result in an inability to differentiate between the treatment arms.42 Studies that have reported the degree of adherence treatment are usually more prone to negative results.43 Of note, treatment with worse adverse effect profiles may falsely prove to be of similar safety if patients are non-adherent due to these adverse effects. Adherence is also a relevant indicator of the acceptance of treatment by patients. Of note, in spite of its obvious importance, treatment adherence is not always consistently reported in clinical trials. In fact, it has been reported that only 33–46% of clinical trials report adherence rates.11,43
One important consideration is that reported adhesion rates are usually elevated in clinical trials compared to those in observational studies. This phenomenon can be related to a possible overestimation of adherence in the context of research.11,43 This may be associated with the additional attention that patients receive during the study, patient selection and the observer effect that alters the behavior of patients.
Adherence in the real world
Adherence to inhaled medication is poor in the real world and shows great variability, ranging from as low as 20% to over 60%.44–48 An observational, cross-sectional, multinational Latin American study involving 795 COPD patients (The LASSYC study) based on the TAI and the MASS-8 questionnaires reported good adherence rates in 54.1% and 51% of patients, respectively.21 In an Austrian retrospective analysis of patients admitted to hospital for severe acute exacerbation of COPD, follow-up data showed 33.6% of patients had adequate adherence to therapy (33.2% of men, 34.4% of women).49 In the Copenhagen General Population Study, the authors found that adherence varied from 29% to 56% depending on the GOLD 1–4 lung function impairment groups for fixed-dose combinations of inhaled corticosteroids (ICS) with long-acting ß2 agonists (LABA), from 51% to 68% across GOLD grades for long-acting muscarinic antagonists (LAMA), and from 25% to 62% for LABA alone.50
Interestingly, a decrease in adherence to treatment appears early in the first year of treatment, with a steep decrease from the beginning, which persists thereafter. A cohort of 3,268 patients with COPD with LABD newly prescribed for 24 months showed that during the first year, adherence dropped to 40% for LAMA and 29% for LABA. Thereafter, in the second year, adherence kept decreasing at a slower pace to 33% for LAMA and 24% for LABA.51 This pattern suggests that measures to improve adherence over time should be established from the beginning of treatment.52 Interestingly, the subjective evaluation of the physician in charge may not match with reality,53 which suggests we should encourage clinicians to employ more objective measures of medication adherence.
Factors associated with treatment adherence
The assessment of the factors associated with appropriate therapeutic adherence in patients with COPD needs a specific comment, as numerous studies have described the association of a considerable number of variables with insufficient adherence (Box 1). Different factors, including age, gender, education, race, number of concomitant treatments, disease severity or smoking status, have been described as being associated with adherence.54–57 However, there are a few specific variables associated with treatment adherence in COPD.
Box 1.
Factors associated with treatment adherence
| Age |
| Gender |
| Education |
| Race |
| Number of concomitant treatments |
| Disease severity |
| Smoking status |
| Satisfaction with the inhaler |
| Type of inhaler device |
| Number of inhalers |
| Satisfaction with drug efficacy |
| Satisfaction with clinician expertise |
| Socioeconomic factors |
| Social/familial support |
| Dose regimen |
| Specific comorbidities |
| Health literacy |
Satisfaction with the inhaler
In respiratory medicine, inhaler management represents a key aspect and a potential barrier.58 A recent study evaluated 373 surveys of COPD patients by 134 physicians and reported complete trust in adequate device usage in 22% of patients and 17% of physicians.59 Interestingly, better confidence was related to higher self-reported adherence. A similar study reported data from 1443 COPD patients and found that patients’ satisfaction with their inhaler was significantly associated with treatment compliance, as well as male gender and fewer maintenance drugs.60 Interestingly, age and breathlessness severity were not associated with treatment compliance.
Type of inhaler device
The impact of the type of inhaler on adherence is controversial. A retrospective multicenter study based on a review of medical registries reported that COPD patients treated with a dry powder inhaler were less likely to have good medication adherence than those treated with a pressurized metered dose inhaler.61 However, other trials have shown different results with regard to the effect of the inhaler device.12,62 In France, a survey of 153 patients with COPD identified that the strongest drivers of preference of inhalation devices were the shape, the dose counter and the reusability.63 Altogether, it seems that adherence to inhalation medication may be device-related in patients with COPD, and efforts should be made to identify the optimal type of inhaler for the patient.64
Number of inhalers
The quantity of inhalers also has an impact on medication adherence in COPD as in the oral medication of other chronic conditions. A retrospective analysis evaluating 23,494 patients matched in pairs revealed that multiple-inhaler users had a significantly higher discontinuation rate than that of single-inhaler users after adjusting for confounding factors.65 These data suggest that fixed dose combinations should improve adherence.
