Abstract
Purpose:
To determine the effects of left gastric artery embolization (LGAE) on computed tomography (CT) body composition change.
Materials and methods:
Sixteen overweight or obese patients who had abdominal CT scans before and after LGAE for gastric bleeding were retrospectively reviewed. Body composition analysis was performed with semiautomated imaging processing algorithms (MATLAB 13.0, Math Works, MA). Adipose tissue and lean skeletal muscle were measured using threshold attenuation values. Total body fat index (BFI), subcutaneous fat index (SFI), visceral fat index (VFI), intramuscular fat index (IMFI), and skeletal muscle index (SMI) were determined ([tissue area (cm)]2/[height (m)]2). Excess body weight (EBW) was determined based on the Lorentz formula for ideal body weight.
Results:
Mean follow-up was 1.5 ± 0.8 months. Following LGAE, patients experienced significantly decreased body weight (p=0.003), BMI (p=0.005), EBW (p=0.003), BFI (p=0.03), SFI (p=0.03), and SMI (p<0.001). Changes in VFI and IMFI did not significantly change (p=0.13 and p=0.83, respectively).
Conclusions:
Patients who underwent LGAE had significant unintended weight loss as a result of decreased body fat and skeletal muscle. Body composition analysis can readily assess the extent of fat loss and identify muscle wasting.
Introduction
Selective left gastric artery embolization (LGAE) has gained attention as a potential minimally invasive endovascular bariatric procedure, although data on efficacy remains limited [1,2]. While the potential for this procedure to result in desired weight loss is appealing, at present, LGAE procedures are still typically performed for the indication of gastric hemorrhage. Understanding the implications of potential undesired weight loss after LGAE is essential. Multiple animal studies as well as early human trials have demonstrated substantial weight loss after embolization of the left gastric artery with or without embolization of the gastroepiploic artery [3–6]. This procedure may decrease caloric intake through induced ischemia of oxyntic cells in the gastric fundus that produce ghrelin, a potent appetite hormone.
A recent study demonstrated significant weight loss occurred after LGAE performed for gastric bleeding. While patient weight may decrease after LGAE, it is unclear what components of body composition, such as subcutaneous fat, visceral fat or skeletal muscle, are contributing to the observed changes in body mass. Studies have demonstrated lean tissue, specifically skeletal muscle, is often compromised after bariatric intervention. Muscle depletion leading to sarcopenia is associated with significant negative outcomes. Computed tomography (CT) has been widely studied and validated as a tool for body composition assessment [7–10]. This study aims to determine how LGAE affects different body compositions contributing to weight loss.
Materials and methods
Institutional review board approval was obtained. Informed consent was waived for this retrospective study.