Satisfaction with drug efficacy and safety
Satisfaction with drug efficacy has also been shown to be related to adherence. A study evaluating pharmacy records designed to estimate adherence to ICS, ipratropium bromide, and LABA over two consecutive six-month periods in 2,730 patients showed poor adherence, with 19.8% of patients adherent to ICS, 30.6% adherent to LABA, and 25.6% adherent to ipratropium bromide. Interestingly, adherence at the baseline was the strongest predictor of future adherence to that same medication but not to other classes of medications.46 These results have been replicated in similar trials, in which a subjective sense of relief after the use of inhaled drugs was a factor improving patients’ compliance.66 Additionally, concerns about inhaled medications has been shown to be independently associated with non-adherence in COPD.67 Of note, a cross-sectional analysis evaluating 100 COPD patients showed that most of the patients that discontinued medication was because of side effects.68
Satisfaction with clinician expertise
Despite the fact that the determinants of adherence may differ by medication class, some studies also indicate that patient perception of clinician expertise is a relevant factor that is strongly associated with adherence to inhaled medication. Therefore, the patient’s perception of their medical provider as an expert is associated with greater adherence to LABA and ICS.69 Interestingly, a cohort study enrolling 13,178 patients discharged from hospital for COPD between 2007 and 2011 in Italy showed that adherence was higher for patients discharged from pneumology wards with a reduction in the variability after entering the hospital level.70
Socioeconomic factors
Socioeconomic factors, including unemployment, low-income status, immigration status, and living alone, have been shown to influence adherence and to be related to the nonuse of medication.71
Social/familial support
Caregivers, especially spouses, may improve adherence in patients with COPD. This was shown in a study evaluating adherence measured by pharmacy refill data according to the presence of a caregiver. The results showed that adherence to LABA was stronger and fewer patients were current smokers in the spousal caregiver group than in the other groups.72
Dose regimen
The impact of dosing on inhaler adherence is a matter of controversy. Some studies have indicated that the number of daily respiratory drug doses is relevant to inhaled drug adherence.44,73 Interestingly, the number of inhalations per administration is also relevant. A recent analysis based on interviews found that the number of inhalations per administration was an independent risk factor for poor adherence.12 Altogether, these findings suggest that less frequent dosing regimens could be an effective method of enhancing adherence to respiratory therapy. However, other authors have reported that medication adherence is poor regardless of the dose regimen and that 12 hr medication dosing has a similar level of adherence to that of 24 hr regimens.74 In any case, having various dose regimens available is an advantage for clinicians because it allows adaptation of the treatment regimen to the patient’s preferences.
Specific comorbidities
The presence of specific comorbidities may also be relevant not only because of the effect on the number of medications but also because of the impact of these comorbidities on COPD. The best example is the relationship between the presence of depressed mood and adherence; this relationship is stronger than those between adherence and disease severity or sociodemographic factors.75 Cognitive impairment might also be an important parameter that can affect inhalation device technique and compliance.76 Additionally, certain comorbidities may influence the initial prescription. It has been described how COPD patients with concurrent cardiovascular disease are less likely to be prescribed bronchodilators.77 Interestingly, it has been described that adherence to COPD medications seems to be positively related to non-COPD medications,78 which suggests that the need to take medications prescribed for comorbid conditions does not adversely impact adherence to COPD medications.
Health literacy
Health literacy is considered the ability of a person to manage health situations, make sense of health information they receive or even provide health information to others. Health literacy is also important because of its influence on adherence. A recent study in a cohort of individuals with COPD showed that low health literacy was common and was associated with disease-related opinions that predict low adherence.79
Consequences of low adherence
The lack of adherence to COPD treatment has direct implications for the management of the disease. In clinical trials, a relationship between adherence and the results of clinical trials has been shown. In a subanalysis of the TOwards a Revolution in COPD Health (TORCH) trial, a randomized controlled trial evaluating 6112 patients with COPD over 3 years, the authors found associations between adherence and mortality and between adherence and hospital admissions.80
Although the majority of studies have analyzed data from administrative databases, the findings are similar. A cross-sectional study included 33,816 Medicare beneficiaries and found that the proportion of days covered was associated with hospitalization rates and costs (–$2,185). Similarly, a population-based study in 12,124 individuals over 5 years found that consistent LABA use was associated with better survival than was occasional LABA/ICS.81 Furthermore, poor adherence to inhaled therapy has now been clearly established as a main risk factor for the exacerbation of COPD, and the effect on the hospitalization rate has been replicated in other analyses.66,82
As a consequence, the economic impact of poor adherence is also worth noting. A systematic analysis highlighted the associations between adherence and both clinical and economic outcomes.83 In the PHARMACOP trial, an interventional study in community pharmacies designed to improve adherence, the overall cost difference per patient in one year was €227, which additionally resulted in the prevention of hospital-treated exacerbations.82 A similar finding was obtained in a retrospective study in Korea, which showed that the adherent group had a 10.4% lower all-cause cost (p<0.001) and an 11.7% lower COPD-related cost (p<0.0001) versus those of the non-adherent group.84
Interventions to improve adherence
Given the short-term and long-term clinical implications of poor therapeutic adherence for patients with COPD, improving adherence emerges as a necessity in COPD care.
Patient education
In a chronic, progressive, disabling disease such as COPD that also has periods of exacerbation that may be common, it is important that the patient understands the disease and its evolution over time and learns to handle incidents that may occur. To this end, self-management plans have been designed that include improving the inhalation technique and understanding the importance of maintaining inhaled medication over time.85 It is important for clinicians to be alert when interacting with these patients; each time the patient contacts the health care system should be considered an opportunity to educate that patient about the value of therapeutic adherence. Recurrent instruction regarding inhalation techniques may also contribute to adherence to therapeutic regimens.86 If disease control is not achieved due to poor adherence, one possibility is switching to other inhaler devices.87 This may have a double effect on the management of the inhaler and a potential improvement in drug efficacy due to idiosyncrasy.16 The switching of devices is acknowledged as a strategy in current recommendation guidelines,88 and it has been shown to be feasible, as long as the choice is carefully explained by the physician.89
Motivational intervention
A recent randomized controlled trial in a primary care setting including 146 COPD patients explored the impact of an intervention consisting of group and individual interviews that was designed to explore motivational aspects related to adherence.90 The authors reported a change in adherence from 41.9% to 32.4% in the control group and 40.3% to 48.6% in the intervention group, with a need-to-treat number of 6.37. Motivational interventions should include caregivers as well.
Out-of-pocket expenses
Although reduced out-of-pocket expenses have been shown to improve medication adherence for chronic conditions,91 in patients with COPD, unless it is accompanied by adequate training of the patient, switching from one device to another can be associated with worse clinical outcomes and a consequently increased use of health care resources.92 Therefore, these decisions should be patient-tailored and agreed on with the patient and caregiver with close follow-up.
Clinical pharmacist consultation
The role of the community pharmacy in the diagnosis of COPD has been demonstrated in numerous studies. In particular, the performance of spirometry has been shown to improve the diagnosis of patients and to be an appropriate tool for identifying cases that are cost-effective.93,94 Additionally, a prospective randomized controlled study evaluating the impact of a pharmacist-managed clinic on medication adherence in patients with COPD showed significantly better adherence compared with the baseline and with an improvement in the medication refill adherence compared with the control group.95 Additionally, pharmacists can influence nonpharmacological interventions and improve vaccination rates by recognizing these influencing factors and by acting as advocates, counselors, and administrators of the influenza vaccine.96
Medication-taking reminders
A recent systematic review identified medication-taking reminders as one intervention that successfully improves adherence to different chronic conditions. Reminders sent via text messages, telephone calls or electronic devices had an impact on adherence.97 Interestingly, personal or interactive reminders were more effective than generic or prerecorded reminders. In airway diseases, both text messaging and audiovisual reminders have been shown to be effective in the short term.98 However, their impact in the long term has not been established, and there is concern that frequent reminders may become a nuisance to patients.99 In this regard, digital technologies will probably be an aid in improving adherence in patients with respiratory diseases,100 but we will need to learn about their implementation in different patient types.
Conclusions
In summary, the evaluation of therapeutic adherence to inhaled medication represents an essential element in the therapeutic strategy for patients with COPD and a current challenge in the clinic. It is necessary to be clear about the different concepts used in the medical literature to use a uniform language in this evaluation of therapeutic adherence. In addition, the consideration of the factors that are associated with a lack of adherence in clinical practice can help the clinician to identify patients at risk of low adherence, which, in turn, can serve to reinforce the importance of a correct adherence in certain populations. Health care professionals should be aware of the issues pertaining to adherence and take the opportunity to educate patients each time they contact the health care system.
Disclosure
JLLC has received honoraria for lecturing, providing scientific advice, participating in clinical studies or writing for publications during the last three years for (in alphabetical order): AstraZeneca, Boehringer Ingelheim, Chiesi, CSL Behring, Esteve, Ferrer, Gebro, GlaxoSmithKline, Grifols, Menarini, Novartis, Rovi, and Teva. The authors report no other conflicts of interest in this work.