Study cohorts
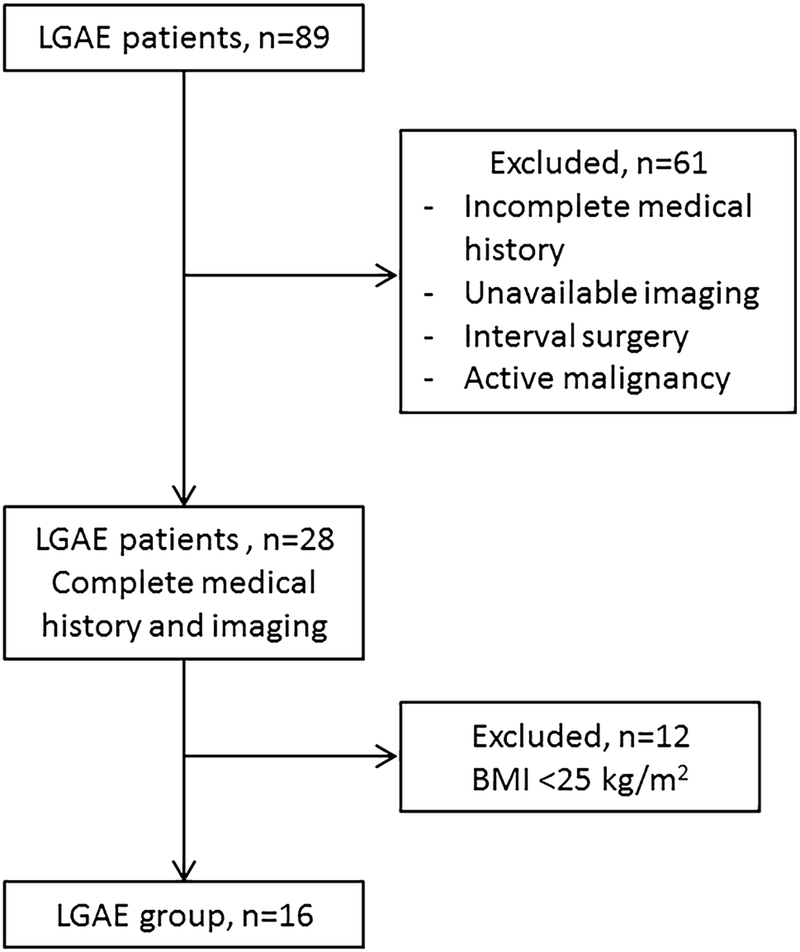
Eighty-nine patients who underwent LGAE for gastric bleeding between 1/2006 and 3/2018 were retrospectively reviewed. Of these, 61 patients were excluded for unavailable pre-LGAE and/or post-LGAE CT scan or unavailable follow-up, active malignancy or surgery during the time between CT scans. Twelve more patients were excluded for body mass index (BMI) below 25 kg/m2. The remaining 16 patients with CT scans before 1–2 months pre-LGAE and 1–2 months post-LGAE were included for analysis (Fig. 1).
Fig. 1.
Cohort selection diagram
CT technique
Due to the retrospective design of the study, there was some variability in CT scan technique. Fourteen of the 16 patients in the LGAE group underwent a portal venous phase CT scan of the abdomen and pelvis, 1 patient underwent a noncontrast CT scan of the abdomen and pelvis, and 1 patient underwent a CT angiogram of the abdomen and pelvis. In the latter 2 patients, both the pre-LGAE and post-LGAE CT scans were the same technique (i.e., non-contrast CT or CT angiogram for both the preintervention CT and postintervention CT, respectively).
Portal venous phase CT scans were performed using 140–200 mL of intravenous Iohexol 300 based on weight, 120 kVp, 300 mAs (quality reference), 0.8 pitch, 0.5 second rotation time, and 64 × 0.6 collimation, with images acquired after a 70 second delay from the start of contrast administration. Noncontrast CT scans were performed with 120 kVp, 300 mAs (quality reference), 0.8 pitch, 0.5 second rotation time, and 64 × 0.6 collimation. The CT angiograms were performed using 140–200 mL of intravenous Iohexol 350 based on weight, 120 kVp, 350 mAs (quality reference), 0.8 pitch, 0.5 second rotation time, and 64 × 0.6 collimation. Image acquisition was triggered once the attenuation of the aorta surpassed the enhancement threshold of 150 Hounsfield units (HU).
Body composition analysis
Excess body weight (EBW) was determined based on the Lorentz formula for ideal body weight [11]:
For males:
For females:
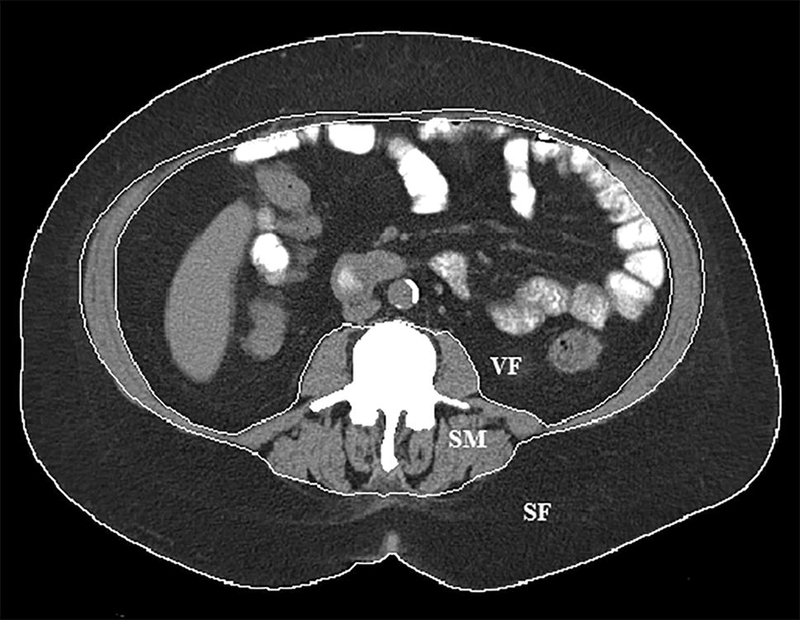
The CT body composition parameters were analyzed at the L1, L3 and L5 lumbar levels using semiautomated imaging processing algorithms (MATLAB 13.0, Math Works, MA) as previously described [12]. Measurements were performed by a single investigator blinded to clinical data and outcome. Adipose tissue and lean skeletal muscle area at each lumbar level were measured using threshold attenuation values between −190 to −30 HU and −29 to +150 HU, respectively. Subcutaneous fat area was determined using the outer boundary of the abdominal wall muscles and paraspinal muscles (Fig. 2). Visceral fat area was determined using the inner boundary of abdominal wall muscle sand paraspinal muscles. Skeletal muscle area was determined using the paraspinal muscles and abdominal wall muscles. Intramuscular fat area was determined using the threshold attenuation values for fat within the skeletal muscle