References
- 1.Bogart M, Stanford RH, Laliberte F, Germain G, Wu JW, Duh MS. Medication adherence and persistence in chronic obstructive pulmonary disease patients receiving triple therapy in a USA commercially insured population. Int J Chron Obstruct Pulmon Dis. 2019;14:343–352. doi: 10.2147/COPD.S184653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Watts RW, McLennan G, Bassham I, el-Saadi O. Do patients with asthma fill their prescriptions? A primary compliance study. Aust Fam Physician. 1997;26(Suppl 1):S4–S6. [PubMed] [Google Scholar]
- 3.Sabaté E, editor. Adherence to Long-Term Therapies: Evidence for Action. Geneva, Switzerland: World Health Organization; 2003. [Google Scholar]
- 4.Cramer JA, Roy A, Burrell A, et al. Medication compliance and persistence: terminology and definitions. Value Health. 2008;11(1):44–47. doi: 10.1111/j.1524-4733.2007.00213.x [DOI] [PubMed] [Google Scholar]
- 5.Hess LM, Raebel MA, Conner DA, Malone DC. Measurement of adherence in pharmacy administrative databases: a proposal for standard definitions and preferred measures. Ann Pharmacother. 2006;40(7–8):1280–1288. [DOI] [PubMed] [Google Scholar]
- 6.Rand CS. Patient adherence with COPD therapy. Eur Respir Rev. 2005;14(96):97–101. [Google Scholar]
- 7.Van Ganse E, Price D. Respiratory medication adherence: toward a common language and a shared vision. J Allergy Clin Immunol Pract. 2016;4(5):799–801. [DOI] [PubMed] [Google Scholar]
- 8.Vrijens B, De Geest S, Hughes DA, et al. A new taxonomy for describing and defining adherence to medications. Br J Clin Pharmacol. 2012;73(5):691–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vrijens B, Dima AL, Van Ganse E, et al. What we mean when we talk about adherence in respiratory medicine. J Allergy Clin Immunol Pract. 2016;4(5):802–812. [DOI] [PubMed] [Google Scholar]
- 10.Brown MT, Bussell JK. Medication adherence: WHO cares? Mayo Clin Proc. 2011;86(4):304–314. doi: 10.4065/mcp.2010.0575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gossec L, Tubach F, Dougados M, Ravaud P. Reporting of adherence to medication in recent randomized controlled trials of 6 chronic diseases: a systematic literature review. Am J Med Sci. 2007;334(4):248–254. doi: 10.1097/MAJ.0b013e318068dde8 [DOI] [PubMed] [Google Scholar]
- 12.Imamura Y, Kawayama T, Kinoshita T, et al. Poor pharmacological adherence to inhaled medicines compared with oral medicines in Japanese patients with asthma and chronic obstructive pulmonary disease. Allergol Int. 2017;66(3):482–484. doi: 10.1016/j.alit.2016.10.008 [DOI] [PubMed] [Google Scholar]
- 13.Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353(5):487–497. doi: 10.1056/NEJMra050100 [DOI] [PubMed] [Google Scholar]
- 14.Hameed MA, Dasgupta I. Medication adherence and treatment-resistant hypertension: a review. Drugs Context. 2019;8:212560. doi: 10.7573/dic.212560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Donohue JF, Singh D, Munzu C, Kilbride S, Church A. Magnitude of umeclidinium/vilanterol lung function effect depends on monotherapy responses: results from two randomised controlled trials. Respir Med. 2016;112:65–74. doi: 10.1016/j.rmed.2016.01.001 [DOI] [PubMed] [Google Scholar]
- 16.Feldman GJ, Sousa AR, Lipson DA, et al. Comparative efficacy of once-daily umeclidinium/vilanterol and tiotropium/olodaterol therapy in symptomatic chronic obstructive pulmonary disease: a randomized study. Adv Ther. 2017;34(11):2518–2533. doi: 10.1007/s12325-017-0626-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garfield S, Clifford S, Eliasson L, Barber N, Willson A. Suitability of measures of self-reported medication adherence for routine clinical use: a systematic review. BMC Med Res Methodol. 2011;11:149. doi: 10.1186/1471-2288-11-149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morisky DE, Green LW, Levine DM. Concurrent and predictive validity of a self-reported measure of medication adherence. Med Care. 1986;24(1):67–74. [DOI] [PubMed] [Google Scholar]
- 19.Barnestein-Fonseca P, Leiva-Fernandez J, Vidal-Espana F, Garcia-Ruiz A, Prados-Torres D, Leiva-Fernandez F. Is it possible to diagnose the therapeutic adherence of patients with COPD in clinical practice? A cohort study. BMC Pulm Med. 2011;11:6. doi: 10.1186/1471-2466-11-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morisky DE, Ang A, Krousel-Wood M, Ward HJ. Predictive validity of a medication adherence measure in an outpatient setting. J Clin Hypertens (Greenwich). 2008;10(5):348–354. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 21.Montes de Oca M, Menezes A, Wehrmeister FC, et al. Adherence to inhaled therapies of COPD patients from seven Latin American countries: the LASSYC study. PLoS One. 2017;12(11):e0186777. doi: 10.1371/journal.pone.0186777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Duarte-de-Araujo A, Teixeira P, Hespanhol V, Correia-de-Sousa J. COPD: understanding patients’ adherence to inhaled medications. Int J Chron Obstruct Pulmon Dis. 2018;13:2767–2773. doi: 10.2147/COPD.S160982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clifford S, Barber N, Horne R. Understanding different beliefs held by adherers, unintentional nonadherers, and intentional nonadherers: application of the necessity-concerns framework. J Psychosom Res. 2008;64(1):41–46. doi: 10.1016/j.jpsychores.2007.05.004 [DOI] [PubMed] [Google Scholar]