compartments. Total body fat area was calculated by the summation of subcutaneous, visceral and intramuscular fat areas. The adipose tissue area and lean skeletal muscle area in each boundary were recorded as cm2. These tissue areas were then divided by body surface area to establish index values. The total body fat index (BFI), subcutaneous fat index (SFI), visceral fat index (VFI), intramuscular fat index (IMFI), and skeletal muscle index (SMI) were determined ([tissue area (cm)]2/[height (m)]2) at each lumbar level and summed.
Fig. 2.
Body composition segmentation at the L3 lumbar vertebral body level. Boundaries for subcutaneous fat (SF), visceral fat (VF) and skeletal muscle (SM) are delineated by the white lines.
Statistical analysis
Statistical analyses were performed using SPSS software version 22 (SPSS Inc., Chicago, IL). Changes in weight and body composition were analyzed with either Wilcoxon signed-rank test or paired Student’s t tests based on the normality of the distributions. A P value < 0.05 was considered statistically significant.
Results
Mean follow-up was 1.5 ± 0.8 months. Among the 16 patients in this study, 11 (68.8%) bleeds were caused by gastric ulcers, 3 (18.8%) bleeds were from traumatic vascular injury, and 2 (12.5%) bleeds were secondary to complications from acute pancreatitis.
Changes in body composition after LGAE are summarized in Table 2. Mean weight and body composition parameters pre-LGAE vs. post-LGAE as well as per cent changes were calculated. Statistically significant changes were observed in body weight (87.9±12.5 vs. 82.3±13.9 kg, −6.4%, p=0.003), BMI (30.0±4.3 vs. 28.3±4.9 kg/m2, −6.3%, p=0.005), EBW (23.3±10.6 vs. 17.7±12.6 kg, −24.1%, p=0.003).
Table 2.
Body weight and CT body composition changes after left gastric artery embolization
| Pre-LGAE | Post-LGAE | % Change | P | |
|---|---|---|---|---|
| Body weight, mean (SD), kg | 87.9(12.5) | 82.3(13.9) | −6.4 | 0.003 |
| Body mass index, mean (SD), kg | 30.0(4.3) | 28.1(4.9) | −6.3 | 0.005 |
| Excess body weight, mean (SD), kg | 23.3(10.6) | 17.7(12.6) | −24.1 | 0.003 |
| Total body fat index, mean (SD), cm2/m2 | 128.6(54.6) | 123.9(59.5) | −3.7 | 0.03 |
| Subcutaneous fat index, mean (SD), cm2/m2 | 81.7(44.5) | 78.4(43.7) | −4.1 | 0.03 |
| Visceral fat index, mean (SD), cm2/m2 | 35.8(17.8) | 34.3(21.6) | −4.2 | 0.13 |
| Intramuscular fat index, mean (SD), cm2/m2 | 10.2(4.8) | 10.1(4.7) | −1.6 | 0.83 |
| Skeletal muscle index, mean (SD), cm2/m2 | 44.5(7.2) | 41.5(6.9) | −6.9 | <0.001 |
LGAE=left gastric artery embolization
Regarding body composition parameters, statistically significant changes were seen in BFI (128.6±54.7 vs 123.9±59.5 cm2/m2, −3.7%, p=0.03), SFI (81.7±44.5 vs. 78.4±43.7 cm2/m2, −4.1%, p=0.03), and SMI (44.5±7.2 vs. 41.5±6.9 cm2/m2, −6.8%, p<0.001). Visceral fat index did not significantly change after LGAE (35.8±17.8 vs. 34.3±21.6 cm2/m2, −4.1%, P=0.13). Similarly, no statistically significant change in IMFI was found (10.2 ± 4.8 vs. 10.1 ± 4.7, −1.6%, p=0.83).