- 24.Hahn SR, Park J, Skinner EP, et al. Development of the ASK-20 adherence barrier survey. Curr Med Res Opin. 2008;24(7):2127–2138. doi: 10.1185/03007990802174769 [DOI] [PubMed] [Google Scholar]
- 25.Matza LS, Park J, Coyne KS, Skinner EP, Malley KG, Wolever RQ. Derivation and validation of the ASK-12 adherence barrier survey. Ann Pharmacother. 2009;43(10):1621–1630. doi: 10.1345/aph.1M174 [DOI] [PubMed] [Google Scholar]
- 26.Toyama T, Kawayama T, Kinoshita T, et al. Differences in adherence barriers to inhaled medicines between japanese patients with chronic obstructive pulmonary disease and asthma evaluated using the “Adherence starts with knowledge 20” (ASK-20) questionnaire. Intern Med. 2019;58(2):175–185. doi: 10.2169/internalmedicine.0488-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kripalani S, Risser J, Gatti ME, Jacobson TA. Development and evaluation of the Adherence to Refills and Medications Scale (ARMS) among low-literacy patients with chronic disease. Value Health. 2009;12(1):118–123. doi: 10.1111/j.1524-4733.2008.00400.x [DOI] [PubMed] [Google Scholar]
- 28.Chabowski M, Luczak J, Dudek K, Jankowska-Polanska B. Sleep disorders and adherence to inhalation therapy in patients with chronic obstructive pulmonary disease. Adv Exp Med Biol. 2019. [DOI] [PubMed] [Google Scholar]
- 29.Tommelein E, Mehuys E, Van Tongelen I, Brusselle G, Boussery K. Accuracy of the Medication Adherence Report Scale (MARS-5) as a quantitative measure of adherence to inhalation medication in patients with COPD. Ann Pharmacother. 2014;48(5):589–595. doi: 10.1177/1060028014522982 [DOI] [PubMed] [Google Scholar]
- 30.George J, Kong DC, Thoman R, Stewart K. Factors associated with medication nonadherence in patients with COPD. Chest. 2005;128(5):3198–3204. doi: 10.1378/chest.128.5.3198 [DOI] [PubMed] [Google Scholar]
- 31.Plaza V, Lopez-Vina A, Cosio BG, en representacion del Comite Cientifico del Proyecto TAI. Test of Adherence to Inhalers. Arch Bronconeumol. 2017;53(7):360–361. doi: 10.1016/j.arbres.2016.08.006 [DOI] [PubMed] [Google Scholar]
- 32.Plaza V, Fernandez-Rodriguez C, Melero C, et al. Validation of the ‘Test of the Adherence to Inhalers’ (TAI) for Asthma and COPD Patients. J Aerosol Med Pulm Drug Deliv. 2016;29(2):142–152. doi: 10.1089/jamp.2015.1212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Plaza V, Lopez-Vina A, Entrenas LM, et al. Differences in adherence and non-adherence behaviour patterns to inhaler devices between COPD and asthma patients. COPD. 2016;13(5):547–554. doi: 10.3109/15412555.2015.1118449 [DOI] [PubMed] [Google Scholar]
- 34.Yin J, Yuan J, Hu Y, Wei X. Association between directly observed therapy and treatment outcomes in multidrug-resistant tuberculosis: a systematic review and meta-analysis. PLoS One. 2016;11(3):e0150511. doi: 10.1371/journal.pone.0150511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.D’Arcy S, MacHale E, Seheult J, et al. A method to assess adherence in inhaler use through analysis of acoustic recordings of inhaler events. PLoS One. 2014;9(6):e98701. doi: 10.1371/journal.pone.0098701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sulaiman I, Cushen B, Greene G, et al. Objective assessment of adherence to inhalers by patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2017;195(10):1333–1343. doi: 10.1164/rccm.201604-0733OC [DOI] [PubMed] [Google Scholar]
- 37.Carpenter DM, Roberts CA, Sage AJ, George J, Horne R. A review of electronic devices to assess inhaler technique. Curr Allergy Asthma Rep. 2017;17(3):17. doi: 10.1007/s11882-017-0684-3 [DOI] [PubMed] [Google Scholar]
- 38.Sentellas S, Ramos I, Alberti J, et al. Aclidinium bromide, a new, long-acting, inhaled muscarinic antagonist: in vitro plasma inactivation and pharmacological activity of its main metabolites. Eur J Pharm Sci. 2010;39(5):283–290. doi: 10.1016/j.ejps.2010.01.004 [DOI] [PubMed] [Google Scholar]
- 39.Sechaud R, Renard D, Zhang-Auberson L, Motte Sde L, Drollmann A, Kaiser G. Pharmacokinetics of multiple inhaled NVA237 doses in patients with chronic obstructive pulmonary disease (COPD). Int J Clin Pharmacol Ther. 2012;50(2):118–128. [DOI] [PubMed] [Google Scholar]
- 40.Clearie KL, Williamson PA, Vaidyanathan S, Du Bois J, Nell H, Lipworth BJ. Systemic bioavailability of hydrofluoroalkane (HFA) formulations of fluticasone/salmeterol in healthy volunteers via pMDI alone and spacer. Br J Clin Pharmacol. 2010;69(6):637–644. doi: 10.1111/j.1365-2125.2010.03655.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hassall D, Brealey N, Wright W, et al. Hair analysis to monitor adherence to prescribed chronic inhaler drug therapy in patients with asthma or COPD. Pulm Pharmacol Ther. 2018;51:59–64. doi: 10.1016/j.pupt.2018.07.001 [DOI] [PubMed] [Google Scholar]
- 42.Cramer J, Rosenheck R, Kirk G, Krol W, Krystal J, Group VANS. Medication compliance feedback and monitoring in a clinical trial: predictors and outcomes. Value Health. 2003;6(5):566–573. doi: 10.1046/j.1524-4733.2003.65269.x [DOI] [PubMed] [Google Scholar]
- 43.Zhang Z, Peluso MJ, Gross CP, Viscoli CM, Kernan WN. Adherence reporting in randomized controlled trials. Clin Trials. 2014;11(2):195–204. doi: 10.1177/1740774513512565 [DOI] [PubMed] [Google Scholar]
- 44.Agh T, Inotai A, Meszaros A. Factors associated with medication adherence in patients with chronic obstructive pulmonary disease. Respiration. 2011;82(4):328–334. doi: 10.1159/000324453 [DOI] [PubMed] [Google Scholar]
- 45.Ismaila A, Corriveau D, Vaillancourt J, et al. Impact of adherence to treatment with tiotropium and fluticasone propionate/salmeterol in chronic obstructive pulmonary diseases patients. Curr Med Res Opin. 2014;30(7):1427–1436. doi: 10.1185/03007995.2014.908828 [DOI] [PubMed] [Google Scholar]
- 46.Huetsch JC, Uman JE, Udris EM, Au DH. Predictors of adherence to inhaled medications among Veterans with COPD. J Gen Intern Med. 2012;27(11):1506–1512. doi: 10.1007/s11606-012-2130-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Arfe A, Nicotra F, Cerveri I, et al. Incidence, predictors, and clinical implications of discontinuing therapy with inhaled long-acting bronchodilators among patients with chronic obstructive pulmonary disease. COPD. 2016;13(5):540–546. doi: 10.3109/15412555.2016.1141877 [DOI] [PubMed] [Google Scholar]
- 48.Mueller S, Wilke T, Bechtel B, Punekar YS, Mitzner K, Virchow JC. Non-persistence and non-adherence to long-acting COPD medication therapy: a retrospective cohort study based on a large German claims dataset. Respir Med. 2017;122:1–11. doi: 10.1016/j.rmed.2016.11.008 [DOI] [PubMed] [Google Scholar]
- 49.Humenberger M, Horner A, Labek A, et al. Adherence to inhaled therapy and its impact on chronic obstructive pulmonary disease (COPD). BMC Pulm Med. 2018;18(1):163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ingebrigtsen TS, Marott JL, Nordestgaard BG, et al. Low use and adherence to maintenance medication in chronic obstructive pulmonary disease in the general population. J Gen Intern Med. 2015;30(1):51–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wurst KE, St Laurent S, Mullerova H, Davis KJ. Characteristics of patients with COPD newly prescribed a long-acting bronchodilator: a retrospective cohort study. Int J Chron Obstruct Pulmon Dis. 2014;9:1021–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Penning-van Beest F, van Herk-Sukel M, Gale R, Lammers JW, Herings R. Three-year dispensing patterns with long-acting inhaled drugs in COPD: a database analysis. Respir Med. 2011;105(2):259–265. [DOI] [PubMed] [Google Scholar]
- 53.Kardas P, Lewek P, Strzondala M. Adherence to treatment in asthma and COPD patients in their doctors’ assessment. Pneumonol Alergol Pol. 2015;83(6):436–444. [DOI] [PubMed] [Google Scholar]
- 54.Laforest L, Denis F, Van Ganse E, et al. Correlates of adherence to respiratory drugs in COPD patients. Prim Care Respir J. 2010;19(2):148–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Laforest L, Licaj I, Devouassoux G, Hartwig S, Marvalin S, Van Ganse E. Factors associated with early adherence to tiotropium in chronic obstructive pulmonary disease. Chron Respir Dis. 2013;10(1):11–18. [DOI] [PubMed] [Google Scholar]
- 56.Donner CF, Amaducci S, Bacci E, et al. Inhalation therapy in the next decade: determinants of adherence to treatment in asthma and COPD. Monaldi Arch Chest Dis. 2018;88(1):886. [DOI] [PubMed] [Google Scholar]
- 57.Nishi SPE, Maslonka M, Zhang W, Kuo YF, Sharma G. Pattern and adherence to maintenance medication use in medicare beneficiaries with chronic obstructive pulmonary disease: 2008-2013. Chronic Obstr Pulm Dis. 2018;5(1):16–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Melzer AC, Ghassemieh BJ, Gillespie SE, et al. Patient characteristics associated with poor inhaler technique among a cohort of patients with COPD. Respir Med. 2017;123:124–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Amin AN, Ganapathy V, Roughley A, Small M. Confidence in correct inhaler technique and its association with treatment adherence and health status among US patients with chronic obstructive pulmonary disease. Patient Prefer Adherence. 2017;11:1205–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chrystyn H, Small M, Milligan G, Higgins V, Gil EG, Estruch J. Impact of patients’ satisfaction with their inhalers on treatment compliance and health status in COPD. Respir Med. 2014;108(2):358–365. [DOI] [PubMed] [Google Scholar]