Discussion
Body composition analysis with CT demonstrated significant decreases in BFI, SFI, and SMI after LGAE. These findings indicate that changes in body composition are not evenly distributed throughout the abdomen. Studies have shown that decreased caloric intake results in the activation of complex metabolic pathways and metabolism may vary in different adipose tissue compartments [13,14]. As a result, the decrease in tissue mass correlating to weight loss does not necessarily occur symmetrically throughout the body nor does it solely affect adipose tissue. The findings in this study emphasize two important issues. First, patients who undergo LGAE for gastric bleeding are at high risk for unintended weight loss and deconditioning, which demands proactive monitoring. Second, the potential use of LGAE as an elective weight loss procedure may place patients at risk for sarcopenia and suggests the need for a comprehensive weight management system to ensure optimal outcomes.
Patients in this study underwent LGAE for the indication of acute gastric bleeding, which is a different population than those who seek elective bariatric embolization. Patients in this study, despite being overweight or obese, had lower BMI than typical bariatric candidates. Potential weight loss from acute bleeding, immobilization, or chronic hospitalization may be confounding factors affecting the observed changes in body weight and composition.
Comparison data for the effect of LGAE or bariatric interventions on CT body composition are limited at present. However, it is well-established that significant muscle mass loss is common after weight loss procedures [15,16]. Maimoun and colleagues investigated body composition changes 1 month after sleeve gastrectomy using dual-energy x-ray absorptiometry [17]. They found that percent lean tissue mass loss was greater than fat mass loss in the trunk (9.7% vs 9.5%, respectively) and in whole body composition (9.4% vs 8.3%, respectively). However, the lean tissue mass to fat mass ratio did not significantly change from preintervention levels. As a result of their findings, the authors concluded that early preventive strategies to minimize lean tissue mass loss need to be implemented in bariatric patients. Vassilev et al also found that lean body mass decreased significantly (p<0.001) from 74.7 kg to 63.9 kg at 1 year after bariatric surgery as determined by bioelectrical impedance [18]. In the present study, SMI decreased significantly by approximately 7% after LGAE, greater than the observed changes in BFI, SFI and VFI, and similar to the findings by Maimoun et al [17]. Thus, patients who undergo LGAE, regardless of whether the indication is for upper gastrointestinal bleeding or for weight loss, should receive active care to minimize lean tissue loss postprocedurally.
Patients who underwent LGAE lost a significant amount of body weight, EBW, and BMI. These findings are consistent with previously reported data from the BEAT Obesity trial, which is investigating the efficacy of bariatric embolization [2]. Preliminary data reported by Weiss et al showed mean excess weight loss of 5.9% and 9.0% and 1 month and 3 months, respectively, among patients with a mean BMI of 43.8 kg/m2. The mean EBW loss of 24.1% in the present study was similar to previously reported data on EBW loss of approximately 20% at 1–2 months after bariatric surgery [18,19]. Patients who underwent LGAE had lower mean BMI than patients in these prior studies, which may have contributed to the higher EBW loss observed. Given the short follow-up in this study, the long-term effects on weight loss are unclear. Long-term weight regain has been shown to occur in approximately 50% of patients who undergo gastric bypass within 2 years [20].
The study was limited by its retrospective design, small sample size and inconsistent patient follow-up. Additionally, the follow-up period in this study was only an average of 1.5 months. Any long-term outcome related to left gastric artery embolization is unclear. Furthermore, the effect on comorbidities in this cohort remains indeterminate. However, the aim of this study was to evaluate body composition change after LGAE. The efficacy of LGAE as a bariatric procedure will be best determined through randomized clinical trials.