- 61.Darba J, Ramirez G, Sicras A, Francoli P, Torvinen S, Sanchez-de la Rosa R. The importance of inhaler devices: the choice of inhaler device may lead to suboptimal adherence in COPD patients. Int J Chron Obstruct Pulmon Dis. 2015;10:2335–2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Koehorst-Ter Huurne K, Movig K, van der Valk P, van der Palen J, Brusse-Keizer M. The influence of type of inhalation device on adherence of COPD patients to inhaled medication. Expert Opin Drug Deliv. 2016;13(4):469–475. [DOI] [PubMed] [Google Scholar]
- 63.Chouaid C, Germain N, De Pouvourville G, et al. Patient preference for chronic obstructive pulmonary disease (COPD) treatment inhalers: a discrete choice experiment in France. Curr Med Res Opin. 2019;35(5):785–792. [DOI] [PubMed] [Google Scholar]
- 64.Braido F, Chrystyn H, Baiardini I, et al. “Trying, but failing” - the role of inhaler technique and mode of delivery in respiratory medication adherence. J Allergy Clin Immunol Pract. 2016;4(5):823–832. [DOI] [PubMed] [Google Scholar]
- 65.Yu AP, Guerin A, Ponce de Leon D, et al. Therapy persistence and adherence in patients with chronic obstructive pulmonary disease: multiple versus single long-acting maintenance inhalers. J Med Econ. 2011;14(4):486–496. [DOI] [PubMed] [Google Scholar]
- 66.Wisniewski D, Porzezinska M, Gruchala-Niedoszytko M, Niedoszytko M, Slominski JM, Jassem E. Factors influencing adherence to treatment in COPD patients and its relationship with disease exacerbations. Pneumonol Alergol Pol. 2014;82(2):96–104. [DOI] [PubMed] [Google Scholar]
- 67.Krauskopf K, Federman AD, Kale MS, et al. Chronic obstructive pulmonary disease illness and medication beliefs are associated with medication adherence. COPD. 2015;12(2):151–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shrestha R, Pant A, Shakya Shrestha S, Shrestha B, Gurung RB, Karmacharya BM. A cross-sectional study of medication adherence pattern and factors affecting the adherence in chronic obstructive pulmonary disease. Kathmandu Univ Med J (KUMJ). 2015;13(49):64–70. [DOI] [PubMed] [Google Scholar]
- 69.Cecere LM, Slatore CG, Uman JE, et al. Adherence to long-acting inhaled therapies among patients with chronic obstructive pulmonary disease (COPD). COPD. 2012;9(3):251–258. [DOI] [PubMed] [Google Scholar]
- 70.Di Martino M, Ventura M, Cappai G, et al. Adherence to long-acting bronchodilators after discharge for COPD: how much of the geographic variation is attributable to the hospital of discharge and how much to the primary care providers? COPD. 2017;14(1):86–94. [DOI] [PubMed] [Google Scholar]
- 71.Tottenborg SS, Lange P, Johnsen SP, Nielsen H, Ingebrigtsen TS, Thomsen RW. Socioeconomic inequalities in adherence to inhaled maintenance medications and clinical prognosis of COPD. Respir Med. 2016;119:160–167. [DOI] [PubMed] [Google Scholar]
- 72.Trivedi RB, Bryson CL, Udris E, Au DH. The influence of informal caregivers on adherence in COPD patients. Ann Behav Med. 2012;44(1):66–72. [DOI] [PubMed] [Google Scholar]
- 73.Toy EL, Beaulieu NU, McHale JM, et al. Treatment of COPD: relationships between daily dosing frequency, adherence, resource use, and costs. Respir Med. 2011;105(3):435–441. [DOI] [PubMed] [Google Scholar]
- 74.Izquierdo JL, Paredero JM, Piedra R. Relevance of dosage in adherence to treatment with long-acting anticholinergics in patients with COPD. Int J Chron Obstruct Pulmon Dis. 2016;11:289–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Khdour MR, Hawwa AF, Kidney JC, Smyth BM, McElnay JC. Potential risk factors for medication non-adherence in patients with chronic obstructive pulmonary disease (COPD). Eur J Clin Pharmacol. 2012;68(10):1365–1373. [DOI] [PubMed] [Google Scholar]
- 76.Turan O, Turan PA, Mirici A. Parameters affecting inhalation therapy adherence in elderly patients with chronic obstructive lung disease and asthma. Geriatr Gerontol Int. 2017;17(6):999–1005. [DOI] [PubMed] [Google Scholar]
- 77.Adesanoye DT, Willey CJ. Does cardiovascular comorbidity influence the prescribing of bronchodilators in chronic obstructive pulmonary disease? Ann Pharmacother. 2017;51(10):855–861. [DOI] [PubMed] [Google Scholar]
- 78.Dhamane AD, Schwab P, Hopson S, et al. Association between adherence to medications for COPD and medications for other chronic conditions in COPD patients. Int J Chron Obstruct Pulmon Dis. 2017;12:115–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kale MS, Federman AD, Krauskopf K, et al. The association of health literacy with illness and medication beliefs among patients with chronic obstructive pulmonary disease. PLoS One. 2015;10(4):e0123937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Vestbo J, Anderson JA, Calverley PM, et al. Adherence to inhaled therapy, mortality and hospital admission in COPD. Thorax. 2009;64(11):939–943. [DOI] [PubMed] [Google Scholar]