In conclusion, patients who underwent LGAE for gastric bleeding experienced significant weight loss, which was associated with both decreased abdominal fat and lean skeletal muscle in the short-term follow-up of this study. Alterations in tissue mass after LGAE do not occur symmetrically and are inadequately reflected by changes in gross weight alone. Body composition analysis can readily assess the extent of fat loss as well as identify unintended muscle wasting, which can then be managed appropriately.
Table 1.
Baseline patient characteristics
| LGAE cohort (N=16) | |
|---|---|
| Female Sex, n (%) | 7 (44) |
| Age, mean (SD), years | 57.6 (12.9) |
| Diabetes mellitus, n (%) | 5 (31.2) |
| Coronary artery disease, n (%) | 6 (37.5) |
| Hypertension, n (%) | 9 (56.2) |
| Chronic obstructive pulmonary disease, n (%) | 3 (18.8) |
| Chronic kidney disease, n (%) | 3 (18.8) |
| Time interval between CT scans, mean (SD), months | 1.5 (0.8) |
| Body weight, mean (SD), kg | 87.9 (12.5) |
| Body mass index, mean (SD), kg | 30.0 (4.3) |
| Excess body weight, mean (SD), kg | 23.3 (10.6) |
| Total body fat index, mean (SD), cm2/m2 | 128.6 (54.6) |
| Subcutaneous fat index, mean (SD), cm2/m2 | 81.7 (44.5) |
| Visceral fat index, mean (SD), cm2/m2 | 35.8 (17.8) |
| Intramuscular fat index, mean (SD), cm2/m2 | 10.2 (4.8) |
| Skeletal muscle index, mean (SD), cm2/m2 | 44.5 (7.2) |
Disclosures:
Sanjay Misra receives funding from National Institutes of Health Grant HL098967 from the National Heart, Lung, and Blood Institute and DK107870 from the National Institute of Diabetes and Digestive and Kidney Diseases.
References
- 1.Weiss CR, Gunn AJ, Kim CY, Paxton BE, Kraitchman DL, Arepally A (2015) Bariatric Embolization of the Gastric Arteries for the Treatment of Obesity. J Vasc Interv Radiol 26 (5):613–624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weiss CR, Akinwande O, Paudel K, Cheskin LJ, Holly B, Hong K, Fischman AM, Patel RS, Shin EJ, Steele KE, Moran TH, Kaiser K, Park A, Shade DM, Kraitchman DL, Arepally A (2017) Clinical Safety of Bariatric Arterial Embolization: Preliminary Results of the BEAT Obesity Trial. Radiology 283 (2):598–608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arepally A, Barnett BP, Montgomery E, Patel T (2007) Catheter-directed gastric artery chemical embolization for modulation of systemic ghrelin levels in a porcine model: Initial experience. Radiology 244 (1):138–143 [DOI] [PubMed] [Google Scholar]
- 4.Diana M, Pop R, Beaujeux R, Dallemagne B, Halvax P, Schlagowski I, Liu YY, Diemunsch P, Geny B, Lindner V, Marescaux J (2015) Embolization of arterial gastric supply in obesity (EMBARGO): an endovascular approach in the management of morbid obesity. proof of the concept in the porcine model. Obes Surg 25 (3):550–558 [DOI] [PubMed] [Google Scholar]
- 5.Gunn AJ, Oklu R (2014) A preliminary observation of weight loss following left gastric artery embolization in humans. J Obes 2014:185349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paxton BE, Kim CY, Alley CL, Crow JH, Balmadrid B, Keith CG, Kankotia RJ, Stinnett S, Arepally A (2013) Bariatric Embolization for Suppression of the Hunger Hormone Ghrelin in a Porcine Model. Radiology 266 (2):471–479 [DOI] [PubMed] [Google Scholar]
- 7.Andreoli A, Garaci F, Cafarelli FP, Guglielmi G (2016) Body composition in clinical practice. Eur J Radiol 85 (8):1461–1468 [DOI] [PubMed] [Google Scholar]
- 8.Graffy PM, Pickhardt PJ (2016) Quantification of hepatic and visceral fat by CT and MR imaging: relevance to the obesity epidemic, metabolic syndrome and NAFLD. Br J Radiol 89 (1062):20151024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gibson DJ, Burden ST, Strauss BJ, Todd C, Lal S (2015) The role of computed tomography in evaluating body composition and the influence of reduced muscle mass on clinical outcome in abdominal malignancy: a systematic review. Eur J Clin Nutr 69 (10):1079–1086 [DOI] [PubMed] [Google Scholar]
- 10.Samara A, Ventura EE, Alfadda AA, Goran MI (2012) Use of MRI and CT for fat imaging in children and youth: what have we learned about obesity, fat distribution and metabolic disease risk? Obes Rev 13 (8):723–732 [DOI] [PubMed] [Google Scholar]
- 11.Budzyński J, Tojek K, Czerniak B, Banaszkiewicz Z (2016) Scores of nutritional risk and parameters of nutritional status assessment as predictors of in-hospital mortality and readmissions in the general hospital population. Clin Nutr 35 (6):1464–1471 [DOI] [PubMed] [Google Scholar]
- 12.Kirihara Y, Takahashi N, Hashimoto Y, Sclabas GM, Khan S, Moriya T, Sakagami J, Huebner M, Sarr MG, Farnell MB (2013) Prediction of Pancreatic Anastomotic Failure After Pancreatoduodenectomy The Use of Preoperative, Quantitative Computed Tomography to Measure Remnant Pancreatic Volume and Body Composition. Ann Surg 257 (3):512–519 [DOI] [PubMed] [Google Scholar]
- 13.Laferrere B, Reilly D, Arias S, Swerdlow N, Gorroochurn P, Bawa B, Bose M, Teixeira J, Stevens RD, Wenner BR, Bain JR, Muehlbauer MJ, Haqq A, Lien L, Shah SH, Svetkey LP, Newgard CB (2011) Differential Metabolic Impact of Gastric Bypass Surgery Versus Dietary Intervention in Obese Diabetic Subjects Despite Identical Weight Loss. Sci Transl Med 3 (80) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dadson P, Ferrannini E, Landini L, Hannukainen JC, Kalliokoski KK, Vaittinen M, Honka H, Karlsson HK, Tuulari JJ, Soinio M, Salminen P, Parkkola R, Pihlajamäki J, Iozzo P, Nuutila P (2017) Fatty acid uptake and blood flow in adipose tissue compartments of morbidly obese subjects with or without type 2 diabetes: effects of bariatric surgery. Am J Physiol Endocrinol Metab 313 (2):E175–E182 [DOI] [PubMed] [Google Scholar]
- 15.Katsanos CS, Madura JA, Roust LR (2016) Essential amino acid ingestion as an efficient nutritional strategy for the preservation of muscle mass following gastric bypass surgery. Nutrition 32 (1):9–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carnero EA, Dubis GS, Hames KC, Jakicic JM, Houmard JA, Coen PM, Goodpaster BH (2017) Randomized trial reveals that physical activity and energy expenditure are associated with weight and body composition after RYGB. Obesity 25 (7):1206–1216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maimoun L, Lefebvre P, Jaussent A, Fouillade C, Mariano-Goulart D, Nocca D (2017) Body composition changes in the first month after sleeve gastrectomy based on gender and anatomic site. Surg Obes Relat Dis 13 (5):780–787 [DOI] [PubMed] [Google Scholar]
- 18.Vassilev G, Hasenberg T, Krammer J, Kienle P, Ronellenfitsch U, Otto M (2017) The Phase Angle of the Bioelectrical Impedance Analysis as Predictor of Post-Bariatric Weight Loss Outcome. Obes Surg 27 (3):665–669 [DOI] [PubMed] [Google Scholar]
- 19.Hutcheon DA, Hale AL, Ewing JA, Miller M, Couto F, Bour ES, Cobb WS, Scott JD (2018) Short-Term Preoperative Weight Loss and Postoperative Outcomes in Bariatric Surgery. J Am Coll Surg 226 (4):514–524 [DOI] [PubMed] [Google Scholar]
- 20.Magro DO, Geloneze B, Delfini R, Pareja BC, Callejas F, Pareja JC (2008) Long-term weight regain after gastric bypass: a 5-year prospective study. Obes Surg 18 (6):648–651 [DOI] [PubMed] [Google Scholar]