- 81.Belleudi V, Di Martino M, Cascini S, et al. The impact of adherence to inhaled drugs on 5-year survival in COPD patients: a time dependent approach. Pharmacoepidemiol Drug Saf. 2016;25(11):1295–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.van Boven JF, Tommelein E, Boussery K, et al. Improving inhaler adherence in patients with chronic obstructive pulmonary disease: a cost-effectiveness analysis. Respir Res. 2014;15:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.van Boven JF, Chavannes NH, van der Molen T, Rutten-van Molken MP, Postma MJ, Vegter S. Clinical and economic impact of non-adherence in COPD: a systematic review. Respir Med. 2014;108(1):103–113. [DOI] [PubMed] [Google Scholar]
- 84.Kim JA, Lim MK, Kim K, Park J, Rhee CK. Adherence to inhaled medications and its effect on healthcare utilization and costs among high-grade chronic obstructive pulmonary disease patients. Clin Drug Investig. 2018;38(4):333–340. [DOI] [PubMed] [Google Scholar]
- 85.Barrecheguren M, Bourbeau J. Self-management strategies in chronic obstructive pulmonary disease: a first step toward personalized medicine. Curr Opin Pulm Med. 2018;24(2):191–198. [DOI] [PubMed] [Google Scholar]
- 86.Takemura M, Mitsui K, Itotani R, et al. Relationships between repeated instruction on inhalation therapy, medication adherence, and health status in chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2011;6:97–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Melani AS, Paleari D. Maintaining control of chronic obstructive airway disease: adherence to inhaled therapy and risks and benefits of switching devices. COPD. 2016;13(2):241–250. [DOI] [PubMed] [Google Scholar]
- 88.Vogelmeier CF, Criner GJ, Martinez FJ, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report: gold executive summary. Arch Bronconeumol. 2017;53(3):128–149. [DOI] [PubMed] [Google Scholar]
- 89.Braido F, Baiardini I, Sumberesi M, Blasi F, Canonica GW. Obstructive lung diseases and inhaler treatment: results from a national public pragmatic survey. Respir Res. 2013;14:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Leiva-Fernandez J, Leiva-Fernandez F, Garcia-Ruiz A, Prados-Torres D, Barnestein-Fonseca P. Efficacy of a multifactorial intervention on therapeutic adherence in patients with chronic obstructive pulmonary disease (COPD): a randomized controlled trial. BMC Pulm Med. 2014;14:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Viswanathan M, Golin CE, Jones CD, et al. Interventions to improve adherence to self-administered medications for chronic diseases in the United States: a systematic review. Ann Intern Med. 2012;157(11):785–795. [DOI] [PubMed] [Google Scholar]
- 92.Braido F, Lavorini F, Blasi F, Baiardini I, Canonica GW. Switching treatments in COPD: implications for costs and treatment adherence. Int J Chron Obstruct Pulmon Dis. 2015;10:2601–2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wright D, Twigg M, Thornley T. Chronic obstructive pulmonary disease case finding by community pharmacists: a potential cost-effective public health intervention. Int J Pharm Pract. 2015;23(1):83–85. [DOI] [PubMed] [Google Scholar]
- 94.Castillo D, Burgos F, Guayta R, et al. Airflow obstruction case finding in community-pharmacies: a novel strategy to reduce COPD underdiagnosis. Respir Med. 2015;109(4):475–482. [DOI] [PubMed] [Google Scholar]
- 95.Xin C, Xia Z, Jiang C, Lin M, Li G. The impact of pharmacist-managed clinic on medication adherence and health-related quality of life in patients with COPD: a randomized controlled study. Patient Prefer Adherence. 2016;10:1197–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Arabyat RM, Raisch DW, Bakhireva L. Influenza vaccination for patients with chronic obstructive pulmonary disease: implications for pharmacists. Res Social Adm Pharm. 2018;14(2):162–169. [DOI] [PubMed] [Google Scholar]
- 97.Kini V, Ho PM. Interventions to improve medication adherence: a review. JAMA. 2018;320(23):2461–2473. [DOI] [PubMed] [Google Scholar]
- 98.Normansell R, Kew KM, Stovold E. Interventions to improve adherence to inhaled steroids for asthma. Cochrane Database Syst Rev. 2017;4:Cd012226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Dennison L, Morrison L, Conway G, Yardley L. Opportunities and challenges for smartphone applications in supporting health behavior change: qualitative study. J Med Internet Res. 2013;15(4):e86. doi: 10.2196/jmir.2583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Blakey JD, Bender BG, Dima AL, Weinman J, Safioti G, Costello RW. Digital technologies and adherence in respiratory diseases: the road ahead. Eur Respir J. 2018;52(5). doi: 10.1183/13993003.01675-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]